Abstract
Duchenne muscular dystrophy is a life-limiting muscle disease that has no current effective therapy. Despite mounting evidence that dysregulation of mechanosensitive ion channels is a significant contributor to dystrophy pathogenesis, effective pharmacologic strategies targeting these channels are lacking. GsMTx4, and its enantiomer GsMTx4-D, are peptide inhibitors of mechanosensitive channels with identical activity. In previous studies, acute in vitro application of GsMTx4 to dystrophic murine muscle effectively reduced the excess MSC dependent calcium influx linked to contraction-induced muscle damage. Here we sought to determine if in vivo treatment with GsMTx4-D proffered benefit in the D2.mdx mouse. GsMTx4-D showed a 1-week half-life when administered by subcutaneous injection over four weeks. Informed by these results, D2.mdx mice were then treated by a subcutaneous injection regimen of GsMTx4-D for six weeks followed by determination of muscle mass, muscle susceptibility to eccentric contraction injury and multiple histological indicators of disease progression. The mice showed a reduction in the loss of muscle mass and a decrease in susceptibility to contraction induced injury. These protective effects were realized without reduction in fibrosis, supporting a model where GsMTx4-D acts directly on muscle cells. We propose GsMTx4-D represents a promising new therapy to slow disease progression and may complement other therapies such as anti-inflammatory agents and gene-replacement strategies.
Keywords: GsMTx4, Dystrophy, Treatment, D2.mdx mouse, Eccentric contraction injury
1. Background
DMD is a muscle disease caused by the genetic depletion of dystrophin, a cytoskeletal protein whose functions include the organization of sarcolemmal and cytoskeletal components [1] and regulation of signaling proteins. In muscle from mdx mice (model of human DMD) the absence of dystrophin is linked to an increase in the permeability of the sarcolemma to extracellular calcium (Ca2+ ) [2,3]. Given firm evidence linking excess Ca2+ permeability to the cellular dysfunction that drives the dystrophic phenotype, pharmacologic approaches targeting dysregulated Ca2+ influx have been a major focus towards a strategy to halt or slow disease progression in DMD.
While studies have identified multiple channel candidates that may contribute to the excess Ca2+ permeability in DMD muscle, their targeting has generated mixed results. Preclinical efficacy studies targeting voltage-activated Ca2+ channels in mdx mice have shown inconsistent results [1] and disease progression appears to be unaffected by treatment in most human trials [4,5]. Antimicrobial aminoglycosides, nonselective cationic channel inhibitors, have shown promise in short-term efficacy trials (6 week) in mdx mice; [6], however longer-term treatment (up to 6 months) was associated with an increase in degeneration of the heart and diaphragm. These deleterious effects may result from the non-specific pore blocking of aminoglycosides [7], and their impact on protein expression [8] and the inositol signaling system [9].
In addition to its scaffold function, dystrophin participates in mechanotransduction by linking the dystrophin–glycoprotein complex (DGC) to the underlying cytoskeleton. Recent evidence shows that the loss of dystrophin leads to increased mechanosensitive ion channels (MSCs) activity [10,11], underscored by the increased sarcolemmal fragility [12–14]. GsMTx4 is a 34 amino acid peptide representing a new class of drugs that selectively inhibits nonselective cationic MSCs [15]. GsMTx4 does not bind directly to the channels, but inhibits MSC gating by association with the cell membrane and buffering tension transmission to the channels [16]. In vitro studies have demonstrated that GsMTx4 is effective at reducing excess stretch-dependent sarcolemma Ca2+ influx through these channels [10,12,14,17–21]. GsMTx4-D is a synthetic enantiomeric form of GsMTx4 made from D amino acids and it has the same potency as the WT peptide [22]. Given evidence that GsMTx4 acts as a cardioprotective in ischemic reperfusion injury [23] and mechanically induced atrial fibrillation [24], we reasoned that GsMTx4-D dependent suppression of MSC activity may proffer benefit as treatment in DMD. The goal of this study was to assess the short-term efficacy of GsMTx4-D in a disease-relevant murine model of DMD. If successful, this study would serve as a foundation for exploring GsMTx4-D as a chronic therapy for DMD.
2. Methods
2.1. Animal model
We chose the D2.mdx murine model of DMD because the mdx crossed into the DBA/2 J background (i.e., D2.mdx) yields a severe dystrophic phenotype that models the human pathogenesis; a severity not observed in other commonly used murine dystrophic models [25,26]. Clearance pharmacokinetics and efficacy testing were conducted in male DBA/2 J and D2.mdx mice at 6–9 weeks of age. All mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Animal care and procedures were approved and performed in accordance with the standards set by the University of Maryland Institutional Animal Care and Use Committee and by the National Institutes of Health (NIH).
2.2. Pharmacokinetics
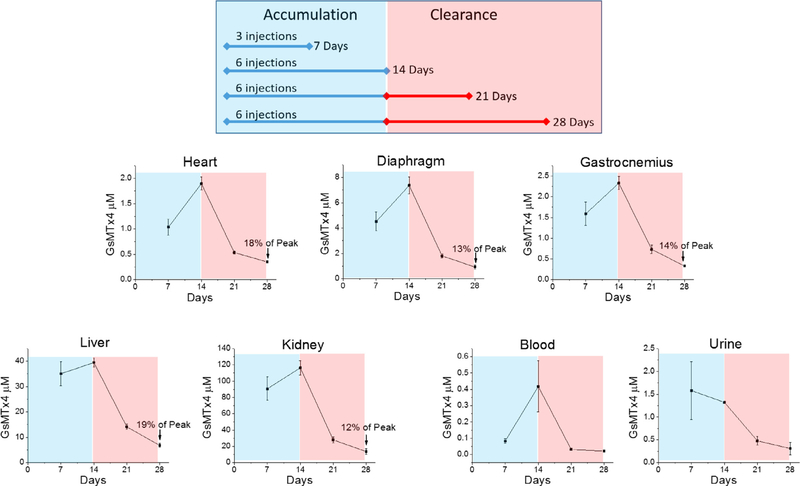
We chose 0.1–5.0 μM in skeletal muscle as our target range based on previous studies [14,18,19,24,27]. We previously demonstrated that a single dose of 50 mg/kg subcutaneous dosing achieved ~1 μM concentration in the heart [23]. Here we conducted accumulation and clearance testing in five key tissues, plasma and urine by a continuous injection regimen. Cohorts of five D2.mdx mice were subcutaneously injected into the back with 10 mg/kg every other day. Dosage times and sacrifice times were as follows:
5 mice injected with 10 mg/kg GsMTx4, 3 times over 7 days – sacrifice on day 7.
5 mice injected with 10 mg/kg GsMTx4, 6 times over 14 days – sacrifice on day 14.
5 mice injected with 10 mg/kg GsMTx4, 6 times over 14 days – sacrifice on day 21.
5 mice injected with 10 mg/kg GsMTx4, 6 times over 14 days – sacrifice on day 28.
Two cohorts were sacrificed at 7 and 14 days during GsMTx4-D accumulation. The peptide injections were ceased at 14 days for the remaining two cohorts which were then sacrificed at 21 and 28 days to determine clearance rate. The kidney, liver, heart, gastrocnemius, diaphragm, blood plasma and urine samples were harvested, flash frozen and shipped to Custom Biologics (Mississauga, Canada) for liquid chromatography coupled with tandem mass spectrometry analysis. Each tissue sample was individually analyzed and the mean concentrations determined.
2.2.1. In vivo muscle strength testing and eccentric contraction-induced injury
Muscle performance was measured in situ with a 305C-FP muscle lever system (Aurora Scientific Inc., Aurora, Canada). Mice were anesthetized (isoflurane; to effect), and placed on a thermostatically controlled table. The knee was stabilized and the foot firmly fixed to the footplate of a torque sensor (Fig. 1). Isometric contractions were elicited from the gastrocnemius muscle with needle electrodes inserted behind the knee in proximity to the sciatic nerve. Optimal isometric twitch torque was determined by increasing the current with a minimum of 30 s between each contraction to avoid fatigue.
Fig. 1.
Experimental setup showing the anesthetized mouse with gastrocnemius muscle exposed and knee and foot in restraints in place for force measurements. The stimulating electrodes are embedded in the muscle and foot restraint is attached to a force torque sensor.
Peak isometric torque: Peak isometric tetanic torque contractions (0.2 ms pulse, 250 ms train, 150 Hz) were determined by taking the peak of three successive contractions elicited 3 min apart. The muscle was then allowed to rest for 5 min.
Injury susceptibility: Mice were injected with Evans Blue dye (EBD) (1 mg/kg i.p.) 24 h prior to the contraction assay. The susceptibility to contraction induced was examined with 20 tetanic contractions (100 Hz, 0.2 ms pulse width, 250 ms duration) elicited where at 200 ms, the muscle was stretched at a rate of 40°/s through a 30° angle [12,13]. The muscle was then passively returned to resting length. Maximal force of the isometric plateau (prior to the eccentric stretch) was measured and used to normalize the force decrease.
2.2.2. Histology
The gastrocnemius muscle from unstimulated (resting) and stimulated (exercised) legs were snap frozen in isopentane cooled in liquid N2 in Cryomatrix. Tissues were sectioned from the muscle midbelly using a cryostat, air dried to a glass slide, and fixed with 4% paraformaldehyde. In exercised legs, muscle permeability post-exercise was assessed by dye incorporation within each muscle as the cross sectional area of EBD containing muscle fibers expressed as a % of total muscle area. To determine the extent of fibrotic inclusions, and central located nuclei, unstimulated sections of muscle were labeled with Texas Red-conjugated wheat germ agglutinin (WGA) to decorate the extracellular matrix and nuclei as described [28]. The mean pixel area of the WGA label, the cross-sectional areas of the muscle fibers, and percentage of those myofibers with central nuclei, were determined in two regions of interest (ROI) per muscle (two ROI’s; one from each head of the gastrocnemius muscle) using Nikon Elements Software analysis tools.
3. Results
3.1. GsMTx4-D tissue accumulation and clearance
Our target concentration of 0.1–5.0 μM in the skeletal muscle was based on multiple studies showing (1) A concentration of 1 μM GsMTx4 inhibited MSC current by > 50% in patch-clamp studies [10,14,15], and (2) reports of in vitro experiments showing 0.1–5.0 μM effective in suppressing MSC activity in various disease models [14,18,19,24,27].
Our previous pharmacokinetic study, aimed at determining rapid tissue exposure to GsMTx4-D in C57BL/6 J mice [23] demonstrated that a single subcutaneous dose of 50 mg/kg achieved ~1 μM concentration in the cardiac muscle within 24 h. Here, we wanted to develop a dosing regimen that could be used for extended periods; so, we performed subcutaneous injections of 10 mg/kg of GsMTx4-D into D2.mdx mice every other day to determine the accumulation (7 or 14 days) and clearance (7 or 14 days) profile of the peptide (Fig. 2). Five tissues and two body fluids (plasma and urine) were followed over both phases. We show that gastrocnemius and cardiac muscle achieved a ~2 μM concentration of GsMTx4-D while diaphragm approached 8 μM. Liver and kidney, two highly vascularized organs, yielded > 10 fold higher concentrations than any of the muscle groups. During the clearance phase all tissues showed a > 50% decrease in concentration after one week that dropped to between 12–19% of the peak concentration after two weeks. Blood concentration dropped rapidly to near 0 after injections ceased, as was previously observed with higher temporal resolution [23]. This suggests that tissue binding sites have higher affinity than plasma binding sites. Urine analysis was limited since samples were not attainable from all animals, though we observed a trend of increased secretion during the accumulation period that decreased after ceasing injections.
Fig. 2.
Pharmacokinetic measurement of GsMTx4-D accumulation and clearance from DBA2-mdx mice. Mice were injected subcutaneously every other day for either 7 or 14 days with 10 mg/kg GsMTx4-D (top panel). Tissues were harvested and fluids were collected at 7, 14, 21 and 28 days. Tissue and body fluid concentrations of GsMTx4-D are shown at the different time points (n = 5 mice/time point). Urine samples were not available from all mice at each time point. Values are means ±SE.
3.2. Six week efficacy study
Based on our published pharmacokinetic analyses [23] and the clearance study conducted here (Fig. 2), we designed a six-week in vivo efficacy study with muscle force and susceptibility to eccentric injury as the primary outcome measure. There were four study groups (n = 10 mice/group): D2.mdx treated with vehicle (saline), or GsMTx4-D at the target concentration (10 mg/kg) or at a 10-fold lower concentration (1 mg/kg) to determine if the target approximates the least effective dose. A group of DBA2 mice treated with vehicle served as a non-diseased control. The clearance data showed that muscle concentrations remained in the target range two weeks after ceasing injections suggesting we could reduce the time between doses following a period of accumulation over the six-week test. Therefore, dosing began at ~8 weeks of age with subcutaneous injections on alternate days for 2 weeks followed by dosing every 4th day for the remaining 4 weeks.
3.2.1. Bodyweight and muscle mass
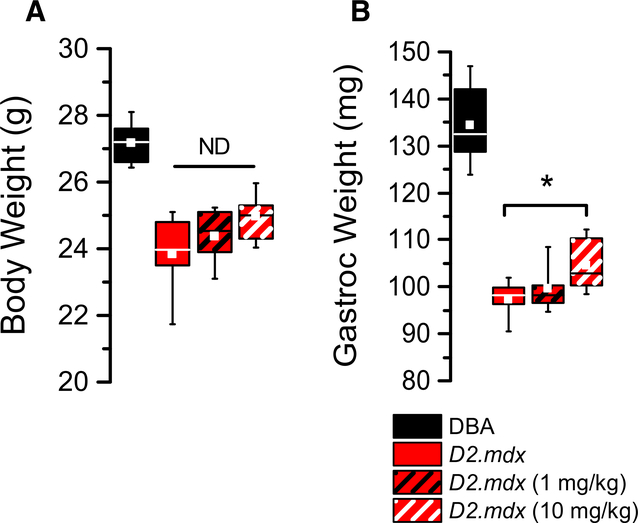
The D2.mdx exhibited significant losses in bodyweight and muscle weight compared to DBA2 controls (Fig. 3A). In response to GsMTx4-D treatment, the D2.mdx exhibited a non-significant trend in protection from loss of bodyweight. In contrast to the impact on bodyweight, the 10 mg/kg treatment was shown to effectively protect the disease driven loss in gastrocnemius muscle weight compared to the untreated D2.mdx (Fig. 3B).
Fig. 3.
Body weight (A) and muscle weight (B) measured at the conclusion of six weeks of high and low dose treatments. (n = 10 mice/cohort, with weights showing means ±SE). *p < 0.05 ANOVA.
3.2.2. Muscle function in vivo
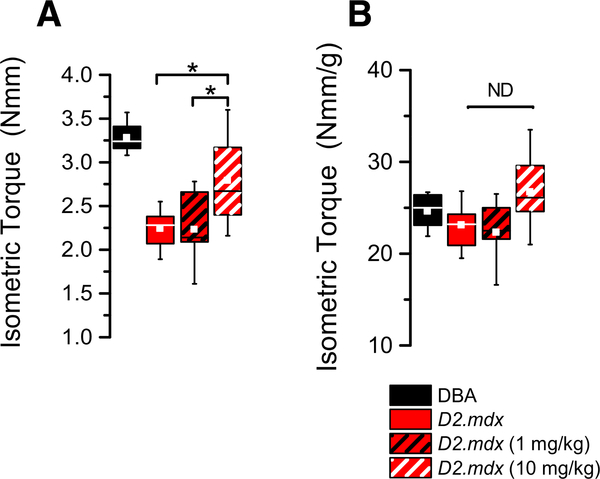
Nerve-evoked isometric torque generated by the plantarflexor group (i.e., gastrocnemius, soleus) was assessed in deeply anesthetized mice as described [12,13]. Consistent with the impact of the dystrophic pathology, we observed a significant deficit in maximal muscle torque in the D2.mdx compared to the DBA2 control (Fig. 4A). Treat-ment of the D2.mdx with 10 mg/kg of GsMTx4-D proffered benefit in isometric torque compared to either the 1 mg/kg or vehicle treated D2.mdx. Given that normalizing the isometric torque to the mass of the muscle accounted for the identified differences in the total torque output (Fig. 4B), we conclude that protection from the loss of muscle mass was responsible for the treatment induced increase in muscle torque.
Fig. 4.
(A) Isometric torque observed in DBA2 and D2.mdx mice. The decline in force generation observed in the D2-mdx was partially rescued by treatment with 1 or 10 mg/kg GsMTx4-D. (B) Normalizing the isometric torque to gastrocnemius weight ablated the disease and treatment effect consistent with the impact of muscle mass on muscle torque output. (n = 10 mice/cohort, with forces showing means ±SE). *= p < 0.05 ANOVA.
3.2.3. Susceptibility to eccentric injury
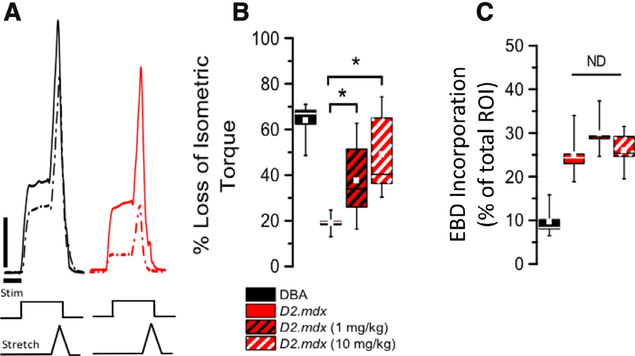
Following the determination of maximal muscle force, we examined the susceptibility to eccentric contraction injury using 20 eccentric contractions (Fig. 5) as described [12,13]. Consistent with dystrophic disease increasing injury susceptibility, the D2.mdx exhibited a ~70% drop in isometric force after 20 eccentric contractions (Fig. 5A) In response to short term treatment with 10 or 1 mg/kg GsMTx4-D, we found a significant protection (~43% and 30%, respectively) from the eccentric contraction induced injury.
Fig. 5.
(A) Following 20 EC contractions D2-mdx (red) show a significant force decline when compared to DBA2 (black; 1st contraction in solid, 20th in dashed) Vertical bar = 0.1 N/cm, and Horizontal bar = 100 ms. (B) Treatment with either the target or low concentration of GsMTx4-D protected the D2.mdx against contraction induced decline in force. (C) The percentage of muscle fibers that accumulated EBD post EC contraction was not impacted by GsMTx4-D treatment. (n = 10 mice/cohort, with forces and dye incorporation showing means ±SE). *= p < 0.05 ANOVA.
Prior to the contractility testing, mice were injected (24 h prior; n = 5 per group; i.p. injected) with Evans Blue dye (EBD). Following the eccentric contraction protocol (15 min post), the gastrocnemius was flash frozen and transversely sectioned to assess EBD accumulation in the muscle fibers. Consistent with the increased susceptibility to eccentric injury seen in the D2.mdx, we identified a significant increase in the percentage of EBD positive muscle fibers in the D2.mdx vs. the DBA. However, GsMTx4 treatment had no significant impact on preventing the eccentric contraction induced EBD dye accumulation (Fig. 5B).
3.2.4. Histopathology
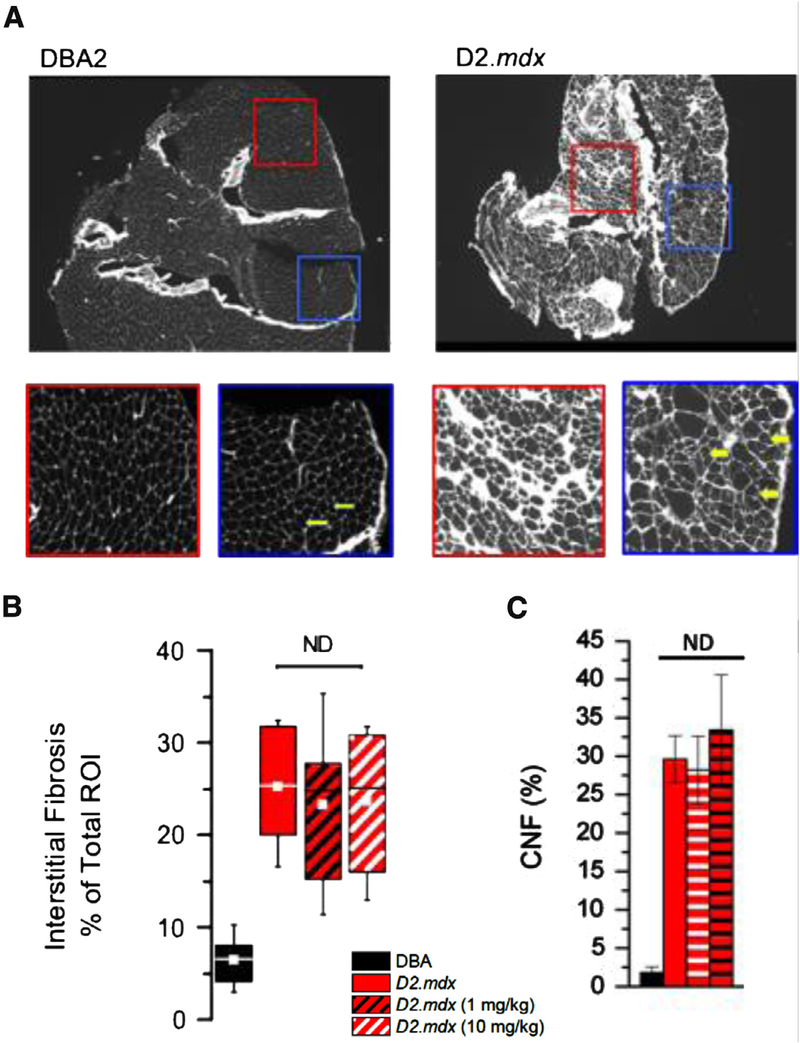
Reports have aligned the enhanced dystrophic phenotype in the D2.mdx to an increase in inflammatory signaling of the DBA2 background. In this strain a mutation in Latent TGF Binding Protein 4 (LTBP4) [25,29] underscores a dramatic increase in fibrosis in the D2.mdx [29]. In muscle cryosections we used fluorescent conjugated wheat germ agglutinin (Texas red) to quantify the abundance of interstitial fibrosis and to identify the presence of centrally nucleated muscle fibers (CNF). As previously reported [25,29], we found significantly greater levels of interstitial fibrosis and CNF’s in the D2.mdx compared to DBA2 (Fig. 6A). Treatment with GsMTx4-D had no impact on the level of fibrosis or CNF’s in the D2.mdx muscle (Fig. 6B and C).
Fig. 6.
WGA staining denotes interstitial fibrotic accumulation and central nuclei. (A) Shows representative cryosections from WGA stained DBA2 and D2.mdx muscle after six weeks of disease progression. Expanded regions of interest (ROI) below the main images highlight the accelerated fibrosis and fiber irregularity in the DBA2-mdx muscle. Central nuclei are readily distinguished in these images and are indicated by arrows. (B) Quantitatively shows the significant increase in fibrosis in DBA2-mdx over DBA2 WT, and that GsMTx4-D treatment has no significant effect on the level of fibrosis. (C) The percent of myofibers with central nucleated fibers’s (CNF) revealed no difference following treatment. (n = 10 mice/cohort, with WGA staining and central nucleation values showing means ±SE).
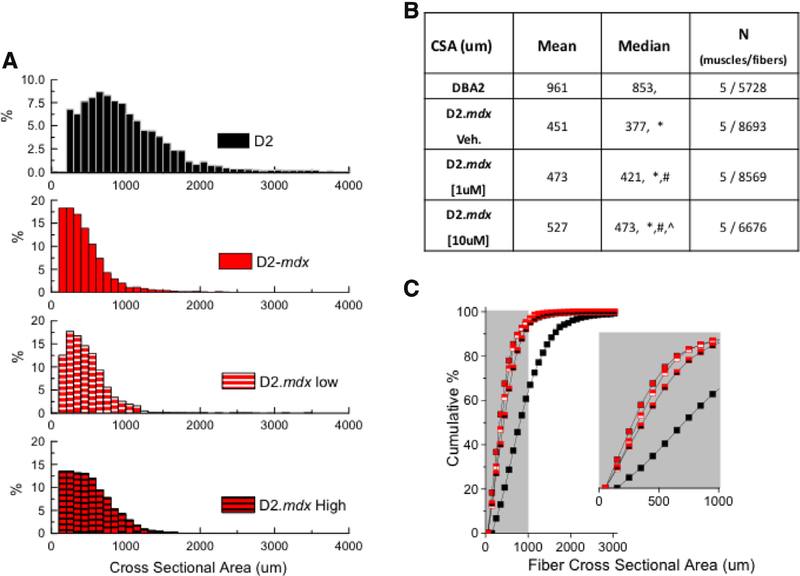
The muscle fiber cross-sectional area (CSA) was quantified to further elucidate the effect of GsMTx4-D on gastrocnemius size. Population histograms of CSA (Fig. 7A) revealed a relative absence of hypertrophic muscle fibers, and an increased percentage of smaller diameter myofibers in the D2.mdx, an effect further highlighted when results were visualized as a plot of CSA cumulative percentage (Fig. 7C). This result aligned with a previous report in the D2.mdx [25]. In response to GsMTx4-D treatment we revealed that myofibers from the [1 μM] group had a larger overall diameter than the non-treated D2.mdx with the [10 uM] treatment group having an even larger impact when compared to the low-dose treated or the non-treated D2.mdx. Taken together, we conclude that GsMTx4-D treatment impacted muscle function by sparring the disease-dependent decrease in muscle fiber CSA.
Fig. 7.
(A) Population histogram of myofibers cross-sectional area (CSA) with mean and median values summarized in (B) Results visualized as plot of CSA cumulative percentage. Non-parametric K-sample analysis (w/ Kruskal Wallis ANOVA; p < 0.05) was used to determine the impact of disease and treatment on muscle fiber CSA. *p < 0.05 vs. DBA2, # p < 0.05 vs. vehicle, ^p < 0.05 vs. 1 μM (GsMTx4).
4. Discussion
4.1. Pharmacokinetics
Our previous [23] and current pharmacokinetic studies demonstrated our ability to achieve a [0.1–5 μM] of GsMTx4-D in skeletal muscle and heart by subcutaneous injections. Most pharmaceutical peptides of less than 70 kDa (kidney filtration cutoff size) suffer from short half-lives due to filtration by the kidney and susceptibility to rapid enzymatic digestion [30] (e.g. 1–2 min for serum half-life of glucagon [31] ). However D amino acid peptides are less susceptible to enzymatic digestion which affords longer half-lives. The wash-out study revealed that concentrations in gastrocnemius, heart and diaphragm muscle were still within ~20% of the peak concentration a week after ceasing injections; concentrations still within the expected therapeutic range. The long half-life of GsMTx4-D in tissues, along with its rapid depletion from the blood, suggests that GsMTx4-D has a higher affinity for tis- sue than for serum proteins, and that GsMTx4-D is resistant to enzymatic digestion or instability.
4.2. Molecular targets
The specific target(s) of GsMTx4 in skeletal muscle need to be rigorously determined. However, both the L and D enantiomers have been shown to inhibit mechanosensitive Piezo channels [32] and multiple TRP channel family members [20,33–35] expressed in heterologous systems. GsMTx4 inhibits endogenous cationic MSCs and exogenously expressed Piezo 1 channels in outside-out patches with a KD of ~0.5 μM [15,32]. The D enantiomer shows strong inhibition of endogenous MSCs in muscle cell types such as chick cardiac cells [22] and murine skeletal muscle myotubes [10,14].
We have shown that GsMTx4 associates superficially with membranes under low tension, and penetrates deeper when membrane tension increases acting as an area/tension clamp for the outer monolayer [16,36]. Electron micrographic studies of the Piezo1 channels place the mechanosensing domains for these channels on the outer monolayer [37], supporting GsMTx4 inhibition of these channels. However, relief of tension in the outer monolayer would transfer tension to the cytoplasmic monolayer. Interestingly, GsMTx4 appears to potentiate K+ selective 2P domain channels [16] which are predicted to have gating elements sensitive to tension changes in the inner monolayer [38,39]. Potentiation of K+ flux would tend to repolarize cells decreasing the activity of voltage gated cationic fluxes.
Functioning as a tension clamp, GsMTx4 may also affect enzymatic mechanosensitive signaling molecules like Nox2 (gp91phox) and gp22phox subunits, that are part of a transmembrane protein complex that generates reactive oxygen species (ROS) as signaling molecules [40]. The active complex responds to mechanical stress in both cardiac and skeletal muscle [41], and we have shown that Nox2 activity is sensitive to sarcolemmal mechanical integrity and microtubule assembly [13]. Indeed, we have shown that GsMTx4-L inhibits the Ca2+ influx pathways triggered by Nox2-ROS production [12]; however, it is unclear whether mechanically sensitive ROS production is inhibited directly by GsMTx4 inhibiting Nox2. Never-the-less, this work and others [12,13,42] support a model in which eccentric contraction-induced force loss is driven by an increase in sarcolemmal Ca2+ influx, a pathway inhibited by GsMTx4.
4.3. Toxicity
There is growing evidence that MSC’s contribute to many physiological functions including arterial pressure regulation [43–46], the skeletal muscle pressor reflex [47], cardiac stretch induced slow force response [20], red blood cell volume regulation [48,49], neuronal touch and pain sensation [50–52], mechanotransduction of sound within the inner ear [53,54], and renal epithelial luminal pressure detection [55]. While we showed that the brain receives almost no GsMTx4-D, a finding consistent with previous studies [23], we showed elevated levels in the kidneys and liver compared to the intended muscular target tissues. Despite the pharmacokinetics analyses suggesting there would be a minimum of 20–60 μM in these organs throughout the six-week study, we observed no overt signs of toxicity. This lack of toxicity aligns with our recently proposed model for GsMTx4-D’s action [16] in which ideal peptide concentrations act to suppress outer monolayer tension that is beyond the bounds of physiological stress, acting solely during conditions of pathological stress. It is important to note that the majority of cell and tissue-based experiments describing GsMTx4 effects on MSC function (i.e. single channel gating in pipette patches, cancer cell migration, atrial fibrillation, blood pressure regulation, peripheral pain sensation, etc.) were conducted under non-physiological stress conditions. While we have some idea of mechanical stress cells experience in vitro, our understanding of the actual stresses and thresholds for cellular mechanosensitivity in situ or in vivo are largely unknown. Nonetheless, it will be important to determine if the peptide elicits adverse effects over longer time frames, and particularly during growth and development, and in mechanically sensitive tissues like the kidney where high levels of GsMTx4-D accumulates.
4.4. GsMTx4-D proffers benefit in D2.mdx mice
Here we show that GsMTx4-D treatment blunts the decline in muscle mass and improves the functional capacity (i.e., the isometric torque) of D2.mdx skeletal muscle. As normalizing isometric torque to the mass of the muscle accounted for the drop in the total torque output, we conclude that GsMTx4-D’s effect was primarily on slowing the disease-dependent decline in muscle mass. Given that this decline is driven by an enhanced calcium-dependent proteolysis and necrosis in dystrophic muscle fibers [42], the GsMTx4-induced suppression of MSC-dependent cation/Ca2+ influx is a parsimonious explanation for these results.
Reduction in contraction injury is a well-established preclinical endpoint in murine DMD [56]. GsMTx4-D also decreased susceptibility of skeletal muscle to EC injury by ~50%, a degree of protection similar to our previous findings with microtubule targeted therapeutics [12,13] or inhibition of Nox2 activity [12] and other signaling pathways linked to excessive MSC activity [57]. Furthermore, these findings are aligned with our recent study demonstrating GsMTx4-D’s function as a cardio-protectant in ischemic reperfusion injury [23]. Given that DMD patients succumb to cardiovascular and pulmonary pathologies, we speculate that GsMTx4-D’s benefit may extend from skeletal muscle to include heart.
Even though potent protection from force loss was observed in GsMTx4-treated D2.mdx mice, we found no difference in EBD incorporation, a classic measure of membrane fragility [58]. While the EBD result is in apparent contrast to the reduced contraction-induced dye accumulation in GsMTx4 treated mdx muscles in vitro [59], the concentration used in vitro (10 μM) was significantly above those reached with chronic in vivo dosing in this study (0.5–2 μM). Furthermore, given that GsMTx4-D had no impact on the level of interstitial fibrosis, and fibrosis changes the mechanical stress to muscle fibers during contraction, it is reasonable to hypothesize that altered mechanics may be a mechanism that drives the incorporation of EBD into the muscle. To this end, EBD has been shown to pass through connexin hemichannels [60]. Given reports of increased connexin permeability in dystrophic muscle [61], a disconnection between the protection of contraction injury and the permeability of EBD may lie within the dysregulated function of connexins.
4.5. GsMTx4-D therapeutic potential
GsMTx4-D is the first member in a class of compounds targeting MSCs. The therapeutic potential for GsMTx4-D is not as a cure, rather as a drug that can act by slowing disease progression. Given growing evidence that dysregulated Ca2+ signals from sarcolemmal MSC’s driving is pathognomonic across several dystrophies, GsMTx4-D may prove effective in other forms of dystrophy as well.
Given the genetic basis of DMD, gene editing and exon skipping therapies that can restore dystrophin expression have the potential to cure the disease. While dystrophin expression to ~10% of normal levels is thought necessary to realize clinical benefits, these levels may be difficult to achieve based on current technology. In fact, phase III trials with eteplirsen, the FDA approved morpholino exon skipping drug, has shown ~1% of normal dystrophin levels over 180 weeks [62]. New evidence that normalizing dysregulated Ca2+ signaling improves exon skipping efficiency [63] raises the intriguing possibility that compunds like GsMTx4-D may be necessary adjuvants to realize effective genetic cures.
5. Conclusion
GsMTx4-D treatment partially protects D2.mdx mice from the disease associated loss of muscle mass and protects from contraction induced injury. These findings, in combination with a recent report of GsMTx4-D’s cardioprotective effects, support further development of GsMTx4-D for treatment of musclar pathology.
Acknowledgments
This work was supported by funding from Tonus Therapeutics and Akashi Therapeutics (where GsMTx4 has been renamed AT-300). We would also like to thank Mr. Jeff Harvey (CSO of Tonus Therapeutics) for his generous financial support for these studies.
References
- [1].Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82(2):291–329. [DOI] [PubMed] [Google Scholar]
- [2].Mallouk N, Jacquemond V, Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc Natl Acad Sci USA 2000;97(9):4950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Backer F, Vandebrouck C, Gailly P, Gillis JM. Long-term study of Ca(2+) homeostasis and of survival in collagenase-isolated muscle fibres from normal and mdx mice. J Physiol. 2002;542(Pt 3):855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Phillips MF, Quinlivan R. Calcium antagonists for Duchenne muscular dystrophy. The Cochrane Library; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ruegg UT, Nicolas-Metral V, Challet C, Bernard-Helary K, Dorchies OM, Wagner S, et al. Pharmacological control of cellular calcium handling in dystrophic skeletal muscle. Neuromuscul Disord. 2002(Suppl 1):S155–61. [DOI] [PubMed] [Google Scholar]
- [6].Jørgensen LH, Blain A, Greally E, Laval SH, Blamire AM, Davison BJ, et al. Long-term blocking of calcium channels in mdx mice results in differential effects on heart and skeletal muscle. Am J Pathol 2011;178(1):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haws CM, Winegar BD, Lansman JB. Block of single L-type Ca2+ channels in skeletal muscle fibers by aminoglycoside antibiotics. J Gen Physiol 1996;107:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Malik V, Rodino-Klapac LR, Viollet L, Mendell JR. Aminoglycoside-induced mutation suppression (stop codon readthrough) as a therapeutic strategy for Duchenne muscular dystrophy. Therap Adv Neurol Disord 2010;3(6):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Du X-J, Anderson KE, Jacobsen A, Woodcock EA, Dart AM. Suppression of ventricular arrhythmias during ischemia-reperfusion by agents inhibiting Ins (1, 4, 5) P 3 release. Circulation 1995;91(11):2712–16. [DOI] [PubMed] [Google Scholar]
- [10].Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J Physiol 2007;581 (Pt 1):369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Franco-Obregon A Jr, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol 1994;481:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, et al. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal 2012;5(236):ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 2015:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562(Pt 2):367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suchyna TM, Johnson JH, Clemo HF, Huang ZH, Gage DA, Baumgarten CM, et al. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks stretch activated channels. J Gen Physiol 2000;115:583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J 2017;112(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol 2006;575(Pt 3):913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gailly P, De Backer F, Van Schoor M, Gillis JM. In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking mdx mice. J Physiol 2007;582(Pt 3):1261–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, et al. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol 2008;28(8):2637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG. Stretch-activated channels in the heart: contributions to length--dependence and to cardiomyopathy. Prog Biophys Mol Biol 2008;97(2–3):232–49. [DOI] [PubMed] [Google Scholar]
- [21].Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol 2007;292(2):H846–55. [DOI] [PubMed] [Google Scholar]
- [22].Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 2004;430(6996):235–40. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Ma Y, Sachs F, Li J, Suchyna TM. GsMTx4-D is a cardioprotectant against myocardial infarction during ischemia and reperfusion. J Mol Cell Cardiol 2016;98:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation – a peptide from spider venom can prevent the heartbeat from losing its rhythm. Nature 2001;409(6816):35–6. [DOI] [PubMed] [Google Scholar]
- [25].Coley WD, Bogdanik L, Vila MC, Yu Q, Van Der Meulen JH, Rayavarapu S, et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum Mol Gen 2016;25(1):130–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fukada S-i, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yam-aguchi M, et al. Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol 2010;176(5):2414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Teichmann MD, Wegner FV, Fink RH, Chamberlain JS, Launikonis BS, Martinac B, et al. Inhibitory control over Ca(2+) sparks via mechanosensitive channels is disrupted in dystrophin deficient muscle but restored by mini-dystrophin expression. PLoS One 2008;3(11):e3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol 2014;117(4):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, et al. Latent TGF-β-binding protein 4 modifies muscular dystrophy in mice. J Clin Invest 2010;120(2):645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diao L, Meibohm B. Pharmacokinetics and pharmacokinetic–pharmacodynamic correlations of therapeutic peptides. Clin Pharmacokinet 2013;52(10):855–68. [DOI] [PubMed] [Google Scholar]
- [31].Strohl WR. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs 2015;29(4):215–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011;50(29):6295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical de- formation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calc 2009;45(1):38–54. [DOI] [PubMed] [Google Scholar]
- [34].Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflügers Arch Eur J Physiol 2010;460(3):571–81. [DOI] [PubMed] [Google Scholar]
- [35].Parpaite T, Cardouat G, Mauroux M, Gillibert-Duplantier J, Robillard P, Quignard J-F, et al. Effect of hypoxia on TRPV1 and TRPV4 channels in rat pulmonary arterial smooth muscle cells. Pflügers Arch Eur J Physiol 2016;468(1):111–30. [DOI] [PubMed] [Google Scholar]
- [36].Nishizawa K, Nishizawa M, Gnanasambandam R, Sachs F, Sukharev SI, Suchyna TM. Effects of Lys to Glu mutations in GsMTx4 on membrane binding, peptide orientation, and self-association propensity, as analyzed by molecular dynamics simulations. Biochim Biophys Acta (BBA) Biomembr 2015;1848(11):2767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018;554(7693):487. [DOI] [PubMed] [Google Scholar]
- [38].Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 1998;17(15):4283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid-and mechano-sensitive K+ ion channel. Science 2012;335(6067):436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Ann Rev Pathol Mech Dis 2014;9:119–45. [DOI] [PubMed] [Google Scholar]
- [41].Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 2011;333(6048):1440–5. [DOI] [PubMed] [Google Scholar]
- [42].Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 2006;33(7):657–62. [DOI] [PubMed] [Google Scholar]
- [43].Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 2006;103(44):16586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fanchaouy M, Bychkov R, Meister JJ, Beny JL. Stretch-elicited calcium responses in the intact mouse thoracic aorta. Cell Calc 2007;41(1):41–50. [DOI] [PubMed] [Google Scholar]
- [45].Gilbert G, Ducret T, Marthan R, Savineau J-P, Quignard J-F. Stretch-induced Ca2+ signaling in vascular smooth muscle cells depend on Ca2+ store segregation. Cardiovasc Res 2014:cvu069. [DOI] [PubMed] [Google Scholar]
- [46].Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, et al. A PLC (gamma) 1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 2014;7(327):ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 2016;594(3):641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vandorpe DH, Xu C, Shmukler BE, Otterbein LE, Trudel M, Sachs F, et al. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One 2010;5(1):e8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stewart AK, Vandorpe DH, Heneghan JF, Chebib F, Stolpe K, Akhavein A, et al. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol 2010;298(2):C283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014;509(7502):622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Park SP, Kim BM, Koo JY, Cho H, Lee CH, Kim M, et al. A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. Pain 2008;137(1):208–17. [DOI] [PubMed] [Google Scholar]
- [52].Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 2009;29(19):6217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 2014;144(1):55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci 2017;20(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep 2013;14(12):1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Musc Nerve 2009;39(5):591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ismail HM, Scapozza L, Ruegg UT, Dorchies OM. Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PloS One 2014;9(10):e110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev 2016;96(1):253–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromusc Disord 2006;16(12):845–54. [DOI] [PubMed] [Google Scholar]
- [60].Cisterna BA, Vargas AA, Puebla C, Sáez JC. Connexin hemichannels explain the ionic imbalance and lead to atrophy in denervated skeletal muscles. Biochim Biophys Acta (BBA) Mol Basis Dis 2016;1862(11):2168–76. [DOI] [PubMed] [Google Scholar]
- [61].Cea LA, Puebla C, Cisterna BA, Escamilla R, Vargas AA, Frank M, et al. Fast skeletal myofibers of mdx mouse, model of Duchenne muscular dystrophy, express connexin hemichannels that lead to apoptosis. Cell Mol Life Sci 2016;73(13):2583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lim KRQ, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Dev Therapy 2017;11:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kendall GC, Mokhonova EI, Moran M, Sejbuk NE, Wang DW, Silva O, et al. Dantrolene enhances antisense-mediated exon skipping in human and mouse models of Duchenne muscular dystrophy. Sci Trans Med 2012;4(164):164ra160. [DOI] [PubMed] [Google Scholar]