Abstract
Intricate life-versus-death decisions are programmed during T cell development, and the regulatory mechanisms that coordinate their activation and repression are still under investigation. Here, HDAC3-deficient DP thymocytes exhibit a severe decrease in numbers. The thymic cortex is rich in ATP, which is released by macrophages that clear apoptotic DP thymocytes that fail to undergo positive selection. We demonstrate that HDAC3 is required to repress expression of the purinergic-receptor P2X7 to prevent DP cell death. HDAC3-deficient DP thymocytes upregulate the P2X7 receptor, increasing sensitivity to ATP-induced cell death. P2rx7/HDAC3-double knockout mice show a partial rescue in DP cell number. HDAC3 directly binds to the P2rx7 enhancer, which is hyperacetylated in the absence of HDAC3. In addition, RORγt binds to the P2rx7 enhancer and promotes P2X7 receptor expression in the absence of HDAC3. Therefore, HDAC3 is a critical regulator of DP thymocyte survival and is required to suppress P2X7 receptor expression.
Introduction
The accurate coordination of transcriptional regulators, chromatin modifiers, and nucleosome remodelers is critical for proper gene expression during T cell development (1). Changes in gene expression occur as thymocytes transition through multiple checkpoints, resulting in different cellular fates. Cell death is a common fate decision, as the majority of thymocytes (especially double positive (DP) thymocytes (2)) fail to complete T cell development. Specifically, TCR signaling controls the fate of DP thymocytes, as an absence of TCR signaling leads to death by neglect, a strong TCR signal leads to death by negative selection, or a weak TCR signal leads to thymocyte survival via positive selection (reviewed by (3)). Each of these cellular fates is coordinated by the activation or repression of different transcriptional programs.
Gene repression is as important as gene activation at each stage of thymocyte development. At the chromatin level, gene repression works through the recruitment of co-repressor complexes. Histone deacetylase 3 (HDAC3) is a co-repressor important in immune cell development (reviewed by (4)). HDAC3 belongs to the class I family of histone deacetylases, and functions to remove acetyl groups from both histone tails and non-histone proteins (5). HDAC3 acts as the catalytic component of the N-CoR co-repressor complex and facilitates gene repression through its recruitment to promoters or enhancers (6). The HDAC3/N-CoR complex does not have a DNA-binding domain, therefore it must be recruited to specific sites in the genome via its interaction with different transcription factors.
Previously, HDAC3 was shown to be critical for thymocyte positive selection (7, 8). When HDAC3 is conditionally deleted in thymocytes using CD2-icre (named HDAC3-cKO mice hereafter), there is a marked reduction in the number of DP and single-positive (SP) thymocytes (7). The reduction in SP thymocytes is due to a block in positive selection and could not be rescued by an OT-II TCR transgene. OT-II HDAC3-cKO mice also exhibit a positive selection block, comparable to HDAC3-cKO mice. Mechanistically, HDAC3 is required to repress RORγt during positive selection, as RORγt is normally downregulated at this stage and constitutive expression of RORγt leads to a similar block in positive selection (9). Deletion of RORγt, in conjunction with a Bcl-xl transgene (necessitated by the dependence of Bcl-xl expression on RORγt (10)), alleviated the block in positive selection resulting from HDAC3 deficiency (RORγt -KO Bcl-xl Tg HDAC3-cKO, hereafter called “RB3”). In addition, DP cellularity was restored in RB3 mice, although the mechanism was not known. The focus of this paper is the mechanism by which HDAC3 regulates DP thymocyte survival.
Here, we find one cause for the survival defect in DP thymocytes from HDAC3-cKO mice. HDAC3-deficient DP thymocytes exhibit increased expression of the purinergic receptor P2X7 (encoded by the P2rx7 gene). Cells that express P2X7 receptor are more sensitive to high concentrations of extracellular ATP, which results in large pore formation and loss of membrane integrity (reviewed by (11)). The regulation of P2rx7 expression is coordinated by HDAC3 and RORγt at the P2rx7 enhancer. HDAC3 deletion leads to an increase in histone acetylation at the P2rx7 gene locus and deletion of RORγt normalizes P2X7 receptor expression in HDAC3-deficient DP thymocytes. Therefore, HDAC3 is required to suppress P2X7 receptor expression in DP thymocytes and promote DP survival.
Materials and Methods
Mice.
HDAC3 fl/fl mice were provided by S. Hiebert (Vanderbilt (12)). Human Bcl-2 Tg mice were generated by S. Korsmeyer (13) and provided by A. Singer (National Institutes of Health). Bcl-xL Tg mice (14), RORγt -KO mice (15), CD2-icre mice (16), and P2rx7-KO (17)–(18) mice were purchased from The Jackson Laboratory. OT-II (19) mice were purchased from Taconic. Mice were housed in barrier facilities and experiments were performed at the Mayo Clinic with Institutional Animal Care and Use Committee approval. Mice were analyzed between the ages of 4 and 8 weeks with either littermate or age-matched controls (termed WT), which may include floxed only mice (no Cre), CD2-icre, or WT mice, as no differences were observed between these mice.
Flow Cytometry.
FACS analysis was performed on an Attune NxT cytometer (Thermo Fisher) and analyzed with FlowJo (TreeStar). Experiments were acquired live or fixed (BD Cytofix/Cytoperm Fixation and Permeabilization kit (BD Biosciences)). Bcl-xl staining used the Foxp3/Transcription Factor Staining Buffer kit (Tonbo). All analyses included size exclusion (forward scatter [FSC] area/ side scatter [SSC] area), doublet exclusion (FSC height/FSC area), and dead cell exclusion (Ghost Dye Red 780, Tonbo). Antibodies used were: CD4 (GK1.5 or RM4–5), CD8α (53–6.7 or 2.43), CD11b (M1/70), CD45.2 (104), CD45.1 (A20), Bcl-xl (7B2.5), P2X7 receptor (polyclonal, Enzo Life Sciences), RORγt (AFKJS-9), B220 (RA3–62B), CD19 (6D5), CD11c (N418), NK1.1 (PK136), Gr-1 (RB6–8C5), Ter119 (TER-119), and TCRβ (H57–597).
Bone Marrow Mixed Chimeras.
Mixed bone marrow chimeras were generated by intravenously injection of 4×106 cells from 50:50 mixes of either WT (CD45.2+)/B6.SJL (CD45.1+) or CD2-icre HDAC3-cKO (CD45.2+)/B6.SJL (CD45.1+) mice into lethally irradiated congenic B6.SJL (CD45.1+) recipients. Mice received enrofloxacin in their drinking water for 3 weeks and analyzed after 8 weeks.
Ex vivo stimulation.
Thymocytes were cultured at 4×106 cells/mL with/without 1mM ATP (Sigma) or 100μM BzATP (Tocris) in culture medium (RPMI 1640, 10% FCS, penicillin/streptomycin/glutamine). For experiments using A438079 (Abcam), cells were pre-treated with 10μM or 100μM A438079 for 1 hour before addition of ATP or BzATP. After 15 minutes of stimulation, cells were harvested, washed, stained for Annexin V binding (Apoptosis Detection kit, BD Biosciences). To measure pore formation, 2μM YO-PRO-1 was added to the culture for the last 5 minutes before harvesting, washing, and staining.
Downloaded datasets.
The following datasets were retrieved from GEO series: GSM726991 (RNA polymerase II), GSM1556287 (H3K27ac), GSM726994 (H3K4me3), GSM945565 (H3K27me3), GSM726993 (H3K4me1), GSE63731 (CapSTARR-seq), and GSM2354271 (RORγt). Sequencing data was imaged using the Integrative Genomics Viewer software (IGV, Broad Institute).
Quantitative ChIP (qChIP).
For qChIP, DP thymocytes were enriched using the EasySep Mouse Streptavidin RapidSpheres Isolation kit (#19860, StemCell Technologies) to remove SP, DN, and ©δ thymocytes with biotin-conjugated anti-CCR7 (4B12), anti-IL-7Rα (A7R34), anti-H2K (AF6–88.5), anti-CD44 (IM7), and anti-CD25 (PC61.5) and anti-TCR©δ (UC7–13D5). DP thymocyte purity was at least 95%. Cells were fixed with 1% formaldehyde for 10 mins (H3K27ac qChIP) or 15mins (HDAC3 qChIP) and quenched with 125 mM glycine for 10 mins. H3K27ac ChIP was performed according to (20) using anti-H3K27ac (Abcam #ab4729). HDAC3 ChIP was performed according to (21), using anti-HDAC3 (Cell Signaling Technologies #85057), with the following adjustments: After cell lysis and brief sonication (4 mins, 30 sec on/30 sec off; Bioruptor Pico (Diagenode, Inc.)), equal volume of 2× MNase buffer (35mM Tris-HCl pH 7.5, 25mM NaCl, 120mM KCl, 2mM CaCl2) was added to each sonication sample and digested with MNase (Cell Signaling Technologies #10011S) at 37 °C for 15 mins. Isolated DNA was used to perform real-time PCR. Graphs depict fold enrichment to regions without H3K27ac (Intergenic primers) or HDAC3 binding (Rpl30 primers). Primers used for H3K27ac qChIP are: P2rx7 enhancer forward, GGTGGGGTGACGAAGTTAGG; P2rx7 enhancer reverse, GAATTCCACGGCACTCACCT; Intergenic forward, CCTGCTGCCTTGTCTCTCTC; Intergenic reverse, ATGGCCTAGGGATTCCAGCA. Primers used for HDAC3 qChIP are: P2rx7 promoter forward, AGACTGTGTGCCTCCCTTTG; P2rx7 promoter reverse, CCCTTATCTCTGTGGGAGCC; P2rx7 enhancer forward, GAACAGTTCCTGCGGCTTTG; P2rx7 enhancer reverse, CTTTTGAAACCAGCCGTGGG, Rpl30 purchased from Cell Signaling Technologies (#7015).
Statistical analysis.
Two-tailed unpaired Student’s t test (GraphPad Prism) was used to compare groups. Boxes on boxplots encompass the 25th to 75th percentile and whiskers extend to the minimum and maximum values.
Results and Discussion
Decreased viability of HDAC3-deficient DP thymocytes is cell-intrinsic and not rescued by Bcl-xl or Bcl-2 transgenes
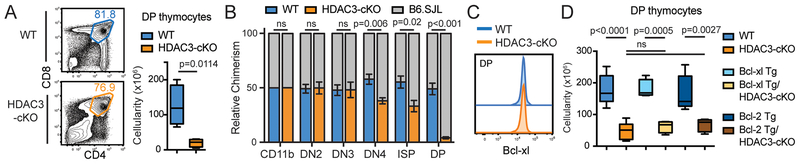
HDAC3-cKO mice had approximately 80% fewer DP thymocytes as compared to WT mice ((7); Figure 1A). The reduction was unlikely due to the positive selection block in HDAC3-cKO mice because lack TCRα, MHC, or key TCR signaling molecules (ex. Zap70) do not have compromised DP thymocytes (22–24). To determine whether the DP reduction was cell intrinsic, 50/50 mixed bone marrow chimeric mice were generated. Chimerism frequency was measured at the DN-to-DP stages, with splenic CD11b+ cells used as a control. Chimerism frequency was slightly reduced in HDAC3-deficient DN4 and ISP thymocytes, while HDAC3-deficient DP thymocytes exhibited a large reduction in chimerism (Figure 1B), demonstrating that the effect is cell intrinsic. While HDAC3 protein deletion starts at DN3 in HDAC3-cKO mice, there was not a deficiency in DN cellularity, β-selection, and proliferation in DN3 and DN4 thymocytes (7). Therefore, it is unlikely that the deficit in DP cell number is due to a defect prior to the DP stage.
Figure 1. HDAC3-deficient DP thymocytes exhibit a Bcl-xl/Bcl-2-independent survival defect.
(A) Representative FACS plots of DP thymocytes from WT and HDAC3-cKO mice. Boxplots show DP cellularity with 4 mice per group. (B) 50/50 mixed bone marrow chimeras of B6.SJL (CD45.1+) bone marrow mixed with WT (CD45.2+) or HDAC3-cKO (CD45.2+). Plots depict mean ± SEM percent chimerism (n=5/group) of splenic CD11b+ cells as well as DN and DP thymocytes between CD45.1+ and CD45.2+ cells. DN2-DN4 thymocytes were identified as kit+CD25+ (DN2), c-kit−CD25+ (DN3) and c-kit−CD25− (DN4) after gating from CD3− lineage− (B220/CD19, CD11b, CD11c, NK1.1, Gr-1, Ter119, CD4, CD8, TCRβ). (C) Representative FACS plot depicting Bcl-xl expression in DP thymocytes from 3 WT and 3 HDAC3-cKO mice. (D) Number of DP thymocytes from WT, HDAC3-cKO, Bcl-xl tg, Bcl-xl tg/HDAC3-cKO, Bcl-2 tg, and Bcl-2 tg/HDAC3-cKO mice. Expression of both Bcl-xl and Bcl-2 transgenes are driven by the proximal Lck promoter. Plots show 4–8 mice per group from 4 independent experiments.
Bcl-xl is required for DP cell survival (25), however Bcl-xl protein expression in DP thymocytes was similar between WT and HDAC3-cKO mice (Figure 1C). To determine whether overexpression of the Bcl-2 family anti-apoptotic protein Bcl-xl or Bcl-2 could rescue DP cell number from HDAC3-cKO mice, Bcl-xl and Bcl-2 transgenes were introduced. However, no increase in DP cell number from Bcl-xl Tg/HDAC3-cKO mice or Bcl-2 Tg/HDAC3-cKO mice was observed as compared to HDAC3-cKO mice (Figure 1D). Thus, the DP survival defect in HDAC3-cKO mice cannot be compensated by overexpression of Bcl-xl or Bcl-2 (7).
HDAC3-deficient DP thymocytes are susceptible to P2X7 receptor-induced cell death
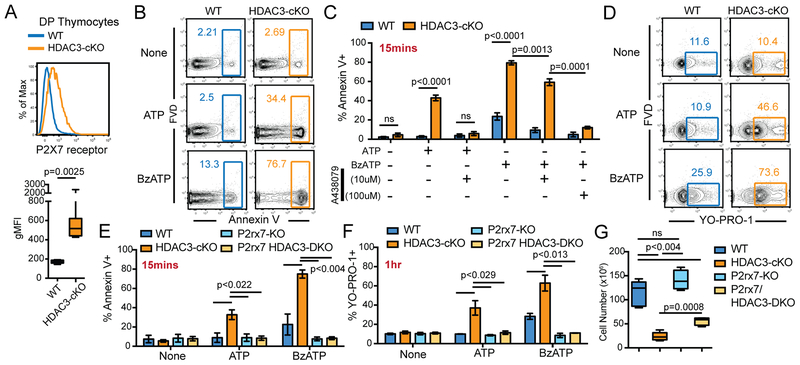
The purinergic receptor P2X7 induces thymocyte cell death upon stimulation with high doses of ATP (26). The thymic cortex is believed to be an ATP-rich environment, as resident macrophages release ATP as a result of phagocytosing DP thymocytes undergoing cell death (27). WT DP thymocytes express low levels of P2X7 receptor compared to DN and SP thymocytes and are thus relatively insensitive to extracellular ATP-induced cell death (Figure 2A, Supplementary Figure 1). However, HDAC3-deficient DP thymocytes significantly upregulated P2X7 receptor as compared to WT DP thymocytes (Figure 2A) and were more sensitive to P2X7 receptor-induced cell death from an ex vivo culture with the P2X7 receptor ligand ATP or the P2X7 receptor agonist BzATP (Figure 2B). Preincubation of HDAC3-deficient thymocytes with the P2X7 receptor-specific antagonist A438079 abrogated the increase in Annexin V binding caused by ATP treatment (Figure 2C), demonstrating that the ATP-induced cell death was not due to stimulation of other purinergic receptors co-expressed by HDAC3-deficient DP thymocytes. HDAC3-deficient DP thymocytes required a higher dose of A438079 to abrogate BzATP-induced Annexin V staining (Figure 2C), again suggesting that HDAC3-deficient DP thymocytes are more sensitive to P2X7 receptor ligands. Strong stimulation of P2X7 receptor can also induce cell membrane pore-mediated cell death (28). YO-PRO-1 is a large (~600Da) nucleic acid stain that labels cells with compromised plasma membranes and is therefore a surrogate marker for pore formation (29). After one-hour stimulation of thymocytes with ATP or BzATP, HDAC3-deficient DP thymocytes exhibited an increase in YO-PRO-1 staining compared to unstimulated, etoposide stimulation, or WT controls (Figure 2D, Supplementary Figure 2), demonstrating that P2X7 receptor induced cell death in HDAC3-cKO mice occurs via pore formation.
Figure 2. HDAC3-deficient DP thymocytes are susceptible to cell death mediated by the P2X7 receptor.
(A) P2X7 receptor expression on DP thymocytes from 5 WT and 7 HDAC3-cKO mice from 3 independent experiments. (B-C) Frequency of Annexin V+ DP thymocytes stimulated for 15 minutes ex vivo with 1mM of ATP or 100μM of BzATP from WT and HDAC3-cKO mice, with or without a 1 hour pre-treatment with the P2X7 receptor antagonist A438079. Plots show mean ± SEM of 3–4 mice per group from 3 independent experiments. (D) Frequency of YO-PRO-1+ DP thymocytes, from WT and HDAC3-cKO mice, stimulated for 1 hour ex vivo with 1mM ATP or 100μM BzATP. Data is representative of 3–4 mice from 3 independent experiments. (E-F) Frequency of DP thymocytes that are Annexin V+ (E) or YO-PRO-1+ (F) after ex vivo stimulation with 1mM of ATP or 100μM of BzATP for 15 minutes or 1 hour, respectively. DP thymocytes are from WT, HDAC3-cKO, P2rx7-KO, or P2rx7/HDAC3-DKO mice. Plot shows mean ± SEM of 3–4 mice from 3 independent experiments. (G) Number of DP thymocytes from WT, HDAC3-cKO, P2rx7-KO or P2rx7/HDAC3-DKO mice. Box plots depict 4–5 mice from 4 independent experiments.
To understand the contribution of the P2X7 receptor to reduced DP cell survival in HDAC3-cKO mice, P2rx7/HDAC3-double knockout (DKO) mice were generated. Loss of the P2X7 receptor protected HDAC3-deficient DP thymocytes from ATP- and BzATP-induced cell death (Figure 2E). Similarly, knocking out P2rx7 abrogated the increase in YO-PRO-1 staining in response to either ATP or BzATP in P2rx7/HDAC3-DKO mice compared to HDAC3-cKO mice (Figure 2F), demonstrating that pore formation is specifically induced by the P2X7 receptor. Examination of DP cell number from P2rx7/HDAC3-DKO mice revealed a two-fold increase in cell number compared to HDAC3-cKO mice (Figure 2G), however the number of DP thymocytes from P2rx7/HDAC3-DKO mice was still below WT mice (Figure 2G). This indicates that there must be other causes of DP cell death in addition to increased expression of P2X7.
The P2rx7 gene locus is suppressed by HDAC3 in DP thymocytes
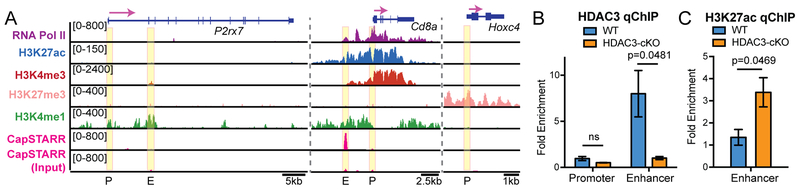
Examination of P2X7 receptor expression during T cell develop revealed that P2rx7 is specifically downregulated at DP stage compared to DN and CD4SP thymocytes (Supplementary Figure 1). Since deletion of HDAC3 in DP thymocytes leads to P2X7 receptor upregulation (Figure 2A), publicly available genome sequencing datasets were used to examine the chromatin state of the P2rx7 gene locus in WT thymocytes. Cd8a and Hoxc7 gene loci were used as controls for highly expressed genes and repressed genes in thymocytes, respectively. Compared to Cd8a, the P2rx7 locus showed a low signal for RNA polymerase II, H3K27ac, and H3K4me3 (Figure 3A), indicating that the P2rx7 locus does not show chromatin marks of active gene expression in WT thymocytes. This is consistent with low P2X7 receptor expression in WT thymocytes (Figure 2A). Interestingly, the repressive mark H3K27me3 was not enriched at the P2rx7 locus (Figure 3A), suggesting that P2rx7 is not actively repressed by a PRC-regulated mechanism. In addition, H3K4me1 ChIP-seq and CapSTARR-seq were utilized to identify enhancers in WT thymocytes and examine their activity (30, 31), respectively. Within intron 2 of P2rx7, an enhancer was revealed by enrichment of H3K4me1 (Figure 3A), which is consistent with previous reports (32). The combination of H3K27ac and H3K4me1 marks identifies active enhancers (30), however the P2rx7 enhancer lacked H3K27ac (Figure 3A), indicating that the P2rx7 enhancer is not active in WT thymocytes. To validate P2rx7 enhancer activity in thymocytes, we used publicly available CapSTARR-seq (31). Consistent with the absence of H3K27ac at this enhancer, the CapSTARR-seq signal was also absent at the P2rx7 enhancer (Figure 3A), confirming that the enhancer in inactive in WT DP thymocytes. Therefore, the P2rx7 gene locus is suppressed in WT thymocytes.
Figure 3. The P2rx7 gene locus is repressed by HDAC3 in DP thymocytes.
(A) ChIP-seq and CapSTARR-seq snapshots at P2rx7, Cd8a, and Hoxc7. Yellow boxes identify previously characterized promoter (P) and enhancer (E) regions (32). (B) HDAC3 qChIP at the P2rx7 promoter and enhancer in DP thymocytes from WT and HDAC3-cKO mice. Plots show mean ± SEM of 3–4 mice per group from 3 independent experiments. (C) H3K27ac qChIP at the P2rx7 enhancer in DP thymocytes from WT and HDAC3-cKO mice. Plots show mean ± SEM of 3 mice per group from 3 independent experiments.
HDAC3 regulates gene expression upon recruitment to gene promoters or enhancers. To determine whether HDAC3 binds to either of these regions of the P2rx7 gene, HDAC3 quantitative ChIP (qChIP) was performed on DP thymocytes from WT mice, with HDAC3-cKO mice used as a negative control for HDAC3 binding. HDAC3 qChIP revealed that HDAC3 bound to the P2rx7 enhancer but not the P2rx7 promoter (Figure 3B), indicating that HDAC3 directly regulates P2rx7 expression. Since HDAC3 deacetylates histones, H3K27Ac was analyzed at the P2rx7 enhancer by qChIP in WT and HDAC3-deficient DP thymocytes. While DP thymocytes from WT mice exhibited low levels of acetylation at the P2rx7 enhancer (similar to Figure 3A), deletion of HDAC3 increased acetylation at the P2rx7 enhancer in DP thymocytes from HDAC3-cKO mice (Figure 3C), demonstrating HDAC3 directly regulates histone acetylation at the P2rx7 gene locus.
RORγt promotes P2X7 receptor expression in HDAC3-deficient DP thymocytes
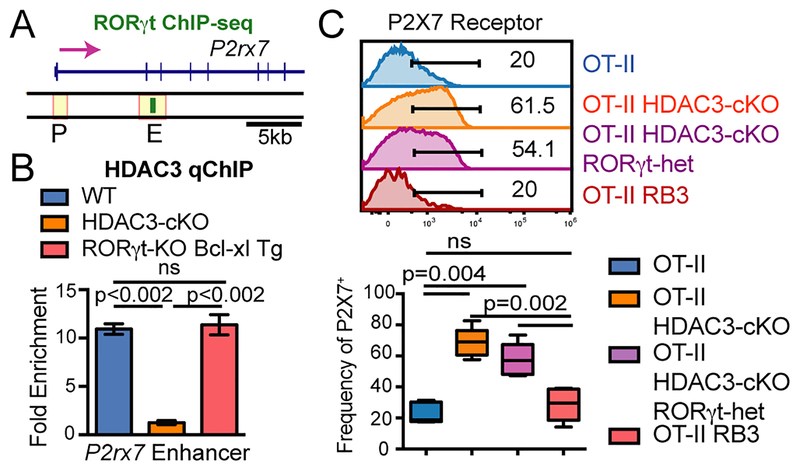
HDAC3 is required to repress RORγt during positive selection (7), as RORγt is normally downregulated at this stage and constitutive expression of RORγt leads to a similar block in positive selection as observed in HDAC3-cKO mice (9). Deletion of RORγt rescues the block in positive selection in RB3 mice as well as DP cellularity (7), suggesting that RORγt may regulate P2X7 receptor expression. A previous study identified retinoic acid response elements (RAREs) in the P2rx7 intronic enhancer and RARα binding to this enhancer in CD4+ T cells (32). RORγt belongs to the RAR-related orphan receptor (ROR) family of transcription factors that show sequence homology to retinoic-acid receptor (RAR) family of proteins (33). Therefore, RORγt may bind to the P2rx7 enhancer in WT thymocytes. Publicly available RORγt ChIP-seq dataset of WT thymocytes demonstrates that RORγt associates with the P2rx7 enhancer in WT thymocytes (Figure 4A). HDAC3 does not have a DNA binding domain, therefore HDAC3 must be recruited by transcription factors to perform its repressive function. RORγt may function to recruit HDAC3 to the P2rx7 enhancer. To test this, HDAC3 qChIP was performed in RORγt-KO Bcl-xl tg DP thymocytes, with the Bcl-xl transgene used to compensate for reduction the number of DP thymocytes produced by RORγt-deficiency (10). Interestingly, HDAC3 binding still occurred at the P2rx7 enhancer in RORγt-deficient DP thymocytes (Figure 4B), demonstrating that HDAC3 is not recruited to the P2rx7 enhancer via RORγt.
Figure 4. RORγt promotes P2X7 receptor expression in HDAC3-deficient DP thymocytes.
(A) RORγt ChIP-seq snapshot at P2rx7 in WT thymocytes. (B) HDAC3 qChIP at the P2rx7 enhancer in DP thymocytes from WT, HDAC3-cKO, and RORγt -KO Bcl-xl tg mice. Plot shows mean ± SEM of 2–3 mice group. (C) Frequency of P2X7 receptor+ DP thymocytes from OT-II, OT-II HDAC3-cKO, OT-II HDAC3-cKO RORγt-het and OT-II RB3 (OT-II RORγt-KO Bcl-xl HDAC3-cKO) mice. Boxplot depicts 4–5 mice from 4 independent experiments.
To determine whether RORγt regulates P2rx7 expression, P2X7 receptor expression in RB3 (RORγt-KO Bcl-xl tg HDAC3-cKO) and HDAC3-deficient DP thymocytes was examined. In these experiments, RB3 and HDAC3-cKO mice contained the OT-II transgene. OT-II HDAC3-cKO DP thymocytes exhibited an increased in the frequency of P2X7 receptor positive cells compared to OT-II thymocytes (Figure 4C), consistent with results in HDAC3-cKO and WT DP thymocytes (Figure 2A). Loss of RORγt expression in OT-II RB3 mice restored P2X7 receptor expression to levels comparable to WT mice (Figure 4C). Interestingly, mice with heterozygous RORγt deficiency (OT-II RORγt-het HDAC3-cKO mice) showed an intermediate frequency of P2X7-positive cells, demonstrating that the frequency of P2X7-positive cells is exquisitely sensitive to RORγt expression (Figure 4C). Thus, RORγt promotes P2rx7 expression in HDAC3-deficient DP thymocytes.
In summary, we have identified a novel role for HDAC3 in DP thymocytes. We demonstrate that HDAC3 is required to repress expression of the purinergic receptor P2X7 to prevent DP cell death. HDAC3-deficient DP thymocytes upregulate the P2X7 receptor, increasing sensitivity to ATP-induced cell death. P2rx7/HDAC3-DKO mice show a partial restoration in DP cell number, with twice as many DP thymocytes as HDAC3-cKO mice. Mechanistically, HDAC3 directly binds to the P2rx7 enhancer, which is hyperacetylated in the absence of HDAC3. In addition, RORγt binds to the P2rx7 enhancer and promotes P2X7 receptor expression in HDAC3-deficient DP thymocytes (model in Supplementary Figure 3). Therefore, HDAC3 is a critical regulator of DP thymocyte survival and is required to suppress P2rx7 expression.
Supplementary Material
Acknowledgements
We thank Dr. Michael Shapiro for thoughtful discussions and critical reading of the manuscript. We also thank the Epigenomics Development Lab (Mayo Clinic) for allowing us to use their Diagenode Sonicator. The authors declare no competing financial interests.
This work was supported by National Institutes of Health Grant R56 AI122746 and T32 AI007425, Center for Biomedical Discovery at Mayo Clinic, Mayo Graduate School funds to R.L.P, and Mayo Foundation funds to V.S.S.
Abbreviations
- BzATP
2 ,3 -O-(benzoyl-4-benzoyl)-ATP
- CapSTARR-seq
capturing self-transcribing active regulatory region sequencing
- ChIP
chromatin immunoprecipitation
- cKO
conditional knockout
- DN
double-negative
- DP
double-positive
- FSC
forward scatter
- HDAC
histone deacetylase
- H3K27ac
acetylated histone H3 Lysine-27
- H3K27me3
trimethylated histone H3 Lysine-27
- H3K4me3
trimethylated histone H3 Lysine-4
- H3K4me1
monomethylated histone H3 Lysine-4
- KO
knockout
- P2XR
purinergic 2X receptors
- RB3
RORγt-KO Bcl-xL Tg HDAC3-cKO
- ROR
retinoic acid–related orphan receptor
- SP
single-positive
- SSC
side scatter
- Tg
transgenic
- WT
wild-type
- YO-PRO-1
quinolinium, 4-(3-methyl-2[H]-benzoxazolyli-dene)methyl)-1–1(3-(triethylammonio)propyl) diodide
References
- 1.Shapiro MJ, and Shapiro VS. 2011. Transcriptional repressors, corepressors and chromatin modifying enzymes in T cell development. Cytokine 53: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scollay RG, Butcher EC, and Weissman IL. 1980. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. European journal of immunology 10: 210–218. [DOI] [PubMed] [Google Scholar]
- 3.Hogquist KA, Baldwin TA, and Jameson SC. 2005. Central tolerance: learning self-control in the thymus. Nature reviews. Immunology 5: 772–782. [DOI] [PubMed] [Google Scholar]
- 4.Ellmeier W, and Seiser C. 2018. Histone deacetylase function in CD4(+) T cells. Nature reviews. Immunology 18: 617–634. [DOI] [PubMed] [Google Scholar]
- 5.Emiliani S, Fischle W, Van Lint C, Al-Abed Y, and Verdin E. 1998. Characterization of a human RPD3 ortholog, HDAC3. Proceedings of the National Academy of Sciences of the United States of America 95: 2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenther MG, Barak O, and Lazar MA. 2001. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Molecular and cellular biology 21: 6091–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philips RL, Chen MW, McWilliams DC, Belmonte PJ, Constans MM, and Shapiro VS. 2016. HDAC3 Is Required for the Downregulation of RORgammat during Thymocyte Positive Selection. Journal of immunology 197: 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stengel KR, Zhao Y, Klus NJ, Kaiser JF, Gordy LE, Joyce S, Hiebert SW, and Summers AR. 2015. Histone Deacetylase 3 Is Required for Efficient T Cell Development. Mol. Cell. Biol 35: 3854–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He YW, Beers C, Deftos ML, Ojala EW, Forbush KA, and Bevan MJ. 2000. Down-regulation of the orphan nuclear receptor ROR gamma t is essential for T lymphocyte maturation. J. Immunol 164: 5668–5674. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, and Littman DR. 2000. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 288: 2369–2373. [DOI] [PubMed] [Google Scholar]
- 11.Di Virgilio F, Giuliani AL, Vultaggio-Poma V, Falzoni S, and Sarti AC. 2018. Non-nucleotide Agonists Triggering P2X7 Receptor Activation and Pore Formation. Front Pharmacol 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, and Hiebert SW. 2008. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. The EMBO journal 27: 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, and Korsmeyer SJ. 1991. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67: 879–888. [DOI] [PubMed] [Google Scholar]
- 14.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, and Korsmeyer SJ. 1995. Bcl-XL and Bcl-2 repress a common pathway of cell death. J. Exp. Med 182: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, and Littman DR. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 16.Zhumabekov T, Corbella P, Tolaini M, and Kioussis D. 1995. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. Journal of immunological methods 185: 133–140. [DOI] [PubMed] [Google Scholar]
- 17.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, Hippen KL, Blazar BR, Masopust D, Kelekar A, Vulchanova L, Hogquist KA, and Jameson SC. 2018. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, and Gabel CA. 2001. Altered cytokine production in mice lacking P2X(7) receptors. The Journal of biological chemistry 276: 125–132. [DOI] [PubMed] [Google Scholar]
- 19.Barnden MJ, Allison J, Heath WR, and Carbone FR. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell. Biol 76: 34–40. [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Ye Z, Lenz SW, Clark CR, Bharucha A, Farrugia G, Robertson KD, Zhang Z, Ordog T, and Lee JH. 2017. Purification of nanogram-range immunoprecipitated DNA in ChIP-seq application. BMC Genomics 18: 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pchelintsev NA, Adams PD, and Nelson DM. 2016. Critical Parameters for Efficient Sonication and Improved Chromatin Immunoprecipitation of High Molecular Weight Proteins. PloS one 11: e0148023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, and et al. 1992. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature 360: 225–231. [DOI] [PubMed] [Google Scholar]
- 23.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, and Schwartzberg PL. 2006. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25: 93–104. [DOI] [PubMed] [Google Scholar]
- 24.Palacios EH, and Weiss A. 2007. Distinct roles for Syk and ZAP-70 during early thymocyte development. The Journal of experimental medicine 204: 1703–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, and Thompson CB. 1995. Bclx regulates the survival of double-positive thymocytes. Proceedings of the National Academy of Sciences of the United States of America 92: 4763–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BD, Liu QH, Gaulton G, Kotlikoff MI, Hescheler J, and Fleischmann BK. 1999. ATP-evoked Ca2+ transients and currents in murine thymocytes: possible role for P2X receptors in death by neglect. European journal of immunology 29: 1635–1646. [DOI] [PubMed] [Google Scholar]
- 27.Szondy Z, Garabuczi E, Toth K, Kiss B, and Koroskenyi K. 2012. Thymocyte death by neglect: contribution of engulfing macrophages. European journal of immunology 42: 1662–1667. [DOI] [PubMed] [Google Scholar]
- 28.Surprenant A, Rassendren F, Kawashima E, North RA, and Buell G. 1996. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272: 735–738. [DOI] [PubMed] [Google Scholar]
- 29.Cankurtaran-Sayar S, Sayar K, and Ugur M. 2009. P2X7 receptor activates multiple selective dye-permeation pathways in RAW 264.7 and human embryonic kidney 293 cells. Mol Pharmacol 76: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 30.Zentner GE, Tesar PJ, and Scacheri PC. 2011. Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res 21: 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanhille L, Griffon A, Maqbool MA, Zacarias-Cabeza J, Dao LT, Fernandez N, Ballester B, Andrau JC, and Spicuglia S. 2015. High-throughput and quantitative assessment of enhancer activity in mammals by CapStarr-seq. Nat Commun 6: 6905. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto-Hill S, Friesen L, Kim M, and Kim CH. 2017. Contraction of intestinal effector T cells by retinoic acid-induced purinergic receptor P2X7. Mucosal Immunol 10: 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fauber BP, and Magnuson S. 2014. Modulators of the Nuclear Receptor Retinoic Acid Receptor-Related Orphan Receptor-gamma (RORgamma or RORc). Journal of medicinal chemistry. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.