Abstract
Our previous study revealed that remote ischemic conditioning (RIC) reduced the incidence of stroke or TIA in octo- and nonagenarians with intracranial atherosclerotic stenosis (ICAS). Herein, we aimed to investigate whether RIC would influence the progression of white matter hyperintensities (WMHs) and cognitive impairment in the same group of patients. Fifty-eight patients with ICAS were randomly assigned in a 1:1 ratio to receive standard medical treatment with RIC (n=30) versus sham-RIC (n=28). The RIC protocol consisted of 5 cycles of alternating 5-min ischemia and 5-min reperfusion applied in the bilateral upper arms twice daily for 300 days. The efficacy outcomes included WMHs change on T2 FLAIR sequences, estimated by the Fazekas scale and Scheltens scale, cognitive change as assessed by the MMSE and MoCA, and some clinical symptoms within 300-day follow-up. Compared with the baseline, RIC treatment significantly reduced Fazekas and Scheltens scores at both 180-day (both p<0.05) and 300-day (both p<0.01) follow-ups, whereas no such reduction was observed in the control group. In the RIC group, Fazekas scores were significantly lower at 300-day follow-up (p<0.001) while Scheltens scores were significantly lower at both 180-day and 300-day follow-ups (both p<0.001), as compared with the control group. There were statistically significant between-group differences in the overall MMSE or MoCA scores, favoring RIC at 180-day and 300-day follow-ups (all p<0.05). RIC may serve as a promising adjunctive to standard medical therapy for preventing the progression of WMHs and ameliorating cognitive impairment in very elderly patients with ICAS.
Keywords: remote ischemic conditioning, octo- and nonagenarians, intracranial atherosclerotic stenosis, white matter hyperintensities, cognitive impairment
INTRODUCTION
White matter hyperintensities (WMHs), also known as leukoaraiosis, are not uncommon neuroimaging findings in elderly individuals, particularly in those with known vascular risk factors and associated cerebrovascular diseases [1, 2]. Anatomically, blood vessels supplying the white matter (WM) are mainly long, penetrating arterioles with few anatomic branches, and the WM receives less blood flow than that in the gray matter. These vascular features may render the WM more vulnerable to ischemic insult. In addition, a group of aging-related vascular changes, such as increased tortuous arterioles, thickened vascular walls due to collagen deposition, decreased vascular density and impaired cerebral blood flow (CBF) autoregulation could further exacerbate WM damage [3, 4]. Clinically, a high WMH burden is considered to be associated with progressive cognitive impairment, increased risk of stroke and dementia, gait disturbance, depression and even death [2, 5, 6].
Although the pathophysiological mechanisms underlying WMHs remain unclear, endotheial dysfunction and chronic hypoperfusion secondary to atherosclerosis appear to play a central role in the initiation and progression of WMHs [7, 8]. Recently, intracranial atherosclerotic stenosis (ICAS) has been suggested as a major cause of chronic cerebral ischemia (CCI) worldwide, notably in Asians [9, 10]. ICAS-induced CCI is the basis for vascular cognitive impairment and can promote the occurrence and recurrence of stroke. In addition, a growing body of evidence indicates a strong association between ICAS and WMHs in stroke patients as well as in an asymptomatic population [7, 8, 11, 12]. Severe stenosis or occlusion of intracranial arteries can cause diffuse cerebral hypoperfusion, and if this CBF reduction is continuous, WMHs may develop, followed by stroke and impaired cognition. Other possible explanations for a high prevalence of WMHs in ICAS patients include artery-to-artery embolism from atherosclerotic lesions and shared risk factors by ICAS and WMHs [7, 8].
To date, despite decades of exploration, effective treatment strategies for reversing or preventing WMHs are still lacking. Preclinical drugs with great efficacy in experimental models have frequently failed to yield encouraging outcomes, which raise doubts about the translational value of these initial findings. Additionally, the conclusions from clinical trials on whether risk factor management in elderly patients could prevent WMHs progression or ameliorate cognitive impairment are mixing and inconsistent [2, 13].
Limb remote ischemic conditioning (RIC) refers to an approach that brief, repetitive and sublethal ischemia-reperfusion applied to a limb or limbs can induce systemic endogenous tolerance, whereby preventing remote organs or tissues from subsequent (detrimental) ischemic injury [14]. Animal studies have demonstrated that daily limb RIC is effective in reducing WM damage, promoting angiogenesis and improving cognitive function after CCI [15–17]. In a proof-of-concept randomized controlled trial conducted by our study group, we found that chronic RIC was able to alleviate WMHs and slow down cognitive decline in patients with cerebral small-vessel disease (CSVD) [18]. Importantly, regarding the patients with ICAS either above or beneath 80 years of age, chronic RIC is safe and shown to decrease the incidence of stroke recurrence, possibly via enhancing cerebral perfusion and ameliorating plasma biomarkers of coagulation and inflammation [19, 20]. However, whether RIC can alter WMHs in octo- and nonagenarians with ICAS has not been reported yet. Herein, the aim of this study was to investigate whether long-term RIC could prevent the progression of WMHs and improve cognition in octo- and nonagenarians with ICAS.
RESULTS
Patient characteristics
A total of 58 patients, including 30 in the RIC group and 28 in the control group, were included in the final analysis. The mean age was 83.5±2.3 years and 84.2±1.6 years for the RIC group and control group, respectively. As shown in Table 1, none of the baseline demographic and clinical characteristics differed significantly between the two groups. There were no statistically significant between-group differences in the Fazekas scale and Scheltens scale scores (Fazekas scores: 3.37±1.03 for RIC vs. 2.93±1.09 for control; Scheltens scores: 14.60±2.79 for RIC vs. 14.86±3.05 for control; all p>0.05). The mean MMSE and MoCA scores were similar between groups (MMSE: 24.27±4.65 for RIC vs. 23.82±5.74 for control; MoCA: 22.20±4.42 for RIC vs. 20.89±5.78 for control; all p>0.05).
Table 1. Baseline characteristics of the 58 patients.
| Variable | RIC group | Control group | p-value |
|---|---|---|---|
| No. of patients | 30 | 28 | - |
| Gender (male/female) | 18/12 | 17/11 | 0.956 |
| Age, years (mean±SD) | 83.5±2.3 | 84.2±1.6 | 0.187 |
| Education (junior middle school level of education and above) | 29 (96.7) | 28 (100.0) | 1.000 |
| Smoking | 2 (6.7) | 3 (10.7) | 0.936 |
| Diabetes | 13 (43.3) | 11 (39.2) | 0.754 |
| Hypertension | 20 (66.7) | 18 (64.3) | 0.849 |
| hyperlipidemia | 22 (73.3) | 20 (71.4) | 0.871 |
| Previous stroke | 16 (53.3) | 16 (57.1) | 0.771 |
| Previous TIA | 15 (50.0) | 15 (53.6) | 0.786 |
| Locations of stenosis | |||
| Right ICA | 6 (20.0) | 5 (17.9) | 0.835 |
| Left ICA | 4 (13.3) | 5 (17.9) | 0.910 |
| Right MCA | 5 (16.7) | 5 (17.9) | 1.000 |
| Left MCA | 5 (16.7) | 4 (14.3) | 1.000 |
| BA | 2 (6.7) | 2 (7.1) | 1.000 |
| Multifocal stenosis | 8 (26.6) | 7 (25.0) | 0.885 |
| Medical management | |||
| ACE inhibitors | 2 (6.7) | 3 (10.7) | 0.665 |
| ARBs | 3 (10.0) | 2 (7.1) | 1.000 |
| ARBs plus CCBs | 11 (36.7) | 10 (35.7) | 0.940 |
| CCBs | 4 (13.3) | 3 (10.7) | 1.000 |
| Antidiabetic oral therapy | 9 (30.0) | 8 (28.6) | 1.000 |
| Antidiabetic insulin therapy | 4 (13.3) | 3 (10.7) | 1.000 |
| Anticoagulation and statin therapy before enrollment | |||
| Aspirin | 11 (36.7) | 12 (42.9) | 0.630 |
| Clopidogrel | 1 (3.3) | 2 (7.1) | 0.951 |
| Aspirin plus clopidogrel | 0 | 0 | - |
| Statins | 2 (6.7) | 3 (10.7) | 0.936 |
| Headache (HIT-6) (median IQR) | 54 (36–58) | 54 (36–56) | 0.924 |
| Dizziness | 25(83.3) | 22 (78.6) | 0.644 |
| Sleeping disorder | 17 (56.7%) | 14 (50%) | 0.611 |
| Fazekas scores (mean±SD) | 3.37±1.03 | 2.93±1.09 | 0.112 |
| Scheltens scores (mean±SD) | 14.60±2.79 | 14.86±3.05 | 0.702 |
| Overall MMSE scores (mean±SD) | 24.27±4.65 | 23.82±5.74 | 0.925 |
| Cognition dysfunction evaluated by MMSE* | 13 (43.3%) | 12 (42.9%) | 0.971 |
| Overall MoCA scores (mean±SD) | 22.20±4.42 | 20.89±5.78 | 0.599 |
| Cognition dysfunction evaluated by MoCA** | 25 (83.3%) | 25 (89.3%) | 0.783 |
| NIHSS scores (mean±SD) | 11.3±2.3 | 11.1±2.6 | 0.757 |
| mRS scores (mean±SD) | 3.4±0.6 | 3.5±0.5 | 0.495 |
Values are given as n. (%) unless otherwise indicated. *Cognition dysfunction was defined as MMSE score<24 in patients with higher than or equal to junior middle school level of education and MMSE score<20 in patients with primary school level of education. **Cognition dysfunction was defined as MoCA<26. Abbreviation: TIA=transient ischemic attack; ICA=internal carotid artery; MCA=middle cerebral artery; BA=basilar artery.
WMHs evaluation
Fazekas scores
In the RIC group, Fazekas scores were significantly lower than those in the control group at 300-day follow-up (both unadjusted and adjusted p<0.001), (Table 2). Compared with the baseline, Fazekas scores in the RIC group were significantly decreased at both 180-day and 300-day follow-ups (p<0.05 and p<0.01). Moreover, 300-day RIC treatment was effective as 180-day RIC treatment in terms of decreasing the Fazekas scores (p>0.05). By contrast, there was no significant difference in the baseline, 180-day and 300-day Fazekas scores in the control group (p>0.05).
Table 2. WMHs and Cognition evaluation at baseline, 180-day and 300-day.
| RIC group | Control group | p-value | Adjusted p-value | |
|---|---|---|---|---|
| WMHs evaluation | ||||
| Fazekas scores | ||||
| 180-day | 2.53±0.63* | 2.86±0.97 | 0.275 | 0.089 |
| 300-day | 2.07±0.25** | 2.82±0.86 | <0.001 | <0.001 |
| Scheltens scores | ||||
| 180-day | 12.87±2.33* | 15.29±2.84 | 0.001 | <0.001 |
| 300-day | 11.07±2.15** | 16.93±3.42** | <0.001 | <0.001 |
| Cognition evaluation | ||||
| Overall MMSE scores | ||||
| 180-day | 27.10±2.95** | 24.68±4.95 | 0.030 | 0.006 |
| 300-day | 27.70±2.48*** | 24.11±4.86 | 0.001 | <0.001 |
| Overall MoCA scores | ||||
| 180-day | 26.70±2.65*** | 23.11±5.02*** | <0.001 | <0.001 |
| 300-day | 26.80±2.22*** | 22.71±4.84*** | <0.001 | <0.001 |
| Other clinical symptoms | ||||
| HIT-6 scores | ||||
| 180-day | 36 (36–37) | 46 (36–48) | <0.001 | <0.001 |
| 300-day | 36 (36–36) | 46 (36–48) | <0.001 | <0.001 |
| Dizziness, n (%) | ||||
| 180-day | 7 (23.3) | 18 (64.3) | 0.002 | 0.001 |
| 300-day | 5 (16.7) | 20 (71.4) | <0.001 | <0.001 |
| Sleeping disorder, n (%) | ||||
| 180-day | 9 (30.0) | 15 (53.6) | 0.069 | 0.024 |
| 300-day | 7 (23.3) | 16 (57.1) | 0.009 | 0.003 |
The discrepancies between the two groups were analyzed through Mann-Whitney U test or Chi-square analysis. Post-treatment vs. pretreatment was processed using Friedman test (*p<0.05, **p<0.01, ***p<0.001). Adjust p-value was adjusted for hypertension, diabetes and hyperlipidemia.
Scheltens scores
As demonstrated in Table 2, both 180-day (unadjusted p=0.001, adjusted p<0.001) and 300-day (both unadjusted and adjusted p<0.001) RIC treatments significantly decreased scores on the Scheltens scale, as compared with the control group. We also found a statistically significant reduction in the Scheltens scores of the RIC group with time and over days (180-day vs. baseline, p<0.05; 300-day vs. baseline, p<0.01), Figure 1. There was no significant difference in the Scheltens score between the baseline and the 180-day follow-up in the control group, but the score was robustly increased at 300-day (180-day vs. baseline, p>0.05; 300-day vs. baseline, p<0.001), Figure 2.
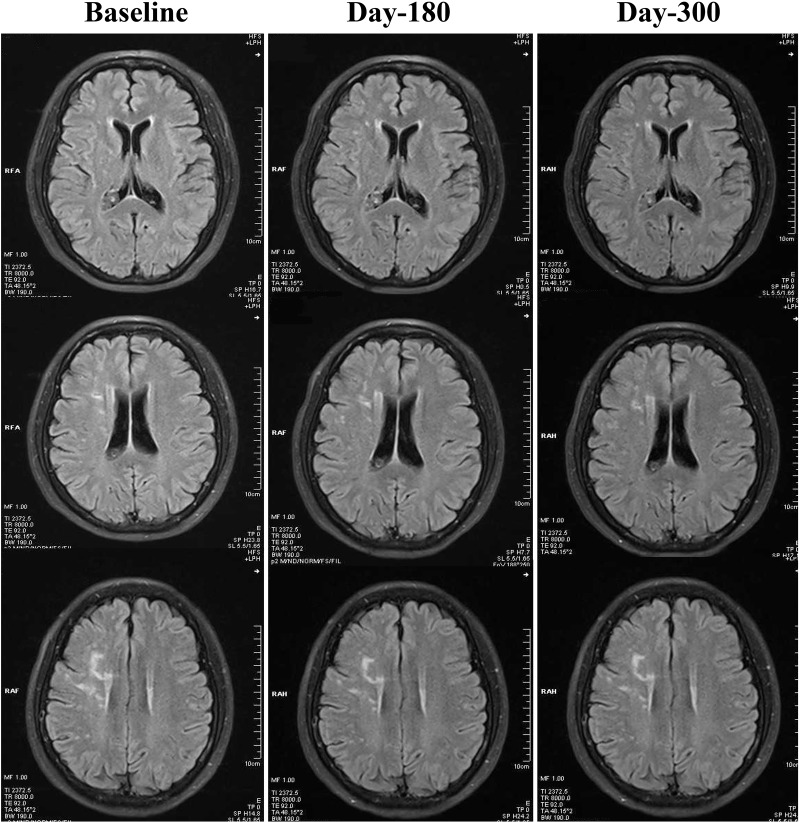
Figure 1.
Changes of WMHs in the RIC group from baseline, day 180 to day 300. Compared with baseline, WMHs in the RIC group were significantly decreased at both day 180 and day 300 follow-ups.
Figure 2.
Changes of WMHs in the control group from baseline, day 180 to day 300. In the control group, WMHs were not significantly attenuated at day 180 or day 300, when compared with baseline levels. By contrast, WMHs seemed to be increased robustly at day 300.
Cognition evaluation
MMSE scores
With regards to the overall MMSE score, there was a statistically significant difference favoring RIC at both 180-day (unadjusted p=0.03, adjusted p=0.006) and 300-day (unadjusted p=0.001, adjusted p<0.001) follow-ups, Table 2. In addition, there was an increase in the overall MMSE score after 180-day and 300-day RIC treatments in comparison to the baseline level (p<0.01 and p<0.001), Supplementary Table 1. However, no similar improvement was observed in the control group (all p>0.05).
An analysis of MMSE subitems was conducted as well, revealing that patients receiving 180-day RIC treatment had significantly higher delayed recall and calculation subscores (p=0.008 and p=0.018), while those receiving 300-day RIC treatment displayed robustly higher immediate memory, delayed recall and reading subscores (p=0.003, p<0.001 and p=0.033), apart from improved delayed recall and calculation subscores (p<0.001 and p=0.002), when compared to those in controls, Supplementary Table 1. The remaining subitems failed to yield any significant group differences (p>0.05). Regarding the discrimination between the pre-treatment and post-treatment RIC groups, the delayed recall, calculation and immediate memory domains demonstrated the greatest improvement relative to the other cognitive domains, Supplementary Table 1.
MoCA scores
When measured at day 180 and day 300, we noticed significantly increased overall MoCA scores in the RIC group as compared to the control group (all p<0.001), Table 2. Changes in the overall MoCA scores reached statistical significance in both groups from baseline to day 180 or day 300 (both p<0.001), Table 2.
Examining by MoCA subitems, patients in the control group displayed robustly lower delayed recall (p<0.001), attention (p<0.001), language (p=0.034) and orientation (p=0.019) subscores at 180-day follow up when compared with those in the RIC group, and the beneficial effects provided by RIC were sustained at 300-day follow-up, Supplementary Table 2. By day 180, besides the subscores in the four cognitive domains, visuospatial and executive abilities were also improved significantly in the RIC group (all p<0.05), Supplementary Table 2.
Correlations between WMHs visual rating scales and cognition rating scales
The correlations between WMHs (assessed by the Fazekas scale and Scheltens scale) and cognition (assessed by the MMSE and MoCA-BJ) were analyzed by Spearman correlation analysis. As shown in Table 3, the Fazekas and Scheltens scores were significantly related to the overall MMSE and MoCA scores at baseline, 180-day and 300-day (all p<0.001). Moreover, correlation data between the two cognitive scales and between the two WMHs rating scales at different time points also showed strong relevance (all p<0.001), Supplementary Table 3.
Table 3. Spearman correlation analysis between MMSE/MoCA scores vs. Fazekas/Scheltens scores.
| Baseline (r, p-value) | MMSE | MoCA |
|---|---|---|
| Fazekas scores | -0.607, p<0.001 | -0.607, p<0.001 |
| Scheltens scores | -0.548, p<0.001 | -0.559, p<0.001 |
| 180-day (r, p-value) | MMSE | MoCA |
| Fazekas scores | -0.583, p<0.001 | -0.444, p<0.001 |
| Scheltens scores | -0.555, p<0.001 | -0.633, p<0.001 |
| 300-day (r, p-value) | MMSE | MoCA |
| Fazekas scores | -0.667, p<0.001 | -0.622, p<0.001 |
| Scheltens scores | -0.665, p<0.001 | -0.728, p<0.001 |
Clinical symptoms
At both 180-day and 300-day follow-ups, patients in the RIC group showed a significant improvement on the clinical symptoms including headache (Headache Impact Test-6 scores: 180-day, 36 (36–37) vs. 46 (36–48), both unadjusted and adjusted p<0.001; 300-day, 36 (36–36) vs. 46 (36–48), both unadjusted and adjusted p<0.001), dizziness (180-day, 23.3% vs. 64.3%, unadjusted p=0.002 and adjusted p=0.001; 300-day, 16.7% vs. 71.4%, both unadjusted and adjusted p<0.001) and sleeping disorder (180-day, 30.0% vs. 53.6%, unadjusted p=0.069 and adjusted p=0.024; 300-day, 23.3% vs. 57.1%, unadjusted p=0.009 and adjusted p=0.003), compared with those in the control group.
DISCUSSION
The principal findings from the present randomized clinical trial exploring the effect of RIC on WMHs and cognition in octo- and nonagenarians with ICAS are that long-term twice-daily RIC combined with standard medical therapy is clinically effective in ameliorating WMHs and improving cognitive function when compared with standard medical therapy alone.
WMHs of presumed vascular origin are often found in elderly individuals, particularly those with vascular risk factors or vascular diseases. Recent studies have shown that intracranial large artery stenosis may result in WMHs, high loads of which are believed to be associated with increased risk of cognitive impairment, gait abnormalities, stroke, dementia and some unspecific symptoms such as headache, dizziness and sleep disturbance [6, 8, 10, 20]. Measuring the severity and progression of WMHs has been proposed as a useful surrogate marker for predicting therapeutic outcomes. Despite the lack of the optimal method of measuring WMHs, varied visual rating scales and volumetric measurements based on computerized techniques have been used in clinical and research settings. Unlike semi-automated volumetric methods, visual rating scales are relatively less time-consuming and much easier to perform on the MRI scans obtained from different devices. Moreover, there is ample evidence showing that volumetric and visual rating methods for the measurement of WMHs are moderately or highly correlated, and they may yield nearly equivalent estimations of WMHs burden [21, 22]. Hence, in this study, two widely used rating scales including the Fazekas scale and Scheltens scale were adopted to evaluate WMHs amongst elderly patients with ICAS. Importantly, we found that twice-daily RIC therapy for either 180-day or 300-day significantly reduced WMHs scores compared with controls, and longer-term use of RIC may be more effective than shorter-term treatment (lower Fazekas and Scheltens scores at 300-day than 180-day). In contrast, patients treated with medical therapy alone seemed to have more severe WMHs measured at 300-day follow-up relative to that at the baseline. Our results are consistent with previous studies examining the neuroprotective effects of RIC on experimental animal models, which demonstrated that chronic limb RIC could reverse or attenuate WM damage induced by bilateral carotid artery occlusion-related CCI [15, 17]. Additionally, a more recent pilot study enrolling patients with CSVD showed that daily RIC for 1-year was associated with a substantially lower mean WMHs volume than that in the control group [18]. Although we did not explore the detailed mechanisms underlying the RIC-mediated protective effect on WMHs in this study, such benefits may be largely attributable to improved CBF resulting from cerebral angiogenesis, collateral formation and vascular remodeling [14, 17, 19]. Furthermore, RIC has been shown to reduce inflammatory responses, downregulate microglial expression, inhibit the apoptosis of oligodendrocytes, and attenuate production of free radicals, all of which may facilitate the recovery of WMHs derived from ischemic injury [14–17]. Interestingly, our previous study using the same group of patients demonstrated that RIC administered for 180-day markedly reduced the incidence of stroke or TIA recurrence [27]. Given the association between WMHs and stroke or TIA recurrence, we may speculate that this clinical benefit is, at least partially, due to attenuation of WMHs.
Vascular cognitive impairment comprises a clinical spectrum ranging from mild cognitive decline (MCD) to vascular dementia, which poses a great threat to patients’ quality of life. Therefore, early and routine screening for cognitive deficits with sensitive instruments is required, knowing that it allows for timely initiation of interventions to prevent cognitive deterioration. While the MMSE is the most commonly used screening test for cognitive impairment in the world, the MoCA has been proven to be more sensitive than the MMSE for the detection of MCD and mild dementia [23–25]. In the present study, we also observed different percentages of patients with cognitive impairment at baseline depending on the type of screening test used: RIC group, MMSE (13%) vs. MoCA (25%); control group, MMSE (12%) vs. MoCA (25%). Compared with pretreatment, the post-treatment overall MMSE scores in the RIC group were significantly increased, whereas no such change was observed in the control group. Moreover, the overall MoCA scores measured at both 180-day and 300-day follow-ups were substantially increased in both groups, whereas the increases were more pronounced in the RIC group, implying that RIC may confer extra benefits over currently used medical therapies for improving cognition. This result may be supported by the evidence of experimental studies, which demonstrated that long-term RIC could improve cognition via enhancing spatial and working memory after CCI [15–17]. Notably, the result of MMSE and MoCA subitems showed that patients in the RIC group had significantly higher delayed recall, calculation, attention and orientation subscores. Compared with MMSE, MoCA is acknowledged as a potentially more sensitive method to evaluate cognitive domains including visuospatial and executive functions. In our study, although there was no significant difference in the visuospatial and executive subscores between groups, these domains were improved in the RIC group relative to the baseline levels.
Population-based studies have found an association between WMHs and cognition, knowing that increased WMHs burden can contribute to poorer cognitive function [6, 26]. We also noticed a significant negative correlation between WMHs (assessed by the Fazekas scale and Scheltens scale) and cognition (assessed by the MMSE and MoCA-BJ) at baseline, 180-day and 300-day. Therefore, it is reasonable to infer that improved cognition may be secondary to reduced WMHs and enhanced CBF.
Our study is of high clinical relevance due to the following reasons. First of all, to the best of our knowledge, this is the first randomized controlled clinical trial reporting the efficacy of RIC on WMHs reduction and cognition improvement amongst patients with ICAS-induced CCI. ICAS is considered as the most common cause of both stroke and CCI in Chinese and even whole Asian populations. At present, studies designed to explore approaches for slowing down or even reversing the progression of ICAS-associated WMHs are sparse. Second, given the overwhelming role of age on clinical outcomes, uncertainty exists regarding the benefits and risks related to current therapeutic recommendations applicable to elderly patients, particularly octo- and nonagenarians. Hence, clinicians should be intensely cautious in the prevention and treatment of adverse events at this advanced age with functional deterioration and multimorbidity. Unlike other clinical trials of RIC restricted to relatively younger patients, all participants in our study were in the very elderly age (older than 80 years) and showed good compliance within 300-day follow-up. Moreover, the RIC device used in the study provides many advantages, such as simplicity (can be performed automatically), low cost (can be performed repetitively) and mild or no adverse effect, all of which render it an optimal option for inpatients as well as home-care patients.
One of the major limitations of the present study was the small number of patients enrolled from a single center in China. Consequently, this study should be viewed as exploratory and cannot be generalized to the overall or other ethnic populations at this stage. Another limitation was that the follow-up period was not long enough, given the progressive feature of WMHs and cognitive function in the elderly patients. In addition, we only used two visual rating scales to assess the severity of WMHs and did not analyze the microstructural alterations within visible lesions as well as in the surrounding normal-appearing WM. The underlying mechanisms of RIC-mediated neuroprotection, such as WMHs reduction, and cognition or symptoms improvement were not fully investigated. Last but not least, we applied a previously reported RIC protocol in our patients, bur whether modifying RIC protocol in this clinical setting could achieve better outcomes remains unknown. A series of larger prospective clinical trials (Clinical Trials.gov: NCT03105141 and NCT02534545) is ongoing in our study group in an attempt to work out ways to tackle these challenges.
MATERIALS AND METHODS
Study design and participants
This study was a single center, prospective and randomized controlled clinical trial. The study protocol was approved by the Institutional Review Board of Capital Medical University (Beijing, China), and all patients provided written informed consent prior to enrollment. The study design, patient selection and randomization, and part of results have been previously described, (NCT01570231) [27].
Patients were enrolled into the study based on the following criteria: 1) presenting with neurologic symptoms corresponded with the territory of offending vessels; 2) significant stenosis of intracranial arteries (at least one artery stenosed ≥70%) measured by magnetic resonance angiography (MRA) or computed tomography angiography (CTA); 3) age of 80 years or older; 4) TOAST (Trial of Org 10172 in Acute Stroke Treatment) subtype 1, large-artery atherosclerosis; 5) National Institutes of Health Stroke Scale (NIHSS) score less than 15, and modified Rankin Scale (mRS) score of 2 to 4.
Patients were excluded if they met any of the following criteria: 1) presence of significant extracranial arterial stenosis (≥50%); 2) ICAS secondary to non-atherosclerotic etiologies, such as moyamoya disease, artery dissection, intracranial granulomatous arteritis, and any known vasculitic diseases; 3) Intracranial abnormalities such as tumor, vascular malformation, and cerebral venous sinus thrombosis; 4) Intracranial bleeding within 6 months prior to inclusion; 5) Refractory hypertension (systolic blood pressure>200 mmHg) that cannot be controlled by medical intervention; 6) severe renal, hepatic or cardiovascular disorder; 7) Contraindications for RIC, such as soft tissue or vascular injury, fracture and other diseases affecting the upper extremities, and severe subclavian artery stenosis; 8) absence of available MR images identifying WMHs; 9) Lost to follow-up.
Randomization and procedures
Patients were randomly allocated into two groups in a 1:1 ratio: RIC group and control group, using a computer-generated technique with random blocks. Patients or their guardians performing RIC were not blinded to treatment assignment. However, researchers who participated in the data analysis were masked to the assignment.
Patients in the RIC group received bilateral upper arm ischemic conditioning treatment (patent number ZL200820123637.X, China) twice daily for 300 consecutive days. The RIC protocol consisted of 5 cycles of alternating 5-min upper arm ischemia and 5-min reperfusion, at a cuffing pressure of 200 mmHg, enabling a complete blockage of the arterial and venous blood flow through the forearm simultaneously. In the control group, patients underwent the similar procedure, with the exception of the inflating pressure, which was set as 30 mmHg, to maintain the pressure feeling of patients without any effect on the blood flow (Supplementary Figure 1). Standard medical therapy and vascular risk factors were controlled similarly in the two groups.
Brain MRI and assessment of WMHs
Brain MRI was performed on a 3.0T scanner system (Siemens, Verio, Germany) at baseline, at 180-day follow-up and at 300-day follow-up. The imaging protocol was mainly comprised of T1-weighted (T1W), T2-weighted (T2W), T2 fluid-attenuated inversion recovery (T2 FLAIR), T2*-weighted gradient recalled echo (GRE), and diffusion-weighted imaging (DWI) sequences, which were all completed with 5-mm thick slices.
WMHs were defined as punctuate and/or diffuse hyperintense lesions on T2W and T2 FLAIR images, but were not seen as apparent hypointense changes on T1W.
Two visual rating scales including the Fazekas scale and Scheltens scale were used to semiquantitatively assess WMHs on the MRI, primarily T2 FLAIR scans [28, 29]. In the Fazekas scale, periventricular and deep WM hyperintensities were scored separately. A total Fazekas score ranging from 0 to 6 were used for statistical comparison by summing the two separate scores. The Scheltens scale has a range from 0–84, where scores 0–6 can be given in the periventricular regions, 0–24 in the deep WM, 0–30 in the basal ganglia, and 0–24 in the infratentorial regions. Two senior neuroradiologists, who were blinded to patients’ clinical data and vascular conditions, performed all the ratings on baseline and follow-up images in a side-by-side fashion. If inconsistency occurred between the two raters, a third rater would be introduced to re-examine WMHs, and the final decision was made based upon the ratings which have the majority, at least two thirds of the votes.
Assessment of cognitive function
Two screening instruments were used to assess the cognitive function of enrolled patients at baseline and at 180-day as well as 300-day follow-ups, including the Mini-Mental State Examination (MMSE) and Beijing version of the Montreal Cognitive Assessment (MoCA-BJ). Neurologists in our institution who were blinded to the study design and randomization completed all the assessments.
The MMSE is a 30-point questionnaire, which covers the domains of orientation, memory (immediate and delayed recall), calculation, language (naming, execution, repetition, reading and writing) and visuospatial ability [30]. A cutoff score of <24 was chosen to indicate cognitive impairment in patients with higher than or equal to junior middle school level of education [30].
The MoCA-BJ is the most commonly used version of MoCA in Mainland China with a maximal score of 30 [31]. The examination assesses the following cognitive domains: visuospatial and executive skills, naming, short-term memory, attention, memory, abstraction and orientation. Cognitive impairment on the MoCA was defined as a score <26 [31].
Outcomes
We measured the change of WMHs on T2 FLAIR sequences, estimated by the Fazekas scale and Scheltens scale (significant level α was defined as 0.25 for each scale), the alterations of cognition assessed by the MMSE and MoCA, and clinical symptoms such as headache, dizziness and sleeping disorder, at baseline and at 180-day and 300-day follow-ups.
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD) or otherwise as median (interquartile range, IQR). Categorical variables were presented as counts and percents. We used the t test or Mann-Whitney U test to compare continuous variables, and the Pearson χ2 test or Fisher’s exact test to compare categorical variables between the two groups. Furthermore, multivariate analyses such as logistic regression model and linear regression model were used to balance the covariates (including hypertension, diabetes and hyperlipidemia). Intragroup comparisons of mean or median values between baseline and follow-up evaluation were conducted by means of the Friedman test in order to remove the bias derived from repeated measurement. The associations between WMHs (measured by the Fazekas scale and Scheltens scale) and cognition (measured by the MMSE and MoCA-BJ) were evaluated by Spearman correlation analysis. A p value < 0.05 was considered statistically significant. All statistical analyses were done with SPSS version 21 (SPSS Inc., Chicago, IL, USA).
Data availability
Anonymized data are available to any qualified investigator for the purpose of reproducing the results or replicating the procedure.
CONCLUSIONS
In summary, our current findings underline that RIC may prove to be an adjunctive to standard medical therapy for preventing the progression of WMHs and ameliorating cognitive impairment in very elderly patients with ICAS-induced CCI. The clinical implications of this pilot study need to be further explored in future multicenter randomized clinical trials powered for the clinical outcomes.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- WMHs
White matter hyperintensities
- WM
white matter
- CBF
cerebral blood flow
- ICAS
intracranial atherosclerotic stenosis
- CCI
chronic cerebral ischemia
- RIC
remote ischemic conditioning
- CSVD
cerebral small-vessel disease
- MRA
magnetic resonance angiography
- CTA
computed tomography angiography
- NIHSS
National Institutes of Health Stroke Scale
- mRS
modified Rankin Scale
- T2 FLAIR
T2 fluid-attenuated inversion recovery
- MMSE
Mini-Mental State Examination
- MoCA-BJ
Beijing version of the Montreal Cognitive Assessment
- MCD
mild cognitive decline.
Footnotes
AUTHOR CONTRIBUTIONS: Da Zhou and Jiayue Ding: study design, acquisition of data, drafting the manuscript, and analysis and interpretation of data. Jingyuan Ya, Liqun Pan, Chaobo Bai, Jingwei Guan, Zhongao Wang and Kexin Jin: study design, acquisition of data and preparation of tables and figures. Qi Yang and Xunming Ji: study conception and design, analysis and interpretation of data, and critical revision of the manuscript. Ran Meng: acquisition of study funding, study conception and design, analysis and interpretation of data, and critical revision of the manuscript.
CONFLICTS OF INTEREST: Dr. Xunming Ji is one of the inventors of the electric auto-control device, which has been patented in China (ZL200820123637.X). All other authors report no disclosures relevant to the manuscript.
FUNDING: This study was partially supported by the National Key R&D Program (2017YFC1308401), the National Natural Science Foundation (81371289), and the Project of Beijing Municipal Top Talent of Healthy Work (2014–2–015) of China. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
REFERENCES
- 1.Maniega SM, Valdés Hernández MC, Clayden JD, Royle NA, Murray C, Morris Z, Aribisala BS, Gow AJ, Starr JM, Bastin ME, Deary IJ, Wardlaw JM. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015; 36:909–18. 10.1016/j.neurobiolaging.2014.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015; 4:001140. 10.1161/JAHA.114.001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol. 2011; 32:2103–09. 10.3174/ajnr.A2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev. 2017; 34:64–76. 10.1016/j.arr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinter D, Ritchie SJ, Gattringer T, Bastin ME, Hernández MD, Corley J, Maniega SM, Pattie A, Dickie DA, Gow AJ, Starr JM, Deary IJ, Enzinger C, et al. Predictors of gait speed and its change over three years in community-dwelling older people. Aging (Albany NY). 2018; 10:144–53. 10.18632/aging.101365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010; 341:c3666. 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam KW, Kwon HM, Jeong HY, Park JH, Kim SH, Jeong SM, Yoo TG, Kim S. Cerebral white matter hyperintensity is associated with intracranial atherosclerosis in a healthy population. Atherosclerosis. 2017; 265:179–83. 10.1016/j.atherosclerosis.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Kwon HM, Lee J, Kim DS, Ovbiagele B. Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur J Neurol. 2015; 22:44–52. 10.1111/ene.12431 [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Bang OY. Medical Treatment of Intracranial Atherosclerosis: an Update. J Stroke. 2017; 19:261–70. 10.5853/jos.2017.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Meng R. Advances in chronic cerebral circulation insufficiency. CNS Neurosci Ther. 2018; 24:5–17. 10.1111/cns.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chutinet A, Biffi A, Kanakis A, Fitzpatrick KM, Furie KL, Rost NS. Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am J Neuroradiol. 2012; 33:1591–95. 10.3174/ajnr.A3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Kim JS, Lee KS, An JY, Kim W, Kim YI, Kim BS, Jung SL. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. 2008; 8:31. 10.1186/1471-2377-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW, Yang JL, Yip HK. Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens. 2015; 33:1001–13. 10.1097/HJH.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Ding J, Ya J, Pan L, Wang Y, Ji X, Meng R. Remote ischemic conditioning: a promising therapeutic intervention for multi-organ protection. Aging (Albany NY). 2018; 10:1825–55. 10.18632/aging.101527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MB, Hoda MN, Vaibhav K, Giri S, Wang P, Waller JL, Ergul A, Dhandapani KM, Fagan SC, Hess DC. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res. 2015; 6:69–77. 10.1007/s12975-014-0374-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Ren C, Li S, Han R, Gao J, Huang Q, Jin K, Luo Y, Ji X. Limb Remote Ischemic Conditioning Promotes Myelination by Upregulating PTEN/Akt/mTOR Signaling Activities after Chronic Cerebral Hypoperfusion. Aging Dis. 2017; 8:392–401. 10.14336/AD.2016.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan MB, Hafez S, Hoda MN, Baban B, Wagner J, Awad ME, Sangabathula H, Haigh S, Elsalanty M, Waller JL, Hess DC. Chronic Remote Ischemic Conditioning Is Cerebroprotective and Induces Vascular Remodeling in a VCID Model. Transl Stroke Res. 2018; 9:51–63. 10.1007/s12975-017-0555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Meng R, Song H, Liu G, Hua Y, Cui D, Zheng L, Feng W, Liebeskind DS, Fisher M, Ji X. Remote Ischemic Conditioning May Improve Outcomes of Patients With Cerebral Small-Vessel Disease. Stroke. 2017; 48:3064–72. 10.1161/STROKEAHA.117.017691 [DOI] [PubMed] [Google Scholar]
- 19.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012; 79:1853–61. 10.1212/WNL.0b013e318271f76a [DOI] [PubMed] [Google Scholar]
- 20.Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics. 2015; 12:667–77. 10.1007/s13311-015-0358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Kim JS, Chung SW, Kim BS, Ahn KJ, Lee KS. White matter hyperintensities (WMH) are associated with intracranial atherosclerosis rather than extracranial atherosclerosis. Arch Gerontol Geriatr. 2011; 53:e129–32. 10.1016/j.archger.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 22.Tiehuis AM, Vincken KL, Mali WP, Kappelle LJ, Anbeek P, Algra A, Biessels GJ. Automated and visual scoring methods of cerebral white matter hyperintensities: relation with age and cognitive function. Cerebrovasc Dis. 2008; 25:59–66. 10.1159/000111500 [DOI] [PubMed] [Google Scholar]
- 23.Valdés Hernández MC, Morris Z, Dickie DA, Royle NA, Muñoz Maniega S, Aribisala BS, Bastin ME, Deary IJ, Wardlaw JM. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology. 2013; 40:13–22. 10.1159/000341859 [DOI] [PubMed] [Google Scholar]
- 24.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009; 73:1738–45. 10.1212/WNL.0b013e3181c34b47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010; 41:1290–93. 10.1161/STROKEAHA.110.579888 [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Sharma VK, Chan BP, Venketasubramanian N, Teoh HL, Seet RC, Tanicala S, Chan YH, Chen C. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010; 299:15–18. 10.1016/j.jns.2010.08.051 [DOI] [PubMed] [Google Scholar]
- 27.Langen CD, Cremers LG, de Groot M, White T, Ikram MA, Niessen WJ, Vernooij MW. Disconnection due to white matter hyperintensities is associated with lower cognitive scores. Neuroimage. 2018; 183:745–56. 10.1016/j.neuroimage.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 28.Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, Schmidt R. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002. (Suppl 2); 13:31–36. 10.1159/000049147 [DOI] [PubMed] [Google Scholar]
- 29.Scheltens P, Erkinjunti T, Leys D, Wahlund LO, Inzitari D, del Ser T, Pasquier F, Barkhof F, Mäntylä R, Bowler J, Wallin A, Ghika J, Fazekas F, Pantoni L. White matter changes on CT and MRI: an overview of visual rating scales. European Task Force on Age-Related White Matter Changes. Eur Neurol. 1998; 39:80–89. 10.1159/000007921 [DOI] [PubMed] [Google Scholar]
- 30.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993; 269:2386–91. 10.1001/jama.1993.03500180078038 [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012; 12:156. 10.1186/1471-244X-12-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available to any qualified investigator for the purpose of reproducing the results or replicating the procedure.