Abstract
Patients undergoing surgical resection of primary breast tumors confront a risk for metastatic recurrence that peaks sharply 12 to 18 months after surgery. The cause of early metastatic relapse in breast cancer has long been debated, with many ascribing these relapses to the natural progression of the disease. Others have proposed that some aspect of surgical tumor resection triggers the outgrowth of otherwise-dormant metastases, leading to the synchronous pattern of relapse. Clinical data cannot distinguish between these hypotheses, and previous experimental approaches have not provided clear answers. Such uncertainty hinders the development and application of therapeutic approaches that could potentially reduce early metastatic relapse. We describe an experimental model system that definitively links surgery and the subsequent wound-healing response to the outgrowth of tumor cells at distant anatomical sites. Specifically, we find that the systemic inflammatory response induced after surgery promotes the emergence of tumors whose growth was otherwise restricted by a tumor-specific T cell response. Furthermore, we demonstrate that perioperative anti-inflammatory treatment markedly reduces tumor outgrowth in this model, suggesting that similar approaches might substantially reduce early metastatic recurrence in breast cancer patients.
INTRODUCTION
Metastatic dormancy has long been known to complicate treatment for patients diagnosed with breast cancer (1, 2), and patients can relapse with metastatic disease many years after resection of their primary tumors (3, 4). A partial explanation for these outcomes has become clear: in as many as one-third of patients diagnosed with localized breast cancer, carcinoma cells have already disseminated to distant anatomical sites at the time of initial diagnosis (5, 6). The vast majority of these cells reside for extended periods of time in an apparently innocuous quiescent state (2, 5, 6). In a subset of patients, however, a small fraction of such clinically inapparent cancer cells ultimately renew proliferation and spawn life-threatening metastases (1). The specific nature of these cells and the stimuli that trigger their outgrowth remain unresolved.
Although some patients recur many years after tumor resection, a substantial fraction of patients develop overt metastases relatively soon after resection of their primary tumors (3, 4, 7). These patients are represented in a sharp rise in the risk of distant recurrence that begins 6 months after surgery and peaks 6 to 12 months later. The synchronicity and abruptness of these early recurrences stand in sharp contrast to the broad, extended period of relapse in the subsequent years and the low incidence of overt metastases at diagnosis, suggesting a discrete triggering event, most plausibly the surgery itself (7). The timing of early relapses is conserved across subtypes of breast cancer, further suggesting a tumor-extrinsic mechanism (4). Because there is not, and cannot be, an appropriate clinical control group of patients who do not undergo surgery, a causal link between surgery and the outgrowth of dormant metastases has not been demonstrated. The lack of clinical consensus on the causes of these early relapses has inhibited the investigation into and the development of therapeutic approaches that might mitigate potential surgery-induced metastatic relapse. For this reason, we set out to establish a model system in mice that would allow us to test, in a controlled experimental setting, whether the surgical wounding required for tumor resection can trigger the outgrowth of tumors at distant anatomical sites.
One possible mechanism through which surgery could trigger early metastatic relapse may derive from the release of cancer cells from the surgical bed during the process of resection (8). However, the fact that such relapses are observed in patients undergoing complete mastectomy, where physical disruption of the tumor is minimal, argues against this mechanism as a principal cause of early relapse (9). An alternative and more attractive hypothesis posits that some aspect of the surgery itself provokes the outgrowth of previously disseminated cancer cells, releasing such cells from physiologic constraints that previously suppressed their outgrowth (7, 10–12). Clinical data from patients who have undergone delayed surgical breast reconstruction support the notion that surgery and the resulting wound-healing response can trigger the outgrowth of distant metastases (13, 14). Focusing on the latter mechanism, we asked whether surgery, and more specifically the postsurgical wound-healing response, is responsible for the early eruption of previously dormant cells at distant anatomical sites.
Metastatic dormancy has been proposed to be enforced by various mechanisms, including cellular quiescence and insufficient angiogenesis (2). However, the precise mechanism by which dormancy is imposed on disseminated tumor cells in human patients is not known and is likely to be patient-specific. Recent evidence has indicated a prominent role for immune restriction of metastatic outgrowth, causing us to focus on this as a centrally important mechanism imposing meta-static dormancy (15–19). By decoupling surgical wounding from tumor resection, we demonstrate that wounding and the subsequent wound-healing response are sufficient to trigger the outgrowth of distant tumor cells, specifically when such outgrowth is restricted by the adaptive immune system. We demonstrate that peri- and postoperative treatment with anti-inflammatory medication attenuates the impact of surgical wounding on the outgrowth of distant tumors, suggesting that a common and inexpensive treatment may improve breast cancer clinical outcomes.
RESULTS
CD8+ T cells restrict the outgrowth of GFP-labeled carcinoma cells in mice
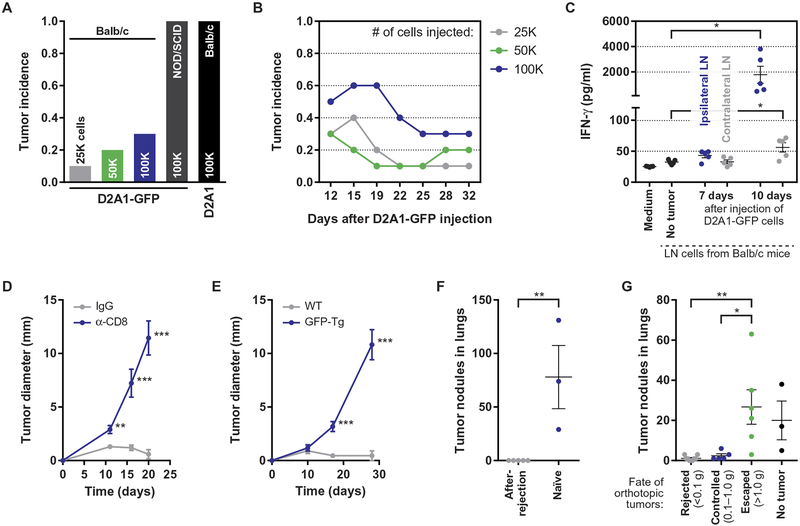
We began our studies by generating a new model of tumor dormancy in which the outgrowth of tumors is restricted by the actions of the adaptive immune system. Wishing to study mammary carcinomas, we developed our model system in Balb/c mice, the strain in which most murine mammary carcinoma models have been established (20, 21). To do so, we took advantage of the fact that green fluorescent protein (GFP) functions as a potent T cell antigen in immunocompetent Balb/c mice (22). Thus, we engineered cells of the aggressive D2A1 murine mammary carcinoma cell line (20) to express GFP, thereby generating immunogenic tumor cells. Whereas parental D2A1 cells generated rapidly growing tumors when injected orthotopically into syngeneic Balb/c mice (Fig. 1A), their GFP-expressing counterparts (D2A1-GFP) produced tumors that, although initiated with comparably high efficiency, were ultimately rejected in 70 to 90% of mice (Fig. 1, A and B, and fig. S1A). D2A1-GFP cells efficiently formed tumors in immunocompromised nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (Fig. 1A), consistent with the adaptive immune system mediating tumor rejection in Balb/c mice.
Fig. 1. The outgrowth of D2A1-GFP tumors in Balb/c mice is restricted by a GFP-specific CD8+ T cell response.
(A) Endpoint tumor incidence, 32 days after the orthotopic injection of 2.5 × 104, 5 × 104, or 1 × 105 D2A1–green fluorescent protein (GFP) cells into syngeneic Balb/c mice; 1 × 105 D2A1-GFP cells injected into immunodeficient nonobese diabetic/severe combined immunodeficient mice; and 1 × 105 unlabeled D2A1 cells injected into Balb/c mice (n = 10 per group). Tumor incidence is reported as the fraction of mice bearing tumors of diameter ≥ 2 mm (see Supplementary Materials and Methods). (B) Fraction of tumor-bearing mice as a function of time after the orthotopic injection of D2A1-GFP cells into Balb/c mice at doses of 2.5 × 104, 5 × 104, or 1 × 105 per injection (n = 8 to 10 per group). (C) Secretion of interferon-γ (IFN-γ) into culture medium during ex vivo coculture of irradiated D2A1-GFP cells with lymph node (LN) cells isolated from the inguinal LNs of tumor-free or D2A1-GFP–bearing Balb/c mice. Data for cells isolated from the ipsilateral (tumor-draining) and contralateral LNs are shown for tumor-bearing mice (n = 5 per group). (D) Tumor diameter after the orthotopic injection of 1 × 105 D2A1-GFP cells into Balb/c mice 1 day after initiating injections of either anti-CD8 antibodies or control immunoglobulin G (IgG) (n = 9 to 10 per group). (E) Tumor diameter after the orthotopic injection of 1 × 105 D2A1-GFP cells into wild-type (WT) or GFP-transgenic (Tg) mice, both on a Balb/c:C57BL/6 F1 background (n = 11 per group). (F) Number of tumor nodules in the lungs after the intravenous injection via the tail vein of 1 × 106 D2A1-GFP cells into Balb/c mice that had previously rejected D2A1-GFP tumors or into naïve mice (n = 3 to 5 per group). (G) Number of tumor nodules in the lungs after the intravenous injection via the tail vein of D2A1-GFP 5 × 105 cells into Balb/c mice bearing D2A1-GFP cells in the mammary fat pad (MFP) at different stages of growth or rejection (n = 3 to 6 per group). For all panels, data are plotted as means ± SEM. P values were calculated using the Mann-Whitney test (*P < 0.05, **P < 0.005, ***P < 0.0005).
To assess the immune response against D2A1-GFP tumors in syngeneic Balb/c hosts, leukocytes from the tumor-draining lymph nodes were harvested either 7 or 10 days after the injection of the tumor cells. When cultured ex vivo with irradiated D2A1-GFP cells, these leukocytes released substantial amounts of interferon-γ, indicative of tumor-specific immune activation (Fig. 1C). Furthermore, D2A1-GFP tumors grew rapidly, without signs of restriction, when injected into mice in which CD8+ T cells had been depleted with neutralizing antibodies (Fig. 1D and fig. S1, B to D). The implied T cell–mediated rejection required the recognition of GFP as a foreign antigen, as D2A1-GFP tumors also grew rapidly when injected into GFP-transgenic hosts in which germ-line expression of the transgene had induced tolerance toward this antigen (Fig. 1E and fig. S1E). Rejection of D2A1-GFP tumors generated immunological memory of the GFP antigen, as evidenced by experiments in which these mice were rechallenged with D2A1-GFP cells injected into the tail vein during an experimental metastasis assay. Whereas tumor nodules formed in the lungs upon tail vein injection of D2A1-GFP cells into tumor-naïve mice, no such nodules were observed upon injection into mice that had previously rejected orthotopic D2A1-GFP tumors (Fig. 1F). Intravenous injection of D2A1-GFP cells failed to generate lung nodules even in mice in which orthotopic D2A1-GFP tumors had been controlled, but not fully rejected, thus indicating the presence of substantial systemic immunity in tumor-bearing mice (Fig. 1G and fig. S1, F and G). Collectively, these data demonstrate that in this model system, the outgrowth of carcinoma cells was restricted by a robust GFP-specific T cell response.
In our model system, after successful initiation of D2A1-GFP tumors, the growth of these tumors stalled, yet the tumors persisted for 2 to 3 weeks before rejection (fig. S1A, right). These data suggest that tumor growth was being countered by the adaptive immune system, such that the tumors had entered transiently into a period of equilibrium with the immune system (23, 24). We hypothesized that this equilibrium state modeled the immune control that might impose dormancy upon—but not rejection of—disseminated tumor cells in breast cancer patients, and that this control might be disrupted by various immune-modulating stimuli. More specifically, given the synchronous pattern of early relapse, we hypothesized that after surgery, the resulting wound-healing response might act systemically to compromise certain actions of the adaptive immune system, thereby permitting the outgrowth of distant tumors that would otherwise be controlled.
Surgical wounding promotes local tumor outgrowth
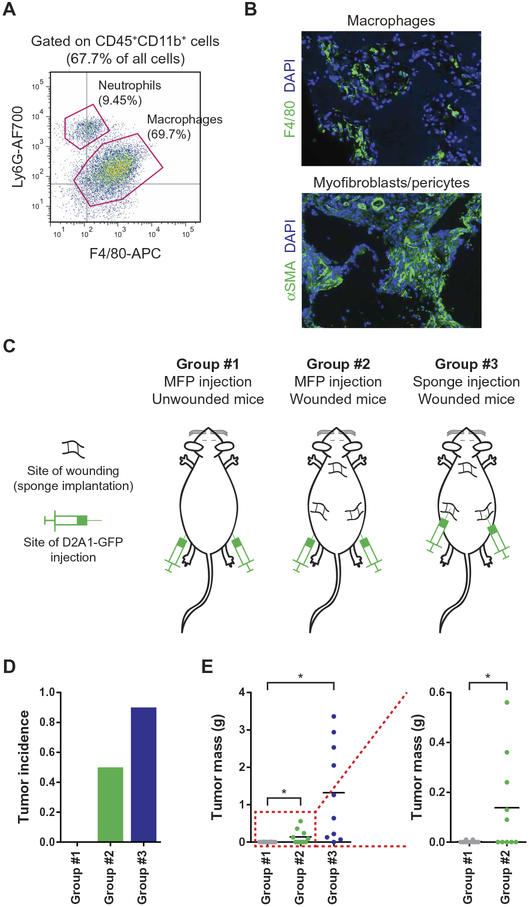
To determine whether surgical wounding could break immune-imposed dormancy and enable tumor outgrowth, we undertook to model surgical wounding and postsurgical wound healing in a robust and reproducible manner. As a crucial consideration, we wished to avoid the confounding issues associated with the surgical removal of primary tumors. These issues include variability in the extent of wounding required to remove each tumor, as well as the idiosyncrasies of specific implanted tumor models, whose systemic effects, lost after their resection, were likely to vary substantially from one tumor type to another. Instead, we focused on the surgical wounding itself and the subsequent wound-healing response that is encountered by all breast cancer patients who have undergone surgery, independent of the nature of their tumors. To do so, we wounded mice by subcutaneously implanting sterile polyvinyl acetate sponges. Sponge implantation represents a well-established model of wound healing, in which the cutaneous incision initiates the wound-healing response and the sponge mesh acts as a bed into which a rich desmoplastic stroma is then recruited over the ensuing days (25, 26). In our hands, this method yielded far more consistent biological responses than did surgical resection of primary tumors, where the extent of wounding and bleeding varied greatly from one tumor resection to another. The response to sponge implantation proceeded according to the canonical wound-healing cascade (27), with the recruitment of neutrophils and macrophages followed by the infiltration of myofibroblasts and extensive vascularization (Fig. 2, A and B). Accordingly, in the work described below, we refer to and equate sites of sponge implantation with sites of surgical wounding and subsequent wound healing.
Fig. 2. The postsurgical wound-healing response protects local immunogenic tumors from immune-mediated destruction.
(A) Identification of sponge-infiltrating myeloid cells using flow cytometry after staining for the presence of neutrophils (CD45+CD11b+Ly6G+) and macrophages (CD45+CD11b+F4/80+), 7 days after sponge implantation. (B) Immunofluorescence staining of tissue sections containing stroma-infiltrated sponges. Sections were stained with anti-F4/80 (macrophages, top) or anti–α–smooth muscle actin (αSMA) (myofibroblasts and blood vessel–lining pericytes, bottom). Representative images at ×10 magnification are shown for sections isolated 14 days after wounding by subcutaneous sponge implantation. DAPI, 4′,6-diamidino-2-phenylindole. (C to E) Injection of immunogenic D2A1-GFP cells into control or wounded Balb/c mice. (C) Schematic illustrating the experimental design in which 1 × 105 tumor cells were injected into the MFP of control mice (left) or mice bearing a local wound (center) or were injected directly into a site of wound healing (right). The incidence (D) and mass (E) of the resulting tumors were determined 30 days after the injection of tumor cells (n = 10 per group). For all panels, data are plotted as means ± SEM. P values were calculated using the Mann-Whitney test (*P < 0.05).
Before examining possible systemic effects of surgical wounding, we considered the local impact of wounding, which has been shown to promote tumor growth in other contexts (28, 29). Thus, we asked whether the wound-healing response after surgery could promote the outgrowth of locally implanted tumors that would otherwise be immune-restricted. To test this possibility, we injected D2A1-GFP cells directly into sponges that had been implanted 1 week earlier and therefore had already been infiltrated by cells that formed a desmoplastic stroma (Fig. 2C, right). D2A1-GFP cells injected into this wound-healing microenvironment formed robustly growing tumors, in contrast to their rejection when these tumor cells were injected orthotopically into the mammary fat pads (MFPs) of unwounded mice (Fig. 2, C to E). Similarly, when D2A1-GFP cells were injected into an MFP directly adjacent to the site of an implanted sponge, tumors once again grew out, albeit to a lesser extent (Fig. 2, C to E). Thus, the wound-healing microenvironment could trigger the local outgrowth of tumors that would otherwise be suppressed by the adaptive immune system.
Surgery triggers the outgrowth of distant immunogenic tumors
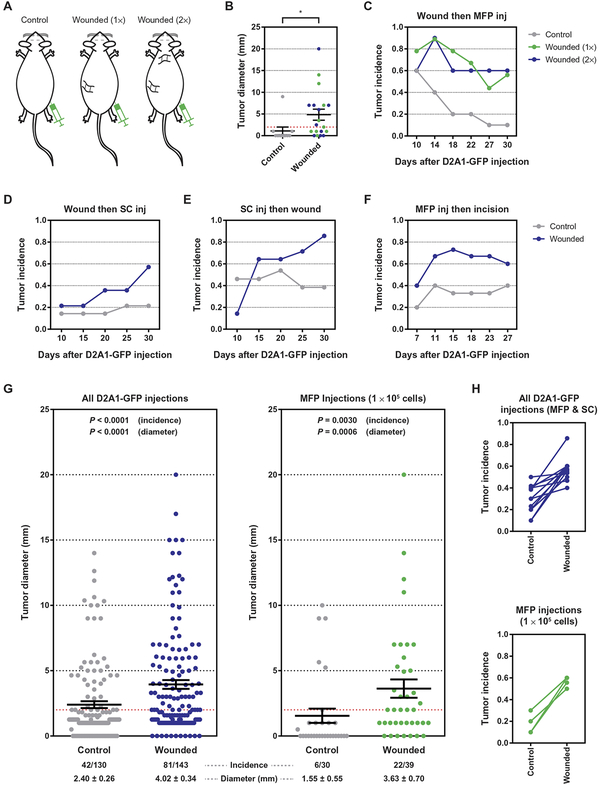
We next asked whether, paralleling the local promotion of tumor outgrowth, surgical wounding would also initiate a systemic response that could similarly affect tumor cells at distant anatomical sites, such as might occur clinically after tumor resection, leading to the outgrowth of metastases. To determine whether surgery sufficed to trigger the outgrowth of distant, immune-controlled tumors, we orthotopically injected D2A1-GFP cells contralateral to sites of surgical wounding, ensuring that any interactions between the tumor and the wound-healing response would be systemic in nature (Fig. 3A). In keeping with the earlier experiments that focused on local wounding, tumor cells were initially injected into mice 1 week after surgical wounding had been modeled by sponge implantation. In wounded mice, 60% of tumors grew out, whereas the remaining tumors were rejected (Fig. 3, B and C, and fig. S2A). This high tumor incidence was in sharp contrast to tumor growth in unwounded, control mice, in which only 10% of tumors persisted (Fig. 3, B and C, and fig. S2A). The extent of wounding beyond an apparent threshold had little impact on eventual tumor outgrowth, as tumor formation was comparable in mice wounded via the implantation of either one or two sponges (Fig. 3, B and C). The effect of surgery on tumor outgrowth depended upon immune restriction of tumor growth, as wounding did not affect the growth of distant D2A1-GFP tumors in immunocompromised NOD/SCID mice (fig. S2B).
Fig. 3. The systemic response to surgery triggers the outgrowth of immunogenic tumor cells at distant anatomical sites.
(A to C) Injection of immunogenic D2A1-GFP cells into syngeneic Balb/c mice wounded at distant sites. (A) Schematic illustrating the experimental design in which 1 × 105 D2A1-GFP cells were injected into the MFP of unwounded mice or into mice that had been previously wounded by sponge implantation at one or two distant sites (1× and 2×, respectively). (B) Tumor diameter at the conclusion of the experiment and (C) tumor incidence as a function of time are shown (n = 9 to 10 per group). The dashed red line indicates a tumor diameter of 2 mm, the threshold for tumor incidence (see Materials and Methods). inj, injection. (D to F) Tumor incidence as a function of time for three experiments in which tumors and surgical wounds were inflicted at contralateral sites: (D) mice were wounded by sponge implantation 7 days before the subcutaneous (SC) injection of 1 × 105 tumor cells; (E) mice were wounded by sponge implantation 7 days after the subcutaneous injection of 1 × 105 tumor cells; and (F) mice were wounded by a cutaneous incision 7 days after the orthotopic injection of 2 × 105 tumor cells into the MFP (n = 12 to 14 per group in each experiment). (G) Meta-analysis of tumor diameter and tumor incidence for experiments in which D2A1-GFP cells were injected into unwounded Balb/c mice or into mice surgically wounded at distant anatomical sites. Data are shown for all experiments with D2A1-GFP cells (left) and for experiments in which 1 × 105 D2A1-GFP cells were injected orthotopically into an MFP (right). (H) Linkage plots of tumor incidence for experiments in which D2A1-GFP cells were injected into unwounded mice or into mice surgically wounded at distant anatomical sites. Data sets are the same as in (G). For all panels, data are plotted as means ± SEM. P values were calculated using the Mann-Whitney test (B) (*P < 0.05) or Fisher’s exact test (G).
Wishing to extend the above results, we undertook to model more closely the clinical scenario in which the postsurgical wound-healing response influences the outgrowth of previously disseminated tumor cells. We first modified the experimental protocol by injecting D2A1-GFP cells into mice subcutaneously rather than orthotopically, doing so to avoid any confounding inflammation resulting from the small incision required for orthotopic injection. Similar to previous experiments, tumor outgrowth was enhanced in wounded mice relative to control mice (Fig. 3D and fig. S2C).
Furthermore, we considered the likelihood that, in patients, interactions between disseminated tumor cells and the adaptive immune system likely begin upon the initial arrival of the tumor cells at a distant organ, before tumor resection. According to this scenario, wounding associated with tumor resection would occur after the distant tumor cells encountered an immune response. To more faithfully model this situation, we subcutaneously injected D2A1-GFP cells 1 week before contralateral sponge implantation, reversing the previously used order of manipulations. We reasoned that this delay between tumor cell injection and surgery provided sufficient time for the tumors to engage the adaptive immune system but not to be fully rejected (Fig. 1, B and C). Tumor outgrowth was again substantially increased, on this occasion by subsequent sponge implantation (Fig. 3E and fig. S2D). This result indicated that the effects of surgical wounding were sufficient to affect tumors that were likely to have already engaged the adaptive immune system, rather than simply preventing the initiation of antitumor immune activity.
Finally, we wished to determine whether a more subtle wound than that induced by sponge implantation could also trigger the outgrowth of tumor cells that had previously been introduced at a distant site. Accordingly, we subjected mice to a 2-cm-long cutaneous incision 1 week after the injection of D2A1-GFP cells. For this experiment, we returned to the orthotopic injection of D2A1-GFP cells to ensure that the cells were lodged within the MFP and were thus sequestered from the wound site. Strikingly, wounding via a substantial cutaneous incision was also able to promote the outgrowth of contralateral D2A1-GFP tumors (Fig. 3F and fig. S2E). These results confirmed that the repeatedly observed systemic impact of wounding on the outgrowth of distant tumor cells was not an artifact of sponge implantation but was instead a general consequence of surgical wounding and, presumably, the postsurgical wound-healing response.
Collectively, our data clearly demonstrated that surgical wounding at one anatomical site could promote the outgrowth of an immunologically restricted tumor at a distant site. A meta-analysis across all of our experiments performed using the D2A1-GFP system (273 mice) demonstrated a marked increase in both the incidence and size of D2A1-GFP tumors in mice that had been wounded at a distant site, relative to unwounded mice (Fig. 3, G and H, and fig. S3, A and B). As is observed in many immuno-oncology models, we note that, when comparing independent experiments, there was some degree of variability in tumor outgrowth in the absence of wounding and, therefore, in the extent to which wounding triggered tumor outgrowth. This variability can be minimized, but not eliminated, by precisely titering the dosage of injected tumor cells to achieve the optimal balance between tumor growth and rejection. Thus, we observed the most marked results when transplanting 1 × 105 and 5 × 105 cells for orthotopic and subcutaneous injections, respectively (Fig. 3, G and H, and fig. S3, A and B). Even when using a suboptimal dose (orthotopic injection of 2 × 105 cells) that increased the frequency of tumor outgrowth in unwounded mice, we still observed a clear impact of surgical wounding on tumor outgrowth (fig. S3, A and B). In every individual experiment that we undertook, tumor outgrowth was, without exception, observed in a larger proportion of mice within the wounded cohort than within the control group (Fig. 3H and fig. S3B). When considered in aggregate, the statistical power of these experiments unambiguously demonstrated the impact of surgical wounding on the outgrowth of anatomically distant, immune-restricted tumors.
Surgical wounding was also able to promote the growth of immune-restricted tumors in a second, independent model system using B16 melanoma cells in syngeneic C57BL/6 host mice. In these experiments, we used the well-established B16-GVAX model of immune activation, in which vaccination of mice with irradiated B16 cells that express granulocyte-macrophage colony-stimulating factor (GM-CSF) initiates a tumor-specific T cell response that attenuates the growth of tumors arising from nonirradiated B16 cells (30). We confirmed that, as previously reported, vaccination delayed tumor growth in unwounded mice (fig. S4, A and B). However, surgical wounding at a distant site enabled B16 tumors in vaccinated mice to grow as rapidly as B16 tumors in unvaccinated mice (fig. S4, C and D). Thus, consistent with our previous findings using D2A1-GFP cells, surgical wounding after the injection of tumor cells was able to overcome the effect of vaccination and promote the growth of distant B16 tumors. These experiments indicate that the systemic components of the wound-healing response permit tumors to resist an otherwise effective adaptive immune response in a second, independent tumor model.
The systemic mobilization of myeloid cells mediates surgery-induced tumor outgrowth
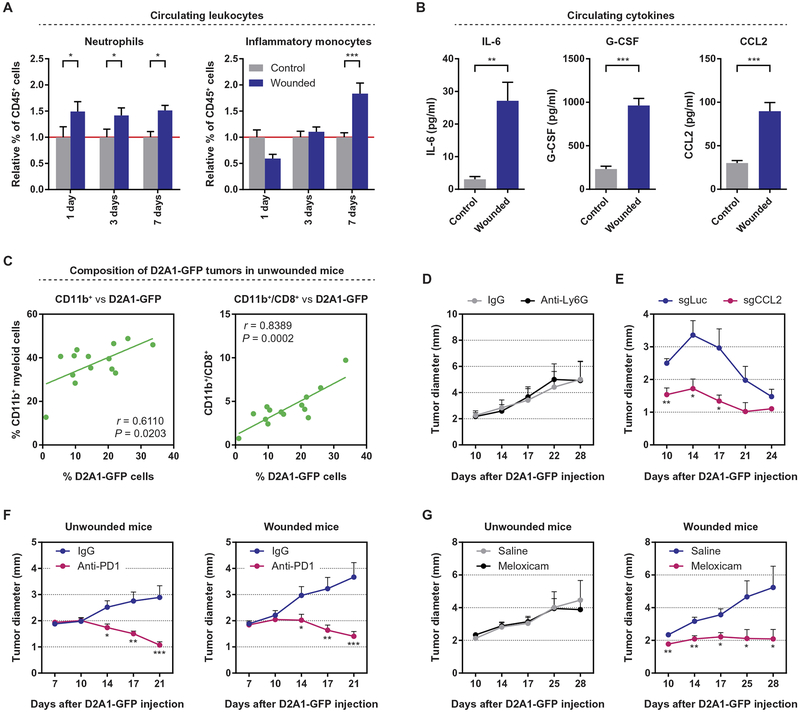
Having demonstrated a causal link between surgery and tumor outgrowth, we endeavored to identify aspects of the systemic wound-healing response that might trigger tumor outgrowth in the face of an otherwise effective CD8+ T cell response. Therefore, we analyzed the blood of wounded and control mice to identify potential systemic mediators of such a response. Analysis of leukocytes in the blood of both Balb/c and C57BL/6 mice revealed that surgical wounding induced an elevation in the number of circulating neutrophils followed by a strong elevation in the number of inflammatory (Ly6Chi) monocytes (Fig. 4A and fig. S5, A to C). No changes in the numbers of circulating lymphocytes or of noninflammatory (Ly6Clo) monocytes were observed (fig. S5, B, D, and E). Consistent with the mobilization of myeloid cell populations, we detected elevated circulating levels of the key inflammatory cytokine interleukin-6, as well as of granulocyte colony-stimulating factor (G-CSF) and CCL2 (Fig. 4B). Specifically, G-CSF and CCL2 induce the egress, respectively, of neutrophils and inflammatory monocytes from the bone marrow (31, 32). Hence, surgical wounding initiates a systemic inflammatory response that, at a cellular level, parallels the local response observed within the wound site (Fig. 2, A and B). Furthermore, the observed mobilization of myeloid cells reflects the response seen in breast cancer patients after surgery (33), indicating the potential for a similar effect of surgery on metastatic outgrowth in patients.
Fig. 4. Surgery initiates a systemic inflammatory response that triggers the outgrowth of distant immunogenic tumors and can be inhibited by perioperative anti-inflammatory treatment.
(A) Relative proportion of circulating neutrophils and inflammatory (Ly6Chi) monocytes in wounded and control Balb/c mice, 1, 3, and 7 days after surgery. The proportion of each cell type in the circulation was determined as a percentage of CD45+ leukocytes, and the values were normalized to those of control mice on each collection day (n = 4 to 6 per group). (B) Concentrations of interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), and CCL2 in the circulation of control and wounded Balb/c mice, 24 hours after surgery, as detected by enzyme-linked immunosorbent assay (n = 6 per group). (C) Correlation between the percentage of tumor-infiltrating CD11b+ myeloid cells (left) or the myeloid-to-CD8+ T cell ratio (right) and the percentage of D2A1-GFP cells within orthotopic tumors, 17 days after the injection of tumor cells into unwounded Balb/c mice. (D) Tumor diameter after the subcutaneous injection of 5 × 105 D2A1-GFP cells into Balb/c mice that were subsequently treated with anti-Ly6G or isotype-control antibodies (n = 12 per group). (E) Tumor diameter after the orthotopic injection of 1 × 106 D2A1-GFP–sgLuciferase (sgLuc) or D2A1-GFP–sgCCL2 cells into Balb/c mice (n = 5 per group). (F) Tumor diameter after the orthotopic injection of 1 × 105 D2A1-GFP cells into previously unwounded (left) or wounded (right) Balb/c mice that were subsequently treated with anti-PD1 or isotype-control antibodies (n = 15 mice per group). PD1, programmed cell death protein 1. (G) Tumor diameter after the orthotopic injection of 1 × 105 D2A1-GFP cells into previously unwounded (left) or wounded (right) Balb/c mice treated peri- and postoperatively with saline or meloxicam (n = 15 mice per group). For all panels, data are plotted as means ± SEM. P values were calculated using Student’s t test (A and B) or the Mann-Whitney test (C to G) (*P < 0.05, **P < 0.005, ***P < 0.0005).
We wished to determine whether the types of myeloid cells mobilized in response to surgery might play a functional role in promoting tumor outgrowth. To do so, we initially characterized the tumor-infiltrating leukocytes present within D2A1-GFP tumors of diverse sizes in unwounded mice, reasoning that informative correlations between subsets of leukocytes could be identified in the absence of wounding. Consistent with T cell–mediated tumor rejection (Fig. 1), the proportion of tumor-infiltrating CD8+ T cells was inversely correlated with tumor size and, even more strongly, with the proportion of D2A1-GFP cells within each tumor (fig. S6A). Similarly, the infiltration of CD4+ T cells was inversely related to the proportion of D2A1-GFP cells (fig. S6B). Intriguingly, the numbers of CD11b+ myeloid cells were negatively correlated with the proportion of CD8+ T cells (fig. S6C) but positively correlated with the number of D2A1-GFP cells (Fig. 4C), suggesting that myeloid cells might promote tumor outgrowth by countering T cell–mediated growth restriction. The ratio of myeloid cells to CD8+ T cells was a strong predictor of D2A1-GFP cellularity within a tumor, as were ratios of both neutrophils and macrophages to CD8+ T cells (Fig. 4C and fig. S6D), further associating these myeloid cell subsets with tumor outgrowth.
We next explored potential functional roles for tumor-infiltrating myeloid cells in promoting tumor outgrowth. Here, we found that the sustained infiltration of neutrophils was dispensable for resistance to immune attack, as the systemic depletion of neutrophils using anti-Ly6G antibodies beginning 7 days after the introduction of D2A1-GFP cells had no impact on tumor growth (Fig. 4D and fig. S6E). Unlike the depletion of neutrophils, the sustained depletion of inflammatory monocytes from bloodstream is fraught with challenges. Therefore, we opted instead to prevent the infiltration of macrophages into D2A1-GFP tumors, using CRISPR/Cas9-mediated editing to disrupt the CCL2-encoding gene in the D2A1-GFP carcinoma cells. CCL2 is a chemokine for inflammatory monocytes and is commonly secreted by breast cancer cells, recruiting monocytes into the tumor where they subsequently differentiate into tumor-associated macrophages (TAMs) (34). We found that loss of CCL2 expression in carcinoma cells reduced the growth of D2A1-GFP tumors before their ultimate rejection (Fig. 4E and fig. S6, F to H). These data indicate that TAMs may well promote the growth of immunogenic D2A1-GFP tumors, consistent with their immunosuppressive properties that have been reported in other contexts. As we have demonstrated, inflammatory monocytes, the precursors of TAMs, are clearly mobilized systemically in response to surgical wounding (Fig. 4A), increasing their availability for recruitment into tumors and suggesting a mechanism by which systemic inflammation after surgery may directly function to promote the outgrowth of distant tumors.
On the basis of the preceding experiments, we examined tumor-infiltrating macrophages to determine whether they exhibited immunosuppressive properties that might promote tumor outgrowth. Programmed cell death-ligand 1 (PD-L1), a potent mediator of immune suppression, was expressed at elevated levels on the surface of macrophages within immunogenic D2A1-GFP tumors, but not on macrophages within parental D2A1 tumors (fig. S7A). These data suggest that PD-L1 expression by macrophages was induced during the course of the antitumor immune response, as has been demonstrated in many contexts (35). To determine whether the observed PD-L1 functioned to promote tumor outgrowth, tumor-bearing mice were treated with anti-PD1 antibodies to prevent PD-L1 from signaling through PD1 on the surface of cytotoxic T cells, which causes, in turn, their functional inactivation (35). As we found, the administration of anti-PD1 antibodies led to the near-complete rejection of D2A1-GFP tumors in both wounded and unwounded mice, indicating that PD-L1 expression is essential for the outgrowth of D2A1-GFP tumors (Fig. 4F). We noted that, in addition to its expression on TAMs, PD-L1 is comparably induced on the surface of D2A1-GFP cells within the tumor (fig. S7A), indicating the potential for carcinoma cell–intrinsic immunosuppression. However, macrophages typically outnumber carcinoma cells in this model (Fig. 4C), suggesting a prominent role for TAM-associated PD-L1 in immunosuppression and tumor outgrowth.
Anti-inflammatory treatment prevents surgery-induced tumor outgrowth
Given the potential role of systemic inflammation in mediating surgery-induced tumor outgrowth, we considered whether perioperative treatment with meloxicam, a nonsteroidal anti-inflammatory drug (NSAID), might attenuate the outgrowth of tumors in response to surgery. We noted that, encouragingly, a retrospective analysis of breast cancer outcomes suggested that the use of anti-inflammatory analgesics, rather than opioids, following tumor resection surgery reduced the incidence of early metastatic relapse in these patients (36–39). However, in the cited study, the mechanism of action of the anti-inflammatory agents could not be inferred unambiguously, simply because such agents have been demonstrated to also directly inhibit tumor growth (40–42). Hence, it was unclear whether these patients responded to the effect of NSAIDs on systemic inflammation following surgery or to direct effects of NSAIDs on metastatic deposits of tumor cells. The potential for such confounding results was highlighted in our own model system, where meloxicam directly affected preexisting D2A1-GFP tumors, reducing tumor growth even in unwounded mice (fig. S7B).
To directly assess the effect of NSAID treatment on surgery-induced tumor outgrowth, we used an experimental strategy in which mice were wounded before the injection of tumor cells (as performed in Fig. 3, A to C). This approach allowed meloxicam, administered peri- and postoperatively, to be cleared from the mice before the introduction of tumor cells (fig. S7C). Mice were treated with meloxicam or, as control, saline, beginning 2 hours before surgical wounding, and dosing was repeated twice daily for 3 days after surgery. Notably, treatment with meloxicam did not appear to impede wound healing in these mice. Seven days after surgical wounding (4 days after the cessation of either meloxicam or saline treatment), D2A1-GFP cells were orthotopically injected contralateral to the wound site. Meloxicam treatment had no effect on tumor growth in the absence of surgical wounding (Fig. 4G, left). In contrast, in wounded groups, tumors in meloxicam-treated mice were significantly smaller than tumors in wounded mice treated with saline (P < 0.05; Fig. 4G, right). Surprisingly, tumors in wounded, meloxicam-treated mice were even smaller than tumors in unwounded, untreated mice.
To investigate the mechanism of NSAID action, we considered the impact of meloxicam treatment on both circulating and tumor-infiltrating myeloid cells. We were surprised to find that the administration of meloxicam did not reduce the mobilization of myeloid cells into the circulation of wounded mice (fig. S7D). In contrast, treatment of wounded mice with meloxicam appeared to alter the phenotype of TAMs within D2A1-GFP tumors. In the absence of meloxicam, distant surgical wounding induced an upward trend in the expression of CD206 on the surface of TAMs, indicative of a protumor M2 polarization that is often associated with immunosuppressive properties (fig. S7E) (43). Treatment of wounded mice with meloxicam prevented the elevation in CD206 expression and instead led to an increase in PD-L1 expression on TAMs (fig. S7E). Although PD-L1 functions as an immunosuppressive protein, its expression is known to be induced in response to antitumor immune activity (fig. S7A) (44), suggesting that tumors in meloxicam-treated mice experienced a heightened immune attack, consistent with reduced tumor outgrowth in this group (Fig. 4G). Collectively, our studies with meloxicam provide a strong indication that the inflammation triggered by surgical wounding is responsible for triggering the outgrowth of distant immunogenic tumors, potentially via its impact on the function of tumor-infiltrating myeloid cells.
DISCUSSION
The current study offers direct evidence that the systemic consequences of surgery can promote the outgrowth of tumor cells at distant anatomical sites. We focused on tumor cell deposits whose outgrowth was restricted by the adaptive immune system. To this end, we developed a new immuno-oncology model based on the ectopic expression of GFP in D2A1 murine mammary carcinoma cells. When these cells were injected orthotopically into syngeneic Balb/c hosts, GFP acted as a tumor antigen that triggered an antitumor T cell response, resulting in restricted tumor outgrowth and, ultimately, complete tumor rejection in the majority of mice. As we repeatedly observed, surgically wounding tumor-bearing mice at a distant anatomical site triggered a substantial increase in the outgrowth of these immunologically restricted tumors. Surgery-induced tumor outgrowth was associated with a local and systemic inflammatory response characterized by the release of cytokines and the mobilization of myeloid cells into the circulation of wounded mice. Specifically, our data implicated inflammatory monocytes, which differentiate into macrophages inside tumors, as likely functional mediators of the systemic response to surgery. Collectively, our results indicate that systemic inflammation initiated as part of the wound-healing response following tumor resection surgery is likely to contribute significantly to the sharp peak in early relapse.
We undertook to model a clinical phenomenon that occurs at low frequency and arises only with delayed kinetics in patients. We therefore used hundreds of mice to ensure that we had sufficient statistical power to draw firm conclusions. In considering these conclusions, we do not wish to suggest that tumor resection surgery be avoided because of the potentially negative side effects suggested previously by clinical data and demonstrated here experimentally. Instead, we argue that coupling surgery with short-term anti-inflammatory treatments may substantially improve patient outcomes by mitigating the systemic consequences of surgical breast cancer resection. Of critical importance, perioperative treatment with the anti-inflammatory drug meloxicam potently inhibited the impact of wounding on tumor growth. Furthermore, our study suggests that the treatment of breast cancer patients with anti-inflammatory agents during and after surgical re-section of primary tumors may yield substantial benefits by reducing the incidence of early metastatic relapse. These findings parallel the results of a retrospective analysis that demonstrated that perioperative anti-inflammatory analgesics reduced the incidence of early metastatic recurrence in breast cancer patients (36, 37). Our results provide a mechanistic explanation for these clinical outcomes and offer strong support for a prospective study testing the impact of perioperative anti-inflammatory treatment on early metastatic relapse (45), the results of which may have profound implications for the future treatment of breast cancer.
Here, we relied on a well-established model of surgical wounding: implantation of a sterile synthetic sponge (25, 26). In doing so, we were able to avoid the resection of a large primary tumor while still faithfully mimicking the tissue damage and inflammation associated with surgical tumor resection. This experimental protocol ensured that the extent of wounding was highly consistent across large numbers of mice. Equally important, we were able to decouple surgery itself from the removal of primary tumors, enabling an understanding of systemic responses that are common to all breast cancer patients undergoing surgery and not dependent on the idiosyncrasies of individual resected tumors. For example, certain primary tumors release factors systemically that affect the outgrowth of distant tumors; the identity and effects of these factors appear to be tumor-specific, either promoting or inhibiting the outgrowth of distant tumors depending on the cancer cells used (46–48).
Using this approach to understand the specific role of surgery in facilitating tumor outgrowth, we were able to clarify and extend the small body of literature that has addressed similar questions. Different from our approach, most of these studies used surgery that involved the removal of primary tumors (49–51). Hence, in addition to triggering a systemic postsurgical wound-healing response, such resection also resulted in the loss of tumor-derived systemic factors. Because the loss of these tumor-specific factors was cited as the primary explanation for their results, it was difficult to draw broadly applicable conclusions from these studies about the impact of surgery on the outgrowth of distant tumors (51). In the one study that directly explored the impact of surgical wounding on tumor outgrowth, cancer cells were seeded in the liver via the portal vein, and their outgrowth was promoted by surgical wounding in the form of a laparoscopy (11). However, because the liver is a central participant in the acute-phase response to inflammation (52), the particular tissue in which the tumor cells were deposited was likely to respond directly to surgery, suggesting that local rather than systemic factors may have promoted tumor outgrowth.
The experiments presented here provide strong evidence for the systemic effects of surgery on deposits of immunogenic tumor cells. However, due to the challenges of developing new model systems, important questions remain to be explored. For example, using our system, we could not address how surgery would affect tumor cells seeded as single cells or as small clusters of cells in the bone marrow, lung, or brain. Nor could we examine how tumor cells that had disseminated from a primary tumor, through a true metastatic process, might respond to a distant surgical wound. In addition, although our experimental model system demonstrates the ability of surgical wounding to overcome immunologically imposed dormancy, our study cannot directly address how surgery would affect other postulated mechanisms of metastatic dormancy, such as the lack of sufficient angiogenesis or the intrinsic quiescence of tumor cells in a foreign and potentially inhospitable tissue microenvironment (2). Each mechanism of dormancy has its own complex features, and how these will be affected by the systemic consequences of surgery cannot be predicted. Nonetheless, TAMs—derived from the circulating inflammatory monocytes—have been demonstrated in other studies to promote tumor angiogenesis and to secrete tumor-promoting mitogens (53–55). Hence, it is reasonable to expect that the inflammatory myeloid cells mobilized in response to surgical wounding might additionally expedite the outgrowth of tumor cells that are held in check by alternative mechanisms of dormancy.
MATERIALS AND METHODS
Study design
The experiments in this study were designed to determine whether surgical wounding would trigger the outgrowth of anatomically distant tumor cells whose growth was otherwise restricted by the adaptive immune system. All experiments were performed in mice. Minimum sample size was determined using Fisher’s exact test. For certain experiments, this number of mice could not be practically achieved. In such cases, data from multiple experiments were combined for statistical analysis, as noted in the relevant figure legends. In all experiments, mice of similar age and size were used across all groups. For all experiments in which comparisons were made between control and wounded mice, half of the mice in each cage were wounded, whereas the other half were not, such that differences between groups could not be attributed to any potential sources of cage-to-cage variation. For experiments in which tumor cells were implanted before a surgical wounding, tumor size was very similar across all mice at the time of wounding, such that further randomization was unnecessary. During the analysis of tumor size, researchers were blinded to the identity of groups when animals were treated with either antibodies or small-molecule inhibitors. Researchers could not be blinded with regard to surgical wounding, because the presence or absence of a subcutaneous sponge implant clearly identified mice within each group. Primary data are reported in table S2.
Statistical analysis
Data are presented as means ± SEM. Tumor diameter and tumor mass data, which did not fit a normal distribution, were analyzed using the Mann-Whitney test. Tumor incidence was analyzed using Fisher’s exact test. Analysis of cytokine levels and circulating immune cells was performed using Student’s t test, with similar variance assumed across independent groups. All statistical analyses were performed as two-tailed tests.
Supplementary Material
www.sciencetranslationalmedicine.org/cgi/content/full/10/436/eaan3464/DC1
Materials and Methods
Fig. S1. The outgrowth of D2A1-GFP tumors in Balb/c mice is restricted by a GFP-specific CD8+ T cell response.
Fig. S2. Surgical wounding triggers the outgrowth of tumor cells at distant anatomical sites.
Fig. S3. Meta-analyses demonstrate that surgical wounding promotes the outgrowth of distantly implanted tumor cells.
Fig. S4. Surgical wounding overcomes the effect of a tumor vaccine to promote the growth of distant B16 tumors.
Fig. S5. Surgical wounding triggers a systemic inflammatory response characterized by the mobilization of inflammatory myeloid cells into the circulation.
Fig. S6. Myeloid cells infiltrate D2A1-GFP tumors and promote tumor growth.
Fig. S7. NSAIDs alter the polarization of tumor-infiltrating macrophages.
Table S1. Components of flow cytometry antibody cocktails.
Reference (56)
Table S2. Primary data.
Acknowledgments:
We would like to thank R. Goldsby and members of the Weinberg Laboratory for helpful discussions, E. B. Krall for critical reading of the manuscript, T. Shibue for D2A1-GFP cells, and J. Rastelli for introducing us to the sponge model of surgical wounding. We thank M. Retsky for many conversations that stimulated our thinking about using experimental approaches to address this important clinical question. We thank G. Bell and the Whitehead Institute (WIBR) Bioinformatics & Research Computing group for assistance with statistical analysis. We thank P. Wisniewski and the Flow Cytometry Core (WIBR), W. Salmon and the Keck Microscopy Facility (WIBR), and the Histology Core at the Massachusetts Institute of Technology (MIT) Koch Institute (Swanson Biotechnology Center) for assistance in the handling and processing of biological samples. G. Goyal and G. Dranoff provided B16 and B16 GM-CSF cells and advice about working with the B16-GVAX system.
Funding: This research was funded by the Transcend Program (a partnership between the Koch Institute and Janssen Pharmaceuticals Inc.), the Breast Cancer Research Foundation, the Ludwig Center for Molecular Oncology at MIT, the Advanced Medical Research Foundation, and the Samuel Waxman Cancer Research Foundation. R.A.W. is an American Cancer Society Professor and a Daniel K. Ludwig Foundation Professor for Cancer Research. J.A.K. was supported by postdoctoral fellowships from Hope Funds for Cancer Research and from the Charles A. King Trust. D.R.P. was supported by a C. J. Martin Overseas Biomedical Fellowship from the National Health and Medical Research Council of Australia (NHMRC APP1071853) and by a K99/R00 Pathway to Independence Award (NIH/NCI 1K99CA201574–01A1). A.W.L. was supported by an America Cancer Society Ellison Foundation Postdoctoral Fellowship (PF-15-131-01-CSM). B.B. was supported by a postdoctoral fellowship from the U.S. Department of Defense Breast Cancer Research Program (W81XWH-10-1-0647).
Footnotes
Competing interests: The authors declare that they have no competing financial interests.
REFERENCES AND NOTES
- 1.Giancotti FG, Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre-Ghiso JA, Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, Goldhirsch A, Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J. Clin. Oncol 34, 927–935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, Rowan PJ, Buchholz TA, Giordano SH, Hazard of recurrence among women after primary breast cancer treatment—A 10-year follow-up using data from SEER-medicare. Cancer Epidemiol. Biomarkers Prev. 21, 800–809 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Brakenhoff RH, Brandt B, Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA, Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Demicheli R, Retsky MW, Hrushesky WJM, Baum M, Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat. Clin. Pract. Oncol 4, 699–710 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Eschwège P, Blanchet P, Benoit G, Jardin A, Dumas F, Le Maire V, Lacour B, Loric S, Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 346, 1528–1530 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Demicheli R, Abbattista A, Miceli R, Valagussa P, Bonadonna G, Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: Further support about the concept of tumor dormancy. Breast Cancer Res. Treat 41, 177–185 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Retsky MW, Demicheli R, Hrushesky WJM, Baum M, Gukas ID, Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 116, 730–741 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Fisher ER, Experimental studies of factors influencing hepatic metastases: III. Effect of surgical trauma with special reference to liver injury. Ann. Surg 150, 731–744 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum M, Chaplain MAJ, Anderson AR, Douek M, Vaidya JS, Does breast cancer exist in a state of chaos? Eur. J. Cancer 35, 886–891 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Dillekås H, Demicheli R, Ardoino I, Jensen SAH, Biganzoli E, Straume O, The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases. Breast Cancer Res. Treat 158, 169–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillekås H, Transeth M, Pilskog M, Assmus J, Straume O, Differences in metastatic patterns in relation to time between primary surgery and first relapse from breast cancer suggest synchronized growth of dormant micrometastases. Breast Cancer Res. Treat 146, 627–636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP, Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 166, 1117–1131.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake SJ, Stannard K, Liu J, Allen S, Yong MCR, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, Martinet L, Colonna M, Takeda K, Kühnel F, Gurlevik E, Bernhardt G, Teng MWL, Smyth MJ, Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 6, 446–459 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Eyles J, Puaux A-L, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, Kato M, Prévost-Blondel A, Chow P, Yang H, Abastado J-P, Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest 120, 2030–2039 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massagué J, Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF, Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531, 513–517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris VL, Koop S, MacDonald IC, Schmidt EE, Grattan M, Percy D, Chambers AF, Groom AC, Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin. Exp. Metastasis 12, 357–367 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Aslakson CJ, Miller FR, Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992). [PubMed] [Google Scholar]
- 22.Gambotto AA, Dworacki GT, Cicinnati V, Kenniston TW, Steitz JA, Tüting T, Robbins PL, DeLeo AB, Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: Identification of an H2-Kd-restricted CTL epitope. Gene Ther. 7, 2036–2040 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD, Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Andrade SP, Ferreira MAND, The sponge implant model of angiogenesis. Methods Mol. Biol 467, 295–304 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Bailey PJ, Sponge implants as models. Methods Enzymol. 162, 327–334 (1988). [DOI] [PubMed] [Google Scholar]
- 27.Singer AJ, Clark RAF, Cutaneous wound healing. N. Engl. J. Med 341, 738–746 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ, Wounding and its role in RSV-mediated tumor formation. Science 230, 676–678 (1985). [DOI] [PubMed] [Google Scholar]
- 29.Antonio N, Bønnelykke‐Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P, The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 34, 2219–2236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC, Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. U.S.A. 90, 3539–3543 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina NV, Pamer EG, Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol 7, 311–317 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC, G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17, 413–423 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V, Naor R, Beilin B, Yardeni IZ, Ben-Eliyahu S, Immune perturbations in patients along the perioperative period: Alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav. Immun 24, 376–386 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJH, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo J-L, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ, Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. U.S.A. 109, 2491–2496 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH, Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol 34, 539–573 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Forget P, Vandenhende J, Berliere M, Machiels J-P, Nussbaum B, Legrand C, De Kock M, Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg 110, 1630–1635 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Demicheli R, Hrushesky WJ, Forget P, De Kock M, Gukas I, Rogers RA, Baum M, Sukhatme V, Vaidya JS, Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: New findings and a review. Curr. Med. Chem 20, 4163–4176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Retsky M, Demicheli R, Hrushesky WJM, Forget P, De Kock M, Gukas I, Rogers RA, Baum M, Pachmann K, Vaidya JS, Promising development from translational or perhaps anti-translational research in breast cancer. Clin. Transl. Med 1, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Retsky M, Rogers R, Demicheli R, Hrushesky WJM, Gukas I, Vaidya JS, Baum M, Forget P, DeKock M, Pachmann K, NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: Particular relevance to triple negative subgroup. Breast Cancer Res. Treat 134, 881–888 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, Chen F, Roh TT, Lay E, Ho PL, Chan KS, Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517, 209–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis e Sousa C, Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H-J, Reinhardt F, Herschman HR, Weinberg RA, Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2, 840–855 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V, Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol 12, 253–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ketorolac in breast cancer surgery (KBCt); https://clinicaltrials.gov/ct2/show/study/NCT01806259
- 46.Castaño Z, Marsh T, Tadipatri R, Kuznetsov HS, Al-Shahrour F, Paktinat M, Greene-Colozzi A, Nilsson B, Richardson AL, McAllister SS, Stromal EGF and IGF-I together modulate plasticity of disseminated triple-negative breast tumors. Cancer Discov. 3, 922–935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA, Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133, 994–1005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J, Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell 79, 315–328 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Fisher B, Gunduz N, Saffer EA, Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 43, 1488–1492 (1983). [PubMed] [Google Scholar]
- 50.Gunduz N, Fisher B, Saffer EA, Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 39, 3861–3865 (1979). [PubMed] [Google Scholar]
- 51.Holmgren L, O’Reilly MS, Folkman J, Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med 1, 149–153 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Moshage H, Cytokines and the hepatic acute phase response. J. Pathol 181, 257–266 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Wynn TA, Chawla A, Pollard JW, Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coussens LM, Pollard JW, Leukocytes in mammary development and cancer. Cold Spring Harb. Perspect. Biol 3, a003285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian B, Pollard JW, Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibue T, Weinberg RA, Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A 106, 10290–10295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencetranslationalmedicine.org/cgi/content/full/10/436/eaan3464/DC1
Materials and Methods
Fig. S1. The outgrowth of D2A1-GFP tumors in Balb/c mice is restricted by a GFP-specific CD8+ T cell response.
Fig. S2. Surgical wounding triggers the outgrowth of tumor cells at distant anatomical sites.
Fig. S3. Meta-analyses demonstrate that surgical wounding promotes the outgrowth of distantly implanted tumor cells.
Fig. S4. Surgical wounding overcomes the effect of a tumor vaccine to promote the growth of distant B16 tumors.
Fig. S5. Surgical wounding triggers a systemic inflammatory response characterized by the mobilization of inflammatory myeloid cells into the circulation.
Fig. S6. Myeloid cells infiltrate D2A1-GFP tumors and promote tumor growth.
Fig. S7. NSAIDs alter the polarization of tumor-infiltrating macrophages.
Table S1. Components of flow cytometry antibody cocktails.
Reference (56)
Table S2. Primary data.