Abstract
Hyperuricemia is an important risk factor for gout. Isorhamnetin (3′-O-methylquercetin) is an O-methylated flavonol, which occurs in onion, almond and sea buckthorn. It is also one of the metabolites of quercetin in mammals. In the present study, we investigated anti-hyperuricemic effect of isorhamnetin adopting both cultured hepatocytes and mice with hyperuricemia induced by purine bodies. In cultured hepatocytes, isorhamnetin as well as quercetin significantly and dose-dependently inhibited uric acid (UA) production. We also examined the inhibitory effects on UA production of other mono-methylquercetins, i.e., tamarixetin, 3-O-methylquercetin, azaleatin, and rhamnetin in addition to isorhamnetin for studying their structure–activity relationships. From the results obtained, hydroxyl groups at C-3, C-5, and especially C-7, but not C-3′ and C-4′ of quercetin are demonstrated to play a critical role in suppressing UA production in the AML12 hepatocytes. Oral administration of isorhamnetin significantly reduced plasma and hepatic UA levels in the hyperuricemic model mice. Isorhamnetin also decreased hepatic xanthine oxidase (XO) activity without changes in XO protein expression, indicating that anti-hyperuricemic effect of isorhamnetin could be, at least partly, attributable to suppression of UA production by directly inhibiting XO activity in the liver. These findings demonstrate that isorhamnetin has a potent anti-hyperuricemic effect and may be a potential candidate for prevention and remediation of hyperuricemia.
Keywords: Isorhamnetin, AML12 hepatocyte, Hyperuricemia, Uric acid
Introduction
Hyperuricemia is the high blood uric acid (UA) state and results from the UA overproduction in the liver and/or its underexcretion from the kidney. Hyperuricemia is accepted as an important risk factor for gout (Thottam et al. 2017). Likewise, hyperuricemia is thought to be risk factors for metabolic syndrome (Babio et al. 2015). High dietary intake of purine-rich foods such as meats, seafood, purine-rich vegetables triggers the increase of UA levels in the blood (Choi et al. 2004). Purine nucleotides such as guanosine-5′-monophosphate (GMP) and inosine-5′-monophosphate (IMP), both of which stimulate umami taste, are metabolized into uric acid via several steps of reactions catalyzed by various enzymes like 5′-nucleotidase, purine nucleoside phosphorylase, guanine deaminase and xanthine oxidase (XO) (Ishikawa et al. 2013). Allopurinol and febuxostat are potent XO inhibitors prescribed in the treatment of hyperuricemia and gouts. However, allopurinol has some of undesirable side effects such as hepatitis, nephropathy and allergy, and febuxostat has some of the effects such as abnormalities in liver function, diarrhea and rash (Kong et al. 2000; Frampton 2015). Therefore, it is anticipated to quest for novel and safe factors in foods and natural resources that suppress hyperuricemia. Isorhamnetin (3′-O-methylquercetin) is an O-methylated flavonol, which occurs in onion, sea buckthorn berry, almond, and wine (La Torre et al. 2006; Chen et al. 2007; Teets et al. 2009; Olsson et al. 2010). It is one of the metabolites of quercetin in mammals (Manach et al. 1998; Morand et al. 1998). Quercetin is a well-known flavonoid that is found naturally in such as onion and strawberry. Isorhamnetin possesses a number of health benefits including anti-inflammatory, anti-cancer and anti-oxidation (Dong et al. 2014; Chen et al. 2015b; Li et al. 2015). Many flavonoids like quercetin and taxifolin are reported to effective against hyperuricemic model mice (Mo et al. 2007; Adachi et al. 2017b). A recent study has revealed that quercetin decreases plasma UA concentration in pre-hyperuricemic adults (Shi and Williamson 2016). However, it remains unclear whether or not isorhamnetin possesses an anti-hyperuricemic action.
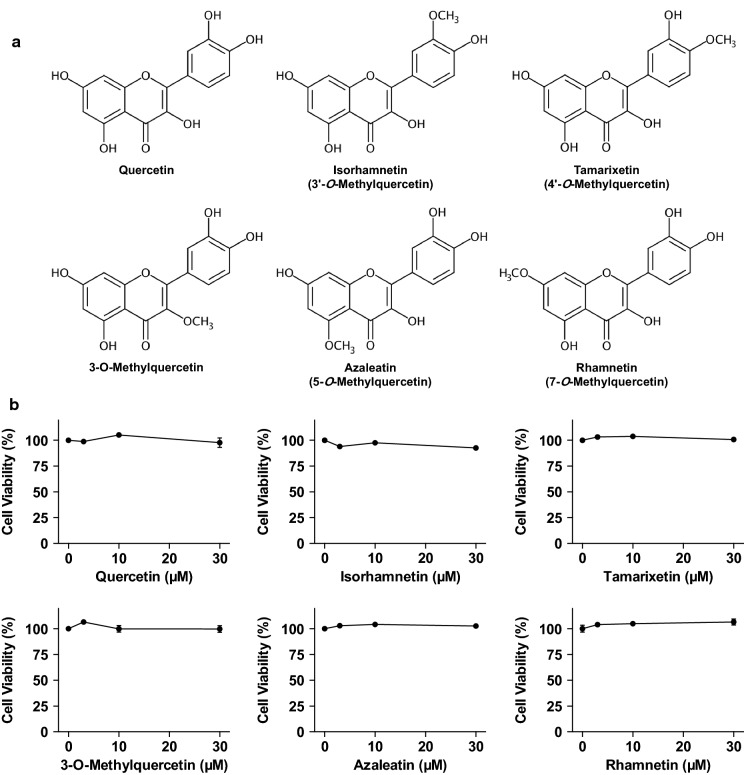
In the present study, we have attempted to examine the effect of isorhamnetin on UA production in cultured hepatocytes and in mice with hyperuricemia induced by purine-bodies administration. We first assessed suppressive action of isorhamnetin on UA production in an in vitro assay system employing the cultured hepatocytes. In order to investigate the structure–activity relationships of mono-methylated quercetins as inhibitors of UA production, we have also tested the inhibitory effects in the AML12 hepatocytes of the other mono-methylquercetins, namely, 3-O-methylquercetin, azaleathin (5-O-methylquercetin), rhamnetin (7-O-methylquercetin) and tamarixetin (4′-O-methylquercetin) (Fig. 1a).
Fig. 1.
Chemical structures of quercetin and mono-methylated quercetin isorhamnetin, tamarixetin, 3-O-methylquercetin, azaleatin and rhamnetin (a). Effects of quercetin and methylquercetin on cell viability in AML12 cells (b). The cells were incubated in BSS with or without various concentrations of quercetin and methylquercetin (0, 3, 10, 30 µM) for 2 h. WST-8 reagent was added 1 h before measurement of OD 450 nm. Data are expressed as a percentage of the vehicle control (DMSO). Each value represents mean ± SEM for six wells
Materials and methods
Materials
AML12 cells were provided by American Type Culture Collection (ATCC® CRL2254, Manassas, VA, USA). Isorhamnetin (3′-O-methylquercetin) was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA), azaleatin (5-O-methylquercetin) from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), DMEM/F-12 from Life Technologies (Grand Island, NY, USA), fetal bovine serum (FBS) from Hyclone (Logan, UT, USA), penicillin and streptomycin from Nacalai Tesque, Inc. (Kyoto, Japan), Pierce™ BCA Protein Assay kit from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Guanosine-5′-monophosphate (GMP) and inosine-5′-monophosphate (IMP) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and 3-O-methylquercetin, rhamnetin (7-O- methylquercetin) and tamarixetin (4′-O-methylquercetin) from Extrasynthese (Lyon, France). Quercetin, selenium, guanosine and inosine were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Allopurinol, dexamethasone, carboxymethyl cellulose sodium (CMC-Na), dimethylsulfoxide (DMSO), recombinant human insulin, transferrin from human blood, uric acid and uric acid assay kit (Uric acid C-test Wako) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Cell counting kit-8 was purchased from Dojindo Laboratories (Kumamoto, Japan). The anti-xanthine oxidase antibody and anti-GAPDH antibody were obtained from Santa Cruz Biotechnology, Inc., horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody from GE Healthcare (Buckinghamshire, UK). The other regents were purchased from Wako Pure Chemical Industries, Ltd., and they were of guaranteed reagent grade.
Culture of AML12 cells
AML12 cells were cultured in DMEM/F-12 supplemented with 10% FBS, 5 µg/ml recombinant human insulin, 5 µg/ml transferrin from human blood, 3 ng/ml selenium, 40 ng/ml dexamethasone, 100 U/ml penicillin and 100 µg/ml streptomycin (10% FBS/DMEM/F-12) under an atmosphere of 5% CO2/95% humidified air at 37 °C as described previously (Petrie et al. 2013) with slight modifications. The cells (1.0 × 105 cells/well) into 24-place multiwell plates and grown for 72 h in 10% FBS/DMEM/F-12, and then kept for 24 h in serum-free DMEM/F-12.
Effects of quercetin and methylquercetin on uric acid production in AML12 cells
UA production by AML12 hepatocytes was evaluated as described previously (Adachi et al. 2017a). In brief, after 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with phosphate buffered saline without calcium or magnesium [PBS (–)] and incubated in 200 µl of balanced salt solution (BSS) including 188 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.8 mM CaCl2, 25 mM NaHCO3, 1 mM NaH2PO4, 10 mM HEPES and 5 mM glucose (Petrie et al. 2013). Furthermore, BSS contained guanosine and inosine (100 µM each) in combination (GI mixture) as UA precursors in the absence or presence of quercetin, isorhamnetin, 3-O-methylquercetin, azaleatin, rhamnetin, or tamarixetin (0, 3, 10, 30 µM) at the final DMSO concentration of 0.15%. On the termination of 2 h incubation, 200 µl of BSS was collected for determination of UA. UA detected in BSS was considered to be an index for UA productivity (Adachi et al. 2017a). The hepatocytes were washed once with PBS (–) and scraped into 300 µl of buffer including 50 mM Tris and 1 mM sodium phosphate (pH 7.5). After being sonicated and centrifuged (12,000×g, 5 min, 4 °C), the supernatants were subjected to protein determination with a Pierce™ BCA Protein Assay kit. UA levels in the BSS were determined by the uricase methods (Uric acid C-test Wako). UA production was expressed as nmol per 2 h per mg cellular protein (nmol/2 h/mg protein).
Cell viability assay
Cell viability assays was assessed with a cell counting kit-8 according to the manufacture’s protocol with slight modifications. AML12 cells were plated in 96-place multiwell plates at 5 × 103 cells per well and incubated for 72 h in 10% FBS/DMEM/F-12, and then kept for 24 h in serum-free DMEM/F-12. After 24 h, the cells were washed once with PBS (–) and incubated in 100 µl of GI mixture in the absence or presence of isorhamnetin, quercetin, 3-O-methylquercetin, azaleatin, rhamnetin, or tamarixetin (0, 3, 10, 30 µM) for 2 h. After washed once with BSS, the hepatocytes were incubated in 100 µl of BSS containing 10 µL of WST-8 reagent for 1 h. The optical density at 450 nm was read with Spark 10 M (Tecan Group Ltd., Männedorf, Switzerlamd). The cell viability was expressed as a percentage of the value of cells treated without samples as 100%.
Animals
Male ICR mice (Charles River Japan, Inc., Yokohama, Japan) at 4 weeks of age were housed in plastic cages in a room with a 12-h light–dark cycle (dark phase of 18:00–6:00) and constant temperature (22 °C). They were housed in groups of four mice for 7 days to acclimatize to the environment. The mice were maintained on tap water and regular diet (CRF-1, Oriental Yeast Co., Tokyo, Japan) ad libitum. This experiment was carried out in accordance with the guideline for Animal Experiments of Utsunomiya University Animal Research Committee (ethic approval number: A14-0017).
Isorhamnetin administration to hyperuricemic model mice
Anti-hyperuricemic effect in the model mice was estimated as described previously (Adachi et al. 2017b). Briefly, after acclimatization to the environment for 1 week, the mice were divided into five groups with similar body weight: normal group (n = 8), hyperuricemic model group (n = 12), allopurinol group (n = 8), low-dose of isorhamnetin group (n = 8) and high-dose of isorhamnetin group (n = 8). In addition, we confirmed anti-hyperuricemic effect of quercetin, a well-known anti-hyperuricemic polyphenol: normal control group (n = 8), hyperuricemic model group (n = 10), allopurinol group (n = 8), low-dose of quercetin group (n = 8) and high-dose of quercetin group (n = 8). Isorhamnetin, quercetin and allopurinol were suspended in 0.5% CMC-Na. After 4 h fasting, allopurinol at 10 mg/kg body weight, isorhamnetin at 100 mg/kg (low-dose group) and 300 mg/kg body weight (high-dose group) and quercetin at 100 mg/kg (low-dose group) and 300 mg/kg body weight (high-dose group) were orally given to the mice once a day for the three consecutive days. Mice of normal control and hyperuricemic model control group were orally given 0.5% CMC-Na alone for 3 days. On day 3, the mice were intraperitoneally injected with both GMP and IMP (300 mg each/kg body weight) to induce hyperuricemia 1 h after allopurinol, isorhamnetin, quercetin, or the vehicle (the model control). GMP and IMP were dissolved in PBS (–). The normal control group was injected with the PBS (–) alone as a vehicle. One hour after GMP and IMP injection, the blood was collected under isoflurane anesthesia from the inferior vena cava in the microtube with heparin sodium and the liver was excised. The blood samples were centrifuged at 5000×g for 10 min at 4 °C to obtain the plasma. The plasma was stored at − 80 °C until analyzed. The excised liver was washed with saline, cut into three pieces, frozen in liquid nitrogen, and stored at –80 °C until analyzed (Adachi et al. 2017b).
Liver sample preparation
Liver sample preparation was performed according to the methods previously described (Adachi et al. 2017b) with slight modifications. One piece of the liver sample was homogenized in ice-cold 100 mM Tris–HCl (pH 7.5), sonicated, and centrifuged (10,000×g, 5 min, 4 °C). The supernatant fraction was used for determination of UA. Another piece was homogenized in ice-cold 100 mM Tris–HCl (pH 7.5) containing 1 mM EDTA-Na, sonicated, and centrifuged (10,000×g, 5 min, 4 °C). The supernatant fraction was used to determine XO activity. The other piece was homogenized in ice-cold RIPA lysis buffer (Nacalai Tesque) and centrifuged (10,000×g, 5 min, 4 °C). The supernatant fraction was subjected to Western blotting.
Determination of uric acid level of plasma and liver
Plasma and liver UA levels were measured by the uricase method (Uric acid C-test Wako). Protein contents in the liver homogenates were determined with a Pierce™ BCA Protein Assay kit. Liver UA levels were expressed as mg per g liver protein.
Liver xanthine oxidase activity assay
Liver XO activity assay was carried out according to the procedure as previously described (Mo et al. 2007) with slight modifications using 96 well multi-plates (Adachi et al. 2017b). Liver homogenates (40 µL) and ice-cold 100 mM Tris–HCl (pH 7.5) containing 1 mM EDTA-2Na (30 µL) were applied into 96-well plates. The reaction was initiated by the addition of 180 µl of 150 µM xanthine in the same buffer. Immediately after the addition of the substrate buffer, the absorbance at 295 nm and 37 °C was measured with Spark 10 M for 30 min. UA production was calculated from the increase of the absorbance for 30 min based on the UA standard curve. Protein concentrations in the liver homogenates were determined with Pierce™ BCA Protein Assay kit. XO activity was expressed as µmol UA produced per min mg protein.
Western blotting
Immunoblotting was processed as previously described (Minakawa et al. 2011), with minor modifications. In each group, we selected four mice that had plasma uric acid levels close to the mean value of the group. Liver protein samples were separated by SDS-PAGE, transferred to a PVDF membrane which was blocked for 1 h with 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween-20 (Sigma-Aldrich Chemical Co.), and incubated overnight at 4 °C with the primary antibody. The primary anti-body was detected with HRP-conjugated anti-mouse secondary antibody and visualized by using Amersham™ ECL™ Western blotting detection reagent (GE healthcare).
Statistical analysis
Data are expressed as means ± SEM. Data on cell viability and UA production in AML12 cells were analyzed by one-way ANOVA and Tukey’s multiple-comparisons test as a post hoc test. The results of the animal experiments were analyzed by one-way ANOVA with Dunnett’s multiple-comparisons test. P values < 0.05 were considered statistically significant. These analyses were conducted by using the Prism 6 software package (GraphPad, San Diego, CA, USA).
Results
Effects of quercetin and methylquercetins on cell viability in AML12 cells
The effects of quercetin and mono-methylated quercetins on the viability of AML12 cells were first determined using WST-8 assay. The treatment of AML12 cells with quercetin, isorhamnetin, tamarixetin, 3-O-methylquercetin, azaleatin, and rhamnetin did not show any cytotoxic effects up to the concentration of 30 µM (Fig. 1b). Based on these results, we adopted the 30 µM of quercetin and methylquercetins for estimating the inhibitory effects on UA production in the hepatocytes without causing cellular damage.
Effects of quercetin and methylquercetin on uric acid in AML12 cells
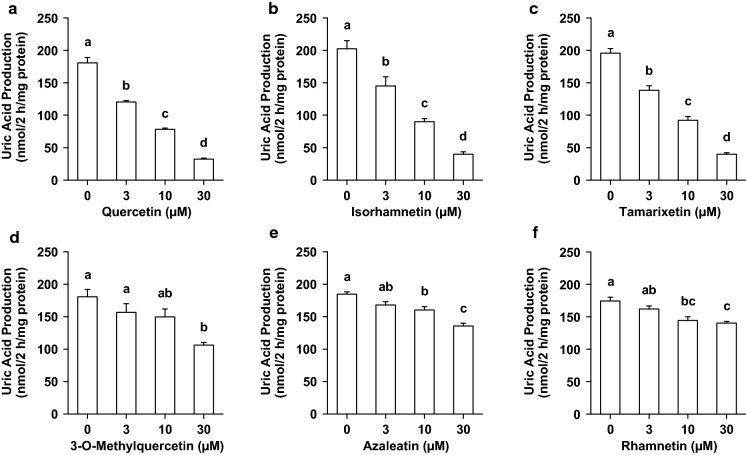
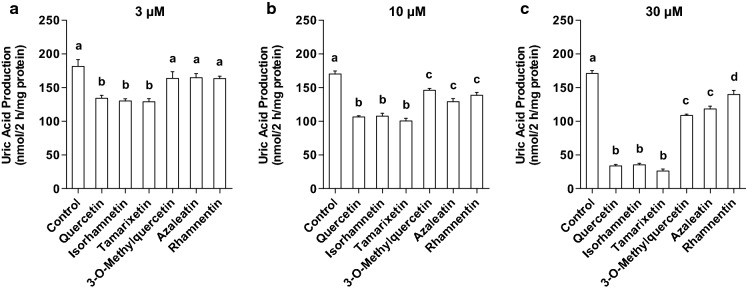
UA production in AML12 cells treated with 3, 10 and 30 µM quercetin was lower than that treated without quercetin (0 µM) (Fig. 2a). Similarly, the treatment of hepatocytes with isorhamnetin, tamarixetin, 3-O-methylquercetin, azaleatin and rhamnetin significantly and dose-dependently decreased the UA production in the hepatocytes (Fig. 2b, c, d, e, f). At the identical concentration of 3 µM, UA production by AML12 hepatocytes treated with quercetin, isorhamnetin and tamarixetin was significantly lower than that of the control (0 µM sample) (Fig. 3a). On the other hand, 3-O-methylquercetin, azaleatin and rhamnetin at dose of 3 µM did not inhibit UA production (Fig. 3a). At the concentration of 10 µM, quercetin and all the mono-methylquercetins significantly suppressed UA production (Fig. 3b). Inhibitory effects of 10 µM quercetin, isorhamnetin and tamarixetin on UA production were stronger than those of 3-O-methylquercetin, azaleatin and rhamnetin (Fig. 3b). Hepatocytes treated with quercetin and all the mono-methylquercetins at 30 µM significantly decreased UA production (Fig. 3c). Quercetin, isorhamnetin and tamarixetin significantly suppressed the production as compared with 3-O-methylquercetin, azaleatin and rhamnetin (Fig. 3c). Inhibitory effects of 3-O-methylquercetin and azaleatin at 30 µM were more notable than that of rhamnetin (Fig. 3c).
Fig. 2.
Effects of quercetin and mono-methylated quercetin on UA production in AML12 cells. AML hepatocytes were treated with 0, 3, 10 and 30 µM querctin (a), isorhamnetin (b), tamrixetin (c), 3-O-methylquerctin (d), azaleatin (e) and rhamnetin (f) for 2 h in BSS containing guanosine + inosine (100 µM each). Each value represents mean ± SEM for six wells (duplicate measurement per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey’s test)
Fig. 3.
Comparison of effects of quercetin and mono-methylquercetin at the same concentration on UA production in AML12 hepatocytes. AML cells were treated with querctin, isorhamnetin, tamrixetin, 3-O-mrthylquerctin, azaleatin and rhamnetin at 3 (a), 10 (b) and 30 (c) µM for 2 h in BSS containing guanosine + inosine (100 µM each). DMSO alone was used as control. Each value represents mean ± SEM for six wells (duplicate measurement per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey’s test)
Effect of isorhamnetin on the plasma uric acid level in hyperuricemic model mice
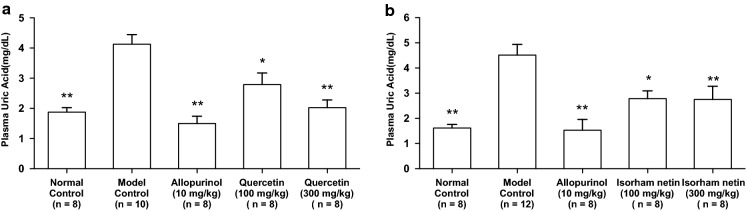
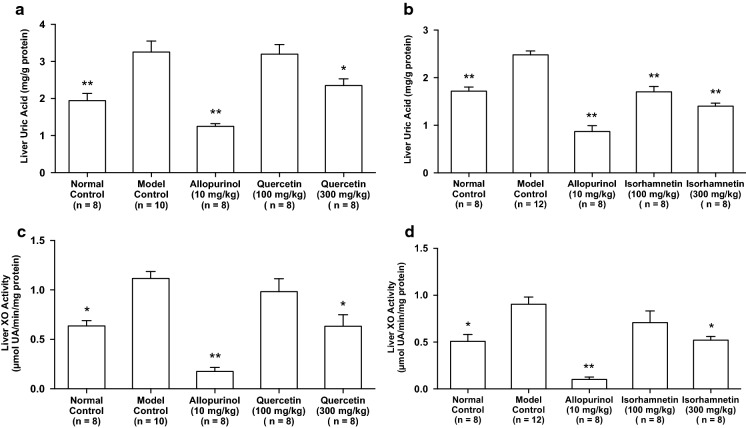
The intraperitoneal injection of GMP and IMP in combination into mice significantly increased the plasma UA concentration (model control group) as compared with that of mice injected with PBS (–) alone (normal control group, Fig. 4a, b). The oral administration of allopurinol at 10 mg/kg body weight, isorhamnetin and quercetin at 100 and 300 mg/kg body weight significantly suppressed the increase in plasma UA concentration as compared with that of model control group (Fig. 4a, b).
Fig. 4.
Effects of quercetin and isorhamnetin on plasma UA levels in hyperuricemic model mice. The mice were perorally administered with quercetin (a) or isorhamnetin (b) at the different doses indicated. The mice were then intraperitoneally injected with both IMP and GMP (300 mg/kg body weight) to induce hyperuricemia. Normal control and model control groups were treated with 0.5% CMC-Na instead of test samples. Normal group was injected with PBS (–) instead of nucleotides. Each value represents mean ± SEM for 8–12 mice (duplicate measurement per mouse). For statistical significance, *P < 0.05 and **P < 0.01 when the treated groups were compared with the model control group (Dunnett’s test)
Effect of isorhamnetin on the liver uric acid level and xanthine oxidase activity in hyperuricemic model mice
The liver UA level in model control group was significantly higher than that in normal control group. The oral administration of allopurinol at 10 mg/kg body weight, isorhamnetin at 100 and 300 mg/kg body weight and quercetin at 300 mg/kg body weight significantly cancelled the increase in hepatic UA concentration as compared with that of model control group (Fig. 5a, b).
Fig. 5.
Effects of quercetin and isorahmnetin on hepatic uric acid levels and xanthine oxidase (XO) activity in hyperuricemic mice. a and b Indicate liver uric acid levels in the mice treated with quercetin and isorhamentin, respectively. c and d Show liver XO activity in the mice treated with quercetin and isorhamentin, respectively. Each value represents mean ± SEM for 8–12 mice (duplicate measurement per mouse). For statistical significance, *P < 0.05 and **P < 0.01 when the treated groups were compared with the model control group (Dunnett’s test)
The intraperitoneal injection of GMP and IMP into mice significantly promoted the liver XO activity than that of PBS (–) alone (normal control group vs. model control group, Fig. 5c, d). The XO activities in allopurinol (10 mg/kg body weight), high-dose (300 mg/kg body weight) of isorhamenetin and high-dose (300 mg/kg body weight) of quercetin groups were significantly lower than that of model control group (Fig. 5c, d).
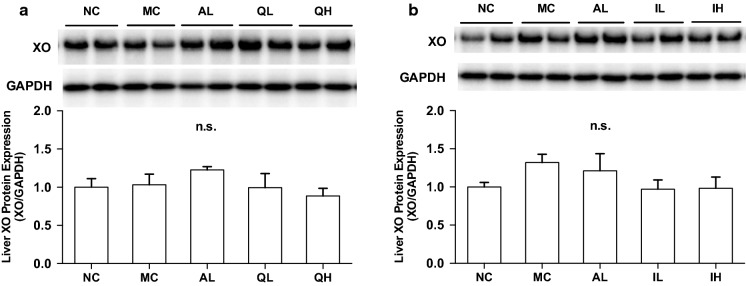
Effects of quercetin and isorhamnetin on liver xanthine oxidase (XO) protein expression in hyperuricemic mice
There was no difference in the hepatic XO protein expression levels among normal control, model control, allopurinol, low-dose (100 mg/kg body weight), and high-dose (300 mg/kg body weight) of quercetin administration (Fig. 6a). Likewise, there was no difference in the XO protein levels among normal control, model control, allopurinol, low-dose (100 mg/kg body weight), and high-dose (300 mg/kg body weight) of isorhamnetin (Fig. 6b).
Fig. 6.
Effects of quercetin and isorhamnetin on liver xanthine oxidase (XO) protein expression in hyperuricemic mice by Western blot analysis. a and b Indicate liver XO protein levels in the mice treated with quercetin and isorhamentin, respectively. Each value represents mean ± SEM for 4 mice
Discussion
We reported that UA levels in BSS increased dose-dependently and significantly by addition of UA precursors, while the UA levels in AML12 hepatocytes were constant (Adachi et al. 2017a). Thus, the extracellular UA level was considered to be a simple index of UA production in the hepatocytes. Employing the cells, we have contrived assay system in vitro for screening compounds that possess inhibitory effects of UA production and constructed in vivo purine bodies-induced hyperuricemic model mice. Allopurinol is an XO inhibitor prescribed clinically for the treatment of hyperuricemia and gout. Recently, quercetin has been reported to lower plasma UA levels in pre-hyperuricemic humans (Shi and Williamson 2016). We have confirmed that both allopurinol and quercetin dose-dependently reduce UA production in the AML12 cells and suppress hyperuricemia induced by UA precursor purine bodies in mice, indicating that our in vitro and in vivo assay systems work adequately. Moreover, we found that taxifolin, present in Siberian larch and strawberries, decreased UA production in the AML12 hepatocytes and inhibited the rises in the plasma UA levels in the model mice by the assay systems (Adachi et al. 2017b).
Quercetin has been found in a wide variety of foods, such as onions and strawberries. Isorhamnetin, tamarixetin, 3-O-methylquercetin, azaleatin and rhamnetin are O-methylated quercetin, and present in food and natural substances. Isorhamnetin (3′-O-methylquercetin) is reported to be present in onion, sea buckthorn berry, almond and wine. Tamarixetin (4′-O-methylquercetin), 3-O-methylquercetin, azaleatin (5-O-methylquercetin) and rhamnetin (7-O-methylquercetin) are isolated from Chromolaena odorata, Nicotiana tabacum L., Rhododendron dauricum L. and Cassia sophera Linn, respectively (Phan et al. 2001; Zhang et al. 2010; Mondal et al. 2013; Schilaty et al. 2014). In this study, we have examined inhibitory effects of these mono-methylated quercetins on UA production by the cultured AML12 hepatocytes when quercetin was used as the positive control. All the mono-methylated quercetins dose-dependently and significantly reduced UA production in the cells (Fig. 2). When compared among the compounds at the identical concentration, isorhamnetin and tamarixetin exhibited equivalently strong inhibitory effects on UA production to quercetin at 3, 10 and 30 µM (Fig. 3). In contrast, the suppressive effects of 3-O-methylquercetin, azaleatin and rhamnetin were lower than that of quercetin. Additionally, at 30 µM, rhamnetin had the lowest inhibitory effect on UA production among the mono-methylquercetins. As above-mentioned, the inhibitory action on UA production of quercetin was weakended by O-methylation of C-3, C-5, or C-7 position, whereas O-methylation of C-3′ or C-4′ position did not affect the inhibitory effect of quercetin. These results strongly suggest that C-3, C-5, and especially C-7, but not C-3′ and C-4′ hydroxyl groups of quercetin play an important role in inhibition of UA production in AML12 cells. UA production in AML12 hepatocytes was reported to be suppressed via inhibition of XO, catalyzing oxidation of hypoxanthine and xanthine to UA (Tsushima et al. 2013). Therefore, in this study, suppression of UA production by quercetin and methylquercetins in AML12 hepatocyte is considered to result from direct inhibition of XO by them. It was reported that the hydroxyl groups at C-3, C-5 and C-7 were important for inhibition of XO by flavonoids including quercetin (Cos et al. 1998; Nessa et al. 2010). On the other hands, O-methylatoion on C-3′ or C-4′ position of quercetin was demonstrated not to be influence its XO inhibition (Cos et al. 1998; Nessa et al. 2010). The results on suppressive effects on UA production in the present study correspond well with these previous reports. Zhang et al. have shown that two hydrogen bonds were formed between hydroxyl groups of quercetin and the hydrogen atoms on amino acid residues of XO, and the hydroxyl group at C-5 and C-7 of quercetin was connected with Glu263 and Arg394 of XO, respectively by molecular docking analysis, indicating that the hydrogen bonding may play a key role in the XO inhibitory process of quercetin (Zhang et al. 2018). In our experiment, diminution of inhibitory effects on UA production by O-methylation at C-5 and C-7 hydroxyl groups of quercetin may be due to the loss of the hydrogen bonds between quercetin and XO.
As shown in vitro study, isorhamnetin and tamarixetin possessed strong inhibitory effects on UA production in AML12 hepatocytes compared to the other mono-methylated quercetins. When rats were orally given quercetin, approximately 63% and 4% of the quercetin were reported to be methylated to isorhamnetin and tamarixetin, respectively (Morand et al. 2000). Thus, we have investigated the effect of isorhamnetin on hyperuricemia in the present study. As a matter of course, hypouricemic effect of tamarixetin should be clarified in the future. Isorhamnetin at dose of 100 and 300 mg/kg body weight, as well as quercetin and allopurinol for the positive control drugs in this study, dose-dependently and significantly cancelled the rise in plasma UA levels in purine bodies-induced hyperuricemia (Fig. 4). Thus, these findings provide evidence that isorhamnetin has a hypouricemic potential for the first time. Intraperitoneally injection of GMP and IMP in combination also caused rises in the mice liver UA levels and enhanced hepatic XO activities (Fig. 5). Similarly to quercetin (300 mg/kg body weight) and allopurinol (10 mg/kg body weight), isorhamnetin (100 and 300 mg/kg body weight) dose-dependently and significantly reduced hepatic UA levels in hyperuricemic mice (Fig. 5a, b). Furthermore, isorhamnetin (300 mg/kg body weight) as well as quercetin (300 mg/kg body weight) and allopurinol (10 mg/kg) suppressed significantly hepatic XO activities (Fig. 5c, d). On the other hands, XO protein expression levels were not significantly increased by GMP and IMP combination (normal control group vs. model control group, Fig. 6). Isorhamnetin, quercetin, and allopurinol had no effects on XO protein expression levels (Fig. 6). These results indicate that isorhamnetin shows anti-hyperuricemic effect by directly suppressing XO activity in the hyperuricemic model mice induced by GMP and IMP.
Flavonoids, including quercetin and isorhamnetin have been described as health-promoting, disease-preventing dietary supplements. In addition, flavonoids themselves are considered to have no or little toxic effects and have a long history of human consumption (Middleton et al. 2000). Indeed, supplementation with quercetin at 500 mg/day for 4 weeks progressively decreased plasma UA levels in pre-hyperuricemic adults without inducing changes in BMI, in fasting blood glucose or showing any adverse effects (Shi and Williamson 2016). In addition, quercetin supplementation at 730 mg/day for 4 weeks has been reported to reduce blood pressure in hypertensive subjects with no side-effects (Edwards et al. 2007). As above-mentioned, isorhamnetin is one of the metabolites of quercetin in mammals, suggesting the possibility of safety of isorhamnetin. In this study, body weight loss or adverse effects have not been observed in the mice administered quercetin and isorhamnetin.
The increases of plasma and liver UA levels may be regulated also by other systems, such as UA production from adipose tissues and cells (Tsushima et al. 2013). In addition, morin, one of flavonols, and polyphenols from green tea demonstrated anti-hyperuricemic effects by inhibiting UA reabsorption and enhancing UA secretion in the kidney of the potassium oxonate-induced hyperuricemic model mice (Wang et al. 2010; Chen et al. 2015a). Apart from suppressing UA production via XO inhibition in the liver, there is a possibility that isorhamnetin may promote UA excretion from the kidney. In this study, quercetin at lower dose (100 mg/kg body weight) repressed the rise in the plasma UA levels, but not hepatic UA levels or XO activity. Moreover, isorhamnetin at the low dose of 100 mg/kg body weight inhibited the rise in not XO activity but the plasma and liver UA levels. These inconsistencies may be due to the reason that the rises in the plasma and liver may be modulated through above-mentioned other systems. Further studies are needed to elucidate precise mechanism for anti-hyperuricemic effect of isorhamnetin.
In summary, isorhamnetin has a high inhibitory action of UA production in AML12 hepatocytes. Structure–activity relationship of mono-methylated quercetins indicates that hydroxyl groups at C-3, C-5, and especially C-7, but not C-3′ and C-4′ of quercetin play a critical role in suppressing UA production in the cells. Furthermore, we have demonstrated that isorhamnetin inhibits the rises in the plasma and hepatic UA levels in mice with hyperuricemia induced by purine bodies, at least partly, due to its effect on hepatic XO activity. Although further studies are required to clarify precise mechanisms involved, these findings suggest that isorhamnetin may be a potential candidate for prevention and remediation of hyperuricemia.
Acknowledgements
This work was supported in part by the Reginal Innovation Strategy Support Program, MEXT, Japan, and in part by JSPS KAKENHI Grant Nos. JP16K16273 and JP15K07424. Authors are grateful to Keiichiro Numao, Kento Kobayashi, Kazusa Narita, Yuki Takami for their excellent technical assistance.
Abbreviations
- BSS
Balanced salt solution
- GMP
Guanosine-5′-monophosphate
- IMP
Inosine-5′-monophosphate
- UA
Uric acid
- XO
Xanthine oxidase
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Adachi S, Yoshizawa F, Yagasaki K. Assay systems for screening food and natural substances that have anti-hyperuricemic activity: uric acid production in cultured hepatocytes and purine bodies-induced hyperuricemic model mice. Cytotechnology. 2017;69:435–442. doi: 10.1007/s10616-016-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Nihei K, Ishihara Y, Yoshizawa F, Yagasaki K. Anti-hyperuricemic effect of taxifolin in cultured hepatocytes and model mice. Cytotechnology. 2017;69:329–336. doi: 10.1007/s10616-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babio N, Martínez-González MA, Estruch R, Wärnberg J, Recondo J, Ortega-Calvo M, Serra-Majem L, Corella D, Fitó M, Ros E, Becerra-Tomás N, Basora J, Salas-Salvadó J. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2015;25:173–180. doi: 10.1016/j.numecd.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang H, Xiao W, Yong ZP, Bai N. High-performance liquid chromatographic fingerprint analysis for different origins of sea buckthorn berries. J Chromatogr A. 2007;1154:250–259. doi: 10.1016/j.chroma.2007.03.097. [DOI] [PubMed] [Google Scholar]
- Chen G, Tan ML, Li KK, Leung PC, Ko CH. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J Ethnopharmacol. 2015;175:14–20. doi: 10.1016/j.jep.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Chen TL, Zhu GL, Wang JA, Zhang GD, Liu HF, Chen JR, Wang Y, He XL. Protective effects of isorhamnetin on apoptosis and inflammation in TNF-α-induced HUVECs injury. Int J Clin Exp Pathol. 2015;8:2311–2320. [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Vanden Berghe D. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–76. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- Dong GZ, Lee JH, Ki SH, Yang JH, Cho IJ, Kang SH, Zhao RJ, Kim SC, Kim YW. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur J Pharmacol. 2014;740:634–640. doi: 10.1016/j.ejphar.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- Frampton JE. Febuxostat: a review of its use in the treatment of hyperuricaemia in patients with gout. Drugs. 2015;75:427–438. doi: 10.1007/s40265-015-0360-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Aw W, Kaneko K. Metabolic interactions of Purine derivatives with human ABC transporter ABCG2: genetic testing to assess gout risk. Pharmaceuticals (Basel) 2013;6:1347–1360. doi: 10.3390/ph6111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LD, Cai Y, Huang WW, Cheng CHK, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/S0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- La Torre GL, Saitta M, Vilasi F, Pellicanò T, Dugo G. Direct determination of phenolic compounds in Sicilian wines by liquid chromatography with PDA and MS detection. Food Chem. 2006;94:640–650. doi: 10.1016/j.foodchem.2005.02.007. [DOI] [Google Scholar]
- Li Q, Ren F-Q, Yang C-L, Zhou LM, Liu YY, Xiao J, Zhu L, Wang ZG. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J Cancer Prev. 2015;16:3035–3042. doi: 10.7314/APJCP.2015.16.7.3035. [DOI] [PubMed] [Google Scholar]
- Manach C, Morand C, Crespy V, Demigné C, Texier O, Régérat F, Rémésy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/S0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- Middleton E, Theoharides T, Kandaswami C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Minakawa M, Kawano A, Miura Y, Yagasaki K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J Clin Biochem Nutr. 2011;48:237–244. doi: 10.3164/jcbn.10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo S-F, Zhou F, Lv Y-Z, Hu QH, Zhang DM, Kong LD. Hypouricemic action of selected flavonoids in mice: structure–activity relationships. Biol Pharm Bull. 2007;30:1551–1556. doi: 10.1248/bpb.30.1551. [DOI] [PubMed] [Google Scholar]
- Mondal A, Rajalingam D, Kumar Maity T. Anti-inflammatory effect of O-methylated flavonol 2-(3,4-dihydroxy-phenyl)- 3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J Ethnopharmacol. 2013 doi: 10.1016/j.jep.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Morand C, Crespy V, Manach C, Besson C, Demigné C, Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol. 1998;275:R212–R219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- Morand C, Manach C, Crespy V, Remesy C. Respective bioavailability of quercetin aglycone and its glycosides in a rat model. BioFactors. 2000;12:169–174. doi: 10.1002/biof.5520120127. [DOI] [PubMed] [Google Scholar]
- Nessa F, Ismail Z, Mohamed N. Xanthine oxidase inhibitory activities of extracts and flavonoids of the leaves of Blumea balsamifera. Pharm Biol. 2010;48:1405–1412. doi: 10.3109/13880209.2010.487281. [DOI] [PubMed] [Google Scholar]
- Olsson ME, Gustavsson K-E, Vågen IM. Quercetin and isorhamnetin in sweet and red cultivars of onion (Allium cepa L.) at harvest, after field curing, heat treatment, and storage. J Agric Food Chem. 2010;58:2323–2330. doi: 10.1021/jf9027014. [DOI] [PubMed] [Google Scholar]
- Petrie JL, Patman GL, Sinha I, Alexander TD, Reeves HL, Agius L. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. Am J Physiol Endocrinol Metab. 2013;305:E1255–E1265. doi: 10.1152/ajpendo.00214.2013. [DOI] [PubMed] [Google Scholar]
- Phan TT, Wang L, See P, Grayer RJ, Chan SY, See ST. Phenolic compounds of Chromolaena odorata protect cultured skin cells from oxidative damage: implication for cutaneous wound healing. Biol Pharm Bull. 2001;24:1373–1379. doi: 10.1248/bpb.24.1373. [DOI] [PubMed] [Google Scholar]
- Schilaty ND, Hedges DM, Jang EY, Folsom RJ, Yorgason JT, McIntosh JM, Steffensen SC. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2014;349:559–567. doi: 10.1124/jpet.113.211490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr. 2016;115:800–806. doi: 10.1017/S0007114515005310. [DOI] [PubMed] [Google Scholar]
- Teets AS, Minardi CS, Sundararaman M, Hughey CA, Were LM. Extraction, identification, and quantification of flavonoids and phenolic acids in electron beam-irradiated almond skin powder. J Food Sci. 2009;74:298–305. doi: 10.1111/j.1750-3841.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- Thottam GE, Krasnokutsky S, Pillinger MH. Gout and metabolic syndrome: a tangled web. Curr Rheumatol Rep. 2017;19:60. doi: 10.1007/s11926-017-0688-y. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-P, Wang X, Zhang X, Shi YW, Liu L, Kong LD. Morin improves urate excretion and kidney function through regulation of renal organic ion transporters in hyperuricemic mice. J Pharm Pharm Sci. 2010;13:411. doi: 10.18433/J3Q30H. [DOI] [PubMed] [Google Scholar]
- Zhang BN, Hou YL, Liu BJ, Liu QM, Qiao GF. The Rhododendron dauricum L. flavonoids exert vasodilation and myocardial preservation. Iran J Pharm Res IJPR. 2010;9:303–311. [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang R, Zhang G, Gong D. Mechanistic insights into the inhibition of quercetin on xanthine oxidase. Int J Biol Macromol. 2018;112:405–412. doi: 10.1016/j.ijbiomac.2018.01.190. [DOI] [PubMed] [Google Scholar]