Abstract
Background
Converging lines of evidence implicate an important role for the immune system in schizophrenia. Microglia are the resident immune cells of the central nervous system and have many functions including neuroinflammation, axonal guidance and neurotrophic support. We aimed to provide a quantitative review of in vivo PET imaging studies of microglia activation in patients with schizophrenia compared with healthy controls.
Methods
Demographic, clinical and imaging measures were extracted from each study and meta-analysis was conducted using a random-effects model (Hedge's g). The difference in 18-kDa translocator protein (TSPO) binding between patients with schizophrenia and healthy controls, as quantified by either binding potential (BP) or volume of distribution (VT), was used as the main outcome. Sub-analysis and sensitivity analysis were carried out to investigate the effects of genotype, ligand and illness stage.
Results
In total, 12 studies comprising 190 patients with schizophrenia and 200 healthy controls met inclusion criteria. There was a significant elevation in tracer binding in schizophrenia patients relative to controls when BP was used as an outcome measure, (Hedge's g = 0.31; p = 0.03) but no significant differences when VT was used (Hedge's g = −0.22; p = 0.29).
Conclusions
In conclusion, there is evidence for moderate elevations in TSPO tracer binding in grey matter relative to other brain tissue in schizophrenia when using BP as an outcome measure, but no difference when VT is the outcome measure. We discuss the relevance of these findings as well as the methodological issues that may underlie the contrasting difference between these outcomes.
Key words: Microglia, neuroinflammation, schizophrenia, TSPO
Introduction
Converging lines of genetic, epidemiological and clinical evidence indicate that inflammatory pathways are altered in schizophrenia. Over 20 epidemiological studies show that people with a history of infection or autoimmune diseases have an increased risk of schizophrenia (Benros et al., 2011, 2014; Khandaker et al., 2012, 2013; Miller et al., 2013). Variants in genes of the immune pathways have also been associated with an increased risk of schizophrenia (Stefansson et al., 2009). Moreover, the largest genome-wide association study in schizophrenia to date found a highly significant association between risk of schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics, 2014) and a locus linked to the major immunohistocompatibility complex with subsequent work implicating microglial and complement activation in this pathway (Sekar et al., 2016). Similarly, elevated levels of a number of immune markers have been observed in schizophrenia (Tourjman et al., 2013). Studies have repeatedly (although not invariably) shown that patients with schizophrenia have increased serum concentrations of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α (Upthegrove et al., 2014; Dickerson et al., 2016). Meta-analyses show that these are elevated in medication-naïve first-episode patients (Upthegrove et al., 2014) and in later stages of illness (Dickerson et al., 2016), with large effect sizes (e.g. Hedge's g > 2.2 for IL-6 and >1.1 for IL-1β) (Upthegrove et al., 2014). Moreover, studies of cerebrospinal fluid (CSF) have shown increased levels of pro-inflammatory markers in schizophrenia patients when compared with healthy controls, including IL-1β, IL-6 and S100B (Schmitt et al., 2005; Soderlund et al., 2009; Sasayama et al., 2013; Schwieler et al., 2015). While there is some evidence for increased inflammatory markers in blood and CSF in schizophrenia, this cannot be taken to suggest neuro-inflammation. The evidence for increased cerebral activation of the immune system is scarce. Post-mortem studies have demonstrated elevated markers for microglia, and morphological changes indicating microglial activation in schizophrenia patients (Bayer et al., 1999; Radewicz et al., 2000; Trepanier et al., 2016) although this was not seen in all brain regions (Steiner et al., 2006) and there are still a large number of null studies (Trepanier et al., 2016). However, a recent meta-analysis of post-mortem studies by van Kesteren et al. confirmed overall increased microglia density in schizophrenia, together with increased concentrations of pro-inflammatory proteins (van Kesteren et al., 2017).
Microglia are the resident immune cells of the central nervous system and act as major mediators of neuroinflammation. In the healthy brain, microglia retain a ‘quiescent’ phenotype where processes extend through the local environment to detect context-specific changes. In this stage, microglia cells produce neurotrophic factors, provide axonal guidance and regulate local cell proliferation. However, in response to inflammatory stimuli, the cells become activated, undergoing morphological changes and releasing pro-inflammatory cytokines. A number of risk factors for schizophrenia, notably pre-natal infection and psychosocial stress, are known to induce microglial activation (Juckel et al., 2011; Calcia et al., 2016). When microglia are activated, it increases the expression of the 18-kDa translocator protein (TSPO) (Cosenza-Nashat et al., 2009). TSPO can be measured in vivo with positron emission tomography (PET) radiotracers and so far a number of PET studies have investigated microglia activation in schizophrenia-spectrum disorders. However, findings have been inconsistent and so far they have only been partially reviewed quantitatively (Plaven-Sigray et al., 2018). We therefore aimed to synthesize PET imaging findings of microglial activation in patients with schizophrenia-spectrum disorders and healthy controls, and to discuss the implications of these findings in relation to both the pathophysiology of the disorder and drug development efforts.
Methods
Data source and study selection
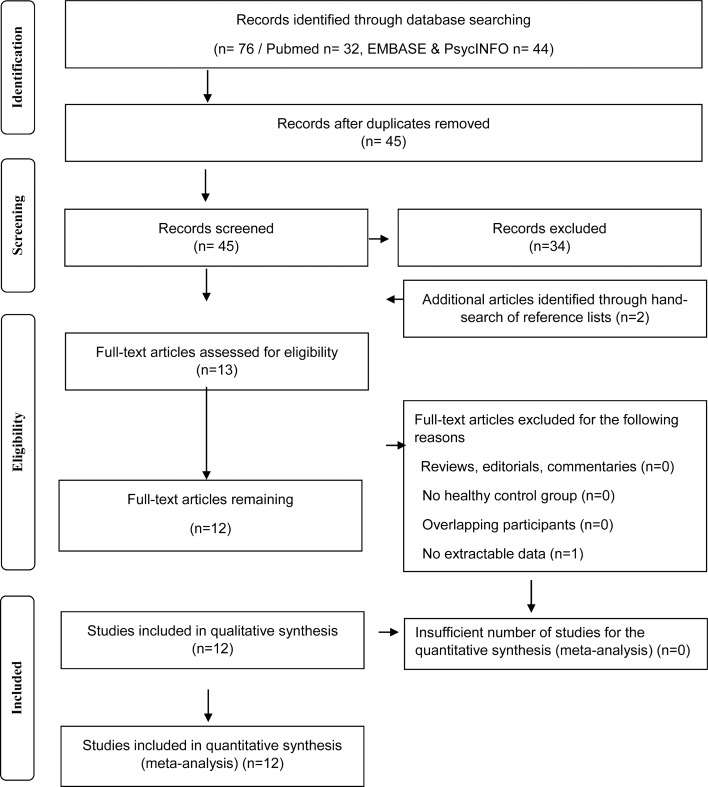
The entire PubMed, EMBASE and PsycINFO databases were searched to identify manuscripts published from inception date until 12 January 2018. To be included in the meta-analysis, a study needed to report in vivo TSPO PET imaging data in patients with schizophrenia-spectrum disorders and in a healthy control group. All studies needed to report the mean and standard deviations for both groups (see Fig. 1).
Fig. 1.
Flowchart showing the inclusion of studies for the meta-analysis.
Data extraction
The main outcome measure was the difference in the TSPO imaging index between patients with schizophrenia-spectrum disorders and healthy controls. For all studies, we extracted the following variables: authors, year of publication, subject characteristics for the patient and healthy control group (group size, age, sex, diagnosis, duration of illness, antipsychotic medication, psychopathology rating scale scores), imaging characteristics (method, radiotracer) and modelling method. The estimation of pooled standard deviation was performed using the statstodo software (http://statstodo.com/ComMeans_Pgm.php). In order to extract data from studies where data were available only in a plot format, we have used the plot digitiser software (http://plotdigitizer.sourceforge.net/).
Data analysis
The main outcome measure was the effect size determined using the TSPO tracer measure and quantified by either BPND, BP−P or VT in the total grey matter in patients with schizophrenia-spectrum disorders and healthy controls using a random-effects model. A minimum of three studies were required to run a meta-analysis. The group mean and error measures were not reported by Banati and Hickie (2009), and although we requested the data from the authors, we were unable to obtain them. Bloomfield et al. (2016a, 2016b) reported the data in both VT and distribution volume ratio (DVR) (using a multivariate analysis approach, where VT in whole brain or cerebellum was used, along with age and TSPO genotype, as covariates in an analysis of covariance producing a marginal mean). The DVR method used in this study is not equivalent to the measures used in the other studies. Also Ottoy et al. (2018) reported VT values only accounting for the vascular component (2TCM-1K) (Ottoy et al., 2018). In view of this, we requested the data for this meta-analysis and both authors provided the VT values not accounting for the vascular component, determined in the same way as other studies (i.e. not covaried for age and gender). Therefore, the main analysis of VT values included six studies using the 2TCM model.
A genetic variant at rs6971 in the TSPO gene, causing a non-conservative amino acid substitution, has been found to affect the binding of some TSPO PET tracers (Owen et al., 2012). Subjects who are homozygotes (LL) have low-affinity binding and have negligible TSPO binding in vivo. Those who are heterozygotes (HL) express both mixed affinity for TSPO (MAB), while those without the polymorphism (HH) have high-affinity binding (HAB) for TSPO (Guo et al., 2012). As on average MABs and HABs have a 22% difference in TSPO binding (Kreisl et al., 2013), we extracted the data for patients who are HABs and MABs separately to explore the effect of genotype in a sub-analysis.
Publication bias was assessed using visual inspection of funnel plots as well as regression test. Where potential publication bias was suspected, trim and fill analysis was conducted to correct for putatively missing studies. Heterogeneity was estimated using the I2 value (I2 values <50% indicate low-to-moderate heterogeneity, whereas I2 >50% indicate moderate-to-high heterogeneity). Leave-one-out sensitivity analyses were conducted to investigate the potential effect of an individual study on the outcome measure. A p value <0.05 (two-tailed) was taken as a significance level. The statistical analysis of the extracted data was conducted using the R statistical programming language version 3.2.2 with the ‘metafor’ package.
Search strategy
The PubMed, EMBASE and PsycINFO databases were searched without language restrictions. The electronic search using EMBASE and PsycINFO were carried out together using Ovid. The following keywords were used: ‘(Positron Emission Tomography OR PET OR Single photon emission tomography OR SPET OR Single Photon Emission Computed Tomography OR SPECT) AND (schizophrenia OR schizophreniform OR psychosis) AND (microglia* OR microglia* activation OR TSPO OR Translocator protein OR peripheral benzodiazepine receptor OR peripheral benzodiazepine binding site)’. Review papers were also screened to search for additional studies.
Inclusion and exclusion criteria
The inclusion criteria were: original studies in (1) patients with a diagnosis of schizophrenia or related psychotic diagnoses (including schizophreniform disorder; psychotic disorder not otherwise specified, brief psychosis), (2) reporting PET measures using a TSPO-specific ligand and (3) reporting data for the whole grey matter or grey matter regions. Studies that did not have a control group were excluded. Where there was sample overlap between studies, we included the largest one and excluded the other to avoid double counting.
Outcome measures
The primary outcome measure was the effect size for the difference in TSPO PET measure in total grey matter between patients with schizophrenia-spectrum disorders and healthy controls. Where several studies only reported values for grey matter sub-regions, we averaged the grey matter regions to estimate the value for the whole grey matter. The PET studies predominantly reported the outcome either as binding potential (BP) or volume of distribution (VT). As these give different information, we conducted separate meta-analyses of these outcomes. The studies that used BP as an outcome measure have used either values obtained using microparameters from Simplified Reference Tissue Model (Holmes et al., 2016; van der Doef et al., 2016; Di Biase et al., 2017), but van Berckel et al. (2008) and Doorduin et al. (2009) have calculated BP using microparameters derived from 2TCM model. Despite this difference, the results are comparable as reviewed in PET receptor imaging consensus (Innis et al., 2007).
Results
TSPO binding
There were a total of 12 studies measuring TSPO tracer binding in 190 patients with schizophrenia-spectrum disorders and 200 healthy controls. Five of these studies were in chronic patients, seven in patients within the first 5 years of diagnosis, and two studies also included subjects at ultra-high risk for psychosis. See Table 1 for a summary of sample and study method characteristics. The rationale behind individual study exclusion is documented in supplementary information.
Table 1.
Subject and methodological characteristics of the in vivo imaging studies of TSPO binding in schizophrenia compared with healthy controls (BP = 6; VT = 6)
| Author Year |
Patients/controls (n) | Patient age mean (SD) years | Diagnosis | Duration of illness mean (SD) years | Current antipsychotic treatment? | Region(s) of interest (ROI) | Tracer | Genotyping | Outcome measures | Results in patients compared with controls |
|---|---|---|---|---|---|---|---|---|---|---|
| van Berckel et al. (2008) | 10/10 | 24 (2) | Recent-onset schizophrenia (<5 years of illness) |
3.1 (1.7) | Yes | Total grey matter | [11C]-PK11195 | No | BP−P | ↑ |
| Doorduin et al. (2009) | 7/8 | 31.2 (7.2) | Recent-onset schizophrenia (<5 years of illness)/psychotic disorder not otherwise specified | 5.4 (5.4) | Yes | Total grey matter Frontal Cx/occipital Cx/temporal Cx/parietal Cx/basal ganglia/thalamus/hippocampus/midbrain/cerebellum/pons |
[11C]-PK11195 | No | BP | ↑ (Hippocampus) ↔ (total grey matter and all ROI) |
| Takano et al., 2010 | 14/14 | 43.8 (7.4) | Chronic Schizophrenia | 18.8 (12.2) | Yes | Total cortical regions Medial frontal cortex, dorsolateral frontal cortex, medial temporal cortex, lateral temporal cortex, parietal cortex, occipital cortex, thalamus, striatum, cerebellum, anterior cingulate cortex and posterior cingulate cortex |
[11C]-DAA1106 | No | BPND | ↔ (All regions) |
| Kenk et al. (2015) | 18/27 | 42.6 (14.1) | Chronic schizophrenia | 14.8 (8.8) | Yes | Hippocampus/mPFC Cx/temporal Cx/DLPFC Cx/V striatum/corpus callosum/cingulum/SLF/PLIC | [18F]-FEPPA | Yes | VT | ↔ (All regions) |
| Bloomfield et al. (2016a, 2016b) | 14/14 | 24.3 (5.4) | Ultra high risk for psychosis | N/A | No | Total grey matter/frontal Cx/temporal/cerebellum/brain stem | [11C]-PBR28 | Yes | DVR VT |
DVR: ↑ (total grey matter, frontal Cx, temporal Cx); ↔ (all other regions) VT: ↔ (all regions) |
| 14/14 | 47.0 (9.3) | Chronic schizophrenia | Not specified | Yes | Total grey matter/frontal Cx/temporal Cx/cerebellum/brain stem | DVR: ↑ (total grey matter, frontal Cx, temporal Cx); ↔ (all other regions) VT: ↔ (all regions) |
||||
| Hafizi et al. (2016) | 19/20 | 27.5 (6.7) | First-episode psychosis: schizophreniform disorder/delusional disorder/schizophrenia/psychosis NOS | 2.8 (3.3) | No | Whole brain Total grey matter DLPFC/mPFC/Temporal Cx/hippocampus |
[18F]-FEPPA | Yes |
VT
DVR |
↔ (All regions) |
| van der Doef et al. (2016) | 19/17 | 26 (4) | First-episode psychosis (<5 years of illness) |
1.3 (1.1) | Yes | Total grey matter Frontal Cx/temporal Cx/parietal Cx/striatum/thalamus |
[11C]-PK11195 | No | BPND | ↔ (All regions) |
| Coughlin et al. (2016) | 12/14 | 24.1 (3.1) | Recent-onset schizophrenia (<5 years of illness) |
2.2 (1.4) | Yes | Total grey matter Insula Cx/cingulate Cx/parietal Cx/frontal Cx/temporal Cx/occipital Cx/hippocampus/amygdala |
[11C]- DPA-713 |
Yes | VT | ↔ (All regions) |
| Holmes et al. (2016) | 8/16 | 33 (9) | Schizophrenia | 15 (7) | Yes | DLPFC/VLPFC/OFC/anterior cingulate Cx/parietal Cx | [11C]-PK11195 | No | BPND | ↑ DLPFC, VLPFC and ACC ↔ (all other regions) |
| 8/16 (Shared control group) | Schizophrenia | 4 (2) | No | ↔ (all other regions) | ||||||
| Collste et al. (2017) | 16/16 | 28.5 (8.4) | First-episode psychosis: schizophrenia/schizophreniform psychosis/psychosis NOS/brief psychosis | 7.9 (9.6) | No | Total grey matter White matter/frontal Cx/temporal Cx/hippocampus |
[11C]-PBR28 | Yes | VT | ↓ (Total grey matter, frontal Cx, temporal Cx, hippocampus) ↔ (white matter) |
| Di Biase et al. (2017) | 18/15 | 20.6 (5.5) | Recent-onset schizophrenia | 1.5 (1.0) | Yes | Dorsal frontal Cx/orbital frontal Cx/anterior cingulate Cx/medial temporal Cx/thalamus/insular Cx | [11C]-PK11195 | No | BPND | ↔ (All regions) |
| 15/12 | 35.2 (6.6) | Chronic schizophrenia | 13.6 (8.8) | Yes | Dorsal frontal Cx/orbital frontal Cx/anterior cingulate Cx/medial temporal Cx/thalamus/insular Cx | [11C]-PK11195 | No | BPND | ↔ (All regions) | |
| Ottoy et al., (2018) | 11/17 | 30.0 (7.0) | Chronic schizophrenia | N/A | Yes | Cortical grey matter/cortical white matter/cerebellum/brainstem/thalamus/basal ganglia/hippocampus/amygdala | [18F]-PBR111 | Yes | VT | ↔ (All regions) |
Studies reporting outcome as BP
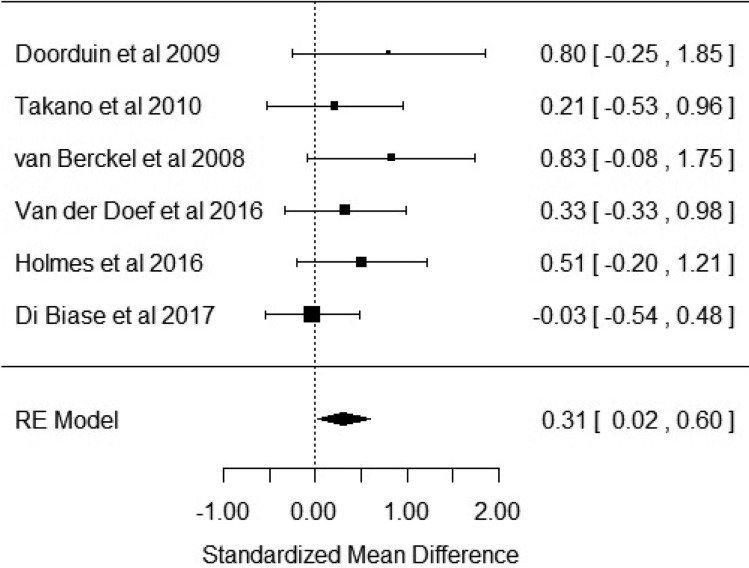
Six studies reported outcome measures as BP. Our results showed that BP was significantly elevated in patients with schizophrenia when compared with healthy controls with an effect size of 0.31 [Hedge's g = 0.31; z = 2.1; p = 0.03; 95% confidence interval (CI) 0.02–0.6] (Fig. 2). The I2 test revealed low heterogeneity (I2 = 0.58%; 95% CI 0–79%). Visual inspection of the funnel plot suggested asymmetry (online Supplementary Fig. S1). The regression test was significant (t = 4.5; df = 4; p = 0.01). The trim and fill analysis showed three putatively missing studies on the left side. The results were not significant after correcting for these studies (Hedge's g = 0.13; z = 0.96; p = 0.34; 95% CI −0.14 to 0.4). The results were significant in two out of six in the leave-one-out analysis, with effect sizes varying from 0.27 to 0.47. Five out of the six studies used the [11C]-PK11195 ligand, with the sub-analysis of these studies revealing a significant elevation of BP in patients with schizophrenia with an effect size of 0.35 (CI 0.01–0.7; p = 0.046) (online Supplementary Fig. S2).
Fig. 2.
Forest plot showing the effect sizes for in vivo microglia measures in schizophrenia patients compared with controls as measured by translocator protein binding potential (BP) in total grey matter. There was a significant elevation in schizophrenia with an effect size = 0.31 (p = 0.03).
Studies reporting the outcome as volume of distribution (VT)
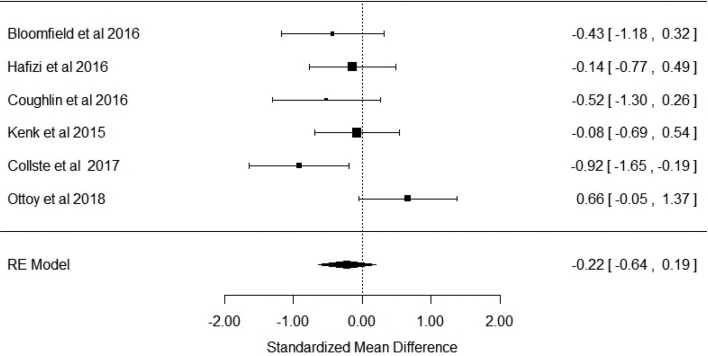
Six studies reported the outcome measure as VT. Figure 3 shows that there was no difference in VT in patients with schizophrenia when compared with healthy controls (Hedge's g = −0.22; p = 0.296; CI −0.64 to 0.19). The I2 test revealed moderate–high heterogeneity (I2 = 53%; 95% CI 0–92%). Visual inspection of funnel plot suggested asymmetry (online Supplementary Fig. S3). However, the regression test was not significant (t = −0.73; df = 4; p = 0.5). Trim and fill analysis showed two missing studies in the right side. The effect sizes varied from −0.08 to −0.37 in the leave-one-out analysis. We extracted data for high- (HABs) and mixed-affinity binders (MABs) separately from these studies to conduct a sub-analysis stratified by genotype. Bloomfield et al. reported only one patient who was a MAB, precluding accurate estimation of the effect size. Thus, this study was not included in the sub-analysis of MAB subjects (Bloomfield et al., 2016b). There was no significant difference between patients and controls in the high-affinity binder sub-analyses (effect size −0.27; p = 0.19; CI −0.68 to 0.13). However, there was a significant difference in the MAB (effect size −0.56; p = 0.03; CI −1.08 to −0.03).
Fig. 3.
Forest plot showing effect sizes for in vivo microglia measures in schizophrenia patients compared with controls as measured by volume of distribution of translocator radiotracer (VT) in total grey matter. There were no significant changes in patients compared with controls (effect size = −0.22, p = 0.296).
Discussion
Our main findings are that TSPO PET tracer binding is significantly elevated in patients with schizophrenia relative to controls when BP is used as an outcome measure, with a small-to-moderate effect size (Hedge's g = 0.31; p = 0.03), but there is no significant difference when the tracer volume of distribution (VT) is used as the outcome measure (Hedge's g = −0.22; p = 0.296). In the following section, we consider methodological factors and the implications of our findings.
Methodological considerations
We identified potential publication bias for both outcome measures, and in the case of BP meta-analysis, the results were no longer significant when adjusted for putative missing studies, which if present could potentially affect our findings. Heterogeneity was low for those studies that used BP as an outcome measure and moderate to high in those that used VT as an outcome measure. For this meta-analysis, we used a random-effects model, which is robust to between-study variations. We acknowledge that four of the 12 studies used in this meta-analysis analysed patients with schizophreniform disorder, psychotic disorder not otherwise specified, and brief psychosis as well as patients with schizophrenia (Doorduin et al., 2009; van der Doef et al., 2016; Collste et al., 2017). Two of the studies do not provide a break-down of individual patient participant diagnoses, other than to classify patients as presenting with first-episode psychosis (van der Doef et al., 2016; Hafizi et al., 2017), thus up to 52/190 (27%) of patients included in the meta-analysis are defined as presenting with a schizophreniform disorder, rather than a definitive diagnosis of schizophrenia. Therefore, our results could be influenced by the inclusion of other psychotic disorders, although the low heterogeneity observed for the meta-analysis using BP as an outcome measure at least suggest that including individuals with broader psychotic diagnoses alongside schizophrenia did not have a major impact on the results. Five out of the six studies included in the BP meta-analysis used the first-generation tracer [11C]-PK11195, whereas the VT studies used second-generation tracers. Thus, the difference between BP and VT outcomes could reflect tracer differences, as [11C]-PK11195 is known to have low brain penetration and high non-specific binding, which is a significant limitation of this tracer relative to the second-generation tracers (Fujita et al., 2008). However, although the outcome of the BP study that used a different tracer ([11C]-DAA1106) is negative, the results using this tracer are not an outlier, suggesting findings may not entirely be accounted for by tracer differences. It has been suggested that TSPO expression may change during the course of the disorder, which could account for the differences in findings between studies (Notter et al., 2018). However, a recent study by Di Biase et al. using BP as an outcome measure showed no differences between at-risk mental state individuals, recent onset schizophrenia and chronic schizophrenia (Di Biase et al., 2017), and our BP and VT meta-analyses both included studies of chronic and recent onset illness, suggesting that this does not clearly explain the differences between our BP and VT findings. Ultimately, longitudinal studies in patients are required to determine if there are changes during the course of the illness. Other differences between the studies using BP and VT, such as differences in tracer and modelling approaches, could contribute to the differences between our BP and VT meta-analytic findings. Another potential issue is that not all studies accounted for a genetic variant at rs6971 in the TSPO gene. Six out of the 12 studies included in this meta-analysis did not report genotyping (van Berckel et al., 2008; Doorduin et al., 2009; Takano et al., 2010; Holmes et al., 2016; van der Doef et al., 2016; Di Biase et al., 2017). Out of these six studies, five used the [11C]-PK11195 tracer while one study used the tracer [11C]-DAA1106. However, genotyping has been shown not to be necessary in studies using [11C]-PK11195, as in vitro studies have shown that this tracer binds to a different site on the TSPO to the locus affected by the rs6971 variant and, consequently, there is no difference in affinity to TSPO between high-affinity and low-affinity binders (Owen et al., 2012). However, our sub-analyses for the other tracers stratified by genotype showed group differences only remained significant in the MAB groups. We caution against overinterpretation of this finding given the small sample, but suggest it warrants further investigation. Another potential methodological limitation is that a number of studies did not account for partial volume effects. However, as brain volumes tend to be reduced in schizophrenia, partial volume effects would not explain the elevation in BP and, if anything, would tend to reduce the effect size, which means our results may underestimate the true effect. Additionally, in those cases where total grey matter TSPO levels were not available, we have averaged the grey matter regions to estimate the value for the whole grey matter. Although this is a common procedure in PET meta-analyses (Howes et al., 2012; Kambeitz et al., 2014; Ashok et al., 2017), it can constitute a potential limitation of this study. Finally, most of the studies in this meta-analysis included patients who were being treated with antipsychotics. Preclinical studies in vitro have found antipsychotic treatment to reduce microglial activation (Zheng et al., 2008), although recent in vivo work has found an increase after olanzapine, but a reduction with risperidone (Zhu et al., 2014; Cotel et al., 2015; Crum et al., 2016). Critically, none of these studies measured TSPO levels, so it remains unknown if antipsychotics alter TSPO expression. Interestingly, both the studies by Holmes et al. and Di Biase et al. showed that unmedicated patients have lower BP when compared with antipsychotic-treated patients and healthy controls (Holmes et al., 2016; Di Biase et al., 2017). However, this analysis was based only on data provided by 12 patients and further work is thus needed to understand whether antipsychotic treatment could have affected our findings.
Interpretation of findings
The non-displaceable BP (BPND) measures the tracer binding in the tissue of interest relative to another brain region selected to have negligible specific binding, to give specific binding in the region of interest (Mintun et al., 1984). An issue for the measurement of BPND is that there is no brain region with negligible TSPO expression, and consequently no ideal reference region for TSPO tracers (Turkheimer et al., 2015; Bloomfield et al., 2016a; Narendran and Frankle, 2016). Thus the elevation in BP we report could reflect an increase in specific binding and/or a reduction in non-specific binding in grey matter relative to the reference brain tissue. In contrast, the volume of distribution (VT) measures the total amount of tracer in the brain region relative to that in the blood (Innis et al., 2007). Volume of distribution is generally the preferred method of quantification of PET tracers where there is no reference region but, critically for group comparisons, assumes blood tracer binding is unaltered between groups (Innis et al., 2007). However, TSPO tracers may bind to a number of sites in the blood, including acute phase and inflammatory plasma proteins, such as α1-acid glycoprotein (AGP) that are known to be elevated in schizophrenia (Telford et al., 2012; Tourjman et al., 2013). As TSPO tracers bind to AGP (Lockhart et al., 2003), there is the potential for a systematic bias between patients and controls that could affect the quantification of VT, and potentially mask an elevation in the patients’ brain as compared with controls. There is inconsistency in findings in schizophrenia, with one study showing elevation in the plasma concentration levels of [11C]-PBR28 in patients with schizophrenia relative to controls (Bloomfield et al., 2016a), while two others using [18F]-FEPPA and [11C]-PBR28 show no differences in plasma concentration levels (Hafizi et al., 2016; Collste et al., 2017). There is also evidence for reduced levels of binding of TSPO tracers to TSPO (previously known as the peripheral benzodiazepine receptor) on platelets in schizophrenia, with reductions of ~30% reported in some studies (Gavish et al., 1986; Weizman et al., 1986), although potentially only in certain sub-types of schizophrenia (Wodarz et al., 1998). Thus, it is possible that changes in either plasma protein binding and/or platelet binding could systematically affect VT values in the disorder, but it should be recognized that there is no direct evidence that plasma binding alters VT values in humans (Cumming et al., 2018). Importantly, in a recent study where protein binding was measured, no significant group differences between drug-naive first-episode psychosis patients and healthy controls were observed, suggesting that VT changes are not explained by an effect of protein binding (Collste et al., 2017). Further studies are thus needed to clarify if there is an impact of this on the measurement of VT in schizophrenia. Nevertheless, while this is a potential concern for studies using VT as the outcome measure, blood binding should not affect the studies that use a ratio approach, as these methods report tracer uptake relative to another brain region rather than blood. Furthermore, TSPO is expressed on the endothelial cells of brain blood vessels as well as on the outer layer of mitochondria in microglia (Rizzo et al., 2014), and both can be accounted for in the PET analysis (Turkheimer et al., 2015). Few of the studies included in this meta-analysis have accounted for endothelial binding. In view of this, in our meta-analysis, we have included the results not accounting for this compartment. A separate meta-analysis was conducted including the data accounting for the endothelial binding and the results were very similar suggesting this is unlikely to be a major influence on our findings.
Taken together, our meta-analytic findings suggest an elevation in TSPO tracer binding in total grey matter relative to other brain tissue, but not relative to blood, with the caveat that the relative increase is largely based on studies using PK11195, which has a lower specific signal. Thus, this could reflect an increase in TSPO in grey matter or a reduction in TSPO in the reference region, or altered non-specific binding in the brain (Cumming et al., 2018). It should also be noted that reductions in TSPO have been reported in some pro-inflammatory states (Narayan et al., 2017). Finally, TSPO may also be expressed on astrocytes (Cosenza-Nashat et al., 2009). While altered TSPO expression on astrocytes may contribute to the differences, the post-mortem findings of unaltered astrocytic but elevated microglial markers (Trepanier et al., 2016), including elevated TSPO binding (Kreisl et al., 2013), are more suggestive of a microglia activity increase in schizophrenia. However, until further work has been conducted to determine if changes in translocator protein expression in schizophrenia are specific to microglia, conclusions about the specificity of changes to microglia should be treated cautiously.
Implications of our findings and future directions
Our different findings depending on the outcome measure used point towards the existence of potential methodological problems in TSPO imaging, raising questions over the interpretation of the elevation in grey matter TSPO binding relative to a reference region in patients with schizophrenia when compared with healthy controls. A recent individual participant data meta-analysis of second-generation radioligand studies, with VT as the outcome measure, showed a reduction in VT in patients relative to healthy controls (Plaven-Sigray et al., 2018). Although these results seem to be in contrast to the absence of differences in VT in our meta-analysis, our meta-analysis included one more second-generation study showing no differences in VT between schizophrenia patients and healthy controls (Ottoy et al., 2018), which may justify the differences in results between meta-analysis. Ultimately, the definitive test of the importance of TSPO and/or microglia activation in schizophrenia will be to pharmacologically target them with a selective drug or monoclonal antibody combined with PET imaging to confirm target engagement and evaluate the relationship between change in microglial activation and symptomatic improvement. Secondly, it remains to be determined if microglia activity is altered across the different stages of the disorder. Epidemiological and preclinical evidence which indicates that neuroinflammation in utero and early development may predispose to schizophrenia (Meyer, 2013). However, the PET results have been inconsistent, with one study using the tracer [11C]-PBR28 showing an elevation in relative TSPO binding in people at ultra high risk of psychosis using the ratio but not with the VT approach (Bloomfield et al., 2016b), while two studies using VT as the outcome measure, using the tracer [18F]-FEPPA and [11C]-PK11195, showed no differences in TSPO binding between healthy controls and in individuals at ultra high risk of psychosis for psychosis (Hafizi et al., 2016; Di Biase et al., 2017). Unfortunately, there were not sufficient studies to proceed with a sub-analysis of studies conducted in the early phase of illness. Future research should focus on at-risk mental state individuals and early in the development of the illness and use longitudinal designs to determine the role of microglial activity in the different stages of the disorder. Ideally, these studies should be conducted before antipsychotic medication initiation, as it would also help to clarify the potential role of antipsychotics in microglia activity. Interestingly, it has been shown that pro-inflammatory cytokines are elevated in first-episode patients who do not respond to antipsychotic treatment relative to those who do respond (Mondelli et al., 2015), which suggests that increases in microglia activity may be specific to a sub-group of patients, consistent with neurobiological sub-types of schizophrenia (Howes and Kapur, 2014). Studies that focus on patients that do not respond to conventional antipsychotic medication should be able to shed light on this topic. When interpreting our results is also important to note that this meta-analysis focused on total grey matter TSPO binding, and there was insufficient data for a meta-analysis of specific brain regions. Although TSPO is ubiquitous and expressed across the whole brain, we cannot exclude that microglia activation in schizophrenia is differentially expressed in specific regions within the brain. Indeed, early studies by Doorduin et al. (2009) and van Berckel et al. (2008) suggest increased TSPO binding in the medial temporal cortex, and Bloomfield et al. (2016b) using the DVR approach also found evidence of this in people at ultra high risk of psychosis. Finally, future studies are needed to address the methodological issues and sources of variance discussed above. This could be potentially achieved by obtaining individual patient data from each individual study and applying both ratio and volume of distribution models to determine if PET modelling or study differences drive the opposing BP and volume of distribution findings. In addition, we recommend that new studies present results for both modelling approaches so that consistency of findings can be compared and support future meta-analysis, while the role of vascular binding in PET TSPO quantification should be clarified.
Conclusion
In conclusion, there is evidence for a moderate effect size elevation in TSPO tracer binding in grey matter in schizophrenia-spectrum disorders when using BP as an outcome measure, but no changes when VT is the outcome measure used. These results suggest that potential methodological differences between TSPO studies need to be accounted for and addressed in future studies and keep open the discussion over the existence of an increase in microglia activity in patients with schizophrenia-spectrum disorders.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291718003057.
click here to view supplementary material
References
- Ashok AH, Mizuno Y, Volkow ND and Howes OD (2017) Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R and Hickie IB (2009) Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Medical Journal of Australia 190, S26–S32. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L and Falkai P (1999) Evidence for activation of microglia in patients with psychiatric illnesses. Neuroscience Letters 271, 126–128. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO and Mortensen PB (2011) Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. American Journal of Psychiatry 168, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Benros ME, Eaton WW and Mortensen PB (2014) The epidemiologic evidence linking autoimmune diseases and psychosis. Biological Psychiatry 75, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Howes OD, Turkheimer F, Selvaraj S and Veronese M (2016a) Response to Narendran and Frankle: the interpretation of PET microglial imaging in schizophrenia. American Journal of Psychiatry 173, 537–538. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V and Howes OD (2016b) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. American Journal of Psychiatry 173, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T and Howes OD (2016) Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berlin) 233, 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collste K, Plaven-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S; Karolinska Schizophrenia Project (KaSP) consortium, Erhardt S, Halldin C, Flyckt L, Farde L and Cervenka S (2017) Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [(11)C]PBR28. Molecular Psychiatry 22, 850–856. [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S and Lee SC (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathology and Applied Neurobiology 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotel MC, Lenartowicz EM, Natesan S, Modo MM, Cooper JD, Williams SC, Kapur S and Vernon AC (2015) Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. European Neuropsychopharmacology 25, 2098–2107. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A and Pomper MG (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Translational Psychiatry 12, e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum WR, Danckaers F, Huysmans T, Cotel MC, Natesan S, Modo MM, Sijbers J, Williams SC, Kapur S and Vernon AC (2016) Chronic exposure to haloperidol and olanzapine leads to common and divergent shape changes in the rat hippocampus in the absence of grey-matter volume loss. Psychological Medicine 46, 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Burgher B, Patkar O, Breakspear M, Vasdev N, Thomas P, Liu GJ and Banati R (2018) Sifting through the surfeit of neuroinflammation tracers. Journal of Cerebral Blood Flow & Metabolism 38, 204–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase MA, Zalesky A, O'Keefe G, Laskaris L, Baune BT, Weickert CSL, Olver J, McGorry PD, Amminger GP, Nelson B, Scott AM, Hickie I, Banati R, Turkheimer F, Yaqub M, Everall IP, Pantelis C and Cropley V (2017) PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Translational Psychiatry 7, e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Schroeder J, Katsafanas E, Schweinfurth L, Savage C, Khushalani S and Yolken R (2016) Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophrenia Bulletin 42, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA and Klein HC (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of Nuclear Medicine 50, 1801–1807. [DOI] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW and Innis RB (2008) Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 40, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish M, Weizman A, Karp L, Tyano S and Tanne Z (1986) Decreased peripheral benzodiazepine binding sites in platelets of neuroleptic-treated schizophrenics. European Journal of Pharmacology 121, 275–279. [DOI] [PubMed] [Google Scholar]
- Guo Q, Owen DR, Rabiner EA, Turkheimer FE and Gunn RN (2012) Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. Neuroimage 60, 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, Houle S, Rusjan PM and Mizrahi R (2016) Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. American Journal of Psychiatry 174, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Da Silva T, Gerritsen C, Kiang M, Bagby RM, Prce I, Wilson AA, Houle S, Rusjan PM and Mizrahi R (2017) Imaging Microglial Activation in Individuals at Clinical High Risk for Psychosis: an In Vivo PET Study with [18F]FEPPA. Neuropsychopharmacology 42, 2474–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A and Talbot PS (2016) In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Molecular Psychiatry 21, 1672–1679. [DOI] [PubMed] [Google Scholar]
- Howes OD and Kapur S (2014) A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic). British Journal of Psychiatry 205, 1–3. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A and Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of General Psychiatry 69, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF and Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow & Metabolism 27, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Juckel G, Manitz MP, Brune M, Friebe A, Heneka MT and Wolf RJ (2011) Microglial activation in a neuroinflammational animal model of schizophrenia – a pilot study. Schizophrenia Research 131, 96–100. [DOI] [PubMed] [Google Scholar]
- Kambeitz J, Abi-Dargham A, Kapur S and Howes OD (2014) Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. British Journal of Psychiatry 204, 420–429. [DOI] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S and Mizrahi R (2015) Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophrenia Bulletin 41, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Dalman C, Lewis G and Jones PB (2012) Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophrenia Research 139, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G and Jones PB (2013) Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychological Medicine 43, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ and Innis RB and Biomarkers Consortium PET Radioligand Project Team (2013) A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. Journal of Cerebral Blood Flow & Metabolism 33, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Davis B, Matthews JC, Rahmoune H, Hong G, Gee A, Earnshaw D and Brown J (2003) The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant alpha1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nuclear Medicine and Biology 30, 199–206. [DOI] [PubMed] [Google Scholar]
- Meyer U (2013) Developmental neuroinflammation and schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 42, 20–34. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Graham KL, Bodenheimer CM, Culpepper NH, Waller JL and Buckley PF (2013) A prevalence study of urinary tract infections in acute relapse of schizophrenia. Journal of Clinical Psychiatry 74, 271–277. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF and Welch MJ (1984) A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Annals of Neurology 15, 217–227. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, Marques TR, Zunszain PA, Morgan C, Murray RM, Pariante CM and Dazzan P (2015) Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. Schizophrenia Bulletin 41, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan N, Mandhair H, Smyth E, Dakin SG, Kiriakidis S, Wells L, Owen D, Sabokbar A and Taylor P (2017) The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLoS ONE 12, e0185767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R and Frankle WG (2016) Comment on analyses and conclusions of “microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study”. American Journal of Psychiatry 173, 536–537. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A and Meyer U (2018) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Molecular Psychiatry 23, 323–334. [DOI] [PubMed] [Google Scholar]
- Ottoy J, De Picker L, Verhaeghe J, Deleye S, Wyffels L, Kosten L, Sabbe B, Coppens V, Timmers M, van Nueten L, Ceyssens S, Stroobants S, Morrens M and Staelens S (2018) [(18)F]PBR111 PET imaging in healthy controls and schizophrenia: test–retest reproducibility and quantification of neuroinflammation. Journal of Nuclear Medicine 59, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA and Rubio JP (2012) An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. Journal of Cerebral Blood Flow & Metabolism 32, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaven-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Mizrahi R, Pomper MG, Rusjan P, Veronese M, Wang Y and Cervenka S (2018) Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biological Psychiatry 84, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM and Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. Journal of Neuropathology & Experimental Neurology 59, 137–150. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE and Bertoldo A (2014) Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. Journal of Cerebral Blood Flow & Metabolism 34, 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N and Kunugi H (2013) Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. Journal of Psychiatric Research 47, 401–406. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Bertsch T, Henning U, Tost H, Klimke A, Henn FA and Falkai P (2005) Increased serum S100B in elderly, chronic schizophrenic patients: negative correlation with deficit symptoms. Schizophrenia Research 80, 305–313. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S and Engberg G (2015) Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia – significance for activation of the kynurenine pathway. Journal of Psychiatry & Neuroscience 40, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Daly MJ, Carroll MC, Stevens B and McCarroll SA (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, Erhardt S and Engberg G (2009) Activation of brain interleukin-1beta in schizophrenia. Molecular Psychiatry 14, 1069–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Børglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Böttcher Y, Olesen J, Breuer R, Möller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Réthelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Genetic Risk and Outcome in Psychosis (GROUP), Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jönsson EG, Terenius L, Agartz I, Petursson H, Nöthen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K and Collier DA (2009) Common variants conferring risk of schizophrenia. Nature 460, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG and Bogerts B (2006) Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathologica 112, 305–316. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y and Suhara T (2010) Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. International Journal of Neuropsychopharmacology 13, 943–950. [DOI] [PubMed] [Google Scholar]
- Telford JE, Bones J, McManus C, Saldova R, Manning G, Doherty M, Leweke FM, Rothermundt M, Guest PC, Rahmoune H, Bahn S and Rudd PM (2012) Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. Journal of Proteome Research 11, 3743–3752. [DOI] [PubMed] [Google Scholar]
- Tourjman V, Kouassi E, Koue ME, Rocchetti M, Fortin-Fournier S, Fusar-Poli P and Potvin S (2013) Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophrenia Research 151, 43–47. [DOI] [PubMed] [Google Scholar]
- Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N and Bazinet RP (2016) Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Molecular Psychiatry 21, 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A and Veronese M (2015) The methodology of TSPO imaging with positron emission tomography. Biochemical Society Transactions 43, 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N and Barnes NM (2014) Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophrenia Research 155, 101–108. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA and Kahn RS (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological Psychiatry 64, 820–822. [DOI] [PubMed] [Google Scholar]
- van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, de Haan L, Eriksson J, Lammertsma AA, Kahn RS and van Berckel BN (2016) In vivo (R)-[(11)C]PK11195 PET imaging of 18 kDa translocator protein in recent onset psychosis. NPJ Schizophrenia 2, 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren CF, Gremmels H, de Witte LD, Hol EH, Van Gool AH, Falkai PH, Kahn RS and Sommer IE (2017) Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on post-mortem brain studies. Translational Psychiatry 7, e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman R, Tanne Z, Karp L, Tyano S and Gavish M (1986) Peripheral-type benzodiazepine-binding sites in platelets of schizophrenics with and without tardive dyskinesia. Life Sciences 39, 549–555. [DOI] [PubMed] [Google Scholar]
- Wodarz N, Rothenhofer C, Fischer R, Stober G, Kiehl B, Jungkunz G, Riederer P and Klein HE (1998) Peripheral-type benzodiazepine receptors in diagnostic subtypes of schizophrenic patients. Psychiatry Research 81, 363–369. [DOI] [PubMed] [Google Scholar]
- Zheng LT, Hwang J, Ock J, Lee MG, Lee WH and Suk K (2008) The antipsychotic spiperone attenuates inflammatory response in cultured microglia via the reduction of proinflammatory cytokine expression and nitric oxide production. Journal of Neurochemistry 107, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Ding YQ, Liu Y, Zhang X, Wu R, Guo X and Zhao J (2014) Minocycline and risperidone prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. PLoS ONE 9, e93966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291718003057.
click here to view supplementary material