Abstract
Glycosaminoglycans (GAGs), dermatan sulfate (DS), heparan sulfate (HS), and keratan sulfate (KS), are the primary biomarkers in patients with mucopolysaccharidoses (MPS); however, little is known about other biomarkers. To explore potential biomarkers and their correlation with GAGs, blood samples were collected from 46 MPS II patients, 34 MPS IVA patients, and 5 MPS IVB patients. We evaluated the levels of 8 pro-inflammatory factors (EGF, IL-1β, IL-6, MIP-1α, TNF-α, MMP-1, MMP-2, and MMP-9), collagen type II, and DS, HS (HS0S, HSNS), and KS (mono-sulfated, di-sulfated) in blood.
Eight biomarkers measured were significantly elevated in untreated MPS II patients, compared with those in normal controls: EGF, IL-1β, IL-6, HS0S, HSNS, DS, mono-sulfated KS, and di-sulfated KS. The same eight biomarkers remained elevated in ERT-treated patients. However, only three biomarkers remained elevated in post-HSCT MPS II patients: EGF, mono-sulfated KS, and di-sulfated KS. Post-HSCT patients with MPS II showed that IL-1β and IL-6 were normalized as HS and DS levels decreased. Eight biomarkers were significantly elevated in untreated MPS IVA patients: EGF, IL-1β, IL-6, MIP-1α, MMP-9, HSNS, mono-sulfated KS, and di-sulfated KS, and four biomarkers were elevated in MPS IVA patients under ERT: IL-6, TNF-α, mono-sulfated KS, and di-sulfated KS. There was no reduction of KS in the ERT-treated MPS IVA patient, compared with untreated patients. Two biomarkers were significantly elevated in untreated MPS IVB patients: IL-6 and TNF-α.
Reversely, collagen type II level was significantly decreased in untreated and ERT-treated MPS II patients and untreated MPS IVA patients.
In conclusion, selected pro-inflammatory factors can be potential biomarkers in patients with MPS II and IV as well as GAGs levels.
Keywords: Morquio syndrome, Hunter syndrome, Glycosaminoglycans, Cytokines, Inflammation
Highlights
-
•
ERT can not normalize DS, HS, and KS levels in MPS II and IVA.
-
•
HSCT reduces DS and HS levels more than ERT.
-
•
GAG storage in MPS leads to elevation of some cytokines and decrease of collagen type II in blood.
-
•
Selected pro-inflammatory factors and collagen type II, as well as GAGs, can be biomarkers in MPS II and MPS IV.
-
•
Relation between biomarkers and clinical improvements needs to be addressed with a longitudinal data.
1. Introduction
Mucopolysaccharidoses (MPS) are a group of lysosomal storage diseases, characterized by the accumulation of glycosaminoglycans (GAGs) and resulting in progressive cellular damage and successive multiple organ failures. There are five types of accumulated GAGs in tissue, dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), chondroitin sulfate, and hyaluronan, depending on the type of MPS.
MPS II, also known as Hunter syndrome, is the only MPS with X-linked inheritance, caused by a deficiency of iduronate-2-sulfatase (I2S) enzyme and progressive accumulation of DS and HS. MPS II patients present multiple progressive symptoms: coarse face, central nervous system (CNS) involvement, airway obstruction, sleep apnea, cardiomyopathy, hearing loss, stiff joints, umbilical and inguinal hernias, hepatosplenomegaly, and skeletal deformities [1].
MPS IV, also known as Morquio syndrome, is divided into two subtypes: MPS IVA and MPS IVB, caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) and β-galactosidase (β-Gal), respectively. KS accumulates in MPS IVA and MPS IVB due to the lack of these enzymes. In MPS IVA patients, disrupted cartilage leads to unique skeletal features: short stature, short neck, odontoid hypoplasia, spinal cord compression, tracheal obstruction, pectus carinatum, kyphoscoliosis, platyspondyly, coxa valga, genu valgum, waddling gait, and laxity of joints [2]. In general, MPS IVB patients exhibit phenotypes milder than those found in MPS IVA. MPS IVB is characterized by skeletal dysplasia, corneal clouding, cardiac insufficiency and increased urinary excretion of KS. There is no CNS involvement in both MPS IVA and MPS IVB [3].
Simonaro et al. showed that GAG storage in MPS leads to cytokine elevation in affected cells and animals [4]. When the inflammatory cytokines stimulate MPS connective tissue cells, it causes enhanced secretion of several matrix-degrading metalloproteinases (MMPs), leading to degradation of extracellular matrix (ECM) components, as well as increased apoptosis of connective tissue cells and cartilage destruction [[4], [5], [6]]. A further study found that GAGs bind TLR4 receptors and activate the TLR4 pathway, leading to cytokines such as TNF-α and IL-1β release. Inactivation of this pathway in MPS VII mice lacking TLR4 corrected many clinical and pathological features of the disease, and an anti-TNF-a drug in MPS VI rats reduced levels of inflammatory cytokines and improved joint pathology [7]. In addition to anti-TNF-α therapy, pentosan polysulfate (PPS) has been studied as well. PPS has a potent anti-inflammatory effect and is approved by the FDA as an oral medication (Elmiron®). Previous studies demonstrated that PPS in MPS VI rats reduced inflammation and GAG levels and improved mobility and organ system pathologies [8,9]. A small clinical trial in 4 adult patients with MPS I indicated that PPS was well tolerated and that PPS decreased GAG excretion and improved joint mobility and pain [10]. Orii et al. also demonstrated that PPS improved the range of joint motion of adult patients with MPS II [11]. Thus, these findings confirm the critical role of inflammation in MPS and a strong positive effect of anti-inflammatory therapies. Polgreen et al. indicated that TNF-α was associated with pain and physical disability, despite treatment under ERT and/or HSCT [12]. Jacques et al. showed the elevation of TNF-α and IL-1β in plasma from MPS II patients under ERT, compared to controls [13]. Donida et al. also indicated that pro-inflammatory and pro-oxidant states occur in MPS IVA patients even under ERT [14]. These studies confirmed the persistence of inflammation despite therapies such as ERT.
Here we examine the levels of pro-inflammatory factors in patients with MPS II and IV and evaluate the correlation between pro-inflammatory factors and GAGs accumulation. Considering that pro-inflammatory factors and GAGs play an important role in inflammation and that few studies have focused on the correlation between pro-inflammatory factors and GAGs, we suggest that this study may help better understanding of pathology in MPS.
2. Materials and methods
2.1. Subjects
There are no exclusion criteria, and inclusion criteria are that the patient is diagnosed as MPS II, IVA or IVB. We provide the table as supplementary for demographics of 85 patients including 46 MPS II patients, 34 MPS IVA patients, and 5 MPS IVB patients. Twenty-five out of 26 MPS II patients from Japan are defined as severe, but the phenotype in 20 MPS II patients is unknown from Brazil. Thirty-two out of 34 MPS IVA patients are defined as severe while two patients are as attenuated. Five MPS IVB are defined as classic. In this study, we collected blood samples from 46 MPS II patients with ages ranging between 2 and 25 years (mean ± SD; 10.0 ± 5.53), 34 MPS IVA patients with ages ranging between 3 and 56 years (16.4 ± 12.6), 5 MPS IVB patients with ages ranging between 12 and 18 years (15.8 ± 2.8), and 61 normal controls in total with ages ranging between 0 and 32 years (6.58 ± 6.93) with informed consent. Six MPS IVA patients were under ERT for 8.83 ± 3.31 months. Sixteen MPS II patients are under ERT (at least over one month). Five MPS II patients are under HSCT for 9.00 ± 4.27 years. This study was approved by IRB at Nemours/Alfred I. duPont Hospital for Children.
Some of the pro-inflammatory factors have been analyzed with a limited number of the sample because of shortness of samples, leading to a limitation of interpretation in this study.
2.2. Immunoassays
EGF, TNF-α, IL-1β, IL-6, and MIP-1α were measured in plasma or serum by Milliplex Human Cytokine / Chemokine Non-Magnetic 96-well Plate, performed according to manufacturer's instruction (Cat #; MPXHCYTO-60 K). MMP-1, MMP-2, and MMP-9 were measured in plasma or serum by Milliplex map kit, according to the manufacturer's instruction. The plasma dilution for MMPs (MMP-1, MMP-2, and MMP-9) was established as 1:20.
Type IIA collagen N-Propeptide (PIIANP) was measured in serum by enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer's instruction. The ELISA plate was read absorbance at 450 nm and 590 nm. The serum dilution for PIIANP was established as 1:10.
2.3. Glycosaminoglycan assay
We developed a highly accurate, sensitive, and cost-effective liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to measure the four disaccharides produced from GAGs; CS, DS, HS and KS [[15], [16], [17]]. Briefly, DS, HS, and KS in human serum and plasma were digested to disaccharides by chondroitinase B, heparitinase, and keratanase II, respectively. Analysis of disaccharides [DiHS0S (HS0S), DiHSNS (HSNS), DiHS6S (DS), mono-sulfated KS, di-sulfated KS] was performed by LC-MS/MS with multiple reactions monitoring in the negative ion mode. Separation of LC was performed on a Hypercarb (2.0 mm i.d. × 150 mm, 5 μm) with a gradient elution of acetonitrile–0.01 M ammonium bicarbonate (pH 10). The mobile phase flow rate was 0.2 ml/min. The 6460 triple quad mass spectrometer with a 1290 Infinity LC system (Agilent Technologies, Palo Alto, CA) was used to determine each GAG in the serum or plasma of MPS patients and controls.
2.4. Statistical analysis
Multiple comparison tests were performed by one-way ANOVA with Tukey's post-hoc test using GraphPad Prism 5. The statistical significance of difference was considered as p < .05. Pearson's correlation coefficient test was used to find the correlation between each pro-inflammatory factors and GAGs, between age and BMI, and between biomarkers and age.
3. Results
3.1. Pro-inflammatory factors and collagen type II
Pro-inflammatory factors (EGF, TNF-α, IL-1β, IL-6, MIP-1α, MMP-1, MMP-2, and MMP-9), as well as type IIA collagen (PIIANP), were assayed.
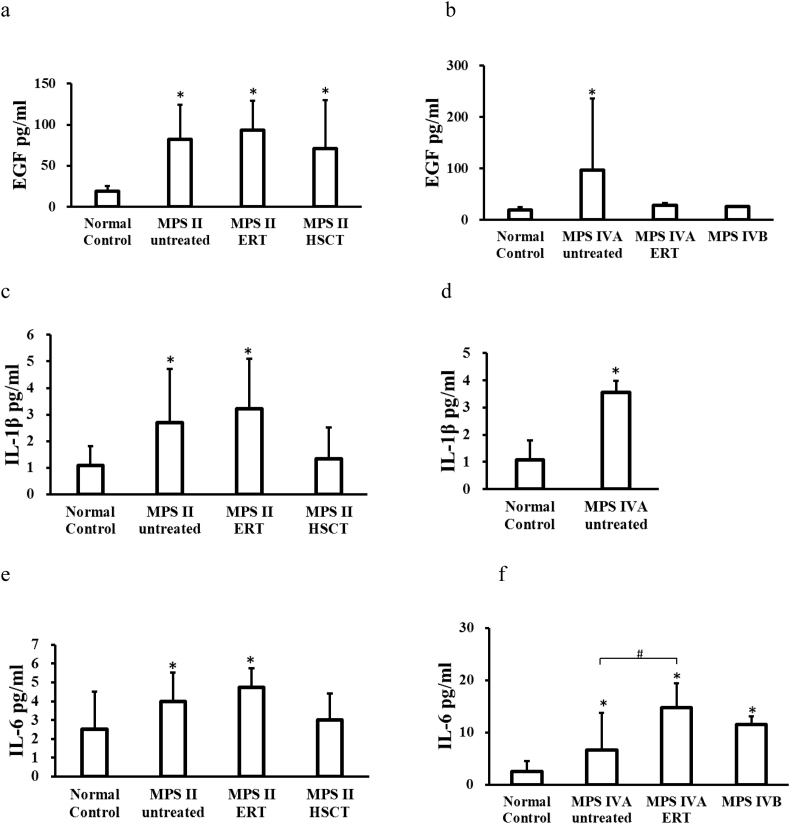
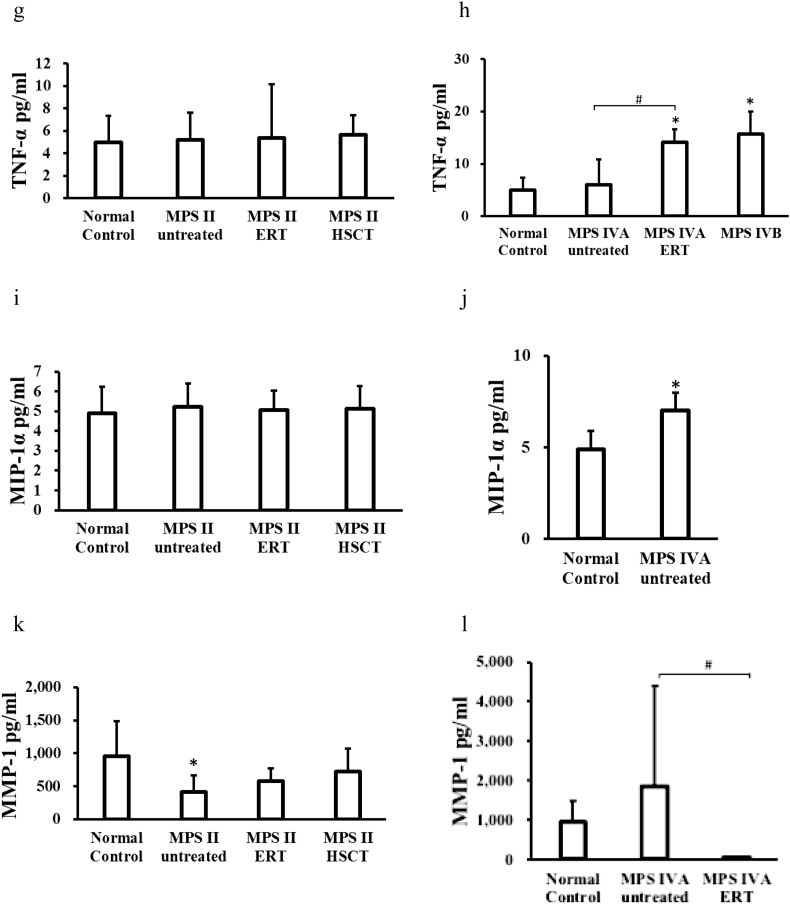
Three pro-inflammatory biomarkers were significantly elevated in untreated and ERT-treated MPS II patients, compared to those in normal controls: EGF, IL-1β, and IL-6. The EGF level was also elevated in MPS II patients treated with HSCT, but the level was lowered, compared with untreated MPS II patients. The only EGF level was elevated in MPS II patients treated with HSCT, but the level was lowered, compared with untreated and ERT-treated MPS II patients. The MMP-1 level was decreased in untreated patients, compared to that in normal controls.
Five pro-inflammatory biomarkers measured were significantly elevated in untreated MPS IVA patients, compared to normal controls: EGF, IL-1β, IL-6, MIP-1α, and MMP-9. Levels of IL-6 and TNF-α were significantly elevated in MPS IVA patients under ERT and untreated MPS IVB patients, compared to those in normal controls (two biomarkers, IL-1β and MIP-1α, could not be evaluated in this study because of the shortage of samples). Two pro-inflammatory biomarkers (IL-6, TNF-α) were significantly elevated in MPS IVA patients under ERT compared with untreated MPS IVA patients while MMP-1 was elevated in untreated MPS IVA.
PIIANP level was significantly decreased in untreated and under ERT MPS II patients and untreated MPS IVA patients, compared to that in normal controls (Fig. 1). ERT-treated and post-HSCT MPS II patients and ERT-treated MPS IVA patients tended to a lower PIIANP level.
Fig. 1.
Blood levels of pro-inflammatory factors and collagen type II in patients with MPS and normal control. (a) EGF, (c) IL-1β, (e) IL-6, (g) TNF-α, (i) MIP-1α, (k) MMP-1, (m) MMP-2, (o) MMP-9 and (q) Collagen type II levels in patients with MPS II. (b) EGF, (d) IL-1β, (f) IL-6, (h) TNF-α, (j) MIP-1α, (l) MMP-1, (n) MMP-2, (p) MMP-9 and (r) Collagen type II levels in patients with MPS IV. *p < .05 vs normal control group. #p < .05 vs MPS IVA untreated patients.
3.2. GAG levels
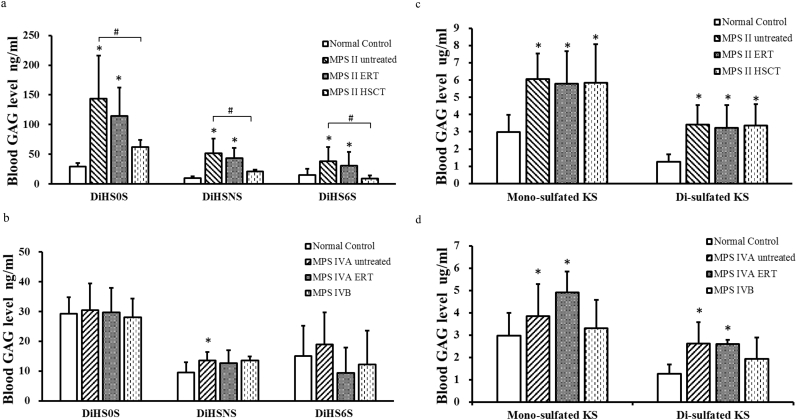
Levels of DiHS0S, DiHSNS, and DS were significantly increased in patients with MPS II (untreated and under ERT), compared to the levels in normal controls. Levels of DiHS0S, DiHSNS, and DS were significantly lower in MPS II patients treated with HSCT than those in untreated MPS II patients. MPS II patients with HSCT had lower levels of DiHS0S, DiHSNS, and DS than patients with ERT.
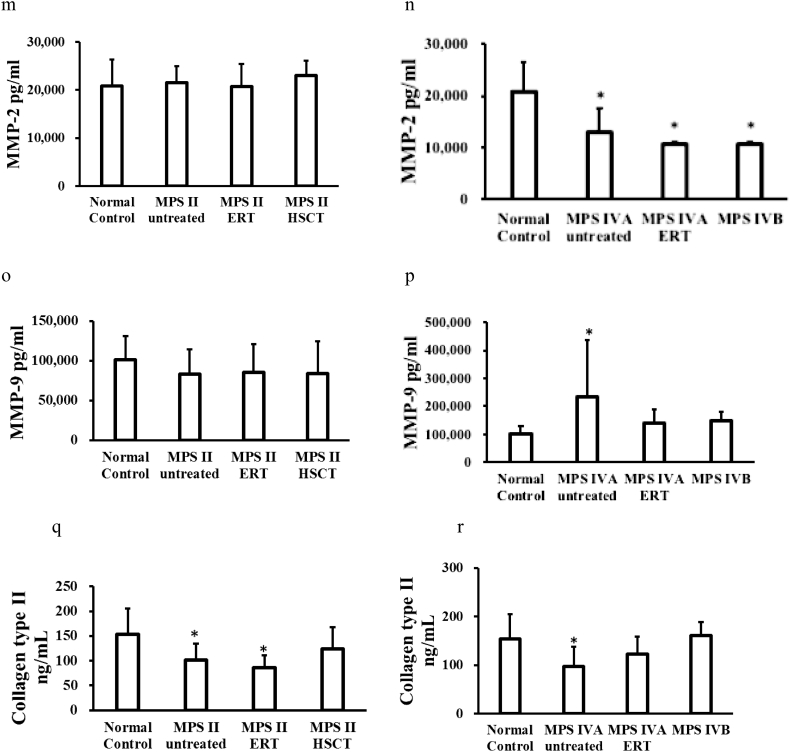
Levels of mono-sulfated KS and di- sulfated KS were significantly elevated in patients with MPS II (untreated, ERT-treated, and post-HSCT) and MPS IVA (untreated and ERT-treated), compared to those in normal controls (Fig. 2). There was no tendency of decrease of KS in both ERT-treated and post-HSCT patients with MPS II as well as ERT-treated patients with MPS IVA, compared with KS in untreated MPS II or MPS IVA patients.
Fig. 2.
Blood GAGs levels in patients with MPS and normal control. (a) HS0S, HSNS, and DS levels in patients with MPS II, (b) HS0S, HSNS, and DS levels in patients with MPS IV, (c) KS levels in patients with MPS II, (d) KS levels in patients with MPS IV. *p < .05 vs normal control group. #p < .05 vs MPS II untreated patients.
3.3. Correlation between biomarkers
We have investigated the correlation between pro-inflammatory factors or PIIANP and specific GAGs. In all patients with MPS II, IVA, and IVB, EGF moderately correlated with DiHS0S, DiHSNS, and DS (Pearson's r; 0.5954, 0.6329, and 0.4037, respectively) while there was no correlation with KS.
PIIANP had a weak to moderate negative correlation with DiHS0S, DiHSNS, and DS (Pearson's r; −0.3829, −0.4011, and − 0.3273, respectively) while there was no correlation with KS.
3.4. Correlation between biomarkers and age
In all MPS patients, mono-sulfated KS correlated negatively with age (Pearson's r; −0.5255).
In normal controls, EGF, DiHS0S, and mono-sulfated KS correlated negatively with age (Pearson's r; −0.4372, −0.535, −0.5405 respectively). In untreated MPS II patients, MMP-2 and DiHS6S correlated negatively with age, and Col II correlated positively with age (Pearson's r; −0.4442, −0.4726, 0.5131 respectively). In MPS II patients under ERT, IL-6 and DiHS0S correlated negatively with age (Pearson's r; −0.6723, −0.5607 respectively). In untreated MPS IVA patients, mono-sulfated KS, and di-sulfated KS correlated negatively with age (Pearson's r; −0.6714, −0.4272 respectively). In MPS II patients post-HSCT, MPS IVB patients and MPS IVA patients under ERT, no biomarkers correlated with age.
3.5. Correlation between biomarkers and BMI
In MPS II patients, a positive relationship was seen between BMI and DiHS0S, DiHSNS, DS and Di-sulfated KS (Pearson's r; 0.723, 0.703, 0.732 and 0.642, respectively) while there was no correlation between BMI and biomarkers in MPS IVA patients.
4. Discussion
In this study, we have demonstrated that pro-inflammatory factors are potential biomarkers in patients with MPS II and IVA and that there is a correlation between specific pro-inflammatory factors and GAGs.
HS and DS levels in blood were significantly elevated in both untreated and ERT-treated MPS II patients, compared to those in normal controls while HS and DS levels were significantly reduced in post-HSCT MPS II patients, compared with untreated patients (Fig. 2a). This finding indicates that HSCT could provide more therapeutic efficacy than ERT, compatible with the previous data [18,19]. However, in post-HSCT MPS II patients, HS0S and HSNS levels were still elevated, compared to those in healthy controls while DS level was normalized. HS is involved in multiple biological functions including modulating the activity of growth factors [20]. MPS I, II, III, and VII patients cannot properly degrade HS and its accumulation in the brain causes neurological symptoms. Tomatsu et al. indicated that blood HS levels in MPS I, II, III, VI, and VII are elevated with a sandwich ELIZA method [21] and that patients with a severe form had a higher elevation of HS in blood [22]. Patients with untreated MPS II had higher levels of DS and HS in blood while untreated MPS III had higher levels of HS in blood than age-matched controls [18]. The levels of DS and HS were higher in all newborn dried blood spots of MPS I and II patients, compared to control newborns [15,23,24]. Thus, our results were matched with previous studies, proving that HS is a useful biomarker in MPS II patients and that HS can be valuable for monitoring therapeutic efficacy.
Our results showed that mono-sulfated KS and di-sulfated KS were significantly elevated in patients with MPS II (untreated, under ERT, and post-HSCT) and MPS IVA (untreated and under ERT), compared with those in normal controls, suggesting that ERT and/or HSCT did not reduce KS levels in the blood. There was no improvement in the bone lesion in MPS IVA patients with ERT in this study, indicating that the measurement of blood KS is essential as a biomarker of the bone lesion as previously suggested [18]. Bone lesions can be evaluated by growth (height) [[25], [26], [27], [28], [29], [30]], skeletal survey with X-rays, CT and MRI [2,27,[31], [32], [33]], BMD [34], surgical history [35,36], and pathological findings from surgical remnants [37,38]. In MPS I, IVA, and VII mice, the severity of skeletal disease displayed by micro-CT, radiographs, and histopathology correlated with the level of KS elevation [39]. KS level was normalized after neonatal bone marrow transplantation in MPS I mice [40]. Patients with MPS II had a secondary elevation of KS in spite of the normal levels of enzymes required to digest KS [20].
MPS IVA and IVB patients cannot properly degrade KS due to a deficiency of GALNS (MPS IVA) and β-galactosidase (MPS IVB). The accumulation of KS disrupts cartilage, resulting in the release of more KS into circulation, which is a critical biomarker for MPS IVA. Blood KS levels in MPS IVA patients were significantly increased when compared to those in age-matched controls [41]. KS level in MPS IVA patients varied with age and clinical severity in patients [[42], [43], [44], [45],56]. Blood KS levels in patients with severe MPS IVA were higher than in the attenuated form [41]. Plasma KS levels varied with age in both control and MPS I, II, III, VI, and VII patients [46,47,48]. Both blood and urine KS are only valuable as biomarkers in younger patients before the growth plate is closed or destroyed since the synthesis of KS decreases clearly after teenage and levels of KS in MPS IVA patients are naturally normalized or subnormal by the age of 20 years [49]. Shimada et al. indicated that blood mono- and di-sulfated KS levels in patients with MPS IVA are significantly higher than those in age-matched control subjects, that the level of blood di-sulfated KS distinguishes control subjects and patients with MPS IVA more clearly than that of mono-sulfated KS, and that blood mono- and di-sulfated KS levels are significantly higher in patients with MPS II than those in age-matched control subjects [50]. Our results were matched with those in these studies, confirming that mono-sulfated KS and di-sulfated KS are a clear biomarker to reflect bone lesions and therapeutic efficacy in both MPS II and MPS IVA.
Our findings showed that EGF levels are higher in patients with MPS II (untreated, under ERT, and post-HSCT) and untreated MPSIVA, compared with those in normal controls and that these patients have higher GAGs levels as well. Pisano et al. indicated that stimulation of ornithine decarboxylase by EGF and prostaglandins leads to an increase in the synthesis of GAGs in embryonic palate mesenchymal cells [51]. Banecka et al. also showed that an excessive EGF stimulates GAG synthesis in MPS fibroblasts, as it shows that under such conditions, accumulation of GAGs is more prominent in untreated patients with various types of MPS [52]. Genistein is a specific inhibitor of the tyrosine-specific protein kinase activity of the EGF receptor [52], which reduce GAG synthesis [9]. Taken together, the current results confirmed those in previous studies, suggesting that EGF increases GAG synthesis and should be an excellent biomarker to reflect GAG level in patients with MPS.
In this study, we also found in pro-inflammatory factors that 1) the TNF-α level was significantly higher in patients with ERT-treated MPS IVA and untreated MPS IVB, compared with those in normal controls, that 2) IL-1β levels were significantly higher in patients with MPS II (untreated and under ERT) and untreated MPS IVA, compared with those in normal controls, that 3) IL-6 levels were significantly higher in patients with MPS II (untreated and under ERT), MPS IVA (untreated and under ERT), and MPS IVB, compared with those in normal controls, and that 4) macrophage inflammatory protein 1 alpha (MIP-1α) level was significantly higher in patients with untreated MPS IVA, compared with that in normal controls.
In 2005, Simonaro et al. showed that GAG storage in MPS leads to cytokine elevation in MPS cells and animals. Cytokines are produced by MPS chondrocytes, synoviocytes, and macrophages, and significantly up-regulate MMP gene expression [4]. A further study found that GAGs bind TLR4 receptors and activate the TLR4 pathway, leading to TNF-α and another cytokine release [7]. IL-1 affects almost every cell type, often in conjunction with TNF [53]. IL-1 and TNF are pro-inflammatory cytokines which induce autophagy to improve infection controls [5]. Polgreen et al. indicated that the TNF-α level was significantly higher in children and adolescents with MPS, compared to the levels in healthy children. TNF-α was associated with pain and physical disability, despite treatment with ERT and/or HSCT [12]. Jacques et al. also measured pro-inflammatory cytokines and showed the elevation of TNF-α and IL-1β in plasma from MPS II patients with ERT, compared to controls [13]. Donida et al. also indicated that the plasma levels of IL-6 were higher in MPS IVA patients under ERT, compared with controls [14]. MIP-1α is an inflammatory chemokine playing an important role in macrophage recruitment, inducing monocytes to infiltrate the CNS and expanding the activated macrophage-microglial subpopulation. MIP-1α level was significantly higher in MPS IIIB mouse serum, compared to that in normal mice [54]. PPS reduced serum MIP-1α levels in MPS VI rats [8]. MIP-1α levels in MPS IVA patients were significantly higher, compared to healthy controls [55]. Our findings were matched with these previous findings, proving that inflammation with an increase of TNF-α, IL-1β, IL-6 and/or MIP-1α indeed occurs in patients with MPS II and/or IVA.
This study has also demonstrated that MMP-9 levels were significantly higher in patients with untreated MPS IVA, compared with those in normal controls, and that MMP-1 levels were significantly higher in patients with untreated MPS IVA, compared with those in MPS IVA patients under ERT.
Simonaro et al. showed that when the inflammatory cytokines stimulate MPS connective tissue cells, it causes enhanced secretion of several matrix-degrading metalloproteinases (MMPs) [4]. MMPs are proteinases that lead to degradation of extracellular matrix components, as well as increased apoptosis of connective tissue cells and cartilage destruction [5,6]. MMPs are involved in both normal and pathological processes. MMP-1 cleaves interstitial collagens I, II, and III, and digest a number of other ECM and non-ECM molecules [6]. Enhanced expression of MMP-2 and MMP-9 was observed in the MPS animals, associated with abnormal expression of TIMP-1. Expression of MMPs was significantly enhanced in articular chondrocytes [4]. MMPs are involved in numerous neuro-inflammatory diseases, and overproduction of MMPs is coupled with disruption of the blood-brain barrier [56]. MMP-2 and MMP-9 are a suitable biomarker demonstrating CNS involvement. Batzios et al. have indicated that MMP-2 expression was significantly increased in serum, whereas a significant decrease in serum MMP-9 was observed in MPS III patients. In MPS VI patients, the activity and protein levels of MMP-9 are decreased by ERT up to 4 months after starting of the treatment. In a MPS II patient, there was a 2.6-fold increase of MMP-2 level in serum and a 0.25-fold decrease in MMP-9 level [57]. In MPS IVA patients, the MMP-2 level was significantly higher when compared to unaffected controls [55].
Thus, our findings were not compatible with the previous findings in that MMP-2 levels were significantly higher in untreated MPS IVA, and it remains unclear that there was no increase of MMPs in untreated MPS II patients.
In addition, we found that collagen type II was significantly decreased in patients with MPS II (untreated and under ERT) and untreated MPS IVA, compared with those in normal controls. Collagen Type II represents 90% to 95% of the collagen in the extracellular matrix and forms fibrils and fibers intertwined with proteoglycan aggregates [58]. Previous studies have proved that increased apoptosis of MPS chondrocytes leads to a depletion of proteoglycans and total collagen in the cartilage of MPS animals [59]. The study by de Francheschi et al. showed low expression of collagen type II at the protein and molecular levels in MPS IVA patients [60]. Our findings were compatible with the previous results, showing that collagen type II can be as a potential biomarker in patients with MPS II and IVA. It is critical to know whether the increase of collagen type II in patients with post-HSCT MPS II and ERT-treated MPS IVA correlates with specific clinical improvement like skeletal dysplasia.
Overall, in both untreated and under ERT MPS II patients, the same eight biomarkers were significantly elevated, compared with those in normal controls while in post-HSCT MPS II patients, four biomarkers (IL-1β, IL-6, MMP-1, PIIANP) were normalized as HS and DS levels decreased and three biomarkers (EGF, mono-sulfated KS, di-sulfated KS) were still elevated, suggesting HSCT could provide more impact to normalize biomarkers correlating with clinical improvements.
Meanwhile, three biomarkers (IL-6, TNF-α, MMP-1) were significantly different between untreated MPS IVA patients and ERT-treated MPS IVA patients; however, there was no reduction of KS in the ERT-treated patient. This discrepancy between pro-inflammatory factors and blood KS level cannot explain by the currently limited data.
In this study, there was a correlation between several biomarkers and age. In both patients and controls, mono-sulfated KS correlated negatively with age while other biomarkers correlated with age in some specific groups negatively or positively. It is required to consider the age as potential confounders longitudinally when we compare the values of some specific biomarkers between controls and patients. BMI in patients with MPS IVA correlated with age, which was compatible with the previous results [35]. In MPS II patients, a positive relationship was observed between BMI and GAGs. Therefore, it is also imperative to assess BMI longitudinally as a confounder.
It is critical to investigate whether these biomarkers correlate with therapeutic efficacy and its clinical improvement to be used for the potential clinical endpoint. The current data are based on a cross-sectional study, and a limitation of our current study refers to a small number of patients with MPS. Therefore, we need to compare the longitudinal data with large numbers of patients with MPS between the biomarkers and clinical severity or therapeutic efficacy (growth, BMD, pulmonary function, IQ/DQ, ADL, skeletal survey with X-rays, surgical history, and pathological findings from surgical remnants) and correlation between age and the biomarkers. The current data needs to be confirmed by age-dependent groups as well.
5. Conclusion
In conclusion, pro-inflammatory factors and collagen type II, as well as GAGs, can be biomarkers in patients with MPS, depending on the type of MPS and treatment method.
Conflict of interest
All the authors contributed to the Review Article and had no conflict of interest with any other party. Honoka Fujitsuka, Hira Peracha, Kazuki Sawamoto, William Mackenzie, Hironori Kobayashi, Seiji Yamaguchi, Yasuyuki Suzuki, Kenji Orii, Tadao Orii, Toshiyuki Fukao, Robert W. Mason, and Shunji Tomatsu declare that they have no conflict of interests.
Contributions to the project
Honoka Fujitsuka has contributed to the concept and planning of the project, collection of data from the publications, the draft of the manuscript, and reporting of the work described as the primary author.
Kazuki Sawamoto has contributed to the concept and planning of the project, collection of data, data analysis, the draft of the manuscript, and reporting of the work described.
Hira Peracha has contributed to the concept and planning of the project, collection of data from the publications, the draft of the manuscript, and reporting of the work described as the primary author.
Robert W. Mason has contributed to the concept and planning of the project, collection of data, data analysis, the draft of the manuscript, and reporting of the work described.
William Mackenzie has contributed to the concept and planning of the project, spine and knee surgeries, and reporting of the work described.
Hironori Kobayashi has contributed to the concept and planning of the project, interpretation of GAG data, and reporting of the work described.
Seiji Yamaguchi has contributed to the concept and planning of the project, interpretation of GAG data, and reporting of the work described.
Yasuyuki Suzuki has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Kenji Orii has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Tadao Orii has contributed to the concept and planning of the project, interpretation of clinical pictures, images, and GAG data, interpretation of published data and reporting of the work described.
Toshiyuki Fukao has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Shunji Tomatsu is a Principal Investigator for this project and has contributed to the concept and planning of the project, interpretation of published data, and reporting of the work described.
Acknowledgments
This work was supported by grants from The Carol Ann Foundation, Angelo R. Cali & Mary V. Cali Family Foundation, Inc., The Vain and Harry Fish Foundation, Inc, The Bennett Foundation, Jacob Randall Foundation, Austrian and Japanese MPS societies, and Nemours Funds. This work was supported by the project for baby and infant in research of health and development to Adolescent and young adult from Japan Agency for Medical Research and development, AMED, under grant number JP18gk0110017. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of National Institutes of Health (NIH) under grant number P30GM114736. The content of the article has not been influenced by the sponsors.
References
- 1.Tomatsu S., Kubaski F. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 10, Mucopolysaccharidosis Type II: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 165–209. [Google Scholar]
- 2.Sawamoto K., Alméciga-Díaz C.J. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 12, Mucopolysaccharidosis Type IVA: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 235–271. [Google Scholar]
- 3.Higaki K., Ninomiya H. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 13, Mucopolysaccharidosis Type IVB: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 273–283. [Google Scholar]
- 4.Simonaro C.M., D'Angelo M., Haskins M.E., Schuchman E.H. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr. Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 5.Simonaro C.M. vol. 1. Nova Science Publishers; New York: 2018. Chapter 6, Inflammation and Its Role in the Lysosomal Storage Disorders, Mucopolysaccharidoses Update vol.1; pp. 75–86. [Google Scholar]
- 6.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 7.Simonaro C.M., Ge Y., Eliyahu E., He X., Jepsen K.J., Schuchman E.H. Involvement of the Toll-like receptor 4 pathway and use of TNF- antagonists for treatment of the mucopolysaccharidoses. Proc. Natl. Acad. Sci. 2010;107:222–227. doi: 10.1073/pnas.0912937107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuchman E.H., Ge Y., Lai A., Borisov Y., Faillace M., Eliyahu E., He X., Iatridis J., Vlassara H., Striker G., Simonaro C.M. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonaro C.M., D'Angelo M., He X., Eliyahu E., Shtraizent N., Haskins M.E., Schuchman E.H. Mechanism of glycosaminoglycan-mediated bone and joint disease implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennermann J.B., Gökce S., Solyom A., Mengel E., Schuchman E.H., Simonaro C.M. Treatment with pentosan polysulphate in patients with MPS I: results from an open label, randomized, monocentric phase II study. J. Inherit. Metab. Dis. 2016;39(6):831–837. doi: 10.1007/s10545-016-9974-5. [DOI] [PubMed] [Google Scholar]
- 11.Orii K., Tomatsu S., Suzuki Y., Solyom A., Radsak M., Schuchman E.H., Simonaro C.M., Orii T., Fukao T. Safety study of sodium pentosan polysulfate for adult patients with mucopolysaccharidosis type II. Mol. Genet. Metab. 2016;117(2):S88. doi: 10.3390/diagnostics9040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polgreen L.E., Vehe R.K., Rudser K., Kunin-Batson A., Utz J.J., Dickson P., Shapiro E., Whitley C.B. Elevated TNF-α is associated with pain and physical disability in mucopolysaccharidosis types I, II, and VI. Mol. Genet. Metab. 2016;117:427–430. doi: 10.1016/j.ymgme.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques C.E., Donida B., Mescka C.P., Rodrigues D.G., Marchetti D.P., Bitencourt F.H., Burin M.G., de Souza C.F., Giugliani R., Vargas C.R. Oxidative and nitrative stress and pro-inflammatory cytokines in Mucopolysaccharidosis type II patients: effect of long-term enzyme replacement therapy and relation with glycosaminoglycan accumulation. Biochim. Biophys. Acta Mol. basis Dis. 2016;1862:1608–1616. doi: 10.1016/j.bbadis.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Donida B., Marchetti D.P., Biancini G.B., Deon M., Manini P.R., da Rosa H.T., Moura D.J., Saffi J., Bender F., Burin M.G., Coitinho A.S., Giugliani R., Vargas C.R. Oxidative stress and inflammation in mucopolysaccharidosis type IVA patients treated with enzyme replacement therapy. Biochim. Biophys. Acta Mol. basis Dis. 2015;1852:1012–1019. doi: 10.1016/j.bbadis.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tomatsu S., Fujii T., Fukushi M., Oguma T., Shimada T., Maeda M., Kida K., Shibata Y., Futatsumori H., Montaño A.M., Mason R.W., Yamaguchi S., Suzuki Y., Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oguma T., Tomatsu S., Montano A.M., Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007;368(1):79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Shimada T., Tomatsu S., Yasuda E., Mason R.W., Mackenzie W.G., Shibata Y., Kubaski F., Giugliani R., Yamaguchi S., Suzuki Y., Orii K., Orii T. Chondroitin6-Sulfate as a Novel Biomarker for Mucopolysaccharidosis IVA and VII. JIMD Rep. 2014;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S.A., Mason R.W., Giugliani R., Orii K., Fukao T., Suzuki Y., Yamaguchi S., Kobayashi H., Orii T., Tomatsu S. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018;125(1–2):44–52. doi: 10.1016/j.ymgme.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubaski F., Yabe H., Suzuki Y., Seto T., Hamazaki T., Mason R.W., Xie L., Onsten T.G.H., Leistner-Segal S., Giugliani R., Dũng V.C., Ngoc C.T.B., Yamaguchi S., Montaño A.M., Orii K., Fukao T., Shintaku H., Orii T., Tomatsu S. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol Blood Marrow Transplant. 2017;23(10):1795–1803. doi: 10.1016/j.bbmt.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapraeger A.C., Krufka A., Olwin B.B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 21.Tomatsu S., Gutierrez M.A., Ishimaru T., Peña O.M., Montaño A.M., Maeda H., Velez-Castrillon S., Nishioka T., Fachel A.A., Cooper A., Thornley M., Wraith E., Barrera L.A., Laybauer L.S., Giugliani R., Schwartz I.V., Frenking G.S., Beck M., Kircher S.G., Paschke E., Yamaguchi S., Ullrich K., Isogai K., Suzuki Y., Orii T., Noguchi A. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:743–757. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 22.Tomatsu S., Shimada T., Mason R., Montaño A., Kelly J., LaMarr W., Kubaski F., Giugliani R., Guha A., Yasuda E., Mackenzie W., Yamaguchi S., Suzuki Y., Orii T. Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Meta. 2014;4:655–679. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Ruijter J., de Ru M.H., Wagemans T., Ijlst L., Lund A.M., Orchard P.J., Schaefer G.B., Wijburg F.A., van Vlies N. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol. Genet. Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Kubaski F., Mason R.W., Nakatomi A., Shintaku H., Xie L., van Vlies N.N., Church H., Giugliani R., Kobayashi H., Yamaguchi S., Suzuki Y., Orii T., Fukao T., Montaño A.M., Tomatsu S. Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J. Inherit. Metab. Dis. 2017;40(1):151–158. doi: 10.1007/s10545-016-9981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomatsu S., Alméciga-Díaz C.J., Montaño A.M., Yabe H., Tanaka A., Dung V.C., Giugliani R., Kubaski F., Mason R.W., Yasuda E., Sawamoto K., Mackenzie W., Suzuki Y., Orii K.E., Barrera L.A., Sly W.S., Orii T. Therapies for the bone in mucopolysaccharidoses. Mol. Genet. Metab. 2015;114(2):94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomatsu S., Montaño Adriana M., Oikawa H., Giugliani R., Harmatz P., Smith M., Suzuki Y., Orii T. Springer Publications; London: 2011. Chapter 126: impairment of body growth in mucopolysaccharidoses, handbook of growth monitoring and health and disease. [Google Scholar]
- 27.Montaño A.M., Tomatsu S., Brusius A., Smith M., Orii T. Growth charts for patients affected with Morquio a disease. Am. J. Med. Genet. A. 2008;146A(10):1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 28.Chinen Y., Higa T., Tomatsu S., Suzuki Y., Orii T., Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yabe H., Tanaka A., Chinen Y., Kato S., Sawamoto K., Yasuda E., Shintaku H., Suzuki Y., Orii T., Tomatsu S. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol. Genet. Metab. 2016;117(2):84–94. doi: 10.1016/j.ymgme.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendriksz C.J., Harmatz P., Beck M., Jones S., Wood T., Lachman R., Gravance C.G., Orii T., Tomatsu S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013;110(1–2):54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomatsu S., Averill L.W., Sawamoto K., Mackenzie W.G., Bober M.B., Pizarro C., Goff C.J., Xie L., Orii T., Theroux M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016;117(2):150–156. doi: 10.1016/j.ymgme.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro C., Davies R.R., Theroux M., Spurrier E.A., Averill L.W., Tomatsu S. Surgical reconstruction for severe tracheal obstruction in morquio A syndrome. Ann. Thorac. Surg. 2016;102(4):329–331. doi: 10.1016/j.athoracsur.2016.02.113. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi A., Montaño A.M., Colón J.E., Oguma T., Luisiri A., Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Acta Paediatr. 2009;98(5):910–912. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 34.Kecskemethy H.H., Kubaski F., Harcke H.T., Tomatsu S. Bone mineral density in MPS IV A (Morquio syndrome type A) Mol. Genet. Metab. 2016;117(2):144. doi: 10.1016/j.ymgme.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda E., Suzuki Y., Shimada T., Sawamoto K., Mackenzie W.G., Theroux M.C., Pizarro C., Xie L., Miller F., Rahman T., Kecskemethy H.H., Nagao K., Morlet T., Shaffer T.H., Chinen Y., Yabe H., Tanaka A., Shintaku H., Orii K.E., Orii K.O., Mason R.W., Montaño A.M., Fukao T., Orii T., Tomatsu S. Activity of daily living for Morquio A syndrome. Mol. Genet. Metab. 2016;118(2):111–122. doi: 10.1016/j.ymgme.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montaño A.M., Tomatsu S., Gottesman G.S., Smith M., Orii T. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007;30(2):165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 37.Doherty C., Averill L.W., Theroux M., Mackenzie W.G., Pizarro C., Mason R.W., Tomatsu S. Natural history of Morquio A patient with tracheal obstruction from birth to death. Mol Genet Metab Rep. 2018;14:59–67. doi: 10.1016/j.ymgmr.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda E., Fushimi K., Suzuki Y., Shimizu K., Takami T., Zustin J., Patel P., Ruhnke K., Shimada T., Boyce B., Kokas T., Barone C., Theroux M., Mackenzie W., Nagel B., Ryerse J.S., Orii K.E., Iida H., Orii T., Tomatsu S. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013;109(3):301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Rowan D.J., Tomatsu S., Grubb J.H., Montaño A.M., Sly W.S. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013;36(2):235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pievani A., Azario I., Antolini L., Shimada T., Patel P., Remoli C., Rambaldi B., Valsecchi M.G., Riminucci M., Biondi A., Tomatsu S., Serafini M. Neonatal bone marrow transplantation prevents bone pathology in a mouse model of mucopolysaccharidosis type I. Blood. 2015;125:1662–1671. doi: 10.1182/blood-2014-06-581207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomatsu S., Montaño A.M., Oguma T., Dung V.C., Oikawa H., de Carvalho T.G., Gutiérrez M.L., Yamaguchi S., Suzuki Y., Fukushi M., Kida K., Kubota M., Barrera L., Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J. Inherit. Metab. Dis. 2010;33:35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 42.Tomatsu S., Okamura K., Taketani T., Orii K.O., Nishioka T., Gutierrez M.A., Velez-Castrillon S., Fachel A.A., Grubb J.H., Cooper A., Thornley M., Wraith E., Barrera L.A., Giugliani R., Schwartz I.V., Frenking G.S., Beck M., Kircher S.G., Paschke E., Yamaguchi S., Ullrich K., Isogai K., Suzuki Y., Orii T., Kondo N., Creer M., Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr. Res. 2004;55(4):592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 43.Hintze J.P., Tomatsu S., Fujii T., Montaño A.M., Yamaguchi S., Suzuki Y., Fukushi M., Ishimaru T., Orii T. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark. Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomatsu S., Montaño A.M., Oguma T., Dung V.C., Oikawa H., de Carvalho T.G., Gutiérrez M.L., Yamaguchi S., Suzuki Y., Fukushi M., Kida K., Kubota M., Barrera L., Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J. Inherit. Metab. Dis. 2010;33:35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 45.Shimada T., Tomatsu S., Mason R.W., Yasuda E., Mackenzie W.G., Hossain J., Shibata Y., Montaño A.M., Kubaski F., Giugliani R., Yamaguchi S., Suzuki Y., Orii K.E., Fukao T., Orii T. Di-sulfated keratan sulfate as a novel biomarker for mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan S.A., Mason R.W., Giugliani R., Orii K., Fukao T., Suzuki Y., Yamaguchi S., Kobayashi H., Orii T., Tomatsu S. Glycosaminoglycans analysis in blood and urine of mucopolysaccharidoses by tandem mass spectrometry. Mol. Genet. Metab. 2018;125(1–2):44–52. doi: 10.1016/j.ymgme.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomatsu S., Okamura K., Taketani T., Orii K.O., Nishioka T., Gutierrez M.A., Velez-Castrillon S., Fachel A.A., Grubb J.H., Cooper A., Thornley M., Wraith E., Barrera L.A., Giugliani R., Schwartz I.V., Frenking G.S., Beck M., Kircher S.G., Paschke E., Yamaguchi S., Ullrich K., Isogai K., Suzuki Y., Orii T., Kondo N., Creer M., Noguchi A. Keratan sulfate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 48.Kubaski F., Suzuki Y., Orii K., Giugliani R., Church H.J., Mason R.W., Dũng V.C., Ngoc C.T., Yamaguchi S., Kobayashi H., Girisha K.M., Fukao T., Orii T., Tomatsu S. Glycosaminoglycan levels in dried blood spots of patients with mucopolysaccharidoses and mucolipidoses. Mol. Genet. Metab. 2017;120(3):247–254. doi: 10.1016/j.ymgme.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomatsu S., Sawamoto K., Shimada T., Bober M.B., Kubaski F., Yasuda E., Mason R.W., Khan S., Alméciga-Díaz C.J., Barrera L.A., Mackenzie W.G., Orii T. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin. Orphan Drugs. 2015;3:1279–1290. doi: 10.1517/21678707.2015.1086640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada T., Tomatsu S., Mason R.W., Yasuda E., Mackenzie W.G., Hossain J., Shibata Y., Montaño A.M., Kubaski F., Giugliani R., Yamaguchi S., Suzuki Y., Orii K.E., Fukao T., Orii T. Di-sulfated keratan sulfate as a novel biomarker for mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisano M.M., Greene R.M. Epidermal growth factor potentiates the induction of ornithine decarboxylase activity by prostaglandins in embryonic palate mesenchymal cells: Effects on cell proliferation and glycosaminoglycan synthesis. Dev. Biol. 1987;122(2):419–431. doi: 10.1016/0012-1606(87)90306-x. [DOI] [PubMed] [Google Scholar]
- 52.Jakóbkiewicz-Banecka J., Piotrowska E., Narajczyk M., Barańska S., Wegrzyn G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factordependent pathway. J. Biomed. Sci. 2009;16(1):26. doi: 10.1186/1423-0127-16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 54.Natale P.D., Domenico C.D., Napoli D.D. Serum MIP-1 α level: a biomarker for the follow-up of lentiviral therapy in mucopolysaccharidosis IIIB mice. J. Inherit. Metab. Dis. 2010;33:159–165. doi: 10.1007/s10545-010-9051-4. [DOI] [PubMed] [Google Scholar]
- 55.Martell L., Lau K., Mei M., Burnett V., Decker C., Foehr E.D. Biomarker analysis of Morquio syndrome: identification of disease state and drug responsive markers. Orphanet J Rare Dis. 2011;6:84. doi: 10.1186/1750-1172-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mun-Bryce S., Rosenberg G.A. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am. J. Phys. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. http://www.ncbi.nlm.nih.gov/pubmed/9644031 [DOI] [PubMed] [Google Scholar]
- 57.Batzios S.P., Zafeiriou D.I., Vargiami E., Karakiulakis G., Papakonstantinou E. Differential expression of matrix metalloproteinases in the serum of patients with mucopolysaccharidoses. JIMD Rep. 2012;3:59–66. doi: 10.1007/8904_2011_58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox A.J.S., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simonaro C.M., Haskins M.E., Schuchman E.H. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab. Investig. 2001;81:1319–1328. doi: 10.1038/labinvest.3780345. http://www.ncbi.nlm.nih.gov/pubmed/11555679 [DOI] [PubMed] [Google Scholar]
- 60.Franceschi L.D., Roseti L., Desando G., Facchini A., Yz M.D., Grigolo B. A molecular and histological characterization of cartilage from patients with Morquio syndrome. Osteoarthr. Cartil. 2007;15:1311–1317. doi: 10.1016/j.joca.2007.04.008. [DOI] [PubMed] [Google Scholar]