There is a need to develop effective antibacterial therapies for mitigating bacterial pathogens in food systems. We used a 3D checkerboard assay to ascertain a safe synergistic combination of food-grade components: vitamin C, copper, and the essential oil linalool. Individually, these constituents have to be added in large amounts to exert their antibacterial effect, which leads to unwanted organoleptic properties. The triple combination could exceptionally inhibit foodborne Gram-negative pathogens like Vibrio fluvialis and Salmonella enterica subsp. enterica serovar Typhi at low concentrations (linalool, 1.298 mM; vitamin C, 8 mM; copper, 16.3 μM) and displayed potent microbial inhibition in acidic beverages. We found increased susceptibility in deletion mutants of oxidative stress regulators (oxyR, katG, ahpC, and sodA mutants) due to ROS generation by Fenton’s chemistry. The results of this study show that it may be possible to use plant-based antimicrobials in synergistic combinations to control microbial contaminants.
KEYWORDS: essential oil, ROS, Salmonella enterica subsp. enterica serovar Typhi, Vibrio fluvialis, antimicrobial agents, copper, foodborne pathogens, oxidative stress, vitamin C
ABSTRACT
Inappropriate and disproportionate use of antibiotics is contributing immensely to the development of antibiotic resistance in bacterial species associated with food contamination. The use of natural products in combination can be a potent alternative hurdle strategy to inactivate foodborne pathogens. Here, we explored the pro-oxidant properties of essential oil linalool and vitamin C in combination with copper (LVC) in combating the foodborne pathogens Vibrio fluvialis and Salmonella enterica subsp. enterica serovar Typhi using a three-dimensional (3D) checkerboard microdilution assay. Antibacterial activity in terms of the MIC revealed that the triple combination exerted a synergistic effect compared to the effects of the individual constituents. The bactericidal effect of the triple combination was confirmed by a live/dead staining assay. Reactive oxygen species (ROS) measurements with the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay and scanning electron microscopy imaging strongly suggested that the increase in ROS production is the underlying mechanism of the enhanced antibacterial potency of the LVC combination (linalool [1.298 mM], vitamin C [8 mM], copper [16.3 μM]). In addition, the hypersensitivity of oxidative stress regulator mutants (oxyR, katG, ahpC, and sodA mutants) toward LVC corroborated the involvement of ROS in cell death. Live/dead staining and changes in cellular morphology revealed that oxidative stress did not transform the cells into the viable but nonculturable (VBNC) state; rather, killing was associated with intracellular and extracellular oxidative burst. Furthermore, the LVC combination did not display toxicity to human cells, while it effectively reduced the pathogen levels in acidic fruit juices by 3 to 4 log CFU/ml without adversely altering the organoleptic properties. This study opens a new outlook for combinatorial antimicrobial therapy.
IMPORTANCE There is a need to develop effective antibacterial therapies for mitigating bacterial pathogens in food systems. We used a 3D checkerboard assay to ascertain a safe synergistic combination of food-grade components: vitamin C, copper, and the essential oil linalool. Individually, these constituents have to be added in large amounts to exert their antibacterial effect, which leads to unwanted organoleptic properties. The triple combination could exceptionally inhibit foodborne Gram-negative pathogens like Vibrio fluvialis and Salmonella enterica subsp. enterica serovar Typhi at low concentrations (linalool, 1.298 mM; vitamin C, 8 mM; copper, 16.3 μM) and displayed potent microbial inhibition in acidic beverages. We found increased susceptibility in deletion mutants of oxidative stress regulators (oxyR, katG, ahpC, and sodA mutants) due to ROS generation by Fenton’s chemistry. The results of this study show that it may be possible to use plant-based antimicrobials in synergistic combinations to control microbial contaminants.
INTRODUCTION
Infections caused by foodborne bacterial pathogens pose a serious public health problem worldwide and result in approximately 1.8 million deaths annually (1). Gastrointestinal diseases associated with Gram-negative enteric pathogens are increasing at an alarming level each year, costing billions of dollars in medical care and lost productivity (2). Thus, the global burden of foodborne diseases calls for multipronged strategies for controlling such infections (2, 3). The food industry, in association with the pharmaceutical industry, is constantly engaged in developing new methods for combating pathogens. Certain Gram-negative bacterial strains, such as those within such genera as Vibrio, Salmonella, and Escherichia, are major bacterial pathogens implicated in foodborne illness (4, 5). The emergence of antibiotic resistance among bacterial pathogens commonly found in foods that will be immediately consumed has prompted researchers to discover alternatives to antibiotics which are safe for human usage and, thus, can be applicable to food (6). One such strategy could be the use of a hurdle technology incorporating antimicrobial plant metabolites and food-grade chemicals at concentrations that, collectively, synergistically mitigate microbial contamination (7). Essential oils (EOs) are well-known for their antibacterial activity (8); however, their weak antibacterial activity leads to the incorporation of higher doses in order to achieve an antibacterial effect, which renders their usage limited to specific conditions only (9).
Vitamin C is an essential micronutrient in the human diet which is used as a cofactor by several biosynthetic enzymes (10, 11). Additionally, vitamin C is an important dietary antioxidant which significantly decreases the adverse effect of reactive oxygen species (ROS) and reactive nitrogen species. These reactive species cause oxidative damage to macromolecules, such as lipids, DNA, and proteins (12, 13). Recently, the role of vitamin C as a pro-oxidant antibacterial molecule against virulent strains of Mycobacterium tuberculosis was reported (14). This surprising revelation that vitamin C (a known antioxidant) acts as a pro-oxidant motivated several researchers to investigate the suitability of vitamin C as an anticancer and antibacterial molecule (12–16). Since vitamin C is naturally present in several foods, we reasoned that a combination of EOs with vitamin C and other safe ingredients, like metals, can be used to control foodborne pathogens.
The essential role of copper in maintaining good health in animals and humans is well established. As an essential trace element, copper plays a vital role in critical enzyme systems (15). Copper is readily available through food, but in several cases of acquired copper deficiency, copper needs to be supplemented (17, 18).
The antimicrobial properties of EOs are well-known and continue to be the subject of several studies that evaluate their antimicrobial potential as alternatives to chemical agents in the food industry (16, 19–21). Constituents of EOs belong to a diverse family of low-molecular-weight organic compounds which have a wide antimicrobial spectrum (16, 22, 23). EOs, like carvacrol, eugenol, thymol, linalool, and cinnamaldehyde, have been studied for their antimicrobial applications against foodborne pathogens and food spoilage by bacteria (24, 25). Linalool is a generally recognized as safe (GRAS) terpene alcohol which is used as a fragrance in cosmetics and as a flavoring agent in the food industry (4, 26). Linalool is produced by more than 200 plants, like holy basil (Ocimum sanctum), coriander (Coriandrum sativum), palmarosa (Cymbopogon martinii), sweet orange (Citrus sinensis), and lavender (Lavandula officinalis) (26), and has also been documented to have holistic effects on human and animal health in Ayurveda (4, 27).
In this study, the pro-oxidant properties of vitamin C in combination with EOs and copper were explored in an effort to mitigate Gram-negative pathogens. Initially, we tested the antibacterial activity of a combination of copper, vitamin C, and EOs (carvacrol, eugenol, cinnamaldehyde, and linalool) against these pathogens. Interestingly, it was observed that linalool showed significantly enhanced antibacterial activity against both Salmonella enterica subsp. enterica serovar Typhi and Vibrio fluvialis when used in combination with vitamin C and copper. We expanded our conceptual understanding of the killing mechanism of the triple combination and investigated the cellular mechanism of inhibition using a set of biochemical and genetic studies. The effectiveness of a triple combination of EOs, an essential micro-trace element in the form of copper, and vitamin C against foodborne pathogens was also tested in acidic fruit beverages to validate its potential to control three Gram-negative foodborne pathogens, Salmonella enterica subsp. enterica serovar Typhi, V. fluvialis, and Escherichia coli.
RESULTS AND DISCUSSION
The synergistic combination of linalool with vitamin C and Cu(II) inhibits Gram-negative enteric pathogens.
EOs contain a wide variety of secondary metabolites that are capable of inhibiting diverse groups of bacteria with varied modes of action (28–30). Carvacrol, eugenol, cinnamaldehyde, and linalool work with different potencies against bacterial pathogens (16). The antibacterial potencies of EOs (carvacrol, eugenol, cinnamaldehyde, and linalool) against a set of Gram-negative bacterial pathogens were ascertained. All the EOs showed MICs above 500 μg/ml against Escherichia coli, Salmonella enterica subsp. enterica serovar Typhi, Vibrio fluvialis, and Campylobacter jejuni. The primary concern in using only EOs as food preservatives is that the concentration of EOs required for effective antimicrobial activity leads to negative organoleptic effects and alters the natural properties of foods, making them unacceptable to the consumers (31). Thus, a combinatorial approach with acceptable dietary ingredients, vitamin C and copper, to enhance the antibacterial activity of EOs was tested. To ascertain a safe concentration of the triple combination (EOs, vitamin C, and copper), a three-dimensional (3D) checkerboard microdilution assay was performed with EOs (carvacrol, eugenol, cinnamaldehyde, and linalool) in combination with vitamin C and Cu(II). The MICs of vitamin C, linalool, and Cu(II), individually, for pathogens (V. fluvialis, Salmonella enterica subsp. enterica serovar Typhi, and E. coli) were 32 mM, 7.5 mM, and 212 μM, respectively. Carvacrol, eugenol, cinnamaldehyde, and linalool showed fractional inhibitory concentration indexes (FICI) of 0.614, 0.714, 0.781, and 0.48, respectively, against the pathogens in combination with Cu(II) and vitamin C. Carvacrol, eugenol, and cinnamaldehyde were not pursued further, as these did not show synergistic inhibitory activity against the pathogens. Cu(II),, vitamin C, and linalool showed synergistic activity at 16.3 μM, 8 mM, and 1.298 mM, respectively, against V. fluvialis, Salmonella enterica subsp. enterica serovar Typhi, E. coli, and C. jejuni (Fig. 1A), and these concentrations were considered to be 1× MIC of the combination linalool, vitamin C, and copper (LVC). The minimal bactericidal concentration (MBC) against the pathogens was determined to be 2× MIC of LVC. The triple combination at synergistic concentrations (1× MIC) showed no toxicity to human embryonic kidney (HEK293) cells (Fig. 1B) and thus was carried forward for further experimentation. The antibacterial activity of the combination LVC against a panel of probiotic strains, Lactococcus lactis NZ9000, Lactobacillus brevis, Lactobacillus casei, and Lactobacillus plantarum, did not show any inhibition (see Fig. S1 in the supplemental material). Since the combination is ineffective against lactic acid bacteria, the beneficial probiotic gut bacteria belonging to this category are unlikely to be affected significantly upon the usage of LVC in food. The resistance of lactic acid bacteria to LVC can be attributed to differences in cell wall structure, ROS-protective chemicals and enzymes, efflux pumps, ion-chelating agents, and differences in metabolism (32).
FIG 1.
(A) Assessment of antimicrobial efficacy of LVC. 3D checkerboard synergy showing isoboles of MICs of and in vitro growth in double and triple combinations of linalool (Lin), vitamin C (Vit C), and copper [Cu(II)]. Colored lines within each panel indicate the MICs of the double combinations [vitamin C and linalool, linalool and Cu(II), and vitamin C and Cu(II)]. Black points indicate the MICs of the triple combination of linalool, vitamin C, and copper (LVC). The black circle indicates the synergistic concentration of all three compounds in the combination (1× MIC of LVC). The fractional inhibitory concentration (FIC) of the triple compound combination was 0.48. (B) Cytotoxic effect of LVC on cells of the HEK293 cell line. The percent viability of cells of the HEK293 cell line in the presence of different concentrations of LVC was evaluated with respect to the viability of control cells by the MTT assay. Cell viability in the presence of different concentrations (0.5× to 10× MIC) of LVC showed a dose-responsive decrease. Sixty percent of HEK293 cells were viable in the presence of 5× MIC of LVC, which corresponds to 6.49 mM linalool, 40 mM vitamin C, and 81.5 μM Cu(II). The experiment was done in triplicate. Error bars represents the standard deviation of the mean. A statistically significant difference from the results for untreated cells was tested using a one-way ANOVA (***, P < 0.001). (C to F) Analysis of killing potential of LVC using live/dead staining by flow cytometry of untreated V. fluvialis cells (C), V. fluvialis cells treated with 1× MIC of LVC (D), untreated Salmonella enterica subsp. enterica serovar Typhi cells (E), and Salmonella enterica subsp. enterica serovar Typhi cells treated with 1× MIC of LVC (F). The lower left (LL) region shows unstained cells, the upper left (UL) region represents live cells, and dead cells are seen in the upper right (UR) region. LR, lower right. Fifty-two percent of V. fluvialis cells and 45% of Salmonella enterica subsp. enterica serovar Typhi cells were killed after 3 h of treatment with 1× MIC of LVC. (G) In vitro time-kill curves of V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi treated with the synergistic concentration of LVC (1× MIC). A 2.5-log reduction of the number of cells was observed in the cases of both V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi cells when they were treated with 1× MIC of LVC. The inhibition of V. fluvialis was comparatively slower than that of Salmonella enterica subsp. enterica serovar Typhi. The experiment was done in triplicate. Error bars represent the standard deviation of the mean.
The antibacterial activities of LVC (triple combination), the double combinations, and the individual components against the bacterial pathogens were tested with a primary inoculum of 105 CFU/ml. It was observed that LVC was able to produce a significant 5-log reduction of Salmonella enterica subsp. enterica serovar Typhi counts, whereas there was a 4.5-log reduction of V. fluvialis counts (Fig. S2). However, other sets of combinations [linalool and Cu(II), Cu(II) and vitamin C, linalool and vitamin C] did not show significant antibacterial activity. To evaluate the extent to which bacterial cells were killed after treatment with the triple combination, live/dead staining of the target bacteria (108 CFU/ml) was carried out after 2 h of incubation with LVC (1× MIC). The stained bacterial cells were analyzed by flow cytometry. After treatment, 52% (≤1 log) of the V. fluvialis cells and 45% (≤1 log) of the Salmonella enterica subsp. enterica serovar Typhi cells were gated in the upper right region, which signified dead (propidium iodide [PI]-stained) cells (Fig. 1C to F). The rapid and effective reduction of viable cells observed using real-time analysis confirms the bactericidal activity of LVC. The constituents separately and in combinations of two could not induce any damage, which corroborates the synergistic activity of the triple-compound combination.
To check the potency of LVC, kill kinetic assays against Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis were carried out. The growth kinetics of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis were studied at a concentration of 1× MIC of LVC. The kill kinetic profiles (Fig. 1G) showed the significant (P < 0.001) bactericidal activity of LVC toward both pathogens. Salmonella enterica subsp. enterica serovar Typhi a showed ≥1-log reduction in the viable cell count relative to the initial inoculum after 1 h of exposure and a ≥2-log reduction after 2 h of exposure. In the case of V. fluvialis, the reduction of the viable cell count was ≥1 log after 2 h of exposure and ≥2 log after 3 h of exposure in comparison to the count for the control. The rate of growth inhibition of V. fluvialis was slightly lower than that of Salmonella enterica subsp. enterica serovar Typhi upon LVC treatment, which could be due to their differences in their metabolic activity and cellular interaction with LVC.
The effect of LVC treatment on bacterial morphology was studied by scanning electron microscopy (SEM), which revealed severe damage to the cell membrane at 1× MIC (Fig. 2A and D; Fig. S3A and B). Exposure of V. fluvialis cells to 0.25× and 0.5× MIC of LVC resulted in elongation and filamentation (Fig. 2B and C). Bacterial filamentation can be attributed to the stress response due to inhibition of cell division, DNA damage, and the SOS response (33). Stress induces the viable but nonculturable (VBNC) state in various bacteria, which is characterized by very low metabolic activity and the arrest of cell division, yet these cells stay alive, maintain cellular integrity, and have the ability to become culturable once resuscitated (34). Flow cytometry coupled with propidium iodide-SYTO9 staining and scanning electron microscopy were employed for the detection of cell viability (35). The collapse of cellular integrity (Fig. 2D), DNA damage (see Fig. 5B), and a 1.5- to 2-log reduction of cells upon 2 h of LVC exposure (Fig. 1C to F) ruled out the possibility that the cells transformed into the VBNC state, and this was subsequently confirmed by the bactericidal nature of the compounds.
FIG 2.
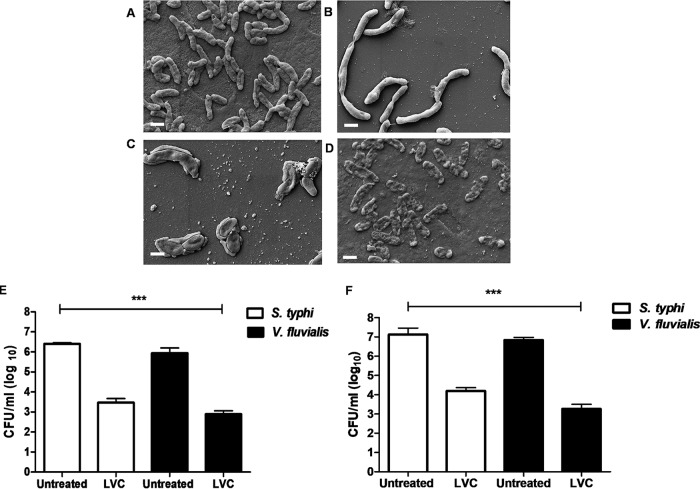
(A to D) Bacterial morphology observed by SEM. Untreated V. fluvialis cells (A), V. fluvialis cells treated with 0.25× MIC of LVC (B), V. fluvialis cells treated with 0.5× MIC of LVC (C), and V. fluvialis cells treated with MIC of LVC (D) showed severe membrane damage. Both 0.5× MIC and 0.25× MIC showed alterations in cellular morphology and a weakened cell envelope. Bars, 1 μm. (E and F) Antibacterial activity of LVC in citrus juice (E) and in pomegranate juice (F). After 12 h of incubation, LVC could effectively reduce 3 to 3.5 log units of cells of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis in comparison to the amount in the untreated beverages, whereas linalool alone and the combination of linalool and copper had no effect on the amounts of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis. The experiment was done in triplicate. Error bars represent the standard deviation of the mean. Statistically significant differences from the results for untreated cells were tested using a one-way and a two-way ANOVA (***, P < 0.001).
FIG 5.

DNA damage study of V. fluvialis. (A) Assessment of genotoxicity of LVC using the sulAp::GFP biosensor. The graph shows the induction of the sulA promoter upon exposure to LVC. The experiment was done in triplicate. Error bars represent the standard deviation of the mean. A statistically significant difference from the results for untreated cells was tested using a one-way ANOVA (***, P < 0.001). (B) Histogram of TUNEL assay results showing DNA fragmentation in untreated V. fluvialis cells (black), V. fluvialis cells treated with LVC (red), and V. fluvialis cells treated with thiourea as an ROS quencher (blue). A reduction of the shift in thiourea-treated cells in comparison to cells treated only with LVC explains the inhibition of DNA fragmentation induced by oxidative stress by LVC upon quenching of ROS. (C) In vitro DNA-nicking assay. Lane 1, pBR322 only; lane 2, pBR322 and H2O2; lane 3, pBR322, H2O2, and 0.5× LVC incubated for 1 min; lane 4, pBR322, H2O2, and 0.5× LVC incubated for 3 min; lane 5, pBR322, H2O2, and 0.5× LVC incubated for 8 min; lane 6, pBR322, H2O2, and 0.5× LVC incubated for 15 min; lane 7, pBR322 and 0.5× LVC incubated for 15 min; lane 8, pBR322, H2O2, 0.5× LVC, and 100 mM thiourea; lane 9, linear pBR322; lane 10, 1-kb ladder. This figure shows that LVC could effectively nick supercoiled DNA, whereas thiourea could inhibit the oxidative effect of LVC, keeping the supercoiled structure of the DNA intact. SD, supercoiled DNA; RD, relaxed DNA; LD, linear DNA.
The LVC combination shows biocompatibility with mammalian cells and red blood cells (RBCs).
Assessment of the toxicity of LVC to mammalian cells and the compatibility of LVC with mammalian cells was carried out using a standard 3-(4,5-dimethyl-2- thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)-based cell viability assay. More than 80% of HEK293 cells were viable at 3-fold the MIC of LVC. The cell viability dropped to 50% at 10-fold the MIC of LVC (Fig. 1B). The LVC triple combination exhibited low hemolysis at up to 5-fold the MIC (65% survival) (Fig. S4). The increased toxicity of LVC beyond the MICs could be due to the oxidation of hemoglobin (Fe2+ to Fe3+) via Fenton’s reaction, lipid peroxidation, the formation of methemoglobin, or osmotic pressure (36). The combination can be considered safe for the mammalian system since the toxicity was observed well beyond the MIC values.
LVC treatment effectively inhibits pathogens in citrus and pomegranate beverages.
Many outbreaks of food poisoning have been linked to the spoilage of fruit beverages by enteric bacteria. Even though fruit juices contain natural constituents which provide protection against microbes to some extent, juices significantly account for foodborne illnesses (37). We evaluated the ability of the triple combination to control food pathogens in unfiltered fruit beverages. The LVC combination reduced the number of viable cells of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis by 3 and 3.5 logs, respectively, in both citrus (Fig. 2E) and pomegranate (Fig. 2F) juices. The other sets of double combinations did not affect the growth of the bacterial cells. Citrus and pomegranate juices alone showed some inhibition of the spiked Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis cells. The influence of LVC on the sensory attributes (color, sweetness-acidity, flavor, taste, and acceptability) of the fruit juices was evaluated using a 9-point hedonic scale. No significant change (P ≥ 0.05) between untreated juices and LVC-treated juices was observed by the panelists (Fig. S5). However, the panelists perceived a minor change in the flavor of the treated juices. The usage of very low concentrations of the compounds (MICs) can be the reason for the insignificant change in the juices’ attributes. The high overall acceptance rate by the panelists confirms that LVC addition to the acidic juices does not significantly alter the organoleptic characteristics.
Synthetic lethality screen reveals that LVC is a potent activator of genes implicated in oxidative stress regulation.
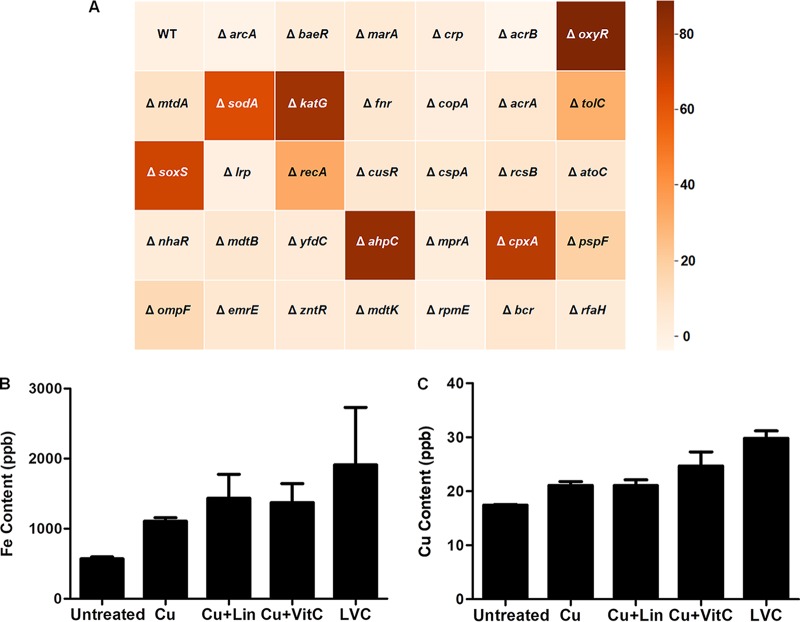
Since vitamin C and divalent metals are known to generate free radicals through Fenton’s chemistry, we reasoned that ROS could play a significant role in inhibiting the bacterial cells by causing oxidative damage to nucleic acids, peroxidation of lipids, and ionization of carbohydrates and proteins (38). To understand the genetic basis of bacterial killing by the triple combination, a battery of E. coli knockout strains of oxygen stress regulators, efflux pumps, and DNA repair proteins (Table S1) was exposed to 0.5× MIC of the triple combination, and growth inhibition of E. coli was observed (Fig. 3A). Interestingly, among the single knockout strains of E. coli, the ΔoxyR, ΔahpC, and ΔkatG strains exhibited a high sensitivity and displayed 80% to 90% growth inhibition in the presence of LVC. Growth inhibition to a lesser extent (50% to 70%) was also observed in the ΔsodA, ΔsoxS, and ΔcpxP mutants, whereas the ΔtolC and ΔrecA E. coli strains were inhibited by up to 50%.
FIG 3.
Synthetic lethality screening and determination of probable mode of action. (A) Heat map showing the sensitivity of E. coli knockout strains to 0.5× MIC of LVC. The percent growth inhibition of strains with knockouts of transcription regulators, DNA repair regulators, and efflux pumps treated with 0.5× MIC of LVC was ascertained. Oxidative stress regulators showed the highest response to LVC, followed by DNA repair regulators and efflux pumps. Inhibition of growth in ΔoxyR, ΔkatG, ΔsodA, and ΔsoxS E. coli cells indicates triggering of oxidative damage by LVC. WT, wild type. (B and C) The cellular iron (B) and copper (C) content in the cells was analyzed by ICP-MS. Cells were treated with copper alone and in different combinations with linalool and vitamin C to see the effect of ROS on the cellular metal content. Four- and 2-fold increases in the intercellular iron and copper content, respectively, account for the steady influx of these metals due to ROS generation. The experiment was done in triplicate. Error bar represents the standard deviation of the mean.
OxyR and SoxR regulators respond primarily to H2O2 and superoxide, respectively (39, 40). H2O2 inactivates Fe-S clusters and activates Fenton’s reaction. Due to the loss of iron and copper from enzyme clusters, enzymes lose their activity, leading to cellular damage. Enzymes like KatG, AhpC, and KatE scavenge H2O2 and balance the action of ROS inside the cell (41, 42); hence, they could account for the lethality seen in case of the ΔoxyR, ΔkatG, and ΔahpC strains of E. coli on LVC treatment.
The SoxS regulon system protects Gram-negative bacterial cells against superoxide radicals, which eventually leads to hydroxyl radical formation (43, 44). The synthetic lethality against LVC treatment observed in the case of the ΔsodA and ΔsoxS mutants was not as severe as that observed with the knockout strains of the oxyR regulon. The SoxS-SoxR system is also known to activate the ΔtolC-acrAB efflux system, which pumps out redox cycling compounds during oxidative stress (45). The ΔtolC strain showed 20% to 30% growth inhibition when exposed to LVC, which could have been due to the accumulation of redox cycling compounds in the cell in the absence of the efflux pump.
We reasoned that LVC treatment might lead to iron uptake inside the bacterial cells and, hence, used inductively coupled plasma mass spectrometry (ICP-MS) to measure the cellular influx of the two metals (iron and copper). During peroxide stress, iron import into the cell has been reported to increase due to overexpression of Fur proteins (46, 47). The iron and copper levels in the cells increased by 4- and 2-fold, respectively, upon LVC treatment but not upon treatment with the individual components (Fig. 3B and C). The increased concentration of iron and copper inside the cell could be a probable reason for the enhanced cell death. The CpxRA two-component system regulon responds to cell envelope damage (48, 49). CpxA activates the genes responsible for oxidative stress, which can account for the high level of synthetic lethality (80% inhibition) observed in the ΔcpxA E. coli strain on LVC treatment. This was further corroborated by membrane permeability studies, which confirmed envelop stress upon LVC treatment. The LVC combination was effective in permeabilizing both the outer and the inner membranes of E. coli ML-35p (Fig. S6A and B), which was monitored by the use of nitrocefin (for outer membrane permeability) and o-nitrophenyl-β-d-galactopyranoside (ONPG; for inner membrane permeability) as probes. Other combinations did not show significant permeabilization.
LVC treatment leads to hydroxyl radical formation.
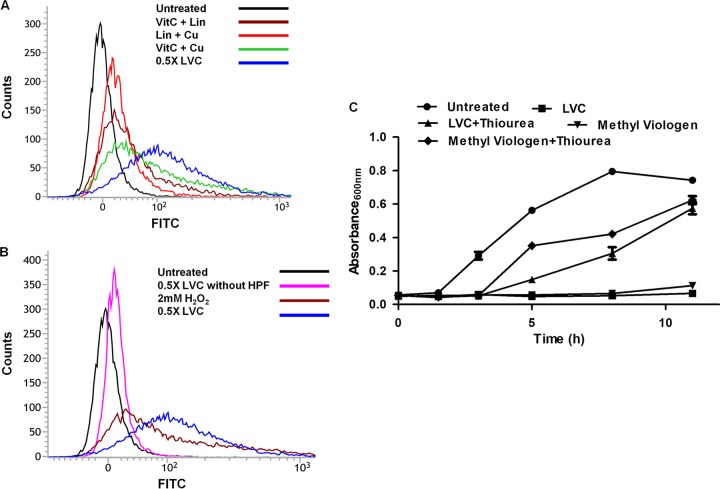
Growth inhibition in the ΔoxyR, ΔsoxS, ΔkatG, and ΔsodA strains by the LVC triple combination prompted us to investigate the status of hydroxyl ion (·OH) production in vivo. ·OH production in vivo was studied by measuring fluorescence using the hydroxyphenyl fluorescein (HPF) dye (50). Forty-four percent of V. fluvialis cells showed an increase in ROS levels after exposure to 0.5× MIC of LVC. A minor increase in ROS levels was observed when the cells were treated with the combination of vitamin C (8 mM) and Cu(II) (16.3 μM) (Fig. 4A; Fig. S7 and S8). Only a small percentage of cells exhibited autofluorescence without dye upon LVC treatment (Fig. 4B). A minor change in cellular morphology may account for the low autofluorescence of the cells (51).
FIG 4.
(A) Determination of ·OH radical generation via detection of HPF by flow cytometry. Hydroxyl radical generation in V. fluvialis cells treated with LVC alone and with different combinations of linalool, vitamin C, and copper was plotted. V. fluvialis cells showed a significant increase in ·OH levels after exposure to 0.5× MIC of LVC. Only minor shifts were observed when the cells were treated with the double combination of vitamin C (8 mM) and Cu(II) (16.3 μM). The shifts in the peaks showed significant ROS generation by LVC. (B) Only a small percentage of cells showed autofluorescence when treated with LVC (no HPF dye). (C) ROS quencher-mediated growth recovery. The growth profile of V. fluvialis showed cell growth recovery after addition of the ROS quencher. Thiourea at 100 mM could protect the cells from LVC-induced damage. Methyl viologen was used as a positive control (ROS producer). The experiment was done in triplicate. Error bars represent the standard deviation of the mean.
The likely involvement of iron and copper in Fenton’s chemistry is presented in equations 1 and 2.
| (1) |
| (2) |
The generation of ROS in response to LVC treatment indicates that oxidative stress is the most probable reason for the lethality of the triple combination against the pathogens tested in this study.
Thiourea alleviates the effect of ROS in LVC-treated cells.
In order to confirm if the inhibition of the enteric pathogens by LVC was dependent on ROS, the ability of thiourea (a hydroxyl radical quencher) to scavenge ROS was tested (50). The bacterial cells treated with LVC and thiourea (100 mM) showed a revival of growth, whereas the cells treated only with LVC did not (Fig. 4C). Also, thiourea- and LVC-treated cells showed longer lag and log phases than the control cells. The revival of the cells in the presence of thiourea further confirmed ROS generation by LVC.
LVC induces DNA damage in target cells.
The elongation of V. fluvialis cells treated with the LVC combination, as observed by SEM (Fig. 2B), could be an indication of an SOS response and inhibition of cell division (33). To monitor the effect of the triple combination on the SOS response, sulA::GFP cells were used as a reporter (52). Upon exposure to LVC, considerable induction of green fluorescent protein (GFP) compared to that for the controls was observed (Fig. 5A). The cell elongation and activation of the sulA promoter upon LVC exposure point toward inhibition of cell division, which is known to occur by the SOS response via inhibition of FtsZ ring formation (53). To further evaluate the DNA damage in V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi after LVC treatment, we performed a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (54). After LVC treatment, the proportion of TUNEL-positive V. fluvialis cells was 35% of the number of TUNEL-positive untreated cells (Fig. 5B). In the case of treatment with thiourea-supplemented LVC, the proportion of TUNEL-positive cells was less than 5%, which indicated that quenching of ROS by thiourea led to the decrease in DNA fragmentation. Taken together, these results highlight the role of the triple combination on DNA fragmentation and the SOS response in V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi which could be attributed to ROS production.
An in vitro nicking assay with supercoiled plasmid DNA revealed time-dependent DNA nicking, which was inhibited in the presence of the free radical scavenger thiourea (Fig. 5C). This corroborates the earlier observation that LVC leads to ROS production, resulting in DNA damage.
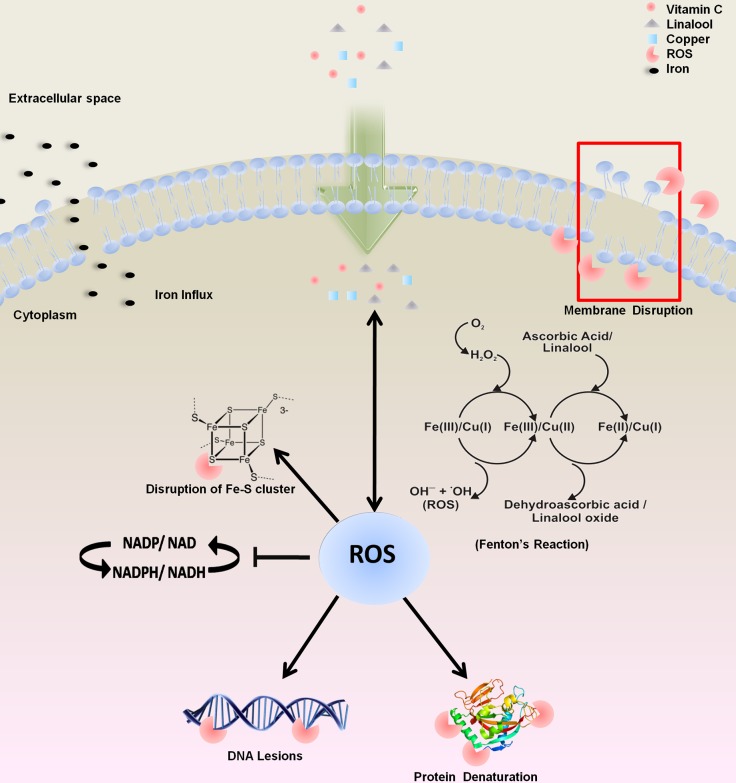
Gram-negative bacterial pathogens pose a formidable challenge for antibacterial therapies. The development of antibiotic resistance further compounds this problem. Since conventional antibiotics cannot be added to food systems to control food spoilage by bacteria, there is a need to develop safe, acceptable chemicals and natural products as alternatives to antibiotics. Due to their mild antibacterial activity, EOs are required to be added at high concentrations, which lead to the deterioration of organoleptic properties. In the present study, we propose a combination of three food-grade ingredients which proved effective in controlling V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi. Linalool (considered a weak antibacterial) acts in a synergistic manner with vitamin C and copper as an effective antibacterial combination. Using biochemical and synthetic lethality data, we deciphered the mechanism for the efficacy of the triple combination. The proposed molecular mechanism of triple synergy was demonstrated by free radical generation through Fenton’s reaction and its aftereffects (membrane disintegration, DNA damage, inhibition of cell division apparatus, disruption of the iron-sulfur cluster, the influx of iron, and energy imbalance) (Fig. 6). Triple synergy between GRAS status ingredients has not been explored to control contaminating bacterial species. The advantage of triple synergy obviates the need for a high dosage of the individual antibacterial chemicals to be added to the food for effective bacterial inhibition. The levels of the three components used are well below the safe limits and did not alter the organoleptic properties significantly. In the presence of vitamin C and linalool, Fenton’s reaction is catalyzed, which leads to bactericidal ·OH (hydroxyl radical) formation. The effect of LVC on compromising the membrane integrity, the generation of lethal free radicals, and degradation of the DNA of target bacterial cells ensures that cells are not able to transform into a viable but nonculturable (VBNC) state. The effectiveness of this antibacterial regimen was also demonstrated in fresh acidic beverages. This study also provides an alternative to conventional chemical preservatives, like acids, hydrogen peroxide, and chelators, in food and that can be extended to replace parabens in cosmetics. Though we have shown the safety of this regimen for human cell lines and RBCs, further toxicological studies are warranted to boost the confidence in the proposed antibacterial combination. It is also tempting to speculate that such combinations may also have potential as therapeutics, which should be followed by efficacy assessments in animal models.
FIG 6.
Schematic representation of the probable mode of action of bacterial cell inhibition using LVC. Exposure of LVC to the target cells leads to membrane damage. Upon entering the cells, vitamin C and linalool in combination with copper produce reactive oxygen species. Copper is much more catalytically efficient than iron in generating hydroxyl radicals (71). The increased pro-oxidant activity of vitamin C and linalool leads to an influx of iron inside the cells, which further sustains Fenton’s reaction. The generation of hydroxyl radicals via Fenton’s reaction causes DNA lesions and disrupts the iron-sulfur cluster of proteins and the redox balance of the cell.
MATERIALS AND METHODS
Media, chemicals, and bacterial strains.
All EOs (linalool, carvacrol, eugenol, cinnamaldehyde) and antibiotics used in this study were obtained from Sigma-Aldrich, USA. Copper sulfate [CuSO4; Cu(II)], ascorbic acid (vitamin C), Luria-Bertani (LB) broth, and Mueller-Hinton (MH) broth were obtained from Merck, Germany. E. coli knockout strains were taken from the Keio Collection library (55). The bacterial strains E. coli (ATCC 25922) and Salmonella enterica subsp. enterica serovar Typhi (ATCC 14901) were obtained from the American Type Culture Collection (ATCC), USA. V. fluvialis L-15318 (Ampr Nalr Neor Sulr Trir Tetr Chlr Cipr Kanr Norr Oxar Vanr), a multiple-antibiotic-resistant clinical strain, was a kind gift from Amit Ghosh of the National Institute of Cholera and Enteric Diseases, Kolkata, India. E. coli ML-35p was a kind gift from Liam Good of the Royal Veterinary College, University of London, London, United Kingdom (56). The pANO1-based E. coli sulA::GFP reporter strain was a kind gift from Soren J. Sorensen, University of Copenhagen, Copenhagen, Denmark (52). Lactococcus lactis NZ9000 was obtained from MoBiTec, Germany. Lactobacillus brevis, Lactobacillus plantarum, and Lactobacillus casei were isolated from Himalayan yak cheese, and Campylobacter jejuni was obtained from the Central Avian Research Institute (CARI; Izzatnagar, India). The strains were identified using 16S rRNA gene sequencing.
Growth conditions and growth inhibition of bacterial strains.
The antibacterial activity of natural products and combinations was determined by broth microdilution assays (CLSI, 2009) with inoculation of approximately 105-CFU/ml suspensions of the bacterial cultures in MH broth (57). Natural products were prepared as a 1:10-diluted stock in dimethyl sulfoxide (DMSO), subsequently diluted with water, and emulsified by sonication. Cellular growth in the presence of EOs was observed by determination of the optical density (OD) at 600 nm (OD600) after 12 h. Growth at a level above 10% of the OD600 for the positive control (lacking any antimicrobial agent) was considered uninhibited growth. The lowest concentration of the compound which prevented the visible growth of the bacteria was determined to be the MIC, and subsequently, 10 μl was plated on MH agar to determine the minimal bactericidal concentration (MBC) (58). The positive-control well also contained DMSO at a concentration of 0.15%, and this concentration was used in all antimicrobial experiments performed in this study. The percentage of bacterial growth inhibition was calculated using the following formula:
Determination of triple-synergy activity of LVC.
The MIC of linalool, vitamin C, and copper (LVC) was determined using two-dimensional (2D) and 3D checkerboard microdilution methods in MH broth with a final inoculum of approximately 105 CFU/ml. Microdilution plates for the evaluation of triple drug combinations were set up using a microtiter plate containing no linalool and increasing concentrations of Cu(II) ranging from 0.379 μM to 388 μM on the x axis and increasing concentrations of vitamin C ranging from 0.25 to 32 mM on the y axis. To each of the subsequent five plates, a fixed concentration of linalool (0.324 mM, 0.694 mM, 1.298 mM, 2.596 mM, 5.189 mM) was added, along with increasing concentrations of Cu(II) ranging from 0.379 μM to 388 μM on the x axis and increasing concentrations of vitamin C ranging from 0.25 to 32 mM on the y axis. Likewise, the concentrations of carvacrol (in place of linalool) were kept at 0.662 mM, 1.324 mM, 1.986 mM, 2.684 mM, and 3.31 mM, and the concentrations of cinnamaldehyde and eugenol were kept at 0.751 mM, 1.502 mM, 3.004 mM, 3.775 mM, and 5.27 mM. MICs and fractional inhibitory concentrations (FICs) were determined after 12 h of growth. The MIC of LVC was defined as the concentration in the well in the microtiter plate with the lowest drug combination at which no visible growth was observed. A fractional inhibitory concentration index (FICI) value lower than 0.5 indicates synergistic activity, a FICI value of >0.5 to <1 indicates partial synergy, a FICI value of 1.0 indicates an additive effect, and a FICI value of >1 indicates an antagonistic effect (59, 60). The synergy of the antibiotic combinations was calculated using the FICI, where the MIC of the antibiotic compound in the combination is divided by the MIC of the compound alone, yielding the fractional contribution of each drug component in the combination. Synergy for all compounds in a combination is summed, and drug interactions were scored using the following formula:
where MIC A combA-B-C, MIC B combA-B-C, and MIC C combA-B-C are the MICs of compounds A, B, and C in the combination of compounds A, B, and C (combA-B-C), respectively, and MICA, MICB, and MICC are the MICs of compounds A, B, and C alone.
Viability analysis of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis treated with the MIC of LVC.
To study the bactericidal effect of LVC on Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis, live/dead staining of the pathogens was carried out. Suspended cells (108 CFU/ml) were treated with the MIC values of LVC [1.298 mM linalool, 8 mM vitamin C, and 16.3 μM Cu(II)], whereas untreated cells were taken as the control. After incubation for 2 h, bacterial samples were centrifuged at 5,000 rpm for 10 min at 4°C, the supernatant was discarded, and the cell pellet was resuspended in 100 μl of 0.9% phosphate-buffered saline (PBS). Untreated and treated samples were stained with 1 μl of 1:1 mixture of solutions A and B of the live/dead staining kit (LIVE/DEAD BacLight bacterial viability kit; Thermo Scientific, USA). An untreated and unstained sample was used as the control. The samples were analyzed using flow cytometry (BD FACSVerse flow cytometer; BD Biosciences, USA) with excitation at a wavelength of 488 nm and emission at a wavelength of 527 to 532 nm for SYTO9-stained (live) cells and excitation at a wavelength of 526 nm and emission at a wavelength of 575 nm for PI-stained (dead) cells. For each sample, 10,000 cells were collected. The photomultiplier tube (PMT) voltage settings used were as follows: for forward scatter (FSC), 450 to 500 V; for side scatter (SSC), 350 to 400 V; for fluorescein isothiocyanate (FITC), 650 to 700 V; and for phycoerythrin (PE), 545 V. Sphero rainbow calibration particles were used for instrument calibration.
In vitro time-kill kinetics.
The antibacterial activity of the LVC combination against Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis cells was assessed by time-kill analysis as described previously (61, 62). Cells of each bacterial strain (105 CFU/ml) were incubated with the MIC of LVC at 37°C and shaking at 250 rpm. Aliquots were removed from each tube at 0, 30, 60, 90, 120, 150, and 180 min after addition of the MIC of LVC, and diluted samples were spread on MH agar plates in triplicate. The plates were incubated at 37°C for 12 h, and the colonies were counted.
Characterization of bacterial morphology by SEM.
To understand the morphological changes in the bacterial cells upon treatment with 1× MIC, 0.5× MIC, and 0.25× MIC of LVC, scanning electron microscopy (SEM) analysis was performed. Treated and untreated cells were washed twice with PBS and were harvested by centrifugation at 5,000 rpm for 10 min. The cells were primarily fixed with 2% glutaraldehyde, followed by gradual dehydration with an ethanol gradient (25%, 50%, 70%, 80%, 90%, and 100%). The prepared samples were coated with a layer of gold and observed under an SEM (Ultra Plus Field Emission; Carl Zeiss Meditec AG, Germany) (63).
Screening of Keio Collection E. coli knockout mutants.
E. coli mutant strains from the Keio Collection were grown on fresh LB medium plates containing 20 μg/ml kanamycin (all bacterial strains from the Keio Collection are kanamycin resistant). Hypersensitive E. coli knockout mutants were selected on the basis of the percent growth inhibition of the strains treated with 0.5× MIC of LVC in culture tubes containing 5 ml MH broth. The percent growth inhibition of the knockout strain was computed as follows:
Determination of ROS by flow cytometry.
For in vivo measurements of hydroxyl radical generation, a fluorescent reporter dye, hydroxyphenyl fluorescein (HPF; Invitrogen, USA), which is oxidized by hydroxyl radicals with a high specificity, was used for the detection of free radicals. Overnight cultures of Salmonella enterica subsp. enterica serovar Typhi and V. fluvialis were subcultured until an OD600 of 0.5 was attained. The cells were diluted to 105 CFU/ml, treated with 0.5× MIC of LVC, and incubated at 37°C with shaking at 250 rpm. After treatment, 100-μl samples were collected and centrifuged at 5,000 rpm at 4°C. The cells were washed twice with PBS and resuspended in PBS and 5 μM HPF. Samples were incubated in the dark at room temperature for 1 h. After incubation, the cells were washed twice with 500 μl of PBS to remove the excess dye and resuspended in PBS for fluorometric analysis. Hydroxyl radical generation was analyzed by flow cytometry with excitation at a wavelength of 488 nm and emission at a wavelength of 527 to 532 nm. For each sample, 10,000 events were recorded. The photomultiplier tube (PMT) voltage settings used were as follows: for FSC, 450 V; for SSC, 350 V, and for FITC, 700 V. Sphero rainbow calibration particles (BD Biosciences, USA) were used for instrument calibration.
ROS quenching assay.
For hydroxyl radical quenching experiments, overnight cultures of V. fluvialis were subcultured until an OD600 of 0.5 was attained. The cells were diluted to 105 CFU/ml and incubated with 100 mM thiourea and the MIC of LVC at 37°C with shaking at 250 rpm. Bacterial growth was observed, and the OD600 was determined after fixed time intervals. Methyl viologen (0.5× MIC) was used as a positive control (64, 65).
Determination of cellular metal content.
To determine the cellular metal content, inductively coupled plasma mass spectrometry (ICP-MS) analysis was carried out. Three milliliters of 105 CFU/ml V. fluvialis cells was incubated with the components of LVC at 0.5× MIC, viz., Cu (8.15 μM), linalool plus Cu (1.292 mM and 8.15 μM, respectively), vitamin C plus Cu (8 mM and 8.15 μM, respectively), and linalool plus vitamin C and Cu (1.292 mM, 4 mM, and 8.15 μM, respectively), in LB broth. Cells were grown for 6 to 8 h, and 1 ml was harvested, washed twice with cold 50 mM Tris-Cl (pH 7.5), and resuspended in 70% nitric acid. The samples were then heated to 100°C until the acid vaporized and again resuspended in 1 ml 3% nitric acid solution. The samples were analyzed by ICP-MS (PerkinElmer, USA) at the Institute Instrumentation Centre, Indian Institute of Technology (IIT) Roorkee (66).
SOS response to LVC.
To monitor DNA damage by LVC, a sulA reporter strain (pNAOI-sulA::GFP) which expresses GFP as a reporter of DNA damage inside the cell was used (52). Cells were grown at 37°C and 250 rpm in LB medium until an OD600 of 0.2 was reached. Bacterial cell cultures were induced with 0.5× MIC of LVC. To quantify the fluorescence intensity, 1 ml of bacterial culture was harvested after 3 h of induction, washed twice, and resuspended in 500 μl PBS. Expression of GFP was measured using a SpectraMax M2e microplate reader in a black opaque 96-well plate (Corning 96; Corning, USA) at an excitation wavelength of 485 nm and an emission wavelength of 520 nm. The numbers of relative fluorescence units (RFU) were calculated by dividing individual fluorescence values by the respective optical densities (49). The number of RFU was normalized to that achieved with nalidixic acid using OriginPro 8 software (version 8.0274).
Determination of DNA fragmentation using TUNEL assay.
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed according to the manufacturer's instructions (Roche Molecular Biochemicals, Germany). V. fluvialis was grown in MH broth at 37°C with constant shaking at 250 rpm. For DNA fragmentation studies, subcultured cells were diluted to 105 CFU/ml and pretreated for 2 h with 0.5× MIC of LVC. After incubation, the cells were washed thrice in PBS and fixed in freshly prepared 4% formaldehyde in PBS for 2 h at room temperature. The cells were washed with PBS and permeabilized in 0.1% Triton X-100 and 0.1% sodium citrate. The cells were placed in 50 μl of freshly prepared TUNEL reagent and incubated in the dark for 1 h at 37°C in a humidified chamber. After incubation, the cells were washed thrice with PBS, and samples were analyzed by flow cytometry at the FITC filter settings described above. Thiourea at a concentration of 100 mM was used to study the effect of the ROS quencher on DNA damage.
DNA-nicking assay.
An in vitro DNA-damaging assay was performed using the pBR322 plasmid purified by use of a Nuclepore plasmid isolation kit (Nuclepore, Genetix, India). The total reaction was carried out in 10 μl of Tris-EDTA (TE) buffer (10 mM Tris-Cl, 1 mM EDTA) containing 100 ng the pBR322 plasmid. Nine reaction mixtures were set up using only pBR322 incubated for 20 min; 1 mM H2O2 and pBR322 incubated for 20 min; 1 mM H2O2, pBR322, and 0.5× MIC of LVC incubated for 1, 3, 8, and 20 min; pBR322 and 0.5× MIC of LVC incubated for 15 min; 1 mM H2O2, pBR322, 0.5× MIC of LVC, and 100 mM thiourea incubated for 20 min; and pBR322 digested with SalI (Thermo Scientific, USA). The samples were run in a 1% agarose gel at 80 V for 30 min and stained with 10 μg/ml ethidium bromide. The gel was photographed and documented with a Bio-Rad Gel Doc XR+ system (Bio-Rad, USA).
Outer cell membrane permeabilization assay.
Outer membrane permeability was determined as described by Eriksson et al. (56). Briefly, E. coli strain ML-35p cells, which carry a plasmid-borne β-lactamase gene, were grown in LB medium to an OD600 of 0.4; washed twice with PBS (pH 7.4), followed by centrifugation at 5,000 rpm; and suspended in 10 mM HEPES buffer (pH 7.4) to an OD600 of 0.5. The permeabilization assays were carried out in 96-well microtiter plates with wells containing 100 μl of 5 mM HEPES (pH 7.4), 20 μg/ml nitrocefin, and bacterial cells diluted to an OD600 of 0.1. Following addition of the test compounds [Cu(II) alone, linalool alone, vitamin C alone, Cu(II) and linalool, Cu(II) and vitamin C, linalool and vitamin C, 0.5× MIC of LVC, 1× MIC of LVC], nitrocefin cleavage was monitored by measuring the absorbance at 486 nm.
Inner cell membrane permeabilization assay.
Permeabilization of the inner membrane was assessed by measuring the access of ortho-nitrophenyl-β-d-galactopyranoside (ONPG) to the cytoplasm as described by Eriksson et al. (56). E. coli ML-35p cells were prepared as described above. Briefly, ONPG was added at a concentration of 100 μg/ml, and substrate cleavage by β-galactosidase in the presence of permeabilizers [Cu(II) alone, linalool alone, vitamin C alone, Cu(II) and linalool, Cu(II) and vitamin C, linalool and vitamin C, 0.5× MIC of LVC, 1× MIC of LVC] was monitored by measuring the absorbance at 420 nm. Polymyxin B sulfate (0.5 μg/ml) was used as a positive control.
Cytotoxicity assay.
A cytotoxic activity assay was performed according to a slight modification of the procedure reported by Mosman (67). Human embryonic kidney 293 (HEK293) cells (5 × 103 cells/well) were plated in 96-well plates and treated with 0.5×, 1×, 2×, 5×, or 10× MIC of LVC for 4 h at 37°C in 5% CO2 with 80% humidity. Control groups received the same amount of PBS. The growth of HEK293 cells was determined by examination of the ability of the living cells to reduce the yellow dye 3-(4,5-dimethyl-2- thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a blue formazan product. At the end of 4 h of incubation, the medium in each well was replaced by fresh medium containing 1 mg/ml of MTT. After 4 h of treatment, the formazan product of MTT reduction was dissolved in DMSO and the absorbance was measured at 570 nm. The absorbance of the untreated cells was taken as 100% viability as a reference. The effect of the different concentrations of LVC was quantified as the percent reduction in viability in comparison with that of the control cells. Experiments were performed in triplicate.
Hemolytic assay.
The hemolytic assay was done as described by Malagoli (68) with slight modifications. Blood samples were drawn from volunteers per human ethics considerations by trained technical staff of the pathology lab of IIT Roorkee. Blood was centrifuged at 1,500 rpm, and the pellet was washed three times with PBS. After each washing, the cells were pelleted by centrifugation at 900 rpm for 5 min and the supernatant was discarded. The final pellet was diluted 1:9 (vol/vol) in PBS and subsequently diluted to 1:24 (vol/vol) in PBS, pH 7.0. Red blood cell suspensions (final volume, 1 ml) were incubated with 0.5×, 1×, 2×, 3×, 5×, or 10× MIC of LVC. The samples were incubated for 4 h at 37°C. Total lysis of the erythrocyte suspension was obtained by incubating the cells with 1% (vol/vol) Triton X-100. In order to evaluate the degree of spontaneous lysis, tubes containing exclusively the red blood cell suspension in PBS were set. After incubation, the cell suspensions were centrifuged at 1,500 rpm for 10 min and the supernatant was carefully collected. The absorbance at 540 nm of the supernatant was taken using the SpectraMax M2e microplate reader. The value of the absorbance of erythrocytes maintained exclusively in PBS was taken as the negative control. Hemolysis levels were expressed as a percentage of RBC survival. The absorbance of cells treated with Triton X-100 was taken as 100% hemolysis. The percent survival of RBCs treated with different concentrations of drug was calculated by subtracting the percent hemolysis of treated cells from that of cells treated with Triton X-100. Protocol (BT/HE/NKN/2016-17/001) for using human samples for biomedical research purpose has been duly approved by the Institutional Ethics Committee of the Indian Institute of Technology Roorkee, India.
Antimicrobial activity assay of LVC in fruit beverages.
To ascertain the suitability of LVC in fruit beverages, the antimicrobial efficacy of LVC against V. fluvialis and Salmonella enterica subsp. enterica serovar Typhi was assayed. Fresh citrus and pomegranate fruits were washed, peeled, and blended. A bacterial suspension (104 CFU/ml) was spiked in 50 ml juice containing 1× MIC of LVC. The samples were incubated at 37°C for 12 h. After incubation, samples were diluted and 100 μl of the sample was spread on MH agar plates. Cells were counted after 12 h of incubation at 37°C (69).
Sensory evaluation of fruit juices.
Consumer acceptability of the LVC-treated juice was assessed as described by Walkling-Ribeiro et al. (70). Twenty untrained panelists ages 20 to 35 years were selected for the study, and the results were compared in terms of product-specific odor, sweetness, color, acidity, flavor, and overall acceptability on a 9-point hedonic scale, with 1 being the lowest acceptability and 9 being the highest acceptability. Nonsalted crackers and water were provided to eliminate the residual taste between samples. LVC at 1× MIC (linalool, 1.298 mM; vitamin C, 8 mM; copper, 16.3 μM) was homogeneously mixed with fresh citrus and pomegranate juice, and the mixture was evaluated for alterations in sensory attributes.
Statistical analysis.
Analysis for differences between the treatment and control arms was performed using the chi-square test and one-way and two-way analysis of variance (ANOVA). P values of <0.05 were considered significant. Statistical calculations were performed using GraphPad Prism (version 5) software.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by the National Agricultural Science Fund of the Indian Council of Agricultural Research (NASF-ICAR), grant code number NASF/Minimization-6024/2017-18, and the Ministry of Human Resources and Development (MHRD), government of India, to N.K.N.
We thank Amit Ghosh of the National Institute of Cholera and Enteric Diseases, Kolkata, India, for providing Vibrio fluvialis L-15318, Liam Good of the Royal Veterinary College, University of London, London, United Kingdom, for the E. coli ML-35p strain, and Soren J. Sorensen, University of Copenhagen, Copenhagen, Denmark, for the pANO1-based E. coli sulA::GFP reporter strain.
N.K.N. and R.P. designed the research; T.G., S.K.S., A.G., A.K., and P.K. performed the research; N.K.N., R.P., and A.S.Y. analyzed the data; and N.K.N. and T.G. wrote the paper.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02487-18.
REFERENCES
- 1.World Health Organization. 2015. WHO estimates of the global burden of foodborne diseases. Foodborne Disease Burden Epidemiology Reference Group 2007-2015, World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 3.Käferstein FK, Motarjemi Y, Bettcher D. 1997. Foodborne disease control: a transnational challenge. Emerg Infect Dis 3:503. doi: 10.3201/eid0304.970414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier RC, Byrd JA, Kubena LF, Hume ME, McReynolds JL, Anderson RC, Nisbet DJ. 2014. Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: effects on poultry. Poult Sci 93:267–272. doi: 10.3382/ps.2013-03254. [DOI] [PubMed] [Google Scholar]
- 5.Buzby JC, Roberts T, Lin C-TJ, MacDonald JM. 1996. Bacterial foodborne disease. Medical costs and productivity losses. Report 741 USDA/ERS, Washington, DC. [Google Scholar]
- 6.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJV, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. 2011. Tackling antibiotic resistance. Nat Rev Microbiol 9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leistner L. 2000. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol 55:181–186. doi: 10.1016/S0168-1605(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 8.Preedy VR. 2015. Essential oils in food preservation, flavor and safety. Academic Press, San Diego, CA. [Google Scholar]
- 9.Silva F, Domingues FC. 2017. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit Rev Food Sci Nutr 57:35–47. doi: 10.1080/10408398.2013.847818. [DOI] [PubMed] [Google Scholar]
- 10.Mandl J, Szarka A, Banhegyi G. 2009. Vitamin C: update on physiology and pharmacology. Br J Pharmacol 157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englard S, Seifter S. 1986. The biochemical functions of vitamin C. Annu Rev Nutr 6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Frei B. 1999. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 13.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. 1996. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilchèze C, Hartman T, Weinrick B, Jacobs WR Jr. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapiero H, Townsend D, Tew K. 2003. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 57:386–398. doi: 10.1016/S0753-3322(03)00012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Jaiser SR, Winston GP. 2010. Copper deficiency myelopathy. J Neurol 257:869–881. doi: 10.1007/s00415-010-5511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlap WM, James GW, Hume DM. 1974. Anemia and neutropenia caused by copper deficiency. Ann Intern Med 80:470–476. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Marshall MR, Wei C-I. 1995. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem 43:2839–2845. doi: 10.1021/jf00059a013. [DOI] [Google Scholar]
- 20.Fisher K, Phillips C. 2008. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol 19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- 21.Harvey AL. 2008. Natural products in drug discovery. Drug Discov Today 13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Shiratsuchi H, Chang S, Wei A, H El-Ghorab A, Shibamoto T. 2012. Biological activities of low-molecular weight compounds found in foods and plants. J Food Drug Anal 20:359–365. [Google Scholar]
- 23.Murbach Teles Andrade BF, Nunes Barbosa L, Bérgamo Alves FC, Albano M, Mores Rall VL, Sforcin JM, Fernandes AAH, Fernandes Júnior A. 2016. The antibacterial effects of Melaleuca alternifolia, Pelargonium graveolens and Cymbopogon martinii essential oils and major compounds on liquid and vapor phase. J Essential Oil Res 28:227–233. doi: 10.1080/10412905.2015.1099571. [DOI] [Google Scholar]
- 24.Heck J, Vollmuth T, Cifone M, Jagannath D, Myhr B, Curren R. 1989. An evaluation of food flavoring ingredients in a genetic toxicity screening battery. Toxicologist 9:257–272. [Google Scholar]
- 25.Patil SD, Sharma R, Srivastava S, Navani NK, Pathania R. 2013. Downregulation of yidC in Escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PLoS One 8:e57370. doi: 10.1371/journal.pone.0057370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Biotechnology Information. 2018. Compound summary for CID 6549. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/6549. Accessed 23 August 2018.
- 27.Rastogi S, Kalra A, Gupta V, Khan F, Lal RK, Tripathi AK, Parameswaran S, Gopalakrishnan C, Ramaswamy G, Shasany AK. 2015. Unravelling the genome of holy basil: an “incomparable” “elixir of life” of traditional Indian medicine. BMC Genomics 16:413. doi: 10.1186/s12864-015-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer KA, Carson C, Riley T. 1999. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.Cowan MM. 1999. Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon RA. 2001. Natural products and plant disease resistance. Nature 411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 31.Fisher K, Phillips CA. 2006. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol 101:1232–1240. doi: 10.1111/j.1365-2672.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- 32.Holloway AC, Gould SW, Fielder MD, Naughton DP, Kelly AF. 2011. Enhancement of antimicrobial activities of whole and sub-fractionated white tea by addition of copper (II) sulphate and vitamin C against Staphylococcus aureus; a mechanistic approach. BMC Complement Altern Med 11:115. doi: 10.1186/1472-6882-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi E, Lutkenhaus J. 1993. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol 175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MMT, Pyle BH, Camper AK. 2010. Specific and rapid enumeration of viable but nonculturable and viable-culturable Gram-negative bacteria by using flow cytometry. Appl Environ Microbiol 76:5088–5096. doi: 10.1128/AEM.02932-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayrapetyan M, Oliver JD. 2016. The viable but non-culturable state and its relevance in food safety. Curr Opin Food Sci 8:127–133. doi: 10.1016/j.cofs.2016.04.010. [DOI] [Google Scholar]
- 36.Fernandes A, Mira ML, Azevedo MS, Manso C. 1988. Mechanisms of hemolysis induced by copper. Free Radic Res Commun 4:291–298. doi: 10.3109/10715768809066894. [DOI] [PubMed] [Google Scholar]
- 37.Vantarakis A, Affifi M, Kokkinos P, Tsibouxi M, Papapetropoulou M. 2011. Occurrence of microorganisms of public health and spoilage significance in fruit juices sold in retail markets in Greece. Anaerobe 17:288–291. doi: 10.1016/j.anaerobe.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. 2012. Oxidative stress and antioxidant defense. World Allergy Organ J 5:9. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boehm DE, Vincent K, Brown OR. 1976. Oxygen and toxicity inhibition of amino acid biosynthesis. Nature 262:418. doi: 10.1038/262418a0. [DOI] [PubMed] [Google Scholar]
- 41.Jang S, Imlay JA. 2007. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem 282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi H-J, Kim S-J, Mukhopadhyay P, Cho S, Woo J-R, Storz G, Ryu S-E. 2001. Structural basis of the redox switch in the OxyR transcription factor. Cell 105:103–113. doi: 10.1016/S0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A 87:6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giro M, Carrillo N, Krapp AR. 2006. Glucose-6-phosphate dehydrogenase and ferredoxin-NADP (H) reductase contribute to damage repair during the soxRS response of Escherichia coli. Microbiology 152:1119–1128. doi: 10.1099/mic.0.28612-0. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Vulić M, Keren I, Lewis K. 2012. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyer K, Imlay JA. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A 93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng M, Doan B, Schneider TD, Storz G. 1999. OxyR and SoxRS regulation of fur. J Bacteriol 181:4639–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duguay AR, Silhavy TJ. 2004. Quality control in the bacterial periplasm. Biochim Biophys Acta 1694:121–134. doi: 10.1016/j.bbamcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava SK, Lambadi PR, Ghosh T, Pathania R, Navani NK. 2014. Genetic regulation of spy gene expression in Escherichia coli in the presence of protein unfolding agent ethanol. Gene 548:142–148. doi: 10.1016/j.gene.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 51.Renggli S, Keck W, Jenal U, Ritz D. 2013. Role of autofluorescence in flow cytometric analysis of Escherichia coli treated with bactericidal antibiotics. J Bacteriol 195:4067–4073. doi: 10.1128/JB.00393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norman A, Hansen LH, Sørensen SJ. 2005. Construction of a ColD cda promoter-based SOS-green fluorescent protein whole-cell biosensor with higher sensitivity toward genotoxic compounds than constructs based on recA, umuDC, or sulA promoters. Appl Environ Microbiol 71:2338–2346. doi: 10.1128/AEM.71.5.2338-2346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee A, Lutkenhaus J. 1998. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J 17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohwer F, Azam F. 2000. Detection of DNA damage in prokaryotes by terminal deoxyribonucleotide transferase-mediated dUTP nick end labeling. Appl Environ Microbiol 66:1001–1006. doi: 10.1128/AEM.66.3.1001-1006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: the Keio Collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eriksson M, Nielsen PE, Good L. 2002. Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli. J Biol Chem 277:7144–7147. doi: 10.1074/jbc.M106624200. [DOI] [PubMed] [Google Scholar]
- 57.Wikler MA. 2006. Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. M100-S16 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 58.Groenink J, Walgreen-Weterings E, van 't Hof W, Veerman ECI, Nieuw Amerongen AV. 1999. Cationic amphipathic peptides, derived from bovine and human lactoferrins, with antimicrobial activity against oral pathogens. FEMS Microbiol Lett 179:217–222. doi: 10.1111/j.1574-6968.1999.tb08730.x. [DOI] [PubMed] [Google Scholar]
- 59.Yoon J, Urban C, Terzian C, Mariano N, Rahal JJ. 2004. In vitro double and triple synergistic activities of polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 48:753–757. doi: 10.1128/AAC.48.3.753-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzales PR, Pesesky MW, Bouley R, Ballard A, Biddy BA, Suckow MA, Wolter WR, Schroeder VA, Burnham C-AD, Mobashery S, Chang M, Dantas G. 2015. Synergistic, collaterally sensitive [beta]-lactam combinations suppress resistance in MRSA. Nat Chem Biol 11:855–861. doi: 10.1038/nchembio.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chin JN, Rybak MJ, Cheung CM, Savage PB. 2007. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob Agents Chemother 51:1268–1273. doi: 10.1128/AAC.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Credito K, Lin G, Appelbaum PC. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob Agents Chemother 51:1504–1507. doi: 10.1128/AAC.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambadi PR, Sharma TK, Kumar P, Vasnani P, Thalluri SM, Bisht N, Pathania R, Navani NK. 2015. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int J Nanomedicine 10:2155. doi: 10.2147/IJN.S72923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang D, Lim Y-H. 2015. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci Rep 5:10029. doi: 10.1038/srep10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dewachter L, Herpels P, Verstraeten N, Fauvart M, Michiels J. 2016. Reactive oxygen species do not contribute to ObgE*-mediated programmed cell death. Sci Rep 6:33723. doi: 10.1038/srep33723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhitnitsky D, Rose J, Lewinson O. 2017. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci Rep 7:44554. doi: 10.1038/srep44554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 68.Malagoli D. 2007. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebrate Survival J 4:92–94. [Google Scholar]
- 69.Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O. 2006. Antimicrobial activity of essential oils on Salmonella enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J Food Prot 69:1579–1586. doi: 10.4315/0362-028X-69.7.1579. [DOI] [PubMed] [Google Scholar]
- 70.Walkling-Ribeiro M, Noci F, Cronin D, Lyng J, Morgan D. 2009. Shelf life and sensory evaluation of orange juice after exposure to thermosonication and pulsed electric fields. Food Bioproducts Processing 87:102–107. doi: 10.1016/j.fbp.2008.08.001. [DOI] [Google Scholar]
- 71.Chumakov A, Batalova V, Slizhov Y. 2016. Electro-Fenton-like reactions of transition metal ions with electrogenerated hydrogen peroxide. AIP Conference Proceedings 1772:040004. doi: 10.1063/1.4964563. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.