Abstract
Rationale: CC16 (club cell secretory protein-16), a member of the secretoglobin family, is one of the most abundant proteins in normal airway secretions and has been described as a serum biomarker for obstructive lung diseases.
Objectives: To determine whether low CC16 is a marker for airway pathology or is implicated in the pathophysiology of progressive airway damage in these conditions.
Methods: Using human data from the birth cohort of the Tucson Children’s Respiratory Study, we examined the relation of circulating CC16 levels with pulmonary function and responses to bronchial methacholine challenge from childhood up to age 32 years. In wild-type and CC16−/− mice, we set out to comprehensively examine pulmonary physiology, inflammation, and remodeling in the naive airway.
Measurements and Main Results: We observed that Tucson Children’s Respiratory Study participants in the lowest tertile of serum CC16 had significant deficits in their lung function and enhanced airway hyperresponsiveness to methacholine challenge from 11 years throughout young adult life. Similarly, CC16−/− mice had significant deficits in lung function and enhanced airway hyperresponsiveness to methacholine as compared with wild-type mice, which were independent of inflammation and mucin production. As compared with wild-type mice, CC16−/− mice had significantly elevated gene expression of procollagen type I, procollagen type III, and α-smooth muscle actin, areas of pronounced collagen deposition and significantly enhanced smooth muscle thickness.
Conclusions: Our findings support clinical observations by providing evidence that lack of CC16 in the lung results in dramatically altered pulmonary function and structural alterations consistent with enhanced remodeling.
Keywords: CC16, CCSP, uteroglobin, asthma, COPD
At a Glance Commentary
Scientific Knowledge on the Subject
Although CC16 (club cell secretory protein 16) has been described as a serum biomarker for obstructive lung diseases, a distinct mechanism of action for CC16 in the lung has remained elusive. We sought to determine whether low CC16 is a marker for airway pathology or is implicated in the pathophysiology of progressive airway damage in these conditions.
What This Study Adds to the Field
Participants in the lowest tertile of serum CC16 had significant deficits in their lung function and enhanced airway hyperresponsiveness to methacholine challenge from 11 years throughout young adult life. CC16−/− mice had significant deficits in lung function and enhanced airway hyperresponsiveness to methacholine as compared with wild-type mice. In mice these associations seem to be attributable to increased airway remodeling and suggest that low CC16 is not only a biomarker for airway pathology but may be implicated in the pathophysiology of the progressive airway damage that characterizes obstructive lung diseases.
CC16 (club cell secretory protein 16), also known as Clara cell secretory protein and uteroglobin, has been the topic of much study in the last several years as a biologic marker of lung function alterations. CC16 is a homodimeric pneumoprotein encoded by the SCGB1A1 gene and, although produced predominantly by club cells and nonciliated epithelial cells in the distal airways, it is easily detectable in the circulation (1, 2). Although CC16 is one of the most abundant proteins in BAL fluid and is increased by several proinflammatory cytokines, oxidant-rich air pollutants, such as ozone and cigarette smoke, lead to decreased CC16 levels (for a review, see Reference 3).
Although the biologic functions of CC16 in the lung have not been completely described, mounting evidence suggests that this protein is critical in mediating antiinflammatory and antioxidant functions within the lung and, by virtue of these activities, may protect against development of obstructive lung diseases. Several clinical and epidemiologic studies have shown decreased serum CC16 levels in individuals with asthma (4, 5), chronic obstructive pulmonary disease (COPD) (6, 7), and lung function deficits (8). In a recent study, we examined several independent epidemiologic cohorts and found that low circulating CC16 levels predicted accelerated lung function decline in adult life (9). We also found that CC16 deficits at age 6 years were associated with lower levels of FEV1 by age 16. However, no study has addressed the relationship of serum CC16 to lung function from childhood into adult life. In addition, despite the previously mentioned epidemiologic evidence, mechanistic insight defining the function of CC16 in the lung environment that would protect against lung function deficits has been elusive.
Therefore, whether low CC16 is a marker for airway pathology or is implicated in the pathophysiology of progressive airway damage in these conditions remains unclear. To gain a better understanding of the role of CC16 in establishment of lung function we used data from the birth cohort of the Tucson Children’s Respiratory Study (TCRS) and examined the relationship of circulating CC16 levels with pulmonary function and responses to bronchial methacholine challenge from childhood up to age 32 years. In parallel, we set out to comprehensively examine pulmonary physiology in mice sufficient or deficient in CC16 and determine if any underlying inflammation or remodeling in the naive airway would lead to altered physiology.
Methods
Studies in Humans: the TCRS Birth Cohort
The human data of the present study come from the longitudinal TCRS birth cohort, which recruited 1,246 healthy infants between 1980 and 1984 (10) and followed them up to age 32 years. At ages 11, 16, 22, 26, and 32 years, standardized questionnaires on respiratory health and spirometric lung function tests were completed as previously described (11). Lung function was measured before and after administration of albuterol via a valved chamber holding device (180 μg at ages 11, 16, and 22; and 360 μg at ages 26 and 32). Active physician-diagnosed asthma was defined at each survey as a positive report on questionnaire of physician-confirmed asthma plus at least one asthma attack and/or wheezing episode in the previous year. Participants completed confidential questionnaires on active smoking starting at age 11. Consistent with the National Center for Health Statistics (12), at each survey we defined as never-smokers participants who had never smoked or who had smoked less than 100 cigarettes in their lifetime.
Methacholine challenge tests were performed at ages 11, 16, 22, and 26 with a controlled inhalation protocol using a dosimetric method modified after Chai and coworkers (13). This protocol doubles the cumulative methacholine dose from 0.004 to 2.048 mg with the endpoint defined as a 20% drop in FEV1 or completion of the final dose. Participants who did not have a 20% drop in FEV1 were defined as negative for airway hyperresponsiveness (AHR). For those who had a 20% drop, the provocative concentration of methacholine causing a 20% fall in FEV1 was calculated using the formula described by Dell and coworkers (14) and tertiles of mild, moderate, and severe AHR using the provocative concentration of methacholine causing a 20% fall in FEV1 were generated at each survey.
Studies in Humans: Blood Assays
Blood samples were collected from participants by venipuncture at ages 11, 16, 22, 26, and 32. Circulating CC16 levels were measured in serum using a commercially available ELISA kit (BioVendor). At each survey, serum CC16 levels were log-transformed and z-scored to normalize their distribution and CC16 tertiles (low, medium, high) were generated to test for nonlinear relationships.
Total IgE levels were assayed by Phadebas Radioimmunosorbent Test using commercially available kits (Pharmacia Diagnostics) at Year 11; by Pharmacia AutoCAP (Pharmacia/Upjohn) at Years 16 and 22 until its discontinuation; and subsequently by Immulite 2000 (Siemens Medical Solutions) for the rest of Year 22, and at Years 26 and 32.
Pulmonary Function Studies in Mice
All experiments were done in accordance with University of Arizona on Institutional Animal Care and Use Committee approved animal protocols. Age-matched (8–10 wk) wild-type (WT) (n = 16), and CC16−/− (15) (n = 16) male and female mice on a C57BL/6J background were analyzed for pulmonary mechanics on the Flexivent (SCIREQ Inc.) system for both baseline differences (no methacholine challenge). An additional set of age-matched (8–10 wk) male mice (n = 17 WT; n = 19 CC16−/−) were examined for responses to methacholine challenge as previously described (16, 17). Pancuronium bromide (0.8 mg/ml in saline; Sigma P1918), was administered to anesthetized mice via intraperitoneal injection at a volume of 10 μl/g of body weight to prevent any interference from the animal during the pulmonary function tests. Following a brief equilibration period under default mechanical ventilation settings (150 breaths/min, tidal volume of 10 ml/kg, and a positive end-expiratory pressure of 3 cm H2O), two maneuvers (inflation to a standard pressure of 30 cm H2O over 3 s and holding for an additional 3 s) were performed to open closed lung areas and standardize lung volume history. Single frequency (Snapshot-150; 2.5 Hz) and broadband (Quick Prime-3; 1–20.5 Hz) forced oscillation technique measurements were alternated a few seconds apart for a total of 12 measurements per perturbation over a period of approximately 3 minutes.
This protocol was repeated a total of four times with increasing concentrations of methacholine (0, 10, 30, 100 mg/ml) as previously described (18). Experiments were conducted with naive mice and the maximum value for each parameter was used to compare the differences between the two groups (WT and CC16−/−) of mice. Rt is total respiratory system resistance. G is tissue damping, which reflects a measure of the amount of energy that is lost within the tissues as a result of friction. H is tissue elastance, which is an index of tissue stiffness and represents the ability of the tissues to retract to its original shape.
Analysis of Pulmonary Inflammation in Mice
After mice were assessed for pulmonary function tests, lungs were lavaged with 0.1 mM ethylenediaminetetraacetic acid to analyze inflammatory cells. Cytospins were examined from each mouse and inflammatory cells were assessed by differential staining with hematoxylin-eosin. Additionally, lungs were harvested from the same group of naive mice and markers of inflammation, TNF-α (tumor necrosis factor-α) (forward: CAT CTT CTC AAA ATT CGA GTG ACA A; reverse: TGG GAG TAG ACA AGG TAC AAC CC), and Muc5AC (forward: GAG GGC CCA GTG AGC ATC TCC; reverse: TGG GAC AGC AGT ATT CAG T) were examined by RT-PCR using a Sybr-green compatible system.
One lobe from each mouse was sent for histologic examination by periodic acid–Schiff and pentachrome staining. Mucin production as assessed by periodic acid–Schiff stained formalin-fixed and inflated lung sections were quantified as previously described (19, 20).
Morphometric Studies to Measure Airspace Enlargement in Mice
After mice were humanely killed, the lungs were inflated with phosphate-buffered saline to 25 cm H2O pressure, removed, fixed in 10% formalin, and embedded in paraffin. Mid-sagittal lung sections (8 μm thick) were stained with Gill stain. For each mouse, all areas of well-inflated sections of lung in both lungs were captured at ×100 magnification using a Nikon microscope. Scion Image software (Scion Corp.) was used to measure the alveolar chord length for each animal, as described previously (21).
Analysis of Airway Remodeling Factors in Mice
Histologic slides at ×10 magnification were photographed and examined in a blinded manner by a noncoauthor for smooth muscle thickness. Briefly, a large airway was identified for each slide that was sent for histologic analysis and if a large airway was present a representative photograph was taken for further analysis. From the representative picture of the large airway, five random measurements were taken that spanned the smooth muscle layer adjacent to the large airway by ImageJ software. The average of the five random measurements was graphed from all large airways that were available for analysis. Data shown are the mean ± SEM (n = 8 WT; n = 7 CC16−/−) for each large airway and statistical analysis was conducted in Prism software.
Factors involved in remodeling, procollagen 1 (forward: AGA CAT GCT CAG CTT TGT GGA TAC; reverse: CGT ACT GAT CCC GAT TGC AAA T), procollagen 3 (forward: GCC CAC AGC CTT CTA CAC; reverse: CCA GGG TCA CCA TTT CTC), α-smooth muscle actin (forward: TC GTC CAC CGC AAA TGC; reverse: AAG GAA CTG GAG GCG CTG), transforming growth factor-β (forward: GTG CGG CAG CTG TAC ATT GAC TTT; reverse: TGT ACT GTG TGT CCA GGC TCC AAA), and platelet-derived growth factor subunit B (forward: AGC TAC ATG GCC CCT TAT GA; reverse: GGA TCC CAA AAG ACC AGA CA) were examined in lung tissue from naive mice by RT-PCR using a Sybr-green compatible system.
Statistical Analysis for Human and Mice Studies
In the TCRS cohort, the relation of serum CC16 to lung function parameters (FEV1, FVC, FEV1/FVC, and forced expiratory flow between 25% and 75% of the FVC [FEF25–75]) was tested in multivariate regression models with subject-clustered sandwich estimators of SE to control for the serial correlation of repeated intrasubject observations. Because in the mice models the phenotype was evaluated at age 8–10 weeks (equivalent to young adult life in humans) and because in humans at ages 11–16 years lung function is still growing, primary analyses included lung function parameters between ages 22 and 32 years. However, additional models were performed using complete lung function data from ages 11 to 32 years. For these models, to control for the substantially lower levels of lung function in childhood than adult life, we first z-scored each lung function parameter within each survey. These z-scored lung function data were then used as the dependent variable of regression models. Because AHR was categorized in four groups (negative, mild, moderate, and severe), multinomial logistic regression models were used to determine the association of serum CC16 levels with different AHR levels. In these models, subject-clustered sandwich estimators of SE were also used.
All regression and multinomial logistic regression models were adjusted for sex, survey year, ethnicity, parental education, and maternal smoking at Year 6, which we recently reported as significant predictors of trajectories of circulating CC16 from childhood into adult life (22). To rule out the possibility that associations of serum CC16 with lung function and AHR were caused by confounding by asthma and active smoking, results from all models were also confirmed after restricting analyses to participants with no asthma and participants who did not smoke. Statistical analyses were performed with STATA version 15.
For all mouse experiments, statistics were analyzed using Prism 7 (GraphPad). For lung function analysis of naive mice, WT and CC16 baseline measures were compared using Student’s t test. For analysis of data collected during methacholine challenges that were recorded on separate experimental days, resistance data were log10 transformed and differences between WT and CC16−/− determined by Student’s t test at each respective methacholine dose according to previous publications (23).
In both human and mice studies, two-sided P values of less than 0.05 were regarded as significant.
Results
TCRS Participants with Circulating CC16 Deficits Have Lower Lung Function and Enhanced AHR from Age 11 to 32 Years
Overall, 707 TCRS participants had available serum CC16 and lung function data in one or more surveys at ages 11, 16, 22, 26, and 32 years and were included in this study. Their characteristics at each survey are shown in Table 1. As compared with the 539 TCRS participants without these data that were excluded from analyses, they were more likely to be Hispanic and to have older mothers and higher parental education, but there were no significant differences between included and excluded participants in relation to sex, body mass index, active asthma, or maternal smoking (data not shown).
Table 1.
Characteristics of TCRS Participants with Serum CC16 and Lung Function Data Available at Different Surveys
| Year 11 (n = 540) | Year 16 (n = 425) | Year 22 (n = 424) | Year 26 (n = 321) | Year 32 (n = 248) | |
|---|---|---|---|---|---|
| Serum CC16, ng/ml, geometric mean (n) | 7.52 (540) | 8.50 (425) | 11.21 (424) | 9.52 (321) | 10.63 (248) |
| Age, yr, mean ± SD (n) | 10.7 ± 0.561 (539) | 16.5 ± 0.552 (420) | 21.8 ± 0.681 (423) | 26.3 ± 0.678 (319) | 31.7 ± 1.01 (248) |
| Male, % (n) | 49.4 (267) | 50.4 (214) | 48.6 (206) | 47.7 (153) | 44.4 (110) |
| Parental ethnicity, % (n) | |||||
| NHW/NHW | 58.2 (314) | 60.2 (256) | 62.5 (265) | 62.0 (199) | 60.9 (151) |
| NHW/Hispanic | 14.3 (77) | 13.4 (57) | 14.6 (62) | 14.0 (45) | 12.9 (32) |
| Hispanic/Hispanic | 14.3 (77) | 12.2 (52) | 11.6 (49) | 11.8 (38) | 12.9 (32) |
| Other | 13.3 (72) | 14.1 (60) | 11.3 (48) | 12.2 (39) | 13.3 (33) |
| BMI, mean ± SD (n) | 19.1 ± 3.98 (540) | 23.2 ± 5.45 (425) | 25.7 ± 6.68 (424) | 27.4 ± 7.69 (321) | 29.1 ± 8.91 (248) |
| Height, cm, mean ± SD (n) | 145 ± 8.20 (540) | 170 ± 9.30 (425) | 171 ± 9.68 (424) | 172 ± 10.0 (321) | 171 ± 10.3 (248) |
| Active smoking, % (n/N) | |||||
| Nonsmoking | 98.0 (432/441) | 69.1 (282/408) | 44.6 (189/424) | 43.0 (137/319) | 44.4 (110/248) |
| Current | 0.23 (1) | 10.3 (42) | 29.0 (123) | 25.7 (82) | 16.1 (40) |
| Former | 1.81 (8) | 20.6 (84) | 26.4 (112) | 31.4 (100) | 39.5 (98) |
| Active asthma, % (n/N) | 16.1 (86/533) | 21.3 (89/417) | 19.0 (80/422) | 21.0 (66/315) | 20.2 (50/248) |
| Maternal age, yr, mean ± SD (n) | 27.5 ± 4.56 (540) | 27.6 ± 4.35 (425) | 27.7 ± 4.54 (424) | 27.3 ± 4.77 (321) | 27.6 ± 4.37 (248) |
| Mother smoked at Year 6, % (n/N) | 19.5 (103/529) | 18.1 (76/420) | 17.5 (72/412) | 18.7 (58/311) | 19.1 (46/241) |
| Parental education, % (n/N) | |||||
| Both >12 yr | 62.6 (329/526) | 65.2 (272/417) | 65.6 (274/418) | 66.4 (211/318) | 66.5 (163/245) |
| Only mother >12 yr | 9.89 (52) | 8.87 (37) | 9.33 (39) | 10.1 (32) | 10.2 (25) |
| Only father >12 yr | 8.56 (45) | 10.1 (42) | 9.33 (39) | 8.81 (28) | 8.98 (22) |
| Neither >12 yr | 19.0 (100) | 15.8 (66) | 15.8 (66) | 14.8 (47) | 14.3 (35) |
Definition of abbreviations: BMI = body masss index; CC16 = club cell secretory protein 16; NHW = non-Hispanic white; TCRS = Tucson Children’s Respiratory Study.
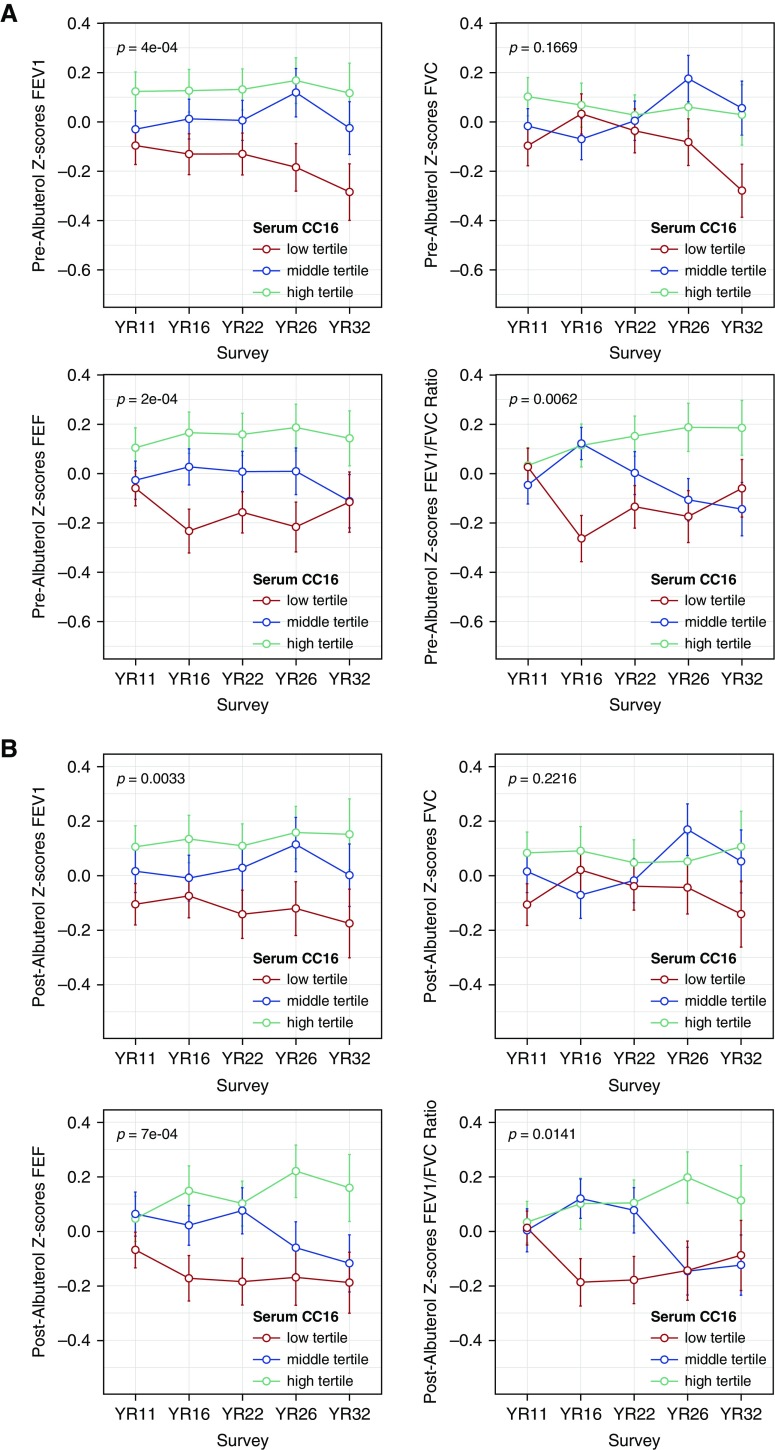
As we reported previously (22), serum CC16 levels increased from childhood (geom. means: 7.5 and 8.5 ng/ml at ages 11 and 16, respectively) into adult life (11.2, 9.5, and 10.6 ng/ml at ages 22, 26, and 32, respectively). Both in male and female participants, lower tertiles of CC16 were associated with lower levels of most lung function indices and these associations were particularly evident in adult life (see Figure E1A in the online supplement). After adjusting for sex, survey year, ethnicity, parental education, maternal smoking, and height, we found serum CC16 levels to be positively associated with lung function levels attained between ages 22 and 32 years for FEV1, FEF25–75, and the FEV1/FVC ratio, but not FVC (Table 2). As compared with participants in the highest CC16 tertile, those in the lowest CC16 tertile had on average deficits of 133 ml (P = 0.002), 304 ml/s (P = 0.001), and 2% (P = 0.002) for FEV1, FEF25–75, and FEV1/FVC, respectively. Subjects with medium CC16 had intermediate levels of lung function.
Table 2.
| Prealbuterol (n Subjects = 505; n Observations = 952) |
||||||||
|---|---|---|---|---|---|---|---|---|
| FEV1 (ml) |
FVC (ml) |
FEF25–75 (ml/s) |
FEV1/FVC Ratio |
|||||
| z-Scores CC16‡ | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value |
| Continuous CC16 | 56.5 (21.8 to 91.3) | 0.001 | 19.7 (−23.0 to 62.3) | 0.365 | 122 (47.1 to 197) | 0.001 | 0.78 (0.25 to 1.31) | 0.004 |
| Category | ||||||||
| High CC16 | (Reference group) |
|||||||
| Middle CC16 | −35.2 (−111 to 40.3) | 0.360 | 46.5 (−36.7 to 130) | 0.273 | −170 (−330 to −9.8) | 0.038 | −1.49 (−2.59 to −0.40) | 0.008 |
| Low CC16 | −133 (−218 to −47.9) | 0.002 | −41.0 (−139 to 57.4) | 0.414 | −304 (−479 to −129) | 0.001 | −1.98 (−3.20 to −0.76) | 0.002 |
| Postalbuterol (n Subjects = 488; n Observations = 901) |
||||||||
|---|---|---|---|---|---|---|---|---|
| FEV1 (ml) |
FVC (ml) |
FEF25–75 (ml/s)§ |
FEV1/FVC Ratio |
|||||
| z-Scores CC16‡ | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value |
| Continuous CC16 | 44.1 (9.4 to 78.8) | 0.013 | 17.0 (−26.9 to 60.9) | 0.446 | 116 (40.8 to 192) | 0.003 | 0.57 (0.11 to 1.03) | 0.014 |
| Category | ||||||||
| High CC16 | (Reference group) |
|||||||
| Middle CC16 | −13.4 (−86.8 to 59.9) | 0.719 | 31.2 (−55.7 to 118) | 0.480 | −153 (−323 to 15.9) | 0.076 | −0.88 (−1.84 to 0.07) | 0.070 |
| Low CC16 | −103 (−187 to −19.3) | 0.016 | −31.4 (−133 to 69.9) | 0.543 | −316 (−501 to −131) | 0.001 | −1.56 (−2.64 to −0.47) | 0.005 |
Definition of abbreviations: CC16 = club cell secretory protein 16; CI = confidence interval; Coef = coefficient; FEF25–75 = forced expiratory flow between 25% and 75% of the FVC.
Lung function was assessed before and after albuterol.
Subject-clustered sandwich estimators of SE were used in linear regression models to adjust for within-subject correlation. All models were adjusted for sex, survey year, ethnicity, parental education, maternal smoking at Year 6, and height (cm).
Circulating CC16 levels were included as z-scores in the model.
n subjects = 487; n observations = 895.
Similar results were obtained when we analyzed post-bronchodilator lung function (bottom part of Table 2; see Figure E1B), when we restricted analyses to participants with no asthma (see Table E1) and participants who did not smoke (see Table E2), and when we further adjusted for total IgE levels (see Table E3). In additional analyses completed on standardized levels of lung function across the entire age range 11–32 years (see Methods section and Figures 1A and 1B), we were able to confirm the direct association of serum CC16 with FEV1, FEF25–75, and FEV1/FVC both in the total population (see Table E4) and among participants with no asthma (see Table E5) and participants who did not smoke (see Table E6).
Figure 1.
z-Scores of FEV1, FVC, forced expiratory flow between 25% and 75% of the FVC, and FEV1/FVC ratio. (A) Before bronchodilator (n subjects = 706) and (B) after bronchodilator (n subjects = 689) from age 11 to age 32 years by serum CC16 (club cell secretory protein 16) tertile categories. Lung function indices were standardized separately for males and females. Error bars indicate the standard error. P values refer to the overall comparison of lung function levels across the three CC16 tertiles. FEF25–75 = forced expiratory flow between 25% and 75% of the FVC.
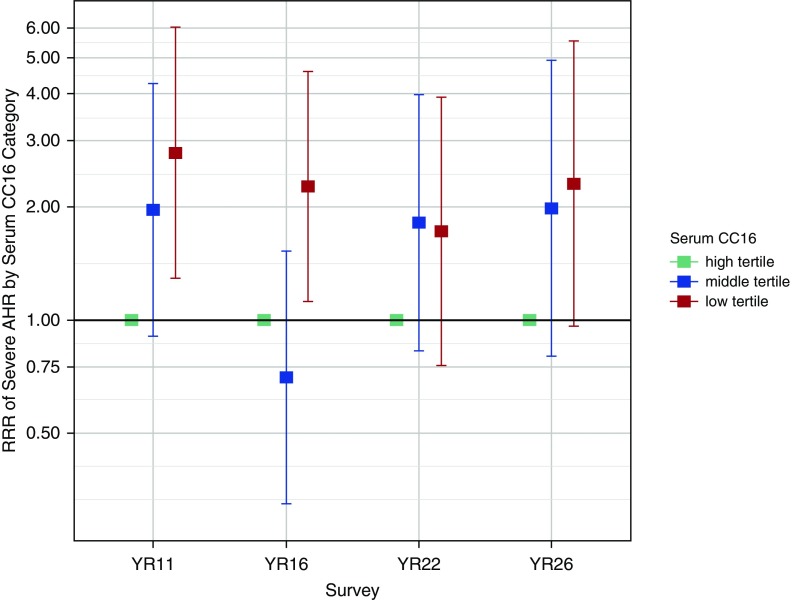
Similar protective associations of serum CC16 were found against AHR. In multivariate multinomial logistic regression models, each SD increase in serum CC16 was associated with a greater than 30% reduction in the risk for severe AHR (P < 0.001) and participants in the lowest CC16 tertile had a 130% increase in their risk for severe AHR as compared with participants in the highest CC16 tertile (adjusted relative risk ratio, 2.30; P < 0.001) (Table 3 and Figure 2). Consistent with the lung function results, these associations with AHR were confirmed when analyses were restricted to participants with no asthma and participants who did not smoke (Table 3) and when models were further adjusted for total IgE levels (see Table E7).
Table 3.
Multinomial Logistic Regression Models for Airway Hyperresponsiveness as Measured from Age 11 to Age 26 Years*
| Airway Hyperresponsiveness | z-Score CC16 | All Participants (n Subjects = 555; n Observations = 1,228) |
No Asthma (n Subjects = 493; n Observations = 1,033) |
Nonsmoker (n Subjects = 485; n Observations = 894) |
|||
|---|---|---|---|---|---|---|---|
| adjRRR (95% CI) | P Value | adjRRR (95% CI) | P Value | adjRRR (95% CI) | P Value | ||
| Continuous CC16† | |||||||
| No drop | (Base outcome) |
||||||
| Mild | 0.92 (0.77–1.10) | 0.352 | 0.89 (0.73–1.09) | 0.270 | 0.88 (0.70–1.09) | 0.232 | |
| Moderate | 0.75 (0.63–0.89) | 0.001 | 0.76 (0.62–0.92) | 0.006 | 0.63 (0.50–0.80) | <0.001 | |
| Severe | 0.66 (0.55–0.81) | <0.001 | 0.63 (0.50–0.79) | <0.001 | 0.58 (0.45–0.73) | <0.001 | |
| Category† | |
||||||
| No drop | (Base outcome) |
||||||
| Mild | High CC16 | (Reference group) |
|||||
| Middle CC16 | 1.18 (0.80–1.73) | 0.404 | 1.22 (0.81–1.82) | 0.343 | 1.41 (0.91–2.18) | 0.126 | |
| Low CC16 | 1.19 (0.77–1.84) | 0.426 | 1.21 (0.77–1.91) | 0.405 | 1.38 (0.82–2.32) | 0.221 | |
| Moderate | High CC16 | (Reference group) |
|||||
| Middle CC16 | 1.25 (0.84–1.86) | 0.274 | 1.25 (0.81–1.94) | 0.317 | 1.53 (0.94–2.48) | 0.088 | |
| Low CC16 | 1.86 (1.22–2.85) | 0.004 | 1.88 (1.19–2.98) | 0.007 | 2.62 (1.57–4.39) | <0.001 | |
| Severe | High CC16 | (Reference group) |
|||||
| Middle CC16 | 1.45 (0.96–2.18) | 0.078 | 1.80 (1.12–2.89) | 0.015 | 1.52 (0.97–2.39) | 0.067 | |
| Low CC16 | 2.30 (1.46–3.61) | <0.001 | 2.64 (1.54–4.51) | <0.001 | 3.05 (1.80–5.18) | <0.001 | |
Definition of abbreviations: adjRRR = adjusted relative risk ratio; CC16 = club cell secretory protein 16; CI = confidence interval.
Subject-clustered sandwich estimators of SE were used in multinomial logistic regression models to adjust for within-subject correlation. All models were adjusted for sex, survey year, ethnicity, parental education, and maternal smoking at Year 6. Airway hyperresponsiveness severity (mild, moderate, severe) was defined at each survey based on tertiles of provocative concentration of methacholine causing a 20% fall in FEV1 (see Methods section for more information).
Circulating CC16 levels were included as z-scores in the model.
Figure 2.
Adjusted relative risk ratios for severe airway hyperresponsiveness (AHR). From multinomial logistic regression models by serum CC16 (club cell secretory protein 16) tertile categories from age 11 to age 26 years (n subjects = 555; n observations = 1,228). High CC16 is the reference category. Error bars indicate 95% confidence intervals. Estimates for mild and moderate AHR are not presented in the figure, but they are reported in Table 3. No AHR is the reference group for both estimates presented in the figure and in Table 3. RRR = relative risk ratio.
CC16-Deficient Mice Have Altered Pulmonary Function at Baseline
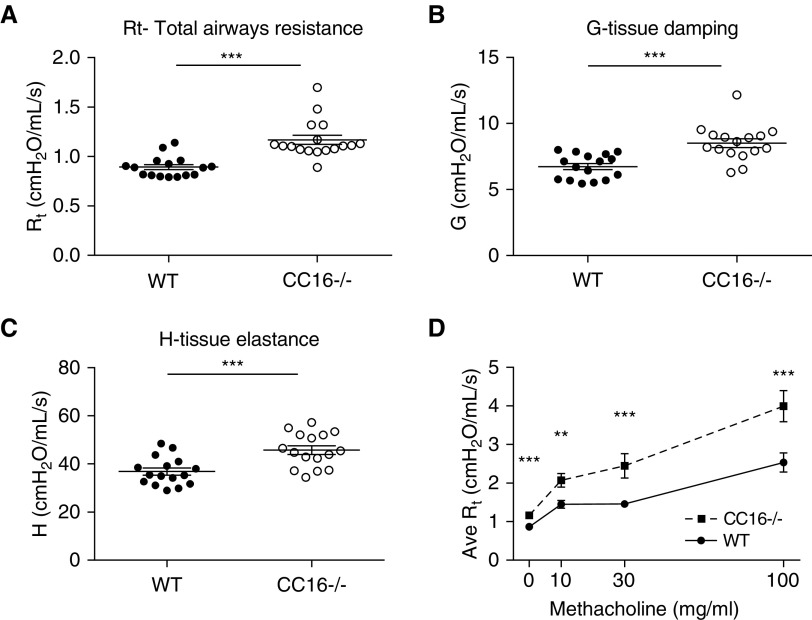
Next, we characterized mice deficient in CC16 and found them to have significantly elevated total airway resistance (Rt average, 1.17 ± SEM 0.04 cm H2O/ml/s) as compared with WT control mice (Rt average, 0.89 ± SEM 0.03 cm H2O/ml/s) (Figure 3A; P < 0.0001). In addition, CC16-deficient mice demonstrated higher resistance in the peripheral airways as detected by increased G-tissue resistance (WT average G, 6.7 ± SEM 0.23; CC16−/− average G, 8.5 ± 0.34 cm H2O/ml/s) (Figure 3B, P = 0.0002) and H-tissue elastance (WT average H, 36.84 ± SEM 1.45; CC16−/− average H, 45.77 ± SEM 1.77 cm H2O/ml/s) (Figure 3C; P = 0.0005) as compared with WT mice.
Figure 3.
Pulmonary mechanics are altered in CC16 (club cell secretory protein 16)-deficient mice. Age-matched naive mice either sufficient (wild-type [WT], n = 16) or deficient in CC16 (CC16−/−; n = 16) were assessed using a Flexivent device for airway physiology measurements of (A) total airway resistance, (B) tissue damping, and (C) tissue elastance. ***P < 0.001 by Student’s t test. (D) An additional set of age-matched naive male mice either sufficient (WT, n = 17) or deficient in CC16 (CC16−/−, n = 19) were assessed using a Flexivent device for airway physiology measurements during increasing doses of aerosolized methacholine, **P < 0.01, ***P < 0.001 by Student’s t test. Data shown are the mean ± SEM. Ave = average; G = tissue damping; H = tissue elastance; Rt = total airway resistance.
CC16-Deficient Mice Have Enhanced Airway Resistance to Methacholine Challenge
An additional set of mice were analyzed for total airway resistance during methacholine challenge. As observed in the previously mentioned baseline measurements of unchallenged mice, CC16−/− mice had significantly higher baseline resistance as compared with WT mice (P < 0.001). With increasing doses of methacholine, CC16−/− mice displayed higher total airway resistance as compared with WT mice at all doses tested: 10 mg/ml (P < 0.01), 30 mg/ml (P < 0.001), and 100 mg/ml (P < 0.05) (Figure 3D).
CC16-Deficient Mice Do Not Display Signs of Lung Inflammation
BAL fluid was collected from the naive mice that were assessed on the Flexivent for pulmonary function. There were no significant differences in leukocytes in the BAL fluid between the WT and CC16−/− mice (see Figures E2A–E2C). Most BAL cells were macrophages (>95%) and less than 5% of the total BAL cells were neutrophils in both groups.
Inflammation in the lung tissue was also examined by RT-PCR for the proinflammatory cytokine, TNF-α, and for mucin gene, Muc5AC. No differences were observed for either TNF-α or Muc5AC in the lung tissue of WT and CC16−/− mice (see Figures E2D and E2E). TNF-α protein in BAL was not detectable by ELISA from either WT or CC16−/− mice (data not shown). Mucin expression did not differ between WT and CC16−/− mice as determined by periodic acid–Schiff score from histologic sections (see Figure E2F).
CC16 Deficiency Does Not Alter Mean Alveolar Airspace in Naive Mice
Similar to observations previously described for CC16−/− mice 14 weeks of age or younger (19), the mean distal airspace size was similar in the naive adult WT and CC16−/− adult mice in which the pulmonary function tests were conducted (see Figures E2G and E2H).
CC16-Deficient Mice Display Enhanced Remodeling Features
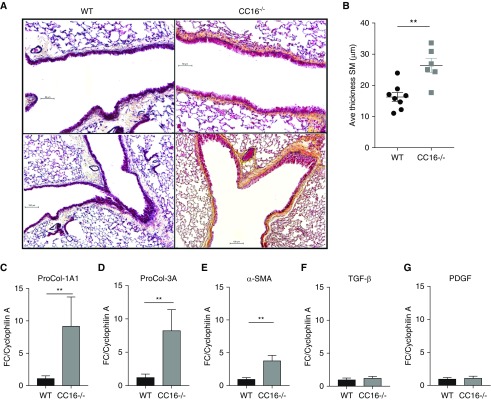
Pentachrome stained histologic sections from CC16-deficient mice revealed large areas of thickened basement membrane and smooth muscle and visibly more collagen deposition (yellow stain) as compared with WT mice (Figure 4A). To quantitate these differences, smooth muscle thickness was measured on histologic sections using ImageJ software and RT-PCR of lung tissue was performed to assess gene expression of factors associated with collagen deposition and lung remodeling. On average, mice lacking CC16 had significantly greater smooth muscle thickness (mean ± SEM, 26.4 ± 2.3 μm) as compared with WT mice (mean ± SEM, 16.3 ± 1.4 μm) of the same sex and age (Figure 4B). In addition, PCR analysis revealed that collagen type I (Col1A1), collagen type III (Col3A1), and α-smooth muscle actin transcripts were all significantly elevated in the lungs of CC16−/− mice compared with WT mice (Figures 4C–4E). Other factors associated with remodeling, transforming growth factor-β and platelet-derived growth factor, were not elevated in CC16−/− mice as compared with WT control animals (Figures 4F and 4G).
Figure 4.
Remodeling factors are increased in the lungs of naive CC16 (club cell secretory protein 16) knockout mice. (A) Representative pentachrome-stained lung sections from wild-type (WT) and CC16−/− naive mice. Scale bars: upper panels, 50 μm; lower panels, 100 μm. (B) Quantitative analysis of smooth muscle thickness was measured on pictures taken at ×10 magnification using ImageJ software as described in the Methods section. n = 8 and 7 WT and CC16−/−, respectively. (C–G) Quantitative RT-PCR analysis for Pro-Col1A1 (C), Pro-Col3A (D), α-sma (E), Tgf-β (F), and Pdgf (G) gene expression in lung tissue from naive WT and CC16−/− mice. Graphed gene expression data are the mean ± SD, **P < 0.01 by Student’s t test. n = 12 and 16 WT and CC16−/−, respectively. Ave = average; FC = fold over control; PDGF = platelet-derived growth factor; Pro-Col = procollagen; SM = smooth muscle; SMA = smooth muscle actin; TGF = transforming growth factor.
Discussion
Although the biologic functions of CC16 have not been completely described, mounting evidence suggests that this protein is associated with protection against development of obstructive lung diseases in humans. However, whether this association is causal remains unclear. To the best of our knowledge, we are the first to report that naive CC16−/− mice have significantly impaired lung function and heightened sensitivity to methacholine, which we observed by 8 weeks of age independent of detectable inflammation and mucin production. These results paralleled our observations of impaired lung function and enhanced AHR in participants with CC16 deficits from the TCRS birth cohort. Indeed, in this same cohort we had previously reported that CC16 levels at age 6 years predicted lung function levels attained by age 16 (9). Here, by analyzing new extensive CC16 data from age 11 to 32 years, we demonstrated that serum CC16 is directly related to both higher lung function and reduced AHR in humans from childhood well into adult life. Furthermore, we determined that the decreased pulmonary function detected inunder CC16−/− mice was accompanied by airway remodeling, which was evident in histologic sections and with a significant upregulation of genes known to accompany this process.
Previous studies have shown that airway and circulating levels of CC16 are diminished in individuals with lung function impairment (8, 9) and in patients with the two most common obstructive lung diseases: asthma (4, 5) and COPD (6, 7). Animal studies suggest a possibly causal nature for these associations. Although with some conflicting results (24), mice studies have shown that CC16−/− animals are more likely to develop COPD-like phenotypes following exposure to cigarette smoking as compared with CC16-sufficient mice (19, 25). Similarly, CC16-deficient mice have markedly increased susceptibility, airway inflammation, and tissue remodeling when exposed to a variety of insults (15, 26–33), including airway infections by respiratory syncytial virus, adenovirus, and Pseudomonas aeruginosa (30, 34). These animal data are also consistent with human studies showing that acute environmental exposures can cause transitory increases in systemic CC16 levels, but repeated noxious exposures (e.g., cigarette smoking) result in chronic deficits of serum CC16, which in turn are associated with poor lung health outcomes (2, 35–41). Thus, there is a need to better understand whether low CC16 levels in the lung simply reflect the epithelial damage and airway remodeling that accompany obstructive lung diseases, in which CC16-producing cells may be present in fewer numbers (42), or whether loss of CC16 is a direct contributing factor that may precede the effects of environmental exposures in the establishment and progression of these diseases.
In support of a causal role are recent reports that show that CC16 inhibits serum amyloid-A-driven inflammation by direct interaction with the lipoxin A4 receptor (43), which is present on airway epithelial cells and is upregulated on macrophages in severe asthma (44). Serum amyloid-A, an apolipoprotein secreted during the acute phase of inflammation, is known to recruit immune cells to sites of inflammation via upregulation of vascular cell adhesion molecule-1 and is associated with severe asthma (44). Previous studies had also shown inhibitory effects of CC16 on the activity of secretory and intracellular phospholipase A2 (45, 46), a regulator of the eicosanoid pathway and, in turn, of its downstream inflammatory mediators. In addition, CC16 has been shown to bind fibronectin and inhibit the adhesion and migration of endothelial cells (47), which may have implications in cancer, and some mechanistic insight has been attributed to CC16 by virtue of its modulating action on transglutaminase (48). Future studies are needed to determine if these or others mechanisms are involved in the role of CC16 in lung diseases.
Two previous studies examined lung mechanics in CC16−/− mice and warrant discussion in relation to our studies (19, 32). The first study used noninvasive whole-body plethysmography (Buxco) to measure airway reactivity/resistance (measured as Penh) in WT and CC16−/− mice on a 129 background and reported differences between the CC16−/− and WT control mice at the highest dose of methacholine challenge (32). Although Penh has largely been questioned as a valid measurement of airway resistance in mice (49, 50), their finding of baseline differences between WT and CC16−/− naive mice is in line with our findings. A more recent study reported no differences in pressure–volume flow loops using a Flexivent device in CC16−/− versus WT mice at approximately 8 months of age (19). However, it is difficult to fully compare results from this study with ours because overall airway resistance and sensitivity to methacholine were not assessed and because mice were substantially older than those evaluated in our study. These studies did not find differences in alveolar chord length in mice that were 8 weeks old, which is consistent with our findings (19).
Despite the differences we detected in lung function in mice sufficient and deficient in CC16, we did not find any difference in underlying inflammation between the groups. Attributes typically reported for such studies, such as increases in inflammatory cells in the BAL fluid, mucin production, and the proinflammatory cytokine TNF-α, were not different in CC16-deficient and CC16-sufficient mice under unchallenged conditions. Interestingly, a recent report shows that aged CC16−/− mice (18 mo) develop accelerated rates of pulmonary function test abnormalities, which are associated with enhanced NF-κB–driven inflammation (51). Because inflammation was not apparent in our young mice (8–10 wk), we sought to determine if the structure of the airways was affected in the absence of CC16. Differences in the alveolar areas of the lung were also not evident. We were, however, able to detect increased collagen staining in histologic sections from naive CC16−/− mice as compared with WT mice. Quantitatively, gene expression from a representative lung lobe of CC16−/− mice revealed that several remodeling associated factors, (i.e., Pro-Col1A1, Pro-Col3A, and α-smooth muscle actin) were all significantly upregulated as compared with WT lung tissue. In addition, smooth muscle thickness was also significantly greater in CC16−/− mice as compared with WT mice in the unchallenged state. Of note, two factors known to be mitogenic for smooth muscle cells, TNF-α and platelet-derived growth factor (52, 53), were not different between the CC16-deficient and WT mice.
We propose that the measures of lung remodeling in CC16−/− mice are likely key contributing factors to the altered lung function in mice, which is independent of inflammation. Therefore, if similar structural and remodeling lung alterations are associated with CC16 deficits in humans, they could affect early airway growth, associate with trajectories of persistently low lung function (54, 55), and in turn play a role in the inception and progression of airway obstruction.
Future studies to determine the specific receptors for CC16 in both the lung and blood compartments will be vital to shed more light on the mechanisms by which CC16 may be protective against impaired lung function and airway remodeling. Should these associations be causal in humans, a better understanding of ways to either endogenously increase CC16 production (56) or deliver a replacement for CC16 into the airways might also have translational relevance to large populations affected by (or at risk for) obstructive lung diseases. Along these lines, a recent report shows that delivery of recombinant CC16 to a smoke-induced model of COPD in mice ameliorates pathologic damage in the lungs and reduced indices of inflammation associated with the model. These effects were mediated by inhibition of DNA binding of smoke-induced nuclear factor-κB/p65 in lung tissues and its nuclear translocation in BAL fluid cells and epithelial cells (57).
In this context, however, it should be noted that although our study and others suggest that higher CC16 levels associate with better lung function, this is not the case in all lung diseases. In a study of idiopathic pulmonary fibrosis (IPF), CC16 was significantly increased in serum and BAL of patients with IPF as compared with patients without IPF and healthy control subjects (58). CC16 levels in patients with IPF were increased despite the fact that most of the subjects with IPF were smokers, a habit typically associated with reduced CC16 in the serum (58).
Among the limitations of our study are the lack of a replication cohort for the human studies and the lack of information on renal function in the TCRS cohort, which has been shown to affect levels of circulating CC16 (59). The strengths of our study include the population-based nature of the TCRS cohort, the phenotypic characterization and long follow-up well into adult life of this birth cohort, and the inclusion of studies of increased methacholine sensitivity in humans in the lowest tertile of CC16 levels and in mice deficient in CC16.
In conclusion, our findings build on previous clinical association studies in which decreased serum CC16 has been associated with the presence, risk, and progression of obstructive lung diseases. In the current study we complemented findings from a human birth cohort, in which participants with low serum CC16 were found to have lung function deficits and increased AHR from childhood into their young adult life, with animal studies in which mice deficient in CC16 had increased airway resistance and AHR as compared with mice sufficient in CC16. Based on our findings, in mice these associations seem to be attributable to increased airway remodeling and suggest that low CC16 is not only a biomarker for airway pathology but may be implicated in the pathophysiology of the progressive airway damage that characterize obstructive lung diseases.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dave Francisco and Stephanie Delgado for technical support, Laurie Ellerman for assistance with histologic sections, and Dr. Aprile Pilon for helpful discussions.
Footnotes
Supported by NIH grants HL111151, HL125602, HL132523, and AI135108.
Author Contributions: S.G., F.D.M., M.K., and J.G.L. designed the initial concept. S.G. and J.G.L. designed the experiments. K.J.A., M.I., W.P., A.D., and C.A.O. performed the experiments and/or analyzed the data. S.G., J.Z., D.A.S., and D.L.S. performed the statistical analysis. W.M., A.L.W., M.H., and F.D.M. aided in collection of and provided access to the longitudinal respiratory study samples for human analysis. S.G. and J.G.L. wrote the manuscript. J.Z., M.I., K.J.A., D.A.S., W.P., A.D., J.R.-Q., C.A.O., D.L.S., W.M., A.L.W., M.H., F.D.M., M.K., S.G., and J.G.L. reviewed the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201807-1345OC on December 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 2.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12:445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 3.Laucho-Contreras ME, Polverino F, Tesfaigzi Y, Pilon A, Celli BR, Owen CA. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD) Expert Opin Ther Targets. 2016;20:869–883. doi: 10.1517/14728222.2016.1139084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shijubo N, Itoh Y, Yamaguchi T, Sugaya F, Hirasawa M, Yamada T, et al. Serum levels of Clara cell 10-kDa protein are decreased in patients with asthma. Hai. 1999;177:45–52. doi: 10.1007/pl00007626. [DOI] [PubMed] [Google Scholar]
- 5.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martin RJ. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol. 2016;138:932–934. doi: 10.1016/j.jaci.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 7.Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer REvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort Thorax 2008631058–1063.18757456 [Google Scholar]
- 8.Rava M, Tares L, Lavi I, Barreiro E, Zock JP, Ferrer A, et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol. 2013;132:230–232. doi: 10.1016/j.jaci.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 11.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. National Health Interview Survey, special topics, adult tobacco use information, glossary [accessed 2018 May]. Available from: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm.
- 13.Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975;56:323–327. doi: 10.1016/0091-6749(75)90107-4. [DOI] [PubMed] [Google Scholar]
- 14.Dell SD, Bola SS, Foty RG, Marshall LC, Nelligan KA, Coates AL. Provocative dose of methacholine causing a 20% drop in FEV1 should be used to interpret methacholine challenge tests with modern nebulizers. Ann Am Thorac Soc. 2015;12:357–363. doi: 10.1513/AnnalsATS.201409-433OC. [DOI] [PubMed] [Google Scholar]
- 15.Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol. 1997;17:147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- 16.Hartney JM, Robichaud A. Assessment of airway hyperresponsiveness in mouse models of allergic lung disease using detailed measurements of respiratory mechanics. Methods Mol Biol. 2013;1032:205–217. doi: 10.1007/978-1-62703-496-8_16. [DOI] [PubMed] [Google Scholar]
- 17.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp. 2013;(75):e50172. doi: 10.3791/50172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addison KJ, Morse J, Robichaud A, Daines MO, Ledford JG. A novel in vivo system to test bronchodilators. J Infect Pulm Dis. 2017;3:1–9. doi: 10.16966/2470-3176.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015;45:1544–1556. doi: 10.1183/09031936.00134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledford JG, Mukherjee S, Kislan MM, Nugent JL, Hollingsworth JW, Wright JR. Surfactant protein-A suppresses eosinophil-mediated killing of Mycoplasma pneumoniae in allergic lungs. PLoS One. 2012;7:e32436. doi: 10.1371/journal.pone.0032436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polverino F, Doyle-Eisele M, McDonald J, Wilder JA, Royer C, Laucho-Contreras M, et al. A novel nonhuman primate model of cigarette smoke-induced airway disease. Am J Pathol. 2015;185:741–755. doi: 10.1016/j.ajpath.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, et al. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med. 2018;198:267–270. doi: 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shalaby KH, Gold LG, Schuessler TF, Martin JG, Robichaud A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir Res. 2010;11:82. doi: 10.1186/1465-9921-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HY, Churg A, Wright JL, Li Y, Tam S, Man SF, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1413–1419. doi: 10.1164/rccm.201305-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS One. 2015;10:e0116159. doi: 10.1371/journal.pone.0116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol. 1998;275:L348–L356. doi: 10.1152/ajplung.1998.275.2.L348. [DOI] [PubMed] [Google Scholar]
- 27.Plopper CG, Mango GW, Hatch GE, Wong VJ, Toskala E, Reynolds SD, et al. Elevation of susceptibility to ozone-induced acute tracheobronchial injury in transgenic mice deficient in Clara cell secretory protein. Toxicol Appl Pharmacol. 2006;213:74–85. doi: 10.1016/j.taap.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am J Respir Cell Mol Biol. 2010;42:161–171. doi: 10.1165/rcmb.2008-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Zhang Z, Mukherjee AB, Linnoila RI. Increased susceptibility of mice lacking Clara cell 10-kDa protein to lung tumorigenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent carcinogen in cigarette smoke. J Biol Chem. 2004;279:29336–29340. doi: 10.1074/jbc.C400162200. [DOI] [PubMed] [Google Scholar]
- 30.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 31.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–L930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 32.Wang SZ, Rosenberger CL, Espindola TM, Barrett EG, Tesfaigzi Y, Bice DE, et al. CCSP modulates airway dysfunction and host responses in an Ova-challenged mouse model. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1303–L1311. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]
- 33.Wendt C, Tram K, Price A, England K, Stiehm A, Panoskaltsis-Mortari A. Club cell secretory protein improves survival in a murine obliterative bronchiolitis model. Am J Physiol Lung Cell Mol Physiol. 2013;305:L642–L650. doi: 10.1152/ajplung.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto T, Fujita M, Hirano R, Uchino J, Tajiri Y, Fukuyama S, et al. Chronic Pseudomonas aeruginosa infection-induced chronic bronchitis and emphysematous changes in CCSP-deficient mice. Int J Chron Obstruct Pulmon Dis. 2016;11:2321–2327. doi: 10.2147/COPD.S113707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocr Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 37.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martinez FD. Serum concentrations of club cell secretory protein (Clara) and cancer mortality in adults: a population-based, prospective cohort study. Lancet Respir Med. 2013;1:779–785. doi: 10.1016/S2213-2600(13)70220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson S, Kristjánsson S, Bjarnarson SP, Wennergren G, Rudin A. Clara cell protein 16 (CC16) serum levels in infants during respiratory syncytial virus infection. Acta Paediatr. 2009;98:579–581. doi: 10.1111/j.1651-2227.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 39.Kurowski M, Jurczyk J, Jarzębska M, Moskwa S, Makowska JS, Krysztofiak H, et al. Association of serum Clara cell protein CC16 with respiratory infections and immune response to respiratory pathogens in elite athletes. Respir Res. 2014;15:45. doi: 10.1186/1465-9921-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomori H, Horio H, Fuyuno G, Kobayashi R, Morinaga S, Hirabayashi Y. Protein 1 (Clara cell protein) serum levels in healthy subjects and patients with bacterial pneumonia. Am J Respir Crit Care Med. 1995;152:746–750. doi: 10.1164/ajrccm.152.2.7633737. [DOI] [PubMed] [Google Scholar]
- 41.Rosas-Salazar C, Gebretsadik T, Carroll KN, Reiss S, Wickersham N, Larkin EK, et al. Urine Club cell 16-kDa secretory protein and childhood wheezing illnesses after lower respiratory tract infections in infancy. Pediatr Allergy Immunol Pulmonol. 2015;28:158–164. doi: 10.1089/ped.2015.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shijubo N, Itoh Y, Yamaguchi T, Imada A, Hirasawa M, Yamada T, et al. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med. 1999;160:930–933. doi: 10.1164/ajrccm.160.3.9803113. [DOI] [PubMed] [Google Scholar]
- 43.Antico G, Aloman M, Lakota K, Miele L, Fiore S, Sodin-Semrl S. Uteroglobin, a possible ligand of the lipoxin receptor inhibits serum amyloid A-driven inflammation. Mediators Inflamm. 2014;2014:876395. doi: 10.1155/2014/876395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, et al. National Heart Lung and Blood Institute’s Severe Asthma Research Program-3 Investigators. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight. 2017;2:93534. doi: 10.1172/jci.insight.93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin SW, Butler JD, Schumacher UK, Wightman PD, Mukherjee AB. Uteroglobin inhibits phospholipase A2 activity. Life Sci. 1986;38:1813–1819. doi: 10.1016/0024-3205(86)90135-9. [DOI] [PubMed] [Google Scholar]
- 46.Lesur O, Bernard A, Arsalane K, Lauwerys R, Bégin R, Cantin A, et al. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am J Respir Crit Care Med. 1995;152:290–297. doi: 10.1164/ajrccm.152.1.7541278. [DOI] [PubMed] [Google Scholar]
- 47.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, et al. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol. 2006;207:553–561. doi: 10.1002/jcp.20604. [DOI] [PubMed] [Google Scholar]
- 48.Yang SH, Shin SJ, Oh JE, Jin JZ, Chung NH, Lim CS, et al. The protective role of uteroglobin through the modulation of tissue transglutaminase in the experimental crescentic glomerulonephritis. Nephrol Dial Transplant. 2008;23:3437–3445. doi: 10.1093/ndt/gfn268. [DOI] [PubMed] [Google Scholar]
- 49.Lundblad LK, Irvin CG, Hantos Z, Sly P, Mitzner W, Bates JH. Penh is not a measure of airway resistance! Eur Respir J. 2007;30:805. doi: 10.1183/09031936.00091307. [DOI] [PubMed] [Google Scholar]
- 50.Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol (1985) 2004;97:286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- 51.Laucho-Contreras ME, Polverino F, Rojas-Quintero J, Wang X, Owen CA. Club cell protein 16 (Cc16) deficiency increases inflamm-aging in the lungs of mice. Physiol Rep. 2018;6:e13797. doi: 10.14814/phy2.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang CM, Luo SF, Wang CC, Chiu CT, Chien CS, Lin CC, et al. Tumour necrosis factor-alpha- and interleukin-1beta-stimulated cell proliferation through activation of mitogen-activated protein kinase in canine tracheal smooth muscle cells. Br J Pharmacol. 2000;130:891–899. doi: 10.1038/sj.bjp.0703359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Vasquez MM, Zhu L, Lizarraga RE, Krutzsch M, Einspahr J, et al. Effects of retinoids on augmentation of club cell secretory protein. Am J Respir Crit Care Med. 2017;196:928–931. doi: 10.1164/rccm.201608-1611LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang M, Liu HY, Li T, Wang D, Hu XY, Zhang XR, et al. Recombinant club cell protein 16 (CC16) ameliorates cigarette smoke-induced lung inflammation in a murine disease model of COPD. Mol Med Rep. 2018;18:2198–2206. doi: 10.3892/mmr.2018.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buendía-Roldán I, Ruiz V, Sierra P, Montes E, Ramírez R, Vega A, et al. Increased expression of CC16 in patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11:e0168552. doi: 10.1371/journal.pone.0168552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermans C, Dong P, Robin M, Jadoul M, Bernard A, Bersten AD, et al. Determinants of serum levels of surfactant proteins A and B and Clara cell protein CC16. Biomarkers. 2003;8:461–471. doi: 10.1080/13547500310001647021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.