Abstract
The American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America jointly sponsored the development of this guideline for the treatment of drug-susceptible tuberculosis, which is also endorsed by the European Respiratory Society and the US National Tuberculosis Controllers Association. Representatives from the American Academy of Pediatrics, the Canadian Thoracic Society, the International Union Against Tuberculosis and Lung Disease, and the World Health Organization also participated in the development of the guideline. This guideline provides recommendations on the clinical and public health management of tuberculosis in children and adults in settings in which mycobacterial cultures, molecular and phenotypic drug susceptibility tests, and radiographic studies, among other diagnostic tools, are available on a routine basis. For all recommendations, literature reviews were performed, followed by discussion by an expert committee according to the Grading of Recommendations, Assessment, Development and Evaluation methodology. Given the public health implications of prompt diagnosis and effective management of tuberculosis, empiric multidrug treatment is initiated in almost all situations in which active tuberculosis is suspected. Additional characteristics such as presence of comorbidities, severity of disease, and response to treatment influence management decisions. Specific recommendations on the use of case management strategies (including directly observed therapy), regimen and dosing selection in adults and children (daily vs intermittent), treatment of tuberculosis in the presence of HIV infection (duration of tuberculosis treatment and timing of initiation of antiretroviral therapy), as well as treatment of extrapulmonary disease (central nervous system, pericardial among other sites) are provided. The development of more potent and better-tolerated drug regimens, optimization of drug exposure for the component drugs, optimal management of tuberculosis in special populations, identification of accurate biomarkers of treatment effect, and the assessment of new strategies for implementing regimens in the field remain key priority areas for research. See the full-text online version of the document for detailed discussion of the management of tuberculosis and recommendations for practice.
Keywords: Mycobacterium tuberculosis, HIV infections, antitubercular agents, case management, public health
EXECUTIVE SUMMARY
The American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC), and Infectious Diseases Society of America (IDSA) jointly sponsored the development of this guideline on the treatment of drug-susceptible tuberculosis, which is also endorsed by the European Respiratory Society (ERS) and the US National Tuberculosis Controllers Association (NTCA). This guideline provides recommendations on the clinical and public health management of tuberculosis in children and adults in settings in which mycobacterial cultures, molecular and phenotypic drug susceptibility tests, and radiographic studies, among other diagnostic tools, are available on a routine basis. Nine PICO (population, intervention, comparators, outcomes) questions and associated recommendations, developed based on the evidence that was appraised using GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology [1, 2], are summarized below. A carefully selected panel of experts, screened for conflicts of interest, including specialists in pulmonary medicine, infectious diseases, pharmacokinetics, pediatrics, primary care, public health, and systematic review methodology were assembled and used GRADE methods to assess the certainty in the evidence (also known as the quality of evidence) and strength of the recommendations (see Supplementary Appendix A: Methods and Table 1). This executive summary is a condensed version of the panel's recommendations. Additional detailed discussion of the management of pulmonary and extrapulmonary tuberculosis is available in the full-text version of this guideline.
Table 1.
Interpretation of “Strong” and “Conditional” Grading of Recommendations Assessment, Development, and Evaluation-Based Recommendations
| Implications for: | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policy | The recommendation can be adopted as policy in most situations. | Policymaking will require substantial debate and involvement of various stakeholders. |
OBJECTIVES OF ANTITUBERCULOSIS THERAPY
Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission of Mycobacterium tuberculosis to other persons, thus, successful treatment of tuberculosis has benefits both for the individual patient and the community in which the patient resides.
The objectives of tuberculosis therapy are (1) to rapidly reduce the number of actively growing bacilli in the patient, thereby decreasing severity of the disease, preventing death and halting transmission of M. tuberculosis; (2) to eradicate populations of persisting bacilli in order to achieve durable cure (prevent relapse) after completion of therapy; and (3) to prevent acquisition of drug resistance during therapy.
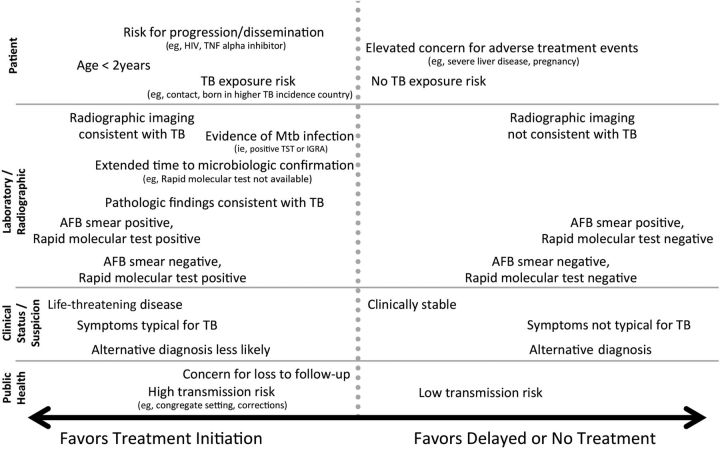
The decision to initiate combination chemotherapy for tuberculosis is based on clinical, radiographic, laboratory, patient, and public health factors (Figure 1). In addition, clinical judgment and the index of suspicion for tuberculosis are critical in making a decision to initiate treatment. For example, in patients (children and adults) who, based on these considerations, have a high likelihood of having tuberculosis or are seriously ill with a disorder suspicious for tuberculosis, empiric treatment with a 4-drug regimen (Tables 2 and 3) should be initiated promptly even before the results of acid-fast bacilli (AFB) smear microscopy, molecular tests, and mycobacterial culture are known.
Figure 1.
Factors to be considered in deciding to initiate treatment empirically for active tuberculosis (TB) (prior to microbiologic confirmation). Abbreviations: AFB, acid-fast bacilli; HIV, human immunodeficiency virus; IGRA, interferon-γ release assay; Mtb, Mycobacterium tuberculosis; TNF, tumor necrosis factor; TST, tuberculin skin test.
Table 2.
Drug Regimens for Microbiologically Confirmed Pulmonary Tuberculosis Caused by Drug-Susceptible Organisms
| Regimen | Intensive Phase |
Continuation Phase |
Range of Total Doses | Commentsc,d | Regimen Effectiveness | ||
|---|---|---|---|---|---|---|---|
| Druga | Interval and Doseb (Minimum Duration) | Drugs | Interval and Doseb,c (Minimum Duration) | ||||
| 1 | INH RIF PZA EMB |
7 d/wk for 56 doses (8 wk), or 5 d/wk for 40 doses (8 wk) |
INH RIF |
7 d/wk for 126 doses (18 wk), or 5 d/wk for 90 doses (18 wk) |
182–130 | This is the preferred regimen for patients with newly diagnosed pulmonary tuberculosis. | Greater Lesser |
| 2 | INH RIF PZA EMB |
7 d/wk for 56 doses (8 wk), or 5 d/wk for 40 doses (8 wk) |
INH RIF |
3 times weekly for 54 doses (18 wk) | 110–94 | Preferred alternative regimen in situations in which more frequent DOT during continuation phase is difficult to achieve. | |
| 3 | INH RIF PZA EMB |
3 times weekly for 24 doses (8 wk) | INH RIF |
3 times weekly for 54 doses (18 wk) | 78 | Use regimen with caution in patients with HIV and/or cavitary disease. Missed doses can lead to treatment failure, relapse, and acquired drug resistance. | |
| 4 | INH RIF PZA EMB |
7 d/wk for 14 doses then twice weekly for 12 dosese | INH RIF |
Twice weekly for 36 doses (18 wk) | 62 | Do not use twice-weekly regimens in HIV-infected patients or patients with smear-positive and/or cavitary disease. If doses are missed, then therapy is equivalent to once weekly, which is inferior. | |
Abbreviations: DOT, directly observed therapy; EMB, ethambutol; HIV, human immunodeficiency virus; INH, isoniazid; PZA, pyrazinamide; RIF, rifampin.
a Other combinations may be appropriate in certain circumstances; additional details are provided in the section “Recommended Treatment Regimens.”
b When DOT is used, drugs may be given 5 days per week and the necessary number of doses adjusted accordingly. Although there are no studies that compare 5 with 7 daily doses, extensive experience indicates this would be an effective practice. DOT should be used when drugs are administered <7 days per week.
c Based on expert opinion, patients with cavitation on initial chest radiograph and positive cultures at completion of 2 months of therapy should receive a 7-month (31-week) continuation phase.
d Pyridoxine (vitamin B6), 25–50 mg/day, is given with INH to all persons at risk of neuropathy (eg, pregnant women; breastfeeding infants; persons with HIV; patients with diabetes, alcoholism, malnutrition, or chronic renal failure; or patients with advanced age). For patients with peripheral neuropathy, experts recommend increasing pyridoxine dose to 100 mg/day.
e See [426]. Alternatively, some US tuberculosis control programs have administered intensive-phase regimens 5 days per week for 15 doses (3 weeks), then twice weekly for 12 doses.
Table 3.
Dosesa of Antituberculosis Drugs for Adults and Childrenb
| Drug | Preparation | Population | Daily | Once-Weekly | Twice-Weekly | Thrice-Weekly |
|---|---|---|---|---|---|---|
| First-line drugs | ||||||
| Isoniazid | Tablets (50 mg, 100 mg, 300 mg); elixir (50 mg/5 mL); aqueous solution (100 mg/mL) for intravenous or intramuscular injection. Note: Pyridoxine (vitamin B6), 25–50 mg/day, is given with INH to all persons at risk of neuropathy (eg, pregnant women; breastfeeding infants; persons with HIV; patients with diabetes, alcoholism, malnutrition, or chronic renal failure; or patients with advanced age). For patients with peripheral neuropathy, experts recommend increasing pyridoxine dose to 100 mg/d. | Adults | 5 mg/kg (typically 300 mg) | 15 mg/kg (typically 900 mg) | 15 mg/kg (typically 900 mg) | 15 mg/kg (typically 900 mg) |
| Children | 10–15 mg/kg | … | 20–30 mg/kg | …b | ||
| Rifampin | Capsule (150 mg, 300 mg). Powder may be suspended for oral
administration. Aqueous solution for intravenous injection. |
Adultsc | 10 mg/kg (typically 600 mg) | … | 10 mg/kg (typically 600 mg) | 10 mg/kg (typically 600 mg) |
| Children | 10–20 mg/kg | … | 10–20 mg/kg | …b | ||
| Rifabutin | Capsule (150 mg) | Adultsd | 5 mg/kg (typically 300 mg) | … | Not recommended | Not recommended |
| Children | Appropriate dosing for children is unknown. Estimated at 5 mg/kg. | |||||
| Rifapentine | Tablet (150 mg film coated) | Adults | 10–20 mg/kge | … | … | |
| Children | Active tuberculosis: for children ≥12 y of age, same dosing as for adults, administered once weekly. Rifapentine is not FDA-approved for treatment of active tuberculosis in children <12 y of age. | |||||
| Pyrazinamide | Tablet (500 mg scored) | Adults | See Table 10 | … | See Table 10 | See Table 10 |
| Children | 35 (30–40) mg/kg | … | 50 mg/kg | …b | ||
| Ethambutol | Tablet (100 mg; 400 mg) | Adults | See Table 11 | … | See Table 11 | See Table 11 |
| Childrenf | 20 (15–25) mg/kg | … | 50 mg/kg | …b | ||
| Second-line drugs | ||||||
| Cycloserine | Capsule (250 mg) | Adultsg | 10–15 mg/kg total (usually 250–500 mg once or twice daily) | There are inadequate data to support intermittent administration. | ||
| Children | 15–20 mg/kg total (divided 1–2 times daily) | |||||
| Ethionamide | Tablet (250 mg) | Adultsh | 15–20 mg/kg total (usually 250–500 mg once or twice daily) | There are inadequate data to support intermittent administration. | ||
| Children | 15–20 mg/kg total (divided 1–2 times daily) | |||||
| Streptomycin | Aqueous solution (1 g vials) for IM or IV administration. | Adults | 15 mg/kg daily. Some clinicians prefer 25
mg/kg 3 times weekly. Patients with decreased renal function may require the 15 mg/kg dose to be given only 3 times weekly to allow for drug clearance. |
|||
| Children | 15–20 mg/kg [427] | … | 25–30 mg/kgi | … | ||
| Amikacin/kanamycin | Aqueous solution (500 mg and 1 g vials) for IM or IV administration. | Adults | 15 mg/kg daily. Some clinicians prefer 25
mg/kg 3 times weekly. Patients with decreased renal function, including older patients, may require the 15 mg/kg dose to be given only 3 times weekly to allow for drug clearance. |
|||
| Children | 15–20 mg/kg [427] | … | 25–30 mg/kgi | … | ||
| Capreomycin | Aqueous solution (1 g vials) for IM or IV administration. | Adults | 15 mg/kg daily. Some clinicians prefer 25
mg/kg 3 times weekly. Patients with decreased renal function, including older patients, may require the 15 mg/kg dose to be given only 3 times weekly to allow for drug clearance. |
|||
| Children | 15–20 mg/kg [427] | … | 25–30 mg/kgi | … | ||
| Para-amino salicylic acid | Granules (4 g packets) can be mixed in and ingested with soft food (granules should not be chewed). Tablets (500 mg) are still available in some countries, but not in the United States. A solution for IV administration is available in Europe. | Adults | 8–12 g total (usually 4000 mg 2–3 times daily) | There are inadequate data to support intermittent administration. | ||
| Children | 200–300 mg/kg total (usually divided 100 mg/kg given 2 to 3 times daily) | |||||
| Levofloxacin | Tablets (250 mg, 500 mg, 750 mg); aqueous solution (500 mg vials) for IV injection. | Adults | 500–1000 mg daily | There are inadequate data to support intermittent administration. | ||
| Children | The optimal dose is not known, but clinical data suggest 15–20 mg/kg [427] | |||||
| Moxifloxacin | Tablets (400 mg); aqueous solution (400 mg/250 mL) for IV injection | Adults | 400 mg daily | There are inadequate data to support intermittent administration.j | ||
| Children | The optimal dose is not known. Some experts use 10 mg/kg daily dosing, though lack of formulations makes such titration challenging. Aiming for serum concentrations of 3–5 µL/mL 2 h postdose is proposed by experts as a reasonable target. | |||||
Abbreviations: FDA, US Food and Drug Administration; HIV, human immunodeficiency virus; IM, intramuscular; INH, isoniazid; IV, intravenous.
a Dosing based on actual weight is acceptable in patients who are not obese. For obese patients (>20% above ideal body weight [IBW]), dosing based on IBW may be preferred for initial doses. Some clinicians prefer a modified IBW (IBW + [0.40 × (actual weight – IBW)]) as is done for initial aminoglycoside doses. Because tuberculosis drug dosing for obese patients has not been established, therapeutic drug monitoring may be considered for such patients.
b For purposes of this document, adult dosing begins at age 15 years or at a weight of >40 kg in younger children. The optimal doses for thrice-weekly therapy in children and adolescents have not been established. Some experts use in adolescents the same doses as recommended for adults, and for younger children the same doses as recommended for twice-weekly therapy.
c Higher doses of rifampin, currently as high as 35 mg/kg, are being studied in clinical trials.
d Rifabutin dose may need to be adjusted when there is concomitant use of protease inhibitors or nonnucleoside reverse transcriptase inhibitors.
e TBTC Study 22 used rifapentine (RPT) dosage of 10 mg/kg in the continuation phase of treatment for active disease [9]. However, RIFAQUIN and PREVENT TB safely used higher dosages of RPT, administered once weekly [164, 210]. Daily doses of 1200 mg RPT are being studied in clinical trials for active tuberculosis disease.
f As an approach to avoiding ethambutol (EMB) ocular toxicity, some clinicians use a 3-drug regimen (INH, rifampin, and pyrazinamide) in the initial 2 months of treatment for children who are HIV-uninfected, have no prior tuberculosis treatment history, are living in an area of low prevalence of drug-resistant tuberculosis, and have no exposure to an individual from an area of high prevalence of drug-resistant tuberculosis. However, because the prevalence of and risk for drug-resistant tuberculosis can be difficult to ascertain, the American Academy of Pediatrics and most experts include EMB as part of the intensive-phase regimen for children with tuberculosis.
g Clinicians experienced with using cycloserine suggest starting with 250 mg once daily and gradually increasing as tolerated. Serum concentrations often are useful in determining the appropriate dose for a given patient. Few patients tolerate 500 mg twice daily.
h Ethionamide can be given at bedtime or with a main meal in an attempt to reduce nausea. Clinicians experienced with using ethionamide suggest starting with 250 mg once daily and gradually increasing as tolerated. Serum concentrations may be useful in determining the appropriate dose for a given patient. Few patients tolerate 500 mg twice daily.
i Modified from adult intermittent dose of 25 mg/kg, and accounting for larger total body water content and faster clearance of injectable drugs in most children. Dosing can be guided by serum concentrations.
j RIFAQUIN trial studied a 6-month regimen. Daily isoniazid was replaced by daily moxifloxacin 400 mg for the first 2 months, followed by once-weekly doses of moxifloxacin 400 mg and RPT 1200 mg for the remaining 4 months. Two hundred twelve patients were studied (each dose of RPT was preceded by a meal of 2 hard-boiled eggs and bread). This regimen was shown to be noninferior to a standard daily administered 6-month regimen [164].
Sixty-five years of investigation, including many clinical trials, have consistently supported the necessity of treating with multiple drugs to achieve these treatment objectives, minimize drug toxicity, and maximize the likelihood of treatment completion [3, 4]. The success of drug treatment, however, depends upon many factors, and numerous studies have found an increased risk of relapse among patients with signs of more extensive disease (ie, cavitation or more extensive disease on chest radiograph) [5–9], and/or slower response to treatment (ie, delayed culture conversion at 2–3 months) [4, 6, 10, 11].
ORGANIZATION AND SUPERVISION OF TREATMENT
Because of the public health implications of prompt diagnosis and effective treatment of tuberculosis, most low-incidence countries designate a government public health agency as legal authority for controlling tuberculosis [12, 13]. The optimal organization of tuberculosis treatment often requires the coordination of public and private sectors [14–16]. In most settings, a patient is assigned a public health case manager who assesses needs and barriers that may interfere with treatment adherence [17]. With active input from the patient and healthcare providers, the case manager, together with the patient, develops an individualized “case management plan” with interventions to address the identified needs and barriers [18–20] (see PICO Question 1 and Supplementary Appendix B, Evidence Profiles 1–3). The least restrictive public health interventions that are effective are used to achieve adherence, thereby balancing the rights of the patient and public safety. Given that tuberculosis treatment requires multiple drugs be given for several months, it is crucial that the patient be involved in a meaningful way in making decisions concerning treatment supervision and overall care. International standards have been developed that also emphasize the importance of using patient-centered approaches to the management of tuberculosis [14–16].
Key considerations when developing a case management plan include (1) improving “treatment literacy” by educating the patient about tuberculosis and its treatment, including possible adverse effects [21, 22]; (2) discussing expected outcomes of treatment, specifically the ability to cure the patient of the disease; (3) reviewing methods of adherence support and plans for assessing response to therapy; and (4) discussing infectiousness and infection control measures using terminology that is appropriate to the culture, language, age, and reading level of the patient [23]. For non-English-speaking patients, the use of medical interpreter services is preferred over using family or friends as interpreters [24]. Relevant information should be reinforced at each visit. Other components of the case management plan include, but are not limited to, setting up patient reminders and systems to follow-up missed appointments [23, 25–29], use of incentives and enablers [30, 31], field and home visits [32], and integration and coordination of tuberculosis care with the patient's primary and specialty care (including mental health services, if appropriate and requested by the patient) (Table 4).
Table 4.
Possible Components of a Multifaceted, Patient-Centered Treatment Strategy
| Enablers | Incentives |
|---|---|
| Interventions to assist the patient in completing therapy [130] | Interventions to motivate the patient, tailored to individual patient wishes and needs and, thus, meaningful to the patient [130] |
| Transportation vouchers [30] | Food stamps or snacks and meals [30] |
| Convenient clinic hours and locations [30] | Restaurant and grocery store coupons [30] |
| Clinic personnel who speak the languages of the populations served [428] | Assistance in finding or provision of housing [429] |
| Reminder systems and follow-up of missed appointments [28] | Clothing or other personal products [30] |
| Social service assistance (referrals for substance abuse treatment and counseling, housing, and other services) [429] | Books [428] |
| Outreach workers (bilingual/bicultural as needed; can provide many services related to maintaining patient adherence, including provision of directly observed therapy, follow-up on missed appointments, monthly monitoring, transportation, sputum collection, social service assistance, and educational reinforcement) [428] | Stipends [30] |
| Integration of care for tuberculosis with care for other conditions [428] | Patient contract [30] |
PICO Question 1: Does adding case management interventions to curative therapy improve outcomes compared to curative therapy alone among patients with tuberculosis? (Case management is defined as patient education/counseling, field/home visits, integration/coordination of care with specialists and medical home, patient reminders, and incentives/enablers).
Recommendation 1: We suggest using case management interventions during treatment of patients with tuberculosis (conditional recommendation; very low certainty in the evidence).
Given the critical importance of chemotherapy, both to the patient and to the public, approaches to ensuring adherence to the treatment regimen are a major focus of the overall management plan. To maximize completion of therapy, management strategies should utilize a broad range of approaches (see “Patient-Centered Care and Case Management” in the full-text version of the guideline). Among these, directly observed therapy (DOT), the practice of observing the patient swallow their antituberculosis medications, has been widely used as the standard of practice in many tuberculosis programs, and deserves special emphasis (see PICO Question 2 and Supplementary Appendix B, Evidence Profile 4). The systematic review conducted to obtain evidence in support of this practice guideline did not find any significant differences between self-administered therapy (SAT) and DOT when assessing several outcomes of interest, including mortality, treatment completion, and relapse. However, DOT was significantly associated with improved treatment success (the sum of patients cured and patients completing treatment) and with increased sputum smear conversion during treatment, as compared to SAT. Because DOT is a multifaceted public health intervention that is not amenable to the conventional clinical trial approaches to assessing benefits, and because participation in DOT can be advantageous for early recognition of adverse drug reactions and treatment irregularities, for allowing providers to establish rapport with the patient and for addressing treatment complications expeditiously, DOT remains the standard of practice in the majority of tuberculosis programs in the United States [33–35] and Europe [15] (Table 5). To be consistent with the principles of patient-centered care noted previously, decisions regarding the use of DOT must be made in concert with the patient [14–16]. For example, DOT can be provided in the office, clinic, or in the “field” (patient's home, place of employment, school, or any other site that is mutually agreeable) by appropriately trained personnel [32].
Table 5.
Examples of Priority Situations for the Use of Directly Observed Therapy
| Patients With the Following Conditions/Circumstances [17, 130, 137, 139, 430, 431]: |
| • Positive sputum smears |
| • Delayed culture conversion (sputum obtained at/after completion of intensive-phase therapy is culture-positive) |
| • Treatment failure |
| • Relapse |
| • Drug resistance |
| • Homelessness |
| • Current or prior substance abuse |
| • Use of intermittent dosing |
| • HIV infection |
| • Previous nonadherence to therapy |
| • Children and adolescents |
| • Mental, emotional or physical disability (ie, cognitive deficits such as dementia; neurological deficits; medically fragile patients; or patients with blindness or severe loss of vision) |
| • Resident at correctional or long-term care facility |
| • Previous treatment for active or latent tuberculosis |
Abbreviation: HIV, human immunodeficiency virus.
PICO Question 2: Does self-administered therapy (SAT) have similar outcomes compared to directly observed therapy (DOT) in patients with various forms of tuberculosis?
Recommendation 2: We suggest using DOT rather than SAT for routine treatment of patients with all forms of tuberculosis (conditional recommendation; low certainty in the evidence).
RECOMMENDED TREATMENT REGIMENS
The preferred regimen for treating adults with tuberculosis caused by organisms that are not known or suspected to be drug resistant is a regimen consisting of an intensive phase of 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) followed by a continuation phase of 4 months of INH and RIF (see Tables 2, 3, 10, 11, and Supplementary Appendix C) [3, 36, 37]. The intensive phase of treatment consists of 4 drugs (INH, RIF, PZA, EMB) because of the current proportion of new tuberculosis cases worldwide caused by organisms that are resistant to INH [38–41]; however, if therapy is being initiated after drug susceptibility test results are known and the patient's isolate is susceptible to both INH and RIF, EMB is not necessary, and the intensive phase can consist of INH, RIF, and PZA only. EMB can be discontinued as soon as the results of drug susceptibility studies demonstrate that the isolate is susceptible to INH and RIF. Pyridoxine (vitamin B6) is given with INH to all persons at risk of neuropathy (eg, pregnant women; breastfeeding infants; persons infected with human immunodeficiency virus [HIV]; patients with diabetes, alcoholism, malnutrition, or chronic renal failure; or those who are of advanced age) [42, 43].
With respect to administration schedule, the preferred frequency is once daily for both the intensive and continuation phases (see PICO Questions 3 and 4 and Supplementary Appendix B, Evidence Profiles 5–11). Although administration of antituberculosis drugs using DOT 5 days a week has been reported in a large number of studies, it has not been compared with 7-day administration in a clinical trial. Nonetheless, on the basis of substantial clinical experience, experts believe that 5-days-a-week drug administration by DOT is an acceptable alternative to 7-days-a-week administration, and either approach may be considered as meeting the definition of “daily” dosing. There are alternative regimens that are variations of the preferred regimen, which may be acceptable in certain clinical and/or public health situations (see “Other Regimens” and “Treatment in Special Situations” in the full-text version of the guideline).
PICO Question 3: Does intermittent dosing in the intensive phase have similar outcomes compared to daily dosing in the intensive phase for treatment of drug-susceptible pulmonary tuberculosis?
Recommendation 3a: We recommend the use of daily rather than intermittent dosing in the intensive phase of therapy for drug-susceptible pulmonary tuberculosis (strong recommendation; moderate certainty in the evidence).
Recommendation 3b: Use of thrice-weekly therapy in the intensive phase (with or without an initial 2 weeks of daily therapy) may be considered in patients who are not HIV infected and are also at low risk of relapse (pulmonary tuberculosis caused by drug-susceptible organisms, that at the start of treatment is noncavitary and/or smear negative) (conditional recommendation; low certainty in the evidence).
Recommendation 3c: In situations where daily or thrice-weekly DOT therapy is difficult to achieve, use of twice-weekly therapy after an initial 2 weeks of daily therapy may be considered for patients who are not HIV-infected and are also at low risk of relapse (pulmonary tuberculosis caused by drug-susceptible organisms, that at the start of treatment is noncavitary and/or smear negative) (conditional recommendation; very low certainty in the evidence). Note: If doses are missed in a regimen using twice-weekly dosing, then therapy is equivalent to once weekly, which is inferior (see PICO Question 4).
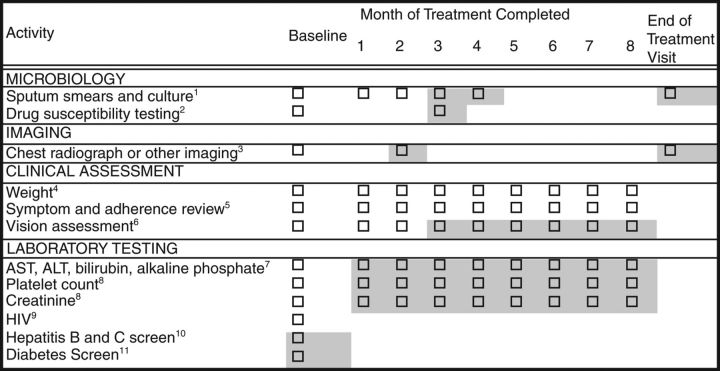
Recommended baseline and follow-up evaluations for patients suspected of having tuberculosis and treated with first-line medications are summarized in Figure 2. During treatment, a sputum specimen for AFB smear and culture are obtained at monthly intervals until 2 consecutive specimens are negative on culture. Duration of the continuation phase regimen hinges on the microbiological status at the end of the intensive phase of treatment; thus, obtaining sputum specimens at the time of completion of 2 months of treatment is critical if sputum culture conversion to negative has not already been documented. The culture result of a sputum specimen obtained at the completion of the intensive phase of treatment (2 months) has been shown to correlate with the likelihood of relapse after completion of treatment for pulmonary tuberculosis, albeit with low sensitivity [9, 44–46]. Cavitation on the initial chest radiograph has also been shown to be a risk factor for relapse [9, 47]. In patients treated for 6 months, having both cavitation and a positive culture at completion of 2 months of therapy has been associated with rates of relapse of approximately 20% compared with 2% among patients with neither factor [9, 45].
Figure 2.
Baseline and follow-up evaluations for patients treated with first-line tuberculosis medications. Shading around boxes indicates activities that are optional or contingent on other information. 1Obtain sputa for smear and culture at baseline, then monthly until 2 consecutive specimens are negative. Collecting sputa more often early in treatment for assessment of treatment response and at end of treatment is optional. At least one baseline specimen should be tested using a rapid molecular test. 2Drug susceptibility for isoniazid, rifampin, ethambutol (EMB), and pyrazinamide should be obtained. Repeat drug susceptibility testing if patient remains culture positive after completing 3 months of treatment. Molecular resistance testing should be performed for patients with risk for drug resistance. 3Obtain chest radiograph at baseline for all patients, and also at month 2 if baseline cultures are negative. End-of-treatment chest radiograph is optional. Other imaging for monitoring of extrapulmonary disease. 4Monitor weight monthly to assess response to treatment; adjust medication dose if needed. 5Assess adherence and monitor improvement in tuberculosis symptoms (eg, cough, fever, fatigue, night sweats) as well as development of medication adverse effects (eg, jaundice, dark urine, nausea, vomiting, abdominal pain, fever, rash, anorexia, malaise, neuropathy, arthralgias). 6Patients on EMB: baseline visual acuity (Snellen test) and color discrimination tests, followed by monthly inquiry about visual disturbance and monthly color discrimination tests. 7Liver function tests only at baseline unless there were abnormalities at baseline, symptoms consistent with hepatotoxicity develop, or for patients who chronically consume alcohol, take other potentially hepatotoxic medications, or have viral hepatitis or history of liver disease, human immunodeficiency virus (HIV) infection, or prior drug-induced liver injury. 8Baseline for all patients. Further monitoring if there are baseline abnormalities or as clinically indicated. 9HIV testing in all patients. CD4 lymphocyte count and HIV RNA load if positive. 10Patients with hepatitis B or C risk factor (eg, injection drug use, birth in Asia or Africa, or HIV infection) should have screening tests for these viruses. 11Fasting glucose or hemoglobin A1c for patients with risk factors for diabetes according to the American Diabetes Association including: age >45 years, body mass index >25 kg/m2, first-degree relative with diabetes, and race/ethnicity of African American, Asian, Hispanic, American Indian/Alaska Native, or Hawaiian Native/Pacific Islander. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In view of this evidence, for patients who have cavitation on the initial chest radiograph and who have positive cultures at completion of 2 months of therapy, expert opinion is to extend the continuation phase with INH and RIF for an additional 3 months (ie, a continuation phase of 7 months in duration, corresponding to a total of 9 months of therapy). Additional factors to be considered in deciding to prolong treatment in patients with either cavitation or a positive culture at 2 months (but not both) might include being >10% below ideal body weight; being an active smoker; having diabetes, HIV infection, or any other immunosuppressing condition; or having extensive disease on chest radiograph [46, 48–52].
Interruptions in therapy are common in the treatment of tuberculosis. When interruptions occur, the person responsible for supervision must decide whether to restart a complete course of treatment or simply to continue as intended originally. In general, the earlier the break in therapy and the longer its duration, the more serious the effect and the greater the need to restart treatment from the beginning (Table 6). Continuous treatment is more important in the intensive phase of therapy when the bacillary population is highest and the chance of developing drug resistance greatest. During the continuation phase, the number of bacilli is much smaller and the goal of therapy is to kill the persisting organisms. The duration of the interruption and the bacteriologic status of the patient prior to and after the interruption are also important considerations (see “Interruptions in Therapy” in the full-text version of the guideline).
Table 6.
Management of Treatment Interruptionsa
| Time Point of Interruption | Details of Interruption | Approach |
|---|---|---|
| During intensive phase | Lapse is <14 d in duration | Continue treatment to complete planned total number of doses (as long as all doses are completed within 3 mo) |
| Lapse is ≥14 d in duration | Restart treatment from the beginning | |
| During continuation phase | Received ≥80% of doses and sputum was AFB smear negative on initial testing | Further therapy may not be necessary |
| Received ≥80% of doses and sputum was AFB smear positive on initial testing | Continue therapy until all doses are completed | |
| Received <80% of doses and accumulative lapse is <3 mo in duration | Continue therapy until all doses are completed (full
course), unless consecutive lapse is >2 mo If treatment cannot be completed within recommended time frame for regimen, restart therapy from the beginning (ie, restart intensive phase, to be followed by continuation phase)b |
|
| Received <80% of doses and lapse is ≥3 mo in duration | Restart therapy from the beginning, new intensive and continuation phases (ie, restart intensive phase, to be followed by continuation phase) |
Abbreviation: AFB, acid-fast bacilli.
a According to expert opinion, patients who are lost to follow-up (on treatment) and brought back to therapy, with interim treatment interruption, should have sputum resent for AFB smear, culture, and drug susceptibility testing.
b The recommended time frame for regimen, in tuberculosis control programs in the United States and in several European countries, is to administer all of the specified number of doses for the intensive phase within 3 months and those for the 4-month continuation phase within 6 months, so that the 6-month regimen is completed within 9 months.
PICO Question 4: Does intermittent dosing in the continuation phase have similar outcomes compared to daily dosing in the continuation phase in patients with drug-susceptible pulmonary tuberculosis patients?
Recommendation 4a: We recommend the use of daily or thrice-weekly dosing in the continuation phase of therapy for drug-susceptible pulmonary tuberculosis (strong recommendation; moderate certainty in the evidence).
Recommendation 4b: If intermittent therapy is to be administered in the continuation phase, then we suggest use of thrice-weekly instead of twice-weekly therapy (conditional recommendation; low certainty in the evidence). This recommendation allows for the possibility of some doses being missed; with twice-weekly therapy, if doses are missed then therapy is equivalent to once weekly, which is inferior.
Recommendation 4c: We recommend against use of once-weekly therapy with INH 900 mg and rifapentine 600 mg in the continuation phase (strong recommendation; high certainty in the evidence). In uncommon situations where more than once-weekly DOT is difficult to achieve, once-weekly continuation phase therapy with INH 900 mg plus rifapentine 600 mg may be considered for use only in HIV-uninfected persons without cavitation on chest radiography.
PRACTICAL ASPECTS OF TREATMENT
Guidance on the practical aspects of tuberculosis treatment, drug–drug interactions, therapeutic drug monitoring (TDM), and management of adverse effects are available in the full-text version of this guideline. In brief, mild adverse effects usually can be managed with treatment directed at controlling the symptoms; severe effects usually require the offending drug(s) to be discontinued, and may require expert consultation on management. If a drug is permanently discontinued, then a replacement drug, typically from a different drug class, is included in the regimen. Patients with severe tuberculosis often require the initiation of an alternate regimen during the time the offending drug(s) are held. In general, for complicated diagnostic or management situations, consultation with local and state health departments is advised. In the United States, the Centers for Disease Control and Prevention's Division of Tuberculosis Elimination funds tuberculosis regional training and medical consultation centers (http://www.cdc.gov/tb/education/rtmc/), which provide medical consultation to programs and health providers on management of tuberculosis. In Europe, the World Health Organization (WHO)/ERS Tuberculosis Consilium (https://www.tbconsilium.org) provides similar consultation services regarding the diagnosis and treatment of tuberculosis.
Gastrointestinal reactions are common, especially early in therapy [53]. The optimum approach to management of epigastric distress or nausea with tuberculosis drugs is not clear. To minimize symptoms, patients receiving SAT may take the medications at bedtime. Gastrointestinal intolerance not associated with hepatotoxicity can be treated with antacids, which have less impact on absorption or peak concentration of first-line drugs than administration with food [54]. Any combination of otherwise unexplained nausea, vomiting, and abdominal pain is evaluated with a physical examination and liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, and alkaline phosphatase to assess for possible hepatotoxicity [55]. Drug-induced hepatitis is the most frequent serious adverse reaction to the first-line drugs. INH, RIF, and PZA can cause drug-induced liver injury (DILI), which is suspected when the ALT level is ≥3 times the upper limit of normal in the presence of hepatitis symptoms, or ≥5 the upper limit of normal in the absence of symptoms. In either situation, hepatotoxic drugs are stopped immediately and the patient is evaluated carefully. Other causes of abnormal liver function tests must be excluded before diagnosing drug-induced hepatotoxicity (Table 7). An official American Thoracic Society statement on the hepatotoxicity of antituberculosis therapy (http://www.thoracic.org/statements/resources/mtpi/hepatotoxicity-of-antituberculosis-therapy.pdf) provides additional details on the management of tuberculosis in the setting of drug-induced liver injury, as well as suggestions on drug rechallenge [56]; however, the optimal approach to reintroducing tuberculosis treatment after hepatotoxicity is still not known [57, 58].
Table 7.
Other Causes of Abnormal Liver Function Tests That Should Be Excluded
| Viral hepatitis (hepatitis A, B, and C in all patients; Epstein-Barr virus, cytomegalovirus, and herpes simplex in immunosuppressed patients) |
| Biliary tract disease |
| Alcohol |
| Other hepatotoxic drugs (eg, acetaminophen, acetaminophen-containing multiagent preparations, lipid-lowering agents, other drugs) |
| Select herbal and dietary supplements |
Source: American Thoracic Society [56].
TREATMENT IN SPECIAL SITUATIONS
Detailed recommendations on the management of tuberculosis in special situations (HIV infection, extrapulmonary tuberculosis, culture-negative pulmonary tuberculosis, advanced age, children, tuberculosis during pregnancy and breastfeeding, renal disease, and hepatic disease, among others) are available in the full-text version of this guideline. Five PICO questions with summary recommendations pertinent to the management of tuberculosis in HIV patients, steroid use in pericardial or meningeal tuberculosis, and culture-negative tuberculosis are summarized below.
HIV Infection
Treatment of tuberculosis in patients with HIV infection has several important differences compared with treatment of patients who do not have HIV infection. The need for antiretroviral therapy (ART), the potential for drug–drug interactions, especially between the rifamycins and antiretroviral agents (Table 8), paradoxical reactions that may be interpreted as clinical worsening, and the potential for developing resistance to rifamycins when using intermittent tuberculosis therapy are some of these differences. Detailed information on these topics is provided in the full-text version of this practice guideline. In regard to duration of treatment for drug-susceptible pulmonary tuberculosis in the presence of HIV infection, our updated systematic review of randomized trials and cohort studies comparing various durations of tuberculosis therapy (6 months vs 8 months or longer), most of which were conducted prior to the era of highly active ART, showed that the risk of recurrence is lower when the continuation phase of treatment is extended. However, it is important to note that the majority of these studies were reports on nonrandomized cohorts, most were completed prior to the era of routine antiretroviral use, many tested intermittent regimens and few distinguished between reinfection and relapse (see “HIV Infection” in the full-text version of the guideline). As discussed below, based on data that show significant reductions in mortality and AIDS-defining illnesses, patients with HIV infection and tuberculosis should receive ART in conjunction with daily antituberculosis medications. For HIV-infected patients receiving ART, we suggest using the standard 6-month daily regimen consisting of an intensive phase of 2 months of INH, RIF, PZA, and EMB followed by a continuation phase of 4 months of INH and RIF for the treatment of drug-susceptible pulmonary tuberculosis. In the uncommon situation in which an HIV-infected patient does not receive ART during tuberculosis treatment, we suggest extending the continuation phase with INH and RIF for an additional 3 months (ie, a continuation phase of 7 months in duration, corresponding to a total of 9 months of therapy) for treatment of drug-susceptible pulmonary tuberculosis (see PICO Question 5 and Supplementary Appendix B, Evidence Profile 12). As is noted for drug-susceptible pulmonary tuberculosis in patients without HIV coinfection, the continuation phase is extended in specific situations that are known to increase risk for relapse, as well as for selected extrapulmonary sites of disease, namely tuberculous meningitis, and bone, joint, and spinal tuberculosis (see “Identification and Management of Patients at Increased Risk of Relapse” and “Extrapulmonary Tuberculosis” in the full-text version of the guideline). Use of intermittent tuberculosis treatment regimens in HIV-infected patients has been associated with high rates of relapse and the emergence of drug resistance [9, 59]. In a trial of rifabutin (RFB)–based antituberculosis therapy in combination with antiretroviral drugs, patients treated with twice-weekly RFB had a relapse rate of 5.3%, but 8 of 9 patients with relapse had acquired rifamycin resistance [60]. Relapse and resistance were associated with low CD4 lymphocyte counts, as all recurrences occurred in patients with baseline CD4 lymphocyte counts <100 cells/µL. In the pharmacokinetic substudy of the trial, lower plasma concentrations of RFB and INH were identified as key risk factors for acquiring rifamycin resistance [61]. More recently, the use of a thrice-weekly RIF-based regimen during the intensive and continuation phases of treatment was associated with a higher rate of relapse and emergence of rifamycin resistance in HIV-infected individuals not receiving antiretrovirals compared with HIV-infected patients also receiving antiretrovirals or HIV-uninfected patients [62]. Based in part on systematic reviews conducted to obtain evidence in support of this guideline, our expert opinion is that treatment of HIV-related tuberculosis be given daily in both the intensive and continuation phases to avoid recurrent disease and the emergence of rifamycin resistance (see “Recommended Treatment Regimens” in the full-text version of the guideline).
Table 8.
Clinically Significant Drug–Drug Interactions Involving the Rifamycinsa
| Drug Class | Drugs Whose Concentrations Are Substantially Decreased by Rifamycins | Comments |
|---|---|---|
| Antiretroviral agents | HIV-1 protease inhibitors (lopinavir/ritonavir, darunavir/ritonavir, atazanavir, atazanavir/ritonavir) | RFB preferred with protease inhibitors. For ritonavir-boosted regimens, give RFB 150 mg daily. Double-dose lopinavir/ritonavir can be used with RIF but toxicity increased. Do not use RIF with other protease inhibitors. |
| NNRTIs Nevirapine Efavirenz Rilpivirine Complera (fixed-dose combination tablet containing emtricitabine, rilpivirine, TDF) Etravirine |
RIF decreases exposure to all NNRTIs. If nevirapine is used with RIF, lead-in nevirapine dose of 200 mg daily should be omitted and 400 mg daily nevirapine dosage given. With RIF, many experts advise that efavirenz be given at standard dosage of 600 mg daily, although FDA recommends increasing efavirenz to 800 mg daily in persons >60 kg. In young children double-dose lopinavir/ritonavir given with RIF results in inadequate concentrations – super-boosted Lopinavir/ritonavir is advised (if available) by some experts. Rilpivirine and etravirine should not be given with RIF. RFB can be used with nevirapine and etravirine at usual dosing. Efavirenz and RFB use requires dose increase of RFB to 600 mg daily, as such RIF is preferred. Rilpivirine should not be given with RFB. | |
| INSTIs Raltegravir Dolutegravir Elvitegravir (coformulated with cobicistat, tenofovir and emtricitabine as Stribild) Genvoya (fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) |
Increase dose of raltegravir to 800 mg twice daily with RIF, although clinical trial data show similar efficacy using 400 mg twice daily. Dolutegravir dose should be increased to 50 mg every 12 h with RIF. Do not use RIF with elvitegravir. RFB can be used with all INSTIs. | |
| CCR5 inhibitors Maraviroc |
RIF should not be used with maraviroc. RFB can be used with maraviroc. | |
| Anti-infectives | Macrolide antibiotics (azithromycin, clarithromycin, erythromycin) | Azithromycin has no significant interaction with rifamycins. Coadministration of clarithromycin and RFB results in significant bidirectional interactions that can increase RFB to toxic levels increasing the risk of uveitis. Erythromycin is a CYP3A4 substrate and clearance may increase in setting of rifamycin use. |
| Doxycycline | May require use of a drug other than doxycycline. | |
| Azole antifungal agents (ketoconazole, itraconazole, voriconazole, fluconazole, posaconazole, isavuconazole) | Itraconazole, ketoconazole, and voriconazole concentrations may be subtherapeutic with any of the rifamycins. Fluconazole can be used with rifamycins, but the dose of fluconazole may have to be increased. | |
| Atovaquone | Consider alternate form of Pneumocystis jirovecii treatment or prophylaxis. | |
| Chloramphenicol | Consider an alternative antibiotic. | |
| Mefloquine | Consider alternate form of malaria prophylaxis. | |
| Hormone therapy | Ethinylestradiol, norethindrone | Women of reproductive potential on oral contraceptives should be advised to add a barrier method of contraception when on a rifamycin. |
| Tamoxifen | May require alternate therapy or use of a non-rifamycin-containing regimen. | |
| Levothyroxine | Monitoring of serum TSH recommended; may require increased dose of levothyroxine. | |
| Narcotics | Methadone | RIF and RPT use may require methadone dose
increase. RFB infrequently causes methadone withdrawal. |
| Anticoagulants | Warfarin | Monitor prothrombin time; may require 2- to 3-fold warfarin dose increase. |
| Immunosuppressive agents | Cyclosporine, tacrolimus | RFB may allow concomitant use of cyclosporine and a rifamycin; monitoring of cyclosporine and tacrolimus serum concentrations may assist with dosing. |
| Corticosteroids | Monitor clinically; may require 2- to 3-fold increase in corticosteroid dose. | |
| Anticonvulsants | Phenytoin, lamotrigine | TDM recommended; may require anticonvulsant dose increase. |
| Cardiovascular agents |
Verapamil, nifedipine, diltiazem (a similar interaction is also predicted for felodipine and nisoldipine) | Clinical monitoring recommended; may require change to an alternate cardiovascular agent. |
| Propranolol, metoprolol | Clinical monitoring recommended; may require dose increase or change to an alternate cardiovascular drug. | |
| Enalapril, losartan | Monitor clinically; may require a dose increase or use of an alternate cardiovascular drug. | |
| Digoxin (among patients with renal insufficiency), digitoxin | TDM recommended; may require digoxin or digitoxin dose increase. | |
| Quinidine | TDM recommended; may require quinidine dose increase. | |
| Mexiletine, tocainide, propafenone | Clinical monitoring recommended; may require change to an alternate cardiovascular drug. | |
| Theophylline | Theophylline | TDM recommended; may require theophylline dose increase. |
| Sulfonylurea hypoglycemics |
Tolbutamide, chlorpropamide, glyburide, glimepiride, repaglinide | Monitor blood glucose; may require dose increase or change to an alternate hypoglycemic drug. |
| Hypolipidemics | Simvastatin, fluvastatin | Monitor hypolipidemic effect; may require use of an alternate antihyperlipidemic drug. |
| Psychotropic drugs | Nortriptyline | TDM recommended; may require dose increase or change to alternate psychotropic drug. |
| Haloperidol, quetiapine | Monitor clinically; may require a dose increase or use of an alternate psychotropic drug. | |
| Benzodiazepines (eg, diazepam, triazolam), zolpidem, buspirone) | Monitor clinically; may require a dose increase or use of an alternate psychotropic drug. |
Abbreviations: CCR5, C chemokine receptor type 5; CYP, cytochrome P450; FDA, US Food and Drug Administration; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; RFB, rifabutin; RIF, rifampin; RPT, rifapentine; TDF, tenofovir disoproxil fumarate; TDM, therapeutic drug monitoring; TSH, thyroid-stimulating hormone.
a See the following useful websites for updated information regarding drug interactions: AIDSinfo, Centers for Disease Control and Prevention, University of California San Francisco, University of Liverpool, Indiana University, and University of Maryland.
PICO Question 5: Does extending treatment beyond 6 months improve outcomes compared to the standard 6-month treatment regimen among pulmonary tuberculosis patients coinfected with HIV?
Recommendation 5a: For HIV-infected patients receiving ART, we suggest using the standard 6-month daily regimen consisting of an intensive phase of 2 months of INH, RIF, PZA, and EMB followed by a continuation phase of 4 months of INH and RIF for the treatment of drug-susceptible pulmonary tuberculosis (conditional recommendation; very low certainty in the evidence).
Recommendation 5b: In uncommon situations in which HIV-infected patients do NOT receive ART during tuberculosis treatment, we suggest extending the continuation phase with INH and RIF for an additional 3 months (ie, a continuation phase of 7 months in duration, corresponding to a total of 9 months of therapy) for treatment of drug-susceptible pulmonary tuberculosis (conditional recommendation; very low certainty in the evidence).
Mortality among patients with HIV and tuberculosis is high, principally due to complications of immunosuppression and occurrence of other HIV-related opportunistic diseases. Co-trimoxazole (trimethoprim-sulfamethoxazole) prophylaxis has been shown to reduce morbidity and mortality in HIV-infected patients with newly diagnosed tuberculosis [63–65]. Whereas co-trimoxazole is recommended by WHO for all HIV-infected people with active tuberculosis disease regardless of the CD4 cell count [66], in high-income countries, co-trimoxazole is primarily used in HIV-infected patients with CD4 counts <200 cells/µL [67]. The use of ART during tuberculosis treatment in persons with HIV infection also reduces mortality rates significantly and decreases the risk of developing AIDS-related conditions. We performed a systematic review and meta-analysis to address the concurrent initiation of ART with tuberculosis treatment. On the basis of high certainty in the evidence that the benefits outweigh the harms, we recommend that patients with tuberculosis and HIV infection receive ART during antituberculosis treatment. ART should ideally be started within 2 weeks for those patients with a CD4 count <50 cells/µL and by 8–12 weeks for those with a CD4 count ≥50 cells/µL (see PICO Question 6 and Supplementary Appendix B, Evidence Profile 13). An exception is HIV-infected patients with tuberculous meningitis, in whom ART is not initiated in the first 8 weeks of antituberculosis therapy (see full-text version of the guideline). The concurrent administration of antiretrovirals and rifamycins is a major therapeutic challenge, and additional details on the coadministration of these medications, including the use of RFB, are available in the full-text version of this guideline.
PICO Question 6: Does initiation of ART during tuberculosis treatment compared to at the end of tuberculosis treatment improve outcomes among tuberculosis patients coinfected with HIV?
Recommendation 6: We recommend initiating ART during tuberculosis treatment. ART should ideally be initiated within the first 2 weeks of tuberculosis treatment for patients with CD4 counts <50 cells/µL and by 8–12 weeks of tuberculosis treatment initiation for patients with CD4 counts ≥50 cells/µL (strong recommendation; high certainty in the evidence). Note: an exception is patients with HIV infection and tuberculous meningitis (see Immune Reconstitution Inflammatory Syndrome).
Patients with HIV infection and tuberculosis are at increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments. These reactions presumably develop as a consequence of reconstitution of immune responsiveness brought about by ART, and are designated as the immune reconstitution inflammatory syndrome (IRIS). Tuberculosis IRIS has been noted to be more common in participants with earlier ART initiation and CD4+ cell counts <50 cells/µL [68]. Signs of IRIS may include high fevers, worsening respiratory symptoms, increase in size and inflammation of involved lymph nodes, new lymphadenopathy, expanding central nervous system lesions, worsening of pulmonary parenchymal infiltrations, new or increasing pleural effusions, and development of intra-abdominal or retroperitoneal abscesses [69]. Such findings are attributed to IRIS only after excluding other possible causes, especially tuberculosis treatment failure from drug-resistant tuberculosis or another opportunistic disease, such as non-Hodgkin lymphoma or infection.
Management of IRIS is symptomatic. Based on expert opinion, for most patients with mild IRIS, tuberculosis and antiretroviral therapies can be continued with the addition of anti-inflammatory agents such as ibuprofen. For patients with worsening pleural effusions or abscesses, drainage may be necessary. For more severe cases of IRIS, treatment with corticosteroids is effective. In a placebo-controlled trial of prednisone for patients with moderate IRIS, prednisone 1.25 mg/kg/day significantly reduced the need for hospitalization or surgical procedures [70]. For patients who develop IRIS, prednisone may be given at a dose of 1.25 mg/kg/day (50–80 mg/day) for 2–4 weeks, with tapering over a period of 6–12 weeks or longer.
Tuberculous Pericarditis
Based on small studies that have shown mortality and morbidity benefits [71–73], corticosteroids have previously been universally recommended in combination with a standard 6-month regimen (Table 2) for treating tuberculosis pericarditis; however, a recent placebo-controlled randomized clinical trial with 1400 participants did not find a difference in the combined primary endpoint of the trial, which included mortality, cardiac tamponade, or constrictive pericarditis, between patients treated with adjunctive corticosteroids vs placebo [74]. A subgroup analysis, however, did suggest a benefit in preventing constrictive pericarditis. Similarly, a systematic review conducted to obtain evidence in support of this guideline did not find a statistically significant benefit in terms of mortality or constrictive pericarditis from the use of corticosteroids [71–75]. Therefore, we suggest that adjunctive corticosteroids should not be used routinely in the treatment of patients with pericardial tuberculosis (see PICO Question 7 and Supplementary Appendix B, Evidence Profile 14). However, selective use of corticosteroids in patients who are at the highest risk for inflammatory complications might be appropriate. Such patients might include those with large pericardial effusions, those with high levels of inflammatory cells or markers in pericardial fluid, or those with early signs of constriction [76].
PICO Question 7: Does the use of adjuvant corticosteroids in tuberculous pericarditis provide mortality and morbidity benefits?
Recommendation 7: We suggest initial adjunctive corticosteroid therapy not be routinely used in patients with tuberculous pericarditis (conditional recommendation; very low certainty in the evidence).
Tuberculous Meningitis
Chemotherapy for tuberculous meningitis is initiated with INH, RIF, PZA, and EMB in an initial 2-month phase. After 2 months of 4-drug therapy, for meningitis known or presumed to be caused by susceptible strains, PZA and EMB may be discontinued, and INH and RIF continued for an additional 7–10 months, although the optimal duration of chemotherapy is not defined. Based on expert opinion, repeated lumbar punctures should be considered to monitor changes in cerebrospinal fluid cell count, glucose, and protein, especially early in the course of therapy. In children with tuberculous meningitis, the American Academy of Pediatrics (AAP) lists an initial 4-drug regimen composed of INH, RIF, PZA, and ethionamide, if possible, or an aminoglycoside, followed by 7–10 months of INH and RIF as the preferred regimen [77]. There are no data from controlled trials to guide the selection of EMB vs an injectable or ethionamide as the fourth drug for tuberculosis meningitis [78]. Most societies and experts recommend the use of either an injectable or EMB. For adults, based on expert opinion, our guideline committee prefers using EMB as the fourth drug in the regimen for tuberculous meningitis.
The role of adjunctive corticosteroid therapy in the treatment of tuberculous meningitis has been reported by numerous studies [79–91], and our updated systematic review found a mortality benefit from the use of adjuvant corticosteroids. Therefore, we recommend adjunctive corticosteroid therapy with dexamethasone or prednisolone tapered over 6–8 weeks for patients with tuberculous meningitis (see PICO Question 8 and Supplementary Appendix B, Evidence Profile 15).
PICO Question 8: Does the use of adjuvant corticosteroids in tuberculous meningitis provide mortality and morbidity benefits?
Recommendation 8: We recommend initial adjunctive corticosteroid therapy with dexamethasone or prednisolone tapered over 6–8 weeks for patients with tuberculous meningitis (strong recommendation; moderate certainty in the evidence).
Culture-Negative Pulmonary Tuberculosis in Adults
Failure to isolate M. tuberculosis from appropriately collected sputum specimens in persons who, because of clinical or radiographic findings, are suspected of having pulmonary tuberculosis does not exclude a diagnosis of active tuberculosis. Some causes of failure to isolate organisms include low bacillary populations, inadequate sputum specimens, temporal variations in the number of expelled bacilli, overgrowth of cultures with other microorganisms, and errors in specimen processing [92]. Alternative diagnoses must be considered and appropriate diagnostic studies undertaken in patients who appear to have culture-negative tuberculosis. At a minimum, patients suspected of having pulmonary tuberculosis have 2 sputum specimens (using sputum induction with hypertonic saline if necessary) for AFB smears and cultures for mycobacteria or for rapid molecular testing for M. tuberculosis as part of the diagnostic evaluation. Other diagnostic procedures, such as bronchoscopy with bronchoalveolar lavage and biopsy, are considered before making a presumptive diagnosis of culture-negative tuberculosis.
Patients who, on the basis of careful clinical and radiographic evaluation, are thought to have pulmonary tuberculosis should have treatment initiated with INH, RIF, PZA, and EMB even when the initial sputum smears are negative. If M. tuberculosis is isolated in culture or a rapid molecular test is positive, treatment for active disease is continued for a full, standard 6-month course (Table 2), if appropriate based on drug susceptibility test results. Patients who have negative cultures but who still are presumed to have pulmonary tuberculosis should have thorough clinical and radiographic follow-up after 2–3 months of therapy [93]. If there is clinical or radiographic improvement and no other etiology is identified, treatment should be continued.
The optimum treatment regimens and duration for smear-negative, culture-negative tuberculosis have not been convincingly established. We performed a systematic review that evaluated treatment regimens of varying durations in adult patients with culture-negative, paucibacillary tuberculosis, and we suggest that a 4-month treatment regimen is adequate for smear-negative, culture-negative pulmonary tuberculosis (see PICO Question 9 and Supplementary Appendix B, Evidence Profile 16). Operationally, treatment is initiated with an intensive phase of INH, RIF, PZA, and EMB daily and continued even when the initial bacteriologic studies are negative. If all cultures on adequate samples are negative (defining culture-negative tuberculosis) and there is clinical or radiographic response after 2 months of intensive phase therapy, the continuation phase with INH and RIF can be shortened to 2 months. Alternatively, if there is concern about the adequacy of workup or the accuracy of the microbiologic evaluations, a standard 6-month regimen remains preferred (see Table 2 and “Culture-Negative Pulmonary Tuberculosis” in the full-text version of the guideline) [14, 15].
PICO Question 9: Does a shorter duration of treatment have similar outcomes compared to the standard 6-month treatment duration among HIV-uninfected patients with paucibacillary tuberculosis (ie, smear negative, culture negative)?
Recommendation 9: We suggest that a 4-month treatment regimen is adequate for treatment of HIV-uninfected adult patients with AFB smear- and culture-negative pulmonary tuberculosis (conditional recommendation; very low certainty in the evidence).
CONCLUSIONS
Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission of M. tuberculosis to other persons, thus, successful treatment of tuberculosis has benefits both for the individual patient and the community in which the patient resides. A 4-drug regimen of INH, RIF, PZA, and EMB remains the preferred initial treatment for drug-susceptible pulmonary tuberculosis. Treatment is initiated promptly even before AFB smear microscopy, molecular tests, and mycobacterial culture results are known in patients with high likelihood of having tuberculosis or those seriously ill with a disorder suspicious for tuberculosis. Initiation of treatment should not be delayed because of negative AFB smears for patients in whom tuberculosis is suspected and who have a life-threatening condition. There are variations of the preferred regimen that are appropriate in certain public health situations or in special clinical situations. Additional detailed and extensively referenced information on treatment of tuberculosis in special situations (patients with renal disease or hepatic disease, those of advanced age, etc), the use of case management strategies (including DOT), regimen and dosing selection in adults and children (daily vs intermittent), the role of TDM, treatment of tuberculosis in the presence of HIV infection (duration of tuberculosis treatment and timing of initiation of ART), treatment of tuberculosis in children, treatment of tuberculosis during pregnancy, and treatment of extrapulmonary tuberculosis, as well as key research priorities, are provided in the full-text version of this practice guideline.
Notes
Acknowledgments. Our sincerest gratitude for the support provided by Kevin Wilson, MD (Chief, Documents and Patient Education, American Thoracic Society); Judy Corn (Director, Documents and Patient Education, American Thoracic Society); Jennifer Padberg (Director of Clinical Affairs, Infectious Diseases Society of America [IDSA]); and Phil LoBue, MD (Director of the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention [CDC]) in the development of this guideline.
Guideline Writing Committee Chairs, Members, And Methodologists. Guideline Committee Chairs: Payam Nahid, MD, MPH, University of California, San Francisco (Chair, American Thoracic Society); Susan E. Dorman, MD, Johns Hopkins University, Maryland (Chair, IDSA); Giovanni Battista Migliori, MD, World Health Organization (WHO) Collaborating Centre for TB and Lung Diseases, Fondazione S. Maugeri Care and Research Institute, Tradate, Italy (Chair, European Respiratory Society); Andrew Vernon, MD, MHS, Division of Tuberculosis Elimination, National Center for Human Immunodeficiency Virus (HIV)/AIDS, Viral Hepatitis, Sexually Transmitted Disease (STD), and TB Prevention, CDC, Georgia (Chair, CDC).
Writing Committee Members. Narges Alipanah, MD, University of California, San Francisco; Pennan Barry, MD, MPH, California Department of Public Health; Jan Brozek, MD, PhD, McMaster University, Ontario, Canada; Adithya Cattamanchi, MD, MAS, University of California, San Francisco; Lelia Chaisson, MSc, University of California, San Francisco; Richard Chaisson, MD, Johns Hopkins University, Maryland; Charles L. Daley, MD, National Jewish Health, Colorado; Susan E. Dorman, MD, Johns Hopkins University, Maryland; Malgosia Grzemska, MD, PhD, World Health Organization, Geneva, Switzerland; Julie Higashi, MD, San Francisco Department of Public Health, TB Control, California; Christine S. Ho, MD, Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, CDC, Georgia; Philip Hopewell, MD, University of California, San Francisco; Salmaan A. Keshavjee, MD, PhD, Harvard Medical School, Massachusetts; Christian Lienhardt, MD, PhD, World Health Organization, Geneva, Switzerland; Richard Menzies, MD, McGill University, Quebec, Canada; Cynthia Merrifield, RN, University of California, San Francisco; Giovanni Battista Migliori, MD, WHO Collaborating Centre for TB and Lung Diseases, Fondazione S. Maugeri Care and Research Institute, Tradate, Italy; Payam Nahid, MD, MPH, University of California, San Francisco; Masahiro Narita, MD, Seattle and King County TB Control and University of Washington; Rick O'Brien, MD, Ethics Advisory Group, International Union against TB and Lung Disease, Paris, France; Charles Peloquin, Pharm. D., University of Florida; Ann Raftery, RN, PHN, MS, University of California, San Francisco; Jussi Saukkonen, MD, Boston University, Massachusetts; H. Simon Schaaf, MD, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa; Giovanni Sotgiu, MD, PhD, University of Sassari, Sassari, Italy; Jeffrey R. Starke, MD, Baylor College of Medicine, Texas; Andrew Vernon, MD, MHS, Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC, Georgia.
GRADE Methodology Group. Narges Alipanah, MD, University of California, San Francisco; Jan Brozek, MD, PhD, McMaster University, Ontario, Canada; Adithya Cattamanchi, MD, MAS, University of California, San Francisco; Lelia Chaisson, MSc, University of California, San Francisco; Richard Menzies, MD, McGill University, Quebec, Canada; Payam Nahid, MD, MPH, University of California, San Francisco; Giovanni Sotgiu, MD, University of Sassari, Sassari, Italy.
Financial support. Support for this guideline was provided by the American Thoracic Society, the IDSA, and the CDC.
Potential conflicts of interest. The authors have reported to the American Thoracic Society the following: P. M. B. reported that his spouse previously owned stocks or options of Merck. R. E. C. reported service as a consultant and ownership of stocks or options for Merck. C. L. D. received research support from Insmed and served on data and safety monitoring boards of Otsuka America Pharmaceutical and Sanofi Pasteur. C. A. P. received research support from Jacobus Pharmaceuticals. J. S. reported service on a data safety and monitoring board of Otsuka Pharmaceuticals. A. V. reported serving as the chief of a CDC clinical research branch doing clinical trials in tuberculosis, which supports and works with the TB Trials Consortium (TBTC) that collaborates with pharmaceutical companies, which may provide support such as drug supplies or laboratory funding for pharmacokinetic substudies. Specifically, Sanofi Aventis has provided approximately $2.8 million in unrestricted grants to the CDC Foundation to facilitate or support TBTC work related to rifapentine, including contract research staff, pharmacokinetic substudies, travel to TBTC scientific meetings for invited speakers, and expenses related to fulfillment of company requests for data and data formats as part of use of TBTC data in support of regulatory filings. TBTC has studies under way involving rifapentine and levofloxacin that may have relevance for future regimens for drug-susceptible tuberculosis and for multidrug-resistant tuberculosis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schunemann HJ, Jaeschke R, Cook DJ et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006; 174:605–14. [DOI] [PubMed] [Google Scholar]
- 2. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999; 3(10 suppl 2):S231–79. [PubMed] [Google Scholar]
- 4. Mitchison D, Davies G. The chemotherapy of tuberculosis: past, present and future. Int J Tuberc Lung Dis 2012; 16:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillespie SH, Crook AM, McHugh TD et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zierski M, Bek E. Side-effects of drug regimens used in short-course chemotherapy for pulmonary tuberculosis. A controlled clinical study. Tubercle 1980; 61:41–9. [DOI] [PubMed] [Google Scholar]
- 7. Phillips PP, Fielding K, Nunn AJ. An evaluation of culture results during treatment for tuberculosis as surrogate endpoints for treatment failure and relapse. PLoS One 2013; 8:e63840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aber VR, Nunn AJ. Short term chemotherapy of tuberculosis. Factors affecting relapse following short term chemotherapy [in French]. Bull Int Union Tuberc 1978; 53:276–80. [PubMed] [Google Scholar]
- 9. Benator D, Bhattacharya M, Bozeman L et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360:528–34. [DOI] [PubMed] [Google Scholar]
- 10. Menzies D, Elwood K. Treatment of tuberculosis disease. Canadian Tuberculosis Standards. 7th ed Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada, 2014. Available at: http://www.respiratoryguidelines.ca/sites/all/files/Canadian_TB_Standards_7th_Edition_ENG.pdf. [Google Scholar]
- 11. Weiner M, Burman W, Vernon A et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003; 167:1341–7. [DOI] [PubMed] [Google Scholar]
- 12. Essential components of a tuberculosis prevention and control program. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep 1995; 44(RR-11):1–16. [PubMed] [Google Scholar]
- 13. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep 2005; 54(RR-12):1–81. [PubMed] [Google Scholar]
- 14. Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis 2006; 6:710–25. [DOI] [PubMed] [Google Scholar]
- 15. Migliori GB, Zellweger JP, Abubakar I et al. European Union standards for tuberculosis care. Eur Respir J 2012; 39:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopewell PC, Fair EL, Uplekar M. Updating the International Standards for Tuberculosis Care. Entering the era of molecular diagnostics. Ann Am Thorac Soc 2014; 11:277–85. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Managing tuberculosis patients and improving adherence. Atlanta, GA: CDC, 2014. [Google Scholar]
- 18. Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007; 4:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tahan HA. A ten-step process to develop case management plans. Lippincotts Case Manag 2002; 7:231–42. [DOI] [PubMed] [Google Scholar]
- 20. Garner P, Smith H, Munro S, Volmink J. Promoting adherence to tuberculosis treatment. Bull World Health Organ 2007; 85:404–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clark PM, Karagoz T, Apikoglu-Rabus S, Izzettin FV. Effect of pharmacist-led patient education on adherence to tuberculosis treatment. Am J Health Syst Pharm 2007; 64:497–505. [DOI] [PubMed] [Google Scholar]
- 22. Liefooghe R, Suetens C, Meulemans H, Moran MB, De Muynck A. A randomised trial of the impact of counselling on treatment adherence of tuberculosis patients in Sialkot, Pakistan. Int J Tuberc Lung Dis 1999; 3:1073–80. [PubMed] [Google Scholar]
- 23. M'Imunya JM, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database Syst Rev 2012; 5:CD006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flores G. The impact of medical interpreter services on the quality of health care: a systematic review. Med Care Res Rev 2005; 62:255–99. [DOI] [PubMed] [Google Scholar]
- 25. Krishnaswami KV, Somasundaram PR, Tripathy SP, Vaidyanathan B, Radhakrishna S, Fox W. A randomised study of two policies for managing default in out-patients collecting supplies of drugs for pulmonary tuberculosis in a large city in South India. Tubercle 1981; 62:103–12. [DOI] [PubMed] [Google Scholar]
- 26. Kunawararak P, Pongpanich S, Chantawong S et al. Tuberculosis treatment with mobile-phone medication reminders in northern Thailand. Southeast Asian J Trop Med Public Health 2011; 42:1444–51. [PubMed] [Google Scholar]
- 27. Tanke ED, Leirer VO. Automated telephone reminders in tuberculosis care. Med Care 1994; 32:380–9. [DOI] [PubMed] [Google Scholar]
- 28. Liu Q, Abba K, Alejandria MM, Sinclair D, Balanag VM, Lansang MA. Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database Syst Rev 2014; 11:CD006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iribarren S, Beck S, Pearce PF et al. TextTB: a mixed method pilot study evaluating acceptance, feasibility, and exploring initial efficacy of a text messaging intervention to support TB treatment adherence. Tuberc Res Treat 2013; 2013:349394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lutge EE, Wiysonge CS, Knight SE, Volmink J. Material incentives and enablers in the management of tuberculosis. Cochrane Database Syst Rev 2012; 1:CD007952. [DOI] [PubMed] [Google Scholar]
- 31. Martins N, Morris P, Kelly PM. Food incentives to improve completion of tuberculosis treatment: randomised controlled trial in Dili, Timor-Leste. BMJ 2009; 339:b4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright CM, Westerkamp L, Korver S, Dobler CC. Community-based directly observed therapy (DOT) versus clinic DOT for tuberculosis: a systematic review and meta-analysis of comparative effectiveness. BMC Infect Dis 2015; 15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA 1998; 279:943–8. [DOI] [PubMed] [Google Scholar]
- 34. Liu SY, Li JH, Schluger NW. DOT and timely treatment completion among Asian-born immigrant tuberculosis patients. Int J Tuberc Lung Dis 2005; 9:884–9. [PubMed] [Google Scholar]
- 35. Stop TB USA Tuberculosis Elimination Plan Committee. A call for action on the tuberculosis elimination plan for the United States. 2010. Available at: http://www.thoracic.org/advocacy/stop-tb/Eliminate_TB_USA.pdf Accessed 23 December 2015.
- 36. Menzies D, Benedetti A, Paydar A et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med 2009; 6:e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menzies D, Benedetti A, Paydar A et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med 2009; 6:e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LoBue PA, Moser KS. Isoniazid- and rifampin-resistant tuberculosis in San Diego County, California, United States, 1993–2002. Int J Tuberc Lung Dis 2005; 9:501–6. [PubMed] [Google Scholar]
- 39. Hoopes AJ, Kammerer JS, Harrington TA, Ijaz K, Armstrong LR. Isoniazid-monoresistant tuberculosis in the United States, 1993 to 2003. Arch Intern Med 2008; 168:1984–92. [DOI] [PubMed] [Google Scholar]
- 40. Jenkins HE, Zignol M, Cohen T. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. PLoS One 2011; 6:e22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuen CM, Jenkins HE, Rodriguez CA, Keshavjee S, Becerra MC. Global and regional burden of isoniazid-resistant tuberculosis. Pediatrics 2015; 136:e50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snider DE., Jr Pyridoxine supplementation during isoniazid therapy. Tubercle 1980; 61:191–6. [DOI] [PubMed] [Google Scholar]
- 43. Visser ME, Texeira-Swiegelaar C, Maartens G. The short-term effects of anti-tuberculosis therapy on plasma pyridoxine levels in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2004; 8:260–2. [PubMed] [Google Scholar]
- 44. Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 1993; 147:1062–3. [DOI] [PubMed] [Google Scholar]
- 45. Jo KW, Yoo JW, Hong Y et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med 2014; 108:654–9. [DOI] [PubMed] [Google Scholar]
- 46. Horne DJ, Royce SE, Gooze L et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang KC, Leung CC, Yew WW, Ho SC, Tam CM. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med 2004; 170:1124–30. [DOI] [PubMed] [Google Scholar]
- 48. Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 2006; 174:344–8. [DOI] [PubMed] [Google Scholar]
- 49. Baker MA, Harries AD, Jeon CY et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang JY, Lee MC, Shu CC et al. Optimal duration of anti-TB treatment in patients with diabetes: nine or six months? Chest 2015; 147:520–8. [DOI] [PubMed] [Google Scholar]
- 51. Leung CC, Yew WW, Chan CK et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J 2015; 45:738–45. [DOI] [PubMed] [Google Scholar]
- 52. Ahmad Khan F, Minion J, Al-Motairi A, Benedetti A, Harries AD, Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis 2012; 55:1154–63. [DOI] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention. Core curriculum on tuberculosis: what the clinician should know. 6th ed Atlanta, GA: CDC, 2013. [Google Scholar]
- 54. Lin MY, Lin SJ, Chan LC, Lu YC. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: systematic review and meta-analysis. Int J Tuberc Lung Dis 2010; 14:806–18. [PubMed] [Google Scholar]
- 55. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006; 354:731–9. [DOI] [PubMed] [Google Scholar]
- 56. Saukkonen JJ, Cohn DL, Jasmer RM et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174:935–52. [DOI] [PubMed] [Google Scholar]
- 57. Sharma SK, Singla R, Sarda P et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis 2010; 50:833–9. [DOI] [PubMed] [Google Scholar]
- 58. Chang KC, Leung CC. The best approach to reintroducing tuberculosis treatment after hepatotoxicity is still open to debate. Clin Infect Dis 2010; 51:366–7; author reply 367–8. [DOI] [PubMed] [Google Scholar]
- 59. Vernon A, Burman W, Benator D, Khan A, Bozeman L. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Tuberculosis Trials Consortium. Lancet 1999; 353:1843–7. [DOI] [PubMed] [Google Scholar]
- 60. Burman W, Benator D, Vernon A et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med 2006; 173:350–6. [DOI] [PubMed] [Google Scholar]
- 61. Weiner M, Benator D, Burman W et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 2005; 40:1481–91. [DOI] [PubMed] [Google Scholar]
- 62. Narendran G, Menon PA, Venkatesan P et al. Acquired rifampicin resistance in thrice-weekly antituberculosis therapy: impact of HIV and antiretroviral therapy. Clin Infect Dis 2014; 59:1798–804. [DOI] [PubMed] [Google Scholar]
- 63. Nunn AJ, Mwaba P, Chintu C et al. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ 2008; 337:a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suthar AB, Granich R, Mermin J, Van Rie A. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ 2012; 90:128C–38C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wiktor SZ, Sassan-Morokro M, Grant AD et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet 1999; 353:1469–75. [DOI] [PubMed] [Google Scholar]
- 66. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach: December 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 67. Masur H, Brooks JT, Benson CA et al. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Luetkemeyer AF, Kendall MA, Nyirenda M et al. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr 2014; 65:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meintjes G, Lawn SD, Scano F et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Meintjes G, Wilkinson RJ, Morroni C et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, Fox W. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transkei. Lancet 1987; 2:1418–22. [DOI] [PubMed] [Google Scholar]
- 72. Strang JI, Kakaza HH, Gibson DG et al. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet 1988; 2:759–64. [DOI] [PubMed] [Google Scholar]
- 73. Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 2000; 84:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mayosi BM, Ntsekhe M, Smieja M. Immunotherapy for tuberculous pericarditis. N Engl J Med 2014; 371:2534. [DOI] [PubMed] [Google Scholar]
- 75. Reuter H, Burgess LJ, Louw VJ, Doubell AF. Experience with adjunctive corticosteroids in managing tuberculous pericarditis. Cardiovasc J S Afr 2006; 17:233–8. [PubMed] [Google Scholar]
- 76. Chaisson RE, Post WS. Immunotherapy for tuberculous pericarditis. N Engl J Med 2014; 371:2535. [DOI] [PubMed] [Google Scholar]
- 77. American Academy of Pediatrics. Committee on Infectious Diseases. 2015 Red Book: Report of the Committee on Infectious Diseases. 30th ed Elk Grove Village, IL: AAP, 2015. [Google Scholar]
- 78. Thwaites G, Fisher M, Hemingway C et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009; 59:167–87. [DOI] [PubMed] [Google Scholar]
- 79. Ashby M, Grant H. Tuberculous meningitis treated with cortisone. Lancet 1955; 268:65–6. [DOI] [PubMed] [Google Scholar]
- 80. O'Toole RD, Thornton GF, Mukherjee MK, Nath RL. Dexamethasone in tuberculous meningitis. Relationship of cerebrospinal fluid effects to therapeutic efficacy. Ann Intern Med 1969; 70:39–48. [DOI] [PubMed] [Google Scholar]
- 81. Escobar JA, Belsey MA, Duenas A, Medina P. Mortality from tuberculous meningitis reduced by steroid therapy. Pediatrics 1975; 56:1050–5. [PubMed] [Google Scholar]
- 82. Girgis NI, Farid Z, Hanna LS, Yassin MW, Wallace CK. The use of dexamethasone in preventing ocular complications in tuberculous meningitis. Trans R Soc Trop Med Hyg 1983; 77:658–9. [DOI] [PubMed] [Google Scholar]
- 83. Girgis NI, Farid Z, Kilpatrick ME, Sultan Y, Mikhail IA. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J 1991; 10:179–83. [DOI] [PubMed] [Google Scholar]
- 84. Kumarvelu S, Prasad K, Khosla A, Behari M, Ahuja GK. Randomized controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis 1994; 75:203–7. [DOI] [PubMed] [Google Scholar]
- 85. Chotmongkol V, Jitpimolmard S, Thavornpitak Y. Corticosteroid in tuberculous meningitis. J Med Assoc Thai 1996; 79:83–90. [PubMed] [Google Scholar]
- 86. Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis 1997; 25:872–87. [DOI] [PubMed] [Google Scholar]
- 87. Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics 1997; 99:226–31. [DOI] [PubMed] [Google Scholar]
- 88. Thwaites GE, Nguyen DB, Nguyen HD et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351:1741–51. [DOI] [PubMed] [Google Scholar]
- 89. Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2008; 1:CD002244. [DOI] [PubMed] [Google Scholar]
- 90. Malhotra HS, Garg RK, Singh MK, Agarwal A, Verma R. Corticosteroids (dexamethasone versus intravenous methylprednisolone) in patients with tuberculous meningitis. Ann Trop Med Parasitol 2009; 103:625–34. [DOI] [PubMed] [Google Scholar]
- 91. Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:223–37. [DOI] [PubMed] [Google Scholar]
- 92. Ho J, Marks GB, Fox GJ. The impact of sputum quality on tuberculosis diagnosis: a systematic review. Int J Tuberc Lung Dis 2015; 19:537–44. [DOI] [PubMed] [Google Scholar]
- 93. Gordin FM, Slutkin G, Schecter G, Goodman PC, Hopewell PC. Presumptive diagnosis and treatment of pulmonary tuberculosis based on radiographic findings. Am Rev Respir Dis 1989; 139:1090–3. [DOI] [PubMed] [Google Scholar]