Abstract
Study Objectives
Insomnia is a common symptom in the clinical course of schizophrenia. There is a robust association between insomnia and suicidality in other psychiatric disorders. Two previous studies found associations between insomnia and suicide attempt or completed suicide in patients with schizophrenia. We hypothesized that greater insomnia would be associated with greater levels of suicidal ideation in patients with schizophrenia and other nonaffective psychoses.
Methods
We recruited 108 inpatients and outpatients age 18–65 between July 2010 and July 2016 with DSM-IV nonaffective psychosis (schizophrenia, schizoaffective disorder, or schizophreniform disorder). We investigated relationships between current insomnia (Insomnia Severity Index [ISI]), suicidal ideation over the past week, and lifetime history of suicide attempt (Beck Scale for Suicide Ideation [BSS]) in regression analyses.
Results
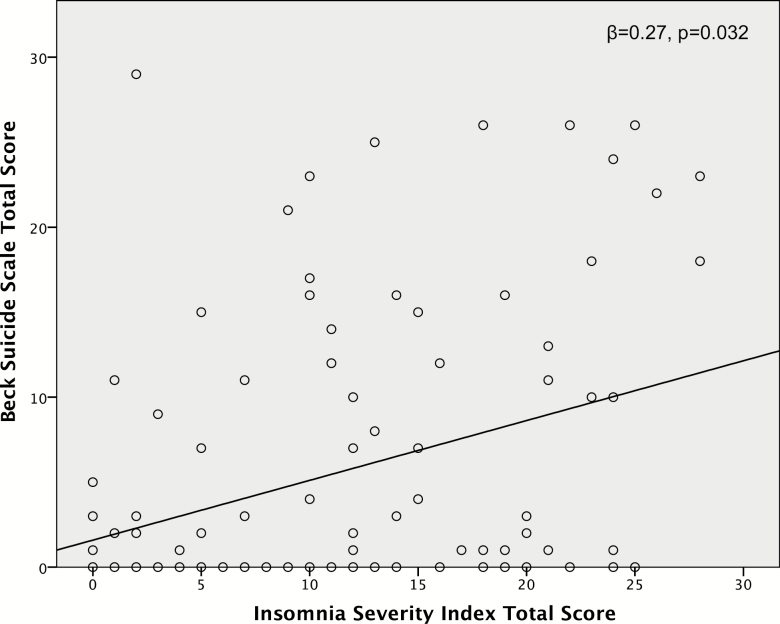
After controlling for potential confounders, insomnia was a significant indicator of suicidal ideation (β = 0.27, p = 0.032). Insomnia was also a significant indicator of a high BSS score (≥16; OR = 1.14, 95% CI: 1.01–1.28, p = 0.029). Furthermore, participants with severe insomnia were almost 15 times more likely to have a lifetime history suicide attempt than participants without current insomnia (OR = 14.8, 95% CI: 1.4–157, p = 0.025). Insomnia was also an indicator of greater PANSS total (β = 0.33, p = 0.001), positive subscale (β = 0.32, p = 0.002), and general subscale (β = 0.40, p < 0.001) scores.
Conclusions
Insomnia is associated with suicidal ideation, lifetime suicide attempt, and greater psychopathology in patients with schizophrenia. Our findings suggest that formal assessment of insomnia may be germane to the clinical care of patients with schizophrenia as a marker of suicide risk and symptom severity.
Keywords: insomnia, psychiatric disorders, schizophrenia, suicide
Statement of Significance
Insomnia is a common symptom in the clinical course of schizophrenia. There is a robust association between insomnia and suicidality in other psychiatric disorders, which has been understudied in patients with schizophrenia. This study found that in patients with schizophrenia, current insomnia is an indicator of current suicidal ideation, lifetime history of suicide attempt, and greater psychopathology. Findings suggest that formal assessment of insomnia may be germane to the clinical care of patients with schizophrenia as a marker of suicide risk and symptom severity.
Introduction
Insomnia is a common symptom in schizophrenia, independent of medication status and clinical course, which may be mediated by dysfunctional and misaligned circadian rhythms [1, 2]. A recent systematic review of patients with early psychosis found that sleep disturbances and abnormalities in sleep architecture and spindles may be correlated with symptom severity [3]. Insomnia is also robustly associated with suicidality in patients with psychiatric disorders; however, there are a paucity of studies on this association in schizophrenia. In a meta-analysis of 19 studies, patients with psychiatric diagnoses and comorbid sleep disturbance were twofold more likely to report suicidal behaviors than those without sleep disturbance [4]. This association was found in patients with depression, posttraumatic stress disorder, panic disorder, and schizophrenia. Findings for schizophrenia were based on a single case–control study of 20 males who died by suicide and 20 living patients treated at the same time and at the same outpatient clinic [5]. In this study, patients with schizophrenia who died by suicide were almost 13 times more likely to have reported insomnia.
More recently, Li et al. [6] performed a naturalistic, longitudinal study in 388 outpatients with schizophrenia-spectrum disorders. Participants completed a sleep questionnaire at baseline, and clinical information was extracted from case notes over an 8 year period of follow-up. They found that baseline frequent insomnia, at least three times per week in the past year, was common (19%) and a significant predictor of increased risk of suicide attempt over the follow-up period (adjusted HR = 4.63, 95% CI: 1.40–15.36). Andriopoulos et al. [7] retrospectively evaluated the prodrome of 106 consecutively admitted patients with recent-onset schizophrenia. They found that patients with sleep disturbance had an increased prevalence of suicidal ideation, but not suicide attempt, during the prodromal period compared with patients without sleep disturbance. Outside of schizophrenia, case–control and longitudinal studies have found that insomnia is associated with increased subclinical psychotic experiences in adolescents, young adults, and adults [8–10]. Experimental studies also show that induction of insomnia is associated with increased psychotic experiences [11].
Schizophrenia is also associated with increased inflammation, including elevated levels of the acute phase protein C-reactive protein (CRP) and cytokines such as interleukin-6 [12, 13]. There is some evidence that inflammation may be associated with insomnia and suicidality in patients with psychiatric disorders, although data in patients with schizophrenia are limited. Patients with psychiatric disorders and suicidality also have higher blood and postmortem brain interleukin-1-β and interleukin-6 levels than patients without suicidality and controls [14]. There is evidence outside of psychosis from experimental sleep deprivation studies that short-term sleep loss is associated with increased CRP and interleukin-6 levels, as well as increased interleukin-6 levels in patients with insomnia versus controls [15]. An actigraphy study in 199 inpatients with schizophrenia found that higher white blood cell counts and the neutrophil-to-lymphocyte ratio, both markers of inflammation, were associated with greater impairments in sleep quality [16].
Although insomnia is robustly associated with suicidality in other psychiatric disorders, data on this association in patients with schizophrenia are limited. Furthermore, to the best of our knowledge, no previous studies have investigated relationships between current insomnia and current suicidal ideation in patients with schizophrenia and other nonaffective psychoses. This gap in the literature has important clinical implications, as a positive association between current insomnia and current suicidal ideation would support a greater emphasis on the clinical assessment of and interventions for insomnia in this patient population. We hypothesized that greater insomnia would be associated with greater levels of suicidal ideation and lifetime history of suicide attempt in patients with nonaffective psychosis. We also hypothesized (exploratory secondary aim) that higher levels of inflammation, as measured by CRP, would be associated with greater insomnia and suicidal ideation in these patients.
Methods
Participants
One hundred eight inpatients and outpatients aged 18–65 and diagnosed with schizophrenia (n = 52) or related nonaffective psychoses, including schizoaffective disorder (n = 51), psychotic disorder not otherwise specified (n = 4), or schizophreniform disorder (n = 1), who also participated in another ongoing study of immune function in nonaffective psychosis, were recruited in the Augusta, GA area between July 2010 and July 2016. The broader category of nonaffective psychosis, which includes schizoaffective disorder, appears to share characteristics of schizophrenia [17, 18]. Participants with nonaffective psychosis were referred to the investigators by their inpatient or outpatient psychiatrist, who asked the patients if they were interested in participating in a research study. Interested participants met with study investigators, who screened them based on the study inclusion/exclusion criteria. Inclusion criteria were males and females; age 18–65; having capacity to give informed consent; and a DSM-IV diagnosis schizophrenia, schizophreniform disorder, brief psychotic disorder, schizoaffective disorder, delusional disorder, or psychotic disorder not otherwise specified. Exclusion criteria were alcohol withdrawal; antibiotic use in the past 14 days; current lower urinary tract infection (based on urinalysis and urine culture); pregnancy; current scheduled use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or other immunomodulatory agents; history of an immune disorder; illicit drug use in the past 30 days; and current use of clozapine. All participants had a negative urine drug screen. Antipsychotic medications were not standardized, but the majority of participants (70%) were treated with second-generation antipsychotic monotherapy.
Procedures
After providing their own written informed consent, participants underwent a laboratory, physical, and psychiatric diagnostic evaluation. Participants had a blood draw between 8 and 9 am after a 10 hr fast for high-sensitivity C-reactive protein (hsCRP). Blood analyses were performed at Clinical Pathology Laboratories Southeast (Augusta, GA). hsCRP levels were measured using an enzyme-linked immunosorbent assay. A urine sample was obtained for a urinalysis, urine culture, and urine drug screen. Vital signs, height/weight, and medical history were obtained. Psychiatric diagnosis was verified using the Structured Clinical Interview for DSM-IV disorders (SCID) psychosis and mood-disorders modules [19]. One experienced rater (B.J.M.) performed all of the SCID interviews. Symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) [20]. Two raters with established inter-rater reliability performed PANSS interviews for participants in this study; one rater (B.J.M.) performed the majority of the interviews (n = 89, 83%). These two raters established inter-rater reliability for both SCID and PANSS on a series of training vignettes and videos prior to the study, as well as additional videos rated every 3 months over the course of the study to ensure continued inter-rater reliability. Data on current antipsychotic medications were used to calculate the participant’s current daily dose in chlorpromazine (CPZ) equivalents [21, 22]. Data on smoking (number of cigarettes per day) and other substance use were obtained using the Dartmouth Assessment of Lifestyle Inventory (DALI) [23]. Data on socioeconomic status (SES) were obtained using the Hollingshead–Redlich Scale [24]. Childhood trauma was assessed using the Adverse Childhood Experiences questionnaire [25]. Current insomnia (over the preceding 2 weeks) was assessed using the Insomnia Severity Index (ISI) [26]. The ISI consists of seven items, each rated between 0 (none) and 4 (very severe); total score range 0–28. Suicidal ideation over the past week and lifetime history of suicide attempt were assessed using the Beck Scale for Suicide Ideation (BSS) [27]. The BSS consists of 19 items, each rated between 0 and 2; total score range 0–38. The study was approved by the IRB’s of both Augusta University and the Georgia Department of Community Health.
Statistical analysis
The data were analyzed using SPSS version 24 (SPSS, Inc.; Chicago, IL). Descriptive statistics (means, standard deviations, and proportions) were calculated for demographic and clinical variables. A one-sample Kolmogorov–Smirnov test was used to examine each variable for normality. ISI scores, BSS scores, and hsCRP were all non-normally distributed. We first calculated bivariate correlation coefficients (Spearman’s rho) between insomnia (ISI total score), suicidal ideation (BSS total score), demographic, and clinical variables, including hsCRP. We then investigated the association between insomnia and suicidal ideation as continuous measures using linear regression models. The assumptions of linear regression were met. Age, sex, race, smoking, BMI, alcohol use, trauma, PANSS total score, and CPZ equivalents were considered as potential confounding and/or moderating factors, and were included in the regression model only if they were correlated with insomnia or suicide with p < 0.10. Given evidence that a BSS total score of ≥16 may represent elevated suicide risk [28], we also investigated the association between insomnia and suicidal ideation as a categorical variable (BSS total score of <16 versus ≥16) using logistic regression, considering the same potential confounding/moderating factors as described above.
We also investigated the association between insomnia as a categorical variable and suicidal ideation. ISI total scores of 0–7 represent no clinically significant insomnia, 8–14 subthreshold insomnia, 15–21 moderate insomnia, and 22–28 severe insomnia. We compared BSS total scores between the four categorical insomnia groups using the Kruskall–Wallis H-test, and post hoc pairwise comparisons using the Mann–Whitney U-test. Lastly, we investigated insomnia as an indicator of lifetime suicide attempt (BSS item 20) as a categorical variable using logistic regression, again considering the same potential confounding/moderating factors. In the analyses of lifetime suicide attempt, we modeled insomnia as both continuous and categorical variables in separate logistic regression analyses. For all analyses, results were considered statistically significant at α = 0.05 level (two-sided).
Results
One hundred eight participants were enrolled in the study. As described in Table 1, in the entire sample, the mean age was 42, 67% were male, 66% were of African descent, and the mean PANSS score was 71. The mean (SD) ISI total score was 11.3 (8.0), and the mean BSS score was 5.3 (8.0). Details for inpatients and outpatients, separately, are also presented in Table 1.
Table 1.
Demographic, clinical, and laboratory characteristics of the study sample
| All participants | Inpatients | Outpatients | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Variables | N = 108 | N = 62 | N = 46 | P* |
| Age | 41.6 (12.9) | 39.6 (12.5) | 44.3 (13.2) | 0.06 |
| Smoking (cigarettes/day) | 7.1 (10.2) | 9.1 (11.1) | 4.5 (8.9) | 0.01 |
| BMI | 31.2 (7.8) | 30.9 (9.0) | 31.4 (7.4) | 0.76 |
| SES | 31.5 (11.4) | 31.4 (12.7) | 31.9 (9.5) | 0.84 |
| CPZ equivalents | 382 (401) | 350 (285) | 445 (528) | 0.26 |
| Age of onset of psychosis | 19.1 (9.9) | 18.1 (9.6) | 20.3 (10.4) | 0.27 |
| hsCRP (mg/L) | 7.5 (9.7) | 7.5 (11.0) | 7.5 (7.7) | 0.47 |
| ISI total score† | 11.3 (8.0) | 13.5 (7.7) | 8.0 (7.3) | <0.01 |
| BSS total score† | 5.3 (8.0) | 8.0 (8.8) | 2.3 (5.7) | <0.01 |
| PANSS Total† | 70.8 (18.1) | 80.8 (14.7) | 58.1 (13.4) | <0.01 |
| PANSS Positive† | 18.2 (6.8) | 21.7 (5.9) | 13.8 (5.2) | <0.01 |
| PANSS Negative† | 16.3 (5.9) | 17.8 (6.4) | 14.5 (4.6) | <0.01 |
| PANSS General† | 36.4 (9.2) | 41.4 (7.1) | 29.7 (7.1) | <0.01 |
| n (%) | n (%) | n (%) | ||
| Diagnosis | 0.31 | |||
| Schizophrenia | 52 (48.1) | 29 (46.8) | 23 (50.0) | |
| Schizoaffective disorder | 51 (47.3) | 28 (45.2) | 23 (50.0) | |
| Psychosis NOS | 4 (3.7) | 4 (6.5) | 0 (0.0) | |
| Schizophreniform disorder | 1 (0.9) | 1 (1.6) | 0 (0.0) | |
| Sex | 0.02 | |||
| Male | 72 (66.7) | 47 (75.8) | 25 (54.3) | |
| Female | 36 (33.3) | 15 (24.2) | 21 (45.7) | |
| Race | 0.52 | |||
| African descent | 71 (65.7) | 38 (61.3) | 34 (73.9) | |
| Caucasian | 33 (30.6) | 21 (33.9) | 11 (21.7) | |
| Hispanic | 2 (1.9) | 1 (1.6) | 1 (2.2) | |
| South Asian | 1 (0.9) | 1 (1.6) | 0 (0.0) | |
| Other | 1 (0.9) | 1 (1.6) | 0 (0.0) |
†Possible ranges for scores for these rating instruments are as follows:
ISI total score: 0–28
BSS total score: 0–38
PANSS Total score: 30–210
PANSS Positive score: 7–49
PANSS Negative score: 7–49
PANSS General score: 16–112
*p-Values for continuous variables are from either Student’s t-test, two-sided, or Mann–Whitney U-test.
p-Values for categorical variables are from chi-square test.
Insomnia and suicidal ideation
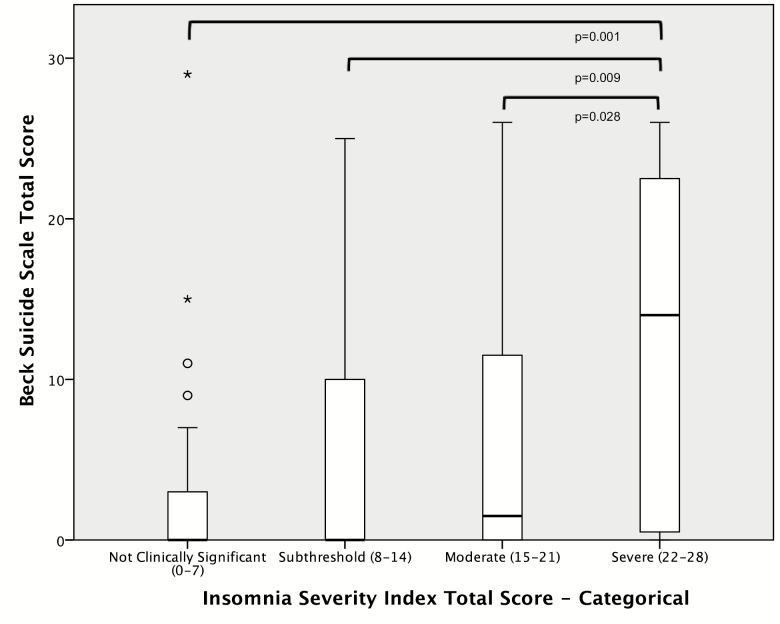
ISI total score was significantly positively correlated with BSS total score (ρ = 0.29, p = 0.002). In a linear regression model controlling for race, alcohol use, trauma, PANSS total score, and CPZ equivalents, insomnia remained a significant indicator of suicidal ideation (β = 0.27, p = 0.032; see also Figure 1). The pattern of the results was unchanged when restricting the analysis to participants with schizophrenia only (data not shown). BSS total scores were significantly different across categorical insomnia groups (H = 0.010). Participants with severe insomnia had significantly higher mean (SD) BSS scores (13.3 [10.3]) than participants with moderate insomnia (4.6 [7.0]), subthreshold insomnia (5.4 [7.9]), or no insomnia (2.8 [5.6]; p ≤ 0.03 for each, see also Figure 2). In a logistic regression model controlling for race, alcohol use, trauma, PANSS total score, and CPZ equivalents, insomnia was a significant indicator of a BSS total score of ≥16 (OR = 1.14, 95% CI: 1.01–1.28, p = 0.029; see also Table 2).
Figure 1.
Linear regression analysis of insomnia as a predictor of suicidal ideation.
Figure 2.
Categorical insomnia severity as a predictor of suicidal ideation.
Table 2.
Logistic regression models of insomnia as a predictor of suicidality
| Final logistic regression model | ||||
|---|---|---|---|---|
| Dependent variables | Predictor | OR (95% CI) | P* | |
| BSS Score ≥ 16 | ISI Total Score (continuous) | 1.14 | (1.01–1.28) | 0.029 |
| Lifetime Suicide Attempt | ISI Total Score (continuous) | 1.10 | (1.03–1.19) | 0.008 |
| ISI Total Score (categorical) | ||||
| Not clinically significant (0–7) | 1.00 | (Reference) | ||
| Subthreshold (8–14) | 1.45 | (0.40–5.27) | 0.577 | |
| Moderate (15–21) | 3.29 | (0.84–12.91) | 0.087 | |
| Severe (22–28) | 14.83 | (1.40– 157.03) | 0.025 | |
*Bolded p-values are significant at the α = 0.05 level.
Insomnia and lifetime history of suicide attempt
Participants with a lifetime history of suicide attempt had significantly higher mean ISI total scores than participants without such a history (14.6 [7.8] versus 8.2 [7.0], p < 0.001). The pattern of the results was unchanged when restricting the analysis to participants with schizophrenia only (data not shown). In a logistic regression model controlling for race, alcohol use, trauma, and PANSS total score, insomnia remained a significant indicator of lifetime suicide attempt (OR = 1.1, 95% CI: 1.03–1.19, p = 0.008; see also Table 2). In logistic regression model controlling for the same potential confounding factors, participants with current severe insomnia were almost 15 times more likely to have a history of lifetime suicide attempt than participants without current clinically significant insomnia (OR = 14.8, 95% CI: 1.4–157, p = 0.025; see also Table 2).
Insomnia and psychopathology
In the analyses of insomnia and suicidal ideation, insomnia was significantly correlated with psychopathology. Therefore, in post hoc analyses, we investigated the relationship between insomnia and PANSS scores. ISI total score was significantly positively correlated with PANSS total (ρ = 0.33, p = 0.001), positive subscale (ρ = 0.37, p < 0.001), and general subscale (ρ = 0.42, p < 0.001) scores. In linear regression analyses controlling for sex, race, CPZ equivalents, trauma, and alcohol use, ISI total score remained a significant indicator of PANSS scores. This association was no longer significant when restricting the analysis to participants with schizophrenia only (data not shown).
In exploratory post hoc analyses, we also repeated the analyses of insomnia, suicidal ideation, and psychopathology in inpatients and outpatients with nonaffective psychosis, separately. In a linear regression model controlling for sex, race, alcohol use, and CPZ equivalents, insomnia was a significant indicator of suicidal ideation (β = 0.41, p = 0.013) in inpatients with nonaffective psychosis. However, in inpatients, insomnia was not correlated with PANSS total or subscale scores.
By contrast, in a linear regression model controlling for race, CPZ equivalents, and PANSS total score, insomnia was an indicator of suicidal ideation at the trend level (β = 0.29, p = 0.056) in outpatients with nonaffective psychosis. However, in outpatients, ISI total score was significantly positively correlated with PANSS total (ρ = 0.38, p = 0.010), positive subscale (ρ = 0.40, p = 0.006), and general subscale (ρ = 0.48, p = 0.001) scores. Insomnia was significantly positively correlated with the following individual PANSS items: delusions, hallucinations, suspiciousness, anxiety, depression, poor impulse control, and active social avoidance (p < 0.05 for each).
Inflammation, insomnia, and suicidal ideation
In the entire sample and in patients with schizophrenia only, hsCRP levels were not correlated with either ISI (ρ = −0.13, p = 0.19) or BSS (ρ = −0.11, p = 0.27) total scores. In outpatients with nonaffective psychosis, blood hsCRP levels were significantly negatively correlated with insomnia (ρ = −0.30, p = 0.04), but not suicidal ideation (ρ = −0.06, p = 0.64). In a linear regression model controlling for race, BMI, trauma, and PANSS total score, hsCRP levels were a significant indicator of insomnia at (β = −0.41, p = 0.039) in these outpatients. By contrast, hsCRP levels were not correlated with insomnia or suicidal ideation in inpatients with nonaffective psychosis.
Discussion
We found that after controlling for potential confounders, current insomnia was a significant indicator of suicidal ideation, both as a continuous and categorical measure, in patients with nonaffective psychosis. Patients with nonaffective psychosis and current severe insomnia were almost 15 times more likely to a lifetime history of suicide attempt than patients without clinically significant insomnia. Furthermore, greater insomnia was also associated with higher levels of psychopathology, including total, positive, and general symptoms, particularly in outpatients. These findings—which underscore the clinical importance of the recognition and treatment of insomnia in this patient population—extend previous associations between sleep disturbance and suicidality in nonaffective psychosis [5–7]. There are several strengths of the present study. We extended previous work in this area by investigating relationships between current insomnia and suicidal ideation. Previous studies have focused on relationships between sleep disturbance and suicide attempt or death by suicide in schizophrenia [5, 6]. We also investigated relationships between inflammation, insomnia, and suicidal ideation, which have not previously been studied. Another strength is that we considered multiple potential confounding factors in the analyses, including age, sex, race, smoking, BMI, alcohol use, trauma, psychopathology, and antipsychotic dose. Importantly, the association between insomnia and suicidal ideation was similar in inpatients and outpatients, and did not appear to be driven by acute exacerbation of psychosis.
Several potential limitations of the present study are the heterogeneity of the sample with respect to clinical status, the nonstandardized antipsychotic treatment, and the absence of objective measurements of insomnia (e.g. actigraphy or polysomnography). Due to the cross-sectional design, our study does not permit inferences regarding the ability of baseline insomnia to predict incident suicide attempt or death by suicide, though we did find an association between current insomnia and lifetime suicide attempt. Perhaps most importantly, our study also does not permit inferences regarding the temporal nature of this association. Although higher levels of insomnia may lead to worsening suicidal ideation, it is equally possible that higher levels of suicidality may lead to worsening insomnia. In linear regression analyses, the strength of the association was similar when considering either insomnia as an indicator of suicidal ideation (β = 0.27) or suicidal ideation as an indicator of insomnia (β = 0.22). Therefore, rigorously designed longitudinal studies are needed to further disentangle these associations. Future studies should also assess for comorbid sleep disorders, such as obstructive sleep apnea and periodic limb movement disorders, which may be prevalent in this patient population. Given that depression is also highly comorbid with insomnia, suicidality, and nonaffective psychosis, its role as a potential moderating factor also warrants additional investigation.
Although we did not find an association between hsCRP levels and insomnia in the entire sample, we did find a significant inverse association between hsCRP levels and insomnia in outpatients with nonaffective psychosis. There is meta-analytic evidence for increased CRP levels in patients with schizophrenia compared with healthy controls [13]. Interestingly, a recent Mendelian randomization analysis found that CRP genetic variants were associated with a decreased risk of schizophrenia [29]. In patients with schizophrenia, higher CRP levels are associated with increased risk of the metabolic syndrome (a constellation of metabolic risk factors associated with the development of atherosclerotic cardiovascular disease) [30] and mortality [31] (Horsdal et al.), as well as greater cognitive impairment [32]. To the best of our knowledge, ours is the first study to explore associations between insomnia and inflammation in nonaffective psychosis. Several studies have reported positive associations between higher levels of CRP (and other inflammatory markers) and sleep disturbance in nonpsychotic populations [33–35]. Our findings—which should be interpreted with caution in light of modest sample size—stand in contrast to these previous studies and warrant replication in a larger sample.
We did not find an association between hsCRP levels and suicidal ideation in the present study. Although there is evidence that higher CRP levels may be associated with suicidal ideation and/or suicide attempt in patients with mood disorders [36–38], there are failures to replicate [39]. One previous study in patients with schizophrenia found significantly higher CRP levels in patients with lifetime history of suicide attempt, but with a suicide attempt in the past month, compared with those without such a history [40]. Again, in light of our modest sample size, the relationship between hsCRP and suicidal ideation in nonaffective psychosis should be investigated in a larger sample.
Our findings contribute to a broader literature regarding associations between insomnia and suicidality. The association between insomnia and suicidality is well-established in depression, including in clinical trials [4, 41]. A meta-analysis also found that insomnia was associated with suicidality in anxiety disorders, including posttraumatic stress disorder and panic disorder [4]. Insomnia is also an emerging mechanistic explanation mediating the association between alcohol use and risk of suicidality [42]. Given associations across multiple, disparate diagnoses, findings suggest that insomnia is a risk factor for suicidality that transcends diagnosis.
What potential mechanisms might mediate the association between insomnia and suicidal ideation? In patients with depression, several psychological and physiological mechanisms have been proposed, including cognitive distortions about sleep, impaired decision-making/cognitive dysfunction, serotonergic hypofunction, and hyperarousal/hypothalamic–pituitary–adrenal (HPA) axis dysfunction [43]. Reasons for insomnia in schizophrenia are likely multifactorial, but these same mechanisms are potentially relevant. Hallucinations and delusions may interfere with sleep, and patients with schizophrenia may also have cognitive distortions about sleep based on past experiences [44]. Cognitive dysfunction is a core symptom domain in schizophrenia; however, several previous studies have found that better cognition is associated with suicidal ideation and suicide attempt in the disorder [45]. There is evidence for serotonergic dysfunction in schizophrenia [46]. Although decreased cerebrospinal fluid concentrations of the serotonin metabolite 5-hydroxyindolacetic acid (5-HIAA) is a well-replicated finding in the neurobiology of suicide, a previous study did not find an association between 5-HIAA and suicidality in schizophrenia [47]. There is also evidence for HPA-axis dysfunction in schizophrenia [48]. Further investigation of these mechanisms in schizophrenia is warranted.
Many sleep disturbances in schizophrenia, including insomnia, may be associated with misalignment of circadian rhythms in sleep–wake and melatonin cycles [1, 2]. There is evidence for altered circadian clock gene expression in patients with schizophrenia [49]. There is also evidence for abnormal circadian rest-activity rhythms in participants at clinical high risk for psychosis [50], and that these abnormalities may predict severity of symptoms and psychosocial impairment over 1 year of follow-up [51]. In an online survey of 111 clinicians regarding the treatment of patients with nonaffective psychosis, although all clinicians reported sleep disturbance in their patients, 82% assessed sleep problems informally (versus standard assessment measures) [52]. Our findings suggest that formal assessment of insomnia may be germane to the clinical care of patients with schizophrenia as a marker of suicidality and symptom severity. Therefore, future studies should use standard instruments to assess both insomnia (such as the ISI) and circadian rhythm dysfunction (such as the Morningness–Eveningness Questionnaire), in order to disentangle these relationships. These assessment scales are brief and easily administered. Additional longitudinal studies of associations between insomnia and suicidality are also warranted.
Given associations between greater insomnia and higher levels of psychopathology, particularly in outpatients, our findings also suggest that insomnia may represent an important treatment target in nonaffective psychosis. A meta-analysis of 31 polysomnographic studies (574 patients and 515 controls) found that patients with schizophrenia have significantly shorter total sleep time, longer sleep onset latency, more wake time after sleep onset, lower sleep efficiency, and decreased stage 4 sleep, slow-wave sleep, and duration and latency of rapid eye movement sleep compared with healthy controls [53]. There is evidence that treatment first- and second-generation antipsychotics is associated with increased sleep time and sleep efficiency [54]. Another study found that patients treated with clozapine monotherapy for more than a year had significant stabilization of rest-activity cycles [55]. Whether beneficial effects on insomnia contribute to the antisuicidal properties of clozapine and other antipsychotics represents another potential area for future study.
Interestingly, one study found that greater baseline insomnia predicted greater worsening of psychotic symptoms following antipsychotic withdrawal [56]. Given the high prevalence of medication nonadherence in schizophrenia, this represents an important potential issue. In the online survey by Rehman et al. [52], the authors found that sleep hygiene and medications were the most common treatment approaches, with infrequent reported use of cognitive behavioral therapy. A pilot-randomized controlled trial in 50 participants with schizophrenia found that after 12 weeks for cognitive behavioral therapy (plus standard care), 41% of participants no longer had insomnia based on ISI scores [57]. A small qualitative study in 14 participants found that participants with schizophrenia reported that CBT was more acceptable, with more perceived benefits and fewer perceived limitations, than pharmacotherapy [58]. By contrast, an 8 week RCT in 39 participants with schizophrenia or schizoaffective disorder and insomnia found that adjunctive eszopiclone was associated with significant improvements in ISI scores and cognition, but not psychiatric symptoms [59]. In addition to its impact on psychiatric symptoms, insomnia may be associated with additional comorbidity in patients with schizophrenia. In a study of 80 patients with schizophrenia, the presence of middle insomnia was associated with a 3.4-fold increased odds of the metabolic syndrome [60]. Another study in 612 Chinese patients with schizophrenia found that insomnia was independently associated with poorer physical quality of life [61].
Taken together, our findings contribute to a growing body of evidence for an association between insomnia, suicidal ideation, and symptom severity in schizophrenia. They also have important potential clinical implications regarding the need for recognition, assessment, and treatment of insomnia in this patient population.
Funding
Direct funding for this research was provided by the National Institute of Mental Health (NIMH; Dr. Miller). The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflict of interest statement. Dr. Miller received grant support for this study from the National Institute of Mental Health (K23MH098014). In the past 12 months, Dr. Miller also received research support from NARSAD, the Stanley Medical Research Institute, Acadia, Alkermes, and Augusta University; and Honoraria from Psychiatric Times. Dr. Parker has nothing to disclose. Dr. Rapaport has nothing to disclose relevant to the present work. In the past 12 months, Dr. Rapaport is a member of the scientific advisory board for Pax, Inc. (unpaid) and the Depression and Bipolar Alternative Therapies Foundation, and a consultant for the American Psychiatric Association. Dr. Buckley has nothing to disclose for this study. In the past 12 months, Dr. Buckley reports grants from Ameritox, grants from Auspex Pharmaceuticals, Inc., grants from Alkermes, Inc., grants from Avanir Pharmaceuticals, Inc., grants from Otsuka Pharmaceuticals, and grants and consultant support from NIMH. Dr. McCall has nothing to disclose for the work under consideration. In the past 12 months, Dr. McCall has received research support from NIMH, the American Foundation for Suicide Prevention, Merck Pharmaceuticals, and the MECTA Corporation. He has been a consultant for Multiple Energy Technologies, and Anthem Insurance. He has been a paid CME speaker for CME Outfitters.
Acknowledgments
The authors wish to thank Niju Philip for assistance.
References
- 1. Monti JM, et al. Sleep and circadian rhythm dysregulation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:209–216. [DOI] [PubMed] [Google Scholar]
- 2. Wulff K, et al. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200(4):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies G, et al. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med Rev. 2017;31:25–38. [DOI] [PubMed] [Google Scholar]
- 4. Malik S, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pompili M, et al. Completed suicide in schizophrenia: evidence from a case-control study. Psychiatry Res. 2009;167(3):251–257. [DOI] [PubMed] [Google Scholar]
- 6. Li SX, et al. Sleep disturbances and suicide risk in an 8-year longitudinal study of schizophrenia-spectrum disorders. Sleep. 2016;39(6):1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andriopoulos I, et al. Suicidality in the “prodromal” phase of schizophrenia. Compr Psychiatry. 2011;52(5):479–485. [DOI] [PubMed] [Google Scholar]
- 8. Cosgrave J, et al. The interaction between subclinical psychotic experiences, insomnia and objective measures of sleep. Schizophr Res. 2017. pii: S0920-9964(17)30397-3. doi: 10.1016/j.schres.2017.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YJ, et al. The relationship between psychotic-like experiences and sleep disturbances in adolescents. Sleep Med. 2012;13(8):1021–1027. [DOI] [PubMed] [Google Scholar]
- 10. Sheaves B, et al. Insomnia and hallucinations in the general population: findings from the 2000 and 2007 British Psychiatric Morbidity Surveys. Psychiatry Res. 2016;241:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reeve S, et al. Disrupting sleep: the effects of sleep loss on psychotic experiences tested in an experimental study with mediation analysis. Schizophr Bull. 2017. doi: 10.1093/schbul/sbx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldsmith DR, et al. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller BJ, et al. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223–230. [DOI] [PubMed] [Google Scholar]
- 14. Black C, et al. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. 2015;78(1):28–37. [DOI] [PubMed] [Google Scholar]
- 15. Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42(2):141–152. [DOI] [PubMed] [Google Scholar]
- 16. Fang SH, et al. Associations between sleep quality and inflammatory markers in patients with schizophrenia. Psychiatry Res. 2016;246:154–160. [DOI] [PubMed] [Google Scholar]
- 17. Lichtermann D, et al. The genetic epidemiology of schizophrenia and of schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci. 2000;250(6):304–310. [DOI] [PubMed] [Google Scholar]
- 18. Tamminga CA, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 19. Fennig S, et al. Best-estimate versus structured interview-based diagnosis in first-admission psychosis. Compr Psychiatry. 1994;35(5):341–348. [DOI] [PubMed] [Google Scholar]
- 20. Kay SR, et al. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23(1):99–110. [DOI] [PubMed] [Google Scholar]
- 21. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. [DOI] [PubMed] [Google Scholar]
- 22. Woods SW. Chlorpromazine Equivalent Doses for Atypical Antipsychotics: An update 2003–2010 2011. http://scottwilliamwoods.com/equivalencesupdate.php. Accessed August 28, 2017.
- 23. Ford P. An evaluation of the Dartmouth assessment of lifestyle inventory and the leeds dependence questionnaire for use among detained psychiatric inpatients. Addiction. 2003;98(1):111–118. [DOI] [PubMed] [Google Scholar]
- 24. Hollingshead AB, et al. Social Class and Mental Illness. New York: Wiley; 1958: 387–397. [Google Scholar]
- 25. Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 26. Bastien CH, et al. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 27. Beck AT, et al. Scale for suicide ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44(4):499–505. [DOI] [PubMed] [Google Scholar]
- 28. McCall WV, et al. A psychometric cut-point to separate emergently suicidal depressed patients from stable depressed outpatients. Indian J Psychiatry. 2013;55(3):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartwig FP, et al. Inflammatory biomarkers and risk of schizophrenia: a 2-Sample Mendelian randomization study. JAMA Psychiatry. 2017;74(12):1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mori N, et al. Total and differential white blood cell counts, inflammatory markers, adipokines, and the metabolic syndrome in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Schizophr Res. 2015;169(1–3):30–35. [DOI] [PubMed] [Google Scholar]
- 31. Horsdal HT, et al. C-reactive protein and white blood cell levels in schizophrenia, bipolar disorders and depression – associations with mortality and psychiatric outcomes: a population-based study. Eur Psychiatry. 2017;44:164–172. [DOI] [PubMed] [Google Scholar]
- 32. Misiak B, et al. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res. 2018;192:16–29. [DOI] [PubMed] [Google Scholar]
- 33. Irwin MR, et al. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liukkonen T, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69(8):756–761. [DOI] [PubMed] [Google Scholar]
- 35. O’Connor MF, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang CC, et al. The relationships of current suicidal ideation with inflammatory markers and heart rate variability in unmedicated patients with major depressive disorder. Psychiatry Res. 2017;258:449–456. [DOI] [PubMed] [Google Scholar]
- 37. Park RJ, et al. Association between high sensitivity CRP and suicidal ideation in the Korean general population. Eur Neuropsychopharmacol. 2017;27(9):885–891. [DOI] [PubMed] [Google Scholar]
- 38. Courtet P, et al. Increased CRP levels may be a trait marker of suicidal attempt. Eur Neuropsychopharmacol. 2015;25(10):1824–1831. [DOI] [PubMed] [Google Scholar]
- 39. Cáceda R, et al. A probe in the connection between inflammation, cognition and suicide. J Psychopharmacol. 2018:269881118764022. doi: 10.1177/0269881118764022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickerson F, et al. The association between immune markers and recent suicide attempts in patients with serious mental illness: a pilot study. Psychiatry Res. 2017;255:8–12. [DOI] [PubMed] [Google Scholar]
- 41. McCall WV, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11(9):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nadorff MR, et al. Explaining alcohol use and suicide risk: a moderated mediation model involving insomnia symptoms and gender. J Clin Sleep Med. 2014;10(12):1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCall WV, et al. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15(9):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waite F, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Delaney C, et al. Preserved cognitive function is associated with suicidal ideation and single suicide attempts in schizophrenia. Schizophr Res. 2012;140(1-3):232–236. [DOI] [PubMed] [Google Scholar]
- 46. Selvaraj S, et al. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–245. [DOI] [PubMed] [Google Scholar]
- 47. Carlborg A, et al. CSF 5-HIAA, attempted suicide and suicide risk in schizophrenia spectrum psychosis. Schizophr Res. 2009;112(1–3):80–85. [DOI] [PubMed] [Google Scholar]
- 48. Bradley AJ, et al. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24 (4 Suppl):91–118. [DOI] [PubMed] [Google Scholar]
- 49. Johansson AS, et al. Altered circadian clock gene expression in patients with schizophrenia. Schizophr Res. 2016;174(1–3):17–23. [DOI] [PubMed] [Google Scholar]
- 50. Castro J, et al. Circadian rest-activity rhythm in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;168(1–2):50–55. [DOI] [PubMed] [Google Scholar]
- 51. Lunsford-Avery JR, et al. Adolescents at clinical-high risk for psychosis: circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophr Res. 2017:pii: S0920-9964(17)30065–8. doi: 10.1016/j.schres.2017.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rehman A, et al. Clinician perceptions of sleep problems, and their treatment, in patients with non-affective psychosis. Psychosis. 2017;9(2):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chan MS, et al. Sleep in schizophrenia: a systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2017;32:69–84. [DOI] [PubMed] [Google Scholar]
- 54. Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939–962. [DOI] [PubMed] [Google Scholar]
- 55. Wirz-Justice A, et al. Disturbed circadian rest-activity cycles in schizophrenia patients: an effect of drugs? Schizophr Bull. 2001;27(3):497–502. [DOI] [PubMed] [Google Scholar]
- 56. Chemerinski E, et al. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Compr Psychiatry. 2002;43(5):393–396. [DOI] [PubMed] [Google Scholar]
- 57. Freeman D, et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry. 2015;2(11):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Waters F, et al. Preferences for different insomnia treatment options in people with schizophrenia and related psychoses: a qualitative study. Front Psychol. 2015;6:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tek C, et al. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res. 2014;160(1–3):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suttajit S, et al. Prevalence of metabolic syndrome and its association with depression in patients with schizophrenia. Neuropsychiatr Dis Treat. 2013;9:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, et al. Quality of life in Chinese patients with schizophrenia treated in primary care. Psychiatry Res. 2017;254:80–84. [DOI] [PubMed] [Google Scholar]