SUMMARY
It has been more than a dozen years since FGF21 burst on the metabolism field in a paper showing that its pharmacologic administration caused weight loss and improved insulin sensitivity and lipoprotein profiles in obese rodents. Since then, FGF21 analogs have advanced all the way to clinical trials, and much progress has been made in understanding FGF21’s pharmacology and physiology. In this perspective, we highlight some of the interesting themes that have emerged from this first dozen years of FGF21 research, including its roles in autocrine/paracrine and endocrine responses to metabolic stress.
ETOC Blurb
This Perspective by Kliewer and Mangelsdorf highlights recent concepts that have emerged from contemporary studies on the physiologic and pharmacologic roles of fibroblast growth factor (FGF) 21.
INTRODUCTION
FGF21 was cloned and named based on its membership in the FGF family (Nishimura et al., 2000). While conventional FGFs act in an autocrine or paracrine manner, FGF21 belongs to an atypical FGF subfamily that can in some circumstances circulate as hormones (Beenken and Mohammadi, 2009). This subfamily also includes FGF15/19, which regulates bile acid homeostasis and other aspects of liver metabolism; and FGF23, which regulates phosphate homeostasis. FGF21 is expressed in liver, endocrine and exocrine pancreas, and adipose tissue. However, under normal physiologic conditions, most if not all FGF21 in the blood is derived from the liver (Markan et al., 2014). While the basis for this selective release from liver is not yet understood, FGF21 lacks the canonical heparan-binding domain found in non-endocrine FGFs, which may allow it to escape from the extracellular matrix into the blood.
FGF21 acts on a cell surface receptor complex comprised of two proteins: a conventional tyrosine kinase FGF receptor (FGFR); and a co-receptor protein, named β-Klotho (KLB). FGF21 binds directly to both of these proteins to activate FGFR signaling activity (Kuro, 2018; Lee et al., 2018). In vitro, FGF21 can act through KLB complexed with either the FGFR1c, FGFR2c, or FGFR3c isoforms. However, gene knockout (KO) analyses and studies with activating antibodies specific for either FGFR1 or the FGFR1/KLB complex suggest that FGFR1c may be particularly important for FGF21’s actions in vivo (Adams et al., 2012; Foltz et al., 2012; Kolumam et al., 2015; Lan et al., 2017; Wu et al., 2011).
FGF21 PHARMACOLOGY
FGF21 has profound and pleiotropic pharmacologic effects. In obese rodents, it causes weight loss and improves insulin sensitivity without causing hypoglycemia or decreasing food intake (Coskun et al., 2008; Kharitonenkov et al., 2005; Xu et al., 2009). It also decreases triglyceride and cholesterol levels. FGF21 itself or long-acting analogs have similar metabolic effects in monkeys (Adams et al., 2013; Kharitonenkov et al., 2007; Talukdar et al., 2016b; Veniant et al., 2012b). Interestingly, however, FGF21 decreases food intake in obese monkeys (Adams et al., 2013; Talukdar et al., 2016b; Thompson et al., 2016). These studies highlight potential differences in FGF21’s mechanism of action across species.
Several human clinical trials have been reported with long-acting FGF21 analogs (Charles et al., 2018; Gaich et al., 2013; Kim et al., 2017; Talukdar et al., 2016b). In two, four-week studies done in obese subjects with type 2 diabetes, FGF21 analogs had favorable effects on body weight and plasma insulin concentrations (Gaich et al., 2013; Talukdar et al., 2016b). Weight loss was not observed in two other trials performed in obese, dyslipidemic people with or without type 2 diabetes (Charles et al., 2018; Kim et al., 2017), which may reflect differences in FGF21 derivatives and dosing regimens. Surprisingly, there was no effect on blood glucose levels in any of these studies. However, FGF21 analogs significantly reduced plasma triglyceride concentrations and increased HDL cholesterol in all studies. In mice, FG21 lowers plasma triglycerides by reducing non-esterified fatty acids and by increasing lipoprotein catabolism in white and brown adipose tissue (Schlein et al., 2016). The molecular basis for the cholesterol lowering effects has yet to be elucidated.
In addition to its use as a potential diabetes and obesity drug, FGF21 has more recently been shown to have a favorable pharmacologic profile in reversing non-alcoholic fatty liver disease and its more severe stage, steatohepatitis (NASH). Several studies in rodents have shown that FGF21 ameliorates many of the sequelae of the disease (Coskun et al., 2008; Fisher et al., 2014; Tanaka et al., 2015; Xu et al., 2009), and FGF21 and its derivatives lower circulating triglycerides and have other favorable effects on lipoprotein profiles in monkeys (Adams et al., 2013; Kharitonenkov et al., 2007; Talukdar et al., 2016b). These promising results have prompted several clinical trials (clinicaltrials.gov), and a recent Phase 2 study showed significant improvements in liver fat and blood biomarkers of fibrosis in patients with NASH (Sanyal et al., 2019).
Pharmacologic studies have also revealed adverse effects. In rodents, but not monkeys, FGF21 or analogs increased blood pressure and blood corticosterone concentrations (Bookout et al., 2013; Kim et al., 2017; Turner et al., 2018). Whereas one group found that FGF21 caused bone loss in mice (Wei et al., 2012), another did not (Li et al., 2017). In mice, FGF21 also caused anovulation and changed circadian behavior, increasing running wheel activity in the light phase and decreasing it in the dark phase (Bookout et al., 2013; Owen et al., 2013). In human clinical trials, FGF21 analogs increased blood pressure and pulse rate in one study (Kim et al., 2017) but not in another (Talukdar et al., 2016b). FGF21 analogs also increased blood markers of bone loss in two of the human studies (Kim et al., 2017; Talukdar et al., 2016b). It is unknown whether long-term FGF21 exposure causes bone loss in people. The most prevalent adverse event in humans was diarrhea, which has not been reported in either rodents or monkeys (Kim et al., 2017; Talukdar et al., 2016b). These adverse event profiles further highlight the species differences in the FGF21 response and underscore the challenges of using preclinical models to predict FGF21’s toxicology in humans.
How does FGF21 mediate its beneficial pharmacologic effects on adiposity, insulin sensitivity and hepatic triglyceride content? In diet-induced obese (DIO) mice, FGF21 induces thermogenesis and energy expenditure by activating brown adipose tissue (BAT) and promoting the browning of white adipose tissue (WAT) (Coskun et al., 2008; Douris et al., 2015; Owen et al., 2014; Xu et al., 2009). FGF21 also stimulates robust glucose uptake into BAT (Ding et al., 2012; Xu et al., 2009) and the secretion of adiponectin from WAT (Holland et al., 2013; Lin et al., 2013). Some of these effects on adipose tissue are direct: KLB and FGFR1c are co-expressed in BAT and WAT, and FGF21’s effect on thermogenic gene expression, glucose uptake and adiponectin secretion can be recapitulated in isolated adipocytes in vitro (Holland et al., 2013; Hondares et al., 2010; Kharitonenkov et al., 2005; Lin et al., 2013). Mechanistically, FGF21 induces the phosphorylation and activation of the transcription factor CREB, which regulates thermogenic gene expression (Wu et al., 2011). FGF21 also inhibits the SUMOylation of PPARγ, which increases its insulin sensitizing activity (Dutchak et al., 2012; Katafuchi et al., 2018). Accordingly, selective disruption of KLB in BAT and WAT of DIO mice inhibited FGF21’s effects on glucose uptake and insulin sensitivity during the first few hours after administration (BonDurant et al., 2017; Ding et al., 2012; Lan et al., 2017). However, FGF21 treatment for 2 weeks still caused weight loss, improved insulin sensitivity and lowered hepatic triglyceride concentrations in these adipose tissue-specific KLB-KO mice (BonDurant et al., 2017; Lan et al., 2017), demonstrating that FGF21 acts on additional tissues.
KLB and FGFR1c are co-expressed in discrete regions of the brain, including the suprachiasmatic nucleus and paraventricular nucleus (PVN) in the hypothalamus and the area postrema and nucleus of the solitary tract in the hindbrain (Bookout et al., 2013; Liang et al., 2014). Notably, direct infusion of FGF21 into the brains of DIO rats increased energy expenditure and insulin sensitivity (Sarruf et al., 2010). Conversely, selective disruption of KLB in neurons eliminated the long-term beneficial effects of either FGF21 or an FGFR1/KLB-specific activating antibody on body weight, glycemia and hepatic triglyceride concentrations in DIO mice (Bookout et al., 2013; Lan et al., 2017; Owen et al., 2014). Knockout of KLB in neurons also eliminated FGF21’s effects on circadian behavior, glucocorticoid concentrations and ovulation (Bookout et al., 2013; Owen et al., 2013). Thus, many—if not most—of FGF21’s pharmacologic actions require that it act directly on neurons.
In DIO mice, FGF21 increases energy expenditure by activating the sympathetic nervous system (SNS). FGF21 injection either peripherally or directly into the brain activated sympathetic nerve activity in BAT through an indirect mechanism involving induction of corticotropin-releasing hormone (Liang et al., 2014; Owen et al., 2014). Likewise, central administration of FGF21 increased norepinephrine turnover in BAT and WAT and the browning of WAT (Douris et al., 2015). These effects were inhibited by administering a β-adrenergic receptor antagonist, and FGF21’s browning activity was eliminated in β-adrenergic receptor-KO mice. Thus, FGF21 signals in the brain to activate the SNS and induce β-adrenergic receptor-mediated thermogenesis in adipose tissue.
Three studies have used uncoupling protein 1 (UCP1)-KO mice to investigate whether the UCP1-mediated futile cycle is required for FGF21’s metabolic actions (Kwon et al., 2015; Samms et al., 2015; Veniant et al., 2015). All three studies demonstrated that FGF21 can cause weight loss in mice lacking UCP1, suggesting the existence of alternative and/or compensatory mechanisms. However, here the findings diverge. Whereas Veniant et al. reported that FGF21-mediated induction of energy expenditure was mostly intact in UCP1-KO mice, Samms et al. showed that nearly all of FGF21’s weight loss effect in UCP1-KO mice was due to reduced food consumption rather than increased energy expenditure. While both groups concluded that UCP1 is not required for FGF21’s long-term beneficial effect on glucose homeostasis, a third group showed that FGF21-mediated stimulation of bolus glucose clearance is lost in UCP1-KO mice (Kwon et al., 2015). The reasons for these seemingly contradictory findings are not yet known, but it is interesting to speculate that they might be due to differences in ambient temperatures. Nevertheless, when taken together, the studies suggest that FGF21 exerts its broad metabolic actions through both UCP1-dependent and UCP1-independent mechanisms.
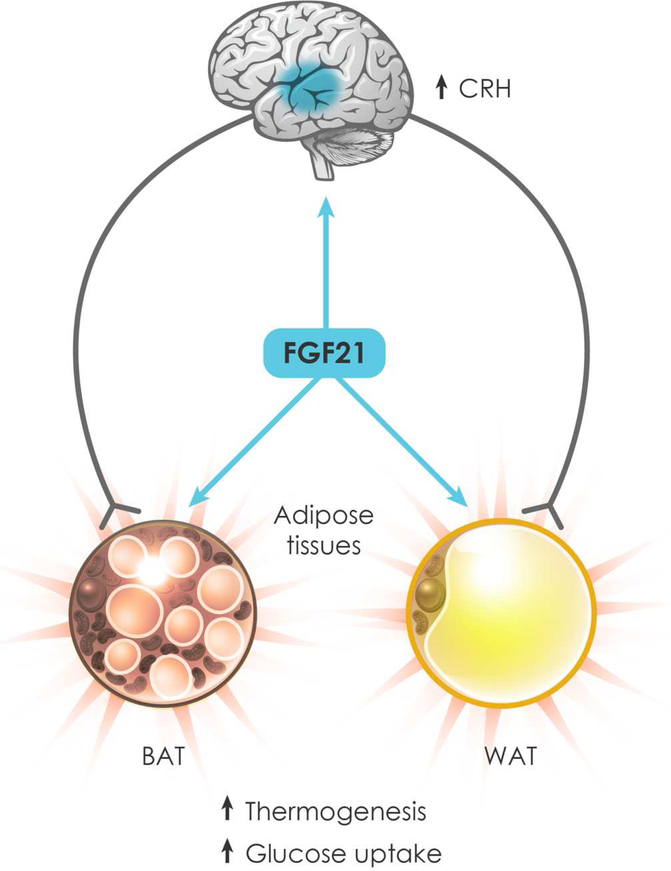
We conclude that FGF21 mediates its beneficial pharmacologic effects by acting through both peripheral and neural mechanisms (Figure 1). In the DIO mouse model, only direct effects on the nervous system are essential for FGF21’s chronic effects on body weight, insulin sensitivity and hepatic triglyceride content, but this probably reflects the importance of weight loss in driving the other metabolic improvements in this particular model. Precisely where in the nervous system FGF21 is acting to mediate its diverse effects remains an open question. The FGF21 receptor complex is expressed both inside (hypothalamus) and outside (hindbrain) the blood-brain barrier (Bookout et al., 2013). While FGF21 can cross the blood-brain-barrier, it does so with only low efficiency (Hsuchou et al., 2007). The finding that direct intracerebroventricular injection of FGF21 into the brain stimulates sympathetic outflow to BAT more quickly than intravenous injection suggests that FGF21 may need to cross the blood-brain barrier (Owen et al., 2014). Likewise, the onset of metabolic effects of an FGFR1/KLB-activating antibody, which crosses the blood-brain barrier only very poorly, is much slower than for native FGF21 (Lan et al., 2017). However, additional studies will be needed to elucidate whether FGF21 is working through central or peripheral neurons or both. An additional key question is whether the preclinical animal model findings will translate to humans. While most adult humans have at least some BAT (Nedergaard et al., 2007), it is unclear whether FGF21 causes weight loss via UCP1-mediated thermogenesis. As discussed above, FGF21 analogs reduce food intake in obese monkeys, suggesting that other mechanisms may also come into play in humans. Additional, long-term clinical studies will be required to assess FGF21’s efficacy— both with respect to its beneficial and adverse effects—and its mechanisms of action in people.
Figure 1.
FGF21 mediates its pharmacologic effects by acting directly on neurons and adipocytes. In the hypothalamus, FGF21 induces corticotropin-releasing hormone (CRH), resulting in sympathetic outflow to brown and white adipose tissue (BAT and WAT, respectively). FGF21 also acts directly on BAT and WAT to stimulate thermogenic gene expression and glucose uptake. The net effect is energy expenditure and weight loss in obese animals.
An important caveat with FGF21-based pharmacology is that the composition, potency and duration of action of these drugs differ greatly from endogenous FGF21. Even in studies using native, recombinant FGF21, the typical pharmacologic dose (typically 100–1000 ng/ml) far exceeds the endogenous circulating concentrations that have been observed in either animal models or humans (maximum levels of less than 30 ng/ml). These differences in concentration and exposure may explain why FGF21 and its analogs elicit pharmacologic effects that are not always consistent with its physiologic actions, which are described next.
FGF21 PHYSIOLOGY
FGF21 expression is induced by a variety of stresses in different tissues, including liver, adipose tissue and pancreas. FGF21 in turn acts to alleviate these stresses. Here, we break down FGF21’s physiology into its autocrine/paracrine and endocrine actions.
Autocrine/paracrine actions
FGF21 expression is regulated by various physiologic stresses in tissues where it is not released into the blood but rather acts in an autocrine/paracrine manner like conventional fibroblast growth factors. Tissues where this has been studied include WAT, BAT and exocrine pancreas.
FGF21 is induced in WAT by fasting/refeeding (Dutchak et al., 2012). However, DIO mice selectively lacking FGF21 in adipose tissue showed only a trend toward decreased adiposity but normal insulin sensitivity (Markan et al., 2014), suggesting that FGF21 has little impact on basal adipose tissue physiology. In contrast, the autocrine action of FGF21 in adipose tissue appears to play a prominent role in cold-induced thermogenesis. Cold exposure markedly elevated FGF21 expression in WAT and to a lesser extent in BAT (Chartoumpekis et al., 2011; Fisher et al., 2012; Hondares et al., 2011). FGF21 knockout in mice decreased cold-induced thermogenic gene expression in WAT but not BAT and compromised the adaptive thermogenic response (Fisher et al., 2012). Mechanistically, this autocrine action of FGF21 has been attributed to the induced expression of the coactivator PGC-1α (Fisher et al., 2012) and to the induction and secretion of the cytokine, CCL11 (Huang et al., 2017), both of which are potent drivers of adipocyte browning.
Acinar cells in the exocrine pancreas synthesize and secrete more protein than any other cell type in the body. They therefore must have mechanisms to minimize proteotoxic stress. FGF21 plays a unique role in this process by acting as a potent secretagogue (Coate et al., 2017). FGF21 is expressed at high levels in acinar cells, and this level is increased further in response to feeding to keep pace with postprandial digestive enzyme synthesis (Coate et al., 2017; Johnson et al., 2009; Singhal et al., 2016). Notably, acinar cells secrete all of their FGF21 into the pancreatic ducts, where it acts on acinar cells in an autocrine/paracrine fashion to stimulate digestive enzyme secretion and thereby prevent protein overload. The physiologic importance of this pathway was demonstrated in mice lacking FGF21 or KLB, which accumulate zymogen granules, have increased ER stress, and are susceptible to cerulein-induced pancreatitis (Coate et al., 2017; Johnson et al., 2009). Conversely, pharmacologic administration or transgenic overexpression of FGF21 protected the pancreas against ER-stress, inflammation and injury in mouse models of pancreatitis (Coate et al., 2017; Johnson et al., 2014; Johnson et al., 2009). In humans, the resolution of acute pancreatitis is correlated with increasing levels of circulating FGF21, further suggesting FGF21 plays a protective role (Shenoy et al., 2016). These studies suggest a potential pharmacologic role for FGF21 in preventing and treating pancreatitis.
Taken together, the autocrine/paracrine actions of FGF21 highlight its important role in metabolic stress responses.
Endocrine actions
An oddity of FGF21 as a fibroblast growth factor is its ability to circulate in the blood and function as a hormone. A study using hepatocyte-specific KO mice showed that liver is the major source of circulating FGF21 under physiologic conditions (Markan et al., 2014). Under certain pathophysiologic conditions, FGF21 is also ectopically expressed and released from nonhepatic tissues such as muscle (Izumiya et al., 2008; Kim et al., 2013) and BAT (Ruan et al., 2018). The molecular basis of the tissue-specific secretion of FGF21 has not been resolved.
FGF21 is induced at the transcriptional level and released from murine liver in response to a remarkable diversity of nutritional stresses including starvation, amino acid restriction, ketogenic and high fat diets, simple sugars and ethanol (BonDurant and Potthoff, 2018; Fisher and Maratos-Flier, 2016). Among the transcription factors involved in FGF21 induction are PPARα, which induces FGF21 in response to starvation and ketogenic diet (Badman et al., 2007; Inagaki et al., 2007), and ChREBP, which induces FGF21 in response to simple sugars (Fisher et al., 2017; Iroz et al., 2017). The mechanism through which FGF21 is induced by alcohol has not been explained. In humans, FGF21 blood levels are rapidly and robustly induced by ethanol, with weaker responses elicited by simple sugars, long-term fasting and obesity (Desai et al., 2017; Soberg et al., 2018; Song et al., 2018). FGF21-KO mice are susceptible to steatosis and other harmful liver effects caused by alcohol, fructose and ketogenic diet, revealing a protective role for FGF21 against these nutrient stressors (Badman et al., 2009; Desai et al., 2017; Fisher et al., 2017; Liu et al., 2016; Zhu et al., 2014). It remains to be determined whether these hepatoprotective effects occur by FGF21 acting through autocrine/paracrine or endocrine mechanisms.
Among its established endocrine actions in mice, FGF21 signals from liver to BAT to stimulate glucose uptake in response to prolonged consumption of a high fat diet, presumably to mitigate peripheral insulin resistance (Markan et al., 2014). FGF21 appears to play an analogous role as an endocrine signal during the transition from the fasted to the fed state: although FGF21 enters the circulation during fasting, it remains in the blood during the early stages of refeeding, when it stimulates glucose disposal (Markan et al., 2014). Endocrine FGF21 is also contributory, but not essential, for various aspects of the adaptive response to starvation, including ketogenesis and the inhibition of female fertility (Badman et al., 2009; Badman et al., 2007; Bookout et al., 2013; Hotta et al., 2009; Inagaki et al., 2007; Owen et al., 2013; Potthoff et al., 2009). The ability of FGF21 to function through both autocrine/paracrine and endocrine mechanisms helps explain some of its seemingly contradictory regulatory patterns across tissues. For example, feeding induces FGF21 in pancreas but suppresses it in liver. However, FGF21 is limited to acting only locally when made by the pancreas.
In 2013, the FGF21 field took an unexpected turn with the publication of two human GWAS studies showing associations between SNPs in and around the FGF21 gene and macronutrient preference (Chu et al., 2013; Tanaka et al., 2013). More specifically, these SNPs were linked to increased carbohydrate consumption and decreased protein and fat consumption. Subsequent GWAS studies showed associations between SNPs in FGF21 and KLB with alcohol consumption (Clarke et al., 2017; Schumann et al., 2016; Soberg et al., 2017). These findings together with the FGF21 induction data suggested the existence of endocrine feedback loops wherein FGF21 suppresses the overconsumption of simple sugars and alcohol.
In two bottle preference experiments, in which mice were given the choice between drinking either pure water or water sweetened with various natural sugars or artificial sweeteners, FGF21 administration markedly decreased sweet intake (Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016). An FGF21 analog also suppressed sweet preference in monkeys (Talukdar et al., 2016a). FGF21-KO mice consumed more glucose, sucrose and fructose than WT mice, and FGF21’s effect on sweet preference was lost in mice selectively lacking KLB in the PVN (von Holstein-Rathlou et al., 2016). Similarly, FGF21 acting on neurons reduced alcohol preference, and neuron-specific KLB-KO mice consumed more alcohol than WT mice at high alcohol concentrations (Schumann et al., 2016; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016). Thus, FGF21 signals from the liver to the brain to limit sugar and alcohol intake. Among its effects in the brain, FGF21 reduced the concentration of the neurotransmitter dopamine in the nucleus accumbens, a region that coordinates reward behaviors, suggesting a possible mechanism of action (Talukdar et al., 2016a).
In addition to decreasing sweet and alcohol preference, FGF21 stimulates water intake in mice and rats. In mice, pharmacologic FGF21 increased water consumption within two hours of administration (Song et al., 2018). Water restriction studies showed that this effect was due to thirst and not secondary to diuresis. The short time required for FGF21 to act and the fact that the effects on water drinking occurred in lean mice, where FGF21 does not induce energy expenditure or weight loss, indicate that the thirst response is not secondary to thermogenesis. Studies performed with FGF21-KO mice showed that FGF21 is essential for increased water intake caused by a ketogenic diet and is contributory to alcohol-induced thirst (Song et al., 2018).
FGF21 does not appear to stimulate thirst through the classical renin-angiotensin-aldosterone or arginine vasopressin (AVP) pathways. This is particularly interesting in the context of alcohol, which had been thought to cause thirst primarily by inhibiting AVP release from the pituitary, thereby stimulating diuresis. FGF21’s effect on thirst is eliminated in neuron-specific KLB-KO mice and partially inhibited by either knocking out KLB in the PVN or co-administering β-adrenergic receptor antagonists (Song et al., 2018). Interestingly, a previous study showed that norepinephrine injection into the PVN stimulates thirst in rats (Leibowitz, 1978). Our findings suggest that FGF21 may act through this pathway.
While an FGF21 analog also stimulated water consumption in rats, albeit only weakly compared to mice, a different conclusion was reached regarding it mechanism of action (Turner et al., 2018). In kinetic studies, the FGF21 analog increased urine output before water intake, indicating diuresis as its primary effect. However, the FGF21 analog had no diuretic effect in a water restriction study, which is the gold standard clinical method for distinguishing primary polyuria. It remains to be determined whether FGF21 induces water consumption through different mechanisms in rats and mice.
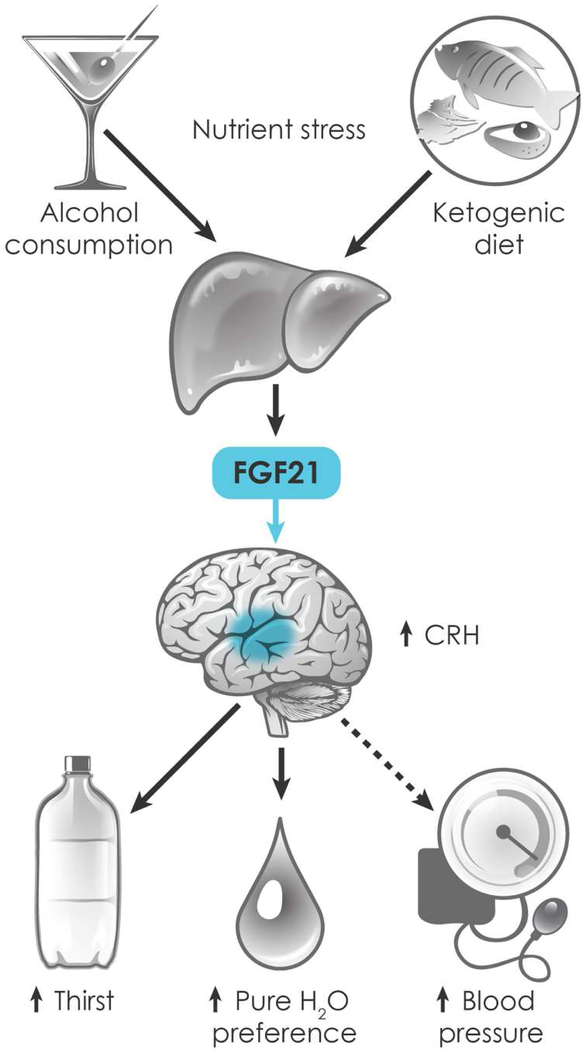
Taken together, the thirst and preference findings suggest a prominent, pre-emptive role for endocrine FGF21 in maintaining water balance in the face of dehydrating nutritional conditions (Figure 2). FGF21 is strongly induced in liver by dehydrating nutrients, most notably alcohol, in both rodents and humans. In mice, FGF21’s subsequent actions are two-fold: First, it stimulates thirst and water intake. Second, it promotes the drinking of pure water in order to optimize hydration. FGF21 mediates both these effects by signaling to the nervous system. The GWAS studies strongly suggest that FGF21’s effect on preference is conserved in humans. Whether its effect on thirst is also conserved awaits further studies.
Figure 2.
Among its physiologic endocrine actions, FGF21 regulates hydration. FGF21 is induced in liver by dehydrating metabolic stresses, including alcohol and ketogenic diet, and circulates to the nervous system, where it stimulates thirst and drinking of pure water. FGF21 also stimulates blood pressure, possibly as a mechanism to prevent dehydration-associated hypotension.
OUTSTANDING QUESTIONS
The physiologic and pharmacologic actions of FGF21 present an interesting conundrum: Why would a hormone that protects against nutritional stressors drive energy expenditure when administered pharmacologically to obese animals? The answer may lie in part in FGF21’s activation of the SNS, which has obvious implications for energy expenditure. However, FGF21-mediated activation of the SNS may also be important for maintaining blood pressure in the face of dehydration and possibly starvation. In support of this hypothesis, FGF21-KO mice had reduced blood pressure when fed a ketogenic diet (Song et al., 2018). Conversely, pharmacologic FGF21 increased blood pressure in both rodents and humans (Kim et al., 2017; Turner et al., 2018). Thus, we speculate FGF21 evolved as an endocrine factor to stimulate sympathetic tone in the vasculature. Why FGF21 does not induce thermogenesis in the context of either dehydration or starvation is another outstanding question. Presumably, there are mechanisms to ensure that thermogenesis occurs only in the context of energy surfeit. Leptin is a candidate mediator of this role (Veniant et al., 2012a). Conversely, why aren’t physiologic concentrations of FGF21 protective against obesity? Is there an FGF21 resistance? And on which neurons does FGF21 act to elicit its array of effects?
In closing, we raise a final, fundamental question: why isn’t FGF21 mitogenic like conventional FGFs, especially since it acts on FGFR1, which is known to drive proliferation? Does KLB alter the signaling pathways downstream of the FGFRs? Given all these questions and many more, we anticipate that the next dozen years of FGF21 research will be as productive and interesting as the last.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01DK067158 and P01AG051459 to D.J.M. and S.A.K.); the Robert A. Welch Foundation (grants I-1558 to S.A.K. and I-1275 to D.J.M.); and the Howard Hughes Medical Institute (D.J.M.).
Footnotes
Declaration of Interests
S.A.K. and D.J.M. collaborate with and have received travel reimbursements from Novo Nordisk. S.A.K. and D.J.M. are authors on a patent filed for the use of FGF21 to treat secretory disorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams AC, Halstead CA, Hansen BC, Irizarry AR, Martin JA, Myers SR, Reynolds VL, Smith HW, Wroblewski VJ, and Kharitonenkov A (2013). LY2405319, an Engineered FGF21 Variant, Improves the Metabolic Status of Diabetic Monkeys. PLoS One 8, e65763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, and Kharitonenkov A (2012). The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Molecular metabolism 2, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Koester A, Flier JS, Kharitonenkov A, and Maratos-Flier E (2009). Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150, 4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, and Maratos-Flier E (2007). Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5, 426–437. [DOI] [PubMed] [Google Scholar]
- Beenken A, and Mohammadi M (2009). The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, Walsh SA, Ornitz DM, and Potthoff MJ (2017). FGF21 Regulates Metabolism Through Adipose-Dependent and -Independent Mechanisms. Cell Metab 25, 935–944.e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, and Potthoff MJ (2018). Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu Rev Nutr 38, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, and Kliewer SA (2013). FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Medicine 19, 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS, and Christian R (2018). Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity (Silver Spring). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, and Papavassiliou AG (2011). Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med 17, 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, and Qi L (2013). Novel locus including FGF21 is associated with dietary macronutrient intake. Human molecular genetics 22, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C, Porteous DJ, Deary IJ, and McIntosh AM (2017). Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry 22, 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate KC, Hernandez G, Thorne CA, Sun S, Le TD, Vale K, Kliewer SA, and Mangelsdorf DJ (2017). FGF21 Is an Exocrine Pancreas Secretagogue. Cell Metab 25, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, and Kharitonenkov A (2008). Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027. [DOI] [PubMed] [Google Scholar]
- Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, Mather ML, Vardeh HG, Douris N, Adams AC, Nasser IA, FitzGerald GA, Flier JS, Skarke C, and Maratos-Flier E (2017). Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab 6, 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, and Kliewer SA (2012). betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell metabolism 16, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic D, Fisher FM, Cisu TI, Chee MJ, Ly Nguyen N, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, and Maratos-Flier E (2015). Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology, en20142001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, and Kliewer SA (2012). Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, Kharitonenkov A, Schuppan D, Flier JS, and Maratos-Flier E (2014). Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147, 1073–1083.e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, Hellerstein MK, Hudgins LC, Maratos-Flier E, and Herman MA (2017). A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab 6, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, and Spiegelman BM (2012). FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development 26, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, and Maratos-Flier E (2016). Understanding the Physiology of FGF21. Annu Rev Physiol 78, 223–241. [DOI] [PubMed] [Google Scholar]
- Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N, Gupte J, Komorowski R, Sekirov L, Hager T, Arora T, Ge H, Baribault H, Wang F, Sheng J, Karow M, Wang M, Luo Y, McKeehan W, Wang Z, Véniant MM, and Li Y (2012). Treating Diabetes and Obesity with an FGF21-Mimetic Antibody Activating the βKlotho/FGFR1c Receptor Complex. Science Translational Medicine 4, 162ra153–162ra153. [DOI] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, and Moller DE (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell metabolism 18, 333–340. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, and Scherer PE (2013). An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metabolism 17, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, and Villarroya F (2011). Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286, 12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, and Villarroya F (2010). Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, and Itoh N (2009). Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150, 4625–4633. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, and Kastin AJ (2007). The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L, Wang Y, Wong CM, and Xu A (2017). The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab 26, 493–508.e494. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, and Kliewer SA (2007). Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5, 415–425. [DOI] [PubMed] [Google Scholar]
- Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, Anthony E, Regnier M, Fouche E, Lukowicz C, Cauzac M, Tournier E, Do-Cruzeiro M, Daujat-Chavanieu M, Gerbal-Chalouin S, Fauveau V, Marmier S, Burnol AF, Guilmeau S, Lippi Y, Girard J, Wahli W, Dentin R, Guillou H, and Postic C (2017). A Specific ChREBP and PPARalpha Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep 21, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, and Walsh K (2008). FGF21 is an Akt-regulated myokine. FEBS Lett 582, 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Mehmood R, Laing SW, Stepniak CV, Kharitonenkov A, and Pin CL (2014). Silencing of the Fibroblast growth factor 21 gene is an underlying cause of acinar cell injury in mice lacking MIST1. Am J Physiol Endocrinol Metab 306, E916–928. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Koester A, and Pin CL (2009). Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology 137, 1795–1804. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Holland WL, Kollipara RK, Kittler R, Mangelsdorf DJ, and Kliewer SA (2018). PPARgamma-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, and Shanafelt AB (2005). FGF-21 as a novel metabolic regulator. J Clin Invest 115, 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, and Etgen GJ (2007). The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781. [DOI] [PubMed] [Google Scholar]
- Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, Chabot JR, Gropp KE, Talukdar S, and Calle RA (2017). Once-weekly administration of a long-acting FGF21 analogue modulates lipids, bone turnover markers, blood pressure, and body weight differently in obese hypertriglyceridemic subjects and in non-human primates. Diabetes Obes Metab. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, and Lee MS (2013). Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Medicine 19, 83–92. [DOI] [PubMed] [Google Scholar]
- Kolumam G, Chen MZ, Tong R, Zavala-Solorio J, Kates L, van Bruggen N, Ross J, Wyatt SK, Gandham VD, Carano RA, Dunshee DR, Wu AL, Haley B, Anderson K, Warming S, Rairdan XY, Lewin-Koh N, Zhang Y, Gutierrez J, Baruch A, Gelzleichter TR, Stevens D, Rajan S, Bainbridge TW, Vernes JM, Meng YG, Ziai J, Soriano RH, Brauer MJ, Chen Y, Stawicki S, Kim HS, Comps-Agrar L, Luis E, Spiess C, Wu Y, Ernst JA, McGuinness OP, Peterson AS, and Sonoda J (2015). Sustained Brown Fat Stimulation and Insulin Sensitization by a Humanized Bispecific Antibody Agonist for Fibroblast Growth Factor Receptor 1/betaKlotho Complex. EBioMedicine 2, 730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro OM (2018). Ageing-related receptors resolved. Nature 553, 409–410. [DOI] [PubMed] [Google Scholar]
- Kwon MM, O’Dwyer SM, Baker RK, Covey SD, and Kieffer TJ (2015). FGF21-Mediated Improvements in Glucose Clearance Require Uncoupling Protein 1. Cell Rep 13, 1521–1527. [DOI] [PubMed] [Google Scholar]
- Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, Holland WL, Kliewer SA, and Mangelsdorf DJ (2017). FGF19, FGF21, and an FGFR1/beta-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab 26, 709–718.e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, and Schlessinger J (2018). Structures of beta-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF (1978). Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav 8, 163–175. [DOI] [PubMed] [Google Scholar]
- Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, Villasenor K, Wang J, Wong P, Boyce R, Dwyer D, Han CY, Chen MM, Liu B, Stolina M, Ke HZ, Ominsky MS, Veniant MM, and Xu J (2017). FGF21 Is Not a Major Mediator for Bone Homeostasis or Metabolic Actions of PPARalpha and PPARgamma Agonists. J Bone Miner Res 32, 834–845. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, and Xu A (2014). FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075. [DOI] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, and Li X (2013). Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism 17, 779–789. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao C, Xiao J, Liu L, Zhang M, Wang C, Wu G, Zheng MH, Xu LM, Chen YP, Mohammadi M, Chen SY, Cave M, McClain C, Li X, and Feng W (2016). Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci Rep 6, 31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, and Potthoff MJ (2014). Circulating FGF21 is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, and Cannon B (2007). Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293, E444–452. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, and Itoh N (2000). Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492, 203–206. [DOI] [PubMed] [Google Scholar]
- Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, and Mangelsdorf DJ (2013). FGF21 contributes to neuroendocrine control of female reproduction. Nature Medicine 19, 1153–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, and Mangelsdorf DJ (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism 20, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, and Burgess SC (2009). FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 106, 10853–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, and Gao PJ (2018). A2A Receptor Activation Attenuates Hypertensive Cardiac Remodeling via Promoting Brown Adipose Tissue-Derived FGF21. Cell Metab 28, 476–489.e475. [DOI] [PubMed] [Google Scholar]
- Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, Kharitonenkov A, Gimeno RE, and Adams AC (2015). Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell Rep 11, 991–999. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, and Christian R (2019). Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 392, 2705–2717. [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, and Schwartz MW (2010). Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J, and Scheja L (2016). FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab 23, 441–453. [DOI] [PubMed] [Google Scholar]
- Schumann G, Liu C, O’Reilly P, Gao H, Song P, Xu B, Ruggeri B, Amin N, Jia T, Preis S, Segura Lepe M, Akira S, Barbieri C, Baumeister S, Cauchi S, Clarke TK, Enroth S, Fischer K, Hallfors J, Harris SE, Hieber S, Hofer E, Hottenga JJ, Johansson A, Joshi PK, Kaartinen N, Laitinen J, Lemaitre R, Loukola A, Luan J, Lyytikainen LP, Mangino M, Manichaikul A, Mbarek H, Milaneschi Y, Moayyeri A, Mukamal K, Nelson C, Nettleton J, Partinen E, Rawal R, Robino A, Rose L, Sala C, Satoh T, Schmidt R, Schraut K, Scott R, Smith AV, Starr JM, Teumer A, Trompet S, Uitterlinden AG, Venturini C, Vergnaud AC, Verweij N, Vitart V, Vuckovic D, Wedenoja J, Yengo L, Yu B, Zhang W, Zhao JH, Boomsma DI, Chambers J, Chasman DI, Daniela T, de Geus E, Deary I, Eriksson JG, Esko T, Eulenburg V, Franco OH, Froguel P, Gieger C, Grabe HJ, Gudnason V, Gyllensten U, Harris TB, Hartikainen AL, Heath AC, Hocking L, Hofman A, Huth C, Jarvelin MR, Jukema JW, Kaprio J, Kooner JS, Kutalik Z, Lahti J, Langenberg C, Lehtimaki T, Liu Y, Madden PA, Martin N, Morrison A, Penninx B, Pirastu N, Psaty B, Raitakari O, Ridker P, Rose R, Rotter JI, Samani NJ, Schmidt H, Spector TD, Stott D, Strachan D, Tzoulaki I, van der Harst P, van Duijn CM, Marques-Vidal P, Vollenweider P, Wareham NJ, Whitfield JB, Wilson J, Wolffenbuttel B, Bakalkin G, Evangelou E, Liu Y, Rice KM, Desrivieres S, Kliewer SA, Mangelsdorf DJ, Muller CP, Levy D, and Elliott P (2016). KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci U S A 113, 14372–14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy VK, Beaver KM, Fisher FM, Singhal G, Dushay JR, Maratos-Flier E, and Flier SN (2016). Elevated Serum Fibroblast Growth Factor 21 in Humans with Acute Pancreatitis. PLoS One 11, e0164351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G, Fisher FM, Chee MJ, Tan TG, El Ouaamari A, Adams AC, Najarian R, Kulkarni RN, Benoist C, Flier JS, and Maratos-Flier E (2016). Fibroblast Growth Factor 21 (FGF21) Protects against High Fat Diet Induced Inflammation and Islet Hyperplasia in Pancreas. PLoS One 11, e0148252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberg S, Andersen ES, Dalsgaard NB, Jarlhelt I, Hansen NL, Hoffmann N, Vilsboll T, Chenchar A, Jensen M, Grevengoed TJ, Trammell SAJ, Knop FK, and Gillum MP (2018). FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab 11, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, Solomon TPJ, Scheele C, Linneberg A, Jorgensen T, Pedersen O, Hansen T, Gillum MP, and Grarup N (2017). FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab 25, 1045–1053.e1046. [DOI] [PubMed] [Google Scholar]
- Song P, Zechner C, Hernandez G, Canovas J, Xie Y, Sondhi V, Wagner M, Stadlbauer V, Horvath A, Leber B, Hu MC, Moe OW, Mangelsdorf DJ, and Kliewer SA (2018). The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol. Cell Metab 27, 1338–1347.e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Muller CP, Tang H, Mangelsdorf DJ, Goodwin B, and Kliewer SA (2016a). FGF21 Regulates Sweet and Alcohol Preference. Cell Metab 23, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, Brenner MB, Trimmer JK, Gropp KE, Chabot JR, Erion DM, Rolph TP, Goodwin B, and Calle RA (2016b). A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab 23, 427–440. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Zhang Y, Krausz KW, Smith PB, Patterson AD, and Gonzalez FJ (2015). Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta 1852, 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, Mikkila V, Renstrom F, Sonestedt E, Zhao JH, Chu AY, Qi L, Chasman DI, de Oliveira Otto MC, Dhurandhar EJ, Feitosa MF, Johansson I, Khaw KT, Lohman KK, Manichaikul A, McKeown NM, Mozaffarian D, Singleton A, Stirrups K, Viikari J, Ye Z, Bandinelli S, Barroso I, Deloukas P, Forouhi NG, Hofman A, Liu Y, Lyytikainen LP, North KE, Dimitriou M, Hallmans G, Kahonen M, Langenberg C, Ordovas JM, Uitterlinden AG, Hu FB, Kalafati IP, Raitakari O, Franco OH, Johnson A, Emilsson V, Schrack JA, Semba RD, Siscovick DS, Arnett DK, Borecki IB, Franks PW, Kritchevsky SB, Lehtimaki T, Loos RJ, Orho-Melander M, Rotter JI, Wareham NJ, Witteman JC, Ferrucci L, Dedoussis G, Cupples LA, and Nettleton JA (2013). Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. The American journal of clinical nutrition 97, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WC, Zhou Y, Talukdar S, and Musante CJ (2016). PF-05231023, a long-acting FGF21 analogue, decreases body weight by reduction of food intake in non-human primates. J Pharmacokinet Pharmacodyn 43, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, Chen X, Zahner M, Opsahl A, DeMarco G, Boucher M, Goodwin B, and Perreault M (2018). FGF21 increases water intake, urine output and blood pressure in rats. PLoS One 13, e0202182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, Busby J, Vonderfecht S, Xu J, and Lloyd DJ (2012a). FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One 7, e40164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, Zhou L, Wada R, Hecht R, and Xu J (2012b). Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 153, 4192–4203. [DOI] [PubMed] [Google Scholar]
- Veniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, and Lloyd DJ (2015). Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab 21, 731–738. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, and Potthoff MJ (2016). FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, and Wan Y (2012). Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proceedings of the National Academy of Sciences of the United States of America 109, 3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, Kates L, van Bruggen N, Leabman M, Wong A, West D, Stern H, Luis E, Kim HS, Yansura D, Peterson AS, Filvaroff E, Wu Y, and Sonoda J (2011). Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Science translational medicine 3, 113ra126. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, and Veniant MM (2009). Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Ma L, Wu Y, Ye X, Zhang T, Zhang Q, Rasoul LM, Liu Y, Guo M, Zhou B, Ren G, and Li D (2014). FGF21 treatment ameliorates alcoholic fatty liver through activation of AMPK-SIRT1 pathway. Acta Biochim Biophys Sin (Shanghai) 46, 1041–1048. [DOI] [PubMed] [Google Scholar]