Abstract
Laryngeal squamous cell carcinoma (LSCC) is a common form of head and neck cancer with poor prognosis. However, the mechanism underlying the pathogenesis of LSCC remains unclear. Here, we demonstrated increased expression of fascin actin-bundling protein 1 (FSCN1) and decreased expression of microRNA-145-5p (miR-145-5p) in a clinical cohort of LSCC. Luciferase assay revealed that miR-145-5p is a negative regulator of FSCN1. Importantly, low miR-145-5p expression was correlated with TNM (tumor, node, metastasis) status and metastasis. Moreover, cases with low miR-145-5p/high FSCN1 expression showed poor prognosis, and these characteristics together served as independent prognostic indicators of survival. Gain- and loss-of-function studies showed that miR-145-5p overexpression or FSCN1 knockdown inhibited LSCC migration, invasion, and growth by suppressing the epithelial-mesenchymal transition along with inducing cell-cycle arrest and apoptosis. Additionally, hypermethylation of the miR-145-5p promoter suggested that repression of miR-145-5p arises through epigenetic inactivation. LSCC tumor growth in vivo could be inhibited by using miR-145-5p agomir or FSCN1 small interfering RNA (siRNA), which highlights the potential for clinical translation. Collectively, our findings indicate that miR-145-5p plays critical roles in inhibiting the progression of LSCC by suppressing FSCN1. Both miR-145-5p and FSCN1 are important potential prognostic markers and therapeutic targets for LSCC.
Keywords: laryngeal squamous cell carcinoma, miR-145-5p, epithelial-mesenchymal transition, FSCN1, promoter methylation
Graphical Abstract
Gao et al. demonstrate that miR-145-5p expression is prognostic for LSCC with low expression associated with poorer outcomes. Underlying this pathology, miR-145-5p suppresses FSCN1 expression, but its inactivation facilitates epithelial-to-mesenchymal transition (EMT), proliferation, and survival. LSCC growth in vivo is inhibited by miR-145-5p agomir, highlighting potential as a biomarker and therapeutic target.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide.1 Laryngeal squamous cell carcinoma (LSCC) has the second highest incidence among these cancers and is especially prevalent in the northern areas of China.2 The limitations of throat anatomy and the richness of submucous lymph readily facilitate local invasion and neck lymphatic metastasis in LSCC and are important risk factors for recurrence and poor prognosis after treatment. Despite therapeutic advances for many cancer types, the outcomes for most patients with advanced LSCC have not improved significantly in the last 30 years.3, 4 Consequently, understanding the molecular mechanisms of the proliferation, invasion, and metastasis of LSCC is essential for its diagnosis, treatment, and prognosis.
An actin-filament bundling oncogene, fascin actin-bundling protein 1 (FSCN1), has been studied in human cancers.5, 6, 7 We previously reported that FSCN1 was frequently upregulated in LSCC tissues as compared with adjacent normal margin (ANM) tissue. Moreover, upregulation of FSCN1 was associated with poor prognosis of LSCC.2 Therefore, understanding the tumor-specific regulation of FSCN1 is crucial and has potential clinical significance.
MicroRNAs (miRNAs) regulate the expression of protein-coding genes at the post-transcription level, and almost 60% of human genes are thought to be regulated by miRNAs.8 Dysregulated miRNAs are associated with the malignant progression of LSCC. The functions of miRNAs in LSCC have been revealed. The expression of miR-155 is upregulated in LSCC, and miR-155 overexpression promotes the proliferation and invasion of LSCC by targeting suppressor of cytokine signaling 1 and signal transducer and activator of transcription 3.9 miR-27a targets polo-like kinase 2 to promote proliferation and suppresses apoptosis in LSCC cells.10 Conversely, miR-1 overexpression suppresses the migration and invasion of Hep-2 LSCC cells.11
Given the critical oncogenic role of FSCN1 in LSCC, we sought to test whether FSCN1 expression is regulated by specific miRNAs, with the hypothesis that the regulatory miRNAs could also be crucial for LSCC pathogenesis. We found that miR-145-5p is a negative regulator of FSCN1 expression in LSCC, and the expression of miR-145-5p was significantly lower in LSCC with poor prognosis. miR-145-5p and FSCN1 levels affected the phenotypes of LSCC cells, including migration, invasion, colony formation, proliferation, cell-cycle, apoptosis, and tumorigenesis both in vitro and in vivo. Moreover, miR-145-5p overexpression or FSCN1 knockdown inhibited the epithelial-to-mesenchymal transitions (EMT) and impaired cytoskeleton organization. Furthermore, miR-145-5p downregulation was attributed to hypermethylation of its promoter.
Results
miR-145-5p Acts as a Negative Regulator of FSCN1 in LSCC
Our microarray data showed that FSCN1 was upregulated in LSCC tissues as compared with ANM tissue (Figure 1A), and we previously reported that differentially high expression of FSCN1 was associated with poor prognosis in LSCC.2 Furthermore, a recent genome-wide analysis of cancer transcriptomes highlighted that FSCN1 expression was prognostic in HNSCC.12 Interrogation of The Cancer Genome Atlas (TCGA) data showed that FSCN1 was not frequently amplified in LSCC (Figure S1A), and we hypothesized that other regulatory mechanisms were involved, particularly that altered expression of miRNAs and affected FSCN1 expression in HNSCC. To address this concept, we turned to our prior analysis of miRNA expression profiles in LSCC determined by genome-wide miRNA microarray assay.13, 14
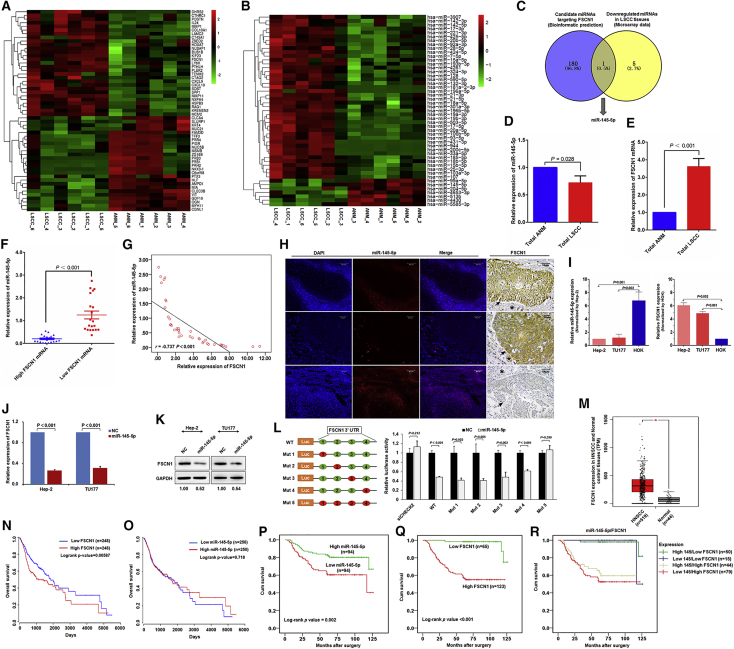
Figure 1.
miR-145-5p Is a Negative Regulator of FSCN1 in LSCC and Is Associated with Prognosis of LSCC Patients
Heatmap of (A) mRNAs and (B) miRNAs most increased and decreased in expression in six LSCC tissues as compared with six matched non-tumor tissues analyzed by Arraystar Chip. (C) Screening of miRNA target FSCN1 in LSCC. Intersection of bioinformatics-predicted miRNAs targeting FSCN1 (181) and miRNAs with decreased expression in LSCC miRNA profiling (6) showing miR-145-5p as a potential negative regulator of FSCN1 in LSCC. (D) miR-145-5p was downregulated in LSCC. qPCR of miR-145-5p level in 40 pairs of fresh LSCC tissue and corresponding ANM tissue. (E) FSCN1 was upregulated in LSCC. qPCR of FSCN1 level in 40 pairs of fresh LSCC tissue and corresponding ANM tissue. (F) Relative miR-145-5p expression in LSCC tissue with high and low FSCN1 expression. (G) Pearson correlation analysis of miR-145-5p and FSCN1 mRNA levels in LSCC tissue. (H) Expression of miR-145-5p and FSCN1 in LSCC tissue determined by fluorescence in situ hybridization and immunohistochemical staining. In immunohistochemical staining, black arrows indicate tumor areas, and black stars indicate interstitial tissues. (I) qPCR analysis of RNA levels of miR-145-5p and FSCN1 in LSCC cells and human oral keratinocyte HOK cells. (J) qPCR analysis of FSCN1 expression in miR-145-5p mimic-transfected Hep-2 and TU177 cells. (K) FSCN1 protein level in miR-145-5p-transfected Hep-2 and TU177 cells examined by western blot analysis. (L) miR-145-5p binds to the 3′ UTR of FSCN1 to inhibit its expression. HEK293T cells were co-transfected with luciferase reporter constructs containing wild-type (WT) or mutated FSCN1 3′ UTRs and miR-145-5p mimic, and relative luciferase activity was measured and normalized to negative control (NC) mimic-transfected cells. Red circle indicates mutation of miR-145-5p binding site. siCHECK2 indicates cells transfected with the empty vector psiCHECK-2, which does not contain the 3′ UTR region of FSCN1. (M) Differential expression analysis of FSCN1 mRNA of head and neck squamous cell carcinoma (HNSCC) patients in the TCGA cohort (tumor group, 519; normal group, 44; *p < 0.01). (N and O) Kaplan-Meier survival curves of HNSCC patients with different FSCN1 (N) and miR-145-5p (O) level in the TCGA cohort via Oncolnc (http://www.oncolnc.org). Upregulated FSCN1 was significantly correlated with poor outcome for patients with HNSCC (log rank test, p = 0.00587), and miR-145-5p level was positively correlated with survival time of HNSCC (log rank test, p = 0.718). (P–R) Kaplan-Meier survival analysis of 188 LSCC patients by expression of miR-145-5p (P), FSCN1 (Q), and a combination of miR-145-5p and FSCN1 (R). p value was determined by log rank test. Error bars represent SD of the indicated clinical samples or three independent assays.
To determine potential miRNA regulators of FSCN1, we performed bioinformatics analysis with miRwalk-2.15 The miRNAs hit by miRwalk-2 were intersected with miRNAs downregulated in LSCC microarray data (Figure 1B; Tables S1 and S2). Notably, miR-145-5p was the only miRNA that was derived in common with the two approaches (Figure 1C). Therefore, we focused on miR-145-5p as a putative regulator of FSCN1 in LSCC.
Next, we compared miR-145-5p and FSCN1 RNA expression across 40 paired samples of fresh LSCC and ANM tissue. For all cases, miR-145-5p was significantly downregulated in LSCC tissue as compared with ANM tissue, and FSCN1 was differentially upregulated (Figures 1D and 1E). As compared with LSCC cases with low FSCN1 level, LSCC cases with high FSCN1 level showed low miR-145-5p level (Figure 1F), with Pearson correlation analyses revealing a clear inverse relation between miR-145-5p expression and FSCN1 mRNA level (r = −0.737; p < 0.001) (Figure 1G). In support, in situ hybridization experiments illustrated that LSCC lesions with high FSCN1 level showed low miR-145-5p level (Figure 1H). In contrast, miR-145-5p expression was readily observed in surrounding cells and ANM tissues (Figure 1H; Figure S1B).
To better understand the association between miR-145-5p and FSCN1, we used cell models of LSCC (Hep-2 and TU177) along with normal HOK cells for comparison. Notably, qRT-PCR-based analysis demonstrated relatively low expression of miR-145-5p in Hep-2 and TU177 cells, and high level of FSCN1 as compared with HOK cells showing the reverse pattern (Figure 1I). Moreover, manipulating miR-145-5p levels with a mimic sharply decreased FSCN1 mRNA and protein levels in both Hep-2 and TU177 cells (Figures 1J and 1K). Together these findings indicate that FSCN1 expression in LSCC depended on miR-145-5p level, but the underlying basis of this relation remained undefined.
We then sought to determine whether FSCN1 mRNA was directly regulated by miR-145-5p binding. Indeed, bioinformatics analysis identified that the 3′ UTR region of FSCN1 contains four putative seed regions with complementarity to miR-145-5p binding (Figure S1C). To verify that miR-145-5p targets FSCN1 via 3′ UTR-mediated regulation, we conducted experiments with HEK293T cells transfected with FSCN1 3′ UTR luciferase reporter constructs. Notably, co-introduction of a miR-145-5p mimic, but not a control, significantly depleted the reporter activity of wild-type FSCN1 3′ UTR construct binding (Figure 1L). Similar results were obtained when each of the four miR-145-5p binding sites in the FSCN1 3′ UTR construct were individually mutated (Figure 1L). Only mutation of all four miR-145-5p binding sites abrogated the ability of miR-145-5p mimics to inhibit reporter activity (Figure 1L), which suggests that all seed regions participate in FSCN1 regulation. Overall, these findings support a mechanism whereby miR-145-5p targets FSCN1 directly, and downregulation of miR-145-5p results in FSCN1 upregulation in LSCC.
Expression of miR-145-5p and FSCN1 Is Associated with Clinical Features and Prognosis in LSCC
To evaluate the clinical and prognostic significance of miR-145-5p expression and FSCN1 protein level in LSCC, we examined their expression in 188 formalin-fixed paraffin-embedded (FFPE) tissue samples (Table S3) by using qRT-PCR and immunohistochemistry. miR-145-5p level was significantly lower in samples from LSCC patients with distant metastases (p = 0.024). On multivariate analysis, the expression of miR-145-5p in LSCC tissue was negatively associated with T staging (p < 0.001), N status (p = 0.018), and clinical stage (p = 0.004; Table 1). Conversely, high FSCN1 protein level in LSCC was positively associated with T staging (p = 0.007), N status (p = 0.005), and clinical stage (p = 0.007; Table S4). Moreover, miR-145-5p expression and FSCN1 protein level were inversely related (p < 0.001; Table 1).
Table 1.
Association between Expression of miR-145-5p/FSCN1 and Clinical Features of LSCC Patients
| miR-145-5p Expression |
FSCN1 Protein Expression |
||||
|---|---|---|---|---|---|
| Parameters | Cases (n) | Average Rank | p Valuea | Average Rank | p Valuea |
| Age (Years) | |||||
| ≤60 | 99 | 95.92 | 0.662 |
88.07 | 0.038 |
| >60 | 89 | 92.92 | 101.65 | ||
| Sex | |||||
| Female | 21 | 87.79 | 0.488 |
100.14 | 0.540 |
| Male | 167 | 95.34 | 93.79 | ||
| Primary Cancer Site | |||||
| Glottic | 101 | 102.41 | 0.043 |
81.40 | <0.001 |
| Supraglottic | 83 | 84.87 | 108.88 | ||
| Subglottic | 4 | 94.50 | 127.00 | ||
| Differentiation | |||||
| High | 72 | 112.78 | <0.001 |
68.25 | <0.001 |
| Medium or low | 116 | 83.16 | 110.79 | ||
| T Staging | |||||
| T1+T2 | 111 | 105.93 | <0.001 |
87.20 | 0.007 |
| T3+T4 | 77 | 78.02 | 105.03 | ||
| Cervical Lymph Node Metastasis | |||||
| N0 | 142 | 99.13 | 0.018 |
89.27 | 0.005 |
| N+ | 46 | 80.20 | 110.65 | ||
| Distant Metastasis | |||||
| M0 | 183 | 95.78 | 0.024 |
93.61 | 0.100 |
| M1 | 5 | 47.50 | 127.00 | ||
| Clinical Stage | |||||
| I+II | 96 | 104.29 | 0.004 |
85.88 | 0.007 |
| III+IV | 92 | 84.28 | 103.50 | ||
| Smoke Preoperatively | |||||
| No | 75 | 100.14 | 0.181 |
84.39 | 0.012 |
| Yes | 113 | 90.76 | 101.21 | ||
| FSCN1 Protein Expression | |||||
| Low | 65 | 119.81 | <0.001 | – | – |
| High | 123 | 81.13 | – | ||
Mann-Whitney U test used for two-group analysis. Kruskal-Wallis H test used for three-group analysis.
To examine FSCN1 expression across HNSCC and normal tissues at the mRNA level, we queried TCGA RNA-sequencing (RNA-seq) data. FSCN1 mRNA level was markedly higher in HNSCC than normal tissues (Figure 1M). Notably, Kaplan-Meier analysis of TCGA cohorts revealed that upregulated FSCN1 was significantly associated with poor outcome with HNSCC (Figure 1N). In contrast, HNSCC patients with upregulated miR-145-5p exhibited longer survival time (Figure 1O).
Our analysis revealed better overall survival (OS) in LSCC patients with comparatively high than low level of miR-145-5p (106.09 ± 4.12 versus 85.01 ± 5.24 months; Figure 1P) and poorer OS with high than low level of FSCN1 (79.74 ± 4.43 versus 122.83 ± 1.77 months; Figure 1Q). Subdividing patients into four groups based on miR-145-5p expression and FSCN1 protein level provided an opportunity to further delineate outcomes associated with biological subtype. As expected, LSCC patients with low miR-145-5p/high FSCN1 levels showed low OS, whereas those with high miR-145-5p/low FSCN1 levels showed the best OS (76.98 ± 5.59 versus 123.16 ± 2.03; p < 0.001; Figure 1R). Furthermore, on regression analysis, the combination of low miR-145-5p expression and high FSCN1 protein level was an independent prognostic indicator for LSCC survival (relative risk = 12.69; 95% confidence interval [CI] 2.83–56.91; p = 0.001; Table S4). Nevertheless, cases with high miR-145-5p/high FSCN1 levels also partitioned with the poor-performing low miR-145-5p/high FSCN1 level cases (Figure 1R). Indeed, FSCN1 level proved to be an independent predictor of LSCC survival (relative risk = 12.27; 95% CI 3.49–43.19; p = 0.001; Table S5), although in contrast, miR-145-5p expression alone was not significant in this analysis.
Collectively, these data support the existence of a high-risk biological subtype of LSCC exhibiting low miR-145-5p/high FSCN1 levels, but further propose that high FSCN1 protein level itself is an independent prognostic indicator for LSCC. Thus, we propose that miR-145-5p and its target FSCN1 are potential biomarkers for the diagnosis and prognosis of LSCC.
Impact of miR-145-5p and FSCN1 Expression on LSCC Growth and Survival
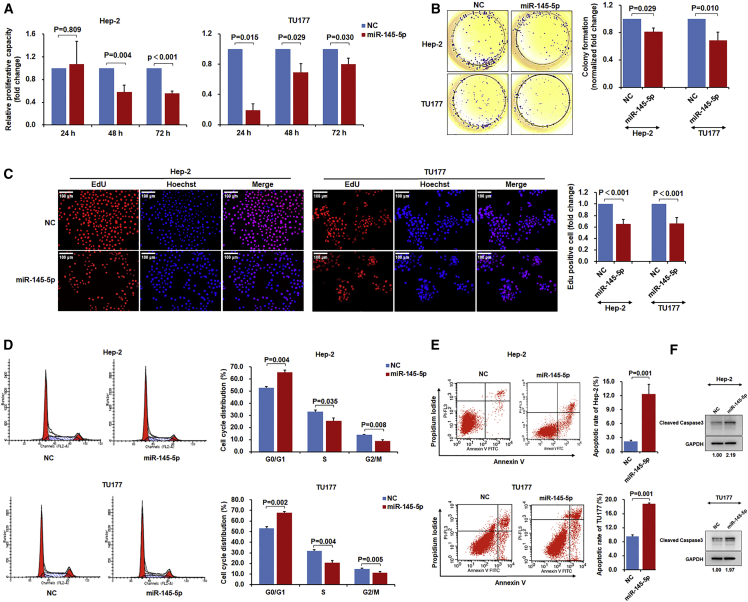
Next, we sought to better understand the functional impact of miR-145-5p and FSCN1 expression on the growth behavior of LSCC cells. First, overexpression of miR-145-5p by using mimics in Hep-2 and TU177 cell lines with low endogenous miR-145-5p expression substantially reduced growth and colony formation (Figures 2A and 2B). Measurement of EdU incorporation established reduced cell proliferation rates (Figure 2C), and cell-cycle analyses established that miR-145-5p overexpression increased the proportion of cells in the G0/G1 stage, with concurrent reduction in the S and G2/M stages (Figure 2D). In addition, the proportion of apoptotic cells increased with the introduction of miR-145-5p mimic (Figure 2E). Cells transfected with miR-145-5p showed increased cleavage of caspase-3 and apoptotic bodies, which was consistent with the induction of apoptosis (Figure 2F; Figure S2A). These data indicate that high miR-145-5p expression in LSCC likely inhibits growth by inducing both G0/G1 phase arrest and apoptosis.
Figure 2.
miR-145-5p Inhibits LSCC Cell Proliferation, Colony Formation, and Induces Cell-Cycle Arrest and Apoptosis
Hep-2 and TU177 cells were transfected with miR-145-5p mimic or negative control (NC) mimic for 48 hr. Cell proliferation was measured by cell counting kit-8 analysis (A), colony formation assay (B), and EdU staining (C); cell cycle (D) and apoptosis (E) were measured by flow cytometry. (F) Hep-2 and TU177 cells were transfected with miR-145-5p mimic or NC mimic for 48 hr. Protein level of cleaved caspase-3 was determined by immunoblotting. Data are mean ± SD of three independent experiments. Error bars represent SD of three independent assays.
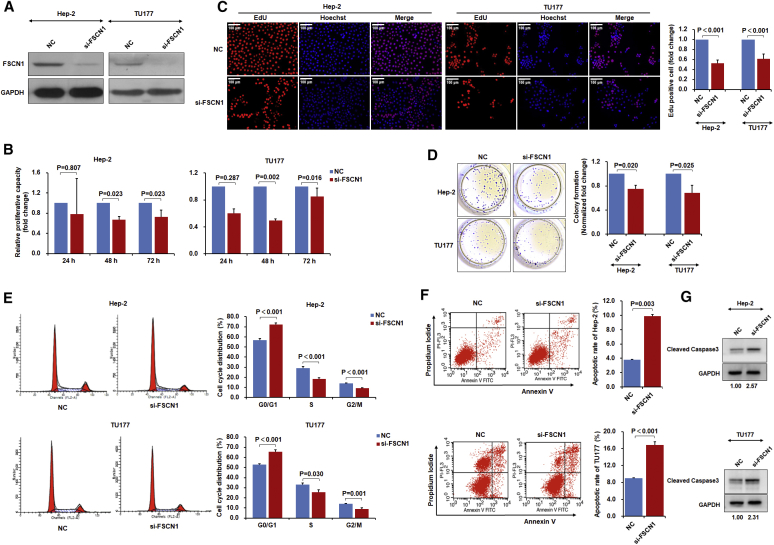
We further conducted similar functional analyses after manipulating FSCN1 levels in LSCC. Both Hep-2 and TU177 cells were depleted of FSCN1 by using an optimized small interfering RNA (siRNA) protocol (Figure S2B); the reduced FSCN1 protein level was confirmed using immunoblotting (Figure 3A). FSCN1 knockdown inhibited both cell proliferation and colony formation of Hep2 and TU177 cells (Figures 3B–3D), thereby largely phenocopying the results obtained with the miR-145-5p mimic. In accordance with these data, FSCN1 knockdown pushed cells toward the G0/G1 phase (Figure 3E) and increased the proportion of cells undergoing apoptosis (Figure 3F). Moreover, immunoblotting results showed that FSCN1 knockdown increased cleavage of caspase-3 (Figure 3G). Thus, these effects of miR-145-5p and FSCN1 in LSCC in vitro reconcile well with the clinical behavior of LSCC tumors in vivo.
Figure 3.
FSCN1 Knockdown Inhibits LSCC Cell Proliferation and Induces Cell-Cycle Arrest and Apoptosis
Hep-2 and TU177 cells were transfected with siRNA targeting FSCN1 (si-FSCN1) or negative control (NC) for 48 hr, and FSCN1 levels were determined by western blot assay (A). Assays of cell proliferation (B), EdU staining (C), colony formation (D), cell cycle (E), and apoptosis (F) after indicated treatments. (G) Hep-2 and TU177 cells were transfected with si-FSCN1 or NC for 48 hr. Protein level of cleaved caspase-3 was determined by immunoblotting. Data are mean ± SD of three independent experiments. Error bars represent SD of three independent assays.
The miR-145-5p/FSCN1 Regulatory Axis Controls the EMT in LSCC
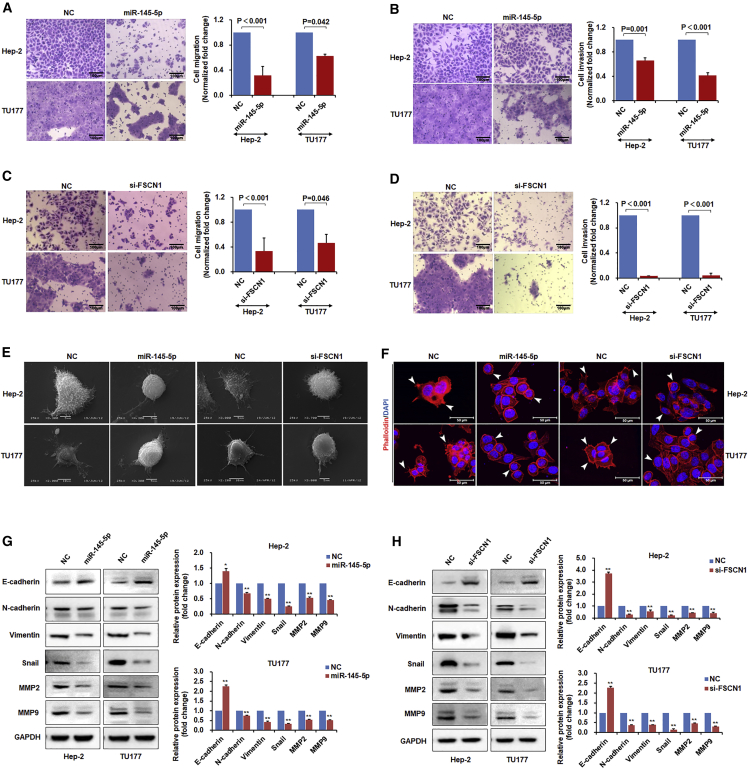
In addition to having effects on cell proliferation and survival, the miR-145-5p mimic appeared to affect the adhesive properties of LSCC cells (Figure S2C). Given the well-known relation between cell adhesion and motility migration, we further investigated this phenomenon. Indeed, overexpression of miR-145-5p with a mimic significantly inhibited cell migration and invasion in Transwell assays as compared with controls (Figures 4A and 4B). Consistent with the mechanistic relation uncovered between miR-145-5p and FSCN1, on repeating these assays with FSCN1 siRNA, Hep-2 and TU177 cells showed decreased migration and invasion (Figures 4C and 4D). Hence our findings propose that FSCN1 is an oncogene in LSCC that promotes cell proliferation and is also involved in promoting migration and invasion.
Figure 4.
miR-145-5p Overexpression or FSCN1 Knockdown Inhibits LSCC Cell Migration, Invasion, and Endothelial-to-Mesenchymal Transition
Hep-2 and TU177 cells were transfected with miR-145-5p mimic or si-FSCN1 for 48 hr. (A and C) Cell migration of miR-145-5p overexpressed (A) and FSCN1 knockdown (C) cells were measured by Transwell assays. (B and D) Cell invasion of miR-145-5p overexpressed (B) and FSCN1 knockdown (D) cells were measured by Transwell assays. (E) Surface structure of cells was observed by scanning electron microscopy. (F) F-actin was stained with rhodamine phalloidin, and nuclei were stained with DAPI. As indicated by white arrows, miR-145-5p mimic or si-FSCN1 treatment decreased cell lamellipodial and filopodial structures as compared with the NC. (G and H) Expression of EMT markers E-cadherin, N-cadherin, Vimentin, Snail, MMP2, and MMP9 in miR-145-5p overexpressed (G) and FSCN1 knockdown (H) cells were determined by western blot assays. Representative images and data from three independent experiments. Data are mean ± SD of three independent experiments. Error bars represent SD of three independent assays. *p < 0.05, **p < 0.01.
A corollary to these findings was the phenotype of LSCC cells observed by scanning electron microscopy. Control cells showed the typical morphology of cultured cancer cells with prominent lamellipodial and filopodial structures, whereas LSCC cells treated with miR-145-5p mimic or si-FSCN1 showed very few lamellipodia and filopodia, for a relatively rounded cell morphology (Figure 4E). Furthermore, impaired F-actin polymerization and filopodium formation were observed after miR-145-5p restoration or FSCN1 knockdown (Figure 4F). Hence, the miR-145-5p/FSCN1 mechanism may be involved in controlling the EMT in LSCC, generally known as a critical determinant of tumor cell metastasis.16
To determine whether the regulation of FSCN1 expression via miR-145-5p and consequent modulation of migration and invasion of LSCC depended on the EMT, we analyzed EMT marker expression. Immunoblotting of Hep-2 and TU177 LSCC cells treated with miR-145-5p mimic or with FSCN1 siRNA revealed increased expression of E-cadherin level along with reduced levels of Vimentin, Snail, N-cadherin, and matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) (Figures 4G and 4H). Hence, miR-145-5p inhibited the EMT, and its target, FSCN1, promoted the EMT in LSCC.
Expression of miR-145-5p Is Regulated by Promoter Hypermethylation in LSCC
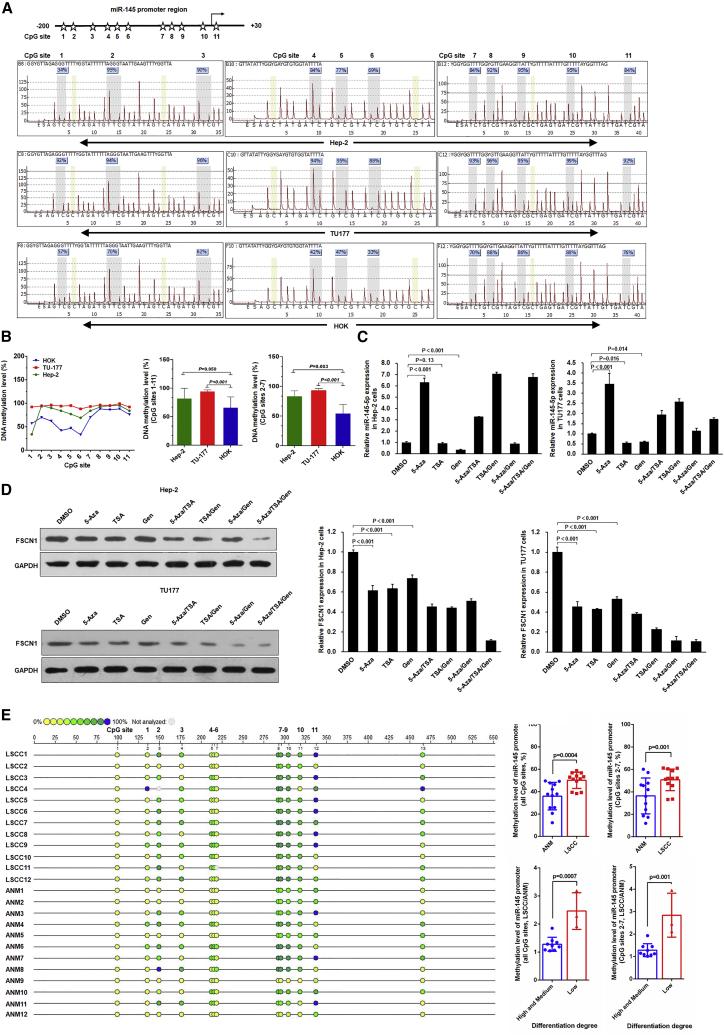
A final question remained as to why downregulation of miR-145-5p occurs in the malignant transition of LSCC cells. To understand this, we analyzed the DNA methylation of the miR-145-5p proximal promoter in LSCC cell lines by using pyrosequencing. As compared with normal control HOK cells, in Hep-2 and TU177 LSCC cells, the CpG sites 2–7 were highly methylated (Figures 5A and 5B), which suggests that promoter hypermethylation underpinned the loss of miR-145-5p expression in LSCC. In support, miR-145-5p expression was significantly increased in Hep-2 and TU177 cells treated with the DNA methyltransferase inhibitor 5-Aza-deoxycytidine (5-Aza) (Figure 5C). In addition, we tested the effect of the histone deacetylase inhibitor trichostatin A (TSA) and chemopreventive agent genistein on miR-145-5p expression. TSA and genistein alone inhibited miR-145-5p expression, whereas their combination increased miR-145-5p expression. Cells treated with 5-Aza combined with TSA and/or genistein showed increased miR-145-5p expression (Figure 5C). Endogenous FSCN1 protein expression exhibited corresponding changes in 5-Aza-treated Hep-2 and TU177 cells (Figure 5D). Notably, genistein treatment reduced both miR-145-5p level and FSCN1 protein level (Figure 5D), which might be due to its different regulatory pathways for miR-145-5p and FSCN1. Moreover, by MassARRAY methylation analysis, we determined the DNA methylation level of miR-145-5p promoter in clinical LSCC tissues separated by laser capture microdissection (LCM) (Figure S3). The methylation level of the miR-145-5p promoter was significantly higher in LSCC tissue than paired ANM tissue (Figure 5E), and the methylation level of the miR-145-5p promoter was inversely associated with the differentiation degree of LSCC (Figure 5E). Together these findings propose that promoter hypermethylation underpins the low expression of miR-145-5p observed in LSCC.
Figure 5.
Hypermethylation of miR-145-5p Promoter Suppresses miR-145-5p Expression in LSCC Cells
(A and B) Pyrosequencing analysis of miR-145-5p promoter methylation status in LSCC cell lines Hep-2 and TU177 and normal control HOK cells. The methylation level in Hep-2 and TU177 cells was normalized to that in HOK cells. Pyrosequencing map (A) and statistical results (B) of methylation levels of Hep-2, TU177 and HOK cells. (C) Hep-2 and TU177 cells were treated with 5-Aza, trichostatin A (TSA), and genistein (Gen) as indicated; the expression of miR-145-5p was determined by qPCR. (D) Hep-2 and TU177 cells were treated with 5-Aza, TSA, and Gen as indicated; FSCN1 protein level was measured by western blot assay. Data are mean ± SD of three independent experiments. (E) Methylation analysis of miR-145-5p in clinical LSCC and paired ANM tissues. DNA purified from LSCC and paired ANM tissues by laser capture microdissection was treated with bisulfite and subjected to MassARRAY methylation analysis. Error bars represent SD of the indicated clinical samples or three independent assays.
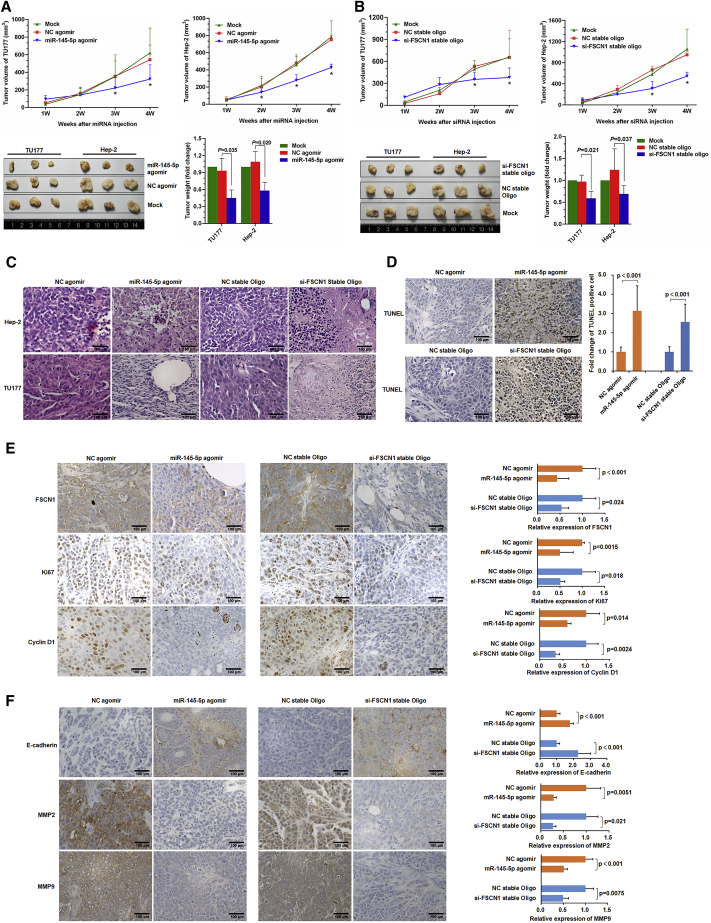
miR-145-5p Suppresses LSCC Tumor Growth In Vivo in a Preclinical Model
To establish the proof of principle that modulating miR-145-5p or FSCN1 expression in LSCC could be an effective therapy, we used a xenograft model. Subcutaneous (s.c.) tumors of Hep-2 or TU177 LSCC cells were established in opposing flanks of nude mice and allowed to grow to 0.5 mm3. Thereafter, miR-145-5p agomir or control (negative control [NC]) agomir was intra-tumorally injected, and tumor growth was monitored over 4 weeks. Compared with controls, injection of miR-145-5p agomir significantly decreased tumor growth rates and tumor size (Figure 6A). Consistent with previous experiments, injecting tumors with FSCN1-targeting oligos also decreased the growth rate and tumor weight of Hep-2 and TU177 tumors (Figure 6B). Moreover, histopathological examination of tissues from miR-145-5p or FSCN1 siRNA-injected tumors showed a higher abundance of shrunken and fragmentized nuclei in sections as compared with control tissues (Figure 6C), which suggests increased rates of apoptosis. This finding was confirmed on TUNEL assay, whereby miR-145-5p overexpression or FSCN1 knockdown increased cell apoptosis as compared with controls (Figure 6D).
Figure 6.
miR-145-5p Overexpression or FSCN1 Knockdown Inhibits LSCC Xenograft Tumor Formation in Mice
Nude mice were subcutaneously injected with Hep-2 and TU177 cells. After tumor formation, miR-145-5p agomir, NC agomir, si-FSCN1 stable oligonucleotides, and corresponding negative oligonucleotides were subcutaneously injected into tumor sites. Tumor volume and weight after injection of miR-145-5p agomir (A) or si-FSCN1 (B) were plotted. Data are mean ± SD. *p < 0.05. (C) Representative H&E staining images of Hep-2 and TU177 xenograft tumors. (D) TUNEL assay of apoptotic cells in xenograft tumors. (E) Representative immunohistochemical staining of FSCN1 and proliferation markers Ki67 and CyclinD1 in Hep-2 and TU177 xenograft tumors. (F) Representative immunohistochemical staining of EMT markers E-cadherin, MMP2, and MMP9 in xenograft tumors. Data are mean ± SD of three independent experiments. Error bars represent SD of three independent experiments.
Further analysis of xenografted LSCC tissues by immunohistochemical staining confirmed that the injection of both miR-145-5p agomir and FSCN1 siRNA decreased FSCN1 protein level (Figure 6E). Moreover, the expression of cyclin-D1 and the proliferation marker Ki67 was decreased in tumors with miR-145-5p agomir and FSCN1 siRNA injection (Figure 6E). Furthermore, as compared with controls, tumors with miR-145-5p agomir or FSCN1 siRNA injection showed upregulated E-cadherin and downregulated MMP2 and MMP9 (Figure 6F). Thus, we established the proof of principle that modulating miR-145-5p expression in vivo could inhibit LSCC tumor growth ostensibly via the same mechanisms revealed in in vitro experiments.
Discussion
The prognostic value of FSCN1 expression has previously emerged across a number of cancer types, with its upregulation known to promote migration and invasion.17, 18 Our previous study involving LSCC found FSCN1 protein overexpression associated with clinical features and poor prognosis.2 However, the regulatory mechanisms of FSCN1 in LSCC remain undefined. In the present study, we found that miR-145-5p functioned as a negative regulator of FSCN1 in LSCC tissue and cells, with decreased miR-145-5p level and accompanying increased FSCN1 level acting as critical determinants of poor prognosis. miR-145-5p and FSCN1 levels may have potential for predicting outcomes with LSCC. Indeed, their applicability as novel biomarkers to identify LSCC patients with distinct clinical features is underscored by our findings relating miR-145-5p expression and FSCN1 protein level with tumor, node, metastasis (TNM) and clinical staging. Moreover, we demonstrated that miR-145-5p has an important regulatory role in LSCC, inhibiting LSCC cell proliferation along with the accompanying cell-cycle arrest and apoptosis. Nevertheless, we also found evidence of a high-risk LSCC group displaying elevated miR-145-5p level with high FSCN1 protein level, likely indicating that non-miR-145-5p mechanism(s) are also responsible for high FSCN1 protein level in some cases.
We initiated our study in response to the clinical impact of FSCN1 expression in LSCC. After identifying miR-145-5p by a bioinformatics approach, we sought to learn more about its role and mechanisms, particularly involving LSCC. We now provide definitive evidence that miR-145-5p robustly targets FSCN1 via a multivalent mechanism involving all four binding sites identified within the 3′ UTR of FSCN1 mRNA. However, although we demonstrated that inhibiting FSCN1 expression in LSCC largely reproduced the experimental findings observed when miR-145-5p expression was restored, we cannot exclude the involvement of other important targets. Indeed, a previous study suggested that miR-145-5p acts in LSCC cells to inhibit stem cell markers SRY-box 2, POU class 5 homeobox 1 (also known as OCT4), Kruppel-like factor 4, and ATP binding cassette subfamily G member 2,19 although the mechanisms need to be further investigated. More generally, these findings expand on the growing evidence of the importance of the miR-145 locus in cancer progression.20, 21, 22, 23
miR-145-5p is found within a cluster of “dual-stranded” miRNAs that also include miR-143-3p, miR-145-3p, and miR-143-5p.24 Recent evidence obtained in colorectal cancer,25 along with prostate cancer,26 has highlighted that miR-145-5p can target FSCN1 with tumor-suppressive effects on cancer cells. Studies have established the role of several other miRNAs in the 143/145 cluster as tumor suppressors. Moreover, miR-143-5p deficiency in gallbladder cancer triggered the EMT and metastasis by targeting HIF-1α in mesothelioma.27 miR-143-3p suppresses the progression of ovarian cancer and esophageal squamous cell carcinoma,28, 29 and miR-145-3p acts as a tumor suppressor in HNSCC by targeting MYO1B.30 Despite some evidence that miRNAs in the 143/145 group may be co-regulated,31 in the context of the current work, we did not evaluate the expression of miRNAs other than miR-145-5p, given our focus on FSCN1. Evaluating the overall impact of each miRNA in the 143/145 cluster on cancer biology is pertinent, as is understanding the hierarchy of their regulatory relationships.
What are the clinical implications of our work? Both in vitro and in vivo experiments demonstrated that miR-145-5p overexpression or FSCN1 knockdown suppressed tumor growth of LSCC via effects on proliferation and cell survival. Notably, using the in vivo models in nude mice, we established that direct injection of miR-145-5p agomir or stable si-FSCN1 oligonucleotides into the tumor significantly retarded growth. This procedure differs from a previous method of directly injecting miRNA-overexpressing cancer cells into nude mice.32 However, our method simulated the process of drug therapy in clinical practice and highlighted the significance of miR-145-5p and FSCN1 as targets of LSCC therapy.
In addition to the effects observed on proliferation and survival, a further biological effect of suppressing miR-145-5p expression in LSCC involved maintaining the cells in a high motility state. Here the re-introduction of miR-145-5p and likewise antagonizing FSCN1 expression inhibited cell migration and invasion with a drastic alteration in cell phenotype. Both miR-145-5p-agomir and si-FSCN1-treated cells lost protrusive structures (lamellipodia, filopodia, and invadopodia) associated with migrating and invading cells. Such structures are formed by spatiotemporal-regulated actin polymerization at the leading edge of the migratory cells, with aberrant regulation leading to malignant progression such as tumor invasion and metastasis.33, 34 Moreover, increased cellular protrusions have been shown to promote invasion and metastasis in oral squamous carcinoma.35 Because FSCN1 is involved in F-actin crosslinking and cytoskeletal organization during cell motility,36, 37, 38 the inhibitory effects on FSCN1 expression may explain how miR-145-5p acts to suppress LSCC migration and invasion.
The EMT plays a crucial role in increased invasion, metastasis, and proliferation for carcinoma. When EMT occurs, the expression of an epithelial marker, such as E-cadherin, is suppressed, whereas that of mesenchymal markers, such as N-cadherin, Vimentin, Snail, MMP2, and MMP9, are enhanced.39 Altering the expression of miR-145-5p and its target, FSCN1, caused EMT reversal in LSCC, as shown by the increased expression of E-cadherin and reduced expression of N-cadherin, Vimentin, Snail, MMP2, and MMP9 both in vitro and in LSCC tumors in vivo. These findings imply that the associated phenotypic and functional changes in LSCC accompanying loss of miR-145-5p are associated with the process of EMT. The EMT of cancer cells accompanies the formation of invasive structures such as lamellipodia and filopodia. These changes depend on the reorganization of actin cytoskeleton, and actin-associated proteins control the polymerization and depolymerization processes to regulate the reorganization of actin cytoskeleton.40, 41 Moreover, recent studies revealed that the actin binding protein Cofilin 1 (CFL1) promotes the EMT process by promoting the formation of invasive structures, as well as by modulating EMT-related gene expression via regulation of actin organization in the nucleus.42 The present results demonstrate that FSCN1 knockdown inhibits invadopodia formation and impairs actin cytoskeleton organization. Therefore, we hypothesized that FSCN1 affects EMT-related gene expression by regulating actin cytoskeleton organization. Further studies are required to elucidate how miR-145-5p/FSCN1 regulates the EMT pathway.
Finally, as observed in some other cancer types,43 we found a differentially lower expression of miR-145-5p in LSCC versus normal tissues. Given the role of miR-145-5p as a tumor suppressor, our study also investigated how its expression is suppressed in LSCC. Promoter-region hypermethylation causing silencing of miR-145-5p was previously observed in cancer,44 and our data similarly support a differentially increased methylation of the miR-145-5p promoter region in LSCC as compared with normal tissues, and high methylation levels of the miR-145-5p promoter indicated low differentiation of LSCC. This observation was functionally evaluated by treating LSCC cells with a variety of epigenetic modifier compounds, with our findings indicating that miR-145-5p expression in LSCC could be readily restored by a number of different treatment approaches. Moreover, consistent with the proposed mechanism (Figure 7), we found largely concurrent reduced expression of FSCN1. However, our findings are not fully concordant with previous data; for example, we found that treatment with TSA plus genistein potently upregulated miR-145-5p expression in LSCC cells, which contrasts with previous data in prostate cancer cells.45 We need to understand the effects of these agents in different cell contexts. Regardless, our findings highlight the potential for these approaches to reverse the methylation of the miR-145-5p promoter region as a potential treatment avenue for LSCC.
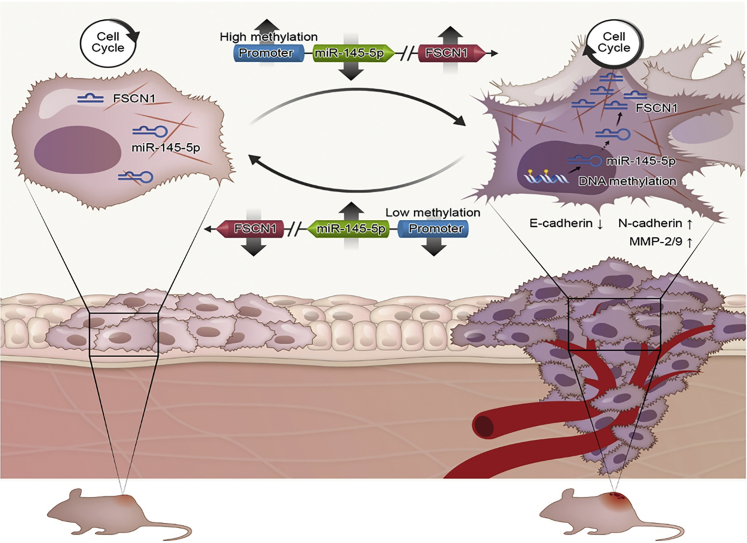
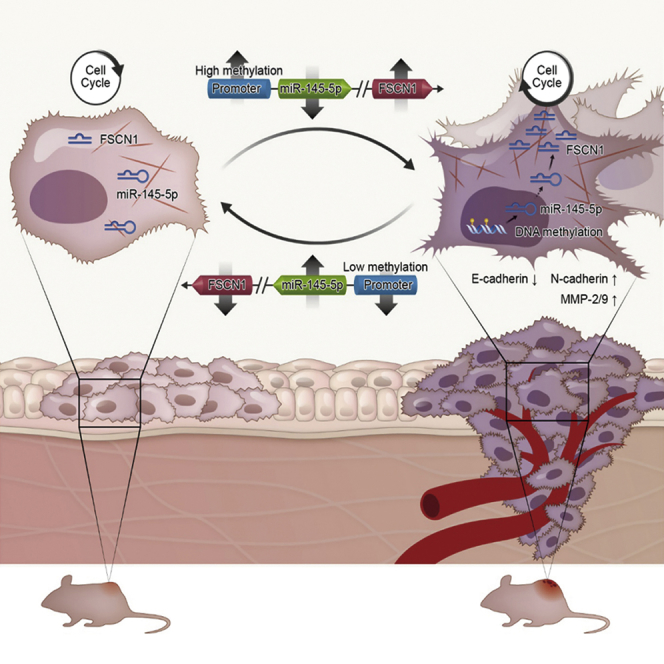
Figure 7.
Proposed Regulatory Model for miR-145-5p/FSCN1 Axis in LSCC Progression
Expression of miR-145-5p was silenced by hypermethylation of its promoter in LSCC. miR-145-5p inhibits malignant phenotypes of LSCC by directly targeting FSCN1 to inhibit the EMT and cytoskeletal organization. Downregulation of miR-145-5p upregulates FSCN1 in LSCC, thus promoting tumorigenesis and progression.
Materials and Methods
Ethical Statement
This study was conducted in accordance with the Helsinki Declaration. All patients signed a written, informed consent before surgery, acknowledging that they understood their rights and obligations. Animal studies were carried out in compliance with our institutional guidelines, and animal experimental procedures were approved by the Animal Care Commission of Experimental Animal Center of Shanxi Medical University. All aspects of the study were approved in full by the Research Ethics Committee at Shanxi Medical University.
Clinical Samples and Patient Population
Forty LSCC tissues and 40 corresponding ANM tissues were obtained from patients undergoing surgery in the Department of Otolaryngology Head & Neck Surgery, The First Hospital Affiliated with Shanxi Medical University. ANM tissues were isolated from surgical specimens at about 1–3 cm from the neoplastic edge. Fresh specimens were frozen by use of liquid nitrogen for qRT-PCR and western blot analysis, and the other part was embedded in paraffin for H&E staining to ensure the diagnosis of LSCC and ANM.
Study Participants
The study involved 188 LSCC patients undergoing surgery in our department from 2000 to 2006. Patients did not receive radiotherapy or chemotherapy before surgery and were without prior associated clinical treatment before diagnosis. The dates of laryngeal cancer surgery and of last follow-up and status at last follow-up (living, lost to follow-up, or deceased) were recorded. Two staff anatomical pathologists provided the diagnosis of laryngeal cancer from paraffin-embedded tissues. Tumor and clinical staging involved the TNM staging system of the American Joint Committee on Cancer (2010). The histological types of LSCC were determined according to the World Health Organization system. Detailed clinical records were available for all participants with follow-up records at three monthly intervals (phone, mail, or outpatient visit) with the final recording in November 2011. Survival time was defined as the time between the date of surgery and patient death or the end of the study period.
Cell Culture and Transfection
The human 293T and LSCC cell line Hep-2 was purchased from the China Center for Type Culture Collection and cultured in DMEM (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (BI, Cromwell, CT, USA). TU177 cells (Bioleaf Biotech, Shanghai) were cultured in MEM supplemented with 10% FBS. Normal human oral mucous-membrane keratinizing basal cells (HOK) were cultured with ScienCell OKM (Cat. No. 2611; ScienCell) and 10% FBS. The micrON Hsa-miR-145-5p mimic (miR10000437-1-5) was from RiboBio (Guangzhou, China) and used in cell transfection experiments. The siRNA and NC were from Genechem (Shanghai). Cells were transfected with Lipofectamine 2000 following the manufacturer’s instructions. FSCN1 siRNA sequences were for si-FSCN1-1: 5′-CGACTATAACAAGGTGGCCAT-3′, si-FSCN1-2: 5′-CAAAGACTCCACAGGCAAA-3′, and si-FSCN1-3: 5′-CAAGTTTGTGACCTCCAAGAA-3′. 5-Aza, TSA, and Genistein were from Sigma-Aldrich (St. Louis, MO, USA) and were dissolved in DMSO. Cells with 50%–60% confluence were treated with concentrations of 5-Aza (10 μM), TSA (100 ng/mL), and Genistein (25 μM); cells treated with DMSO were controls.
DNA Methylation Analysis
For cell sample, DNA was extracted and treated with bisulfite by using the EpiTect Bisulfite Kit (QIAGEN, Germany). An amount of 2 μL of bisulfite-treated DNA was used for PCR to amplify the miR-145 promoter fragment with primers listed in Supplemental Materials and Methods. After purification, PCR products were degenerated for 2 min with the sequencing primer (Supplemental Materials and Methods) at 80°C, followed by pyrosequencing on the PyroMark Q96 instrument (QIAGEN, Germany).
For clinical samples, to precisely separate the tumor and normal tissue frozen sections, we stained frozen sections of LSCC or paired ANM fresh tissue with Histogene staining solution (Thermo Fisher Scientific, Waltham, MA, USA) and microdissected them by using the Leica LMD6500 laser microdissection system (Leica Microsystems CMS, Wetzlar, Germany). DNA was extracted from the microdissected LSCC and ANM tissue fragments. Subsequently, MALDI-TOF-mass spectrometry (MS)-based DNA methylation analysis was performed with MassARRAY Analyzer 4.0 (Agena Bioscience, San Diego, CA, USA) as described previously.46 In brief, the target gene regions were amplified by using bisulfite-modified DNA and the primer pairs described in the Supplemental Materials and Methods. Next, in vitro RNA transcription was performed on the reverse strand, followed by base-specific cleavage. The cleavage products were analyzed by using MALDI-TOF, and a distinct signal pair pattern resulting from the methylated and non-methylated template DNA was obtained and analyzed by using MassARRAY EpiTYPER (Agena Bioscience, San Diego, CA, USA). The clinical features of LSCC samples that underwent MassARRAY methylation analysis are described in Table S6.
Nude Mouse Xenograft Tumor Model
BALB/C nude mice (specific pathogen-free grade, female, 7 weeks old) were obtained from HNJIA (Hunan, China). Mice were maintained in the Medical Experimental Animal Center (Guangzhou, China). Hep-2 or TU177 cells were s.c. injected into the flanks of nude mice to generate xenograft tumors. Agomir is a chemically modified double-strand miRNA mimic, with two phosphorothioates at the 5′ end, four phosphorothioates and one cholesterol group at the 3′ end, and a full-length nucleotide 2′-methoxy added to the antisense strand. Compared with miRNA mimics, agomir exhibits enhanced cellular uptake, stability, and regulatory activity in vivo. Agomir can upregulate corresponding miRNAs by local or systemic injection into animals. Hsa-miR-145-5p agomir and FSCN1 siRNA stable oligonucleotides modified by cholesterol, 2′-OMe, and phosphorothioate were synthesized by GenePharma (Shanghai). When tumors had grown to 0.5 mm3, miR-145-5p agomir and FSCN1 siRNA stable and NC oligonucleotides were s.c. injected into the tumor site as follows: multi-point injection with 0.1 mL oligonucleotides (10 nmol), 2 times/week/position and on the first and fourth day per week. The delivery efficiency and distribution of miR-145-5p agomir or FSCN1 siRNA in tumors were verified (Figure S4). Tumor growth was measured every week. Tumor volume (V) was calculated as V = (L × W2)/2. Four weeks after the first injection, mice were euthanized, and tumors were excised, weighed, and paraffin embedded.
Statistical Analysis
Statistical analysis involved use of SPSS v19.0 (SPSS, Chicago, IL, USA) and R software v3.2.5 (https://www.r-project.org/). Independent Student’s t test was used to compare the differences between two groups. The association between miR-145-5p/FSCN1 expression and clinicopathological variables was assessed by Pearson’s chi-square or Fisher’s exact test. Mann-Whitney test was used for two-group analysis, and Kruskal-Wallis test for three-group analysis. The association between miR-145-5p and FSCN1 RNA expression in clinical samples was assessed by Pearson correlation analysis. OS was defined as the time from surgery date to death from laryngeal carcinoma or date of last contact. Survival was analyzed by the Kaplan-Meier method with a log rank test. Eight possible factors affecting LSCC selected to analyze by Cox proportional-hazards regression included age, T staging, neck lymph node metastasis, differentiation level, clinical stage, smoking preoperatively, miR-145-5p expression, and FSCN1 protein expression. The reported results included relative risk and 95% CIs for all tests. Two-sided p ≤ 0.05 was considered statistically significant.
The following experiments were presented in the Supplemental Materials and Methods: RNA extraction and qRT-PCR analysis, western blot analysis, generation of reporter constructs, luciferase reporter assay, immunohistochemistry staining and analysis, cell proliferation assay, cell adhesion assay, apoptosis analysis, cell-cycle analysis, Transwell migration/invasion assay, microscopy imaging, and staining. Antibody information is shown in the Supplemental Materials and Methods.
Author Contributions
B.W., S.W., Y.W., Y.C., R.F.T., and W.G. conceived the study and participated in the study design; W.G., C.Z., H. Li, W.L., J.S., X.Z., H. Luo, Y.L., Y.S., D.Y., R.Z., Z.L., Y.B., H.G., J.W., M.N., J.H., Z.W., and C.X. developed methods and performed the experiments; C.Z., Q.Z., Y.B., and R.Z. obtained clinical samples and data; W.G., X.Z., H. Luo, J.S., W.L., and Y.W. performed bioinformatics analysis; W.G., C.Z., H. Li, Y.C., and L.Z. analyzed experimental data; W.G., R.F.T., Y.Y.W., S.X.W., and B.Q.W. wrote the manuscript; Y.Z., J.C., and J.L. repeated the experiments. All authors read and approved the final manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81602394, 81402256, and 81572670), China Postdoctoral Science Foundation (grants 2016M591412 and 2017M610174), Natural Science Foundation of Shanxi Province (grants 2014011039-5, 2015021198, and 201601D011087), Shanxi Province Scientific and Technological Achievements Transformation Guidance Foundation (grants 201604D131002 and 201604D132040), Key Technological Innovation Platform Foundation for Head and Neck Cancer Research of Shanxi Province (grant 201605D151003), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (STIP; grants 2016-92 and 2016-93), the Research Project of Shanxi Province Health and Family Planning Commission (grants 201301073, 2014028, 201601037, and 201601038), the Excellent talent science and technology innovation project of Shanxi Province (grants 201605D211029 and 201705D211018), Innovation Fund for Graduate Student of Shanxi Province (grants 2016-119 and 2016-101), Outstanding Youth Development Foundation of The First Hospital Affiliated with Shanxi Medical University (grant YR1601), Youth Foundation of The First Hospital Affiliated with Shanxi Medical University (grant YQ1503), Startup Foundation for Doctors of Shanxi Medical University (grant BS03201624), Startup Foundation for Doctors of Liaoning Province (grant 201601355), and Dalian medical science research project (grant 1711030).
Footnotes
Supplemental Information includes four figures, six tables, and Supplemental Materials and Methods and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.09.018.
Contributor Information
Rick F. Thorne, Email: rickfthorne@gmail.com.
Yongping Cui, Email: cuiyp@sxmu.edu.cn.
Yongyan Wu, Email: wuyongyan@sxent.org.
Shuxin Wen, Email: wsxsx@sxent.org.
Binquan Wang, Email: wbq_xy@sxent.org.
Supplemental Information
Potential miRNA of FSCN1 was predicted with miRwalk-2, the information of potential binding site was shown in the Excel file.
References
- 1.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao W., Zhang C., Feng Y., Chen G., Wen S., Huangfu H., Wang B. Fascin-1, ezrin and paxillin contribute to the malignant progression and are predictors of clinical prognosis in laryngeal squamous cell carcinoma. PLoS ONE. 2012;7:e50710. doi: 10.1371/journal.pone.0050710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H.C., Chen G.G., Vlantis A.C., Tong M.C., van Hasselt C.A. Chemotherapy for laryngeal cancer—an apoptotic approach. Curr. Drug Targets. 2008;9:878–886. doi: 10.2174/138945008785909257. [DOI] [PubMed] [Google Scholar]
- 4.Jenckel F., Knecht R. State of the art in the treatment of laryngeal cancer. Anticancer Res. 2013;33:4701–4710. [PubMed] [Google Scholar]
- 5.Lin C., Zhang S., Wang Y., Wang Y., Nice E., Guo C., Zhang E., Yu L., Li M., Liu C. Functional role of a novel long noncoding RNA TTN-AS1 in esophageal squamous cell carcinoma progression and metastasis. Clin. Cancer Res. 2018;24:486–498. doi: 10.1158/1078-0432.CCR-17-1851. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Lu Y., Zhang C., Huang D., Wu W., Zhang Y., Shen J., Cai Y., Chen W., Yao W. FSCN-1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int. J. Oncol. 2018 doi: 10.3892/ijo.2018.4327. Published online March 20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C.Q., Li Y., Huang B.F., Zhao Y.M., Yuan H., Guo D., Su C.M., Hu G.N., Wang Q., Long T. EGFR conjunct FSCN1 as a novel therapeutic strategy in triple-negative breast cancer. Sci. Rep. 2017;7:15654. doi: 10.1038/s41598-017-15939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X.D., Zhang W., Liang H.J., Ji W.Y. Overexpression of miR -155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS ONE. 2013;8:e56395. doi: 10.1371/journal.pone.0056395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y., Fu S., Qiu G.B., Xu Z.M., Liu N., Zhang X.W., Chen S., Wang Y., Sun K.L., Fu W.N. MicroRNA-27a promotes proliferation and suppresses apoptosis by targeting PLK2 in laryngeal carcinoma. BMC Cancer. 2014;14:678. doi: 10.1186/1471-2407-14-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F., Song G., Liu M., Li X., Tang H. miRNA-1 targets fibronectin1 and suppresses the migration and invasion of the HEp2 laryngeal squamous carcinoma cell line. FEBS Lett. 2011;585:3263–3269. doi: 10.1016/j.febslet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F. A pathology atlas of the human cancer transcriptome. Science. 2017;357 doi: 10.1126/science.aan2507. eaan2507. [DOI] [PubMed] [Google Scholar]
- 13.Gao W., Zhang C., Ma T., Wen S., Fu R., Zhao D., Wu Y., Wang B. Potential biomarkers and their regulatory relationships in laryngeal squamous cell carcinoma with lymph node metastasis revealed by integrating mRNA, microRNA and long non-coding RNA profiles. Int. J. Clin. Exp. Pathol. 2016;9:5103–5116. [Google Scholar]
- 14.Zhang C., Gao W., Wen S., Wu Y., Fu R., Zhao D., Chen X., Wang B. Potential key molecular correlations in laryngeal squamous cell carcinoma revealed by integrated analysis of mRNA, miRNA and lncRNA microarray profiles. Neoplasma. 2016;63:888–900. doi: 10.4149/neo_2016_608. [DOI] [PubMed] [Google Scholar]
- 15.Dweep H., Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat. Methods. 2015;12:697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 16.Pang B., Wu N., Guan R., Pang L., Li X., Li S., Tang L., Guo Y., Chen J., Sun D. Overexpression of RCC2 enhances cell motility and promotes tumor metastasis in lung adenocarcinoma by inducing epithelial-mesenchymal transition. Clin. Cancer Res. 2017;23:5598–5610. doi: 10.1158/1078-0432.CCR-16-2909. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Machesky L.M. Fascin1 in carcinomas: its regulation and prognostic value. Int. J. Cancer. 2015;137:2534–2544. doi: 10.1002/ijc.29260. [DOI] [PubMed] [Google Scholar]
- 18.El-Balat A., Arsenic R., Sänger N., Karn T., Becker S., Holtrich U., Engels K. Fascin-1 expression as stratification marker in borderline epithelial tumours of the ovary. J. Clin. Pathol. 2016;69:142–148. doi: 10.1136/jclinpath-2015-203224. [DOI] [PubMed] [Google Scholar]
- 19.Karatas O.F., Suer I., Yuceturk B., Yilmaz M., Hajiyev Y., Creighton C.J., Ittmann M., Ozen M. The role of miR-145 in stem cell characteristics of human laryngeal squamous cell carcinoma Hep-2 cells. Tumour Biol. 2016;37:4183–4192. doi: 10.1007/s13277-015-4219-z. [DOI] [PubMed] [Google Scholar]
- 20.Lei C., Du F., Sun L., Li T., Li T., Min Y., Nie A., Wang X., Geng L., Lu Y. miR-143 and miR-145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis. 2017;8:e3101. doi: 10.1038/cddis.2017.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larne O., Hagman Z., Lilja H., Bjartell A., Edsjö A., Ceder Y. miR-145 suppress the androgen receptor in prostate cancer cells and correlates to prostate cancer prognosis. Carcinogenesis. 2015;36:858–866. doi: 10.1093/carcin/bgv063. [DOI] [PubMed] [Google Scholar]
- 22.Cioce M., Ganci F., Canu V., Sacconi A., Mori F., Canino C., Korita E., Casini B., Alessandrini G., Cambria A. Protumorigenic effects of mir-145 loss in malignant pleural mesothelioma. Oncogene. 2014;33:5319–5331. doi: 10.1038/onc.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing A.Y., Wang Y.W., Su Z.X., Shi D.B., Wang B., Gao P. Catenin-δ1, negatively regulated by miR-145, promotes tumour aggressiveness in gastric cancer. J. Pathol. 2015;236:53–64. doi: 10.1002/path.4495. [DOI] [PubMed] [Google Scholar]
- 24.Mataki H., Seki N., Mizuno K., Nohata N., Kamikawaji K., Kumamoto T., Koshizuka K., Goto Y., Inoue H. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7:72084–72098. doi: 10.18632/oncotarget.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y., Zhu J., Ou C., Deng Z., Chen M., Huang W., Li L. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br. J. Cancer. 2014;110:2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W., Chang J., Du X., Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed. Pharmacother. 2017;95:1112–1118. doi: 10.1016/j.biopha.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 27.He M., Zhan M., Chen W., Xu S., Long M., Shen H., Shi Y., Liu Q., Mohan M., Wang J. MiR-143-5p deficiency triggers EMT and metastasis by targeting HIF-1α in gallbladder cancer. Cell. Physiol. Biochem. 2017;42:2078–2092. doi: 10.1159/000479903. [DOI] [PubMed] [Google Scholar]
- 28.Shi H., Shen H., Xu J., Zhao S., Yao S., Jiang N. MiR-143-3p suppresses the progression of ovarian cancer. Am. J. Transl. Res. 2018;10:866–874. [PMC free article] [PubMed] [Google Scholar]
- 29.He Z., Yi J., Liu X., Chen J., Han S., Jin L., Chen L., Song H. MiR-143-3p functions as a tumor suppressor by regulating cell proliferation, invasion and epithelial-mesenchymal transition by targeting QKI-5 in esophageal squamous cell carcinoma. Mol. Cancer. 2016;15:51. doi: 10.1186/s12943-016-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Yamada Y., Koshizuka K., Hanazawa T., Kikkawa N., Okato A., Idichi T., Arai T., Sugawara S., Katada K., Okamoto Y., Seki N. Passenger strand of miR-145-3p acts as a tumor-suppressor by targeting MYO1B in head and neck squamous cell carcinoma. Int. J. Oncol. 2018;52:166–178. doi: 10.3892/ijo.2017.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iio A., Takagi T., Miki K., Naoe T., Nakayama A., Akao Y. DDX6 post-transcriptionally down-regulates miR-143/145 expression through host gene NCR143/145 in cancer cells. Biochim. Biophys. Acta. 2013;1829:1102–1110. doi: 10.1016/j.bbagrm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Cui R., Meng W., Sun H.L., Kim T., Ye Z., Fassan M., Jeon Y.J., Li B., Vicentini C., Peng Y. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc. Natl. Acad. Sci. USA. 2015;112:E4288–E4297. doi: 10.1073/pnas.1502068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alblazi K.M., Siar C.H. Cellular protrusions—lamellipodia, filopodia, invadopodia and podosomes—and their roles in progression of orofacial tumours: current understanding. Asian Pac. J. Cancer Prev. 2015;16:2187–2191. doi: 10.7314/apjcp.2015.16.6.2187. [DOI] [PubMed] [Google Scholar]
- 34.Gimona M., Buccione R., Courtneidge S.A., Linder S. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Condeelis J., Segall J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 36.Johnson H.E., King S.J., Asokan S.B., Rotty J.D., Bear J.E., Haugh J.M. F-actin bundles direct the initiation and orientation of lamellipodia through adhesion-based signaling. J. Cell Biol. 2015;208:443–455. doi: 10.1083/jcb.201406102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayo A., Malboubi M., Antoku S., Chang W., Ortiz-Zapater E., Groen C., Pfisterer K., Tootle T., Charras G., Gundersen G.G., Parsons M. Fascin regulates nuclear movement and deformation in migrating cells. Dev. Cell. 2016;38:371–383. doi: 10.1016/j.devcel.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelman J.D., Suarez C., Hocky G.M., Harker A.J., Morganthaler A.N., Christensen J.R., Voth G.A., Bartles J.R., Kovar D.R. Fascin- and α-actinin-bundled networks contain intrinsic structural features that drive protein sorting. Curr. Biol. 2016;26:2697–2706. doi: 10.1016/j.cub.2016.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez D.M., Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izdebska M., Zielińska W., Grzanka D., Gagat M. The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. BioMed Res. Int. 2018;2018:4578373. doi: 10.1155/2018/4578373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J.M., Bera R., Chiou C.Y., Yu M.C., Chen T.C., Chen C.W., Wang T.R., Chiang W.L., Chai S.P., Wei Y. Actin cytoskeleton remodeling drives epithelial-mesenchymal transition for hepatoma invasion and metastasis in mice. Hepatology. 2018;67:2226–2243. doi: 10.1002/hep.29678. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Tao L., Jin F., Gu H., Dai X., Ni T., Feng J., Ding Y., Xiao W., Qian Y., Liu Y. Cofilin 1 induces the epithelial-mesenchymal transition of gastric cancer cells by promoting cytoskeletal rearrangement. Oncotarget. 2017;8:39131–39142. doi: 10.18632/oncotarget.16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kent O.A., McCall M.N., Cornish T.C., Halushka M.K. Lessons from miR-143/145: the importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014;42:7528–7538. doi: 10.1093/nar/gku461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia W., Chen Q., Wang J., Mao Q., Dong G., Shi R., Zheng Y., Xu L., Jiang F. DNA methylation mediated silencing of microRNA-145 is a potential prognostic marker in patients with lung adenocarcinoma. Sci. Rep. 2015;5:16901. doi: 10.1038/srep16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaman M.S., Chen Y., Deng G., Shahryari V., Suh S.O., Saini S., Majid S., Liu J., Khatri G., Tanaka Y., Dahiya R. The functional significance of microRNA-145 in prostate cancer. Br. J. Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrich M., Nelson M.R., Stanssens P., Zabeau M., Liloglou T., Xinarianos G., Cantor C.R., Field J.K., van den Boom D. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc. Natl. Acad. Sci. USA. 2005;102:15785–15790. doi: 10.1073/pnas.0507816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential miRNA of FSCN1 was predicted with miRwalk-2, the information of potential binding site was shown in the Excel file.