Abstract
Neuronal stimulation is an emerging field in modern medicine to control organ function and reestablish physiological homeostasis during illness. The nervous system innervates most of the peripheral organs and provides a fine tune to control the immune system. Most of these studies have focused on vagus nerve stimulation and the physiological, cellular and molecular mechanisms regulating the immune system. Here, we review the new results revealing afferent vagal signaling pathways, immunomodulatory brain structures, spinal cord-dependent circuits, neural and non-neural cholinergic/catecholaminergic signals and their respective receptors contributing to neuromodulation of inflammation in rheumatoid arthritis. These new neuromodulatory networks and structures will allow the design of innovative bioelectronic or pharmacological approaches for safer and low-cost treatment of arthritis and related inflammatory disorders.
Keywords: bioelectronic medicine, neuroimmunomodulation, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by deleterious inflammation in the joints, hyperplasia of synovial tissues and damage of the joint cartilage and bone [1]. RA is the most common type of autoimmune arthritis, affects approximately 0.5–1% of the population worldwide, causes morbidity and reduces mobility and life expectancy [2]. Although its etiology is unknown, RA is a multifactorial process not well understood. Both, inflammatory cells and cytokines are found in the synovial fluid and contribute to joint inflammation and tissue damage in arthritis. Currently, there is no cure for RA and the most effective treatments are the new biological disease-modifying antirheumatic drugs (bDMARDs) that neutralize cytokines (such as TNF, IL-1β and IL-6) or their receptors [3–5]. The most common treatments for RA include the use of monoclonal antibodies that neutralize TNF as Remicade® (infliximab), a chimeric IgG1κ antibody (composed of human constant and murine variable regions), and Humira® (adalimumab), a human monoclonal antibody in rheumatoid arthritis. Likewise, Enbrel® (etanercept), a fusion recombinant protein of the human eTNF receptor 2 and the Fc end of the IgG1, also binds and neutralizes TNF. Biological agents targeting IL-6 (sirukumab, olokizumab and clazakizumab), IL-6 receptor (tocilizumab and sarilumab) or IL-1 receptor antagonist (IL-1Ra; anakinra) have also provided beneficial effects in RA patients [6–10]. These drugs decrease joint inflammation in RA, but they are very expensive and increase the risk of opportunistic infections and immunosuppression [11–13].
Recent studies on neuromodulation revealed the potential of the nervous system to control organ function and re-establish physiological homeostasis during illness [14–18]. These results encouraged investigators to analyze the potential of neuromodulation for treating infectious and inflammatory disorders. The autonomic nervous system can be divided into the enteric, sympathetic and parasympathetic divisions that control physiological homeostasis including the urogenital, cardiovascular and gastrointestinal systems. Likewise, the nervous system modulates the immune system to re-establish immune homeostasis after infections, trauma and other immunological challenges. Nerve stimulation and bioelectronic medicine is an emerging field in modern medicine to control organ function and re-establish physiological homeostasis during illness [14]. Vagus nerve, the main nerve connecting the brain with the viscera, is a bidirectional nerve with afferent signal toward the brain and efferent signals toward the viscera. An initial study showed that efferent vagal projections attenuates inflammation in endotoxemic mice [19], while the afferent vagal fibers activate the hypothalamic–pituitary–adrenal (HPA) axis [20,21]. These results encouraged many investigators to analyze the potential of vagal stimulation in multiple inflammatory disorders such as RA [22–27]. Our results concur with other investigators in showing that neuronal stimulation attenuates inflammation in RA both in experimental and clinical settings [24,28,29]. This article reviews the recent advances in neuroimmune interactions, bioelectronic medicine and the potential of neuronal stimulation for treating arthritis.
Vagal regulation of the innate immune system in endotoxemia
Endotoxemia is the standard experimental model used to study the innate immune responses to bacterial infection [30,31]. The injection of bacterial endotoxin (Lipopolysaccharide; LPS), a cell membrane component of Gram-negative bacteria, activates macrophages to produce inflammatory cytokines such as TNF, IL-1β and IL-6 [30,32]. Overzealous TNF production can be more dangerous than the original infection, and can cause cardiovascular collapse, septic shock and multiple organ failure in severe sepsis [33,34]. The original studies in endotoxemic mice indicated that electrical stimulation of the parasympathetic vagus nerve inhibits the production of proinflammatory, but not anti-inflammatory (e.g., IL-10) cytokines from macrophages [14–16,19,33,35]. Since macrophages M1 and M2 phenotypes are categorized by the production of proinflammatory (e.g., TNF) and anti-inflammatory (e.g., IL-10) cytokines, it has been suggested that vagal signaling can induce macrophage switch from M1 to M2 profile [36–38].
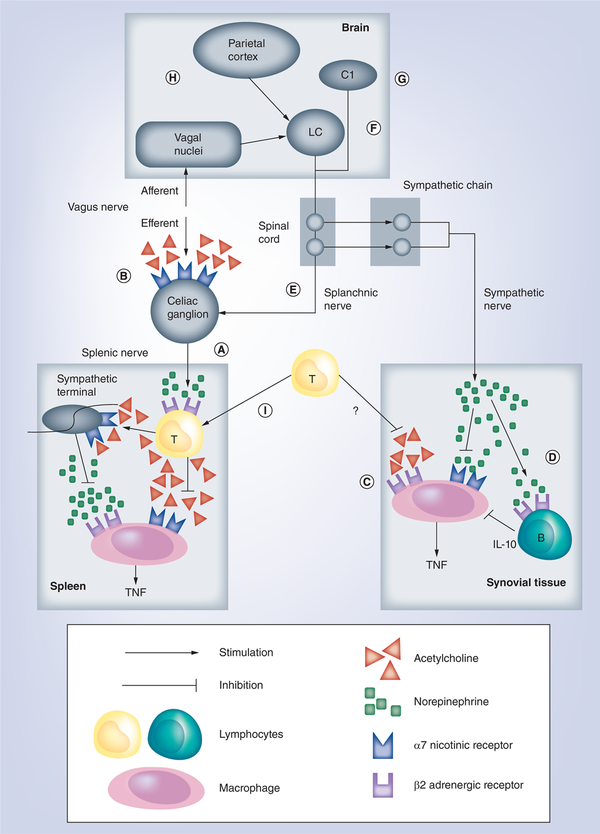
The vagus nerve is the crucial neurosensitive conduit with around 80% of sensory afferent fibers and represents the most studied example of neuroimmune crosstalk. This mechanism can be divided into two different anatomical portions based on vagal pathways. First, the afferent sensitive nerve can be activated by inflammatory factors in the periphery and transmit the information to the brain [15,39]. The brain processes the information and can activate multiple neuronal pathways to control peripheral inflammation, including the HPA axis and the sympathetic nervous system [20,21,40,41]. Second, the brain can also activate efferent pathways such as the efferent motor vagus nerve that innervate specific organs and will contribute to counteract peripheral inflammation [19]. These systems can also interact between them, and the vagus nerve (which does not innervate the spleen) can activate the sympathetic splenic nerve to release norepinephrine in the spleen (Figure 1A) [42–43]. Multiple studies suggest that the preganglionic vagus nerve can activate the post-LPS-ganglionic splenic nerve through the mesenteric ganglia. Although recent studies argue the synaptic connection from vagal preganglionic neurons to splenic postganglionic neurons [44,45], most studies concur in the lack of cholinergic innervations in the spleen and the joints and synovial capsule [46]. Most studies in sepsis suggest that neurogenic splenic norepinephrine activates β2-adrenoceptors of periarteriolar cholinergic lymphocyte sheet surrounding splenic nerve [28,42,47–49]. These lymphocytes can produce acetylcholine, which inhibits TNF production in the macrophages of the marginal zone [15,50,51]. This modulatory signaling depends on α−7 nicotinic acetylcholine receptors (α7nAChRs) expressed in macrophages because electrical vagal stimulation attenuates serum TNF levels in wild-type but not in α7nAChRs-KO mice [52]. In this neuroimmune pathway, the parasympathetic vagus nerve and sympathetic splenic nerve are connected as a sequential part of the same pathway. These models of functional organization of the nervous system can help to design novel therapeutic strategies co-stimulating different neuronal networks to achieve the most effective control of inflammation.
Figure 1. Description of neuroimmune circuits with potential therapeutic use in the treatment of arthritis.
An efferent vagus nerve can inhibit inflammation through the activation of splenic nerve (A) by a neuroimmune pathway constituted by alpha-7 nicotinic acetylcholine receptor (α7nAChR)-expressing mesenteric ganglia (B) and splenic acetylcholine-producing T lymphocytes. Afferent vagal signaling, sympathetic C1 neurons (G) and cortical stimulation (H) can prevent local joint inflammation by a mechanism dependent on activation of LC (F) followed by stimulation of splanchnic nerve (E) or synovial sympathetic innervations (C & D). An articular non-neural cholinergic system improves local inflammation by a mechanism dependent on a7nAchR expressed in macrophage/fibroblast (C) while a neural adrenergic system inhibits local inflammation by direct (β-adrenoceptors) or indirect (IL-10-producing B lymphocytes) mechanisms (D). A recent hypothesis suggests that non-neural acetylcholine can be produced by peripheral T-cells that migrate to organs (e.g., spleen) after vagal stimulation (I). LC: Locus coeruleus.
Efferent vagal & cholinergic regulation of inflammation
The potential of efferent vagal signaling to control inflammation in several critical disorders including infectious diseases [14,34], ischemia and reperfusion [53–55], postoperative trauma [56], hemorrhage, resuscitation [55], pancreatitis [57], endotoxemia [19], septic shock and severe sepsis [58,59] was demonstrated in multiple experimental studies. Most of these studies show that vagal stimulation inhibits TNF production in macrophages. This strategy is similar to using neutralizing anti-TNF antibodies, which were actually first used in endotoxemia to prevent septic shock [60]. Unfortunately, anti-TNF antibodies therapies failed in clinical trials for sepsis for two main reasons. First, sepsis induces an acute ‘early’ production of TNF that peaks within the first 2 h after the infection, and serum TNF levels return to a baseline by the time the patients arrive to the hospital. Thus, anti-TNF therapies are effective to ‘prevent’ experimental sepsis when the treatment is started before the septic challenge, but they provide a very narrow therapeutic time-window in clinical settings. Second, sepsis is characterized by the production of multiple inflammatory factors that can cause sepsis even in TNF-knockout mice [60–63]. Unlike sepsis, RA is characterized by a chronic production of TNF and neutralizing anti-TNF therapies are currently the most effective treatment for arthritis [13].
From a pharmacological perspective, cholinergic agonists such as acetylcholine and nicotine inhibit the production of inflammatory cytokines in macrophages via α−7 nicotinic acetylcholine receptors (α7nAChRs) [35]. Recent studies indicated that α7nAChRs may regulate inflammation through cholinergic mechanisms independent on the vagus nerve, as nicotine can also activate neuronal pathways such as the splenic nerve by binding to neuronal α7nAChRs on the mesenteric ganglia (Figure 1B) [47]. As ganglionic cell bodies express nicotinic receptors, nicotine may act on these neurons to activate the splenic nerve to release neurogenic norepinephrine in the spleen inhibit TNF production in splenic macrophages [33,47,52]. At the cellular level, α7nAChR-agonists can activate several intracellular pathways to control cytokine production in macrophages by inhibiting the NF-κB and Jak2/Stat3 pathways as well as inducing miRNA124 [58,64–66]. Treatment with nicotine inhibits serum TNF and high mobility group box-1 (HMGB1; a late proinflammatory cytokine) levels and improves survival in experimental models of polymicrobial peritonitis [58]. Actually, cholinergic agonists such as nicotine have been previously used in clinical trials to control inflammation in ulcerative colitis [67]. These results suggest that specific cholinergic agonists may provide pharmacological advantages for treating autoimmune disorders.
Pharmacological translation for arthritis
The most standard experimental model of arthritis is challenging the animals with type II collagen, a protein mostly found in cartilages [68]. Collagen induces joint inflammation and pathological markers similar to that observed in human arthritis. Given that vagal stimulation inhibits TNF production in endotoxemic animals [19], investigators analyzed whether cholinergic agonists such as nicotine inhibit TNF production in experimental arthritis. Treatment with nicotine can reduce synovial TNF levels, joint swelling, inflammatory factors and histopathological score including both hyperplasia and bone erosion in collagen-induced arthritis [69–71]. However, the mechanism of these anti-inflammatory effects remains controversial. These results concur with recent studies showing that depletion of α7nAchR worsens inflammation in collagen-induced arthritis [72]. Thus, selective α7nAChR-agonists has been considered for treating arthritis due to their potential to inhibits TNF production and joint inflammation in arthritic mice [70,73,74]. Recent studies suggest that nicotinic agonists modulate macrophages by regulating specific subsets of lymphocytes. For example, nicotinic agonists induce T-helper cells shift to a Th2 anti-inflammatory phenotype [75]. Nicotine can also inhibit inflammation in arthritis by reducing IL-17 production by splenic α7nAChR-expressing Th17 cells [76], or preventing macrophage infiltration into the synovial tissues by inhibiting the expression of adhesion molecules such as ICAM-1 [77]. Indeed, RA was originally considered a Th1-mediated disease due to the high levels of TNF and IFN-γ and the lack of Th2-cytokines such as IL-4. However, recent studies show that Th1-cytokines are not the main effectors of arthritis autoimmunity, but a new subset T cells producing IL17 in human arthritic synovial fluid [78–80]. This lineage of Th17 cells is characterized by the expression of transcriptional factor RAR-related orphan receptor gamma-t (RORγ-t) as compared with classical Th1 (Tbet) and Th2 (GATA3) [81–83]. Thus, inhibiting Th1/Th2/Th17 imbalance can be a potential strategy for treating arthritis [84,85]. Likewise, α7nAChRs in T-regulatory cells can also inhibit cytokine production in macrophages and prevent inflammation in arthritis [86]. Thus, α7nAChR-agonists can limit inflammation even in tissues lacking parasympathetic innervation such as the skin, skeletal muscle and synovial tissue, by regulating non-neuronal cells [46,87–90]. Macrophages and fibroblasts expressing functional α7nAChRs were originally identified in the synovial tissue of the arthritic patients [74,91,92] (Figure 1C). α7nAChR activation in these cells inhibits the nuclear translocation of NF-κB and thereby the production of inflammatory factors such cytokines [93].
Despite the beneficial effects of peripheral nicotinic control of inflammation in experimental sepsis and arthritis, the potential of nicotine-like drugs is limited by their side effects such as toxicity and addiction. Other studies reported that nicotine increases inflammation in experimental arthritis. Nicotine can enhance the formation of neutrophil extracellular traps by human neutrophils and exacerbate inflammation in murine collagen-induced arthritis by inducing autoantigens [94,95]. These results concur with epidemiological studies showing that cigarette smoking can contribute to autoimmune diseases such as arthritis [96]. This discrepancies about the effects of nicotine in arthritic may be due to its administration: pretreatment (before the arthritis induction) with nicotine aggravated adjuvant-induced arthritis severity in rats, whereas the nicotine post-treatment decreased inflammation and clinical score signs of arthritis [97]. Still, the effects and mechanisms of nicotinic agonists on inflammatory diseases such as RA are not totally understood.
Recent studies in murine collagen–induced arthritis depict a local non-neural catecholaminergic system modulating pro- and anti-inflammatory cytokines in the initial asymptomatic and secondary symptomatic phases of arthritis, respectively [98]. In the asymptomatic phase, the sympathetic signaling exacerbates collagen-induced arthritis via CD4+CD25+ T cells [99]. Synovial tissues of the patients with chronic RA show a significant decline in the density of sympathetic nerve fibers [100], similar to that observed in arthritic mice [98]. However, non-neural cells producing catecholamines are found in the synovial tissue and they seem to substitute the sympathetic innervations destroyed during the arthritis progression. These non-neural cells produce catecholamines to reduce local inflammation in the arthritic joints [101]. In the symptomatic phase, catecholamines activate B cells to produce IL-10 and attenuate joint inflammation in arthritis (Figure 1D) [102]. These basic physiological studies on neuromodulation are allowing the design of innovative therapeutic approaches to control inflammation in RA and other inflammatory and infectious disorders.
Vagal afferent signal & central processing of inflammation
Most studies show that the vagus nerve stimulation, focused on the efferent vagal signals toward the periphery, controls systemic inflammation in experimental sepsis by inhibiting splenic TNF production. Although some studies proposed a direct vagal innervation of the spleen [103], most studies suggest that the vagus nerve does not innervate the spleen [44]. Instead, efferent vagal terminals enter the celiac ganglion to activate the splenic nerve [43]. These apparent contradictions from surgical vagotomy and splenectomy provided different results depending on the experimental conditions. For instance, unilateral cervical vagotomy increased inflammation in septic and arthritic mice worsening morbidity and mortality [70,77,104,105].
Selective surgical neurectomies showed that cervical or subdiaphragmatic vagus nerve exerts specific functions transmitting afferent and efferent signals along the anti-inflammatory network. As the cervical vagal trunks are critical for both afferent and efferent nerve signals, its ablation blocks all signals regardless of their origin and processing [106]. The original studies on endotoxemia showed that the spleen has a critical role modulating systemic inflammation by linking both the nervous and immune system [14,51]. The spleen also contributes to sustain the chronic synovial inflammation in peptidoglycan–polysaccharide-induced arthritis in rats [107]. These studies showed that surgical splenectomy prevents the development of collagen-induced arthritis [107,108], but increased acute joint inflammation induced with intra-articular zymosan injection [24,109]. These results show that splenectomy per se has different effects on synovial immune response depending on several factors, as the inflammatory stimulus, the disease outcome and the immune cells stimulated. As RA has different inflammatory patterns along its development, these results may explain the debatable effects of splenectomy on clinical arthritis progression [110–112]. Furthermore, surgical splenectomy did not prevent the anti-inflammatory effect of vagus nerve stimulation in intra-articular zymosan-challenged animals or other models of inflammatory diseases [24,109,113], suggesting the existence of other vagal neuroimmune pathways.
In addition to the efferent vagal signal, the afferent vagal signals toward the brain can also contribute to modulate inflammation. Stimulation of the proximal part of sectioned vagus nerve also controls systemic inflammation in endotoxemic animals [47,114,115]. Neurophysiological studies showed that vagus nerve stimulation modulates splenic nerve activity by an afferent pathway (Figure 1E) [44]. Another example is that electrical stimulation of aortic depressor nerve inhibited joint inflammation, cytokine production and neutrophil infiltration in experimental arthritis [109]. The aortic depressor nerve is a critical component of the afferent vagal system that contributes to the baroreflex system, an autonomic neuronal network that maintains cardiovascular homeostasis. Although the vagus nerve is the principal nerve of the parasympathetic system, morphological studies show a subpopulation of tyrosine hydroxylase positive (sympathetic) fibers at the cervical vagus nerve [116,117]. Moreover, the synovial tissue is innervated by adrenergic but not by cholinergic nerves [46]. Afferent vagal stimulation activates specific brain sympathetic-excitatory structures, especially the locus coeruleus (LC) and the paraventricular hypothalamic nucleus, and reduces knee joint inflammation in an acute model of RA (Figure 1F) [24]. Of note, the synaptic connection between vagal afferent signals (toward the NTS) and the LC (a brain noradrenergic nucleus) was mandatory for the vagal anti-inflammatory effects. This vagus nerve-LC-joint network is completely independent of the spleen and the adrenal glands, but is mediated by central and local sympathetic neural networks and synovial β-adrenergic receptors [24,109]. Several studies concur with these findings, reporting the role of sympathetic nervous system [118,119] and β2-adrenoreceptors [48,120] in the neural regulation of immunity. A similar anti-inflammatory effect in mice was also observed after the stimulation of C1 neurons, a neuronal group located in the medulla oblongata with reciprocal connections with the LC (Figure 1G) [121]. Vagal stimulation has a widespread and stimulatory effect on many specific cortical and subcortical regions of the brain [122–126]. Of note, cortical or vagal stimulation activated similar brain structures: in addition to the LC and paraventricular hypothalamic nucleus, both stimulatory modalities increased the activity of other neural structures involved with autonomic control, as the periaqueductal gray matter, raphe, amygdaloid nuclei and piriform cortex [29]. Actually, stimulation of the piriform cortex reduces joint inflammation in arthritic rats through a LC-dependent sympathetic mechanism. These results reveal, for the first time, a brain map formed by specific neural structures with potential immunomodulatory properties (Figure 1H) [29]. These results concur with clinical studies showing that some arthritic patients that suffered central neural lesions or cerebrovascular accidents, displayed reduced or even absence of arthritis on the affected side [127–130] and clear impairments on the local sympathetic activity and vascular permeability [131,132]. However, the neural or humoral networks between the brain and joint inflammation remained unknown. Further studies indicated that stimulation of primary afferent nociceptors from the inflamed area can attenuate the inflammatory process via a brain feedback toward the HPA axis activation [133–135]. Curiously, this anti-inflammatory effect was potentiated in animals that underwent subdiaphragmatic vagotomy, suggesting that vagal mechanisms are involved in central modulation of peripheral inflammation [134,136]. These results reveal that, in addition to the efferent vagal pathway, afferent vagal signaling modulates peripheral inflammation by activating central neuronal pathways [137,138].
Experimental and clinical studies show that vagal stimulation limits inflammation in RA through central vagal-mediated mechanisms controlling joint arthritis inflammation [27,139]. These physiological mechanisms appear similar to that of the spleen [43]. The vagus nerve can modulate inflammation in the arthritic joints by coordinating with the sympathetic adrenergic system. Unlike the spleen, whose neural activity is modulated via a vagal efferent subdiaphragmatic connection in the celiac-mesenteric ganglia with the splenic nerve, vagal regulation of arthritic joints may be mediated by afferent vagal signals activating central pathways and efferent sympathetic adrenergic networks innervating the joints [140,141].
From a physiological perspective, these studies on neuromodulation depict new models of functional organization of the nervous system to control inflammation [14]. Classically, the sympathetic and parasympathetic systems are described as ‘antagonistic’ mechanisms opposing one another to balance physiological homeostasis. The sympathetic and parasympathetic nervous systems produce antagonistic signals with norepinephrine and acetylcholine to balance both heart beat rate and blood pressure. A characteristic example is the baroreceptor reflex system [142]. Arterial baroreceptors are stretch receptors stimulated by distension of the arterial wall to control blood pressure. If blood pressure falls, baroreceptor firing rate decreases, and the central nervous system activates the sympathetic system to produce norepinephrine and increase the heart rate and blood pressure. Conversely, when blood pressure rises, the baroreceptor reflex activates the parasympathetic nervous system to release acetylcholine and decrease the heart rate and blood pressure. The recent findings of vagal neuromodulation of inflammation in endotoxemia suggest a different level of functional organization through a ‘sequential’ connection between the parasympathetic and sympathetic systems to inhibit cytokines production.
Bioelectronic medicine
Bioelectronic medicine is a new medical field that includes electrical engineering, neurophysiology and molecular biology designing novel treatments and diagnostics by using electronic devices to interface with the body [143]. Electric fields could be used to improve the outcome of patients with cancer [144,145]. Bioelectronics medicine has also been proposed as an innovative strategy to control inflammation and organ function by targeting distinct nerves and brain networks [143,146–149]. In 1997, the US FDA approved the use of a pulse generator implanted under the skin below the clavicle to induce electrical vagal stimulation for treating refractory epilepsy [150]. These treatments are proved safe without major side effects, and similar stimulation procedure was also approved in 2005 by the FDA for drug-resistant depression [151].
Growing preclinical and clinical studies evidence the potential of vagal stimulation to reduce inflammation in arthritis. An initial preclinical study demonstrated that cervical vagal stimulation with implantable electrodes reduced inflammation, articular bone loss and clinical score of collagen-induced arthritis in rats [26]. Electrical vagal stimulation with an implanted device (Cyberonics®/LivaNova) in epilepsy patients (n = 7) decreased the TNF, IL-1β and IL-6 production in whole-blood incubated with lipopolysaccharide (LPS) [27]. Also, vagus nerve stimulation for a short period (maximum time: 4 min/day for 84 days) inhibited cytokine production and improved the clinical score in 12 of 17 RA patients in two cohorts (total n = 17; cohort I: RA patients in the early stage of disease refractory to methotrexate treatment; cohort II: RA patients in the late stages of disease refractory to biological therapy) [27,147].
Recently, transcutaneous noninvasive vagus nerve stimulation has become available to replace permanent device implantation in RA patients by using gammaCore© (a cervical vagus nerve stimulator approved for the treatment of various types of primary headaches, including migraine and cluster headaches) [152,153] and Nemos® (a device of auricular branch vagal stimulation used in drug-resistant patients to decrease the seizure frequency) [154]. A preliminary, randomized and blinded pilot trial demonstrated in whole blood culture of healthy volunteers (n = 20) that gammaCore decrease the release of proinflammatory cytokines and chemokines and increase IL-10 anti-inflammatory cytokine as compared with sham stimulation [155]. The bilateral stimulation of cervical vagal nerve with gammaCore increase the cardiac vagal tone and reduces TNF blood levels in healthy subjects (n =20) [156]. Likewise, transcutaneous auricular vagus nerve stimulation can also inhibit blood levels of proinflammatory cytokines in endotoxemic rats [157].
Future perspective
The design of new neuroimmunomodulatory therapies for arthritis will require the study of: the physiological neural networks modulating the immune system, especially in the joints, the specific neurotransmitters and receptors controlling immune cells and their pharmacological properties, the role of autonomic dysfunction in arthritis and other chronic inflammatory disorders, the design of low-cost bioelectronic devices to control inflammation and organ function through neuronal stimulation; and potential side-effects of these new therapies.
Recent studies indicate that cholinergic regulation of the immune system is not exclusive to the vagus nerve. For instance, high concentrations of norepinephrine (1 mM) can activate splenic lymphocytes to produce acetylcholine, which inhibits cytokine production in splenic macrophages [42,47]. Acetylcholine can also be produced by choline acetyltransferase-expressing T cells that migrate into the spleen after efferent vagal stimulation (Figure 1I) [28]. These results warrant further studies to determine why lymphocytes require such high concentrations of norepinephrine to produce acetylcholine, the specific homing of T cells into the spleen, and their cellular responses. Furthermore, new studies should analyze the role of other organs involved in arthritis, such as the lymph nodes, which modulate the immune response in RA and that are innervated by sympathetic fibers and regulated by β-adrenergic signaling [158].
The stimulation of specific cholinergic pathways by pharmacological agents such as acetyl-cholinesterase inhibitors and β2 adrenergic agonists can be considered as potential strategies to control inflammation in arthritis [159–162]. Recent studies showed that activation of central muscarinic M1 or peripheral M3 receptors reduced inflammation in endotoxemia and delayed the progression of collagen-induced arthritis, respectively [163,164]. It is also known that vagal stimulation releases other neurotransmitters such as the neuropeptide vasoactive intestinal peptide (VIP). VIP has immunomodulatory effects on collagen-induced arthritis and therefore VIP could be used for treating arthritis [165]. The spinal cord is considered the key intermediate between the brain and peripheral sympathetic networks [18,24,166,167]. Therefore, pharmacological or electrical (bioelectronics) strategies modulating the spinal cord excitability could represent therapeutic approaches for treating arthritis. Examples of drugs that modulate spinal neuronal activity and inhibit inflammation in arthritis are GABAb receptor antagonists and cytokine-neutralizing agents, such as p38 MAPK inhibitors and anti-TNF molecules [168].
Heart rate variability (HRV) is a functional measure of the autonomic balance. Multiple studies suggest that HRV correlates with inflammation and may reflect autonomic dysfunction contributing to inflammatory disorders [169]. However, this view is still controversial because parasympathetic and sympathetic signaling are essentially organ-specific [104]. In addition, autonomic dysfunction is a common signal in inflammatory diseases, suggesting that the disruption of the parasympathetic/sympathetic balance is not specific to a neural system. Lower HRV may correlate to the higher incidence of sudden death in the arthritis patients [170]. Indeed, several studies correlated decreased vagal or increased sympathetic tonus with a worse outcome in RA [169,171]. For example, it has been shown an association between reduced vagal tonus and elevated blood levels of HMGB-1, a proinflammatory cytokine, contributing to arthritis [171]. In fact, high HMGB-1 levels were associated with low α7nAChR expression by peripheral circulating monocytes in the arthritic patients, suggesting a cholinergic signaling deficiency [172]. In summary, HRV is a biological signal with promising application to predict arthritis outcome, and to investigate the efficacy of pharmacological treatments, as anti-TNF therapies [173], although its mechanisms need further study.
From a bioelectronic perspective, the stimulation of other nerves (e.g., splanchnic nerve) with noninvasive approaches (e.g., pulsed ultrasound) may also provide therapeutic advantages to control inflammation without the side effects observed with conventional invasive methods [119,137,148,174]. Electroacupuncture is another alternative medical treatment with promising use in RA. Stimulation of the sciatic nerve with electroacupuncture controls systemic inflammation in endotoxemic mice through a vagal-adrenal dependent pathway and dopaminergic modulation of the immune cells [175]. This novel neuroimmune pathway could shed light on the mechanisms of electroacupuncture to alleviate arthritis [176–179]. Moreover, from a pharmacological perspective dopaminergic agonists could be used to control inflammation in arthritis due to their ability to inhibit Th17 cytokines [180].
In addition to the promising results on vagal control of joint inflammation, the potential side effects of vagal stimulation in RA are not known. The current use of vagal stimulation for the treatment of refractory epilepsy appears to induce minor side effects related to cervical surgical implantation, as such local infections, vocal cord paralysis and electrode rejection [181,182]. These clinical studies indicate that vagal stimulation does not produce immunosuppression as found with the biological drugs used in the treatment of arthritis. The patients under antirheumatic drug therapy appear to have more severe side effects mainly immunosuppression [11,12]. On the other hand, it has been proposed that neurological disorders can affect peripheral inflammation. For instance, neurological damages increase the susceptibility to infections triggering chronic autonomic output signals [183–185]. A recent study showed that occlusion of one middle cerebral artery (a classic model of stroke) prevents the progression of arthritis [186]. These studies suggest that vagal stimulation may also re-establish neurological function contributing to potential inflammatory disorders.
Conclusion
Despite its recent identification, a large number of studies show the potential of the vagus nerve to control the immune system and attenuate inflammation in both infectious and inflammatory disorders. A growing number of studies show the beneficial effects of cervical vagal stimulation to control experimental and clinical arthritis, even in patients refractory to current antirheumatic treatments. The local (joints) and/or systemic (spleen) targets of distinct (afferent/efferent) vagal signals may be more complex than anticipate and they need to be described in detail. Moreover, while the sympathetic and parasympathetic nervous systems have been described as opposing functional systems, multilevel interactions between both systems may be responsible for the control/exacerbation of inflammation in arthritis and must be clarified in the future. It is also possible that in the experimental models of arthritis, time and/or intensity of vagal stimulation influence diverse neuroimmune pathways in isolated or integrative (when more than one pathways are activated simultaneously) mechanisms. A recent study shows a central neural arc that includes vagal afferents-LC-sympathetic innervations in the regulation of joint inflammation. In addition, this novel neuronal network involving cortical-LC-sympathetic signaling could be stimulated by noninvasive methods such as electrical and magnetic transcranial techniques. Finally, the presence of catecholaminergic and cholinergic non-neural systems in the synovial tissue can provide new therapeutic targets for designing innovative treatments for arthritis.
Acknowledgements
This study was supported by FAPESP project grants (11/20343–4, 12/04237–2 and 13/08216–2). A Kanashiro is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (118636/2017–0). L Ulloa is supported by the NIH R01-GM114180, Eastern Scholarship JZ2016010, and NSFC #81774429.
Financial & competing interests disclosure
The authors declare no competing financial interests. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 4, S265 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edrees AF, Misra SN, Abdou NI. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-α serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin. Exp. Rheumatol 23:469–474 [PubMed] [Google Scholar]
- 4.Inui K, Koike T. Combination therapy with biologic agents in rheumatic diseases: current and future prospects. Ther. Adv. Musculoskelet. Dis 8, 192–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis Rheumatology (Oxford) 51(Suppl. 6), vi28–vi36 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J. Rheumatol 36, 1118–1125 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Christensen R, Wells GA et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews In: Cochrane Database of Systematic Reviews. Singh JA (Ed.). John Wiley & Sons, Ltd, Chichester, UK, p CD007848 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Devel. Ther 11, 1593–1603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb. Perspect. Biol a028456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery P, Keystone E, Tony HP et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomized placebo-controlled trial. Ann. Rheum. Dis . 67, 1516–1523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favalli EG, Desiati F, Atzeni F et al. Serious infections during anti-TNFα treatment in rheumatoid arthritis patients. Autoimmun. Rev 8, 266–273 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Inanc N, Direskeneli H. Serious infections under treatment with TNF-α antagonists compared to traditional DMARDs in patients with rheumatoid arthritis. Rheumatol. Int 27, 67–71 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka H TNF as a target of inflammation in rheumatoid arthritis. Endocr. Metab. Immune Disord. Drug Targets 15, 129–134 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol. Med 23, 1103–1120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tracey KJ. The inflammatory reflex. Nature 420, 853–859 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46, 927–942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther 179, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldburger J-M, Firestein GS. Regulation of peripheral inflammation by the central nervous system. Curr. Rheumatol. Rep 12, 370–378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borovikova LV, Ivanova S, Zhang M et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Bonaz B, Sinniger V, Pellissier S. Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med 282, 46–63 (2017). [DOI] [PubMed] [Google Scholar]
- 21.De Herdt V, Puimege L, De Waele J et al. Increased rat serum corticosterone suggests immunomodulation by stimulation of the vagal nerve. J. Neuroimmunol 212, 102–105 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Koopman FA, van Maanen MA, Vervoordeldonk MJ, Tak PP. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J. Intern. Med 282, 64–75 (2017). [DOI] [PubMed] [Google Scholar]
- 23.van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat. Rev. Rheumatol 5, 229–232 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Bassi GS, Dias DPM, Franchin M et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain, Behav. Immun 64, 330–343 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tak PP. Interview with Paul-Peter Tak: stimulating the vagus nerve to treat rheumatoid arthritis. Bioelectron. Med 1(1), 17–20 (2018). [Google Scholar]
- 26.Levine YA, Koopman FA, Faltys M et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PloS ONE 9, e104530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koopman FA, Chavan SS, Miljko S et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 113, 8284–8289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci 182, 65–69 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Bassi GS, Ulloa L, Santos VR et al. Cortical stimulation in conscious rats controls joint inflammation. Progress in neuro-psychopharmacology and biological psychiatry. 84, 201–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai B, Dong W, Sharpe S, Deitch EA, Ulloa L. Survival and inflammatory responses in experimental models of hemorrhage. J. Surg. Res 169, 257–266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulloa L, Deitch EA. Neuroimmune perspectives in sepsis. Crit. Care 13, 133 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol. Med 11, 56–63 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Kanashiro A, Sônego F, Ferreira RG et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol. Res 117, 1–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm . 2010, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulloa L The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov 4, 673–684 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen SE, Pfeiffer-Jensen M, Drewes AM et al. Vagal influences in rheumatoid arthritis. Scand. J. Rheumatol 47, 1–11 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Lu Y, Bian H, Guo L, Zhu H. Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am. J. Transl. Res 9, 971–985 (2017). [PMC free article] [PubMed] [Google Scholar]
- 38.Pinheiro NM, Santana FPR, Almeida RR et al. Acute lung injury is reduced by the α7nAChR agonist PNU-282987 through changes in the macrophage profile. FASEB J. 31, 320–332 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Watkins LR, Goehler LE, Relton JK et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett 183, 27–31 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol 309, H1740–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kember G, Ardell JL, Armour JA, Zamir M. Vagal nerve stimulation therapy: what is being stimulated? PLoS ONE 9, e114498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas-Ballina M, Olofsson PS, Ochani M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosas-Ballina M, Ochani M, Parrish WR et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol 97, 1180–1185 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Romanovsky AA. The inflammatory reflex: the current model should be revised. Exp. Physiol 97, 1178–1179 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Grimsholm O, Rantapää-Dahlqvist S, Dalén T, Forsgren S. Unexpected finding of a marked non-neuronal cholinergic system in human knee joint synovial tissue. Neurosci. Lett 442, 128–133 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Vida G, Peña G, Deitch EA, Ulloa L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol 186, 4340–4346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vida G, Peña G, Kanashiro A et al. β2-adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J .25, 4476–4485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pena G, Cai B, Ramos L, Vida G, Deitch EA, Ulloa L. Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis. J. Immunol 187, 718–725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol. Res 63, 38–57 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huston JM, Ochani M, Rosas-Ballina M et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203, 1623–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Yu M, Ochani M et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Bernik TR, Friedman SG, Ochani M et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vas. Surg 36, 1231–1236 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Altavilla D, Guarini S, Bitto A et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-κB activation, blunts TNF-α production, and protects againts splanchic artery occlusion shock. Shock 25, 500–506 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Cai B, Chen F, Ji Y et al. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J. Cell. Mol. Med 13, 3774–3785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grech D, Li Z, Morcillo P et al. Intraoperative low-frequency electroacupuncture under general anesthesia improves postoperative recovery in a randomized trial. J. Acupunct. Meridian Stud 9, 234–241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Westerloo DJ, Giebelen IA, Florquin S et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Liao H, Ochani M et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med 10, 1216–1221 (2004). [DOI] [PubMed] [Google Scholar]
- 59.van Westerloo DJ, Giebelen IAJ, Florquin S et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis 191, 2138–2148 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Tracey KJ, Fong Y, Hesse DG et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 (1987). [DOI] [PubMed] [Google Scholar]
- 61.Carré JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim. Biophys. Acta 1777, 763–771 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 4, 89–95 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol 148, 2724–2730 (1992). [PubMed] [Google Scholar]
- 64.Peña G, Cai B, Liu J et al. Unphosphorylated STAT3 modulates alpha7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol 40, 2580–2589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peña G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J. Mol. Med 88, 851–859 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulloa L The cholinergic anti-inflammatory pathway meets microRNA. Cell Res.23, 1249–1250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pullan RD, Rhodes J, Ganesh S et al. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med 330, 811–815 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Cho Y-G, Cho M-L, Min S-Y, Kim H-Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun. Rev 7, 65–70 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Lindblad SS, Mydel P, Jonsson I-M, Senior RM, Tarkowski A, Bokarewa M. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res. Ther 11, R88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Maanen MA, Lebre MC, van der Poll T et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 60, 114–122 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Li T, Zuo X, Zhou Y et al. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J. Clin. Immunol 30, 213–220 (2010). [DOI] [PubMed] [Google Scholar]
- 72.van Maanen MA, Stoof SP, LaRosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor 7 subunit gene knockout mice. Ann. Rheum. Dis 69, 1717–1723 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Bruchfeld A, Goldstein RS, Chavan S et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J. Intern. Med 268, 94–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waldburger J-M, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 58, 3439–3449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu S, Luo H, Xiao X, Zhang H, Li T, Zuo X. Attenuation of collagen induced arthritis via suppression on Th17 response by activating cholinergic anti-inflammatory pathway with nicotine. Eur. J. Pharmacol 735, 97–104 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, Yang Y, Yang J, Xie R, Ren Y, Fan H. Regulatory effect of nicotine on collagen-induced arthritis and on the induction and function of in vitro-cultured Th17 cells. Mod. Rheumatol 24, 781–787 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Li S, Zhou B, Liu B et al. Activation of the cholinergic anti-inflammatory system by nicotine attenuates arthritis via suppression of macrophage migration. Mol. Med. Rep 14, 5057–5064 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotake S, Udagawa N, Takahashi N et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest 103, 1345–1352 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J. Immunol 182, 3884–3891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ziolkowska M, Koc A, Luszczykiewicz G et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol 164, 2832–2838 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Ivanov II, McKenzie BS, Zhou L et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Park H, Li Z, Yang XO et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. immunol 6, 1133–1141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrington LE, Hatton RD, Mangan PR et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol 6, 1123–1132 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Bettelli E, Carrier Y, Gao W et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Hoeve MA, Savage NDL, de Boer T et al. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol 36, 661–670 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Wang D, Zhou R, Yao Y et al. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J. Pharmacol. Exp. Ther 335, 553–561 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J. Investig. Dermatol 126, 1948–1965 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Leite PEC, Gandía L, de Pascual R et al. Selective activation of α7 nicotinic acetylcholine receptor (nAChRα7) inhibits muscular degeneration in mdx dystrophic mice. Brain Res. 1573, 27–36 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Kurzen H, Berger H, Jäger C et al. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J. Investig. Dermatol 123, 937–949 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Pereira MR, Leite PEC. The involvement of parasympathetic and sympathetic nerve in the inflammatory reflex. J. Cell. Physiol 231, 1862–1869 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Westman M, Engström M, Catrina AI, Lampa J. Cell specific synovial expression of nicotinic alpha 7 acetylcholine receptor in rheumatoid arthritis and psoriatic arthritis. Scand. J. Immunol 70, 136–140 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Maanen MA Van Stoof SP, Zanden EP Van Der et al. The α7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 60, 1272–1281 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Zhou Y, Zuo X, Li Y, Wang Y, Zhao H, Xiao X. Nicotine inhibits tumor necrosis factor-α induced IL-6 and IL-8 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Rheumatol. Int 32, 97–104 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J. Leukoc. Biol 100, 1105–1112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J, Luria A, Rhodes C et al. Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology (Oxford) 56, kew449 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baka Z, Buzás E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res. Ther 11, 238 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu H, Yang Y-H, Rajaiah R, Moudgil KD. Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum. 63, 981–991 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Härle P, Möbius D, Carr DJJ, Schölmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 52, 1305–1313 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Härle P, Pongratz G, Albrecht J, Tarner IH, Straub RH. An early sympathetic nervous system influence exacerbates collagen-induced arthritis via CD4+CD25+ cells. Arthritis Rheum. 58, 2347–2355 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Miller LE, Jüsten HP, Schölmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 14, 2097–2107 (2000). [DOI] [PubMed] [Google Scholar]
- 101.Capellino S, Cosentino M, Wolff C et al. Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Ann. Rheum. Dis 69, 1853–1860 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Pongratz G, Melzer M, Straub RH. The sympathetic nervous system stimulates anti-inflammatory B cells in collagen-type II-induced arthritis. Ann. Rheum. Dis 71, 432–439 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Buijs RM, van der Vliet J, Garidou M-L, Huitinga I, Escobar C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS ONE 3, e3152 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McAllen RM, Cook AD, Khiew HW, Martelli D, Hamilton JA. The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res. Ther 17, 87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li T, Zuo X, Zhou Y et al. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J. Clin. Immunol 30, 213–220 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Székely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am. J. Physiol 275, R63–R68 (1998). [DOI] [PubMed] [Google Scholar]
- 107.Kimpel D, Dayton T, Fuseler J et al. Splenectomy attenuates streptococcal cell wall-induced arthritis and alters leukocyte activation. Arthritis Rheum. 48, 3557–3567 (2003). [DOI] [PubMed] [Google Scholar]
- 108.Rahman J, Staines NA. Contribution of the spleen, lymph nodes and bone marrow to the antibody response in collagen-induced arthritis in the rat. Clin. Exp. Immunol 85, 48–54 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bassi GS, Brognara F, Castania JA et al. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav. Immun 49, 140–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Balint GP, Balint PV. Felty’s syndrome. Best pract. Res. Clin. Rheumatol 18, 631–645 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Bradley JD, Pinals RS. Felty’s syndrome presenting without arthritis. Clin. Exp. Rheumatol 1, 257–259 (1983). [PubMed] [Google Scholar]
- 112.Uluhan A, Sager D, Jasin HE. Juvenile rheumatoid arthritis and common variable hypogammaglobulinemia. J. Rheumatol 25, 1205–1210 (1998). [PubMed] [Google Scholar]
- 113.Matteoli G, Gomez-Pinilla PJ, Nemethova A et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Olofsson PS, Lavine Y, Caravaca A et al. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron. Med 2, 37–42 (2015). [Google Scholar]
- 115.Inoue T, Abe C, Sung SJ et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Investig 126, 1939–1952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verlinden TJM, Rijkers K, Hoogland G, Herrler A. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol. Scand 133, 173–182 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Seki A, Green HR, Lee TD et al. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm 11, 1411–1417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kox M, van Eijk LT, Zwaag J et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl Acad. Sci. USA 111, 7379–7384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martelli D, Yao ST, Mancera J, McKinley MJ, McAllen RM. Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anesthesia. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R1085–R1091 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Elenkov IJ, Haskó G, Kovács KJ, Vizi ES. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J. Neuroimmunol 61, 123–131 (1995). [DOI] [PubMed] [Google Scholar]
- 121.Abe C, Inoue T, Inglis MA et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci 20, 700–707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci 2, 94–98 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Barnes A, Duncan R, Chisholm JA et al. Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy, using 99mTc-HMPAO SPET brain images. Eur. J. Nucl. Med. Mol. Imaging 30, 301–305 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Henry TR, Bakay RA, Votaw JR et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia 39, 983–990 (1998). [DOI] [PubMed] [Google Scholar]
- 125.Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 22, 53–62 (1995). [DOI] [PubMed] [Google Scholar]
- 126.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology 33, 1884–1895 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Thompson M, Bywaters EG. Unilateral rheumatoid arthritis following hemiplegia. Ann. Rheum. Dis 21, 370–377 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glick EN. Asymmetrical rheumatoid arthritis after poliomyelitis. BMJ 3, 26–28 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamilton S Unilateral rheumatoid arthritis in hemiplegia. J. Can. Assoc. Radiol 34, 49–50 (1983). [PubMed] [Google Scholar]
- 130.Lapadula G, Iannone F, Zuccaro C, Covelli M, Grattagliano V, Pipitone V. Recovery of erosive rheumatoid arthritis after human immunodeficiency virus-1 infection and hemiplegia. J. Rheumatol 24, 747–751 (1997). [PubMed] [Google Scholar]
- 131.Stangenberg L, Burzyn D, Binstadt BA et al. Denervation protects limbs from inflammatory arthritis via an impact on the microvasculature. Proc. Natl Acad. Sci. USA 111, 11419–11424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coderre TJ, Chan AK, Helms C, Basbaum AI, Levine JD. Increasing sympathetic nerve terminal-dependent plasma extravasation correlates with decreased arthritic joint injury in rats. Neuroscience 40, 185–189 (1991). [DOI] [PubMed] [Google Scholar]
- 133.Green PG, Miao FJ, Jänig W, Levine JD. Negative feedback neuroendocrine control of the inflammatory response in rats. The J. Neurosci 15, 4678–4686 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miao FJ, Jänig W, Levine JD. Vagal branches involved in inhibition of bradykinin-induced synovial plasma extravasation by intrathecal nicotine and noxious stimulation in the rat. J. Physiol 498 (Pt 2), 473–481 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miao FJ, Green PG, Coderre TJ, Jänig W, Levine JD. Sympathetic-dependence in bradykinin-induced synovial plasma extravasation is dose-related. Neurosci. Lett 205, 165–168 (1996). [DOI] [PubMed] [Google Scholar]
- 136.Miao FJ, Jänig W, Dallman MF et al. Role of vagal afferents and spinal pathways modulating inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine. J. Neurophysiol 72, 1199–1207 (1994). [DOI] [PubMed] [Google Scholar]
- 137.Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol 592, 1677–1686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Willemze RA, Luyer MD, Buurman WA, de Jonge WJ. Neural reflex pathways in intestinal inflammation: hypotheses to viable therapy. Nat. Rev. Gastroenterol. Hepatol 12, 353–362 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Levine YA, Koopman FA, Faltys M et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE 9, e104530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schaible H-G, Straub RH. Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton. Neurosci 182, 55–64 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Sato Y, Schaible HG. Discharge characteristics of sympathetic efferents to the knee joint of the cat. J. Auton. Nerv. Sys 19, 95–103 (1987). [DOI] [PubMed] [Google Scholar]
- 142.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 71, 1733–1738 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Vitale F, Litt B. Bioelectronics: the promise of leveraging the body’s circuitry to treat disease. Bioelectron. Med 1, 3–7 (2018). [Google Scholar]
- 144.Rehman AA, Elmore KB, Mattei TA. The effects of alternating electric fields in glioblastoma: current evidence on therapeutic mechanisms and clinical outcomes. Neurosurg. focus 38, E14 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Shawki MM, Farid A. Low electric field parameters required to induce death of cancer cells. Electromagn. Biol. Med 33, 159–163 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Birmingham K, Gradinaru V, Anikeeva P et al. Bioelectronic medicines: a research roadmap. Nat. Rev. Drug Dis 13, 399–400 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Suzuki K, Nakai A. Immune modulation by neuronal electric shock waves. J. Allergy Clin. Immunol doi: 10.1016/j.jaci.2018.02.027 (2018) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 148.Murray K, Reardon C. The cholinergic anti-inflammatory pathway revisited. Neurogastroenterol. Motil 30, e13288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Botha C, Farmer AD, Nilsson M et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 64, 611–617 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Wheless JW, Baumgartner J. Vagus nerve stimulation therapy. Drugs Today 40, 501–515 (2004). [DOI] [PubMed] [Google Scholar]
- 151.Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14, 716–727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mwamburi M, Liebler EJ, Tenaglia AT. Review of non-invasive vagus nerve stimulation (gammaCore): efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. Am. J. Manag. Care 23, S317–S325 (2017). [PubMed] [Google Scholar]
- 153.Simon B, Blake J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am. J. Manag. care 23, S312–S316 (2017). [PubMed] [Google Scholar]
- 154.Stefan H, Kreiselmeyer G, Kerling F et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 53, e115–e118 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Lerman I, Hauger R, Sorkin L et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation 19, 283–290 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Brock C, Brock B, Aziz Q et al. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol. Motil 29, e12999 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Zhao YX, He W, Jing XH et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid.-Based Complementary Altern. Med 2012, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med 211, 2583–2598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kanashiro A, Talbot J, Peres RS et al. Neutrophil recruitment and articular hyperalgesia in antigen-induced arthritis are modulated by the cholinergic anti-inflammatory pathway. Basic Clin. Pharmacol. Toxicol 119, 453–457 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Gowayed MA, Refaat R, Ahmed WM, El-Abhar HS. Effect of galantamine on adjuvant-induced arthritis in rats. Eur. J. Pharmacol 764, 547–553 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J. Immunol 162, 6278–6283 (1999). [PubMed] [Google Scholar]
- 162.Cobelens PM, Kavelaars A, Vroon A et al. The beta 2-adrenergic agonist salbutamol potentiates oral induction of tolerance, suppressing adjuvant arthritis and antigen-specific immunity. J. Immunol 169, 5028–5035 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Pavlov VA, Ochani M, Gallowitsch-Puerta M et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA 103, 5219–5223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beckmann J, Dittmann N, Schütz I, Klein J, Lips KS. Effect of M3 muscarinic acetylcholine receptor deficiency on collagen antibody-induced arthritis. Arthritis Res. Ther 18, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Delgado M, Abad C, Martinez C et al. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J. Mol. Med 80, 16–24 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Kanashiro A, Franchin M, Bassi GS et al. Inhibition of spinal p38 MAPK prevents articular neutrophil infiltration in experimental arthritis via sympathetic activation. Fundam.Clin. Pharmacol 32, 155–162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bassi GS, do C Malvar D, Cunha TM, Cunha FQ, Kanashiro A. Spinal GABA-B receptor modulates neutrophil recruitment to the knee joint in zymosan-induced arthritis. Naunyn Schmiedeberg Arc. Pharmacol 389, 851–861 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Boyle DL, Jones TL, Hammaker D et al. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 3, e338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bruchfeld A, Goldstein RS, Chavan S et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J. Intern. Med 268, 94–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Evrengul H, Dursunoglu D, Cobankara V et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int 24, 198–202 (2004). [DOI] [PubMed] [Google Scholar]
- 171.Goldstein RS, Bruchfeld A, Yang L et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med 13, 210–215 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koopman FA, Tang MW, Vermeij J et al. Autonomic dysfunction precedes development of rheumatoid arthritis: a prospective cohort study. EBioMedicine 6, 231–237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Holman AJ, Ng E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton. Neurosci 143, 58–67 (2008). [DOI] [PubMed] [Google Scholar]
- 174.Gigliotti JC, Huang L, Ye H et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol 24, 1451–1460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Torres-Rosas R, Yehia G, Pena G et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med 20, 291–295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhu J, Chen X-Y, Li L-B et al. Electroacupuncture attenuates collagen-induced arthritis in rats through vasoactive intestinal peptide signalling-dependent re-establishment of the regulatory T cell/T-helper 17 cell balance. Acupunct. Med 33, 305–311 (2015). [DOI] [PubMed] [Google Scholar]
- 177.Li J, Li J, Chen R, Cai G. Targeting NF-κB and TNF-α activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model rats. Altern. Ther. Health Med 21, 26–34 (2015). [PubMed] [Google Scholar]
- 178.Dong Z-Q, Zhu J, Lu D, Chen Q, Xu Y. Effect of electroacupuncture in ‘Zusanli’ and ‘Kunlun’ acupoints on TLR4 signaling pathway of adjuvant arthritis rats. Am. J. Ther 25, 314–319 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Ouyang B, Gao J, Che J et al. Effect of electro-acupuncture on tumor necrosis factor-α and vascular endothelial growth factor in peripheral blood and joint synovia of patients with rheumatoid arthritis. Chin. J. Integr. Med 17, 505–509 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Lu J-H, Liu Y-Q, Deng Q-W, Peng Y-P, Qiu Y-H. Dopamine D2 receptor is involved in alleviation of Type II collagen-induced arthritis in mice. BioMed Res. Int 2015, 496759 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J. Neurosurg. Pediatr. 18, 97–104 (2016). [DOI] [PubMed] [Google Scholar]
- 182.Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy. Seizure 22, 827–833 (2013). [DOI] [PubMed] [Google Scholar]
- 183.Engel O, Akyüz L, da Costa Goncalves AC et al. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke 46, 3232–3240 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Prass K, Meisel C, Höflich C et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell Type 1–like immunostimulation. J. Exp. Med 198, 725–736 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wong CHY, Jenne CN, Lee W-Y, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011). [DOI] [PubMed] [Google Scholar]
- 186.Irmler IM, Gajda M, Kamradt T. Amelioration of experimental arthritis by stroke-induced immunosuppression is independent of Treg cell function. Ann. Rheum. Dis 73, 2183–2191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]