Abstract
Objective
Many children with autism spectrum disorder (ASD) have feeding and mealtime problems. To address these, we conducted a pilot randomized trial of a new 11-session, individually delivered parent training program that integrated behavioral strategies and nutritional guidance (PT-F).
Methods
Forty-two young children (age: 2 to 7–11 years) with ASD and feeding problems were assigned to 11 sessions of PT-F intervention over 20 weeks or a waitlist control. Outcomes included attendance, parent satisfaction, therapist fidelity, and preliminary assessments of child and parent outcomes.
Results
Of the 21 PT-F families, attendance was high (85%) as was parent satisfaction (94% would recommend to others). Treatment fidelity was also high (97%—therapist integrity; 94%—parent adherence). Compared with waitlist, children whose parents participated in PT-F showed significantly greater reductions on the two parent-completed primary outcomes (Brief Autism Mealtime Behavior Inventory-Revised; Twald = −2.79; p = .003; About Your Child’s Eating; Twald = −3.58; p = .001). On the independent evaluator-completed secondary eating outcome, the Clinical Global Impression-Improvement, 48.8% of the participants in PT-F were rated as “responders” compared with 0% in waitlist (p = .006). General child disruptive behavior outcomes decreased more in PT-F but not significantly. Parent outcomes of caregiver stress showed nonsignificant trends favoring PT-F with moderate to small effect sizes.
Conclusions
This trial provides evidence for feasibility, satisfaction, and fidelity of implementation of PT-F for feeding problems in young children with ASD. Feeding outcomes also appeared favorable and lends support for conducting a larger efficacy trial.
Keywords: autism spectrum disorder, eating habits, feeding problems, food selectivity, parent training
Introduction
Autism spectrum disorder (ASD) is characterized by social communication deficits and repetitive and restrictive behaviors (American Psychiatric Association, 2013). Many children with ASD also have co-occurring behavioral concerns. For example, an estimated 46–89% of these children exhibit problematic feeding and eating habits (Bandini et al., 2010; Curtin et al., 2015) compared with about 13–32% of typically developing children (Laud, Girolami, Boscoe, & Gulotta, 2009). The most common feeding problems in children with ASD include food selectivity based on type, texture or presentation, and disruptive mealtime behaviors (Bandini et al., 2010; Curtin et al., 2015), and many present with both. These problems often emerge in toddler and preschool years before ASD diagnosis (Emond, Emmett, Steer, & Golding, 2010) and remain in adolescence (Kuschner et al., 2015).
In prior studies, which largely had single-subject designs, behaviorally based procedures have been shown to decrease feeding problems in some children with ASD (Laud et al., 2009; Sharp, Jaquess, Morton, & Herzinger, 2010). Procedures are often applied in combination and include antecedent and stimulus control strategies, e.g., slowly fading in new foods, shaping new feeding behaviors, providing structure and establishing discriminant stimuli (Gentry & Luiselli, 2008; Milnes, 2011), contingent and noncontingent reinforcement systems (Gentry & Luiselli, 2008; Najdowski et al., 2010), prompting hierarchies (Najdowski, Tarbox, & Wilke, 2012), extinction of escape-related mealtime problem behaviors usually by nonremoval of spoon (Allison et al., 2012; Bui, Moore, & Anderson, 2013; Peterson, Piazza, & Volkert, 2016), teaching compliance around mealtimes (Meier, Fryling, & Wallace, 2012; Penrod, Gardella, & Fernand, 2012), and increasing motivation by allowing for hunger (Levin & Carr, 2001). Much of this work has been conducted in specialized feeding programs, often as part of an intensive partial day treatment program, with an emphasis on increasing bites accepted or grams consumed as the outcome variables, although some also included a parent/caregiver questionnaire. The involvement of the parent(s) varied greatly across these studies. A few single-subject studies have reported on exclusively parent delivered interventions for feeding problems in children with ASD (Bui et al., 2013; Gale, Eikeseth, & Rudrud, 2011; Gentry & Luiselli, 2008). In the only randomized trial of a feeding intervention for the ASD population, a parent training curriculum delivered in a group format did not significantly improve feeding behaviors (Sharp, Burrell, & Jaquess, 2014). Parent involvement also varies substantially in routine clinical practice with variation in the type of intervention (e.g., behaviorally based or sensory-based approaches) and providers (e.g., speech language pathologists, occupational therapists, or psychologists) (Marshall, Hill & Dodrill, 2013).
Designating a parent as the primary agent to address feeding problems seems most appropriate given the central role that parents play in all areas of a young child’s life and given that they are the caregivers most intimately involved with diets and feeding of their child. This includes up to three meals per day along with a daily snack. Hence, the level of intensity of intervention can be naturally increased if parents play the role of change agent. Furthermore, as a child’s disruptive mealtime behaviors, selective eating, and rigidity have been shown to be correlated with stress and family burden (Thullen & Bonsall, 2017), teaching parents strategies to improve eating and decrease disruptive mealtimes behaviors could result in decreased parental stress as previously suggested (Johnson, Foldes, DeMand, & Brooks, 2015; Sharp et al., 2014). Parent training as an intervention model has been successfully applied to target core social communication deficits, disruptive behaviors, adaptive skills, and sleep problems in ASD (Aman et al., 2009; Bearss et al., 2015; Dawson et al., 2010; Johnson et al., 2013; Scahill et al., 2016). In a study of disruptive behaviors in children with ASD, parent training diminished parental stress and improved parental sense of competence (Iadarola et al., 2017).
In prior work, we drafted a structured parent training manual that integrated behavior change strategies developed and tested in single-subject studies for feeding problems in children with ASD, and we conducted a case series that provided initial evidence of the promise of this parent training for feeding (PT-F) (Johnson et al., 2015). To our knowledge, the study was the first to examine an intervention delivered individually to families for feeding problems in ASD. In that study, parent attendance and satisfaction were high, as was treatment fidelity. Parent-reported feeding problems and disruptive behaviors were significantly reduced. Significant reduction in parenting stress was also observed. As the next step in treatment development, we revised and expanded the manual with additional expert input. We also developed video vignettes, depicting actual families of children with ASD at mealtimes in their homes, to illustrate concepts and techniques and added a nutritional component. This involved obtaining diaries of children’s food intake, analyzing nutritional adequacy with accurate and comprehensive nutrient calculator software, and having a registered dietician review findings with families. Based on these findings, the dietician made recommendations to the parents and therapists to improve nutrition as well as food variety and amount. Here, we report on a pilot randomized clinical trial whose primary aim was to test the feasibility, acceptability, and fidelity of the revised PT-F program. We hypothesized the intervention would be feasible as defined as (1) <15% attrition, (2) >80% attendance at parent training sessions, and (3) acceptable defined as at least 80% of parents rating of recommending the program to others. We also hypothesize that fidelity goals would be 80% or higher for integrity of implementation of therapy sessions and 65% or higher for parent adherence to PT-F activities. A secondary aim was to report preliminary data on the impact of this interdisciplinary PT-F program compared with a waitlist control group on child (feeding and general behavior) and parent outcomes (stress, sense of competency) in a sample of young children with ASD and feeding problems with hypothesis that those participating in PT-F would show more improvements. The long-term goal was to develop a PT-F program that can be implemented by clinicians in outpatient settings, notably pediatric psychologists, who often play pivotal roles on interdisciplinary pediatric feeding teams.
Methods
Participants
Eligible participants were children, 2 to 7–11 years of age, diagnosed with ASD based on expert evaluation using Diagnostic Statistical Manual - 5th edition (DSM-5) criteria, corroborated by the Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994) and the Autism Diagnostic Observation Schedule—second edition (Lord et al., 2012). This age range represents the period when parents of children with ASD are still much involved in mealtimes of their children. Children had to have substantial feeding/mealtime problems, indicated by a score of 54 or greater on the Brief Autism Mealtime Behavior Inventory-Revised (BAMBI-R); this eligibility threshold was selected because it was 1 SD above the mean reported by the original developers for the ASD group (Lukens & Linscheid, 2008). Further, children needed to have a receptive language level of 12 months or greater, based on the Stanford–Binet Intelligence Test, fifth edition (Roid, 2003), or for children who were unable to achieve a basal on the Stanford–Binet, the Mullen Scales of Early Learning (Mullen, 1995). Children on medication were included if the medication had been stable for 6 weeks or more. Children were excluded if parents reported prescribed medication or supplements to target appetite, were being enterally fed, or had significant oral motor dysfunction that impaired chewing and swallowing.
Design
This was a parallel-group trial conducted at two sites (University of Florida and University of Rochester). Randomization was stratified by site in equal numbers to PT-F or waitlist control. The study biostatistician generated the randomization sequence using permuted blocks (block sizes of 2–4) to conceal the allocation sequence from all other study personnel; the study coordinator at each site obtained the assignment for each participant via a Web portal maintained by the data center. Parents and therapists were aware of the assigned treatment condition. Independent evaluators were blinded to treatment assignment. To protect the treatment blinding, we maintained separate study binders and separate clinical space for the therapists and independent evaluators. Parents were instructed to avoid discussing the treatment during assessments with independent evaluators.
Procedures
All procedures were approved by the institutional review board at both recruiting sites. All parents provided written informed consent before study enrollment. Potential participants were preliminarily screened by telephone via a scripted, standardized interview by the study coordinator. If the parent was interested and the child passed the preliminary screen (i.e., child had ASD diagnosis and had significant feeding problems as per telephone administration of the BAMBI-R), an initial clinic screening visit was scheduled to determine study eligibility. Screening measures were completed by study coordinators and/or other trained research team members. Following this visit, parent(s) were instructed on how to complete a 3-Day Food Record (3DFR). Then, a baseline visit was scheduled within 7 days of the clinic screening visit to obtain outcome measures, which included parent-completed rating scales and clinical ratings by an independent evaluator (IE) who was masked to treatment group. Study coordinators were available to clarify the instructions for parent rating scales. Independent evaluators, who were postdoctoral fellows or licensed psychologists, administered standardized tests and provided clinical ratings. Immediately after baseline, participants were randomized to PT-F or waitlist control. PT-F included 11, 60–90 min, individually administered sessions, as well as up to three parent coaching appointments via Healthcare Insurance Portability and Accountability Act-compliant VSee teleconferencing platform (depending on parent and therapist assessed benefit) and one home visit (see “PT-F” below). The first seven PT-F sessions were delivered weekly, and the remainder were delivered every other week; some flexibility was allowed to accommodate family schedules (e.g., rescheduling a session if necessary). Parent training therapists were doctoral-level psychologists and behavior analysts who had experience with children with ASD and training in applied behavior analysis. Training in the PT-F program was provided in the start-up meeting and during weekly cross-site teleconferences. Outcome and safety measures were repeated at Week 10 and Week 20 with study coordinators and the independent evaluator involvement. Participants in the waitlist control group were offered the full PT-F intervention on completion of the Week 20 measures. Total 17 of the 21 waitlist participants took this opportunity.
Parent Training for Feeding Program
The PT-F in this study was modeled after the Research Unit on Pediatric Psychopharmacology (RUPP) and Research Unit on Behavioral Research (RUBI) Autism Network Parent Training programs (Aman et al., 2009; Bearss et al., 2015) but dedicated specifically to feeding issues (Johnson et al., 2015), particularly food selectivity, food refusal, disruptive mealtime behaviors (not sitting at table, tantrums, aggression, and table swiping), and teaching adaptive feeding skills (using utensils and preparing a simple meal). The therapist also made a home visit after the first session to observe the home environment and held up to three parent–child coaching appointments over the course of the intervention via VSEE. The dietitian led the second session to review findings from the food record and make recommendations to the family and therapist regarding possible foods to introduce to boost nutrition. The program addressed current high-priority feeding and mealtime problems and was also intended to equip parents to address future goals and possible problems (see Table I for session topics). For each session, the PT-F manual included a therapist script, video vignettes that illustrated correct and incorrect use of behavioral techniques of parents of children with ASD at mealtimes in the home, in-session parent activity sheets, homework assignment forms, and a summary parent handout. Direct instruction, modeling, and role-playing were used in sessions to promote skill acquisition. A cumulative home feeding/mealtime plan was developed throughout the 20 weeks of the trial with updates at each session, based on changes in the child’s feeding behaviors and introduction of new procedures. After the first two sessions (introduction to program/behavioral principles and nutritional counseling), the therapist had the latitude to individualize the order of sessions to meet the needs of the child and family. For example, the therapist could cover the reinforcement/extinction session before the stimulus shaping session if that appeared important for the particular participant and family. These decisions were discussed in advance on the therapist teleconference calls.
Table I.
Session Topics and Outline
| Sessions | Topics/conceptual areas (Note: Language at 6th grade level) |
|---|---|
| A. Basic behavioral principles |
|
| Home visit scheduled | Observe home feeding environments. |
| B. Nutritional counseling |
|
| C. Antecedent approaches |
|
| D. Reinforcement |
|
| E. Use of stimulus control and fading |
|
| G. Address noncompliance around mealtimes, food acceptance |
|
| H. Teaching II: Use of shaping |
|
| I. Self-feeding and higher -level skills (skills may not be initiated with child but for future use) |
|
| J. Generalization and maintenance |
|
| K. Booster session |
|
Outcome Measures
Primary Feasibility Measures
Attrition rates and percentage of session attendance were measured as indices of feasibility along with other measures below.
Parent Satisfaction. The Parent Satisfaction Questionnaire (PSQ) adapted from the questionnaire developed by an earlier project (RUPP Autism Network, 2007) was completed at the end. Parents rated satisfaction with the number of sessions, usefulness of the teaching tools (e.g., video vignettes and worksheets), and helpfulness of specific session topics on either three- or four-point Likert scales, with higher scores reflecting greater satisfaction. A total percentage score was calculated.
Treatment Fidelity Checklist. Consistent with our prior PT studies (Aman et al., 2009; Bearss et al., 2015; Johnson et al., 2013; Johnson et al., 2015), we measured treatment fidelity as (a) therapist integrity in the delivery of treatment as intended and (b) parent adherence to the delivered treatment. Sessions were all video-recorded. Each session had a Treatment Fidelity Checklist that contained the essential elements of each session for the therapist (see example in supplemental materials). There were also several items assessing parent adherence (completion of homework and implementation of recommended strategies) and understanding of session materials through responses to activity sheets. Therapists rated each element of therapist fidelity and parent adherence during each session on a scale of 0 (not achieved) to 2 (fully achieved) and the therapist provided written comments on reasons for rating of 0. Percentage scores for treatment fidelity and parent adherence were obtained by calculating the sum of scores across all items (5 to 8 items therapist fidelity items and up to 8 parent adherence items per session) divided by the total possible score × 100. A random sample of 10% of each therapist’s video-recorded sessions was examined quarterly by an independent observer to determine inter-rater reliability. Independent observers were doctoral-level psychologists and behavior analysts.
Primary Child Feeding Outcomes
BAMBI—R (Lukens & Linscheid, 2008), as revised by our group based on a factor analysis (DeMand, Johnson, & Foldes, 2015), is a 15-item questionnaire on mealtime behaviors common to children with ASD (e.g., “Is disruptive during mealtime” and “Prefers to have food served in a particular way”). Items are rated on a five-point Likert scale, from 1 (never/rarely) to 5 (at almost every meal). The BAMBI has strong internal consistency correlation (ICC; Cronbach’s α = 0.88), test–retest reliability over 7 months (r = .87), and inter-rater reliability between a parent and teacher or therapist (r = .78). It also correlates with other feeding measures (Crist & Napier-Phillips, 2001) and discriminates between children with ASD and typical controls (Lukens & Linscheid, 2008). The BAMBI-R was one of two primary child outcome measures (ICC = 0.71 for this sample).
About Your Child’s Eating (AYCE) is a 25-item (scored on one to five Likert scale) parent-completed rating scale with adequate psychometrics that asks parents about their child’s eating, parents beliefs and concerns about the child’s eating, parent–child interactions at mealtimes, and parent’s feelings about mealtimes (Davies, Ackerman, Davies, Vannatta, & Noll, 2007). The total score, Feeding Relationship Disturbance, is the average of the three subscales (Child Resistance to Eating, Positive Mealtime Environment [reversed scored to represent Negative Mealtime Environment], and Parent Aversion to Mealtime). The total AYCE score was one of two primary child outcome measures (ICC = 0.86 for current sample).
Secondary Child Feeding Outcome
Clinical Global Impression-Improvement scale (CGI-I) (Guy, 1976) is a clinician-rated, seven-point scale designed to measure overall improvement from baseline. Scores range from 1 (Very Much Improved) to 4 (Unchanged) to 7 (Very Much Worse). An IE masked to group assignment used all available information to judge treatment response. By convention, CGI-I ratings of Much Improved (score of 2) or Very Much Improved (score of 1) are used to classify subjects as positive responders. All other scores classify subjects as nonresponders. An essential contributor to the CGI-I is the content of the semi-structured Parent Target Problem interview. At baseline, the IE asked the parent to nominate the child’s two most important feeding/mealtime problems. Nutritional data obtained based on diet records were also included in the assessment of clinical change. The use of the CGI-I method has been shown to be reliable and valid in other parent training studies of ours (Aman et al., 2009; Bearss et al., 2015). IEs, doctoral-level psychologists or behavior analysts, were trained using case vignettes and procedures adopted from earlier studies.
Secondary Child Behavior Outcomes
Aberrant Behavior Checklist (ABC) is a 58-item parent-report measure with five subscales: Irritability (agitation, aggression, and self-injurious behaviors), Social Withdrawal, Stereotyped behaviors, Hyperactivity, and Inappropriate Speech (Aman, Singh, Stewart, & Field, 1985). The ABC is often used in clinical trials in children with ASD (Aman et al., 2009; Bearss et al., 2015). The Irritability subscale (ABC-I) was used as a measure of child disruptive behavior (ICC = 0.92 for current sample).
Home Situations Questionnaire (HSQ) is a parent-rated scale of noncompliance (Barkley & Murphy, 1998). The parent notes whether the child has difficulties following rules or expectations in 27 everyday situations. Items answered affirmatively are then rated on a one to nine Likert scale, with higher scores indicating greater noncompliance. The scale yields a total severity score with a range of 0–225. We used the modified HSQ developed by the Autism RUPP Network to include items relevant to children with ASD. This measure was chosen based on its sensitivity in other parent training projects and the specific focus on noncompliance in children with ASD (Bearss et al., 2015; Chowdhury et al., 2015). The total mean severity score was used as another indicator of child behavioral disruption (ICC = 0.88 for current sample).
Secondary Parent Outcomes
Parenting Stress Index (PSI) Short Form is a 36-item parent-rated questionnaire for families of children 12 years of age and younger (Abidin, 1995). Items are rated on a five-point scale from 0 (strongly disagree) to 5 (strongly agree), and a higher score reflects higher stress. The PSI is composed of three scales (Parental Distress, Difficult Child Characteristics, and Dysfunctional Paren–Child Interaction) and a Total Stress score that was used in this study. A total score of ≥88 (85th percentile) indicates clinically significant stress (ICC = 0.89 for current sample).
The Parenting Sense of Competence (PSOC) scale is a 17-item scale developed to assess parenting self-esteem (Gibaud-Wallson & Wandersman, 1978). Each item is answered on a six-point scale ranging from strongly disagree to strongly agree. The measure has high internal consistency and test–retest reliability (Gibaud-Wallson & Wandersman, 1978). In a community sample of mothers, the mean total competence score was 61 (Gilmore & Cuskelly, 2009). The total competence score was used in analyses, with a higher score reflecting more competence (ICC = 0.50 for current sample).
Caregiver Strain Questionnaire (CGSQ) measures the burden of caring for a child and the interference on family activities (Brannan, Heflinger, & Bickman, 1997). The questionnaire includes 21 items, rated on a five-point scale ranging from not at all a problem to very much a problem, that assess three dimensions: objective strain, internalized subjective strain, and externalized subjective strain. Additionally, a global mean score is obtained. The mean global score in the original sample of parents of children with unspecified behavioral/emotional disorder was 2.6 (average of the three mean scores of 2 .0 for objective strain, 3.4 for internalized train, and 2.3 for externalized strain). The global mean score was used for analyses with higher score being indicative of more strain (ICC = 0.83 for current sample).
Safety Measures
3DFRs are used widely in research as well as in clinical practice to capture recent dietary intake (Center for Disease Control and Prevention, 2012). All food, beverages, supplements, and medications ingested over a 3-day period were recorded at baseline, Weeks 10 and 20. These data were reviewed by the dietician to determine if there were any nutritional deficiencies that warranted immediate treatment before randomization.
Safety Review and Adverse Events: A Safety Review form was completed by the IE at the three time points, based on parent interview, to probe for any concerns about changes in appetite or gastrointestinal symptoms. Any adverse events were to be documented as they occurred and evaluated to determine whether they appeared to be study-related.
Data Analysis
Because this was a pilot randomized clinical trial (RCT), we were mainly interested in obtaining a sample size that would provide information about feasibility and efficacy but not necessarily determine statistical significance in the comparison of treatment to control conditions. We aimed to enroll 25 children in each group, which would give us experience with the intervention and study protocol while also potentially allowing us to detect a large effect, Specifically, using a two-sided hypothesis test with an alpha level = 0.05, and with outcome data, available for 80% of the enrolled children (n = 40), we calculated 80% power to detect a difference that is ≥0.91*SD between the PT-F and waitlist groups on continuous measures.
The intention-to-treat principle was used, whereby all randomized participants who had been enrolled (and completed baseline assessment) were analyzed according to the intervention to which they were assigned. Descriptive statistics were derived for key demographic variables, feasibility, parent satisfaction, and treatment fidelity (therapist treatment integrity and parent adherence). Percent agreement was calculated to compare ratings by the therapist and independent observer for therapist integrity and parent adherence. To study the association between the treatment and each longitudinal treatment outcome, linear mixed models with subject-specific random effects were fit in R software version 3.4.3 using the lme4 package (Bates, Maechler, Bolker, & Walker, 2014), which accounts for the within-subject correlation and accommodates missing data if the data are missing at random (Little & Rubin, 2014). The longitudinal outcomes included the primary measures (BAMBI and AYCE) as well as the secondary child (ABC-I and HSQ) and parent measures (PSI, PSOC, and CGSQ). The models included fixed effects for treatment (two levels) and time (baseline, 10 and 20 weeks) while controlling for baseline level of the outcome variable and site (Florida and Rochester). The average slope of the regression line over time was compared between the two groups for statistical significance. Cohen’s d effect sizes and 95% confidence interval were calculated between groups at baseline, Week 10 and Week 20. For the categorical variable of responders versus nonresponders derived from the CGI-I, the Fisher’s exact test was used to examine the response difference between treatment groups.
Results
Demographics
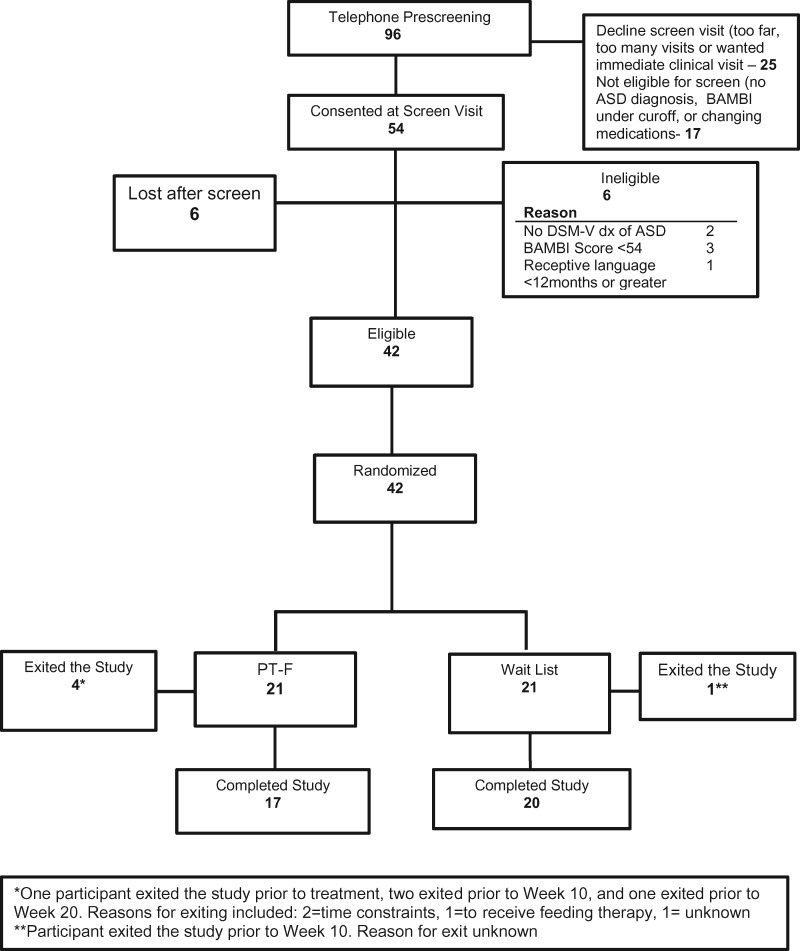
Between December 2014 and February 2017, 54 parents consented at the time of a clinic screening visit. Before this visit, a preliminary telephone screening call was conducted for 96 potential participants. Of this total, 25 parents declined to move forward to the clinic screen visit, citing the distance, the number of visits required, and/or the wish for immediate treatment. Seventeen children were found to be ineligible at the time of the telephone screen and were thus not scheduled. Of those 54 children and parents seen for a clinic screen visit, 6 children were ineligible, and 6 others dropped out after the screen (often citing a preference for community treatment over assignment to waitlist). Participant flow, reasons for exclusion, and information about attrition from the study are provided in the CONSORT chart (Figure 1). The remaining 42 participants were randomized. Two participants were delayed in being randomized because of identified risk for nutritional deficiencies from the baseline 3-day diet records (vitamin C for one participant and multivitamin for another). These participants were randomized after supplementation to address these deficits. Total 5 of the 42 randomized participants dropped out (12%) before study completion. Table II displays the demographic information and baseline scores for the 42 participants. The two groups showed no significant differences at baseline. Participants averaged 5 years, 1 month of age, and all but two were male; 83% were White, and 81% were non-Hispanic. Total 48% had intelligence quotient (IQ) of 70 or above, and 40% were placed in regular (general) education. All but two parents had at least some college education. Both groups of children had high scores on the BAMBI-R (M = 65 for both groups) and AYCE (M = 66 for PT-F and 68 for waitlist) and also had high scores on the ABC-I (M = 15 for PT-F and 17 for waitlist), though not the HSQ (M = 2.0 for PT-F and 2.9 for waitlist). Parents had clinically elevated scores on the PSI (M = 92 for PT-F and 97 for waitlist) and lower PSOC scores (M = 56 for PT-F and 59 for waitlist) than the originally reported by the measurement developers (suggesting lower sense of competence), but the CGSQ scores (M = 2.1 for PT-F and 2.6 for waitlist) were lower than the developers reported.
Figure 1.
CONSORT chart.
Table II.
Baseline Demographic and Clinical Characteristics by Treatment Group
| Parent training (n = 21) |
Waitlist (n = 21) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Study center | ||||
| University of Florida | 10 | 47.6 | 10 | 47.6 |
| University of Rochester | 11 | 52.4 | 11 | 52.4 |
| Child demographics | M | SD | M | SD |
| Age | 5.1 | 1.3 | 5.1 | 1.4 |
| Males | 19 | 90.5 | 21 | 100 |
| Race | ||||
| White | 18 | 85.7 | 16 | 76.2 |
| Black | 1 | 4.8 | 0 | 0 |
| Asian/multiracial | 2 | 9.5 | 4 | 19.0 |
| Other | 0 | 0 | 1 | 4.8 |
| Ethnicity | ||||
| Hispanic | 3 | 14.3 | 5 | 23.8 |
| Non-Hispanic | 18 | 85.7 | 16 | 76.2 |
| IQ <70 | 11 | 52.4 | 11 | 52.4 |
| School program | ||||
| None/day care only | 4 | 19.0 | 2 | 9.5 |
| Regular school* | 9 | 42.9 | 8 | 38.1 |
| Special class in regular school | 4 | 19.0 | 10 | 47.6 |
| Special school | 4 | 19.0 | 1 | 4.8 |
| Family demographics | M | SD | M | SD |
| Maternal age (years) | 37 | 6 | 36 | 5.7 |
| Maternal education | ||||
| Advanced graduate or professional degree | 5 | 23.8 | 4 | 19.0 |
| College graduate | 9 | 42.9 | 7 | 33.3 |
| Some college or post-high school or 2-year degree | 6 | 28.6 | 9 | 42.9 |
| High school graduate or General Equivalency Diploma (GED) | 1 | 4.8 | 1 | 4.8 |
| Family income | N | % | N | % |
| <$20,000 | 1 | 4.76 | 1 | 4.76 |
| $20,001–40,000 | 5 | 23.8 | 4 | 19 |
| $40,001–60,000 | 4 | 19 | 4 | 19 |
| $60,001–90,000 | 5 | 23.8 | 7 | 33.3 |
| >$90,000 | 6 | 28.6 | 5 | 23.8 |
| Baseline clinical scores** | M | SD | M | SD |
| Brief Autism Mealtime Behavior Inventory (BAMBI) | 65.0 | 7.7 | 64.7 | 6.1 |
| About Your Child’s Eating (AYCE) | 66.2 | 10.2 | 67.9 | 12.9 |
| Aberrant Behavior Checklist-Irritability | 14.6 | 10.1 | 17.0 | 8.0 |
| Home Situation Questionnaire (HSQ) | 2.0 | 1.8 | 2.9 | 1.6 |
| Parenting Stress Inventory (PSI) | 91.8 | 17.8 | 96.6 | 21.7 |
| Parent Sense of Competence (PSCO) | 56.4 | 7.5 | 58.6 | 7.5 |
| Caregiver Strain Questionnaire (CGSQ) | 2.1 | 0.53 | 2.6 | 0.82 |
(Regular private preschool, regular public kindergarten, preschool, and school grade)
p values range .25–.91.
Feasibility (Attendance, Attrition, and Parent Satisfaction)
The 21 PT-F families attended 197 of the 231 possible sessions (11 sessions × 21 participants), or 85% attendance at the 11 sessions. Attrition was 12%. Seventeen families completed the study-specific PSQ with 94% (16 of 17) reporting they would recommend the parent training program to others, and they felt confident in dealing with any new feeding problems that might arise. Sessions that parents found most helpful included the first session on behavioral principles and the third session on prevention (88% rated “very helpful”), while the session on shaping of acceptance of new foods was rated by only 59% of parents as “very helpful.”
Treatment Fidelity (Therapist Integrity and Parent Adherence) and Inter-Rater Reliability
The average treatment integrity was 97% with a range of 92–100% and the average parent adherence was 94% (range 70–100%). Percent agreement between ratings by the therapist and independent observer was 91% (range 56–100%) for therapist integrity and 80% (range 33–100%) for parent adherence.
Child Outcomes
The PT-F group made greater improvements across the two assessment time points than those in the waitlist group on the two primary outcomes, the BAMBI (Twald = −3.13; p = .003; Cohen’s d = 0.95 at Week 20) and the AYCE (Twald = −3.58; p = .001; d = 1.12 at Week 20). On the CGI-I, 48.8% of the participants in PT-F were rated as much improved or very much improved (“positive responders”) compared with 0% in the waitlist control group (p = .006). In contrast, general child disruptive behavior outcomes decreased more in PT-F group than waitlist group, but the differences were not statistically significant, and effect sizes were small, with Twald = −0.29; p = .77; d = −0.27 at Week 20 for ABC Irritability and Twald = −0.75; p = .46; d = −0.39 at Week 20 for the HSQ (see Supplemental figures for graphed figures of these data).
Parent Outcomes
Parent outcomes improved in PT-F more than in waitlist, but the group differences were not significant, with Twald = −1.40; p = .17; d = −0.52 at Week 20 for PSI, Twald = −1.51; p = .14; d = −0.84 at Week 20 for CGSQ and Twald = 1.17; p = .25; d = 0.13 at Week 20 for PSOC (see Supplemental figures for graphed figures of these data).
Safety
No study-related adverse events occurred during the trial. As already noted, two participants were delayed in randomization, as diet records indicated the need for vitamin supplementation.
Discussion
To our knowledge, this is the first RCT of an individually delivered, nutritionally informed parent training program specifically for feeding problems in young children with ASD. Results suggest that the intervention is feasible, with high attendance and low attrition. The attrition rate for this study is comparable with prior studies of individually delivered parent training for the ASD population such as Bearss et al., 2015, and much lower than in the only prior RCT of group parent training for feeding (Sharp et al., 2014). Moreover, as evidence of parent satisfaction, the vast majority of parents reported that they would recommend the program to others and finished the program feeling more confident in the ability to address feeding problems in their child. Furthermore, therapist integrity of implementation and parent adherence were high, averaging 97% and 94%, respectively; this indicated that therapists delivered the intervention consistently and that parents participated in the sessions and home assignments. These findings support the overall viability of the PT-F program.
With respect to preliminary data on the impact of the PT-F program, PT-F showed significantly greater improvement than the waitlist control on ratings by parents and a masked IE on measures of feeding problems, including food selectivity, disruptive mealtime behavior, and parents’ perception of their child around mealtimes. The apparent improvements in these problems for children whose parents participated in PT-F are an encouraging finding and central to the goal of PT-F. More general and distal measures of child disruptive behavior and parent functioning improved but not significantly so for the PT-F group compared with the waitlist group. Although these results must be interpreted with caution because of the small sample size, they provide preliminary evidence that PT-F shows promise as a new model for delivering treatment for the most commonly occurring feeding problems in children with ASD. This is notable because P6T-F is much less intensive and involves parents more systematically than many intervention models that have been used in prior intervention research, such as partial day treatment programs. The results also suggest that measures of overall feeding behavior may be sensitive to change and can be used along with or instead of the more “molecular” measures that have been used in single case studies (e.g., grams of novel foods consumed). These more global measures are more practical outside of highly specialized feeding programs.
Limitations
This study is limited by the reliance on parent-completed questionnaires. While we had an outcome measure completed by an independent evaluator, this was still based on information from the parent as informant. Further, this was a small sample of young children with ASD who were predominantly Caucasian and non-Hispanic, from well-educated families. Our sample, by design, did not include children with chewing or swallowing issues secondary to oral motor dysfunction or children who were enterally fed. The parent training program was not intended as the sole treatment for children with these more complex challenges. Hence, generalizability is limited for these children with ASD and their families. Our findings are based only on the 20 weeks of the trial with no follow-up of whether changes maintained over time. The sample size was too small to support analyses of moderators of response to PT-F. Moreover, the number of comparisons in this small sample on multiple outcomes could have led to false-positive findings.
Our waitlist group did not control for time and attention but was viewed as appropriate for this pilot study. The waitlist design also hindered recruitment and retention, as a few families either opted not to enroll or exited early in favor of seeking feeding therapy. Finally, while we spent considerable time and efforts in the development of the PT-F manual and supporting materials to include video vignettes of actual children with ASD and their parents, improvements in PT-F could be made based on parent feedback on the PSQ, and therapist reported experiences in this initial trial. For example, the majority of parents found the initial session on behavioral principles and later sessions on prevention, nutrition, and use of stimulus control helpful, but fewer parents reported that the use of shaping was useful. Therapists’ experience was that the final generalization and maintenance session was not always beneficial because, even at the end of the 2-week trial, these strategies were premature, as the child behavior change was so new. For the video vignettes that depicted children with ASD and a parent, all but one of the children had verbal skills even though they were young. It would be optimal to have children with different skill levels including vignettes of children with minimally verbal ability.
Future Directions
With the findings of this preliminary RCT, the next step is to undertake a larger trial that is adequately powered to evaluate efficacy and identify child and parent characteristics associated with positive response to PT-F. This trial would benefit from more attention to the recruitment of a sample with diverse racial and ethnic backgrounds. In addition, it would optimally include an active comparator as a control such as a psychoeducational parent program that provides valuable information about ASD but does not include direct training in behavioral strategies (Bearss et al., 2015; Johnson et al., 2013). Also worth considering are alternative delivery models such as telehealth platforms that may enhance the ecological validity of the intervention and reduce family burden. While we conducted parent coaching in videoconferences, more components of the program could be delivered via telehealth.
Conclusions
Individually delivered PT-F was feasible, accepted by parents and resulted in significantly more improvements in feeding and mealtime problems compared with the waitlist control in our small group of young children with ASD. This provides initial support that a parent training model for the feeding problems (food selectivity and disruptive mealtime behaviors) frequently seen in young children with ASD holds promise as an alternative approach than the more traditional one of having a therapist from one of many different backgrounds (pediatric psychology, occupational therapy, speech/language pathology, and dietitians) work with a child in an outpatient setting and involve the parents in varying degrees. Pediatric psychologists have a long history of providing parent training; this program specifically for feeding problems offers an expansion of that role. This is particularly relevant at this time when pediatric psychologists are likely to see more children with ASD for a variety of present problems including feeding issue. As pediatric psychologist is likely to provide care for children with ASD in a variety of settings, knowledge of empirically based interventions for this population is of great importance.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Supplementary Material
Acknowledgments
The authors wish to acknowledge and thank Rachel Davis, Katie Brown, Kelsey Lisbon, Patricia Stewart, PhD, and Cristina Whitehouse, PhD, for their work on this project. The views expressed in this article are those of the authors and do not necessarily reflect the official position of the National Institutes of Health (NIH), or any other part of the U.S. Department of Health and Human Services. NIH encourages publication of results and free scientific access to data.
Funding
This work was funded by the National Institute of Mental Health to University of Florida/University of Pittsburgh (MH100253; principal investigator: C.R.J.) and University of Rochester (MH 100254; principal investigator: T.S.).
Conflicts of interest: None declared.
References
- Abidin R. (1995). Parenting Stress Index (PSI) (3rd ed.). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Allison J., Wilder D. A., Chong I., Lugo A., Pike J., Rudy N. (2012). A comparison of differential reinforcement and noncontingent reinforcement to treat food selectivity in a child with autism. Journal of Applied Behavior Analysis, 45, 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman M. G., McDougle C. J., Scahill L., Handen B., Arnold L. E., Johnson C., Stigler K. A., Bearss K., Butter E., Swiezy N. B., Sukhodolsky D. D., Ramadan Y., Pozdol S. L., Nikolov R., Lecavalier L., Kohn A. E., Koenig K., Hollway J. A., Korzekwa P., Gavaletz A., Mulick J. A., Hall K. L., Dziura J., Ritz L., Trollinger S., Yu S., Vitiello B., Wagner A; Research Units on Pediatric Psychopharmacology Autism Network. (2009). Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. Journal of American Academy of Child and Adolescent Psychiatry, 48, 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman M. G., Singh N. N., Stewart A. W., Field C. J. (1985). Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency, 89, 492–502. [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Association Publishing. [Google Scholar]
- Bandini L. G., Anderson S. E., Curtin C., Cermak S., Evans E. W., Scampini R., Maslin M., Must A. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. The Journal of Pediatrics, 157, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. A., Murphy K. R. (1998). Attention-deficit hyperactivity disorder: A clinical workbook (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2014). lme4: Linear mixed-effects models using Eigen and S4. R Package Version, 1, Vienna, Austria. URL http://www.R-project.org/. pp. 1–23.
- Bearss K., Johnson C., Smith T., Lecavalier L., Swiezy N., Aman M., McAdam D. B., Butter E., Stillitano C., Minshawi N., Sukhodolsky D. G., Mruzek D. W., Turner K., Neal T., Hallett V., Mulick J. A., Green B., Handen B., Deng Y., Dziura J., Scahill L. (2015). Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: A randomized clinical trial. Journal of the American Medical Association, 313, 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan A. M., Heflinger C. A., Bickman L. (1997). The Caregiver strain questionnaire: Measuring the impact. Journal of Emotional and Behavioral Disorders, 5, 212–222. [Google Scholar]
- Bui L. T. D., Moore D. W., Anderson A. (2013). Using escape extinction and reinforcement to increase eating in a young child with autism. Behaviour Change, 30, 48–55. [Google Scholar]
- Center for Disease Control and Prevention. (2012, September 19, 2012). National health and nutrition examination survey Retrieved from http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- Chowdhury M., Aman M. G., Lecavalier L., Smith T., Johnson C., Swiezy N., McCracken J. T., King B., McDougle C. J., Bearss K., Deng Y., Scahill L. (2015). Factor structure and psychometric properties of the revised home situations questionnaire for autism spectrum disorder: The home situations questionnaire-autism spectrum disorder. Autism, 20, 528–537. [DOI] [PubMed] [Google Scholar]
- Crist W., Napier-Phillips A. (2001). Mealtime behaviors of young children: A comparison of normative and clinical data. Journal of Developmental and Behavioral Pediatrics, 22, 279–286. [DOI] [PubMed] [Google Scholar]
- Curtin C., Hubbard K., Anderson S. E., Mick E., Must A., Bandini L. G. (2015). Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W. H., Ackerman L. K., Davies C. M., Vannatta K., Noll R. B. (2007). About your child’s eating: Factor structure and psychometric properties of a feeding relationship measure. Eating Behaviors, 8, 457–463. [DOI] [PubMed] [Google Scholar]
- Dawson G., Rogers S., Munson J., Smith M., Winter J., Greenson J., Donaldson A., Varley J. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver model. Pediatrics, 125, e17–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMand A., Johnson C. R., Foldes E. (2015). Psychometric properties of the Brief Autism Mealtime Behaviors Inventory. Journal of Autism and Developmental Disorders, 45, 2667–2673. doi: 10.1007/s10803-015-2435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond A., Emmett P., Steer C., Golding J. (2010). Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics, 126, e337–e342. [DOI] [PubMed] [Google Scholar]
- Gale C., Eikeseth S., Rudrud E. (2011). Functional assessment and behavioural intervention for eating difficulties in children with autism: A study conducted in the natural environment using parents and ABA tutors as therapists. Journal of Autism and Developmental Disorders, 41, 1383–1396. [DOI] [PubMed] [Google Scholar]
- Gentry J., Luiselli J. (2008). Treating a child’s selective eating through parent implemented feeding intervention in the home setting. Journal of Developmental and Physical Disabilities, 20, 63–70. [Google Scholar]
- Gibaud-Wallson J., Wandersman L. P. (1978). Development and utility of the parenting sense of competence scale. Paper presented at the American Psychological Association, Toronto.
- Gilmore L., Cuskelly M. (2009). Factor structure of the parenting sense of competence scale using a normative sample. Child: Care, Health and Development, 35, 48–55. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976). Dosage record and treatment emergent symptoms scale ECDEU assessment manual for psychopharmacology (Revised) (pp. 223–244). Rockville, MD: US Department of Health, Education and Human Welfare publication (ADM; ). [Google Scholar]
- Iadarola S., Levato L., Harrison B., Smith T., Lecavalier L., Johnson C., Swiezy N., Bearss K., Scahill L. (2017). Teaching parents behavioral strategies for autism spectrum disorder (ASD): Effects on stress, strain, and competence. Journal of Autism and Developmental Disorders, 48, 1031–1040. [DOI] [PubMed] [Google Scholar]
- Johnson C. R., Foldes E., DeMand A., Brooks M. (2015). Behavioral parent training to address feeding problems in children with Autism Spectrum Disorder: A pilot trial. Journal of Developmental and Physical Disabilities, 27, 591–607. doi: 10.1007/s10882-015-9437-1. [Google Scholar]
- Johnson C. R., Turner K. S., Foldes E., Brooks M. M., Kronk R., Wiggs L. (2013). Behavioral parent training to address sleep disturbances in young children with autism spectrum disorder: A pilot trial. Sleep Medicine, 14, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner E. S., Eisenberg I. W., Orionzi B., Simmons W. K., Kenworthy L., Martin A., Wallace G. L. (2015). A preliminary study of self-reported food selectivity in adolescents and young adults with autism spectrum disorder. Research in Autism Spectrum Disorders, 15, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laud R. B., Girolami P. A., Boscoe J. H., Gulotta C. S. (2009). Treatment outcomes for severe feeding problems in children with Autism Spectrum Disorder. Behavior Modification, 33, 520–536. [DOI] [PubMed] [Google Scholar]
- Levin L., Carr E. G. (2001). Food selectivity and problem behavior in children with developmental disabilities: Analysis and intervention. Behavior Modification, 25, 443–470. [DOI] [PubMed] [Google Scholar]
- Little R. J. A., Rubin D. B. (2014). Statistical analysis with missing data (Vol. 333). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Lord C., Rutter M., Le Couteur A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M. L., DiLavore P. C., Risi S., Gotham K., Bishop S. (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Torrance, CA Western Psychological Services. [Google Scholar]
- Lukens C., Linscheid T. (2008). Development and validation of an inventory to assess mealtime behavior problems in children with autism. Journal of Autism and Developmental Disorders, 38, 342–352. [DOI] [PubMed] [Google Scholar]
- Marshall J., Hill R. J., Dodrill P. (2013). A survey of practice for clinicians working with children with autism spectrum disorders and feeding difficulties. International Journal of Speech Language Pathology, 15, 279–285. [DOI] [PubMed] [Google Scholar]
- Meier A. E., Fryling M. J., Wallace M. D. (2012). Using high-probability foods to increase the acceptance of low-probability foods. Journal of Applied Behavior Analysis, 45, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes S. M. (2011). The evaluation of a parent-implemented behavioral intervention for the treatment of food selectivity and mealtime behavior problems in children with autism spectrum disorders. Albany, NY: Department of Psychology, University at Albany. [Google Scholar]
- Mullen E. (1995). Mullen scales of early learning (AGS ed.). Circle Pines: American Guidance Service. [Google Scholar]
- Najdowski A. C., Tarbox J., Wilke A. E. (2012). Utilizing antecedent manipulations and reinforcement in the treatment of food selectivity by texture. Education and Treatment of Children, 35, 101–110. [Google Scholar]
- Najdowski A. C., Wallace M. D., Reagon K., Penrod B., Higbee T. S., Tarbox J. (2010). Utilizing a home-based parent training approach in the treatment of food selectivity. Behavioral Interventions, 25, 89–107. [Google Scholar]
- Penrod B., Gardella L., Fernand J. (2012). An evaluation of a progressive high-probability instructional sequence combined with low-probability demand fading in the treatment of food selectivity. Journal of Applied Behavior Analysis, 45, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Piazza C. C., Volkert V. M. (2016). A comparison of a modified sequential oral sensory approach to an applied behavior-analytic approach in the treatment of food selectivity in children with autism spectrum disorder. Journal of Applied Behavioral Analysis, 49, 485–511. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology [RUPP] Autism Network. (2007). Parent training for children with pervasive developmental disorders: A multi-site feasibility trial. Behavior Interventions, 22, 179–199. [Google Scholar]
- Roid G. H. (2003). Stanford-binet intelligence scales: Examiner's manual (5th ed.). Itasca, IL: Riverside. [Google Scholar]
- Scahill L., Bearss K., Lecavalier L., Smith T., Swiezy N., Aman M., Sukhodolsky D. G., McCracken C., Minshawi N., Turner K., Levato L., Saulnier C., Dziura J., Johnson C. R. (2016). Effect of parent training on adaptive behavior in children with autism spectrum disorder and disruptive behavior: Results of a randomized trial. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 602–609. 10.1016/j.jaac.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Sharp W. G., Burrell T. L., Jaquess D. L. (2014). The autism MEAL plan: A parent-training curriculum to manage eating aversions and low intake among children with autism. Autism, 18, 712–722. [DOI] [PubMed] [Google Scholar]
- Sharp W. G., Jaquess D. L., Morton J. F., Herzinger C. V. (2010). Pediatric feeding disorders: A quantitative synthesis of treatment outcomes. Clinical Child and Family Psychology Review, 13, 348–365. [DOI] [PubMed] [Google Scholar]
- Thullen M., Bonsall A. (2017). Co-parenting quality, parenting stress, and feeding challengesin families with a child diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 878–886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.