Abstract
Korean red ginseng is a traditional health food frequently used to prevent or treat various diseases worldwide. In this study, we evaluated the immunomodulatory activities of eleven compounds (1–11) isolated from Korean red ginseng, focusing on T cell function. First, the effects of the eleven compounds were studied on the regulation of IL-2, a potent T cell growth factor. Compounds 5, 7, and 9 significantly increased IL-2 secretion in phorbol 12-myristate 13-acetate (PMA)/ionomycin (Io)-induced EL-4 T cells. Next, we examined the effects of compounds 5, 7, and 9 on the regulation of transcription factors related to IL-2 production in T cells. Compound 9 significantly increased the PMA/Io-induced promoter activity of nuclear factor of activated T cells (NF-AT) in EL-4 T cells, but did not have any significant effects on the promoters of NF- κB. These results suggest that compound 9 activates T cell function via the regulation of NF-AT-mediated IL-2 production.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0428-8) contains supplementary material, which is available to authorized users.
Keywords: Panax ginseng, Korean red ginseng, Ginsenosides, IL-2
Introduction
Korean red ginseng (original scientific name as Panax ginseng, Araliaceae) is the best-known processed ginseng product in Korea. For thousands of years, it has been used as an herbal medicine and health food in countries such as South Korea, China, and Japan (Jia et al., 2009). Dammarane type triterpene saponins (ginsenosides) are known as the major effective component in Korean red ginseng. Ginsenosides have many biological activities including anti-cancer, anti-inflammation, anti-tumor, anti-diabetes, and immunomodulatory effects (Attele et al., 1999; He et al., 2017; Jiang et al., 2017). Recently, more than 80 ginsenosides have been isolated from various parts of P. ginseng. Ginsenosides Rg1 and Rd were reported to have immunomodulatory effects on T cells (Lee and Han, 2006; Yang et al., 2007). Although many ginsenosides have been isolated and their biological activities described, it is still important to find new ginsenosides from Korean red ginseng and to clarify their various biological activities and action mechanisms.
Communication between the innate and adaptive immune systems is crucial for successful immunity to pathogens. The adaptive immune system is strongly influenced by innate effector cells, and adaptive responses to pathogen infection require synergistic collaboration with the innate immune system. T cells are critical for the generation of effective immune reactions to a pathogen. In addition, T helper cells regulate cytokine secretion and engage in aspects of cell-to-cell signaling to promote immune responses (Kara et al., 2014). Interleukin-2 (IL-2) is the main stimulator of T cell differentiation and growth (Read et al., 2016). IL-2 drives the clonal expansion of activated T cells and prompts cell differentiation to acquire effector functions (Smith, 1992). Consequently, the specific production of IL-2 is an important step in T cell-dependent immune responses. The production of IL-2 is mainly regulated at the transcriptional level through multiple transcription factors. The nuclear factor of activated T cells (NF-AT) associated with activator protein-1 (AP-1) has been reported to bind several motifs within the IL-2 promoter. The binding of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the IL-2 promoter activates a canonical site for NF-κB and transcription of the CD28 response element (Macian, 2005). Cyclosporine A and tacrolimus inhibit IL-2 production and represent the mainstay of immunosuppressive therapy in transplantation. Their mechanism of action is the inactivation of the Ca*/calmodulin-dependent serine-threonine phosphatase, calcineurin, leading to the inactivation of NF-AT, a transcription factor that is required for the expression of IL-2, interferon-γ (IFN-γ), and granulocyte–macrophage colony- stimulating factor (GM-CSF) genes (Liu, 2009).
Previously, we isolated eleven compounds, including two new ginsenosides from Korean red ginseng and evaluated their anti-inflammatory activities (Vinh et al., 2017). These results showed that compound 1 (3-oxo-20R-ginsenoside Rh1) significantly reduced the level of proinflammatory cytokines IL-6 and TNF-α secretion and inhibited the expression of COX-2 and iNOS in LPS-activated RAW264.7 cells.
In this study, we evaluated the immunomodulatory activities of the eleven compounds (1–11), focusing on T cell functions. Compounds 5, 7, and 9 significantly increased IL-2 secretion in phorbol 12-myristate 13-acetate (PMA)/ionomycin (Io)-induced EL-4 T cells, and we tested the effects of compounds 5, 7, and 9 on the regulation of transcription factors related to IL-2 production in T cells.
Materials and methods
Cell cultures
EL-4 T cells purchased from the American Type Culture Collection (Manassas, VA, USA) were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Welgene; Daegu, Korea) supplemented with 1% penicillin/streptomycin (Thermofisher Scientific; Waltham, MA, USA)and 10% FBS (Thermofisher Scientific; Waltham, MA, USA) under an atmosphere of 5% CO2 in a humidified 37 °C incubator.
Cell proliferation assay
EL-4 T cells (1 × 104 cells/well) were treated with each compound (5 and 10 μM) for 48 h as previously described (Park et al., 2017a). Viability of EL-4 T cell was assayed by using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)- 5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (CCK-8, Dojindo; Rockville, MD, USA). Absorbance was read with an ELx808 ELISA microplate reader (BioTek Instruments Inc.; Winooski, VT, USA).
Enzyme-linked immunosorbent assay (ELISA)
EL-4 T cells (1 × 105 cells/well) were pretreated with each compound for 4 h followed by the addition of phorbol 12-myristate 13-acetate (PMA, 0.5 ng/mL)(Sigma-Aldrich Chemical Co; St. Louis, MO, USA)/ionomycin (Io, 5 nM)(Sigma-Aldrich Chemical Co; St. Louis, MO, USA) for 48 h as previously described (Park et al., 2017a). The IL-2 cytokines released in the cell culture media were measured using sandwich ELISA kits following the manufacturer’s experimental protocols (R&D Systems; Minneapolis, MN, USA). The absorbance was measured at 450 nm using an ELISA microplate reader within 30 min.
DNA transfection and luciferase reporter assays
NF-AT or NF-κB promoter driven plasmids pNF-AT-Luc and pNF-κB-Luc, respectively, were purchased from Promega (Madison, WI, USA). EL-4 T cells were seeded in a 6-well plate (5 × 105 cells/well) and transfected with the indicated firefly luciferase reporter DNAs (3 μg) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) as previously described (Park et al., 2017a). After a 48 h incubation, the cells were treated with each compound for 4 h, followed by treatment with PMA (0.5 ng/mL)/Io (5 nM) for 24 h. Luciferase activity in the cell lysates was measured with the luciferase reporter system (Promega, Madison, WI, USA).
Results and discussion
Phytochemical analysis
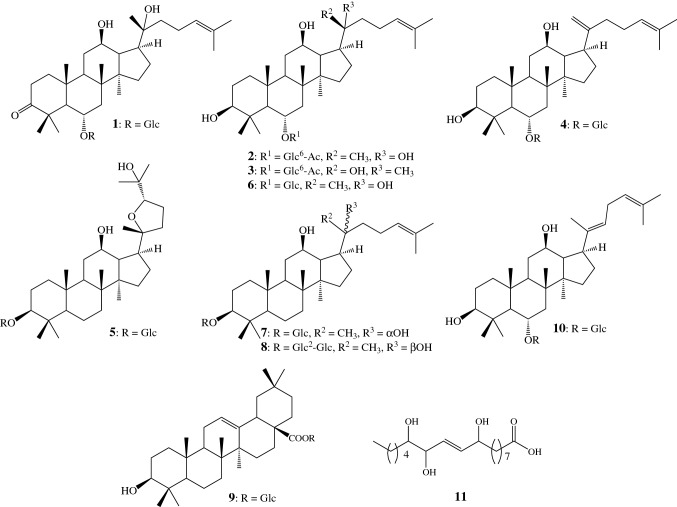
The extract of Korean red ginseng was subjected to various separation procedures and 11 compounds were isolated (1-11). Their structures were identified as 6-O-β-D-glucopyranosyl-20R-protopanaxadiol-3-one (1), 20R-ginsenoside Rh1 6′-acetate (2), 20S-ginsenoside Rh1 6′-acetate (3), ginsenoside Rk3 (4), ginsenoside RT5 (5), 20R-ginsenoside Rh1 (6), 20S-ginsenoside Rh2 (7), 20R-ginsenoside Rg3 (8), oleanolic acid β-D-glucopyranosyl ester (9), ginsenoside Rh4 (10), and 9,12,13-trihydroxy-10E-octadecenoic acid (11) based on spectral and chemical evidence, consistent with previously published paper (Vinh et al., 2017) (Fig. 1).
Fig. 1.
Structure of ginsenosides from Korean red ginseng
Biological activities
To study the immunomodulatory activity, we evaluated the effectiveness of eleven compounds (1–11) on IL-2 cytokine production in PMA/Io-induced EL-4 T lymphocyte cells (ATCC, Manassas, VA, USA). IL-2 plays an essential role in early T lymphocyte clonal differentiation and expansion (Gaffen and Liu, 2004; Rochman et al., 2009).
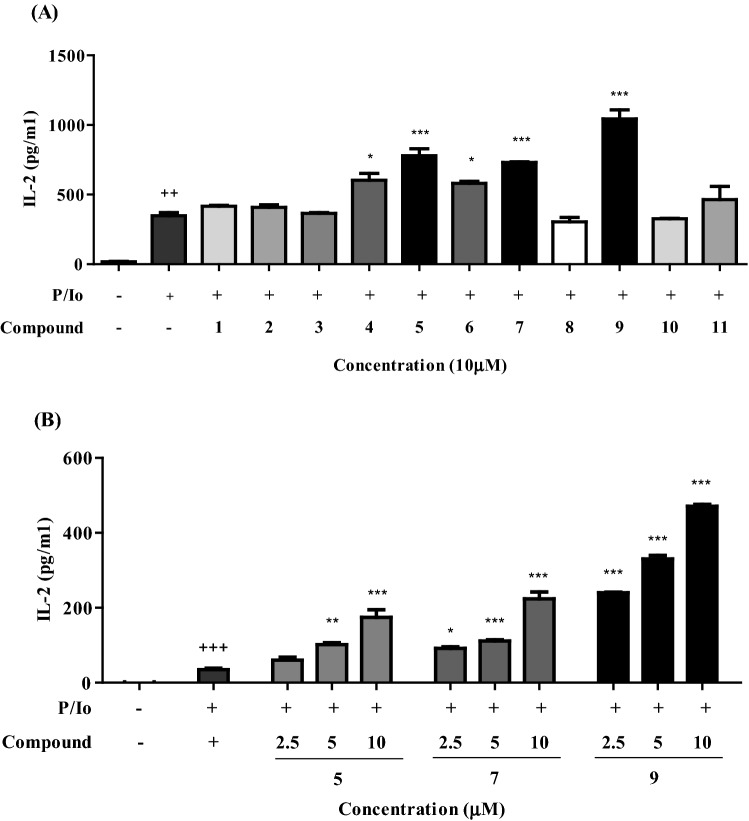
The effect of the eleven compounds isolated from Korean red ginseng was evaluated on the production of a potent T cell growth factor IL-2. Among tested eleven ginsenosides, compounds 5, 7, and 9 significantly increased the production of IL-2 cytokine from PMA/Io-activated EL-4 T cells in a dose-dependent manner [Fig. 2(A), (B)]. Compound 9 at all tested concentrations of 2.5, 5.0, and 10 µM significantly increased IL-2 production from EL-4T cells pre-treated with PMA/Io.
Fig. 2.
Effect of compounds on IL-2 production in PMA/Io-induced EL-4 T cells
Next, we investigated the possibility that IL-2 increase induced by compounds 5, 7, and 9 was related to regulation of transcriptional factors, NF-AT and NF-κB. NF-AT is an essential transcriptional factor for IL-2 expression by activated T cells (Macian, 2005; Maloy and Powrie, 2001). NF-κB also binds to the IL-2 promoter. EL-4 T cells were transfected with each firefly luciferase reporter NF-AT and NF-κB promoters, and then treated with the three compounds for 2 h and PMA/Io for 24 h. Compounds 5 and 7 did not show any effects on NF-AT or NF-κB promoter activity (Fig. 3). Compound 9 (oleanolic acid β-D-glucopyranosyl ester) significantly increased NF-AT promoter activity at a concentration of 10 µM in PMA/Io-activated EL-4 T cells [Fig. 3(A)], while no difference was observed in the NF-κB promoter activity [Fig. 3(B)]. These results suggest that compound 9 exerts its immunomodulatory activity by upregulating T cell growth factor IL-2 production via the regulation of NF-AT. Activated CD4+ and CD8+ T cells are usually regulated by multiple transcription factors including NF-AT, NF-kB, AP-1, and CREB/AFT (Kim et al., 2006). These results suggest that the compounds 5 and 7 could induce IL-2 production by transcription factors other than NF-AT and NF-kB. Further study would elucidate the mechanism by which the compounds 5 and 7 increase IL-2 production from EL-4 T cells.
Fig. 3.
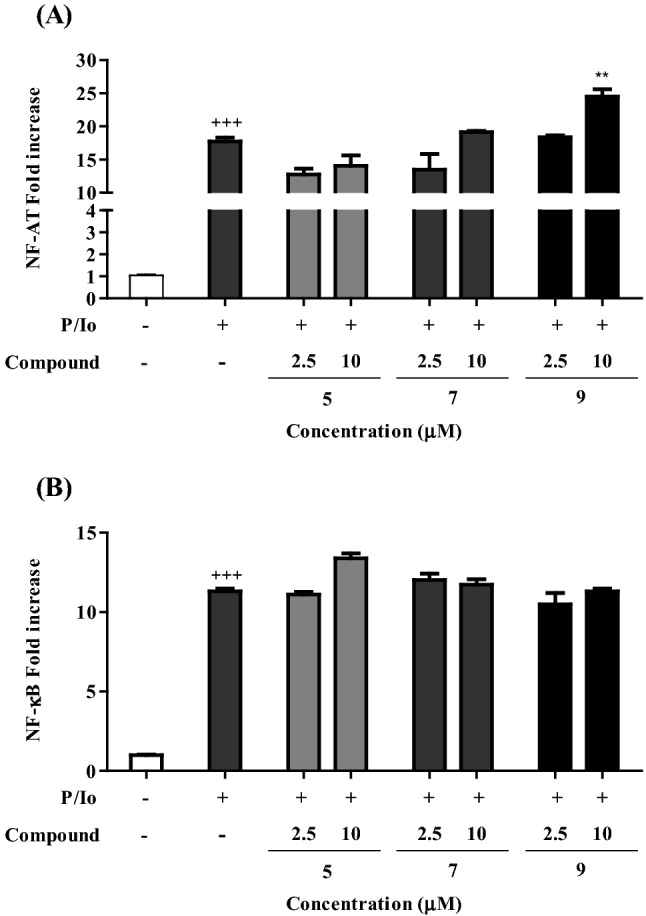
Effect of compounds 5, 7, and 9 on NF-AT and NF-κB promoter activities in PMA/Io-activated EL-4 T cells
In this study, we found for the first time that compounds 5 and 7 had the immunomodulatory activity increasing the production of IL-2 cytokine, but not related with NF-AT and NF-κB promoter activity. Compound 5 (ginsenoside RT5) was reported to have cytotoxic effects (Atopkina et al., 1999). Recently, compound 7 (20S-ginsenoside Rh2) was identified as a major metabolite of ginseng that had antitumor activity and the potential to prevent cancer (Chen et al., 2017; Shi et al., 2016). Moreover, it has been shown to induce the apoptosis and differentiation of acute myeloid leukemia cells (Wang et al., 2017).
It also indicates that oleanane type triterpenoids have greater immunomodulatory activity than does the dammarane type.
We also tested single compounds (5, 7, and 9) on the viability of the T cells by assessing cell metabolic activity (Park et al., 2017b). Compounds 5, 7, and 9 did not show any cytotoxic effect on EL-4 T cells at concentrations of 5 and 10 µM (Fig. 4). In this study, the compounds 5, 7, and 9 increased IL-2 productions from EL-4 T cells (Fig. 2), but did not show any activity on the cell proliferation (Fig. 4). IL-2 cytokine is the main regulator of survival, proliferation, and differentiation of activated T cells and NK cells. IL-2 shows dual functions in the immune response. IL-2 not only induces effector lymphocyte responses but also regulates or inhibits the responses at the same time by driving the expansion and suppressive function of regulatory T cells (Sim et al., 2014; Sim and Radvanyi, 2014; Williams et al., 2006). IL-2 was reported to have a narrow therapeutic window, and the level of dosing should be determined by further study.
Fig. 4.
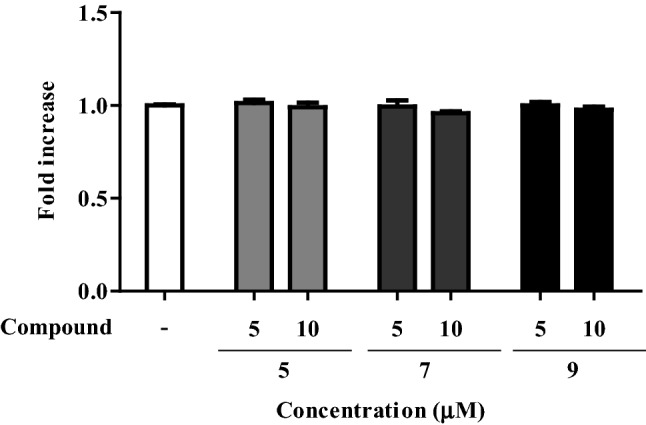
Effects of compounds 5, 7, and 9 on the viability of EL-4 T cells
Recently, studies of oleanane type triterpenoid saponins were investigated with good effects of immunomodulatory, hemolytic, and cytotoxic properties while studies about ginsenosides (dammarane type saponins) have limited in immunomodulatory activities (Sarikahya et al., 2018).
This is the first study reporting of the immunomodulatory activity of compounds 5, 7, and 9 obtained from a Korean red ginseng. In the view point of structure activity relationship, oleanane type triterpenoids have more potent immunomodulatory activity than does the dammarane type.
Immune responses are important in the prevention and treatment of infectious diseases and cancer. Thousands of studies have described the diverse roles of compounds isolated from P. ginseng in physiological processes such as cancer, neurodegenerative disorders, insulin resistance, and hypertension. P. ginseng facilitates homeostasis of the immune system and to enhance resistance to illness or microbial attacks via regulation of the immune system (Kang and Min, 2012).
The present study suggests that compounds 5, 7, and 9 (ginsenosides from Korean red ginseng) show immune enhancing activity through the modulation of T cell function caused by the increased IL-2 production. Considering the important roles of T cells in immune responses, these results suggest that compounds 5, 7, and 9 showing T cell-mediated immune potentiating activity have potential effects for a cure of cancer immune therapy.
Given the results for compounds 5, 7, and 9, these compounds are candidates for further investigation to elucidate their molecular mechanisms of action on specific immunomodulatory targets.
EL-4 T cells were pretreated with each compounds for 4 h and then incubated with PMA (0.5 ng/mL)/Io (5 nM) for 48 h. An ELISA was used to measure IL-2 production in cell culture medium. Compounds 5, 7, and 9 increased IL-2 cytokine production in EL-4 T cells activated with PMA/Io. All statistical calculations were performed using GraphPad Prism ver. 5.01 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance followed by the Bonferroni post hoc test was used for multigroup comparisons. (+P < 0.01 and +++P < 0.001 in comparison with the untreated group. *P < 0.05, **P < 0.01, and ***P < 0.001 correlated with the PMA/Io-treated group.)
EL-4 T cells were transfected with the reporter plasmids pNF-AT-Luc (A) or pNF-κB-Luc (B). The EL-4 T cells were pretreated with each compounds for 4 h and then incubated with PMA (0.5 ng/mL)/Io (5 nM) for 24 h. Luciferase activity in cell lysates were measured with the luciferase reporter system. All statistical calculations were performed using GraphPad Prism ver. 5.01 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance followed by the Bonferroni post hoc test was used for multigroup comparisons. (+P < 0.01 and +++P < 0.001 in comparison with the untreated group. *P < 0.05, **P < 0.01, and ***P < 0.001 correlated with the PMA/Io-treated group.)
EL-4 T cells were exposed to single compounds 5, 7, and 9 for 48 h and the cell cytotoxicity was assayed by CCK-8. Compound 5, 7, and 9 did not show cytotoxic effect on EL-4 T cells at concentrations of 5 and 10 µM. *P < 0.05 and ***P < 0.001 compared to the untreated group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by the Priority Research Center Program (Code: 2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Atopkina LN, Malinovskaya GV, Elyakov GB, Uvarova NI, Woerdenbag HJ, Koulman A, Pras N, Potier P. Cytotoxicity of natural ginseng glycosides and semisynthetic analogues. Planta Med. 1999;65:30–34. doi: 10.1055/s-1999-13957. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan C-S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Chen F, Sun Y, Zheng SL, Qin Y, McClements DJ, Hu JN, Deng ZY. Antitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing mice. J Funct Foods. 2017;32:382–390. doi: 10.1016/j.jff.2017.03.013. [DOI] [Google Scholar]
- Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- He LX, Ren JW, Liu R, Chen QH, Zhao J, Wu X, Zhang ZF, Wang JB, Pettinato G, Li Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017;8:3523–3532. doi: 10.1039/C7FO00957G. [DOI] [PubMed] [Google Scholar]
- Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z, Pan Z, Ren X. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96:378–383. doi: 10.1016/j.biopha.2017.09.129. [DOI] [PubMed] [Google Scholar]
- Kang S, Min H. Ginseng, the’immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, McColl SR. Tailored immune responses: novel effector helper T cell subsets in protective immunity. PLoS Pathog. 2014;10:e1003905. doi: 10.1371/journal.ppat.1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Han Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4 + T cell. Int Immunopharmacol. 2006;6:1424–1430. doi: 10.1016/j.intimp.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Liu JO. Calmodulin-dependent phosphatase, kinases, and transcriptional corepressors involved in T-cell activation. Immunol Rev. 2009;228:184–198. doi: 10.1111/j.1600-065X.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- Park JU, Kang BY, Lee HJ, Kim S, Bae D, Park JH, Kim YR. Tetradecanol reduces EL-4 T cell growth by the down regulation of NF-κB mediated IL-2 secretion. Eur J Pharmacol. 2017;799:135–142. doi: 10.1016/j.ejphar.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Park JU, Kang BY, Lee HJ, Kim S, Bae D, Park JH, Kim YR. Tetradecanol reduces EL-4 T cell growth by the down regulation of NF-κB mediated IL-2 secretion. Eur J Pharmacol. 2017;799:135–142. doi: 10.1016/j.ejphar.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Read Kaitlin A., Powell Michael D., McDonald Paul W., Oestreich Kenneth J. IL-2, IL-7, and IL-15: Multistage regulators of CD4+ T helper cell differentiation. Experimental Hematology. 2016;44(9):799–808. doi: 10.1016/j.exphem.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikahya NB, Nalbantsoy A, Top H, Gokturk RS, Sumbul H, Kirmizigul S. Immunomodulatory, hemolytic and cytotoxic activity potentials of triterpenoid saponins from eight Cephalaria species. Phytomedicine. 2018;38:135–144. doi: 10.1016/j.phymed.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Shi Q, Shi X, Zuo G, Xiong W, Li H, Guo P, Wang F, Chen Y, Li J, Chen DL. Anticancer effect of 20 (S)-ginsenoside Rh2 on HepG2 liver carcinoma cells: Activating GSK-3β and degrading β-catenin. Oncol Rep. 2016;36:2059–2070. doi: 10.3892/or.2016.5033. [DOI] [PubMed] [Google Scholar]
- Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L. IL-2 therapy promotes suppressive ICOS + Treg expansion in melanoma patients. J Clin Investig. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25:377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Smith KA. Interleukin-2. Curr Opin Immunol. 1992;4:271–276. doi: 10.1016/0952-7915(92)90076-Q. [DOI] [PubMed] [Google Scholar]
- Vinh Le Ba, Lee Yunjeong, Han Yoo Kyong, Kang Jong Seong, Park Jung Up, Kim Young Ran, Yang Seo Young, Kim Young Ho. Two new dammarane-type triterpene saponins from Korean red ginseng and their anti-inflammatory effects. Bioorganic & Medicinal Chemistry Letters. 2017;27(23):5149–5153. doi: 10.1016/j.bmcl.2017.10.058. [DOI] [PubMed] [Google Scholar]
- Wang C, He H, Dou G, Li J, Zhang X, Jiang M, Li P, Huang X, Chen H, Li L. Ginsenoside 20 (S)-Rh2 induces apoptosis and differentiation of acute myeloid leukemia cells: Role of Orphan Nuclear Receptor Nur77. J Agric Food Chem. 2017;65:7687–7697. doi: 10.1021/acs.jafc.7b02299. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8 + memory T cells. Nature. 2006;441:890. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen A, Sun H, Ye Y, Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine. 2007;25:161–169. doi: 10.1016/j.vaccine.2006.05.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.