Abstract
Obesity develops, in part, due to frequent overconsumption. Therefore, it is important to identify the regulatory mechanisms that promote eating beyond satiety. Previously, we have demonstrated that an acute microinjection of the neuropeptide PACAP into the nucleus accumbens (NAcc) attenuates palatable food consumption in satiated rats. To better understand the mechanism by which intra-NAcc PACAP selectively blocks palatable food intake, the current work employed a rodent taste reactivity paradigm to assess the impact of PACAP on the hedonic processing of a 1% sucrose solution. Our results revealed that bilateral intra-NAcc PACAP infusions significantly reduced appetitive orofacial responses to sucrose. Interestingly, the effect of PACAP on the expression of aversive responses to sucrose were dependent on the rostral-caudal placement of the microinjection. In a separate group of rats, PACAP was microinjected into the hypothalamus (a region of the brain in which PACAP does not attenuate palatable feeding). Here we found that PACAP had no effect on the hedonic perception of the sucrose solution. Taken together, this dataset indicates that PACAP acts in specific subregions of the NAcc to attenuate palatability-induced feeding by reducing the perceived hedonic value of palatable food.
INTRODUCTION
In the United States of America, an estimated 30% of the population is obese.1 This is troubling as obesity drastically impacts a person’s health by increasing risks for cancer, stroke, hypertension, diabetes and ultimately leading to 300,000 deaths annually.2 Unfortunately, the development of effective therapeutic approaches to treat obesity has remained stagnant due, in part, to the overwhelming complexity of an energy homeostasis system that regulates vastly diverse brain processes ranging from fat metabolism to hedonic drive.3–6 Therefore it remains critical to characterize the mechanisms by which the brain regulates distinct aspects of feeding behavior, as this process may reveal novel therapeutic targets.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a highly conserved 38-amino acid peptide that belongs to the glucagon superfamily of peptides.7, 8 This family of peptides is critically important to a number of biological processes including the regulation of feeding behavior.9 It is well documented that both the hypothalamic ventromedial nuclei (VMN) and nucleus accumbens (NAcc) play crucial roles in the regulation of feeding behavior.11–13 Previously, we have demonstrated that PACAP, acutely microinjected into the rat VMN, suppresses hunger-driven feeding, while PACAP microinjected in the NAcc suppresses palatability-driven feeding.10 However, it is not clear how PACAP signals in these respective brain regions to regulate different aspects of feeding behavior.
Given the preferential actions of PACAP in the NAcc on palatability-driven feeding, and the well-documented involvement of the NAcc in hedonic perception,11 we propose the parsimonious hypothesis that PACAP acts in the NAcc to suppresses palatability-driven feeding because it diminishes the hedonic perception of palatable food. The current studies tested this hypothesis by analyzing the effect of PACAP microinjected into the VMN or NAcc on hedonic processing using a taste reactivity paradigm, as described by Grill and Norgren.12 Our results not only support a selective role for PACAP acting in the NAcc to alter hedonic perception but also a rostral-caudal gradient in the effectiveness of intra-NAcc PACAP. Taken together, the current dataset provides strong evidence that while PACAP exerts its effects at multiple nodes in the energy homeostasis system, it acts specifically in the NAcc to regulate hedonic drive.
MATERIALS & METHODS
Animals
Male Sprague-Dawley rats (Envigo; Indianapolis, IN) weighing between 250-275 grams, were housed individually in standard tub cages in a climate controlled colony room under a 12:12 light/dark cycle. Animals had ad lib access to standard chow (Harlan Diet #8604) throughout the duration of the study. Additionally, food intake and body weight were recorded daily. All animal procedures were approved by the Marquette University Institutional Animal Care and Use Committee.
Surgery
Animals were anesthetized with ketamine/xylazine/acepromazine (77:1.5:1.5 mg/ml/kg; i.p.) and implanted with an intraoral catheter for tastant delivery, as described previously.13 26-gauge guide cannulae (Plastics One; Roanoke, VA) were stereotaxically placed 2 mm above the nucleus accumbens core (NAcc; anterior/posterior +1.2 mm from bregma; medial/lateral +2.2 mm from midline; dorsal/ventral -4.8 mm from the surface of the skull, at 6° angle) or 3 mm above the ventromedial hypothalamic nucleus (VMN; anterior/posterior, −2.5 mm from bregma; medial/lateral, ± 0.6 mm from midline; dorsal/ventral, −6.2 mm from surface of the skull) and then secured to the surface of the skull using acrylic dental cement.14 Animals were given one week to recover prior to experimentation. Following the conclusion of the study, brains were collected and analyzed for cannula placement using Nissl staining, only those with correct placements were included in the studies.
Microinjections
Pituitary adenylate cyclase-activating polypeptide (PACAP; California Peptide Research; Napa, CA) or saline were microinjected into the VMN (50 pmol/.25μl/side; n=11) or NAcc (100 pmol/.5μl/side; n=10) over a two-minute period in gently restrained awake animals followed by an additional minute to prevent backflow.
Taste Reactivity
Design
Following one week to recover from surgery, animals were habituated to the taste reactivity chamber for 3 consecutive days for 30 minutes/day. On test day, a 50 mL syringe containing a 1% sucrose solution was connected to the animal’s intraoral catheter. The syringe was then locked into a syringe pump, which infused the sucrose solution at a rate of 1mL/min over a 1 minute trial. Each trial commenced 15 min following the intracranial microinfusion, and appetitive and aversive orofacial responses were video recorded using a camera fixed beneath the Plexiglas floor of the testing chamber. Animals were then given a 48-hour washout period before receiving a second, counterbalanced, microinjection of either vehicle or PACAP.
Video scoring
Digital video files of each session were analyzed frame-by-frame over the one-minute test trial. Scored in 10 second bins, occurrences of lateral tongue protrusions and paw licks were counted as appetitive taste reactivity while gapes and paw flails were evidence of aversive taste reactivity, as described in previous reports.15 After the six, ten second bins are scored frame-by-frame, an animal is assigned an “appetitive responses” score which is the cumulative number of appetitive response made by that animal over the one minute trial. Similarly, the animal is assigned an “aversive responses” score which reflects the cumulative number of aversive responses made over the one-minute trial. Analyses were conducted on these composite scores.
Calculating PACAP-induced change in aversive response
Within-subject PACAP-induced changes in aversive responses were normalized to vehicle trial aversive responses. To calculate this, we used the following equation:
Using this calculation, a number greater than zero indicates that PACAP increased aversive responses to the sucrose solution, whereas a number below zero would indicate PACAP decreased aversive responses.
Statistics
Using paired t-tests, group differences between hedonic responses to sucrose pre-and post-intracranial microinfusions were compared using Sigma Plot 11 software (Systat Software Inc.; San Jose, CA). P values < 0.05 were considered statistically significant.
RESULTS
VMN
The data are presented as cumulative appetitive (lateral tongue protrusion + paw licks) and aversive (gapes + paw flails) responses to a sucrose solution following either a vehicle or PACAP microinjection into the ventromedial nuclei of the hypothalamus (VMN). Within-subject results demonstrated that PACAP microinjected into the VMN did not affect the expression of either appetitive (Fig. 1A; paired t-test; t= -0.0625; DF= 10 p=0.951) or aversive responses to the sucrose solution compared to the vehicle trial (Fig. 1B; paired t-test; t= -1.203; DF=10; p=0.257).
Figure 1.
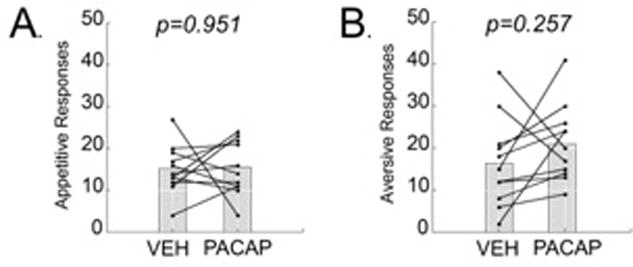
Acute administration of PACAP (50pmol/.25ul) into the VMN did not alter hedonic orofacial responses to a 1% sucrose solution. (A) A VMN PACAP microinjection 15 minutes prior to a taste reactivity test did not significantly alter appetitive responses (lateral tongue protrusions & paw licks) to the sucrose solution. (B) A VMN PACAP microinjection 15 minutes prior to a taste reactivity test did not significantly alter aversive responses (gapes & paw flails) to the sucrose solution. Data from each subject are presented, as well as the group mean. * = p <.05 compared to vehicle injection.
NAcc
A separate group of animals were implanted with cannula targeting the NAcc as well as intraoral catheters and tested in an identical manner. Rats received either PACAP or vehicle 15 minutes prior to taste reactivity testing as described above. Interestingly, PACAP microinjected into the NAcc significantly suppressed appetitive responses (lateral tongue protrusions and paw licks combined) to sucrose (Fig. 2A; paired t-test; t= 3.8; DF=9; p=0.004). Although PACAP did not significantly alter aversive responses to sucrose (gapes and paw flails combined), compared to vehicle (Fig. 2B; paired t-test; t= -1.163; DF=9; p=0.275), the variance in the effect of PACAP appeared to be related to the rostral-caudal placement of the microinjection. Given this observation, and recognizing that pharmacological manipulations have been shown to differentially affect rostral and caudal regions of this nucleus,11 we decided to statistically analyze this trend. A Pearson correlation revealed a strong rostral-caudal influence of PACAP on the change in expression of aversive taste reactivity between vehicle and drug treated groups (r = -0.64, n = 10, p = 0.04; Fig. 2C). Specifically, PACAP microinjected in the caudal NAcc (bregma +1.68 to +1.32) enhanced aversive responses to sucrose, while PACAP microinjected into the rostral NAcc (bregma +2.02 to +1.92) decreased aversive responses.
Figure 2.
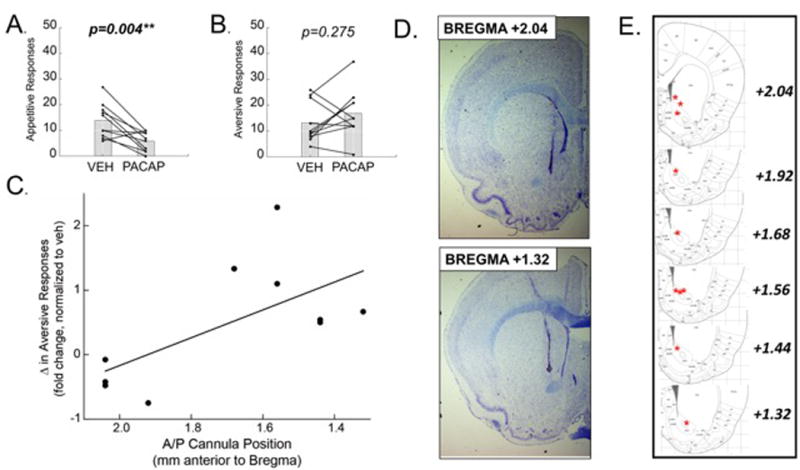
Acute application of PACAP (100pmol/.5ul) into the NAcc significantly decreased appetitive responses to sucrose and increased aversive responses to sucrose in the caudal accumbens. (A) Appetitive responses to 1% sucrose were significantly suppressed following a PACAP microinjection. (B) PACAP did not significantly alter aversive orofacial responses to sucrose. (C) A closer examination of anatomical placements revealed a significant correlation between anatomical placement (rostral-caudal) and fold change in aversive responses, normalized to vehicle treated aversive responses. (D) Representative photomicrographs of coronal (20μm thick) nissl-stained sections demonstrating rostral (TOP) and caudal (BOTTOM) NAcc cannula placements. (E) Representative images adapted from Paxinos & Watson, 2007 identifying cannula tip placement. Data from each subject are presented, as well as the group mean.
DISCUSSION
With overconsumption as a major contributor to the obesity epidemic,16 it reinforces the importance to investigate the mechanisms by which eating beyond satiety is regulated. Previously, we reported that the neuropeptide PACAP acts in the rat NAcc (but not the VMN) to specifically attenuate palatable food intake in satiated rats.10 To better characterize PACAP-induced voluntary reduction in palatable food intake, we used a rodent taste reactivity design to assess if PACAP microinjected into the nucleus accumbens (NAcc) altered hedonic perceptions of sucrose.
In the first experiment, we found that PACAP microinjected into the VMN, an area where PACAP reduces feeding but does not affect palatable food consumption, had no effect on the expression of either positive (appetitive) or negative (aversive) responses to a 1% sucrose solution. This result was not unexpected since our prior work showed that PACAP administered in the VMN only attenuated hunger-driven feeding while having no effect on feeding driven by palatability10. In contrast, administration of PACAP in the NAcc significantly blunted appetitive responses to sucrose without altering the expression of aversive responses overall. However, a closer examination of the anatomical placements of PACAP delivery revealed an additional layer of complexity in the NAcc. By examining the behavioral responses along a rostral-caudal axis, we found that PACAP delivered to the rostral NAcc decreased aversive responses to sucrose, while PACAP microinjected into the caudal NAcc enhanced aversive responses.
PACAP enhancement of aversive taste reactivity in the caudal NAcc is consistent with other studies demonstrating a similar rostral-caudal gradient in the NAcc regulating appetitive and aversive behaviors. For example, pharmacological inactivation of the caudal medial accumbens shell decreases food intake and appetitive responses to sucrose, while increasing aversive responses.11, 17 Whereas, the opposite effect is observed in the rostral NAcc, inactivation stimulates feeding behavior and increases appetitive responses to sucrose while decreasing aversive responses. Moreover, these results support our previous findings that bath application of PACAP in a slice preparation attenuates evoked activity in medium spiny neurons,10 suggesting that PACAP may be producing its region-specific effect on taste reactivity through inhibitory actions.
It is interesting to note that previous research has identified a hedonic regulatory role for GABA, endocannabinoids, and endogenous opioids in the rostromedial NAc shell.18 This report details the effects of PACAP microinfused into the core, based on a prior report that the suppressive effect of PACAP on palatable food intake is restricted to this subregion.10 While there are several possibilities for this apparent discrepancy, a likely explanation is the difference in pharmacological target. This possibility is more intriguing when considered along with the fact that the cell phenotype-specific distribution of PACAP receptors (PAC1R, VPAC1 and VPAC2) in the NAc core and shell subregions is not well characterized. It is likely that resolving receptor distribution and cell-type connectivity will shed light on why PACAP appears to have a unique regulatory role for this signaling pathway in the modulation of hedonic processing. Moreover, it is likely that understanding phenotypic receptor distribution more precisely will reveal insight into the roles of smaller accumbal networks in regulating hedonic processing and motivated behavior.
In order to determine the precise mechanism of action by PACAP in the regulation of motivated behavior, additional studies will be necessary to identify the origin of PACAP projections into this region of the NAcc. One potential source of PACAP-containing afferents to the NAcc is the prefrontal cortex. Interestingly, obese individuals have been reported to display aberrant neural activity in both the prefrontal cortex and the striatum.19, 20 Moreover, transcranial direct current stimulation of the prefrontal cortex in obese individuals has been shown to decrease food intake and weight loss.21 Further defining the contribution of PACAP signaling and its underlying mechanisms to the expression of hedonic perception and motived behavior may yield new therapeutic opportunities for combatting obesity.
Acknowledgments
This work was supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK074734) and the US National Institute on Drug Abuse (NIDA: DA035088). We would like to thank Dr. D. Wheeler for his helpful comments on the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.OECD. OECD Health Data: Non-medical determinants of health. OECD Health Statistics. 2013 [Google Scholar]
- 2.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. Jama. 1999;282(16):1530–8. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008 Dec;37(4):811–23. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saper CB, Chou TC, Elmquist JK. The Need to Feed. Neuron. 36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 6.Matafome P, Seica R. The Role of Brain in Energy Balance. Adv Neurobiol. 2017;19:33–48. doi: 10.1007/978-3-319-63260-5_2. [DOI] [PubMed] [Google Scholar]
- 7.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: From Structure to Functions. Pharmacological Reviews. 2000;52(2):269–324. [PubMed] [Google Scholar]
- 8.Tönshoff C, Hemmick L, Evinger M. Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J Mol Neurosci. 1997;9(2):127–140. doi: 10.1007/BF02736856. [DOI] [PubMed] [Google Scholar]
- 9.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21(6):619–70. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 10.Hurley MM, Maunze B, Block ME, Frenkel MM, Reilly MJ, Kim E, et al. Pituitary Adenylate-Cyclase Activating Polypeptide Regulates Hunger- and Palatability-Induced Binge Eating. Frontiers in Neuroscience. 2016;10(383) doi: 10.3389/fnins.2016.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CY, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: Mapping hotspots of nucleus accumbens and ventral pallidum. Eur J Neurosci. 2014 Nov;40(10):3556–72. doi: 10.1111/ejn.12720. Epub 2014 Sep 17. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS. Carelli Regina M. Behavioral and Electrophysiological Indices of Negative Affect Predict Cocaine Self-Administration. Neuron. 57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler DS, Robble MA, Hebron EM, Dupont MJ, Ebben AL, Wheeler RA. Drug predictive cues activate aversion-sensitive striatal neurons that encode drug seeking. J Neurosci. 2015;35(18):7215–25. doi: 10.1523/JNEUROSCI.4823-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices and activity. Br J Nutr. 2000;83(1):S33–8. doi: 10.1017/s0007114500000933. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21(9):3261–70. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–64. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Meer F, Charbonnier L, Smeets PAM. Food Decision-Making: Effects of Weight Status and Age. Curr Diab Rep. 2016;16:84. doi: 10.1007/s11892-016-0773-z. Epub 2016 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small DM. Individual Differences in the Neurophysiology of Reward and the Obesity Epidemic. Int J Obes (Lond) 2009 Jun;33(Suppl 2):S44–8. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluck ME, Alonso-Alonso M, Piaggi P, Weise CM, Schwartzenberg RJ, Reinhardt M, et al. Noninvasive neuromodulation targeted to the lateral prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2015 Nov;23(11):2149–56. doi: 10.1002/oby.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]


