Abstract
Emotionally traumatic experiences can lead to maladaptive memories that are enduring and intrusive. The goal of exposure-based therapies is to extinguish conditioned fears through repeated, unreinforced exposures to reminders of traumatic events. The extinction of conditioned fear depends upon the consolidation of new memories made during exposure to reminders. An impairment in extinction recall, observed in certain patient populations, can interfere with progress in exposure-based therapies, and the drive to avoid thoughts and reminders of the trauma can undermine compliance and increase dropout rate. Effective adjuncts to exposure- based therapies should improve the consolidation and maintenance of the extinction memory, or improve the tolerability of the therapy. Under stressful conditions, the vagus nerve responds to elevations in epinephrine and signals the brain to facilitate the storage of new memories while, as part of the parasympathetic nervous system, it slows the sympathetic response. We propose that stimulation of the left cervical vagus nerve during exposure to conditioned cues signals the brain to store new memories just as epinephrine or emotional arousal would do, but bypasses the peripheral sympathetic “fight-or-flight” response. In support of this hypothesis, we have found that VNS accelerates extinction and prevents reinstatement of conditioned fear in rats. Here, we review studies relevant to fear extinction, describing the anatomical and functional characteristics of the vagus nerve and mechanisms of VNS-induced memory enhancement and plasticity. Finally, we propose future studies targeting the optimization of stimulation parameters and the search for biomarkers of VNS effectiveness that may improve exposure therapy outcomes.
Keywords: Anxiety, Extinction, Fear, Posttraumatic Stress Disorder, Vagus Nerve Stimulation
“The stream of thought flows on; but most of its segments fall into the bottomless abyss of oblivion. Of some, no memory survives the instant of their passage. Of others, it is confined to a few moments, hours, or days. Others, again, leave vestiges which are indestructible, and by means of which they may be recalled as long as life endures. ”
- William James (1890)
Introduction
The observation made by William James strikes a familiar cord. We all experience rapid storage of long-lasting memories for some experiences whereas other experiences are fleeting. James went on to ask “Can we explain these differences?”. One factor that distinguishes memories that “leave vestiges” from memories that “fall into the bottomless abyss of oblivion” is emotional state. Emotional arousal can serve an evaluative function, determining whether an event is important enough to be stored for the long term (Gold and Van Buskirk 1975; Gold et al. 1975). Threats and challenging environments have been elements of life since the rise of man and, consequently, mankind is well equipped to handle stress (Diamond 1992; Sapolsky 2004). Learning from emotionally arousing experiences is critical for adaptation to life challenges, and in most cases, it is fortunate that these experiences are resistant to forgetting (Joels et al. 2006). However, maladaptive memories of extraordinarily traumatic events can negatively impact health, quality of life, and productivity by intrusively coming to mind when the situation is not appropriate, chronically elevating arousal and anxiety, causing traumatized individuals to perpetually avoid situations where they may be reminded of the trauma, and having degenerative effects on cognition and mood. It is estimated that more than 2/3 of all individuals worldwide will experience a traumatic event at least once in their lives (Benjet et al. 2016), leading many of these individuals to develop psychopathological conditions like trauma- and stress-related disorders. Various forms of cognitive behavioral therapies involve re-exposure to reminders of the trauma. Some attempt to interfere with the reconsolidation of the traumatic memory (Brunet et al. 2018; Haubrich and Nader 2018), others focus on the trauma with the goal of teaching better coping skills (Resick and Schnicke 1992; Kozel et al. 2018). Exposure to reminders of the trauma can result in extinction of conditioned fear, which depends upon the suppression of old learned associations by the formation of new associations (Bouton 2004; Sotres-Bayon et al. 2006; Quirk and Mueller 2008). Thus, extinction does not involve memory erasure, but rather it requires new learning in which cues that were once threatening no longer signal danger.
Though exposure therapies are considered the best evidence-based practice for the treatment of anxiety- and trauma-related disorders (Powers et al. 2010), limitations such as nonresponse, dropout and noncompliance, and relapse leave room for improvements. Non-response rates following treatment are high, which may be a consequence of the extinction impairments that are seen in patients with disorders including: PTSD (Rothbaum and Davis 2003; Davis et al. 2006a; Milad et al. 2008; Milad et al. 2009), OCD (Milad et al. 2013), and specific phobias (Powers et al. 2009). For example, in PTSD patients, non-response rates are as high as fifty percent (Schottenbauer et al. 2008) and patients with PTSD show impaired extinction of conditioned fear even for conditioned cues learned in controlled laboratory studies (Milad et al. 2009), presenting a challenge for a therapy that depends upon extinction learning. Dropout and poor compliance rates during treatment also limit the success of exposure-based therapies. Patients exhibit extreme avoidance of fear-related cues and attempt to avoid recalling the events; such avoidant behaviors can lead to dropout before the goals of treatment are achieved. Dropout rates as high as 68 percent have been reported in veterans with PTSD (Garcia et al. 2011; Najavits 2015). A study of OCD patients revealed that compliance scores were positively correlated with treatment outcomes (Simpson et al. 2011), suggesting that improvements in tolerability and compliance would lead to better outcomes in exposure-based therapies.
Finally, because extinction is not memory erasure but rather a new learning that suppresses an old memory (Quirk and Mueller 2008), patients are susceptible to relapse even after achieving the goals of therapy (Boschen et al. 2009; Vervliet et al. 2013). In preclinical studies, the term relapse describes different forms of fear return (Goode and Maren 2014): spontaneous recovery, where fear returns following the passage of time (Bouton 1993; Rescorla 1997); renewal, where fear returns in a context-dependent manner (Bouton 2004; Maren et al. 2013); and reinstatement, where fear returns following a stressor (Rescorla and Heth 1975; Bouton and Bolles 1979). Adjuncts to exposure-based therapies may be effective if they improve the consolidation and maintenance of the extinction memory, and/or improve the tolerability of the therapy.
Enhancing fear extinction
Preclinical research frequently uses extinction of conditioned fear in non-human animals to model exposure-based therapies. Preclinical studies are designed to investigate the mechanisms and circuitry of fear extinction and to test potential adjunctive strategies that could supplement the effects of exposure-based therapies (Anderson et al. 2004; Ressler et al. 2004). A variety of adjunctive therapies have been examined, including: glutamatergic drugs like D- cycloserine (DCS) and ketamine (Ledgerwood 2003; Davis et al. 2006a; Davis et al. 2006b; Feder et al. 2014), noradrenergic drugs (Davis and Myers 2002; Fitzgerald et al. 2015), cannabinoid receptor agonists (Chhatwal et al. 2005), GABA agonists (Akirav et al. 2006), glucocorticoids (Soravia et al. 2006; Bentz et al. 2010), and electrical and pharmacological stimulation of brain regions important for fear extinction (Ressler and Mayberg 2007; Maroun et al. 2012; Rodriguez-Romaguera et al. 2012) as means to accelerate extinction of fear and potentially benefit exposure-based therapies.
Early preclinical work was based on findings that blockade of NMDA receptors via phosphonopentanoic acid (AP5) infusion into the amygdala prior to extinction training blocked fear extinction learning (Falls et al. 1992). Walker and colleagues hypothesized that DCS, a partial NMDA-agonist, would have the opposite effect. Using fear-potentiated startle as a measure of conditioned fear in rats, they found that systemic DCS, or DCS given directly into the amygdala prior to extinction training enhanced extinction of the learned fear (Walker et al. 2002). Additional preclinical studies using freezing to measure conditioned fear replicated these results and revealed that DCS was also effective in reducing conditioned fear if given immediately after the extinction session, suggesting that DCS affects the consolidation of extinction memory (Ledgerwood 2003). Following these positive preclinical findings, clinicians examined the utility of DCS to augment exposure-based therapies in patients. Early work demonstrated acceleration and augmentation of exposure-based therapies in the treatment of anxiety disorders (Ressler et al. 2004; Hofmann et al. 2006; Guastella et al. 2008; Wilhelm et al. 2008; de Kleine et al. 2012). However, recent meta-analyses indicate that results of DCS effects on exposure-based therapy outcomes were mixed (Rodrigues et al. 2014; Mataix-Cols et al. 2017). In one study of combat veterans with PTSD, those given DCS showed less symptom reduction than placebo controls following therapy (Litz et al. 2012). These pharmacological tools lack temporal specificity and, once in the system, cannot be quickly eliminated. It is possible that targeting enhancement of extinction using systemic pharmacological interventions can enhance the association of the stress response with reminders of the trauma when exposure to reminders provokes a strong negative reaction. Researchers have examined the use of anxiolytic drugs to improve the tolerability of exposure-based therapies. This approach may be counter-productive because anxiolytic drug administration before exposure can impair the consolidation of extinction memories (Clarke et al. 1970; Lucki et al. 1987). On the other hand, studies indicate that systemic administration of the beta-adrenergic antagonist propranolol can reduce the conditioned fear response without impairing the consolidation of extinction in rats (Rodriguez- Romaguera et al. 2009; Fitzgerald et al. 2015). A preclinical study carried out in humans revealed that administration of propranolol prior to extinction training enhanced extinction of conditioned fear (Kroes et al. 2016). Taken together, these findings suggest that the weakening of sympathetic responses during exposure can facilitate the extinction of conditioned fear. Ideally, an adjunctive therapy would reduce the aversive sympathetic response while allowing the stress-induced enhancement of memory consolidation during exposure therapy.
Targeting the stress response to enhance extinction
Stress can enhance memory consolidation via activation of the sympathetic nervous system (for review, see LaLumiere et al. 2017). For example, posttraining administration of epinephrine enhances memory consolidation in rats (Gold and Van Buskirk 1975) and humans (Cahill and Alkire 2003). Studies suggest that a strong sympathetic stress response and heightened emotional arousal during exposure therapy is necessary for establishing an extinction memory that is robust enough to compete with a stress-enhanced traumatic memory (Tuerk et al. 2018). However, epinephrine cannot directly alter memory as it does not readily cross the blood- brain barrier (Weil-Malherbe et al. 1959). One explanation for epinephrine effects on memory is that it acts on glycogen stores in the liver to increase blood glucose levels, thereby providing more energy to the brain (Gold 2014). Another possible route by which epinephrine may signalthe brain is through binding to beta-adrenergic receptors on the vagus nerve, which in turn, increases release of norepinephrine in the brain (Miyashita and Williams 2006; Chen and Williams 2012). Like systemic epinephrine administration, electrical stimulation of the vagus nerve (VNS) enhances memory in rats (Clark et al. 1995; Clark et al. 1998) and humans (Clark et al. 1999). Under stressful conditions, the vagus nerve responds to elevations in epinephrine and signals the brain to rapidly store new memories while, as part of the parasympathetic nervous system, it slows the sympathetic response. In the brain, the LC receives inputs from the vagus nerve through the nucleus tractus solitarius (NTS) in the brain stem. Once activated, the LC provides norepinephrine throughout the brain, including regions like the basolateral amygdala (BLA) and prefrontal cortex (PFC). Systemic administration of epinephrine or sympathetic activation by stress increases levels of norepinephrine in the amygdala (Galvez et al. 1996; Chen and Williams 2012). Norepinephrine administered directly into the amygdala enhances memory consolidation and intra-amygdala injections of the beta-adrenergic antagonist propranolol blocks the memory enhancing effect of systemic epinephrine (Liang et al. 1986). In addition to epinephrine release from the adrenal medulla, the stress response includes the release of glucocorticoids from the adrenal cortex into the bloodstream and, like epinephrine, this glucocorticoid response contributes to the enhancement of emotionally arousing memories (Joels et al. 2006). Exogenous glucocorticoid administration also enhances memory consolidation (Buchanan and Lovallo 2001), however, glucocorticoid enhancement of memory appears to depend on norepinephrine signaling in the basolateral amygdala (Roozendaal et al. 2006; McReynolds et al. 2010). Memory-enhancing VNS also increases norepinephrine levels in the amygdala (Hassert et al. 2004) and intra-basolateral amygdala infusions of norepinephrine enhance the consolidation of conditioned fear extinction (Berlau and McGaugh 2006). Likewise, VNS increases norepinephrine concentration in the medial prefrontal cortex (Follesa et al. 2007), and blocking beta-adrenergic receptors in the infralimbic region of the prefrontal cortex impairs the consolidation of extinction of conditioned fear (Mueller et al. 2008). We propose that stimulation of the left cervical vagus nerve signals the brain to rapidly store new memories just as epinephrine or emotional arousal would do, but bypasses the peripheral sympathetic “fight-or- flight” response (Figure 1). Therefore, pairing extinction with VNS has the potential to make the extinction memory just as robust as the trauma memory by tapping into the mechanisms that enhance the storage of the traumatic memory without the requirement of a sympathetic stress response.
Figure 1. Vagus nerve stimulation harnesses stress response mechanisms to enhance memory consolidation. Left. Traumatic events lead to activation of the sympathetic nervous system (the “fight-or-flight” response).
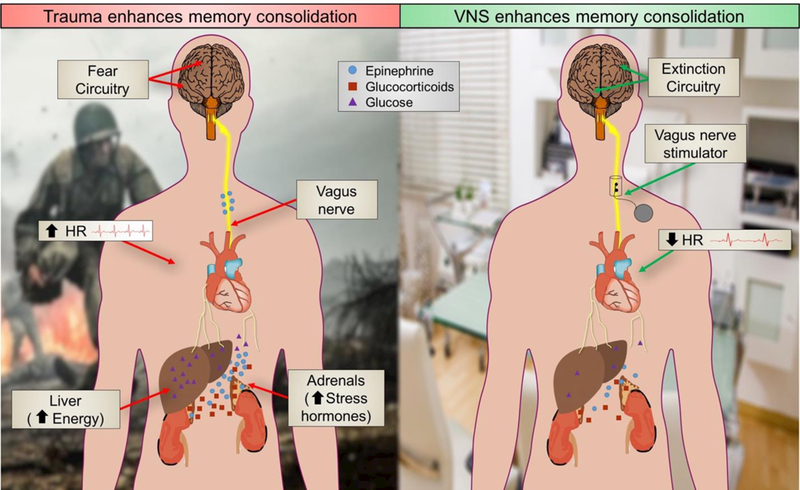
Sympathetic activation involves release of epinephrine (adrenaline) and glucocorticoids from the adrenals and glucose from the liver, increased lung capacity, blood-flow to striated muscles and heart rate. These peripheral changes occur during the memory consolidation window and they are associated with enhanced memory storage. Epinephrine contributes to the enhancement of memory consolidation, but it does not readily cross the blood-brain barrier. Rather, it binds to beta-adrenergic receptors on the vagus nerve. The vagus then signals the brain, through the nucleus tractus solitaries (NTS) in the brain stem, to promote storage of the newly acquired memory. Right. Electrical stimulation of the vagus nerve by implanted cuff signals the brain to store newly acquired memories during exposure therapy. Like the sympathetic stress response, VNS promotes brain plasticity, but VNS bypasses peripheral “fight-or-flight” actions such as increased heart rate. Instead, VNS engages the parasympathetic nervous system, slowing heart rate and increasing gut motility.
In support of this hypothesis, we have found that VNS promotes the rapid storage of new extinction memories in rats (Pena et al. 2013; Pena et al. 2014; Alvarez-Dieppa et al. 2016; Noble et al. 2017a), and others have reported evidence that chronic VNS can reduce anxiety in rats (Shah et al. 2016) and humans (George et al. 2008). This unique combination of effects makes VNS an exciting potential adjuvant to exposure-based therapies. In the next sections, we review studies, relevant to fear extinction, describing the anatomical and functional characteristics of the vagus nerve, prior research on VNS-induced extinction enhancement and current research investigating mechanisms of VNS-induced memory enhancement and plasticity. Finally, we propose future studies targeting the optimization of stimulation parameters and the discovery of biomarkers of VNS effectiveness that may improve outcomes of current VNS applications and expand applications of VNS as an adjunct in exposure therapy.
Functional components of vagus nerve stimulation
Arising from the medulla, the tenth cranial nerve is the longest and most widely distributed visceral sensory nerve in the body. Once known as the “pneumogastric nerve” (Corning 1884), the term vagus (orpar vagum), deriving from the Latin meaning “the wanderer” was first described by the Greek surgeon and anatomist Galen (129 – 210), and it was later studied in detail by the English anatomist Thomas Willis (1664). Initial observations that manual compression and electrical transcutaneous stimulation of the vagus nerve slow the heart rate and reduce seizures (Corning 1884) led to studies exploring the effects of VNS as a treatment for epilepsy. VNS was approved in Europe (1994) and the US (1997) as a treatment for medication- refractory epilepsy, opening the doors for further uses for VNS as a clinical tool.
The vagus transmits information using A, B and C-fibers (Evans and Murray 1954; Agostoni et al. 1957), giving the nerve the capacity to elicit a variety of regulatory effects and feedback about body states. The level of complexity and refinement of the nerve stems from the particular characteristics of its fiber groups, that differ in diameter, conduction velocity, thresholds for activation and afferent-efferent function. The large and myelinated A-fibers (4 – 120 m/sec, activated at intensities between 0.02 to 0.2 mA) carry fast somatic and sensory (pain, stretch, chemical and temperature) information, as well as efferent motor information. The parasympathetic component of the nerve is carried by the small myelinated B-fibers (3 −15 m/sec, activated at intensities between 0.04 to 0.6 mA) from the viscera, while slow visceral afferent information (pain, stretch, chemical and temperature) is carried by the small unmyelinated C-fibers (< 2 m/sec, activated at intensities higher than 2.0 mA) (Foley and DuBois 1937; Ruffoli et al. 2011; Yuan and Silberstein 2016).
For preclinical rodent studies, the most common approach to administering VNS uses surgical implantation of a cuff electrode on the left vagus nerve (for further details, see Childs et al. 2015). Although results of clinical trials indicate that stimulation of the right vagus can be as effective as left vagus stimulation for epilepsy without severe cardiac effects (Krahl 2012), both human and rodent studies suggest that stimulation of the right vagus nerve results in more bradycardia (Randall et al. 1985; Ben-Menachem 2001). Consequently, stimulation of the right vagus nerve is being studied as a possible treatment for heart failure (De Ferrari and Schwartz 2011; Sun et al. 2015).
Central activation by VNS is rapid, and studies demonstrate that VNS triggers scalp- recorded evoked potentials after only 10 milliseconds of stimulation (Usami et al. 2013). Such fast transfer of information is initiated by the vagal afferents that project to the NTS and, from there, to the LC, the dorsal raphe nucleus, and the cholinergic basal forebrain (Krahl and Clark 2012). VNS increases firing activity in the LC and the dorsal raphe nucleus (Manta et al. 2009), and VNS also modulates basal forebrain activity (Detari et al. 1983). These regions have diffuse projections throughout the brain (Groves and Brown 2005) and are responsible for brain-wide release of norepinephrine, dopamine, serotonin, and acetylcholine.
VNS effects on seizure prevention are suppressed by lesions of the LC (Krahl et al. 1998) and VNS-induced cortical desynchronization is dependent on muscarinic acetylcholine receptor signaling (Krahl et al. 1998; Nichols et al. 2011), indicating that the LC and basal forebrain are critically involved in central effects of VNS. These neuromodulatory systems are also implicated in plasticity and memory. Stimulation of the LC (Kasamatsu et al. 1985) or nucleus basalis (Kilgard and Merzenich 1998), paired with visual stimuli or tones, drives cortical plasticity, and lesions of the locus coeruleus or nucleus basalis impair learning (Anlezark et al. 1973; Dunnett 1985). Collectively, these findings provide evidence that VNS can be used to harness neuromodulatory processes that promote plasticity, learning and memory.
The Vagal Brake
The vagus nerve provides information about peripheral arousal to the brain while sending feedback to activate the parasympathetic nervous system and promote adaptive physiological and behavioral strategies (Agostoni et al. 1957; Zagon 2001). In this sense, stimulation of the vagus nerve results in upstream activation of brain areas involved in learning, while it reduces heart rate and controls tachycardia (Hamlin and Smith 1968; Levy 1997; Thompson et al. 1998; Buschman et al. 2006), providing evidence for what Porges describes as the polyvagal theory (Porges 1992). According to the polyvagal theory, low vagal tone is associated with the inability to overcome stress (Porges 1992; 2009) while higher vagal tone correlates with active forms of coping and responsivity to environment challenges (Porges 2009). Vagal tone, or control over heart rate, is typically inferred from measures such as heart rate variability. Consistent with this hypothesis, low vagal tone has been observed in patients who suffer from anxiety disorders (Friedman 2007) and PTSD (Hauschildt et al. 2011). Considering that the vagus nerve dampens sympathetic activity and informs the brain about visceral activity under arousing circumstances to promote self-regulation and optimal behavioral strategies, it is possible that both vagal inputs to the brain and the vagal brake over sympathetic responses are disrupted in PTSD patients. Further studies are necessary to determine whether poor vagal tone is a cause, consequence, or by-product of PTSD. For example, pre-deployment heart rate variability predicted postdeployment PTSD symptom severity in a recent investigation of Army National Guard soldiers (Pyne et al. 2016). In animals, intervention studies can use VNS to increase heart rate variability during conditioned fear learning, retention, or extinction training, to test the hypothesis that vagal tone can influence fear memory.
VNS-induced extinction enhancement reverses PTSD-like symptoms in rats
Evidence from both rat and human studies shows that VNS can enhance memory consolidation (Clark et al. 1995; Clark et al. 1999). In seminal studies, when a single 30-sec 0.4 mA VNS train was delivered following inhibitory avoidance, VNS-treated rats showed higher retention latencies versus sham-treated rats (Clark et al. 1995). Similarly, in humans, the same VNS intensity given following a word learning task increased the number of words participants could recall at retention (Clark et al. 1999). Effects of post-training administration of VNS on memory retention indicate that VNS improves memory consolidation. Because extinction learning requires memory consolidation (Quirk and Mueller 2008), and VNS enhances memory consolidation, we have investigated the effects of VNS on extinction. We administered VNS to fear-conditioned rats during extinction training, temporally pairing the stimulation with exposures to the conditioned stimulus. We found that temporally pairing VNS with exposure to the conditioned stimulus (i.e. an auditory tone) led to decreased levels of conditioned fear of the stimulus on the following day (Figure 2a) (Pena et al. 2013; Pena et al. 2014; Alvarez-Dieppa et al. 2016). Additionally, when extinction training was carried out over sessions, in a fashion that more closely mimics exposure-based therapies, VNS accelerated extinction. Following eleven consecutive days of extinction, five of which were paired with VNS or sham stimulation, only VNS-treated rats reached remission of fear (freezing less than ten percent during CS presentations). VNS-treated rats showed no spontaneous recovery of fear when tested two weeks later. In a separate group of animals, VNS also accelerated extinction of a memory that was learned two weeks prior to extinction sessions (Pena et al. 2013), indicating that the effect is not limited to the extinction of a newly consolidated, or incompletely consolidated memory. This is important because disorders like PTSD are not diagnosed until at least one month following symptom onset, so a treatment that is only effective immediately after the trauma would not be as useful.
Figure 2. Behavioral and physiological changes induced by VNS. A. VNS accelerates extinction of conditioned fear.
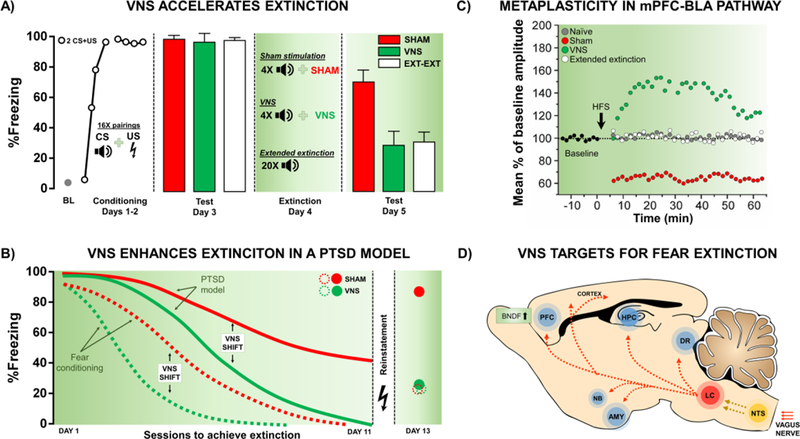
Following auditory fear conditioning (AFC), rats showed similar levels of conditioned fear (day 3). On the following day, rats were then given extinction training consisting of four tones paired with sham stimulation (red), four tones paired with VNS (green), or twenty tones given alone (Extended extinction - EXT-EXT; white). When retested (day 5), rats given VNS showed a significant reduction in conditioned fear versus sham-treated rats. Additionally, four presentations of the tone paired with VNS generated extinction equivalent to twenty presentations of the tone alone (modified from Peña et al. 2013). B. VNS accelerates extinction of conditioned fear in a rat model of PTSD with extinction impairments. VNS accelerated extinction of conditioned fear following AFC (dashed lines). Rats exposed to singleprolonged stress, social isolation, and AFC (PTSD model; solid lines), did not reach remission of fear even following eleven days of extinction training paired with sham stimulation. However, VNS reversed the extinction impairment. VNS-paired extinction also reduced reinstatement. PTSD model rats given sham stimulation showed a complete reinstatement of fear after a single reminder of the footshock. In contrast, VNS-treated rats show reinstatement of fear similar to sham rats who underwent AFC alone (modified from Noble et al. 2017). C) VNS induces metaplasticity in extinction-related circuitry. To examine changes in extinction circuitry, twenty-four hours following the behavior described in panel A, rats were given high-frequency stimulation in the IL and local field potentials were recorded in the BLA. Sham-treated rats showed long-term depression (LTD) in this pathway following high frequency stimulation. In contrast, the same high frequency stimulation induced LTP in VNS-treated rats. Although Ext-Ext rats reached similar levels of extinction as VNS-treated rats, high-frequency stimulation did not produce LTP in the IL-BLA pathway (modified from Peña et al. 2014). D) VNS promotes plasticity through activation of neuromodulatory systems. The vagus nerve projects to the nucleus tractus solitarius (NTS) in the brainstem, which then sends projections to the locus coeruleus (LC). The LC projects to several regions of the brain that are important for fear learning and extinction including the prefrontal cortex (PFC), amygdala (AMY), and the hippocampus (HPC). Additionally, the LC projects to the nucleus basalis (NB) and dorsal raphe nucleus (DR), two important sources of acetylcholine and serotonin, respectively. VNS increases levels of BDNF in the PFC, and these changes may facilitate ongoing experience-dependent plasticity and mediate VNS-induced enhancement of extinction.
To determine if VNS could accelerate extinction in an animal model of stress-induced extinction impairments, we used the single prolonged stress (SPS) rat model of PTSD. We found that SPS-treated rats given fear conditioning (PTSD model rats) showed robust extinction impairments versus rats given fear conditioning alone (Noble et al. 2017a). Following eleven consecutive days of extinction, fear-conditioned control rats showed a full remission of fear. However, PTSD model rats still showed robust conditioned fear. Administration of VNS to PTSD model rats during five of the eleven extinction sessions reversed the extinction impairment; PTSD model rats given VNS exhibited a level of fear that was comparable to that of control rats (Figure 2b). In addition to extinction enhancement, VNS-paired extinction protected against relapse in PTSD model rats. Following a single reminder of the unconditioned stimulus (footshock) PTSD model rats given sham stimulation during extinction sessions showed complete reinstatement of conditioned fear whereas freezing in VNS-treated PTSD model rats was not significantly different from that of controls. In order to test the hypothesis that treatment of the extinction impairment could have beneficial effects on other symptoms of PTSD, separate groups of PTSD model and fear conditioning alone rats underwent the same extinction training protocol and received VNS or sham stimulation, however instead of undergoing a reinstatement protocol, these rats were left undisturbed for one week. Following a week in the home-cage, these rats were tested on a battery of behavioral tests designed to measure PTSD-like symptoms including: anxiety, startle/arousal, avoidance, and social withdrawal. We found that VNS given during extinction, more than one week prior to behavioral testing, reduced anxiety, acoustic startle response, and marble burying, and increased social interaction in PTSD model rats. Our results indicate that by reversing the extinction impairment, VNS reduced other PTSD-like symptoms without the need for symptom-specific treatment (Noble et al. 2017b).
VNS effects on synaptic plasticity
Extensive evidence indicates that VNS is a potent promoter of synaptic plasticity (Kilgard 2012). Repeated pairing of VNS with a sensory stimulus or specific motor movement remodels cortical maps (Engineer et al. 2011; Porter et al. 2012; Shetake et al. 2012). Furthermore, VNS facilitates plasticity-driven rehabilitation following stroke (Khodaparast et al. 2013; Hays et al. 2014; Khodaparast et al. 2016; Meyers et al. 2018), spinal cord injury (Ganzer et al. 2018), and traumatic brain injury (Pruitt et al. 2016). VNS also modulates neural plasticity in the hippocampus. The same VNS that enhances memory consolidation (Clark et al. 1995) enhanced long-term potentiation (LTP) in the dentate gyrus of the hippocampus of freely moving rats (Zuo et al. 2007). The infralimbic region of the prefrontal cortex (IL) to the basolateral amygdala (BLA) pathway is critically involved in extinction of conditioned fear (Sierra-Mercado et al. 2011; Marek et al. 2013). In a study designed to test the effects of VNS on plasticity in extinction-related circuitry, we administered high-frequency stimulation in the IL region and recorded local field potentials in the BLA 48 hours after extinction paired with VNS or sham stimulation. (Pena et al. 2013), Rats given VNS during extinction exhibited LTP in the BLA following high-frequency stimulation in the IL (Pena et al. 2014). In contrast, rats given sham- paired extinction exhibited long-term depression (LTD) in the same pathway following the same high frequency stimulation. High-frequency stimulation did not induce LTP in rats given extended amounts of extinction without VNS, or VNS alone (Figure 2c) (Pena et al. 2014). These findings suggest that VNS pairing with extinction produces metaplasticity in the extinction-related circuit between the IL and the BLA. Consistent with these findings, Alvarez-Dieppa et al. (2016) observed a significant increase in expression of GluN2b protein in the BLA of VNS-treated (vs. sham-treated) rats after extinction training. A change in ratio of expression of the GluN2b to GluN2a subtype of the NMDA receptor in BLA synapses could shift the synaptic state from a predisposition toward depression to a predisposition toward potentiation (Shouval et al. 2002).
In brain regions involved in fear extinction, administration of VNS alters levels of neuromodulators of synaptic plasticity (Figure 2d). The NTS has direct and indirect projections to brain regions that mediate learning and memory. In addition to VNS effects on norepinephrine release from the LC, activity in the basal forebrain is increased following VNS and there is evidence that acetylcholine signaling in the amygdala, hippocampus, and prefrontal cortex acts to modulate memory for fear and extinction (Wilson and Fadel 2017). Stimulation of the vagus nerve also activates the dorsal raphe nucleus (DRN) via indirect projections from the LC and monosynaptic projections from the NTS. Serotonergic DRN neurons send projections throughout the brain (Jacobs and Azmitia 1992) and serotonergic agents such as the antidepressants can influence extinction of conditioned fear (Deschaux et al. 2013). For example, fluoxetine prevented the return of fear when administered together with extinction training (Karpova et al. 2011). VNS also modulates dopamine release as the NTS has direct and indirect projections to the ventral tegmental area (VTA), which then provides dopaminergic input to regions of the brain involved in fear learning and extinction (Pezze and Feldon 2004). It is well established that dopamine is involved in reward learning, however dopamine also plays a role in fear and extinction learning (for review, see Abraham et al. 2014), and recent evidence indicates that dopamine that is co-released from LC terminals in the hippocampus is critical for episodic-like memory (Takeuchi et al. 2016). VNS also increases levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex (Follesa et al. 2007). BDNF engages downstream effectors and pathways related to plasticity (Ying et al. 2002) and plays a critical role in memory consolidation and fear extinction (Bramham and Messaoudi 2005; Chhatwal et al. 2006). VNS also increases phosphorylation of several sites on the BDNF receptor, tyrosine receptor kinase B (TrkB) (Furmaga et al. 2012). Furthermore, multiple studies have demonstrated that infusion of BDNF into the prefrontal cortex is sufficient to generate extinction of fear (Peters et al. 2010; Rosas- Vidal et al. 2014), suggesting that BDNF-driven plasticity may be part of the mechanism by which VNS enhances extinction. VNS acts on several neuromodulatory systems relevant to fear learning and extinction, it is likely that these systems interact to facilitate the consolidation of extinction memories. It is also possible that altering the parameters of VNS stimulation would change the way these neuromodulatory systems are engaged.
Optimizing parameters of VNS
Though VNS is effective across a variety of clinical and preclinical applications, little is known about the effects of altering stimulation parameters. Controlled optimization of stimulation parameters can be performed by varying output current or pulse amplitude (the electrical intensity in mA), frequency of the pulses (in Hz), pulse width (duration of each pulse in |is), number of bursts, and on/off cycle or train duration (in seconds or minutes). In studies investigating targeted plasticity induced by VNS paired with learning, the number of VNS pairings, delay, and the inter-stimulation interval can also be optimized. Studies addressing VNS-induced targeted plasticity suggest that optimizing parameters and timing of stimulation may result in precise engagement of neuromodulatory systems in a nearly physiological fashion that can be delivered in a safe and tolerable way. Optimization of VNS may also allow a high degree of temporal precision and control during therapy (Hays et al. 2013).
Converging evidence suggests that optimization of VNS can be achieved by choosing correct stimulation intensities. Given that increasing VNS intensity might recruit a larger number of afferent fibers and trigger greater release of neuromodulators (Groves and Brown 2005; Roosevelt et al. 2006; Castoro et al. 2011), one might argue that higher currents would result in better plasticity. However, studies addressing memory and plasticity enhancing effects of VNS demonstrate that the effects may bear an inverted-U profile, similar to stress and epinephrine administration, where better effects are observed in moderate (0.4–0.8 mA) than at low or high intensities (0.2 or 1.6 mA) (Clark et al. 1998; Clark et al. 1999; Borland et al. 2016). Clark et al (Clark et al. 1995; 1998) demonstrated that a single 30-sec stimulation of the vagus nerve immediately after the acquisition of inhibitory avoidance in rats resulted in better retention only when stimulation was applied at 0.4 mA, but not 0.2 or 0.8 mA. A similar profile of effects was found in humans studies, where a 30-sec train at 0.5 mA was able to enhance recognition memory, and intensities ranging from 0.75 – 1.5 mA failed (Clark et al. 1999). In a recent study, Borland and coworkers (2016) found that a 9 kHz tone paired with a 20-day VNS training (2.5 h/day, 0.5-sec pairings with 30-sec interval) induced cortical map plasticity when stimulations were moderate (0.4 – 0.8 mA) but not high (1.2 – 1.6 mA), supporting the notion that memory enhancing effects of VNS follow a non-monotonic relationship with intensity. Thus, while higher stimulation intensity is described to improve the effects of VNS in epilepsy (Handforth et al. 1998) and treatment-resistant depression (Aaronson et al. 2013), likely by recruiting more fibers and driving greater release of neuromodulators (Roosevelt et al. 2006; Follesa et al. 2007; Castoro et al. 2011), memory and plasticity enhancement driven by VNS administration are less effective at low or higher intensities.
A growing body of evidence suggests that VNS paired with sensory and motor events reorganizes cortical maps and restores impaired function (Engineer et al. 2011; Porter et al. 2012; Khodaparast et al. 2013; Hays et al. 2014; Pruitt et al. 2016; Ganzer et al. 2018; Meyers et al. 2018). However, one important difference between these studies and the ones describing evidence that VNS facilitates memory extinction is that the duration of pairings is generally 0.5 second in the cortical plasticity studies, in contrast with the 30-second stimulation used in the extinction studies (Clark et al. 1995; Clark et al. 1998; Pena et al. 2013; Pena et al. 2014; Noble et al. 2017a). In addition to the inverted-U function for stimulation intensity, recent evidence indicates that the timing of VNS administration is critical for maximizing plasticity and recovery following spinal cord injury (Ganzer et al. 2018). This suggests that rather than increasing training duration or stimulation intensity, precisely timed VNS-paired extinction may be critical for optimizing therapeutic outcome. Until studies using parametric variations of VNS intensities and timing during fear extinction are performed, it remains unclear which VNS parameters are optimal.
Vagus nerve stimulation as a clinical tool:
VNS has been FDA approved for over two decades. It has been safely used in tens of thousands of patients and it is well-tolerated (Englot et al. 2011; Englot et al. 2012). Current pharmacological tools cannot provide the temporal control that VNS offers, and trains of stimulation as short as 0.5 second can drive pairing-specific plasticity in rats (Engineer et al. 2011; Khodaparast et al. 2013; Borland et al. 2016; Ganzer et al. 2018; Meyers et al. 2018). Pharmacological tools that both augment the effects of exposure therapy and reduce anxiety are lacking. This unique combination of benefits observed in preclinical studies of VNS could improve treatment efficacy and tolerability, giving VNS potential utility for a variety of disorders that are treated with exposure-based therapies, especially those characterized by extinction impairments and/or poor vagal tone.
Limitations and Future Directions:
The invasive nature of a minor surgery carries some risk and discomfort, and it limits treatment availability. Recent research has investigated the use of less invasive methods to stimulate the vagus nerve, including transcutaneous VNS (tVNS). During tVNS, an apparatus is placed on the left concha of the ear to stimulate the auricular branch of the vagus (Peuker and Filler 2002; Van Leusden et al. 2015; Burger et al. 2016; Burger et al. 2017). Some research showed evidence that tVNS accelerates extinction, evidenced by decreased US expectancy ratings, in fear conditioned human participants (Verkuil et al. 2017). However, results of tVNS effects on extinction have been mixed. Other studies found that, though tVNS facilitated extinction by decreasing US expectancy ratings, tVNS did not enhance retention of extinction (Burger et al. 2016; Burger et al. 2017). In these tVNS studies researchers also measured physiological responses such as skin conductance responses, heart rate responses, and fear potentiated startle responses. No physiological differences were seen between tVNS and sham stimulation (Burger et al. 2016; Burger et al. 2017; Verkuil et al. 2017). Future research continuing to improve tolerability and effectiveness of tVNS could help to improve the availability of vagal stimulation to greater patient populations.
Since tVNS patients did not show any measurable physiological changes, it is possible that stimulation was not delivered effectively. Research and treatment could benefit from identification of a physiological marker of VNS effectiveness. Many of the currently-used biomarkers of effective stimulation of descending vagal fibers, such as measureable reductions in inflammatory mediators or alterations in heart rate, can be used as indirect markers of afferent vagal stimulation across individuals. However, other measures are more clearly related to central nervous system effects. For example, P3 event-related potentials (ERPs) are sensitive to VNS (De Taeye et al. 2014; Schevernels et al. 2016), and new VNS electrodes are fMRI conditional (e.g. the Vivistim, from Microtransponder Inc., and the ReStore wireless vagus nerve stimulator in development by Texas Biomedical Device Center at UT Dallas, Dr. Robert Rennaker’s personal communication). These insights could provide direction to clinicans to alter VNS parameters on a patient-by-patient basis.
Though VNS shows promise as a potential adjunct for exposure-based therapies, more research is needed to identify mechanisms of action, optimal VNS parameters, and biomarkers that could facilitate more effective VNS. Understanding the precise mechanisms of VNS- accelerated extinction could improve effectiveness and utilization and aid in discovering new targets for therapy development. Identifying the optimal parameters for VNS-accelerated extinction could accelerate treatments even further and potentially produce more robust and longer-lasting extinction. Chronic VNS can reduce anxiety, but it remains untested whether acute VNS has an immediate effect on anxiety, which would improve the tolerability of exposure- based therapies when VNS is used as an adjunct. Future research examining VNS as a potential tool to supplement exposure-based therapies could benefit from these, and other critical questions.
Conclusions:
Evidence indicating that VNS can drive plasticity, augments extinction, prevent relapse, and reduce anxiety make the treatment an exciting potential adjunct to exposure-based therapies. This unique combination of effects could improve treatment outcomes as well as tolerability of therapy. We have described preclinical evidence that VNS can accelerate extinction of conditioned fear for both recently learned and remote memories. Additionally, VNS reverses extinction impairments seen in rat models of pathology and improves their PTSD-like symptoms more than one week following VNS-paired extinction training. Importantly, VNS protects against relapse including reinstatement and spontaneous recovery, which could function to maintain extinction memories in patients following therapy. VNS is a potent promoter of plasticity and it is likely that the persistence of the extinction memory seen in VNS-treated rats is due to VNS effects on plasticity in the extinction circuitry. VNS is generally well- tolerated by epilepsy and depression patients and it has been used in humans for decades. Taken together, this evidence indicates that VNS shows promise for improving the lives of those who need exposure- based therapies.
Acknowledgments and Disclosure
This work was sponsored by the National Institutes of Mental Health (NIMH - MH10 MH105014) and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) ElectRx program under the auspices of Dr. Doug Weber and Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001–15-2–4057. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the funding agencies. Lindsey J. Noble and Rimenez R Souza reported no biomedical financial interests or potential conflicts of interest. Christa K. McIntyre is an author of a patent entitled “Enhancing Fear Extinction using Vagus Nerve Stimulation”.
References
- Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, Moreno FA, Dunner DL, Lesem MD, Thompson PM, Husain M, Vine CJ, Banov MD, Bernstein LP, Lehman RB, Brannon GE, Keepers GA, O’Reardon JP, Rudolph RL, Bunker M (2013) Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul 6: 631–40. [DOI] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM (2014) Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem 108: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni E, Chinnock JE, De Daly MB, Murray JG (1957) Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol 135: 182–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M (2006) Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 23: 758–64. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dieppa AC, Griffin K, Cavalier S, McIntyre CK (2016) Vagus Nerve Stimulation Enhances Extinction of Conditioned Fear in Rats and Modulates Arc Protein, CaMKII, and GluN2B-Containing NMDA Receptors in the Basolateral Amygdala. Neural Plast 2016: 4273280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Jacobs C, Rothbaum BO (2004) Computer-supported cognitive behavioral treatment of anxiety disorders. J Clin Psychol 60: 253–67. [DOI] [PubMed] [Google Scholar]
- Anlezark GM, Crow TJ, Greenway AP (1973) Impaired learning and decreased cortical norepinephrine after bilateral locus coeruleus lesions. Science 181: 682–4. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E (2001) Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol 18: 415–8. [DOI] [PubMed] [Google Scholar]
- Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, Shahly V, Stein DJ, Petukhova M, Hill E, Alonso J, Atwoli L, Bunting B, Bruffaerts R, Caldas-de-Almeida JM, de Girolamo G, Florescu S, Gureje O, Huang Y, Lepine JP, Kawakami N, Kovess-Masfety V, Medina-Mora ME, Navarro-Mateu F, Piazza M, Posada-Villa J, Scott KM, Shalev A, Slade T, ten Have M, Torres Y, Viana MC, Zarkov Z, Koenen KC (2016) The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med 46: 327–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ, Wilhelm FH (2010) Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. J Anxiety Disord 24: 223–30. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL (2006) Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86: 123–32. [DOI] [PubMed] [Google Scholar]
- Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, Engineer CT, Kilgard MP (2016) Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 9: 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen MJ, Neumann DL, Waters AM (2009) Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Aust N Z J Psychiatry 43: 89–100. [DOI] [PubMed] [Google Scholar]
- Bouton ME (1993) Context, Time, and Memory Retrieval in the Interference Paradigms of Pavlovian Learning. Psychological Bulletin 114: 80–99. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11: 485–94. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC (1979) Role of Conditioned Contextual Stimuli in Reinstatement of Extinguished Fear. J Exp Psychol Anim B 5: 368–378. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology 76: 99–125. [DOI] [PubMed] [Google Scholar]
- Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK (2018) Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry 175: 427–433. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR (2001) Enhanced memory for emotional material following stress- level cortisol treatment in humans. Psychoneuroendocrinology 26: 307–17. [DOI] [PubMed] [Google Scholar]
- Burger A, Verkuil B, van Diest I, van der Does W, Thayer J, Brosschot J (2016) The Effects of Transcutaneous Vagus Nerve Stimulation on Conditioned Fear Extinction in Humans. Psychophysiology 53: S8–S8. [DOI] [PubMed] [Google Scholar]
- Burger AM, Verkuil B, Fenlon H, Thijs L, Cools L, Miller HC, Vervliet B, Van Diest I (2017) Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behaviour Research and Therapy 97: 64–74. [DOI] [PubMed] [Google Scholar]
- Buschman HP, Storm CJ, Duncker DJ, Verdouw PD, van der Aa HE, van der Kemp P (2006) Heart rate control via vagus nerve stimulation. Neuromodulation 9: 214–20. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT (2003) Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem 79: 194–8. [DOI] [PubMed] [Google Scholar]
- Castoro MA, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, Grill WM (2011) Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol 227: 62–8. [DOI] [PubMed] [Google Scholar]
- Chen CC, Williams CL (2012) Interactions between epinephrine, ascending vagal fibers, and central noradrenergic systems in modulating memory for emotionally arousing events. Front Behav Neurosci 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Maguschak KA, Davis M, Ressler KJ (2005) Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Biol Psychiat 57: 113s–113s. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ (2006) Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci 9: 870–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Alvarez-Dieppa AC, McIntyre CK, Kroener S (2015) Vagus Nerve Stimulation as a Tool to Induce Plasticity in Pathways Relevant for Extinction Learning. Jove-J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA (1995) Post-Training Unilateral Vagal-Stimulation Enhances Retention Performance in the Rat. Neurobiology of Learning and Memory 63: 213–216. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2: 94–8. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA (1998) Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem 70: 364–73. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Eccersley PS, Frisby JP, Thornton JA (1970) The amnesic effect of diazepam (Valium). Br J Anaesth 42: 690–7. [DOI] [PubMed] [Google Scholar]
- Corning JL (1884) Electrization of the sympathetic and pneumogastric nerves, with simultaneous bilateral compression of the carotids. The New York Medical Journal 39: 4. [Google Scholar]
- Davis M, Myers KM (2002) The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry 52: 998–1007. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ (2006a) Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics 3: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R (2006b) Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 60: 369–75. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Schwartz PJ (2011) Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev 16: 195–203. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A (2012) A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry 71: 962–8. [DOI] [PubMed] [Google Scholar]
- De Taeye L, Vonck K, van Bochove M, Boon P, Van Roost D, Mollet L, Meurs A, De Herdt V, Carrette E, Dauwe I, Gadeyne S, van Mierlo P, Verguts T, Raedt R (2014) The P3 event- related potential is a biomarker for the efficacy of vagus nerve stimulation in patients with epilepsy. Neurotherapeutics 11: 612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaux O, Zheng X, Lavigne J, Nachon O, Cleren C, Moreau JL, Garcia R (2013) Postextinction fluoxetine treatment prevents stress-induced reemergence of extinguished fear. Psychopharmacology (Berl) 225: 209–16. [DOI] [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T (1983) Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiol Hung 61: 147–54. [PubMed] [Google Scholar]
- Diamond JM (1992) The third chimpanzee : the evolution and future of the human animal, 1st edn. HarperCollins, New York, NY [Google Scholar]
- Dunnett SB (1985) Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology (Berl) 87: 357–63. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP (2011) Reversing pathological neural activity using targeted plasticity. Nature 470: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Chang EF, Auguste KI (2011) Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 115: 1248–55. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Rolston JD, Wang DD, Hassnain KH, Gordon CM, Chang EF (2012) Efficacy of vagus nerve stimulation in posttraumatic versus nontraumatic epilepsy. J Neurosurg 117: 970–7. [DOI] [PubMed] [Google Scholar]
- Evans DH, Murray JG (1954) Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat 88: 320–37. [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M (1992) Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12: 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS (2014) Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71: 681–8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S (2015) Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A 112: E3729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JO, DuBois F (1937) Quantitative studies of the vagus nerve in the cat - I. The ratio of sensory to motor fibers. Journal of Comparative Neurology 67: 49–67. [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G (2007) Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Research 1179: 28–34. [DOI] [PubMed] [Google Scholar]
- Friedman BH (2007) An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol 74: 185–99. [DOI] [PubMed] [Google Scholar]
- Furmaga H, Carreno FR, Frazer A (2012) Vagal Nerve Stimulation Rapidly Activates Brain- Derived Neurotrophic Factor Receptor TrkB in Rat Brain. Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL (1996) Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory 66: 253–257. [DOI] [PubMed] [Google Scholar]
- Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, Adcock KS, James JT, Jeong HS, Becker AM, Goldberg MP, Pruitt DT, Hays SA, Kilgard MP, Rennaker RL 2nd, (2018) Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HA, Kelley LP, Rentz TO, Lee S (2011) Pretreatment Predictors of Dropout From Cognitive Behavioral Therapy for PTSD in Iraq and Afghanistan War Veterans. Psychological Services 8: 1–11. [Google Scholar]
- George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, Anderson B, Kose S, Howland RH, Goodman WK, Ballenger JC (2008) A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimulation 1: 112–121. [DOI] [PubMed] [Google Scholar]
- Gold PE (2014) Regulation of memory - from the adrenal medulla to liver to astrocytes to neurons. Brain Res Bull 105: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB (1975) Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13: 145–53. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB, McGaugh JL (1975) Effects of hormones on time-dependent memory storage processes. Prog Brain Res 42: 210–1. [DOI] [PubMed] [Google Scholar]
- Goode TD, Maren S (2014) Animal Models of Fear Relapse. Ilar Journal 55: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Brown VJ (2005) Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience & Biobehavioral Reviews 29: 493–500. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR (2008) A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiat 63: 544–549. [DOI] [PubMed] [Google Scholar]
- Hamlin RL, Smith CR (1968) Effects of vagal stimulation on S-A and A-V nodes. Am J Physiol 215: 560–8. [DOI] [PubMed] [Google Scholar]
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW (1998) Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51: 48–55. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL (2004) The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 118: 79–88. [DOI] [PubMed] [Google Scholar]
- Haubrich J, Nader K (2018) Memory Reconsolidation . Curr Top Behav Neurosci 37: 151–176. [DOI] [PubMed] [Google Scholar]
- Hauschildt M, Peters MJ, Moritz S, Jelinek L (2011) Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol Psychol 88: 215–22. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL 2nd, Kilgard MP (2014) Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 45: 3097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, Kilgard MP (2013) Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 207: 275–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW (2006) Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63: 298–304. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72: 165–229. [DOI] [PubMed] [Google Scholar]
- James W (1890) The principles of psychology H. Holt and company, New York, [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ (2006) Learning under stress: how does it work? Trends Cogn Sci 10: 152–8. [DOI] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, Antila H, Popova D, Akamine Y, Bahi A, Sullivan R, Hen R, Drew LJ, Castren E (2011) Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 334: 1731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Watabe K, Heggelund P, Scholler E (1985) Plasticity in cat visual cortex restored by electrical stimulation of the locus coeruleus. Neurosci Res 2: 365–86. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL 2nd, Kilgard MP (2013) Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 60: 80–8. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL 2nd, Hays SA (2016) Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabilitation and neural repair 30: 676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP (2012) Harnessing plasticity to understand learning and treat disease. Trends Neurosci 35: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279: 1714–8. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Motes MA, Didehbani N, DeLaRosa B, Bass C, Schraufnagel CD, Jones P, Morgan CR, Spence JS, Kraut MA, Hart J (2018) Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. J Affect Disord 229: 506–514. [DOI] [PubMed] [Google Scholar]
- Krahl SE (2012) Vagus nerve stimulation for epilepsy: A review of the peripheral mechanisms. Surg Neurol Int 3: S47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Clark KB (2012) Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg Neurol Int 3: S255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA (1998) Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39: 709–14. [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Tona KD, den Ouden HEM, Vogel S, van Wingen GA, Fernandez G (2016) How Administration of the Beta-Blocker Propranolol Before Extinction can Prevent the Return of Fear. Neuropsychopharmacology 41: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, McGaugh JL, McIntyre CK (2017) Emotional Modulation of Learning and Memory: Pharmacological Implications. Pharmacol Rev 69: 236–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L (2003) D-Cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Aust J Psychol 55: 84–84. [Google Scholar]
- Levy MN (1997) Neural control of cardiac function. Baillieres Clin Neurol 6: 227–244. [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL (1986) Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res 368: 125–33. [DOI] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG (2012) A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res 46: 1184–90. [DOI] [PubMed] [Google Scholar]
- Lucki I, Rickels K, Giesecke MA, Geller A (1987) Differential effects of the anxiolytic drugs, diazepam and buspirone, on memory function. Br J Clin Pharmacol 23: 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P (2009) Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharm 19: 250–255. [DOI] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P (2013) The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 591: 2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience 14: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Kavushansky A, Holmes A, Wellman C, Motanis H (2012) Enhanced Extinction of Aversive Memories by High-Frequency Stimulation of the Rat Infralimbic Cortex. Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Fernandez de la Cruz L, Monzani B, Rosenfield D, Andersson E, Perez-Vigil A, Frumento P, de Kleine RA, Difede J, Dunlop BW, Farrell LJ, Geller D, Gerardi M, Guastella AJ, Hofmann SG, Hendriks GJ, Kushner MG, Lee FS, Lenze EJ, Levinson CA, McConnell H, Otto MW, Plag J, Pollack MH, Ressler KJ, Rodebaugh TL, Rothbaum BO, Scheeringa MS, Siewert-Siegmund A, Smits JAJ, Storch EA, Strohle A, Tart CD, Tolin DF, van Minnen A, Waters AM, Weems CF, Wilhelm S, Wyka K, Davis M, Ruck C, and the DCSAC, Altemus M, Anderson P, Cukor J, Finck C, Geffken GR, Golfels F, Goodman WK, Gutner C, Heyman I, Jovanovic T, Lewin AB, McNamara JP, Murphy TK, Norrholm S, Thuras P (2017) D-Cycloserine Augmentation of Exposure-Based Cognitive Behavior Therapy for Anxiety, Obsessive-Compulsive, and Posttraumatic Stress Disorders: A Systematic Review and Meta-analysis of Individual Participant Data. JAMA Psychiatry 74: 501–510. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK (2010) Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem 93: 312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL 2nd, Kilgard MP, Hays SA (2018) Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 49: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S (2013) Deficits in Conditioned Fear Extinction in Obsessive-Compulsive Disorder and Neurobiological Changes in the Fear Circuit. Jama Psychiatry 70: 608–618. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang YC, Rauch SL, Pitman RK (2008) Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res 42: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009) Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiat 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Williams CL (2006) Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral beta-adrenergic receptors. Neurobiol Learn Mem 85: 116–24. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ (2008) Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28: 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM (2015) The problem of dropout from “gold standard” PTSD therapies. F1000Prime Reports 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M (2011) Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189: 207–14. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramanathan KR, Meyers E, Kilgard MP, Rennaker RL, McIntyre CK (2017a) Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl Psychiat 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramanathan KR, Meyers E, Kilgard MP, Rennaker RL, McIntyre CK (2017b) Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl Psychiatry 7: e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S (2014) Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena DF, Engineer ND, McIntyre CK (2013) Rapid Remission of Conditioned Fear Expression with Extinction Training Paired with Vagus Nerve Stimulation. Biol Psychiat 73: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ (2010) Induction of Fear Extinction with Hippocampal-Infralimbic BDNF. Science 328: 1288–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuker ET, Filler TJ (2002) The nerve supply of the human auricle. Clinical Anatomy 15: 35–37. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J (2004) Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 74: 301–20. [DOI] [PubMed] [Google Scholar]
- Porges SW (1992) Vagal tone: a physiologic marker of stress vulnerability. Pediatrics 90: 498–504. [PubMed] [Google Scholar]
- Porges SW (2009) The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 76 Suppl 2: S86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL 2nd, Kilgard MP (2012) Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex 22: 2365–74. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB (2010) A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review 30: 635–641. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JAJ, Otto MW, Sanders C, Emmelkamp PMG (2009) Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders 23: 350–356. [DOI] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL (2016) Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma 33: 871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne JM, Constans JI, Wiederhold MD, Gibson DP, Kimbrell T, Kramer TL, Pitcock JA, Han X, Williams DK, Chartrand D, Gevirtz RN, Spira J, Wiederhold BK, McCraty R, McCune TR (2016) Heart rate variability: Pre-deployment predictor of post-deployment PTSD symptoms. Biol Psychol 121: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall WC, Ardell JL, Becker DM (1985) Differential responses accompanying sequential stimulation and ablation of vagal branches to dog heart. Am J Physiol 249: H133–40. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1997) Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Animal Learning & Behavior 25: 99–107. [Google Scholar]
- Rescorla RA, Heth CD (1975) Reinstatement of Fear to an Extinguished Conditioned Stimulus. J Exp Psychol-Anim B 104: 88–96. [PubMed] [Google Scholar]
- Resick PA, Schnicke MK (1992) Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol 60: 748–56. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10: 1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M (2004) Cognitive enhancers as adjuncts to psychotherapy - Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiat 61: 1136–1144. [DOI] [PubMed] [Google Scholar]
- Rodrigues H, Figueira I, Lopes A, Goncalves R, Mendlowicz MV, Coutinho ES, Ventura P (2014) Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta analysis. PLoS One 9: e93519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FH, Quirk GJ (2012) Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A 109: 8764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ (2009) Systemic Propranolol Acts Centrally to Reduce Conditioned Fear in Rats Without Impairing Extinction. Biol Psychiat 65: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA (2006) Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 1119: 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL (2006) Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A 103: 6741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ (2014) Hippocampal-Prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology 39: 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M (2003) Applying learning principles to the treatment of post-trauma reactions. Roots of Mental Illness in Children 1008: 112–121. [DOI] [PubMed] [Google Scholar]
- Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F (2011) The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat 42: 288–96. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2004) Why zebras don’t get ulcers: A Guide to Stress, Stress-Related Diseases and Coping, 3rd edn. Times Books, New York [Google Scholar]
- Schevernels H, van Bochove ME, De Taeye L, Bombeke K, Vonck K, Van Roost D, De Herdt V, Santens P, Raedt R, Boehler CN (2016) The effect of vagus nerve stimulation on response inhibition. Epilepsy Behav 64: 171–179. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH (2008) Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry- Interpersonal and Biological Processes 71: 134–168. [DOI] [PubMed] [Google Scholar]
- Shah AP, Carreno FR, Wu H, Chung YA, Frazer A (2016) Role of TrkB in the anxiolytic-like and antidepressant-like effects of vagal nerve stimulation: Comparison with desipramine. Neuroscience 322: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP (2012) Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol 233: 342–9. [DOI] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN (2002) A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A 99: 10831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011) Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36: 529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Maher MIJ, Wang YJ, Bao YY, Foa EB, Franklin M (2011) Patient Adherence Predicts Outcome From Cognitive Behavioral Therapy in Obsessive-Compulsive Disorder. J Consult Clin Psych 79: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ (2006) Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A 103: 5585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE (2006) Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol Psychiat 60: 329–336. [DOI] [PubMed] [Google Scholar]
- Sun J, Lu Y, Huang Y, Wugeti N (2015) Unilateral vagus nerve stimulation improves ventricular autonomic nerve distribution and functional imbalance in a canine heart failure model. Int J Clin Exp Med 8: 9334–40. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RG (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GW, Levett JM, Miller SM, Hill MR, Meffert WG, Kolata RJ, Clem MF, Murphy DA, Armour JA (1998) Bradycardia induced by intravascular versus direct stimulation of the vagus nerve. Ann Thorac Surg 65: 637–42. [DOI] [PubMed] [Google Scholar]
- Tuerk PW, Wangelin BC, Powers MB, Smits JAJ, Acierno R, Myers US, Orr SP, Foa EB, Hamner MB (2018) Augmenting treatment efficiency in exposure therapy for PTSD: a randomized double-blind placebo-controlled trial of yohimbine HCl. Cogn Behav Ther: 1–21. [DOI] [PubMed] [Google Scholar]
- Usami K, Kawai K, Sonoo M, Saito N (2013) Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul 6: 615–23. [DOI] [PubMed] [Google Scholar]
- Van Leusden JWR, Sellaro R, Colzato LS (2015) Transcutaneous Vagal Nerve Stimulation (tVNS): a new neuromodulation tool in healthy humans? Frontiers in Psychology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B, Burger AM, van Diest I, Vervliet B, van der Does W, Thayer JF, Brosschot JF (2017) Transcutaneous vagal nerve stimulation to promote the extinction of fear. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 10: 395. [Google Scholar]
- Vervliet B, Craske MG, Hermans D (2013) Fear extinction and relapse: state of the art. Annu Rev Clin Psychol 9: 215–48. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M (2002) Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 22: 2343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H, Axelrod J, Tomchick R (1959) Blood-brain barrier for adrenaline. Science 129: 1226–7. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL (2008) Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiat 165: 335–341. [DOI] [PubMed] [Google Scholar]
- Willis T (1664) Cerebri anatome: cui accessit nervorum descriptio et usus . Typis Tho. Roycroft, impensis Jo. Martyn & Ja. Allestry [Google Scholar]
- Wilson MA, Fadel JR (2017) Cholinergic regulation of fear learning and extinction. J Neurosci Res 95: 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR (2002) Brain- derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. Journal of Neuroscience 22: 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD (2016) Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I . Headache 56: 71–8. [DOI] [PubMed] [Google Scholar]
- Zagon A (2001) Does the vagus nerve mediate the sixth sense? Trends Neurosci 24: 671–3. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Smith DC, Jensen RA (2007) Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav 90: 583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


