Abstract
Autism-spectrum disorder (ASD) is a neurodevelopmental disorder characterized by persistent deficits in social communication and repetitive patterns of behavior. ASD is, however, often associated with medical comorbidities and gastrointestinal (GI) dysfunction is among the most common. Studies have demonstrated a correlation between GI dysfunction and the degree of social impairment in ASD. The etiology of GI abnormalities in ASD is unclear, though the association between GI dysfunction and ASD-associated behaviors suggest that overlapping developmental defects in the brain and the intestine and/or a defect in communication between the enteric and central nervous systems (ENS and CNS, respectively), known as the gut-brain axis, could be responsible for the observed phenotypes. Brain-gut abnormalities have been increasingly implicated in several disease processes, including ASD. As a critical modulator of ENS and CNS development and function, serotonin may be a nexus for the gut-brain axis in ASD. This paper reviews the role of serotonin in ASD from the perspective of the ENS. A murine model that has been demonstrated to possess brain, behavioral and GI abnormalities mimicking those seen in ASD harbors the most common serotonin transporter (SERT) based mutation (SERT Ala56) found in children with ASD. Discussion of the gut-brain manifestations in the SERT Ala56 mice, and their correction with developmental administration of a 5-HT4 agonist, are also addressed in conjunction with other future directions for diagnosis and treatment.
Introduction
Autism-spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by persistent deficits in social communication and repetitive patterns of behavior [1]. The prevalence of ASD among school-age children in the US has more than doubled over the past decade, with recent data from the Center for Disease Control estimating a prevalence rate of 1 in 68 [2, 3]. Despite the rapid increase in prevalence, the precise etiologies of most cases of ASD remain unknown.
Though classified as a neurodevelopmental condition, ASD is often associated with multiple medical comorbidities and gastrointestinal (GI) problems are among the most common [4–6]. Of the GI conditions experienced in ASD, constipation is reported most frequently [6, 7]. GI symptoms occur at a four-fold greater rate in ASD when compared to the general population [7] and studies have demonstrated a correlation between the degree of social impairment and the likelihood of constipation [8]. Further, subsets of individuals with functional constipation and ASD exhibit more severe behavioral problems, including self-injury, aggression, and rigid-compulsivity [9–11]. The etiology of constipation and GI dysfunction in ASD is unclear. The association between GI dysfunction and ASD-associated behaviors raise questions as to whether an abnormal gut-brain connection exists in ASD and/or a dysregulation of factors that critically affect enteric and central nervous system (ENS and CNS, respectively) development is present.
The gut-brain axis is defined as the bidirectional communication between the CNS and ENS. One of the factors that affects this axis, the intestinal microbiome, has received considerable attention [12–14]. Dysregulation of the interactions between the brain, the intestine and the enteric microbiome has been implicated in several disease processes involving both the CNS and ENS, including, in addition to ASD [15–17], functional GI disorders such as irritable bowel syndrome [18, 19], psychiatric disorders such as anxiety and depression [20, 21], as well as Parkinson’s disease [22].
Serotonin as a link in the gut-brain axis
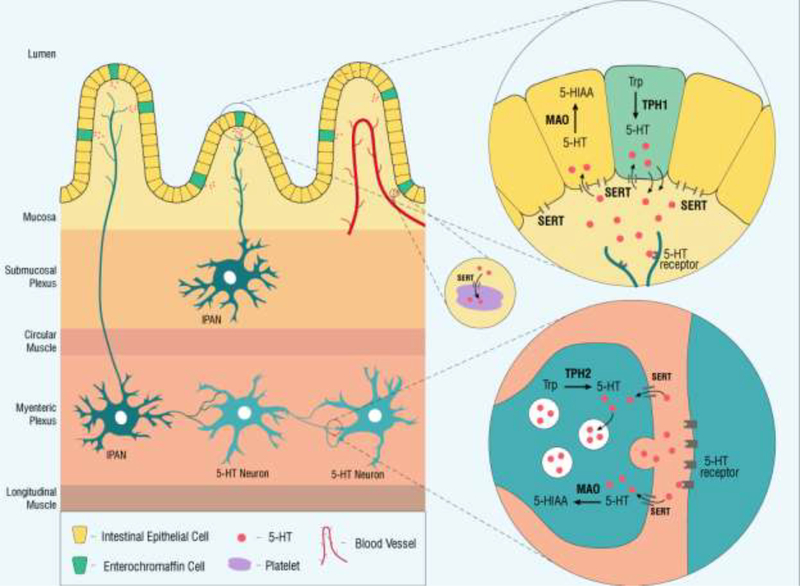
There is evidence to suggest that serotonin (5-hydroxytryptamine, 5-HT) may be a nexus for the gut-brain-microbiome axis [23]. During CNS development, 5-HT plays critical roles in the regulation of neuronal differentiation and migration, as well as axonal outgrowth, myelination and synapse formation [24, 25]. Enteric 5-HT, which accounts for >90% of the body’s 5-HT stores, is contained in two primary reservoirs: in the intestinal epithelium, where it is produced by enterochromaffin (EC) cells, and in neurons of the ENS [26]. Enteric 5-HT production is controlled by two different isoforms of the same rate-limiting enzyme, tryptophan hydroxylase (TPH), based on its locales (Figure 1). Synthesis of neuronal 5-HT, both in the CNS and ENS, is regulated by TPH2, while EC-cell derived 5-HT is produced by TPH1 [26].
Figure 1. Schematic of the synthesis, inactivation and degradation of 5-HT in the intestinal epithelium and ENS.
5-HT is synthesized in the intestinal epithelium by enterochromaffin (EC) cells from tryptophan (Trp) by the rate-limiting tryptophan hydroxylase 1 (TPH1). Luminal distention results in the basal release of 5-HT into the interstitial space of the lamina propria, leading to activation of 5-HT receptors on intrinsic primary afferent neurons (IPANs) in both the submucosal and myenteric plexuses. 5-HT is inactivated through the actions of the serotonin reuptake transporter (SERT), which is expressed by intestinal epithelial cells. Once intracellular, 5-HT is degraded by monoamine oxidase (MAO) into 5-hydroxyindoleacetic acid (5-HIAA). Platelets also express SERT; they are thought to reflect stores of intestinal epithelial 5-HT that are picked up as platelets pass through enteric circulation. The ENS contains serotonin-synthesizing neurons. Enteric neurons utilize a different isoform of tryptophan hydroxylase, TPH2, to synthesize serotonin from tryptophan, which is then stored in synaptic vesicles. Secreted 5-HT activates post-synaptic receptors and is then inactivated through pre-synaptic reuptake by SERT, where it can be packaged into vesicles once again for release or degraded by MAO into 5-HIAA.
Although the vast majority of intestinal 5-HT is located in the EC cells, it is the neuronal pool of 5-HT that is most critical for ENS development and motility [26, 27]. During ENS development, serotonergic neurons are among the first inhabitants of the ENS, where they impact neurogenesis and guide the development and survival of late-born neurons, including those expressing dopamine, gamma-Aminobutyric acid (GABA) and calcitonin gene-related peptide (CGRP) [28]. Further, genetic deletion of TPH2 leads to a reduction of neuron density in both neuronal plexuses of the ENS, as well as slowed intestinal transit both in vivo and in vitro [27]. GI motility was initially thought to be entirely dependent on neuronal 5-HT because no in vivo motility abnormalities in GI transit were detected in mice lacking epithelial 5-HT (TPH1KO) [27]. Motility abnormalities in TPH1KO mice, however, have more recently been observed; generation of peristaltic reflexes do not occur in TPH1KO mice with mucosal stimulation and instead require stretching of the gut wall. Further, even when they are produced, these contractions demonstrate deficiencies in propagation [29]. This may be because the absence of 5-HT in EC cells interferes with mucosal activation of peristaltic reflexes [30]. Because, however, peristalsis can be evoked with stretching, it is also possible that other paracrine messengers or direct stimulation of stretch-sensitive nerve fibers in the bowel are able to substitute for the role of 5-HT in TPH1KO mice [31].
5-HT is inactivated by monoamine oxidase (MAO); because, however, MAO is located intracellularly, secreted 5-HT needs to be taken back up into the cell before it can be inactivated. Because 5-HT is a highly charged molecule, it cannot passively diffuse across cell membranes and thus requires transport. The serotonin reuptake transporter (SERT; Slc6a4) is primarily responsible for this intracellular transport (Figure 1). Increased or decreased levels of SERT can thus lead to decreased or increased levels, respectively, of the 5-HT available for neurotransmission in the brain and the intestine [32]. Murine models possessing abnormalities in SERT during development, such as SERT knockout mice, as well as mice that receive selective serotonin reuptake inhibitors during pregnancy and breastfeeding, exhibit ENS hyperplasia as well as long-lasting deficits in ENS-mediated functions, including in vivo and in vitro gastrointestinal motility [33].
Because 5-HT impacts the development of both the central and enteric nervous systems, 5-HT and/or its modulators (e.g., SERT) could play an important role in the both the brain and intestinal anomalies in ASD. 5-HT has been associated with ASD since the 1960s [34] when studies revealed that approximately 30% of individuals with ASD exhibited an increase in whole-blood 5-HT levels, termed hyperserotonemia [35, 36]. Whole-blood 5-HT, which is primarily a measure of 5-HT stored in platelets, is likely to be derived almost entirely from the intestine [37]; although platelets express SERT, they do not possess the enzymes necessary to synthesize 5-HT [38]. Instead, 5-HT is synthesized in EC cells by TPH1, secreted into the gut lumen, and thought to be incorporated into platelets as they pass through the enteric circulation [26]. In this subset of ASD patients, therefore, elevations of blood 5-HT levels may be indicative of dysregulation of gastrointestinal 5-HT secretion. In fact, higher whole-blood 5-HT levels have been shown to correlate with constipation in children with ASD [39]. It is also plausible, however, that other peripheral anomalies in 5-HT production and regulation may contribute to hyperserotonemia, such as changes in platelet 5-HT uptake or alterations in the proportion of free 5-HT cleared through the hepatic and/or pulmonary systems [40]. The precise connection(s) between hyperserotonemia and ASD pathophysiology, however, remain enigmatic. Although studies demonstrate a strong heritability of whole-blood 5-HT levels [41–44], the clinical correlates between hyperserotonemia and ASD-associated behaviors (e.g., stereotypy and self-injury) have been inconsistent [36, 45–47].
The serotonin reuptake transporter (SERT) in ASD
Investigators have begun to elucidate the role(s) of Slc6a4, the gene which encodes SERT, in individuals with ASD, in addition to the link between Slc6a4 variants and hyperserotonemia [48, 49]. A genome-wide study of whole-blood 5-HT as a quantitative trait demonstrated associations with Slc6a4 [49] and linkage studies in ASD have implicated the 17q11.2 region containing Slc6a4 [50, 51]. Because common Slc6a4 variants have a limited connection to ASD, investigators screened Slc6a4 for rare variants in multiplex families that demonstrated strong linkage to 17q11.2. They identified five variants that were overexpressed in the individuals with ASD; each variant was a gain-of-function mutation that conferred a hyperactivity to SERT [36, 52]. The most common of these variants, SERT Ala56, was found to be associated with both rigid-compulsive behavior and sensory aversion [53, 54]. The mutation was subsequently incorporated into a murine model and entitled the “SERT Ala56 mouse.” SERT Ala56 mice exhibit whole-blood hyperserotonemia as well as brain and behavioral anomalies, including increased 5-HT clearance in the CNS, hypersensitivity of CNS 5-HT1A and 5-HT2A receptors, and behavioral anomalies emulating those in ASD including repetitive behaviors, delayed communication and deficits in social interactions [55]. It was more recently determined that the SERT Ala56 mice also possess abnormalities in ENS development [56]. Specifically, the ENS of the SERT Ala56 mice exhibits a markedly lower total neuron count, with a particular deficit in late-born neuronal subsets whose development are under serotonergic control [33]. As a consequence of abnormal ENS development, SERT Ala56 mice have abnormally slowed in vivo and in vitro motility throughout the entire intestine, including a decrease in the frequency and speed of colonic peristaltic contractions, which are thought to be under almost exclusive control of the ENS [33]. Further, these mice also exhibit differences in the intestinal mucosa, including an increase in EC cells as well as Tph1 transcripts, both of which may be contributory to the demonstrated increase in whole blood 5-HT levels [33].
Interestingly, it may be possible to bypass the abnormalities caused by the SERT Ala56 mutation, by modulation of the 5-HT4 receptor. The 5-HT4 receptor has been implicated in ENS neurogenesis and colonic motility [57–59]. It was recently demonstrated that administration of the highly selective 5-HT4 agonist, prucalopride during critical periods of neurodevelopment, including gestation and breastfeeding, normalized enteric neuronal numbers in the SERT Ala56 mice and also provided long-term rescue of colonic motility [33].
Serotonin and the gut-brain-microbiome axis in ASD
Emerging evidence is supportive of the idea that specific intestinal bacteria may play important roles in regulating levels of EC cell-derived 5-HT; levels of whole-blood 5-HT, which may largely reflect production of 5-HT in EC cells, are decreased in germ-free mice [60]. This may be a direct result of impaired bacterial production or secondary to the effects of intestinal metabolites produced by the microbiota. For example, short-chain fatty acids, which are produced by enteric bacteria that ferment dietary saccharides, including Clostridial species, have been shown to increase levels of Tph1 mRNA in EC cells and, subsequently, increase intestinal 5-HT levels without changing SERT expression [61]. Further, fecal metabolites produced by spore-forming bacteria, and particularly Clostridial species, have been shown to increase 5-HT levels in EC cell cultures as well as in the colon and blood of germ-free mice [62]. Conversely, 5-HT stimulates the growth of the commensal facultative intestinal anaerobes Enterococcus faecalis and Escherischia coli in cell culture [63, 64].
Several transgenic mouse models possessing both ASD-associated behaviors and GI anomalies further support the notion that an enteric serotonin-microbiome connection exists. SERT Ala56 mice demonstrate abnormalities in the intestinal microbiome that correlate with slowed GI transit (unpublished data). Further, the BTBR T+ Itpr3tf/J (BTBR) mouse model, which exhibits core behavioral features of ASD including deficits in social interactions and engagement in repetitive behaviors [65], also demonstrates changes in intestinal microbiota that correlate with slowed gastrointestinal transit and impaired production of intestinal 5-HT [66].
Genetic abnormalities may not be the only etiology underlying the connection between the gut-brain-microbiome axis and 5-HT. A prominent environmental risk factor for ASD may be in utero exposure to fever or infection, particularly of viral origin, with recent data suggesting that children with ASD and GI problems may be particularly likely to have been exposed [56, 67–70]. Additionally, immune system impairment has been noted in both mothers of ASD patients and in ASD patients themselves [71]. ASD patients with comorbid GI dysfunction specifically exhibit altered innate immune responses and distinct pro-inflammatory transcriptional profiles compared to ASD patients without GI dysfunction [72, 73]. The relationship between maternal infection, immunity and a gut-brain link in ASD has been replicated in mouse models using the maternal immune activation (MIA) paradigm. Changes in behavior, CNS neurodevelopment, intestinal permeability and the microbiome that mimic ASD have been reported in mice exposed to MIA via administration of polyinosinic:polycytidylic acid (poly I:C), which models viral infection [74, 75]. Recent data from this mouse model also show alterations in CNS and intestinal 5-HT levels which accompany the changes demonstrated in the GI microbiome and ASD-relevant behaviors [75, 76, 77, 78]. In one such model, investigators noted a dysbiosis in the stool of MIA progeny mice, particularly in the classes Clostridia and Bacteroidia. Interestingly, treating the dysbiosis in these mice with Bacteroides fragilis corrected both the GI permeability defects and ASD-related behavioral abnormalities [75]. Further, MIA offspring display significantly increased levels of serum indolepyruvate, a key molecule involved in tryptophan metabolism; this imbalance was also corrected with treatment with B. fragilis [75].
The link between specific species of the enteric microbiome, 5-HT and GI symptoms has also recently been demonstrated in children with ASD. In the only multi-omics study of its kind thus far, distinct connections were made between the GI microbiome, intestinal 5-HT levels and gastrointestinal pain. Specifically, the investigators identified significant associations between several enteric mucosa-associated Clostridial species and levels of either tryptophan or serotonin in mucosal supernatant [79].
Although distinct microbial differences have been noted individuals with ASD, studies have been small, utilized very different methods of analysis and have evaluated dissimilar patient populations [80]. These may be some of the reasons why no precise microbial composition has been identified. A likely reason, however, why a unifying microbial composition is unlikely to be found is due to the large heterogeneity of ASD phenotypes [81]. This issue may be addressed more adequately in a large-scale study that takes ASD phenotypes into account.
Future directions
There is an urgent need to develop novel therapies for ASD. Although over 70% of these individuals are taking medication(s) [82], existing treatments are used primarily for specific symptoms (e.g., irritability and agitation) [83], without the ability to address the underlying neurobehavioral etiologies. Efficacy-based studies have failed to demonstrate an impactful benefit of pharmacotherapy to the underlying disorder [84, 85, 83]. Although exploration into the role of 5-HT and SERT as a gut-brain link in ASD has revealed novel insights, many questions remain. For example, in addition to its presence in the central and enteric nervous systems, SERT is also found in the epithelial cells of the intestine [86], pulmonary endothelial cells [87], and in organs and cells that do not synthesize 5-HT, including platelets and T and B lymphocytes [32]. The specific importance of CNS and ENS-derived 5-HT, in addition to 5-HT from these other systems, must be determined to enhance our ability to create focused, effective therapies.
The gut phenotype rescued in the SERT Ala56 mice by pharmacological modulation of the 5-HT4 receptor highlights the potentially important role that this receptor plays in the ENS development and function [33]. Whether 5-HT4 agonism will result in the rescue of the brain or behavioral abnormalities demonstrated in the SERT Ala56 mice remains to be determined. The expression of 5-HT4 receptors in ASD-relevant regions in the CNS, however, makes this a worthwhile pursuit [88]. It should also be noted that nonselective 5-HT4 agonists have been linked to a greater frequency of cardiovascular adverse events in clinical trials; although the newer, more selective 5-HT4 agonists have an improved safety profile, an analysis of the risks and benefits of their use must be considered in clinical settings [89]. Further, while agonism of the 5-HT4 receptor remains a promising strategy from a preclinical perspective, human studies are required to determine the safety and efficacy of this approach.
Although microbiome studies thus far demonstrate a bidirectional relationship between 5-HT and the enteric microbiota, the precise nature of the in vivo mechanisms governing this relationship have yet to be determined. For example, whether gut microbiome or metabolome abnormalities can cause immune dysregulation, or whether the reverse is true, remains unanswered. Further, findings in murine studies require confirmation in humans. Some therapies involving microbial manipulation may have promise, including those involving probiotics or fecal transplant. Mice administered Lactobacillus reuteri demonstrate improvements in GI and behavioral manifestations [90, 91]. Findings in humans, however, have been limited, and large prospective trials are warranted. Pilot studies evaluating the role of other probiotics are ongoing; an early-phase trial is currently analyzing whether a probiotic mixture of Lactobacilli and Bifidobacteria, particularly L. acidophilus, L. plantarum, L. helveticus, L. paracasei, B. breve and B. lactis can improve GI symptoms and the quality of life in ASD patients [92]. A different study is evaluating the safety and efficacy of a Bifidobacterium-based probiotic (BB-12; B. lactis) in ASD, in addition to whether it improves maladaptive behaviors as well as GI symptoms [93]. Although the single study evaluating GI and behavioral outcomes after fecal transplant in ASD was highly successful [94], the study was small and open-label. Based on these data, however, the investigators have begun recruiting participants for a phase 2 double-blind placebo-controlled trial [95].
There is currently no distinct microbiome profile characteristic of ASD or a specific profile that is associated with its neurobehavioral manifestations. Moreover, whether changes in the microbiome are caused by gastrointestinal dysfunction or, alternatively, whether changes in the microbiome are causative of gastrointestinal problems, remains unclear. A study utilizing a multi-omics approach similar to the one described above, but with a larger cohort is ongoing [96] and may help to identify significant associations between the microbiome, 5-HT, and gastrointestinal dysfunction in ASD [79].
Highlighting the underlying mechanisms by which ASD phenotypes are guided by the developmental impact of serotonin on the ENS, CNS and the enteric microbiome will allow for maturation of our understanding of ASD and will bring us closer to the development of novel therapeutics. Based on its interactions within the CNS, ENS and enteric microbiome, further study of 5-HT as a link in the gut-brain-microbiome axis in ASD is warranted. Despite the current evidence, however, many questions remain.
Acknowledgments
The authors would like to acknowledge funding from the following sources: NIH KO8 DK093786 (KGM), The Department of Defense PR160365 (KGM), The Center for Discovery (KGM) and NIA T35AG044303 (NI).
Footnotes
Neither author has any conflicts of interest regarding the data presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Posar A, Resca F, Visconti P, Autism according to diagnostic and statistical manual of mental disorders 5(th) edition: The need for further improvements, J Pediatr Neurosci 10(2) (2015) 146–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Newschaffer CJ, Falb MD, Gurney JG, National autism prevalence trends from United States special education data, Pediatrics 115(3) (2005) e277–82. [DOI] [PubMed] [Google Scholar]
- [3].Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee LC, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M, Centers for Disease C, Prevention, Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012, MMWR Surveill Summ 65(3) (2016) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Doshi-Velez F, Ge Y, Kohane I, Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis, Pediatrics 133(1) (2014) e54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aldinger KA, Lane CJ, Veenstra-VanderWeele J, Levitt P , Patterns of Risk for Multiple Co-Occurring Medical Conditions Replicate Across Distinct Cohorts of Children with Autism Spectrum Disorder, Autism Res 8(6) (2015) 771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, Bickel J, Wattanasin N, Spence S, Murphy S, Churchill S, The co-morbidity burden of children and young adults with autism spectrum disorders, PLoS One 7(4) (2012) e33224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McElhanon BO, McCracken C, Karpen S, Sharp WG, Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis, Pediatrics 133(5) (2014) 872–83. [DOI] [PubMed] [Google Scholar]
- [8].Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P, Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors, Autism Res 5(2) (2012) 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H, Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report, Pediatrics 125 Suppl 1 (2010) S1–18. [DOI] [PubMed] [Google Scholar]
- [10].Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, Macklin EA, Levitt P, Margolis KG, Beversdorf DQ, Veenstra-VanderWeele J, Association of Rigid-Compulsive Behavior with Functional Constipation in Autism Spectrum Disorder, J Autism Dev Disord 47(6) (2017) 1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaidez V, McNiven S, Vosti SA, Kaiser LL, Sweetened food purchases and indulgent feeding are associated with increased toddler anthropometry, J Nutr Educ Behav 46(4) (2014) 293–8. [DOI] [PubMed] [Google Scholar]
- [12].Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF, Microbiota and neurodevelopmental windows: implications for brain disorders, Trends Mol Med 20(9) (2014) 509–18. [DOI] [PubMed] [Google Scholar]
- [13].Carabotti M, Scirocco A, Maselli MA, Severi C, The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems, Ann Gastroenterol 28(2) (2015) 203–209. [PMC free article] [PubMed] [Google Scholar]
- [14].Collins SM, Surette M, Bercik P, The interplay between the intestinal microbiota and the brain, Nat Rev Microbiol 10(11) (2012) 735–42. [DOI] [PubMed] [Google Scholar]
- [15].Li Q, Zhou JM, The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder, Neuroscience 324 (2016) 131–9. [DOI] [PubMed] [Google Scholar]
- [16].Cryan JF, Dinan TG, Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour, Nat Rev Neurosci 13(10) (2012) 701–12. [DOI] [PubMed] [Google Scholar]
- [17].Mayer EA, Padua D, Tillisch K, Altered brain-gut axis in autism: comorbidity or causative mechanisms?, Bioessays 36(10) (2014) 933–9. [DOI] [PubMed] [Google Scholar]
- [18].Mayer EA, Tillisch K, Gupta A, Gut/brain axis and the microbiota, J Clin Invest 125(3) (2015) 926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rhee SH, Pothoulakis C, Mayer EA, Principles and clinical implications of the brain-gut-enteric microbiota axis, Nat Rev Gastroenterol Hepatol 6(5) (2009) 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Evrensel A, Ceylan ME, The Gut-Brain Axis: The Missing Link in Depression, Clin Psychopharmacol Neurosci 13(3) (2015) 239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foster JA, McVey Neufeld KA, Gut-brain axis: how the microbiome influences anxiety and depression, Trends Neurosci 36(5) (2013) 305–12. [DOI] [PubMed] [Google Scholar]
- [22].Klingelhoefer L, Reichmann H, Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors, Nat Rev Neurol 11(11) (2015) 625–36. [DOI] [PubMed] [Google Scholar]
- [23].O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF, Serotonin, tryptophan metabolism and the brain-gut-microbiome axis, Behav Brain Res 277 (2015) 32–48. [DOI] [PubMed] [Google Scholar]
- [24].Gaspar P, Cases O, Maroteaux L, The developmental role of serotonin: news from mouse molecular genetics, Nat Rev Neurosci 4(12) (2003) 1002–12. [DOI] [PubMed] [Google Scholar]
- [25].Homberg JR, Kolk SM, Schubert D, Editorial perspective of the Research Topic “Deciphering serotonin’s role in neurodevelopment”, Front Cell Neurosci 7 (2013) 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gershon MD, 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract, Curr Opin Endocrinol Diabetes Obes 20(1) (2013) 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD, Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons, J Neurosci 31(24) (2011) 8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD, Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation, J Neurosci 24(6) (2004) 1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK, Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1, J Physiol 591(23) (2013) 5939–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith TK, Gershon MD, CrossTalk proposal: 5-HT is necessary for peristalsis, J Physiol 593(15) (2015) 3225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ, CrossTalk opposing view: 5-HT is not necessary for peristalsis, J Physiol 593(15) (2015) 3229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mawe GM, Hoffman JM, Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets, Nat Rev Gastroenterol Hepatol 10(8) (2013) 473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD, Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function, J Clin Invest 126(6) (2016) 2221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schain RJ, Freedman DX, Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children, J Pediatr 58 (1961) 315–20. [DOI] [PubMed] [Google Scholar]
- [35].Gabriele S, Sacco R, Persico AM, Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis, Eur Neuropsychopharmacol 24(6) (2014) 919–29. [DOI] [PubMed] [Google Scholar]
- [36].Muller CL, Anacker AMJ, Veenstra-VanderWeele J, The serotonin system in autism spectrum disorder: From biomarker to animal models, Neuroscience 321 (2016) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rao M, Gershon MD, The bowel and beyond: the enteric nervous system in neurological disorders, Nat Rev Gastroenterol Hepatol 13(9) (2016) 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mercado CP, Kilic F, Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels, Mol Interv 10(4) (2010) 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, Macklin EA, Levitt P, Gillespie CH, Anderson GM, Margolis KG, Beversdorf DQ, Veenstra-VanderWeele J, Brief Report: Whole Blood Serotonin Levels and Gastrointestinal Symptoms in Autism Spectrum Disorder, J Autism Dev Disord 46(3) (2016) 1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Janusonis S, Origin of the blood hyperserotonemia of autism, Theor Biol Med Model 5 (2008) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Abney M, McPeek MS, Ober C, Broad and narrow heritabilities of quantitative traits in a founder population, Am J Hum Genet 68(5) (2001) 1302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abramson RK, Wright HH, Carpenter R, Brennan W, Lumpuy O, Cole E, Young SR, Elevated blood serotonin in autistic probands and their first-degree relatives, J Autism Dev Disord 19(3) (1989) 397–407. [DOI] [PubMed] [Google Scholar]
- [43].Leventhal BL, Cook EH Jr., Morford M, Ravitz A, Freedman DX, Relationships of whole blood serotonin and plasma norepinephrine within families, J Autism Dev Disord 20(4) (1990) 499–511. [DOI] [PubMed] [Google Scholar]
- [44].Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni MC, Launay JM, Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives, Biol Psychiatry 45(2) (1999) 158–63. [DOI] [PubMed] [Google Scholar]
- [45].McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, Shapiro T, Cohen DJ, Effects of diagnosis, race, and puberty on platelet serotonin levels in autism and mental retardation, J Am Acad Child Adolesc Psychiatry 37(7) (1998) 767–76. [DOI] [PubMed] [Google Scholar]
- [46].Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB, Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates, J Am Acad Child Adolesc Psychiatry 43(4) (2004) 491–9. [DOI] [PubMed] [Google Scholar]
- [47].Kolevzon A, Newcorn JH, Kryzak L, Chaplin W, Watner D, Hollander E, Smith CJ, Cook EH Jr., Silverman JM, Relationship between whole blood serotonin and repetitive behaviors in autism, Psychiatry Res 175(3) (2010) 274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Devlin B, Cook EH Jr., Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD, Network CG, Autism and the serotonin transporter: the long and short of it, Mol Psychiatry 10(12) (2005) 1110–6. [DOI] [PubMed] [Google Scholar]
- [49].C. International Molecular Genetic Study of Autism, A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p, Am J Hum Genet 69(3) (2001) 570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Weiss LA, Abney M, Cook EH Jr., Ober C, Sex-specific genetic architecture of whole blood serotonin levels, Am J Hum Genet 76(1) (2005) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Carneiro AM, Cook EH, Murphy DL, Blakely RD, Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans, J Clin Invest 118(4) (2008) 1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD, Enhanced activity of human serotonin transporter variants associated with autism, Philos Trans R Soc Lond B Biol Sci 364(1514) (2009) 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD, Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors, Am J Hum Genet 77(2) (2005) 265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB, Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes, Nat Genet 27(4) (2001) 435–8. [DOI] [PubMed] [Google Scholar]
- [55].Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD, Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior, Proc Natl Acad Sci U S A 109(14) (2012) 5469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Isaksson J, Pettersson E, Kostrzewa E, Diaz Heijtz R, Bolte S, Brief Report: Association Between Autism Spectrum Disorder, Gastrointestinal Problems and Perinatal Risk Factors Within Sibling Pairs, J Autism Dev Disord 47(8) (2017) 2621–2627. [DOI] [PubMed] [Google Scholar]
- [57].Liu MT, Kuan YH, Wang J, Hen R, Gershon MD, 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice, J Neurosci 29(31) (2009) 9683–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gershon MD, Liu MT, Serotonin and neuroprotection in functional bowel disorders, Neurogastroenterol Motil 19 Suppl 2 (2007) 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Manabe N, Wong BS, Camilleri M, New-generation 5-HT4 receptor agonists: potential for treatment of gastrointestinal motility disorders, Expert Opin Investig Drugs 19(6) (2010) 765–75. [DOI] [PubMed] [Google Scholar]
- [60].Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G, Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites, Proc Natl Acad Sci U S A 106(10) (2009) 3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC, Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells, FASEB J 29(4) (2015) 1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY, Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis, Cell 161(2) (2015) 264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Oleskin AV, Kirovskaia TA, Botvinko IV, Lysak LV, [Effect of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms], Mikrobiologiia 67(3) (1998) 305–12. [PubMed] [Google Scholar]
- [64].Tsavkelova EA, Klimova S, Cherdyntseva TA, Netrusov AI, [Hormones and hormone-like substances of microorganisms: a review], Prikl Biokhim Mikrobiol 42(3) (2006) 261–8. [PubMed] [Google Scholar]
- [65].Meyza KZ, Defensor EB, Jensen AL, Corley MJ, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ, The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers, Behav Brain Res 251 (2013) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG, Cryan JF, Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism, EBioMedicine 24 (2017) 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, Hirtz D, Gunnes N, Lie KK, Magnus P, Mjaaland S, Reichborn-Kjennerud T, Schjolberg S, Oyen AS, Levin B, Susser ES, Stoltenberg C, Lipkin WI, Prenatal fever and autism risk, Mol Psychiatry (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mahic M, Mjaaland S, Bovelstad HM, Gunnes N, Susser E, Bresnahan M, Oyen AS, Levin B, Che X, Hirtz D, Reichborn-Kjennerud T, Schjolberg S, Roth C, Magnus P, Stoltenberg C, Suren P, Hornig M, Lipkin WI, Maternal Immunoreactivity to Herpes Simplex Virus 2 and Risk of Autism Spectrum Disorder in Male Offspring, mSphere 2(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, Yang F, Deng M, Ruan B, Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis, Brain Behav Immun 58 (2016) 165–172. [DOI] [PubMed] [Google Scholar]
- [70].Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET, Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders, J Autism Dev Disord 40(12) (2010) 1423–30. [DOI] [PubMed] [Google Scholar]
- [71].Goines P, Van de Water J, The immune system’s role in the biology of autism, Curr Opin Neurol 23(2) (2010) 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ashwood P, Anthony A, Torrente F, Wakefield AJ, Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10, J Clin Immunol 24(6) (2004) 664–73. [DOI] [PubMed] [Google Scholar]
- [73].Jyonouchi H, Geng L, Streck DL, Toruner GA, Children with autism spectrum disorders (ASD) who exhibit chronic gastrointestinal (GI) symptoms and marked fluctuation of behavioral symptoms exhibit distinct innate immune abnormalities and transcriptional profiles of peripheral blood (PB) monocytes, J Neuroimmunol 238(1–2) (2011) 73–80. [DOI] [PubMed] [Google Scholar]
- [74].Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP, Maternal immune activation and abnormal brain development across CNS disorders, Nat Rev Neurol 10(11) (2014) 643–60. [DOI] [PubMed] [Google Scholar]
- [75].Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK, Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders, Cell 155(7) (2013) 1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, Bonnin A, Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain, J Neurosci 36(22) (2016) 6041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Parker-Athill EC, Tan J, Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway, Neurosignals 18(2) (2010) 113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lombardo MV, Moon HM, Su J, Palmer TD, Courchesne E, Pramparo T, Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder, Mol Psychiatry (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC, Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder, Cell Mol Gastroenterol Hepatol 3(2) (2017) 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Navarro F, Liu Y, Rhoads JM, Can probiotics benefit children with autism spectrum disorders?, World J Gastroenterol 22(46) (2016) 10093–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jeste SS, Geschwind DH, Disentangling the heterogeneity of autism spectrum disorder through genetic findings, Nat Rev Neurol 10(2) (2014) 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG, A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders, J Autism Dev Disord 39(9) (2009) 1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J, A systematic review of medical treatments for children with autism spectrum disorders, Pediatrics 127(5) (2011) e1312–21. [DOI] [PubMed] [Google Scholar]
- [84].Krishnaswami S, McPheeters ML, Veenstra-Vanderweele J, A systematic review of secretin for children with autism spectrum disorders, Pediatrics 127(5) (2011) e1322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Williams K, Brignell A, Randall M, Silove N, Hazell P, Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD), Cochrane Database Syst Rev (8) (2013) CD004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Spohn SN, Mawe GM, Non-conventional features of peripheral serotonin signalling - the gut and beyond, Nat Rev Gastroenterol Hepatol 14(7) (2017) 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Maclean MR, Dempsie Y, The serotonin hypothesis of pulmonary hypertension revisited, Adv Exp Med Biol 661 (2010) 309–22. [DOI] [PubMed] [Google Scholar]
- [88].Samuels BA, Mendez-David I, Faye C, David SA, Pierz KA, Gardier AM, Hen R, David DJ, Serotonin 1A and Serotonin 4 Receptors: Essential Mediators of the Neurogenic and Behavioral Actions of Antidepressants, Neuroscientist 22(1) (2016) 26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Muller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH, Stanghellini V, Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders, Aliment Pharmacol Ther 35(7) (2012) 745–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M, Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring, Cell 165(7) (2016) 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liu Y, Fatheree NY, Mangalat N, Rhoads JM, Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation, Am J Physiol Gastrointest Liver Physiol 299(5) (2010) G1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Arnold LE. Probiotics for Quality of Life in Autism Spectrum Disorders. ClinicalTrials.gov NCT02903030 [https://clinicaltrials.gov/ct2/show/NCT02903030] (accessed 5 March 2018)
- [93].Rhoads JM. Road to Discovery for Combination Probiotic BB- 12 with LGG in Treating Autism Spectrum Disorder. ClinicalTrials.gov NCT02674984 [https://clinicaltrials.gov/ct2/show/NCT02674984] (accessed 5 March 2018)
- [94].Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R, Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study, Microbiome 5(1) (2017) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Adams JB. Microbiota Transfer Therapy for Adults with Autism Spectrum Disorder (ASD) Who Have Gastrointestinal Disorders (MTT-ASD). ClinicalTrials.gov NCT03408886 [https://clinicaltrials.gov/show/NCT03408886] (accessed 5 March 2018)
- [96].Luna RA. Identifying Biomarkers of GI Morbidity in Autism Spectrum Disorders: Linking Multi-Omics and Human Behavior. [https://www.bcm.edu/research/clinical-trials/h-35071] (accessed 5 March 2018)