Acute myeloid leukemia (AML) in adults aged 60 years and older has a poor prognosis. The goal of this study was to describe the perceptions of older patients with AML regarding treatment decisions and the potential risks and benefits of treatment. Patient‐clinician concordance in estimated treatment risk and the likelihood of cure with intensive and nonintensive treatment was also examined.
Keywords: Decision making, Perception of prognosis, Prognostic understanding, Acute myeloid leukemia, Treatment risk
Abstract
Background.
Older patients (≥60 years) with acute myeloid leukemia (AML) face difficult decisions regarding treatment with “intensive” chemotherapy that carries significant toxicity for a small chance of a cure versus “nonintensive” chemotherapy to control the disease, but with fewer side effects. However, studies of how these patients understand the risks and benefits of such treatments are lacking.
Methods.
We conducted a longitudinal study of older patients newly diagnosed with AML assessing patients’ (n = 100) and oncologists’ (n = 11) perceptions of treatment‐related mortality at enrollment and prognosis at 1 month. We examined concordance between patients’ and oncologists’ perceptions using Cohen's kappa (κ < 0.10 indicates little/no concordance).
Results.
We enrolled patients within 72 hours of initiating intensive (n = 50) or nonintensive (n = 50) chemotherapy. Whereas 91% of patients reported that they were “somewhat” to “extremely likely” to die from treatment, oncologists estimated that only 12% were at high risk of dying because of treatment (κ = −0.09). Ninety percent of patients reported that they were “somewhat” or “very likely” to be cured of their AML, whereas oncologists estimated this chance of cure for only 31% of patients (κ = 0.05). Among patients receiving intensive chemotherapy, 98% reported that they were “somewhat” or “very likely” to be cured, whereas their oncologists estimated this likelihood of cure for only 49% (κ = 0.04); among those receiving nonintensive chemotherapy and their clinicians, these proportions were 82% and 13%, respectively (κ = 0.03). Patients who indicated a lower likelihood of cure reported significantly higher depression symptoms (p = .03).
Conclusion.
Older patients with AML overestimate the risks and benefits of treatment. Interventions to facilitate communication and enhance patients’ understanding of the goals of therapy and treatment risk are needed.
Implications for Practice.
Older patients with acute myeloid leukemia (AML) are confronted with challenging decisions regarding treatment with “intensive” chemotherapy that carries significant toxicity for a small chance of a cure versus “nonintensive” chemotherapy to control the disease, but with fewer side effects. A clear understanding of the likely outcome and risks of the various treatment strategies is essential for these patients to make informed decisions about their care. This article reports that older patients with AML overestimate both the risks and benefits of treatment and have substantial misperceptions about their prognosis. Interventions to enhance patients’ understanding of their prognosis and treatment risk are needed.
Introduction
Acute myeloid leukemia (AML) in adults over the age of 60 has a poor prognosis and is associated with a low chance for long‐term disease‐free survival [1], [2]. Oncologists lack consensus regarding the optimal initial treatment strategy for older patients with AML, especially those with comorbidities or poor performance status. Before the regulatory approval of several new agents for AML‐related indications in 2017, treatment options included (a) intensive chemotherapy using a combination of cytarabine and an anthracycline (“7+3” regimen or equivalent), which requires a prolonged 4–6 week hospitalization [3], [4]; (b) nonintensive therapy with low‐dose cytarabine or the hypomethylating agents decitabine or azacitidine, which can be often given in the outpatient setting [3], [5], [6]; (c) clinical trial enrollment [3]; or (d) supportive care alone without any disease‐modifying therapy [3]. In the academic settings, oncologists typically recommend intensive therapy, which has a higher risk of morbidity and mortality, with the hope of attaining a complete remission to allow for potentially curative allogeneic hematopoietic stem cell transplantation for the medically fit patients versus nonintensive therapy or supportive care for patients who are older or frail [1], [7].
Consequently, older patients with AML are often confronted with challenging decisions that require them to understand the chance of cure or death with the different treatment strategies and balance these risks and benefits [7]. Data demonstrate that patients’ perceptions of their prognosis impact their medical decision making. For example, among patients with advanced solid tumors, patients’ understanding of the likelihood of cure is associated with their willingness to accept chemotherapy [8], [9], [10], and those who overestimate their survival are more likely to prefer aggressive medical care at the end of life [11], [12]. Thus, a clear understanding of the likely outcome and risks of the various treatment strategies is essential for older patients with AML to make informed decisions about their cancer care. Unfortunately, studies are lacking regarding patients’ perceptions of the risks and benefits of intensive versus nonintensive therapy [13], [14]. In one study, investigators assessed prognostic understanding in older patients with leukemias but included only small numbers of patients with AML [7]. Studies are needed to examine comprehensively how older patients with AML understand the potential risks and benefits of treatment and to explore differences in these perceptions among those receiving intensive versus nonintensive therapy.
Data are also lacking assessing the relationship between patients’ prognostic awareness and their quality of life (QOL) and mood [15]. In a study of patients with advanced solid cancers, those who acknowledged the terminal nature of their illness reported lower QOL and worse anxiety symptoms compared with those with overly optimistic and inaccurate prognostic understanding [15]. Older patients with AML endure substantial distress as they confront their sudden, shocking, and life‐threatening diagnosis [16], [17], [18], [19], [20], [21]. Therefore, the relationship between prognostic awareness, QOL, and mood in these individuals is critically important given their high physical and psychological symptom burden.
The goal of this study was to describe the perceptions of older patients with AML regarding their treatment decision making and the potential risks and benefits of treatment. We also examined patient‐clinician concordance in their estimates of treatment risk and the likelihood of cure with intensive and nonintensive treatment. Finally, we explored associations between patients’ perceptions of their likelihood of cure and their QOL and mood.
Materials and Methods
Participants
We recruited 100 consecutive patients aged ≥60 years with a new diagnosis of AML, including 50 initiating intensive therapy and 50 initiating nonintensive therapy, and their oncology physicians (n = 11). Intensive therapy was defined as 7+3 or a similar intensive chemotherapy regimen requiring 4–6 weeks’ hospitalization. Nonintensive therapy included hypomethylating agents, low‐dose cytarabine, or other nonintensive chemotherapy treatments that do not require a prolonged hospitalization. Similar to other academic centers, oncologists at our institutions typically recommend intensive therapy for the medically fit patients and nonintensive therapy for those who are older or frail. Patients were required to be able to read questions in English or willing to complete questionnaires with the assistance of an interpreter. As the goal of this study was to examine treatment decision making pertaining to intensive and nonintensive chemotherapy, we excluded patients receiving only supportive care, including hydroxyurea. We also excluded patients with significant uncontrolled psychiatric disorders, or other comorbid diseases, which the oncologist believed inhibited their ability to complete the study procedures.
Study Design and Procedures
This prospective, longitudinal study of older patients with a new diagnosis of AML was approved by the Dana Farber Harvard Cancer Center Institutional Review Board. From July 1, 2014, to August 1, 2016, we screened the inpatient leukemia census and the outpatient leukemia clinic schedules at two institutions in Boston to identify potentially eligible patients with a new diagnosis of AML. The research assistant (RA) then informed the primary oncologist that we planned to approach the patient for study participation and inquired about any concerns regarding his or her participation. Once permission was obtained, the RA approached eligible patients for study participation within 72 hours of their initiating intensive or nonintensive therapy. The RA reviewed the consent form with patients in a private setting and obtained written informed consent. Patients were required to complete baseline self‐report assessments within 72 hours of initiating therapy for AML.
Study Measures
Decision‐Making Preferences and Perception of Treatment Risk and Prognosis.
At baseline, we assessed patients’ decision‐making preferences and their perceptions of their treatment‐related mortality using three items that have been previously validated and used in the literature [7], [22], [23]. Specifically, patients reported the role they prefer when making decisions about their leukemia treatment (i.e., “prefer to make decisions about treatment with little or no input from my oncologist,” “prefer to make decisions after considering my oncologist's opinion,” “prefer that my oncologist make the decision after considering my opinion,” or “prefer that my oncologist make the decision with little or no input from me”). We also asked patients about the role they actually played when making the decision about treatment for their AML using similar response categories. We asked patients to report all the treatment options that were offered to them, including “intensive chemotherapy requiring 4–6‐week hospital stay,” “less intensive chemotherapy,” “clinical trial,” “blood transfusions and focusing on managing symptoms alone,” and “not sure.” We also asked patients to report the likelihood that a patient would die during treatment for leukemia as a result of their treatment, using a 7‐point scale (ranging from “extremely likely,” >90%, to “almost no chance,” less than 10%). We categorized their responses into three categories: (a) very unlikely or almost no chance, <10%; (b) unlikely, somewhat, or moderately likely, 10–74%; and (c) very or extremely likely, >75% chance.
We used the Prognosis and Treatment Perception Questionnaire (PTPQ) to assess participants’ preferences for information about their treatment and their understanding of likelihood of cure [15], [24]. We developed the PTPQ using items from a number of different measures and conducted cognitive interviews with patients to ensure its content validity, readability, and acceptability [15], [22], [25]. As the prognosis of patients with AML is dependent on molecular and cytogenetic factors that are not available at the time of diagnosis, we asked patients to complete the PTPQ at 1 month after enrollment. Specifically, patients rated the importance of knowing about their prognosis on a 5‐point scale (ranging from “extremely important” to “not at all important”). Additionally, patients reported the frequency of having a conversation with their oncologist about prognosis on a 5‐point scale (ranging from “never” to “very often”). We categorized patient's responses into three categories: (a) never or rarely, (b) sometimes, and (c) very often or often. Finally, patients rated the likelihood that they would be cured of their leukemia on a 7‐point scale (ranging from “very unlikely, <10%,” to “extremely likely, >90%”). We grouped responses into three categories: (a) extremely or very likely to be cured, 75%–100%; (b) moderately, somewhat, or unlikely to be cured, 10%–74%); and (c) very unlikely to no chance of cure, <10%.
QOL and Mood.
We assessed patient‐reported QOL and mood at baseline and 1 month after enrollment. We used the Functional Assessment of Cancer Therapy–Leukemia (FACT‐Leuk) questionnaire to assess patients’ QOL [26]. The FACT‐Leuk consists of five subscales assessing physical, functional, emotional, and social well‐being, as well as leukemia‐specific concerns, during the past week. Higher scores indicate better QOL. We measured patients’ anxiety and depression symptoms with the 14‐item Hospital Anxiety and Depression Scale (HADS). The HADS consists of two subscales assessing anxiety and depression symptoms in the past week, with subscale scores ranging from 0 (no distress) to 21 (maximum distress) [27].
Demographic and Clinical Factors.
Patients completed a demographic questionnaire that included age, gender, race, ethnicity, marital status, income, and educational level. We reviewed the electronic health records to obtain data on AML diagnosis and cytogenetic and molecular profile. We used the European LeukemiaNet risk stratification to classify disease risk [4], [28].
Oncologists’ Perceptions of Treatment Options, Treatment Risk, and Likelihood of Cure.
At the time of patient enrollment, we asked patients’ oncologists to complete the same items that patients completed regarding the treatment options they discussed with the patient and their assessment of the patients’ risk of treatment‐related mortality. At 1 month after enrollment, oncologists also completed the PTPQ item inquiring about the likelihood of cure for each patient.
Statistical Analysis.
We performed statistical analyses using STATA (version 9.3; StataCorp LLC, College Station, TX). We first calculated descriptive statistics, including means or medians for continuous variables, depending on the normality of the data, and proportions for categorical variables. To examine patients’ understanding of the risks and benefits of treatment, we reported responses (frequencies) to PTPQ items as well as decision‐making preferences and treatment risk items. When examining patients’ and oncologists’ perceptions, we included dyads in which we had information from both participants. We assessed concordance between patients’ and oncologists’ responses using Cohen's kappa. A kappa <0.10 indicates very little or no concordance between patients’ and oncologists’ responses, whereas a kappa of 0.8–1 indicates almost perfect concordance [29]. We categorized patients as having an accurate perception of their likelihood of cure if their response matched their oncologist's response on the 3‐point categorical response scale: (a) extremely or very likely to be cured, 75%–100%; (b) moderately, somewhat, or unlikely to be cured, 10%–74%); and (c) very unlikely to no chance of cure, <10%). We explored associations between patient’ perception of their likelihood of cure and QOL and mood at 1 month after enrollment using linear regression models. Given the descriptive objectives of this manuscript, a sample size of a 100 was deemed adequate to provide a representative sample of older patients’ perceptions of their AML treatment decision making and likelihood of cure.
Results
Participant Characteristics
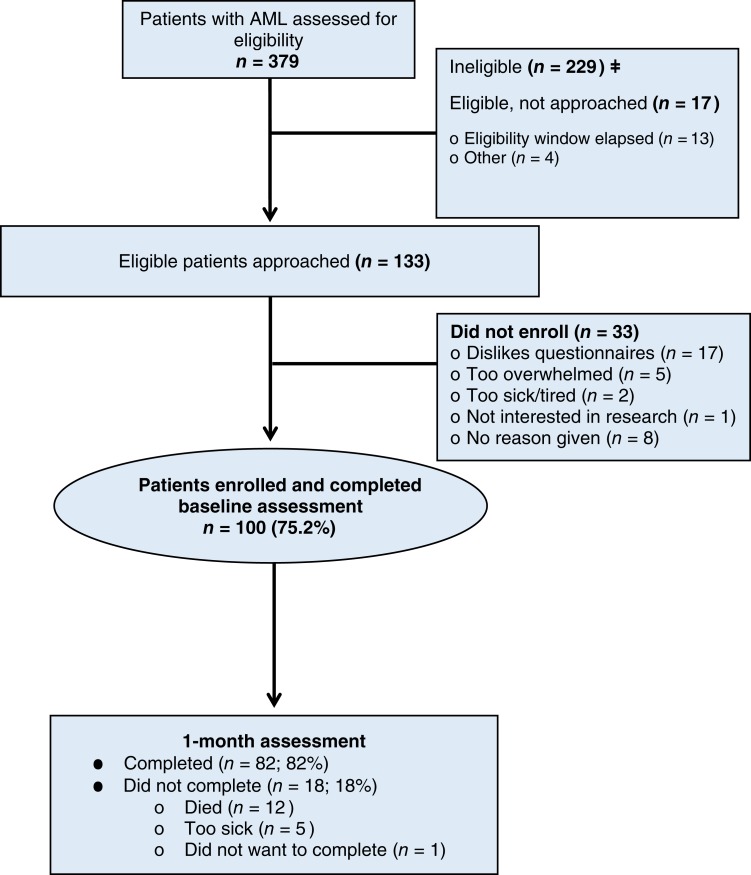
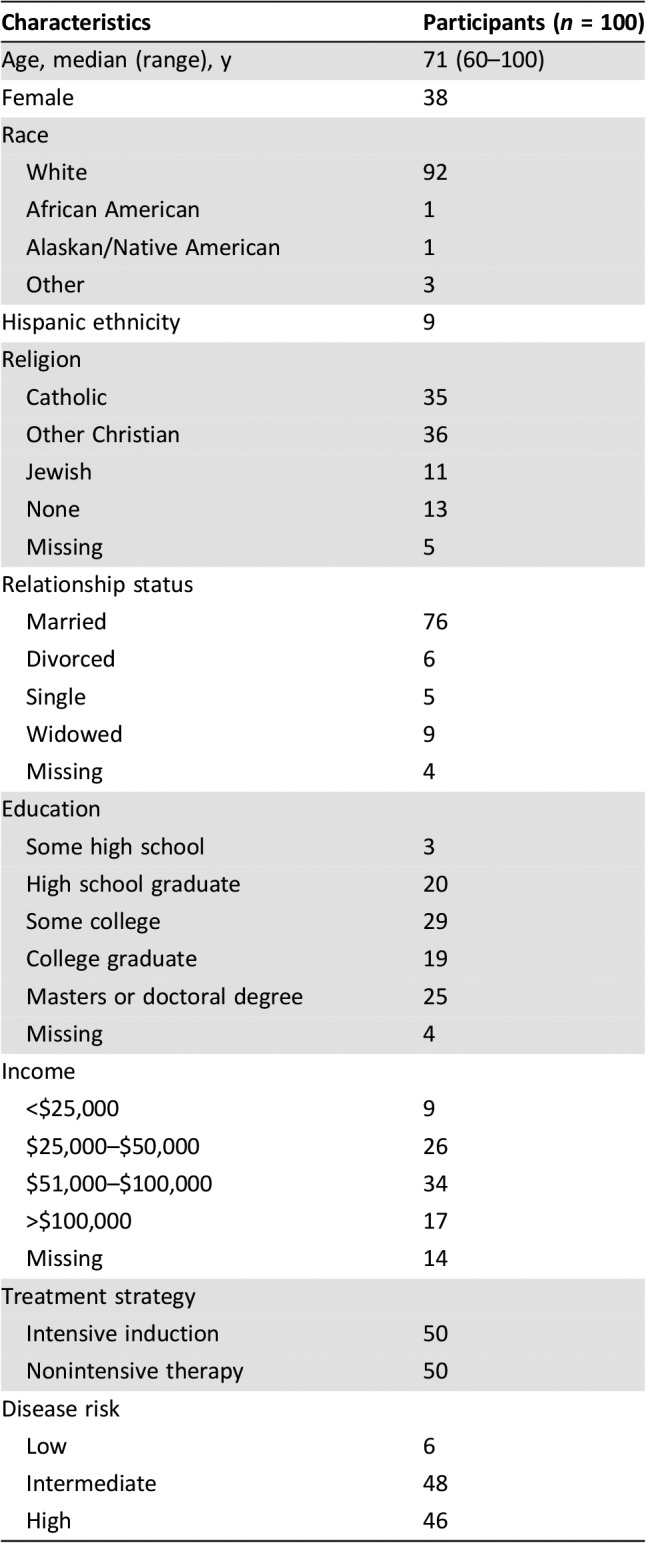
We screened 379 patients with AML and identified 133 eligible patients for study participation (Fig. 1). We enrolled 75% (100/133) of potentially eligible patients receiving intensive therapy (n = 50) or nonintensive therapy (n = 50). All patients completed baseline assessments, and 82% (82/100) completed the assessment at 1 month after enrollment. The most common reason for missing data at 1 month was death (n = 12) or feeling too ill (n = 5). Table 1 depicts patients’ baseline characteristics. Enrolled patients were mostly white (92%) with a median age of 71 years (range, 60–100), and 38% were female.
Figure 1.
Flow diagram. Abbreviation: AML, acute myeloid leukemia.
Table 1. Patient baseline characteristics.
Decision‐Making Preferences
Of 100 patients, 60 stated that they prefer to make their own treatment decisions after considering their oncologist's opinion, whereas 40 reported that they preferred the oncologist to make treatment decisions after considering their opinion. Notably, 28% of patients (28/99) reported discordance between their preferred role in making treatment decisions and the role they actually played in making the decision about their AML treatment.
When asked about the treatment options that their oncologist discussed with them, 68% (68/100) of patients recalled discussing intensive chemotherapy, 49% recalled discussing nonintensive chemotherapy, and only 21% recalled discussing supportive care alone. Oncologists reported that they discussed intensive chemotherapy, nonintensive chemotherapy, and supportive care alone with 73%, 69%, and 59% of patients, respectively.
Perceptions of Treatment Risks and Benefits
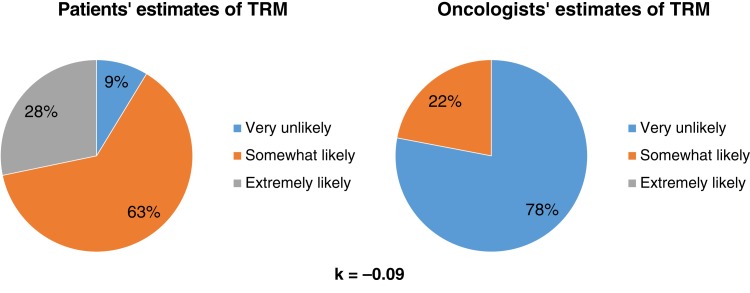
Patients overestimated the risk of treatment‐related mortality compared with their oncologists (Fig. 2). Most patients (91%, 84/92) reported that they were somewhat or extremely likely to die because of the treatment, whereas their oncologists reported the same risk of death for only 12% (11/92) of patients (κ = −0.09, SE = 0.03).
Figure 2.
Patients’ and oncologists’ perceptions of the risk of treatment‐related mortality.Abbreviation: TRM, treatment‐related mortality.
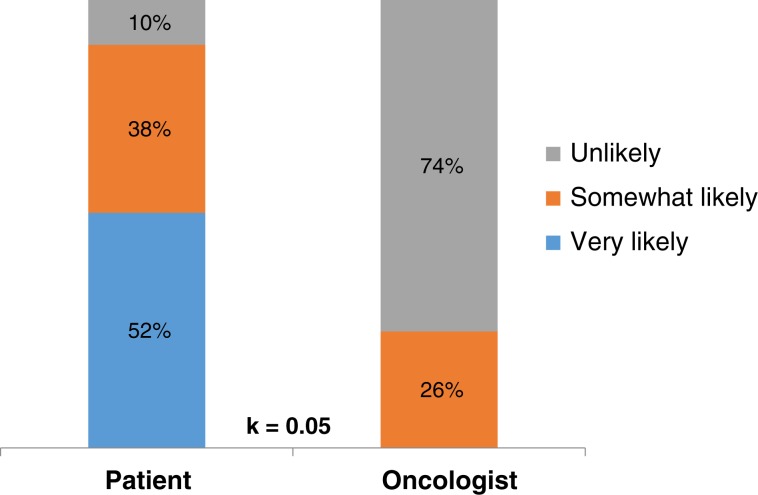
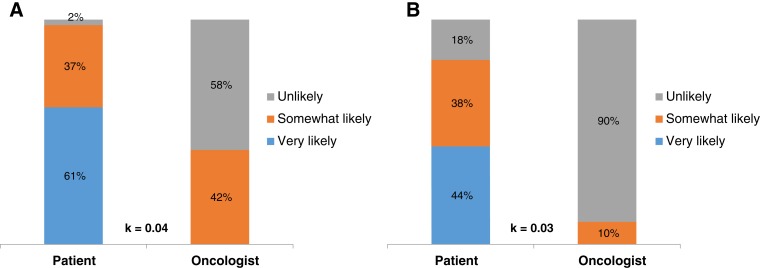
Thirty‐one percent (25/82) of patients reported that they never or rarely had conversations with their oncologists about their prognosis, 35% (29/82) reported having some conversations, and 34% (28/82) reported having frequent conversations. Although 95% of patients (77/81) reported it was very important to know their prognosis, we observed substantial discordance in prognostic understanding between patients and their oncologists. Whereas 90% of patients (72/80) reported that they were somewhat or very likely to be cured of their leukemia, their oncologists reported the same chance of cure for only 31% (25/80) (κ = 0.05, SE = 0.04; Fig. 3). Patients had significant misperceptions about their prognosis regardless of their treatment. Among patients receiving intensive chemotherapy, 98% (40/41) reported that they were very likely or somewhat likely to be cured, whereas oncologists reported the same chance of cure in only 49% (20/41) of patients (κ = 0.04, SE = 0.05; Fig. 4A]. Among patients receiving nonintensive chemotherapy, 82% (32/39) reported that they were very likely or somewhat likely to be cured, whereas oncologists reported the same chance of cure in only 13% (5/39) of patients (κ = 0.03, SE = 0.05; Fig. 4B).
Figure 3.
Patients’ and oncologists’ perceptions of the likelihood of cure of leukemia.
Figure 4.
Patients’ and oncologists’ perceptions of the likelihood of cure. (A): Perceptions among those receiving intensive chemotherapy. (B): Perceptions among those receiving nonintensive chemotherapy.
Association of Prognostic Awareness with QOL and Mood
The mean FACT‐Leuk score for study participants at 1 month from study enrollment was 120.3 (SD = 24.3). The mean HADS depression and anxiety subscale scores at 1 month from study enrollment were 6.93 (SD = 4.68) and 4.29 (SD = 3.51), respectively. Patients who indicated a lower likelihood of cure reported significantly higher depression symptoms (HADS depression: B = 2.29, 95% confidence interval [CI] 0.22–4.36, p = .03) and a trend towards lower QOL (FACT‐Leuk: B = −9.51, 95% CI −20.50 to 147, p = .089) at 1 month after study enrollment (Table 2). Anxiety symptoms did not differ based on patients’ estimate of their likely cure.
Table 2. Association between prognostic awareness and patient‐reported quality of life and mood 1 month after enrollment.
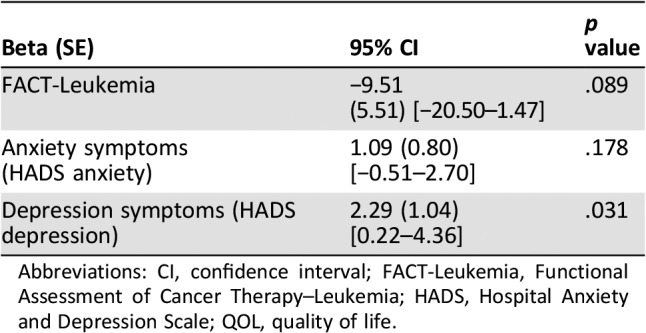
Abbreviations: CI, confidence interval; FACT‐Leukemia, Functional Assessment of Cancer Therapy–Leukemia; HADS, Hospital Anxiety and Depression Scale; QOL, quality of life.
Discussion
In this prospective study, we comprehensively examined the perceptions of older patients with AML regarding treatment decision‐making and the risks and benefits of treatment. Although over half of patients preferred to make their own treatment decisions after considering the oncologist's opinion, a substantial minority preferred to have their oncologists make the decision. Notably, over one quarter of patients indicated that the role they played in choosing their initial leukemia therapy was not aligned with their preferred role in treatment decision making. Moreover, most patients significantly overestimated the risk of treatment‐related mortality compared with their oncologists’ estimates. Despite endorsing the importance of knowing about their prognosis, most patients reported an overly optimistic assessment of the likelihood of cure compared with their oncologists. These data underscore the critical gaps in communication about prognosis, goals of therapy, and treatment risk in this population as well as the need for interventions to improve patient‐clinician communication.
Although in many prior studies, investigators have reported prognostic misperceptions in patients with advanced solid tumors [30], [31], the degree of prognostic misperceptions we observed in older patients with AML is especially striking. Most patients overestimated their likelihood of cure compared with their oncologists. These misperceptions were most pronounced among patients receiving nonintensive therapy, with 82% reporting that they were likely to be cured of their illness despite the palliative nature of their treatment. These data are particularly concerning given the uncertainty regarding the optimal treatment strategy for older patients with AML and thus the importance of helping patients balance the risk and benefits of treatment to ensure informed decision making. Interestingly, patients who reported a more realistic estimate of cure reported higher depression symptoms, which is consistent with our prior studies of patients with advanced solid tumors [15], [22]. Although these findings should not hinder clinicians’ disclosure of prognosis for patients with serious cancers, they do highlight the need for further study of how best to support patients who correctly acknowledge their prognosis.
To our knowledge, this is the first study to report that older patients with AML often overestimate the risk of treatment‐related mortality. Conversely, Sekeres et al. reported that patients with a variety of leukemias underestimated their risk of treatment‐related mortality [7]. The different findings in these two studies likely reflects how the question about treatment‐related mortality was framed. The previous study asked patients to report their own personal risk of dying as a result of their treatment. In contrast, we asked patients to report the likelihood that a patient would die during treatment for leukemia as a result of their treatment. Although patients may cognitively acknowledge the mortality associated with their diagnosis, they often experience emotion or even “magical thinking” when responding to questions about their own illness and prognosis and are thus more likely to choose optimistic responses about their likely outcome with cancer. These findings highlight the implications of cognitive dissonance on patients’ responses to questionnaires about the likely outcome of their illness, which must be taken into consideration when we study patients’ illness and prognostic understanding.
We also demonstrate that patients with AML have varying preferences regarding how to make decisions about their leukemia treatment. Although many patients prefer to make their treatment decisions after discussing their oncologists’ opinions, 40% preferred that their oncologists make the treatment decisions. Yogaparan and colleagues also reported similar findings in a study of 31 patients with AML [32]. Interestingly, studies have shown that most patients with advanced solid tumors prefer to make their own treatment decisions [33], [34]. Because of the often sudden and urgent nature of leukemia, patients may feel overwhelmed by the acuity of their diagnosis, the urgent need to initiate therapy, and the complexity of the decision‐making process, thus preferring that their oncologist take a more active role in making a treatment recommendation [20], [35], [36], [37]. Further research is needed to examine how best to tailor communication about treatment decision making in older patients with AML to ensure the delivery of high‐quality, patient‐centered care.
Our study has several important limitations. First, we included mostly white, highly educated patients drawn from two urban tertiary care centers in the U.S., and therefore our findings may not be generalizable to all older patients with AML. Second, although our results reveal substantial gaps in patients’ understanding of the risks and benefits of treatment, we did not audio‐record patients’ conversations with their oncologists to determine what information was actually communicated to patients. Third, we used the oncologists’ assessment of the patients’ prognosis as the metric to assess whether patients’ estimates of the risk and benefit of treatment were accurate. Studies have shown that oncologists also hold overly optimistic perceptions of their patients’ prognosis [38]; thus, we may be underestimating the frequency of prognostic misperceptions among study participants. Fourth, although our findings suggest an association between patients’ perception of the likelihood of cure and their mood, we cannot establish the causality or direction of this association. It is also possible that patients with higher depression symptoms report more accurate perceptions of their prognosis. Fifth, we asked patients and oncologists about their perception of prognosis at 1 month after initiating therapy, which introduces recall bias. Additionally, patients’ experience and tolerance of therapy during this month may affect their perception of their prognosis as well as their oncologists’ responses. However, given the need for molecular and cytogenetic factors for accurate prognostication in AML, which are not available at the time of diagnosis, we assessed perceptions of prognosis at 1 month from enrollment to allow for these important data to be incorporated into prognostic discussions.
Conclusion
We demonstrated that most older patients with newly diagnosed AML have substantial misperceptions regarding the risk and benefits of their treatment and that their perceptions are highly discordant with those of their treating oncologist. Patients’ misperceptions about the potential outcome of their treatment likely impedes their ability to weigh the pros and cons of their treatment options. Notably, patients with a more realistic estimate of their likelihood of cure reported higher depression symptoms, underscoring the need for psychological support to facilitate effective coping as patients learn about their prognosis. Future research should focus on facilitating patient‐clinician communication to enhance patients’ understanding of their prognosis, goals of therapy, and treatment risk to ensure informed treatment decision making in this population.
Acknowledgments
This work was supported by funds from the National Cancer Institute Federal Share Program (El‐Jawahri) and K24 CA 181253 (Temel).
Author Contributions
Conception/design: Areej El‐Jawahri, Lara Traeger, Gregory A. Abel, Joseph A. Greer, Amir Fathi, David P. Steensma, Richard M. Stone, Jennifer S. Temel
Financial support: Areej El‐Jawahri, Jennifer S. Temel
Administrative support: Richard M. Stone, Jennifer S. Temel
Collection and/or assembly of data: Areej El‐Jawahri, Harry VanDusen, Amir Fathi, David P. Steensma, Daniel DeAngelo, Martha Wadleigh, Gabriela Hobbs, Julia Foster, Andrew Brunner, Philip Amrein, Richard M. Stone
Data analysis and interpretation: Areej El‐Jawahri, Margaret Nelson‐Lowe, Harry VanDusen, Lara Traeger, Gregory A. Abel, Joseph A. Greer, Amir Fathi, David P. Steensma, Thomas W. LeBlanc, Zhigang Li, Daniel DeAngelo, Martha Wadleigh, Gabriela Hobbs, Julia Foster, Andrew Brunner, Philip Amrein, Richard M. Stone, Jennifer S. Temel
Manuscript writing: Areej El‐Jawahri, Margaret Nelson‐Lowe, Lara Traeger, Gregory A. Abel, Joseph A. Greer, David P. Steensma, Jennifer S. Temel
Final approval of manuscript: Areej El‐Jawahri, Jennifer S. Temel
Other (manuscript revision): Areej El‐Jawahri, Margaret Nelson‐Lowe, Harry VanDusen, Lara Traeger, Gregory A. Abel, Joseph A. Greer, Amir Fathi, David P. Steensma, Thomas W. LeBlanc, Zhigang Li, Daniel DeAngelo, Martha Wadleigh, Julia Foster, Andrew Brunner, Philip Amrein, Richard M. Stone, Jennifer S. Temel
Other (statistical analysis): Areej El‐Jawahri, Zhigang Li
Disclosures
Joseph A. Greer: Pfizer, National Comprehensive Cancer Network (RF); Thomas LeBlanc: Celgene, Helsinn Therapeutics, Heron Therapeutics, Amgen, Pfizer, Janssen (C/A), AstraZeneca, Seattle Genetics (RF); Andrew Brunner: Celgene, Takeda Pharmaceutical Co., Novartis (RF), Celgene (C/A); Jennifer S. Temel: Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Appelbaum FR, Gundacker H, Head DR et al. Age and acute myeloid leukemia. Blood 2006;107:3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol 2007;25:1908–1915. [DOI] [PubMed] [Google Scholar]
- 3.Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol 2008;35:430–438. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H, Estey EH, Amadori S et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–474. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Thomas XG, Dmoszynska A et al. Multicenter, randomized, open‐label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low‐dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lübbert M, Suciu S, Baila L et al. Low‐dose decitabine versus best supportive care in elderly patients with intermediate‐ or high‐risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: Final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol 2011;29:1987–1996. [DOI] [PubMed] [Google Scholar]
- 7.Sekeres MA, Stone RM, Zahrieh D et al. Decision‐making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia 2004;18:809–816. [DOI] [PubMed] [Google Scholar]
- 8.Brundage MD, Davidson JR, Mackillop WJ. Trading treatment toxicity for survival in locally advanced non‐small cell lung cancer. J Clin Oncol 1997;15:330–340. [DOI] [PubMed] [Google Scholar]
- 9.Hirose T, Yamaoka T, Ohnishi T et al. Patient willingness to undergo chemotherapy and thoracic radiotherapy for locally advanced non‐small cell lung cancer. Psychooncology 2009;18:483–489. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non‐small cell lung cancer: Descriptive study based on scripted interviews. BMJ 1998;317:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weeks JC, Cook EF, O'Day SJ et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–1714. [DOI] [PubMed] [Google Scholar]
- 12.Mack JW, Weeks JC, Wright AA et al. End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010;28:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiff PJ, Miller LA, Mumby P et al. Patients’ understanding of disease status and treatment plan at initial hematopoietic stem cell transplantation consultation. Bone Marrow Transplant 2006;37:479–484. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Fairclough D, Antin JH et al. Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA 2001;285:1034–1038. [DOI] [PubMed] [Google Scholar]
- 15.El‐Jawahri A, Traeger L, Park ER et al. Association among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014;120:278–285. [DOI] [PubMed] [Google Scholar]
- 16.Rodin G, Yuen D, Mischitelle A et al. Traumatic stress in acute leukemia. Psychooncology 2013;22:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann C, Yuen D, Mischitelle A et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res 2013;37:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zittoun R, Achard S, Ruszniewski M. Assessment of quality of life during intensive chemotherapy or bone marrow transplantation. Psychooncology 1999;8:64–73. [DOI] [PubMed] [Google Scholar]
- 19.El‐Jawahri AR, Abel GA, Steensma DP et al. Health care utilization and end‐of‐life care for older patients with acute myeloid leukemia. Cancer 2015;121:2840–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissim R, Zimmermann C, Minden M et al. Abducted by the illness: A qualitative study of traumatic stress in individuals with acute leukemia. Leuk Res 2013;37:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou C, Johnston B, Themessl‐Huber M. The experience of acute leukaemia in adult patients: A qualitative thematic synthesis. Eur J Oncol Nurs 2013;17:640–648. [DOI] [PubMed] [Google Scholar]
- 22.El‐Jawahri A, Traeger L, Kuzmuk K et al. Prognostic understanding, quality of life and mood in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2015;50:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? JAMA 1984;252:2990–2994. [PubMed] [Google Scholar]
- 24.Mack JW, Wolfe J, Grier HE et al. Communication about prognosis between parents and physicians of children with cancer: Parent preferences and the impact of prognostic information. J Clin Oncol 2006;24:5265–5270. [DOI] [PubMed] [Google Scholar]
- 25.Shin JA, El‐Jawahri A, Parkes A et al. Quality of life, mood, and prognostic understanding in patients with metastatic breast cancer. J Palliat Med 2016;19:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Jensen SE, Webster K et al. Measuring health‐related quality of life in leukemia: The Functional Assessment of Cancer Therapy‐Leukemia (FACT‐Leu) questionnaire. Value Health 2012;15:1051–1058. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 28.Stone RM. Acute myeloid leukemia in first remission: To choose transplantation or not? J Clin Oncol 2012;31:1262–1266. [DOI] [PubMed] [Google Scholar]
- 29.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 30.Temel JS, Greer JA, Admane S et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non‐small‐cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326. [DOI] [PubMed] [Google Scholar]
- 31.Weeks JC, Catalano PJ, Cronin A et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yogaparan T, Panju A, Minden M et al. Information needs of adult patients 50 or older with newly diagnosed acute myeloid leukemia. Leuk Res 2009;33:1288–1290. [DOI] [PubMed] [Google Scholar]
- 33.Bruera E, Sweeney C, Calder K et al. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol 2001;19:2883–2885. [DOI] [PubMed] [Google Scholar]
- 34.Janz NK, Wren PA, Copeland LA et al. Patient‐physician concordance: Preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol 2004;22:3091–3098. [DOI] [PubMed] [Google Scholar]
- 35.Alibhai SM, Leach M, Kermalli H et al. The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol 2007;64:19–30. [DOI] [PubMed] [Google Scholar]
- 36.Alibhai SM, Breunis H, Timilshina N et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol 2015;6:262–271. [DOI] [PubMed] [Google Scholar]
- 37.Burnett AK, Hills RK, Milligan DW et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: Results of the MRC AML12 trial. J Clin Oncol 2010;28:586–595. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan M, Temel JS, Wright AA et al. Predicting life expectancy in patients with advanced incurable cancer: A review. J Support Oncol 2013;11:68–74. [DOI] [PubMed] [Google Scholar]