Abstract
Hyperglycemia induces retinal pigmented epithelial cell apoptosis and mitochondrial stress via poorly understood mechanisms. The goal of our current study is to explore whether mammalian sterile 20-like kinase 1 (Mst1) is involved in the pathogenesis of hyperglycemia-mediated retinal pigmented epithelial cell apoptosis by triggering mitochondrial abnormalities and activating the Smad2 signaling pathway. Retinal pigmented epithelial ARPE-19 cells were presented with a high-glucose challenge in vitro. Cell viability and apoptosis were measured via western blotting, ELISAs, and immunofluorescence assays. Mitochondrial function was detected via JC-1 staining, mitochondrial ROS flow cytometry, western blotting, and ELISAs. Loss- and gain-of-function assays were performed via cell transfection and transduction with Mst1 siRNA and Smad2 adenovirus, respectively. The results indicated that hyperglycemia treatment upregulated the levels of Mst1, an effect that was accompanied by an increase in ARPE-19 cell apoptosis. Loss of Mst1 attenuated hyperglycemia-induced cell apoptosis, and this effect seemed to be associated with mitochondrial protection. In response to hyperglycemia stimulus, mitochondrial stress was noted in ARPE-19 cells, including mitochondrial ROS overproduction, mitochondrial respiratory metabolism dysfunction, mitochondrial fission/fusion imbalance, and mitochondrial apoptosis activation. Further, we provided evidence to support the crucial role played by Smad2 in promoting Mst1-mediated cell apoptosis and mitochondrial stress. Overexpression of Smad2 abrogated the beneficial effects of Mst1 deletion on ARPE-19 cell viability and mitochondrial protection. Altogether, our results identified Mst1 as a novel mediator controlling the fate of retinal pigmented epithelial cells and mitochondrial homeostasis via the Smad2 signaling pathway. Based on this finding, strategies to repress Mst1 upregulation and block Smad2 activation are vital to alleviate hyperglycemia-mediated retinal pigmented epithelial cell damage.
Keywords: Retinal pigmented epithelial cell, Mitochondria, Mst1, Smad2
Introduction
Diabetic retinopathy (DR), known as a diabetic microvascular complication, is one of the main causes of blindness globally (Bikfalvi 2017). Chronic hyperglycemia stress induces blockade of tiny blood vessels and progressively causes retinal ischemia and nutrient deficiencies. Massive apoptosis of retinal pigmented epithelial cells contributes to micro-aneurysm formation, which is closely followed by abnormal blood vessel proliferation and intraretinal hemorrhages, gradually leading to vision impairment (Blackburn et al. 2017). Although several attempts have been made to understand the pathogenesis of DR, the precise molecular mechanism underlying the hyperglycemia-mediated retinal pigmented epithelial cell apoptosis has not been adequately explored (Zhu et al. 2016).
Mammalian sterile 20-like kinase 1 (Mst1) has been originally reported as the apoptotic inducer for several types of cells, such as cardiac microvascular endothelial cells, HepG2 hepatocellular carcinoma cells, neural stem cells, and aortic dissection smooth muscle cells (Buijs et al. 2017; Das et al. 2017; Hambright et al. 2017). Subsequent studies further report that Mst1 is primarily activated by chronic high-glucose stress and that increased Mst1 causes islet cell dysfunction, promotes diabetic cardiomyopathy, and inhibits angiogenesis (Gao et al. 2017; Yang et al. 2017). These information hint to us that Mst1 activation might play a key role in the development of hyperglycemia-mediated retinal pigmented epithelial cell apoptosis and DR progression. However, this notion remains to be confirmed (Kalyanaraman 2017).
At the molecular level, chronic hyperglycemia promotes excessive accumulation of glucose in retinal pigmented epithelial cells (Chang et al. 2017a; Conradi et al. 2017). Increased glucose metabolism enhances ROS production in the mitochondrion, and this process evokes cell oxidative stress. Moreover, to rapidly breakdown glucose, mitochondria divide into several fragments via mitochondrial fission (Sheng et al. 2018). However, abnormal mitochondrial fission produces immature daughter mitochondria with fragmentary mitochondrial DNA and mitochondrial respiratory complex deficiency (Zhou et al. 2017b), ultimately impairing cellular energy metabolism. More severely, aberrant mitochondrial fission activates the caspase-9-related mitochondrial death pathway (Han et al. 2017; Kozlov et al. 2017), leading to loss of functional cells. In the development of DR, mitochondrial stress, such as mitochondrial oxidative stress, mitochondrial DNA base mismatch, mitochondrial autophagy delay, and mitochondrial metabolism reprogramming, have been reported (Ghiroldi et al. 2017; Giatsidis et al. 2018; Iggena et al. 2017). There is strong evidence supporting the role of mitochondria in controlling the fate of retinal pigmented epithelial cells, suggesting that further studies should be conducted to fully explore the upstream mediators of mitochondrial stress under high-glucose stimulus.
The Smad pathway is a classical pathway responsible for hyperglycemia-mediated epithelial-mesenchymal transition in human retinal pigment epithelium cells (Lee et al. 2017; Lee and Back 2017). Moreover, activated Smad promotes retinal fibrosis. In addition, robust data from animal studies and cell experiments have demonstrated a strong correlation between Smad activation and mitochondrial injury in various disease models, such as angiotensin II-induced renal tubular epithelial cell damage, glioblastoma multiforme metastasis, fatty liver disease, and uric acid-mediated kidney inflammation response models (Hong et al. 2017; Romero et al. 2017). Mechanistically, Smad2 has been shown to be a transcription factor that regulates gene expression related to mitochondrial dynamics (Hassanshahi et al. 2017; Hooshdaran et al. 2017). In addition, Smad2 indirectly affects mitochondrial function by repressing mitochondrial Sirt3 activity and improving ROS production (Liu and Desai 2015; Zhou et al. 2018d). However, little evidence is available to explain the detailed role played by Smad2 in hyperglycemia-mediated mitochondrial stress. Altogether, the aim of our study was to determine whether Mst1 modulates the pathogenesis of hyperglycemia-mediated retinal pigmented epithelial cell apoptosis by inducing mitochondrial stress in a manner dependent on the Smad2 signaling pathway (Zhou et al. 2018g).
Methods and material
Cell culture and treatment
Retinal pigmented epithelial ARPE-19 cells (ATCC® CRL-2302™) were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10% FBS under 37 °C/5% CO2 conditions. Control group cells were cultured in normal glucose medium (5.5 mmol/L). To induce high-glucose stress, high-glucose medium (25 mmol/L) was applied for 12 h. Meanwhile, cells cultured in mannitol medium (20 mmol/l mannitol + 5.5 mmol/l glucose, Sigma-Aldrich, USA) served as the control group for high-glucose stress. To inhibit Mst1 expression, ARPE-19 cells were transfected with siRNA against Mst1. In addition, Mst1-deleted cells were infected with Smad2 adenovirus to induce Smad2 overexpression (Kelly et al. 2017).
Western blotting analysis
Cells were scraped in RIPA lysis buffer (Beyotime, Shenzhen, Guangdong, China). The lysates (50–70 μg) were separated by 10% SDS-polyacrylamide gel (10–15%) electrophoresis (SDS-PAGE). Proteins were electrotransferred onto the pure nitrocellulose blotting membrane (Life Sciences) (Millipore, Bedford, MA, USA) and then blocked with 5% nonfat milk for 2 h at room temperature (Ackermann et al. 2017). After washing with TBST three times, the membranes were incubated at 4 °C overnight with the following primary antibodies: Bcl2 (1:1000, Cell Signaling Technology, #3498), Bax (1:1000, Cell Signaling Technology, #2772), caspase-9 (1:1000, Cell Signaling Technology, #9504), pro-caspase-3 (1:1000, Abcam, #ab13847), cleaved caspase-3 (1:1000, Abcam, #ab49822), survivin (1:1000, Cell Signaling Technology, #2808), complex III subunit core (CIII-core2, 1:1000, Invitrogen, #459220), complex II (CII-30, 1:1000, Abcam, #ab110410), complex IV subunit II (CIV-II, 1:1000, Abcam, #ab110268), Drp1 (1:1000, Abcam, #ab56788), Fis1 (1:1000, Abcam, #ab71498), Opa1 (1:1000, Abcam, #ab42364), Mfn2 (1:1000, Abcam, #ab56889), Mff (1:1000, Cell Signaling Technology, #86668), Mst1 (1:1000, Cell Signaling Technology, #3682), Tom20 (1:1000, Abcam, #ab186735), and Smad2 (1:1000, Abcam, #ab33875). After washing with TBST, the membranes were incubated with horseradish peroxidase-coupled secondary antibodies (1:2000; cat. nos. 7074 and 7076; Cell Signaling Technology, Inc.) for 1 h at room temperature. GAPDH was used as a reference to calculate the expression of the target proteins. The proteins were visualized using Pierce enhanced chemiluminescence western blotting substrate (Pierce; Thermo Fisher Scientific, Inc.) and autoradiography. Subsequently, the blots were analyzed using Quantity One 4.6 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) (Karwi et al. 2017; Liu et al. 2017a). The experiments were performed in triplicate and repeated three times with similar results.
ELISA
To analyze changes in caspase-9, caspase-9 activity kits (Beyotime Institute of Biotechnology, China; Catalog No. C1158) were used according to the manufacturer’s protocol. In brief, to measure caspase-9 activity, 5 μl of LEHD-p-NA substrate (4 mM, 200 μM final concentration) was added to the samples for 1 h at 37 °C. Then, the absorbance at 400 nm was recorded via a microplate reader to reflect the caspase-3 and caspase-9 activities. To analyze caspase-3 activity, 5 μl of DEVD-p-NA substrate (4 mM, 200 μM final concentration) was added to the samples for 2 h at 37 °C. The levels of antioxidant factors, including GPX, SOD, and GSH, were measured with ELISA kits purchased from the Beyotime Institute of Biotechnology (Blackburn et al. 2017). The experiments were performed in triplicate and repeated three times with similar results.
Immunofluorescence
Cells were washed twice with PBS, permeabilized in 0.1% Triton X-100 overnight at 4 °C. After the fixation procedure, the sections were cryoprotected in a PBS solution supplemented with 0.9 mol/l of sucrose overnight at 4 °C. The primary antibodies used in the present study were as follows: Tom20 (1:1000, Abcam, #ab186735) and Smad2 (1:1000, Abcam, #ab33875). Subsequently, samples were incubated with Alexa Fluor 488 donkey anti-rabbit secondary antibody (1:1000; cat. no. A-21206; Invitrogen; Thermo Fisher Scientific, Inc.) for ~ 1 h at room temperature. DAPI was used to label the nuclei, and images were captured using an inverted microscope (magnification, × 40; BX51; Olympus Corporation, Tokyo, Japan) (Zhou et al. 2018i).
Quantitative real-time PCR
For mRNA expression analysis, total RNA was isolated using Trizol (Invitrogen, Carlsbad, California, USA) according to a previous study. Then, cDNA was synthesized using 1 mg RNA and the First-Strand Synthesis Kit (Fermentas, Flamborough, Ontario, Canada) according to a previous study (Zhou et al. 2018f; Zhu et al. 2018a). The cycling conditions were as follows: 92 °C for 7 min, 40 cycles of 95 °C for 20 s, and 70 °C for 45 s. β-actin was amplified as an internal standard. All primer sequences are listed below: Drp1 (forward primer 5′-CATGGACGAGCTGGCCTTC-3′, reverse primer 5′-ATCCTGTAGTGATGTATCAGG-3′), Fis1 (forward primer 5′-TGTCCAGTCCGTAACTGAC-3′, reverse primer 5′-TTCGATACCTGACTTAC-3′), Mfn2 (forward primer 5′-CCTCTTGATCCTGATCTTAACGT-3′, reverse primer 5′-GGACTACCTGATTGTCATTC-3′), Mff (forward primer 5′-ATGCAGACAATTAAGTGTGTTGTTGTGGGCGA-3′, reverse primer 5′-reverse primer, TCATAGCAGCACACACCTGCGGCTCTTCTT-3′), OPA1 (forward primer 5′-GCTACTTGTGAGGTCGATTC-3′, reverse primer 5′-GCCGTATACCGTGGTATGTCTG-3′).
Mitochondrial potential analysis
To observe the mitochondrial potential, JC-1 staining (Thermo Fisher Scientific Inc., Waltham, MA, USA; catalog no. M34152) was used (Shi et al. 2018). Then, 10 mg/ml JC-1 was added to the medium for 10 min at 37 °C in the dark to label the mitochondria. Normal mitochondrial potential showed red fluorescence, and damaged mitochondrial potential showed green fluorescence (Zhou et al. 2018b).
Mitochondrial ROS analysis
Flow cytometry was applied as a quantitative method for evaluating mitochondrial ROS levels according to a previous study. Cells were seeded onto 6-well plates and then treated with erlotinib. Subsequently, the cells were isolated using 0.25% trypsin and then incubated with MitoSOX red mitochondrial superoxide indicator (Molecular Probes, USA) for 30 min in the dark at 37 °C. Subsequently, PBS was used to wash cell two times, and then the cells were analyzed with a FACSCalibur flow cytometer. Data were analyzed by FACS Diva software. The experiment was repeated three times to improve the accuracy (Kleinbongard et al. 2017; Korbel et al. 2018).
TUNEL staining and MTT assay
Apoptotic cells were detected with an In Situ Cell Death Detection Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA; catalog no. C1024) according to the manufacturer’s protocol. Briefly, cells were fixed with 4% paraformaldehyde at 37 °C for 15 min. Blocking buffer (3% H2O2 in CH3OH) was added to the wells, and then cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. The cells were incubated with TUNEL reaction mixture for 1 h at 37 °C. DAPI (Sigma-Aldrich, St. Louis, MO, USA) was used to counterstain the nuclei, and the numbers of TUNEL-positive cells were recorded. MTT was used to analyze the cellular viability. Cells (1 × 106 cells/well) were cultured on a 96-well plate at 37 °C with 5% CO2. Then, 40 μl of MTT solution (2 mg/ml; Sigma-Aldrich) was added to the medium for 4 h at 37 °C with 5% CO2. Subsequently, the cell medium was discarded, and 80 μl of DMSO was added to the wells for 1 h at 37 °C with 5% CO2 in the dark. The OD of each well was observed at A490 nm via a spectrophotometer (Epoch 2; BioTek Instruments, Inc., Winooski, VT, USA) (Li et al. 2018).
Transfections
Transfection with siRNA was used to inhibit Mff expression. Two independent siRNAs (siRNA1-Mst1 and siRNA2-Mst1, Yangzhou Ruibo Biotech Co., Ltd., Yangzhou, China) were used to infect cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol (Zhu et al. 2018b). Briefly, the cells were seeded onto 6-well plates and then incubated with Opti-Minimal Essential Medium (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h (Koentges et al. 2017). Then, Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific, Inc.) was added into the medium of cells and supplemented with 5 nmol/l siRNA solution. Transfection was performed for 48 h, and then the cells were collected. Western blotting was used to verify the transfection efficiency (Liu et al. 2017b).
Statistical analysis
Image intensity was quantified using Nikon NIS-Elements-AR software. ImageJ software (NIH, MD, USA) was used to quantify the protein levels on western blots. The data were analyzed using one-way ANOVA followed by Tukeyʼs test for post hoc analysis. The data are presented as the means ± SEM., and differences were deemed significant when p < 0.05.
Results
Mst1 deletion protects ARPE-19 cells against hyperglycemia-induced cell apoptosis
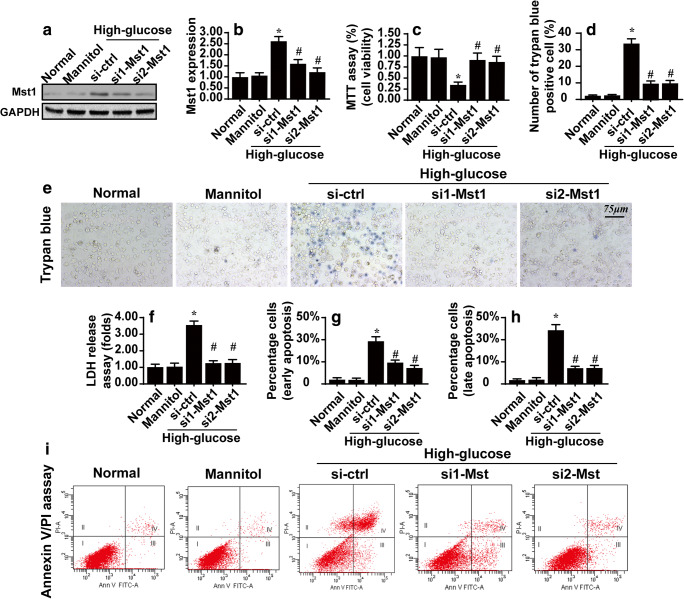
First, ARPE-19 cells were treated with high-glucose medium (25 mmol/l) for 12 h to induce hyperglycemic stress. Meanwhile, to rule out the effect of osmolarity on cell viability, mannitol was added to the medium of control group cells. Then, western blotting was performed to analyze the expression of Mst1. Compared with the control group and mannitol group, the expression of Mst1 was rapidly increased in response to high-glucose treatment (Fig. 1a–b). Subsequently, to establish a relationship between Mst1 upregulation and ARPE-19 cell viability, ARPE-19 cells were transfected with two independent siRNAs against Mst1, and the knockdown efficiency was confirmed via western blotting, as shown in Fig. 1a–b. After silencing of Mst1, the viability of ARPE-19 cells was determined via MTT assays and trypan blue staining. As shown in Fig. 1c–e, compared with the control and mannitol groups, high-glucose stimulus significantly reduced cell viability and increased the number of trypan blue-positive cells. Interestingly, hyperglycemia-mediated cell apoptosis was abolished by Mst1 knockdown, suggesting that Mst1 upregulation accounted for the hyperglycemia-induced apoptosis in ARPE-19 cells. This finding was further validated via an LDH release assay, which demonstrated Mst1 deletion attenuated hyperglycemia-mediated ARPE-19 cell damage (Fig. 1f). Subsequently, flow cytometry was used to further quantify cell apoptosis. As shown in Fig. 1g–i, compared with the control and mannitol groups, the apoptotic index of ARPE-19 cells was significantly increased upon exposure to hyperglycemia stimulus. Interestingly, both the early apoptosis rate and late apoptosis score was reduced by Mst1 deletion (Fig. 1g–i). Altogether, the above data indicate that Mst1, primarily activated by hyperglycemia stress, promotes ARPE-19 cell apoptosis.
Fig. 1.
Loss of Mst1 attenuates hyperglycemia-mediated ARPE-19 cell apoptosis. a–b In vitro, ARPE-19 cells were treated with normal glucose medium (5.5 mmol/l), high-glucose medium (25 mmol/l), or mannitol medium (5.5 mmol/l glucose + 20 mmol/l mannitol) for 12 h. Then, western blotting was performed to analyze changes in Mst1 expression. Meanwhile, ARPE-19 cells were also transfected with siRNAs against Mst1, and western blotting was performed to confirm the siRNAs knockdown efficiency after transfection. c MTT assays were used to observe cell viability after transfection with siRNAs in the setting of hyperglycemia stress. d–e Trypan blue staining for dead cells. ARPE-19 cells were treated with high-glucose stress and transfected with Mst1 siRNAs. Then, the number of trypan blue-positive cells was recorded. f LDH release assay for cell damage in response to Mst1 deletion. g–i Annexin/PI staining for apoptotic cells, followed by flow cytometry analysis. Early apoptosis (annexin+/PI− cells) and late apoptosis (annexin+/PI+ cells) were measured. *p < 0.05
Mst1 activation causes mitochondrial energy metabolism disorder
Mitochondria are the energy centers of cell metabolism, and 90% of cellular ATP is supplied by mitochondria (Zhou et al. 2018h). However, compared with the control and mannitol groups, ATP production was significantly reduced in response to hyperglycemia stress (Fig. 2a), and this effect was reversed by Mst1 deletion. At the molecular level, mitochondria generate ATP via breakdown of glucose into NADH and electrons, which are captured by mitochondria to generate the mitochondrial membrane potential. Subsequently, with the help of the mitochondrial respiratory complex, mitochondrial membrane potential is converted into ATP, and electrons and NADH interact with each other to form H2O. To further observe the mitochondrial energy metabolism, the levels of glucose and lactic acid were measured using ELISAs. As shown in Fig. 2b–c, compared with the control and mannitol group, glucose uptake was obviously repressed by hyperglycemia stress, an effect that was accompanied by a decline in lactic acid production. However, loss of Mst1 reversed the glucose uptake and promoted lactic acid metabolism (Fig. 2b–c), suggesting that hyperglycemia-interrupted energy disorder could be reversed by Mst1 deletion.
Fig. 2.
Mitochondrial respiratory function is regulated by Mst1 under hyperglycemia stimulus. a Cellular ATP production was measured in ARPE-19 cells treated with hyperglycemia and/or transfected with Mst1 siRNA. b–c The levels of glucose uptake and lactic acid production were quantified via ELISAs. d–e Mitochondrial membrane potential was assessed using JC-1. The fluorescence intensity of the red-to-green ratio was used to quantify the mitochondrial membrane potential. ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. f–i After treatment, proteins were isolated, and the expression levels of mitochondrial respiratory complex components were determined using western blotting. ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs.*p < 0.05
In addition, we found that mitochondrial membrane potential was reduced in hyperglycemia-treated ARPE-19 cells (Fig. 2d–e), and this alteration was reversed via transfection with Mst1 siRNAs. Western blotting analysis also illustrated that the expression levels of mitochondrial respiratory complex components were drastically downregulated in response to hyperglycemia (Fig. 2f–i), indicating that high-glucose treatment disturbed mitochondrial respiratory function. However, loss of Mst1 reversed the activity of the mitochondrial respiratory complex. Altogether, these data indicate that hyperglycemia-induced mitochondrial metabolism disturbance is primarily regulated by Mst1 in ARPE-19 cells.
Mst1 elevates mitochondrial fission and represses mitochondrial fusion
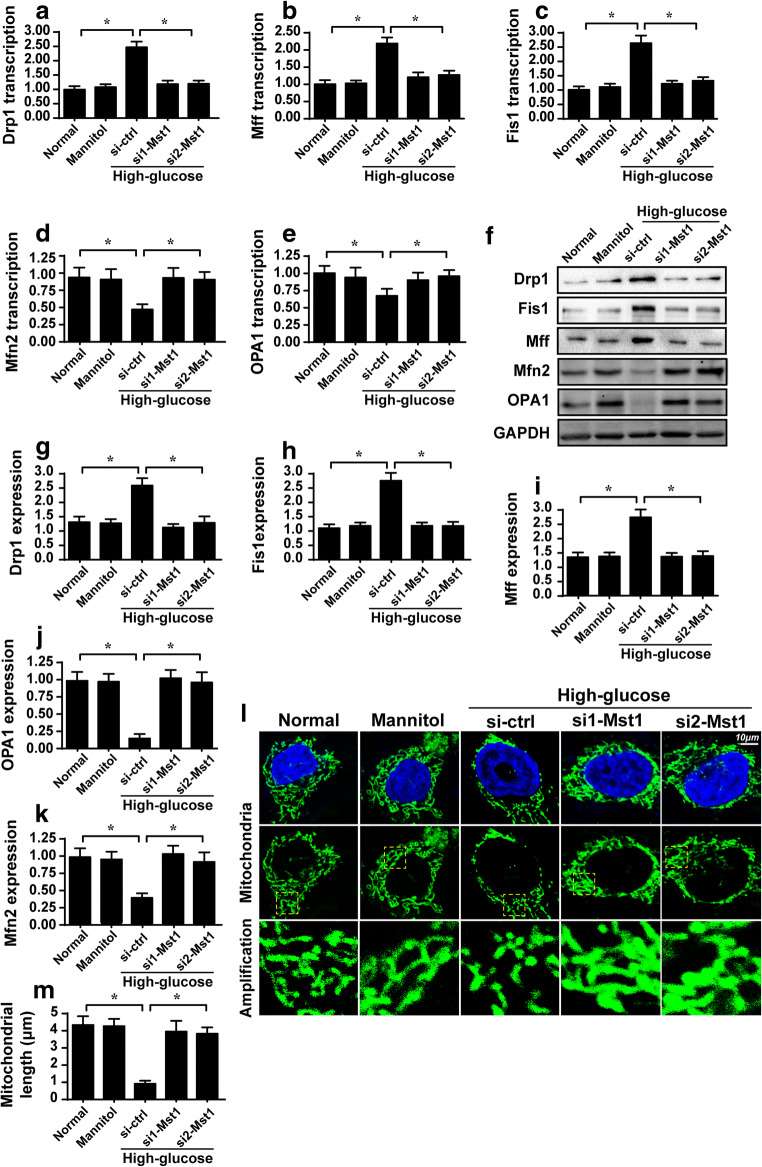
Previous studies have reported that mitochondrial fission is activated by hyperglycemia to produce additional daughter mitochondria to accelerate glucose metabolism (Zhou et al. 2018e). As a consequence of mitochondrial fission activation, interconnective mitochondria are converted to a massive number of fragmented mitochondria, which is harmful for cell viability. In the present study, we asked whether hyperglycemia caused mitochondrial fission in ARPE-19 cells via Mst1. First, qPCR and western blotting were used to detect the transcription and expression of pro-fission proteins. Compared with the control and mannitol groups, the transcription (Fig. 3a–e) and expression (Fig. 3f–k) of Drp1, Mff, and Fis1 were progressively upregulated in response to hyperglycemia. Interestingly, Mst1 deletion prevented upregulation of these proteins. In contrast to mitochondrial fission, mitochondrial fusion is a repair system that corrects abnormal mitochondrial fission. However, the parameters related to mitochondrial fusion, such as OPA1, Mfn1, and Mfn2, were significantly downregulated in hyperglycemia-treated cells compared to control and mannitol group cells, indicating that hyperglycemia impaired mitochondrial fusion. Interestingly, loss of Mst1 reversed the transcription (Fig. 3a–e) and expression (Fig. 3f–k) of mitochondrial fusion factors. Altogether, the above data indicate that mitochondrial fission is activated whereas mitochondrial fusion is repressed by hyperglycemia via Mst1.
Fig. 3.
Mst1 deletion corrects the imbalance between mitochondrial fission and fusion. a–e ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. Then, RNA was isolated, and the transcription of mitochondrial fission/fusion-related proteins was evaluated via qPCR. f–k Western blotting was used to analyze the expression of proteins related to mitochondrial fusion/fission. ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. l–m Immunofluorescence assay of mitochondria stained with Tom 20. The average length of mitochondria was used as a parameter to quantify mitochondrial fission/fusion. *p < 0.05
This finding was further supported by analyzing the average length of mitochondria. Compared with the control and mannitol groups, mitochondria length was reduced to ~ 1.3 μm after exposure to hyperglycemia (Fig. 3l–m). However, loss of Mst1 reversed the decrease in mitochondrial length, extending the average length to ~ 4.6 μm, despite treatment with high-glucose medium. In addition, we observed massive round and fragmented mitochondria in hyperglycemia-treated APRE-19 cells whereas this phenotypic alteration was reversed by Mst1 (Fig. 3l–m). Taken together, these results further confirm that hyperglycemia-modulated mitochondrial fission and fusion via Mst1.
Mst1 activates caspase-9-related mitochondrial apoptosis
Excessive mitochondrial fission has been verified to be associated with mitochondrial apoptosis activation (Nawaz et al. 2018; Zhou et al. 2018a). Based on this information, we observed the alterations in mitochondrial apoptosis in response to Mst1 deletion in the setting of hyperglycemia challenge. Early mitochondrial apoptosis is characterized by overproduction of mitochondrial ROS. With the help of flow cytometry, we found that the mitochondrial ROS levels were significantly increased in hyperglycemia-treated cells (Fig. 4a–b). However, loss of Mst1 attenuated the mitochondrial ROS overload. In addition, ELISAs were used to detect alterations in cellular antioxidant levels. Compared with the control and mannitol group, hyperglycemia treatment obviously reduced the levels of GSH, SOD, and GPX (Fig. 4c–e), suggesting redox imbalance under hyperglycemia stress. However, loss of Mst1 reversed the changes in GSH, SOD, and GPX content. In addition, a feature of late mitochondrial apoptosis is long-lasting opening of the mitochondrial permeability transition pore (mPTP). In the present study, we found that hyperglycemia treatment promoted opening of the mPTP, and this effect was abolished by Mst1 deletion (Fig. 4f). The above data indicated that mitochondrial apoptosis was activated by Mst1 at different stages. Last, western blotting analysis was carried out to evaluate the expression of mitochondrial apoptotic proteins under hyperglycemia stress and Mst1 deletion. As shown in Fig. 4g–l, compared with the control group, the expression of pro-apoptotic factors, such as caspase-9, capsase-3, and Bax, was significantly increased in response to high-glucose treatment. In contrast, the levels of anti-apoptotic factors, including Bcl-2 and survivin (Fig. 4g–l), were rapidly downregulated upon exposure to high-glucose conditions. Notably, loss of Mst1 corrected the anti-apoptotic protein expression and prevented upregulation of pro-apoptotic factors (Fig. 4g–l). Altogether, these results indicate that caspase-9-related mitochondrial apoptosis is activated by hyperglycemia via Mst1 in APRE-19 cells.
Fig. 4.
Mst1 initiates the caspase-9-related mitochondrial apoptotic pathway. a–b Flow cytometry analysis of mitochondrial ROS production. ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. c–e The concentrations of cellular antioxidants, specifically, GSH, SOD, and GPX, were measured using ELISAs in ARPE-19 cells treated with hyperglycemia and/or transfected with Mst1 siRNAs. f The mPTP opening rate in ARPE-19 cells under hyperglycemia treatment and with Mst1 deletion. g–l ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. Then, proteins were isolated, and the expression of mitochondrial apoptosis pathway components was analyzed. *p < 0.05
Smad2 was activated by Mst1 and contributed to hyperglycemia-mediated ARPE-19 cell apoptosis
Subsequently, we explored the mechanism by which Mst1 modulates ARPE-19 cell viability and mitochondrial homeostasis. Previous studies have proposed that Smad2 is a key signal transmitter in the setting of hyperglycemia stress (Koentges et al. 2017; Koopman et al. 2017). Based on this, we asked whether Smad2 is required for Mst1-modulated ARPE-19 apoptosis and mitochondrial stress. First, western blotting demonstrated that the expression of Smad2 was rapidly increased in response to hyperglycemia, and this effect was repressed by Mst1 deletion (Fig. 5a–b). These data suggested that Mst1 could be considered an upstream regulator of Smad2 in the setting of hyperglycemia stress. This finding was further supported via immunofluorescence assays. The fluorescence intensity of Smad2 (Fig. 5c–d) was rapidly elevated upon exposure to hyperglycemia. Subsequently, to verify whether Smad2 is necessary for Mst1-mediated ARPE-19 cell apoptosis, ARPE-19 cells were infected with Smad2-carrying adenovirus. The Smad2 overexpression efficiency was verified via western blotting (Fig. 5a–b) and an immunofluorescence assay (Fig. 5c–d). Then, cell viability was measured via MTT assays in response to Smad2 overexpression. As shown in Fig. 5e, compared with the control and mannitol groups, hyperglycemia-repressed cell viability was reversed by Mst1 deletion. However, overexpression of Smad2 reduced cell viability despite Mst1 deletion (Fig. 5e). These findings were further validated via TUNEL staining. As shown in Fig. 5f–g, after treatment with high-glucose stress, the percentage of TUNEL-positive cells increased by ~ 46% compared with the control and mannitol group cells. However, loss of Mst1 attenuated the cell death ratio whereas Smad2 overexpression elevated the TUNEL-positive ratio compared with the hyperglycemia group (Fig. 5f–g). Altogether, these data indicate that Smad2 is activated by hyperglycemia and contributes to ARPE-19 cell apoptosis.
Fig. 5.
Hyperglycemia increases Smad2 expression via Mst1 and a higher Smad2 level is associated with increased ARPE-19 cell apoptosis. a–b ARPE-19 cells were treated with hyperglycemia and/or transfected with Mst1 siRNAs. Then, the expression of Smad2 was determined via western blotting. Smad2 adenovirus was used to induce Smad2 overexpression in Mst1-deleted cells. The overexpression efficiency was confirmed via western blotting. c–d Immunofluorescence assay for Smad2. The fluorescence intensity of Smad2 staining was recorded in ARPE-19 cells treated with hyperglycemia. e MTT assays for cell viability in response to Mst1 deletion and Smad2 overexpression in ARPE-19 cells. f–g TUNEL staining for apoptotic ARPE-19 cells. The number of TUNEL-positive cells was evaluated. *p < 0.05
Mst1-activated Smad2 also regulates mitochondrial stress
Finally, we sought to examine whether Smad2 was also involved in Mst1-modified mitochondrial stress under hyperglycemia conditions. First, mitochondrial ROS production was detected via flow cytometry. Compared with the control and mannitol groups, the mitochondrial ROS levels were progressively increased in response to hyperglycemia stress, and this effect was negated by Mst1 deletion (Fig. 6a). Interestingly, overexpression of Smad2 re-induced mitochondrial ROS overload (Fig. 6a). Subsequently, ELISA assays demonstrated that hyperglycemia-mediated cellular antioxidant downregulation was reversed by Mst1 deletion (Fig. 6b–d), and this effect was abolished by Smad2 overexpression. In addition, hyperglycemia upregulated the transcription of pro-fission factors (Fig. 6e–f) and repressed the transcription of pro-fusion proteins (Fig. 6g–h). Mst1 deletion reversed the balance between mitochondrial fission and fusion factors in a manner dependent on Smad2 activity (Fig. 6e–h). In addition, mitochondrial apoptosis was measured by analyzing the activity of caspase-9. Notably, compared with the hyperglycemia group, the mitochondrial apoptosis protein caspase-9 was inactivated by Mst1 deletion (Fig. 6i), and this effect was negated by Smad2 overexpression. Altogether, our results indicate that Smad2 is also involved in Mst1-mediated mitochondrial stress in the setting of hyperglycemia challenge in ARPE-19 cells.
Fig. 6.
Smad2 is also involved in Mst1-mediated mitochondrial stress. a Flow cytometry analysis of mitochondrial ROS production. ARPE-19 cells were treated with hyperglycemia, transfected with Mst1 siRNA, and infected with Smad2 adenovirus. b–d The concentrations of the cellular antioxidants GSH, SOD, and GPX were measured using ELISAs in ARPE-19 cells treated with hyperglycemia, transfected with Mst1 siRNA, and infected with Smad2 adenovirus. e–h qPCR assay to examine mitochondrial fission/fusion proteins in ARPE-19 cells treated with hyperglycemia, transfected with Mst1 siRNA, and infected with Smad2 adenovirus. i Caspase-9 activity was determined via ELISA. The relative activity of caspase-9 is presented as the ratio of the activity in the experimental and control groups. *p < 0.05
Discussion
Chronic hyperglycemia stress promotes the death of retinal pigmented epithelial cells through poorly understood mechanisms. In the present study, we provided evidence to support the key role played by Mst1 in inducing apoptosis of retinal pigmented epithelial cells via induction of mitochondrial stress in vitro. We reported that high-glucose treatment upregulated the expression of Mst1 that accounted for mitochondrial dysfunction, including mitochondrial oxidative stress, mitochondrial energy undersupply, mPTP opening, mitochondrial fission upregulation, mitochondrial fusion repression, and mitochondrial apoptosis activation. Subsequently, our data further demonstrated that Mst1 modulated mitochondrial stress and retinal pigmented epithelial cell apoptosis by enhancing Smad2 expression. As far as we know, this is the first study to describe the functional roles of the Mst1-Smad2 signaling pathways in regulating mitochondrial homeostasis and apoptosis in retinal pigmented epithelial cells (Zhou et al. 2017a; Zhou et al. 2018b). Based on our results, approaches to repress Mst1 activation and Smad2 upregulation as well as strategies to sustain mitochondrial function are vital to retard or reverse DR development and progression.
The relationship between Mst1 and cell apoptosis has been extensively explored. For example, in diabetic cardiomyopathy, Mst1 is transferred from cardiac microvascular endothelial cells to cardiomyocytes and promotes the death of functional cardiomyocytes (Peterson et al. 2017). In lung cancer, overexpression of Mst1 affects the feedback loop of p53 and JNK and ultimately exacerbates cancer cell death (Landry et al. 2017). Moreover, Mst1 is a downstream effector of several anti-cancer drugs such as matrine (Cao et al. 2018) and taurine. Recently, careful studies from several laboratories have further demonstrated that Mst1 regulates cell viability by targeting mitochondria (Zhou et al. 2018c). An early report illustrates that Mst1 affects the copying and expression of mitochondrial threonyl-tRNA synthetase (Reddy et al. 2018). Subsequent research identifies an interaction between Mst1 and mitochondrial cyclophilin D (Morell et al. 2017). Recent studies further illustrate that Mst1 modulates mitochondrial apoptosis by inhibiting Bcl-xL phosphorylation and evoking mitochondrial oxidative stress (Yu et al. 2017). In addition, Mst1 disturbs mitophagy and promotes mitochondrial fission in endometriosis (Zhao et al. 2018) and diabetic cardiomyopathy (Zhai et al. 2017). Our observations are consistent with the previous findings (Jin et al. 2018; Li et al. 2018). The difference between our experiments and previously reported data is that we comprehensively explain the role played by Mst1 in mitochondrial stress, including mitochondrial energy metabolism, mitochondrial redox balance, mitochondrial fission/fusion, and mitochondrial apoptosis (Tenreiro et al. 2017). Besides, our findings, which uncovered the molecular mechanism involved in regulating retinal pigmented epithelial cell survival, may answer the following question: what is the key player in induction and aggravation of diabetic retinopathy? Therefore, from a therapeutic perspective, when designing drugs to treat DR, clinicians should bear in mind that Mst1-modified mitochondrial stress is of utmost importance.
Smad2 is a transcription factor that controls gene transcription related to proliferation and fibrosis. In diabetes development, Smad2 participates in muscle remodeling, myocardial fibrosis, glomerular endothelial cell apoptosis, retinal pigment epithelial cell fibrosis (Chang et al. 2017b), and islet β-cell apoptosis (Pryds et al. 2017). In addition, Smad2 induces mitochondrial stress in several disease models. For example, in human glioma cells, mitochondrial membrane potential and mitochondrial apoptosis is regulated via a Smad2-dependent pathway (Schluter et al. 2017). In chronic obstructive pulmonary disease, Smad2 is activated by miRNA-542-3p/5p and contributes to mitochondrial dysfunction (Turner et al. 2017), including mitochondrial oxidative stress and mitochondrial ribosomal dysfunction. Moreover, mitochondrial fusion is negatively regulated by the Smad2-RIN1-Mfn2 signaling pathway in neurodegenerative disorders (Zhou et al. 2017c). In the present study, we found that Smad2 was signaled by Mst1 and Smad2 upregulation was associated with mitochondrial stress and retinal pigmented epithelial cell apoptosis (Zhu et al. 2018b). This information lays the foundation to help us further understand the role played by Smad2 in diabetic complications.
Taken together, our results reveal functional implications of Mst1 in retinal pigmented epithelial cell apoptosis under constant hyperglycemia stimulation. Meanwhile, the robust regulatory potential of Mst1 in retinal epithelial cell death is achieved by improving Smad2 expression and evoking mitochondrial stress. This finding may provide a new entry point for treating DR by targeting the Mst1-Smad2-mitochondrial stress-signaling pathway.
Authors’ contributions
WB, XDH, and MW made substantial contributions to the concept and design of the present study, XDH, WB, MW, and WH contributed to the performance of experiments, data analysis and interpretation, and manuscript writing.
Funding
This work is supported by the 2016 Liaoning Provincial Natural Science Fund Guidance Plan Project (No. 201602383).
Compliance with ethical standards
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s12192-021-01226-0
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/25/2021
A Correction to this paper has been published: 10.1007/s12192-021-01226-0
References
- Ackermann M, Kim YO, Wagner WL, Schuppan D, Valenzuela CD, Mentzer SJ, Kreuz S, Stiller D, Wollin L, Konerding MA. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis. 2017;20:359–372. doi: 10.1007/s10456-017-9543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A. History and conceptual developments in vascular biology and angiogenesis research: a personal view. Angiogenesis. 2017;20:463–478. doi: 10.1007/s10456-017-9569-2. [DOI] [PubMed] [Google Scholar]
- Blackburn NJR, Vulesevic B, McNeill B, Cimenci CE, Ahmadi A, Gonzalez-Gomez M, Ostojic A, Zhong Z, Brownlee M, Beisswenger PJ, Milne RW, Suuronen EJ. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res Cardiol. 2017;112:57. doi: 10.1007/s00395-017-0646-x. [DOI] [PubMed] [Google Scholar]
- Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, Houdijk AP, Geller DA, van Leeuwen PA. A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis. 2017;20:557–565. doi: 10.1007/s10456-017-9567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wei R, Yao S (2018) Matrine has pro-apoptotic effects on liver cancer by triggering mitochondrial fission and activating Mst1-JNK signalling pathways. J Physiol Sci. 10.1007/s12576-018-0634-4 [DOI] [PMC free article] [PubMed]
- Chang SH, Yeh YH, Lee JL, Hsu YJ, Kuo CT, Chen WJ. Transforming growth factor-beta-mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol. 2017;112:58. doi: 10.1007/s00395-017-0647-9. [DOI] [PubMed] [Google Scholar]
- Chang YC, Lin CW, Hsieh MC, Wu HJ, Wu WS, Wu WC, Kao YH. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell Signal. 2017;40:248–257. doi: 10.1016/j.cellsig.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Conradi LC, Brajic A, Cantelmo AR, Bouché A, Kalucka J, Pircher A, Brüning U, Teuwen LA, Vinckier S, Ghesquière B, Dewerchin M, Carmeliet P. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis. 2017;20:599–613. doi: 10.1007/s10456-017-9573-6. [DOI] [PubMed] [Google Scholar]
- Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res 62. 10.1111/jpi.12404 [DOI] [PubMed]
- Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res 62. 10.1111/jpi.12380 [DOI] [PubMed]
- Ghiroldi A, Piccoli M, Ciconte G, Pappone C, Anastasia L. Regenerating the human heart: direct reprogramming strategies and their current limitations. Basic Res Cardiol. 2017;112:68. doi: 10.1007/s00395-017-0655-9. [DOI] [PubMed] [Google Scholar]
- Giatsidis G, Cheng L, Haddad A, Ji K, Succar J, Lancerotto L, Lujan-Hernandez J, Fiorina P, Matsumine H, Orgill DP. Noninvasive induction of angiogenesis in tissues by external suction: sequential optimization for use in reconstructive surgery. Angiogenesis. 2018;21:61–78. doi: 10.1007/s10456-017-9586-1. [DOI] [PubMed] [Google Scholar]
- Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q (2017) Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res 63. 10.1111/jpi.12431 [DOI] [PubMed]
- Hassanshahi M, Hassanshahi A, Khabbazi S, Su YW, Xian CJ. Bone marrow sinusoidal endothelium: damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis. 2017;20:427–442. doi: 10.1007/s10456-017-9577-2. [DOI] [PubMed] [Google Scholar]
- Hong H, Tao T, Chen S, Liang C, Qiu Y, Zhou Y, Zhang R. MicroRNA-143 promotes cardiac ischemia-mediated mitochondrial impairment by the inhibition of protein kinase Cepsilon. Basic Res Cardiol. 2017;112:60. doi: 10.1007/s00395-017-0649-7. [DOI] [PubMed] [Google Scholar]
- Hooshdaran B, Kolpakov MA, Guo X, Miller SA, Wang T, Tilley DG, Rafiq K, Sabri A. Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res Cardiol. 2017;112:62. doi: 10.1007/s00395-017-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggena D, Winter Y, Steiner B (2017) Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J Pineal Res 62. 10.1111/jpi.12397 [DOI] [PubMed]
- Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B. Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi: 10.1016/j.redox.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwi QG, Bice JS, Baxter GF. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: a systematic review and meta-analysis. Basic Res Cardiol. 2017;113:6. doi: 10.1007/s00395-017-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Denver P, Satchell SC, Ackermann M, Konerding MA, Mitchell CA. Microvascular ultrastructural changes precede cognitive impairment in the murine APPswe/PS1dE9 model of Alzheimer’s disease. Angiogenesis. 2017;20:567–580. doi: 10.1007/s10456-017-9568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol. 2017;113:3. doi: 10.1007/s00395-017-0660-z. [DOI] [PubMed] [Google Scholar]
- Koentges C, Pepin ME, Müsse C, Pfeil K, Alvarez SVV, Hoppe N, Hoffmann MM, Odening KE, Sossalla S, Zirlik A, Hein L, Bode C, Wende AR, Bugger H. Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans. Basic Res Cardiol. 2017;113:8. doi: 10.1007/s00395-017-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman CD, Zimmermann WH, Knopfel T, de Boer TP. Cardiac optogenetics: using light to monitor cardiac physiology. Basic Res Cardiol. 2017;112:56. doi: 10.1007/s00395-017-0645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbel C, Gerstner MD, Menger MD, Laschke MW. Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis. 2018;21:37–46. doi: 10.1007/s10456-017-9580-7. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Lancaster JR, Jr, Meszaros AT, Weidinger A. Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol. 2017;13:170–181. doi: 10.1016/j.redox.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry NM, Cohen S, Dixon IMC (2017) Periostin in cardiovascular disease and development: a tale of two distinct roles. Basic Res Cardiol 113(1). 10.1007/s00395-017-0659-5 [DOI] [PubMed]
- Lee HJ, Jung YH, Choi GE, Ko SH, Lee SJ, Lee SH, Han HJ. BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol. 2017;13:426–443. doi: 10.1016/j.redox.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Back K (2017) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res 62. 10.1111/jpi.12392 [DOI] [PubMed]
- Li R, Xin T, Li D, Wang C, Zhu H, Zhou H. Therapeutic effect of sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, Desai LP. Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gan L, Luo D, Sun C (2017a) Melatonin promotes circadian rhythm-induced proliferation through clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res 62. 10.1111/jpi.12383 [DOI] [PubMed]
- Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S, Sun C (2017b) Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J Pineal Res 63. 10.1111/jpi.12414 [DOI] [PubMed]
- Morell M, Burgos JI, Gonano LA, Vila Petroff M. AMPK-dependent nitric oxide release provides contractile support during hyperosmotic stress. Basic Res Cardiol. 2017;113:7. doi: 10.1007/s00395-017-0665-7. [DOI] [PubMed] [Google Scholar]
- Nawaz IM, Chiodelli P, Rezzola S, Paganini G, Corsini M, Lodola A, di Ianni A, Mor M, Presta M. N-tert-butyloxycarbonyl-Phe-Leu-Phe-Leu-Phe (BOC2) inhibits the angiogenic activity of heparin-binding growth factors. Angiogenesis. 2018;21:47–59. doi: 10.1007/s10456-017-9581-6. [DOI] [PubMed] [Google Scholar]
- Peterson YK, Nasarre P, Bonilla IV, Hilliard E, Samples J, Morinelli TA, Hill EG, Klauber-DeMore N. Frizzled-5: a high affinity receptor for secreted frizzled-related protein-2 activation of nuclear factor of activated T-cells c3 signaling to promote angiogenesis. Angiogenesis. 2017;20:615–628. doi: 10.1007/s10456-017-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryds K, Nielsen RR, Jorsal A, Hansen MS, Ringgaard S, Refsgaard J, Kim WY, Petersen AK, Bøtker HE, Schmidt MR. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol. 2017;112:67. doi: 10.1007/s00395-017-0658-6. [DOI] [PubMed] [Google Scholar]
- Reddy KRK, Dasari C, Duscharla D, Supriya B, Ram NS, Surekha MV, Kumar JM, Ummanni R. Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA) Angiogenesis. 2018;21:79–94. doi: 10.1007/s10456-017-9587-0. [DOI] [PubMed] [Google Scholar]
- Romero CA, Remor A, Latini A, De Paul AL, Torres AI, Mukdsi JH. Uric acid activates NRLP3 inflammasome in an in-vivo model of epithelial to mesenchymal transition in the kidney. J Mol Histol. 2017;48:209–218. doi: 10.1007/s10735-017-9720-9. [DOI] [PubMed] [Google Scholar]
- Schluter KD, Wolf A, Weber M, Schreckenberg R, Schulz R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way. Basic Res Cardiol. 2017;112:63. doi: 10.1007/s00395-017-0650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Li H, Dai Q, Lu C, Xu M, Zhang J, Feng J. DUSP1 recuses diabetic nephropathy via repressing JNK-Mff-mitochondrial fission pathways. J Cell Physiol. 2018;234:3043–3057. doi: 10.1002/jcp.27124. [DOI] [PubMed] [Google Scholar]
- Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018;14:59–71. doi: 10.1016/j.redox.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro MM, Correia ML, Brito MA. Endothelial progenitor cells in multiple myeloma neovascularization: a brick to the wall. Angiogenesis. 2017;20:443–462. doi: 10.1007/s10456-017-9571-8. [DOI] [PubMed] [Google Scholar]
- Turner CJ, Badu-Nkansah K, Hynes RO. Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis. 2017;20:519–531. doi: 10.1007/s10456-017-9563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, Chen Y, Gao CY, Cui ZT, Yao JM. Protective effects of microRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell Physiol Biochem. 2017;42:506–518. doi: 10.1159/000477597. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang X, Geng P, Tang X, Xiang L, Lu X, Li J, Ruan Z, Chen J, Xie G, Wang Z, Ou J, Peng Y, Luo X, Zhang X, Dong Y, Pang X, Miao H, Chen H, Liang H (2017) Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J Pineal Res 63. 10.1111/jpi.12405 [DOI] [PubMed]
- Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, Gao E, Yang Y, Liang H, Jin Z, Yu S (2017) Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res 63. 10.1111/jpi.12419 [DOI] [PubMed]
- Zhao Q, Ye M, Yang W, Wang M, Li M, Gu C, Zhao L, Zhang Z, Han W, Fan W, Meng Y. Effect of Mst1 on endometriosis apoptosis and migration: role of Drp1-related mitochondrial fission and Parkin-required mitophagy. Cell Physiol Biochem. 2018;45:1172–1190. doi: 10.1159/000487450. [DOI] [PubMed] [Google Scholar]
- Zhou H, du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018a) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res 64. 10.1111/jpi.12450 [DOI] [PubMed]
- Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017a) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63. 10.1111/jpi.12438 [DOI] [PubMed]
- Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res. 2018;65:e12503. doi: 10.1111/jpi.12503. [DOI] [PubMed] [Google Scholar]
- Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018c) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res 64. 10.1111/jpi.12471 [DOI] [PubMed]
- Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21:599–615. doi: 10.1007/s10456-018-9611-z. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang J, Hu S, Zhu H, Toanc S, Ren J (2018e) BI1 alleviates cardiac microvascular ischemia-reperfusion injury via modifying mitochondrial fission and inhibiting XO/ROS/F-actin pathways. J Cell Physiol. 10.1002/jcp.27308 [DOI] [PubMed]
- Zhou H, Wang J, Zhu P, Hu S, Ren J. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018;45:12–22. doi: 10.1016/j.cellsig.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018g) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113(23). 10.1007/s00395-018-0682-1 [DOI] [PubMed]
- Zhou H, Wang S, Hu S, Chen Y, Ren J. ER-mitochondria microdomains in cardiac ischemia-reperfusion injury: a fresh perspective. Front Physiol. 2018;9:755. doi: 10.3389/fphys.2018.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2017;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23:101–113. doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, Li Y, Zhou H, Chen Y. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018;16:157–168. doi: 10.1016/j.redox.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YW, Yan JK, Li JJ, Ou YM, Yang Q. Knockdown of radixin suppresses gastric cancer metastasis in vitro by up-regulation of E-cadherin via NF-kappaB/snail pathway. Cell Physiol Biochem. 2016;39:2509–2521. doi: 10.1159/000452518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.