This article reports on the prognostic value of the progesterone receptor by tumor proliferation using tumor grade as a surrogate for the proliferative activity of estrogen receptor‐positive HER2‐negative breast cancer.
Keywords: Breast cancer, Progesterone receptor, Subtype, Age, Prognostic value, Luminal
Abstract
Background.
In estrogen receptor‐positive (ER+), human epidermal growth factor receptor 2 (HER‐2) negative breast cancers, the progesterone receptor (PR) is an independent prognostic marker. Little is known about the prognostic value of PR by tumor grade. We assessed this in two independent datasets.
Patients and Methods.
Women with primary operable, invasive ER+ HER‐2 negative breast cancer diagnosed between 2000 and 2012, treated at University Hospitals Leuven, were included. We assessed the association of PR status and subtype (grade 1–2 vs. grade 3) with distant recurrence‐free interval (DRFI) and breast cancer‐specific survival. The interaction between PR status and subtype was investigated, and associations of PR status by subtype were calculated. The BIG 1‐98 data set was used for validation.
Results.
In total, 4,228 patients from Leuven and 5,419 from BIG 1‐98 were analyzed. In the Leuven cohort, the adjusted hazard ratio (HR) of PR‐positive versus PR‐negative tumors for DRFI was 0.66 (95% confidence interval [CI], 0.50–0.89). For the interaction with subtype (p = .34), the HR of PR status was 0.79 (95% CI, 0.61–1.01) in luminal A‐like and 0.59 (95% CI, 0.46–0.76) in luminal B‐like tumors. In luminal A‐like tumors, observed 5‐year cumulative incidences of distant recurrence were 4.1% for PR‐negative and 2.8% for PR‐positive tumors, and in luminal B‐like 18.7% and 9.2%, respectively. In the BIG 1‐98 cohort, similar results were observed; for the interaction with subtype (p = .12), the adjusted HR of PR status for DRFI was 0.88 (95% CI, 0.57–1.35) in luminal A‐like and 0.58 (95% CI, 0.43–0.77) in luminal B‐like tumors. Observed 5‐year cumulative incidences were similar.
Conclusion.
PR positivity may be more protective against metastatic relapse in luminal B‐like versus luminal A‐like breast cancer, but no strong conclusions can be made. In absolute risk, results suggest an absent PR is clinically more important in high compared with low proliferative ER+ HER‐2 negative tumors.
Implications for Practice.
An absent progesterone receptor (PR) predicts a worse outcome in women treated for an estrogen receptor‐positive, human epidermal growth factor receptor 2 negative breast cancer. As low proliferative tumors lacking PR are now also classified high risk, the prognostic value of PR across risk groups was studied. Despite a negative test for interaction of the prognostic value of PR by tumor grade, the magnitude of an absent PR on breast cancer relapse is much larger in high than in low proliferative breast cancers.
摘要
背景。在雌激素受体阳性 (ER+)、人表皮生长因子受体 2 (HER‐2) 阴性乳腺癌中,孕激素受体 (PR) 是一种独立的预后指标。我们对于根据肿瘤级别估计的 PR 预后价值知之甚少。在 2 个独立的数据集中,我们对此进行了评估。
患者和方法。研究中包含在 2000 年至 2012 年期间被诊断出患有原发性可手术、浸润性 ER+ HER‐2 阴性乳腺癌并在鲁汶大学医院接受治疗的女性。利用无远端复发间期 (DRFI) 和乳腺癌相关生存率,我们评估了 PR 状态和亚型(1–2 级与 3 级)之间的关联。我们调查了 PR 状态和亚型之间的互相作用并推测出根据亚型估计的 PR 状态的关联。BIG 1‐98 数据集用于进行验证。
结果。我们一共分析了 4,228 名来自鲁汶的患者和 5,419 名来自 BIG 1‐98 的患者。在鲁汶队列中,针对 DRFI 的 PR 阳性肿瘤与 PR 阴性肿瘤的校正危害比 (HR) 为 0.66(95% 置信区间 [CI],0.50–0.89)。就与亚型的相互作用而言 (p = .34),在管腔 A 型肿瘤和管腔 B 型肿瘤中,PR 状态的 HR 为分别为 0.79(95% CI,0.61–1.01)和 0.59(95% CI,0.46–0.76)。在管腔 A 型肿瘤中,对于 PR 阴性肿瘤和 PR 阳性肿瘤,观察到的远端复发的 5 年累积发生率分别为 4.1% 和 2.8%,而在管腔 B 型肿瘤中,该发生率分别为 18.7% 和 9.2%。在 BIG 1‐98 队列中,我们观察到了相似的结果;就与亚型的相互作用而言 (p = .12),在管腔 A 型肿瘤和管腔 B 型肿瘤中,针对 DRFI 的 PR 状态的校正 HR 分别为 0.88(95% CI,0.57–1.35)和 0.58(95% CI,0.43–0.77)。观察到的 5 年累积发生率相似。
结论。在管腔 B 型乳腺癌与管腔 A 型乳腺癌中,PR 阳性可能对转移性复发更具防护力,但是,我们未能得出有力的结论。在绝对风险方面,研究结果表明,与低增生性 ER+ HER‐2 阴性肿瘤相比,缺乏 PR 在高增生性 ER+ HER‐2 阴性肿瘤中具有更高的临床重要性。
对临床实践的提示:缺乏孕激素受体 (PR) 可以预测女性接受雌激素受体阳性、人表皮生长因子受体 2 阴性乳腺癌治疗的较差效果。由于缺乏 PR 的低增生性肿瘤现在也已被划分为高风险,所以,我们研究了各风险组之间的 PR 预后价值。尽管根据肿瘤等级对 PR 预后价值的相互作用进行了阴性检测,但是,就乳腺癌复发而言,在高增生性乳腺癌中 PR 的缺乏程度要远远高于低增生性乳腺癌。
Introduction
Worldwide, breast cancer is the most common malignancy among women [1]. The prognosis depends on demographic and pathologic factors such as age at diagnosis, tumor type and grade, mitotic activity, tumor size, vascular space invasion, and the number of lymph nodes involved. In addition, expression of the estrogen receptor (ER), the progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER‐2) are also prognostic [2]. In ER+ HER‐2 negative breast cancers, an absent PR is an independent prognostic marker for poor prognosis [3], [4], [5], [6], [7], [8].
According to the 2015 St. Gallen guidelines, both PR and Ki‐67 need to be favorable to classify ER+ HER‐2 negative breast cancers low risk. Administration of adjuvant chemotherapy can then be omitted, at least if ER is strongly positive with a low tumor burden and no more than three lymph nodes are involved [9]. Other guidelines, such as those of the American Society of Clinical Oncology and the National Comprehensive Cancer Network, have different recommendations in which chemotherapy is almost always recommended in tumors with positive lymph nodes; the European Society for Medical Oncology guidelines also refer to the St. Gallen guidelines [10], [11], [12]. Patients carrying ER+ HER‐2 negative tumors with low or no PR expression or with high Ki‐67 expression are considered to have a high risk of relapse and therefore often receive adjuvant chemotherapy. However, very often, PR is high with low Ki‐67 expression or, vice versa, PR is low or absent with high Ki‐67 expression. In these situations, chemotherapy is often administered, as St. Gallen classifies these tumors as high risk. PR expression in ER+ HER‐2 negative breast cancer is dependent on age and menopausal status, with lower expression rates in postmenopausal and older women [13], [14], [15]. Prat and collaborators have also recently shown that low proliferating luminal A‐like tumors with a low PR expression carry a poor prognosis [16].
We hypothesized that a high‐grade lesion might do well if PR is positive. Our research objective was to study the prognostic value of PR by tumor proliferation using tumor grade as a surrogate for the proliferative activity of ER+ HER‐2 negative breast cancer [17].
Patients and Methods
Patient and Tumor Characteristics
Records of women with primary operable, invasive ER+ HER‐2 negative breast cancer diagnosed between January 1, 2000, and December 31, 2012, and treated at the University Hospitals Leuven were retrieved from our prospectively managed Multidisciplinary Breast Centre database. Follow‐up was provided until November 2015. All patients were treated according to standard of care. Patients with primary metastatic breast cancer were excluded from the analysis. We further excluded patients who received neoadjuvant therapy, as the number of patients with ER+, HER‐2 negative breast cancer who received neoadjuvant treatment was rather small (n = 393). Also, the exact TNM stage of these patients was not known. This was important as we adjusted for tumor size and lymph node status in the multivariable analysis. We also excluded patients with ER‐negative PR‐positive breast cancer, as this group was very small [18]. ER+ HER‐2 negative breast cancer subtypes were defined based on the definition by Brouckaert et al. [19]: luminal A‐like if the tumor was ER+ HER‐2 negative with tumor grade 1 or 2; luminal B‐like if the tumor was ER+ HER‐2 negative with tumor grade 3.
The expression of molecular markers was measured by immunohistochemistry. ER and PR were classified positive if the receptor was expressed in 1% or more of the tumor cells. HER‐2 expression was considered negative when the immunohistochemistry score was 0 or 1 or when fluorescent in situ hybridization was negative following an immunohistochemistry score equal to or more than 2. Tumor grade was measured using the Nottingham grading system [6].
The primary endpoint was distant recurrence‐free interval (DRFI), and the secondary endpoint was breast cancer‐specific survival (BCSS). DRFI was defined as the time from breast cancer diagnosis to the first recording of a distant recurrence. Patient follow‐up continued after a local or regional breast cancer recurrence or a second, nonbreast primary cancer; therefore, it was possible to assess directly time to metastatic disease. BCSS was based on the cause of death as recorded in the database.
The BIG 1‐98 data set was used to validate our findings. Details of the BIG 1‐98 cohort can be found elsewhere [20], [21]. The BIG 1‐98 data set only included postmenopausal women. The primary endpoint in this cohort was disease‐free survival, defined as the time from random assignment to the earliest time of invasive recurrence in local, regional, or distant sites; a new invasive breast cancer in the contralateral breast; any second (nonbreast) malignancy; or death from any cause. The secondary endpoints were overall survival, time to distant recurrence, and safety. Luminal A‐ and B‐like were defined based on the criteria of Cheang et al. [22]: luminal A‐ if the tumor was ER+ HER‐2 negative and Ki‐67 less than 14%, and luminal B‐like if the tumor was ER+ HER‐2 negative and Ki‐67 equal to or more than 14%. ER, PR, and HER‐2 expressions were defined as in the Leuven cohort. This study was approved by the ethics committee of the University Hospitals Leuven.
Statistical Analysis
To investigate the differential prognostic effects of PR by subtype, we looked at DRFI and BCSS. For patients who were alive and free from distant metastases at the time of the database lock (November 2015 and December 2010 for Leuven and the BIG 1‐98, respectively), follow‐up was censored at the last follow‐up visit. Information about BCSS was based on the cause of death recorded in the follow‐up data in the database. In data from the BIG 1‐98, this information was based on the primary cause of death data recorded on the follow‐up data collection forms. Follow‐up of patients who were alive at the time of the database lock was censored at the time of the last follow‐up visit. DRFI was modeled using Cox proportional hazards regression. BCSS was modeled using Fine & Gray proportional subdistribution hazards regression, with death before metastasis or breast cancer‐unrelated death as a competing event.
Candidate predictors for the multivariate model in the data set from Leuven were age at diagnosis, tumor size, subtype (luminal A‐ versus luminal B‐like, i.e., grade 1/2 versus grade 3), PR status (positive if the receptor was expressed in 1% or more of the tumor cells measured by immunohistochemistry), nodal status (lymph node status unknown or negative, one to three positive nodes, or four or more positive nodes), adjuvant endocrine therapy, radiotherapy, and adjuvant chemotherapy. Vascular invasion was not included in the analysis, as this was underreported in the Leuven database. Patients with missing data were excluded from the analysis. There was specific interest in the interaction between PR status and subtype. This interaction was investigated with a single likelihood ratio test [23].
In the BIG 1‐98 analysis, covariates in the multivariable models were age at study enrolment, PR status (positive or negative), subtype (luminal A‐ or luminal B‐like), tumor size (≤2 cm, 2–5 cm, or ≥5 cm), local therapy received, use of (neo)adjuvant chemotherapy, nodal status (lymph node status unknown or negative, one to three positive nodes, or four or more positive nodes), and peritumoral vascular invasion (yes or no). Regression models for BIG 1‐98 were stratified by randomization option (2‐arm or 4‐arm), chemotherapy use, and randomized treatment assignment.
The probability of distant recurrence was displayed using Kaplan‐Meier curves; the probability of death from breast cancer was displayed using cumulative incidence functions.
Hazard ratios (HR) for Cox models and subdistribution hazard ratios (sHR) for Fine‐Gray models were calculated with 95% confidence intervals (CIs). Statistical analysis of the Leuven data was performed using SAS University Edition (SAS Institute, Cary, NC, USA) and R version 3.3 (www.r-project.org).
Results
Patient and Tumor Characteristics
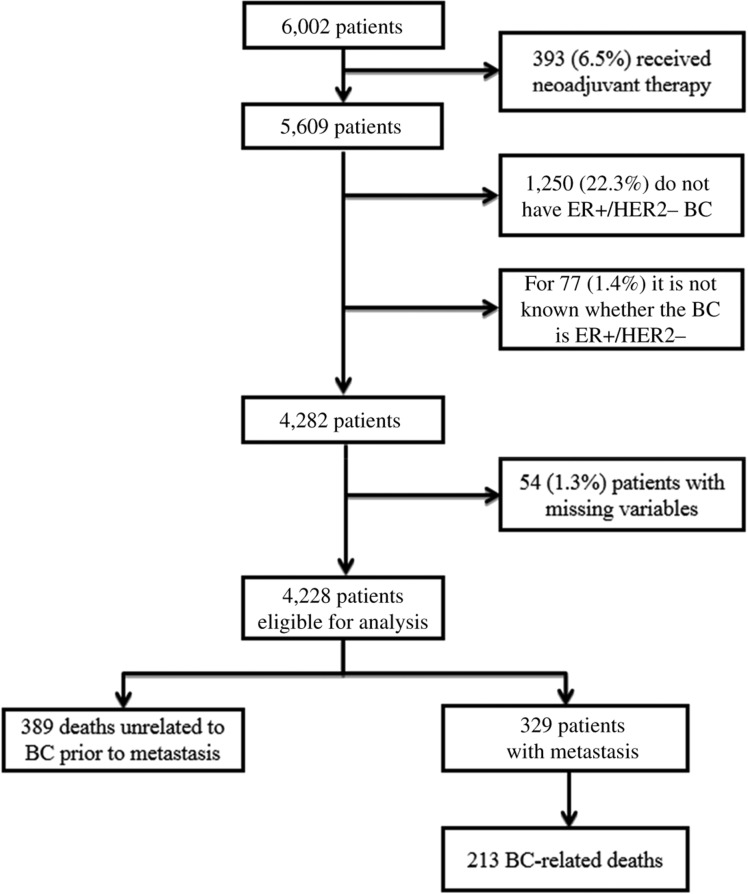
In this retrospective study, 5,609 patients were diagnosed with primary operable breast cancer at the University Hospitals Leuven between 2000 and 2012, and 4,282 patients (76%) were luminal HER‐2 negative. These variables were not available for 77 (1%) patients. Only 54 patients (1.3%) had missing covariate data, leaving 4,228 patients eligible for statistical analysis (Fig. 1). Patient and tumor characteristics can be found in Table 1. The median age at diagnosis was 58 years with ages ranging from 22 to 95 years; 3,062 patients (72%) were aged above 50 years at diagnosis. The cohort consisted of 3,093 patients (73%) with luminal A‐like subtype and 1,135 patients (27%) with luminal B‐like subtype. Lymph node status was predominantly negative (62%). Median tumor size was 21 mm with a range from 0.1 to 160 mm. Most of the patients received adjuvant endocrine therapy (96%) and/or radiotherapy (87%). Adjuvant chemotherapy was administered to 29% of the patients. The median follow‐up period was 8.6 years with a 5‐year overall survival probability of 93.5% (95% CI, 92.7–94.2%). In 3,753 cases (89%), PR was positive.
Figure 1.
Patient inclusion diagram. Abbreviations: BC, breast cancer; ER+, estrogen receptor positive; HER2−, human epidermal growth factor receptor 2 negative.
Table 1. Patient and tumor characteristics.
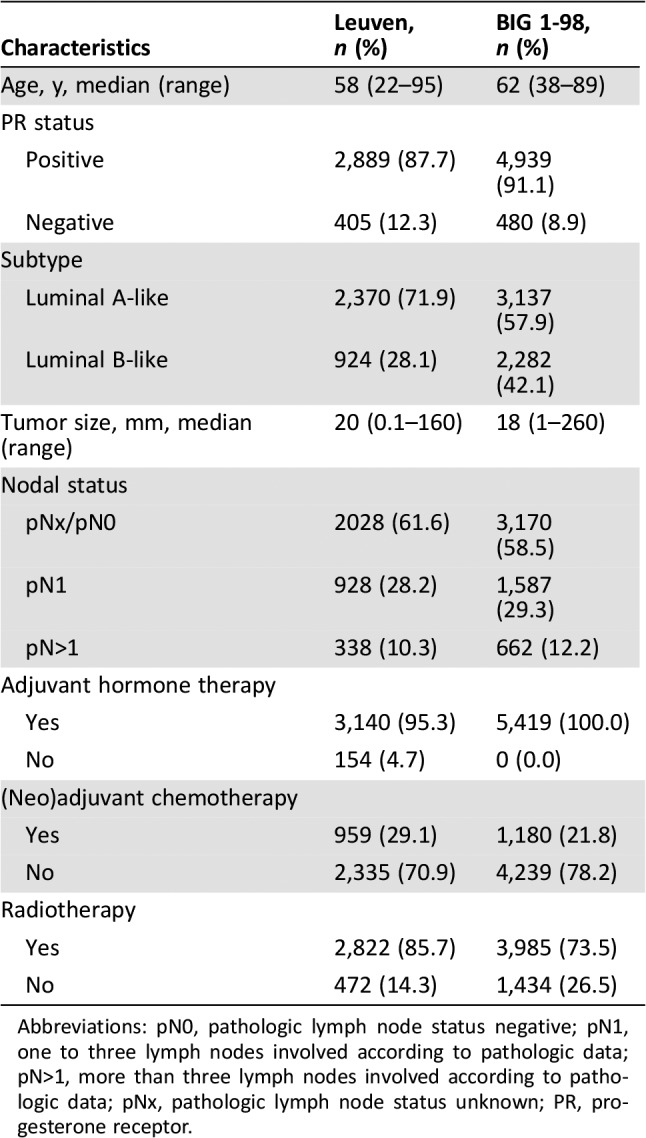
Abbreviations: pN0, pathologic lymph node status negative; pN1, one to three lymph nodes involved according to pathologic data; pN>1, more than three lymph nodes involved according to pathologic data; pNx, pathologic lymph node status unknown; PR, progesterone receptor.
The BIG 1‐98 cohort consisted of 5,538 postmenopausal patients with central pathology data. One hundred nineteen (2.1%) patients were removed from the analysis because of missing covariate data or lack of ER or PR positivity in central review. Consequently, 5,419 patients were analyzed. Patient and tumor characteristics are shown in Table 1. The luminal A‐like and luminal B‐like groups consisted of 3,137 (57.9%) and 2,282 (42.1%) patients, respectively. Median age at diagnosis and enrolment was 62 years, with a range of 38–89 years. Most patients were lymph node‐negative (58.5%). Median tumor size was 18 mm, ranging from 1 to 260 mm. All patients received endocrine therapy, 73.5% of the patients were treated with radiotherapy, and 21.8% received chemotherapy. The median follow‐up in this cohort was 8.1 years. The 5‐year overall survival probability was 93.0% (95% CI, 92.3%–93.7%).
Distant Recurrence‐Free Interval
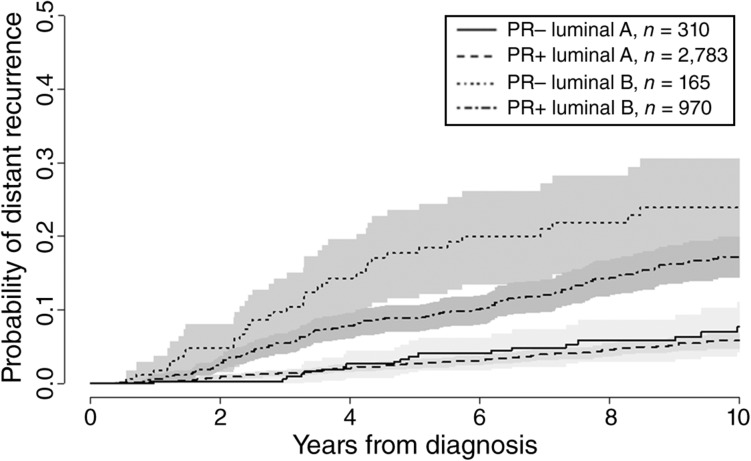
The cumulative probability of distant recurrence by PR and subtype is shown in Figure 2. In the Leuven cohort, 329 of 4,228 patients experienced metastases (7.8%). Among patients with luminal A‐like breast cancer, the 5‐year probability of distant recurrence was 2.8% among PR‐positive tumors (based on 135 events in 2,783 patients) and 4.1% among PR‐negative tumors (based on 20 events in 310 patients). For luminal B‐like breast cancer, the 5‐year probability of distant recurrence was 9.0% among PR‐positive tumors (based on 137 events in 970 patients) and 18.7% among PR‐negative tumors (based on 37 events in 165 patients). In the multivariable analysis, the likelihood ratio test for the interaction between PR status and subtype did not provide strong statistical evidence that the prognostic effect of PR varied with subtype (p = .34). Table 2 shows that positive versus negative PR status was associated with a reduced risk of distant recurrence by 34% (HR, 0.66; 95% CI, 0.50–0.89). The risk of distant recurrence was associated with an increased risk by 184% (HR, 2.84; 95% CI, 2.27–3.56) in patients with luminal B‐like versus luminal A‐like breast cancer. When the interaction between PR status and subtype was added, the estimated HR of PR positivity was 0.79 (95% CI, 0.62–1.01) in luminal A‐like and 0.59 (95% CI, 0.46–0.76) in luminal B‐like breast cancer. The interactions suggest a stronger relationship of PR with DRFI in luminal B‐like breast cancer, but based on the likelihood ratio test, no strong conclusions can be drawn. There was an indication that the effect of subtype was not proportional but decreased with time from diagnosis. This, however, did not influence the result of the interaction with PR.
Figure 2.
Cumulative incidence of distant recurrence by PR status and subtype. Abbreviations: PR+, progesterone receptor positive; PR−, progesterone receptor negative.
Table 2. Multivariable analyses for the Leuven data (n = 4,228).
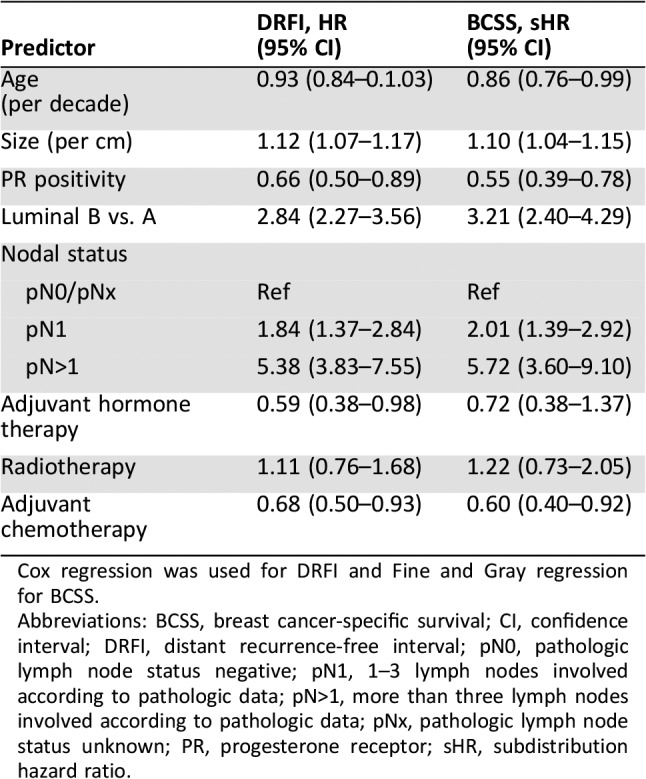
Cox regression was used for DRFI and Fine and Gray regression for BCSS.
Abbreviations: BCSS, breast cancer‐specific survival; CI, confidence interval; DRFI, distant recurrence‐free interval; pN0, pathologic lymph node status negative; pN1, 1–3 lymph nodes involved according to pathologic data; pN>1, more than three lymph nodes involved according to pathologic data; pNx, pathologic lymph node status unknown; PR, progesterone receptor; sHR, subdistribution hazard ratio.
In the BIG 1‐98 cohort, 620 of 5,419 patients experienced metastatic disease. The cumulative probability of distant recurrence is displayed in supplemental online Figure 1. The probability of distant recurrence over time was very similar to that observed in the Leuven data (Fig. 2). In the group of patients with luminal A‐like tumors, 218 of 2,862 with PR‐positive disease developed distant metastasis compared with 23 of 275 in PR‐negative cases. In patients with luminal B‐like disease, 315 of 2,077 PR‐positive cases and 56 of 205 PR‐negative cases experienced a metastatic event. In the multivariable analysis, women with luminal A‐like disease had a 12% reduction in the risk of a metastatic event if PR was expressed in the tumor compared with PR‐negative tumors (HR, 0.88; 95% CI, 0.57–1.35). Patients with PR‐positive luminal B‐like breast cancer had a 42% reduction in the risk of metastasis formation compared with patients with PR‐negative breast cancer (HR, 0.58; 95% CI, 0.43–0.77). The p value of the interaction between PR and subtype was .12.
Breast Cancer‐Specific Survival
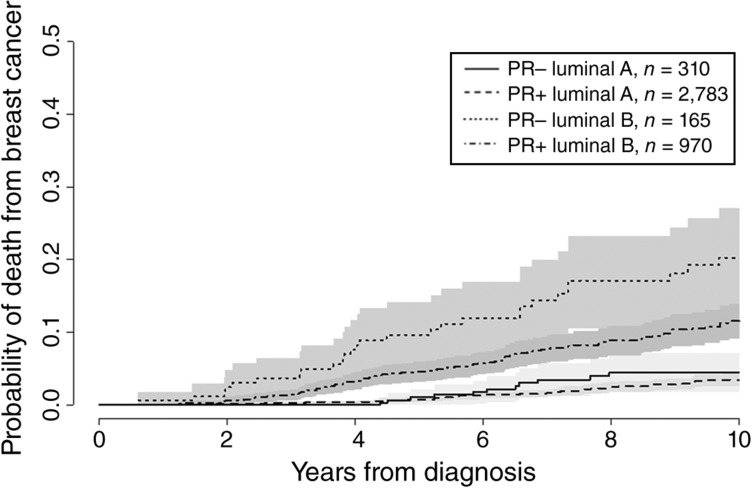
Two hundred and ten of 4,228 patients (5%) in the Leuven cohort died of breast cancer. The probability of death from breast cancer by PR and subtype is shown in Figure 3. For luminal A‐like tumors, the 5‐year probability of death from breast cancer was 0.9% among PR‐positive tumors (based on 78 events in 2,783 patients) and 1.4% among PR‐negative tumors (based on 14 events in 310 patients). In the patients with luminal B‐like tumors, the 5‐year probability of death from breast cancer was 4.6% among PR‐positive tumors (based on 90 events in 970 patients) and 10.4% among PR‐negative tumors (based on 28 events in 165 patients). The likelihood ratio test for the interaction between PR status and subtype suggested little evidence that the prognostic effect of PR varied with subtype (p = .21). In the main effects model (Table 2), positive versus negative PR status was associated with a reduced risk of breast cancer‐related death by 45% (sHR, 0.55; 95% CI, 0.39–0.78). The risk of breast cancer‐related death was increased by 221% for luminal B‐like versus luminal A‐like disease (sHR, 3.21; 95% CI, 2.40–4.29). When the interaction between PR status and subtype was added, the estimated sHR of PR positivity was 0.64 (95% CI, 0.41–1.00) in luminal A‐like and 0.50 (95% CI, 0.32–0.79) in luminal B‐like breast cancer. Similar to DRFI, the interactions suggest a stronger relationship of PR with BCSS in luminal B‐like breast cancer. However, strong conclusions cannot be drawn. There was an indication that the effect of subtype was not proportional but decreased with time from diagnosis. This, however, did not influence the result of the interaction with PR.
Figure 3.
Cumulative incidence of breast cancer‐related death by PR status and subtype. Abbreviations: PR+, progesterone receptor positive; PR−, progesterone receptor negative.
The number of patients in the BIG 1‐98 cohort with death due to breast cancer was 418 (7.7%). The probability of death from breast cancer is displayed in supplemental online Figure 2. This was also remarkably similar to what was observed in the Leuven data (Fig. 3). In patients with PR‐positive luminal A‐like breast cancer, 125 of 2,862 patients died of breast cancer compared with 15 of 275 of PR‐negative cases. Two hundred thirty‐nine of 2,077 PR‐positive luminal B‐like breast cancer patients and 39 of 205 PR‐negative patients died of breast cancer. PR‐positive luminal A‐like breast cancer patients had a 22% lower risk of dying from breast cancer compared with patients with PR‐negative breast cancer (sHR, 0.78; 95% CI, 0.46–1.34). Patients with PR‐positive luminal B‐like disease had a 30% reduction in the hazard of death due to breast cancer compared with PR‐negative patients (sHR, 0.70; 95% CI, 0.50–0.99). The p value for the interaction between PR and subtype was .74.
Discussion
Although PR is prognostic in all ER+ HER‐2 negative breast cancers using relative risk HR, our findings in this retrospective study suggest that PR‐positive lesions are more strongly associated with better prognosis in luminal B‐like than in luminal A‐like breast cancer. When PR (and/or ER) is less expressed, other tumor growth mechanisms predominate. Our original hypothesis that a high‐grade lesion might do well if PR is positive has not been completely confirmed. The interaction between PR and subtype was not significant. Therefore, we cannot provide clear statistical evidence that the prognostic effect of PR in ER+ HER‐2 negative breast cancers is subtype dependent. However, a clear trend is visible indicating that PR is more prognostic in luminal B‐like versus luminal A‐like tumors.
The different effect of PR for high versus low proliferative tumors was observed more for DRFI than for BCSS. Interestingly, the observed cumulative probabilities of distant metastases and death from breast cancer are very similar and are also similar for the Leuven and BIG 1‐98 cohort. The BIG 1‐98 cohort consisted of only postmenopausal patients, whereas the Leuven cohort included patients of all ages.
Despite the large overall sample sizes, some groups were very small such that tests for an interaction between PR status and phenotype were not sufficiently powered. Most notably, the number of patients with PR‐negative BC from Leuven who developed metastasis or died from BC was small. In the Leuven cohort, there were 20 metastases and 14 deaths among 310 patients with luminal A‐like breast cancer and 37 metastases and 28 deaths among 165 patients with luminal B‐like breast cancer. In the BIG 1‐98 cohort, the respective numbers were 23 and 15 (luminal A‐like) and 56 and 39 (luminal B‐like). However, the validation analysis on the larger BIG 1‐98 cohort yielded high similar results for the development of metastasis. For BCSS, the results of the validation analysis went in the same direction but were not of the same magnitude.
Our findings on the prognostic importance of PR expression in patients with hormone receptor‐positive breast cancer is clinically relevant considering current and previous St. Gallen guidelines [9], [24], in which PR is considered an important prognostic as well as surrogate marker for subtyping ER+ HER‐2 negative breast cancers into luminal A‐ or luminal B‐like. We agree PR is important in all ER+ HER‐2 negative lesions; its effect in absolute figures is clearly more obvious in high proliferative disease.
Nowadays, many physicians prefer to discriminate low‐risk from high‐risk tumors by using multigene assays rather than through the use of surrogate histopathological‐based classification [24], [25]. These tests provide prognostic information, and some of them are approved to guide treatment decisions [24], [25], [26]. In many countries, however, these tests are still too expensive to be implemented. Consequently, in settings where patients would benefit from multigene testing, access is only possible through large personal payments. The histopathological surrogate definitions, on the other hand, are widely applicable for women with luminal‐like breast cancer, which makes further optimization of these histopathological‐based definitions increasingly relevant. Furthermore, it remains to be proven whether PGR gene expression (RNA) will show the same prognostic value as PR expression by immunohistochemistry in the Leuven and BIG 1‐98 cohorts. There are some inexpensive statistical prognostic models that are based on histopathological characteristics, such as ER, PR, HER2, and Ki‐67. Examples include the Breast Cancer Recurrence Score Estimator, Memorial Sloan Kettering Simplified Score, the Magee equations, and IHC4 [27], [28], [29], [30], [31]. Our study reaffirms the importance of PR.
The definitions of luminal A‐like and luminal B‐like are different in the training and validation cohorts. In this study, we used tumor grade as a surrogate marker for proliferation activity, whereas in the validation cohort the Ki‐67 was used. Ki‐67 is the most widely used method to compare proliferation between tumor specimens. However, international guidelines for the assessment of Ki‐67 and cutoff values for the interpretation of Ki‐67 are lacking as yet [10]. As a result, in our center, we consider the tumor grade a more robust and more standardized procedure that, moreover, provides indirect information about proliferation activity of breast cancer. Grade remains important for prognosis, even when gene signatures are used to classify tumors and recommend for or against chemotherapy in ER+ HER‐2 negative cases. PR and grade showed highest prediction for low or high recurrence score [32]; even with available recurrence score, grade remains important when added to the recurrence score [33] and PR [34].
Maisonneuve et al. evaluated distant metastasis‐free interval for patients with low (<14%), intermediate (14%–19%), and high (≥20%) Ki‐67 positivity by PR expression [35]. Based on these results, the authors proposed new surrogate definitions to distinguish luminal A‐like from luminal B‐like breast cancers. Lesions with low Ki‐67 expression, irrespective of PR status, and tumors with intermediate Ki‐67 expression and high PR expression (≥20%) had comparably good outcomes. The authors suggested that all such tumors be classified as luminal A‐like. Consequently, an important number of patients were reclassified from the high‐risk into the low‐risk group. The authors thus suggested the use of quantitative PR expression and Ki‐67 for subtyping in ER+ HER‐2 negative breast cancers. We do not have quantitative PR levels to validate these findings. In our series, grade 3, PR‐positive cases do well but still worse than grade 1–2, PR‐negative tumors, so we can agree with a more aggressive approach. In future work, we will include this quantitative analysis of the PR, as it might provide further interesting insight.
Recently, Pan et al. published data regarding late breast cancer recurrence; they investigated the influence of various characteristics of the original tumor on the 20‐year incidence of breast cancer outcomes in women with ER+, early‐stage breast cancer who were scheduled to receive adjuvant endocrine therapy for 5 years. They observed that the PR status was independently prognostic during the first 5 years after diagnosis but not thereafter [36].
Based on our results, no conclusions on the predictive value of PR could be made, as this was not investigated in our study. In the multivariable analysis, adjustments were made for endocrine therapy, chemotherapy, and radiotherapy, but not for type of treatment (tamoxifen vs. aromatase inhibitor vs. switch; type of chemotherapy). All patients were treated according to standard of care. Most patients received 5 years of treatment, and many patients were included in clinical trials. Whether patients received chemotherapy or not was based on their risk of relapse. We assumed that the patients were compliant with endocrine therapy, although there was a possibility that they were not.
Conclusion
Our results suggest that PR expression is prognostic in ER+ HER‐2 negative breast cancer but depends on tumor proliferation. However, the interaction between PR and subtype was not significant, and therefore we cannot provide strong statistical evidence. Our data also suggest that PR status mainly adds prognostic value in high proliferative tumors. Based on these findings, we conclude that the prognostic value of PR in absolute figures is more important in high proliferative lesions and that the relative prognostic effect of an absent PR is independent of any other risk factor.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The BIG 1‐98 trial was funded by Novartis, which contracted with the International Breast Cancer Study Groupfor provision of services related to the conduct and management of the trial and provided partial support for collection and review of tumor blocks.
Contributed equally
Author Contributions
Conception/design: Kathleen Van Asten, Laurence Slembrouck, Hans Wildiers, Giuseppe Floris, Patrick Neven
Provision of study material or patients: Kathleen Van Asten, BIG 1‐98, Anita Giobbie‐Hurder, Meredith M. Regan, Giuseppe Viale, Beat Thürlimann
Collection and/or assembly of data: Kathleen Van Asten, Siel Olbrecht
Data analysis and interpretation: Kathleen Van Asten, Laurence Slembrouck, Anita Giobbie‐Hurder, Meredith M. Regan, Giuseppe Viale, Beat Thürlimann, Ben Van Calster, Patrick Neven
Manuscript writing: Kathleen Van Asten, Laurence Slembrouck, Siel Olbrecht, Lynn Jongen, Olivier Brouckaert, Hans Wildiers, Giuseppe Floris, Erik Van Limbergen, Caroline Weltens, Ann Smeets, Robert Paridaens, Anita Giobbie‐Hurder, Meredith M. Regan, Giuseppe Viale, Beat Thürlimann, Evangelia Christodoulou, Ben Van Calster, Patrick Neven
Final approval of manuscript: Kathleen Van Asten, Laurence Slembrouck, Siel Olbrecht, Lynn Jongen, Olivier Brouckaert, Hans Wildiers, Giuseppe Floris, Erik Van Limbergen, Caroline Weltens, Ann Smeets, Robert Paridaens, Anita Giobbie‐Hurder, Meredith M. Regan, Giuseppe Viale, Beat Thürlimann, Ignace Vergote, Evangelia Christodoulou, Ben Van Calster, Patrick Neven
Disclosures
Meredith M. Regan: Novartis (RF); Giuseppe Viale: Dako (C/A); Beat Thürlimann: Novartis (OI), AstraZeneca (H). The other authors indicated no financial relationship.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Payne SJ, Bowen RL, Jones JL et al. Predictive markers in breast cancer–the present. Histopathology 2008;52:82–90. [DOI] [PubMed] [Google Scholar]
- 3.Purdie CA, Quinlan P, Jordan LB et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: A population‐based study. Br J Cancer 2014;110:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouckaert O, Van Calster B, Paridaens R et al. Prognostic relevance of PR and detection mode in luminal Her‐2 negative breast cancer. Cancer Res 2012;72 (suppl 24):P 6‐07‐19A. [Google Scholar]
- 5.Van Asten K, Van Calster B, Lintermans A et al. Prognostic value of the progesterone receptor by proliferation rate in patients with luminal HER2 negative breast cancer. Cancer Res 2015;75 (suppl 9):P 6‐08‐05A. [Google Scholar]
- 6.Van Belle V, Van Calster B, Brouckaert O et al. Qualitative assessment of the progesterone receptor and HER2 improves the Nottingham Prognostic Index up to 5 years after breast cancer diagnosis. J Clin Oncol 2010;28:4129–4134. [DOI] [PubMed] [Google Scholar]
- 7.Creighton CJ, Kent Osborne C, van de Vijver MJ et al. Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res Treat 2009;114:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardou VJ, Arpino G, Elledge RM et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003;21:1973–1979. [DOI] [PubMed] [Google Scholar]
- 9.Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies‐improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early. Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris LN, Ismaila N, McShane LM et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 1.2018. Fort Washington, PA: National Comprehensive Cancer Network, 2018. [Google Scholar]
- 12.Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26 (suppl 5)v8–v30. [DOI] [PubMed] [Google Scholar]
- 13.Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 1984;2:1102–1109. [DOI] [PubMed] [Google Scholar]
- 14.Thorpe SM, Rose C. Oestrogen and progesterone receptor determinations in breast cancer: Technology and biology. Cancer Surv 1986;5:505–525. [PubMed] [Google Scholar]
- 15.Romain S, Lainé Bidron C, Martin PM et al. Steroid receptor distribution in 47, 892 breast cancers. A collaborative study of 7 European laboratories. The EORTC Receptor Study Group. Eur J Cancer 1995;31A:411–417. [DOI] [PubMed] [Google Scholar]
- 16.Prat A, Cheang MC, Martín M et al. Prognostic significance of progesterone receptor‐positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long‐term follow‐up. Histopathology 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 18.De Maeyer L, Van Limbergen E, De Nys K et al. Does estrogen receptor negative/progesterone receptor positive breast carcinoma exist? J Clin Oncol 2008;26:335–336; author reply 336–338. [DOI] [PubMed] [Google Scholar]
- 19.Brouckaert O, Laenen A, Vanderhaegen J et al. Applying the 2011 St Gallen panel of prognostic markers on a large single hospital cohort of consecutively treated primary operable breast cancers. Ann Oncol 2012;23:2578–2584. [DOI] [PubMed] [Google Scholar]
- 20.Regan MM, Price KN, Giobbie‐Hurder A et al. Interpreting Breast International Group (BIG) 1‐98: A randomized, double‐blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor‐positive, early breast cancer. Breast Cancer Res 2011;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adami HO, Malker B, Holmberg L et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med 1986;315:559–563. [DOI] [PubMed] [Google Scholar]
- 22.Cheang MC, Chia SK, Voduc D et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schemper M. Cox analysis of survival data with non‐proportional hazard functions. J R Stat Soc Ser D 1992;41:455–465. [Google Scholar]
- 24.Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early. Breast Cancer 2013. Ann Oncol 2013;24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chia SK, Bramwell VH, Tu D et al. A 50‐gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 2012;18:4465–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markopoulos C. Overview of the use of Oncotype DX as an additional treatment decision tool in early breast cancer. Expert Rev Anticancer Ther 2013;13:179–194. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Umbricht CB, Illei PB et al. Optimizing the use of gene expression profiling in early‐stage breast cancer. J Clin Oncol 2016;34:4390–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gage MM, Rosman M, Mylander WC et al. A validated model for identifying patients unlikely to benefit from the 21‐Gene Recurrence Score assay. Clin Breast Cancer 2015;15:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eaton AA, Pesce CE, Murphy JO et al. Estimating the Oncotype DX score: Validation of an inexpensive estimation tool. Breast Cancer Res Treat 2017;161:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein ME, Dabbs DJ, Shuai Y et al. Prediction of the Oncotype DX recurrence score: Use of pathology‐generated equations derived by linear regression analysis. Mod Pathol 2013;26:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzick J, Dowsett M, Pineda S et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki‐67, and human epidermal growth factor. receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–4278. [DOI] [PubMed] [Google Scholar]
- 32.Orucevic A, Bell JL, McNabb AP et al. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 2017;163:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik S, Shak S, Tang G et al. A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N Engl J Med 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary LN, Jawa Z, Szabo A et al. Relevance of progesterone receptor immunohistochemical staining to Oncotype DX recurrence score. Hematol Oncol Stem Cell Ther 2016;9:48–54. [DOI] [PubMed] [Google Scholar]
- 35.Maisonneuve P, Disalvatore D, Rotmensz N et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2‐negative) intrinsic breast cancer subtypes. Breast Cancer Res 2014;16:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan H, Gray R, Braybrooke J et al. 20‐year risks of breast‐cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]