Abstract
Toll-like receptors (TLRs) under diabetic conditions trigger inflammation and impair immunity. In the present study, we looked at the expression of TLRs (2 and 4) and their adaptors in Normal Glucose Tolerant (NGT), Newly Diagnosed Type-2 Diabetic (NDD) and Known Type-2 Diabetic (KDM) subjects. We also estimated TLR induced cytokine secretion, cellular activation and apoptosis. Surface expression of TLR2 and 4 was significantly reduced in the B cells of the NDD subjects and was associated with decreased cellular activation and cytokine secretion (TNF-α and IL-6). This impairment was not due to B cell deficiency or apoptosis or immunosuppressive cytokine (IL-10 and TGF-β) secretion. However, the upregulation of immunomodulatory enzymes (Arg-1, HO-1 and IDO) could probably account for the reduced TLR expression. The defective TLR signalling was largely ameliorated in the KDM group which might be due to the use the anti-diabetic drugs which have anti-inflammatory effect.
Keywords: TLR2, TLR4, TNF-α, IL-6, CD69
1. Introduction
Diabetic patients are known to be immunocompromised and have an increased incidence of intestinal, respiratory and genitourinary tract infections indicating severe immune impairment. Toll-like receptors (TLRs) are innate immune receptors which recognise pathogen associated molecular patterns (PAMPs), present in the pathogens and damage associated molecular patterns (DAMPs), present in the host [1]. Out of 10 TLR identified in humans, TLR2 and 4 were found to be strongly associated with diabetes [1–3]. While TLR2 utilizes the Mal-MyD88 pathway, TLR4 utilizes both Mal-MyD88 and TRIF-TRAM pathways resulting in – (a) Cytokine/chemokine secretion, (b) cellular activation and (c) activation driven apoptosis/proliferation [4]. Recently, some of the immunomodulatory effector enzymes have emerged as negative-regulators controlling the expression/activity of the TLRs, forming a negative feed-back loop [5–7]. In the present study,we looked at the expression and activity of TLR2 and 4 in peripheral blood monocytes as well as B cells in the whole blood assay in type-2 diabetic subjects. Further, the expression/activity of TLRs in newly diagnosed versus long standing diabetes was compared.
2. Material and methods
2.1. Study subjects
Study subjects were recruited from the outpatients visiting Dr. Mohan’s Diabetes Specialities Centre, Chennai, India. Institutional ethical approval was obtained from the Madras Diabetes Research Foundation Ethics Committee (Ref No-MDRF-EC/SOC/2009//05) and written informed consent was obtained from all participants and the work was carried out in accordance with the Declaration of Helsinki. The following groups were included in the study: 1. Control subjects with Normal Glucose Tolerance (NGT) (n = 42); 2. Subjects with newly diagnosed Type-2 diabetes and not under anti-diabetic medication (NDD) (n = 34) and 3. Subjects with known Type-2 diabetes and under medication (KDM) (n = 53). All the KDM subjects were on oral hypoglycemic drugs and or insulin (Table 1). The in vitro cytokine analysis was done on all the subjects, while all other experiments were done on 13 NGT, 14 NDD and 15 KDM subjects who were randomly selected from each group. The diagnosis was done following WHO guidelines as NGT (fasting plasma glucose <100 mg/dl or 2 h post glucose value <140 mg/dl) and diabetes (fasting ≥126 mg/dl or 2 h PG value ≥200 mg/dl) based on the Oral Glucose Tolerance Test (OGTT) [8].
Table 1.
Clinical and biochemical characteristics of the study subjects.
| Parameters | NGT (n = 42) | NDD (n = 34) | KD (n = 53) | |
|---|---|---|---|---|
| Age (Years) | 41.3 ± 12.6 | 51.7 ± 11.6 | 47.1 ± 15.2 | |
| Body mass index (kg/m2) | 23.4 ± 4.08 | 25.9 ± 3.5a** | 24.2 ± 2.6 | |
| Systolic BP (mm Hg) | 111 ± 15 | 138 ± 21 | 138 ± 21 | |
| Diastolic BP (mm Hg) | 71 ± 10 | 78 ± 8 | 80 ± 9 | |
| Fasting blood sugar (mg/dL) | 100 ± 11 | 134 ± 33a*** | 144 ± 37b*** | |
| Post prandial blood sugar (mg/dL) | 114 ± 22 | 236 ± 80a*** | 216 ± 86b** | |
| Glycated hemoglobin (%) | 5.5 ± 0.4 (37 mmol/mol) | 7.5 ± 1.6 (58 mmol/mol)a*** | 7.8 ± 1.2 (62 mmol/mol)b*** | |
| Total serum cholesterol (mg/dL) | 167 ± 43 | 191 ± 40 | 154 ± 32 | |
| Serum triglycerides (mg/dL) | 121 ± 40 | 156 ± 90 | 134 ± 55 | |
| HDL-cholesterol (mg/dL) | 40 ± 8 | 44 ± 12 | 38 ± 11 | |
| LDL-cholesterol (mg/dL) | 103 ± 37 | 114 ± 37 | 90 ± 28 | |
| Microalbuminuria (mg/dL) | 12.3 ± 9.1 | 14.3 ± 10 | 15.8 ± 19.3 | |
| Anti-diabetic Medicationsc | Insulin | – | – | 35% |
| Metformin | – | – | 41% | |
| Thiazolidinedione | – | – | 18% | |
| Sulfonylurea | – | – | 41% | |
| Meglitinide | – | – | 18% | |
| Alpha glucosidase inhibitor | – | – | 18% | |
| Mecobalamin | – | – | 18% |
For all the parameters mean ± SD is reported.
Comparison between NGT and NDD.
Comparison between NGT and KD.
Most patients were under the combination of insulin with one or more oral hypoglycemic drugs
P < 0.01.
P < 0.0001.
2.2. Inclusion and exclusion criteria
The inclusion criteria were patients within the normal range of white blood cells. The exclusion criteria were patients with type-1 diabetes and patients with a previous diagnosis of urolithiasis, liver cirrhosis, congestive heart failure, chronic lung diseases, chronic infections or viral hepatitis.
2.3. Biochemical parameters
Blood sugar, cholesterol, triglycerides, LDL, HDL, urea, creatinine and urinary albumin were measured using a Hitachi-912 Autoanalyser (Hitachi, Mannheim, Germany). Glycated hemoglobin (HbA1c) was estimated by high pressure liquid chromatography (Bio-Rad, Hercules, CA). The intra- and inter assay coefficient of variation for the biochemical assays ranged between 3.1% and 5.6%.
2.4. Peripheral blood leukocyte cultures
Whole blood was collected in EDTA coated tubes from the study subjects. 1 ml of blood was stored in Tempus™ Blood RNA Tubes (Lifetechnologies, US) for ex vivo gene expression analysis. 1 ml of blood was processed and fixed with 4% paraformaldehyde (PFA) and stored at −80 °C for Florescent Activated Cell Sorting (FACS) analysis. The remaining blood was centrifuged and the plasma was separated and stored at −80 °C. The packed cell volume was diluted with RPMI medium (1:1 ratio) containing 10% FCS and was used for in vitro culture. Cells were stimulated with TLR2 ligand-PAM3 (100 ng/ml) (InvivoGen, US) or TLR4 ligand-LPS (100 ng/ml) (InvivoGen, US) or were left unstimulated for 24 h in parallel cultures. The supernatants were harvested from all cultures and were stored at −80 °C for cytokine estimation. One set of cell pellets were processed and fixed with 4% PFA (for FACS analysis) while another set was solubilized in RNAzol (for qRT-PCR analysis). All samples were stored at −80 °C till the analysis.
2.5. Gene expression analysis
RNA extraction was performed from stored samples using RNeasy Mini Kit (Qiagen). Real-time PCR was performed using TaqMan probes (Appliedbiosystems, US) specific for MyD88, TRIF, Mal and TRAM. RNA extracted from TLR stimulated cultures were used for estimating arginase (Arg)-1, heamoxygenase (HO)-1, cycloxygenase (Cox)-2 and indoleamine 2,3-dioxygenase (IDO). 18S RNA was used as an internal control.
2.6. Fluorescent-activated cell sorting (FACS)
Fixed and stored cells were permeabilized with saponin (0.01%) and stained with flurochrome conjugated monoclonal antibodies specific for CD3, CD14, CD19, CD11c, CD123 and HLA-DR, TLR2, TLR4, TNF-α, IL-6, CD69 and activated CASP3 and were analyzed on a FACS Canto (BD Biosciences). A detailed description on gating and analysis for both TLR surface stating (ex vivo study) and TNF-α, IL-6, CD69 and activated CASP3 (in vitro study) is provided as supplementary materials and methods.
2.7. Estimation of cytokines by ELISA
The levels of cytokines (TNF-α, IL-6, IL-1β and IL-10) in the cell supernatant were quantified using ELISA following the Manufacturer’s instructions (Invitrogen, US). The lowest detection limits of TNF-α, IL-6, IL-1β and IL-10 is 0.97 pg/ml, 0.97 pg/ml, 1.95 pg/ml and 1.95 pg/ml respectively. The CV was found to be <10%.
2.8. Statistical analysis
Student t-test was used to compare groups for continuous variables, whereas χ2 test or Fisher exact test (as appropriate) was used to compare proportions. Kruskal–Wallis test was used for multiple parameters that did not show normal distribution. Multiple comparisons were corrected using the Holm’s correction. All the analyses were done using GraphPad Prism version 5.0 (GraphPad Software, CA, USA) and SPSS statistical package (Version 20.0; SPSS, Chicago, IL). p Value less than 0.05 was considered significant.
3. Results
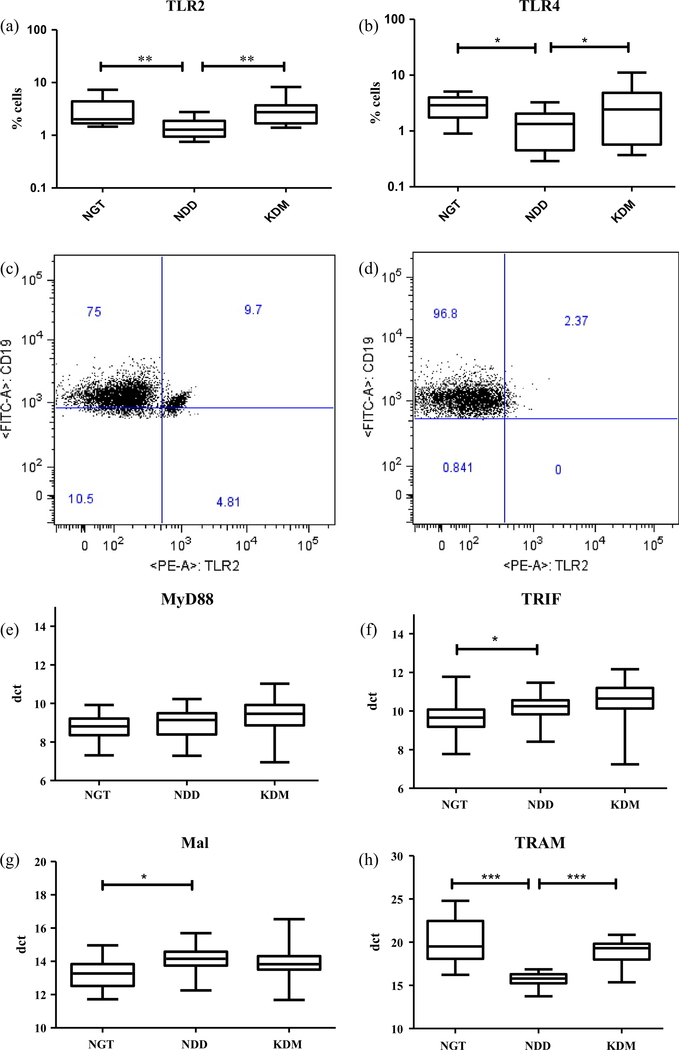
Table 1 shows the clinical and biochemical characteristics of the study subjects. The NDD group had higher BMI compared to NGT and KDM groups. Both the NDD and KDM groups had significantly higher levels of FBS, PPBS and HbA1c than the NGT group. The blood pressure, blood cholesterol and blood triglyceride levels were not significantly different among the groups. None of the study subjects had microalbuminuria. Next, we studied the expression of both TLR2 and TLR4 in dendritic cells, monocytes and B cells and the expression of TLR adaptor molecules (MyD88, TRIF, Mal and TRAM) in the whole blood (Fig. 1, S. Figs. 1–3). Significantly decreased surface expression of both TLR2 (Fig. 1a, c and d) and TLR4 (Fig. 1b) was seen in the NDD group compared to both NGT and KDM groups. This TLR down regulation was predominantly seen only in the peripheral B cell population and not in monocytes and dendritic cells (DCs) (S. Figs. 1–3). The reduced expression of TLRs in B cells was not due to B cell deficiency in the NDD group (S. Table 1). With respect to the expression of TLR adaptors, MyD88 expression was not altered across the groups (Fig. 1c), TRIF and MAL were significantly up regulated in both NDD and KDM groups (Fig. 1d and e) while TRAM was significantly downregulated only in the NDD group (Fig. 1f).
Fig. 1.
Newly diagnosed DM is characterized by decreased surface expression of TLR2 and 4 and deranged expression of TLR adaptors in peripheral blood leukocytes (PBL) as determined by flowcytometry and qRT-PCR respectively. Box and whisker plots showing the levels of expression of TLR2 (a) and TLR4 (b) in NGT/control (n = 13), NDD (n = 14) and KDM (n = 15) subjects. Representative dot plot showing the expression of TLR2+ B cells in NGT (c) and NDD (d) subjects. Box and whisker plots showing the levels of expression of MyD88 (c), TRIF (d), Mal (e) and TRAM (f) in NGT/control, NDD and KDM subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. NGT – normal glucose tolerance, NDD – newly diagnosed diabetic, KDM – known diabetic subjects.
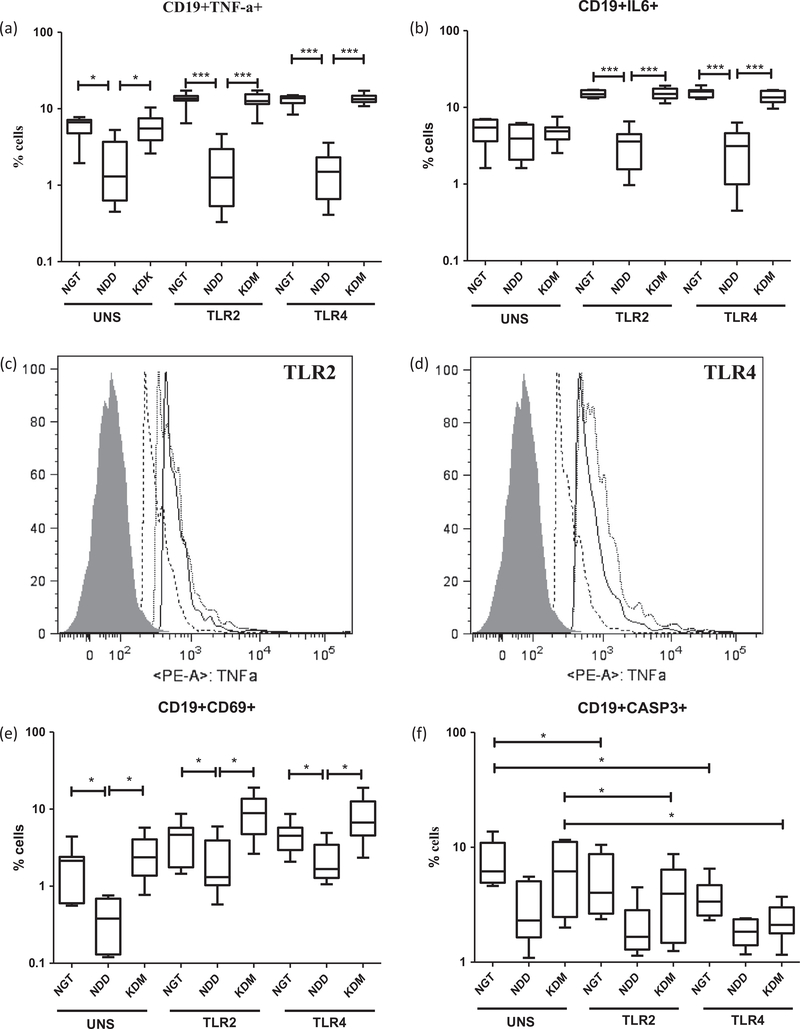
We next studied the induction of both pro-(TNF-α, IL-6 and IL-1β) and anti-(IL-10 and TGF-β) inflammatory cytokine secretion, following TLR stimulation (Fig. 2). Decreased levels of both proand anti-inflammatory cytokines were observed in the NDD group following TLR2 and TLR4 stimulation (Fig. 2a–d). This downregulation was restricted only to the NDD group and was not seen in the KDM group. TLR induced TGF-β secretion could not be detected in the culture supernatant. Combined immunophenotyping with intracellular cytokine staining, identified peripheral B cells (and not the monocytes) as the major cellular source of impaired cytokine secretion (Fig. 3a–d and S. Fig. 4). Intracellular cytokine staining to identify TNF-α and IL-6 secreting DCs in the whole blood culture was not successful due to reduced DC count in the peripheral blood. The reduced expression of cytokines (TNF-α and IL-6) in B cells was associated with diminished cellular activation (Fig. 3e) but not apoptosis (Fig. 3f). In fact, TLR stimulated B cells expressed significantly reduced levels of activated caspase-3 compared to unstimulated cells (Fig. 3d). In contrast to B cells, TLR induced expression of TNF-α and IL-6 in monocytes was unaffected among the study groups (S. Fig. 4a and b). As expected CD69 expression was not upregulated in monocytes following TLR stimulation since it is a lymphocyte activation marker (S. Fig. 4c). Even in monocytes, as like B cells, TLR stimulation significantly reduced the expression of activated caspase-3 (S. Fig. 4d).
Fig. 2.
Newly diagnosed DM is characterized by decreased TLR induced secretion of pro and anti-inflammatory cytokines by peripheral blood leukocytes (PBLs) as determined by ELISA. Box and whisker plots showing the levels of TNF-α (a), IL-6 (b), IL-1β (c) and IL-10 (d) in the supernatants of PBL cultures following TLR2 and 4 stimulation in NGT/control (n = 42), NDD (n = 34) and KDM (n = 53) subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. NGT – normal glucose tolerance, NDD – newly diagnosed diabetic, KDM – known diabetic subjects.
Fig. 3.
Newly diagnosed DM is characterized by decreased expression of IL-6, TNF-α and CD69 in B cells following TLR stimulation as determined by flowcytometry. Box and whisker plots showing the % of B cells expressing TNF-α (a) and IL-6 (b) in NGT/control (n = 13), NDD (n = 14) and KDM (n = 15) subjects are shown. Histogram analysis showing the downregulation of TNF-α in TLR2 (c) and TLR4 (d) stimulated B cells (shaded area-Isotype control; dotted line-NDD; grey line-NGT and black line-KDM. Box and whisker plots showing the % of B cells expressing CD69 (e) and activated caspase-3 (f) in NGT/control, NDD and KDM subjects are shown. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. NGT – normal glucose tolerance, NDD – newly diagnosed diabetic, KDM – known diabetic subjects.
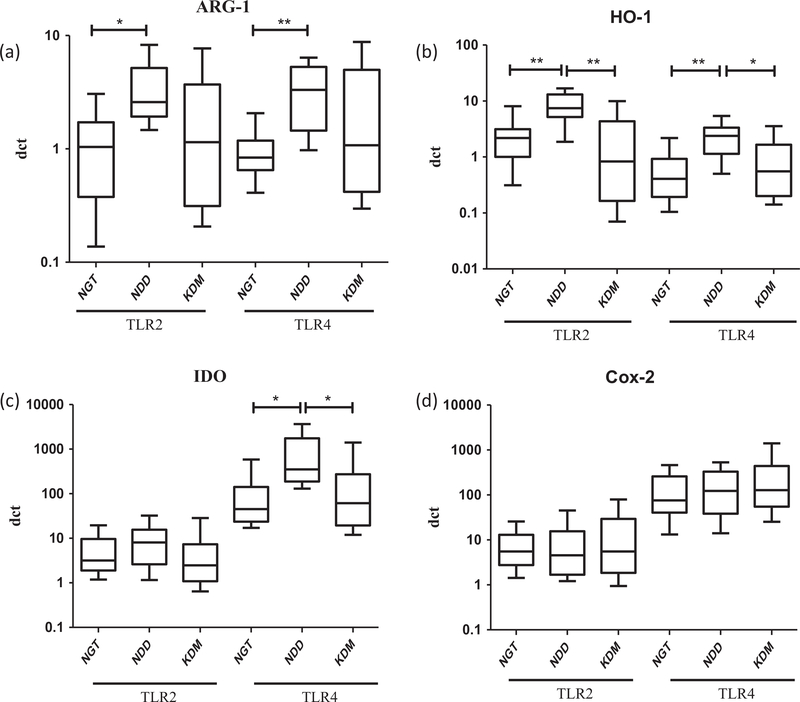
Finally, we evaluated the expression of TLR induced effector enzymes (Fig. 4). Arg-1 was specifically induced by both TLR2 and 4 only in the NDD group (Fig. 4a). TLR2 induced upregulation of HO-1 and TLR4 induced upregulation of HO-1 and IDO were seen only in the NDD group (Fig. 4b and c). TLR4 induced Cox-2 expression was not altered across the groups (Fig. 4d).
Fig. 4.
Newly diagnosed DM is characterized by increased expression of TLR induced immunomodulatory enzymes as determined by qRT-PCR. Box and whisker plots showing the fold change expression of Arg-1 (a), HO-1 (b), IDO (c) and Cox-2 (d) in NGT/control (n = 13), NDD (n = 14) and KDM (n = 15) subjects are shown. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. NGT – normal glucose tolerance, NDD – newly diagnose ddiabetic, KDM – known diabetic subjects.
4. Discussion
TLRs serve as sentinels providing first line defence against invading microbes [4]; but under diabetic conditions they might trigger inflammation and impair immunity [4]. Few studies have previously addressed the involvement of TLRs in diabetes associated inflammation and have yielded contradictory results [9–11]. Further, till date to the best of our knowledge, no study has been done to compare the effect of newly diagnosed versus chronic diabetes on TLR signalling. It is well known that many anti-diabetic drugs have anti-inflammatory effect and vice versa [12,13]. Thus, in the present study, we aimed at comparing the expression and activity of TLRs in newly diagnosed versus long standing diabetes. We hypothesized that with anti-diabetic treatment, the magnitude of inflammation seen in newly diagnosed subjects might subside. The major findings of this study are: 1. Decreased surface expression of TLR2 and 4 in the peripheral B cells (but not monocytes and DCs) of NDD subjects, 2. The decreased TLR expression was associated with impaired cellular activation and cytokine secretion in B cells and 3. Increased expression of immunomodulatory enzymes (Arg-1, HO-1 and IDO) was associated with downregulation of TLRs.
Few studies have previously reported significant upregulation of TLRs in monocytes [9] as well as in B cells [10]. However, in the present study significant downregulation of TLRs was seen in the NDD group. Monocytes, B cells and DCs are the primary immune cells which predominantly express TLRs and also respond to it [4]. Our results indicate significant downregulation of TLRs in B cells, leaving the monocytes and DCs largely unaffected. In accordance with the TLR profile, TLR induced secretion of TNF-α, IL-6, IL-1β and IL-10 were significantly downregulated, in the NDD group. The decreased secretion of the pro-inflamatory cytokines was not mediated by the anti-inflammatory cytokines IL-10 or TGF-β. Imuunophenotyping identified B cells as the primary cells being affected. Previously, Komura et al., had reported significant hyporesponsivemess of monocytes to TLR stimuli in NDD subjects which was largely attributed to ER stress [11]. However, in the present study the hyporesponsiveness seems largely due to TLR downregulation even though the involvement of ER stress cannot be ruled out. The reduced cytokine secretion in B cells was not due to apoptosis but was due to reduced cellular activation. The exact reason for the downregulation of TLRs in the NDD group is currently not known. However the upregulation of immunosuppresive enzymes like arginase-1, HO-1 and IDO in the NDD group could at least partially account for this TLR downregulation.
5. Conclusion
Taken together, our data indicates a defective TLR signalling in B cells in type-2 diabetes. While some of these defects are being reported for the first time (TLR adaptor expression), others (TLR expression and cytokine secretion) are different from what has been previously reported (9–11). The exact reason for this apparent disparity between our report and previous report is currently not known, even though both ethnic (genetic) and environmental factors (intestinal microbiota, infection load, etc) could probably play a role. The other reason could be the difference in the experimental protocol used- while we used whole blood cultures, the other groups have used purified cell types [9–11]. Most of the defects which were seen in TLR signalling in the NDD group were largely ameliorated in the KDM group which might be due to the anti-inflammatory effect of the anti-diabetic drugs [12,13]. It is important to note that at least 35% and 40% of the KDM subjects were on insulin and metformin respectively; both of which have been shown to have strong immunomodulatory effect [14,15]. However, not all oral hypoglycemic drugs have immunomodulatory effect. In most in vitro experiments high glucose was shown to upregulate TLR expression/activity which was downregulated following treatment with insulin, metformin and/or sulfonylurea [14,15]. However, in the present study, at least under in vivo condition, diabetes was shown to suppress TLR expression/activity while the anti-diabetic treatment was shown to rescue this phenomenon making the relevance of these in vitro models questionable. The major limitation of our study is its cross-sectional design, which means that no direct cause and effect relationship can be drawn. Further, in vitro treatment of B cells isolated from NDD subjects with insulin or metformin showed no effect on TLR expression/activity indicating epigenetic modifications, which is beyond the scope of this paper to explore. Nevertheless, this study gains importance in that it provides a complete picture of TLR signalling in a high risk ethnic population and identifies few defects which were previously not known in this population.
Supplementary Material
Acknowledgements
Dr. Vivekanandhan Aravindhan is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The project was funded by Fast Track Scheme for Young Scientists, Department of Science & Technology, New Delhi (SR/FT/LS-105/2009).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cyto.2015.04.010.
References
- [1].Rosa Ramirez S, Ravi Krishna Dasu M. Toll-like receptors and diabetes complications: recent advances. Curr Diabetes Rev 2012;8:480–8. [DOI] [PubMed] [Google Scholar]
- [2].Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the proinflammatory state of diabetes. Cytokine 2011;55:441–5. [DOI] [PubMed] [Google Scholar]
- [3].Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol 2011;31: 1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4: 499–511. [DOI] [PubMed] [Google Scholar]
- [5].Riquelme SA, Bueno SM, Kalergis AM. Carbon monoxide down-modulates TLR4/MD2 expression on innate immune cells and reduces endotoxic shock susceptibility. Immunology 2015;144:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol 2009;183:6303–12. [DOI] [PubMed] [Google Scholar]
- [7].Morris SM Jr. Arginine: master and commander in innate immune responses. Sci Signal 2010;3(135):pe27. [DOI] [PubMed] [Google Scholar]
- [8].World Health O. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: World Health Organization; 2006. p. 1–50. [Google Scholar]
- [9].Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010;33:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 2010;53:1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Komura T, Sakai Y, Honda M, Takamura T, Matsushima K, Kaneko S. CD14+ monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes 2010;59:634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dasu MR, Park S, Devaraj S, Jialal I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 2009;150:3457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Netea MG, Tack CJ, Netten PM, Lutterman JA, Van der Meer JW. The effect of salicylates on insulin sensitivity. J Clin Invest 2001;108: 1723–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tilich M Modulation of toll-like receptors by insulin. Am J Ther 2011;18: e130–7. [DOI] [PubMed] [Google Scholar]
- [15].Soraya H, Farajnia S, Khani S, Rameshrad M, Khorrami A, Banani A, et al. Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: are AMPK and TLRs connected? Int Immunopharmacol 2012;14:785–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.