Abstract
Adeno-associated virus-mediated gene replacement is emerging as a safe and effective means of correcting single-gene mutations affecting the CNS. AAV-mediated progranulin gene (GRN) delivery has been proposed as a treatment for GRN-deficient frontotemporal dementia and neuronal ceroid lipofuscinosis, and recent studies using intraparenchymal AAV-Grn delivery to brain have shown moderate success in histopathologic and behavioral rescue in mouse models. Here, we used AAV9 to deliver GRN to the lateral ventricle to achieve widespread expression in the Grn null mouse brain. We found that, despite a global increase in progranulin, overexpression resulted in dramatic and selective hippocampal toxicity and degeneration affecting neurons and glia. Hippocampal degeneration was preceded by T cell infiltration and perivascular cuffing. GRN delivery with an ependymal-targeting AAV for selective secretion of progranulin into the cerebrospinal fluid similarly resulted in T cell infiltration, as well as ependymal hypertrophy. Interestingly, overexpression of GRN in wild-type animals also provoked T cell infiltration. These results call into question the safety of GRN overexpression in the CNS, with evidence for both a region-selective immune response and cellular proliferative response. Our results highlight the importance of careful consideration of target gene biology and cellular response to overexpression prior to progressing to the clinic.
Keywords: brain gene therapy, AAV, neurodegeneration, frontotemporal dementia, neuronal ceroid lipofuscinosis, CNS gene therapy, progranulin
Graphical Abstract
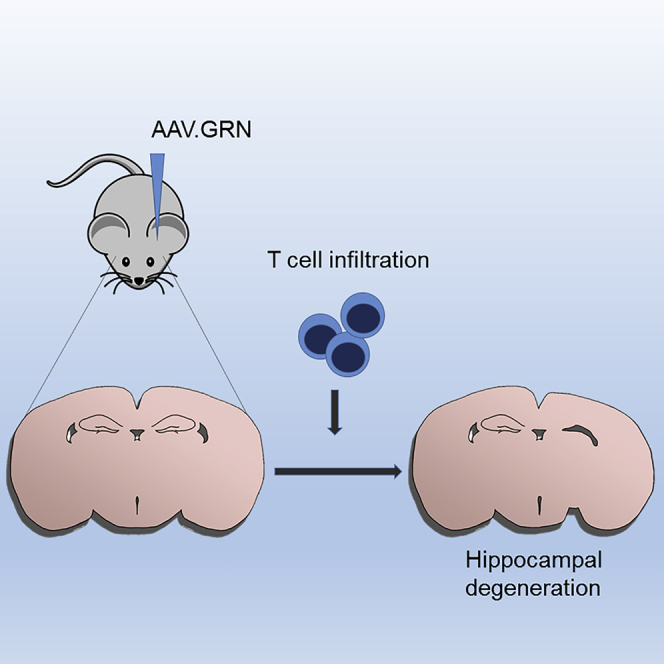
Treatment of progranulin-deficient frontotemporal dementia and neuronal ceroid lipofuscinosis could be achieved by increasing brain progranulin expression. Here, the authors use AAV to deliver progranulin to the CNS in a mouse model of NCL/FTD and unexpectedly find a T cell-mediated inflammatory response accompanied by hippocampal toxicity and unchecked growth.
Introduction
Frontotemporal dementia (FTD)1, 2 and neuronal ceroid lipofuscinosis (NCL) type 11 (CLN11)3 are neurodegenerative diseases resulting from haploinsufficiency or complete deficiency of progranulin (GRN), encoded by the gene GRN. FTD symptoms range from behavioral changes to language deterioration, with death ensuing in 3–5 years.4 Nearly 70 FTD-causing GRN mutations have been identified, accounting for up to 25% of inherited cases, >90% of which are nonsense mutations.1, 2, 5 For poorly understood reasons, GRN deficiency results in the accumulation of Tar-DNA binding protein of 43 kDa (TDP-43)1, 2 in characteristic inclusion bodies, with subsequent neuronal loss and atrophy of frontal and temporal lobes. In CLN11, complete GRN deficiency leads to lysosomal dysfunction and accumulation of lipofuscin, as well as a clinical syndrome of generalized seizures, cognitive dysfunction, vision loss, and cerebellar degeneration.6, 7, 8 Strategies to boost GRN have been under development since its discovery as a major causal mutation for these diseases.9, 10, 11
GRN is a secreted growth factor involved in development, wound healing, and immune modulation.12, 13 In mice, Grn is expressed highly in neurons and is upregulated in activated microglia following injury.14 In human postmortem brain, GRN expression is widespread in both normal and FTD subjects.15 In vitro and in vivo, GRN plays a role in neuronal survival and neurite outgrowth,16, 17, 18 and a neuronal GRN receptor, sortilin, has been identified.19 Based on its growth-promoting properties, GRN augmentation has been considered for treatment of a range of neurodegenerative diseases. Indeed, lentivirus- and adeno-associated virus (AAV)-mediated GRN delivery to the CNS has been investigated in preclinical models of Alzheimer’s disease,20, 21 Parkinson’s disease,22 motor neuron disease,23, 24 and Huntington’s disease.25
Methods to augment GRN expression include enhancing transcription10 or translation,9 increasing extracellular GRN levels,11 or using gene therapy. Among the latter, gene delivery using AAV vectors has risen to the forefront based on its excellent safety and efficacy profile, and it has been used in preclinical models of diverse diseases for several decades. Recent successes in humans include treatment of hemophilia, Leber’s congenital amaurosis, and spinal muscular atrophy.26, 27, 28 To transduce the CNS, AAV can be delivered to brain parenchyma or cerebrospinal fluid (CSF), with therapeutic benefit in preclinical models of both gain- and loss-of-function diseases.29, 30, 31, 32, 33, 34 In contrast to peripheral administration,35, 36 numerous studies have shown minimal innate or adaptive immune response to AAV-mediated gene delivery in the CNS.
A recent study using bilateral injection of AAV1.Grn into the medial prefrontal cortex of Grn null mice demonstrated focal improvements in lipofuscinosis, microgliosis, and lysosomal function.37 This group had previously used this approach in Grn haploinsufficient mice and showed improvement in lysosomal readouts and social dominance deficits.38 Notably, Grn null mice displayed microglial activation at the injection site, with induction of anti-GRN antibodies.37 No other immunologic phenotypes were reported in this short-term study.
While these studies are promising, translation of intraparenchymal gene delivery to the human brain is challenging. Our aim was to deliver GRN globally and sustainably using a method easily translatable to humans; namely, a single intraventricular injection of AAV.GRN. We selected AAV serotype 9 (AAV9) based on its ability to broadly disperse and infect neurons and glia after CSF delivery, as well as its prior track record.28, 34, 39 We also tested AAV serotype 4 (AAV4), due to its ependymal selectivity and safety profile,30, 40, 41 to maximize CSF secretion with the goal of broad CNS uptake through the sortilin receptor. Regardless of serotype, our studies show that overexpression of GRN in brain is deleterious, causing profound neurodegeneration and raising concern about excessive expression of GRN in mammalian brain as a therapy for FTD and/or NCL.
Results
Characterization of the Grn Null Phenotype
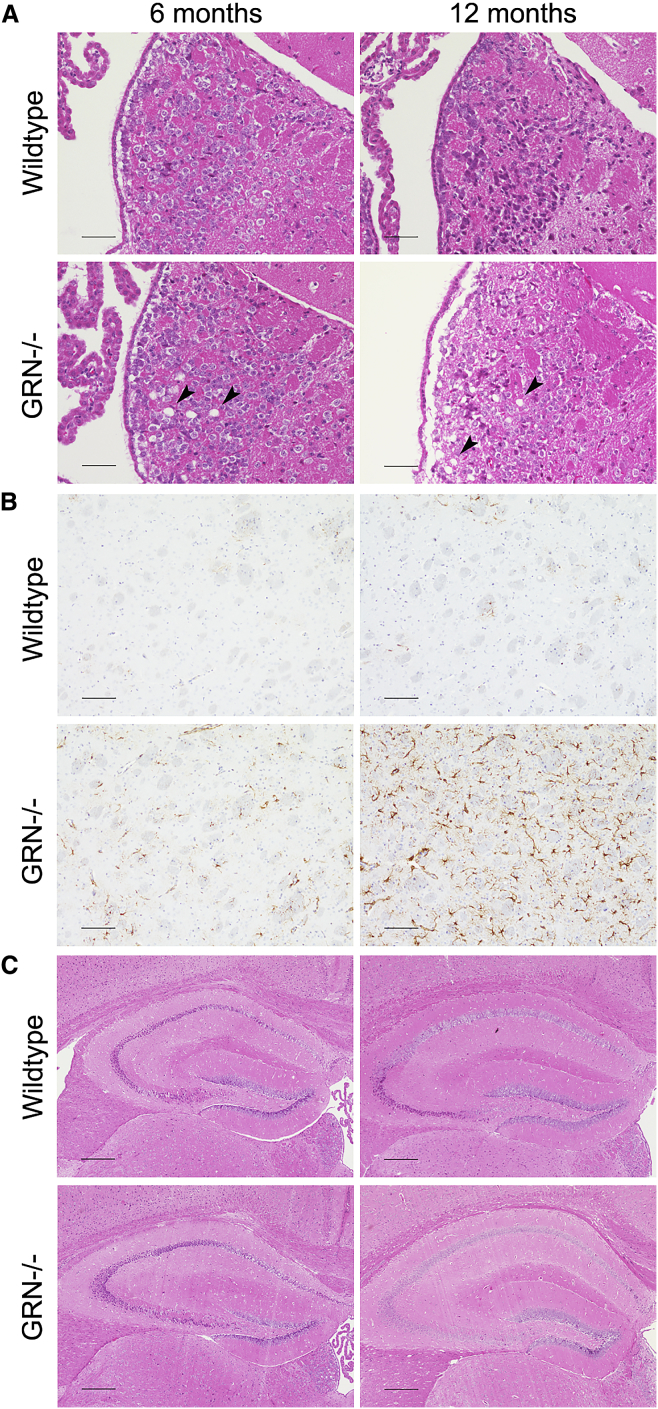
As Grn haploinsufficient mice have minimal phenotoypes, we used Grn null mice for our studies. Mice lacking Grn have an age-dependent histopathologic phenotype consisting of habenular and hippocampal vacuolation and increased ubiquitination starting at 7 months of age, as well as diffusely increased astrogliosis and microgliosis starting at 12 months of age.42, 43, 44 In our Grn null animals, we confirmed the previously reported increase in vacuolation,42 which was most pronounced in the habenula and increased with age (Figure 1A, arrowheads). Additionally, we noted astrocytosis in the Grn null striatum that is present as early as 6 months and progresses with age; this histopathological finding was not present, even in 12-month-old wild-type (WT) mice (Figure 1B), and has not been previously described. Hippocampal morphology was unaffected by genotype at any age (Figure 1C).
Figure 1.
Grn Null Mice Recapitulate Previously Published Histopathologic Findings and Exhibit Previously Undescribed Abnormalities
(A) Grn null mice exhibit vacuolation that is most pronounced in the habenula and increases with age (arrowheads) and is absent from WT mice at all time points. (Scale bars: 50 μm.) (B) Grn null mice demonstrate an age-dependent increase in astrocytosis compared to WT mice, as seen by GFAP staining. Indicated here is the striatum, an area in which astrocytosis in Grn null mice has not been previously described. (Scale bars: 100 μm.) (C) The hippocampus shows no gross morphological differences in Grn null mice compared to WT at 6 or 12 months. (Scale bars: 250 μm.)
AAV-Mediated Gene Transfer Results in Sustained GRN Expression in Grn Null Mice
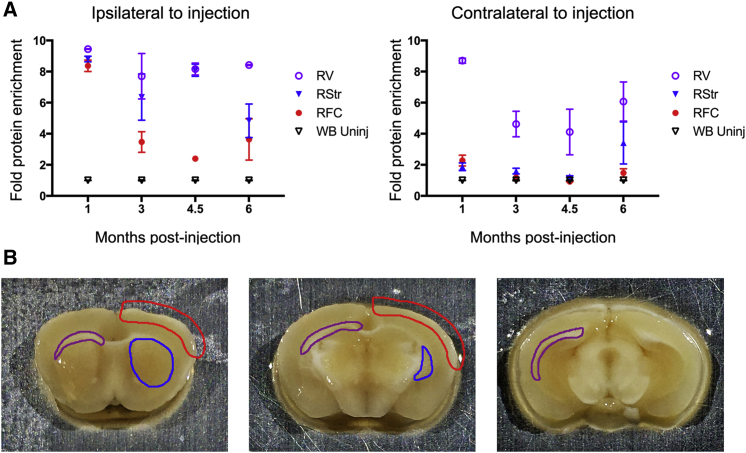
Grn null mice were injected with AAV9 encoding human GRN (AAV9.GRN) (Figure S1) in the right posterior lateral ventricle at 6–7.5 months of age and sacrificed at time points ranging from 1 to 6 months post-injection. We observed the highest levels of GRN expression, as detected by ELISA, in the ipsilateral periventricular region; GRN levels remained undiminished at 1, 3, 4.5, and 6 months post-injection, compared to levels in uninjected whole brain tissue from null mice (Figure 2A, left). We also observed high GRN levels in the ipsilateral striatum and, to a lesser extent, the ipsilateral frontal cortex, although cortical expression diminished over time. Similarly high levels of GRN were detected in the contralateral periventricular region (Figure 2A, right) but with minimal increase in the left striatum or left frontal cortex. GRN was additionally detected at moderate levels in the periventricular region of the third ventricle, the brainstem, and the spinal cord at all time points (Figure S2). These data indicate broad, sustained GRN expression.
Figure 2.
AAV9 Mediates Sustained Expression of GRN in Grn Null Mouse Brain
Grn null mice were injected at 6–7.5 months of age with AAV9.GRN or AAV9.eGFP in the right lateral ventricle and sacrificed 1, 3, 4.5, or 6 months post-injection. Brains were microdissected, and GRN levels were measured by ELISA. (A) Left: GRN levels remained elevated at all time points in the right (injected) peri-ventricular area (RV), right striatum (RStr), and right frontal cortex (RFC) as compared to uninjected homogenized whole brain (WB). (A) Right: GRN levels remained elevated at all time points in the left (uninjected) peri-ventricular area (LV), but far less so in the left striatum (LStr) and left frontal cortex (LFC) as compared to uninjected homogenized whole brain (WB). (B) Schematic illustrating the regions collected by microdissection in blue (striatum), red (cortex), and purple (peri-ventricular area). n = 3 mice per group at each time point.
AAV9-Mediated Overexpression of GRN Causes Progressive Hippocampal Toxicity
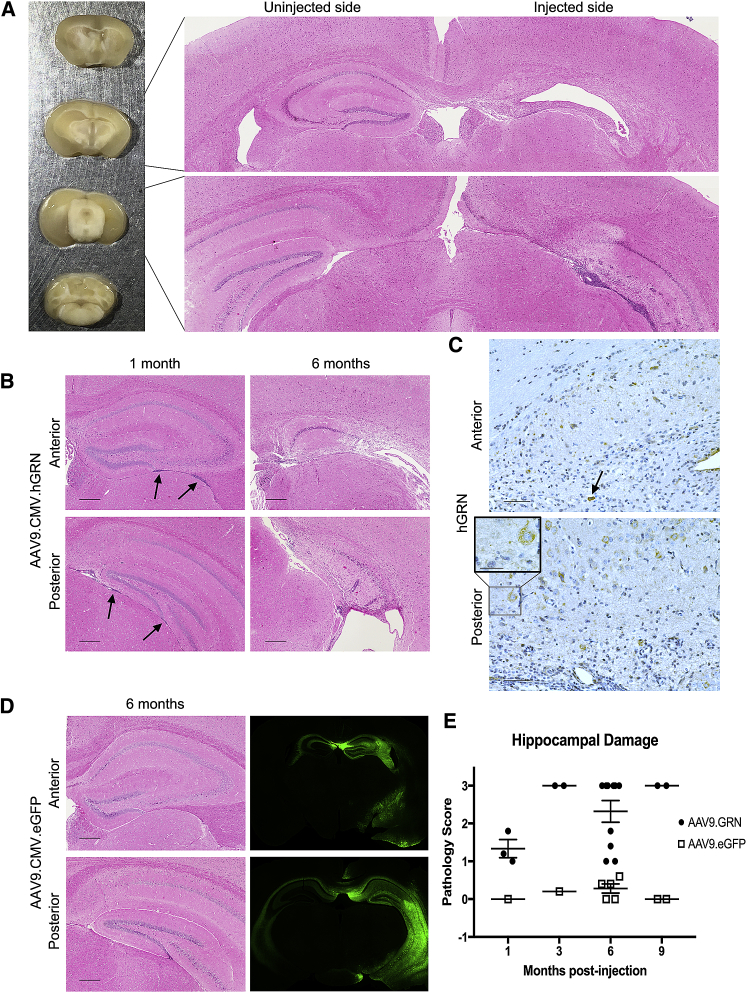
Having established sustained expression of GRN, we next performed detailed histological and immunohistochemical analyses to assess for rescue in our treated mice. Grn null animals injected with AAV9.GRN at 6–7.5 months of age and sacrificed 6 months after injection had striking morphological and histological changes in the hemisphere ipsilateral to injection. Specifically, in 10 out of 10 injected animals, the hippocampus ipsilateral to the injection site showed a hypercellular infiltrate often accompanied by loss of structural integrity (Figure 3A). In all cases, adjacent structures, as well as the contralateral hippocampus, appeared relatively unaffected. Similar hippocampal degeneration occurred in Grn null mice injected with an AAV9 vector delivering mouse Grn (Figure S3), indicating that the response was not specific to delivery of a human gene.
Figure 3.
Overexpression of GRN Is Toxic to Cells of the Hippocampus
Mice were injected at 6–8 months of age and sacrificed 1–9 months post-injection, and brains were either embedded and stained for immunohistochemical analysis (A–E) or sectioned and imaged fluoroscopically (D). (A) Left: histological differences were observed between the injected and uninjected hemispheres in 10 of 10 mice 6 months after injection of AAV9.GRN, in 6 of whom there was grossly visible atrophy of the hippocampus on the injected side. Right: H&E of AAV9.GRN-injected mouse brain revealed marked degeneration of the hippocampus ipsilateral to injection and a dense cellular infiltrate throughout the remaining hippocampal tissue, extending from anterior (upper panel) to posterior (lower panel). (B) The cellular infiltrate was observed along the length of the hippocampus as early as 1 month post-injection of AAV9.GRN, both inferior to (arrows) and within the parenchyma of the hippocampus (left panels; n = 3 mice). By 6 months, histological changes were observed in 10 of 10 mice, with 6 of 10 showing pervasive cell loss (right panels). (Scale bars: 250 μm.) (C) High levels of GRN were detected throughout the hippocampus at all time points, with a diffuse cytoplasmic pattern of expression (inset). In some cases, cells expressing GRN exhibited pyknotic nuclei (arrow). Indicated here is GRN expression 6 months post-injection. (Scale bars: 50 μm; inset: 25 μm.) (D) Left panels: AAV9.eGFP-injected brains did not display cell loss or hypercellular infiltrate at 1 (n = 3), 3 (n = 6), 6 (n = 5), and 9 (n = 4) months post-injection and were similar in appearance to those of uninjected Grn null mice (n = 4 age-matched littermates to 6-month cohort; data not shown). Expression 6 months post-injection is indicated here. (Scale bars, 250 μm.) (D) Right panels: high levels of EGFP expression were detected by fluorescence microscopy adjacent to the injection site, with cross-over to the contralateral side. Expression 3 months post-AAV9.eGFP injection is indicated here. (E) Quantitative assessment of hippocampal toxicity by raters blinded to treatment condition showed toxicity ranging from hypercellular infiltrates to pervasive cell loss in 17/17 (100%) of AAV9.GRN-treated animals, compared to 0/18 (0%) of AAV9.eGFP-treated animals. Data are presented as mean ± SEM.
To better define the timeline of degenerative changes, animals were harvested soon after injection. At 1 month post-injection with AAV9.GRN, a hypercellular infiltrate was noted to extend anteriorly and posteriorly along the entire hippocampus (Figure 3B, left panels) and was most prominent inferior to and within the hippocampal parenchyma. By 6 months post-injection, prominent hypercellular infiltrates and perivascular cuffing accompanied loss of recognizable hippocampal structures (Figure 3B, right panels), and staining confirmed strong GRN expression in these regions (Figure 3C). Positive GRN staining was noted in neurons that appeared to be healthy (Figure 3C, lower inset) and in cells with pyknotic nuclei (Figure 3C, arrow). In contrast, littermate control animals treated with AAV9-delivered eGFP (AAV9.eGFP) showed no pathology at 6 months post-injection (Figure 3D) and appeared similar to uninjected littermates (data not shown), despite high levels of EGFP expression.
Indeed, over 4 time points after injection, in histopathological assessments performed by observers blinded to treatment condition, 17/17 (100%) of the AAV9.GRN-injected animals showed evidence of hippocampal toxicity, with 10/17 (59%) affected severely enough to cause atrophy visible to the naked eye. In contrast, 0/18 (0%) of the AAV9.eGFP-injected animals exhibited evidence of toxicity (Figure 3E).
Responses to hGRN Overexpression Are Region- and Cell-Type Specific
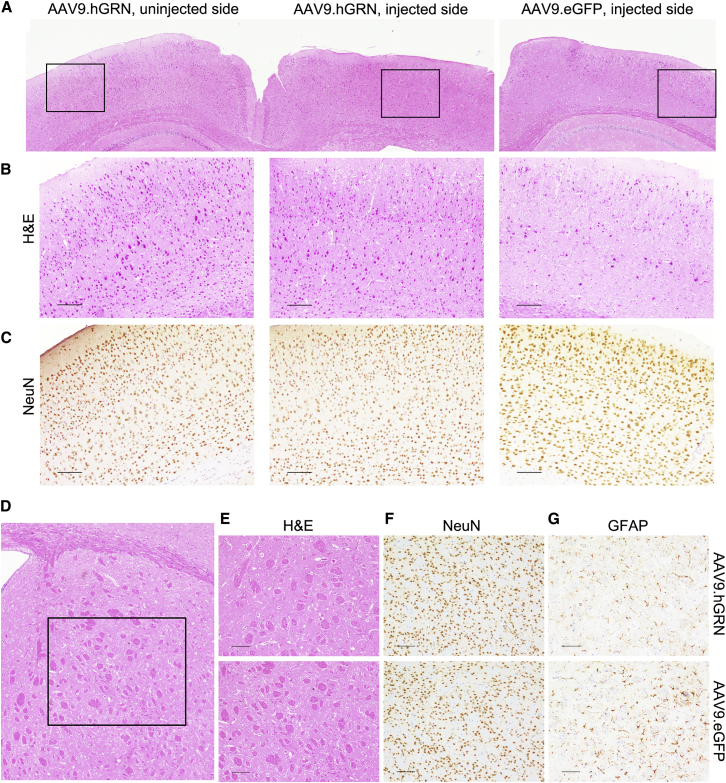
Histological assessments were performed to determine whether the hypercellular infiltrates and degeneration observed after AAV9.GRN delivery were present in other brain regions with high levels of expression. No differences were seen between the cortex ipsilateral to the injection site and either the contralateral cortex or that of AAV9.eGFP-injected controls at 6 months post-injection (Figures 4A and 4B), despite moderately high GRN levels in cortical brain isolates (Figure 2). Indeed, cortical neuron organization remained intact ipsilateral to the site of AAV9.GRN injection (Figure 4C), and no obvious changes in architecture, neuronal number, or gliosis were found in the ipsilateral striatum (Figures 4D–4G), an area with high GRN levels (Figure 2). Brain tissue samples from AAV9.eGFP-injected animals and from uninjected animals were similar in appearance across all parameters (data not shown). These results suggest that GRN overexpression selectively affects hippocampal brain regions.
Figure 4.
The Cortex and Striatum Are Unaffected by GRN Overexpression
(A) The ipsilateral cortex immediately adjacent to the degenerated hippocampus appeared unaffected by AAV9.GRN overexpression when compared to the contralateral cortex and to cortex ipsilateral to AAV9.eGFP-injected brain. Gross morphology, layer organization, and neuronal numbers appeared unremarkable by H&E staining (B; n = 10 AAV9.GRN-injected mice, and n = 5 AAV9.eGFP-injected mice assessed); NeuN staining confirmed intact neuronal appearance (C). (Scale bars: 100 μm.) Similarly, the ipsilateral striatum directly anterior to the degenerated hippocampus (D) appeared unremarkable by H&E (E), NeuN (F), and GFAP (G) staining, compared to AAV9.eGFP-injected controls 6 months post-injection (scale bars: 100 μm), as well as to uninjected controls (data not shown).
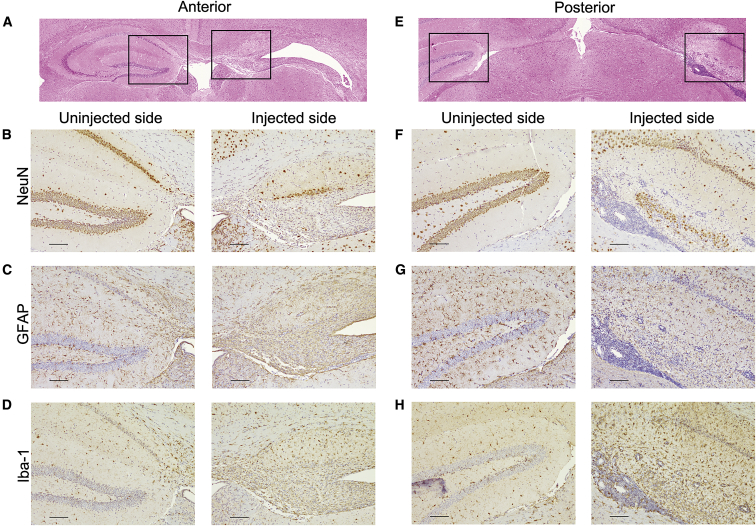
To determine what cell types were affected in the hippocampus anteriorly (Figures 5A–5D) and posteriorly (Figures 5E–5H), tissue sections were stained for the neuronal marker NeuN, the glial marker glial fibrillary acidic protein (GFAP), and the microglial marker Iba-1. In 13/17 (76%) of AAV9.GRN-injected mice, hippocampal neuronal loss was noted (Figures 5B and 5F). Hippocampal astrocytes demonstrated fewer, less robust processes (Figures 5C and 5G), and microglial infiltration ipsilateral to the injection was prominent (Figures 5D and 5H).45 In all cases, the side contralateral to the injection (left panels) appeared similar to the hippocampus of both AAV9.eGFP-injected and uninjected control littermates (data not shown). These data indicate that AAV9-mediated GRN overexpression is toxic to neurons and astrocytes in the hippocampus and provokes a strong local microglial response.
Figure 5.
AAV9.GRN-Overexpressing Mice Undergo Cell-Specific Hippocampal Degeneration
H&E-stained coronal sections indicated hippocampal degeneration 6 months post-injection on the injected side anteriorly (A) and posteriorly (E), with boxes indicating regions that are magnified below, in (B)–(D) and (F)–(H). On the injected side, NeuN staining indicated striking neuronal loss throughout all regions of the hippocampus both anteriorly (B) and posteriorly (F); GFAP staining showed qualitative loss of astrocytic processes in anterior (C) and posterior (G) hippocampus; and Iba-1 staining for microglia showed a dense microglial infiltrate in the hippocampal region anteriorly (D) and posteriorly (H). Throughout the hippocampus, there was a dense cellular infiltrate in the ependymal space underlying the hippocampus (F–H) ipsilateral to injection. (Scale bars: 100 μm.)
A T Cell-Mediated Inflammatory Response Precedes Neuronal Loss and Occurs in Both WT and Grn Null Animals
The hypercellular infiltrate found in AAV9.GRN-injected animals consisted of cells with a high nucleus-to-cytoplasm ratio characteristic of lymphocytes. As such, sections were stained for the cell proliferation marker Ki-67, the B lymphocyte marker B220 (CD45), and the T lymphocyte marker CD3.
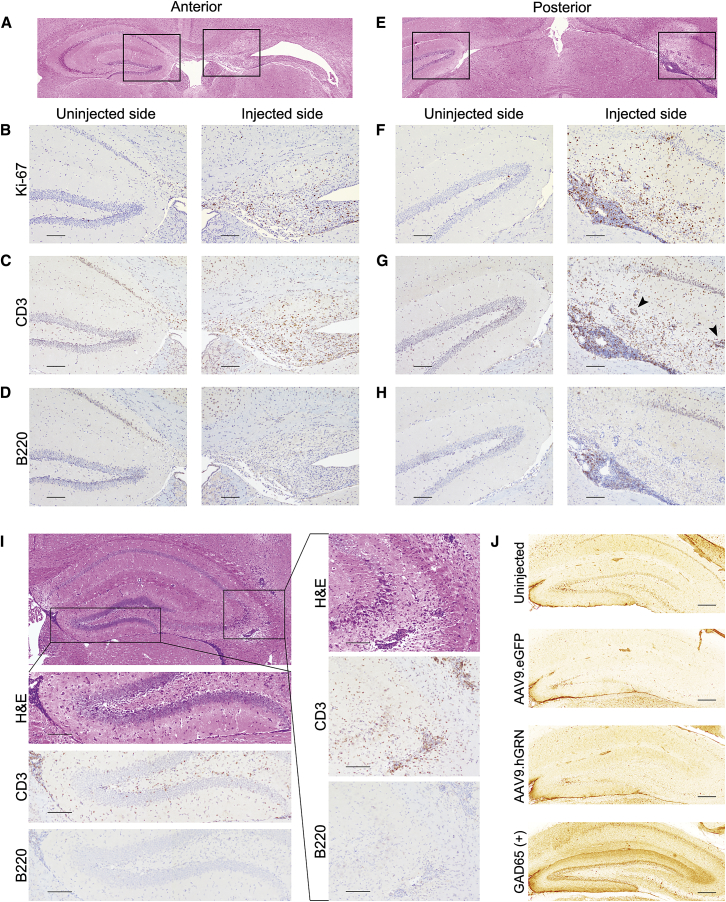
As shown in Figure 6, abundant proliferative cells were noted (Figures 6B and 6F), and the majority of these cells were positive for CD3 (Figures 6C and 6G). In both anterior and posterior sections, there was extensive perivascular cuffing by CD3+ cells both within and adjacent to the hippocampus (Figure 6G, arrowheads). These data collectively indicate a robust T cell infiltration of the hippocampal region, with minimal contribution from B cells.
Figure 6.
Hippocampal Degeneration Is Characterized by a T Cell Inflammatory Response that Precedes Cell Death
Mice were injected with AAV9.GRN at 6–8 months of age and sacrificed 6 months post-injection (A-H) or 1 month post-injection (I). H&E-stained coronal sections indicated hippocampal degeneration 6 months post-injection on the injected side anteriorly (A) and posteriorly (E), with boxes indicating regions magnified below, in (B)–(D) and (F)–(H). On the injected side, Ki-67 staining demonstrated proliferating cells throughout the hippocampus anteriorly (B) and posteriorly (F); CD3 staining identified most of these proliferating cells as T cells (anterior in C and posterior in G), while B220 staining for B cells was largely negative (anterior in D and posterior in H), aside from some positively stained cells in the dense posterior sub-hippocampal infiltrate (H). (Scale bars: 100 μm.) Mice were then examined at 1 month post-injection (I). H&E staining showed a dense infiltrate in the ependymal space inferior to the hippocampus that was CD3+ and B220− (left box and magnifications below; scale bars: 100 μm). In the CA2/3 region, there was also dense hypercellularity with perivascular cuffing that was CD3+ and B220− (right box and magnifications on right; scale bars: 100 μm; n = 3 mice analyzed). (J) Rat brain slices were incubated with mouse serum from uninjected (top, n = 3), AAV9.eGFP-injected (second, n = 2), or AAV9.GRN-injected (third, n = 5) mice at 6 months post-injection, with GAD65+ mouse serum used as a positive control for antibody-based hippocampal reactivity (bottom). There was no immune reactivity in serum collected from mice injected with AAV9.GRN at 6 months (J) or at 1 (n = 3) or 3 (n = 2) months after AAV9.GRN injection (data not shown). (Scale bars: 250 μm.)
To test whether inflammatory infiltrates precede or follow the hippocampal degeneration, brain sections from animals sacrificed at earlier time points after AAV9.GRN injection were characterized. At 1 month post-injection, hippocampal structures were maintained, despite dense cellular infiltration ventral to the hippocampus, with widespread perivascular cuffing lateral to and within the hippocampus (Figure 6I). Infiltrating cells were positive for CD3 and negative for B220, indicating that T cell infiltration precedes hippocampal neurodegeneration.
In human autoimmune encephalitides, hippocampal degeneration often ensues from autoantibodies specific for hippocampal antigens. Triggering events may be the expression of an ectopic antigenic protein by a tumor or the unmasking of a native hippocampal antigen by an inflammatory process.46 Therefore, we tested whether GRN overexpression elicited a similar pathophysiological process in our Grn null animals using a previously described rat hippocampal slice assay.47 As shown in Figure 6J, serum from mice with the hippocampal degeneration phenotype screened negative for anti-hippocampal antibodies, regardless of whether serum was drawn 1, 3, or 6 months after AAV9.GRN injection. Moreover, AAV9.GRN intraventricular delivery into WT animals also elicited perivascular cuffing with infiltration of CD3+ T cells as early as 1 month after gene delivery, which became prominent and was accompanied by loss of hippocampal structures by 3 months post-injection (Figure 7). Taken together, these data support a role for T cell-mediated hippocampal neurodegeneration following GRN overexpression.
Figure 7.
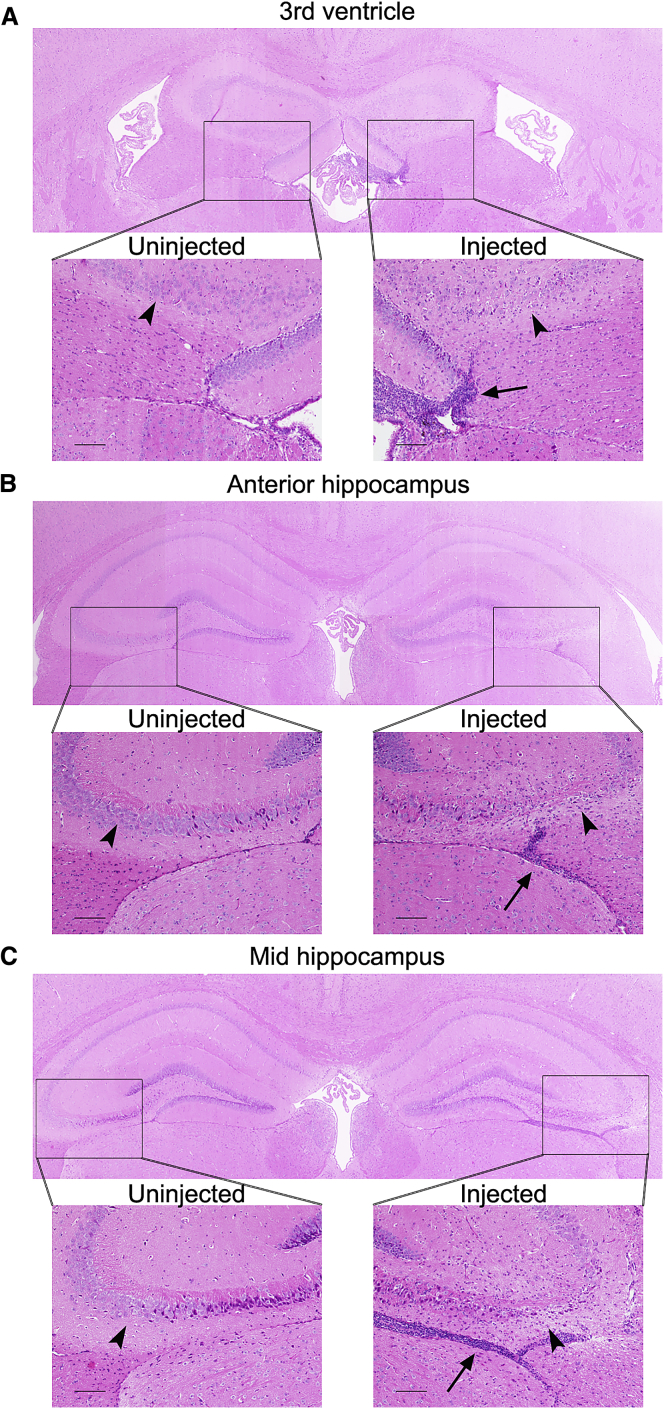
WT Mice Also Mount a T Cell Response Accompanied by Hippocampal Cellular Loss after Injection with AAV9.GRN
WT background-matched mice were injected in the right lateral ventricle at 6 months of age with AAV9.GRN and sacrificed at 1 (data not shown) or 3 months post-injection (n = 2 mice per time point). A hypercellular infiltrate was observed most prominently adjacent to the third ventricle ipsilateral to injection (A) and extending posteriorly throughout the anterior hippocampus (B) and mid-hippocampus (C). The infiltrate extended inferior to the hippocampus (arrows) as well as within the parenchyma, where we observed marked loss of cells in the CA2/3 region of the hippocampus on the injected side compared to the uninjected side (arrowheads; n = 1/2 mice affected at 1 month and 2/2 mice at 3 months). There was no evidence of cell loss or hypercellular infiltrate in AAV9.eGFP-injected WT mice (n = 2 at 3 months; data not shown). (Scale bars: 100 μm.)
hGRN Delivered by the AAV4 Ependymal-Targeting Vector Elicits an Inflammatory Response and Ependymal Hypertrophy
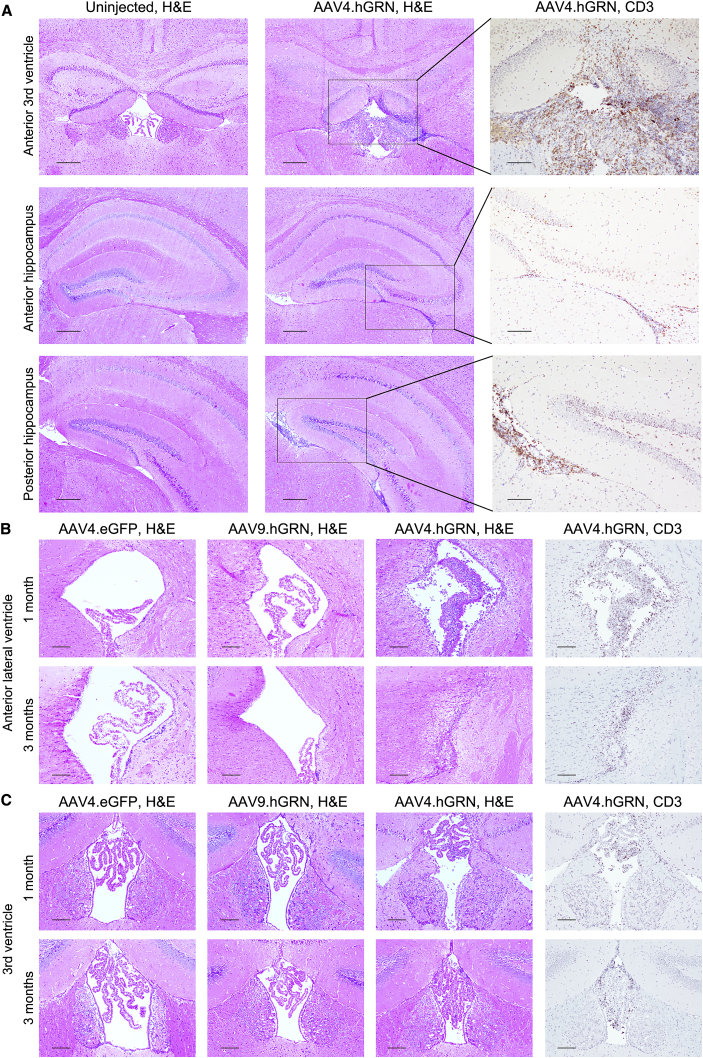
AAV9 transduces multiple cell types, including neurons and glia.48 Therefore, we asked whether the observed inflammatory response and subsequent hippocampal degeneration were serotype specific. For this, we used AAV4, an ependymal-targeting serotype49 that allows secretion of transgene products into the CSF.30, 41, 50 In Grn null mice injected at 6.5–8 months with AAV4.GRN in the right lateral ventricle, at the same dose as our AAV9.GRN-injected animals, GRN was detected throughout the brain by ELISA at mildly increased levels that were greatest in ependyma-rich areas (Figure S4A). A hypercellular infiltrate was present throughout the ventricular system as early as 1 month post-injection (Figure 8A). This infiltrate was not present in AAV4.eGFP-injected controls. Consistent with our previous findings, infiltrating cells were positive for the T cell marker CD3 (Figure 8A, right panels).
Figure 8.
Grn Null Mice Expressing GRN Delivered by an Ependymal-Targeting Vector, AAV4, Show an Inflammatory Response and Ependymal Hypertrophy
Mice were injected at 6.5–8 months of age in the right lateral ventricle with AAV4.GRN and sacrificed 1 or 3 months post-injection. At 1 month, AAV4.GRN-injected mice showed a dense cellular infiltrate throughout the ependyma underlying the hippocampus (A, middle panels) that was not present in uninjected age-matched controls (A, left panels) or in AAV4.eGFP-injected littermates (data not shown). (Scale bars: 250 μm.) The infiltrate was highly positive for the T cell marker CD3 in all regions (A, right panels; scale bars: 100 μm). When compared to age-matched AAV9.GRN-injected mice, AAV4.GRN-injected mice showed unique choroidal and ependymal hyperplasia and thickening and dense T cell infiltration in the ipsilateral (B) and contralateral (data not shown) lateral ventricles that worsened with time to the point of effacement by 3 months post-injection (scale bars: 100 μm). This was also apparent in the third ventricle, where the ependyma were thickened and the choroid nearly filled the ventricle by 3 months, again with a T cell infiltrate (C; scale bars: 100 μm). 3/3 AAV4.GRN-injected mice were affected at each of the 1- and 3-month post-injection time points, while AAV4.eGFP control brains were not affected (n = 2 at 1 month, and n = 1 at 3 months).
There was also marked ependymal and choroidal hypertrophy in the lateral ventricles adjacent to the hippocampus (Figure 8B, upper panel), as well as the third ventricle (Figure 8C, upper panel), with corresponding T cell infiltration. By 3 months post-injection, the effect was so severe as to have ablated the lateral ventricles (Figure 8B, lower panel), with choroidal hypertrophy nearly ablating the third ventricle as well (Figure 8C, lower panel). This effect was not seen in AAV4.eGFP-injected mice at any time point (Figures 8B and 8C), suggesting a direct effect of GRN-induced cellular proliferation. Additionally, ependymal and choroidal hypertrophy were not observed with AAV9-mediated gene delivery at any time (Figures 8B and 8C). As AAV9 does not efficiently transduce ependymal cells, these data indicate a hypertrophic effect on GRN transduced ependymal cells. Of note, the hippocampus was unaffected in AAV4.GRN-treated mice, and GRN was not detected in this area (Figure S4B).
To test whether this is an effect of overexpression of transgenes in animals null for a disease gene, we subsequently examined brain sections from mucopolysaccharidosis type IIIA (MPS IIIA) sulfamidase (SGSH) homozygous knockout mice treated with AAV4 expressing human SGSH, a secreted protein, using the same dose, injection coordinates, and volume as in our AAV4.GRN studies.51 There was no observed toxicity in treated MPS IIIA mice (data not shown), supporting an effect specific to GRN.
Cumulatively (Table S1), our data show that overexpression of progranulin in the brain can trigger robust responses in the CNS that, in turn, can cause cellular damage, regardless of the AAV serotype used for delivery.
Discussion
In these studies we sought to test the safety and efficacy of AAV-mediated GRN expression in a Grn null mouse model, toward the development of a gene replacement strategy for treatment of GRN-deficient FTD and CLN11. To our surprise, we found that AAV9-mediated GRN or Grn overexpression led to hippocampal toxicity in 100% of Grn null mice, with hippocampal neurodegeneration in nearly all, while AAV9-mediated eGFP overexpression had no deleterious effect. The observed degeneration was markedly selective, with sparing of the cortex above, striatum anterior to, and thalamic structures inferior to the hippocampus, despite high GRN levels in these tissues. We also observed a cellular infiltrate primarily composed of T cells as well as perivascular cuffing preceding the onset of hippocampal degeneration and persisting until late stages of degeneration. In addition, we detected a T cell-mediated immune response, regardless of the genetic background of the mouse injected and regardless of the AAV serotype used, as well as a direct hypertrophic effect of GRN following AAV4 delivery to ependyma cells. The consistent observation of toxicity in GRN-injected mice in multiple experimental settings, as well as no evidence of toxicity in any of the eGFP-injected control mice, strongly points to GRN overexpression as the direct cause. These data emphasize the need for caution in pursuing GRN delivery in the human CNS.
Toxicity in our study was region selective, with hippocampal neurodegeneration preceded by T cell infiltration in the case of AAV9-mediated GRN delivery, and severe damage to the ventricular system via ependymal hypertrophy and T cell infiltration when GRN was expressed following AAV4 transduction of ependyma. We considered various explanations for our findings, starting with serotype. The tropism of AAV9 for neurons and glia, and of AAV4 for ependymal cells, might have resulted in toxic levels of transgene expression in their target tissues. However, in contrast to GRN, EGFP delivered by either AAV9 or AAV4 vectors did not elicit a degenerative or inflammatory response. This suggests that the choice of serotype and high levels of transgene expression alone are not sufficient to explain our findings. This is supported by many others’ works in which overexpression by these serotypes in a variety of null or haploinsufficient models has not led to robust immune responses.
Second, the intraventricular delivery route chosen here may have triggered immunogenicity and downstream tissue destruction. In this respect, the recent reports by Arrant et al. demonstrating rescue in mouse models of FTD and CLN11 after intraparenchymal delivery of Grn are noteworthy37, 38; GRN was expressed in mice lacking one or both copies of Grn, but there was no dramatic hippocampal degeneration or ependymal toxicity. It is possible that the intraventricular route exposes particular antigen-presenting cells to GRN, thus provoking the T cell infiltration and inflammation observed in our animals. Our strategy was based on considerations of eventual clinical translation for GRN-deficient human diseases, for which intraparenchymal delivery poses safety and feasibility issues in humans. Moreover, intraventricular CNS delivery of many transgenes has been successfully achieved in preclinical models, including in animals null for the therapeutic gene. In mice, at doses and injection routes similar to ours, gene replacement strategies have consistently been safely achieved in null models; for instance, one group safely delivered the ATP-binding cassette transporter (ABCD1) gene in a mouse model of X-linked adrenoleukodystrophy.39 Similarly, in our studies, overexpression of EGFP, a completely foreign protein, was well tolerated, regardless of serotype. Furthermore, MPS IIIA Sgsh null mice treated with AAV4.SGSH, a secreted protein, using the same approach as our study, showed no sign of hippocampal or ventricular degeneration or of cellular infiltrate.51 Cumulatively, the data suggest that our results are specific to overexpression of GRN as a target gene, rather than route of delivery, dose, expression of a human gene in a null mouse model, or expression of a secreted protein.
While much of the literature in more than 10 years since GRN mutations were first linked to neurodegeneration is concerned with the role of GRN as a neurotrophic factor,14, 16, 17, 18 GRN is also widely expressed in cell types ranging from epithelial cells to hematopoietic cells, macrophages, and T cells.12, 13 Early studies described its role in wound healing and regulation of inflammation, which, while poorly understood, involves an interplay between GRN, which is itself active, and its cleavage products, the granulins, which have opposing effects on a number of immune cell-mediated processes.13 Thus, the existing literature on GRN suggests that tight regulation of its expression, both on the transcriptional side and with respect to the protein cleavage events that generate daughter peptides, may be needed to avoid untoward immunological effects.
There is also extensive literature on the involvement of GRN in cell growth and proliferation, both in normal development and in cancer.12, 13 Indeed, GRN is overexpressed and promotes cell growth in many tumors, including glioblastoma.52, 53 In this respect, our findings using AAV4-mediated GRN delivery are noteworthy. In mice, AAV4 selectively targets ependymal cells and subventricular astrocytes,54 and AAV4-mediated GRN delivery resulted in marked hypertrophy of the ependyma, suggesting a direct effect of GRN on the targeted cells accompanied by T cell infiltration.
Arrant et al.37, 38 recently reported the reversal of phenotypes associated with GRN deficiency using AAV-mediated replacement therapy in Grn null mice, with no reported evidence of T cell infiltration or hippocampal degeneration. Despite differences in our approach (intraventricular versus intraparenchymal), vector (AAV9 or AAV4 versus AAV1), dose (higher in our study), and transgene (our vector does not have a Myc tag and can interact with sortilin), both Arrant et al. and we observed an immune response. In the case of Arrant et al., the response consists of profound microglial activation and major histocompatibility complex class II (MHCII) presentation at the injection site, as well as antibodies to GRN detected in plasma. In our studies, by contrast, we observed T cell infiltration and destruction of neurons and astrocytes in the hippocampus. It is possible that, given a longer period of time, evidence of neuronal degeneration or unchecked growth would emerge in their studies. Indeed, Arrant et al. remarked that the upregulation of MHCII is typically associated with a T cell response and speculated that a longer exposure could result in T cell-mediated neuronal degeneration and functional deficits.
These findings raise concerns regarding how to safely translate these studies to humans. Specifically, our combined data suggest that GRN replacement could be highly immunogenic in CLN11 (GRN null) patients. For FTD due to haploinsufficiency, high levels of GRN may be required to reverse disease, which, in turn, may induce either tumorigenic growth effects of GRN or an immune response to transduced cells. To tease apart whether toxicity is dependent on the growth-promoting properties of GRN, overexpression in target cells, or host naiveté, additional studies are needed in heterozygous models with progranulin variants. As a final consideration, future pharmacological toxicity studies of the WT human protein may remain a challenge, as overexpression will be problematic in rodents.
In summary, while GRN-associated FTD and NCL are attractive targets for gene replacement therapy, our results suggest that concerns stemming from the identity and function of the transgene—GRN—are paramount in considerations of a path to human intervention. Specifically, work elucidating the mechanisms by which GRN modulates inflammatory and growth responses, particularly in the CNS, are needed. Our work highlights the potential for inflammatory and tumorigenic effects of GRN overexpression, to which specific attention should be paid in preclinical models. More broadly, these findings call into question our current conception of the brain as an immune privileged organ broadly tolerant of transgene overexpression.
Materials and Methods
Viral Vector Constructs
AAV9 and AAV4 were used for these studies. AAV9 vectors contained a cytomegalovirus (CMV) promoter, while AAV4 vectors contained a CAG promoter. The human GRN cDNA (GenBank: BC000324) was amplified from a Hek293 cell cDNA library, and the mouse Grn cDNA (GenBank: NM_008175) was cloned using gBlocks (Integrated DNA Technologies; IDT). The transgenes or an eGFP reporter gene was inserted into the G0347 pFB.AAV.CMV.bHGpA plasmid or the G0347 pFB.AAV.CAG.bHGpA plasmid (Iowa Vector Core, Iowa City, IA, USA) and were then used to produce AAV vectors by the Children’s Hospital of Philadelphia (CHOP) Research Vector Core (Philadelphia, PA, USA). All constructs were verified by Sanger sequencing (CHOP NAPCORE, Philadelphia, PA, USA).
Mice
Generation of progranulin null (Grn null) mice on a C57BL/6J background through targeted disruption of the Grn gene has been reported previously.55 Grn null mice were provided to the University of Pennsylvania from the Nishihara laboratory at the University of Tokyo, and the colony was expanded and maintained. Male and female WT C57BL/6J (WT; Jackson Laboratories, Bar Harbor, ME, USA) and Grn null mice ages 6–15 months were used in these studies, as well as age-matched WT C57BL/6J controls. Mice were housed in a controlled-temperature environment on a 12-hr:12-hr light:dark cycle and were given free access to food and water. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. MPS IIIA Sgsh null and heterozygous mice were generated and maintained as previously described,51 and studies were approved by the Institutional Animal Care and Use Committee of CHOP.
Stereotaxic Delivery
Mice were deeply anesthetized with isoflurane and immobilized in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) installed with both a digital stereotaxic control panel (Leica Biosystems, Buffalo Grove, IL) and a microinjection robot (KD Scientific, Holliston, MA, USA) for motorized injections. Mice were injected unilaterally by a team of the same two operators in the posterior right lateral ventricle via a Hamilton syringe using the following coordinates: anterior-posterior (AP), −2.18 mm; medial-lateral (ML), −2.9 mm; and dorsal-ventral (DV), −3.5 mm (from bone) relative to bregma. Each mouse received 10 μL vector at a concentration of 5e+12 viral genomes per milliliter (vg/mL), for a total dose of 5e+10 vg, with the exception of AAV4.eGFP, which was delivered at a concentration of 4e+11 vg/mL, for a total dose of 4e+9 vg. Vectors were infused at a rate of 0.5 μL/min, with a 3-min wait time post-infusion prior to withdrawal of the trochanter. All vector dilutions were prepared in parallel using the same diluent, and experimental and control mice were injected on the same day in a mixed order. Mice were injected between 6 and 8 months of age and euthanized between 1 and 9 months post-injection. MPS IIIA Sgsh null and heterozygous mice were injected at 2 months and euthanized at 5.5 months, as previously described.51
Mouse Brain Isolation
Mice were sacrificed at indicated ages by anesthetizing with a ketamine-xylazine-acepromazine mixture, followed by transcardial perfusion with 15 mL ice-cold 0.9% PBS. Brains were quickly removed from the skull. Those used for fluorescent imaging studies were fixed whole in 4% paraformaldehyde overnight at 4°C followed by placement in a 30% sucrose-0.05% sodium azide solution for cryoprotection at 4°C. Those used for immunohistochemistry or for GRN quantification by ELISA were blocked into 2-mm-thick coronal slices and then either fixed in 4% paraformaldehyde overnight at 4°C or microdissected and flash-frozen in liquid nitrogen, respectively.
Protein Extraction
Frozen isolated brain tissues were weighed, transferred to a 500-μL Potter-Elvehjem Dounce homogenizer (Sigma-Aldrich, Allentown, PA, USA), and manually homogenized in 1% radioimmunoprecipitation buffer (RIPA buffer; 50 mM Tris, 150 mM NaCl, 5 mM EDTA, 0.5% sodium deoxycholate, 1% NP-40, and 0.1% SDS [pH 8.0]) with 0.2% PMSF and 0.1% protease inhibitors (Penn Center for Neurodegenerative Research [CNDR], Philadelphia, PA, USA). Homogenates were centrifuged at 21,380 × g RCF for 30 min, supernatant was harvested, and protein concentration was measured by the Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA). GRN was quantified by using a human progranulin ELISA kit (Adipogen, San Diego, CA, USA). 118 μg total protein was plated per well, with samples run in duplicate. Plates were read on a TriStar vTI LB 941 multimode reader (Berthold Technologies, Bad Wildbad, Germany) at a wavelength of 450 nm, and GRN concentration was calculated using the provided standard curve per kit instructions. Graphs were prepared using GraphPad Prism Version 7.00c for MacOS X and GraphPad software (La Jolla, CA, USA; www.graphpad.com).
Antibodies
Primary antibodies used for immunohistochemistry included the following: anti-human GRN rabbit polyclonal antibody (0.01 mg/mL; developed by CNDR, Philadelphia, PA, USA, as previously described15), anti-NeuN rabbit polyclonal antibody (0.5 μg/mL; ABN78; Sigma-Aldrich, St. Louis, MO, USA), anti-GFAP rabbit polyclonal antibody (0.58 μg/mL; Z0334; Dako-Agilent, Santa Clara, CA, USA), anti-Iba1 rabbit polyclonal antibody (0.25 μg/mL; Saf5299; Wako Pure Chemical Industries, Richmond, VA), anti-Ki67 rabbit monoclonal antibody (dilution 1:1,000; ab16667; Abcam, Cambridge, UK), anti-CD45R rat monoclonal antibody (5 μg/mL; RA3-6B2; Invitrogen, Carlsbad, CA, USA), and anti-CD3e rabbit monoclonal antibody (dilution 1:150; MA1-90582; Invitrogen, Carlsbad, CA, USA). Biotinylated goat anti-rabbit and goat anti-rat immunoglobulin G (IgG) secondary antibodies (Vector Laboratories, Burlingame, CA, USA) was used at a concentration of 1.5 μg/mL in all cases except for anti-GRN, for which the secondary antibody concentration was 7.5 μg/mL. In the autoantibody studies, biotinylated goat anti-mouse IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA) was used at a concentration of 0.75 μg/mL.
Section Preparation and Immunohistochemistry
Fixed coronal brain slices were serially ethanol dehydrated and paraffin embedded. Blocks were sectioned coronally at 6 μm and incubated at 37–42°C overnight. Sections were deparaffinized in xylene and rehydrated in serially dilute ethanol solutions, and they either were stained with H&E (Thermo Scientific, Kalamazoo, MI, USA; and Fisher Chemical, Waltham, MA, USA) or underwent immunohistochemical (IHC) staining as follows: sections underwent deactivation of endogenous peroxidase with 5% hydrogen peroxide in methanol for 30 min as well as microwave antigen retrieval using antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA), followed by washing in Tris buffer then blocking against nonspecific binding sites with 2% fetal bovine serum for 30 min at room temperature. Sections were then incubated in primary antibody overnight at 4°C in humidified chambers, blocked again, and incubated in biotinylated secondary antibody for 1 hr at room temperature. Sections were again blocked, followed by treatment for 1 hr with the VECTASTAIN ABC Kit (Vector Laboratories, Burlingame, CA, USA) for avidin binding and peroxidation, and then treated with Vector ImmPACT 3,3′-diaminobenzidine (DAB) peroxidase substrate solution for detection (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with hematoxylin and dehydrated prior to coverslipping. Bright-field images were taken on a Nikon 80i upright fluorescence microscope and analyzed with Nikon NIS-Elements AR Imaging software.
Quantitation of Hippocampal Toxicity
A 4-point scoring system was developed for rating the degree of hippocampal toxicity, with 0 representing normal hippocampus, 1 representing presence of infiltrate without cell loss, 2 representing focal cell loss, and 3 representing pervasive cell loss (Figure S5). Five evaluators experienced in mouse brain histopathology were presented with an anterior and a posterior H&E-stained slide from each mouse and were blinded with regard to time point and treatment. Each evaluator independently assigned each animal a score ranging from 0 to 4, using the scale described as a reference, and an average score was computed for each mouse. These scores were then averaged for each treatment group at each time point and plotted along with the SEM. Graphs were prepared using GraphPad Prism Version 7.00c for Mac OS X and GraphPad software (La Jolla, CA, USA; www.graphpad.com).
Section Preparation and Fluorescence Imaging
Fixed, cryoprotected brains were sectioned at 60 μm on a freezing microtome and stored at −20°C in a cryoprotectant solution (30% ethylene glycol, 15% sucrose, 0.05% sodium azide in PBS) until use. Sections were imaged using a DM6000B Leica microscope equipped with a Hamamatsu ORCA-Flash 4.0 camera.
Autoantibody Detection
Studies were performed as previously described.56 Adult female Wistar rats were anesthetized and decapitated. Brains were removed and washed in 1× PBS and then bisected sagitally and fixed in 4% paraformaldehyde in PBS at 4°C for 1 hr. Brains were then transferred to 40% sucrose in 0.1 M PBS for 48 hr, followed by embedding in Tissue-Tek O.C.T. Compound embedding medium (Sakura Finitek, Torrance, CA, USA), and then snap-frozen in 2-methylbutane cooled with liquid nitrogen. Sections were cut sagitally at 7 μm, mounted on glass slides, and stored at −20°C until use. Endogenous peroxidase was quenched with 0.3% hydrogen peroxide in PBS for 15 min. Sections were blocked in 5% goat normal serum for 1 hr, after which serum samples were applied to sections at a dilution of 1:200 in 5% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and incubated overnight at 4°C in humidified chambers. Sections were incubated in biotinylated goat anti-mouse secondary antibody for 2 hr at room temperature and then were treated for 1 hr with the VECTASTAIN Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) for avidin binding and peroxidation. Slides were incubated for 30 s in 0.5% Triton X solution in PBS and then treated with the Vector ImmPACT DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA) for detection. Slides were counterstained with 50% hematoxylin and dehydrated prior to coverslipping. Bright-field images were taken on a Nikon 80i upright fluorescence microscope and analyzed with Nikon NIS-Elements AR Imaging software.
Author Contributions
Conceptualization, A.S.C.-P., B.L.D., D.A.A., and J.M.R.; Methodology, A.S.C.-P., B.L.D., D.A.A., J.M.R., and E.L.; Investigation, D.A.A., J.M.R., F.D., P.H.-C., A.S., J.T.M., and J.Z.; Writing – Original Draft, D.A.A. and J.M.R.; Writing – Review & Editing, A.S.C.-P., B.L.D., D.A.A., J.M.R., J.Z., J.T.M., and E.L.; Funding Acquisition, A.S.C.-P., B.L.D., and D.A.A.; Resources and Supervision, A.S.C.-P. and B.L.D.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Masugi Nishihara for providing us with Grn null animals and Virginia Lee for providing GRN antibody reagents. Sources of support for this project include the Pechenik Montague Award Fund (to A.S.C.-P.), the Benaroya Award Fund (to A.S.C.-P.), and a Brody Family Medical Trust Fund fellowship (to D.A.A.).
Footnotes
Supplemental Information includes five figures and one table and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.11.013.
Contributor Information
Beverly L. Davidson, Email: davidsonbl@email.chop.edu.
Alice S. Chen-Plotkin, Email: chenplot@pennmedicine.upenn.edu.
Supplemental Information
References
- 1.Baker M., Mackenzie I.R., Pickering-Brown S.M., Gass J., Rademakers R., Lindholm C., Snowden J., Adamson J., Sadovnick A.D., Rollinson S. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 2.Cruts M., Gijselinck I., van der Zee J., Engelborghs S., Wils H., Pirici D., Rademakers R., Vandenberghe R., Dermaut B., Martin J.J. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 3.Smith K.R., Damiano J., Franceschetti S., Carpenter S., Canafoglia L., Morbin M., Rossi G., Pareyson D., Mole S.E., Staropoli J.F. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman M. Frontotemporal dementia: a review. J. Int. Neuropsychol. Soc. 2002;8:566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- 5.Chen-Plotkin A.S., Martinez-Lage M., Sleiman P.M., Hu W., Greene R., Wood E.M., Bing S., Grossman M., Schellenberg G.D., Hatanpaa K.J. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch. Neurol. 2011;68:488–497. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canafoglia L., Morbin M., Scaioli V., Pareyson D., D’Incerti L., Fugnanesi V., Tagliavini F., Berkovic S.F., Franceschetti S. Recurrent generalized seizures, visual loss, and palinopsia as phenotypic features of neuronal ceroid lipofuscinosis due to progranulin gene mutation. Epilepsia. 2014;55:e56–e59. doi: 10.1111/epi.12632. [DOI] [PubMed] [Google Scholar]
- 7.Almeida M.R., Macário M.C., Ramos L., Baldeiras I., Ribeiro M.H., Santana I. Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol. Aging. 2016;41:200.e1–200.e5. doi: 10.1016/j.neurobiolaging.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Kousi M., Lehesjoki A.E., Mole S.E. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum. Mutat. 2012;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 9.Capell A., Liebscher S., Fellerer K., Brouwers N., Willem M., Lammich S., Gijselinck I., Bittner T., Carlson A.M., Sasse F. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J. Neurosci. 2011;31:1885–1894. doi: 10.1523/JNEUROSCI.5757-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenik B., Sephton C.F., Dewey C.M., Xian X., Wei S., Yu K., Niu W., Coppola G., Coughlin S.E., Lee S.E. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J. Biol. Chem. 2011;286:16101–16108. doi: 10.1074/jbc.M110.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee W.C., Almeida S., Prudencio M., Caulfield T.R., Zhang Y.J., Tay W.M., Bauer P.O., Chew J., Sasaguri H., Jansen-West K.R. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum. Mol. Genet. 2014;23:1467–1478. doi: 10.1093/hmg/ddt534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh H., Chitramuthu B.P., Bennett H.P., Bateman A. Structure, function, and mechanism of progranulin; the brain and beyond. J. Mol. Neurosci. 2011;45:538–548. doi: 10.1007/s12031-011-9569-4. [DOI] [PubMed] [Google Scholar]
- 13.Jian J., Konopka J., Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J. Leukoc. Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkau T.L., Neal S.J., Orban P.C., MacDonald J.L., Hill A.M., Lu G., Feldman H.H., Mackenzie I.R., Leavitt B.R. Progranulin expression in the developing and adult murine brain. J. Comp. Neurol. 2010;518:3931–3947. doi: 10.1002/cne.22430. [DOI] [PubMed] [Google Scholar]
- 15.Chen-Plotkin A.S., Xiao J., Geser F., Martinez-Lage M., Grossman M., Unger T., Wood E.M., Van Deerlin V.M., Trojanowski J.Q., Lee V.M. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol. 2010;119:111–122. doi: 10.1007/s00401-009-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme P., Van Hoecke A., Lambrechts D., Vanacker P., Bogaert E., van Swieten J., Carmeliet P., Van Den Bosch L., Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J. Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X., Joselin A.P., Wang L., Kar A., Ray P., Bateman A., Goate A.M., Wu J.Y. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3β. Protein Cell. 2010;1:552–562. doi: 10.1007/s13238-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beel S., Moisse M., Damme M., De Muynck L., Robberecht W., Van Den Bosch L., Saftig P., Van Damme P. Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Hum. Mol. Genet. 2017;26:2850–2863. doi: 10.1093/hmg/ddx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu F., Padukkavidana T., Vægter C.B., Brady O.A., Zheng Y., Mackenzie I.R., Feldman H.H., Nykjaer A., Strittmatter S.M. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami S.S., Min S.W., Krabbe G., Wang C., Zhou Y., Asgarov R., Li Y., Martens L.H., Elia L.P., Ward M.E. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat. Med. 2014;20:1157–1164. doi: 10.1038/nm.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Kampen J.M., Kay D.G. Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer’s disease. PLoS ONE. 2017;12:e0182896. doi: 10.1371/journal.pone.0182896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Kampen J.M., Baranowski D., Kay D.G. Progranulin gene delivery protects dopaminergic neurons in a mouse model of Parkinson’s disease. PLoS ONE. 2014;9:e97032. doi: 10.1371/journal.pone.0097032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird A.S., Van Hoecke A., De Muynck L., Timmers M., Van den Bosch L., Van Damme P., Robberecht W. Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PLoS ONE. 2010;5:e13368. doi: 10.1371/journal.pone.0013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitramuthu B.P., Kay D.G., Bateman A., Bennett H.P. Neurotrophic effects of progranulin in vivo in reversing motor neuron defects caused by over or under expression of TDP-43 or FUS. PLoS ONE. 2017;12:e0174784. doi: 10.1371/journal.pone.0174784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tauffenberger A., Chitramuthu B.P., Bateman A., Bennett H.P., Parker J.A. Reduction of polyglutamine toxicity by TDP-43, FUS and progranulin in Huntington’s disease models. Hum. Mol. Genet. 2013;22:782–794. doi: 10.1093/hmg/dds485. [DOI] [PubMed] [Google Scholar]
- 26.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359:eean4672. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 28.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 29.Sands M.S., Davidson B.L. Gene therapy for lysosomal storage diseases. Mol. Ther. 2006;13:839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Katz M.L., Tecedor L., Chen Y., Williamson B.G., Lysenko E., Wininger F.A., Young W.M., Johnson G.C., Whiting R.E., Coates J.R., Davidson B.L. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci. Transl. Med. 2015;7:313ra180. doi: 10.1126/scitranslmed.aac6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.H., Chang M., Davidson B.L. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteys A.M., Ebanks S.A., Keiser M.S., Davidson B.L. CRISPR/Cas9 editing of the mutant huntingtin allele in vitro and in vivo. Mol. Ther. 2017;25:12–23. doi: 10.1016/j.ymthe.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer K., Ferraiuolo L., Schmelzer L., Braun L., McGovern V., Likhite S., Michels O., Govoni A., Fitzgerald J., Morales P. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol. Ther. 2015;23:477–487. doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haurigot V., Marcó S., Ribera A., Garcia M., Ruzo A., Villacampa P., Ayuso E., Añor S., Andaluz A., Pineda M. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J. Clin. Invest. 2013;123:3254–3271. doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingozzi F., High K.A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 36.Vandamme C., Adjali O., Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arrant A.E., Onyilo V.C., Unger D.E., Roberson E.D. Progranulin gene therapy improves lysosomal dysfunction and microglial pathology associated with frontotemporal dementia and neuronal ceroid lipofuscinosis. J. Neurosci. 2018;38:2341–2358. doi: 10.1523/JNEUROSCI.3081-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arrant A.E., Filiano A.J., Unger D.E., Young A.H., Roberson E.D. Restoring neuronal progranulin reverses deficits in a mouse model of frontotemporal dementia. Brain. 2017;140:1447–1465. doi: 10.1093/brain/awx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Y., Mu D., Prabhakar S., Moser A., Musolino P., Ren J., Breakefield X.O., Maguire C.A., Eichler F.S. Adenoassociated virus serotype 9-mediated gene therapy for X-linked adrenoleukodystrophy. Mol. Ther. 2015;23:824–834. doi: 10.1038/mt.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G., Martins I., Wemmie J.A., Chiorini J.A., Davidson B.L. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J. Neurosci. 2005;25:9321–9327. doi: 10.1523/JNEUROSCI.2936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodge J.C., Treleaven C.M., Fidler J.A., Hester M., Haidet A., Handy C., Rao M., Eagle A., Matthews J.C., Taksir T.V. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol. Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed Z., Sheng H., Xu Y.F., Lin W.L., Innes A.E., Gass J., Yu X., Wuertzer C.A., Hou H., Chiba S. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am. J. Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wils H., Kleinberger G., Pereson S., Janssens J., Capell A., Van Dam D., Cuijt I., Joris G., De Deyn P.P., Haass C. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J. Pathol. 2012;228:67–76. doi: 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- 44.Ghoshal N., Dearborn J.T., Wozniak D.F., Cairns N.J. Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol. Dis. 2012;45:395–408. doi: 10.1016/j.nbd.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster E., Martinez-Hernandez E., Titulaer M.J., Boulos M., Weaver S., Antoine J.C., Liebers E., Kornblum C., Bien C.G., Honnorat J. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancaster E., Dalmau J. Neuronal autoantigens--pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson B.L., Stein C.S., Heth J.A., Martins I., Kotin R.M., Derksen T.A., Zabner J., Ghodsi A., Chiorini J.A. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudry E., Dashkoff J., Roe A.D., Takeda S., Koffie R.M., Hashimoto T., Scheel M., Spires-Jones T., Arbel-Ornath M., Betensky R. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci. Transl. Med. 2013;5:212ra161. doi: 10.1126/scitranslmed.3007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Zheng S., Tecedor L., Davidson B.L. Overcoming limitations inherent in sulfamidase to improve mucopolysaccharidosis IIIA gene therapy. Mol. Ther. 2018;26:1118–1126. doi: 10.1016/j.ymthe.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liau L.M., Lallone R.L., Seitz R.S., Buznikov A., Gregg J.P., Kornblum H.I., Nelson S.F., Bronstein J.M. Identification of a human glioma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res. 2000;60:1353–1360. [PubMed] [Google Scholar]
- 53.Menges C.W., Chen Y., Mossman B.T., Chernoff J., Yeung A.T., Testa J.R. A Phosphotyrosine proteomic screen identifies multiple tyrosine kinase signaling pathways aberrantly activated in malignant mesothelioma. Genes Cancer. 2010;1:493–505. doi: 10.1177/1947601910375273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu G., Martins I.H., Chiorini J.A., Davidson B.L. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 55.Kayasuga Y., Chiba S., Suzuki M., Kikusui T., Matsuwaki T., Yamanouchi K., Kotaki H., Horai R., Iwakura Y., Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav. Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 56.McCracken L., Zhang J., Greene M., Crivaro A., Gonzalez J., Kamoun M., Lancaster E. Improving the antibody-based evaluation of autoimmune encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e404. doi: 10.1212/NXI.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.