Significance
The development, repair, and regeneration of anisotropic connective tissues (e.g., tendon, ligament, meniscus) involve deposition of aligned fibrillar collagen by cells. However, the intracellular signaling mechanisms mediating this process are not fully understood. We show that the mechanosensitive cation channel transient receptor potential vanilloid 4 (TRPV4) plays a critical role in controlling aligned collagen assembly by mesenchymal stem cells. Specifically, inhibiting TRPV4 activity in mesenchymal stem cells disrupts aligned collagen matrix assembly, and conversely, activating TRPV4 accelerates collagen deposition. Additionally, TRVP4 activity modulates force transmitted across vinculin, a key mechanosensitive protein within cell–matrix adhesions, where cell-generated forces are critical in fibrillar collagen assembly. Understanding and controlling specific cell-signaling mechanisms underlying aligned matrix assembly could lead to improved tissue regeneration outcomes.
Keywords: mechanobiology, mechanotransduction, tendon, calcium, fibrocartilage
Abstract
Microarchitectural cues drive aligned fibrillar collagen deposition in vivo and in biomaterial scaffolds, but the cell-signaling events that underlie this process are not well understood. Utilizing a multicellular patterning model system that allows for observation of intracellular signaling events during collagen matrix assembly, we investigated the role of calcium (Ca2+) signaling in human mesenchymal stem cells (MSCs) during this process. We observed spontaneous Ca2+ oscillations in MSCs during fibrillar collagen assembly, and hypothesized that the transient receptor potential vanilloid 4 (TRPV4) ion channel, a mechanosensitive Ca2+-permeable channel, may regulate this signaling. Inhibition of TRPV4 nearly abolished Ca2+ signaling at initial stages of collagen matrix assembly, while at later times had reduced but significant effects. Importantly, blocking TRPV4 activity dramatically reduced aligned collagen fibril assembly; conversely, activating TRPV4 accelerated aligned collagen formation. TRPV4-dependent Ca2+ oscillations were found to be independent of pattern shape or subpattern cell location, suggesting this signaling mechanism is necessary for aligned collagen formation but not sufficient in the absence of physical (microarchitectural) cues that force multicellular alignment. As cell-generated mechanical forces are known to be critical to the matrix assembly process, we examined the role of TRPV4-mediated Ca2+ signaling in force generated across the load-bearing focal adhesion protein vinculin within MSCs using an FRET-based tension sensor. Inhibiting TRPV4 decreased tensile force across vinculin, whereas TRPV4 activation caused a dynamic unloading and reloading of vinculin. Together, these findings suggest TRPV4 activity regulates forces at cell-matrix adhesions and is critical to aligned collagen matrix assembly by MSCs.
Tendon, meniscus, intervertebral disc. cartilage, and other connective tissues play critical roles in resisting and transferring loads throughout the body. The ability of these tissues to perform their mechanical function depends in large part upon the presence of highly aligned collagen fibrils, organized hierarchically into larger collagen fibers, that are primarily responsible for the tensile properties, and in turn, the anisotropy and nonlinear properties of these tissues (1–3). During development, these tissues are assembled by fibroblast-like or chondrocytic cells in a process that involves cell–matrix interactions, cell-generated tensile forces, and multicellular cooperation (4–6). In response to injury or degeneration, however, these tissues generally exhibit poor repair capabilities and form “scar” tissues that lack aligned collagen organization, and therefore have compromised functional properties (7–10). Promoting effective tissue repair or engineering tissue replacements with functional mechanical properties depends upon the ability to direct cells to generate and maintain an aligned fibrillar collagen matrix.
In this regard, a number of tissue engineering approaches have attempted to produce tissue replacements with enhanced collagen alignment. In particular, mesenchymal stem cells (MSCs) provide a promising cell source for the regeneration and repair of aligned connective tissues (e.g., refs. 11–13). MSCs are now believed to exist in vivo as a population of pericytes within multiple tissue types (14), but exhibit multipotent capabilities ex vivo (15) and have been shown to sense and respond to microenvironmental cues in engineered tissues. When cultured on or within biomaterials featuring aligned nano- or microscale architectural cues, MSCs can align with the cue direction and assemble an aligned fibrillar collagen matrix (16, 17). Cellular mechanotransduction and downstream responses to alignment cues that regulate the process of collagen fibril matrix assembly have been shown to involve a number of interacting pathways, including actin cytoskeletal alignment (18, 19), focal adhesion alignment and oriented growth (20), integrin-mediated fibronectin polymerization (4, 21), anisotropic cell-generated contractile forces and other mechanical stresses (20, 22, 23), and cell signaling involving Rho and nonmuscle myosin IIA (21, 22). However, the signaling mechanisms that regulate aligned collagen matrix assembly are not fully understood, and these processes are relatively difficult to study within complex in vivo or 3D matrix scaffolds.
Notably, a number of mechanical signaling pathways involve transient changes in intracellular Ca2+ levels that subsequently regulate a diverse set of cell processes, ranging from cell motility to transduction of extracellular stimuli, to gene transcription and protein expression (reviewed in ref. 24). MSCs exhibit spontaneous Ca2+ oscillations (25, 26) and respond to varying mechanical environments with changes in Ca2+ signals (27–29). These Ca2+ signals play roles in a number of cell processes in MSCs [e.g., migration (30), differentiation (31)], but the role of MSC Ca2+ signaling in aligned collagen matrix formation has not been established. In recent years, the mechanosensitive Ca2+-permeable ion channel transient receptor potential vanilloid 4 (TRPV4) has been shown to play key roles in mechanotransduction and tissue development in a variety of contexts (32). In humans, TRPV4 mutations that alter channel function disrupt normal skeletal development and joint health (33–35), and in chondrocytes, TRPV4 plays a critical role in sensing and responding to dynamic mechanical loading (36, 37). In MSCs, TRPV4 has recently been identified to be involved in the sensing of fluid flow shear that stimulates early osteogenic differentiation (38).
The objective of this study was to examine the hypothesis that TRPV4-mediated Ca2+ signaling synergizes with substrate architecture to regulate aligned collagen matrix assembly by MSCs. We utilized a microphotopatterning (μPP) in vitro model system with aligned cell-adhesive cues, which are potent regulators of collagen assembly (17), to precisely arrange MSCs into multicellular units and induce the formation of aligned fibrillar collagen. Our primary hypothesis was that Ca2+ signaling via TRPV4 is critical to the formation of aligned collagen by MSCs, and that this process could be manipulated by altering TRPV4 activity. To better understand the mechanism underlying this process, we used an FRET-based intracellular tension sensor to examine the effects of TRPV4 activity on tension across the protein vinculin within focal adhesions, where matrix assembly occurs and requires cell-generated force. Additionally, using different pattern geometries where MSCs do/do not assemble aligned fibrillar collagen, we investigated whether MSC Ca2+ signaling was dependent on the pattern microenvironment or exhibited feedback from the developing collagen matrix. We find that TRPV4 plays a critical role in the formation of aligned collagen matrix assembly as well as in modulating vinculin tensile forces within focal adhesions at the cell–ECM interface. Furthermore, by specific inhibition or activation of TRPV4 activity, we found that aligned collagen assembly can be either inhibited or accelerated, respectively. Our data suggest that TRPV4-dependent Ca2+ signaling in MSCs occurs in a cell-autonomous fashion that, in combination with substrate-mediated control of cell shape and position, controls aligned collagen fibril assembly.
Results
MSCs Express Functional TRPV4 Channels.
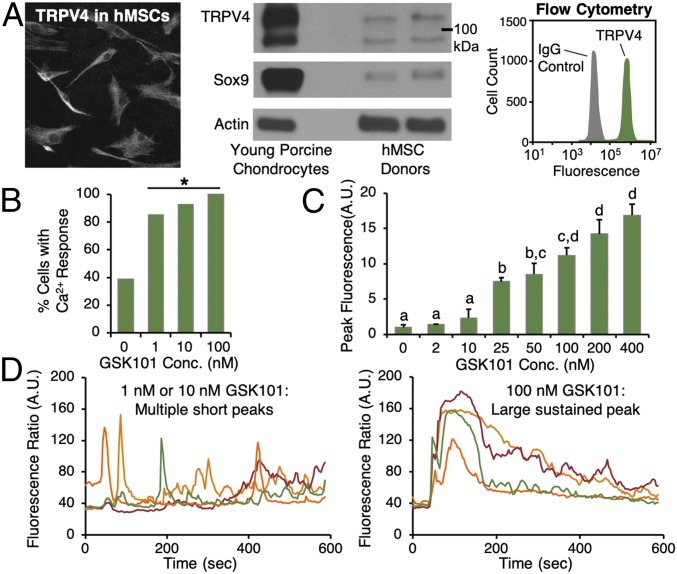
We first investigated whether TRPV4 was present and functional in human MSCs. MSCs showed consistent, positive expression for TRPV4, as assessed via immunofluorescence (Fig. 1A, Left), Western blot (Fig. 1A, Center), and flow cytometry (Fig. 1A, Right), where 96.4 ± 2.6% of MSCs were TRPV4 positive. When exposed to the TRPV4-specific chemical agonist GSK1016790 (GSK101), a high percentage of MSCs responded with Ca2+ signaling (92.9% cells signaling in response to 10 nM GSK101, Fig. 1B), and peak Ca2+ signal intensity was found to be dependent on the GSK101 dose (Fig. 1C). TRPV4 activation with low concentrations of GSK101 (1, 10 nM) induced multiple short peaks of MSC Ca2+ signaling (Fig. 1D, Left; traces represent individual cells), whereas higher concentrations (100 nM, Fig. 1D, Right) yielded large, sustained peaks that gradually decayed. These data clearly demonstrate that MSCs express functional TRPV4 channels.
Fig. 1.
MSCs express functional TRPV4. (A) Human MSCs show positive expression for TRPV4 via immunofluorescence (Left), Western blot (Center), and flow cytometry (Right, representative histogram shown, 99.8% positive). (Magnification: 20×.) (B) MSCs respond with calcium signaling when exposed to TRPV4 agonist GSK101 (*different from control, P < 0.05, bars represent percentage of cells signaling for the entire cell population per condition and thus do not have error bars, semiconfluent monolayer culture). Peak calcium signal intensity in response to GSK101 stimulation (C) is dose-dependent (treatments not connected by the same letter are different, P < 0.05). (D) TRPV4 activation with low doses of GSK101 (Left, 1, 10 nM) results in multiple short peaks of calcium in MSCs (traces are individual cells), whereas larger doses (Right, 100 nM) yield large, sustained peaks that gradually decay.
TRPV4 Mediates MSC Ca2+ Signaling Early in Aligned Matrix Development and Is Critical for Aligned Collagen Formation.
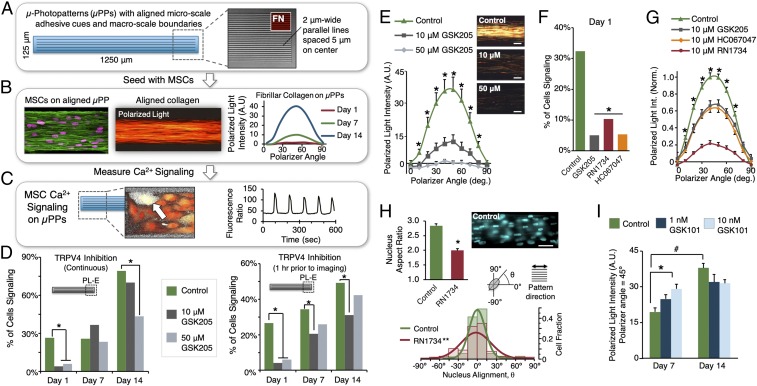
We utilized a multicellular patterning model system to induce aligned fibrillar collagen assembly by MSCs and observe intracellular Ca2+ signaling events during this process. MSCs were cultured on μPP substrates presenting cell-adhesive cues at two different length scales (Fig. 2A): “microscale” architecture providing subcellular-scale alignment cues (e.g., 2-μm-wide parallel lines), and “macroscale” pattern boundaries that are larger than individual cells but can align cells via multicellular confinement and packing. Consistent with previous findings (17), MSCs cultured on μPPs with alignment cues at both length scales align and assemble significant quantities of aligned fibrillar collagen over 2 wk in culture (Fig. 2B). MSC Ca2+ signaling was assessed via live-cell ratiometric fluorescence imaging at different times during collagen matrix development (days 1, 7, 14). MSCs cultured on μPPs exhibited spontaneous oscillatory Ca2+ signaling (Fig. 2C and Movie S1), and with time in culture the percentage of cells with a detectable Ca2+ signal increased 50–100% relative to day 1 (Fig. 2D and SI Appendix, Fig. S1B) and the time between signal peaks decreased 28–44% (i.e., increasing signal frequency; SI Appendix, Fig. S1B, Right).
Fig. 2.
TRPV4 mediates Ca2+ signaling early in MSC aligned matrix formation, and aligned collagen formation is inhibited by blocking TRPV4 and accelerated by daily TRPV4 stimulation. (A) µPPs with microscale cell-adhesive architectures and macroscale boundaries. (B) Human MSCs cultured on µPPs with aligned micro/macrocues align with pattern direction and assemble fibrillar collagen over 14 d. (C) MSCs exhibit spontaneous calcium signaling on multicellular µPPs. (D) MSCs were cultured on µPPs in the presence of TRPV4-specific antagonist GSK205 continuously (Left) or for 1 h before imaging (Right). GSK205 treatment nearly abolished MSC Ca2+ signaling at day 1, but later (days 7, 14) signaling was only partially inhibited (*different from control at given day, χ2, P < 0.05, n = 62–124 cells per condition per time; bars represent percentage of cells signaling for the entire population per condition and thus do not have error bars; see SI Appendix, Supplementary Methods for details; signaling measured at PL-E pattern position). (E) Fibrillar collagen deposition (day 14) was significantly reduced in the (continuous) presence of GSK205 (*different at given polarizer angle, P < 0.05, n = 3 patterns per condition; scale = 50 μm). (F) Treatment with alternate TRPV4 antagonists RN1734 (10 μM) and HC067047 (10 μM) showed similar reductions in Ca2+ signaling (*different from control, P < 0.001, χ2, day 1, n = 75–154 cells per condition, PL-E pattern position) and (G) fibrillar collagen deposition (*control different from other groups at given polarizer angle, n = 4–13 patterns per condition, day 14). (H) TRPV4 inhibition also disrupted cellular organization, reducing nuclear aspect ratio (*different from control, P < 0.0001, t test, n = 83–88 per condition) and nucleus alignment with pattern (**sig. difference, Kolmogorov–Smirnov test, α = 0.025; lines: normal fit). (Scale bar, 50 μm.) (I) MSCs stimulated with TRPV4 agonist GSK101 (30 min/d) showed increased collagen deposition at day 7, but not at day 14 [*different from control within time point, number different between days within treatment group, P < 0.05, n = 4 patterns per condition per time, data shown at peak polarizer angle (45°) for clarity]. # indicates day 7 significantly differs from day 14. (Magnification: B and C, 40×.)
Using this system, we investigated whether TRPV4 mediates Ca2+ signaling in MSCs during aligned fibrillar collagen formation. At early culture periods (day 1), blocking TRPV4 activity [via the TRPV4-specific chemical inhibitor GSK205 (37)] abolished nearly all MSC Ca2+ signaling [Fig. 2D, Left, all data shown for the patterned long-end (PL-E) pattern region], with the percent of signaling cells reduced from 26.6% (control) to less than 6% in the presence of inhibitor. At later stages in aligned matrix development (days 7, 14), blocking TRPV4 had less effect: no differences were detected between controls and those cultured in GSK205 at day 7, and only the highest dose (50 μM) of GSK205 decreased signaling (to 54% of control levels) at day 14. We confirmed that this effect was not simply due to GSK205 desensitization (due to continuous exposure) over the culture period, as constructs grown for 7 or 14 d without inhibitor again showed only partial reduction in Ca2+ signaling when exposed to GSK205 for 1 h before imaging (Fig. 2D, Right, 37–40% less than control levels for 10 μM GSK205). To confirm that these results were TRPV4-specific and not due to off-target effects of GSK205, we blocked TRPV4 activity at day 1 (when TRPV4 signaling was found to be greatest) using two other TRPV4-specific inhibitors (RN-1734, HC-067047) and observed very similar reductions in Ca2+ signaling (Fig. 2F). Altogether, these results suggest that TRPV4 is a primary mediator of Ca2+ signaling early in MSC multicellular culture on aligned patterns, but other mechanisms contribute to Ca2+ signaling at later times.
We then examined whether Ca2+ signaling mediated by TRPV4 played a functional role in aligned collagen formation. Blocking TRPV4 significantly decreased aligned fibrillar collagen formation after 14 d in culture at both inhibitor concentrations (10, 50 μM GSK205, Fig. 2E). Alternate TRPV4-specific inhibitors (RN-1734, HC-067047) yielded similar or greater reductions in fibrillar collagen (Fig. 2G). We also measured the shape and alignment of cell nuclei within our constructs after 2 wk with/without TRPV4 inhibition (using RN-1734, Fig. 2H), and found clear reductions in nuclear alignment and elongation with inhibition, indicating a disruption of cell and matrix organization. MSCs cultured in the presence of TRPV4 inhibitors covered μPPs in a manner similar to the controls, with no observed differences in overall cell numbers or gross morphology. Conversely, when TRPV4 was activated via low, daily doses of GSK101 (30 min/d, 1–10 nM), aligned collagen formation increased relative to control after 7 d of culture (Fig. 2I, Left). At 14 d, however, there was no detectable difference between TRPV4-stimulated and control groups (Fig. 2I, Right). Overall, we found that aligned collagen formation is inhibited by blocking TRPV4 and accelerated by activating TRPV4, indicating a key role for TRPV4-mediated Ca2+ signaling in MSC aligned fibrillar collagen matrix formation.
MSC Ca2+ Signaling Is Insensitive to Pattern Microenvironment and Developing Collagen Matrix.
We next investigated the influence of pattern shape and the associated accumulation of aligned collagen on MSC Ca2+ signaling on different pattern geometries where MSCs either do (pattern type PL, SI Appendix, Fig. S1A) or do not (pattern types: unpatterned square, US and patterned square) assemble significant amounts of fibrillar collagen over time (SI Appendix, Fig. S1A and ref. 17). While the percentage of signaling cells increased and period of signaling decreased with culture time across all pattern types, differences between pattern types were not detected (SI Appendix, Fig. S1B). Additionally, TRPV4 inhibition showed similar reductions in Ca2+ signaling across all pattern types (SI Appendix, Fig. S1C), nearly abolishing signaling at day 0 while having a reduced effect at day 14.
As MSCs have been shown to generate spatially varying substrate traction forces when confined in multicellular patterns (39), as well as respond to mechanical stimuli with changes in Ca2+ signaling (27, 28), we also investigated whether cell position within the pattern (distance from cell centroid to pattern edge, SI Appendix, Fig. S1D) affected MSC Ca2+ signaling. At early times (days 1, 7), no relationship between position and signaling frequency was detected on any pattern configuration (day 1 shown in SI Appendix, Fig. S1D, Left). By day 14, only a weak correlation (R2 = 0.085, P = 0.02) was observed on US patterns, with cells near the edge signaling with greater frequency (SI Appendix, Fig. S1D, Right; other pattern configurations did not show significant correlations at any time point). Thus, spatially varying signaling differences within patterns were minimal across all pattern types and time points. Taken together, these findings suggest that in MSCs, oscillating Ca2+ signaling (including the portion specifically mediated by TRPV4) is insensitive to the specific patterned microenvironment, subpattern spatial position, or the presence of an aligned fibrillar collagen matrix.
Ca2+ Signaling at Later Stages of Aligned Collagen Matrix Formation Does Not Require Extracellular Ca2+ Influx and Is Insensitive to Actin Cytoskeletal State.
We further investigated the source of oscillatory Ca2+ signals at later time points that were independent of TRPV4 signaling. Depletion of intracellular Ca2+ stores via thapsigargin eliminated Ca2+ signaling at all time points (SI Appendix, Fig. S2A), indicating all observed MSC Ca2+ signaling involved intracellular Ca2+ store release. When extracellular Ca2+ was removed from imaging media (SI Appendix, Fig. S2B), signaling was abolished at day 1, but only partially reduced at later times (80% and 74% of control at days 7 and 14, respectively). This result is consistent with our finding of a reduced effect of blocking TRPV4 at later time points on collagen alignment and suggests that the additional Ca2+ signaling observed at later times does not involve extracellular Ca2+ influx via an alternate (i.e., non-TRPV4) Ca2+ channel. Inhibition of inositol triphosphate (IP3) receptors via 2-aminoethoxydiphenyl borate (2-APB) in Ca2+-free imaging media (SI Appendix, Fig. S2B) further reduced Ca2+ signaling at later time points (day 7) or completely abolished Ca2+ signaling (day 14), suggesting IP3 as a mediator of intracellular store release at later times, independent of TRPV4. Disruption of the actin cytoskeleton via cytochalasin D (SI Appendix, Fig. S2C) resulted in a modest decrease of Ca2+ signaling at days 1 and 14 (24–31% of control, no difference at day 7). Altogether, these findings suggest that the TRPV4-independent portion of MSC Ca2+ signaling observed at later time points does not require extracellular Ca2+ influx and is insensitive to the state of the actin cytoskeleton.
TRPV4 Modulates Force Across the Focal Adhesion Protein Vinculin.
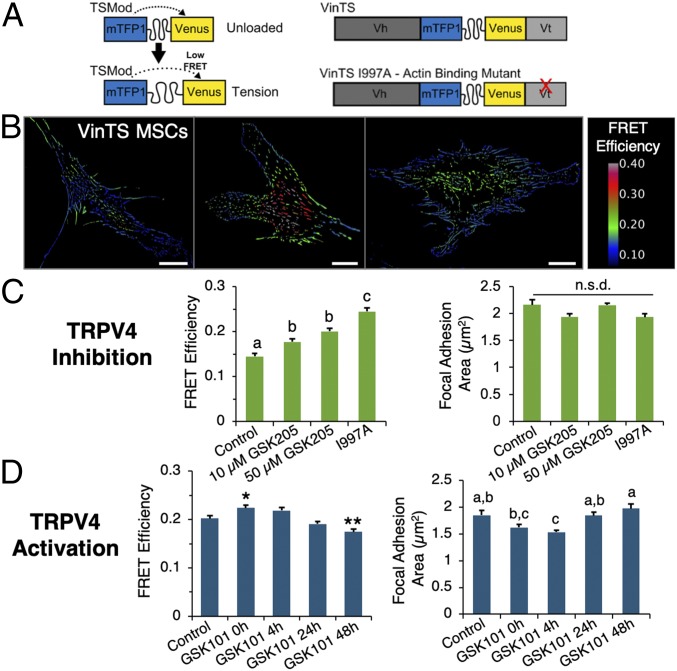
As the assembly of fibrillar collagen is dependent upon cell-generated contractile forces (22), and Ca2+ signaling has been linked to MSC mechanical force sensing (28), we investigated whether blocking or stimulating TRPV4 affected intracellular forces across vinculin, a key load-bearing protein within focal adhesions, by using an FRET-based molecular tension sensor (40). The vinculin tension sensor (VinTS, Fig. 3A) consists of a tension sensor module (TSMod, with the FRET pair mTFP and Venus separated by a [GGSGGS]9 linker sequence) inserted between the head and tail domains of the vinculin protein. Individual MSCs stably expressing VinTS exhibit spatially varying patterns of VinTS FRET efficiency (Fig. 3B), which is inversely related to load across vinculin. Following 3 d of continuous TRPV4 inhibition (via GSK205 treatment), vinculin load in MSCs was significantly reduced relative to controls (FRET efficiency increased, Fig. 3C), but retained higher average load than a VinTS mutant that fails to bind actin (VinTS I997A) (41, 42). However, inhibition of TRPV4 did not induce detectable differences in focal adhesion size (Fig. 3C, Right). We then examined the short-term vinculin-loading response to TRPV4 activation. Following exposure to GSK101 (10 nM, 30-min exposure), vinculin load initially decreased (FRET efficiency increased), as shown in Fig. 3D, Left, GSK 101 0 h), and then over the next 48 h increased beyond the initial control state (GSK101 48 h, Fig. 3D). The size of focal adhesions also followed a similar pattern, with an initial decrease and then recovery following TRPV4 activation (Fig. 3D, Right). Altogether, these findings indicate that sustained blocking of TRPV4 in MSCs leads to a long-term decrease in tensile loading across vinculin, whereas a pulse of TRPV4 activation results in a dynamic unloading and reloading of vinculin, as well as a decrease and then recovery in focal adhesion size.
Fig. 3.
TRPV4 signaling modulates tension across vinculin. (A) FRET-based VinTS consisting of a TSMod inserted between head (Vh) and tail (Vt) domains of the focal adhesion protein vinculin. A mutant sensor which fails to bind actin (VinTS I997A) was utilized as a control. (B) MSCs stably expressing VinTS (FRET efficiency inversely related to load). (Scale bars, 20 μm.) (C) Inhibiting TRPV4 (GSK205, 3 d) increases vinTS FRET efficiency (decreases force; different letters significantly different, P < 0.05, n = 20–22 images fields per condition, >16,700 focal adhesions per condition), but does not affect focal adhesion size (P = 0.124). (D) Stimulating TRPV4 with a single dose of GSK101 (10 nM, 30 min) increased VinTS FRET efficiency (Left) immediately following activation (0 h), followed by recovery over 48 h (*,** higher/lower than control, P < 0.05; n = 21–27 image fields per condition, >17,000 focal adhesions per condition). Correspondingly, focal adhesion size decreased and then recovered following TRPV4 activation (Right, conditions with different letters different, P < 0.05). n.s.d., no significant differences.
Discussion
The formation of highly ordered fibrillar collagenous tissues of the musculoskeletal system requires multicellular alignment and coordination. Although several key cellular and subcellular processes involved in collagen fibrillogenesis have been previously documented (4, 43), the details of the cell-signaling mechanisms underlying aligned collagen matrix assembly by cells in general, and by MSCs specifically, remain to be determined. Here, using a defined micropatterned geometry to induce controlled formation of aligned collagen, we found that Ca2+ signaling mediated by the TRPV4 ion channel is an important regulator of vinculin tension and necessary for aligned fibrillar collagen formation by MSCs (summarized in SI Appendix, Fig. S3). Furthermore, we found that by modulating signaling through this ion channel using TRPV4-specific inhibitors or agonists, aligned collagen matrix formation could be either inhibited or accelerated, respectively. These findings indicate that TRPV4-mediated Ca2+ signaling is necessary for aligned collagen formation by MSCs in response to microarchitectural cues.
Oscillating Ca2+ signaling has previously been observed in MSCs (26), as well as a variety of other cell types. In myofibroblasts, these Ca2+ oscillations have been shown to directly correlate with microcontractions that directionally move ECM-coated beads attached to the cell surface and are distinct from long-lived isometric forces mediated by actin stress fibers, with the two contraction modes potentially working in concert to drive ECM remodeling (44). Ca2+ oscillations in myofibroblasts were observed every ∼100 s (44), in close agreement with our MSC findings (SI Appendix, Fig. S1B), and increasing myofibroblast oscillation frequency was shown to increase microcontraction frequency, which may relate to our finding of accelerated matrix assembly with TRPV4 activation (Fig. 2I). Interestingly, oscillating mechanical contractions with periods in this same range (60–100 s) have recently been observed in tendon fibroblasts during in vitro collagen fibrillogenesis (45). In combination, our findings and these previous studies raise the intriguing possibility that oscillating contractions may be due to TRPV4-mediated calcium oscillations, although further work is required to verify this hypothesis. Furthermore, Ca2+ oscillations in MSCs have also been linked to other processes which may affect matrix assembly, including migration (30), transcription factor activation and differentiation (25, 31), and mechanosensing (28, 29).
TRPV4 has previously been shown to play a critical role in matrix biosynthesis by chondrocytes (36), with TRPV4 activation/inhibition (in combination with dynamic compressive loading) leading to large increases/decreases in neocartilage matrix accumulation, respectively. Here we observed that a daily pulse of TRPV4 activation promoted greater aligned collagen formation at 7 d, but not at 14 d. This finding was consistent with our observation of the major contribution that TRPV4 makes to MSC Ca2+ signaling early in aligned matrix formation (as shown in Fig. 2D). Alternatively, the downstream effects of TRPV4 stimulation, including our findings of the dynamic unloading/reloading of vinculin and disassembly/reassembly of focal adhesions (Fig. 3D), could be beneficial early in the matrix formation process (promoting dynamic changes during cell realignment, migration, etc.) but detrimental at later times (possibly disrupting established cell/cytoskeleton organization and force generation). We also found that blocking TRPV4 interfered with MSC aligned collagen assembly (Fig. 2D). TRPV4 inhibition did not cause overt changes in MSC proliferation or cell morphology, but significantly reduced both matrix accumulation and cell organization (as evidenced by polarized light as well as nuclear shape, Fig. 2H). Despite having much less of an effect on Ca2+ signaling at later culture times, TRPV4 inhibition significantly impaired aligned matrix formation over the course of 2 wk, suggesting that early signaling may be particularly critical to establishing the initial alignment for ongoing collagen fibril formation.
Cell-generated actomyosin-driven tension is a Ca2+-dependent process that is critical to the assembly of both fibronectin and collagen fibrils (22, 46, 47) and may be modulated via TRPV4. In fibroblasts, Ca2+ influx mediated by TRPV4 channels localized to cell–matrix adhesions enables the generation of cell extensions essential for collagen remodeling by regulating the interaction between the actin-binding protein flightless-1 and nonmuscle myosin IIA (48). Here, using a molecular tension sensor that measures piconewton-scale forces inside of cells, we found that blocking TRPV4 reduced tensile loading across vinculin, a focal adhesion protein that mediates load transfer between the cytoskeleton and ECM, but did not alter focal adhesion size (Fig. 3C). This finding suggests that TRPV4 inhibition may decrease the overall contractile state in MSCs, and/or modify local focal adhesion assembly or signaling, both of which are regulated by vinculin loading (40). In contrast, we found that a pulse of TRPV4 activation first led to a decrease in vinculin tension to levels approaching those of a mutant sensor that fails to bind actin (I997A, Fig. 3D) and the unloaded sensor [FRET efficiency of ∼0.28 (49)], and was followed by recovery above the original baseline (control) tension levels after 48 h. Additionally, this activation pulse resulted in an initial decrease in focal adhesion size, followed by subsequent recovery. Altogether, these findings suggest TRPV4 can regulate forces at cell–matrix adhesions (as summarized in SI Appendix, Fig. S3B) and may affect the mechanics of collagen matrix assembly.
Given that TRPV4 may be regulated mechanically (36, 50–53), we investigated whether Ca2+ signaling in our system might vary spatially within a given multicellular pattern [due to possible differences in cell-generated traction stresses (39)], or between pattern types where distinct differences in fibrillar collagen content and alignment develop over time (17) and might contribute to altered mechanical signaling. We found that differences in Ca2+ signaling among pattern types where fibrillar collagen is/is not produced (i.e., long/square patterns, respectively) were not significantly different at any time point (SI Appendix, Fig. S1B), and that TRPV4’s contribution to this signaling was similar across pattern types (SI Appendix, Fig. S1C). Additionally, differences based on subpattern spatial position were minimal (SI Appendix, Fig. S1D), and signaling persisted even when the actin cytoskeleton was disrupted (SI Appendix, Fig. S2C). Thus, our data suggest that MSC oscillating Ca2+ signaling is relatively insensitive to external microenvironment mechanical cues or feedback from developing collagen. We find that this TRPV4-dependent signaling mechanism is necessary for the assembly of aligned collagen (Fig. 2 E and G), but not sufficient in the absence of physical (microarchitectural) cues that force multicellular alignment (SI Appendix, Fig. S1) and end-to-end cell positioning [overall pattern lengths > ∼500 μm (17)]. As such, we propose fibril assembly may be considered an emergent behavior arising from a group of properly arranged and autonomously signaling cell “units."
Overall, our findings show that TRPV4-mediated Ca2+ signaling regulates vinculin tension and the formation of aligned fibrillar collagen by MSCs on patterned substrates, and that modulation of this signaling could be used to inhibit or enhance aligned collagen formation. This work may have useful ramifications in understanding the development of aligned collagenous tissues, as well as for improving or accelerating tissue regeneration.
Methods
μPP.
Cell-adhesive patterns with microscale architecture and macroscale boundaries (Fig. 2A) were created via two-photon photoablation of thin (∼150 nm) polyvinyl alcohol hydrogel layers (17, 54). Ablated cell-adhesive regions were functionalized with fibronectin (20 μg/mL) via adsorption. Patterns are stable in culture for over 2 wk.
Cell Culture.
Human MSCs isolated from bone marrow aspirates (cells pooled from three donors) were expanded in monolayer (passage 5) and seeded onto μPP substrates, and cultured (5% CO2, 37 °C) for 1–14 d on μPPs (full details in SI Appendix).
TRPV4 Expression and Activity.
MSC TRPV4 protein expression was assessed via immunostaining, Western blot analysis, and flow cytometry using a TRPV4-specific antibody (ACC-034; Alamone Labs) and standard protocols (SI Appendix). Dose–response to TRPV4-specific chemical agonist GSK101 (GSK1016790A; Sigma-Aldrich) was assessed using a fluorometric imaging plate reader (Molecular Devices). MSCs were plated in a 96-well plate, cultured overnight (unpatterned, semiconfluent cultures), and loaded with the Ca2+-sensitive dye Fluo-4 No Wash (Sigma-Aldrich). GSK101 solutions (2–400 nM) were added to each well and fluorescence measured over 70 s. Peak fluorescence intensity for each well was recorded and normalized to vehicular control.
Ca2+ Signaling on μPPs.
Cells grown on μPPs (for 1, 7, or 14 d) were loaded with fura-red and fluo-4 calcium–sensitive dyes for ratiometric Ca2+ confocal imaging as described previously (37, 55). Square patterns were imaged entirely; long patterns were imaged in two pattern regions, middle and end (Fig. 1C). The mean fluorescence ratio was measured for each cell in the image over 5 min of imaging (∼2 s acquisition intervals). A Ca2+ signal was defined as an increase in fluorescence ratio greater than 3 SDs above baseline. For TRPV4 inhibition experiments, μPPs were cultured with GSK205 (10 or 50 μM), RN-1734 (10 μM; Sigma-Aldrich), HC067047 (10 μM; Tocris Bioscience), or vehicle control (DMSO), continuously (up to 14 d) or for 1 h before imaging. For continuous inhibition experiments, fresh inhibitor was added with each media change (2–3 d). To further elucidate the calcium signaling pathway, MSCs grown on μPPs (1–14 d) were treated with the following compounds (80 min pretreatment before imaging): thapsagargin (1 μM), 2-APB (100 μM in Ca2+-free HBSS), cytochalasin D (2 μM).
Polarized Light Analysis.
Fibrillar collagen deposition was assessed using polarized light. Samples were fixed (4% formaldehyde), stained with picrosirius red, and imaged via a polarized light microscope equipped with rotating polarizer/analyzer (see SI Appendix for full details). To assess the effects of TRPV4 inhibition, MSCs on μPPs were cultured continuously with GSK205 (10 or 50 μM), RN-1734 (10 μM), HC-067047 (10 μM) for 14 d, then fixed and imaged. For TRPV4 activation experiments, MSCs on μPPs were treated with GSK101 (1 or 10 nM, or DMSO control) for 30 min/d (in culture media) for 7 or 14 d.
VinTS.
MSCs were transduced to stably express an FRET-based intracellular biosensor designed to measure force across the focal adhesion protein vinculin [VinTS (40)]. A mutant sensor that fails to bind actin (VinTS I997A, ref. 42) was used as a control. VinTS MSCs were seeded in a semiconfluent monolayer on fibronectin-coated (10 μg/mL) glass and cultured (3 d) with or without TRPV4 inhibitor GSK205 (10 μM, 50 μM, or DMSO control). Treated VinTS MSCs were fixed, imaged, and focal adhesions analyzed for FRET via sensitized emission (ref. 49, see SI Appendix details). In a separate experiment, TRPV4 was activated for 30 min (10 nM GSK101), with cells allowed to recover for various times (0, 4, 24, 48 h) before fixation and analysis.
Statistical Analysis.
All data are presented as mean ± SEM unless otherwise noted, with differences considered significant where P < 0.05. Full details of statistical methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants AR065888, AR48182, AR50245, AG15768, AR48852, AG46927, AR065764, AR057235, AR073752, the Arthritis Foundation, the AO Foundation, NSF Grant 1638442, and the Nancy Taylor Foundation.
Footnotes
Conflict of interest statement: F.G. and W.L. have filed patents on inhibitors of TRPV4.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811095116/-/DCSupplemental.
References
- 1.Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: Experimental measurement and material model predictions. J Biomech Eng. 2001;123:256–263. doi: 10.1115/1.1374202. [DOI] [PubMed] [Google Scholar]
- 2.LeRoux MA, Setton LA. Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of the meniscus in tension. J Biomech Eng. 2002;124:315–321. doi: 10.1115/1.1468868. [DOI] [PubMed] [Google Scholar]
- 3.Fang F, Lake SP. Experimental evaluation of multiscale tendon mechanics. J Orthop Res. 2017;35:1353–1365. doi: 10.1002/jor.23488. [DOI] [PubMed] [Google Scholar]
- 4.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development. 2015;142:4191–4204. doi: 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014;47:214–222. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beredjiklian PK, et al. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 8.Howell K, et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep. 2017;7:45238. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AP, Anderson DR, Daniels AU, Dales MC. Mechanics of the healed meniscus in a canine model. Am J Sports Med. 1989;17:164–175. doi: 10.1177/036354658901700205. [DOI] [PubMed] [Google Scholar]
- 10.Lee NM, et al. Polymer fiber-based models of connective tissue repair and healing. Biomaterials. 2017;112:303–312. doi: 10.1016/j.biomaterials.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad HA, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 12.Baker BM, et al. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci USA. 2012;109:14176–14181. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher MB, et al. Engineering meniscus structure and function via multi-layered mesenchymal stem cell-seeded nanofibrous scaffolds. J Biomech. 2015;48:1412–1419. doi: 10.1016/j.jbiomech.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan AI. New MSC: MSCs as pericytes are sentinels and gatekeepers. J Orthop Res. 2017;35:1151–1159. doi: 10.1002/jor.23560. [DOI] [PubMed] [Google Scholar]
- 15.Gimble JM, et al. In vitro differentiation potential of mesenchymal stem cells. Transfus Med Hemother. 2008;35:228–238. doi: 10.1159/000124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrist CL, Ruch DS, Little D, Guilak F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials. 2014;35:10015–10024. doi: 10.1016/j.biomaterials.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manwaring ME, Walsh JF, Tresco PA. Contact guidance induced organization of extracellular matrix. Biomaterials. 2004;25:3631–3638. doi: 10.1016/j.biomaterials.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Wang JH, Jia F, Gilbert TW, Woo SL. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech. 2003;36:97–102. doi: 10.1016/s0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 20.Ray A, et al. Anisotropic forces from spatially constrained focal adhesions mediate contact guidance directed cell migration. Nat Commun. 2017;8:14923. doi: 10.1038/ncomms14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Van Den Diepstraten C, D’Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol. 2003;163:1045–1056. doi: 10.1016/s0002-9440(10)63464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalson NS, et al. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc Natl Acad Sci USA. 2013;110:E4743–E4752. doi: 10.1073/pnas.1314348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Licup AJ, et al. Stress controls the mechanics of collagen networks. Proc Natl Acad Sci USA. 2015;112:9573–9578. doi: 10.1073/pnas.1504258112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 25.Kawano S, et al. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium. 2006;39:313–324. doi: 10.1016/j.ceca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Kawano S, et al. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32:165–174. doi: 10.1016/s0143416002001240. [DOI] [PubMed] [Google Scholar]
- 27.Kim TJ, et al. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. eLife. 2015;4:e04876. doi: 10.7554/eLife.04876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TJ, et al. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218:285–293. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TJ, Sun J, Lu S, Qi YX, Wang Y. Prolonged mechanical stretch initiates intracellular calcium oscillations in human mesenchymal stem cells. PLoS One. 2014;9:e109378. doi: 10.1371/journal.pone.0109378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci. 2017;74:3697–3710. doi: 10.1007/s00018-017-2545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007;21:1472–1480. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- 32.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang SS, Shin SH, Auh CK, Chun J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp Mol Med. 2012;44:707–722. doi: 10.3858/emm.2012.44.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamandé SR, et al. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat Genet. 2011;43:1142–1146. doi: 10.1038/ng.945. [DOI] [PubMed] [Google Scholar]
- 35.Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. doi: 10.1038/embor.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan MN, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu K, Sun H, Gui B, Sui C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2017;91:841–848. doi: 10.1016/j.biopha.2017.04.094. [DOI] [PubMed] [Google Scholar]
- 39.He S, et al. Dissecting collective cell behavior in polarization and alignment on micropatterned substrates. Biophys J. 2015;109:489–500. doi: 10.1016/j.bpj.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thievessen I, et al. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J Cell Biol. 2013;202:163–177. doi: 10.1083/jcb.201303129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothenberg KE, Scott DW, Christoforou N, Hoffman BD. Vinculin force-sensitive dynamics at focal adhesions enable effective directed cell migration. Biophys J. 2018;114:1680–1694. doi: 10.1016/j.bpj.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadler KE. Fell Muir Lecture: Collagen fibril formation in vitro and in vivo. Int J Exp Pathol. 2017;98:4–16. doi: 10.1111/iep.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castella LF, Buscemi L, Godbout C, Meister JJ, Hinz B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci. 2010;123:1751–1760. doi: 10.1242/jcs.066795. [DOI] [PubMed] [Google Scholar]
- 45.Holmes DF, et al. Synchronized mechanical oscillations at the cell-matrix interface in the formation of tensile tissue. Proc Natl Acad Sci USA. 2018;115:E9288–E9297. doi: 10.1073/pnas.1801759115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubow KE, et al. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun. 2015;6:8026. doi: 10.1038/ncomms9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong C, et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arora PD, Di Gregorio M, He P, McCulloch CA. TRPV4 mediates the Ca2+ influx required for the interaction between flightless-1 and non-muscle myosin, and collagen remodeling. J Cell Sci. 2017;130:2196–2208. doi: 10.1242/jcs.201665. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberg KE, Neibart SS, LaCroix AS, Hoffman BD. Controlling cell geometry affects the spatial distribution of load across vinculin. Cell Mol Bioeng. 2015;8:364–382. [Google Scholar]
- 50.Matthews BD, et al. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Y, et al. Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2015;87:65–73. doi: 10.1016/j.yjmcc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Rahaman SO, et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124:5225–5238. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mrkonjić S, et al. TRPV4 participates in the establishment of trailing adhesions and directional persistence of migrating cells. Pflugers Arch. 2015;467:2107–2119. doi: 10.1007/s00424-014-1679-8. [DOI] [PubMed] [Google Scholar]
- 54.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993;14:359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.