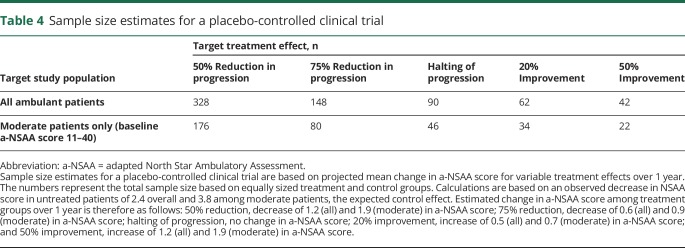
Ursula Moore
Ursula Moore, MBBchir
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Marni Jacobs
Marni Jacobs, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Meredith K James
Meredith K James, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Anna G Mayhew
Anna G Mayhew, PT, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Roberto Fernandez-Torron
Roberto Fernandez-Torron, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Jia Feng
Jia Feng, MSc
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Avital Cnaan
Avital Cnaan, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Michelle Eagle
Michelle Eagle, PT, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Karen Bettinson
Karen Bettinson, MSc
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Laura E Rufibach
Laura E Rufibach, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Robert Muni Lofra
Robert Muni Lofra, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Andrew M Blamire
Andrew M Blamire, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Pierre G Carlier
Pierre G Carlier, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Plavi Mittal
Plavi Mittal, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Linda Pax Lowes
Linda Pax Lowes, PT, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Lindsay Alfano
Lindsay Alfano, DPT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Kristy Rose
Kristy Rose, PT, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Tina Duong
Tina Duong, MPT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Katherine M Berry
Katherine M Berry, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Elena Montiel-Morillo
Elena Montiel-Morillo, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Irene Pedrosa-Hernández
Irene Pedrosa-Hernández, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Scott Holsten
Scott Holsten, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Mohammed Sanjak
Mohammed Sanjak, PT, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Ai Ashida
Ai Ashida, DPT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Chikako Sakamoto
Chikako Sakamoto, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Takayuki Tateishi
Takayuki Tateishi, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Hiroyuki Yajima
Hiroyuki Yajima, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Aurélie Canal
Aurélie Canal, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Gwenn Ollivier
Gwenn Ollivier, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Valerie Decostre
Valerie Decostre, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Juan Bosco Mendez
Juan Bosco Mendez, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Nieves Sánchez-Aguilera Praxedes
Nieves Sánchez-Aguilera Praxedes, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Simone Thiele
Simone Thiele, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Catherine Siener
Catherine Siener, PT, MHS
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Jeanine Shierbecker
Jeanine Shierbecker, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Julaine M Florence
Julaine M Florence, PT, MHS, DPT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Bruno Vandevelde
Bruno Vandevelde
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Brittney DeWolf
Brittney DeWolf, PT, DPT, PCS
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Meghan Hutchence
Meghan Hutchence, BSc, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Richard Gee
Richard Gee, MPT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Juliana Prügel
Juliana Prügel, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Elke Maron
Elke Maron, PT
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Heather Hilsden
Heather Hilsden, BA
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Hanns Lochmüller
Hanns Lochmüller, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Ulrike Grieben
Ulrike Grieben, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Simone Spuler
Simone Spuler, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Carolina Tesi Rocha
Carolina Tesi Rocha, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
John W Day
John W Day, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Kristi J Jones
Kristi J Jones, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Diana X Bharucha-Goebel
Diana X Bharucha-Goebel, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Emmanuelle Salort-Campana
Emmanuelle Salort-Campana, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Matthew Harms
Matthew Harms, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Alan Pestronk
Alan Pestronk, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Sabine Krause
Sabine Krause, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Olivia Schreiber-Katz
Olivia Schreiber-Katz, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Maggie C Walter
Maggie C Walter, MD, MA
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Carmen Paradas
Carmen Paradas, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Jean-Yves Hogrel
Jean-Yves Hogrel, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Tanya Stojkovic
Tanya Stojkovic, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Shin'ichi Takeda
Shin'ichi Takeda, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Madoka Mori-Yoshimura
Madoka Mori-Yoshimura, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Elena Bravver
Elena Bravver, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Susan Sparks
Susan Sparks, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Jordi Díaz-Manera
Jordi Díaz-Manera, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Luca Bello
Luca Bello, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Claudio Semplicini
Claudio Semplicini, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Elena Pegoraro
Elena Pegoraro, MD, PhD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Jerry R Mendell
Jerry R Mendell, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Kate Bushby
Kate Bushby, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,
Volker Straub
Volker Straub, MD
1From the John Walton Muscular Dystrophy Research Centre (U.M., M.K.J., A.G.M., R.F.-T., M.E., K.B., R.M.L., H.H., H.L., K.B., V.S.), Newcastle University and Newcastle Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, Central Parkway, Newcastle Upon Tyne, UK; Center for Translational Science (M.J., J.F., A. Cnaan), Division of Biostatistics and Study Methodology, Cooperative International Neuromuscular Research Group (T.D., B.D.), and Department of Neurology (D.X.B.-G.), Children's National Health System; Pediatrics, Epidemiology and Biostatistics (M.J., A. Cnaan), George Washington University, Washington, DC; Neuromuscular Area (R.F.-T.), Biodonostia Health Research Institute, Neurology Service, Donostia University Hospital, Donostia-San Sebastian, Spain; Jain Foundation (L.E.R., P.M.), Seattle, WA; Magnetic Resonance Centre (A.M.B.), Institute of Cellular Medicine, Newcastle University, Newcastle Upon Tyne, UK; AIM & CEA NMR Laboratory (P.G.C.), Institute of Myology, Pitié-Salpêtrière University Hospital, 47-83, Paris, France; Research Institute at Nationwide Children's Hospital (L.P.L., L.A., K.M.B., J.R.M.), The Ohio State University, Columbus; Institute for Neuroscience and Muscle Research (K.R., M. Hutchence, K.J.J.), Children's Hospital at Westmead, University of Sydney, Australia; Lucile Salter Packard Children's Hospital at Stanford (T.D.), 24349, Neurology, Palo Alto, CA; Physical Medicine and Rehabilitation (E.M.-M., I.P.-H.), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Neuroscience Institute (S.H., M.S., E.B., S. Sparks), Carolinas Neuromuscular/ALS-MDA Center, Carolinas HealthCare System, Charlotte, NC; Department of Physical Rehabilitation (A.A., C. Sakamoto, T.T., H.Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Institut de Myologie (A. Canal, G.O., V.D., J.-Y.H., T.S.), AP-HP, GH Pitié-Salpêtrière, Paris, France; Neurorehabilitation Unit (J.B.M.), Rehabilitation Hospital Universitario Virgen del Rocío Sevilla; Neurophysiotherapy Department (N.S.-A.P.), Hospital Universitario Virgen del Rocío, Seville, Spain; Friedrich-Baur-Institute (S. Thiele, S.K., O.S.-K. M.C.W.), Department of Neurology, Ludwig-Maximilians-University of Munich, Germany; Department of Neurology (C. Siener, J.S., J.M.F., M. Harms, A.P.), Washington University School of Medicine, St. Louis, MO; Centre de Reference des Maladies Neuromusculaires PACA Réunion Rhone Alpes (B.V., E.S.-C.), Hopital de la Timone, Aix-Marseille Université, France; ELAN-PHYSIO (J.P., E.M.), Praxis für Physiotherapie Maron; Charite Muscle Research Unit (U.G., S. Spuler), Experimental and Clinical Research Center, a joint cooperation of the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany; Department of Neurology and Neurological Sciences (C.T.R., J.W.D.), Stanford University School of Medicine, CA; NIH (D.X.B.-G.), Bethesda, MD; Neuromuscular Unit (C.P.), Department of Neurology, Hospital U. Virgen del Rocío/Instituto de Biomedicina de Sevilla, Spain; Department of Neurology (S. Takeda, M.M.-Y.), National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan; Centro de Investigación Biomédica en Red en Enfermedades Raras (J.D.-M.); Neuromuscular Disorders Unit (J.D.-M.), Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; and Department of Neuroscience (L.B., C. Semplicini, E.P.), University of Padova, Italy.
1,✉;
for the Jain COS Consortium1