Abstract
The chaperome is the collection of proteins in the cell that carry out molecular chaperoning functions. Changes in the interaction strength between chaperome proteins lead to an assembly that is functionally and structurally distinct from each constituent member. In this review, we discuss the epichaperome, the cellular network that forms when the chaperome components of distinct chaperome machineries come together as stable, functionally integrated, multimeric complexes. In tumors, maintenance of the epichaperome network is vital for tumor survival, rendering them vulnerable to therapeutic interventions that target critical epichaperome network components. We discuss how the epichaperome empowers an approach for precision medicine cancer trials where a new target, biomarker, and relevant drug candidates can be correlated and integrated. We introduce chemical biology methods to investigate the heterogeneity of the chaperome in a given cellular context. Lastly, we discuss how ligand–protein binding kinetics are more appropriate than equilibrium binding parameters to characterize and unravel chaperome targeting in cancer and to gauge the selectivity of ligands for specific tumor-associated chaperome pools.
Keywords: cancer biology, chaperone, chemical biology, stress response, biomarker, cancer therapy, heat shock protein 90 (Hsp90), 70 kilodalton heat shock protein (Hsp70), chaperome, chemical probes, epichaperome, protein networks
Introduction
Like its better-known cousins, the genome and the proteome, the chaperome is a large collection of biological macromolecules at work in the cell. Unlike the first two, which in the simplest sense can be defined by their composition, the chaperome is both a structural and functional definition, comprising proteins that carry out the molecular chaperoning functions of the cell (1, 2). The term chaperome was introduced in 2006 (3), but like our understanding of the component members themselves, its precise definition is evolving as our understanding of these proteins develops (4–6). A 2013 study of the human chaperome identified 147 predicted members (1). This list included heat shock protein 90s (HSP90s), HSP70s, HSP60s, HSP110s, HSP40s (also known as DNAJ proteins), HSP10, the small heat shock proteins, their co-chaperones, and members of the folding peptidylprolyl isomerase and protein-disulfide isomerase enzymes. A later study expanded this list to 332 members (4, 5) and included tetratricopeptide repeat domain–containing proteins selected based on their functional interactions with chaperones. Some but not all chaperome members are heat-inducible (1, 2).
The chaperome is among the most abundant protein collections in human cells (1, 2). The HSP90s comprise 2.8% of the total cellular protein mass. The HSP70s add another 2.7%, and the HSP60 and HSP110 classes account for ∼3.3% of the total protein mass. Chaperones perform their functions aided by a specific subset of co-chaperones, and this assembly of chaperome members is often referred to as a machinery (7–9). Co-chaperones are substoichiometric to the chaperones they assist. Thus, for example, HOP (HSP70–HSP90 organizing protein, also called STIP1) is 10–20 times less abundant than HSP90, even in cancer cells (1).
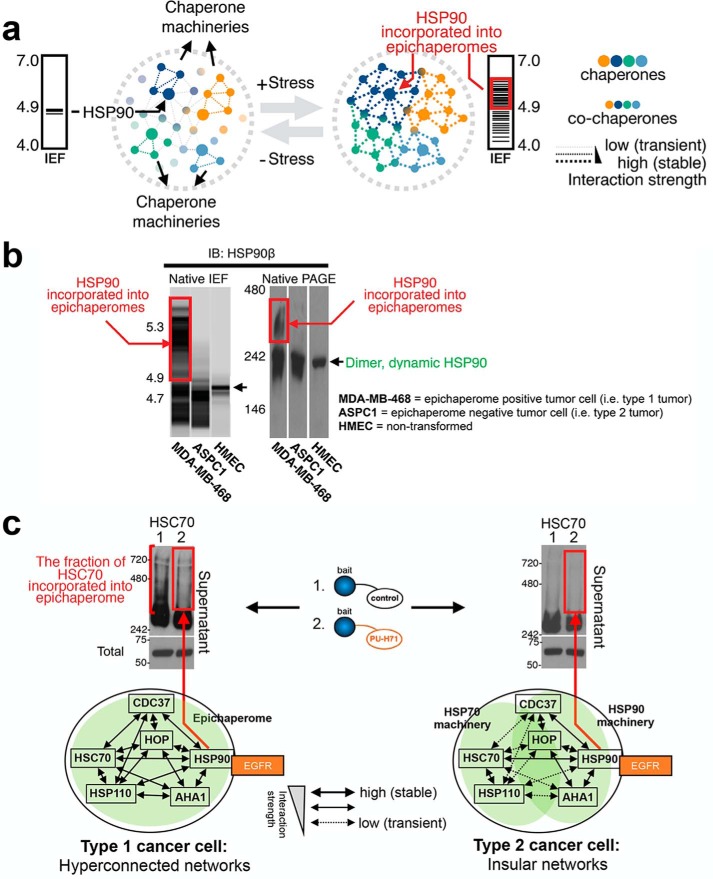
Under normal physiological conditions, the chaperome is required for a variety of housekeeping functions, including de novo protein folding during nascent polypeptide synthesis, transport of proteins to specific cellular locations, unfolding and disaggregation of misfolded proteins, and the assembly of protein complexes (10–14). Because of the inherently disordered nature of the unfolded or partially folded proteins acted upon by chaperones and their helpers, chaperones have evolved to interact with these proteins, also referred to as “clients,” in a highly dynamic manner (15, 16). The interaction of chaperone machineries with clients is believed to occur in a hierarchical order, where, for example, an emerging polypeptide chain is transferred from the ribosome to TCP-1 ring complex (TRiC, also called CCT for chaperonin containing TCP-1) or to the HSP70 chaperone machinery, whereas the HSP90 machinery may participate in client regulation at a later stage of the client folding process (7, 8, 17–20). The different roles of the chaperone machineries give rise to a functional network, with partially overlapping functions and specificities (Fig. 1a) (21).
Figure 1.
Proteome stress biochemically remodels the chaperome in cancer. a, dynamic and hierarchical interactions between the chaperone machineries govern normal cellular proteostasis. Here, different chaperone machineries give rise to a network, with partially overlapping functions and specificities. Cellular stress, such as induced by Myc hyperactivation, increases the connectivity between distinct chaperone machineries; this is executed by an increase in the interaction strength among chaperones, co-chaperones, and other factors. A functionally and structurally interconnected chaperome network, as opposed to that of individual chaperone machineries, is formed. Because the formation of the interconnected chaperome creates an entity that is distinct, both thermodynamically and functionally, from its constituent chaperome units, this network was coined the epichaperome. b, tumors with the interconnected chaperome, i.e. epichaperome expressors, were termed type 1, whereas those with partly overlapping chaperome machineries were named type 2. The biochemical signature of HSP90 when part of the epichaperome is exemplified in the MDA-MB-468 breast cancer cell homogenates. Conversely, HSP90 of type 2 tumors is exemplified in ASPC-1 cells. IB, immunoblot. c, in type 1 tumors, but not in type 2, HSC70 is an abundant component of stable HSP90 chaperome complexes; this HSC70 pool, but not others, is depleted by the PU-H71 bait, a binder of HSP90 when incorporated into the epichaperome. Adapted from Ref. 46.
Chaperome under cellular stress
The dynamic interactions between chaperones, co-chaperones, and clients governing normal cellular function, and the partial functional overlap among the component machineries of the chaperome, are both altered under stress (22). This has been seen in yeast (23, 24), plants (25–27), and other organisms (28). For example, Hsp82, the yeast HSP90, forms stable complexes with most chaperones mainly after exposure to a stress condition such as the introduction of the exogenous mammalian proteins v-Src or the glucocorticoid receptor (23, 29). Following this stress, Hsp82 can be affinity-purified with Ssa proteins, the yeast homologs of HSP70, Sti1, the yeast HOP, and a 46-kDa immunophilin homolog, among others. Several co-chaperones of the HSP70 machinery, including Ydj1, the yeast HSP40 and Sse1, and the yeast HSP110, are also involved in the regulation of exogenous v-Src.
In addition to an increase in the biochemical overlap between the HSP90 and HSP70 machineries, stress also increases their functional overlap. For example, yeast with a defective Ydj1 fails to enable a functional v-Src. Overexpression of Sse1, the more abundant of the two yeast HSP110s, rescues this phenotype (30). This has also been seen following heat shock. Yeast that tolerate the lack of Sti1 and Sse1 at 30 °C could not grow at 37 °C. Deletion of Sse1, when also associated with loss of Hsp82, was toxic even at 30 °C (31). Furthermore, Sti1 and Sse1 mutant strains exhibit markedly increased sensitivity to inhibition of HSP90 under conditions in which the WT strain remained unaffected by these drugs (31). Overall, these studies indicate that stress enhances both the physical and the functional overlap between chaperome machineries with the goal of enhancing cellular survival.
Studies in human cells recapitulate these observations from simple organisms. Pioneering work on human chaperones in the 1980s and 1990s discovered that the association of mammalian HSP90 with co-chaperones and client proteins was dynamic (32–34). The isolation and characterization of these multiprotein complexes were possible only after their stabilization by oxyanions such as molybdate, vanadate, and tungstate. Researchers soon reported that cellular stress could also stabilize these interactions, and the isolation of “oligomeric” HSP90 or HSP70 species was often observed on native PAGE when cells were exposed to stress. For example, the stress-inducible HSP70 isoform, HSP72, and the constitutively-active isoform, HSC70, formed oligomeric structures in response to heat stress (35). BiP, the ER5 resident HSP70, also known as glucose-regulated protein 78 (GRP78), self-oligomerized in response to glucose deprivation (36), and GRP94, the ER HSP90, formed multimeric complexes with BiP in cells exposed to a toxin such as antimycin A (37).
Mammalian HSP90 can also oligomerize under conditions of thermal stress (38–40). Although all members of the HSP90 family are believed to exist primarily as homodimers, a monomeric fraction has also been reported, while others have noted a tendency of HSP90 to self-associate when purified from cells under thermal stress. For example, although HSP90 purified to homogeneity from chick embryos appeared as a single band on SDS-PAGE and as a dimer under native PAGE, thermal stress produced several oligomeric forms (41). These HSP90 oligomers were not insoluble aggregates but rather soluble high-molecular-mass assemblies that retained their binding to co-chaperones such as activators of HSP90 ATPase protein 1 (AHA1). In fact, AHA1 appears to prefer binding to oligomeric HSP90 over the dimer (42).
Thus, cellular stress can change the interaction strength between both chaperome members as well as between chaperome machineries. This “rewiring” of the chaperome by higher-order reorganization may enable new functions that are silent under normal conditions but that may be needed to counteract the deleterious effects of stress. By bringing nominally independent component chaperome machineries together, stress also increases the functional overlap and functional inter-dependence of the chaperome (Fig. 1a). This mechanism appears to extend beyond the chaperome, as the heat-induced “reversible aggregation” or oligomerization of several cellular proteins was recently reported to reflect an adaptive, autoregulatory process that aids cellular survival under thermal stress (43).
Epichaperome–A chaperome entity formed under stress
The cellular stress of human disease also alters the interactions of chaperome units and chaperome machineries. We will next review such instances in cancer. High-affinity complexes between components of the chaperome were noted in tumor cells as early as the 1990s. For example, stable, ATP-independent oligomeric forms of HSP70/HSP110 were identified in a mouse C3H mammary carcinoma in 1998 (44), and high-affinity complexes between HSP90, HOP, and p23 (also known as PTGES3) were reported in tumor cells in 2003 (45). At the time, the function of these stabilized complexes was not understood, but, more recently, Rodina et al. (46) systematically analyzed the biochemical behavior of chaperome members in several different human cancer cells and found that stable oligomeric complexes containing HSP90, HSC70, HOP, HSP110, CDC37, AHA1, and others were detected in some, but not all, cancer cells. Unlike the dynamic HSP90 complexes found under normal conditions (see for example the human mammary epithelial cells in Fig. 1b), these chaperome complexes remained stable during native PAGE and had a distinct isoelectric focusing signature (Fig. 1b, exemplified by MDA-MB-468 cells). Moreover, unlike the HSP90 found in dynamic complexes, which dissociate under native PAGE and appear as dimers, these high-affinity HSP90 complexes have distinct molecular masses, reflecting the incorporation of HSP90 into stable protein complexes with chaperones, co-chaperones, and other proteins.
Although the abundant chaperones HSP90 and HSC70 nucleate these chaperome complexes (Fig. 1b), these stable oligomers represent only a fraction of the total chaperome in the cell. Despite being a minor species, however, their presence is a distinguishing trait, and not all cancer cells form these stable chaperome complexes. The incorporation of the chaperome into the stable complexes is independent of the expression level of HSP90 and other chaperome members, HSP90 client proteins and anti-apoptotic molecules, tissue or origin or causal genetic mutations (22, 46). Using HSP90 incorporation as a marker, tumors from 95 cancer cell lines, 40 primary acute myeloid leukemia, and 23 primary breast tumors, ex vivo, and 51 solid tumors and lymphomas, in human patients could be categorized into “type 1” tumors that contained the stable chaperome complexes (Fig. 1b, exemplified by MDA-MB-468 cells) and “type 2” that did not (Fig. 1b, exemplified by ASPC1 cells).
To understand why differentiating tumors on the basis of their chaperome pool content is significant, it is worthwhile to consider the concepts of protein networks and gene essentiality. Cellular function can be described by the structural organization of proteins into networks. A network is composed of multiple nodes (herein proteins) connected by edges (herein protein–protein interactions). Biological networks fit the so-called scale-free network organization model composed of a small number of highly connected nodes (hubs) and a large number of poorly connected nodes (nonhubs) (47). Each tumor has an intrinsic need for a well-defined organization of protein networks. Providing a backbone for the structuring of these networks is the chaperome (48, 49). Such function places the chaperome in a central position of cellular networks, which is the hub. This placement is of importance in cancer because deleting a highly-connected protein node (hub) is more likely to be lethal to a cancer cell than deleting a lowly-connected node (nonhub), a phenomenon known as the centrality–lethality rule (50).
An essential hub chaperone is HSP90 (51, 52). Mapping the interaction network of this chaperone in yeast and mammalian systems placed HSP90 at the epicenter of multiple pathways and cellular processes (53–57). Among these processes are important signaling, transcriptional, and metabolomic pathways that are often dysregulated in cancer. These findings have driven the push for the introduction of HSP90 inhibitors in cancer (58, 59). Yet, despite strong preclinical data showing that HSP90 inhibition in tumors led to the depletion of multiple oncogenic pathways, and the entry of an array of inhibitors of diverse chemotypes into clinical investigation, this approach has so far failed to produce favorable clinical outcomes.
Puzzled by the disconnect between the potential of this target and the failure to deliver upon its promise, we analyzed HSP90 and its interactome in tumors (46). Our goal was to see whether the placement or connectivity of HSP90 in protein networks, rather than its oncogenic protein interactors, could explain the observed tumor sensitivity (or the lack of) to HSP90 inhibition. The prevailing view in the field was that HSP90 and other key chaperones, such as HSP70, were hubs of protein networks but that they functioned separately, each with its co-chaperone subset and each as a hub of its own protein network (60). In this view, only limited connectivity existed between the two machineries, mainly carried out by the ubiquitous HOP and CHIP co-chaperones (60). We found that, indeed, some tumors were characterized by such “insular” chaperome networks. In these tumors, HSP90 inhibition could be overcome despite depletion of HSP90-dependent oncoproteins such as oncogenic kinases. These are the type 2 tumors (46).
In contrast to the type 2 tumors, we found that HSP90 became essential in cancer when its network connections increased through its incorporation into complexes with other chaperome machineries such as HSC70, the constitutive HSP70 paralog (46). These increased interactions allowed the previously nonessential HSP90 to become a member of global (as opposed to insular) protein pathways. These are the type 1 tumors. Indeed, it was in these tumors, and not those of type 2, that targeting co-chaperones of either HSP90 or HSP70 was toxic to the cancer cells (see Fig. 2 and sections below).
Figure 2.
Functional integration of the chaperome in type 1 tumors. a, in type 1 tumors, epichaperome integrity is maintained, even after a drastic reduction in HSP90 levels, by increasing the total expression levels and the participation in the epichaperome of other key epichaperome components. In these conditions of low HSP90 but high epichaperome, the function of oncogenic protein networks (see EGFR levels) and cell viability remained unaffected. At an inflection point, and as HSP90 continues to decrease, epichaperome networks collapse, oncogenic proteins are depleted, and cell death ensues. b, in type 2 tumors, HSP90 depletion results in immediate client (EGFR) depletion, but no remodeling of the chaperome takes place (HSC70 shown). IB, immunoblot. c and d, in type 1 but not in type 2 tumors, siRNA directed to HSP110, a co-chaperone of HSP/C70, depletes the HSP90 client protein EGFR and results in cell death. e, summary schematic of individual chaperome member inhibition and its outcome in type 1 and 2 cancer cells. a–d are adapted from Ref. 46 and show a representative result or a mean value, as appropriate.
We coined the term epichaperome to describe these hyperconnected HSP90–HSC70 chaperome networks (46). Thus, epichaperomes are cellular networks that form when the chaperome components of distinct chaperome machineries come together as stable, functionally integrated multimeric complexes.
What are the components of the epichaperome? We performed a series of unbiased large-scale proteomic interrogations of the chaperome in type 1 tumors. These analyses found that co-chaperones and other factors, which were usually associated with either HSP90 or HSP70, were now integrated into large stable complexes (Figs. 1 and 2) (46). Hyperconnecting the chaperome by strengthening the interactions between individual chaperome members of distinct machineries resulted in the functional integration of these distinct chaperome networks. Showing this functional interdependence in type 1 (i.e. epichaperome expressors), but not in type 2 cancer cells, knock down of HSP110 (an HSP70 co-chaperone) and HOP was sufficient to diminish the activity or expression of HSP90 kinases, such as p-S6K, p-ERK, and EGFR, in a fashion similar to knock down of HSP90α/β and AHA1 (a direct HSP90 activator) (Fig. 2) (46).
To conclude this section, the distinction between types 1 and 2 tumors is key to understanding the sensitivity or lack of sensitivity of tumors to chaperome therapies. Epichaperome formation, which is a characteristic of type 1 tumors, arises from the increased network connections of HSP90 with chaperome members of other chaperome machineries; it is effected by enhancing the stability of such protein complexes. This mechanism is not restricted to cancer. In the context of Parkinson's disease, we have recently reported how pathogenic protein network rewiring is driven by remodeling the interaction strength between chaperome members and the stability of such protein complexes (61).
Kinetic binding drives inhibitor selectivity
Small molecules may sense the structural and dynamic differences between chaperome and epichaperome complexes, and these characteristics are the basis for the use of such chemicals in the detection and analysis of chaperome heterogeneity in disease (Figs. 3–6) (46).
Figure 3.
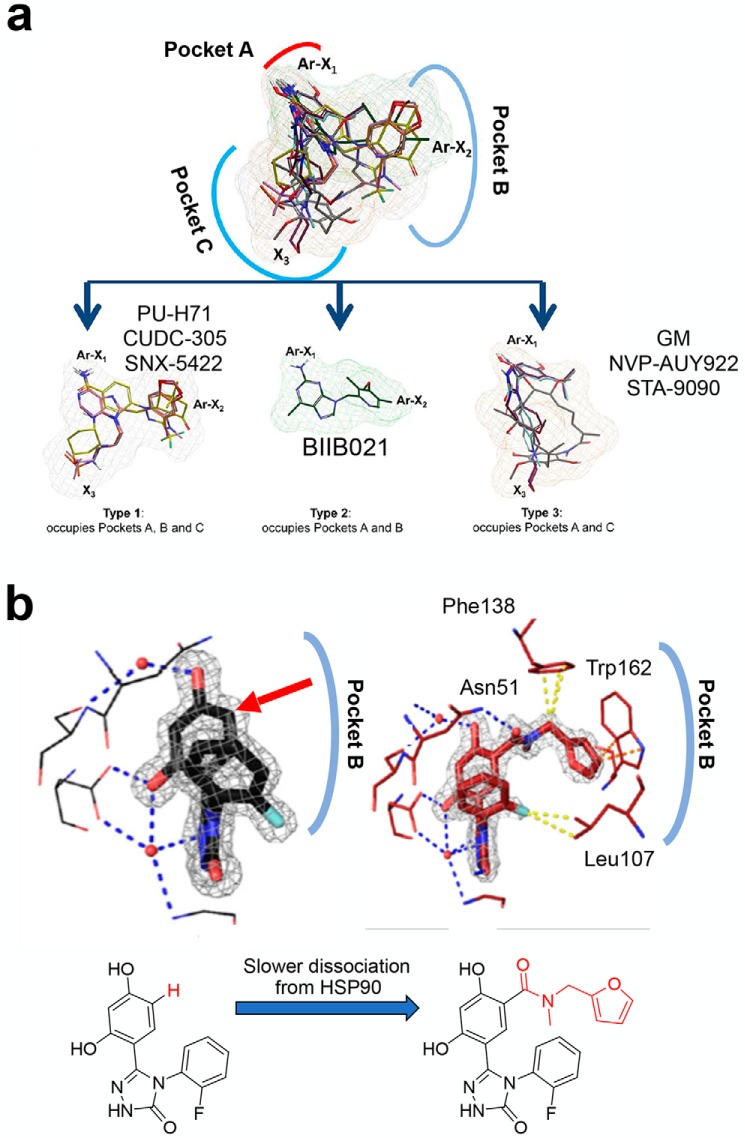
ATP-competitive HSP90 inhibitor subtypes. a, overlay of the indicated inhibitors and their segregation based on subpocket occupancy. Adapted from Ref. 94. b, protein–ligand interactions representative of the loop-in conformation ligands (left) and of the helical conformation ligands (right). Chemical structure of the ligands is shown below. Adapted from Ref. 69.
Figure 4.
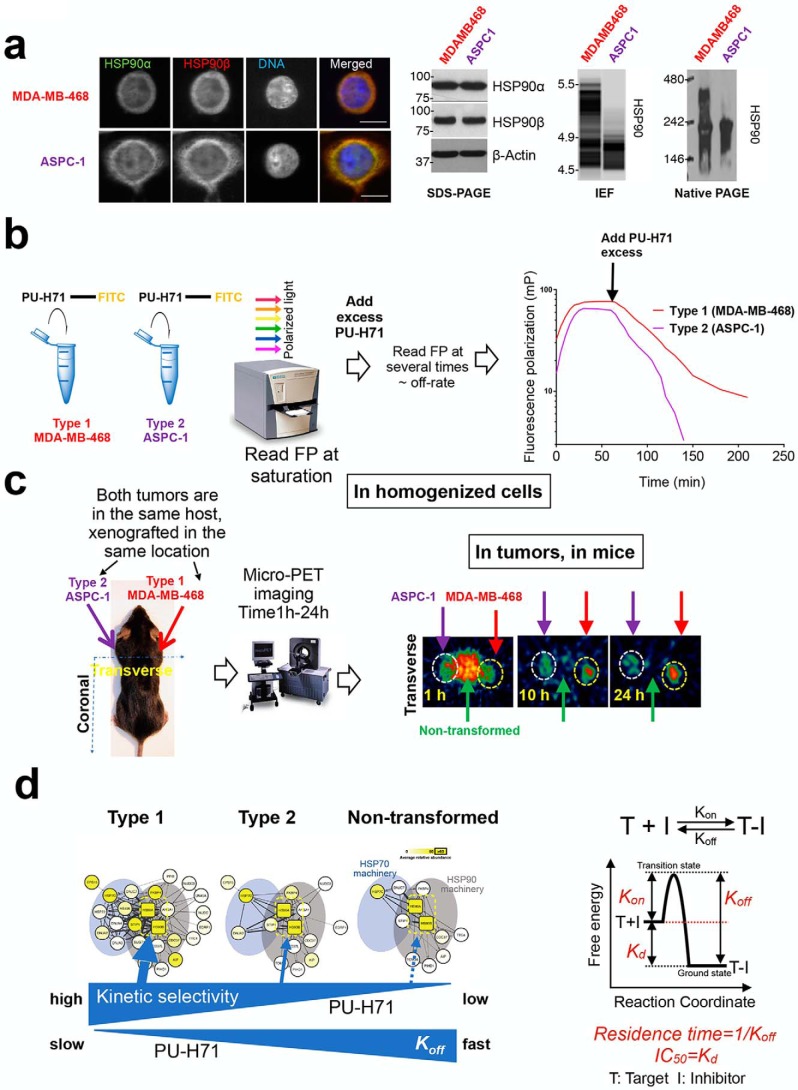
Binding of PU-H71 to different HSP90 pools. a, biochemical characterization of HSP90 in MDA-MB-468 and ASPC-1 cancer cells. Both cell lines contain similar total HSP90 levels (evaluated by immunofluorescence and immunoblot). MDA-MB-468 but not ASPC-1 contains HSP90 incorporated into epichaperome networks (evaluated by native PAGE and IEF). b and c, methods to evaluate the dissociation reaction kinetics of a ligand from different cellular HSP90 pools. One way is to compete a fluorescent or radioligand ligand from its receptor with a large excess of unlabeled ligand. The fluorescent ligand dissociates at a rate determined by Koff and does not rebind, because the unlabeled ligand takes its place. b, another way is to dilute an equilibrium mixture of ligand (A), receptor (B), and ligand–receptor complex (AB) and observe the time course of the dissociation of AB to establish new equilibrium concentrations of A and B. Monitoring of in vivo PK is in essence a surrogate of such a method. Adapted from Ref. 46. d, kinetic binding provides the selectivity of PU-H71 for epichaperome over other HSP90 pools. Reaction coordinates for a one-step binding event are shown on the right side to clarify the nature of equilibrium and kinetic binding parameters.
Figure 5.
Clinical assays to detect the stress-modified chaperome in cancer patients. a, PET scan is used to detect epichaperome-expressing solid tumors in cancer patients; b, flow cytometry-based assays is used to detect the epichaperome in liquid tumors; and c, native protein separation and analysis by isoelectric focusing followed by immunoblotting with native cognate antibodies are used for minute biopsy specimens. For the PET scan, the patient receives a minute dose of 124I-labeled PU-H71 and then undergoes PET imaging to determine epichaperome expression (or the lack of) in the tumor. HSP90, involved in dynamic housekeeping chaperome complexes, although abundant and expressed essentially in all cells in the body, does not image in the scan. For the flow cytometry assay, the peripheral blood or bone marrow sample is stained with PU-FITC, and the single-cell signal (i.e. epichaperome expression or the lack of) is detected in a flow cytometer upon sorting of cell populations. Here, for example, blasts (the malignant population in a leukemia patient) are detected to be high epichaperome expressors. Conversely, normal cells (lymphocytes in the same leukemia patient), while high HSP90 expressors, do not stain with PU-FITC. For the biopsy, the frozen biospecimen is applied for IEF-IB analysis by the NanoPro IEF platform.
Figure 6.
Chemical biology approach for the investigation of the epichaperome. A combination of chemical biology probes, functional and biochemical assays applied to cells in their native environment, and complemented with classical biology approaches can be used to investigate epichaperome biology in cancer.
The interaction of small molecules with proteins is typically described using affinity indicators measured at equilibrium (i.e. Kd and IC50/EC50). These have been the mainstay of drug discovery and were assumed to be valid indicators of drug activity and predictors of in vivo efficacy. As we have discussed, they fall short in defining the properties of a safe and active anti-HSP90 agent (22, 62–64).
We will discuss below why kinetic, and not equilibrium binding analyses are better descriptors of HSP90–ligand interactions in tumors. The kinetic approach is more revealing because the kinetics of the association or dissociation may themselves provide the preference for a specific protein pool. For instance, in the context of a drug treatment regimen where the concentration of the drug at the target is continually changing, a drug with a longer residence time in one HSP90 pool can kinetically select a given pool over another, even if the affinity for both is comparable at equilibrium.
Is there a structural basis for inhibitor selectivity (or the lack of) for the several HSP90 pools? HSP90s are highly dynamic and flexible proteins, and their conformation and activity are modulated by regulatory nucleotides, association with conformation-specific co-chaperones, and post-translational modifications (65–68). The N-terminal domain of HSP90 may exist in a combination of different conformational states in its free state with the lid segment undergoing internal motions on the microsecond–millisecond time scale (69, 70). The binding of a compound may alter the protein's conformational distribution by increasing the pool of conformational states that are rare in the unbound state. For example, ligand binding may redistribute the population toward more stable conformations. Alternatively, but not exclusive of the prior scenario, the ligand may favor specific, already present, populations driven by either a particular HSP90 conformation, a binding partner(s), or fluctuations in the cellular environment. Inhibitors may take advantage of the high flexibility in the N-terminal lid structure to obtain either specificity for one paralog over another or to select for a specific HSP90 pool (69, 71–73).
For the former, for example, we reported ligands that take advantage of the conformational flexibility of GRP94 to “freeze” the protein in a state that unveils a unique allosteric site that arises from a shift in Phe-199 (71). This unique conformation is not accessible to cytosolic HSP90, providing a rationale for the design of GRP94-selective ligands. Others have used this feature to design ligands that are selective for HSP90 over the organelle paralogs or selective for HSP90 of other organisms over that expressed in humans (71–74).
To understand the latter, we will review the structural observations of ligand binding to HSP90. Crystal structures of ligand-bound HSP90 became available soon after each inhibitor class was reported (75–93), and in 2013 we employed these structures to investigate the influence of inhibitors on HSP90 pocket configuration (94). We observed that the ATP-binding pocket could be subdivided in three major subpockets (we referred to them as A, B, and C) and that inhibitors occupied some or all of these subpockets (Fig. 3a). The binding of geldanamycin (GM) and several resorcinols was restricted to subpockets A and C. The configuration that exposed pocket B was absent in crystal structures of apo-HSP90. This indicated that exposure of pocket B might be transient, that only a small fraction of free HSP90 populates this conformational state, or that it is inhibitor-induced. Whichever the reason, pocket B became exposed upon PU-H71 binding, and this ligand occupied all three available subpockets. Pocket B was unveiled by PU-H71 by inducing a conformational change in helix 3 of HSP90, creating a new binding channel (Protein Data Bank code 2FWZ). Specifically, the 8-aryl moiety rearranges helix 3, exposing a lipophilic pocket (B), whereby the phenyl ring of the ligand becomes stacked between Phe-138 and Leu-107 and forms further favorable interactions with Met-98 and Leu-103. The N9 chain is oriented toward an exit pocket (C) that is narrowed somewhat by Leu-107 (Fig. 3a).
In 2017, Amaral et al. (69) attached larger moieties onto resorcinolic scaffolds in such a way that they pointed toward pocket B (see Fig. 3b, arrow pointing to attachment site). They showed that such ligands could also induce the pocket rearrangement observed with PU-H71. They characterized this subpocket as a “continuous helical conformation,” and the ligands inducing this conformation were termed “helix-binders.” The GM and resorcinol type binders that insert into pockets A and C, were named “loop-binders.” They next performed surface plasmon resonance and computational studies to show that the majority of the helix-binders had lower association and dissociation rate constants for binding to HSP90 than the loop-binders, a result that supported different binding mechanisms. Compounds that bound to the helical conformation could reach up to 650-fold slower dissociation rates when compared with compounds that bound to the loop conformation. Helix-binders had up to 230-fold slower association rates, when compared with loop-binders, indicating a higher transition state energy barrier for helix-binders.
This study demonstrates the relationship between ligand-induced protein rearrangements and ligand-binding kinetics, and it provides an in vitro correlate to the observations reported by us in cells, mice and humans, as we detail below. This study also stresses that whereas tighter interactions make binding more favorable, the thermodynamic signature of a strong binder does not have to be dominated by an enthalpic term. Furthermore, this study supports occupancy of site B as an important determinant of the time the ligand spends bound to target.
The simple system described above, composed of purified proteins and ligands, highlights the complexity of ligand–HSP90 association and the effect protein conformation and dynamics have on ligand binding (and vice versa). In contrast, cells are biochemically more complex, especially if we consider the contribution of chaperone stoichiometries in larger complexes, variations in their components, changes in the binding affinity between chaperome complex components, and the post-translational modifications they carry.
By 2004, it had become clear to us that equilibrium binding parameters were insufficient to characterize the interaction of these agents with HSP90 in tumors (95). When we studied the in vivo properties of PU24FCl, an early generation probe and a precursor to the clinical agent PU-H71, we observed that the binding of the agent was rapid in all tissues, but its dissociation from tumors was slow (95). This “tumor retention” characteristic, or long tumor residence time, was not the result of drug stickiness to tumor tissue or other nontarget-related factors, because oncogenic clients remained suppressed in tumors as long as the agent also remained in the tumor. The magnitude of oncogenic client suppression also corresponded to the concentration of PU24FCl measured in the tumor. In other words, tumor pharmacokinetics mirrored tumor pharmacodynamics, and the off-rate or the dissociation constant from the tumor determined the biological activity of this agent in tumor-bearing mice.
Tumor PK parameters, with extended tumor retention, were reported soon after this study for all agents with a claim for in vivo antitumor activity. Interestingly, although some varying degree of tumor retention was reported for most agents, tumor PK did not always translate into tumor PD (63). In other words, although several agents appeared to be retained in the tumor, the levels of the inhibitor and its duration of stay in the tumor did not correlate with how long the target was suppressed in the tumor. Thus, if tumor PK and PD values lack correlation, an extended retention of the agent in the tumor does not reflect specific target engagement.
In addition to in vivo kinetic analyses, we have recently shown (46) through a number of alternative methods using cell homogenates, live cells, and tumors in mice that kinetic binding, with a slowest off-rate from epichaperomes, provides the selectivity of PU-H71 for such tumor HSP90 pools (see examples in Fig. 4). We have recently discussed, and in detail in Fig. 5, how this property of PU-H71 can be used to noninvasively detect tumors in cancer patients characterized by epichaperome abundance (46). We have also shown that this property of PU-H71 can be used at the single-cell level to detect those cells characterized by the presence of the epichaperome (46, 96). Finally, we have used an unbiased large-scale approach to show that this property of PU-H71 can be used to identify the interactome and function of this stress-specific HSP90 pool (22, 46).
In recent years, studies from a variety of targets have pointed to the kinetics of drug–target binding as an important hallmark of drug efficacy and safety (97–102). The rate constant for the dissociation of the ligand from the target is usually the main determinant of the affinity because many association reactions are fast, with rate constants in the range of 106 to 107 m−1 s−1. Thus, for example, dissociation rate constants will be on the order of 1 s−1 for a low-affinity reaction with a Kd of 1 μm, and only 0.001 s−1 for a high-affinity reaction with a Kd of 1 nm. In other words, when binding is very tight, complexes can persist for hours to days. In addition to providing selectivity, such binding profiles may also increase the therapeutic index of an inhibitor, as we have recently discussed (22).
Thus, both ground state stabilization (i.e. drug–target affinity, Kd) and transition state destabilization (Koff) influence the time the ligand spends bound to protein (Fig. 4). These observations require a change in our thinking of how we assess, discover, and investigate HSP90 drugs. Currently, most such studies use tissue culture treatments with HSP90 agents added for 24–72 h. These settings, however, reflect a state of equilibrium binding. Over the long time of the experiment, the compound resides inside the cell at a constant concentration, and the ligand may occupy and inhibit the phenotype-determining pool regardless of its kinetic binding preference. Under these conditions, and as often reported, molecular and cellular outcomes are similar for many of the HSP90 agents (46, 103), irrespective of their kinetic binding-defined HSP90 pool preference.
How is HSP90 in the epichaperome different from the HSP90 pool of type 2 tumors or of nontransformed cells so that it influences ligand dissociation kinetics? The answer to this question is as yet unknown. However, it has been shown that the dynamics and the strength of interaction between HSP90 and other chaperome members, as well as the constitution of the complexes in the two tumor types, differs. In the epichaperome, the dense and complex associations surrounding the resident HSP90 may limit the conformational flexibility of the chaperone, in turn unmasking or stabilizing the exposure of a site that is absent in free HSP90, giving rise to a new protein–protein interaction platform and a new quaternary structure. What this conformation is, however, remains to be elucidated.
Proteome imbalance drives epichaperome formation
Recent work has shown that a significant driver of epichaperome formation is proteome imbalances (46, 61). We identified one factor, MYC hyperactivation, to be partly responsible for driving the “rewiring” of the chaperome into the epichaperome in cancer (22, 46, 104). A significantly higher MYC transcriptional activity characterized epichaperome-positive cells, when compared with epichaperome-negative (or low) cancer cells. Knockdown of MYC converted type 1 cells into type 2. The introduction of a functional MYC gene into a type 2 cancer cell was sufficient to rewire it into a type 1.
However, transformation with bona fide HSP90 client proteins v-Src or mutant MET kinase, which led to an increase in the cellular levels of several chaperones and co-chaperones, such as HSP70, HSC70, HOP, HSP110, HSP40, and AHA1, failed to substantially influence epichaperome formation (46). Recently, NOTCH was found to be an epichaperome inducer, presumably through its role as an upstream MYC activator in T-cell acute lymphoblastic leukemia (T-ALL) (104). Blocking NOTCH activity, by preventing its cleavage at the cell surface with γ-secretase inhibitors, reduced the epichaperome.
A discussion of the formation and role of the epichaperome has been published recently (22, 61), but an illustration of these concepts can be seen in a recent publication by Kishinevsky et al. (61), where the effects of rotenone treatment, a toxic stress, or Parkin mutation, a genetic stress, or a combination of the two on the chaperome were evaluated in midbrain dopaminergic neurons. Both rotenone and Parkin mutations induce mitochondrial dysfunction in neurons and are associated with Parkinson's disease (105). Because each stress was “titrated” into a normal proteostasis background, this study allows us to appreciate the influence of specific stresses on the chaperome.
In the nonstressed neurons that had normal function and phenotype, the chaperome was characteristic of nontransformed cells (46). In these neurons, HSP90 complexes disassembled under native PAGE, immobilized PU-H71 isolated little HSP90, and few co-chaperones were observed bound to HSP90. These cells were largely refractory to PU-H71, and even high concentrations of PU-H71 (50 μm over 72 h) showed minimal toxicity in neurons. This profile is identical to what we have observed for nonmalignant cells, both primary and cultured, and this is a state that defines normal cellular proteostasis (46).
Both toxic and genetic stresses augmented the number of proteins forming stable complexes with HSP90 (61). However, it was the genetic stress, and not the acute toxin treatment, that induced the most dramatic chaperome remodeling. Furthermore, each stress induced a distinct signature in the chaperome indicating that chaperome network composition may represent a stress-specific fingerprint. For example, HSP60 was recruited to the HSP90 chaperome network under the toxic stress. In contrast, a number of HSP70 machinery-associated chaperome members, but not HSP60, were tightly bound to HSP90 following the genetic stress.
Why was epichaperome formation necessary under each stress? We observed that under toxic stress these epichaperomes remodeled dopamine production pathways, whereas under genetic stress they enhanced the fitness of inflammatory pathways along with other mechanisms (61). Thus, as we discussed in cancer (22), epichaperomes act by maintaining the functionality and fitness of pathogenic protein pathways and networks, rather than the folding of individual clients. It remains to be elucidated how epichaperomes execute such functions, but it was recently hypothesized that epichaperomes act as scaffolding platforms that enable a restructuring of protein–protein interactions and therefore the rewiring of cellular protein networks (22).
Epichaperomes as a tumor survival mechanism
The existence of the epichaperome is vital for the survival of type 1 tumors (22, 46, 104). In these cancer cells, but not in those of type 2, inhibition of the epichaperome-participating members HSP90, AHA1, HOP, and HSP110 is toxic (Fig. 2) (46). As the epichaperome fraction of the tumor cell increases, the more sensitive the tumor cell becomes to HSP90 inhibition or siRNA knockdown of HSP90, HSP110, HOP, or AHA1. In epichaperome-high T-ALL, where NOTCH activates MYC, which in turn augments the epichaperome levels (see above), blocking NOTCH, which de-activates MYC, reduced the epichaperome fraction of the cell. These cells, in turn, exhibited reduced sensitivity to inhibition of HSP90, a key component of the epichaperome (104).
In type 1 cancer cells, but not in those of type 2, a reduction in the total HSP90 levels to as little as 5% of its normal amount remodeled the remaining HSP90 into epichaperome complexes by increasing the cellular concentration of other epichaperome components (AHA1, CDC37, HSP110, HSC70, and HOP were evaluated) (Fig. 2) (46). In these conditions of low HSP90 but high epichaperome, the function of oncogenic protein networks and cell viability remained unaffected. However, as HSP90 levels continued to drop, an inflection was observed. At this point, the epichaperomes collapsed; oncogenic proteins were depleted, and cell death ensued. The conclusion from these experiments was that epichaperomes are key for the survival of type 1 tumors and that their abundance determines tumor vulnerability to its inhibition. Finally, they show that when the level of a key chaperome member becomes limiting, such as by the 95% depletion of HSP90 described above, an increase in the expression level of other key chaperome members may retain network structure and functionality, and in turn foster cancer cell survival.
Epichaperome as a tumor target and biomarker
Chaperome members are abundant in all cells in the human body. Biochemical analyses of tumors and associated surrounding normal tissues, as well as whole body scans of mouse and human cancer patients, however, demonstrated the exclusive presence of epichaperomes in certain tumors but not all (Figs. 4 and 5) (https://clinicaltrials.gov/identifier NCT01269593) (46). This was irrespective of tissue of origin, tumor subtype, or genetic background.
Because the sensitivity of tumors to inhibition of key epichaperome components correlates with the cellular abundance of the epichaperome, the epichaperome is a biomarker. Clinical assays were therefore developed to identify the high epichaperome expressing tumors as these tumors are more likely to benefit from chaperome therapy, such as from HSP90 inhibitors. Three such assays have been developed and translated to clinic: PU-PET for solid tumors, to detect the epichaperome biomarker by PET imaging (Fig. 5a) (https://clinicaltrials.gov/identifier NCT01269593) (46, 106); PU-FITC for liquid tumors, to detect the epichaperome by flow cytometry (Fig. 5b) (46, 96); and IEF for biopsies, to detect the epichaperome by native IEF chromatography (Figs. 1 and 5c) (46).
Because the epichaperome is vital for the survival of tumors that express it, the epichaperome is also a tumor target. Although a variety of pharmacological inhibitors and siRNA reagents are useful and appropriate to study its function in cultured cells (46), this is not the case for its inhibition in humans. To target the epichaperome in clinic, inhibitors that discriminate between a single chaperome and a chaperome incorporated into epichaperomes (that is HSP90 versus HSP90-containing epichaperomes for example) are preferred for treatment as they may offer better target engagement and a safer profile (22, 46). Although acute chaperome inhibition may not be toxic to normal cells, chronic suppression may have unwanted effects (107, 108). A number of review articles have been written on the discovery, identity, and mode of action of small molecule inhibitors of HSP90, HSP70, and others (62, 109–117), and the reader is referred to these sources for further expansion on this topic.
An often-heard misconception is that all the small-molecule inhibitors of chaperome members are identical and that they will behave similarly in clinic. This view overlooks the reality that agents directed toward chaperome members in cancer cells are in fact being directed toward a heterogeneous cellular chaperome, where a multitude of biochemically and functionally distinct complexes co-exist (22). It also overlooks the reality that humans are not culture dishes where drugs are kept at constant concentrations for days on end and that factors such as drug residence time, pharmacokinetics, and pharmacodynamics greatly influence what chaperome species an inhibitor can engage while inside a human body (Figs. 3–5) (22).
In addition to the discussion above, the kinetics of binding, the evaluation of these factors, and their influence on inhibitor efficacy and safety have been recently reviewed elsewhere (22). Because of the complexity of the tumor cell chaperome, equilibrium binding metrics, and those evaluated with the use of recombinant proteins, are poorly suited to understand, or deduce, the biological activity of a chaperome inhibitor, and they are even less appropriate to gauge the safety and potency of such inhibitors in the clinic.
Reagents and methods for epichaperome analysis
The biochemical and functional heterogeneity of the chaperome, combined with its stress-specific remodeling, highlights the limitations of methods that either perceive the chaperome as a monolithic entity or disrupt and engineer the cellular environment to study its function. Methods and reagents that recognize such chaperome heterogeneity, and also enable the study of disease-specific chaperome species, are therefore needed for a proper understanding of chaperome biology in disease. Chemical biology has emerged as a complementary yet powerful approach to tackle complex biological problems. By modifying the probe (chemical tool) rather than the system (protein or cell), chemical biology enables the analysis of the proteome in the nonengineered, native state, thus providing information most closely resembling the reality of a disease. Chemical biology uses chemical probes to detect and analyze proteins. Chemical probes are small molecules, and by definition, small molecules can sense and detect changes in proteins.
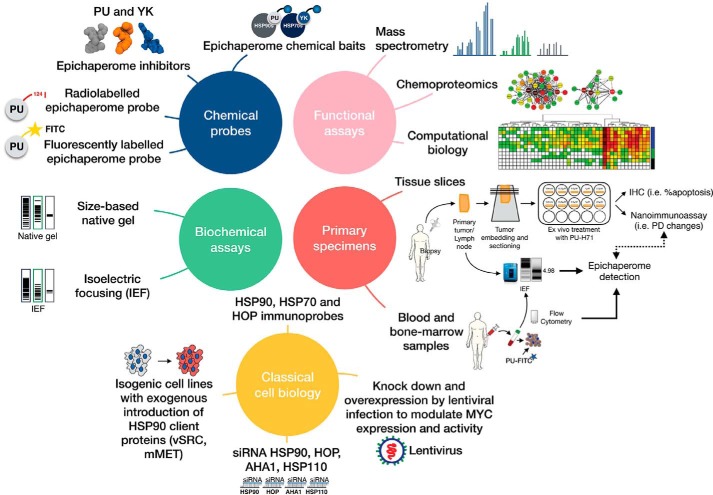
By taking advantage of kinetic selectivity, chemical probes may be designed to differentiate between the dynamic and the stable chaperome complexes and between the many distinct chaperome conformations. Our lab has spent the last decade developing chemical probes and methods for the investigation of the chaperome in disease (46, 56, 71, 74, 118–126), and we will highlight below several that enable the study of the epichaperome (Fig. 6).
Chemical probes
PU-H71 is an inhibitor of HSP90 specifically when HSP90 is part of the stable chaperome complexes of epichaperome networks formed under stress (46, 56, 127). The more HSP90 is incorporated into stable epichaperome networks, the higher the binding affinity of PU-H71 for HSP90, and the slower its dissociation (off-rate) from the stable epichaperome complexes (which provide the biochemical basis for the epichaperome networks) (Fig. 4) (46, 61). This property of PU-H71 is the basis for the use of labeled PU-H71 to differentiate epichaperome-expressing tumors from those that are not and to measure the expression of the epichaperome. While initially discovered as an HSP90 inhibitor, later studies have shown PU-H71 prefers “a tumor-enriched HSP90” or a “stress HSP90” (56, 96, 128–133). Later studies have identified this HSP90 species to be the epichaperome (46). Its kinetic selectivity for the epichaperome over HSP90 has been shown in cell homogenates, in live cells, in mice, and in humans and through a number of alternative methods (46). Therefore, PU-H71 and related chemical probes (i.e. fluorescently labeled, radiolabeled, and solid support immobilized) can be used as chemical sensors to recognize the tumor stress-modified HSP90 pool incorporated into epichaperome networks and, moreover, capture and identify the disease-related protein networks it regulates. Because of these features, PU-H71 itself is used in the clinic to treat epichaperome-addicted tumors (https://clinicaltrials.gov/identifiers NCT01393509, NCT03166085, and NCT03373877) (134), to detect the epichaperome by flow cytometry (see the PU-FITC assay, Fig. 5), and to detect epichaperomes in solid tumors by PET imaging (see PU-PET assay, Fig. 5). In addition to PU-type probes, we have developed the YK-probes that act on the epichaperome via HSP70s (when HSC70 is incorporated into the epichaperome) (46). When using these chemical probes, one must appreciate and consider the discussion above regarding equilibrium and kinetic binding conditions and understand that selectivity comes from the kinetics of binding.
Biochemical assays
To identify and separate chaperome complexes in tumors, and to overcome the limitations of classical protein chromatography methods for resolving complexes of similar composition and size, we implemented a capillary-based assay that combines IEF with immunoblotting capabilities (Fig. 1) (46). Based on the NanoPro 1000 system from ProteinSimple (135), it uses an immobilized pH gradient to separate native multimeric protein complexes based on their pI and allows for subsequent probing of immobilized complexes with native cognate antibodies. Proteins separated by capillary IEF remain in a native state, which may influence the ability of antibodies to recognize the proteins, and thus we screened a panel of antibodies to identify those that best capture the epichaperome complexes. The method uses only minute amounts of sample, enabling the interrogation of primary specimens. We also developed a similar method for the analysis of epichaperome complexes by native PAGE (Fig. 1) (46).
Functional assays
To investigate the epichaperomes, and their composition and function in the specific tumor context, we have developed chaperome proteomics and computational methods (46, 56, 119, 133). These enable large-scale nonbiased analyses of the epichaperome, including the composition and post-translational makeup of its constituent chaperomes, the identity and function of its interactome, and the global cellular pathways and networks dependent on and driving epichaperome formation. The discovery of MYC hyperactivation as a driver of epichaperome formation in cancer and the discovery of epichaperome formation as the basis of tumor sensitivity to PU-H71 were based on hypothesis-building findings derived from the use of such large-scale functional assays (see Ref. 46 for details). Understanding how the epichaperome restructures under specific stresses was also derived from the incorporation of this assay (56, 61).
Primary specimens
To closely recapitulate the human disease and to capture the epichaperome as expressed in native tumors, we have developed methods to detect epichaperomes in minute samples and to test the activity of epichaperome inhibitors in tissue slices (for solid tumors, see Ref. 46) and in blood and bone marrow samples (for liquid tumors, see Ref. 46, 56, 96). These retain the tumor environment and architecture (stroma, immune cells, etc.). We have also developed streamlined protocols for the evaluation of drugs in fresh solid-tumor tissue slices (46, 123) and in leukemic specimens from clinic (96).
A combination of these numerous methods and probes, along with classical cell biology approaches (engineered cells, isogenic lines, siRNAs, antibodies, etc., see also Ref. 46) is best used to investigate the nature and significance of the epichaperome in tumors (Fig. 6).
Summary and future outlook
We have summarized recent work demonstrating how stresses associated with disease can remodel the chaperome so that major chaperome machineries become structurally and functionally integrated. Such chaperome remodeling is accomplished by increasing the interaction strength between the members of distinct chaperome machineries and between the chaperome and the proteome it regulates. These changes appear largely independent of overall chaperome levels, suggesting that increased connectivity is important during cellular alterations induced by proteome stresses.
Several landmark studies have indicated that a remodeling of the chaperome in both cancer and neurodegenerative diseases may also be associated with changes in the expression of subsets of chaperome members (5, 6, 111, 136–138), indicating that intricate chaperome remodelings, as reflected by changes in both expression and interaction strength among members, are hallmarks of diseases.
We have also discussed how equilibrium binding parameters are inadequate to characterize chaperome targeting in cancer and to appreciate the selectivity of a ligand for specific tumor-associated chaperome pools. It is now clear that targets other than the chaperome can also benefit from understanding drug–target kinetics. Indeed, the role of binding kinetics is being explored in programs such as the K4DD Innovative Medicines Initiative (69, 97–102, 139).
Although the discovery of the epichaperome in cancer; the development of reagents and methods to detect this chaperome-addicted state in cells, animals, and patients; the development of inhibitors that enable its inhibition in clinic, and the clinical proof-of-principle that epichaperome modulation may lead to effective treatment have all proceeded apace, many questions remain.
How do epichaperomes form?
It is likely that cellular stress favors conformations of chaperome members that are more permissive of oligomerization. Whether these conformations become stabilized by post-translational modifications in constituent chaperomes or even in interacting proteins, or both, remains to be determined.
How do epichaperomes foster tumor cell survival?
Evidence so far indicates that epichaperomes may regulate the cellular complement of oncogenic proteins and pathways, a function recently reviewed (22). Unlike individual chaperomes, epichaperomes may function as complex scaffolds, providing oncogenic cellular components with a platform on which they can work more efficiently than they would without epichaperome participation (22). Future studies are needed to address the biochemical mechanisms of these functions, but the formation of epichaperome complexes appears to redirect the chaperome toward functions other than folding or disaggregation.
Are epichaperomes other than those nucleating around HSP90–HSC70 also forming in disease?
One may imagine that not all stresses may be regulated by HSP90–HSC70 epichaperomes and that other varieties may form in a stress-dependent manner. Organelle-specific networks may also form in conditions of disease, similar to those described here for the cytosolic chaperome.
What are the fundamental factors and mechanisms that drive or inhibit epichaperome formation?
Although MYC is a driver in cancer, it is likely that other cells may have distinct vulnerabilities to stress and stressors, as we have noted recently in the context of Parkinson's disease (61).
How may we influence the formation of the epichaperome in situ?
Because stress is a factor in epichaperome formation, artificially augmenting epichaperomes by the exogenous application of stress may be a route to increasing the sensitivity of tumors to epichaperome-targeted therapies. Our team is currently investigating certain chemotherapies for inducing proteome stress and, in turn, epichaperomes in cancer (NCT03166085).
What therapies positively (or negatively) influence epichaperome formation?
Drugs that positively influence epichaperomes may augment sensitivity of tumors to epichaperome therapies, and identification of such agents will be of importance in the future clinical development. Just as important is to identify those agents that may negatively influence epichaperomes. On the one hand, these may be indirect epichaperome inhibitors, and thus may be used as such to treat epichaperome-addicted tumors. On the other hand, one may imagine that such agents limit the efficacy of epichaperome inhibitors. Cancer treatment does not occur in a vacuum but rather in patients co-treated for a number of possible morbidities, such as high blood pressure or a bacterial infection, and any such drugs could interfere with epichaperome formation through yet unknown mechanisms.
What is the composition and the design of a combination therapy that targets the epichaperome for maximal therapeutic benefit?
Drugs may augment or inhibit epichaperomes, and such factors must be taken in account when designing combination therapies, the backbone of cancer treatment. The timing and the sequence in which these drugs are administered also become of great importance in such therapies, and these factors will have to be carefully investigated.
How is epichaperome inhibition affecting and is affected by the tumor microenvironment and the immune system? Are tumor-associated cells also using epichaperomes as a mechanism of transformation?
Although cancer research has been heavily focused in the past on the tumor itself, it is now appreciated that several factors outside the tumor greatly influence the efficacy of cancer therapies, including the immunosuppressive cell populations that aid in promoting tumor progression and immune evasion. The influence of these factors in epichaperome therapies remains to be resolved. Studies so far also highlight the need to study the epichaperome in the context of native cellular states. Therefore, the use of chemical biology methods becomes important in the study of epichaperomes in disease.
Overall, the discovery of the epichaperome offers the potential for precision medicine, and it emphasizes that the properties of chaperome networks, not genetics or individual client proteins, should drive chaperome therapy implementation. Future development of epichaperome inhibitors thus requires a shift in our thinking, with a focus on patient selection and drug implementation based on mechanisms of tumor addiction to epichaperomes.
This is the ninth article in the JBC Reviews series “Molecular chaperones and protein quality control.” G. C. has partial ownership in Samus Therapeutics Inc., which develops epichaperome inhibitors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ER
- endoplasmic reticulum
- IEF
- isoelectric focusing
- PET
- positron emission tomography
- GM
- geldanamycin
- EGFR
- epidermal growth factor receptor
- PK
- pharmacokinetics
- PD
- pharmacodynamics
- T-ALL
- T-cell acute lymphoblastic leukemia.
References
- 1. Finka A., and Goloubinoff P. (2013) Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones 18, 591–605 10.1007/s12192-013-0413-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finka A., Mattoo R. U., and Goloubinoff P. (2011) Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones 16, 15–31 10.1007/s12192-010-0216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X., Venable J., LaPointe P., Hutt D. M., Koulov A. V., Coppinger J., Gurkan C., Kellner W., Matteson J., Plutner H., Riordan J. R., Kelly J. W., Yates J. R. 3rd., and Balch W. E. (2006) Hsp90 co-chaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 127, 803–815 10.1016/j.cell.2006.09.043 [DOI] [PubMed] [Google Scholar]
- 4. Brehme M., and Voisine C. (2016) Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis. Model. Mech. 9, 823–838 10.1242/dmm.024703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brehme M., Voisine C., Rolland T., Wachi S., Soper J. H., Zhu Y., Orton K., Villella A., Garza D., Vidal M., Ge H., and Morimoto R. I. (2014) A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 9, 1135–1150 10.1016/j.celrep.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadizadeh Esfahani A., Sverchkova A., Saez-Rodriguez J., Schuppert A. A., and Brehme M. (2018) A systematic atlas of chaperome deregulation topologies across the human cancer landscape. PLoS Comput. Biol. 14, e1005890 10.1371/journal.pcbi.1005890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schopf F. H., Biebl M. M., and Buchner J. (2017) The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 18, 345–360 10.1038/nrm.2017.20 [DOI] [PubMed] [Google Scholar]
- 8. Kampinga H. H., and Craig E. A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 10.1038/nrm2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bukau B., and Horwich A. L. (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92, 351–366 10.1016/S0092-8674(00)80928-9 [DOI] [PubMed] [Google Scholar]
- 10. Kim Y. E., Hipp M. S., Bracher A., Hayer-Hartl M., and Hartl F. U. (2013) Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 10.1146/annurev-biochem-060208-092442 [DOI] [PubMed] [Google Scholar]
- 11. Miller S. B., Mogk A., and Bukau B. (2015) Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J. Mol. Biol. 427, 1564–1574 10.1016/j.jmb.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 12. Sontag E. M., Samant R. S., and Frydman J. (2017) Mechanisms and functions of spatial protein quality control. Annu. Rev. Biochem. 86, 97–122 10.1146/annurev-biochem-060815-014616 [DOI] [PubMed] [Google Scholar]
- 13. Kakihara Y., and Houry W. A. (2012) The R2TP complex: discovery and functions. Biochim. Biophys. Acta 1823, 101–107 10.1016/j.bbamcr.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 14. Horwich A. L. (2013) Chaperonin-mediated protein folding. J. Biol. Chem. 288, 23622–23632 10.1074/jbc.X113.497321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiller S., and Burmann B. M. (2018) Chaperone-client complexes: a dynamic liaison. J. Magn. Reson. 289, 142–155 10.1016/j.jmr.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 16. Koldewey P., Horowitz S., and Bardwell J. C. (2017) Chaperone–client interactions: non-specificity engenders multifunctionality. J. Biol. Chem. 292, 12010–12017 10.1074/jbc.R117.796862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lackie R. E., Maciejewski A., Ostapchenko V. G., Marques-Lopes J., Choy W. Y., Duennwald M. L., Prado V. F., and Prado M. A. (2017) The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front. Neurosci. 11, 254 10.3389/fnins.2017.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaner L., and Morano K. A. (2007) All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones 12, 1–8 10.1379/CSC-245R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bracher A., and Verghese J. (2015) The nucleotide exchange factors of Hsp70 molecular chaperones. Front. Mol. Biosci. 2, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z., Hartl F. U., and Bracher A. (2013) Structure and function of Hip, an attenuator of the Hsp70 chaperone cycle. Nat. Struct. Mol. Biol. 20, 929–935 10.1038/nsmb.2608 [DOI] [PubMed] [Google Scholar]
- 21. Echtenkamp F. J., and Freeman B. C. (2012) Expanding the cellular molecular chaperone network through the ubiquitous co-chaperones. Biochim. Biophys. Acta 1823, 668–673 10.1016/j.bbamcr.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 22. Joshi S., Wang T., Araujo T. L. S., Sharma S., Brodsky J. L., and Chiosis G. (2018) Adapting to stress-chaperome networks in cancer. Nat. Rev. Cancer 18, 562–575 10.1038/s41568-018-0020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang H. C., and Lindquist S. (1994) Conservation of Hsp90 macromolecular complexes in Saccharomyces cerevisiae. J. Biol. Chem. 269, 24983–24988 [PubMed] [Google Scholar]
- 24. Dey B., Caplan A. J., and Boschelli F. (1996) The Ydj1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol. Biol. Cell 7, 91–100 10.1091/mbc.7.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cha J. Y., Ahn G., Kim J. Y., Kang S. B., Kim M. R., Su'udi M., Kim W. Y., and Son D. (2013) Structural and functional differences of cytosolic 90-kDa heat-shock proteins (Hsp90s) in Arabidopsis thaliana. Plant Physiol. Biochem. 70, 368–373 10.1016/j.plaphy.2013.05.039 [DOI] [PubMed] [Google Scholar]
- 26. Lee J. R., Lee S. S., Jang H. H., Lee Y. M., Park J. H., Park S. C., Moon J. C., Park S. K., Kim S. Y., Lee S. Y., Chae H. B., Jung Y. J., Kim W. Y., Shin M. R., Cheong G. W., et al. (2009) Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Natl. Acad. Sci. U.S.A. 106, 5978–5983 10.1073/pnas.0811231106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee S. S., Jung H. S., Park S. K., Lee E. M., Singh S., Lee Y., Lee K. O., Lee S. Y., and Chung B. Y. (2015) Enhancement of chaperone activity of plant-specific thioredoxin through γ-ray mediated conformational change. Int. J. Mol. Sci. 16, 27302–27312 10.3390/ijms161126019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson A. D., Bernard S. M., Skiniotis G., and Gestwicki J. E. (2012) Visualization and functional analysis of the oligomeric states of Escherichia coli heat shock protein 70 (Hsp70/DnaK). Cell Stress Chaperones 17, 313–327 10.1007/s12192-011-0307-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang H. C., Nathan D. F., and Lindquist S. (1997) In vivo analysis of the Hsp90 co-chaperone Sti1 (p60). Mol. Cell. Biol. 17, 318–325 10.1128/MCB.17.1.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goeckeler J. L., Stephens A., Lee P., Caplan A. J., and Brodsky J. L. (2002) Overexpression of yeast Hsp110 homolog Sse1p suppresses ydj1–151 thermosensitivity and restores Hsp90-dependent activity. Mol. Biol. Cell 13, 2760–2770 10.1091/mbc.02-04-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X. D., Morano K. A., and Thiele D. J. (1999) The yeast Hsp110 family member, Sse1, is an Hsp90 co-chaperone. J. Biol. Chem. 274, 26654–26660 10.1074/jbc.274.38.26654 [DOI] [PubMed] [Google Scholar]
- 32. Dahmer M. K., Housley P. R., and Pratt W. B. (1984) Effects of molybdate and endogenous inhibitors on steroid-receptor inactivation, transformation, and translocation. Annu. Rev. Physiol. 46, 67–81 10.1146/annurev.ph.46.030184.000435 [DOI] [PubMed] [Google Scholar]
- 33. Csermely P., Kajtár J., Hollósi M., Jalsovszky G., Holly S., Kahn C. R., Gergely P. Jr., Söti C., Mihály K., and Somogyi J. (1993) ATP induces a conformational change of the 90-kDa heat shock protein (hsp90). J. Biol. Chem. 268, 1901–1907 [PubMed] [Google Scholar]
- 34. Hutchison K. A., Stancato L. F., Jove R., and Pratt W. B. (1992) The protein–protein complex between pp60v-src and hsp90 is stabilized by molybdate, vanadate, tungstate, and an endogenous cytosolic metal. J. Biol. Chem. 267, 13952–13957 [PubMed] [Google Scholar]
- 35. Angelidis C. E., Lazaridis I., and Pagoulatos G. N. (1999) Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur. J. Biochem. 259, 505–512 10.1046/j.1432-1327.1999.00078.x [DOI] [PubMed] [Google Scholar]
- 36. Freiden P. J., Gaut J. R., and Hendershot L. M. (1992) Interconversion of three differentially modified and assembled forms of BiP. EMBO J. 11, 63–70 10.1002/j.1460-2075.1992.tb05028.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuznetsov G., Bush K. T., Zhang P. L., and Nigam S. K. (1996) Perturbations in maturation of secretory proteins and their association with endoplasmic reticulum chaperones in a cell culture model for epithelial ischemia. Proc. Natl. Acad. Sci. U.S.A. 93, 8584–8589 10.1073/pnas.93.16.8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yonehara M., Minami Y., Kawata Y., Nagai J., and Yahara I. (1996) Heat-induced chaperone activity of HSP90. J. Biol. Chem. 271, 2641–2645 10.1074/jbc.271.5.2641 [DOI] [PubMed] [Google Scholar]
- 39. Nemoto T., and Sato N. (1998) Oligomeric forms of the 90-kDa heat shock protein. Biochem. J. 330, 989–995 10.1042/bj3300989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nemoto T. K., Ono T., and Tanaka K. (2001) Substrate-binding characteristics of proteins in the 90-kDa heat shock protein family. Biochem. J. 354, 663–670 10.1042/bj3540663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chadli A., Ladjimi M. M., Baulieu E. E., and Catelli M. G. (1999) Heat-induced oligomerization of the molecular chaperone Hsp90–inhibition by ATP and geldanamycin and activation by transition metal oxyanions. J. Biol. Chem. 274, 4133–4139 10.1074/jbc.274.7.4133 [DOI] [PubMed] [Google Scholar]
- 42. Lepvrier E., Moullintraffort L., Nigen M., Goude R., Allegro D., Barbier P., Peyrot V., Thomas D., Nazabal A., and Garnier C. (2015) Hsp90 oligomers interacting with the Aha1 co-chaperone: an outlook for the Hsp90 chaperone machineries. Anal. Chem. 87, 7043–7051 10.1021/acs.analchem.5b00051 [DOI] [PubMed] [Google Scholar]
- 43. Wallace E. W., Kear-Scott J. L., Pilipenko E. V., Schwartz M. H., Laskowski P. R., Rojek A. E., Katanski C. D., Riback J. A., Dion M. F., Franks A. M., Airoldi E. M., Pan T., Budnik B. A., and Drummond D. A. (2015) Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298 10.1016/j.cell.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hatayama T., Yasuda K., and Yasuda K. (1998) Association of HSP105 with HSC70 in high molecular mass complexes in mouse FM3A cells. Biochem. Biophys. Res. Commun. 248, 395–401 10.1006/bbrc.1998.8979 [DOI] [PubMed] [Google Scholar]
- 45. Kamal A., Thao L., Sensintaffar J., Zhang L., Boehm M. F., Fritz L. C., and Burrows F. J. (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425, 407–410 10.1038/nature01913 [DOI] [PubMed] [Google Scholar]
- 46. Rodina A., Wang T., Yan P., Gomes E. D., Dunphy M. P., Pillarsetty N., Koren J., Gerecitano J. F., Taldone T., Zong H., Caldas-Lopes E., Alpaugh M., Corben A., Riolo M., Beattie B., et al. (2016) The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 538, 397–401 10.1038/nature19807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albert R. (2005) Scale-free networks in cell biology. J. Cell Sci. 118, 4947–4957 10.1242/jcs.02714 [DOI] [PubMed] [Google Scholar]
- 48. Palotai R., Szalay M. S., and Csermely P. (2008) Chaperones as integrators of cellular networks: changes of cellular integrity in stress and diseases. IUBMB Life 60, 10–18 [DOI] [PubMed] [Google Scholar]
- 49. Rizzolo K., and Houry W. A. (2018) Multiple functionalities of molecular chaperones revealed through systematic mapping of their interaction networks. J. Biol. Chem. 293, 10.1074/jbc.TM118.002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeong H., Mason S. P., Barabási A. L., and Oltvai Z. N. (2001) Lethality and centrality in protein networks. Nature 411, 41–42 10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- 51. Taipale M., Jarosz D. F., and Lindquist S. (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- 52. Radons J. (2016) The Hsp90 chaperone machinery: an important hub in protein interaction networks. Br. J. Med. Med. Res. 14, 1–32 [Google Scholar]
- 53. Zhao R., and Houry W. A. (2007) Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol. 594, 27–36 10.1007/978-0-387-39975-1_3 [DOI] [PubMed] [Google Scholar]
- 54. Echeverría P. C., Bernthaler A., Dupuis P., Mayer B., and Picard D. (2011) An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PLoS ONE 6, e26044 10.1371/journal.pone.0026044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taipale M., Krykbaeva I., Koeva M., Kayatekin C., Westover K. D., Karras G. I., and Lindquist S. (2012) Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150, 987–1001 10.1016/j.cell.2012.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moulick K., Ahn J. H., Zong H., Rodina A., Cerchietti L., Gomes DaGama E. M., Caldas-Lopes E., Beebe K., Perna F., Hatzi K., Vu L. P., Zhao X., Zatorska D., Taldone T., Smith-Jones P., et al. (2011) Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat. Chem. Biol. 7, 818–826 10.1038/nchembio.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weidenauer L., Wang T., Joshi S., Chiosis G., and Quadroni M. R. (2017) Proteomic interrogation of HSP90 and insights for medical research. Exp. Rev. Proteomics 14, 1105–1117 10.1080/14789450.2017.1389649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miyata Y., Nakamoto H., and Neckers L. (2013) The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 19, 347–365 10.2174/138161213804143725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Butler L. M., Ferraldeschi R., Armstrong H. K., Centenera M. M., and Workman P. (2015) Maximizing the therapeutic potential of HSP90 inhibitors. Mol. Cancer Res. 13, 1445–1451 10.1158/1541-7786.MCR-15-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taipale M., Tucker G., Peng J., Krykbaeva I., Lin Z. Y., Larsen B., Choi H., Berger B., Gingras A. C., and Lindquist S. (2014) A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 158, 434–448 10.1016/j.cell.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kishinevsky S., Wang T., Rodina A., Chung S. Y., Xu C., Philip J., Taldone T., Joshi S., Alpaugh M. L., Bolaender A., Gutbier S., Sandhu D., Fattahi F., Zimmer B., Shah S. K., et al. (2018) HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nat. Commun. 9, 4345 10.1038/s41467-018-06486-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taldone T., Ochiana S. O., Patel P. D., and Chiosis G. (2014) Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 35, 592–603 10.1016/j.tips.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel H. J., Modi S., Chiosis G., and Taldone T. (2011) Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin. Drug Discov. 6, 559–587 10.1517/17460441.2011.563296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shrestha L., Bolaender A., Patel H. J., and Taldone T. (2016) Heat shock protein (HSP) drug discovery and development: targeting heat shock proteins in disease. Curr. Top. Med. Chem. 16, 2753–2764 10.2174/1568026616666160413141911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Obermann W. M., Sondermann H., Russo A. A., Pavletich N. P., and Hartl F. U. (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 143, 901–910 10.1083/jcb.143.4.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krukenberg K. A., Street T. O., Lavery L. A., and Agard D. A. (2011) Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 44, 229–255 10.1017/S0033583510000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li J., Soroka J., and Buchner J. (2012) The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta 1823, 624–635 10.1016/j.bbamcr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 68. Mollapour M., and Neckers L. (2012) Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta 1823, 648–655 10.1016/j.bbamcr.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amaral M., Kokh D. B., Bomke J., Wegener A., Buchstaller H. P., Eggenweiler H. M., Matias P., Sirrenberg C., Wade R. C., and Frech M. (2017) Protein conformational flexibility modulates kinetics and thermodynamics of drug binding. Nat. Commun. 8, 2276 10.1038/s41467-017-02258-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dollins D. E., Immormino R. M., and Gewirth D. T. (2005) Structure of unliganded GRP94, the endoplasmic reticulum Hsp90. Basis for nucleotide-induced conformational change. J. Biol. Chem. 280, 30438–30447 10.1074/jbc.M503761200 [DOI] [PubMed] [Google Scholar]
- 71. Patel P. D., Yan P., Seidler P. M., Patel H. J., Sun W., Yang C., Que N. S., Taldone T., Finotti P., Stephani R. A., Gewirth D. T., and Chiosis G. (2013) Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 9, 677–684 10.1038/nchembio.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang T., Bisson W. H., Mäser P., Scapozza L., and Picard D. (2014) Differences in conformational dynamics between Plasmodium falciparum and human Hsp90 orthologues enable the structure-based discovery of pathogen-selective inhibitors. J. Med. Chem. 57, 2524–2535 10.1021/jm401801t [DOI] [PubMed] [Google Scholar]
- 73. Ernst J. T., Neubert T., Liu M., Sperry S., Zuccola H., Turnbull A., Fleck B., Kargo W., Woody L., Chiang P., Tran D., Chen W., Snyder P., Alcacio T., Nezami A., et al. (2014) Identification of novel HSP90α/β isoform selective inhibitors using structure-based drug design. demonstration of potential utility in treating CNS disorders such as Huntington's disease. J. Med. Chem. 57, 3382–3400 10.1021/jm500042s [DOI] [PubMed] [Google Scholar]
- 74. Shrestha L., Patel H. J., and Chiosis G. (2016) Chemical tools to investigate mechanisms associated with HSP90 and HSP70 in disease. Cell Chem. Biol. 23, 158–172 10.1016/j.chembiol.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stebbins C. E., Russo A. A., Schneider C., Rosen N., Hartl F. U., and Pavletich N. P. (1997) Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89, 239–250 10.1016/S0092-8674(00)80203-2 [DOI] [PubMed] [Google Scholar]
- 76. Chiosis G., Timaul M. N., Lucas B., Munster P. N., Zheng F. F., Sepp-Lorenzino L., and Rosen N. (2001) A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol. 8, 289–299 10.1016/S1074-5521(01)00015-1 [DOI] [PubMed] [Google Scholar]
- 77. Wright L., Barril X., Dymock B., Sheridan L., Surgenor A., Beswick M., Drysdale M., Collier A., Massey A., Davies N., Fink A., Fromont C., Aherne W., Boxall K., Sharp S., et al. (2004) Structure–activity relationships in purine-based inhibitor binding to HSP90 isoforms. Chem. Biol. 11, 775–785 10.1016/j.chembiol.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 78. Brough P. A., Barril X., Beswick M., Dymock B. W., Drysdale M. J., Wright L., Grant K., Massey A., Surgenor A., and Workman P. (2005) 3-(5-Chloro-2,4-dihydroxyphenyl)-pyrazole-4-carboxamides as inhibitors of the Hsp90 molecular chaperone. Bioorg. Med. Chem. Lett. 15, 5197–5201 10.1016/j.bmcl.2005.08.091 [DOI] [PubMed] [Google Scholar]
- 79. Immormino R. M., Kang Y., Chiosis G., and Gewirth D. T. (2006) Structural and quantum chemical studies of 8-aryl-sulfanyl adenine class Hsp90 inhibitors. J. Med. Chem. 49, 4953–4960 10.1021/jm060297x [DOI] [PubMed] [Google Scholar]
- 80. Proisy N., Sharp S. Y., Boxall K., Connelly S., Roe S. M., Prodromou C., Slawin A. M., Pearl L. H., Workman P., and Moody C. J. (2006) Inhibition of Hsp90 with synthetic macrolactones: synthesis and structural and biological evaluation of ring and conformational analogs of radicicol. Chem. Biol. 13, 1203–1215 10.1016/j.chembiol.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 81. Huth J. R., Park C., Petros A. M., Kunzer A. R., Wendt M. D., Wang X., Lynch C. L., Mack J. C., Swift K. M., Judge R. A., Chen J., Richardson P. L., Jin S., Tahir S. K., Matayoshi E. D., et al. (2007) Discovery and design of novel HSP90 inhibitors using multiple fragment-based design strategies. Chem. Biol. Drug Des. 70, 1–12 10.1111/j.1747-0285.2007.00535.x [DOI] [PubMed] [Google Scholar]
- 82. Brough P. A., Aherne W., Barril X., Borgognoni J., Boxall K., Cansfield J. E., Cheung K. M., Collins I., Davies N. G., Drysdale M. J., Dymock B., Eccles S. A., Finch H., Fink A., Hayes A., et al. (2008) 4,5-Diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer. J. Med. Chem. 51, 196–218 10.1021/jm701018h [DOI] [PubMed] [Google Scholar]
- 83. Brough P. A., Barril X., Borgognoni J., Chene P., Davies N. G., Davis B., Drysdale M. J., Dymock B., Eccles S. A., Garcia-Echeverria C., Fromont C., Hayes A., Hubbard R. E., Jordan A. M., Jensen M. R., et al. (2009) Combining hit identification strategies: fragment-based and in silico approaches to orally active 2-aminothieno[2,3-d]pyrimidine inhibitors of the Hsp90 molecular chaperone. J. Med. Chem. 52, 4794–4809 10.1021/jm900357y [DOI] [PubMed] [Google Scholar]
- 84. Murray C. W., Carr M. G., Callaghan O., Chessari G., Congreve M., Cowan S., Coyle J. E., Downham R., Figueroa E., Frederickson M., Graham B., McMenamin R., O'Brien M. A., Patel S., Phillips T. R., et al. (2010) Fragment-based drug discovery applied to Hsp90. Discovery of two lead series with high ligand efficiency. J. Med. Chem. 53, 5942–5955 10.1021/jm100059d [DOI] [PubMed] [Google Scholar]
- 85. Roughley S. D., and Hubbard R. E. (2011) How well can fragments explore accessed chemical space? A case study from heat shock protein 90. J. Med. Chem. 54, 3989–4005 10.1021/jm200350g [DOI] [PubMed] [Google Scholar]
- 86. Vallée F., Carrez C., Pilorge F., Dupuy A., Parent A., Bertin L., Thompson F., Ferrari P., Fassy F., Lamberton A., Thomas A., Arrebola R., Guerif S., Rohaut A., Certal V., et al. (2011) Tricyclic series of heat shock protein 90 (Hsp90) inhibitors part I: discovery of tricyclic imidazo[4,5-c]pyridines as potent inhibitors of the Hsp90 molecular chaperone. J. Med. Chem. 54, 7206–7219 10.1021/jm200784m [DOI] [PubMed] [Google Scholar]
- 87. Bussenius J., Blazey C. M., Aay N., Anand N. K., Arcalas A., Baik T., Bowles O. J., Buhr C. A., Costanzo S., Curtis J. K., DeFina S. C., Dubenko L., Heuer T. S., Huang P., Jaeger C., et al. (2012) Discovery of XL888: a novel tropane-derived small molecule inhibitor of HSP90. Bioorg. Med. Chem. Lett. 22, 5396–5404 10.1016/j.bmcl.2012.07.052 [DOI] [PubMed] [Google Scholar]
- 88. Davies N. G., Browne H., Davis B., Drysdale M. J., Foloppe N., Geoffrey S., Gibbons B., Hart T., Hubbard R., Jensen M. R., Mansell H., Massey A., Matassova N., Moore J. D., Murray J., et al. (2012) Targeting conserved water molecules: design of 4-aryl-5-cyanopyrrolo[2,3-d]pyrimidine Hsp90 inhibitors using fragment-based screening and structure-based optimization. Bioorg. Med. Chem. 20, 6770–6789 10.1016/j.bmc.2012.08.050 [DOI] [PubMed] [Google Scholar]
- 89. Suda A., Koyano H., Hayase T., Hada K., Kawasaki K., Komiyama S., Hasegawa K., Fukami T. A., Sato S., Miura T., Ono N., Yamazaki T., Saitoh R., Shimma N., Shiratori Y., and Tsukuda T. (2012) Design and synthesis of novel macrocyclic 2-amino-6-arylpyrimidine Hsp90 inhibitors. Bioorg. Med. Chem. Lett. 22, 1136–1141 10.1016/j.bmcl.2011.11.100 [DOI] [PubMed] [Google Scholar]
- 90. Kitson R. R., Chang C. H., Xiong R., Williams H. E., Davis A. L., Lewis W., Dehn D. L., Siegel D., Roe S. M., Prodromou C., Ross D., and Moody C. J. (2013) Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat. Chem. 5, 307–314 10.1038/nchem.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Taldone T., Zatorska D., Patel H. J., Sun W., Patel M. R., and Chiosis G. (2013) Preparation of a diverse purine-scaffold library via one-step palladium catalyzed cross-coupling. Heterocycles 87, 91–113 10.3987/COM-12-12613 [DOI] [Google Scholar]
- 92. Casale E., Amboldi N., Brasca M. G., Caronni D., Colombo N., Dalvit C., Felder E. R., Fogliatto G., Galvani A., Isacchi A., Polucci P., Riceputi L., Sola F., Visco C., Zuccotto F., and Casuscelli F. (2014) Fragment-based hit discovery and structure-based optimization of aminotriazoloquinazolines as novel Hsp90 inhibitors. Bioorg. Med. Chem. 22, 4135–4150 10.1016/j.bmc.2014.05.056 [DOI] [PubMed] [Google Scholar]
- 93. McBride C. M., Levine B., Xia Y., Bellamacina C., Machajewski T., Gao Z., Renhowe P., Antonios-McCrea W., Barsanti P., Brinner K., Costales A., Doughan B., Lin X., Louie A., McKenna M., et al. (2014) Design, structure-activity relationship, and in vivo characterization of the development candidate NVP-HSP990. J. Med. Chem. 57, 9124–9129 10.1021/jm501107q [DOI] [PubMed] [Google Scholar]
- 94. Taldone T., Patel P. D., Patel M., Patel H. J., Evans C. E., Rodina A., Ochiana S., Shah S. K., Uddin M., Gewirth D., and Chiosis G. (2013) Experimental and structural testing module to analyze paralogue-specificity and affinity in the Hsp90 inhibitors series. J. Med. Chem. 56, 6803–6818 10.1021/jm400619b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vilenchik M., Solit D., Basso A., Huezo H., Lucas B., He H., Rosen N., Spampinato C., Modrich P., and Chiosis G. (2004) Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem. Biol. 11, 787–797 10.1016/j.chembiol.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 96. Zong H., Gozman A., Caldas-Lopes E., Taldone T., Sturgill E., Brennan S., Ochiana S. O., Gomes-DaGama E. M., Sen S., Rodina A., Koren J. 3rd., Becker M. W., Rudin C. M., Melnick A., Levine R. L., et al. (2015) A hyperactive signalosome in acute myeloid leukemia drives addiction to a tumor-specific Hsp90 species. Cell Rep. 13, 2159–2173 10.1016/j.celrep.2015.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bruce N. J., Ganotra G. K., Kokh D. B., Sadiq S. K., and Wade R. C. (2018) New approaches for computing ligand-receptor binding kinetics. Curr. Opin. Struct. Biol. 49, 1–10 10.1016/j.sbi.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 98. Cusack K. P., Wang Y., Hoemann M. Z., Marjanovic J., Heym R. G., and Vasudevan A. (2015) Design strategies to address kinetics of drug binding and residence time. Bioorg. Med. Chem. Lett. 25, 2019–2027 10.1016/j.bmcl.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 99. de Witte W. E. A., Danhof M., van der Graaf P. H., and de Lange E. C. M. (2016) In vivo target residence time and kinetic selectivity: the association rate constant as determinant. Trends Pharmacol. Sci. 37, 831–842 10.1016/j.tips.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 100. Folmer R. H. A. (2018) Drug target residence time: a misleading concept. Drug Discov. Today 23, 12–16 10.1016/j.drudis.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 101. Hothersall J. D., Brown A. J., Dale I., and Rawlins P. (2016) Can residence time offer a useful strategy to target agonist drugs for sustained GPCR responses? Drug Discov. Today 21, 90–96 10.1016/j.drudis.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 102. Tautermann C. S. (2016) Impact, determination and prediction of drug-receptor residence times for GPCRs. Curr. Opin. Pharmacol. 30, 22–26 10.1016/j.coph.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 103. Wu Z., Gholami A. M., and Kuster B. (2012) Systematic identification of the HSP90 candidate regulated proteome. Mol. Cell. Proteomics 11, M111.016675 10.1074/mcp.M111.016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kourtis N., Lazaris C., Hockemeyer K., Balandrán J. C., Jimenez A. R., Mullenders J., Gong Y., Trimarchi T., Bhatt K., Hu H., Shrestha L., Ambesi-Impiombato A., Kelliher M., Paietta E., Chiosis G., et al. (2018) Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat. Med. 24, 1157–1166 10.1038/s41591-018-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pickrell A. M., and Youle R. J. (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Taldone T., Zatorska D., Ochiana S. O., Smith-Jones P., Koziorowski J., Dunphy M. P., Zanzonico P., Bolaender A., Lewis J. S., Larson S. M., Chiosis G., and Pillarsetty N. V. (2016) Radiosynthesis of the iodine-124 labeled Hsp90 inhibitor PU-H71. J. Labelled Comp. Radiopharm. 59, 129–132 10.1002/jlcr.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kanamaru C., Yamada Y., Hayashi S., Matsushita T., Suda A., Nagayasu M., Kimura K., and Chiba S. (2014) Retinal toxicity induced by small-molecule Hsp90 inhibitors in beagle dogs. J. Toxicol. Sci. 39, 59–69 10.2131/jts.39.59 [DOI] [PubMed] [Google Scholar]
- 108. Zhou D., Liu Y., Ye J., Ying W., Ogawa L. S., Inoue T., Tatsuta N., Wada Y., Koya K., Huang Q., Bates R. C., and Sonderfan A. J. (2013) A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol. Appl. Pharmacol. 273, 401–409 10.1016/j.taap.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 109. Ferraro M., D'Annessa I., Moroni E., Morra G., Paladino A., Rinaldi S., Compostella F., and Colombo G. (2018) Allosteric modulators of HSP90 and HSP70: dynamics meets function through structure-based drug design. J. Med. Chem. 10.1021/acs.jmedchem.8b00825 [DOI] [PubMed] [Google Scholar]
- 110. Zuehlke A. D., Moses M. A., and Neckers L. (2018) Heat shock protein 90: its inhibition and function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160527 10.1098/rstb.2016.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chatterjee S., and Burns T. F. (2017) Targeting heat shock proteins in cancer: a promising therapeutic approach. Int. J. Mol. Sci. 18, E1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li X., Shao H., Taylor I. R., and Gestwicki J. E. (2016) Targeting allosteric control mechanisms in heat shock protein 70 (Hsp70). Curr. Top. Med. Chem. 16, 2729–2740 10.2174/1568026616666160413140911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gewirth D. T. (2016) Paralog specific Hsp90 inhibitors–a brief history and a bright future. Curr. Top. Med. Chem. 16, 2779–2791 10.2174/1568026616666160413141154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Woodford M. R., Dunn D., Miller J. B., Jamal S., Neckers L., and Mollapour M. (2016) Impact of posttranslational modifications on the anticancer activity of Hsp90 inhibitors. Adv. Cancer Res. 129, 31–50 10.1016/bs.acr.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 115. Kumar S., Stokes J. 3rd, Singh U. P., Scissum Gunn K., Acharya A., Manne U., and Mishra M. (2016) Targeting Hsp70: a possible therapy for cancer. Cancer Lett. 374, 156–166 10.1016/j.canlet.2016.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Khandelwal A., Crowley V. M., and Blagg B. S. J. (2016) Natural product inspired N-terminal Hsp90 inhibitors: from bench to bedside? Med. Res. Rev. 36, 92–118 10.1002/med.21351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bhat R., Tummalapalli S. R., and Rotella D. P. (2014) Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem. 57, 8718–8728 10.1021/jm500823a [DOI] [PubMed] [Google Scholar]
- 118. Taldone T., Gomes-DaGama E. M., Zong H., Sen S., Alpaugh M. L., Zatorska D., Alonso-Sabadell R., Guzman M. L., and Chiosis G. (2011) Synthesis of purine-scaffold fluorescent probes for heat shock protein 90 with use in flow cytometry and fluorescence microscopy. Bioorg. Med. Chem. Lett. 21, 5347–5352 10.1016/j.bmcl.2011.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rodina A., Taldone T., Kang Y., Patel P. D., Koren J. 3rd., Yan P., DaGama Gomes E. M., Yang C., Patel M. R., Shrestha L., Ochiana S. O., Santarossa C., Maharaj R., Gozman A., Cox M. B., et al. (2014) Affinity purification probes of potential use to investigate the endogenous Hsp70 interactome in cancer. ACS Chem. Biol. 9, 1698–1705 10.1021/cb500256u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Taldone T., Zatorska D., Patel P. D., Zong H., Rodina A., Ahn J. H., Moulick K., Guzman M. L., and Chiosis G. (2011) Design, synthesis, and evaluation of small molecule Hsp90 probes. Bioorg. Med. Chem. 19, 2603–2614 10.1016/j.bmc.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Taldone T., Rodina A., DaGama Gomes E. M., Riolo M., Patel H. J., Alonso-Sabadell R., Zatorska D., Patel M. R., Kishinevsky S., and Chiosis G. (2013) Synthesis and evaluation of cell-permeable biotinylated PU-H71 derivatives as tumor Hsp90 probes. Beilstein J. Org. Chem. 9, 544–556 10.3762/bjoc.9.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Patel H. J., Patel P. D., Ochiana S. O., Yan P., Sun W., Patel M. R., Shah S. K., Tramentozzi E., Brooks J., Bolaender A., Shrestha L., Stephani R., Finotti P., Leifer C., Li Z., et al. (2015) Structure–activity relationship in a purine-scaffold compound series with selectivity for the endoplasmic reticulum Hsp90 paralog Grp94. J. Med. Chem. 58, 3922–3943 10.1021/acs.jmedchem.5b00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Corben A. D., Uddin M. M., Crawford B., Farooq M., Modi S., Gerecitano J., Chiosis G., and Alpaugh M. L. (2014) Ex vivo treatment response of primary tumors and/or associated metastases for preclinical and clinical development of therapeutics. J. Vis. Exp. 2014, e52157 10.3791/52157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Taldone T., Kang Y., Patel H. J., Patel M. R., Patel P. D., Rodina A., Patel Y., Gozman A., Maharaj R., Clement C. C., Lu A., Young J. C., and Chiosis G. (2014) Heat shock protein 70 inhibitors. 2. 2,5′-Thiodipyrimidines, 5-(phenylthio)pyrimidines, 2-(pyridin-3-ylthio)pyrimidines, and 3-(phenylthio)pyridines as reversible binders to an allosteric site on heat shock protein 70. J. Med. Chem. 57, 1208–1224 10.1021/jm401552y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kang Y., Taldone T., Patel H. J., Patel P. D., Rodina A., Gozman A., Maharaj R., Clement C. C., Patel M. R., Brodsky J. L., Young J. C., and Chiosis G. (2014) Heat shock protein 70 inhibitors. 1. 2,5′-Thiodipyrimidine and 5-(phenylthio)pyrimidine acrylamides as irreversible binders to an allosteric site on heat shock protein 70. J. Med. Chem. 57, 1188–1207 10.1021/jm401551n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rodina A., Patel P. D., Kang Y., Patel Y., Baaklini I., Wong M. J., Taldone T., Yan P., Yang C., Maharaj R., Gozman A., Patel M. R., Patel H. J., Chirico W., Erdjument-Bromage H., et al. (2013) Identification of an allosteric pocket on human hsp70 reveals a mode of inhibition of this therapeutically important protein. Chem. Biol. 20, 1469–1480 10.1016/j.chembiol.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. He H., Zatorska D., Kim J., Aguirre J., Llauger L., She Y., Wu N., Immormino R. M., Gewirth D. T., and Chiosis G. (2006) Identification of potent water-soluble purine-scaffold inhibitors of the heat shock protein 90. J. Med. Chem. 49, 381–390 10.1021/jm0508078 [DOI] [PubMed] [Google Scholar]
- 128. Goldstein R. L., Yang S. N., Taldone T., Chang B., Gerecitano J., Elenitoba-Johnson K., Shaknovich R., Tam W., Leonard J. P., Chiosis G., Cerchietti L., and Melnick A. (2015) Pharmacoproteomics identifies combinatorial therapy targets for diffuse large B cell lymphoma. J. Clin. Invest. 125, 4559–4571 10.1172/JCI80714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kucine N., Marubayashi S., Bhagwat N., Papalexi E., Koppikar P., Sanchez Martin M., Dong L., Tallman M. S., Paietta E., Wang K., He J., Lipson D., Stephens P., Miller V., Rowe J. M., et al. (2015) Tumor-specific HSP90 inhibition as a therapeutic approach in JAK-mutant acute lymphoblastic leukemias. Blood 126, 2479–2483 10.1182/blood-2015-03-635821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bhagwat N., Koppikar P., Keller M., Marubayashi S., Shank K., Rampal R., Qi J., Kleppe M., Patel H. J., Shah S. K., Taldone T., Bradner J. E., Chiosis G., and Levine R. L. (2014) Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood 123, 2075–2083 10.1182/blood-2014-01-547760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ojala P. M. (2013) Naughty chaperone as a target for viral cancer. Blood 122, 2767–2768 10.1182/blood-2013-08-522425 [DOI] [PubMed] [Google Scholar]
- 132. Nayar U., Lu P., Goldstein R. L., Vider J., Ballon G., Rodina A., Taldone T., Erdjument-Bromage H., Chomet M., Blasberg R., Melnick A., Cerchietti L., Chiosis G., Wang Y. L., and Cesarman E. (2013) Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood 122, 2837–2847 10.1182/blood-2013-01-479972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Darby J. F., and Workman P. (2011) Chemical biology: many faces of a cancer-supporting protein. Nature 478, 334–335 10.1038/478334b [DOI] [PubMed] [Google Scholar]
- 134. Speranza G., Anderson L., Chen A. P., Do K., Eugeni M., Weil M., Rubinstein L., Majerova E., Collins J., Horneffer Y., Juwara L., Zlott J., Bishop R., Conley B. A., Streicher H., et al. (2018) First-in-human study of the epichaperome inhibitor PU-H71: clinical results and metabolic profile. Invest. New Drugs 36, 230–239 10.1007/s10637-017-0495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Padhan N., Yan J., Boge A., Scrivener E., Birgisson H., Zieba A., Gullberg M., Kamali-Moghaddam M., Claesson-Welsh L., and Landegren U. (2017) Highly sensitive and specific protein detection via combined capillary isoelectric focusing and proximity ligation. Sci. Rep. 7, 1490 10.1038/s41598-017-01516-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Calderwood S. K. (2013) Molecular co-chaperones: tumor growth and cancer treatment. Scientifica (Cairo) 2013, 217513 10.1155/2013/217513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Carman A., Kishinevsky S., Koren J. 3rd., Lou W., and Chiosis G. (2013) Chaperone-dependent neurodegeneration: a molecular perspective on therapeutic intervention. J. Alzheimers Dis. Parkinsonism 2013, 007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lindberg I., Shorter J., Wiseman R. L., Chiti F., Dickey C. A., and McLean P. J. (2015) Chaperones in neurodegeneration. J. Neurosci. 35, 13853–13859 10.1523/JNEUROSCI.2600-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tonge P. J. (2018) Drug-target kinetics in drug discovery. ACS Chem. Neurosci. 9, 29–39 10.1021/acschemneuro.7b00185 [DOI] [PMC free article] [PubMed] [Google Scholar]