Abstract
Colorectal cancer is the abnormal growth of cells in colon or rectum. Recent findings have acknowledged the role of bacterial infection and chronic inflammation in colorectal cancer initiation and progression. In order to detect and treat precancerous lesions, new tools are required, which may help to prevent or identify colorectal cancer at an early stage. To date, several different screening tests are available, including endoscopy, stool-based blood tests, and radiology-based tests. However, these analyses either lack sensitivity or are of an invasive nature. The use of fluorescently labeled probes can increase the detection sensitivity. However, autofluorescence, photobleaching, and photodamage are commonly encountered problems with fluorescence imaging. Upconverting nanoparticles (UCNPs) are recently developed lanthanide-doped nanocrystals that can be used as light-triggered luminescent probes and in drug delivery systems. In this review, we comprehensively summarize the recent developments and address future prospects of UCNP-based applications for diagnostics and therapeutic approaches associated with intestinal infection and colorectal cancer.
Keywords: near infrared, imaging, bacteria, photodynamic therapy, anti-Stokes emission, inflammation
Introduction
Colorectal cancer is the third most common cancer worldwide and accounts for almost 10% of cancer-related deaths in Western countries.1 The growing evidence suggests that bacteria can play an important role in the initiation and progression of colorectal neoplasm by inducing chronic inflammation and by the release of carcinogenic metabolites.2 Some of the most commonly associated bacteria with colon cancer includes Fusobacteium nucleatum,3 Bacteroides fragilis, and Escherichia coli.4 Inflammatory bowel disease (IBD)-associated colorectal cancer is a classic example of an inflammation-induced cancer form.5
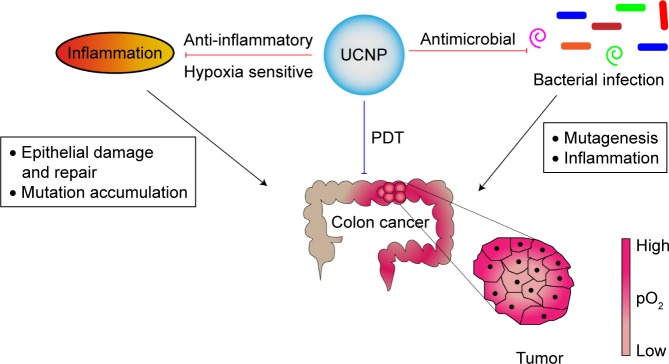
Early detection of precancerous polyps can prevent the onset of colorectal neoplasm or increase the chances of a successful treatment. Currently, several different screening tests are available including endoscopy, stool-based blood tests, and radiology-based tests. However, these protocols lack either sensitivity or sufficient invasiveness, one of the reasons why colorectal cancer is still a major cause of cancer-related deaths in Western countries. The use of fluorescent labeling probes for cancer imaging increases the detection sensitivity. However, autofluorescence, photobleaching, and photodamage are the most commonly encountered problems with fluorescence imaging (FI). Recent reports have identified upconverting nanoparticles (UCNPs) as options to overcome these limitations.6,7 UCNPs are light-triggered lanthanide (Ln)-doped nanocrystals that can be used to detect and treat colorectal cancer and other intestinal related pathologies, as depicted in Figure 1. UCNPs convert long-wavelength near infrared (NIR) excitation light into short-wavelength emission.8 At NIR, the nonspecific absorption and scattering of light by endogenous chromophores are at minimum.9 This leads to deeper penetration of light and high signal-to-noise ratio (SNR). Furthermore, with different modifications, UCNPs can be used to detect bacterial infection and inflammation, which appear to precede colorectal cancer.
Figure 1.
In vivo applications of UCNPs in intestinal infection, inflammation, and colorectal cancer.
Notes: In this figure, we depict potential (red arrows) and current (blue arrow) target areas where UCNPs can be used either for imaging or treating infection, inflammation, and colon cancer. Bacterial infection and/or inflammation leading to IBD and colorectal cancer are depicted with black arrows. UCNPs loaded with antimicrobial and anti-inflammatory compounds can be used to treat infection, inflammation, and cancer. Furthermore, hypoxia-sensitive UCNPs can be used to monitor the hypoxic state of the gastrointestinal tract. Due to the NIR excitation range, UCNPs are currently being used for localized PDT in certain cancer forms.
Abbreviations: IBD, inflammatory bowel disease; NIR, near infrared; PDT, photodynamic therapy; UCNPs, upconverting nanoparticles.
In this review, we provide an updated summary and future prospects of UCNP-based applications in diagnostics and therapeutics for intestinal infection and colorectal cancer.
Colorectal cancer
Cancer is defined as an uncontrolled cell proliferation induced by the accumulation of genetic or epigenetic mutations. Improvement in the average standard of living and basic health care facilities has considerably reduced the incidence of communicable diseases. These advances have led to an increase in average life expectancy in almost every region of the world. Although the incidence of communicable diseases is decreased as a result of improved medical facilities, cancer mortality rates have increased by almost 40% in the last few decades. Cancer diagnoses increased by 33% between 2005 and 2015, and in 2015, over 8.7 million people globally have died due to cancer. It is projected that the number is expected to reach 13 million people by 2030.1 Colorectal cancer is the second most common form of cancer in women and the third most common in men, and globally, it is the third most common cancer, with 1.4 million new cases diagnosed in 2012 alone.10
Both genetic and environmental factors play a role in the etiology of colorectal cancer. Patients affected by hereditary colorectal cancer syndrome accounts for 5%–10% of reported colorectal cancer cases. Among these, Lynch syndrome, which is caused by a mutation in the DNA mismatch-repair genes, such as EPCAM, PMS2, MLH1, MLH2, and MLH6, is the most common cancer syndrome.11,12 Familial adenomatous polyposis is the second most common hereditary syndrome, which is caused by a mutation in the adenomatous polyposis coli (APC), a component of the Wnt-signaling pathway.12 Furthermore, lifestyle factors such as smoking,13 alcohol consumption,14 and high body mass index15 may lead to an increase risk of colorectal cancer development. To a certain extent, these lifestyle factors can explain the geographical and socioeconomic distribution of colorectal cases.16
In the last decade, it has become evident that the inflammatory microenvironment is essential for tumor development and progression. Hallmarks of cancer-inducing inflammation include the presence of inflammatory cells, inflammatory mediators such as cytokines and chemokines, tissue repair and remodeling, and fibrosis.17 Inflammation is the key component of the host immune system’s fight against foreign invaders such as bacteria and viruses. Acute inflammation, if resolved properly, results in the tissue repair and healing and maintenance of homeostasis. However, uncontrolled chronic inflammation may lead to malignancy. Notably, patients with early-onset IBD develop long-term chronic intestinal inflammation and, therefore, present an increased risk of developing colon cancer.18
Bacteria and colorectal cancer
The intestinal tract acts as a reservoir for various microbial species, collectively known as the intestinal microbiota.19 In the last two decades, accumulating evidence has indicated that the gut microbiota plays a critical role in providing nutrients to the gut mucosa, in the development of the mucosal immune system, and in preventing pathogen colonization.20 The mucosal immune system’s main tasks are to mount an immune response against pathogenic microbes and to maintain tolerance against food and commensal microbial antigens. Loss of tolerance to commensal enteric microorganisms ultimately leads to uncontrolled chronic inflammation exemplified by that seen in patients with IBD. In addition, since the colon carries 1012 bacteria/mL, compared to 102 bacteria/mL in the small intestine, the colon presents a 12-fold higher risk of developing tumors.21 Indeed, recent findings suggest that microbes such as F. nucleatum,22 enterotoxigenic B. fragilis, Streptococcus bovis, E. coli, and Klebsiella pneumoniae can play an important role in colon cancer development.23,24 These gut-associated bacteria can increase the risk of tumor malignancy by several mechanisms including mutagenesis, secretion of mutagenetic metabolites, and the risk of promoting inflammation. Lately, a link between gut bacteria and the efficacy of anti-PD-1 immunotherapy has also been uncovered.25–27 Collectively, these studies establish an important link between bacteria and colorectal cancer pathogenesis.
Upconverting nanoparticles
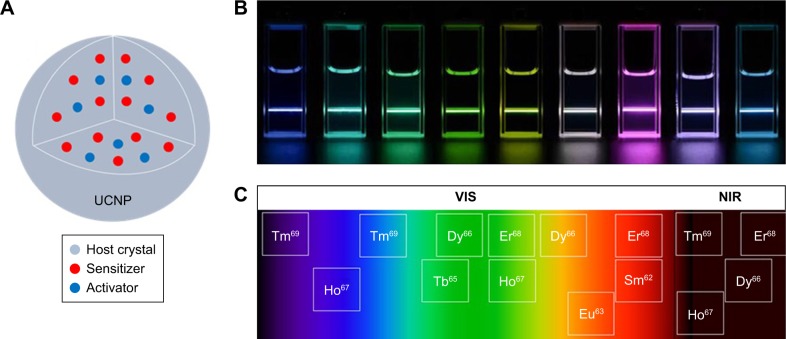
As stated previously, development of new tools to image or even treat diseases such as colon cancer are required. Among these, UCNPs have been shown to have advantageous properties compared to other available probes. UCNPs are a unique class of photoluminescent materials capable of exploiting photon upconversion (UC).28 Two or multiple excitation photons with lower energy are converted into one emitted photon with higher energy. In general, NIR light is converted into ultraviolet (UV), visible (VIS), and anti-Stokes shifted NIR light.29 A typical luminescent UCNP consists of an inorganic host crystal and doped Ln3+ ions used as sensitizers and activators, as illustrated in Figure 2A. Numerous UC mechanisms in Ln-doped crystals were recognized and formulated including excited-state absorption, energy transfer UC (ETU), photo avalanche, cooperative sensitization UC, and cross-relaxation.30,31 Among all of these processes, ETU has the highest two-photon UC efficiency.30 In a typical ETU process, a sensitizer ion sequentially absorbs incoming photons by donating its energy at its excited state to an activator ion. Through the sequential energy transfers from the sensitizers, the activator ion undergoes multi-step excitation, which results in UC luminescence.
Figure 2.
UCNP characteristics.
Notes: (A) A cross-sectional schematic representation of a typical UCNP. This includes an optically inert host crystal where optically active Ln3+ activator and sensitizer ions are embedded by replacing the cations of host matrix in the crystallization process. (B) Lanthanide-based UCNPs in colloidal solution are able to convert NIR light into different wavelengths in the VIS region of the EM. Reproduced from Zhong Y, Tian G, Gu Z, et al. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd3+-Sensitized Nanoparticles. Adv Mater. 2013;26(18):2831–2837.29 Publisher: John Wiley and Sons. © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) Ln3+ ions exhibit different emission spectral bands under NIR excitation, spanning from NIR to VIS and UV regions as each Ln3+ has its unique energy level structure.
Abbreviations: EM, electromagnetic; NIR, near infrared; UCNP, upconverting nanoparticle; UV, ultraviolet; VIS, visible.
To date, several different crystal compositions have been investigated as host crystals, including oxides, fluorides, vanadates, sulfides, and chlorides.32–37 Among the reported host crystals, fluoride-based NaREF4 crystals (in particular NaYF4) are the most favorable host materials due to their low photon energy and high chemical stability.38,39 Lns, a special group of elements in the periodic table, offer unique energy level structure, which are beneficial for use in UCNP. UCNPs embedded with Ln3+ ions produce sharp emission spectral peaks varying from UV to NIR region of electromagnetic (EM) spectrum, as shown in Figure 2B.29
Among all Lns, trivalent thulium (Tm3+), erbium (Er3+), and holmium (Ho3+) are the most favorable activators used as luminescent centers due to their higher UC emission efficiency when they are doped with trivalent ytterbium (Yb3+), an efficient NIR sensitizer. Another commonly used sensitizer is trivalent neodymium (Nd3+) ion, which has relatively high molar extinction coefficient in the NIR region. Due to the unique energy level structure of each Ln3+ ion, UC emission wavelength is dependent on the type of Ln3+ ion used as an activator ion.40 Typical activator Ln3+ ions with their observed UC emission wavelengths in the EM are shown in Figure 2C. Moreover, UC emission varies depending on the host crystal type, concentration ratio between activator and sensitizer dopants, and the crystalline phase of the nanocrystal.40–42
Inexpensive low power continuous wave NIR light sources are adequate to generate UC emission in Ln-based UCNPs. NIR excitation of UCNPs enables deeper tissue penetration.43,44 Most of the intrinsic tissue biomolecules are relatively weak scatters and absorbers in the NIR region.45 Thus, tissue autofluorescence is minimized, which yields superior sensitivity and higher SNR. Furthermore, narrow emission bandwidths of Ln3+ ions originating from the electronic transitions within 4f energy states have relatively long emission lifetime ranging from microseconds to milliseconds.46 Unlike quantum dots (QDs), another class of photoluminescent materials, Ln-based UCNPs do not display photoblinking and UC emission wavelength is not susceptible to changes in particle size47 – thus long-term continuous imaging is feasible. Moreover, Ln-based UCNPs do not suffer from photobleaching and offer high photochemical stability as compared to conventional organic fluorophores and QDs.48
Although UCNPs with their unique optical properties offer numerous advantages in bioimaging, an ideal Ln-based UCNP also requires various physical and chemical criteria for efficient use in biological applications. Hydrophilicity and high colloidal stability are critical functionalities. Functionalization of UCNPs surface is crucial for further interaction with various bio-entities. The introduction of functional groups such as carboxyl (–COOH), thiol (–SH), hydroxyl (–OH), maleimide (MA), amine (–NH), and amino (–NH2) to the surface of UCNPs provides capabilities for covalent attachment and bio-conjugation with a variety of biomolecules including proteins, antibodies, oligonucleotides, and cell receptors.49
There are still some hurdles to overcome to allow a wider use of UCNPs. For example, quantum yield (QY), a parameter proportional to brightness, is relatively low, particularly at the low optical power regime that is required to avoid any photodamage to biological specimens. The UC QYs of different kinds of UCNPs are summarized deftly by Wilhelm.50 In addition, although no or some toxicity of UCNPs has been reported in rodents,51,52 no reports in human studies are yet available and there are still no adequate research studies to evaluate the biodistribution of UCNPs in vivo in humans.53 In addition, nonspecific accumulation of UCNPs in reticuloendothelial system including liver and spleen is still a big hurdle in the field.54
Surface modification of UCNPs for biological applications
There are a number of methods to synthesize UCNPs, including co-precipitation, thermal decomposition, hydro(solvo) thermal, and microwave-assisted synthesis, all of which have been reviewed in details in various articles.55–58 In general, hydrophobic ligands, oleylamine or oleic acid, are favored during the synthesis of UCNPs to control the crystal growth, phase, shape, and size, in order to yield high-quality monodispersed UCNPs with uniform size and shape. These hydrophobic ligands form a layer upon the surface of UCNPs, hence they become dispersible in nonpolar organic solvents such as hexane and toluene. However, UCNPs capped with hydrophobic surfactant molecules lack biocompatibility. They cannot be utilized directly in biomedical applications, as they cannot disperse in water.
Biocompatibility of UCNPs in vivo and in vitro is governed by their surface properties. Fortunately, the surface nature of Ln-based UCNPs can be changed by the following surface modification approaches. Ligand interaction, ligand oxidization, layer-by-layer deposition, and ligand exchange are the most common strategies to alter the surface characteristics of UCNPs59 and result in the gain of hydrophilic characteristics by hydrophobic UCNPs. Moreover, as a consequence of these modifications, the surface of UCNPs hosts functional groups, which enables conjugation with other biomolecules such as antibodies.
UCNPs functionalized with carboxylic acid group are particularly suitable for conjugating with biomolecules containing amine group through covalent linkage. Kumar et al60 demonstrated the conjugation of mercaptopropionic acid-capped NaYF4:Gd3+, Yb3+, Er3+ UCNPs with anti-claudin-4 antibody for imaging cancer cells. Similarly, the amine group present on the UCNPs’ surface can bind to molecules containing the carboxylic group. Zhan et al61 successfully targeted carcinoembryonic antigen (CEA) on the surface of HeLa cells in vitro by employing polyallylamine hydrochloride-coated mercaptosuccinic acid-capped NaYbF4:Yb3+, Ho3+ and NaYbF4:Yb3+, Tm3+ UCNPs immunolabeled with anti-CEA8 antibodies.
Furthermore, Raphaela et al reported that MA-functionalized PEG-UCNPs can conjugate with γ-globulin via thioether linkage.62 Also, Xiong et al63 reported the binding of MA group to amine-capped NaYF4:Yb3+, Er3+ (Tm3+) UCNPs. In addition, Feng et al64 reported a method to synthesize chitosan-capped LaF3:Eu3+, which is water soluble and contains hydroxyl and amine groups. Additionally, Li et al65 modified the surface of hydrophobic OM-capped NaYF4:Yb3+, Er3+ by using thioglycollic acid (TGA), which results in hydrophilic UCNPs containing carboxyl and thiol groups.
Therefore, surface modifications play a crucial role to resolve biocompatibility issues arising from conventional synthesis methods of UCNPs. As a consequence of appropriate modifications, UCNPs can gain new surface properties, such as hydrophilicity, biocompatibility, colloidal stability, and bio-targeting, which are crucial for UCNPs to be more extensively used in biological applications.
Optical imaging (OI)
Molecular imaging plays a significant role in medicine, providing essential diagnostic and prognostic information of a disease by the visualization and characterization of biological processes at cellular and/or subcellular levels. The benefit of molecular imaging techniques is the evaluation of molecular changes rather than morphological abnormalities.
OI is a rapidly emerging molecular imaging method where light–tissue interactions such as absorption, scattering, and fluorescence are investigated. Due to the cost-effectiveness and simplicity, FI has been a method of choice for biological researchers. This technique relies on the endogenous or exogenous fluorescent characteristics of biomolecules. A fluorescent biomolecule is able to absorb excitation light at different wavelengths, with varying strengths, and re-emit light at a longer wavelength than that of the excitation light, which is known as fluorescence emission. The biomolecules with this ability are considered as promising molecular contrast agents for in vivo and in vitro applications. However, endogenous contrast agents generally exhibit inadequate spectroscopic characteristics. Therefore, exogenous fluorescent contrast agents including organic dyes (eg, indocyanine green [ICG], fluorescein sodium, and methylene blue), fluorescent proteins (eg, green fluorescent protein), and QDs (eg, CdSe2, Ag2, and CuInS2) are externally administered to enhance image contrast. Despite the remarkable optical properties of the exogenous fluorescent contrast agents, their use in molecular imaging is limited. This is due to several reasons. First, they suffer from relatively poor spatial resolution due to the overlap of excitation and fluorescence emission spectra of major fluorescent tissue components (eg, nicotinamide adenine dinucleotide, collagen, elastin, and flavins), ie, tissue auto-fluorescence. Second, exogenous fluorescent contrast agents generally require UV or VIS light excitation, which limits the light penetration depth due to a relatively high absorption and scattering nature of bio-entities inside the tissue. Hence, deep tissue imaging with high sensitivity becomes difficult. While employing QDs, cytotoxicity appears a major challenge due to the presence of toxic heavy metals commonly used including cadmium, silver, and copper. They also exhibit photoblinking effects upon continuous irradiation, which hinders long-term imaging. Moreover, organic dyes and fluorescent proteins suffer from photobleaching effect; the fluorescence emission fades after relatively short time as the molecule starts to degrade under continuous irradiation, which also impedes long-term imaging. To date, among all reported exogenous fluorescent contrast agents, only two NIR fluorescent molecules, ICG and IRDye800CW conjugate, are approved by Food and Drug Administration (FDA).66 Noura et al showed the feasibility of a lateral region sentinel node biopsy of lower colorectal rectal cancer guided by ICG.67 Additionally, Gong et al68 used IRDye800CW to image the tumors in colorectal cancer.
Considering the aforementioned issues associated with the existing fluorescent contrast agents, Ln-based UCNPs with their previously stated remarkable optical features can be considered as promising photoluminescent contrast markers. Ln-based UCNPs with their NIR excitation ability can avail of optical transparency window, thus maximizing high light penetration depths. In addition, their photon UC ability gives them anti-Stokes shifted characteristics, which minimizes tissue autofluorescence. As they show high photochemical stability, they do not create photoblinking and photobleaching effects under long-term continuous irradiance.
In the next sections, we will be focusing on UCNPs’ applications of bacterial, anti-microbial detection as well as tumor imaging.
Bacterial detection using UCNPs
The well-known bacterial detection techniques, culture-based or DNA-based using PCR, present both benefits and deficiencies. Various drawbacks in these methodologies, which make them less suitable for on-site diagnosis, include enrichment steps and inability to simultaneously detect two or more bacteria or amplification steps, which makes process more time-consuming.69 Recently, bacterial detection using UCNPs (Table 1 and Figure 3A) has been shown to be successful by improving the sensitivity and the speed of the detection process.
Table 1.
List of published studies where UCNPs were used to detect bacteria
| Type of UCNPs’ system used | UCNPs’ conjugation | Bacteria/bacterial metabolite detected | Advantage | Reference |
|---|---|---|---|---|
| Multicolor UCNPs (NaYF4:Yb, Tm NaYF4:Yb, Ho NaYF4:Yb, Er/Mn) | Antibacteria aptamer | S. aureus, Vibrio parahemolyticus, and S. typhimurium | High specificity, simultaneous detection | Wu et al69 |
| NaYF4:Yb, Er | Anti-E. coli antibody | E. coli | High photostability | Ong et al70 |
| NaYF4:Yb, Er | Anti-E. coli antibody | E. coli | Low detection limit – 10 CFU/mL | Pan et al71 |
| NaYF4:Yb0.2, Tm 0.02 | Anti-ochratoxin A aptamer | Ochratoxin A | High sensitivity | Dai et al 72 |
| NaYF4:Tm@SiO2, NaYF4:Er@SiO2 | Anti-P. gingivalis antibody | P. gingivalis | Low detection limit – 10 CFU/mL | Qin et al73 |
| LET, Mn2+-doped NaYF4:Yb, Tm as donor and Au nanorod as acceptor | Anti-Salmonella aptamer | S. typhimurium | Low detection limit – 11 CFU/mL | Cheng et al74 |
| FRET, Au NP as donor and NaYF4:Yb/Er as acceptor | Antiaptamer cDNA | E. coli, B. subtilis, and S. aureus | Detect E. coli in real food and water samples | Jin et al75 |
| Optical trapping, KLu2F7:Yb3+, Er3+ | None | E. coli | Single bacteria detection | Xin et al76 |
| Bio-microlens to enhance the upconversion fluorescence of NaYF4:Yb3+/Tm3+ | None | E. coli | Single bacteria detection | Li et al77 |
Abbreviations: B. subtilis, Bacillus subtilis; E. coli, Escherichia coli; FRET, fluorescence resonance energy transfer; LET, luminescence energy transfer; NP, nanoparticle; P. gingivalis, Porphyromonas gingivalis; S. aureus, Staphylococcus aureus; S. typhimurium, Salmonella typhimurium; UCNPs, upconverting nanoparticles.
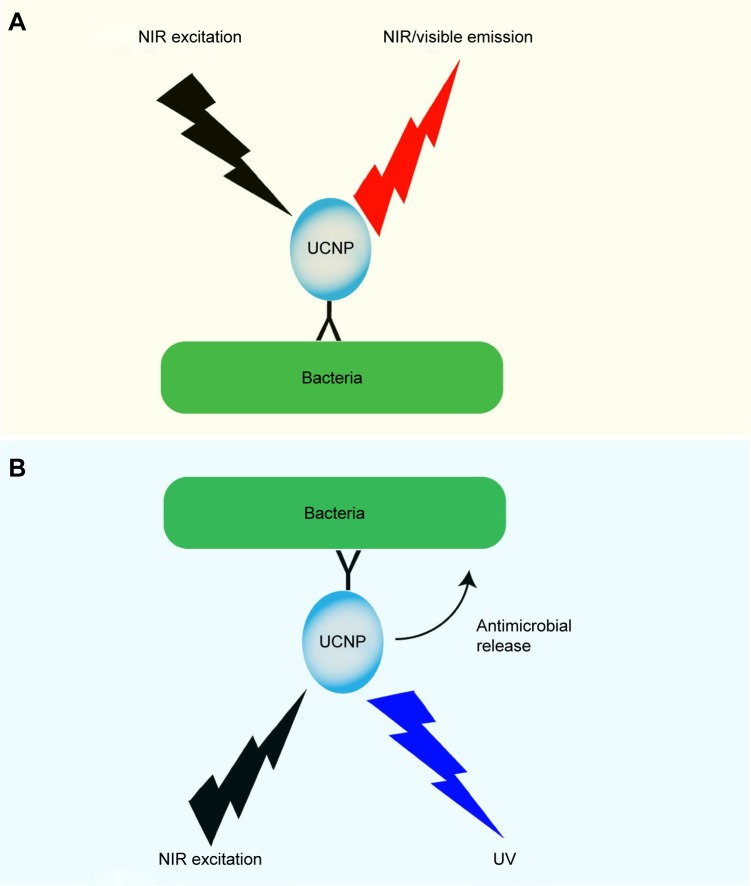
Figure 3.
Mode of action of UCNPs in visualizing and killing bacteria.
Notes: In this figure, we summarize published work on UCNPs’ role in visualizing and killing bacteria. (A) Functionalized UCNPs targeting bacteria with NIR/VIS emission range is used to image the bacteria. (B) UCNPs loaded with antimicrobial compounds or with an UV emission range are used for localized antimicrobial activity.
Abbreviations: NIR, near infrared; UCNPs, upconverting nanoparticles; UV, ultraviolet; VIS, visible.
Ong et al reported the first in vitro study showing that UCNPs coupled with an anti-E. coli antibody successfully detected E. coli as a consequence of superior photostability of the UCNP-labeled bacteria as compared to the GFP-expressing bacteria.70 Moreover, using a dendritic cell and bacteria co-culture infection system, the potential of UCNPs’ imaging for long-term bacterial trafficking was also demonstrated. Additionally, Pan et al71 were able to reduce the detection level of E. coli as low as 10 CFU/mL, when using UCNPs functionalized with an anti-E. coli antibody. In an in vitro study, Wu et al,69 improved the existing technology using multicolor UCNPs, doped in various rare-earth metals to obtain different emission peaks, coupled with bacteria-specific aptamers, and confirmed simultaneous detection of three different bacterial strains, such as Staphylococcus aureus, Vibrio parahemolyticus, and Salmonella typhimurium. The authors also tested if their UCNPs’ conjugates were able to detect the three bacteria in real food samples, providing evidence for the potential use of UCNPs in bioassays for food safety. Dai et al72 also measured the level of ochratoxin, a group of mutagenic and teratogenic compounds produced by Aspergillus and Penicillium fungal species, in beer samples by using UCNPs–aptamer complexes. More recently, the periodontal pathogen Porphyromonas gingivalis was also detected by UCNPs and, similar to E. coli studies, the P. gingivalis detection limit was 10 CFU/mL.73 Furthermore, Cheng et al designed a luminescence energy transfer system, where anti-Salmonella aptamer-conjugated UCNPs served as energy donor and gold nanorods served as acceptor allowing the detection of Salmonella in an aqueous buffer with a detection limit of 11 CFU/mL. In the absence of Salmonella, the electrostatic interaction between the negatively charged UCNPs and the positively charged gold particles shorten the distances between these two, leading to luminescence quenching. However, addition of Salmonella into the system restores the luminescence by increasing the distance between the donor and the acceptor due to the binding of Salmonella to the UCNPs through anti-Salmonella aptamer.74 Jin et al75 also developed a similar fluorescence resonance energy transfer (FRET) system by using gold nanoparticle aptamers as the donor and UCNPs coupled with cDNA as the acceptor. Recently, Xin et al76 and Li et al77 reported that a single bacteria resolution was achieved by enhancing the UC fluorescence of UCNPs using the trapped optical fiber on yeast and human cells, respectively, as form of a microlens. These collective data demonstrate that UCNPs can be used as a luminescent probe to detect bacterial signal as low as 1 CFU, which can be of relevance for a number of different areas including diagnostics and food industry.
Antimicrobial applications of UCNPs
As illustrated in Figure 3, the use of UCNPs has not only been limited to bacterial detection. For example, NIR-triggered release of the antibiofilm agent d-amino acid and other bactericides was used to eradicate bacteria and biofilms.78 UCNPs coated with a thin layer of TiO2, with an emission frequency in the UV region, is linked to d-amino acid through a UV cleavable linkage. Upon excitation at NIR, UCNPs emit photons in UV range, which subsequently lead to the controlled release of ROS from TiO2 and d-amino acid. Furthermore, Li et al79 developed a FRET system using UCNPs as energy donors and poly[9,9-bis(6,6-(N,N,N-trimethylaminium)-fluorene-2,7-ylenevinylene)-co-alt-2,5-dicyano-1,4-phenylene] (PFVCN), a conjugate polymer (CP), as energy acceptor. CPs are organic polymers that have emerged as alternative antibiotics and therefore can led to the generation of ROS by absorbing UV or VIS light. However, UV light in itself can damage the cells and VIS light is too weak to penetrate deeper into the tissue. To overcome these limitations, the authors used the UCNPs/PFVCN FRET system. In this system, UCNPs convert the NIR light into localized UV light, which is subsequently absorbed by PFVCN and generates ROS. In addition, to kill multidrug-resistant S. aureus and β-lactamase-producing E. coli, a photodynamic system, where UCNPs were coated with N-octyl chitosan loaded with ROS producing zinc phthalocyanine as photosentsitizer, was developed.80 Collectively, these reports highlight further the use for UCNPs as a light-triggered antimicrobial agent (Table 2 and Figure 3B), which could potentially replace conventional antimicrobial drugs.81
Table 2.
Antimicrobial actions of UCNPs
| Type of UCNPs’ system used | Antimicrobial | Target bacterium | Reference |
|---|---|---|---|
|
| |||
| β-NaYF4:Tm3+ coated with TiO2 | d-amino acid (d-tyrosine) and ROS | Individual B. subtilis and biofilm | Wei et al78 |
| β-NaYF4:Yb, Tm | PFVCN | E. coli | Li et al79 |
| Oleic acid capped β-NaYF4:23%Yb, 2%Er@NaYF4 zinc phthalocyanine as photosensitizer | OC and ROS inducing zinc phthalocyanine | Multidrug-resistant S. aureus and β-lactamase-producing E. coli | Li et al80 |
Abbreviations: B. subtilis, Bacillus subtilis; E. coli, Escherichia coli; OC, N-octyl chitosan; PFVCN, poly(9,9-bis[6,6-{N,N,N-trimethylaminium}-fluorene]-2,7-ylenevinylene)-co-alt-2,5-dicyano-1,4-phenylene]; S. aureus, Staphylococcus aureus; UCNPs, upconverting nanoparticles.
Detection and treatment of colon cancer by UCNPs
Due to the low fluorescence background in the NIR region, NIR fluorescent nanoparticles and dyes in conjugation with tumor-targeting ligands have been preferentially used for in vivo imaging of colorectal cancer (Table 3). However, commonly encountered issues with dyes or other luminescent materials include low penetration depth, photobleaching, autofluorescence, and high background signal. To overcome these limitations, tumor-targeting UCNPs have been developed (Table 3). The feasibility of UCNPs to act as a contrasting agent was initially reported by Liu et al82 who successfully imaged the mouse gastrointestinal tract using UCNPs. Next, tumor-targeting UCNPs were developed with the aim to detect primary gastric tumors and lymphatic metastasis using an orthotopic tumor model.83 Biocompatible tumor targeting UCNPs were used to detect primary colorectal cancer in Kunming mice.84 To date, UCNPs are commonly administered intravenously for colorectal imaging in mice. However, a study by Gao et al85 comparing biodistribution and accumulation of UCNPs in cancerous and normal tissues demonstrated that intra-peritoneal administration of UCNPs in a colorectal cancer model appeared to be a superior route when compared with intravenous administration.
Table 3.
Colorectal cancer detection and treatment using UCNPs
| Type of UCNPs | UCNPs’ conjugation | Ligand specificity | Tumor model | Route of UCNPs’ administration | Type of tumor detected | Anticancer property used | Reference |
|---|---|---|---|---|---|---|---|
|
| |||||||
| NaGdF4:Yb, Er@NaGdF4 coated with PEG | FA | Folate-receptor overexpressing melanoma cells | Chemically induced primary colorectal cancer model | Intravenous injection | Primary colorectal cancer | None | Liu et al84 |
| NaLuF4:Yb, Tm@NaLuF4 nanocrystals modified with citrates | None | Passive targeting | Kunming mice injected with human colorectal cancer LOVO cell line | Intraperitoneal and intravenous injected | Colorectal tumor | None | Gao et al85 |
| PEGylated NaYF4 (Y/Yb/Er=78:20:2) | Ce6, a photosensitizer, and imiquimod (R837), a toll-like-receptor-7 agonist and an anti-CTLA-4, checkpoint inhibitor | None | BALB/c mice, subcutaneously injected CT26 cells | Intratumor injection | Colorectal tumor | PDT with checkpoint inhibitor | Xu et al86 |
Abbreviations: Ce6, Chlorin e6; FA, folic acid; PDT, photodynamic therapy; PEG, polyethylene glycol.
Recently, UCNPs loaded with an immune adjuvant, a toll-like receptor-7 agonist were used with the FDA-approved check-point inhibitor anti-cytotoxic T lymphocyte-associated protein 4 antibody to treat a primary tumor in an animal model of colorectal cancer.86 NIR exposure triggers photo-dynamic destruction of the primary tumor. Subsequently to photodynamic therapy (PDT), the cancer-associated antigens released from the cancerous tissue induce an anticancer immune response with the help of the immune adjuvant. The antitumor response is further potentiated by the checkpoint inhibitor.
The importance of the tumor microenvironment has been recently recognized as an attractive target for cancer treatment. Hypoxia, low pH, extracellular matrix, the tumor vascular system, immune cells, and tumor-associated fibroblasts collectively form the tumor microenvironment. UCNPs can be used to target the tumor microenvironment for therapeutic purposes. Indeed, a recent in vitro study reported a targeted killing of tumor cells by affecting specific tumor markers such as EGF by Ln-UCNPs.87 Overall, these findings identify a potential use of UCNPs in colorectal cancer diagnosis and treatment.
Conclusion and future perspectives
In vitro and preclinical studies to date indicate that, for colorectal cancer, UCNPs are an enhanced and better imaging strategy as compared with luminescent or fluorescence probes. Additional analyses have demonstrated that UCNPs can indeed target-specific bacteria strains and even exert antimicrobial actions upon NIR triggering, suggesting their utilities as new potential treatment strategies in colorectal cancer and other intestinal disorders.
The healthy gut maintains a state of hypoxia on and around the mucosal surface of the colon. However, in a chronically inflamed intestine (which often precedes a colorectal cancer finding in IBD patients), the concentration of oxygen on the colonic surface increases due to an increase in blood flow around the inflamed tissue. By utilizing a hypoxia-sensitive UCNPs’ imaging, the inflammatory state of the gut could be imaged with minimal invasiveness (Figure 1). Earlier identification of individuals predisposed to develop colorectal cancer or intestinal inflammation (eg, IBD) may result in better clinical outcomes.
Although several studies have demonstrated the involvement of microbes in IBD and cancer progression, the mechanistic insights into how these bacteria actually lead to these conditions or their potential role in relapse of disease are yet to be discovered. UCNPs can therefore be used to visualize host–microbe interactions at the cellular and molecular levels to establish a cause and effect relationship for these chronic conditions.
Photodynamic therapy involves the generation of ROS resulting from the interaction of photosensitizer and VIS light. However, VIS light is too weak to penetrate deep into the tissue. Also, ROS production is limited due to the hypoxic environment of tumor and the colon tissue. Furthermore, ROS production consumes most of the oxygen available to an induced hypoxic environment in the tissue, which further potentiates tumorigenesis. Talaporifin sodium (TS), a light-activated drug/photosensitizer, has been approved in Japan for the treatment of early-state endobronchial cancer.88 Activation of TS with a 664 nm VIS range light generates a single oxygen species, resulting in the induction of apoptotic cell death.88 In a Phase II trial of TS in patients with colorectal cancer and metastasis to the liver, the efficacy of the treatment depended on the number of excitation sources used to activate the drug.89 This study shows that the treatment efficacy depends on the penetration of cancer tissue by the excitation light with enough photons to activate the photosensitizer. UCNPs with emission at UV range could be used to overcome these limitations associated with VIS light and ROS production. Deep penetrating NIR light can be used to excite the UCNPs, and the localized emission of UV can be used to kill the surrounding carcinogenic cells.
However, even though NIR is nontoxic for biological tissues, high intensity NIR can indeed overheat cells and result in photodamage90 of not only cancerous cells but also healthy and noncancerous cells. A way to reduce these issues is using 800 nm excited Nd3+-based UCNPs, which leave cells intact due to less absorption of water90 or by lowering the laser power.91 In summary, we are confident that future studies will resolve the gap between the bench and clinical translation, resulting in the beneficial use of UCNPs in humans in several biological applications including diagnostics and treatment purposes.
Acknowledgments
The authors thank Dr Amanda Lohan for proofreading the article.
Footnotes
Disclosure
Professor Stefan Andersson-Engels, Mr Raminder Singh, and Mr Gokhan Dumlupinar receive financial support from Science Foundation Ireland (SFI) (Research Professor Award), grant number SFI/15/RP/2828, and the APC Microbiome Ireland, and Dr Silvia Melgar receives financial support from SFI, grant number SFI/12/RC/2273. The authors report no other conflicts of interest in this work.
References
- 1.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:1–25. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi JR, Dutilh BE, Hall N, et al. Towards the Human Colorectal Cancer Microbiome. PLoS One. 2011;6(5):e20447–e20447. doi: 10.1371/journal.pone.0020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang F-M, Liu H-L. Fusobacterium nucleatum and colorectal cancer: A review. World journal of gastrointestinal oncology. 2018;10(3):71. doi: 10.4251/wjgo.v10.i3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejea CM, Fathi P, Craig JM, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi CHR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14(4):218–229. doi: 10.1038/nrgastro.2017.1. [DOI] [PubMed] [Google Scholar]
- 6.Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS nano. 2011;5(11):8488–8505. doi: 10.1021/nn202378b. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Hou Y, Gao M. Are rare-earth nanoparticles suitable for in vivo applications? Adv Mater. 2014;26(40):6922–6932. doi: 10.1002/adma.201305535. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Qiu H, Prasad PN, Chen X. Upconversion Nanoparticles : Design, Nanochemistry, and Applications in Theranostics. Chem Rev. 2015;114(10):5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmer E, Benayas A, Légaré F, Vetrone F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016;1(3):168–184. doi: 10.1039/c5nh00073d. [DOI] [PubMed] [Google Scholar]
- 10.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: Clinical perspectives. Qjm. 2016;109(3):151–158. doi: 10.1093/qjmed/hcv137. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HFA, Tomlinson I, Castells A. Clinical management of hereditary colorectal cancer syndromes. Nat Rev Gastroenterol Hepatol. 2015;12(2):88–97. doi: 10.1038/nrgastro.2014.229. [DOI] [PubMed] [Google Scholar]
- 13.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and Colorectal Cancer. Jama. 2008;300(23):2765–2765. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 14.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: An overall and dose-Response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 15.Hanyuda A, Ogino S, Qian ZR, et al. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer. 2016;139(4):854–868. doi: 10.1002/ijc.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. JNCI. 2012;104(18):1353–1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Waldner MJ, Neurath MF. Mechanisms of Immune Signaling in Colitis-Associated Cancer. Cmgh. 2015;1(1):6–16. doi: 10.1016/j.jcmgh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang M, Martin A. Microbiome and colorectal cancer: Unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin Immunol. 2017 Apr;32:3–13. doi: 10.1016/j.smim.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat Rev Microbiol. 2012;10(8):575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 22.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonic V, Stojadinovic A, Kester KE, et al. Significance of infectious agents in colorectal cancer development. J Cancer. 2013;4(3):227–240. doi: 10.7150/jca.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopalakrishnan V, Spencer C, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 28.Suyver JF, Aebischer A, Biner D, et al. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Optical Materials. 2005;27(6):1111–1130. [Google Scholar]
- 29.Zhong Y, Tian G, Gu Z, et al. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd3+-Sensitized Nanoparticles. Adv Mater. 2013;26(18):2831–2837. doi: 10.1002/adma.201304903. [DOI] [PubMed] [Google Scholar]
- 30.Auzel F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem Rev. 2004;104(1):139–174. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 31.Chivian JS, Case WE, Eden DD. The photon avalanche: A new phenomenon in Pr3+-based infrared quantum counters. Applied Physics Letters. 1979;35(2):124–125. [Google Scholar]
- 32.Cao YC. Synthesis of Square Gadolinium-Oxide Nanoplates. J Am Chem Soc. 2004;126(24):7456–7457. doi: 10.1021/ja0481676. [DOI] [PubMed] [Google Scholar]
- 33.Stouwdam JW, van Veggel FCJM. Near-infrared Emission of Redispersible Er3+, Nd3+, and Ho3+ Doped LaF3 Nanoparticles. Nano Letters. 2002;2(7):733–737. [Google Scholar]
- 34.Riwotzki K, Haase M. Wet-Chemical Synthesis of Doped Colloidal Nanoparticles: YVO4:Ln (Ln = Eu, Sm, Dy) The Journal of Physical Chemistry B. 1998;102(50):10129–10135. [Google Scholar]
- 35.Mirkovic T, Hines MA, Nair PS, Scholes GD. Single-Source Precursor Route for the Synthesis of EuS Nanocrystals. Chemistry of Materials. 2005;17(13):3451–3456. [Google Scholar]
- 36.Li Y, Wei X, Yin M. Synthesis and upconversion luminescent properties of Er3+ doped and Er3+–Yb3+ codoped GdOCl powders. Journal of Alloys and Compounds. 2011;509(41):9865–9868. [Google Scholar]
- 37.Chen G, Qiu H, Prasad PN, Chen X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem Rev. 2014;114(10):5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menyuk N, Dwight K, Pierce JW. NaYF4 : Yb,Er—an efficient upconversion phosphor. Applied Physics Letters. 1972;21(4):159–161. [Google Scholar]
- 39.Diamente PR, Raudsepp M, van Veggel FCJM. Dispersible Tm3+-Doped Nanoparticles that Exhibit Strong 1.47 μm Photoluminescence. Advanced Functional Materials. 2007;17(3):363–368. [Google Scholar]
- 40.Dong H, Sun L-D, Yan C-H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chemical Society Reviews. 2015;44(6):1608–1634. doi: 10.1039/c4cs00188e. [DOI] [PubMed] [Google Scholar]
- 41.Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D. Advances in highly doped upconversion nanoparticles. Nature Communications. 2018;9(1):2415. doi: 10.1038/s41467-018-04813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krämer KW, Biner D, Frei G, Güdel HU, Hehlen MP, Lüthi SR. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chemistry of Materials. 2004;16(7):1244–1251. [Google Scholar]
- 43.Liu Q, Sun Y, Yang T, Feng W, Li C, Li F. Sub-10 nm Hexagonal Lanthanide-Doped NaLuF4 Upconversion Nanocrystals for Sensitive Bioimaging in Vivo. J Am Chem Soc. 2011;133(43):17122–17125. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]
- 44.Dong N-N, Pedroni M, Piccinelli F, et al. NIR-to-NIR Two-Photon Excited CaF2:Tm3+,Yb3+ Nanoparticles: Multifunctional Nano-probes for Highly Penetrating Fluorescence Bio-Imaging. ACS Nano. 2011;5(11):8665–8671. doi: 10.1021/nn202490m. [DOI] [PubMed] [Google Scholar]
- 45.Liu T-M, Conde J, Lipiński T, Bednarkiewicz A, Huang C-C. Smart NIR linear and nonlinear optical nanomaterials for cancer theranostics: Prospects in photomedicine. Progress in Materials Science. 2017;88:89–135. [Google Scholar]
- 46.Berezin MY, Achilefu S. Fluorescence Lifetime Measurements and Biological Imaging. Chem Rev. 2010;110(5):2641–2684. doi: 10.1021/cr900343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park YI, Kim JH, Lee KT, et al. Nonblinking and Nonbleaching Upconverting Nanoparticles as an Optical Imaging Nanoprobe and T1 Magnetic Resonance Imaging Contrast Agent. Adv Mater. 2009;21(44):4467–4471. [Google Scholar]
- 48.Wu S, Han G, Milliron DJ, et al. Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals. Proceedings of the National Academy of Sciences. 2009;106(27):10917. doi: 10.1073/pnas.0904792106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnach A, Bednarkiewicz A. Lanthanide-doped up-converting nanoparticles: Merits and challenges. Nano Today. 2012;7(6):532–563. [Google Scholar]
- 50.Wilhelm S. Perspectives for Upconverting Nanoparticles. ACS Nano. 2017;11(11):10644–10653. doi: 10.1021/acsnano.7b07120. [DOI] [PubMed] [Google Scholar]
- 51.Xiong L, Yang T, Yang Y, Xu C, Li F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials. 2010;31(27):7078–7085. doi: 10.1016/j.biomaterials.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 52.Jalil RA, Zhang Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials. 2008;29(30):4122–4128. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA. Upconverting nanoparticles: assessing the toxicity. Chemical Society Reviews. 2015;44(6):1561–1584. doi: 10.1039/c4cs00177j. [DOI] [PubMed] [Google Scholar]
- 54.Cheng L, Yang K, Shao M, Lu X, Liu Z. In vivo pharmacokinetics, long-term biodistribution and toxicology study of functionalized upconversion nanoparticles in mice. Nanomedicine. 2011;6(8):1327–1340. doi: 10.2217/nnm.11.56. [DOI] [PubMed] [Google Scholar]
- 55.Zhengquan L, Yong Z. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF 4 :Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology. 2008;19(34):345606. doi: 10.1088/0957-4484/19/34/345606. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y-W, Sun X, Si R, You L-P, Yan C-H. Single-Crystalline and Monodisperse LaF3 Triangular Nanoplates from a Single-Source Precursor. J Am Chem Soc. 2005;127(10):3260–3261. doi: 10.1021/ja042801y. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature. 2005;437:121. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- 58.Wang H-Q, Nann T. Monodisperse Upconverting Nanocrystals by Microwave-Assisted Synthesis. ACS Nano. 2009;3(11):3804–3808. doi: 10.1021/nn9012093. [DOI] [PubMed] [Google Scholar]
- 59.Sedlmeier A, Gorris HH. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chemical Society Reviews. 2015;44(6):1526–1560. doi: 10.1039/c4cs00186a. [DOI] [PubMed] [Google Scholar]
- 60.Kumar R, Nyk M, Ohulchanskyy TY, Flask CA, Prasad PN. Combined Optical and MR Bioimaging Using Rare Earth Ion Doped NaYF4 Nanocrystals. Advanced Functional Materials. 2009;19(6):853–859. [Google Scholar]
- 61.Zhan Q, Qian J, Liang H, et al. Using 915 nm Laser Excited Tm3+/Er3+/Ho3+-Doped NaYbF4 Upconversion Nanoparticles for in Vitro and Deeper in Vivo Bioimaging without Overheating Irradiation. ACS Nano. 2011;5(5):3744–3757. doi: 10.1021/nn200110j. [DOI] [PubMed] [Google Scholar]
- 62.Raphaela BL, Tero S, Otto SW, Hans HG. Maleimide activation of photon upconverting nanoparticles for bioconjugation. Nanotechnology. 2012;23(48):485103. doi: 10.1088/0957-4484/23/48/485103. [DOI] [PubMed] [Google Scholar]
- 63.Xiong L, Chen Z, Tian Q, Cao T, Xu C, Li F. High Contrast Upconversion Luminescence Targeted Imaging in Vivo Using Peptide-Labeled Nanophosphors. Anal Chem. 2009;81(21):8687–8694. doi: 10.1021/ac901960d. [DOI] [PubMed] [Google Scholar]
- 64.Feng W, Yong Z, Xianping F, Minquan W. One-pot synthesis of chitosan/LaF 3 :Eu 3+ nanocrystals for bio-applications. Nanotechnology. 2006;17(5):1527. [Google Scholar]
- 65.Li D, Dong B, Bai X, Wang Y, Song H. Influence of the TGA Modification on Upconversion Luminescence of Hexagonal-Phase NaYF4:Yb3+, Er3+ Nanoparticles. The Journal of Physical Chemistry C. 2010;114(18):8219–8226. [Google Scholar]
- 66.Nagaya T, Nakamura YA, Choyke PL, Kobayashi H. Fluorescence-Guided Surgery. Frontiers in oncology. 2017;7:314–314. doi: 10.3389/fonc.2017.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noura S, Ohue M, Seki Y, et al. Feasibility of a Lateral Region Sentinel Node Biopsy of Lower Rectal Cancer Guided by Indocyanine Green Using a Near-Infrared Camera System. Annals of Surgical Oncology. 2010;17(1):144–151. doi: 10.1245/s10434-009-0711-2. [DOI] [PubMed] [Google Scholar]
- 68.Gong L, Ding H, Long NE, et al. A 3E8.scFv.Cys-IR800 Conjugate Targeting TAG-72 in an Orthotopic Colorectal Cancer Model. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2018;20(1):47–54. doi: 10.1007/s11307-017-1096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S, Duan N, Shi Z, Fang C, Wang Z. Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal Chem. 2014;86(6):3100–3107. doi: 10.1021/ac404205c. [DOI] [PubMed] [Google Scholar]
- 70.Ong LC, Ang LY, Alonso S, Zhang Y. Bacterial imaging with photostable upconversion fluorescent nanoparticles. Biomaterials. 2014;35(9):2987–2998. doi: 10.1016/j.biomaterials.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 71.Pan W, Zhao J, Chen Q. Fabricating Upconversion Fluorescent Probes for Rapidly Sensing Foodborne Pathogens. J Agric Food Chem. 2015;63(36):8068–8074. doi: 10.1021/acs.jafc.5b02331. [DOI] [PubMed] [Google Scholar]
- 72.Dai S, Wu S, Duan N, Wang Z. A near-infrared magnetic aptasensor for Ochratoxin A based on near-infrared upconversion nanoparticles and magnetic nanoparticles. Talanta. 2016;158:246–253. doi: 10.1016/j.talanta.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 73.Qin W, Zheng B, Yuan Y, et al. Sensitive detection of Porphyromonas gingivalis based on magnetic capture and upconversion fluorescent identification with multifunctional nanospheres. Eur J Oral Sci. 2016;124(4):334–342. doi: 10.1111/eos.12286. [DOI] [PubMed] [Google Scholar]
- 74.Cheng K, Zhang J, Zhang L, Wang L, Chen H. Aptamer biosensor for Salmonella typhimurium detection based on luminescence energy transfer from Mn2 +-doped NaYF4:Yb, Tm upconverting nanoparticles to gold nanorods. Spectrochim Acta A Mol Biomol Spectrosc. 2017;171:168–173. doi: 10.1016/j.saa.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Jin B, Wang S, Lin M, et al. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens Bioelectron. 2017;90:525–533. doi: 10.1016/j.bios.2016.10.029. September 2016. [DOI] [PubMed] [Google Scholar]
- 76.Xin H, Li Y, Xu D, Zhang Y, Chen CH, Li B. Single Upconversion Nanoparticle–Bacterium Cotrapping for Single-Bacterium Labeling and Analysis. Small. 2017;13(14):1–10. doi: 10.1002/smll.201603418. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Liu X, Yang X, Lei H, Zhang Y, Li B. Enhancing Upconversion Fluorescence with a Natural Bio-microlens. ACS Nano. 2017;11(11):10672–10680. doi: 10.1021/acsnano.7b04420. [DOI] [PubMed] [Google Scholar]
- 78.Wei W, Bing W, Ren J, Qu X. Near infrared-caged D-amino acids multifunctional assembly for simultaneously eradicating biofilms and bacteria. Chem Commun. 2015;51(63):12677–12679. doi: 10.1039/c5cc04729c. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Zhao Q, Shi F, Liu C, Tang Y. NIR-Mediated Nanohybrids of Upconversion Nanophosphors and Fluorescent Conjugated Polymers for High-Efficiency Antibacterial Performance Based on Fluorescence Resonance Energy Transfer. Adv Healthc Mater. 2016;5(23):2967–2971. doi: 10.1002/adhm.201600868. [DOI] [PubMed] [Google Scholar]
- 80.Li S, Cui S, Yin D, et al. Dual antibacterial activities of a chitosan-modified upconversion photodynamic therapy system against drug-resistant bacteria in deep tissue. Nanoscale. 2017;9(11):3912–3924. doi: 10.1039/c6nr07188k. [DOI] [PubMed] [Google Scholar]
- 81.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. PT. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z, Ju E, Liu J, et al. Direct visualization of gastrointestinal tract with lanthanide-doped BaYbF5upconversion nanoprobes. Biomaterials. 2013;34(30):7444–7452. doi: 10.1016/j.biomaterials.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 83.Qiao R, Liu C, Liu M, et al. Ultrasensitive in vivo detection of primary gastric tumor and lymphatic metastasis using upconversion nanoparticles. ACS Nano. 2015;9(2):2120–2129. doi: 10.1021/nn507433p. [DOI] [PubMed] [Google Scholar]
- 84.Liu C, Qi Y, Qiao R, et al. Detection of early primary colorectal cancer with upconversion luminescent NP-based molecular probes. Nanoscale. 2016;8(25):12579–12587. doi: 10.1039/c5nr07858j. [DOI] [PubMed] [Google Scholar]
- 85.Gao Y, Zhu X, Zhang Y, et al. In vivo biodistribution and passive accumulation of upconversion nanoparticles in colorectal cancer models via intraperitoneal injection. RSC Adv. 2017;7(50):31588–31596. [Google Scholar]
- 86.Xu J, Xu L, Wang C, et al. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano. 2017;11(5):4463–4474. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 87.Stochaj U, Burbano DCR, Cooper DR, Kodiha M, Capobianco JA. The effects of lanthanide-doped upconverting nanoparticles on cancer cell biomarkers. Nanoscale. 2018;10(30):14464–14471. doi: 10.1039/c8nr01451e. [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Bromley E, Xu L, Chen JC, Keltner L. Talaporfin sodium. Expert opinion on pharmacotherapy. 2010;11(1):133–140. doi: 10.1517/14656560903463893. [DOI] [PubMed] [Google Scholar]
- 89.Kujundžić M, Vogl T, Stimac D, et al. A Phase II safety and effect on time to tumor progression study of intratumoral light infusion technology using talaporfin sodium in patients with metastatic colorectal cancer. Journal of surgical oncology. 2007;96(6):518–524. doi: 10.1002/jso.20832. [DOI] [PubMed] [Google Scholar]
- 90.Xie X, Gao N, Deng R, Sun Q, Xu Q-H, Liu X. Mechanistic investigation of photon upconversion in Nd3+-sensitized core–shell nanoparticles. J Am Chem Soc. 2013;135(34):12608–12611. doi: 10.1021/ja4075002. [DOI] [PubMed] [Google Scholar]
- 91.He S, Krippes K, Ritz S, et al. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem Commun. 2015;51(2):431–434. doi: 10.1039/c4cc07489k. [DOI] [PubMed] [Google Scholar]