Abstract
Purpose
Early intensification with methotrexate (MTX) is a key component of acute lymphoblastic leukemia (ALL) therapy. Two different approaches to MTX intensification exist but had not been compared in T-cell ALL (T-ALL): the Children’s Oncology Group (COG) escalating dose intravenous MTX without leucovorin rescue plus pegaspargase escalating dose, Capizzi-style, intravenous MTX (C-MTX) regimen and the Berlin-Frankfurt-Muenster (BFM) high-dose intravenous MTX (HDMTX) plus leucovorin rescue regimen.
Patients and Methods
COG AALL0434 included a 2 × 2 randomization that compared the COG-augmented BFM (ABFM) regimen with either C-MTX or HDMTX during the 8-week interim maintenance phase. All patients with T-ALL, except for those with low-risk features, received prophylactic (12 Gy) or therapeutic (18 Gy for CNS3) cranial irradiation during either the consolidation (C-MTX; second month of therapy) or delayed intensification (HDMTX; seventh month of therapy) phase.
Results
AALL0434 accrued 1,895 patients from 2007 to 2014. The 5-year event-free survival and overall survival rates for all eligible, evaluable patients with T-ALL were 83.8% (95% CI, 81.2% to 86.4%) and 89.5% (95% CI, 87.4% to 91.7%), respectively. The 1,031 patients with T-ALL but without CNS3 disease or testicular leukemia were randomly assigned to receive ABFM with C-MTX (n = 519) or HDMTX (n = 512). The estimated 5-year disease-free survival (P = .005) and overall survival (P = .04) rates were 91.5% (95% CI, 88.1% to 94.8%) and 93.7% (95% CI, 90.8% to 96.6%) for C-MTX and 85.3% (95% CI, 81.0%–89.5%) and 89.4% (95% CI, 85.7%–93.2%) for HDMTX. Patients assigned to C-MTX had 32 relapses, six with CNS involvement, whereas those assigned to HDMTX had 59 relapses, 23 with CNS involvement.
Conclusion
AALL0434 established that ABFM with C-MTX was superior to ABFM plus HDMTX for T-ALL in approximately 90% of patients who received CRT, with later timing for those receiving HDMTX.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common form of cancer in children. Approximately 15% have T-cell ALL (T-ALL), which is more common in older adolescents and African Americans.1 Historically, T-ALL has had inferior event-free survival (EFS) and overall survival (OS) compared with precursor B-cell ALL (B-ALL).1-4 Patients with T-ALL often present with high-risk clinical features, including older age, higher WBC count, and extramedullary disease, especially in the CNS. Compared with B-ALL, those with T-ALL frequently display slower kinetics of blast clearance after initiation of therapy.5-9 Relapse commonly occurs during active therapy, frequently involves the CNS, and has a dismal salvage rate.1,6,10,11
Although treatment intensification has improved survival for children with ALL,12 the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate (MTX), which seem to be particularly important for T-ALL, remain unclear.9,13-16 Two different MTX intensification strategies commonly are used in pediatric ALL trials: high-dose MTX (HDMTX) with leucovorin rescue and Capizzi-style escalating intravenous MTX without leucovorin rescue plus pegaspargase Capizzi-style, intravenous MTX (C-MTX).
We have reported previously that HDMTX is superior to C-MTX for children and adolescents with high-risk B-ALL.17 Because disease sensitivity to MTX and pegaspargase differ between B-ALL and T-ALL,5,14,15,18 we designed COG AALL0434, conducted in parallel with AALL0232, to compare C-MTX and HDMTX in T-ALL. AALL0434 was a 2 × 2 pseudofactorial trial with a second randomized question that tested the addition of six 5-day cycles of nelarabine. Because of concerns about high rates of CNS relapse in T-ALL, approximately 90% of patients received presymptomatic cranial radiation therapy (CRT) given during consolidation (month 2 of therapy) in the C-MTX regimen or during delayed intensification (DI; month 7 of therapy) for those who received HDMTX.19-21 We report the results of the AALL0434 MTX randomization. The results of the nelarabine randomization will be reported separately.
PATIENTS AND METHODS
Patient Characteristics
COG AALL0434 enrolled participants from January 2007 to July 2014. Eligibility included newly diagnosed, untreated (except corticosteroids) patients with T-ALL ages 1 to 31 years. AALL0434 was amended in 2010 to include those with lymphoblastic lymphoma (T-LLy). This report is limited to patients with T-ALL because those with T-LLy did not participate in the MTX randomization study. Enrollment in the COG classification/biology studies AALL03B1 or AALL08B1 was required for study entry. Minimal residual disease (MRD) testing was performed at the University of Washington (B.L.W.) using established methodologies.22 Before receiving systemic therapy, CSF was obtained for stratification into CNS1 (no blasts in the CSF), CNS2 (CSF WBC < 5/μL with blasts), and CNS3 (CSF WBC ≥ 5/μL with blasts or clinical symptoms of cranial nerve palsies, brain/eye involvement, or hypothalamic syndrome).17 Adjustments for CSF red cell contamination were determined using the Steinherz/Bleyer algorithm.23 AALL0434 was approved by the National Cancer Institute, Food and Drug Administration, the pediatric central institutional review board, and institutional review boards at each participating center. Informed consent/assent was obtained from study participants and, when appropriate, their legal guardians, in accordance with the Declaration of Helsinki.
Treatment
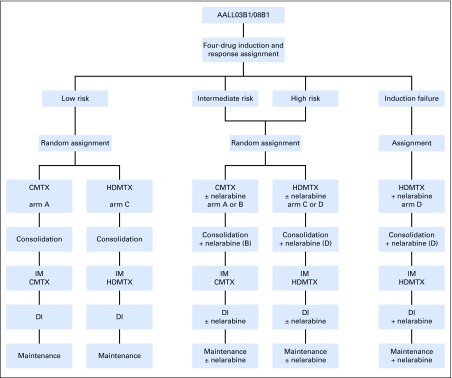
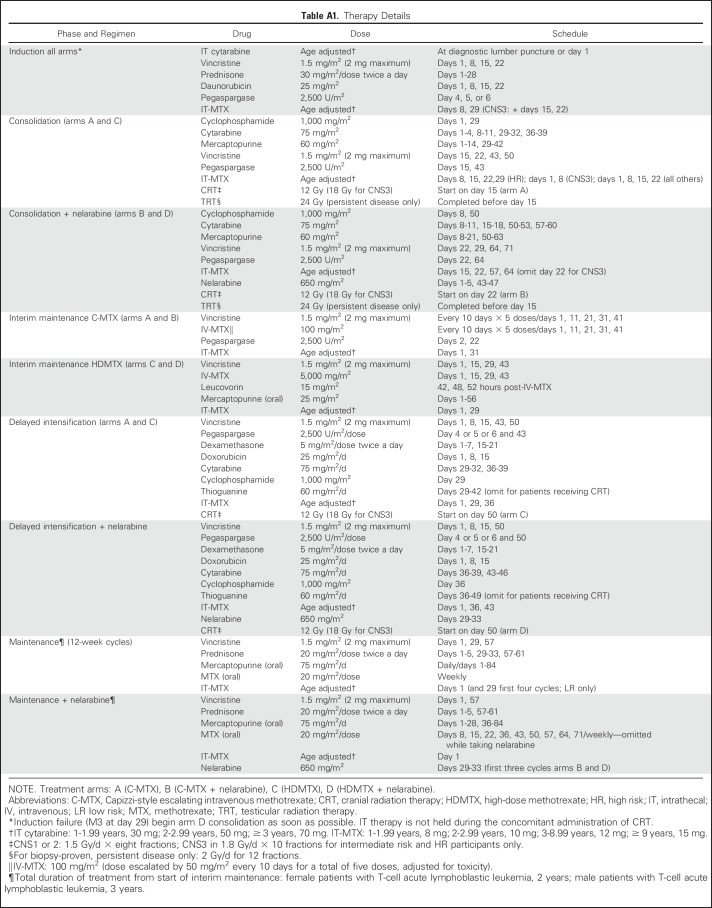
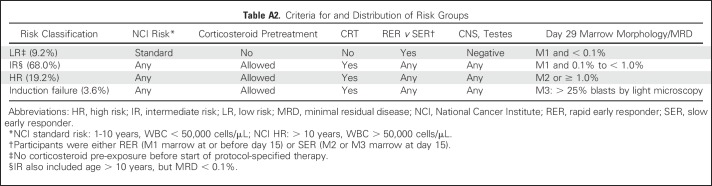
AALL0434 used a COG-augmented Berlin-Frankfurt-Muenster (ABFM) regimen to compare the efficacies of HDMTX versus C-MTX with or without nelarabine in a 2 × 2 pseudofactorial design16,17 (Appendix Fig A1, online only). After receiving a 28-day, prednisone-based, four-drug induction (Appendix Table A1, online only), patients with T-ALL were classified into low-risk (LR), intermediate-risk (IR), and high-risk (HR) groups or as those who experienced induction failure.16 Patients with LR T-ALL were ages 1 to 9.99 years with an initial WBC ≤ 50,000/μL, CNS1 status, and no testicular leukemia (males), rapid early responders (RERs) with an M1 marrow (< 5% blasts) by induction day 15, and < 0.1% day 29 MRD. Slow early responders (SERs) had an M2/M3 marrow at induction day 15 or ≥ 0.1% to 1% day 29 MRD. Patients with HR T-ALL had a day 29 M2 marrow (5% to 25% blasts) or MRD levels ≥ 1%. All other patients were classified as IR (Appendix Table A2, online only). All except the LR patients were assigned to receive CRT (12 Gy for CNS1/CNS2 and 18 Gy for CNS3).
AALL0434 used two consents, one for induction and a second for the postinduction random assignments among arms A (C-MTX/without nelarabine), B (C-MTX/nelarabine), C (HDMTX/without nelarabine), and D (HDMTX/nelarabine). After induction, all patients received an ABFM consolidation phase (with or without nelarabine), with CRT delivered during this phase for those assigned to the C-MTX arm16 (Appendix Table A2). During the 8-week interim maintenance (IM) phase, patients received either C-MTX with escalating intravenous MTX without leucovorin plus two doses of pegaspargase or HDMTX with leucovorin rescue without pegaspargase.17 The IM phases also included vincristine (VCR; four with HDMTX, five with C-MTX), intrathecal MTX (two for both), and oral 6-mercaptopurine (6-MP; days 1 to 56 with HDMTX only).
After completion of IM, patients received a single DI) phase, with CRT given during the second half of DI to those assigned to HDMTX. Patients then received maintenance therapy until 2 (females) or 3 (males) years after the start of IM.
The CNS3 patients were nonrandomly assigned to receive HDMTX and 1.8 Gy CRT given during DI. All boys with persistent testicular leukemia after day 29 received 2.4 Gy of testicular radiation during consolidation and were nonrandomly assigned to receive HDMTX. Participants who received corticosteroid pretreatment for > 48 hours before diagnosis were excluded from the LR cohort. Participants with a prior seizure disorder that required anticonvulsant therapy were randomly assigned between the MTX arms but excluded from the nelarabine randomization.24 Treatment-related adverse events were graded using Common Terminology Criteria for Adverse Events (version 4). Patients with Down syndrome or the Philadelphia chromosome were ineligible. No participants were assigned treatment on the basis of cytogenetics,25 genomic alterations,26-31 or the early T-precursor phenotype.32,33
Statistical Analysis
EFS was defined as time from study enrollment (first consent) to first event (induction failure, induction death, relapse, second malignant neoplasm, remission death) or date of last contact for those who were event free. Disease-free survival (DFS) was defined as time from postinduction random assignment (second consent) to first event or date of last contact. OS was defined as time from study enrollment or from postinduction random assignment, as appropriate, to death or date of last of contact.
Using a two-sided α of 5%, there was 85.3% power to detect an improvement in 4-year DFS from 82% to 89% between the two randomized MTX regimens, with a total of 980 patients accrued over the life of the study (total expected events, 142), with a minimum follow-up of 3 years. Study accrual duration was driven by the time needed to meet accrual targets for the nelarabine randomization. Interim analyses for efficacy and futility occurred when approximately 20%, 40%, 60%, 80%, and 100% of the expected events were observed. An alpha - t2 spending function with truncation at 3 standard deviations was used for interim monitoring.
Data current as of March 31, 2017, are included in this report. Survival rates were estimated by using the Kaplan-Meier method with standard errors of Peto et al.34 Survival rates and hazard ratios are presented with 95% CIs. Because the study was designed for the comparison of the randomized arms (C-MTX v HDMTX), no adjustments were made for multiple comparisons among participant subsets. Two-sided log-rank tests were used for comparison of survival curves. Proportions were compared between groups using a χ2 or Fisher’s exact test. Cumulative incidence rates were computed using the cumulative incidence function for competing risks, and comparisons were made using the K-sample test.35 P < .05 was considered significant for all comparisons. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC). Graphics were generated with R version 2.13.1 (http://www.r-project.org).
RESULTS
Participants
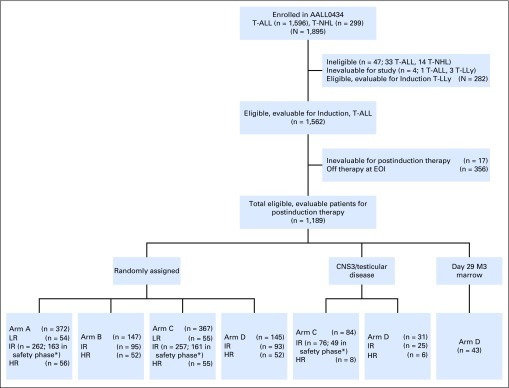
AALL0434 enrolled 1,895 patients; 47 were ineligible (19 because of timing of therapy initiation, 11 for incorrect disease type, seven for inaccurate disease staging, and 10 for other reasons), four were inevaluable (one for a nonstudy drug shortage and three for lack of baseline MRD testing), and 282 had T-LLy. The remaining 1,562 patients with T-ALL were eligible and evaluable for induction therapy (Fig 1). At the second consent stage, 17 participants with T-ALL were inevaluable for postinduction therapy, and 356 with T-ALL (23%) came off the study almost universally because the physician or patient did not wish to participate in the randomization. The remaining 1,189 eligible, evaluable participants with T-ALL were risk classified at the end of induction as LR (n = 109 [9.2%]), IR (n = 808 [68.0%]), HR (n = 229 [19.2%]), and induction failure (n = 43 [3.6%]; Appendix Table A2). Among the randomly assigned participants, 10.6% were LR, 68.6% were IR, and 20.8% were HR. A total of 519 participants were randomly assigned to receive C-MTX, and 512 to receive HDMTX, with the randomization stratified by risk group.
Fig 1.
CONSORT diagram for risk-stratified therapy. (*) Includes IR patients not randomly assigned to receive nelarabine during the safety phase (assigned to arms A and C only). EOI, end of induction; HR, high risk; IR, intermediate risk; LR, low risk; T-ALL, T-cell acute lymphoblastic leukemia; T-LLy, T-cell non-Hodgkin lymphoma.
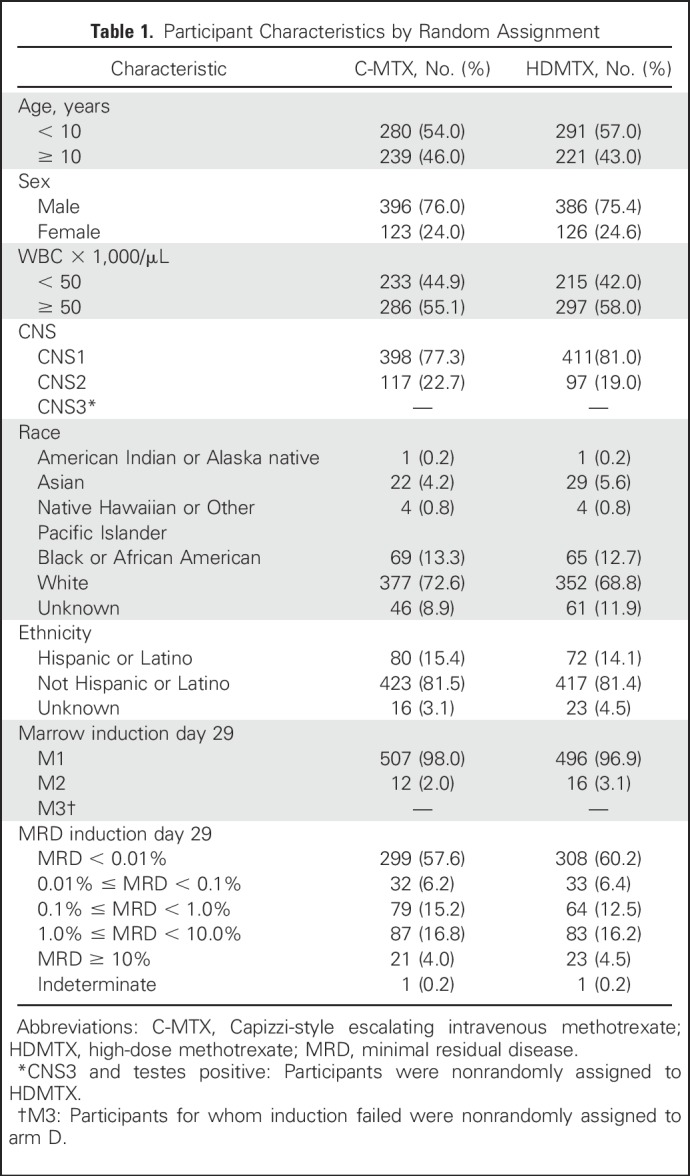
Patient age ranged from 1 to 30 years, with 52.2% 1 to 9 years of age, 44.9% 10 to 20 years of age, and 2.9% 21 to 30 years of age. Adolescents and young adults ages 15 to 30 years comprised 20.7% of the study population. Seventy-four percent were male and 26% were female. African American enrollment was 13.5%, and Hispanic enrollment was 14.4%. At study entry, 72.8% of participants were classified as CNS1 and 19.7% as CNS2; all participants with CNS3 (7.5%) were nonrandomly assigned to HDMTX (Table 1).
Table 1.
Participant Characteristics by Random Assignment
Outcomes of the MTX Randomization
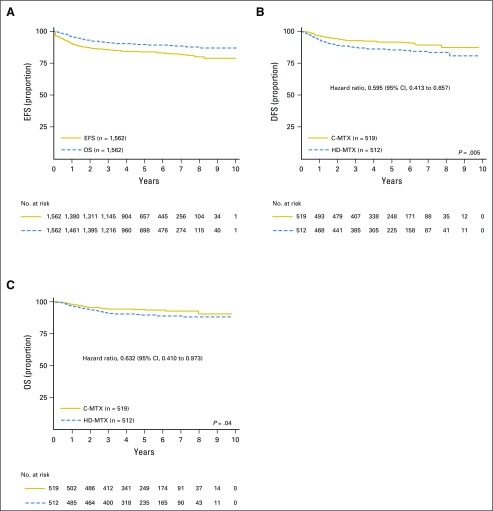
Among the 1,844 eligible and evaluable participants, 40 experienced death as a first event (seven died during induction and 33 during remission). The 5-year EFS and OS rates for all eligible, evaluable participants with T-ALL were 83.8% (81.2% to 86.4%) and 89.5% (87.4% to 91.7%), respectively (Fig 2A). For the 1,031 participants with T-ALL who participated in the MTX randomization, the 5-year DFS rate was 88.4% (85.7% to 91.1%), and the OS rate was 91.6% (89.2% to 94.0%). At the time of interim monitoring in April 2015, a predetermined efficacy monitoring boundary was crossed, with estimated 4-year DFS rates of 92.5% (89.0% to 96.0%) for C-MTX and 86.1% (81.4% to 90.8%) for HDMTX (P = .017). Because all participants who were randomly assigned to receive HDMTX had completed IM when the monitoring boundary was met, no alterations were made to therapy. At the time of final analyses, the estimated 5-year DFS (hazard ratio, 0.595; P = .005) and OS (hazard ratio, 0.632; P = .036) rates were 91.5% (88.1% to 94.8%) and 93.7% (90.8% to 96.6%) for C-MTX and 85.3% (81.0% to 89.5%) and 89.4% (85.7% to 93.2%) for HDMTX (Figs 2B and 2C). No significant qualitative interaction was found between the MTX and nelarabine randomizations (P = .41). For participants assigned to receive HDMTX for CNS3 or testicular disease, the 5-year DFS rate was 76.7% (65.1% to 88.4%) and 89.5% (70.5% to 100%), and the OS rate was 85.4% (75.9% to 94.9%) and 89.2% (70.0% to 100%).
Fig 2.
Event-free survival (EFS), disease-free survival (DFS), and overall survival (OS) curves overall and by regimen. (A) EFS and OS curves for all patients with T-cell acute lymphoblastic leukemia; 5-year EFS and OS rates were 83.8% (81.2% to 86.4%) and 89.5% (87.4% to 91.7%), respectively. (B) DFS curves for Capizzi-style escalating intravenous methotrexate (C-MTX) versus high-dose methotrexate (HDMTX) randomly assigned cohorts; 5-year DFS rate was 91.5% (88.1% to 94.8%) for C-MTX and 85.3% (81.0% to 89.5%) for HDMTX. (C) OS curves for C-MTX versus HDMTX randomly assigned cohorts; 5-year OS was 93.7% (90.8% to 96.6%) for C-MTX and 89.4% (85.7% to 93.2%) for HDMTX.
Patterns of Treatment Failure
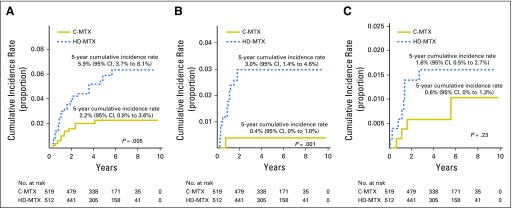
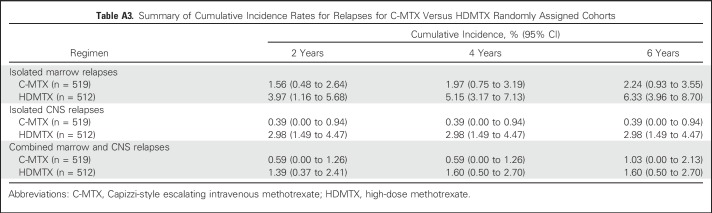
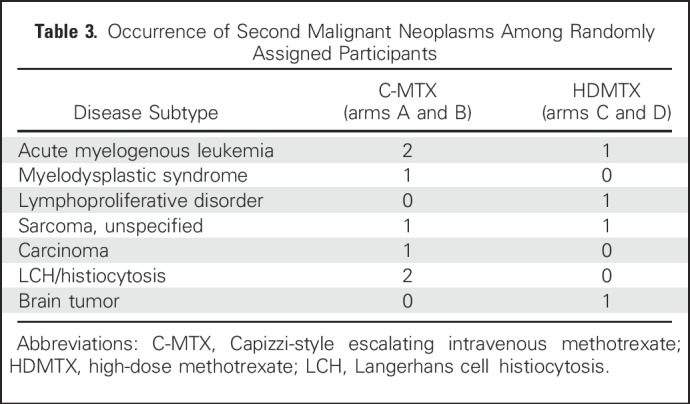
Approximately 90% of AALL0434 participants with T-ALL in the MTX randomization received 12 Gy prophylactic CRT for CNS1 or CNS2 disease. CRT was administered at week 8 (consolidation) in the C-MTX arm versus week 26 (DI) in the HDMTX arm. A total of 122 events (Table 2) occurred in the randomized T-ALL cohort, including 12 second malignant neoplasms (seven in C-MTX; five in HDMTX; P = .58; Tables 2 and 3) and 19 deaths in remission (eight in C-MTX; 11 in HDMTX; P = .47). There were 91 relapses (32 in C-MTX [six that involved the CNS] and 59 in HDMTX [23 that involved the CNS]). The 5-year cumulative incidence rates of isolated marrow relapse (2.2% [0.8% to 3.6%] for C-MTX v 5.9% [3.7% to 8.1%] for HDMTX; P = .005) and isolated CNS relapse (0.4% [0% to 1.0%] for C-MTX v 3.0% [1.4% to 4.6%] for HDMTX; P = .001) were significantly higher in those assigned to HDMTX who experienced more relapses (Appendix Table A3, online only; Appendix Figs A2A to A2C, online only).
Table 2.
DFS Event Summary by Randomized Regimen
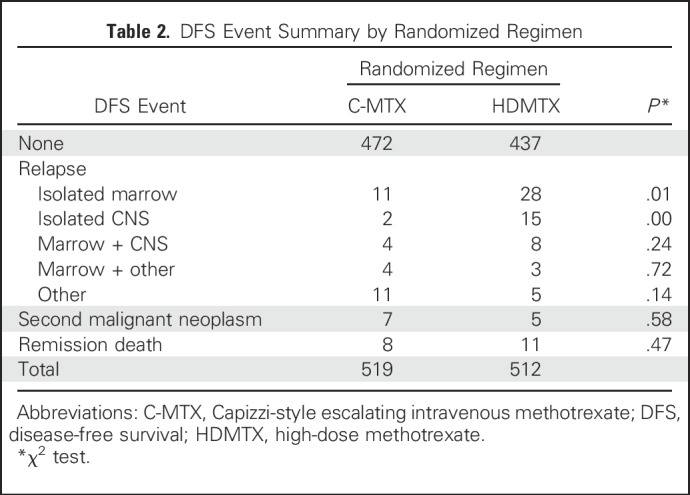
Table 3.
Occurrence of Second Malignant Neoplasms Among Randomly Assigned Participants
Outcomes Defined by Risk Groups and Early Response
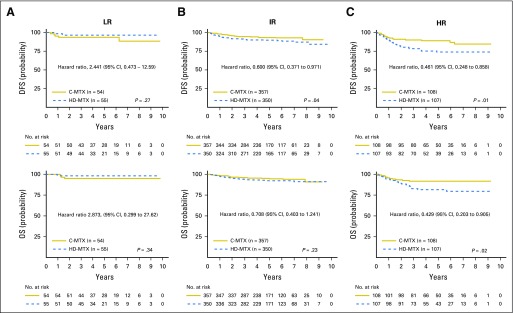
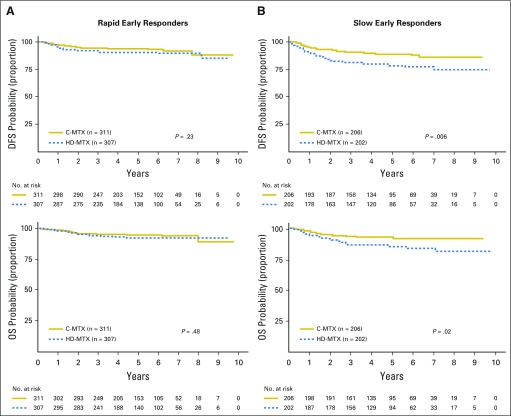
For participants with LR T-ALL, the 5-year DFS rate was 92.6% (83.3% to 100%) for C-MTX versus 96.2% (88.2% to 100%) for HDMTX (P = .27), and the OS rate was 94.4% (86.2% to 100%) versus 98.1% (92.3% to 100%; P = .34; Fig 3). For IR patients, the 5-year DFS rate was 92.5% (88.7% to 96.3%) for C-MTX versus 88.3% (83.7% to 92.9%) for HDMTX (P = .04), and the OS rate was 94.6% (91.2% to 97.9%) versus 91.3% (87.2% to 95.3%; P = .23). For HR patients, the 5-year DFS rate was 87.4% (78.8% to 96.0%) for C-MTX versus 70.0% (57.9% to 82.0%) for HDMTX (P = .01), and the OS rate was 90.5% (82.8% to 98.2%) versus 79.1% (68.3% to 89.9%; P = .02). Similar comparisons were made for all participant RER/SERs (Appendix Figs A3A and A3B, online only). Compared with LR and IR patients, those with HR or who are SERs had relatively greater improvements in DFS and OS with C-MTX therapy.
Fig 3.
Disease-free survival (DFS) and overall survival (OS) for low-risk (LR), intermediate-risk (IR), and high-risk (HR) groups by regimen. (A) LR T-cell acute lymphoblastic leukemia (T-ALL): 5-year DFS rate was 92.6% (83.3% to 100%) for Capizzi-style escalating intravenous methotrexate (C-MTX) versus 96.2% (88.2% to 100%) for high-dose methotrexate (HDMTX), and the OS rate was 94.4% (86.2% to 100%) for C-MTX versus 98.1% (92.3% to 100%) for HDMTX. (B) IR T-ALL: 5-year DFS rate was 92.5% (88.7% to 96.3%) for C-MTX versus 88.3% (83.7% to 92.9%) for HDMTX, and the OS rate was 94.6% (91.2% to 97.9%) for C-MTX versus 91.3% (87.2% to 95.3%) for HDMTX. (C) HR T-ALL: 5-year DFS rate was 87.4% (78.8% to 96.0%) for C-MTX versus 70.0% (57.9% to 82.0%) for HDMTX, and the OS rate was 90.5% (82.8% to 98.2%) for C-MTX versus 79.1% (68.3% to 89.9%) for HDMTX.
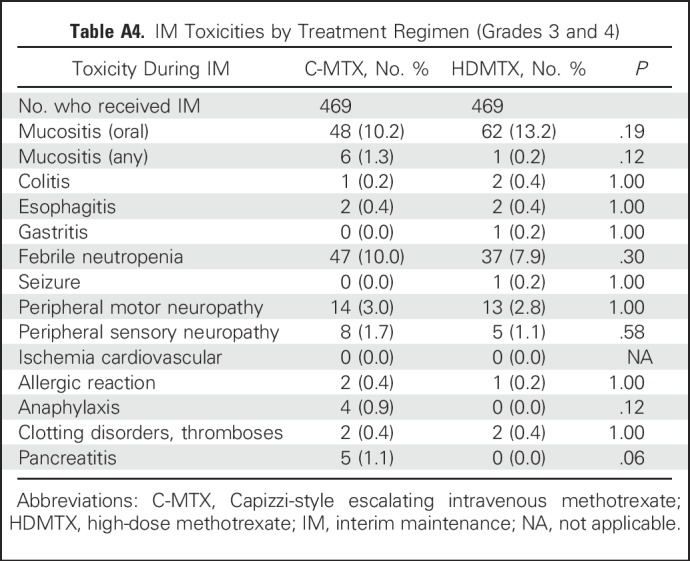
Toxicities During the IM Phase
No clinically significant differences were found between C-MTX and HDMTX with respect to grade 3 and 4 febrile neutropenia, seizures, and peripheral motor and sensory neuropathies. The C-MTX regimen included two additional doses of pegaspargase during the IM phase, which did not result in significant differences in the occurrence of grade 3 and 4 clotting/coagulation events, pancreatitis (five with C-MTX, none with HDMTX; P = .062), allergic reactions, or anaphylaxis (Appendix Table A4, online only).
DISCUSSION
To our knowledge, COG AALL0434 is the largest trial of T-ALL ever conducted. It showed a 5-year EFS rate of 83.8% and OS rate of 89.5% among eligible, evaluable participants. In comparison, the 5-year OS rate for T-ALL was 80.7% in COG trials conducted from 1995 to 1999 (n = 624) and 81.6% (n = 429) in 2000 to 2005.1 The AIEOP-BFM-ALL-2000 trial included 464 patients with T-ALL enrolled from 2000 to 2006 who had 5-year EFS and OS rates of 76.3% and 81.2%, respectively.5 The outcomes (EFS and OS) for T-ALL in AALL0434 were better than those observed in past trials.
Although MTX has long been recognized for its importance in the treatment of ALL, questions about its dose and integration into multiagent therapy have merited continued investigation.17,36,37 On the basis of improved outcomes with C-MTX in B- and T-ALL study CCG 1961 (1996 to 2002) and with HDMTX in T-ALL study 9404 (1996 to 2001), the COG tested whether C-MTX versus HDMTX differentially affected outcomes for HR B-ALL (AALL0232) and T-ALL (AALL0434) when given during the IM phase.16,17 In AALL0232, we found that HDMTX was superior to C-MTX in patients with HR B-ALL, with a significantly better 5-year EFS rate (79.6% [76.5% to 82.7%] v 75.2% [71.9% to 78.5%]; P = .008) and OS rate (88.9% [86.5% to 91.3%] v 86.1% [83.4% to 88.8%]; P = 0.025) because of reductions in both marrow and CNS relapse.16 Of note, AALL0434 showed the opposite effect, with the C-MTX regimen being superior to HDMTX with a significantly higher 5-year DFS rate (91.5% [88.1% to 94.8%] v 85.3% [81.0% to 89.5%]; P = .005) and OS rate (93.7% [90.8% to 96.6%] v 89.4% [85.7% to 93.2%]; P = .036), also because of reductions in both marrow and CNS relapse. How can these findings be reconciled?
Like AALL0232, AALL0434 was not a strict comparison of two different MTX schedules because the doses of pegaspargase, 6-MP, and VCR and the timing of CRT differed between the two ABFM arms during the 2-month IM phase. Use of ABFM therapy, with additional doses of VCR and no leucovorin rescue with C-MTX showed similar DFS and OS rates for LR (no CRT) and IR (CRT) participants. However, similar to other studies,5 SERs and participants with HR T-ALL had more relapses but with better OS with C-MTX than with HDMTX, where postinduction therapy had its greatest effect for those who historically experienced relapse more often than lesser-risk participants. The health care costs and time burden associated with C-MTX are substantially less than for HDMTX,38 which may have affected adherence in some participants.
Leukemic involvement of the CNS is a common problem in T-ALL either at presentation or at relapse.11 The preceding phase III COG T-ALL trials took different approaches to CRT. In POG 9904, all patients received CRT at week 30.15 In CCG 1961, only patients with CNS3 status or an SER on the basis of an M3 marrow on induction day 8 received CRT, which was delivered during the first 2 weeks of consolidation therapy.15,39 Because 35% to 40% of T-ALL relapses in CCG 1961 involved the CNS, AALL0434 was designed to include CRT for all IR and HR patients, who comprised approximately 90% of participants. Because the C-MTX regimen was the same as that used in the ABFM arm of CCG 1961, CRT was delivered during the second month of therapy during consolidation. In contrast, CRT delivered in the HDMTX arm was given during DI in the seventh month, similar to AEIOP-BFM-2000, not only to preserve the timing of CRT administration in POG 9404 but also to reduce the risk of neurotoxicities when CRT is given before HDMTX. In the AALL0434 C-MTX cohort, fewer CNS, marrow, or mixed relapse events were observed. Because T cells traffic between medullary and sanctuary sites, prophylactic CRT may have prevented both local and systemic relapses when given during consolidation.40,41 The differential timing of CRT between the study arms of AALL0434 possibly affected the observed differences in EFS and OS.
The C-MTX arm included two additional doses of pegaspargase (seven total, two during IM) compared with the HDMTX arm (five total, none during IM). Although pegaspargase does not cross the blood-brain barrier, its asparagine-depleting effects equilibrate across the blood-brain barrier.42,43 AALL0434 was unique among contemporary frontline COG ALL studies because Erwinia asparaginase was commonly available for the approximately 15% of participants who developed grade 3 (or higher) hypersensitivity reactions to pegaspargase.44,45 Enhanced asparagine depletion with C-MTX also may have prevented relapse events, including those that involve the CNS.
Although the AALL0434 C-MTX regimen was superior to the HDMTX regimen for treating pediatric T-ALL, this finding is in the context of approximately 90% of randomly assigned patients who received CRT. Because CRT is associated with secondary malignancies,46 cognitive impairment,47 and other long-term health issues,48,49 many groups have evaluated alternate strategies for controlling CNS disease.
Either singly or in combination, others either replaced or reduced CRT with dose-intensified pegaspargase,14,50 HDMTX,5,51 dexamethasone during induction,51,52 triple intrathecal therapies,53,54 and first remission hematopoietic cell transplantation for MRD-defined HR patients.53 Whether similar differences between C-MTX and HDMTX would be seen if patients did not receive CRT is not known.
Data from previous COG studies have shown that National Cancer Institute risk status, recurring cytogenetic features, and other traditional prognostic factors in B-ALL have limited value in T-ALL,13-15 but results from the I-BFM-SG MRD6 and AEIOP-BFM-20005 studies have shown that end-induction and end-consolidation MRD levels could portend risk for relapse. Although cytogenetic and phenotypic findings in T-ALL have not been useful for treatment assignment, these and other biomarkers, in combination with MRD, may better risk stratify HR disease, as demonstrated in the FRALLE2000T study.12,55 The improved survival rates observed for COG AALL0434 may better position us to include biomarkers with MRD to further improve survival in future trials.
ACKNOWLEDGMENT
We thank the Cancer Therapeutic Evaluation Program for support of AALL0434, the study’s data safety and monitoring committee, and the pediatric central institutional review board. We appreciate the important intellectual contributions that James Nachman, MD, provided in the creation of this study. We especially acknowledge all COG investigators and the patients and their families for participating in the study; without them this report would not have been possible.
Appendix
Fig A1.
Randomization and assignment strategies. (*) Participants with CNS3 and testicular disease were nonrandomly assigned to high-dose methotrexate (HDMTX). C-MTX, Capizzi-style escalating intravenous methotrexate; DI, delayed intensification.
Fig A2.
Cumulative incidence rate of relapse by site for Capizzi-style escalating intravenous methotrexate (C-MTX) and high-dose methotrexate (HDMTX) randomly assigned cohorts. (A) Isolated bone marrow relapse. (B) Isolated CNS relapse. (C) Combined bone marrow and CNS relapse.
Fig A3.
Disease-free survival (DFS) and overall survival (OS) by early response status. (A) Rapid early responders: 5-year DFS was 93.3% (89.4% to 97.1%) for Capizzi-style escalating intravenous methotrexate (C-MTX) versus 90.0% (85.1% to 94.6%) for high-dose methotrexate (HDMTX; hazard ratio, 0.722 [0.421 to 1.238]; P = .23), and OS was 94.5% (91.0% to 98.0%) for C-MTX versus 92.1% (87.8% to 96.4%) for HDMTX (hazard ratio, 0.801 [0.429 to 1.493]; P = .48). (B) Slow early responders: 5-year DFS was 88.7% (82.7% to 94.7%) for C-MTX versus 78.1% (70.4% to 85.8%) for HDMTX (hazard ratio, 0.501 [0.305 to 0.824]; P = .001), and OS was 92.4% (87.3% to 97.5%) for C-MTX versus 85.2% (78.6% to 91.8%) with HDMTX (hazard ratio, 0.506 [0.276 to 0.928]; P = .03).
Table A1.
Therapy Details
Table A2.
Criteria for and Distribution of Risk Groups
Table A3.
Summary of Cumulative Incidence Rates for Relapses for C-MTX Versus HDMTX Randomly Assigned Cohorts
Table A4.
IM Toxicities by Treatment Regimen (Grades 3 and 4)
Footnotes
Supported by National Institutes of Health Grants No. U10 CA98543, U10 CA98413, U10 CA180886, 1U24 CA196173, and U10 CA180899 and by the St Baldrick’s Foundation. M.L.L. is the University of California, San Francisco, Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia.
Clinical trial information: NCT00408005.
AUTHOR CONTRIBUTIONS
Conception and design: Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Robert J. Hayashi, Barbara L. Asselin, Paul S. Gaynon, Mignon L. Loh, Naomi J. Winick, William L. Carroll, Stephen P. Hunger
Collection and assembly of data: Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Brent L. Wood, Zhiguo Chen, Nancy Eisenberg, Robert J. Hayashi, Julie M. Gastier-Foster, Andrew J. Carroll, Nyla A. Heerema, Barbara L. Asselin, Michael J. Borowitz, Mignon L. Loh, Karen R. Rabin, Patrick A. Zweidler-Mckay, Stephen P. Hunger
Data analysis and interpretation: Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Brent L. Wood, Natia Esiashvili, Zhiguo Chen, Nancy Eisenberg, Nikki Briegel, Robert J. Hayashi, Julie M. Gastier-Foster, Barbara L. Asselin, Paul S. Gaynon, Mignon L. Loh, Karen R. Rabin, Elizabeth A. Raetz, Naomi J. Winick, William L. Carroll, Stephen P. Hunger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children’s Oncology Group AALL0434 Methotrexate Randomization
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Stuart S. Winter
Stock or Other Ownership: Amgen
Consulting or Advisory Role: Jazz Pharmaceuticals
Kimberly P. Dunsmore
Employment: TypeZero Technologies (I)
Leadership: TypeZero Technologies(I)
Stock or Other Ownership: TypeZero Technologies (I)
Speakers’ Bureau: Tandem (I)
Research Funding: Novo Nordisk
Travel, Accommodations, Expenses: Novo Nordisk, Tandem (I), TypeZero Technologies (I)
Meenakshi Devidas
Honoraria: Pfizer, Novartis, PSI
Travel, Accommodations, Expenses: PSI
Brent L. Wood
Honoraria: Seattle Genetics (I), Amgen, Beckman Coulter
Consulting or Advisory Role: Amgen
Research Funding: Amgen (Inst), Juno Therapeutics (Inst), Seattle Genetics (Inst), BioLineRx (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Beckman Coulter, Amgen, Seattle Genetics
Natia Esiashvili
Honoraria: Varian Medical Systems
Speakers’ Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems, IBA Worldwide
Zhiguo Chen
No relationship to disclose
Nancy Eisenberg
No relationship to disclose
Nikki Briegel
No relationship to disclose
Robert J. Hayashi
Consulting or Advisory Role: Otonomy (Inst)
Julie M. Gastier-Foster
Research Funding: Bristol-Myers Squibb (Inst), Incyte (Inst)
Andrew J. Carroll
No relationship to disclose
Nyla A. Heerema
No relationship to disclose
Barbara L. Asselin
Consulting or Advisory Role: Sigma Tau Pharmaceuticals
Paul S. Gaynon
Honoraria: Shire
Consulting or Advisory Role: Shire
Speakers’ Bureau: Shire
Michael J. Borowitz
Honoraria: Beckman Coulter
Research Funding: Becton Dickinson, Bristol-Myers Squibb
Mignon L. Loh
No relationship to disclose
Karen R. Rabin
Consulting or Advisory Role: Adaptive Biotechnologies Corporation
Travel, Accommodations, Expenses: Adaptive Biotechnologies Corporation
Elizabeth A. Raetz
No relationship to disclose
Patrick A. Zweidler-Mckay
Employment: ImmunoGen
Stock or Other Ownership: ImmunoGen
Naomi J. Winick
No relationship to disclose
William L. Carroll
No relationship to disclose
Stephen P. Hunger
Stock or Other Ownership: Express Scripts, Amgen, Merck (I), Amgen (I), Pfizer (I)
Honoraria: Jazz Pharmaceuticals, Spectrum Pharmaceuticals, ERYTECH Pharma
Consulting or Advisory Role: Novartis
REFERENCES
- 1.Hunger SP, Lu X, Devidas M, et al. : Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. J Clin Oncol 30:1663-1669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinherz PG, Gaynon PS, Breneman JC, et al. : Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features: Randomized controlled trial from the Children’s Cancer Group. Cancer 82:600-612, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Uckun FM, Sensel MG, Sun L, et al. : Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood 91:735-746, 1998 [PubMed] [Google Scholar]
- 4.Pui CH, Yang JJ, Hunger SP, et al. : Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol 33:2938-2948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrappe M, Valsecchi MG, Bartram CR, et al. : Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 118:2077-2084, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Willemse MJ, Seriu T, Hettinger K, et al. : Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood 99:4386-4393, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Nachman J, Sather HN, Gaynon PS, et al. : Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: A report from the Children’s Cancer Group. J Clin Oncol 15:2222-2230, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Nachman JB, Sather HN, Sensel MG, et al. : Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med 338:1663-1671, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Dunsmore KP, Devidas M, Linda SB, et al. : Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol 30:2753-2759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunger SP, Mullighan CG: Acute lymphoblastic leukemia in children. N Engl J Med 373:1541-1552, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Nachman J, Sather HN, Cherlow JM, et al. : Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: A report from the Children’s Cancer Group. J Clin Oncol 16:920-930, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Patrick K, Vora A: Update on biology and treatment of T-cell acute lymphoblastic leukaemia. Curr Opin Pediatr 27:44-49, 2015 [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1002/pbc.20429. Winter SS, Holdsworth MT, Devidas M, et al: Antimetabolite-based therapy in childhood T-cell acute lymphoblastic leukemia: A report of POG study 9296. Pediatr Blood Cancer, 46:179-186, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Amylon MD, Shuster J, Pullen J, et al. : Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group study. Leukemia 13:335-342, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Asselin BL, Devidas M, Wang C, et al. : Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: A randomized study by the Children’s Oncology Group (POG 9404). Blood 118:874-883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter SS, Dunsmore KP, Devidas M, et al. : Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatr Blood Cancer 62:1176-1183, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen EC, Devidas M, Chen S, et al. : Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group Study AALL0232. J Clin Oncol 34:2380-2388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barredo JC, Synold TW, Laver J, et al. : Differences in constitutive and post-methotrexate folylpolyglutamate synthetase activity in B-lineage and T-lineage leukemia. Blood 84:564-569, 1994 [PubMed] [Google Scholar]
- 19.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Spiegler BJ, Kennedy K, Maze R, et al. : Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol 24:3858-3864, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kellie SJ, Chaku J, Lockwood LR, et al. : Late magnetic resonance imaging features of leukoencephalopathy in children with central nervous system tumours following high-dose methotrexate and neuraxis radiation therapy. Eur J Cancer 41:1588-1596, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Wood BL: Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol 999:123-136, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Steinherz PG: CNS leukemia: Problem of diagnosis, treatment, and outcome. J Clin Oncol 13:310-313, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Berg SL, Blaney SM, Devidas M, et al. : Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: A report from the Children’s Oncology Group. J Clin Oncol 23:3376-3382, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Heerema NA, Sather HN, Sensel MG, et al. : Frequency and clinical significance of cytogenetic abnormalities in pediatric T-lineage acute lymphoblastic leukemia: A report from the Children’s Cancer Group. J Clin Oncol 16:1270-1278, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Matlawska-Wasowska K, Kang H, Devidas M, et al. : MLL rearrangements impact outcome in HOXA-deregulated T-lineage acute lymphoblastic leukemia: A Children’s Oncology Group Study. Leukemia 30:1909-1912, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrando AA, Look AT: Gene expression profiling in T-cell acute lymphoblastic leukemia. Semin Hematol 40:274-280, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ferrando AA, Herblot S, Palomero T, et al. : Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood 103:1909-1911, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Weng AP, Ferrando AA, Lee W, et al. : Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306:269-271, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Palomero T, Odom DT, O’Neil J, et al. : Transcriptional regulatory networks downstream of TAL1/SCL in T-cell acute lymphoblastic leukemia. Blood 108:986-992, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez A, Dahlberg SE, Neuberg DS, et al. : Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol 28:3816-3823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coustan-Smith E, Mullighan CG, Onciu M, et al. : Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 10:147-156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrick K, Wade R, Goulden N, et al. : Outcome for children and young people with early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 166:421-424, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 35:1-39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988. [Google Scholar]
- 36.Gaynon PS, Angiolillo AL, Carroll WL, et al. : Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children’s Oncology Group Report. Leukemia 24:285-297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matloub Y, Bostrom BC, Hunger SP, et al. : Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 118:243-251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiNofia AM, Seif AE, Devidas M, et al. : Cost comparison by treatment arm and center-level variations in cost and inpatient days on the phase III high-risk B acute lymphoblastic leukemia trial AALL0232. Cancer Med 7:3-12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibel NL, Steinherz PG, Sather HN, et al. : Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 111:2548-2555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. doi: 10.1038/leu.2016.272. Oruganti SR, Torres DJ, Krebsbach S, et al: CARMA1 is a novel regulator of T-ALL disease and leukemic cell migration to the CNS. Leukemia, 31:255-258, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchey AK, Pollock BH, Lauer SJ, et al. : Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: A Pediatric Oncology Group study. J Clin Oncol 17:3745-3752, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Henriksen LT, Nersting J, Raja RA, et al. : Cerebrospinal fluid asparagine depletion during pegylated asparaginase therapy in children with acute lymphoblastic leukaemia. Br J Haematol 166:213-220, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Panosyan EH, Wang Y, Xia P, et al. : Asparagine depletion potentiates the cytotoxic effect of chemotherapy against brain tumors. Mol Cancer Res 12:694-702, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asselin BL: The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol 457:621-629, 1999 [PubMed] [Google Scholar]
- 45.Burke MJ, Devidas M, Maloney K, et al. : Severe pegaspargase hypersensitivity reaction rates (grade ≥3) with intravenous infusion vs. intramuscular injection: Analysis of 54,280 doses administered to 16,534 patients on Children’s Oncology Group (COG) clinical trials. Leuk Lymphoma 59:1624-1633, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Relling MV, Rubnitz JE, Rivera GK, et al. : High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet 354:34-39, 1999 [DOI] [PubMed] [Google Scholar]
- 47. doi: 10.1200/JCO.2007.10.8464. Waber DP, Turek J, Catania L, et al: Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol 25:4914-4921, 2007 [Erratum: J Clin Oncol 26:694, 2008] [DOI] [PubMed] [Google Scholar]
- 48.Pui CH, Cheng C, Leung W, et al. : Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med 349:640-649, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Mody R, Li S, Dover DC, et al. : Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood 111:5515-5523, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg JM, Silverman LB, Levy DE, et al. : Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium experience. J Clin Oncol 21:3616-3622, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Möricke A, Zimmermann M, Valsecchi MG, et al. : Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 127:2101-2112, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Veerman AJ, Kamps WA, van den Berg H, et al. : Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10:957-966, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Pui CH, Campana D, Pei D, et al. : Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360:2730-2741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark B, Avrahami G, Nirel R, et al. : Extended triple intrathecal therapy in children with T-cell acute lymphoblastic leukaemia: A report from the Israeli National ALL-Studies. Br J Haematol 147:113-124, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Petit A, Trinquand A, Chevret S, et al. : Oncogenetic mutations combined with MRD improve outcome prediction in pediatric T-cell acute lymphoblastic leukemia. Blood 131:289-300, 2018 [DOI] [PubMed] [Google Scholar]