Abstract
Electromagnetic fields (EMF) in the intermediate frequency (IF) range are generated by many novel electrical appliances, including electric vehicles, radiofrequency identification systems, induction hobs, or energy supply systems, such as wireless charging systems. The aim of this systematic review is to evaluate whether cardiovascular implantable electronic devices (CIEDs) are susceptible to electromagnetic interference (EMI) in the IF range (1 kHz–1 MHz). Additionally, we discuss the advantages and disadvantages of the different types of studies used to investigate EMI. Using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, we collected and evaluated studies examining EMI in in vivo studies, in vitro studies (phantom studies, benchmark tests), and simulation studies. Our analysis revealed that cardiac implants are susceptible to malfunction induced by EMF in the IF range. Electromagnetic interference may in particular be provoked by security systems and induction hobs. The results of the studies evaluated in this systematic review further indicate that the likelihood for EMI is dependent on exposure-related parameters (field strength, frequency, and modulation) and on implant- as well as on lead-related parameters (model, type of implant, implant sensitivity setting, lead configuration, and implantation site). The review shows that the factors influencing EMI are not sufficiently characterized and EMF limit values for CIED patients cannot be derived yet. Future studies should therefore, consider exposure-related parameters as well as implant- and lead-related parameters systematically. Additionally, worst-case scenarios should be considered in all study types where possible.
Keywords: Electromagnetic interference, Implantable cardioverter-defibrillator, Cardiac pacemaker, Electric fields, Magnetic fields, Intermediate frequency, Systematic review
Introduction
In recent years, the number of patients that have been fitted with cardiovascular implantable electronic devices (CIEDs) such as cardiac pacemakers (PMs) or implantable cardioverter-defibrillators (ICDs) has strongly increased. In the USA, while 9000 CIEDs were implanted in 1990,1 its number increased to 368 829 in 2009.2 Over 4.2 million primary CIED implantations were performed between 1993 and 2008.3 In Europe, 547 586 PMs and 105 730 ICDs were implanted in 2016.4 Additionally, novel CIEDs like leadless PMs, subcutaneous ICDs, and heart failure devices are gaining more and more importance.
At the same time, with the success of the CIED technology during the past decades, exposure to external electric, magnetic, and electromagnetic fields (EMF) has increased, at least in the intermediate frequency (IF) and radiofrequency (RF) range.5–8 Electromagnetic fields are used e.g. to transmit communication signals or arise along power transmission lines. Other sources of EMF are electrical appliances. Electromagnetic fields are classified according to their wavelength and frequency. For example, power lines or electrical household devices emit EMF with a lower frequency (LF) while mobile phones, Wi-Fi, or microwave ovens produce EMF of a higher frequency. Electromagnetic fields in the IF range are generated by many novel electrical appliances, including electric vehicles, RFID (RF identification) systems, induction hobs, or energy supply systems, such as wireless charging systems.
Cardiovascular implantable electronic devices are known to be susceptible to malfunction in the presence of strong EMF.9–12 Many researchers have studied electromagnetic interference (EMI), i.e. potential, undesirable effects of EMF on the operation of CIEDs. The EMF-Portal (www.emf-portal.org), the most comprehensive scientific literature database on biological and health-related effects of EMF provided by our institute currently comprises 639 records on EMI (June 2017). The Manufacturer and User Facility Device Experience (MAUDE) database of the American Food and Drug Administration13 identified 2843 cases of malfunctions of medical devices induced by EMI between January 2010 and March 2017. However, this may be an underestimation of events as reporting of such incidents is not mandatory and some physicians may misjudge EMI episodes e.g. as atrial fibrillation. A survey of physicians in France showed that 16% of them were concerned about patients who reported EMI at least once a year, e.g. oversensing of noise signals due to EMF exposure is a phenomenon regularly seen in daily practice.14 Napp et al.9 demonstrated the general mechanisms of effects in CIED caused by EMF, e.g. heating of the implant or lead by RF fields or induction of electric currents within the human body by LF fields leading to e.g. disturbance of the sensing capabilities of the implant. Additionally, Beinart and Nazarian10 showed potential everyday sources of EMI and documented typical effects, e.g. damage to CIED circuitry, PM inhibition, asynchronous pacing, or inappropriate ICD shocks.
Standard organizations have not proposed limit values to EMF exposure for patients with CIEDs. The American National Standards Institute (ANSI),15,16 the International Commission on Non-Ionizing Radiation Protection (ICNIRP),17 and the European Union18,19 did not consider patients with CIEDs in their safety guidelines for the protection of humans exposed to EMF. Consequently, it is often difficult for physicians and patients to identify sources of EMF which pose a risk and to determine appropriate safety distances which should be respected. In some cases, the applied safety measures in occupational environments might result in a ban from workplaces for CIED carriers.
To date, no systematic analysis has been done for EMI in the IF range (1 kHz–1 MHz). The aim of this systematic review is therefore to evaluate whether CIEDs are susceptible to EMI in the IF range. In particular, we consider the results from different types of studies (in vivo and phantom studies, benchmark test) and outline their advantages and disadvantages. Additionally, we identify the type of study which is most appropriate to further investigate the various parameters (implant setting, lead configuration, and individual parameters) that influence the likelihood for EMI.
Methods
Literature search strategy and general information
As prescribed by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement,20 we conducted a systematic literature search to identify relevant studies published from inception to October 2016 using our thematically specialized open-access literature database EMF-Portal (www.emf-portal.org). The EMF-Portal is the most comprehensive scientific literature database on biological and health-related effects of EMF and has been approved by the WHO as a reference database.21 It has been publicly available for more than 15 years and comprises currently 25 900 publications22 (January 2018). Our search in the EMF-Portal for the current systematic review was based in a first step on the more general search term ‘electromagnetic interference’ (for a link to the search string, see Supplementary material online: search strategy). Additionally, we performed a more specific search in the frequency range <10 MHz and in the category ‘electromagnetic interference’. The lists of results were corrected for double publications.
Eligibility criteria and study selection
Articles were included when they reported experimental studies on EMI with CIED in the frequency range of 1 kHz–1 MHz. We accepted benchmark tests, studies with phantoms, in vivo studies, and numerical simulations. Only articles written in English or German and published in a peer-reviewed journal were considered. There was no restriction regarding the year of publication.
Excluded were studies that focused on CIED-programmer interference, CIED-interference with further (cardiac) implant, EMI with other implants (e.g. neurostimulator), or EMI induced by current application (e.g. by medical devices). Furthermore, studies without specification of the tested frequency range were excluded. Review articles, case studies, editorials, commentaries, and unpublished or clearly not peer-reviewed articles were also excluded.
Two authors independently (S.D. and D.S.) screened the studies for eligibility based on inclusion/exclusion criteria. Articles were screened in two stages. First, titles and abstracts were reviewed to identify potentially relevant articles. For those abstracts which met the inclusion criteria, the full text was retrieved and independently reviewed in the second stage of assessment. The two review authors made a joint decision about inclusion of the articles.
Data extraction
The data from the studies included were extracted independently by two authors (S.D. and D.S.). The extraction protocol was defined and agreed upon before the start of the project. Extracted data included bibliographical data, study type (e.g. phantom, in vivo study), exposure parameters (field source, i.e. electrical appliance, frequency, and field strength if provided), number of patients/CIED, CIED characteristics, and outcome (disturbance). Additionally, for in vivo studies, implant settings and lead polarities were extracted. Disagreements and uncertainties were discussed and resolved between the two review authors.
Results
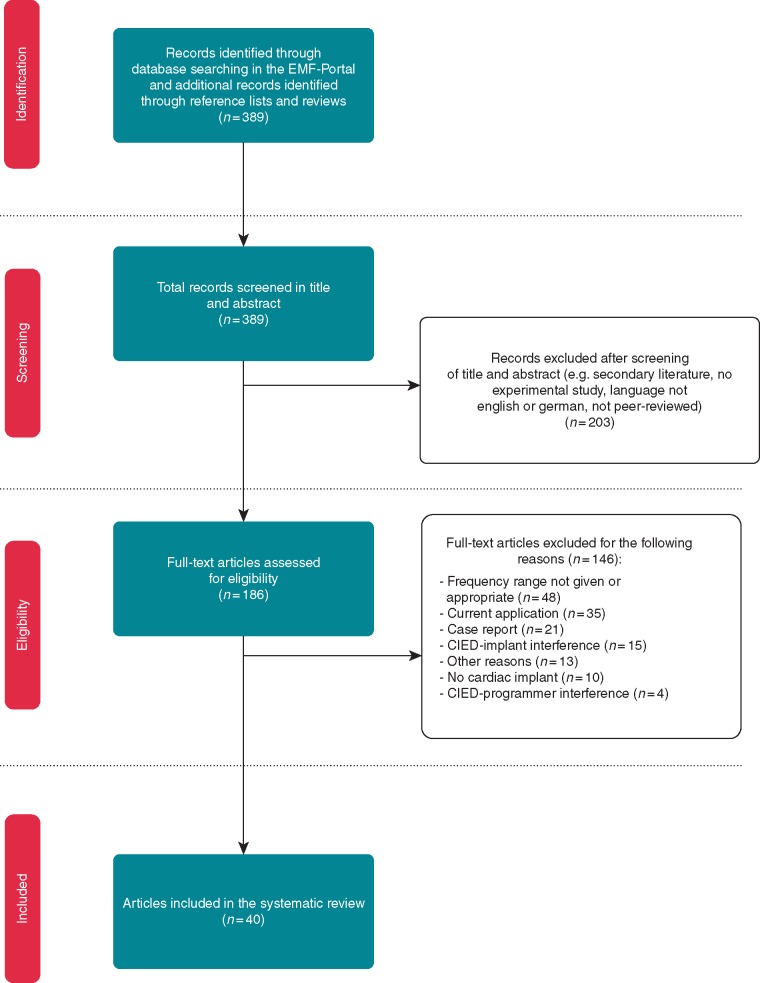
The systematic literature search identified 389 articles that matched the search criteria. After screening the title and abstract, 203 articles were excluded for various reasons (e.g. secondary literature, not dealing with EMI). The full text was obtained for the remaining 186 articles to check for eligibility to be included in our analysis. Of these, 146 articles were excluded for the following reasons: frequency range not provided or appropriate (n = 48), current application (n = 35), case report (n = 21), CIED-implant interference (n = 15), no CIED (n = 10), CIED-programmer interference (n = 4), or other reasons (n = 13). Forty articles fulfilled the eligibility criteria and were included in this review (see Figure 1). Of these, most studies (n = 15) used combinations of several methods to investigate EMI (e.g. phantom and benchmark), 13 studies used phantoms only, 10 articles investigated EMI in patients (in vivo), and one study used a benchmark test only. Additionally, the search identified one simulation study (see Figure 2).
Figure 1.
Flow diagram of literature search, eligibility, and inclusion process. Adapted from Moher et al.20 CIED, cardiovascular implantable electronic device; EMF, electromagnetic field.
Figure 2.
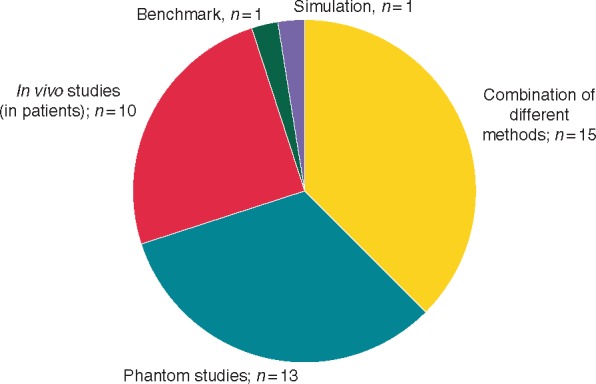
Study types used for EMI investigation in the IF range. EMI, electromagnetic interference; IF, intermediate frequency.
Most of the studies investigated EMI on PMs only (n = 20), while four studies considered ICDs only. Ten studies considered different types of CIEDs [PMs, ICDs, and implantable loop recorder (ILR)]. Five studies did not use a CIED but a modified CIED case to measure the induced voltage at its terminals. One study investigated EMI on ILRs only.23
In some studies, additional frequency ranges or electrical appliances outside the IF range were investigated, but these data on EMI are not considered in this review—if not stated otherwise.
Table 1 provides detailed information on the most important technical terms which are used in this review.
Table 1.
Technical terms used in this review
| Terms | Explanation |
|---|---|
| EMI | Disturbance of CIEDs’ operation by induction of intracorporal voltage caused by electric, magnetic, or EMF |
| External EMF | EMF emitted by an electrical appliance, e.g. magnetic field (measured in T or A/m) or electric field (measured in V/m) |
| Induced voltage | Intracorporal voltage occurring at the terminals of a CIED induced by external EMF |
| Interference thresholds | Minimum field strength of an external EMF required to cause EMI |
| Disturbance/interfering signal | Noise signal that may disturb the regular operation of a CIED |
| CW, AM, PW, and pulses | Waveforms (CW, AM, PW, and pulses) of an external EMF or a disturbing/interfering signal; the waveform can significantly influence the response of a CIED |
| Detection level | Voltage of a disturbing/interfering signal which causes disturbance of CIEDs’ operation |
| Performance limits | Minimum detection levels of CIEDs defined in product standards, given in mV |
A/m, Ampere/meter; AM, amplitude modulation; CIED, cardiovascular implantable electronic device; CW, continuous wave; EMF, electromagnetic fields; EMI, electromagnetic interference; mV, millivolts; PW, pulsed modulation; T, Tesla; V/m, Volt/meter.
In vivo studies
In in vivo studies, patients with CIEDs are directly exposed to EMF to assess the electromagnetic compatibility of CIEDs. As such, individual interference thresholds of the CIED can be determined for specific exposure conditions.
In the current review, 10 in vivo studies were evaluated which exposed patients with CIEDs to EMF (Supplementary material online, Table S1). Additional five studies used a combined methods approach, i.e. they conducted also benchmark or phantom tests (Supplementary material online, Table S3).
Altogether, potential EMI was investigated in 1084 patients that had been fitted with CIEDs (769 PMs, 313 ICDs, and 2 ILRs). The nine studies providing details on PM and ICD types included 369 single chamber (217 PM, 152 ICD), 433 dual chamber (361 PM, 72 ICD), and 42 resynchronization therapy devices (13 PM, 29 ICD). Eight of the 15 studies included CIED carriers with both unipolar and bipolar leads, whereas three studies24–26 tested only patients with one lead configuration (unipolar OR bipolar). Four studies did not provide any details on lead configuration.27–30 Eleven of the 15 studies left the CIED sensitivity unchanged or investigated different sensitivities settings (e.g. maximum, nominal), whereas two studies29,31 tested under maximum sensitivity only. Two studies did not provide any details on sensitivity.28,32
All included studies used real-life electrical appliance exposure such as security systems [electronic article surveillance (EAS) systems or metal detector gates, n = 8], medical devices (n = 4), induction hobs (n = 3), or avalanche transceivers (n = 1). One study tested both an EAS system and an induction hob (counted separately in each category).31 None of the included in vivo studies were performed under a standardized exposure set-up, i.e. with e.g. a Helmholtz coil. Furthermore, not all of the studies provided details on the field strengths which actually occurred at the height of the implant (chest area). At least, one study measured the magnetic field strength at a distance from the patient to the security device (1.6–2.7 A/m, 50 cm to the security gates)33 and another study performed comprehensive field measurements (14–310 A/m), but the field strength required to induce EMI remained unclear.34
The data of three studies showed that the acoustomagnetic EAS system (58 kHz) could disturb PMs.31,32,34 There was no evidence that ICDs could be disturbed. However, only 38 ICD patients were included in these studies compared with 265 PM patients (whereof the PMs of 72 patients were disturbed). Electromagnetic interference with PMs was also found with other security systems operating at higher (120 kHz) or lower (10 kHz) frequencies.33,35 Importantly, the study by Wilke et al.33 provided evidence that PMs can be negatively affected by security systems below the ICNIRP limits for the general public (i.e. 21 A/m).
Two further studies did not observe EMI following exposure to metal detectors36 or EAS systems23; with the latter being one of the two studies investigating ILRs. In a further study it remained unclear whether EMI was induced by a 100 Hz or 1 kHz metal detector.24
Three studies on potential EMI of induction hobs25,26,31 showed that the patients’ safety with CIEDs was guaranteed when minimum distances were respected, i.e. no EMI was observed at distances of 20–35 cm. It was, however, not documented in these studies whether disturbances occurred in closer proximity to the induction hobs.
Other devices such as an electromagnetic articulography device,29 an ultrasonic dental scaler,30 a magnetic endoscope imager,27 or avalanche transceivers37 did not cause EMI under the used conditions. Some dental devices appeared to have the potential to disturb PMs,28 however, the frequency was provided only for one device and it is unclear whether the other dental devices emitted EMF in the IF range. The relevance of this data is, however, debatable for today’s applications, because the study28 was published in 1975.
Altogether, EMI in the IF range was revealed in 6 out of the 15 studies resulting in e.g. sensing anomalies (e.g. undersensing or oversensing),31–34 asynchronous pacing,33,34 increased pacing rate,24,34 pacing inhibition,24,32–35 and mode switch.32 In McIvor et al.34 EMI was accompanied by symptoms in patients, e.g. palpitations and presyncope.
For a general risk assessment, in vivo studies with exposures to a single device, such as avalanche transceivers37 have only a limited significance due to the lack of a proper dosimetry. Additionally, the applicability of the data to other exposure situations is limited. Studies performed under standardized exposure set-ups, i.e. using e.g. a Helmholtz coil or antenna settings are better suited, because EMF can be homogenously generated and EMF at different frequencies and field strengths can be applied systematically. That way, more general data for various applications can be obtained.
Phantom studies
Phantoms simulate the human body or parts of the human body including different tissue characteristics.38 Experimental in vitro studies using phantoms examine either directly the disturbance (EMI) of CIEDs or they are used to determine the intracorporal voltage induced by external EMF at the terminals of CIEDs. In phantoms, both the response of CIEDs to different EMF and the impact of the lead can be tested.
Potential EMI with CIEDs was investigated in 15 studies and five studies measured the induced voltage. An additional five studies considered the development of a coupling model (Supplementary material online, Tables S2 and S3). In Babouri et al.,39 the phantom served to validate detection levels recorded in benchmark tests. Therefore, this study is discussed in the ‘Benchmark tests and test in air’ section.
Studies investigating electromagnetic interference
In the 15 studies on potential EMI, altogether, 185 CIEDs were investigated [100 PMs, 60 ICDs, and 25 PM/ICDs (not further specified40)]. Only 4 out of the 15 studies used a standardized exposure set-up, e.g. a Helmholtz coil setting,41–44 whereas the remaining 11 studies investigated potential EMI in phantoms upon exposure to real-life electrical appliances [RFID/security systems (n = 5), induction hobs (n = 2), medical devices (n = 2), wireless power transfer (WPT) systems (n = 1), or a magnetically levitated linear motor car (n = 1)]. Only one study provided precise data on the correlation of EMI and exposure characteristics.45
The data of two studies46,47 on RFID systems showed that the majority of the investigated PMs (67–83%) and ICDs (47–71%) could be disturbed by different 134 kHz RFID systems at a distance of up to 61.3 cm. There was no clear correlation between EMI and lead configuration and no difference between maximum and nominal sensitivity,46 most likely due to the high intensity field strength of the RFID system. Mattei et al.45 investigated typical exposure patterns of RFID systems and identified EMI from 40 A/m at 125 kHz for a pulsed signal and from 60 A/m for a continuous wave (CW) signal.
Two further studies on security systems showed that EMI was induced by an anti-theft device of 120 kHz33 and by different EAS signals (100 Hz–8 kHz, CW or pulsed48). Kainz et al.48 found that the interference level of a pulsed signal was lower than that of a CW.
Two studies on induction hobs25,49 revealed that EMI occurred both as a function of the distance to the induction hob and dependent on the presence/absence of the pot or the position of the pot. However, the exact field strengths at specific distances were not clear from both studies.
The data on security systems or induction hobs showed that some devices in our everyday life may induce EMI in CIEDs and thus confirm the findings of in vivo studies.
Hikage et al.40 investigated 14 different WPT systems. Electromagnetic interference occurred in 5 of the 12 WPT systems for mobile application with modulated fields at a maximum distance of ≤2 cm for PMs and at a distance of ≤1 cm for ICDs. The two WPT systems for electric vehicle charging provoked no EMI. However, no field strengths were provided and no details were shown which WPT system caused which kind of EMI.
No EMI was induced under the specific study conditions for a microtron device used for cancer therapy,50 nor for an electromagnetic navigational bronchoscopy device51 or for a magnetically levitated linear motor car52.
The four phantom studies on potential EMI using a standardized exposure set-up41–44 found that EMI depended on field frequency, CIED type, and programmed sensitivity.
For a general risk assessment, the data of the evaluated phantom studies on potential EMIs are limited as are the data of the discussed in vivo studies. Although there are many studies using a comprehensive study design, the applicability of the data to other electrical appliances or comparable exposure scenarios is limited due to the lack of sufficient dosimetric data or a missing correlation of those data to EMI.25,33,49 Likewise, phantom investigations with exposures to only a single device, such as a magnetically levitated linear motor car52 only contribute in a limited way to a general risk assessment. The same applies to studies using standardized conditions if EMI is not systematically tested for various frequencies and field strengths.
Determination of induced voltage
Besides the direct measurement of EMI, phantom studies can also serve to determine the voltage induced by external EMF at the terminals of CIEDs. The induced voltage is the critical measurement parameter for electromagnetic compatibility testing of CIEDs and can be compared with international product standards, e.g. Ref.53 These CIED standards set performance limits up to 3 GHz with the objective of preventing malfunctions induced by EMF. The performance limits increase linearly in the IF range (3 kHz–167 kHz) and vary between unipolar (9–500 mV) and bipolar (0.9–50 mV) testing.53–55
The induced voltage was investigated in five studies.
Bassen56 investigated different iPods but the induced voltage was below the noise level of their measurement instruments. They concluded that no EMI would be expected.
Irnich and Bernstein57 investigated 11 induction hobs and the induced voltage was between 6 and 800 mV dependent on the distance and the position of the pot. The combination of their phantom study with a benchmark test showed that 14.8% of PMs would be disturbed under worst-case conditions and never at a minimum distance of 35 cm to the thorax. These results confirm the findings of phantom studies on EMI25,49 and the findings of in vivo studies25,26,31 in that induction hobs appear to be safe at a specific distance.
Seckler et al.58 investigated a WPT system and compared the data with a standardized exposure set-up using Helmholtz coils (111 kHz, both systems). Under the standardized exposure condition the performance limit (i.e. 333 mV for CIEDs with unipolar leads and 33.3 mV for bipolar leads) was already exceeded at 11 µT and thus below the ICNIRP limit17 of 27 µT; whereas with the WPT system, the limits—even with the WPT system touching the phantom—were not exceeded. This comprehensive study approach demonstrates the significant difference between the voltage induced by a homogenous field of Helmholtz coils and by inhomogenous fields of an electronic device. Moreover, this study highlights the importance to characterize the emitted field patterns (e.g. CW and AM) and dosimetric data of electronic devices.
Mattei et al.59 used an antenna design at 125 kHz to emulate RFID systems and measured a maximum induced voltage for a unipolar lead of 62.2 mV and 19.8 mV for a bipolar lead, thus indicating that the performance limits (i.e. 375 mV for CIEDs with unipolar and 37.5 mV for bipolar leads) were not exceeded under the specific exposure conditions. In a later study by the same authors,45 however, EMI was detected and thus, the performance limits seemed to be exceeded by exposure of RFID systems (see section ‘phantom studies—Studies investigating electromagnetic interference’). Exceeding of the performance levels under consideration of EAS-similar exposure scenarios was also demonstrated by numerical simulations of Leitgeb et al.60 who calculated induced voltages between 3.2- and 13.5-fold above the performance limits in the IF range (60 kHz–5 kHz, respectively) under worst-case conditions (Supplementary material online, Table S5).
Gustrau et al.61 identified induced voltages at the PM terminals of 0.126 mV–131 mV (1 kHz–1 MHz, at 1 A/m, i.e. 1.26 µT). Gustrau et al.,61 Seckler et al.,58 and Mattei et al.59 found a dependence of the induced voltage on lead configuration.
Coupling model
A further motivation to perform phantom studies, is the development of a coupling model (transfer function). Transfer functions demonstrate the relationship between the strength of an external EMF and the induced intracorporal voltage at the terminals of a CIED. The transfer functions of the five studies included were determined by numerical or analytical approaches based on data gained in phantom studies or benchmark tests.
Hedjiedj et al.62 developed a transfer function based on a simple phantom and found detection levels from >55/104 mV at 10/25 kHz for a sensitivity level of 0.7 mV. The authors also included benchmark tests and reported detection levels in two out of five PMs of >150/130 mV at 10/25 kHz for a sensitivity level of 1 mV.
In two comprehensive studies, Andretzko et al. presented a numerical model for the determination of transfer functions between electric fields63 or magnetic fields64 and the induced voltage. The results obtained by numerical simulation were in agreement with experimental data from benchmark tests and phantom studies. The detection levels increased with increasing PM sensitivity values; additionally, the interference thresholds depended on the loop area formed by the CIED with its lead, i.e. for a large (300 cm2) loop area interference threshold occurred from 20 µT and for a standard loop area (225 cm2) from 26 µT (unipolar lead).
In a further study by the same research group,43 the realistic lowest interference thresholds were calculated and given with 33.36 µT (25 kHz) or 79.18 µT (10 kHz), respectively, for unipolar lead settings (200 cm2). However, the coupling model was not validated and tests in air and tests with a phantom yielded different results. The result of this study together with the results of the other three studies suggest that the detection levels of the considered CIEDs were significantly above the performance limits recommended by international product standards,53 thus indicating compliance with the proposed standards, although the applied magnetic fields (30–85.4 µT) exceeded the limit value recommended by ICNIRP (i.e. 27 µT).43,64 Whether the transfer function presented by this research group could serve as a solid basis for the calculation of induced voltages from external EMF should be validated by comprehensive realistic data obtained from benchmark tests, phantom, and in vivo studies.
Finally, in a study by van Wijk van Brievingh et al.,65 the authors calculated—according to their coupling model—interference thresholds between 0.1 and 100 A/m (i.e. 0.126–126 µT) for 100 Hz and 250 kHz. However, no specific values for induced voltages were provided.
In general, the data of these five studies indicate that interference thresholds depend on the loop area formed by the CIED with its lead, on the frequency of the applied external EMF, the sensitivity setting of the CIED and the device itself and thus confirm the findings of several other phantom studies. Universal transfer function therefore, may provide a helpful tool to estimate induced voltages under specific conditions (e.g. lead type) and in dependence of specific exposure scenarios (frequency and field strength).
Benchmark tests and tests in air
Manufactures of CIEDs are obligated to test their implants for compliance with product standards in order to obtain approval for the European market (CE marking) or from the Food and Drug Administration (FDA) for the US market. Regarding electromagnetic compatibility performance limits as well as test methodologies (benchmark tests) are defined in ISO 14117:201253 for the US market and in EN 45502-2-1:200455 (PMs) and EN 45502-2-2:200854 (ICD) for the European market. In benchmark tests disturbance signals are fed directly into the pace/sense channel of the CIED by galvanic coupling in order to analyse the CIED’s response and detection levels. The simple methodology is a major advantage of benchmark tests. Cardiovascular implantable electronic devices of different manufacturers and with different settings can be tested and evaluated by any number of various disturbance signals. However, a disadvantage of benchmark tests is that individual parameters of the patient or the lead are not considered and the data cannot be transferred to external EMF of e.g. a certain electrical appliance.
Although benchmark tests are a well-accepted method, many researchers preferred to perform tests in air. In such tests, implants are equipped with leads and located within the exposure area of a standardized set-up or close to a specific electrical appliance, comparable to phantom studies but without a phantom. Thus, potential EMI can be correlated with field strengths of an external EMF. In the current review, benchmark tests were used in six studies, whereas tests in air were performed in seven studies (Supplementary material online, Tables S3 and S4). Andretzko et al.64 conducted both benchmark tests and tests in air (counted in each category separately). Altogether, in the 12 studies included interference was evaluated in >286 PMs (exact number not given in van Wijk van Brievingh et al.65), in one ICD and in three ILRs. The results of benchmark studies generally indicated compliance with the performance limits set by product standards. Additionally, the results of some benchmark tests and tests in air showed the dependence of potential EMI on frequency, the CIED and its sensitivity level.39,64,66
In 4 of the 12 studies included, benchmark tests or tests in air were combined with other methods in order to establish a transfer function.62–65 These studies are discussed in detail in the ‘Phantom Studies—Coupling Model’ section. Nevertheless, due to the data of these studies it can be summarized that the lowest detection levels were found from 55 mV (10 kHz)62 and 95 mV (25 kHz)64 depending on the sensitivity level of the PM.64 Comparable detection levels were also found in an earlier study by the same research group39 combining benchmark tests and phantom investigations.
A further publication with tests in air43 reported interference thresholds from 1.09 µT at 10 kHz (single-chamber PM) and from 0.54 µT at 25 kHz (dual-chamber PM); however, this was found under artificial loop conditions (90 turns-lead).
Irnich and Bernstein57 performed benchmark tests in order to investigate the impact of a typical signal of induction hobs (24 kHz) and reported detection levels below the performance limits. It has to be noted, however, that the data was recorded for an older PM model which was released before 1998.
Three out of four studies performing tests in air on security systems found that EMI was caused by different EAS systems and EAS signals.35,48,66 Only the study by de Cock et al.23 did not report EMI. Potential EMI provoked by EAS systems was also found in in vivo studies e.g. Refs31,32,34,35 and in phantom studies e.g. Refs46,47. The strengths of external EMF were only provided for EAS systems used in Dodinot et al.35 and for signals used in Kainz et al.48 where interference thresholds were reached from 1.13 mT and approximately 15 A/mpeak-to-peak, respectively. Additionally, the study by Lucas et al.66 indicated that unipolar PMs were affected more often than bipolar PMs.
Finally, Corbett et al.27 investigated a medical magnetic endoscope imager and did not find EMI with different CIEDs. The same result was obtained in their experiment with patients (see In Vivo Studies section).
Discussion
The aim of this systematic review was to evaluate whether CIEDs are susceptible to EMI in the IF range generated by many novel electrical appliances, including electric vehicles, induction hobs, or wireless charging systems.
Forty articles fulfilled the eligibility criteria and were included in this review. Most of the studies investigated EMI on PMs only (n = 20), while four studies considered ICDs only. Ten studies considered PMs, ICDs, and ILRs. Five studies did not use a CIED but a modified CIED case to measure the induced voltage at its terminals. One study investigated EMI on ILRs only.23
There is only limited data on EMI with CIEDs in the IF range and only a few of the evaluated studies correlated the documented EMI with exposure data (e.g. Refs33,45,58). Likewise, the studies that did not report EMI, rarely provided detailed data on exposure conditions.26,29,31,41,42,51
More than one-third of the studies investigated CIEDs which were exposed to security systems, including EAS, metal detectors, and RFID (n = 15), and five studies investigated potential EMI in the proximity of induction hobs. Single studies also investigated other electronic appliances, such as iPods,56 a magnetically levitated linear motor car,52 WPT systems,40,58 or avalanche transceivers.37
There is evidence that EMF sources of everyday life such as security systems may induce EMI.31–34,45,46 Also, induction hobs appear to provoke EMI in close proximity.25,26,31,49 For other electronic appliances, EMI or exceeding of performance levels was found only for WPT systems.40,58 However, it cannot be concluded that the other investigated electrical appliances that did not reveal any EMI adhere to safety standards in general, because some studies investigated only a few CIEDs or a few patients (7 ICD51 or 3 PM, 1 ICD52) or the exposure parameters were insufficiently described.37,56
We evaluated benchmark tests, simulation, phantom, and in vivo studies. As shown in Table 2, the studies evaluated in this review used different methods (e.g. phantom and benchmark) to investigate EMI for various electrical appliances. For RFID/EAS systems, EMI was consistently reported in in vivo studies, phantom studies, and benchmark tests, whereas for induction hobs and wireless charging systems, EMI was found only in phantom studies.
Table 2.
Types of studies used for EMI investigation in different electrical appliances
| Electrical appliance | In vivo studies | Phantom studies | Benchmark tests and tests in air | Simulation |
|---|---|---|---|---|
| Standardized exposure set-up | X39,41–44,58,61–65 | O39,43,62–65 | O43,61,63,64 | |
| RFID/EAS systems | X23,31–35 | X33,45–48,59 | X23,35,48,66 | O60 |
| Metal detector | O23,24,36 | O48 | O23,48 | |
| Induction hobs | O25,26,31 | X25,49,57 | O57 | |
| Wireless charging systems | X40,58 | O40 | ||
| Different medical devices (articulography device, dental devices, microtron device, navigational bronchoscopy device, and magnetic endoscope imager) | O28–30 | O50,51 | O27 | |
| Different iPods | O56 | |||
| Avalanche transceivers | O37 | |||
| Magnetically levitated linear motor car | O52 |
EAS, electronic article surveillance; EMI, electromagnetic interference; O, no EMI was found in this category; RFID, radiofrequency identification; X, EMI was reported for this study type in at least one study.
Clinical relevance
Previous studies have shown that oversensing of noise signals due to EMF exposure occurs in everyday life and physicians caring for CIED patients are regularly confronted with EMI.14,67,68 In the studies included in this review sensing anomalies (e.g. undersensing, oversensing),31–34 asynchronous pacing,33,34 increased pacing rate,24,34 pacing inhibition,24,32–35 and mode switch32 were reported.
Oversensing in the atrial channel can be misinterpreted by CIEDs as atrial fibrillation and cause a change in the pacing mode to either VVI(+R) or DDI(+R) mode, which results in atrioventricular dysynchrony. The event may remain unnoticed and has no clinical consequence if the interference is brief. However, in the case of extended atrioventricular dysynchrony, patients with a high ventricular pacing percentage may develop the pacemaker syndrome, including symptoms of palpitations, dizziness, and reduced physical capacity.69 Additionally, inappropriate mode switch episodes could lead physicians to initiate therapeutic oral anticoagulation if the episodes are not correctly identified as EMI. If atrial oversensing occurs and if mode switch is disabled, inappropriate ventricular pacing may be triggered up to the upper tracking rate.9
Oversensing in the ventricular channel may result in pacing inhibition with subsequent severe bradyarrhythmias, (near-)syncope, or asystole in PM-dependent patients.70 Additionally, sustained ventricular oversensing in ICD patients may lead to inappropriate shock delivery. Inappropriate shocks are not only painful and can result in psychological distress but they can be potentially proarrhythmic and are associated with adverse overall survival.71 In the case of strong EMF exposure, PMs/ICDs may switch to noise mode with asynchronous pacing (VOO/DOO).72 In noise mode, the subsequent loss of sensing of the intrinsic signal prevents the detection of the underlying intrinsic rhythm faster than the pacing frequency resulting in a risk of T-wave stimuli as well as the perception of ventricular arrhythmias. Thus, anti-tachycardia therapy of ICDs would be withheld with potential lethal consequences.
Precautionary methods
Cardiologists can reduce the risk of EMI for CIED patients by evaluating and programming the sensitivity settings. The lowest possible sensitivity should be selected, which still ensures an appropriate sensing of intrinsic signals. Features like automatic capture measurement or adaptive sensitivity control may lead to inappropriate automatic reprogramming of the sensitivity and should therefore, be switched off in patients with foreseeable strong EMF exposure or documented EMI. In the case of ICDs, defibrillator testing with ventricular fibrillation induction may be necessary to evaluate appropriate sensing of fibrillation waves with sensitivity settings lower than the manufacturer’s recommendations. Prolonged detection intervals and elevation of the VT/VF zones as mentioned in several studies73–75 may prevent inappropriate shocks without impairing the outcome of the patients.
Programming to VVI mode is an additional option to prevent atrial oversensing which usually occurs before the ventricular channel is affected due to the small intrinsic atrial signals and the corresponding high sensitivity setting (poor signal-to-noise ratio). Therefore, to be able to programme a lower sensitivity it is important to achieve a stable anchoring of the lead with good sensing amplitudes during the implantation procedure.
In a systematic investigation on the CIED’s lead location, we found that a medial position and horizontal orientation of a bipolar lead’s distal end as well as a short lead’s tip-to-ring spacing makes CIEDs less susceptible to EMI.76 We, therefore, recommend the implantation of true bipolar leads and programming the sensing configurations appropriately in all patients, if possible. When changing the sensing configuration or in case of pre-existing unipolar leads, physicians, and patients should be aware of a higher likelihood of EMI.
Patients with recent EMI events, should first be advised to maintain a greater distance (usually >30 cm) to the source of EMF, followed by a careful evaluation of the technical integrity of the CIED. In addition, an in-depth analysis of the situation of EMI including field measurements, e.g. at the workplace, should be performed and a history of earlier device disturbances should be obtained. Furthermore, remote monitoring of devices may be of great help for early EMI detection.
Research needs
For future studies, we recommend using standardized exposure set-ups and to conduct different types of studies in order to achieve a comprehensive risk assessment for patients with CIEDs.
Exposure set-up and characterization
Only 11 of the 40 studies included in this review used a standardized exposure set-up, e.g. a Helmholtz coil or an antenna setting, while most of the studies conducted experiments with a single electronic appliance. For a general risk assessment, however, studies with single device exposure have only a limited value, often due to a lack of a proper dosimetry. In contrast, under standardized conditions, EMF of a defined frequency and field strength can be generated and applied. In such a setting, it is possible to determine exactly the exposure parameters for which EMI is likely or unlikely to be induced. When conducting studies with single devices a complete dosimetry should be performed such that the results are applicable to different exposure scenarios, including new technologies. It is important to characterize the field strength and distribution as well as the frequency and modulation (i.e. waveform such as CW vs. pulses) of EMF sources in the vicinity of the patient or implant. This view is supported by McIvor et al.34 and Seidman et al.47 who noted that the field strength, frequency and modulation are the crucial parameters for EMI. From their findings, McIvor et al.34 further concluded that susceptibility to interference was enhanced by the 60 Hz pulsed signal. Additionally, Hikage et al.40 found that EMI were more likely when CIEDs were exposed to pulsed signals with a repetition time close to the physiological heart rhythm.
Different types of studies
Beyond the use of standardized exposure set-ups and the characterization of the exposure parameters, we recommend performing benchmark tests, phantom, and in vivo studies. The combination of different types of studies will help to systematically evaluate the influence of CIED-, lead-, and patient-related factors. However, it has to be noted that each type of study has several advantages and disadvantages.
Benchmark tests are highly suited for investigating the influence of different CIED types and sensitivity settings. The disturbance signals are fed directly into the pace/sense channel of the CIED by galvanic coupling in order to analyse the CIED’s response and detection levels. Several studies included in this review have shown that the likelihood for disturbance depends on the CIED type (i.e. PM or ICD, model41,46,47) and CIED sensitivity settings.32,66 The simple methodology is a major advantage of benchmark tests. However, a disadvantage is that individual parameters of the patient or the lead cannot be considered.
The influence of the lead can best be examined in phantom studies. Additionally, with phantom studies, the intracorporal voltage induced by external EMF in the CIED-lead system can also be determined. The induced voltage is an important measurement parameter for electromagnetic compatibility testing of CIEDs because it can directly be compared with the performance limits set in international product standards, e.g. Ref.53 Several studies included in this review have shown that the likelihood for EMI depends on the lead parameters (bipolar and unipolar42), lead configuration (i.e. loop area formed by implant housing with its lead wire), and implantation site.58,59 In clinical practice, the susceptibility of CIEDs to EMI has been reduced by using bipolar instead of unipolar leads. However, bipolar leads are still susceptible to interference in the presence of strong EMF.76
Individual parameters, including height or physique, which are also affecting the interference threshold,77 can however, neither be considered in phantom studies nor in benchmark tests. Therefore, in vivo studies should be performed in which patients with CIEDs are directly exposed to EMF and in which individual interference thresholds of the CIED can be determined for specific exposure conditions. Data obtained from in vivo studies need no additional validation and can be transferred directly to real-life exposure situations. Therefore, results from benchmark tests and phantom studies should always be validated by in vivo studies. A disadvantage of in vivo studies is, however, that they are time-consuming and that a large number of patients has to be tested to identify patient-related, CIED-related, and lead-specific predictors.
The combination of benchmark tests, phantom, and in vivo studies allows the development of a coupling model (transfer function). Transfer functions demonstrate the relationship between the strength of an external EMF and the induced intracorporal voltage at the terminals of a CIED. A solid transfer function which serves as the basis for the calculation of the induced voltages from various external EMF can be derived by using comprehensive data obtained from the different types of studies. Establishing a transfer function is necessary to define limit values.
It is of great importance to define limit values for patients with CIEDs because the current EMF limit values (e.g. ICNIRP,17 27 µT and 21 A/m for 3 kHz–10 MHz) proposed for the general public may be exceeded by everyday electrical appliances emitting EMF in the IF range. According to Leitgeb et al.,60 EAS systems may exceed the limits by a factor of 13 compared with ICNIRP’s recommendation published in 201017 and even by a factor of 60 with regard to ICNIRP’s recommendation published in 1998.78 Induction hobs may also exceed ICNIRP limit values in close proximity and depending on the position of the pot.25 This does not suggest that the general public (including people with CIEDs) is automatically at risk but caution is warranted for worst-case exposure scenarios.60 Vice versa, compliance with ICNIRP does not suggest that the safety of patients with CIEDs is guaranteed, because ICNIRP does not consider people fitted with electronic implants in their recommendation. Furthermore, the results of single studies in the present review provide evidence that EMI may even be induced below the proposed ICNIRP limit values.33,58 Thus, the establishment of limit values for patients with CIEDs will contribute to estimate which electronic appliances can be considered safe for CIEDs carriers and which distances to various electronic appliances should be respected in order to prevent EMI.
Conclusion
There are several studies investigating EMI of CIEDs by novel electrical appliances emitting EMF in the IF range. However, the current data do not allow a general risk assessment for CIED carriers regarding common or future potential interferers, especially due to the lack of a proper dosimetry in most of the studies or the missing correlation of dosimetric data with EMI. The findings were only consistent for security systems and induction hobs for which EMI in CIEDs could be demonstrated in close proximity to the appliances. The results of the studies evaluated in this systematic review and the results of studies on EMI in other frequency ranges indicate that the likelihood for EMI is dependent on exposure-related parameters (field strength, frequency, modulation) and on implant- as well as on lead-related parameters (model, type of implant, implant sensitivity setting, lead configuration, and implantation site). To better characterize the factors influencing EMI, future studies should consider all these factors systematically by conducting different types of studies. Benchmark test and phantom studies should be performed according to international standards.53–55 Concerning in vivo studies, where no comparable recommendations exist, good experiences70,72 were had in co-operation between cardiologists with profound knowledge in electrophysiology and electrical engineers with profound knowledge in exposure set-ups.
Additionally, worst-case scenarios should be considered in all study types where possible (i.e. unipolar sensing, maximum sensitivity, atrium sensing, sustained pacing of the CIED, left-sided implantation, lateral lead’s tip position, vertical lead’s tip orientation, homogeneous field exposure, and thorax perpendicular to the magnetic field exposure). That way, it might be possible to derivate EMF limit values for CIED patients in the future.
Funding
This work was supported by the European Research Group on Environment and Health in the Transport Sector (EUGT) e.V., Germany.
Conflict of interest: A.N. received travel grants by Biotronik, Boston Scientific, Medtronic, and St. Jude Medical (now Abbott). All other authors have no conflict of interest.
Supplementary Material
References
- 1. Lahor-Soler E, Miranda-Rius J, Brunet-Llobet L, Sabate de la Cruz X.. Capacity of dental equipment to interfere with cardiac implantable electrical devices. Eur J Oral Sci 2015;123:194–201. [DOI] [PubMed] [Google Scholar]
- 2. Mond HG, Proclemer A.. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia's Project. Pacing Clin Electrophysiol 2011;34:1013–27. [DOI] [PubMed] [Google Scholar]
- 3. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 4. Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H. et al. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017;19:ii1–90. [DOI] [PubMed] [Google Scholar]
- 5. Gajsek P, Ravazzani P, Wiart J, Grellier J, Samaras T, Thuroczy G.. Electromagnetic field exposure assessment in Europe radiofrequency fields (10 MHz-6 GHz). J Expo Sci Environ Epidemiol 2015;25:37–44. [DOI] [PubMed] [Google Scholar]
- 6. Urbinello D, Joseph W, Verloock L, Martens L, Röösli M.. Temporal trends of radio-frequency electromagnetic field (RF-EMF) exposure in everyday environments across European cities. Environ Res 2014;134:134–42. [DOI] [PubMed] [Google Scholar]
- 7. Calin MD, Ursachi C, Helerea E. Electromagnetic environment characteristics in an urban area. In IEEE 4th International Symposium on Electrical and Electronics Engineering (ISEEE) October 11-13, 2013, Galaţi, Romania.
- 8. SCENHIR. Potential health effects of exposure to electromagnetic fields (EMF). European Commission 2015. https://doi.org/10.2772/75635.
- 9. Napp A, Stunder D, Maytin M, Kraus T, Marx N, Driessen S.. Are patients with cardiac implants protected against electromagnetic interference in daily life and occupational environment? Eur Heart J 2015;36:1798–804. [DOI] [PubMed] [Google Scholar]
- 10. Beinart R, Nazarian S.. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation 2013;128:2799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misiri J, Kusumoto F, Goldschlager N.. Electromagnetic interference and implanted cardiac devices: the nonmedical environment (part I). Clin Cardiol 2012;35:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Misiri J, Kusumoto F, Goldschlager N.. Electromagnetic interference and implanted cardiac devices: the medical environment (part II). Clin Cardiol 2012;35:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manufacturer and User Facility Device Experience (MAUDE). http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM (31 May 2017, date last accessed).
- 14. Hours M, Khati I, Hamelin J.. Interference between active implanted medical devices and electromagnetic field emitting devices is rare but real: results of an incidence study in a population of physicians in France. Pacing Clin Electrophysiol 2014;37:290–6. [DOI] [PubMed] [Google Scholar]
- 15. IEEE C95.6-2002. IEEE Standard for Safety Levels with Respect to Human Exposure to Electromagnetic Fields, 0-3 kHz New York, USA: The Institute of Electrical and Electronics Engineering; 2002.
- 16. IEEE C95.1-2005. IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz New York, USA: The Institute of Electrical and Electronics Engineering; 2006.
- 17. International Commission on Non-Ionizing Radiation Protection . Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys 2010;99:818–36. [DOI] [PubMed] [Google Scholar]
- 18.1999/519/EG. Council Recommendation of 12 July 1999 on the Limitation of Exposure of the General Public to Electromagnetic Fields (0 Hz to 300 GHz). Brussels, Belgium: Council of the European Union (CONSILIUM); 1999. Official Journal of the European Communities.
- 19.2013/35/EU. Directive 2013/35/EU of the European Parliament and of the Council of 26 June 2013 on the Minimum Health and Safety Requirements Regarding the Exposure of Workers to the Risks Arising from Physical Agents (electromagnetic fields) and Repealing Directive 2004/40/EC. Brussels, Belgium; 2013. Official Journal of the European Union. doi:10.3000/19770677.L_2013.179.eng.
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. http://www.who.int/peh-emf/research/database/en/index1.html (2 June 2017, date last accessed).
- 22.EMF-Portal. www.emf-portal.org (02 June 2017, date last accessed).
- 23. de Cock CC, Spruijt HJ, van Campen LM, Plu AW, Visser CA.. Electromagnetic interference of an implantable loop recorder by commonly encountered electronic devices. Pacing Clin Electrophysiol 2000;23:1516–8. [DOI] [PubMed] [Google Scholar]
- 24. Keshishian JM, Smyth NP, Hood OC, Hoffman AA, Baker NR, Podolak E. et al. The behavior of triggered unipolar pacemakers in active magnetic fields. J Thorac Cardiovasc Surg 1972;64:772–8. [PubMed] [Google Scholar]
- 25. Binggeli C, Rickli H, Ammann P, Brunckhorst C, Hufschmid U, Luechinger R. et al. Induction ovens and electromagnetic interference: what is the risk for patients with implantable cardioverter defibrillators? J Cardiovasc Electrophysiol 2005;16:399–401. [DOI] [PubMed] [Google Scholar]
- 26. Rickli H, Facchini M, Brunner H, Ammann P, Sagmeister M, Klaus G. et al. Induction ovens and electromagnetic interference: what is the risk for patients with implanted pacemakers? Pacing Clin Electrophysiol 2003;26:1494–7. [DOI] [PubMed] [Google Scholar]
- 27. Corbett GD, Lim YC, Lee JC, Chernolesskiy A, Pugh PJ, Cameron EA.. Safety of the colonoscope magnetic imaging device (ScopeGuide) in patients with implantable cardiac devices. Endoscopy 2014;46:135–8. [DOI] [PubMed] [Google Scholar]
- 28. Simon AB, Linde B, Bonnette GH, Schlentz RJ.. The individual with a pacemaker in the dental environment. J Am Dent Assoc 1975;91:1224–9. [DOI] [PubMed] [Google Scholar]
- 29. Joglar JA, Nguyen C, Garst DM, Katz WF.. Safety of electromagnetic articulography in patients with pacemakers and implantable cardioverter-defibrillators. J Speech Lang Hear Res 2009;52:1082–7. [DOI] [PubMed] [Google Scholar]
- 30. Maiorana C, Grossi GB, Garramone RA, Manfredini R, Santoro F.. Do ultrasonic dental scalers interfere with implantable cardioverter defibrillators? An in vivo investigation. J Dent 2013;41:955–9. [DOI] [PubMed] [Google Scholar]
- 31. Tiikkaja M, Aro AL, Alanko T, Lindholm H, Sistonen H, Hartikainen JE. et al. Electromagnetic interference with cardiac pacemakers and implantable cardioverter-defibrillators from low-frequency electromagnetic fields in vivo. Europace 2013;15:388–94. [DOI] [PubMed] [Google Scholar]
- 32. Mugica J, Henry L, Podeur H.. Study of interactions between permanent pacemakers and electronic antitheft surveillance systems. Pacing Clin Electrophysiol 2000;23:333–7. [DOI] [PubMed] [Google Scholar]
- 33. Wilke A, Kruse T, Hesse H, Funck R, Maisch B.. Interactions between pacemakers and security systems. Pacing Clin Electrophysiol 1998;21:1784–8. [DOI] [PubMed] [Google Scholar]
- 34. McIvor ME, Reddinger J, Floden E, Sheppard R, Johnson D, Becker GI. et al. Study of pacemaker and implantable cardioverter defibrillator triggering by electronic article surveillance devices (SPICED TEAS). Pacing Clin Electrophysiol 1998;21:1847–61. [DOI] [PubMed] [Google Scholar]
- 35. Dodinot B, Godenir J-P, Costa AS, Zeller C, Broschart M.. Electronic article surveillance: a possible danger for pacemaker patients. Pacing Clin Electrophysiol 1993;16:46–53. [DOI] [PubMed] [Google Scholar]
- 36. Kolb C, Schmieder S, Lehmann G, Zrenner B, Karch MR, Plewan A. et al. Do airport metal detectors interfere with implantable pacemakers or cardioverter-defibrillators? J Am Coll Cardiol 2003;41:2054–9. [DOI] [PubMed] [Google Scholar]
- 37. Dorenkamp M, Blaschke F, Voigt K, Fleck E, Goetze S, Roser M.. Electromagnetic interference of avalanche transceivers with cardiac pacemakers and implantable cardioverter defibrillators. Pacing Clin Electrophysiol 2013;36:931–8. [DOI] [PubMed] [Google Scholar]
- 38. Mobashsher A, Abbosh A.. Artificial human phantoms: human proxy in testing microwave apparatuses that have electromagnetic interaction with the human body. IEEE Microwave Mag 2015;16:42–62. [Google Scholar]
- 39. Babouri A, Hedjiedj A, Guendouz L, Andretzko JP.. The behavior of dual-chamber pacemakers exposed to a conducted low-frequency disruptive signal. Physiol Meas 2006;27:725–36. [DOI] [PubMed] [Google Scholar]
- 40. Hikage T, Nojima T, Fujimoto H.. Active implantable medical device EMI assessment for wireless power transfer operating in LF and HF bands. Phys Med Biol 2016;61:4522–36. [DOI] [PubMed] [Google Scholar]
- 41. Tiikkaja M, Alanko T, Lindholm H, Hietanen M, Toivonen L, Hartikainen J.. Interference of low frequency magnetic fields with implantable cardioverter-defibrillators. Scand Cardiovasc J 2012;46:308–14. [DOI] [PubMed] [Google Scholar]
- 42. Tiikkaja M, Alanko T, Lindholm H, Hietanen M, Hartikainen J, Toivonen L.. Experimental study on malfunction of pacemakers due to exposure to different external magnetic fields. J Interv Card Electrophysiol 2012;34:19–27. [DOI] [PubMed] [Google Scholar]
- 43. Babouri A, Hedjeidj A, Guendouz L.. Experimental and theoretical investigation of implantable cardiac pacemaker exposed to low frequency magnetic field. J Clin Monit Comput 2009;23:63–73. [DOI] [PubMed] [Google Scholar]
- 44. Buzduga V, Witters DM, Casamento JP, Kainz W.. Testing the immunity of active implantable medical devices to CW magnetic fields up to 1 MHz by an immersion method. IEEE Trans Biomed Eng 2007;54:1679–86. [DOI] [PubMed] [Google Scholar]
- 45. Mattei E, Lucano E, Censi F, Triventi M, Calcagnini G.. Provocative testing for the assessment of the electromagnetic interference of RFID and NFC readers on implantable pacemaker. IEEE Trans Electromagn Compat 2016;58:314–22. [Google Scholar]
- 46. Seidman SJ, Ruggera PS, Brockman RG, Lewis B, Shein MJ.. Electromagnetic compatibility of pacemakers and implantable cardiac defibrillators exposed to RFID readers. Int J Radio Freq Ident Tech Appl 2007;1:237–46. [Google Scholar]
- 47. Seidman SJ, Brockman R, Lewis BM, Guag J, Shein MJ, Clement WJ. et al. In vitro tests reveal sample radiofrequency identification readers inducing clinically significant electromagnetic interference to implantable pacemakers and implantable cardioverter-defibrillators. Heart Rhythm 2010;7:99–107. [DOI] [PubMed] [Google Scholar]
- 48. Kainz W, Casamento JP, Ruggera PS, Chan DD, Witters DM.. Implantable cardiac pacemaker electromagnetic compatibility testing in a novel security system simulator. IEEE Trans Biomed Eng 2005;52:520–30. [DOI] [PubMed] [Google Scholar]
- 49. Hirose M, Hida M, Sato E, Kokubo K, Nie M, Kobayashi H.. Electromagnetic interference of implantable unipolar cardiac pacemakers by an induction oven. Pacing Clin Electrophysiol 2005;28:540–8. [DOI] [PubMed] [Google Scholar]
- 50. Salmi J, Eskola HJ, Pitkanen MA, Malmivuo JA.. The influence of electromagnetic interference and ionizing radiation on cardiac pacemakers. Strahlenther Onkol 1985;3:81–156. [PubMed] [Google Scholar]
- 51. Magnani A, Matheoud R, Brambilla M, Valzano S, Occhetta E, Marino P. et al. In vitro tests of electromagnetic interference of electromagnetic navigational bronchoscopy to implantable cardioverter defibrillators. Europace 2012;14:1054–9. [DOI] [PubMed] [Google Scholar]
- 52. Fukuta M, Mizutani N, Waseda K.. Influence of electromagnetic interference on implanted cardiac arrhythmia devices in and around a magnetically levitated linear motor car. J Artif Organs 2005;8:154–60. [DOI] [PubMed] [Google Scholar]
- 53. ISO 14117:2012. Active Implantable Medical Devices—Electromagnetic Compatibility—EMC Test Protocols for Implantable Cardiac Pacemakers, Implantable Cardioverter Defibrillators and Cardiac Resynchronization Devices Geneva, Switzerland: International Organization for Standardization (ISO); 2012. ISO Standards Catalogue.
- 54. EN 45502-2-2:2008. Active Implantable Medical Devices—Part 2-2: Particular Requirements for Active Implantable Medical Devices Intended to Treat Tachyarrhythmia (Includes Implantable Defibrillators) Brussels, Belgium: European Committee for Electrotechnical Standardization (CENELEC), Central Secretariat; 2008.
- 55. EN 45502-2-1:2004. Active Implantable Medical Devices—Part 2-1: Particular Requirements for Active Implantable Medical Devices Intended to Treat Bradyarrhythmia (Cardiac Pacemakers) Brussels, Belgium: European Committee for Electrotechnical Standardization (CENELEC), Central Secretariat; 2004.
- 56. Bassen H. Low frequency magnetic emissions and resulting induced voltages in a pacemaker by iPod portable music players. Biomed Eng Online 2008;7:7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Irnich W, Bernstein AD.. Do induction cooktops interfere with cardiac pacemakers? Europace 2006;8:377–84. [DOI] [PubMed] [Google Scholar]
- 58. Seckler T, Jagielski K, Stunder D.. Assessment of electromagnetic interference with active cardiovascular implantable electronic devices (CIEDs) caused by the Qi A13 design wireless charging board. Int J Environ Res Public Health 2015;12:5886–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mattei E, Censi F, Delogu A, Ferrara A, Calcagnini G.. Setups for in vitro assessment of RFID interference on pacemakers. Phys Med Biol 2013;58:5301–16. [DOI] [PubMed] [Google Scholar]
- 60. Leitgeb N, Niedermayr F, Loos G.. Impact of EAS systems on implanted cardiac pacemakers and defibrillators. J Electromagn Anal 2013;05:67–73. [Google Scholar]
- 61. Gustrau F, Bahr A, Goltz S, Eggert S.. Active medical implants and occupational safety—measurement and numerical calculation of interference voltage. Biomed Tech (Berl) 2002;47:656–9. [DOI] [PubMed] [Google Scholar]
- 62. Hedjiedj A, Goeury C, Nadi M.. A methodological approach for the characterization of cardiac pacemaker immunity to low frequency interferences: case of 50 Hz, 60 Hz, 10 kHz and 25 kHz led disruptions. J Med Eng Technol 2002;26:223–7. [DOI] [PubMed] [Google Scholar]
- 63. Andretzko JP, Hedjiedj A, Guendouz L.. Calculation of the induced voltage at the terminals of cardiac pacemakers submitted to conducted disturbances. Physiol Meas 2007;28:363–72. [DOI] [PubMed] [Google Scholar]
- 64. Andretzko JP, Hedjiedj A, Guendouz L.. A model for determining the induced voltage at the terminals of a pacemaker exposed to a low frequency magnetic field. Physiol Meas 2008;29:1121–32. [DOI] [PubMed] [Google Scholar]
- 65. van Wijk van Brievingh RP, Hoeskstra A, de Bakker JM, Hemelaar A.. Measurement techniques for assessing the influence of electromagnetic fields on implanted pacemakers. Med Biol Eng 1974;12:42–9. [DOI] [PubMed] [Google Scholar]
- 66. Lucas EH, Johnson D, McElroy BP.. The effects of electronic article surveillance systems on permanent cardiac pacemakers: an in vitro study. Pacing Clin Electrophysiol 1994;17:2021–6. [DOI] [PubMed] [Google Scholar]
- 67. von Olshausen G, Rondak IC, Lennerz C, Semmler V, Grebmer C, Reents T. et al. Electromagnetic interference in implantable cardioverter defibrillators: present but rare. Clin Res Cardiol 2016;105:657–65. [DOI] [PubMed] [Google Scholar]
- 68. Kolb C, Zrenner B, Schmitt C.. Incidence of electromagnetic interference in implantable cardioverter defibrillators. Pacing Clin Electrophysiol 2001;24:465–8. [DOI] [PubMed] [Google Scholar]
- 69. Ausubel K, Boal BH, Furman S.. Pacemaker syndrome: definition and evaluation. Cardiol Clin 1985;3:587–94. [PubMed] [Google Scholar]
- 70. Stunder D, Seckler T, Joosten S, Zink MD, Driessen S, Kraus T. et al. In vivo study of electromagnetic interference with pacemakers caused by everyday electric and magnetic fields. Circulation 2017;135:907–9. [DOI] [PubMed] [Google Scholar]
- 71. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P. et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol 2008;51:1357–65. [DOI] [PubMed] [Google Scholar]
- 72. Napp A, Joosten S, Stunder D, Knackstedt C, Zink M, Bellmann B. et al. Electromagnetic interference with implantable cardioverter-defibrillators at power frequency: an in vivo study. Circulation 2014;129:441–50. [DOI] [PubMed] [Google Scholar]
- 73. Sedláček K, Ruwald A-C, Kutyifa V, Mcnitt S, Thomsen PB, Klein H. et al. The effect of ICD programming on inappropriate and appropriate ICD Therapies in ischemic and nonischemic cardiomyopathy: the MADIT-RIT trial. J Cardiovasc Electrophysiol 2015;26:424–33. [DOI] [PubMed] [Google Scholar]
- 74. Saeed M, Hanna I, Robotis D, Styperek R, Polosajian L, Khan A. et al. Programming implantable cardioverter-defibrillators in patients with primary prevention indication to prolong time to first shock: results from the PROVIDE study. J Cardiovasc Electrophysiol 2014;25:52–9. [DOI] [PubMed] [Google Scholar]
- 75. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB. et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903–11. [DOI] [PubMed] [Google Scholar]
- 76. Seckler T, Stunder D, Schikowsky C, Joosten S, Zink MD, Kraus T. et al. Effect of lead position and orientation on electromagnetic interference in patients with bipolar cardiovascular implantable electronic devices. Europace 2017;19:319–28. [DOI] [PubMed] [Google Scholar]
- 77. Joosten S, Pammler K, Silny J.. The influence of anatomical and physiological parameters on the interference voltage at the input of unipolar cardiac pacemakers in low frequency electric fields. Phys Med Biol 2009;54:591–609. [DOI] [PubMed] [Google Scholar]
- 78. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys 1998;74:494–522. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.