Abstract
Obstructive Sleep Apnea (OSA) is a prevalent condition thought to increase in the future. Being mostly undiagnosed, the most serious complications are cardiovascular diseases, among which are arrhythmias. Controversy remains as to whether OSA is a primary etiologic factor for ventricular arrhythmias, because of the high incidence of cardiovascular comorbidities in OSA patients. Howev-er, there is mostly a strong evidence of a relation between OSA and ventricular arrhythmias. A few mechanisms have been proposed to be responsible for this association and some electrocardiographic changes have also been demonstrated to be more frequent in OSA patients. Treatment of OSA with Continuous Positive Airway Pressure (CPAP) has the potential to reduce arrhythmias and confer a mortality benefit.
Keywords: Obstructive sleep apnea, ventricular arrhythmias, premature ventricular contractions, ventricular tachycardia, sudden cardiac death, continuous positive airway pressure
1. INTRODUCTION
Searching for Obstructive Sleep Apnea (OSA) in patients presenting with Atrial Fibrillation (AF) is a common and recommended practice [1] due to the strong evidence of an association between these two entities [2-6]. While most studies have focused on the links between OSA and AF, associations with Ventricular Arrhythmias (VA) have also been characterized, although pooling and meta-analysis of studies have not been possible due to the heterogeneity of data [7]. A few mechanisms have been proposed to be responsible for this association and some electrocardiographic changes have also been demonstrated to be more frequent in OSA patients.
2. OBSTRUCTIVE SLEEP APNEA
OSA is a highly prevalent disease, affecting 4% of men and 2% of women [8]. Due to its association with obesity, the prevalence of which is rising, OSA will represent an escalating public health burden.
OSA is characterized by repetitive upper airway collapses during sleep resulting in intermittent hypoxia and hypercapnia, sleep fragmentation and repetitive intrathoracic pressure changes due to increased respiratory efforts against occluded upper airway. The mechanism by which the upper airway collapses is not fully understood but is multifactorial and includes obesity, craniofacial changes, alteration in upper airway muscle function, pharyngeal neuropathy and fluid shift towards the neck [9].
A detailed medical history (including the patient’s partner as he or she can provide important information about what occurs during the night) and clinical examination are important in the evaluation of patients suspected of having OSA. Some predictive information can be obtained from self-reported questionnaires intended to measure daytime sleepiness [Epworth Sleepiness Scale (ESS) [10], the Berlin Questionnaire [11] or Stop-Bang Questionnaire [12]].
Polysomnography (PSG) is the preferred method to diagnose OSA. Apnea is defined as a cessation of airflow for >10 seconds, while hypopnea is a reduction in but not complete cessation of airflow to <50% of normal, usually in association with a reduction in oxyhemoglobin saturation. The most commonly used index to diagnose OSA and define its severity is the Apnea/Hypopnea Index (AHI), calculated as the number of obstructive events per hour of sleep. A diagnosis is made if there are more than five predominantly obstructive respiratory AHI in a symptomatic patient [13]. Symptoms and clinical signals include excessive daytime sleepiness, non-restorative sleep, fatigue or insomnia; waking up with choking, breath holding or gasping; headaches in the morning, witnessed habitual snoring and/or breathing interruptions; and hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive Heart Failure (HF), AF or type 2 diabetes mellitus [13]. Importantly, sleep apnea discovered in a sleep recording without any symptoms is usually not considered to be OSA, except if the AHI is >15 [14]. In this review, otherwise indicated, we use the following cutoffs for graduating OSA: mild (5 < AHI < 15), moderate (15 < AHI < 30) and severe (AHI ≥ 30) OSA.
Continuous Positive Airway Pressure (CPAP) is the primary treatment modality in patients with moderate to severe OSA. It is a treatment that uses mild air pressure to keep the airways open. The beneficial effect of CPAP on symptoms and quality of life is obtained after only a few days of treatment [15, 16] but it depends on adherence [17, 18], Which is not easy to achieve.
3. CARDIAC ARRHYTHMIAS IN OSA PATIENTS
Cardiac arrhythmias are reportedly in 30%-60% of patients with OSA and include sinus arrest and second-degree atrioventricular conduction block, AF and flutter, atrial and ventricular extrasystoles, nonsustained and sustained Ventricular Tachycardia (VT) and even Sudden Cardiac Death (SCD) [19-27]. The higher the number of AHI and severity of the associated hypoxemia, the higher the prevalence of such arrhythmias [28-33].
VA, primarily premature ventricular contractions (PVCs), have been reported in up to two-thirds of patients with OSA, which is significantly higher than the rates reported in persons without OSA (0% to 12%) [20, 21]. In most OSA patients, VA appear most often during sleep, with the greatest frequency occurring during apneic periods [29, 34, 35]. This is opposed to the pattern of VA distribution in individuals without OSA. Furthermore, VA (particularly PVCs) occur more frequently during the apneic phases than during hyperpnea in OSA patients, which is in contrast to those patients with Central Sleep Apnea (CSA), in whom ventricular ectopy was noted to occur more frequently during hyperpneas than apneas [36].
4. MECHANISMS OF OSA-INDUCTED VENTRICULAR ARRHYTHMIAS
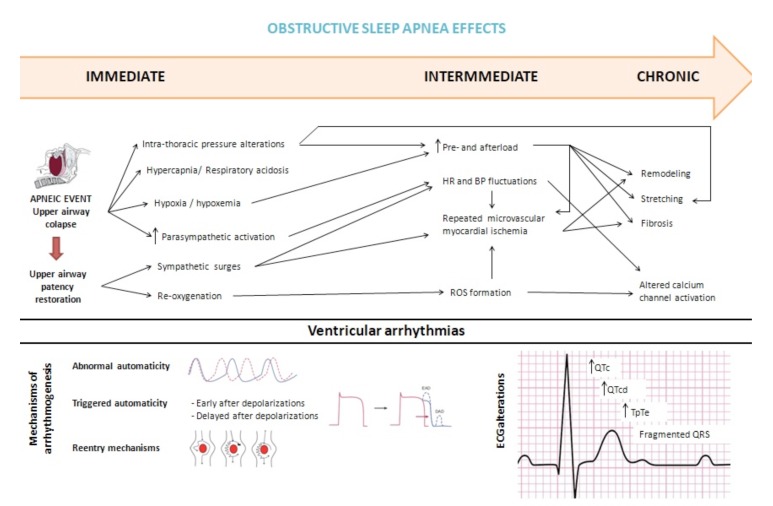
OSA itself causes a complex impairment of myocardium through multifactorial mechanisms. Key findings implicate OSA-related autonomic nervous system fluctuations typified by enhanced parasympathetic activation during and sympathetic surges subsequent to respiratory events, which contribute to augmented arrhythmic propensity. Other more immediate pathophysiologic influences of OSA enhancing arrhythmogenesis include intermittent hypoxia and hypercapnia/acidemia, sleep fragmentation and intrathoracic pressure swings leading to myocardial stretch. Intermediate pathways by which OSA may trigger arrhythmia include increased systemic inflammation, oxidative stress, enhanced pro-thrombotic state and vascular dysfunction [37]. These mechanisms lead to ventricular hypertrophy and dysfunction at the organ level, presented at the tissue and cellular levels as multifocal infarcts, myocyte hypertrophy and apoptosis and inflammatory infiltrations [38]. Long-term OSA sequelae such as hypertension, ventricular hypertrophy, fibrosis and coronary artery disease also predispose to cardiac arrhythmia [37].
4.1. Sympathovagal Imbalance
In OSA patients, there are sequential autonomic alterations which lead to enhanced arrhythmia susceptibility. First, enhanced vagal efferent outflow to the heart leads to the bradycardia observed during the apneic event (i.e. the diving reflex due to increased respiratory efforts of progressive magnitude to achieve restoration of airway patency). After upper airway patency restoration, strong sympathetic nervous system responses are elicited secondary to the interacting effects of central respiratory sympathetic coupling, hypoxia, hypercapnia and absence of sympatho-inhibition from normal lung initiation reflexes [37, 39]. Local cardiac stretch reflexes and baroreflexes might also play a role [39].
4.2. Hypoxia and Hypoxemia
Apneas and hypopneas impair gas exchange resulting in hypoxia and hypoxemia. Hypoxemia directly stimulates chemoreceptors in the carotid body, precipitating increased ventilation and sympathetic discharges [40-42]. In addition, hypoxia leads to peripheral vasoconstriction which increases both preload and afterload, alters ventricular repolarization and increases expression of Left Ventricle (LV) endocardial calcium channels [43].
Furthermore, re-oxygenation after termination of upper airway obstruction may lead to the formation of hazardous Reactive Oxygen Species (ROS). ROS generation has been linked with arrhythmogenesis, as a result of changes in calcium channel activity and by the promotion of microvascular ischemia [44, 45].
4.3. Intrathoracic Pressure Alterations
In healthy individuals, intrathoracic pressure during inspiration is normally about -8 cmH2O. In patients with OSA, upper airway occlusion generates negative intrathoracic pressures of less than -30 cmH2O. These large pressure shifts during obstructive apneas increase venous return to the right heart and LV afterload, decrease LV compliance and increase cardiac wall stress [46, 47], which appear to be sufficient to cause ventricular remodeling [37]. Supporting this hypothesis, healthy subjects who performed the Müller maneuver (inspiration against an occluded mouthpiece) simulating increased intrathoracic pressures were found to have an acute increase in LV afterload [48]. In animal experiments, intrathoracic pressure swings during obstructive apneas contribute to changes in ventricular repolarization, which are not observed with central apneas and are mainly driven by sympathetic activation [49].
4.4. Myocardial Ischemia
The combination of hypoxemia, increased Heart Rate (HR) and Blood Pressure (BP) and increased LV afterload - due to augmented sympathetic nerve activity and increased intra-thoracic pressure - leads to an imbalance between increased myocardial oxygen consumption and decreased oxygen supply. All could lead to myocardial ischemia, which could predispose to VA and SCD.
4.5. Changes in Cardiac Structure
The repetitive fluctuations in HR, BP and intrathoracic pressure during sleep and increased sympathetic nerve activity during sleep and wakefulness, lead to remodeling of the ventricles over time, which then could lead to right and left ventricular hypertrophy and subsequently to systolic and diastolic dysfunction. Also, the repetitive ischemic insults may promote ventricular fibrosis [50]. Indeed, mechanical effects of OSA promote cardiac stretching and can elucidate a mechano-electrical mechanism responsible to predispose to arrhythmias [51].
Due to the pathological abnormalities occurring in OSA, several mechanisms of arrhythmogenesis can be implicated:
Abnormal automaticity involves spontaneous cardiac impulse formation and may arise due to hypoxemia and respiratory acidosis accompanying apneic events [52].
Reentry mechanisms may occur through the vagal stimulation that results from respiration against a partially occluded airway.
Triggered automaticity may occur due to enhanced sympathetic nervous system activity associated with a respiratory event–related hypoxemia and arousal [53].
Triggered activity precipitants via early after-depolarizations include hypoxia, acidosis and ventricular hypertrophy.
Delayed after-depolarizations often occur in response to increased catecholamine levels [37].
Indeed, acute and chronic hemodynamic, autonomic, electrical and structural myocardial changes can all contribute to cardiac arrhythmias in OSA patients (Fig. 1).
Fig. (1).
Proposed mechanisms responsible for enhanced arrhythmogenesis in Obstructive Sleep Apnea (OSA) patients. BP: Blood Pressure. DAD: Delayed After-Depolarizations. EAD: Early After-Depolarizations. HR: Heart Rate. ROS: Reactive Oxygen Species.
5. ECG PREDICTORS OF VENTRICULAR ARRHYTHMIAS IN OSA PATIENTS
Parameters that can be used to evaluate ventricular repolarization are QT interval (QT), corrected QT interval (QTc), QT dispersion (QTd) and transmural dispersion of repolarization, which can be assessed using the time interval from the peak to the end of the T wave (TpTe interval). TpTe/QT and TpTe/QTc ratios are among the other electrocardiographic indices representing ventricular arrhythmogenic potential. It has been extensively reported these parameters are associated with VA and SCD [23-26, 54-62]. Since increased heterogeneity in ventricular recovery time and repolarization time are correlated with VA, these studies provide a mechanistic basis for OSA as a predisposing factor for VA and SCD [37].
5.1. QT Interval Prolongation
The QT interval represents the electrocardiographic correlate of ventricular de- and repolarization, including the vulnerable period for reentry tachycardia and it is considered a marker of ventricular electrical instability and a risk factor for the occurrence of malignant cardiac arrhythmias and SCD [63].
Previous explained pathophysiologic mechanisms suggest that apneic episodes in OSA are associated with both significant QT prolongation due to increased vagal activity and abrupt QT shortening during post-apnea due to increased sympathetic tone and/or vagal withdrawal [64].
In fact, QTc interval was revealed to show a significant prolongation during the apnea period in patients with mild to severe OSA [64-66]. Several mechanisms responsible for prolongation in action potential duration (thus QTc prolongation) have been proposed. According to Schalatzer et al., the QTc interval increased more at the end and even further after the release of the Mueller maneuver, suggesting enhanced sympathetic tone and also intrathoracic pressure swings and associated acute cardiac volume changes are responsible for QTc prolongation [67]. Hypoxia, hypercapnia and acidemia also play a role in QTc prolongation. Hypoxia has been shown to impact the activity of all of the channels involved in cardiac action potential generation (INa, ICaL, slow component of IKs) [68]. Low pH depolarizes the voltage dependence of INa channel activation and inactivation [69].
In what concerns QTc interval behavior during post-apnea hyperventilation period, conflicting results were published: some authors found an abruptly decrease [64], others demonstrated no differences [70] and others found QTc interval persisted to be prolonged in the post-apnea hyperventilation period [65]. The latter authors proposed increased sympathetic activation and rapid alterations in the intrathoracic pressure during this period as mechanisms by which persistent prolongation of the QTc occurred even in the post-apnea hyperventilation period [65]. Also in daytime ECG, Shamazzuman found a significant increase in QTc [66].
5.2. QT Dispersion
QTd is the difference between the maximum and minimum QT intervals on ECG and reflects inhomogeneity in ventricular repolarization and myocardial electrical instability [71]. An increased corrected QT dispersion (QTcd) > 60 ms is a strong and independent risk factor for cardiac mortality [62].
QTcd was found to be increased in severe OSA patients without hypertension [72] and contrasting with no-OSA patients, it increased during sleep (65.0 ± 14.6 ms) compared with before sleep (57.0 ± 13.5 ms, p < 0.0001) [73]. However, on the contrary, Barta el al found that QTd and QTcd did not increase during the nighttime period [74]. The high value of AHI in the former studies (42.4±17.6/ h [72] and 51.9 ± 18.5/h [73], respectively) can justify this different result (25.8 ±18.5/ h in Barta study [74]). Probably the AHI and the severity of apnea-hypopnea-related hypoxemia had a significant effect on the QTcd during sleep in OSA patients [75, 76]. A correlation between QTcd during sleep and AHI (r=0.38, P=0.009) and the percentage of time that SaO2 was inferior to 90% (r=0.34, p=0.018) was demonstrated by Nakamura et al. Other factors may contribute to QTcd increasing during sleep: CO2 retention/academia [75], change in parasympathetic nerve activity [77, 78] and fluctuating intrathoracic pressure, which induces myocardial wall stress, cardiac distortion and changes in venous return.
5.3. TpTe interval
The interval between the electrocardiographic T-wave peak and end (TpTe) has been studied as a measure of cardiac dispersion and repolarization. The TpTe interval is a measure of cardiac transmural dispersion of repolarization, which is explained by a gradient of action potential duration from endo- (longest) to epicardial cells (shortest) [79, 80]. A prolongation of the TpTe interval leads to increased vulnerability for the occurrence of early afterdepolarizations and has been associated with ventricular tachycardia and an increased risk for SCD [25, 81-83]. Specifically, recent findings from Panikkath et al. suggest that patients with an uncorrected TpTe interval >100 ms in the resting ECG are at increased risk of SCD [25]. Comparisons of OSA patients versus controls identified increased TpTe in those with OSA [43, 70, 84, 85]. Camen et al. investigating the effect of simulated obstructive apnea and hypopnea on arrhythmic potential on a healthy group of individuals, reported a prolongation in QTc and TpTe intervals, suggesting negative intrathoracic pressure changes as a contributory mechanism. Schalatzer et al. found the increase in TpTec was only significant after release of the Mueller manoeuvre, but not during the manoeuvre, suggesting a key contribution of enhanced sympathetic tone and intrathoracic pressure swings [67]. The impact of hypoxia and hypercapnia/acidemia on the transmural refractory behavior needs to be investigated.
In addition to the prolonged QTc and TpTe intervals, an increased TpTe/QT and TpTe/QTc ratios, a measure of disproportional prolongation of global dispersion relative to the QT interval, may have an important role in arrhythmogenesis [86]. These ratios have the advantage of more reliably eliminating the confounding effects of the heart rate variability in the ECG and the inter-individual variation in the length of the QT interval [83]. They are prolonged in patients with moderate and severe OSA patients and correlated with AHI index [70]. They were found to be increased during the apnea period compared to pre-apnea period, and decreased significantly in the post-apnea hyperventilation period [65]. Rossi et al. demonstrated an increase in the TpTe/QT ratio after CPAP withdrawal, further substantiating a potential risk for arrhythmias in this situation [87].
5.4. Fragmented QRS
Not only repolarization was found to be impaired in OSA patients. Fragmented QRS (fQRS) complexes are markers of depolarization abnormality and reflect disordered electrical activation of ventricles through the inhomogeneous substrate and/or localized intramyocardial/ intraventricular conduction blocks [88, 89]. Abnormal impulse conduction creates a milieu for VA through reentry mechanisms and fQRS complexes are a predictor of cardiovascular death in patients with structural heart disease [88, 90]. Recent studies found fQRS in patients with OSA (independently of obesity), suggestive of electrical myocardial remodeling [87, 91, 92]. One of the mechanisms thought to be responsible for fQRS is cellular apoptosis and interstitial fibrosis in cardiac structure, which may be secondary to chronic hypoxia, metabolic abnormalities and oxidative stress [87, 91, 92]. Adar et al. found fQRS is an independent predictor of subclinical LV dysfunction in patients with OSA, suggesting it could identify OSA patients who could be at risk for developing overt cardiac dysfunction. Also, a higher C-reactive Protein (CRP) level in OSA patients with fragmented QRS may suggest that inflammation could also play a role in the alterations of the QRS morphology [92].
6. EVIDENCE-BASED VENRICULAR ARRHYTHMIAS IN OSA PATIENTS
6.1. Premature Ventricular Contractions
The frequency of PVCs normally falls during nonrapid eye movement (NREM) sleep, which comprises approximately 85% of total sleep time. This decline parallels the decrease in sympathetic outflow and the increase in vagal outflow to the heart that accompanies the transition from wakefulness to NREM sleep [93]. Conversely, the frequency of PVCs tends to rise just before and after waking, a time when cardiac sympathetic tone increases [94]. Indeed, it is expectable that PVCs frequency is higher in patients with OSA due to sympathovagal imbalance.
PVCs were demonstrated to occur in a characteristic cyclic fashion that is synchronous with respiratory oscillations [29] and there was a significantly greater frequency of PVCs during apnea than during hyperpnea in OSA patients [36, 95] (contrary to patients with CSA) [95]. This is probably related to the generation of negative intrathoracic pressure and hypoxia, hypercapnia and recurrent arousals, which induce repetitive surges in sympathetic neural outflow, HR and BP. Study of Camen et al. support these mechanisms, since the authors found simulated obstructive apnea and hypopnea in healthy individuals are associated with an increase of PVCs [96].
Abe et al. (2010) found PVCs (Lown IVa, IVb or V) were more frequent when increasing severity of OSA patients: 0 in non-OSA; 0.5% in mild OSA; 3.0% in moderate OSA and 4.2% in severe OSA (p= 0.004) [30]. In sleeping breathing disorders (SBD) patients (including OSA and CSA) similar results were found [21, 28]. Mehra et al. found a significant relationship between SDB and PVCs/h during sleep period (p=0.0003), but only in patients with severe SBD (AHI>30/h) [97]. In another study, AHI was independently associated with an increased prevalence of PVCs not only at night [Odds Ratio (OR) per 1-U increase of log-transformed AHI 1.5, 95% confidence interval (CI) 1.1 to 2.0, p=0.008] but also during the day (OR 1.37, 95% CI 1.0 to 1.8, p=0.035) after adjusting for relevant confounders and even in middle-aged patients with mainly mild or moderate OSA [98]. Although the authors did not provide possible mechanisms responsible for the higher number of PVCs during the day [98], the long-term effects in cardiac structure in OSA patients could be involved in arrhythmogenesis in daytime.
The prevalence of frequent PVCs in OSA patients depends on the cutoff value used to define it. More than 35 years ago, Guilleminault et al. found 19% of patients with SBD had frequent PVCs (>2/min) [20]. More recently, other studies addressed this question. When considering the cutoff ≥5 PVCs/h, PVCs were almost two times more prevalent in subjects with OSA comparing with those with no-OSA during night [98, 99] and also during day [98]. When a cutoff of >30PVCs/h was used, a non-significant higher proportion of patients with OSA had frequent PVCs comparing with non-OSA patients [100]. There are other studies which do not confirm the high frequency of PVCs in OSA patients [21, 85, 99, 101, 102]. Of note, in the majority of these studies [21, 85, 99, 102], the non-OSA/SBD patients had a relatively high prevalence of arrhythmias, which can be explained by a “particular” control group (patients referred for PSG in whom OSA was not confirmed).
6.2. Complex Ventricular Ectopy
Almenneessier et al. found non-isolated PVCs (bi-,tri- and quadrigeminism) in 10.8% of patients with OSA comparing to 2.3% in patients with no-OSA (p=0.04) [99]. Mehra et al. achieved similar results but they studied patients with SDB: almost twice the odds of complex ventricular ectopy [OR 1.74; 95% CI, 1.11–2.74 after adjusting for age, sex, body mass index, and prevalent coronary heart disease] [103]. Tilkian et al. found complex PVCs occurred in 10 of 15 patients with OSA [22].
6.3. Non-sustained Ventricular Tachycardia
Non-sustained VT (NSVT) was defined as three or more consecutive PVCs with duration less than 30 seconds. Only two studies addressed NSVT in OSA patients. According to Abe et al., NSVT were not more frequent in OSA patients: 0 in non-OSA; 1.0% in mild OSA; 1.5% in moderate OSA and 1.3% in severe OSA (p= 0.417) [30]. Aydin et al. found no NSVT in OSA (and non-OSA) patients [100]. In SBD patients, a few studies found a high prevalence of NSVT [28, 104]. Mehra et al. demonstrated that, compared with those without SDB and adjusting for age, sex, body mass index, and prevalent coronary heart disease, individuals with SDB had three times the odds of NSVT (OR 3.40; 95% CI, 1.03–11.20) [97].
All NSVT studied by Guilleminault et al. happened during an apneic event in SBD patients20. Monahan et al. studied SBD patients and cardiac arrhythmias, including NSVT and AF in the same analysis, and they found a direct temporal association between arrhythmia and a preceding respiratory event, enhancing a very probably causal inference. Although the absolute arrhythmia rate is low, the relative risk of NSVT and AF during sleep is markedly increased shortly after a respiratory disturbance (within a 90-second hazard period): the odds of an arrhythmia following a respiratory disturbance were nearly 18-times (OR 17.5; 95% CI 5.3–58.4) the odds of an arrhythmia occurring following normal breathing. The absolute rate of arrhythmia associated with respiratory disturbances was low (1 excess arrhythmia/40000 respiratory disturbances). Only 57 patients with OSA (AHI 5-30/h) and arrhythmias (62 arrhythmias) contributed to these results [104], but it is the first study supporting a direct temporal link between OSA events and the development of arrhythmias.
6.4. Sustained Ventricular Tachycardia
Most studies did not detect sustained VT in patients with OSA [30, 85, 98, 99]. Javaheri et al. found no correlation between sustained VT and OSA (percentage of patients with sustained VT was similar in the control and the OSA groups) [35].
6.5. Appropriate ICD Therapy
No published studies exclusively on OSA patients were found, but studies on SDB patients (including a significant proportion of OSA patients) demonstrated an association between Implantable Cardioverter-defibrillator (ICD) shocks and SBD [31, 32, 105, 106]. In patients with ICD, VA were significantly more often associated with apneas/hypopneas than with normal breathing [95, 105]. The risk for VA [32], anti-tachycardia pacing therapy (ATP) [106] and ICD shocks [106, 107] was higher in SBD patients due to an increase in events occurring between midnight and 6 a.m., with no discernible effect on appropriate ICD therapy during nonsleeping hours. The presence of SBD was an independent predictor for appropriate ICD therapy (hazard ratio 4.05, 95% CI 1.20 to 13.65, p=0.015 [102]) and the severity of OSA correlates with the risk of nocturnal arrhythmias [32]. ICD appropriate shocks in patients with HF are independently associated with AHI as a continuous variable and inversely and independently associated with minimum oxygen saturation during the night [31].
Recently, the relationship between OSA and VA burden has been evaluated by means of a device allowing simultaneous nasal pressure recordings, as a surrogate for PSG, and ECG, in 214 patients with an ICD or Cardiac Resynchronization Therapy (CRT). This study confirmed that the number of VA was significantly higher in patients with moderate or severe OSA than in those with mild or non-OSA [105].
6.6. Sudden Cardiac Death
In the general population, the circadian distribution of cardiovascular events follows the circadian pattern of the autonomic nervous system: they are suppressed during sleep in parallel with the nocturnal nadir of sympathetic activity and vagal predominance [108] and increase in the morning hours [109].
The first study suggesting an association between OSA and SCD was carried out by Gami et al. in 2005. They reviewed the death certificates of 112 patients who suddenly died from cardiovascular causes and had previously undergone a PSG. They demonstrated that from midnight to 6 a.m., SCD occurred in 46% of people with OSA, as compared with 21% of people without OSA (p=0.01) and 16% of the general population (p<0.001). The authors concluded that people with OSA have a peak in SCD from cardiac causes during sleeping hours (from midnight to 6 a.m.) [110], which contrasts with the nadir of SCD from cardiac causes in patients without OSA and in general population. Later on, a longitudinal study of more than 10.000 patients conducted by the same author found that OSA predicted SCD (including ICD shocks as a surrogate of SCD in some patients) and the magnitude of the risk was predicted by parameters that characterize OSA severity: AHI (>20), lowest and mean nocturnal oxygen desaturation (<78% and <93%, respectively). However, in this study SCD was not inevitably due to VT/VF since it included deaths due to acute myocardial infarction (13%) or acute pulmonary embolism (1%) [33].
7. OSA IN “IDIOPHATIC” VENRICULAR ARRHYTMIAS PATIENTS
Koshino et al. found that approximately 51% of patients with idiopathic VA (VT or ≥300 PVCs/h) had OSA (≥5 AHI), which suggests a strong association between OSA and VA in patients without heart failure. Of interest, in this study, there were no differences in ESS score, showing that patients with OSA had no symptoms of daytime sleepiness [111].
In HF patients with VA, the prevalence of OSA is particularly high [112], but an impaired LV function itself may be a substrate for VA.
8. TREATMENT OF VENTRICULAR ARRHYTHMIAS IN OSA PATIENTS
Continuous positive airway pressure (CPAP) is the primary treatment modality in patients with severe OSA, whereas oral appliances are also widely used in mild to moderate forms. Other treatment options include weight loss, avoidance of alcohol and sedatives and surgery [19]. Whether these treatment modalities have a role in VA of OSA patients is still controversial.
8.1. Continuous Positive Airway Pressure
CPAP is a treatment that delivers positive pressure through a mask to maintain the opening of the upper airways during sleep, usually indicated in patients with moderate or severe OSA. Adherence and compliance to the treatment are the main problems faced by clinicians, mainly due to mask application and difficulty dealing with the equipment [113].
CPAP alleviates apnea-related hypoxia and arousals from sleep and abolishes exaggerated negative intrathoracic pressure swings [114]. The possible mechanisms of action of CPAP in VA may include improved myocardial oxygen delivery, decreased sympathetic activity, LV transmural pressure and afterload [115]. By reducing oxygen demand and increasing oxygen supply, CPAP could attenuate PVCs either by preventing ischaemia in patients with ischaemic heart disease or by improving ventricular repolarization [116]. A second potential mechanism is through a reduction in cardiac sympathetic nerve traffic [117]. This is supported by the finding of reduced overnight urinary norepinephrine in the CPAP treated group [118, 119]. Another mechanism may involve unloading of the ventricles with the elimination of their transient mechanical distension and a consequent alleviation of electrical-mechanical dissociation [120].
Relevant studies that addressed CPAP effects on VA in OSA patients are presented in Table 1. A few studies were not included due to the reduced number of patients with OSA (<10 patients) [34, 35, 121, 122]. Of note, the majority of studies support a protective effect of CPAP against the occurrence of VA, but definitive evidence is still lacking. In studies that did not confirm a reduction in arrhythmia frequency after CPAP, the low number of events (patients with arrhythmia) is likely the reason for the absence of significantly favorable results.
Table 1.
Principal characteristics of studies regarding CPAP effects on ventricular arrhythmias (VA) in obstructive sleep apnea (OSA) patients.
| Author, Year | Study Design | Patients | AHI Before Treatment; Mean (SD) | Findings |
|---|---|---|---|---|
| Abe [30] 2010 |
Observational, prospective | 632 pts, suspected of having OSA. 316 had moderate to severe OSA (>20AHI) and were treated with CPAP during average 3.9 weeks | 50.3 (22.3) | After CPAP treatment, PVCs were less frequent (p=0.016) but no significant reduction in ventricular arrhythmias (PVCs or NSVT) were found |
| Craig [129] 2009 |
Interventional, randomized controlled trial | 83 pts with moderate to severe OSA (AHI ND). 43 with therapeutic CPAP and 40 with non-therapeutic CPAP* during 1 month | 41.2 (24.3) | CPAP therapy reduces mean 24-h heart rate possibly due to reduced sympathetic activation, but did not result in a significant decrease in dysrhythmia frequency. A trend toward a fewer daytime VT events |
| Dediu [130] 2015 |
Interventional, non-controlled | 15 pts with OSA (AHI>5) and PVCs. 8 with CPAP and pharmacological therapy and 7 with no-CPAP during 6 months. | N.D. | More patients with class II Lown ventricular extrasystoles passed in class I Lown in those with CPAP (non-significant). |
| Dursunoglu [131] 2007 | Observational,prospective | 30 pts with moderate to severe OSA (AHI≥15). 18 compliant** with nasal CPAP and 11 non-compliant, during 6 months | 50.1 (11.6) | The QTcd at baseline [54.5 (8.7) ms] significantly decreased after CPAP therapy [35.5 (4.2) ms, p<0.001] and it did not significantly change in 11 non-compliant patients. |
| Nakamura [70] 2004 | Observational, prospective | 48 pts with moderate to severe OSA (AHI≥20) after one night and 1 month of nasal CPAP | 51.9 (18.5) | After 1 night and after one month of nCPAP therapy, the QTcd during sleep [50.6 (11.4) ms] decreased from that before treatment (p < 0.0001) |
| Peled [132] 1999 |
Observational, prospective | 15 pts with OSA (AHI>5) treated with CPAP during 1 night, of whom 9 had nocturnal ischemia | 35.1(6.2) | Treatment with CPAP significantly ameliorated the nocturnal ST depression time from 78 min to 33 min (p < 0.001) |
| Roche [133] 2005 |
Observationalprospective | 38 pts with moderate to severe OSA (≥15) before and after CPAP (and 38 pts with no OSA - control group) | 56.9 (28.4) | QT length related to heart rate significantly improved with the treatment of the OSAS [−0.151(0.051); p<0.01 vs pretreatment status]. There was no significant impact of CPAP therapy on PVCs. |
| Rossi [87] 2012 |
Interventional, randomized controlled trial | 41 pts with OSA (severity N.D) and previously CPAP. 20 continued CPAP and 21 received placebo-CPAP during 2 weeks | 36.0 (17.3) (CPAP group) vs. 45.3 (22.3) (control) NS | CPAP withdrawal is associated with the prolongation of the QTc and TpTec intervals and TpTe/QT ratio |
| Ryan [116] 2005 |
Interventional, randomized | 18 HF pts with moderate to severe OSA (AHI>20) and >10PVCs/h. 10 treated with CPAP during 1 month | 29.3 (4.8) (CPAP group) vs. 57.9 (5.50) (control) (p=0.017) |
A 58% significant reduction in the frequency of PVCs during total sleep [from 170 (65) to 70 (28) per hour, p=0.011] after 1 month of CPAP treatment. |
| Seyis [134] 2018 |
Observational, prospective | 80 HF pts with newly diagnosed moderate to severe OSAS and >30PVCs/h. 40 pts accepted CPAP and 40 did not. |
35.85 (8.61) (CPAP group) vs. 32.45 (8.88) (control) NS | CPAP treatment significantly reduced the frequency of PVCs, T-peak to T-end, QTc, QTcd, and T-peak to T-end/corrected QT ratio |
*Subtherapeutic CPAP was physically identical to therapeutic CPAP except the pressure was less than 1 cmH2O, and inadequate to splint open the pharynx as previously described.
**Patients were considered to be compliant if they used CPAP an average 3.5 hours per night at the six-month follow-up.
N.D. no data. CPAP: continuous positive airway pressure. nCPAP: nasal CPAP. NS: non-significant. QTcd: QT corrected dispersion. Pts: patients. VT: ventricular tachycardia.
Overall, the duration of CPAP application, compliance with treatment, baseline severity of OSA and cardiac pathology are important confounding factors that influence the effect of CPAP treatment. The small sample sizes that limit extrapolation, as well as the inconsistencies in methodologies for the measurement of outcomes and variables of interest, indicate that larger controlled randomized studies will provide the homogeneity warranted to promote a more useful synthesis of the current evidence.
8.2. Anti-arrhythmics
No studies addressed the efficacy of drug therapy in VA and if a particular class of Antiarrhythmic Drug (AAD) is more effective than the others in OSA patients. In what concerns AF, patients with severe OSA are less likely to respond to AAD therapy than those with milder forms of OSA [123] and possibly the same is true in VA.
8.3. Catheter Ablation
Koshino et al. demonstrated that VA in patients with OSA (defined as AHI ≥10) have a higher rate of recurrence of VT/PVCs after catheter ablation compared with non-OSA patients (45% versus 6%, p=0.02) during a mean follow-up period of 13.5 (7.3) months [111]. However, this study had some important limitations: the sample size was small (n=44), patients with AHI 0-9 were considered as having no OSA and the authors did not explain if OSA patients were treated with CPAP after OSA diagnosis.
8.4. Other Therapies
The role of oxygen treatment in preventing VA in OSA patients had been proposed but there are insufficient studies with a low number of patients [120, 124].
Weight loss is likely to be an effective measure, not only due to beneficial effects on OSA itself but also on cardiovascular comorbidities, very frequent in these patients. Alcohol consumption is also not advised since it is associated per si to cardiac arrhythmias [125, 126].
9. GAPS IN EVIDENCE / LIMITATIONS
Due to the nature of the outcome and the presence of comorbidities in OSA patients, the observational studies demonstrating an association between OSA and VA are not enough to prove a causal relationship.
The definition for OSA and its severity (regarding AHI cutoffs) were not uniform across all studies, which may be responsible for some conflicting results.
Given the small number of studies available, studies including not only obstructive but also CSA (SDB) were analysed. Although we only used OSA sub-analysis, in some cases, it was difficult to definitely separate the outcomes. OSA is characterized by repetitive collapse of the upper airway, whereas the hallmark of CSA is recurrent complete or partial withdrawal of central respiratory drive. Only a few of the possible mechanisms responsible for arrhythmogenesis are shared by these two entities. In CSA, the increased respiratory effort is absent, and only the presence of peripheral mechanisms such as intermittent hypoxia, increased catecholamines, and frequent arousals are responsible for the increased sympathetic activation [127, 128].
CONCLUSION
OSA is a prevalent condition thought to increase in the future. Being mostly undiagnosed, the most serious complications are cardiovascular diseases, among which are arrhythmias. Treatment of OSA has the potential to reduce arrhythmias and confer a mortality benefit.
The controversies regarding the relationships between OSA and VA can be explained by selection bias and inhomogeneity in OSA definition and disease severity. Also, OSA is a complex syndrome that involves hypoxemia, endothelial dysfunction, inflammation and sympathetic stimulation and is commonly present in patients with other cardiovascular risk factors. In general, the evidence suggests OSA is associated with VA. From our point of view, the main questions are “Is this association significant enough to establish OSA as a risk factor for SCD due to VA?” and “Is the available evidence strong enough to come up with new treatment strategies in patients with VA and OSA?”
We propose that patients with VA should be screened for OSA due to the lack of symptoms in this population and the possible high prevalence of OSA in patients with “idiopathic” VA. However, not all patients with arrhythmias need to undergo PSG to establish OSA diagnosis. The authors suggest considering for evaluation for possible OSA patients with (1) Nocturnal arrhythmias (2) Arrhythmias refractory to standard therapy or (3) Other clinical indicators of OSA such as obesity, disruptive snoring, witnessed apnea or gasping and hypersomnolence.
In some particular conditions, the authors advocate CPAP therapy may be considered to prevent VA. For example, CPAP may be indicated with respect to an increased QTc or QTcd or when VA is detected at night, because the evidence suggests they are independent risks factors for cardiac mortality and CPAP may reduce them.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Gami A.S., Hodge D.O., Herges R.M., et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Vizzardi E., Sciatti E., Bonadei I., D’Aloia A., Curnis A., Metra M. Obstructive sleep apnoea-hypopnoea and arrhythmias: New updates. J. Cardiovasc. Med. (Hagerstown) 2017;18(7):490–500. doi: 10.2459/JCM.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 4.Digby G.C., Baranchuk A. Sleep apnea and atrial fibrillation; 2012 update. Curr. Cardiol. Rev. 2012;8:265–272. doi: 10.2174/157340312803760811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.K., Lai M.S., Chen Y.C., et al. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin. Sci. (Lond.) 2012;122:121–132. doi: 10.1042/CS20110178. [DOI] [PubMed] [Google Scholar]
- 6.Linz D. Atrial fibrillation in obstructive sleep apnea: atrial arrhythmogenic substrate of a different sort. Am. J. Cardiol. 2012;110:1071. doi: 10.1016/j.amjcard.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Raghuram A., Clay R., Kumbam A., Tereshchenko L.G., Khan A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J. Clin. Sleep Med. 2014;10(10):1155–1160. doi: 10.5664/jcsm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 9.Levy P., Kohler M., McNicholas W.T., et al. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 10.Johns M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Netzer N.C., Stoohs R.A., Netzer C.M., Clark K., Strohl K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Abrishami A., Khajehdehi A., Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can. J. Anaesth. 2010;57:423–438. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 13.Jelic S., LeJemtel T.H. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc. Med. 2008;18:253–260. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Sateia M.J. International classification of sleep disorders — third edition: highlights and modifications. . Chest. 2014;146:1387–94. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 15.Lamphere J., Roehrs T., Wittig R., Zorick F., Conway W.A., Roth T. Recovery of alertness after CPAP in apnea. Chest. 1989;96:1364–1367. doi: 10.1378/chest.96.6.1364. [DOI] [PubMed] [Google Scholar]
- 16.Pépin J.L., Krieger J., Rodenstein D., et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am. J. Respir. Crit. Care Med. 1999;160:1124–1129. doi: 10.1164/ajrccm.160.4.9802027. [DOI] [PubMed] [Google Scholar]
- 17.Antic N.A., Catcheside P., Buchan C., et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep (Basel) 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver T.E., Maislin G., Dinges D.F., et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers V.K., White D.P., Amin R., et al. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C., Connolly S.J., Winkle R.A. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am. J. Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 21.Hoffstein V., Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest. 1994;106:466–471. doi: 10.1378/chest.106.2.466. [DOI] [PubMed] [Google Scholar]
- 22.Tilkian A.G., Guilleminault C., Schroeder J.S., Lehrman K.L., Simmons F.B., Dement W.C. Sleep-induced apnea syndrome. Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am. J. Med. 1977;63:348–358. doi: 10.1016/0002-9343(77)90272-8. [DOI] [PubMed] [Google Scholar]
- 23.Sicouri S., Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. Mol Cell Circ Res. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 24.Hevia J.C., Antzelevitch C., Bárzaga F.T., et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ ventricular fibrillation in patients with the brugada syndrome. J. Am. Coll. Cardiol. 2006;47(9):1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panikkath R., Reinier K., Uy-Evanado A., et al. Prolonged Tpeak to Tend interval on the resting electrocardiogram is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–447. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X., Xie Z., Chu Y., et al. Association between Tp-e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Clin. Cardiol. 2012;35:559–564. doi: 10.1002/clc.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cintra F.D., Leite R.P., Storti L.J., et al. Sleep apnea and nocturnal cardiac arrhythmia: A populational study. Arq. Bras. Cardiol. 2014;103(5):368–374. doi: 10.5935/abc.20140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selim B.J., Koo B.B., Qin L., et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: The DREAM Study. J. Clin. Sleep Med. 2016;12(6):829–837. doi: 10.5664/jcsm.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard J.W., Jr, Garrison M.W., Grither D.A., Dolan G.F. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest. 1985;88(3):335–340. doi: 10.1378/chest.88.3.335. [DOI] [PubMed] [Google Scholar]
- 30.Abe H., Takahashi M., Yaegashi H., et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25:63–69. doi: 10.1007/s00380-009-1164-z. [DOI] [PubMed] [Google Scholar]
- 31.Tomaello L., Zanolla L., Vassanelli C., LoCascio V., Ferrari M. Sleep disordered breathing is associated with appropriate implantable cardioverter defibrillator therapy in congestive heart failure patients. Clin. Cardiol. 2010;33(2):E27–E30. doi: 10.1002/clc.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeidan-Shwiri T., Aronson D., Atalla K., et al. Circadian pattern of life-threatening ventricular arrhythmia in patients with sleep-disordered breathing and implantable cardioverter-defibrillators. Heart Rhythm. 2011;8(5):657–662. doi: 10.1016/j.hrthm.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Gami A.S., Olson E.J., Shen W.K., et al. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbison J., O’Reilly P., McNicholas W.T. Cardiac rhythm disturbances in the obstructive sleep apnea syndrome: Effects of nasal continuous positive airway pressure therapy. Chest. 2000;118(3):591–595. doi: 10.1378/chest.118.3.591. [DOI] [PubMed] [Google Scholar]
- 35.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 36.Ryan C.M., Juvet S., Leung R., Bradley T.D. Timing of nocturnal ventricular ectopy in heart failure patients with sleep apnea. Chest. 2008;133(4):934–940. doi: 10.1378/chest.07-2595. [DOI] [PubMed] [Google Scholar]
- 37.May A.M., VanWagoner D.R., Mehra R. Obstructive sleep apnea and cardiac arrhythmogenesis: Mechanistic insights. Chest. 2017;151(1):225–241. doi: 10.1016/j.chest.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farré R., Montserrat J.M., Navajas D. Morbidity due to obstructive sleep apnea: Insights from animal models. Curr. Opin. Pulm. Med. 2008;14:530–536. doi: 10.1097/mcp.0b013e328312ed76. [DOI] [PubMed] [Google Scholar]
- 39.Leung R. Sleep-disordered breathing: Autonomic mechanisms and arrhythmias. Prog. Cardiovasc. Dis. 2009;51(4):324–338. doi: 10.1016/j.pcad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Daly M.D., Scott M.J. The cardiovascular responses to stimulation of the carotid body chemoreceptors in the dog. J. Physiol. 1963;165:179–197. doi: 10.1113/jphysiol.1963.sp007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Daly M.B., Scott M.J. The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J. Physiol. 1958;144(1):148–166. doi: 10.1113/jphysiol.1958.sp006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souvannakitti D., Kumar G.K., Fox A., Prabhakar N.R. Contrasting effects of intermittent and continuous hypoxia on low O(2) evoked catecholamine secretion from neonatal rat chromaffin cells. Adv. Exp. Med. Biol. 2009;648:345–349. doi: 10.1007/978-90-481-2259-2_39. [DOI] [PubMed] [Google Scholar]
- 43.Morand J., Arnaud C., Pepin J.L., Godin-Ribuot D. Chronic intermittent hypoxia promotes myocardial ischemiarelated ventricular arrhythmias and sudden cardiac death. Sci. Rep. 2018;8(1):2997. doi: 10.1038/s41598-018-21064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown D.A., O’Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc. Res. 2010;88(2):241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong E.M., Liu M., Sturdy M., et al. Metabolic stress, reactive oxygen species, and arrhythmia. J. Mol. Cell. Cardiol. 2012;52(2):454–463. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somers V.K., Dyken M.E., Skinner J.L. Autonomic and hemodynamic responses and interactions during the Mueller maneuver in humans. J. Auton. Nerv. Syst. 1993;44:253–259. doi: 10.1016/0165-1838(93)90038-v. [DOI] [PubMed] [Google Scholar]
- 47.Virolainen J., Ventila M., Turto H., Kupari M. Effect of negative intrathoracic pressure on left ventricular pressure dynamics and relaxation. J. Appl. Physiol. 1995;79:455–460. doi: 10.1152/jappl.1995.79.2.455. [DOI] [PubMed] [Google Scholar]
- 48.Orban M., Bruce C.J., Pressman G.S., et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am. J. Cardiol. 2008;102(11):1557–1561. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linz D., Denner A., Illing S., et al. Impact of obstructive and central apneas on ventricular repolarisation: lessons learned from studies in man and pigs. Clin. Res. Cardiol. 2016;105(8):639–647. doi: 10.1007/s00392-016-0961-5. [DOI] [PubMed] [Google Scholar]
- 50.Gami A.S., Somers V.K. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J. Cardiovasc. Electrophysiol. 2008;19(9):997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 51.Franz M.R. Mechano-electrical feedback in ventricular myocardium. Cardiovasc. Res. 1996;32:15–24. [PubMed] [Google Scholar]
- 52.Zipes D. Autonomic modulation of cardiac arrhythmias. In: Jalife J., Zipes D.P., editors. Cardiac electrophysiology: From cell to bedside. 2nd ed. Philadelphia: WB Saunders; 1995. pp. 441–454. [Google Scholar]
- 53.Wit A.L., Rosen A.R. After depolarizations and triggered activity. In: Fossard H.A., Haber E., Jenning R.B., Katz M., Morgan E., editors. The heart and cardiovascular system. New York: Raven Press; 1986. pp. 1449–1490. [Google Scholar]
- 54.Kors J.A., Ritsema van Eck H.J., van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J. Electrocardiol. 2008;41:575–580. doi: 10.1016/j.jelectrocard.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Peters R.W., Byington R.P., Barker A., Yusuf S. Prognostic value of prolonged ventricular repolarization following myocardial infarction: The BHAT experience. J. Clin. Epidemiol. 1990;43:167–172. doi: 10.1016/0895-4356(90)90180-w. [DOI] [PubMed] [Google Scholar]
- 56.Munger R.G., Prineas R.J., Crow R.S., et al. Prolonged QT interval and risk of sudden death in South-East Asian men. Lancet. 1991;338(8762):280–281. doi: 10.1016/0140-6736(91)90419-p. [DOI] [PubMed] [Google Scholar]
- 57.Algra A., Tijssen J.G., Roelandt J.R., Pool J., Lubsen J. QTc prolongation measured by standard 12- lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 58.Wheelan K., Mukharji J., Rude R.E., et al. Sudden death and its relation to QT-interval prolongation after acute myocardial infarction: Two-year follow-up. Am. J. Cardiol. 1986;57(10):745–750. doi: 10.1016/0002-9149(86)90606-5. [DOI] [PubMed] [Google Scholar]
- 59.Gupta P., Patel C., Patel H., et al. Tp-e/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Kuo C.S., Reddy C.P., Munakata K., Surawicz B. Mechanism of ventricular arrhythmias caused by increased dispersion of repolarization. Eur. Heart J. 1985;6:63–71. doi: 10.1093/eurheartj/6.suppl_d.63. [DOI] [PubMed] [Google Scholar]
- 61.Perkiomaki J., Koistinen M.J., Yli-Mayry S., Huikuri H.V. Dispersion of the QT interval in patients with andwithout susceptibility to ventricular tachyarrhythmias after previous myocardial infarction. J. Am. Coll. Cardiol. 1995;26:174–179. doi: 10.1016/0735-1097(95)00122-g. [DOI] [PubMed] [Google Scholar]
- 62.de Bruyne M.C., Hoes A.W., Kors J.A., Hofman A., van Bemmel J.H., Grobbee D.E. QTc dispersion predicts cardiac mortality in the elderly: The Rotterdam-study. Circulation. 1998;97:467–472. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 63.Moss A.J. QTc prolongation and sudden cardiac death: The association is in the detail. J. Am. Coll. Cardiol. 2006;47:368–369. doi: 10.1016/j.jacc.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 64.Gillis A.M., Stoohs R., Guilleminault C. Changes in the QT interval during obstructive sleep apnea. Sleep. 1991;14(4):346–350. doi: 10.1093/sleep/14.4.346. [DOI] [PubMed] [Google Scholar]
- 65.Sökmen E, Özbek SC, Çelik M, Sivri S, Metin M, Avcu M. Changes in the parameters of ventricular repolarization during preapnea, apnea, and postapnea periods in patients with obstructive sleep apnea. 2018. [DOI] [PubMed]
- 66.Shamsuzzaman A., Somers V., Knilans T., Ackerman M., Wang Y., Amin R. Obstructive sleep apnea in patients with congenital long QT syndrome: Implications for increased risk of sudden cardiac death. Sleep (Basel) 2015;38(7):1113–1119. doi: 10.5665/sleep.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlatzer C., Schwarz E., Sievi N., et al. Intrathoracic pressure swings induced by simulated obstructive sleep apnoea promote arrhythmias in paroxysmal atrial fibrillation. EP Europace. 2016;18(1):64–70. doi: 10.1093/europace/euv122. [DOI] [PubMed] [Google Scholar]
- 68.Shimoda L., Polak J. Hypoxia and ion channel function. Am. J. Physiol. Cell Physiol. 2011;300(5):C951–C967. doi: 10.1152/ajpcell.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones D., Peters C., Tolhurst D., Claydon T., Ruben P. Extracellular proton modulation of the cardiac voltage-gated sodium channel, NaV1.5. Biophys. J. 2011;101(9):2147–2156. doi: 10.1016/j.bpj.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilicaslan F., Tokatli A., Ozdag F., et al. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin. Electrophysiol. 2012;35(8):966–972. doi: 10.1111/j.1540-8159.2012.03439.x. [DOI] [PubMed] [Google Scholar]
- 71.Malik M., Batchvarov V.N. Measurement, interpretation and clinical potential of QT dispersion. J. Am. Coll. Cardiol. 2000;36:1749–1766. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 72.Dursunoglu D., Dursunoglu N., Evrengül H., et al. QT interval dispersion in obstructive sleep apnoea syndrome patients without hypertension. Eur. Respir. J. 2005;25(4):677–681. doi: 10.1183/09031936.05.00067104. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura T., Chin K., Hosokawa R., Takahashi K., Sumi K., Ohi M., Mishima M. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125(6):2107–2114. doi: 10.1378/chest.125.6.2107. [DOI] [PubMed] [Google Scholar]
- 74.Barta K., Szabó Z., Kun C., et al. The effect of sleep apnea on QT interval, QT dispersion, and arrhythmias. Clin. Cardiol. 2010;33(6):E35–E39. doi: 10.1002/clc.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarubbi B., Esposito V., Ducceschi V., et al. Effect of blood gás derangement on QTc dispersion in severe chronic obstructive pulmonary disease: Evidence of an electropathy? Int. J. Cardiol. 1997;58:287–292. doi: 10.1016/s0167-5273(96)02876-8. [DOI] [PubMed] [Google Scholar]
- 76.Kiely D.G., Cargill R.I., Grove A., Struthers A.D., Lipworth B.J. Abnormal myocardial repolarisation in response to hypoxemia and fenoterol. Thorax. 1995;50:1062–1066. doi: 10.1136/thx.50.10.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishida S., Nakagawa M., Fujino T., Yonemochi H., Saikawa T., Ito M. Circadian variation of QT interval dispersion: correlation with heart rate variability. J. Electrocardiol. 1997;30:205–210. doi: 10.1016/s0022-0736(97)80005-2. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu M., Ino H., Okeie K., et al. Increased QT dispersion does not reflect the increased regional variation of cardiac sympathetic nervous activity in hypertrophic cardiomyopathy. Am. Heart J. 2001;142:358–362. doi: 10.1067/mhj.2001.116765. [DOI] [PubMed] [Google Scholar]
- 79.Antzelevitch C. Cellular basis for the repolarization waves of the ECG. Ann. N. Y. Acad. Sci. 2006;1080:268–281. doi: 10.1196/annals.1380.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia Y., Liang Y., Kongstad O., Holm M., Olsson B., Yuan S. Tpeak–Tend interval as an index of global dispersion of ventricular repolarization: Evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J. Interv. Card. Electrophysiol. 2005;14:79–87. doi: 10.1007/s10840-005-4592-4. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi M., Shimizu M., Ino H., et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: A new index for arrhythmogenicity. Clin. Sci. (Lond.) 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 82.Topilski I., Rogowski O., Rosso R., et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J. Am. Coll. Cardiol. 2007;49:320–328. doi: 10.1016/j.jacc.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 83.Gupta P., Patel C., Patel H., et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008;41(6):567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Voigt L., Haq S., Mitre C., Lombardo G., Kassotis J. Effect of obstructive sleep apnea on QT dispersion: A potential mechanism of sudden cardiac death. Cardiology. 2011;118:68–73. doi: 10.1159/000324796. [DOI] [PubMed] [Google Scholar]
- 85.Roche F., Xuong A.N., Court-Fortune I., et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin. Electrophysiol. 2003;26(3):669–677. doi: 10.1046/j.1460-9592.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 86.Yan G.X., Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 87.Rossi V.A., Stoewhas A.C., Camen G., et al. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: Data from a randomized controlled trial. Eur. Heart J. 2012;33(17):2206–2212. doi: 10.1093/eurheartj/ehs073. [DOI] [PubMed] [Google Scholar]
- 88.Hayashi T., Fukamizu S., Hojo R., et al. Fragmented QRS predicts cardiovascular death of patients with structural heart disease and inducible ventricular tachyarrhythmia. Circ. J. 2013;77:2889–2897. doi: 10.1253/circj.cj-13-0335. [DOI] [PubMed] [Google Scholar]
- 89.Jain R., Singh R., Yamini S., Das M.K. Fragmented ECG as a risk marker in cardiovascular diseases. Curr. Cardiol. Rev. 2014;10:277–286. doi: 10.2174/1573403X10666140514103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Das M.K., El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr. Opin. Cardiol. 2010;25(1):59–64. doi: 10.1097/HCO.0b013e328333d35d. [DOI] [PubMed] [Google Scholar]
- 91.Bacharova L., Triantafyllou E., Vazaios C., Tomeckova I., Paranicova I., Tkacova R. The effect of obstructive sleep apnea on QRS complex morphology. J. Electrocardiol. 2015;48(2):164–170. doi: 10.1016/j.jelectrocard.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 92.Adar A., Kırış A., Bülbül Y., Bektaş H., Acat M., Casim H., et al. Association of Fragmented QRS with Subclinical Left Ventricular Dysfunction in Patients with Obstructive Sleep Apnea. Med. Princ. Pract. 2015;24(4):376–381. doi: 10.1159/000382077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Somers V.K., Dyken M.E., Mark A.L., Abboud F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 94.Muller J.E., Tofler G.H., Verrier R.L. Sympathetic activity as the cause of the morning increase in cardiac events. A likely culprit, but the evidence remains circumstantial. Circulation. 1995;91(10):2508–2509. doi: 10.1161/01.cir.91.10.2508. [DOI] [PubMed] [Google Scholar]
- 95.Fichter J., Bauer D., Arampatzis S., Fries R., Heisel A., Sybrecht G.W. Sleep-related breathing disorders are associated with ventricular arrhythmias in patients with an implantable cardioverter-defibrillator. Chest. 2002;122(2):558–561. doi: 10.1378/chest.122.2.558. [DOI] [PubMed] [Google Scholar]
- 96.Camen G., Clarenbach C.F., Stöwhas A.C., et al. The effects of simulated obstructive apnea and hypopnea on arrhythmic potential in healthy subjects. Eur. J. Appl. Physiol. 2013;113(2):489–496. doi: 10.1007/s00421-012-2457-y. [DOI] [PubMed] [Google Scholar]
- 97.Mehra R., Benjamin E.J., Shahar E., et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Namtvedt S.K., Randby A., Einvik G., et al. Cardiac arrhythmias in obstructive sleep apnea (from the Akershus Sleep Apnea Project). Am. J. Cardiol. 2011;108(8):1141–1146. doi: 10.1016/j.amjcard.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 99.Almeneessier A.S., Alasousi N., Sharif M.M. Seithikurippu Rp, Hersi AS, Bahammam A. Prevalence and predictors of arrhythmia in patients with obstructive sleep apnea. Sleep Sci. 2017;10(4):142–146. doi: 10.5935/1984-0063.20170025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aydin M., Altin R., Ozeren A., Kart L., Bilge M., Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex. Heart Inst. J. 2004;31(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 101.Miller W.P. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am. J. Med. 1982;73(3):317–321. doi: 10.1016/0002-9343(82)90716-1. [DOI] [PubMed] [Google Scholar]
- 102.Flemons W.W., Remmers J.E., Gillis A.M. Sleep apnea and cardiac arrhythmias. Is there a relationship? Am. Rev. Respir. Dis. 1993;148(3):618–621. doi: 10.1164/ajrccm/148.3.618. [DOI] [PubMed] [Google Scholar]
- 103.Mehra R., Stone K.L., Varosy P.D., et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monahan K., Storfer-Isser A., Mehra R., et al. Triggering of nocturnal arrhythmias by sleep disoirdered breathing events. J. Am. Coll. Cardiol. 2009;54(19):1797. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anselme F., Maounis T., Mantovani G., et al. Severity of sleep apnea syndrome correlates with burden of ventricular tachyarrhythmias in unselected ICD patients. Heart Rhythm. 2013 [abstract]. [Google Scholar]
- 106.Bitter T., Fox H., Dimitriadis Z., et al. Circadian variation of defibrillator shocks in patients with chronic heart failure: the impact of Cheyne-Stokes respiration and obstructive sleep apnea. Int. J. Cardiol. 2014;176(3):1033–1035. doi: 10.1016/j.ijcard.2014.07.294. [DOI] [PubMed] [Google Scholar]
- 107.Serizawa N., Yumino D., Kajimoto K., et al. Impact of sleep-disordered breathing on life-threatening ventricular arrhythmia in heart failure patients with implantable cardioverter-defibrillator. Am. J. Cardiol. 2008;102(8):1064–1068. doi: 10.1016/j.amjcard.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 108.Furlan R., Guzzetti S., Crivellaro W., et al. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 109.Muller J.E., Ludmer P.L., Willich S.N., et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 110.Gami A.S., Howard D.E., Olson E.J., Somers V.K. Day-night pattern of sudden death in obstructive sleep apnea. N. Engl. J. Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 111.Koshino Y., Satoh M., Katayose Y., et al. Sleep apnea and ventricular arrhythmias: Clinical outcome, electrophysiologic characteristics, and follow-up after catheter ablation. J. Cardiol. 2010;55(2):211–216. doi: 10.1016/j.jjcc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Koshino Y., Satoh M., Katayose Y., et al. Association of sleep-disordered breathing and ventricular arrhythmias in patients without heart failure. Am. J. Cardiol. 2008;101(6):882–886. doi: 10.1016/j.amjcard.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 113.Weaver T., Grunstein R. Adherence to continuous positive airway pressure therapy. The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaneko Y., Floras J.S., Usui K., et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 115.Dursunoglu N., Dursunoglu D., Ozkurt S., Gür S., Ozalp G., Evyapan F. Efects of CPAP on right ventricular myocardial performance index in obstructive sleep apnea patients without hypertension. Respir. Res. 2006;7:22. doi: 10.1186/1465-9921-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryan C.M., Usui K., Floras J.S., Bradley T.D. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaye D.M., Mansfield D., Aggarwal A., Naughton M.T., Esler M.D. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103:2336–2338. doi: 10.1161/01.cir.103.19.2336. [DOI] [PubMed] [Google Scholar]
- 118.Mansfield D.R., Gollogly N.C., Kaye D.M., Richardson M., Bergin P., Naughton M.T. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am. J. Respir. Crit. Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 119.Sukegawa M., Noda A., Sugiura T., et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clin. Cardiol. 2005;28(11):519–522. doi: 10.1002/clc.4960281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Franz M.R., Cima R., Wang D., Profitt D., Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 121.Vitulano N., Giubilato G., Santangeli P., et al. Continuous positive airway pressure treatment in addition to optimal medical therapy for ventricular ectopy in a patient with heart failure and sleep-related breathing disorder. J. Cardiovasc. Med. (Hagerstown) 2013;14(9):673–676. doi: 10.2459/JCM.0b013e3283356e70. [DOI] [PubMed] [Google Scholar]
- 122.Jyothula S.S., Ramachandran S. Reversible ventricular arrhythmia in REM sleep associated with hypoxic sleep-disordered breathing. Sleep Med. 2006;7(1):81–82. doi: 10.1016/j.sleep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Monahan K., Brewster J., Wang L., et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am. J. Cardiol. 2012;110(3):369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki J., Ishihara T., Sakurai K., et al. Oxygen therapy prevents ventricular arrhythmias in patients with congestive heart failure and sleep apnea. Circ. J. 2006;70(9):1142–1147. doi: 10.1253/circj.70.1142. [DOI] [PubMed] [Google Scholar]
- 125.Kupari M., Koskinen P. Alcohol, cardiac arrhythmias and sudden death. Novartis Found. Symp. 1998;216:68–79. doi: 10.1002/9780470515549.ch6. [DOI] [PubMed] [Google Scholar]
- 126.Brunner S., Herbel R., Drobesch C., et al. Alcohol consumption, sinus tachycardia, and cardiac arrhythmias at the Munich Octoberfest: results from the Munich Beer Related Electrocardiogram Workup Study (MunichBREW). Eur. Heart J. 2017;38(27):2100–2106. doi: 10.1093/eurheartj/ehx156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spicuzza L., Bernardi L., Calciati A., Di Maria G.U. Autonomic modulation of heart rate during obstructive versus central apneas in patients with sleepdisordered breathing. Am. J. Respir. Crit. Care Med. 2003;167:902–910. doi: 10.1164/rccm.200201-006OC. [DOI] [PubMed] [Google Scholar]
- 128.Passino C., Sleight P., Valle F., Spadacini G., Leuzzi S., Bernardi L. Lack of peripheral modulation by analysis of heart rate variability activity during apneas in humans. Am. J. Physiol. 1997;272:H123–H129. doi: 10.1152/ajpheart.1997.272.1.H123. [DOI] [PubMed] [Google Scholar]
- 129.Craig S., Pepperell J.C., Kohler M., Crosthwaite N., Davies R.J., Stradling J.R. Continuous positive airway pressure treatment for obstructive sleep apnoea reduces resting heart rate but does not affect dysrhythmias: A randomised controlled trial. J. Sleep Res. 2009;18(3):329–336. doi: 10.1111/j.1365-2869.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 130.Dediu G.N., Dumitrache-Rujinski S., Lungu R., et al. Positive pressure therapy in patients with cardiac arrhythmias and obstructive sleep apnea. Pneumologia. 2015;64(1):18–22. [PubMed] [Google Scholar]
- 131.Dursunoglu D., Dursunoglu N. Effect of CPAP on QT interval dispersion in obstructive sleep apnea patients without hypertension. Sleep Med. 2007;8(5):478–483. doi: 10.1016/j.sleep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Peled N., Abinader E.G., Pillar G., Sharif D., Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J. Am. Coll. Cardiol. 1999;34(6):1744–1749. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 133.Roche F., Barthélémy J.C., Garet M., Duverney D., Pichot V., Sforza E. Continuous positive airway pressure treatment improves the QT rate dependence adaptation of obstructive sleep apnea patients. Pacing Clin. Electrophysiol. 2005;28(8):819–825. doi: 10.1111/j.1540-8159.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- 134.Seyis S., Usalan A.K., Rencuzogullari I., Kurmus O., Can Gungen A. the effects of continuous positive airway pressure on premature ventricular contractions and ventricular wall stress in patients with heart failure and sleep apnea. Can. Respir. J. 2018;3:1–8. doi: 10.1155/2018/2027061. [DOI] [PMC free article] [PubMed] [Google Scholar]