Abstract
PURPOSE
Breast cancer is the most common cancer in women and a leading cause of cancer-related mortality worldwide. South Africa has the largest global burden of HIV infection and the largest anti-retroviral treatment (ART) program. This study aimed to analyse the association of HIV and ART use with breast cancer clinico-pathological characteristics.
METHODS
Study participants were females, newly diagnosed from May 2015 through September 2017 with invasive breast cancer at two academic Surgical Breast Units in Johannesburg, South Africa at the Charlotte Maxeke Johannesburg Academic Hospital and Chris Hani Baragwanath Academic Hospital. We compared HIV-positive and HIV negative patients’ demographic and clinical-pathological characteristics at the time of breast cancer diagnosis.
RESULTS
Of 1050 patients enrolled, 1016 (96.8%) had known HIV status, with 226 (22.2%) being HIV positive. HIV positive patients were younger (median (IQR) age 45 (40–52) years), than HIV-negative patients (median (IQR) age 57 (46–67)) (p<0.001). HIV positive patients were more likely to be diagnosed with late stage breast cancer(p=0.01). However, HIV positive patients receiving ART at the time of breast cancer diagnosis were less likely to present with metastatic disease than those not on ART (p=0.05).
CONCLUSION
HIV-positive patients present with breast cancer at a younger age and later stage disease than HIV-negative patients. Neither the duration of HIV infection nor ART use was associated with clinicopathological characteristics of breast cancer.
Keywords: BREAST CANCER, HUMAN IMMUNODEFICIENCY VIRUS (HIV), ANTI-RETROVIRAL THERAPY (ART)
INTRODUCTION
Breast cancer is the most common malignancy and the leading cause of cancer-related deaths among women worldwide1. For at least two decades, reported incidence rates of breast cancer have increased worldwide and now account for 24.2% of all cancers and 15% of cancer-related deaths among women1. In South Africa, breast cancer is the most common female malignancy, accounting for about 22% of all malignancies2. It has an age-standardised rate of 33.35 per 100 000 population with a lifetime risk (before the age of 74) of 1 in 27 women2.
South Africans carry 20% of the global HIV burden3, with 15% of new HIV infections and 11% of AIDS related deaths3. From 2002 through 2016, the total number of persons living with HIV in South Africa increased from 4 million to 7.03 million4. The prevalence of HIV in the entire population is 12.8%, which is even higher in adults aged 15–49 years (19.1%)5. The prevalence of HIV among people above 50 years of age has also increased over the years, with 80% of them residing in low-middle income countries6,7.
In 2016, South Africa was reported to have the largest anti-retroviral treatment (ART) program in the world. More than 50% of people living with HIV (PLWH) are receiving treatment and about 45% of those on treatment have a viral load below detectable levels3. The number of those on treatment is expected to increase due to the change of the treatment guidelines, recommending ART in all patients diagnosed with HIV regardless of the CD 4 count or clinical stage of the disease8. The availability and effectiveness of ART has led to a decrease in HIV-associated mortality and has prolonged the life-expectancy of people living with HIV5. A standard fixed dose combination (FDC) is currently being used as a first line regime and consists of 2 Nucleoside reverse transcriptase inhibitors (NRTI), tenofivir disoprostil fumarate (TDF) & emtricitabine (FTC)/lamivudine(3TC) and a Non-nucleoside reverse transcriptase inhibitor (NNRTI), efavirenz(EFV)8.
HIV is not an oncogenic virus but rather a permissive virus which indirectly predisposes infected patients to the development of certain malignancies through suppression of T-cell function9,10. An estimated 3040% of HIV infected patients are expected to have cancer in their lifetimes11, although the risk is mitigated by the use of ART. Malignancies may account for more than one-third of deaths among patients living with HIV12.
In the past few years, several studies have explored associations between HIV infection and breast cancer. Molecular and genetic studies have demonstrated possible interaction between HIV and breast cancer, however, there is no proven direct link between them13. Some studies suggest HIV infection may modify breast cancer growth and progression while other studies have postulated that HIV may be protective against the development and growth of breast cancer13.
This study aims to investigate the association of HIV infection and ART, with the clinico-pathological presentation of breast cancer in a South African urban female population with known high HIV prevalence.
PATIENTS AND METHODS
This observational, descriptive study draws on data from the South African Breast Cancer and HIV Outcome (SABCHO), a cohort of breast cancer patients diagnosed and treated at five hospitals in Gauteng and KwaZulu Natal, South Africa. Our study participants were diagnosed at the Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) Surgical Breast Unit and the Batho Pele Breast Unit of the Chris Hani Baragwanath Academic Hospital (CHBAH), from 15 May 2015 through 31 September 2017. Both are public hospitals based in an urban setting that serve the socio-economically disadvantaged majority of the population. The CMJAH Surgical Breast Unit is based in central Johannesburg and diagnoses about 250 patients with breast cancer yearly. The Batho Pele Breast Unit serves patients from Soweto and surrounding areas and diagnoses about 350 patients with breast cancer yearly14.
All consenting female patients aged 18 years and older, newly diagnosed with invasive breast cancer, were enrolled on the study. Patients who were HIV-unknown were counselled about HIV and were asked to give informed consent for testing. Patients newly diagnosed with HIV were offered post-test counselling. All HIV positive were asked to provide blood samples for CD 4 counts and HIV viral load testing. We recorded the time span since HIV diagnosis, as per the first positive HIV serological test recorded on the National Health Laboratory system, and initiation of ART prior to histologically-confirmed breast cancer diagnosis. HIV positive patients not yet on ART were sent to the HIV/ART clinic for initiation of ART prior to cancer treatment.
Our clinical staging of breast cancer followed the American Joint Committee on Cancer (AJCC) system15. We also categorised patients into early (Stage I/II) and late (Stage III/IV) stage disease. Pre-treatment pathology reporting included the histological diagnosis, tumour subtype, grade, oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki 67. The Allred score was calculated from the intensity and proportion scores for oestrogen and progesterone receptors; specimens scored 3–8 were regarded as hormone receptor positive16. HER2 was regarded as positive when the test showed 3+ and negative when it was 1+. A HER2 score of 2+ was regarded as equivocal, and HER2 positive result was confirmed using in situ hybridization (FISH or SISH)16. Ki 67 is a nuclear antigen used as a marker of cell proliferation; we categorized specimens in which < 14% of cells expressed Ki67 as having low expression, as per the St Gallen 2011 guidelines17,18. The breast tumours were categorized based on IHC 4 subtype into: Luminal A [ER and/or PR positive, HER2 negative, Ki 67 < 14%]; Luminal B [ER and/or PR positive and HER2 negative with Ki 67% ≥ 14% OR HER2 positive with any Ki 67]; HER2 positive subtype [ER and PR negative, HER2 positive]; and triple negative breast cancer (TNBC) [ ER, PR, and HER2 negative]18.The Modified Bloom & Richardson grading system was used which is based on tubule formation, mitosis, and nuclear pleomorphism; a score of ≤ 5 denoted a Grade 1 (well differentiated) tumour, 6–7 a Grade 2 (moderately differentiated) tumour, and 8–9 a Grade 3 (poorly differentiated) tumor19.
Patients were categorised as HIV positive, HIV negative or HIV unknown. Only 3.2% of the patients were HIV unknown and were excluded from further analysis. Demographic and clinico-pathological data were categorised and comparisons between HIV positive and HIV negative patients performed using χ2 tests. We obtained CD 4 counts and HIV viral loads for the HIV-infected patients from the South African National Health Laboratory Service (NHLS) (www.nhls.ac.za) using results of tests performed closest to the date of breast cancer diagnosis. Viral load was categorised as either detectable (>50 copies/ml) or below detectable limits (≤50 copies/ml). Age, CD 4 count, and viral load (when detectable) were nonnormally distributed and were thus represented as medians and inter-quartile ranges (IQRs) and compared using a Kruskal-Wallis test. Generalised linear models with binary outcomes and a log link function were used to determine prevalence ratios for non-missing variables to assess the relationship between stage at diagnosis, tumour grade, IHC4 subtype and presence or absence of metastatic disease with HIV status controlling for age at diagnosis (continuous) and ethnicity (black vs non-black). Collected data were analysed using STATA v12.1.
RESULTS
Of the 1050 patients newly diagnosed with invasive breast cancer enrolled in the study, 34 had unknown HIV status and were excluded from the analysis. The demographic and clinico-pathological characteristics of 1016 patients with and without HIV are shown in Table 1. Of these, 226 (22.2%) were HIV positive, 855 (84.2%) patients were self-reported as black. The median (interquartile range, IQR) age at diagnosis of those analysed was 54 (IQR 44–64) years, and 560 (55.1%) were diagnosed at late stage disease (stage III/IV).
Table 1 –
Demographic and clinico-pathological characteristics of patients with known HIV status* at breast cancer diagnosis.
| Total n (%) | HIV positive n (%) | HIV negative n (%) | p value** | |
|---|---|---|---|---|
| Total*** | 1016 (100%) | 226 (22.2%) | 790 (77.8%) | |
| Age at Breast Cancer Diagnosis | ||||
| 20–39 | 145 (14.3%) | 56 (24.8%) | 89 (11.3%) | |
| 40–49 | 258 (25.4%) | 103 (45.6%) | 155 (19.6%) | |
| 50–59 | 236 (23.2%) | 43 (19.0%) | 193 (24.4%) | |
| 60–69 | 202 (19.9%) | 19 (8.4%) | 183 (23.2%) | |
| 70–79 | 127 (12.5%) | 4 (1.8%) | 123 (15.6%) | |
| ≥80 | 48 (4.7%) | 1 (0.4%) | 47 (6.0%) | |
| Age at Breast Cancer Diagnosis (median (IQR)) | 54(44–64) | 45 (40–52) | 57(46–67) | <0.001‡ |
| Ethnicity | ||||
| Black | 855 (84.2%) | 219 (97.3%) | 636 (80.6%) | |
| White | 82 (8.1%) | 1 (0.4%) | 81 (10.3%) | |
| Coloured | 60 (5.9%) | 5 (2.2%) | 55 (7.0%) | |
| Asian | 17 (1.7%) | 0 | 17 (2.2%) | |
| Stage at Diagnosis | ||||
| Stage I | 62 (6.2%) | 6 (2.7%) | 56 (7.2%) | |
| Stage II | 384 (38.2%) | 77 (34.2%) | 307 (39.3%) | |
| Stage III | 401 (40.1%) | 103 (45.8%) | 298 (38.2%) | |
| Stage IV | 159 (15.5%) | 39 (17.3%) | 120 (15.4%) | |
| Early Stage | 446 (43.9%) | 83 (36.9%) | 363 (46.5%) | 0.01 |
| Late Stage | 560 (55.1%) | 142 (63.1%) | 418 (53.5%) | |
| Metastasis | ||||
| No known metastasis | 887 (84.5%) | 186 (82.3%) | 669 (84.7%) | 0.39 |
| Metastasis | 163 (15.5%) | 40 (17.7%) | 121 (15.3%) | |
| Site of Metastasis | ||||
| Visceral | 70 (45.5%) | 20 (54.1%) | 49 (42.6%) | 0.17 |
| Non-visceral | 43 (27.9%) | 6 (16.2%) | 37 (32.2%) | |
| Visceral and non-visceral | 41 (26.6%) | 11 (29.7%) | 29 (25.2%) | |
| Tumour grade at diagnosis | ||||
| 1 | 57 (5.8%) | 11 (5.2%) | 44 (5.9%) | 0.34 |
| 2 | 496 (50.2%) | 116 (54.5%) | 363 (48.8%) | |
| 3 | 435 (44.0%) | 86 (40.4%) | 337 (45.3%) | |
| ER status at diagnosis | ||||
| Positive | 769 (75.6%) | 162 (74.0%) | 580 (75.9%) | 0.56 |
| Negative | 248 (24.4%) | 57 (26.0%) | 184 (24.1%) | |
| PR status at diagnosis | ||||
| Positive | 661 (65.1%) | 137 (62.8%) | 500 (65.5%) | 0.46 |
| Negative | 354 (34.8%) | 81 (37.2%) | 263 (34.5%) | |
| Her2 status at diagnosis | ||||
| Positive | 257 (25.6%) | 65 (30.1%) | 179 (23.8%) | 0.06† |
| Negative | 738 (73.6%) | 149 (69.0%) | 570 (75.7%) | |
| Ki67 status at diagnosis | ||||
| <14% | 164 (16.4%) | 30 (14.0%) | 127 (16.8%) | 0.31 |
| ≥14% | 838 (83.6%) | 185 (86.1%) | 627 (83.2%) | |
| IHC4 subtype at diagnosis | ||||
| Luminal A | 136 (13.8%) | 23 (10.9%) | 107 (14.4%) | 0.47 |
| Luminal B | 639 (64.9%) | 138 (65.1%) | 481 (64.8%) | |
| Her2-positive | 59 (6.0%) | 13 (6.1%) | 43 (5.8%) | |
| Triple Negative | 151 (15.3%) | 38 (17.9%) | 111 (15.0%) | |
| Luminal B (ER+/PR+; Her2-; Ki67≥14%) | 432 (69.8%) | 86 (62.3%) | 346 (71.9%) | |
| Luminal B (ER+/PR+; Her2+) | 187 (30.2%) | 52 (37.7%) | 135 (28.1%) | |
34 patients (3.2%) had unknown HIV status and were excluded from the analysis.
Chi square test or
Mann Whitney test comparing HIV positive to HIV negative proportions for non-missing values.
Of 1016 patients, 10 (1.1%) were missing stage at diagnosis; 2 (0.2% were missing ethnicity; 62 (6.1%) (n=62) were missing tumour grade; 33 (3.2%) were missing ER status; 35 (3.4%) were missing PR status; 47 (4.6%) were missing Her2 status; 48 (4.7%) were missing Ki67 status; 65 (6.4%) were missing IHC4 subtype. Nine of 163 patients with known metastatic disease (5.5%) were missing the site of metastasis.
Comparison for positive and negative HER2 values only.
HIV positive patients were younger at diagnosis (median age 45 (IQR 40–52) years) than HIV negative ones (57 (IQR 46–67) years, p<0.001). They were also more likely to be self-reported as being black (p<0.001) and to be diagnosed at a late stage of breast cancer (p=0.02) than HIV negative patients (Table 1). However, in a model adjusted for age and ethnicity, tumour stage was not associated with HIV status (Table 2). IHC 4 breast cancer subtype and tumour grade did not differ between HIV positive and HIV negative patients, with or without adjustment for age and ethnicity, except in the unadjusted comparison within subgroups of Luminal B (Table 2).
Table 2 -.
Prevalence ratios (PRs) of HIV by breast cancer stage, grade, metastatic disease status, and IHC subtype.
| Unadjusted PR | p-value | Adjusted PR* | p-value | |
|---|---|---|---|---|
| Stage at Diagnosis | ||||
| Early Stage | 1 (Ref) | 1 (Ref) | ||
| Late Stage | 1.36 (1.07–1.73) | 0.01 | 1.12 (0.90–1.39) | 0.31 |
| Metastatic disease | ||||
| No metastasis | 1 (Ref) | 1 (Ref) | ||
| At least 1 known site of metastasis | 1.14 (0.85 – 1.54) | 0.38 | 1.26 (0.96 – 1.65) | 0.10 |
| Tumour grade at diagnosis | ||||
| 1 | 1 (Ref) | 1 (Ref) | ||
| 2 | 1.21 (0.70 – 2.11) | 0.49 | 1.27 (0.79 – 2.04) | 0.33 |
| 3 | 1.01 (0.58 – 1.78) | 0.96 | 1.00 (0.61 – 1.63) | 0.99 |
| IHC4 subtypes at diagnosis | ||||
| Luminal A | 1 (Ref) | 1 (Ref) | ||
| Luminal B | 1.26 (0.85 – 1.88) | 0.26 | 0.88 (0.61 – 1.27) | 0.49 |
| Her2-positive | 1.31 (0.72 – 2.40) | 0.38 | 1.00 (0.58 – 1.70) | 0.99 |
| Triple Negative | 1.44 (0.91 – 2.29) | 0.12 | 1.10 (0.73 – 1.66) | 0.64 |
| Luminal B (ER+/PR+; Her2-; Ki67≥14%) | 1 (Ref) | 1 (Ref) | ||
| Luminal B (ER+/PR+; Her2+) | 1.40 (1.04 – 1.88) | 0.03 | 1.19 (0.91 – 1.57) | 0.21 |
Adjusted for age (linear) and ethnicity (black vs non-black)
Patients with HIV
Of the 226 patients with HIV infection, 129 (57.1%) knew their HIV status at the time of their breast cancer diagnosis. The duration (median (IQR) of known HIV status prior to breast cancer diagnosis was 4 years. (0–9) years).
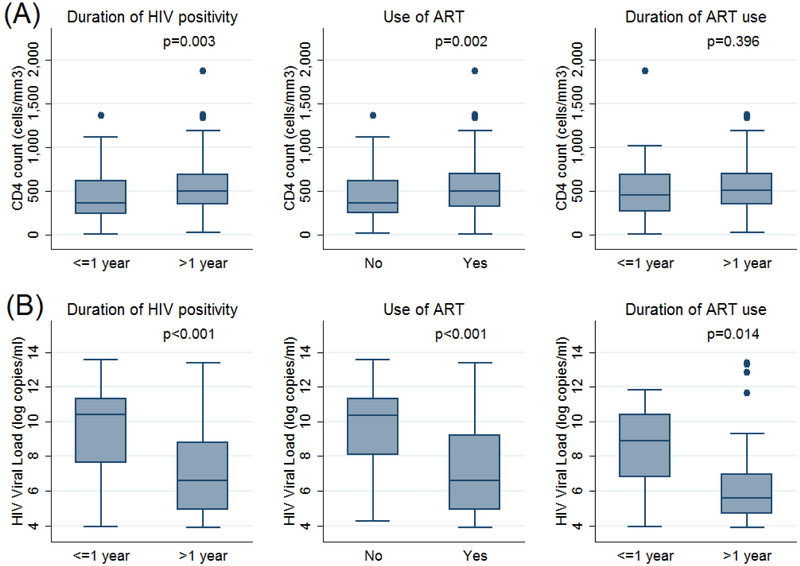
The HIV-infected patients had a median (IQR) CD 4 count of 477 (287–670) cells/mm3 and 59.4% of patients on ARTs had a viral load below detectable levels (i.e. viral load below 50 copies/ml). Among patients with detectable viral loads, the median (IQR) viral load was 4757.5 (268.5–58609.5) copies/ml. The duration of seropositivity and ART use, CD 4 cell count and viral load were not associated with the clinical stage and pathological characteristics of the breast cancer (Tables 3 & 5; Figure 1). Patients not on ARTs were diagnosed at a later stage than those on ARTs; the difference was of marginal statistical significance (Table 4). Patients who had been diagnosed with HIV more than a year prior to their breast cancer diagnosis, and those on ART had lower viral loads and higher CD4 cell counts than other patients.
Table 3 -.
Clinical characteristics of HIV positive patients by time since HIV diagnosis.
| Less than or equal to 1 year (n=68) | More than 1 year (n=158) | P-value* | |
|---|---|---|---|
| Stage at BC diagnosis | |||
| Early Stage | 26 (38.2%) | 57 (36.3%) | 0.78 |
| Late Stage | 42 (61.8%) | 100 (63.7%) | |
| Metastatic disease | |||
| No metastasis | 51 (75%) | 135 (85.4%) | 0.06 |
| Metastasis | 17 (25%) | 23 (14.6%) | |
| IHC4 subtypes at diagnosis | |||
| Luminal A | 8 (12.5%) | 15 (10.1%) | 0.51‡ |
| Luminal B | 45 (70.3%) | 93 (62.8%) | |
| Her2-positive | 3 (4.7%) | 10 (6.8%) | |
| Triple Negative | 8 (12.5%) | 30 (20.3%) | |
| Luminal B (ER+/PR+; Her2-; Ki67≥14%) | 29 (64.4%) | 57 (61.3%) | 0.72 |
| Luminal B (ER+/PR+; Her2+) | 16 (35.6%) | 36 (38.7%) | |
| Viral Load | |||
| Detectable | 46 (74.2%) | 44 (33.6%) | <0.001 |
| Below detectable levels | 16 (25.8%) | 87 (66.4%) | |
| CD 4 count (median (IQR)) | 362 (236 – 616) | 495.5 (337.5 – 687.5) | 0.003# |
| Viral load when detectable (median (IQR)) | 32500 (2000–84000) | 738 (135.5 – 6755) | <0.001# |
Chi square test,
Fisher’s exact test and
Mann Whitney test comparing duration of HIV positivity.
Table 5-.
Clinical characteristics of HIV positive patients by duration of anti-retroviral therapy (ART) at breast cancer diagnosis
| Less than or equal to 1 year (n=30) ** | More than 1 year (n=130) ** | P-value* | |
|---|---|---|---|
| Stage at BC diagnosis | |||
| Early Stage | 13 (43.3%) | 52 (40.3%) | 0.76 |
| Late Stage | 17 (56.7%) | 77 (59.7%) | |
| Metastatic disease | |||
| No metastasis | 23 (76.7%) | 114 (87.7%) | 0.12 |
| Metastasis | 7 (23.3%) | 16 (12.3%) | |
| IHC4 subtypes at diagnosis | |||
| Luminal A | 5 (17.2%) | 13 (10.7%) | 0.19‡ |
| Luminal B | 21 (72.4%) | 73 (60.3%) | |
| Her2-positive | 1 (3.5%) | 8 (6.6%) | |
| Triple Negative | 2 (6.9%) | 27 (22.3%) | |
| Luminal B (ER+/PR+; Her2-; Ki67≥14%) | 15 (71.4%) | 42 (57.5%) | 0.25 |
| Luminal B (ER+/PR+; Her2+) | 6 (28.6%) | 31 (42.5%) | |
Chi square test,
Fisher’s exact test and
Mann Whitney test comparing duration of ART.
Duration of ART missing for 66 HIV positive patients (29.2%).
Figure 1:
CD 4 count and HIV viral load. Box and whisker plots of CD 4 cell counts (cells/mm3) and HIV viral loads (log copies/ml) categorized by duration of HIV seropositivity, use of ARTs and the duration of ART use. Boxes represent interquartile ranges with the median line drawn. Outliers are shown as dots. p-values were calculated using the Mann Whitney test.
Table 4-.
Clinical characteristics of HIV positive patients by anti-retroviral (ART) use at breast cancer diagnosis
| Not on ART (n=63) ** | On ART (n=160) ** | P-value* | |
|---|---|---|---|
| Stage at BC diagnosis | |||
| Early Stage | 18 (28.6%) | 65 (40.9%) | 0.09 |
| Late Stage | 45 (71.4%) | 94 (59.1%) | |
| Metastatic disease | |||
| No metastasis | 47 (74.6%) | 137 (85.6%) | 0.05 |
| Metastasis | 16 (25.4%) | 23 (14.4%) | |
| IHC4 subtypes at diagnosis | |||
| Luminal A | 5 (8.3%) | 18 (12.0%) | 0.74‡ |
| Luminal B | 42 (70.0%) | 94 (62.7%) | |
| Her2-positive | 4 (6.7%) | 9 (6.0%) | |
| Triple Negative | 9 (15.0%) | 29 (19.3%) | |
| Luminal B (ER+/PR+; Her2-; Ki67≥14%) | 28 (66.7%) | 57 (60.6%) | 0.50 |
| Luminal B (ER+/PR+; Her2+) | 14 (33.3%) | 37 (39.4%) | |
Chi square test,
Fisher’s exact test and
Mann Whitney test comparing use of ART.
ART use missing for 3 HIV positive patients (1.3%).
DISCUSSION
Patients in low-and middle-income countries diagnosed with breast cancer have been reported to present with more advanced disease (Stage III/IV) than patients in high income countries20–22. Both patient-dependent (low socio-economic status, lack of awareness of breast cancer, patients’ belief system) and health care system-dependent (travelling distance to the treating hospital, number of health facilities visited prior to the treating hospital) factors have been found to play a significant role in late presentation20–22. Moreover, low level of education and visiting more than 2 health care facilities before breast cancer diagnosis were shown to contribute to the advanced disease stage at the time of diagnosis22. In several countries of sub-Saharan Africa, more than 75% of patients have stage III/IV disease at diagnosis20,23, compared to less than 20% in high income countries24. In this study, 55.1% of patients presented with stage III/IV disease, which is lower than other countries in sub-Saharan Africa. This finding could be explained by the fact that the study was done on an urban population which has good access to health care. Moreover, the study sites operate open-access clinics, whereby not only patients referred from other health professionals are seen, but self-referred patients and ‘walk-in’ patients are also seen on the day of presentation. It has been shown that a system that only allows referral of patients from another health professional and referral secondary hospitals may create a barrier to time to diagnose and treat as there may be delay in getting an appointment, patients may need to be seen by multiple health professionals before the diagnosis is made and the patient may thus incur added costs22,25. This may lead to some patients being discouraged along the process, even reaching the breast specialists for diagnosis and treatment. Though at the time of the study, there were no formal national screening programmes in place, but an extensive breast cancer awareness programmes offered by various non-governmental organisations. The South African National Department of Health has recently instituted a Breast Cancer Policy recommending routine Clinical Breast Examination (CBE) at Primary Health Care (PHC) level26.
More than 75% of breast tumours in the current study were oestrogen receptor positive, the histopathological parameter associated with a favourable clinical outcome and responsiveness to adjuvant hormonal therapy27. The Luminal B subtype was predominant. The human epidermal growth factor (HER2) oncogene was positive in 24.4% of cases, a larger proportion than reported in other populations (18–20%)27. HER2 positive result in breast cancer is associated with aggressive clinico-pathological outcome27. In areas whereby HER2 targeted therapy is not available, as was the case in this cohort, the overall survival is worse than for other cancer subtypes276. However, the HER2 targeted therapy has recently become available in the adjuvant setting for patients with HER2 positive breast cancer, in the Public Hospitals in South Africa
As a non-AIDS defining malignancy, the incidence of breast cancer in people living with HIV is expected to rise in the era of anti-retroviral therapy28. The accessibility and effectiveness of ART has led to an improved life expectancy of people living with HIV, a decline in the incidence of AIDS-defining malignancies but a steady increase in the incidence of non-AIDS defining malignancies28–29. There have been conflicting findings in the literature about the true incidence of breast cancer in HIV positive patients. In the current study, the prevalence of breast cancer in HIV positive patients was similar to the general population. Previous studies have shown similar findings30,31. On the contrary, two South African studies conducted in the era of ART have demonstrated a higher incidence of breast cancer in HIV positive patients31,32, however, one of these studies indicated that about 50% of the population studied had unknown HIV status32.
Among the HIV positive patients in the current study, breast cancer was diagnosed at a younger age than among HIV negative patients. This finding was demonstrated by several other studies as well29–35. However, when age-stratified, in patients younger than 50 years of age there was no difference in the median age of presentation between the HIV positive and HIV negative patients. Recent studies show that HIV prevalence in women younger than 50 is declining but steadily increasing among women older than 506,36, with one study showing more than half of adults tested sero-converting after the age of 5036. This finding indicates the need for prospective studies to investigate the association of HIV and breast cancer specifically in women older than 50 where central adiposity associated both with obesity and prolonged ART may influence breast cancer treatment outcomes.
In this study, immunosuppression was not severe among our HIV infected patients; their median CD 4 cell count was 477 cells/mm3. Similar counts have been reported in other studies, such as that by Shaaban et al., who found a median CD4 count of 410 cells/mm3,29–38. The level of CD 4 count was not associated with the stage at the diagnosis, tumour grade or the tumour subtype found in other studies29,30.
The duration of HIV sero-positivity was not associated with the pathological characteristics of breast cancer. Patients diagnosed with breast cancer within 1 year of HIV diagnosis were more likely to have metastases than those diagnosed more than a year previously, but the difference was only marginally statistically significant. In a model adjusted for age and ethnicity, HIV positive and HIV negative patients did not differ in tumour stage at presentation, IHC 4 breast cancer subtype, or tumour grade. Several other studies have also demonstrated no difference between the two groups in terms of staging and pathological characteristics of the tumour29,30,39. In Uganda, HIV positive patients were found to be diagnosed with cancer at an earlier stage than HIV negative patients40, perhaps because they were already participating in the health care system for HIV treatment providing opportunity for incidental identification of their breast cancer symptoms.
15.5% of our patients had metastatic disease at diagnosis. We may have missed other patients with metastatic breast cancer because not all patients with metastatic disease present to the Surgical Breast Units. Moreover, our patients with breast cancer have a staging CT scan only if they have a suspicious chest x-ray and/or liver ultrasound. However, we have no reason to think that the missed patients were more or less likely to be infected with HIV than those included in our sample.
More than 70% of HIV positive patients were on ART at the time of diagnosis, and more than 50% had a viral load below the detectable level. Patients who were not on ART at the time of breast cancer diagnosis were referred to the HIV/ART clinic to be initiated on ART before starting cancer treatment. The use of ART did not affect the clinico-pathological characteristics of breast cancer even though there was a trend shown among those not on ARTs to present with metastatic disease.
The limitations of this study include the possible bias in detecting metastasis because not all patients diagnosed with breast cancer receive a staging CT scan at the study sites and some patients with metastatic disease may be referred directly to Medical Oncology Unit, bypassing the Surgical Breast Units completely. Future studies might recruit patients with metastatic breast cancer diagnosed in Medical Oncology Unit or detected in other units of the participating hospitals, including the HIV clinics. Finally, although the duration of HIV seropositivity and ART use within 1 year of breast cancer diagnosis were not associated with the clinical-pathological characteristics of breast cancer, it would be interesting to see if there is any effect within 6 months of HIV diagnosis and initiation of ART on the outcome of breast cancer. We did not have information regarding the month, only year, of HIV diagnosis or ART initiation in our patient cohort. Furthermore, we did not have the data on the co-existence of the co-morbidities and co-infection as well as the treatment outcome of our patients. However, the latter is been considered for future research projects.
CONCLUSION
HIV-positive patients present with breast cancer at a younger age and later stage disease than HIV-negative patients. The use of ART did not affect the clinico-pathological characteristics of breast cancer even though there was a trend shown among those not on ARTs to present with metastatic disease.
HIGHLIGHTS.
CLINICO-PATHOLOGICAL CHARACTERISTICS AMONG SOUTH AFRICAN WOMEN WITH BREAST CANCER RECEIVING ANTI-RETROVIRAL THERAPY FOR HIV
Fifty-five percent of patients presented with late stage disease (Stage III/IV)
HIV positive patients with breast cancer presented at a younger age
HIV positive patients presented with late stage disease
Use of ART did not affect the clinicopathological characteristics of breast cancer
Duration of HIV infection did not affect the characteristics of breast cancer
ACKNOWLEDGEMENTS
The authors wish to thank the participants for their willingness to be part of the study. We would also like to thank the medical personal who entered data into the database for further study.
FUNDING
The authors wish to acknowledge:
NIH of USA National Cancer Institute (Grant no: R01-CA192627 and P30-CA13696) to Drs Jacobson, Joffe, Neugut and Ruff.
University of Witwatersrand / South African Medical Research Council Common Epithelial Cancer Research Centre Grant (CECRC) to Dr Ruff
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ETHICS CLEARANCE
Ethics clearance for this study was obtained from the Human Research Ethics Committee (Medical) at the University of Witwatersrand (clearance number: M161130 and M150351).
REFERENCES
- 1.Bray F; Ferlay J; Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 0:1–31 doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Registry. Summary statistics of cancer diagnosed histologically in 2014. Female-All population groups combined. www.nicd.ac.za/wp-content/uploads/2017/03/2014-NCRtables
- 3.Mid-year population estimates for South Africa. Statistical release P0302. 2016. https://www.stassa.gov.za/publications/P0302/P03022016.pdf
- 4.The AIDS Foundation of South Africa. www.aids.org.za
- 5.South Africa’s National Strategic Plan for HIV, TB and STIs 2017–2022. http://sanac.org.za/wpcontent/uploads/2017/05/NSP_FullDocument_FINAL.pdf
- 6.Mahy M; Autenrieth CS; Stanecki K; Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS 2014, 28(Suppl 4): S453– S459. doi: 10.1097/QAD.000000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swai SJ; Damian DJ; Urassa S et al. Prevalence and risk factors for HIV among people aged 50 years and older in Rombo district, Northern Tanzania. Tanzania J Health Res. 2017, 19(2). doi: 10.4314/thrb.v19i2.2 [DOI] [Google Scholar]
- 8.Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. S Afr J HIV Med. 2017;18(1), a776 10.4102/sajhivmed.v18i1.776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper GM. The cell: A molecular approach 2nd Edition. Sunderland (MA) Sinauer Associates, 2000Tumor Viruses. https://www.ncbi.nlm.nih.gov/books/NBK9929 [Google Scholar]
- 10.Hessol NA, Pipkin S, Schwarz S et al. The impact of Highly Active Antiretroviral therapy on nonAIDS defining cancers among adults with AIDS. Am J Epidemiol 2007; 165:1143–1153. doi: 10.1093/aje/kwm017 [DOI] [PubMed] [Google Scholar]
- 11.Mournier N, Katlama C, Costagliola et al. Drug interactions between antineoplastic and antiretroviral therapy: implications and management for clinical practice. Crit Rev Onc/Hematol 2009; 72:10–20. doi: 10.1016/j.critrevonc.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Ulrike K, Markus H, Thomas H et al. NNRTI-based antiretroviral therapy may increase the risk of radiation induced side effects in HIV-1infected patients. Radiotherapy and Oncology 2015; 116:323–330. doi: 10.1016/j.radonc.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 13.Grover S; Martei YM; Puri P et al. Breast cancer and HIV in Sub-Saharan Africa: A complex relationship. J Glob Oncol. 2017; 00 10.1200/JGO.2016.006585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cubasch H, Ruff P, Joffe M et al. South African Breast Cancer and HIV Outcomes study: Methods and Baseline assessment. J Glob Oncol 2016. doi: 10.1200/JGO.2015.002675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Joint Cancer Staging Manual, 7th Edition [Google Scholar]
- 16.Fitzgibbons PL, Dillon DA, Alsabeh R et al. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014; 138:595601. doi: 10.5858/arpa.2013-0566-CP [DOI] [PubMed] [Google Scholar]
- 17.Juhasz-Böss I, Mavrova R, Moga S, et al. , “Can Ki-67 Play a Role in Prediction of Breast Cancer Patients’ Response to Neoadjuvant Chemotherapy?” BioMed Research International, vol. 2014, Article ID 628217, 7 pages, 2014. 10.1155/2014/628217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Wood WC, Coates AS et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy or early breast cancer 2011. Ann of Onc 2011,22:1736–1747. Doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer JS, Alvarez C, Milikowski C et al. Breast carcinoma malignancy grading by BloomRichardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Modern Pathol 2015, 18:1067–1078. doi: 10.1038/modpathol.3800388 [DOI] [PubMed] [Google Scholar]
- 20.Dickens C, Joffe M, Jacobson J et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a peri-urban setting: A South African public hospital case series of over 1000 women. Int J Cancer 2014. 135(9):2173–2181. doi: 10.1002/ijc.28861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace LE, Mounga T, Hategekimana V et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centres in Rwanda. The Oncologist. 2015; 20: 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joffe M, Ayeni O, Norris SA et al. Barriers to early stage presentation of breast cancer among women in Soweto, South Africa. PLoS ONE 2018. 13(2): e0192071 10.1371/journal.pone.0192071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal J, Ginsburg O, Rochon P et al. Differences in breast cancer stage at diagnosis and cancerspecific survival by race and ethnicity in the United States. JAMA. 2015; 313 (2):165–173. doi: 10.1001/jama.2014.17322 [DOI] [PubMed] [Google Scholar]
- 24.Ries L, Eisner M, Kosary C et al. SEER Cancer statistics review.1975–2001 [Google Scholar]
- 25.Rayne S, Lince-Deroche N, Hendrickson C et al. Characterising breast conditions at an openaccess breast clinic in South Africa: a model that is more than cancer care for a resource-limited setting. BMC Health Serv Res. 2017. January 21;17(1):63. doi: 10.1186/s12913-016-1959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breast Cancer Prevention and Control Policy- National Department of Health. www.health.gov.za.2015
- 27.Vogel M, Friedrich O, Luchters G et al. Cancer risk in HIV infected individuals on HAART is largely attributed to oncogenic infections and state of immunocompetence. Eur J Med Res 2011. 16:101–7. doi: 10.1186/2047-783X-16-3-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiels MS, Cole SR, Kirk DG et al. A Meta-Analysis of the incidence of Non-AIDS cancers in HIV-Infected Individuals. J. Acquired Immun Defic Syndr 2009; 52 (5):611–622. doi: 10.1097/QAI.0b013e3181b327ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cubasch H, Joffe M, Hanisch R et al. Breast Cancer characteristics and HIV among 1092 women in Soweto, South Africa. Breast Cancer Res Treat. July 2013; 140: 177–186. doi: 10.1007/s10549013-2606-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phakathi BP, Basson G, Karusseit VOL et al. The effect of HIV infection on the surgical, chemo- and radiotherapy management of breast cancer. A prospective cohort study. Int J Surgery. 2016; 34:109–115. doi: 10.1016/j.ijsu.2016.08.520 [DOI] [PubMed] [Google Scholar]
- 31.Ngidi S, Magula N, Sartorius B et al. Incidence of chemotherapy-induced neutropenia in HIV infected and uninfected patients with breast cancer receiving neoadjuvant chemotherapy. S Afr Med J 2017; 107(7):595–601. doi: 10.7196/SAMJ.2017.12309 [DOI] [PubMed] [Google Scholar]
- 32.Van Zyl N, Minné C, Mokone DH. Human immunodeficiency virus infection in breast cancer patients: The prevalence thereof and its effect on breast cancer characteristics at Dr. George Mukhari Academic Hospital Breast Clinic, Ga-Rankuwa, South Africa S Afr J Rad. 2018; 22(2),361 10.4102/sajr.v22i2.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz M, Davis H. Breast Cancer in HIV-infected patients: A retrospective single-institution study. J In Assoc Physicians AIDS Care 2011; 10(1):30–34. doi: 10.1177/1545109710385002. [DOI] [PubMed] [Google Scholar]
- 34.Amir H, Koaya EE, Kwesigabo G, Kiitinya JN. Breast Cancer before and during the AIDS epidemic in women and men: A study of Tanzanian Cancer Registry Data 1968–1996. J Natl Med Assoc. 2009; 92: 301–305 [PMC free article] [PubMed] [Google Scholar]
- 35.Spano J, Lanoy E, Mounier N et al. Breast Cancer among HIV infected individuals from the ONCOVIH study in France: Therapeutic Implications. Eur J of Cancer 2012; 48: 3335–3341. doi: 10.1016/j.ejca.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 36.McCormack VA1, Febvey-Combes O1, Ginsburg O2, Dos-Santos-Silva I3. Breast cancer in women living with HIV: A first global estimate. Int J Cancer. 2018. July 11. doi: 10.1002/ijc.31722. [DOI] [PubMed] [Google Scholar]
- 37.Smit M, Olney J, Ford NP et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS 2018,32: 773–782. doi: 10.1097/QAD0000000000000001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaaban HS, Modi Y, Guron G. Is there an association between human immunodeficiency virus infection and breast cancer? Med Oncol 2012; 29: 446–447. doi: 10.1007/s12032-011-9856-5 [DOI] [PubMed] [Google Scholar]
- 39.Hurley J; Franco S; Gomez-Fernandez et al. Breast cancer and Human Immunodeficiency Virus: A report of 20 cases. Clinical Breast Cancer 2001; 2(3): 215–220. doi: 10.3816/CBC.2001.n.024 [DOI] [PubMed] [Google Scholar]
- 40.Menon MP, Coghill A, Mutyaba I et al. Association between HIV infection and cancer stage at presentation at the Uganda Cancer Institute. J Glob Oncol 2017: 00. doi: 10.1200/JGO.17.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]