SYNOPSIS
Non-celiac wheat sensitivity (NCWS), also commonly referred to as non-celiac gluten sensitivity, is a condition characterized by gastrointestinal and extra-intestinal symptoms following the ingestion of gluten-containing cereals, i.e., wheat, rye, and barley, in subjects without celiac disease or wheat allergy. The exact identity of the molecular triggers responsible for the associated symptoms is not entirely clear yet. Besides gluten, other components have been suggested to contribute to symptoms, including additional proteins, such as α-amylase/protease inhibitors, and short chain carbohydrates that are poorly absorbed in the small intestine, such as fructan. Recent research has identified a biological basis for the condition, with the presence of systemic immune activation in response to microbial translocation that appears to be linked to intestinal barrier defects in affected individuals. NCWS is at the present reliably diagnosed only via double-blind placebo-controlled crossover trials. Ongoing research efforts are aimed at further delineating the etiology, mechanism, and biomarkers of the condition.
Keywords: Gluten, fructan, innate immunity, microbiota, antibody to native gliadin, biomarkers, double-blind placebo-controlled crossover trial, systemic immune activation
Introduction
Over the past two decades, the incidence of diseases believed to be induced by the ingestion of wheat and related gluten-containing cereals, including rye and barley, has increased (1). This trend is believed to be due not only to a significant improvement in diagnostic tools (2,3), but also appears to indicate an actual increase in disease incidence (4). The reasons for such a rise are not entirely clear. Improved hygiene, exposure to certain infectious agents, gut microbial dysbiosis resulting from the use of antibiotics or other drugs and the changing dietary habits, and alterations in the cultivation, preparation, and processing of gluten-containing cereals have been researched or discussed in this context, but firm conclusions have not been reached (3).
This background sets the basis for discussing a much-debated condition within the scientific community, and in particular, among experts gathered in four recent consensus conferences held in London, Munich, Salerno and Merano from 2011 to 2016 (5–8). Distinct from celiac disease (CD) (and its primary related autoimmune disorder, dermatitis herpetiformis) and wheat allergy, a new condition has been identified and referred to as nonceliac gluten sensitivity (NCGS) or non-celiac wheat sensitivity (NCWS), a term that has been coined primarily to distinguish the condition from the clinically overlapping CD. NCWS is now recognized as a condition triggered by an adverse reaction to certain wheat components and characterized by gastrointestinal, namely irritable bowel syndrome (IBS)-like, symptoms, and by extra-intestinal manifestations, occurring a few hours or days after the ingestion of foods made with gluten-containing cereals, i.e., wheat, rye, or barley. The associated symptoms improve with the withdrawal of the offending cereals and relapse after re-challenge. Exclusion of both CD and wheat allergy by established tests is a requirement for suspecting NCWS in patients on a gluten-containing diet. Although gluten has been the chief suspect as the triggering component of symptoms in NCWS, other components of wheat and related cereals may also be involved in symptom generation, either solely or possibly in conjunction with gluten (5–8). Growing interest has been devoted to a group of αamylase/protease inhibitors, commonly referred to as amylase/trypsin inhibitors (ATIs), and to the so-called fermentable oligo-, di-, and mono-saccharides, and polyols (FODMAPs) (9,10). As such, NCWS may be etiologically heterogeneous as clinically characterized currently, with subsets of patients responding to different components of wheat and related cereals. The diagnosis of NCWS relies on clinical criteria due to the lack of established biomarkers, making the diagnosis of this condition a clinical challenge (5–8). Self-diagnosed NCWS may also be attributable to a placebo effect in a subset of individuals (11, 12). However, the use of validated questionnaires has been shown to be helpful in assessing the symptom variation before and after the exclusion of gluten-containing cereals, thus allowing the identification of patients with true NCWS (7). Although inconvenient in daily practice, a double-blind, placebo-controlled cross-over trial (DBPCC) is a particularly useful tool to establish and confirm the diagnosis (7,8). Regardless of the apparently normal villous architecture, as detected by current technology and methods, a significant proportion of patients with NCWS appears to display mild intestinal malabsorption resulting in low levels of vitamin D3, ferritin, and folic acid (13). The mechanism leading to selective malabsorption is likely related to inflammatory changes in the small intestinal mucosa caused by innate immune activation, epithelial barrier impairment, and possible deleterious changes in the gut microbial population (14). Recent research suggests that a combination of serologic markers of immune activation and intestinal cell damage may have utility in aiding the diagnosis of the condition in the near future (15).
Because of the inadequate level of knowledge about the condition and the unmet needs in clinical practice, the present review aims to provide physicians with a thorough account and practical indications related to various aspects concerning NCWS, including pathogenesis, clinical picture, diagnosis, treatment, and future directions for research.
Pathogenic mechanisms
The pathogenesis of NCWS is likely to be multifactorial, with the innate immune response playing a key role. Several studies have identified an altered expression of innate immune components in response to wheat consumption in heterogeneous cohorts of wheatsensitive individuals, including mucosal Toll-like receptor 2 (TLR2) (16,17), PBMC-derived interleukin-10 (IL-10), granulocyte-colony stimulating factor (GCSF), transforming growth factor-α (TGF-α), and the chemokine CXCL-10 (18–20). While wheat-sensitive individuals lack a significant increase in intraepithelial lymphocyte infiltration, a characteristic histology in CD, an elevated frequency of interferon-γ (IFN-γ)-producing type 1 innate lymphoid cells (ILC1) in the rectal mucosa after oral wheat challenge has been shown in NCWS patients (21). In line with these findings, increased mRNA levels of IFN-γ have been detected in the mucosal tissue of NCWS patients upon gluten challenge (22). However, it is not clear whether the increased IL-10 mRNA levels are mediated by the activation of innate or adaptive immune cells. The production of gliadin-specific antibodies in wheat-sensitive individuals (15, 23, 24) is suggestive of a concomitant activation of the adaptive immune response in NCWS, though the lack of CD-specific markers (anti-TG2 antibodies and antideamidated gliadin antibodies) point to a mechanism that is significantly different from that in CD.
An impaired intestinal epithelial barrier has also been demonstrated in vivo (by lactulosemannitol test) and ex vivo (altered tight junction protein expression on colonic mucosal biopsies) in a subset of patients exhibiting HLA-DQ2/DQ8 haplotypes and in whom wheatevoked IBS-like symptoms is found (18). Other data supporting the so-called ‘leaky gut’ aspect in the context of NCWS include increased duodenal myosin light chain kinase activity and elevated colonocyte claudin-15 expression (25). Notably, these alterations were reversible in NCWS patients after the withdrawal of gluten-containing food and were accompanied by symptom remission. In addition, intestinal dysbiosis might contribute to epithelial barrier dysfunction and associated inflammatory response to gluten, thereby contributing to the pathogenesis of NCWS, similarly to what has been shown in other disorders, such as CD, IBS, and inflammatory bowel disease (26–29).
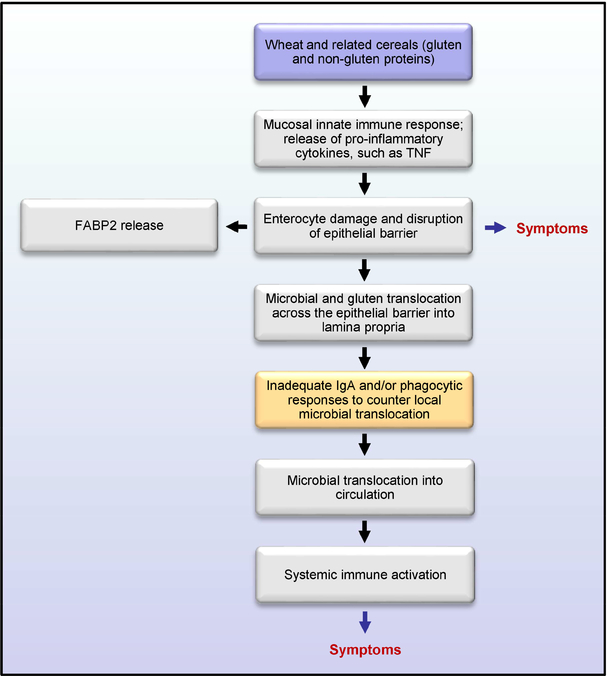
A recent study by Uhde et al. has provided compelling evidence for the existence of a compromised intestinal barrier in a well-defined cohort of NCWS patients that results in a systemic immune response to microbial and dietary antigens (15). Serum levels of lipopolysaccharide (LPS)-binding proteins (sCD14 and LPS binding protein - LBP) and antibody reactivity to microbial products (LPS, flagellin) were found to be elevated in patients with NCWS, and to correlate with circulating levels of intestinal fatty acid-binding protein (FABP2), suggesting a compromised gut barrier and microbial translocation. Upon the introduction of a diet free of wheat and related cereals, the increase in markers of immune activation and epithelial cell damage changed significantly towards normalization in affected individuals, demonstrating a link between wheat-containing diet, a dysfunctional intestinal barrier, and systemic immune activation as underlying mechanistic components in NCWS. These results have demonstrated the presence of objective markers of systemic immune activation and gut epithelial cell damage in individuals who report sensitivity to wheat in the absence of celiac disease, providing a biological basis to explain both the intestinal and extraintestinal manifestations of the condition (15). The presumed sequence of events leading to the systemic immune activation and associated symptoms in NCWS, as suggested by these data, is shown in Figure 1.
Figure 1:
Diagrammatic representation of the proposed sequence of events in non-celiac wheat sensitivity (NCWS) based on the available data.
Clinical picture
Epidemiological data on NCWS are not available as the diagnosis of this condition is still uncertain due to lack of biomarkers. Depending on the clinical setting, the prevalence of NCWS has been suggested to vary from 0.6% in primary care to 6% in tertiary care (5, 30), whereas in a multicenter study, the observed prevalence was 3.2% among more than 12.000 patients examined in 38 tertiary care centers (31). This survey showed that NCWS was much more prevalent in young adult females than in men (female to male ratio = 5:1, mean age 38 years), while being rare in childhood and elderly. The study also found a similar frequency for both NCWS and CD (NCWS:CD = 1.14). The clinical presentation of NCWS resembles IBS, although extra-intestinal manifestations may be substantially more prevalent in NCWS (16, 31). Symptoms occur after a few hours to days following the ingestion of wheat and related cereals, i.e., gluten-containing foods, while usually dissipating relative rapidly after the withdrawal of the offending foods, and returning soon after challenge. Before establishing NCWS, patients should be tested for CD serology and specific IgE antibodies to wheat allergens in order to rule out both CD and wheat allergy while they are still on a gluten/wheat-containing diet (7–10). Patients with NCWS have been reported to complain of gastrointestinal symptoms, such as bloating and abdominal pain in more than 80% of cases (31). Diarrhea is reported by about half of cases, whereas 20 to 30% of NCWS patients complain of alternating bowel and even constipation (31). Other manifestations affecting the GI tract, such as gastro-esophageal reflux disease, nausea, vomiting, aerophagia, and aphthous stomatitis are reported by 30 to 50% of NCWS patients (7, 16). The extra-intestinal manifestations in NCWS encompass a rather broad spectrum of symptoms, among which fatigue, headache, anxiety, and cognitive difficulties feature prominently, affecting some 30 to 50% of patients (31, 32). Fibromyalgia-like symptoms, including diffuse and migratory joint and muscle pain were observed in up to 30% of cases (33). Although both manifestations of presumed neurologic (32) and rheumatologic (33) origin can also be detected in IBS, skin complaints appear to be more specific to NCWS (34). Skin rash and dermatitis have been found to be reported by up to 30% of patients (31, 34). Regarding laboratory abnormalities in NCWS patients, malabsorption features such as low levels of vitamin D3 (up to 16%) leading to osteopenia (35), ferritin (up to 23%), and folic acid (up to 11%), which can be due to small intestinal micro-inflammation, have been reported (13). About one fifth of NCWS patients has been reported to have a family history of CD (31, 36, 37). Based on these data, serological screening for CD may be recommended in first degree relatives of NCWS patients. Similarly to IBS, a large proportion of NCWS patients displays lactose and, less frequently, fructose intolerance (31). About 20% of NCWS patients are positive for IgE antibodies to inhalants and foods including mites, graminaceae, shellfish, and other alimentary molecules (31). In line with these findings, a subset of NCWS patients may complain of multiple food hypersensitivities, including food antigens other than those from wheat and related cereals (38). In contrast with initial studies that ruled out an association between NCWS and autoimmunity (17), more recent data point to a high prevalence of autoimmune markers (anti-nuclear antibodies - ANA) and autoimmune disorders (mainly Hashimoto’s thyroiditis) among NCWS patients (39, 40) (Table 1). In contrast to CD, current data do not point to an association between NCWS and the development of complications, such as small intestinal lymphoma, small bowel adenocarcinoma and ulcerative jejunoileitis (4–8).
Table 1: Immunologic, genetic, and histopathological features in NCWS patients.
Antibodies to native gluten, genetic haplotype, duodenal histology, autoimmune features and familiarity for celiac disease in patients with non-coeliac wheat sensitivity.
| References | Antibodies to native gliadin | HLA-DQ2/DQ8 | Marsh 1 at duodenal biopsy | Antinuclear antibodies | Autoimmune disorders | CD family history |
|---|---|---|---|---|---|---|
| Sapone et al.17 | 48% | 46% | 84% | - | - | - |
| Carroccio et al.24, 40 | 50% | 75% | 96% | 46% | 29% | 14% |
| Francavilla et al.44 | 66% | 46% | 26% | - | - | - |
| Aziz et al.37 | - | 53% | - | - | 10% | 12% |
| Kabbani et al.38 | - | 42% | - | - | 12% | 13% |
| Volta et al.23, 32, 41 | 56% | 46% | 46% | 37% | 14% | 18% |
Notes: CD, celiac disease; Marsh 1: intraepithelial lymphocytes (IELs) > 25 / 100 epithelial cells at high power field; HLA, human leukocyte antigen.
Diagnostic criteria
Significant effort has been directed at identifying specific criteria for NCWS diagnosis. Earlier in this effort, the diagnostic approach relied only on exclusion criteria since symptomatic patients were labeled as NCWS after the exclusion of CD (i.e., absence of antitissue transglutaminase (TG2) / endomysial antibodies and of villous atrophy) (41) and wheat allergy (i.e., negative serum IgE antibodies to wheat allergens and relevant skin prick tests) (42). Although the exclusion of these two disorders remains a mandatory pre-requisite, during the 3rd Consensus Conference on NCWS, a panel of experts made a significant step forward by introducing positive criteria, including 1) the evaluation of symptoms before and after gluten/wheat exclusion from the diet by means of a modified version of the Gastrointestinal Symptom Rating Scale (GSRS) integrated with extraintestinal manifestations; 2) the identification of potential biomarkers; and 3) the standardization of DBPCC for confirming the diagnosis (7). The first step of the diagnostic work-up for NCWS is based on the symptom assessment (scored from 1 - very mild - to 10 - very severe) at baseline (when patients are still on a gluten/wheat-containing diet) and weekly for six weeks on gluten-free diet (GFD) by using the modified GSRS questionnaire (7). A GFD-dependent symptom decrease of >30% compared with baseline in at least three symptoms is regarded as a criterion to suspect NCWS.
The identification of established biomarkers for NCWS is still eagerly awaited. A number of studies have reported elevated IgG anti-gluten antibodies (AGA) in over half of NCWS patients (Table 1) (23, 24, 43), although these antibodies lack specificity for NCWS, being also found in CD, certain other autoimmune disorders, and some apparently healthy subjects (44). Nonetheless, their detection in symptomatic patients may support the diagnosis of NCWS (45). The diagnostic potential of antibody reactivity to gluten as a possible biomarker of NCWS has been recently expanded by the finding of increased levels of the IgM class AGA in the sera of NCWS patients in comparison to healthy individuals (15).
A series of intestinal cell damage and systemic immune activation markers have been recently described as having the potential to identify NCWS (15). Serum levels of sCD14 and LPS-binding protein, both related to acute-phase innate immune response to bacterial components, as well as antibody reactivity to bacterial antigens, have been found to be significantly increased in NCWS, suggesting systemic immune activation in response to microbial translocation. In addition, serum levels of FABP2, a marker of increased intestinal epithelial cell injury and turnover rate, were shown to be elevated in NCWS compared to controls, pointing to a compromised intestinal barrier against antigen translocation from the gut lumen. Of particular significance, all of the aforementioned biomarkers decreased significantly towards normalization after the exclusion of wheat and related cereals from diet, which was associated with improvement in symptoms. Although no single biomarker can be considered diagnostic, the principal component analysis of the above biomarkers, including various antibody isotype reactivity to gluten, allowed for the differentiation of NCWS individuals from CD and healthy controls (15, 46). The data establish the presence of objective markers of systemic immune activation and epithelial cell damage in NCWS, suggesting that a combination of biomarkers may have utility for identifying patients or specific patient subsets. A recent study utilized these same markers to identify a significant subset of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and gastrointestinal symptoms as potentially having NCWS (47).
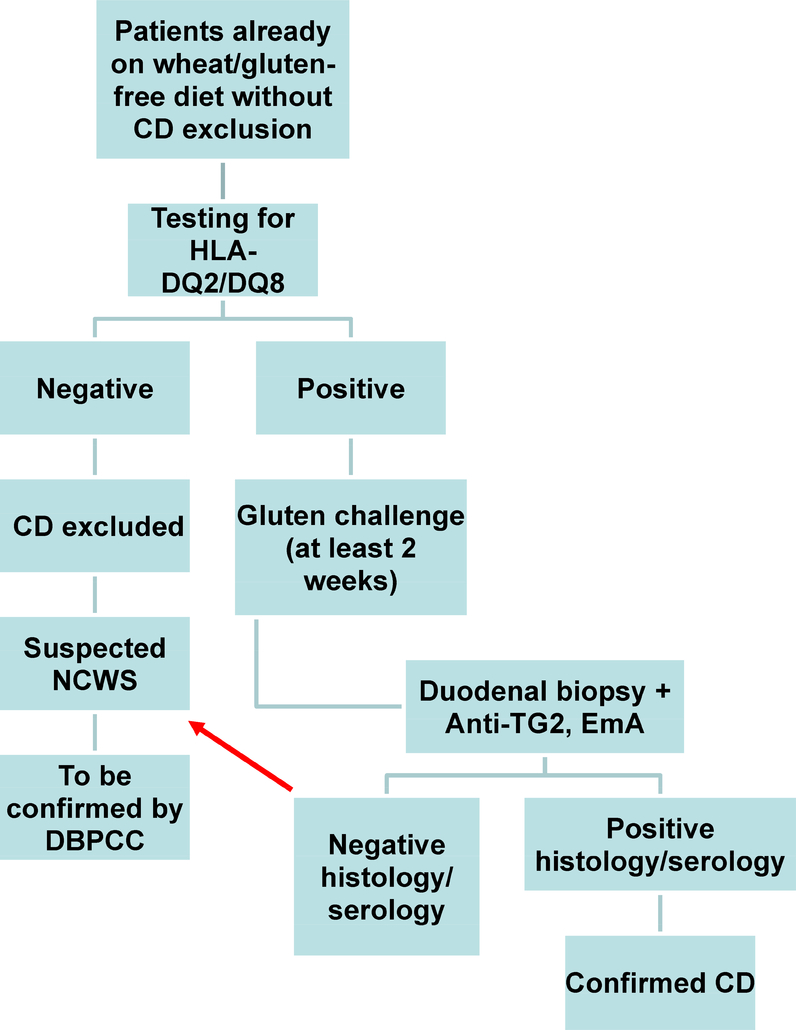
Another interesting aid in the diagnosis of NCWS might be the chemokine (CXCL10). In both children and adults with suspected NCWS, the in vitro stimulation of peripheral blood mononuclear cells (PBMC) by wheat extracts (Manitoba) resulted in significantly increased secretion of this chemokine, which was undetectable in control sera (19, 20). Preliminary data have also shown significantly higher serum levels of zonulin in NCWS patients than in IBS patients and healthy controls. Although serum zonulin levels in NCWS did not differ from those found in CD, they tended to normalize more rapidly in the former than in the latter condition after GFD (48). Another interesting finding comes from the study of mast cell density in the duodenal mucosa/submucosa. Our unpublished results indicate a higher mast cell density in NCWS in comparison to healthy controls and CD patients. This increased mast cell number in NCWS seems to be closely related with the presence of IBS-like symptoms such as bloating, abdominal pain, and impaired bowel function. Moreover, the close vicinity of mast cells and nerve fibers observed in these patients may have a role in the generation of symptoms, e.g., abdominal pain, via a neuroimmune mechanism. No association has yet been identified between NCWS and specific genetic markers. The only certainty is that NCWS is not associated with HLA-DQ2 and/or -DQ8, which have a high negative predictive value for CD. The frequencies of HLA-DQ2 and/or -DQ8 are generally not found to be substantially different between NCWS and healthy individuals (Table 1) (17, 23, 31, 36, 37, 39). Nevertheless, genetic testing for these alleles is useful for patients who have already commenced a restricted diet without ruling out CD diagnosis. In these patients, negativity for both DQ2 and DQ8 allows for the exclusion of CD (5–8). Obviously, a gluten challenge remains mandatory for the diagnosis of patients who are positive for the DQ2/DQ8 genetic predisposition for CD (Figure 2).
Figure 2:
Suggested diagnostic algorithm for patients adhering to a gluten free diet (GFD) without having previously ruled out celiac disease.
From a histopathological standpoint, the intestinal mucosa in NCWS displays a normal villous/crypt ratio (>3:1), with a preserved villous architecture. An increase in intraepithelial lymphocytes (IELs), consistent with lesion type 1 according to the Marsh-Oberhüber classification, has been reported with a highly variable prevalence ranging from 26% to 96% of these patients (Table 1) (17, 24, 32, 44). In contrast to CD (49), the IEL increase in NCWS is usually very mild ranging from 25 to 40 lymphocytes in a high-power field (31). Also the distribution of lymphocytes might be helpful for differentiating NCWS from CD, since in the former these cells are located in the deeper part of the mucosa with a palisade pattern and in clusters in the villi, whereas in CD they are more closely confined to the epithelium with a diffuse distribution (50).
Due to the current lack of established biomarkers, DBPCC is still regarded as the gold standard for confirming the diagnosis of NCWS (5–8). However, this approach is not well accepted by patients who are already on a diet that excludes gluten-containing foods, while being time-consuming and difficult to implement. A review of 10 DBPCCs, including 1,312 adults with IBS and/or suspected NCWS revealed the lack of standardization (51). In fact, the trials differed from one another for the selection of patients, the dosage of gluten or wheat, the duration of challenge (from one day to six weeks) and the composition of placebo (gluten-free products, xylose, whey protein, rice, or corn starch containing fermentable carbohydrates) (52). This systematic review of the literature suggested that only 16% of the patients evaluated by DBPCC for suspected NCWS experienced gluten-specific symptoms (triggered with gluten, but not with placebo ingestion) (51). The results emerging from the single studies confirmed a large variability in the rate of NCWS. Carroccio et al. reported a prevalence of wheat sensitivity in seventy (26%) of 270 patients (23), whereas a further trial confirmed this diagnosis in 9 out of 59 (15%) patients, with three NCWS patients (5%) showing a particularly pronounced symptomatic response to gluten challenge (52). More recently, NCWS was reported by means of DBPCC in 14% and 34% out of 98 and 35 patients, respectively (53, 54). Two trials carried out by the same investigative group produced somewhat different results with a first study (performed without cross-over) confirming NCWS in 68% of cases (55), and a second one (with cross-over) finding it in only 8% (56). Another recent study on a cohort of NCWS patients suggested that fructan oligosaccharides (a FODMAP) are the more likely culprits in the generation of intestinal symptoms (specifically, bloating) when compared with gluten proteins (57). The fructan used in the study originated from chicory roots, so it remains to be seen whether the actual fructo-oligosaccharide in wheat have the same effect. The study’s findings also suggested that neither gluten proteins nor FODMAPs are by themselves significant triggers of the abdominal pain or extra-intestinal symptoms associated with NCWS. Indeed, fructans can plausibly contribute to certain IBS-like symptoms, such as bloating, but they are less likely to be directly linked to immune activation or the extra-intestinal symptoms associated with NCWS (58). Furthermore, a role for the FODMAP (e.g., fructans) component of wheat as the sole trigger for symptoms is somewhat doubtful, because many patients with NCWS report resolution of symptoms after the withdrawal of wheat and related cereals, while continuing to ingest vegetables and fruits with high FODMAP content in their diets (59). On the whole, it is conceivable that more than one culprit may be involved in symptoms of NCWS (as they are currently defined), including gluten, other wheat proteins, and FODMAPs (60–62). Five out of six DBPCC trials enrolling a total of 558 patients have confirmed the existence of NCWS, while suggesting that the frequency of the condition may be somewhat overestimated (Table 2) due to the associated placebo effect. A further element supporting the existence of such wheat sensitivity comes from a study utilizing confocal laser endomicroscopy (63). The administration of wheat to the duodenal mucosa through the endoscopic route was found to induce small intestinal changes characterized by increased IEL number, epithelial leaks/gaps, and widened intervillous spaces in more than one/third of the patients tested.
Table 2: Study design and results of DBPCC for NCWS diagnosis.
Results of double-blind placebo-controlled trials (DBPCC) in patients with suspected non-celiac wheat sensitivity (NCWS): study design and modality of gluten/wheat administration, duration of the trial, number of patients completing the trial and percentage of patients with confirmed NCWS (complaining of symptoms triggered with wheat/gluten and not with placebo).
| Reference | Study Design | Inclusion criteria | Mode of administration of gluten/wheat | Placebo | Duration of the trial | Number of patients completin g DBPCC | Number of patients Having symptoms triggered with gluten/wheat but not with placebo |
|---|---|---|---|---|---|---|---|
| Biesiekierski et al., 2013, Australia (ref 57) | Cross-over randomized DBPCC | Self-reported NCWS patients | Food with high (16 g/d) or low content (2 g/d) of carbohydratedepleted wheat protein | Gluten-free food with whey protein (16 g/d) | 2 week-run-in period with a low-FODMAPs diet/1 week with high or low-gluten diet or placebo/2-week washout before cross-over for 1 week | 37 | 3 (8%) |
| Carroccio et al, 2012, Italy (ref 24) | Cross-over, randomized DBPCC | IBS and selreported NCWS patients | Wheat flourcontaining capsules (13 g/d) | Xylosecontaining capsules | 2 weeks with one type of capsules/1-week washout/2 weeks with the other type of capsules | 270 | 70 (26%) |
| Zanini et al., 2015, Italy (ref 55) | Cross-over randomized DBPCC | Self-reported NCWS patients | Gluten containing flour | Gluten-free flour | 10 days with one type of flour /2-week washout/ 2 weeks with the other flour | 35 | 12 (34%) |
| Di Sabatino et al., 2015, Italy (ref 53) | Cross-over randomized DBPCC | Self-reported NCWS patients | Gluten containing capsules (4.375 g/d) | Rice starch containing capsules | 1 week with one type of capsules/1week wash-out/1week with the other type of capsules | 59 | 9 (15%) ° |
| Elli et al,, 2016, Italy (ref 54) | Cross-over randomized DBPCC | Patients with functional symptoms, including IBS and functional dyspepsia | Gluten containing capsules (5.6 g/d) | Rice starch containing capsules | 1 week with one type of capsules/1week wash-out/1week with the other type of capsules | 98 | 14 (14%) |
| Skodje GI et al., 2018, Norway (ref 58) | Cross-over randomized DBPCC | Self-reported NCWS patients | Gluten (5.7g/d), fructans (2.1g/d) concealed in muesli bars | Not reported | 1 week with one type of bars (once daily) with gluten or fructans or placebo, followed by 1-week washout | 59 | 0* |
| Overall results | 558 | 108 (19.3%) |
Of these patients, three out of 59 (5%) showed a particularly pronounced symptomatic response to gluten.
Fructans (not gluten) induced the highest symptom scores for bloating compared to placebo, but not for other NCWS-related symptoms. Notes: DBPCC, double-blind, placebo controlled challenge; IBS, irritable bowel syndrome; NCWS, non-celiac wheat sensitivity; FODMAPs, fermentable oligo-, di-, monosaccharides, and polyols.
Management of NCWS
The first recommendation for patients complaining of symptoms after the ingestion of gluten-containing food is to start the diagnostic work-up for CD and wheat allergy prior to any dietary restriction (5–8). As recommended in the 4th Consensus Conference on NCWS (8), only after having ruled out both CD and wheat allergy should these patients be studied by an open trial of wheat/rye/barley-free diet for 6 weeks. Those who respond to the diet with the reduction of at least 30% in three symptoms based on the modified version of the GSRS (7) can be regarded as affected by suspected NCWS and should be kept on a diet that excludes wheat, rye, and barley, while awaiting further confirmation by a standardized DBPCC. The diagnosis of NCWS is excluded in those patients who do not improve after the wheat withdrawal. The DBPCC standardization is still far from being accomplished. In the third Consensus Conference on NCWS, an attempt to standardize a gluten-related challenge was undertaken (7). It was recommended to test the patients with a gluten amount of 8 grams/day, using a muesli bar as vehicle. Moreover, it was established that the ATI content should not be higher than 0.3 g/day and that the gluten vehicle should be without fructans. Another relevant input was that the gluten and placebo preparations should be indistinguishable in look and taste. A one-week gluten challenge was recommended followed by a one-week GFD and crossover to the second one-week challenge. The diagnosis of NCWS could be confirmed when at least a variation of 30% between the gluten and placebo challenge is detected. By using the Salerno criteria, a recent DBPCC confirmed the existence of true NCWS in 11 out of 36 children (39.2%) (64).
Future Directions
Despite the clinical evidence for its existence and strong recent evidence for a biological basis, NCWS is still a “work-in-progress” disorder with many unanswered questions (65). Based on the clinical evidence available, gluten is thought to be relevant, but not the only culprit of this syndrome. Other components of wheat, such as ATIs and wheat germ agglutinins, as well as fructans (i.e., FODMAPs), may have a role in symptom generation (61–63). For this reason, the term NCWS would seem preferable to NCGS (59), with the understanding that “wheat” also represents the closely related rye and barley, in the same way that the term “gluten” is now used to refer to the prolamin proteins of not only wheat, but also rye and barley. Since it is feasible to separate the different fractions of wheat and other related cereals, a DBPCC trail with five arms including pure preparations of gluten, ATIs, and fructans, as well as a complete wheat composition and placebo, could be a major step towards deciphering the impact of the different fractions of wheat in the clinical picture of NCWS. One of the most important challenges for the scientific community is the identification of established biomarkers for confirming the diagnosis without the need for DBPCC as already achieved for CD. Serum intestinal cell damage and immune activation markers have already given promising results, showing that the combination of several markers (AGA, LBP, sCD14, antibodies to LPS and flagellin, and FABP2) allows for the differentiation of NCWS from CD and control subjects with some outliers (15). Other studies dealing with further characterization and validation of these biomarkers and possibly others, including those directly related to the small intestinal mucosa of NCWS patients, can improve the diagnostic work-up of this condition. Other potentially useful markers might be represented by the increased duodenal mast cell density interacting with nerve fibers as a potential trigger for symptom generation. The identification of biomarkers will also allow for clarification of the prevalence of NCWS, which is currently based on estimates using various assumptions (66). Another unanswered question is whether NCWS is a disorder with possible recovery like wheat allergy or a primarily permanent condition like CD. Although still debatable, a recent study suggests that NCWS may be a chronic disorder (67). Of 200 patients with NCWS confirmed by DBPCC and followed up for 8 years, 148 were still on the restricted diet, whereas 52 came back to a gluten-containing diet. Ninety-eight percent of those still on a restricted diet remained symptom-free compared to only 58% of those on a gluten-containing diet, suggesting that the reintroduction of gluten-containing foods was associated with a significantly higher recurrence of symptoms. Table 3 summarizes the comparison between NCWS, CD, and IBS.
Table 3: Comparison for various features in irritable bowel syndrome, non-celiac wheat sensitivity, and celiac disease.
Comparison of epidemiological, pathogenic, clinical, diagnostic, and outcome features of irritable bowel syndrome (IBS), non-celiac wheat sensitivity (NCWS), and celiac disease (CD).
| IBS | NCWS | CD | |
|---|---|---|---|
| Epidemiology | 10–20% | Unknown (possible range 0.6 −6%) | 1% |
| Duration | Chronic | Unknown | Chronic |
| Pathogenesis | Multifactorial (innate immunity involved) | Innate immunity | Innate/adaptive immunity |
| Onset age | 40–50 years | 30–40 years | Any age |
| Gender | Female/male 2:1 | Female/male 5:1 | Female/male 2:1 |
| Familiarity for glutenrelated disorders | Variable | Present | Present |
| Symptoms | Gastrointestinal | Gastrointtestinal/Extraintestinal | Gastrointestinal/Extraintestinal |
| Biomarkers | None | None (AGA?) | Anti-tTG and EmA |
| HLA | None | None | HLA-DQ2 and -DQ8 restricted |
| Duodenal histology | Normal mucosa | Normal mucosa / mild lesions | Villous atrophy |
| Autoimmune disorders | Low prevalence | High prevalence | High prevalence |
| Reduced bone mineral density | Up to 50% | Up to 70% | |
| Food Intolerance | Lactose intolerance | Lactose / fructose intolerance | Lactose / fructose intolerance |
| Outcome (complications) | No complications | No complications | Refractory disease, lymphoma, small bowel adenocarcinoma, ulcerative jejunoileitis |
| DBPCC | Unnecessary for diagnosis | Recommended for diagnosis | Unnecessary for diagnosis |
Notes: IBS, irritable bowel syndrome; NCWS, Non-celiac wheat sensitivity; CD, celiac disease; AGA, antibodies to native gliadins; Anti-tTG, antibodies to tissue transglutaminase; EmA, anti-endomysial antibodies; DBPCC, double blind placebo controlled cross-over challenge.
Conclusions
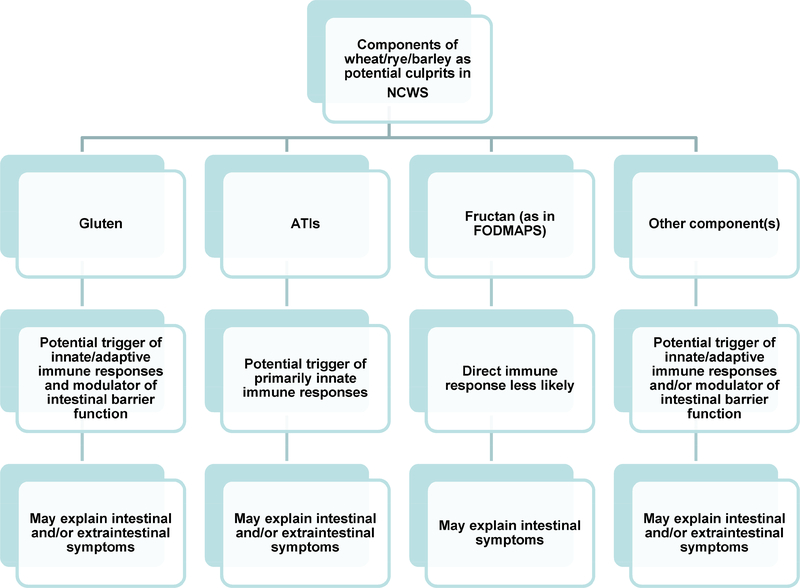
Interest in NCWS within the scientific community is reflected by the increasing number of original papers, reviews, and trials published on this topic in the past 10 years. The initial skepticism surrounding this disorder has gradually given way to a progressing awareness of the existence of NCWS. This diagnosis has been confirmed by DBPCC trials in varying proportions of patients with self-reported NCWS (51). The exact trigger(s) among the various components of wheat and related cereals for the associated symptoms remains unclear, but gluten, ATIs, and fructan, as well as other components, may play a role (60–62). FODMAPs have been reported to contribute to certain intestinal symptoms in some patients with NCWS, but other components of wheat and related cereals, such as specific proteins, are believed to trigger substantial immune activation and/or intestinal barrier dysfunction that would explain both gastrointestinal and extra-intestinal manifestations (Figure 3) (58). The identification and confirmation of established biomarkers is eagerly awaited since their availability will allow for the diagnosis of this condition without the need for time-consuming food challenge, which is not well-accepted by patients. Promising results have been achieved by the analysis of markers of intestinal cell damage and systemic immune activation. Further evaluation of the mechanisms involved in the generation of these markers is likely to add significantly to our understanding of NCWS and the identification of associated diagnostic markers.
Figure 3:
Different components of wheat, such as gluten, fructans (as part of fermentable oligo-, di-, and mono-saccharides, and polyols, FODMAPs), amylase-trypsin inhibitors (ATIs), and other molecules may act as triggers of non-celiac wheat sensitivity (NCWS), including immune system modulation, intestinal barrier disruption, and symptom generation.
Key Points.
Non-celiac wheat sensitivity (NCWS) is a condition characterized by intestinal and extra-intestinal symptoms which occur after the ingestion of gluten-containing foods in patients without celiac disease or wheat allergy.
Gluten, α-amylase/protease inhibitors, and fructan contained in wheat and related cereals are considered as potential triggers or co-triggers of NCWS symptoms.
The pathogenesis of NCWS is likely to be the result of a complex interplay among different factors, including specific components of wheat and related cereals, intestinal barrier function, gut microbiota, and innate and adaptive immunity.
NCWS can present at any age, but its frequency appears to be higher in young adults (3rd−4th decades of life) and in females.
Currently, a double-blind placebo-controlled crossover trial is the only widely accepted method to confirm NCWS, although research is underway to establish biomarkers to aid the diagnosis.
Footnotes
Disclosure: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leonard MM, Sapone A, Catassi C, et al. Celiac disease and nonceliac gluten sensitivity: a review. JAMA 2017;318:647–56. [DOI] [PubMed] [Google Scholar]
- 2.Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70–81. [DOI] [PubMed] [Google Scholar]
- 3.Volta U, De Giorgio R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol 2012;9:295–9. [DOI] [PubMed] [Google Scholar]
- 4.(Lebwohl Ludvigsson et al. 2015).
- 5.Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catassi C, Bai JC, Bonaz B, et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients 2013;5:3839–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015;7:4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catassi C, Alaedini A, Bojarski C, et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients. 2017;9(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 2012;209:2395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut 2016;65:169–78. [DOI] [PubMed] [Google Scholar]
- 11.Di Sabatino A, Corazza GR. Nonceliac gluten sensitivity: sense or sensibility? Ann Intern Med 2012;156:309–11. [DOI] [PubMed] [Google Scholar]
- 12.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995;333:1–4. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Infante J, Santolaria S, Sanders DS, et al. Systematic review: non-coeliac gluten sensitivity. Aliment Pharmacol Ther 2015;41:807–20. [DOI] [PubMed] [Google Scholar]
- 14.Volta U, Caio G, Karunaratne TB, et al. Non-coeliac gluten/wheat sensitivity: advances in knowledge and relevant questions. Expert Rev Gastroenterol Hepatol. 2017;11:9–18. [DOI] [PubMed] [Google Scholar]
- 15.Uhde M, Ajamian M, Caio G, et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016;65:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasano A, Sapone A, Zevallos V, et al. Non-celiac Gluten Sensitivity. Gastroenterology 2015;149:1195–204. [DOI] [PubMed] [Google Scholar]
- 17.Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten associated conditions: celiac disease and gluten sensitivity. BMC Medicine 2011; 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013;144:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valerii MC, Ricci C, Spisni E, et al. Responses of peripheral blood mononucleated cells from non-celiac gluten sensitive patients to various cereal sources. Food Chem 2015;176:167–74. [DOI] [PubMed] [Google Scholar]
- 20.Alvisi P1, De Fazio L2, Valerii MC, et al. Responses of blood mononucleated cells and clinical outcome of non-celiac gluten sensitive pediatric patients to various cereal sources: a pilot study. Int J Food Sci Nutr. 2017;68:1005–12. [DOI] [PubMed] [Google Scholar]
- 21.Di Liberto D, Mansueto P, D’Alcamo A, et al. Predominance of Type 1 Innate Lymphoid Cells in the Rectal Mucosa of Patients With Non-Celiac Wheat Sensitivity: Reversal After a Wheat-Free Diet. Clin Transl Gastroenterol 2016;7:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brottveit M, Beitnes AC, Tollefsen S, et al. Mucosal cytokine response after shortterm gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol 2013;108:842–50. [DOI] [PubMed] [Google Scholar]
- 23.Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol 2012;46:680–5. [DOI] [PubMed] [Google Scholar]
- 24.Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012;107:1898–906. [DOI] [PubMed] [Google Scholar]
- 25.Wu RL, Vazquez-Roque MI, Carlson P, et al. Gluten-induced symptoms in diarrheapredominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab Invest 2017;97:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieterich W, Schuppan D, Schink M, et al. Influence of low-FODMAP and glutenfree diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin Nutr 2018; e-pub. [DOI] [PubMed] [Google Scholar]
- 27.Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol 2012; 2012:654143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011. November;14:1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD . Nat Rev Gastroenterol Hepatol. 2012;9:599–608. [DOI] [PubMed] [Google Scholar]
- 30.DiGiacomo DV, Tennyson CA, Green PH,et al. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol 2013;48:921–5. [DOI] [PubMed] [Google Scholar]
- 31.Volta U, Bardella MT, Calabrò A, et al. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med 2014;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjivassiliou M, Rao DG, Grünewald RA, et al. Neurological dysfunction in coeliac disease and non-coeliac gluten sensitivity. Am J Gastroenterol 2016;111;561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigo L, Blanco I, Bobes J, et al. Effect of one year of a gluten-free diet on the clinical evolution of irritable bowel syndrome plus fibromyalgia in patients with associated lymphocytic enteritis: a case-control study. Arthritis Res Ther 2014;16:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonciolini V, Bianchi B, Del Bianco E, et al. Cutaneous manifestations of non-coeliac gluten sensitivity: clincal, histological and immunopathological features. Nutrients 2015;7:7798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroccio A, Soresi M, D’Alcamo A, et al. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: a prospective observation study. BMC Med. 2014;12:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aziz I, Lewis NR, Hadjivassiliou M, et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol 2014;26:33–9. [DOI] [PubMed] [Google Scholar]
- 37.Kabbani TA, Vanga RR, Leffler DA, et al. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol 2014;109:741–6. [DOI] [PubMed] [Google Scholar]
- 38.Carroccio A, Brusca I, Mansueto P, et al. A comparison between two different in vitro basophil activation tests for gluten- and cow’s milk protein sensitivity in irritable bowel syndrome (IBS)-like patients. Clin Chem Lab Med 2013;51:1257–63. Carroccio A et al., Clin Chem Lab Med 2013 [DOI] [PubMed] [Google Scholar]
- 39.Carroccio A, D’Alcamo A, Cavataio F, et al. High proportions of people with nonceliac wheat sensitivity have autoimmune disease or antinuclear antibodies. Gastroenterology 2015;149:596–603. [DOI] [PubMed] [Google Scholar]
- 40.Volta U, Caio G, De Giorgio R. Is Autoimmunity More Predominant in Nonceliac Wheat Sensitivity Than Celiac Disease? Gastroenterology. 2016;150:282. [DOI] [PubMed] [Google Scholar]
- 41.Volta U, Villanacci V Celiac disease: diagnostic criteria in progress. Cell Mol Immunol 2011;8:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inomata N Wheat allergy. Curr Opin Allergy Clin Immunol 2009;9:238–43. [DOI] [PubMed] [Google Scholar]
- 43.Francavilla R, Cristofori F, Castellaneta S, et al. Clinical, serologic, and histologic features of gluten sensitivity in children. J Pediatr. 2014;164:463–7. [DOI] [PubMed] [Google Scholar]
- 44.Volta U, Granito A, Parisi C, et al. Deamidated gliadin peptide antibodies as a routine test for celiac disease: a prospective analysis. J Clin Gastroenterol 2010;44:186–90. [DOI] [PubMed] [Google Scholar]
- 45.Caio G, Volta U, Tovoli F, De Giorgio R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhde M, Caio G, De Giorgio R, Green PHR, Volta U, Alaedini A. Serologic markers of systemic immune activation and intestinal cell damage in non-celiac wheat sensitivity. Gastroenterology 2017; 152 (S1):S37. [Google Scholar]
- 47.Uhde M, Indart AC, Yu XB et al. Markers of non-coeliac wheat sensitivity in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Gut 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbaro MR, Cremon C, Caio G, et al. Increased zonulin serum levels and correlation with symptoms in non-celiac gluten sensitivity and irritable bowel syndrome with diarrhea. United European J Gastroenterol 2014; 2(suppl. 1):A555. [Google Scholar]
- 49.Rostami K, Marsh MN, Johnson MW, et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut 2017;66:2080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanini B, Villanacci V, Marullo M, et al. Duodenal histological features in suspected non-celiac gluten sensitivity: new into a still undefined condition. Virchows Arch 2018; e-pub [DOI] [PubMed] [Google Scholar]
- 51.Molina-Infante J, Carroccio A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin Gastroenterol Hepatol 2017;15:339–48. [DOI] [PubMed] [Google Scholar]
- 52.Di Sabatino A, Volta U, Salvatore C, et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebocontrolled, cross-over trial. Clin Gastroenterol Hepatol 2015;13:1604–12. [DOI] [PubMed] [Google Scholar]
- 53.Elli L, Tomba C, Bianchi F, et al. Evidence for the presence of non-coeliac gluten sensitivity in patients with functional gastrointestinal symptoms: results form a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanini B, Baschè R, Ferraresi A, et al. Randomised clinical study: gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment Pharmacol Ther 2015;42:968–76. [DOI] [PubMed] [Google Scholar]
- 55.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebocontrolled trial. Am J Gastroenterol 2011;106:508–14. [DOI] [PubMed] [Google Scholar]
- 56.Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320–28. [DOI] [PubMed] [Google Scholar]
- 57.Skodje GI, Sarna VK, Minelle IH, et al. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018;154:529–539. [DOI] [PubMed] [Google Scholar]
- 58.Verbeke K Nonceliac Gluten Sensitivity: What Is the Culprit? Gastroenterology 2018;154:471–3. [DOI] [PubMed] [Google Scholar]
- 59.Prichard R, Rossi M, Muir J, et al. Fermentable oligosaccharide, disaccharide, monosaccharide and polyol content of foods commonly consumed by ethnic minority groups in the United Kingdom. Int J Food Sci Nutr 2016;67:383–90. [DOI] [PubMed] [Google Scholar]
- 60.Schuppan D, Pickert G, Ashfaq-Khan M, et al. Non-celiac wheat sensitivity: differential diagnosis, triggers and implications. Best Pract Res Clin Gastroenterol 2015;29:469–76. [DOI] [PubMed] [Google Scholar]
- 61.de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013;5:771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75. [DOI] [PubMed] [Google Scholar]
- 63.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of paytients with irritable bowel syndrome. Gastroenterology 2014;147:1012–20. [DOI] [PubMed] [Google Scholar]
- 64.Francavilla R, Cristofori F, Verzillo L, et al. Randomized Double-Blind Placebo-Controlled Crossover Trial for the Diagnosis of Non-Celiac Gluten Sensitivity in Children. Am J Gastroenterol 2018. e-pub. [DOI] [PubMed] [Google Scholar]
- 65.Volta U, Caio G, De Giorgio R, et al. Non-celiac gluten sensitivity: a work-inprogress entity in the spectrum of wheat-related disorders. Best Pract Res Clin Gastroenterol 2015;29:477–91. [DOI] [PubMed] [Google Scholar]
- 66.Vasagar B, Cox J, Herion JT, et al. World epidemiology of non-celiac gluten sensitivity. Minerva Gastroenterol Dietol. 2017;63:5–15. [DOI] [PubMed] [Google Scholar]
- 67.Carroccio A, D’Alcamo A, Iacono G, et al. Persistence of Nonceliac Wheat Sensitivity, Based on Long-term Follow-up. Gastroenterology 2017;153:56–58. [DOI] [PubMed] [Google Scholar]