Abstract
Abnormalities in body composition can occur at any body weight. Low muscle mass is a predictor of poor morbidity and mortality and occur in several populations. This narrative review provides an overview of the importance of low muscle mass on health outcomes for patients in inpatient, outpatient, and long-term care clinical settings. A one-year glimpse at publications that showcases the rapidly growing research of body composition in clinical settings is included. Low muscle mass is associated with outcomes such as higher surgical and post-operative complications, longer length of hospital stay, lower physical function, poorer quality of life, and shorter survival. As such, the potential clinical benefits of preventing and reversing this condition in patients are likely to impact patient outcomes and resource utilization/health care costs. Clinically viable tools to measure body composition are needed for routine screening and intervention. Future research studies should elucidate the effectiveness of multimodal modalities to counteract low muscle mass for optimal patient outcomes across the healthcare continuum.
Keywords: body composition, low muscle mass, sarcopenia, general surgery, neoplasms, cardiovascular diseases, pulmonary disease, critical illness, liver diseases, primary health care, long-term care
Introduction
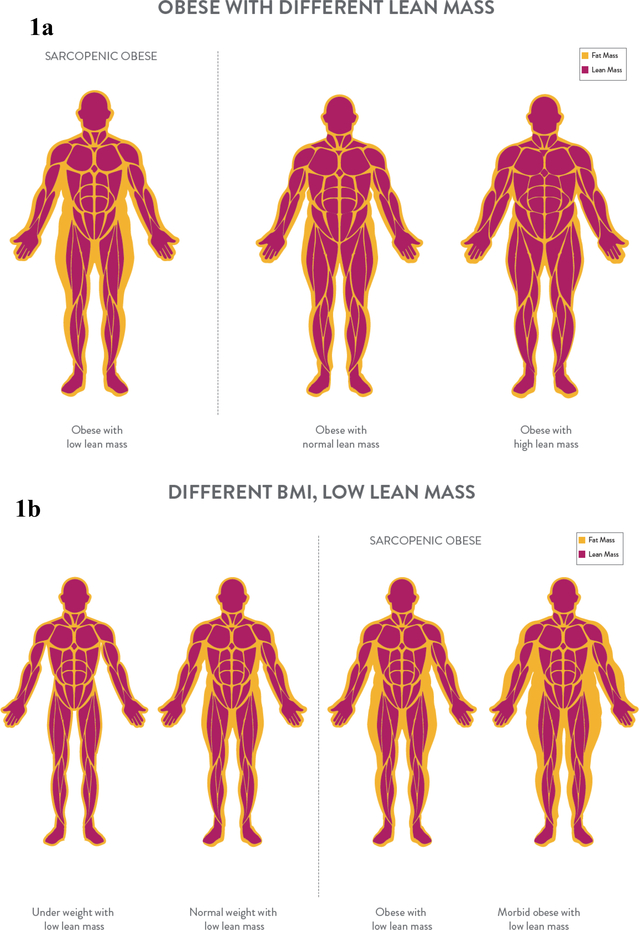
Measures of body mass such as weight and body mass index (BMI) have long been regarded as practical and sensitive for the prediction of health risks and outcomes. Although of value, these measurements do not depict an individual’s variability in body composition (i.e., lean versus adipose tissue). Body composition can be variable among individuals of the same body size, confounding the association between body weight and health, Figures 1a and 1b. Abnormalities in body composition such as low muscle mass are powerful predictors of morbidity and mortality, particularly in clinical settings where the disease or illness itself can lead to this condition (1, 2). In this narrative review, we provide an overview of the importance of low muscle mass on health outcomes for patients in inpatient, outpatient, and long-term care clinical settings, as well as a past year review of the literature.
Figures 1a and 1b.
Body composition across the body weight continuum. Low lean mass can occur in people obesity (1a) and at any body weight (1b).
Low Muscle Mass: A New Face of an Old Problem
From a theoretical standpoint, skeletal muscle is a primary driver of the relationship between body composition and clinical outcomes, as it is involved in mobility, strength and balance. However, there are several methods to measure body composition, and as a result there are many constructs and terms that ultimately reflect this compartment in the research hereby discussed (Table 1). Therefore, for clarity, we will refer to “low muscle mass” when providing an overview and discussion of the topic and will use the terminology described in Table 1 when referring to specific research findings. The latter will reflect the compartment being assessed by the body composition technique used in the study.
Table 1.
Commonly used terminology in body composition research
| Terminology | Description | Technique |
|---|---|---|
| Fat-free mass | Sum of skeletal and non-skeletal muscle, organs, connective tissue and bone | DXA, ADP, BIA* |
| Lean soft tissue or lean mass† | Sum of all lean tissues includes protein, water, carbohydrates, non-fat lipids, soft tissue minerals (excludes bone and fat) | DXA |
| Skeletal muscle mass | The total mass of skeletal muscle | US, MRI, CT |
| Appendicular skeletal muscle mass | Lean soft tissue (or lean mass) from arms and legs which is skeletal muscle (except from a negligible amount of skin). | DXA |
| Muscle radiodensity or attenuation | Reflective of intermuscular adipose tissue or ‘quality’ of skeletal muscle; low muscle radiodensity/attenuation are reflective of higher amount of fat infiltration | CT, MRI |
| Sarcopenia | Low muscle mass in combination with low strength, muscular performance, or physical performance | Several techniques are available‡ |
BIA estimates this compartment (versus direct measurement). Additionally, equations can be used to estimate skeletal muscle from BIA measurements.
The term lean body mass is not specific and should no longer be used
Consensus definitions of sarcopenia have employed several methods, although the accuracy of these techniques varies.
ADP: air displacement plethysmography; BIA: bioelectrical impedance analysis; CT: computerized tomography; DXA: dual X-ray absorptiometry; MRI: magnetic resonance imaging; US: ultrasound
Several cutpoints have been used to define “low” muscle mass. We present the most commonly used ones in Table 2, although this list is not exhaustive. The term “sarcopenia” has often been used to describe low muscle mass in clinical settings. However, the term is now widely accepted to also include measures of strength, muscular performance, or physical performance (1, 2) and usually used in context of aging. Notably, cutoffs for low muscle mass are mostly derived from relatively healthy cohorts and hence are of limited applicability to different patient populations and clinical settings. However, they provide a benchmark for quantifying degrees of muscle depletion as it correlates to changes in health status. The absence of standardized diagnostic criteria for low muscle mass precludes a more comprehensive characterization of the prevalence and significance of this condition among different cohorts of patients, an issue discussed later in this review.
Table 2.
Common cut-points to define low muscle mass
| Method | Cut-points |
|---|---|
| Computerized tomography | L3 skeletal muscle index (cm2/m2): • <52.4 for men, <38.5 for women (109) • <43 for men with BMI ≤ 24.9 kg/m2, <53 for men with BMI >24.9 kg/m2, <41 for all women (222) • Study-specific cut-points (e.g. tertiles) |
| Cross-sectional area of psoas muscle at L4 • Study-specific cut-points |
|
| L2 skeletal muscle size (cm2)Study-specific cut-points | |
| Dual X-ray absorptiometry | Appendicular skeletal muscle index (kg/m2): • 7.26 for men, 5.54 for women (223) •<7.25 for men, 5.67 for women (224) •<7.23 for men, 5.67 for women (225) |
| Bioelectrical impedance analysis | Muscle mass calculated from resistance or manufacturer proprietary calculations • Appendicular skeletal muscle index: <8.87 kg/m2 for men. <6.42 kg/m2 for women (226) • Skeletal muscle index: <10.76 kg/m2 for men, <6.76 kg/m2 for women (227) |
BMI: body mass index
Low muscle mass is not restricted to individuals with a small frame, Figures 1a and 1b. The concurrent appearance of low muscle mass with high adiposity (also termed sarcopenic obesity) is the new face of an old problem, common in people who face chronic diseases. This condition has a compounding impact on health and outcomes. To date, medical practice relies on measures of body size such as BMI; however, BMI does not necessarily relate to muscle mass, a limitation that may impact the ability of health care professionals to carry out targeted interventions directed at addressing low muscle mass rather than interventions focused on addressing adiposity. The prevalence of low muscle mass relates to age, sex, diet, physical activity, disease state, and hormonal regulation and other metabolic abnormalities in both obese and non-obese states.
Although most studies to date have only quantified muscle mass, the “quality” of the muscle is also a determinant of outcomes. This feature is assessed by the amount of fat infiltration in skeletal muscle (myosteatosis) using computerized tomography (CT) or magnetic resonance imaging. CT images provide measures of macroscopic intermuscular fat and muscle attenuation, the latter of which is inversely associated with muscle fat content (3) The lower the muscle attenuation or radiodensity, the higher the amount of fat infiltration into muscle. This feature is an emerging prognostic indicator although primarily studied under the auspices of cancer, where imaging techniques are available for its assessment.
Selected techniques available for the estimation of the muscle mass compartment have been described elsewhere (4, 5). The reader is referred to previous articles for a comprehensive discussion of the benefits and limitations of available body composition techniques (6).
Muscle Loss Across the Continuum of Care: An Under-recognized Phenomenon
Each sub-section of this article begins with an overview of the literature (expert opinion) followed by a summary of a year of recent publications. Our approach to focus on 12 months of publications was to highlight how the field has evolved into the current state of knowledge and how this has shaped current understanding of the role of low muscle mass in health care.
We included only studies using body composition techniques and not those that using anthropometry (through equations or direct measures). As the purpose of this manuscript was to provide an overview of the most recent literature rather than a systematic review, criteria for selection of the publications were more lenient. Observational studies, longitudinal studies, and clinical trials were included. No exclusion criteria were set other than mentioned above.
The search for January 2016 – January 2017 publications was conducted exclusively on PubMed yielding 2,350 articles. Search terms included those related to muscle mass and clinical outcomes, Supplementary Material.
One hundred and forty-three relevant articles in a single year were selected and are included in our discussion below, highlighting emerging research on the impact of muscle mass in various cohorts (Table 3). Articles were organized by clinical care settings: inpatient (surgery, cardiovascular disease, renal disease, chronic obstructive pulmonary disease [COPD], critical illness, “other”), outpatient (cancer, liver conditions, and primary care/population) and long-term care. Not all publications provided sufficient details regarding the study cohort; as such, samples of individuals undergoing surgery or any other procedure that typically requires at least one inpatient day are discussed in the context of inpatient (vs outpatient) settings. As discussed above, the terminology herein will refer to specific compartments being measured/estimated when discussing specific study findings (per Table 1), except when discussing the assimilated data in general terms/collection of evidence.
Table 3.
Summary of Studies in a Single Year Assessing the Effect of Muscle Mass on Clinical Outcomes
| Muscle mass/radiodensity as a positive impact | Muscle mass/quality as a null impact | |
|---|---|---|
| Inpatient | ||
| Surgery | Survival Post-operative complications Length of stay and readmission Morbidity Surgical complications Physical function Quality of life Inflammation Dischanrge destination Hospitalization costs | Survival Post-operative complications Quality of life |
| Heart disease/ conditions | Survival Length of hospital stay Physical function Disease-specific variables Ventilator support | Survival |
| Renal | SurvivalPhysical function | Survival |
| COPD | Disease-specific variables Osteopenia/osteoporosis | |
| Critical care/ICU | Survival Length of hospital stay Post-operative morbidity | Length of hospital stay Ventilator support |
| “Other” | Survival Physical function Disease-specific variables Dysphagia Length of hospital stay | |
| Outpatient | ||
| Cancer | Survival Treatment toxicityResponse to therapyNumber of treatment cycles Disease progression | Survival Treatment toxicity Response to therapy Disease progression |
| Liver conditions | Survival Physical function Disease-specific variables Albumin | |
| Primary care | Survival Physical functionQuality of life Balance Strength Exercise capacity Disease-specific variables Albumin Cardiovascular risk factors Fibrosis Hospitalization Fractures Pulmonary function Renal function | Quality of life |
| Population | Survival Physical function Bone mineral density Fracture/osteoporosis risk Frailty Morbidity Pulmonary function | Healthcare utilization |
| Long-term care | ||
| Long term care | Survival Physical function Nutritional status Alzheimer’s severity | Physical function Nutritional status Cognitive function |
COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; NYHA: New York Heart Association
Inpatient Settings
Low muscle mass in hospitalized patients is a prevalent phenomenon that worsens with increased time spent admitted to a hospital (7). This is led by a variety of reasons ranging from hyper-catabolism, inadequate food intake, and/or immobility, depending on the condition being investigated. The ability to conduct in-depth body composition assessments in inpatient settings has contributed to a large number of investigations, including recent ones. We identified 81 articles that examined the relationship between muscle mass and outcomes published within the timeline described above. These included the following settings and conditions: surgical (n=50), heart conditions (n=10), renal disease (n=4), chronic obstructive pulmonary disease (COPD) (n=2), critical illness (n=6), and other conditions not otherwise specified (n=7) among hospitalized patients.
Surgery:
Approximately 9.7 million inpatient surgical procedures occur in the United States each year (8). Identifying potential modifiable risk factors for complications and mortality is essential for optimal post-surgical care, such as immune status, nutritional status, and muscle mass. Surgery invokes a catabolic environment and alterations in nutritional status possibly driven by nausea, vomiting, decreased appetite, etc. (9), creating an environment conducive to muscle loss.
Low muscle mass is an economic burden to the healthcare system, as this condition is associated with an average increase of over $14,000 in total hospital costs per patient undergoing major abdominal surgery compared to patients with normal muscle amounts (10). Post-surgical complications such as sepsis, infection, prolonged ventilation, and pneumonia are reported to occur at a higher rate and survival is shorter in individuals with low muscle mass undergoing different types of surgery (11). In patients with colorectal cancer and documented low muscle mass, hospital length of stay is longer, the infection risk is greater, and more inpatient rehabilitation care is required than in patients with normal muscle mass (12). Furthermore, having low muscle is an independent predictor of post-operative infections and rehabilitation care among hospitalized older adults (≥ 65 years old) (12). Low muscle mass is also associated with a higher incidence of surgical complications and shorter survival in numerous different cancer types (13). In response to the significant burden of this condition, some programs have implemented preoperative lifestyle interventions that incorporate exercise, nutrition interventions, stress reduction, smoking cessation, and spirometer exercises (14). The effectiveness of each intervention is still unknown, but has been embraced by patients awaiting surgery (14).
A Year at a Glance:
Fifty studies reported the impact of muscle mass on outcomes in surgical patients; most (n=46, 92.0%) used CT images, three used bioelectrical impedance analysis (BIA, reporting fat-free mass or estimated skeletal muscle) and one used dual energy X-ray absorptiometry (DXA, reporting fat-free mass). The majority of these publications (n=26, 50%) were conducted in individuals with cancer. The remaining studies included diverse patient populations comprising of individuals undergoing surgery for liver or lung transplant, upper abdominal surgery, spine surgery, proctocolectomy, hip arthroplasty, intrahepatic portosystemic shunt placement, pancreaticoduodenectomy, pancreatic resection, aneurysm, or general gastrointestinal resection. Of these, 28 studies investigated the impact of muscle mass on survival and most (n=22; 79.0%) reported a positive association between survival and muscle mass (15–36). One publication reported that low muscle mass was not associated with survival in multivariate analysis (only in univariate analysis) (37), and another found that low muscle mass was prognostic of survival only in the presence of obesity (38), suggesting that disease factors and/or excess adiposity might work synergistically with muscle to predict outcomes. Four other publications reported no association between the amount of muscle mass and survival in individuals undergoing nephrectomy for kidney cancer (39), pancreatoduodenectomy (40), esophagectomy (41), or left ventricular assist device implantation (42). The lack of an association could be partially explained by the patients included, who may have experienced body composition changes after neoadjuvant therapy (41) or who had advanced cancer (39). One of the studies that found no association between muscle mass and overall survival also reported that higher muscle attenuation (i.e. better muscle quality) was associated with longer survival (40).
Post-operative complications were additional common outcomes found to be associated with low muscle mass or attenuation in 18 studies (28, 32, 34, 36, 38–40, 43–53), except for one (43), possibly due to the high rates of complication in the entire sample. Low muscle attenuation (24) and low muscle mass (47, 54, 55) were associated with longer length of hospital stay and hospital readmissions (34, 44, 48, 56, 57). Other outcomes that were reported to be associated with low muscle mass included general morbidity (24, 34, 41), surgical complications (58), reduced physical function (57, 59), lower quality of life (59), higher inflammatory response after surgery (60), discharge destination to a nursing or rehabilitation facility (34, 56), and higher hospitalization costs (10, 61, 62). One study reported a null association between muscle mass and quality of life post-discharge (63); although the population was large (n=215), diagnoses within the sample were diverse (cancer and non-cancer patients), which could have concealed significant sub-group associations.
In sum, individuals undergoing surgery have diverse clinical backgrounds. Nevertheless, low muscle mass and attenuation have been associated with shorter survival and worse post-operative complications, among other negative outcomes, in most studies.
Cardiovascular Disease/Conditions:
Cardiovascular disease is a leading cause of death worldwide. Heart failure in particular is highly prevalent, affecting approximately one in five adults during their lifetime (64) and might trigger substantial loss of muscle mass.
Obesity is a risk factor for heart disease, and many patients therefore have obesity at disease presentation. However, body weight may be artificially elevated due to edema and can conceal underlying low muscle mass. This influences body composition measurement since hydration may affect some measures of muscle mass. Nevertheless, the presence of low muscle mass is estimated to occur in almost 20% of patients with stable heart failure (65). It is associated with a decline in several functional parameters such as strength (handgrip and quadriceps), total peak VO2, walking distance (6-meter walk tests) (65), abnormal cardiac parameters, and cardiac perfusion (66).
A Year at a Glance:
Ten articles investigated the association of low muscle mass with clinical outcomes in heart diseases/conditions in acute care settings. All studies except one (DXA) reported muscle mass with using CT images. The patient populations of interest included individuals undergoing procedures such as transcatheter aortic valve implantation, aortic aneurysm repair, or left ventricular assist device implantation. Higher muscle mass was associated with improved overall survival in most (30–33, 50, 67), but not all studies (42). Though low muscle mass was not associated with overall survival in one publication, patients with lower muscle had higher rates of inpatient death or prolonged length of hospital stay (42). Compared to low muscle, having a higher muscle mass was also positively associated with better physical function (49, 68, 69), New York Heart Association score (33), fewer post-operative complications (32, 50), shorter length of stay (42, 49, 69), and less ventilator support (32). Among these discussed, low muscle mass was a negative prognostic factor in cardiovascular disease.
Renal Disease:
In individuals with chronic kidney disease (CKD), systemic inflammation, transient catabolic comorbidities, nutrient losses during dialysis, endocrine abnormalities (such as resistance to insulin, growth hormone, and insulin-like growth factor), hyperglycemia, hyperparathyroidism, and loss of blood during hemodialysis are prevalent (70). Additionally, reduced protein diets of 0.6–0.8 g/kg/day may be recommended to patients not on dialysis. These factors contribute to muscle wasting, which is usually reported under the auspices of protein energy wasting (70). In individuals undergoing dialysis, old age, comorbidities, subjective global assessment score >1, inactivity, low albumin, and inflammation (C-reactive protein) were associated with low hand grip strength but not with low muscle mass measured by DXA (71). In the same study, body composition alone was not associated with poorer survival; however, low strength alone (hazard ratio [HR] 1.98, 95% confidence interval [CI]: 1.01 to 3.87, p=0.04) or in combination with low muscle mass (HR: 1.93, 95% CI: 1.01 to 3.71, p=0.04) was more strongly associated with higher mortality (71). These findings suggest that strength and muscle mass – while highly related – are two different entities differently affecting outcomes in this population.
A Year at a Glance:
A variety of body composition techniques were used in patients with CKD including CT images, DXA, BIA, bioelectrical impedance spectroscopy, and magnetic resonance imaging. In these studies, higher muscle mass was associated with better physical function (72, 73); both the amount of muscle mass (74) and muscle attenuation (75) were indicative of better survival, although another study reported no associations (76). In the latter publication (76), all patients had sarcopenic obesity and were actively undergoing maintenance hemodialysis, and no standard cut points of low muscle mass could predict survival. Overall, these studies suggest that muscle mass is associated with poorer outcomes in renal disease patients, but the effect of obesity and other confounders such stage of disease might mitigate these associations.
COPD:
Weight loss is common in individuals with COPD, instigated by the difficulty of eating (77) and higher energy expenditure (78, 79). Fat-free mass represents a large portion of this weight loss, as 15–40% of COPD patients are reported to have low fat-free mass (80, 81). Patients with COPD are also three times more likely to have low fat-free mass with obesity (sarcopenic obesity), poor physical performance (i.e., lower 6-minute walk test distance) and higher systemic inflammation (82).
A Year at a Glance:
Recent clinical evidence demonstrates that individuals with COPD with higher muscle mass (measured by BIA or DXA) experienced better outcomes (e.g., improved forced expiratory volume and COPD assessment test score, and reduced dyspnea) (83) and lower occurrence of osteopenia/osteoporosis than among COPD patients with low muscle mass (84). Importantly, the prevalence of low muscle mass was higher in men with COPD compared to matched males with normal lung function. There was a relationship between muscle mass and airflow limitation (84), which suggests that disease severity may induce or exacerbate the muscle loss in individuals with COPD. The studies in the past year show that muscle mass is a predictor of important disease-specific outcomes.
Critical Illness
Almost 6 million individuals are admitted to intensive care units (ICU) in the United States each year. The most frequent reasons for admission include respiratory system issues with ventilator support required, acute myocardial infarction, intracranial hemorrhage or cerebral infarction, percutaneous cardiovascular procedure with stent, and sepsis (85).
The stress response to trauma induces a negative protein balance and resistance to anabolic signals; this reaction, along with physical inactivity [immobility] ultimately leads to proteolysis and loss of muscle mass (86). Up to 63% of individuals admitted to the ICU on ventilator support have low muscle mass (87), and this percentage is higher in patients ≥ 65 years old (88). Muscle wasting is likely to worsen during the hospital stay due to considerable systemic inflammation, pre-existing comorbidities, multi-organ dysfunction, and prolonged bed rest (89). Numerous studies have shown that low muscle mass is associated with lower ventilator-free and ICU days (88) and shorter survival (87, 88, 90).
A Year at a Glance:
In six studies evaluating the critical illness population, muscle mass was usually measured via CT images; one study used BIA-derived phase angle, which is an indicator of cell membrane function and nutritional status (91). Higher muscle mass or muscle attenuation was unanimously associated with better survival (91–96). More specifically, greater muscle attenuation was associated with lower 6-month mortality (HR per 10 Hounsfield units: 0.640; 95% CI:0.55–0.72, p<0.001) in mechanically ventilated critically ill patients, after adjustment for the Acute Physiological, Age, and Chronic Health Evaluation (APACHE) II score, BMI, and muscle mass (94). Phase angle was predictive of 28-day mortality (OR: 0.63, 95% CI: 0.78–0.96, p=0.008) in individuals in the ICU (91). Trauma patients (n=23,622) in the lowest quartile of muscle mass had over 9 times greater odds of death compared to those in the highest quartile (OR: 9.15, 95% CI not reported, p<0.001) (93). In terms of outcomes other than survival, two studies reported an inverse association between length of stay and muscle mass (92) and density (94). In emergency surgery, higher muscle mass was predicative of lower surgical morbidity, but not survival in multivariate analysis (92); however, this study used only psoas muscle, which may not be representative of whole-body skeletal muscle (97). Overall, both the amount of muscle mass and attenuation are usually indicative of survival and other clinical outcomes.
Other Conditions:
Individuals with aspiration pneumonia, abdominal wall hernias, and pancreatic disease are also inpatients and at risk for muscle loss (measured by BIA in four studies and CT images in two studies). In general, higher muscle mass was associated with better outcomes such as physical function (98), disease-specific outcomes (pancreatic exocrine insufficiency) (99), less dysphagia (100, 101), shorter length of stay (102), and survival (103, 104).
Outpatient Settings
Cancer:
Individuals with cancer make up a large proportion of patients receiving care in outpatient settings. The tumor-bearing state often induces metabolic alterations such as anorexia, hypoanabolism or hypercatabolism (105, 106), which might elicit dramatic changes in body composition.
As obesity is a risk factor for certain types of cancer, many newly diagnosed patients have obesity, and up to 15% of patients with obesity have low muscle mass (107). Low muscle is associated with worse prognosis including poorer quality of life and function (108), severe treatment toxicity, more postoperative infections and complications, incidence of hospitalization, longer length of hospital stay, and shorter survival (108–110).
CT images are regularly obtained for diagnostic and follow-up purposes and are an opportunistic method to accurately assess muscle mass, usually using the 3rd lumbar vertebra as a landmark (111). The availability of these images allows for a large number of studies exploring the association between muscle mass and patient outcomes. Additionally, the assessment of muscle attenuation is also possible; CT-assessed low muscle attenuation is of emerging importance as it may relate to equivalent of even higher indication of poor patient prognosis, as discussed elsewhere (110, 112, 113).
A Year at a Glance:
Twenty-five of the studies in outpatient populations were in oncology; all of them included measurement of muscle mass using CT images. Many of these publications found that higher amounts of muscle mass were associated with improved survival (114–127). Others reported a statistically significant association between survival and intramuscular adipose tissue (125, 126, 128–131). Few studies found a significant association only in sub-groups of patients, such as those with a high neutrophil/lymphocyte ratio in males with small cell lung cancer (132), disease with lymph node involvement in individuals with esophageal squamous cell carcinoma (58) or pancreatic cancer patients with a BMI ≥ 22 kg/m2 (133), suggesting that disease status and body weight might influence the impact of muscle mass on survival. One study reported only a trend towards significance for the association between muscle mass and survival (134), and another found that muscle mass was predictive of survival only in univariate analysis (97). Within the outpatient oncology setting, only one investigation reported no association between body composition and survival in advanced non-small cell lung cancer (135).
Treatment toxicity (135, 136), poorer radiographic and objective response to therapy (117), fewer treatment cycles completed (134), and shorter time to tumor progression (135) were also associated with low muscle mass. Some studies reported no association of muscle mass or attenuation and the following outcomes: time to disease progression (135), toxicity to chemotherapy (135) and objective response to therapy (117). However, these studies were either carried out in advanced cancer patients with almost 10% weight loss in 6 months (135) or were in a sample that reported high muscle density was associated with radiographic complete response, but not objective response rates (117). Therefore, body composition is still a useful tool for predicting outcomes in outpatient cancer settings.
Cancer research in one year shows that while low muscle mass might predict lower survival in most studies, factors such as inflammation, disease stage, and BMI might mitigate this relationship. Body composition might also relate to other prognostic outcomes.
Liver Diseases:
Liver conditions largely comprise of cirrhosis and liver disease. Cirrhosis is a highly catabolic condition due to insufficient liver glycogen storage, metabolic patterns are similar to that observed in healthy individuals after 2–3 days of starvation (137). Of particular concern is high use of amino acids as fuel, leading to the breakdown of muscle tissue.
Up to 43% of patients with cirrhosis have low muscle mass, 20% have low muscle concurrent to obesity (sarcopenic obesity), and 52% have low muscle attenuation (138). Importantly, patients with any body composition abnormality have shorter survival compared to patients with normal body composition (138).
A Year at a Glance:
Five studies investigated the impact of muscle mass on outcomes in liver conditions, using muscle mass from CT images (n=3), estimated muscle mass from BIA data, (n=1), or both (n=1). In these individuals, low muscle mass was associated with worse albumin levels (139), Child-Pugh scores (139), physical function (140) (correlation only), and shorter survival (138, 141, 142). Body composition in these studies was therefore a useful tool for prognostication.
Primary Care and Population
Body composition abnormalities such as low muscle mass can occur across age, sex, and BMI groups (143). Hence, it is plausible that a seemingly healthy individual might have low muscle mass. Research on the implications of low muscle mass is limited in younger adults. Instead, community-dwelling older adults are the most common population studied in primary care and population-level investigations. As such, low muscle mass has been associated with increased risk of falls, osteoporosis, hospital re-admission, and difficulties in activities of daily living (1). At the same time, fat mass increases until the sixth decade of life (143), increasing the risk of these individuals to develop sarcopenic obesity.
Among older adults, men often have higher rates of low muscle mass than women (144), due in part to the higher amounts of muscle men have earlier in life. However, the mortality risk is higher in women with low muscle (144). This is likely because women have higher fat mass and lower muscle mass and strength than men, increasing their risk for developing functional decline.
Physical function is also critical in older adults, as measures such as grip strength and gait speed are inversely associated with disability and mortality (145, 146). In fact, some suggest that these measures are a different construct than muscle mass (147), and including measures of physical function might be more predictive of negative outcomes than measures/estimations of muscle mass alone (146, 148). The term sarcopenia is used in this setting when both low mass and function/performance are taken into account (1, 2).
A Year at a Glance:
Twenty articles investigated low muscle mass in primary care, mainly in healthy community dwelling older adults. Muscle mass was measured using BIA (n=9), DXA (n=7), CT images (n=3), or magnetic resonance imaging (n=1). Higher muscle mass and not having sarcopenia was positively associated with serum albumin levels (149) better physical function (150–159), higher quality of life (157, 159), and longer survival (154, 160–162). Higher muscle mass was also negatively associated with cardiovascular disease risk factors (163), idiopathic pulmonary fibrosis (154), low bone mineral density (164), bone fractures (165), hospitalization (161), poor pulmonary function (166); sarcopenia was associated with poorer disease specific outcomes such as ankylosing spondylitis (164) and poor renal function (167). Better muscle attenuation was associated with improved balance (150), quality of life (159), and survival (168). Notably, one study (169) found no association between estimated muscle mass (from BIA) and quality of life in older adults; only physical activity, handgrip strength, and balance were associated with overall quality of life. In older individuals, strength declines more rapidly than muscle mass and factors such as muscle attenuation, metabolism, aerobic capacity, insulin resistance, fibrosis, and neural activation might be more relevant for physical function and impaired mobility (and thus quality of life) than muscle mass alone (170–172).
The impact of muscle mass and sarcopenia on patient/health care outcomes has also been investigated on a population level (mainly community-dwelling middle-age or older adults) in fourteen studies, which primarily utilized DXA alone (n=10) or in combination with air displacement plethysmography (n=1), followed by BIA (n=2), and CT images (n=1). Survival was longer in those with more muscle mass (173–178). Sarcopenia was associated with lower bone mineral density (179) and osteoporosis risk (180), and low muscle mass was associated with poor pulmonary function (181) and physical function (179, 182), and higher fracture risk (183, 184), frailty (182), morbidity (185). One study reported that slow walking speed, but not low muscle mass was associated with increased risk of hospitalization or higher likelihood of short-term nursing facility stay in women aged ≥65 years (186).
The outcomes assessed in this group of individuals are diverse. Although low muscle mass is predictive of many of these outcomes in primary care and population level settings, the interplay between other aging-related factors (e.g. hormonal changes, muscle energetics, neural connectivity, muscle blood flow, etc.) are also thought to contribute to mobility-disability in this population.
Long-term Care Settings
The pathophysiology of low muscle mass in long-term care residents is multi-factorial and consists of morphological changes (e.g. type II muscle fiber loss), hormonal changes (e.g. lower testosterone, estrogen, and growth hormone), higher inflammation/oxidative stress, and lifestyle influences (low physical activity and energy intake) (187). Comorbidities such as heart disease, dementia, and Parkinson’s disease are common and contribute to further nutritional decline (187).
Low muscle mass in long-term care is most often investigated as a construct of sarcopenia (ie. low mass and function). Sarcopenia in individuals in long-term care is more prevalent than in community-dwelling adults (188). In individuals age 70 years and older, over one-third have sarcopenia and this condition is associated with a higher risk of death compared to residents without sarcopenia after adjusting for age, sex, physical impairment, BMI, and a number of comorbidities (HR 2.34, 95% CI 1.04 to 5.24)(189).
A Year at a Glance:
Dual X-ray absorptiometry (n=5) and BIA (n=2) were the only measurement methods used in seven studies in the long-term care setting. Cognitive function was the most frequently reported outcome, and most did not find any association between sarcopenia and this parameter (190–192). Activities of daily living were found to be associated with muscle mass and sarcopenia in two studies (193, 194), but not another (192). Mixed findings were also apparent when the relationship between muscle mass (194) or sarcopenia (192) and nutritional status as measured by the Mini Nutritional Assessment were investigated (positive relationship (194); null (192)). Individuals with lower muscle mass had worse Alzheimer’s severity (195) and individuals with sarcopenia had poorer survival (196). A year of research had no conclusive evidence for a diverse array of outcomes.
Treating Low Muscle Mass: Addressing an Unmet Medical Need
The above discussion, complimented by a one-year glimpse at publications, showcases the rapidly growing research of body composition in clinical settings. As we discuss, low muscle mass is prevalent across the continuum of care and is a predictor of poor outcomes. Therefore, the potential clinical benefits of preventing and reversing low muscle mass in patients are likely to impact not only patient outcomes but also resource utilization/health care costs (61, 197–199).
Some clinicians may question the anabolic potential of patients who suffer from acute or chronic conditions associated with muscle loss. However, evidence suggests that interventions can help to maintain or rebuild muscle in these patients (200). For instance, cancer patients were found to have a normal anabolic response to nutrition interventions (201), not differently than healthy persons (202), even after surgery (203). Also, in patients with COPD, the anabolic response to nutrition is not different from that of healthy individuals (204).
New Perspectives
The availability of body composition techniques is still limited in clinical settings. Nonetheless, as the value of assessing muscle mass and mitigating muscle loss emerges, so will the recognition of its use for adequate patient screening and monitoring, and we anticipate a surge of technological developments and financial investments. The recent establishment of ICD-10-CM code for sarcopenia by the Centers for Disease Control and Prevention highlights the momentum for such change in perspective and practice. With the establishment of the ICD-10 code in the US, muscle loss as sarcopenia is now recognized as a condition that can be documented and reported within the health care setting and for data collection. The code, M62.84, is now available for use by the medical community effective October 1, 2016. The new code designation for this condition has the potential to affect research and development of treatments. It calls into action a need to establish clear clinical guidelines for diagnosis, early intervention and treatment of muscle loss. It will potentially enable development and reimbursement of diagnostic tools to measure muscle mass. It also opens new avenues for development of novel therapeutics targeting muscle loss that could receive FDA approval. Having an ICD-10 code will also enable data capture from electronic medical records, death certificates, and other system data sources, which will help the understanding of health economics associated with this condition, also helping researchers to access this data more easily. Overall, the ICD-10 code gives validity to the importance of muscle mass in driving long term health benefits across the continuum of care.
Where Do We Go from Here?
With this increasing evidence on the importance of maintaining muscle mass in various clinical settings, it is critical to develop effective health screening tools to identify people at risk of losing muscle. Tools such as the SARC-F (strength, assistance in walking, rise from a chair, climb stairs, falls) questionnaire (205) are quick and valid (206) in older individuals, but the psychometric properties of such instruments in clinical populations has not been determined. In addition, there is a need for clinically viable tools to measure muscle mass (and attenuation) at the bed side and develop early intervention strategies to mitigate muscle loss. Currently body composition measurement tools (e.g. DXA, CT,) although available to specific clinical settings are not widely available to the general population. Hand held tools such as ultrasound and BIA show promise (207) and are in various stages of validation in different clinical settings. Measurements of muscle function such as grip strength are very good predictors of mobility limitations in the older adults, as previously discussed (208). Therefore, regulatory agencies have suggested improvement in both muscle mass and physical function or survival as endpoints, although these outcomes are ambiguous (209, 210).
For such tools to become routine in clinical practice, healthcare professionals need to be educated on the importance of muscle loss, and how to incorporate routine screening and intervention for these conditions into their clinical practice. For example, screening for muscle loss could be included as part of the Welcome to Medicare and annual Medicare wellness preventive exams in the United States.
In order to provide a foundation for clinical measurement of body composition, a consensus definition of low muscle is needed for various clinical populations. The use of CT images has been recently popularized due to its wide spread availability. Total muscle cross-sectional area at the 3rd lumbar vertebrae is highly correlated with whole body muscle mass (211) and is often used to identify low muscle. The use of a single muscle group (e.g. psoas) is a new trend in body composition assessment. However, this methodology is poorly correlated with total skeletal area and may impede correct interpretation of prognosis (97). Furthermore, this particular muscle might atrophy due to comorbidities such as low back pain or hip osteoarthritis independent of surrounding musculature (212, 213). Some research suggests using linear measures of two muscle groups (psoas and paraspinal muscles) in combination with age and sex can accurately identify individuals with low muscle mass (214), although alternative measurements of total muscle cross-sectional area have been strongly discouraged (215).
Although CT images using total muscle cross-sectional area at the third lumbar vertebra offer an accurate assessment of whole body skeletal muscle mass, certain conditions (i.e. critical care settings) limit weight bearing activity and are associated with disproportional muscle atrophy in the lower limbs (216). Therefore, the accuracy of CT images for whole-body muscle may be compromised, although little is known on this topic. Furthermore, CT imaging may not be sensitive enough to detect body composition changes in short time periods (217). Ultrasound is emerging as the method of choice for such settings where selected muscle groups can be assessed individually (218), although protocols for accurate assessment are still underway.
There is considerable need to counteract the loss of muscle mass, strength, and physical function. Possible dietary interventions might include protein/amino acid formulas, creatine, β-hydroxy-β-methylbuthyrate, and micronutrients, among others. Exercise typically consists of strength or aerobic activity in a supervised or home-based intervention. A recent systematic review concluded that exercise improves muscle mass, strength, and physical performance in healthy adults 60 years and older with additional benefit of nutrition in a small proportion of studies (219). The purported limited effectiveness of nutrition could be due to the great heterogeneity in the type and duration of dietary supplement protocols. Future well-designed clinical trials that combine some form of nutrition and exercise such as the Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC) (220) and Nutrition and Exercise in Critical Illness (NEXIS) (221) will elucidate the potential feasibility and efficacy of methods to counteract muscle loss.
Supplementary Material
Key messages.
Low muscle mass is associated with several negative outcomes across the healthcare continuum.
Modalities to identify and counteract low muscle mass in clinical settings are needed.
Acknowledgments
Funding details: Research reported in this publication was supported by the National Institute of Environmental Health Sciences under grant number P30ES023512 and the National Heart, Lung, and Blood Institute under grant number R01HL132887 of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Carla M. Prado is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award and the Campus Alberta Innovates Research Chair Program.
Footnotes
Disclosure of interest: Carolyn Alish and Suzette Pereira are employees of Abbott. Carla Prado has received speaker honorarium from Abbott. Nicolaas E. Deutz has received research funding and speaker honorarium from Abbott. Bret H. Goodpaster has received research funding from Abbott. Steven B. Heymsfield serves on Medical Advisory Board for Tanita Corporation.
References:
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210(3):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawthon PM. Assessment of Lean Mass and Physical Performance in Sarcopenia. J Clin Densitom. 2015;18(4):467–71. [DOI] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74(4):355–66. [DOI] [PubMed] [Google Scholar]
- 6.Prado CM, Pinto CM, Gonzalez MC, Heymsfield SB. Techniques for Assessment of Body Composition in Health Scientific American Nutrition. Hamilton, Ontario, Canada: Decker Intellectual Properties; 2017. [Google Scholar]
- 7.Koukourikos K, Tsaloglidou A, Kourkouta L. Muscle atrophy in intensive care unit patients. Acta Informatica Medica. 2014;22(6):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality. Inpatient vs. Outpatient Surgeries in U.S. Hospitals Rockville, MD, USA March 2015. Available from: www.hcup-us.ahrq.gov/reports/infographics/inpt_outpt.jsp.
- 9.Bisgaard T, Kehlet H. Early oral feeding after elective abdominal surgery—what are the issues? Nutrition. 2002;18(11):944–8. [DOI] [PubMed] [Google Scholar]
- 10.Gani F, Buettner S, Margonis GA, Sasaki K, Wagner D, Kim Y, et al. Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery. 2016;160(5):1162–71. [DOI] [PubMed] [Google Scholar]
- 11.Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256(2):255–61. [DOI] [PubMed] [Google Scholar]
- 12.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107(6):931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and somplications in surgical sncology: A review of the current literature. J Surg Oncol. 2015;112(5):503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015;30(2):175–9. [DOI] [PubMed] [Google Scholar]
- 15.Hale AL, Twomey K, Ewing JA, Langan EM 3rd, Cull DL, Gray BH. Impact of sarcopenia on long-term mortality following endovascular aneurysm repair. Vasc Med. 2016;21(3):217–22. [DOI] [PubMed] [Google Scholar]
- 16.Higashi T, Hayashi H, Taki K, Sakamoto K, Kuroki H, Nitta H, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol. 2016;21(2):310–9. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Paik HC, Haam SJ, Lee CY, Nam KS, Jung HS, et al. Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients. J Thorac Dis. 2016;8(8):2011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima H, Nakanishi Y, Kataoka M, Tobisu KI, Koga F. Postoperative changes in skeletal muscle mass predict survival of patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. Clin Genitourin Cancer. 2016;15(2):e229–38. [DOI] [PubMed] [Google Scholar]
- 19.Hervochon R, Bobbio A, Guinet C, Mansuet-Lupo A, Rabbat A, Regnard JF, et al. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103(1):287–95. [DOI] [PubMed] [Google Scholar]
- 20.Hirasawa Y, Nakashima J, Yunaiyama D, Sugihara T, Gondo T, Nakagami Y, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2016;23(Suppl 5):1048–54. [DOI] [PubMed] [Google Scholar]
- 21.Huang DD, Chen XX, Chen XY, Wang SL, Shen X, Chen XL, et al. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol. 2016;142(11):2347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: a retrospective multi-institution study. Int J Clin Oncol. 2016;22(1):136–44. [DOI] [PubMed] [Google Scholar]
- 23.Itoh S, Yoshizumi T, Kimura K, Okabe H, Harimoto N, Ikegami T, et al. Effect of sarcopenic obesity on outcomes of living-donor liver transplantation for hepatocellular carcinoma. Anticancer Res. 2016;36(6):3029–34. [PubMed] [Google Scholar]
- 24.Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103(5):572–80. [DOI] [PubMed] [Google Scholar]
- 25.Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24(4):1037–45. [DOI] [PubMed] [Google Scholar]
- 26.Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba-Ssalamah A, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–84. [DOI] [PubMed] [Google Scholar]
- 27.Psutka SP, Boorjian SA, Moynagh MR, Schmit GD, Costello BA, Thompson RH, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. 2016;195(2):270–6. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Okamoto T, Fujishita T, Katsura M, Akamine T, Takamori S, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–7. [DOI] [PubMed] [Google Scholar]
- 29.van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2016;8(2):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drudi LM, Phung K, Ades M, Zuckerman J, Mullie L, Steinmetz OK, et al. Psoas muscle area predicts all-cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52(6):764–9. [DOI] [PubMed] [Google Scholar]
- 31.Mok M, Allende R, Leipsic J, Altisent OA, Del Trigo M, Campelo-Parada F, et al. Prognostic Value of Fat Mass and Skeletal Muscle Mass Determined by Computed Tomography in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am J Cardiol. 2016;117(5):828–33. [DOI] [PubMed] [Google Scholar]
- 32.Paknikar R, Friedman J, Cron D, Deeb GM, Chetcuti S, Grossman PM, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151(3):745–50. [DOI] [PubMed] [Google Scholar]
- 33.Saji M, Lim DS, Ragosta M, LaPar DJ, Downs E, Ghanta RK, et al. Usefulness of psoas muscle area to predict mortality in patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2016;118(2):251–7. [DOI] [PubMed] [Google Scholar]
- 34.Bokshan SL, Han AL, DePasse JM, Eltorai AE, Marcaccio SE, Palumbo MA, et al. Effect of sarcopenia on postoperative morbidity and mortality after thoracolumbar spine surgery. Orthopedics. 2016;39(6):e1159–e64. [DOI] [PubMed] [Google Scholar]
- 35.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101(3). [DOI] [PubMed] [Google Scholar]
- 36.Izumi T, Watanabe J, Tohyama T, Takada Y. Impact of psoas muscle index on short-term outcome after living donor liver transplantation. Turk J Gastroenterol. 2016;27(4):382–8. [DOI] [PubMed] [Google Scholar]
- 37.Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets-Tan RG, Massuger LF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43(4):717–24. [DOI] [PubMed] [Google Scholar]
- 38.Boer BC, de Graaff F, Brusse-Keizer M, Bouman DE, Slump CH, Slee-Valentijn M, et al. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis. 2016;31(6):1117–24. [DOI] [PubMed] [Google Scholar]
- 39.Peyton CC, Heavner MG, Rague JT, Krane LS, Hemal AK. Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage III and IV kidney cancer? J Endourol. 2016;30(2):229–36. [DOI] [PubMed] [Google Scholar]
- 40.Van Rijssen LB, van Huijgevoort NC, Coelen RJ, Tol JA, Haverkort EB, Nio CY, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol. 2017;24(1):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. 2016;40(11):2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heberton GA, Nassif M, Bierhals A, Novak E, LaRue SJ, Lima B, et al. Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol. 2016;118(9):1363–7. [DOI] [PubMed] [Google Scholar]
- 43.Chemama S, Bayar MA, Lanoy E, Ammari S, Stoclin A, Goere D, et al. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2016;23(12):3891–8. [DOI] [PubMed] [Google Scholar]
- 44.Lou N, Chi CH, Chen XD, Zhou CJ, Wang SL, Zhuang CL, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: A prospective study. Eur J Surg Oncol. 2017;43(1):188–95. [DOI] [PubMed] [Google Scholar]
- 45.Makiura D, Ono R, Inoue J, Kashiwa M, Oshikiri T, Nakamura T, et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J Geriatr Oncol. 2016;7(6):430–6. [DOI] [PubMed] [Google Scholar]
- 46.Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113(6):678–84. [DOI] [PubMed] [Google Scholar]
- 47.Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: A prospective study. Ann Surg Oncol. 2016;23(2):556–64. [DOI] [PubMed] [Google Scholar]
- 48.Lindqvist C, Majeed A, Wahlin S. Body composition assessed by dual-energy X-ray absorptiometry predicts early infectious complications after liver transplantation. J Hum Nutr Diet. 2017;30(3):284–91. [DOI] [PubMed] [Google Scholar]
- 49.Dahya V, Xiao J, Prado CM, Burroughs P, McGee D, Silva AC, et al. Computed tomography-derived skeletal muscle index: A novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J. 2016;182:21–7. [DOI] [PubMed] [Google Scholar]
- 50.Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119(3):457–60. [DOI] [PubMed] [Google Scholar]
- 51.Fujikawa H, Araki T, Okita Y, Kondo S, Kawamura M, Hiro J, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today. 2017;47(1):92–8. [DOI] [PubMed] [Google Scholar]
- 52.Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2016;15(6):934–6. [DOI] [PubMed] [Google Scholar]
- 53.Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20(9):1586–94. [DOI] [PubMed] [Google Scholar]
- 54.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2016;8(1):113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsaousi G, Kokkota S, Papakostas P, Stavrou G, Doumaki E, Kotzampassi K. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur J Cancer Care (Engl). 2017;26(6). [DOI] [PubMed] [Google Scholar]
- 56.Onesti JK, Wright GP, Kenning SE, Tierney MT, Davis AT, Doherty MG, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016;16(2):284–9. [DOI] [PubMed] [Google Scholar]
- 57.Weig T, Milger K, Langhans B, Janitza S, Sisic A, Kenn K, et al. Core muscle size predicts postoperative outcome in lung transplant candidates. Ann Thorac Surg. 2016;101(4):1318–25. [DOI] [PubMed] [Google Scholar]
- 58.Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(6):627–33. [DOI] [PubMed] [Google Scholar]
- 59.Jo S, Park SB, Kim MJ, Kim T, Park KI, Sung J, et al. Comparison of balance, proprioception and skeletal muscle mass in total hip replacement patients with and without fracture: a pilot study. Ann Rehabil Med. 2016;40(6):1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reisinger KW, Derikx JP, van Vugt JL, Von Meyenfeldt MF, Hulsewe KW, Olde Damink SW, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35(4):924–7. [DOI] [PubMed] [Google Scholar]
- 61.Sousa AS, Guerra RS, Fonseca I, Pichel F, Ferreira S, Amaral TF. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016;70(9):1046–51. [DOI] [PubMed] [Google Scholar]
- 62.van Vugt JLA, Buettner S, Levolger S, Coebergh van den Braak RRJ, Suker M, Gaspersz MP, et al. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PLoS One. 2017;12(10):e0186547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aahlin EK, Trano G, Johns N, Horn A, Soreide JA, Fearon KC, et al. Health-related quality of life, cachexia and overall survival after major upper abdominal surgery: a prospective cohort study. Scand J Surg. 2016;106(1):40–6. [DOI] [PubMed] [Google Scholar]
- 64.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature reviews Cardiology. 2011;8(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34(7):512–9. [DOI] [PubMed] [Google Scholar]
- 66.Uematsu M, Akashi YJ, Ashikaga K, Yoneyama K, Kida K, Suzuki K, et al. Association between heart rate at rest and myocardial perfusion in patients with acute myocardial infarction undergoing cardiac rehabilitation - a pilot study. Arch Med Sci. 2012;8(4):622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsubara Y, Matsumoto T, Inoue K, Matsuda D, Yoshiga R, Yoshiya K, et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J Vasc Surg. 2016;65(5):1390–7. [DOI] [PubMed] [Google Scholar]
- 68.Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–6. [DOI] [PubMed] [Google Scholar]
- 69.Zuckerman J, Ades M, Mullie L, Trnkus A, Morin JF, Langlois Y, et al. Psoas muscle area and length of stay in older adults undergoing cardiac operations. Ann Thorac Surg. 2016;103(5):1498–504. [DOI] [PubMed] [Google Scholar]
- 70.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. [DOI] [PubMed] [Google Scholar]
- 71.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clinical Journal of the American Society of Nephrology : CJASN. 2014;9(10):1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsiao SM, Tsai YC, Chen HM, Lin MY, Chiu YW, Chen TH, et al. Association of fluid status and body composition with physical function in patients with chronic kidney disease. PLoS One. 2016;11(10):e0165400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segura-Orti E, Gordon PL, Doyle JW, Johansen KL. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res. 2017;27(5):579–96. [DOI] [PubMed] [Google Scholar]
- 74.Barros A, Costa BE, Mottin CC, d’Avila DO. Depression, quality of life, and body composition in patients with end-stage renal disease: a cohort study. Rev Bras Psiquiatr. 2016;38(4):301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locke JE, Carr JJ, Nair S, Terry JG, Reed RD, Smith GD, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant. 2017;31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malhotra R, Deger SM, Salat H, Bian A, Stewart TG, Booker C, et al. Sarcopenic obesity definitions by body composition and mortality in the hemodialysis patients. J Ren Nutr. 2017;27(2):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J. 2014;44(6):1504–20. [DOI] [PubMed] [Google Scholar]
- 78.Slinde F, EllegÅRd L, GrÖNberg AM, Larsson S, Rossander-HulthÉN L. Total energy expenditure in underweight patients with severe chronic obstructive pulmonary disease living at home. Clin Nutr. 2003;22(2):159–65. [DOI] [PubMed] [Google Scholar]
- 79.Baarends EM, Schols AM, Westerterp KR, Wouters EF. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax. 1997;52(9):780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. [DOI] [PubMed] [Google Scholar]
- 81.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–6. [DOI] [PubMed] [Google Scholar]
- 82.Joppa P, Tkacova R, Franssen FM, Hanson C, Rennard SI, Silverman EK, et al. Sarcopenic obesity, functional outcomes, and systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2016;17(8):712–8. [DOI] [PubMed] [Google Scholar]
- 83.Pothirat C, Chaiwong W, Phetsuk N, Liwsrisakun C, Bumroongkit C, Deesomchok A, et al. The Relationship between Body Composition and Clinical Parameters in Chronic Obstructive Pulmonary Disease. J Med Assoc Thai. 2016;99(4):386–93. [PubMed] [Google Scholar]
- 84.Hwang JA, Kim YS, Leem AY, Park MS, Kim SK, Chang J, et al. Clinical implications of sarcopenia on decreased bone density in men with chronic obstructive pulmonary disease. Chest. 2016;151(5):1018–27. [DOI] [PubMed] [Google Scholar]
- 85.Society of Critical Care Medicine. Critical Care Patients. Mount Prospect, IL: 2016. [Google Scholar]
- 86.Preiser J-C, van Zanten ARH, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Critical Care. 2015;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Journal of Parenteral and Enteral Nutrition. 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 90.Akahoshi T, Yasuda M, Momii K, Kubota K, Shono Y, Kaku N, et al. Sarcopenia is a predictive factor for prolonged intensive care unit stays in high-energy blunt trauma patients. Acute Medicine & Surgery. 2016;3:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thibault R, Makhlouf AM, Mulliez A, Cristina Gonzalez M, Kekstas G, Kozjek NR, et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study Phase Angle Project. Intensive Care Med. 2016;42(9):1445–53. [DOI] [PubMed] [Google Scholar]
- 92.Dirks RC, Edwards BL, Tong E, Schaheen B, Turrentine FE, Shada A, et al. Sarcopenia in emergency abdominal surgery. J Surg Res. 2017;207:13–21. [DOI] [PubMed] [Google Scholar]
- 93.Leeper CM, Lin E, Hoffman M, Fombona A, Zhou T, Kutcher M, et al. Computed tomography abbreviated assessment of sarcopenia following trauma: The CAAST measurement predicts 6-month mortality in older adult trauma patients. J Trauma Acute Care Surg. 2016;80(5):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Twisk JW, Oudemans-van Straaten HM, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shibahashi K, Sugiyama K, Kashiura M, Hamabe Y. Decreasing skeletal muscle as a risk factor for mortality in elderly patients with sepsis: a retrospective cohort study. J Intensive Care. 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wallace JD, Calvo RY, Lewis PR, Brill JB, Shackford SR, Sise MJ, et al. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: Comparing the masseter to the psoas using computed tomography. J Trauma Acute Care Surg. 2017;82(1):65–72. [DOI] [PubMed] [Google Scholar]
- 97.Rutten IJG, Ubachs J, Kruitwagen RFPM, Beets-Tan RGH, Olde Damink SWM, Van Gorp T Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(4):630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur J Clin Nutr. 2016;70(5):595–601. [DOI] [PubMed] [Google Scholar]
- 99.Shintakuya R, Uemura K, Murakami Y, Kondo N, Nakagawa N, Urabe K, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017;17(1):70–5. [DOI] [PubMed] [Google Scholar]
- 100.Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2016;16(4):515–21. [DOI] [PubMed] [Google Scholar]
- 101.Maeda K, Takaki M, Akagi J. Decreased skeletal muscle mass and risk factors of sarcopenic dysphagia: A prospective observational cohort study. J Gerontol A Biol Sci Med Sci. 2016;72(9):1290–4. [DOI] [PubMed] [Google Scholar]
- 102.Rinaldi JM, Geletzke AK, Phillips BE, Miller J, Dykes TM, Soybel DI. Sarcopenia and sarcopenic obesity in patients with complex abdominal wall hernias. Am J Surg. 2016;212(5):903–11. [DOI] [PubMed] [Google Scholar]
- 103.Maeda K, Akagi J. Muscle mass loss is a potential predictor of 90-day mortality in older adults with aspiration pneumonia. J Am Geriatr Soc. 2017;65(1):e18–e22. [DOI] [PubMed] [Google Scholar]
- 104.Perez-Zepeda MU, Sgaravatti A, Dent E. Sarcopenia and post-hospital outcomes in older adults: A longitudinal study. Arch Gerontol Geriatr. 2017;69:105–9. [DOI] [PubMed] [Google Scholar]
- 105.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–9. [DOI] [PubMed] [Google Scholar]
- 106.Purcell SA, Elliott SA, Baracos VE, Chu QSC, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70(11):1230–8. [DOI] [PubMed] [Google Scholar]
- 107.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. [DOI] [PubMed] [Google Scholar]
- 108.Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer. 2013;21(6):1569–77. [DOI] [PubMed] [Google Scholar]
- 109.Prado CMM, Mourtzakis M, Baracos V, Reiman T, Sawyer MB, McCargar LJ. Overweight and obese patients with solid tumors may have sarcopenia, poor prognosis and early features of cachexia. Int J Body Compos Res. 2010;8(1):7–15. [Google Scholar]
- 110.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 111.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 112.Xiao J, Caan BJ, Weltzien E, Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Associations of pre‐existing co‐morbidities with skeletal muscle mass and radiodensity in patients with non‐metastatic colorectal cancer. Journal of Cachexia, Sarcopenia and Muscle.0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daly L, Prado CM, Ryan A. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc. 2018;77(2):135–51. [DOI] [PubMed] [Google Scholar]
- 114.Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore). 2016;95(13):e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Begini P, Gigante E, Antonelli G, Carbonetti F, Iannicelli E, Anania G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16(1):107–14. [DOI] [PubMed] [Google Scholar]
- 116.Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 117.Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J Cachexia Sarcopenia Muscle. 2017;8(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daly LE, Power DG, O’Reilly A, Donnellan P, Cushen SJ, O’Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol. 2016;195(1):26–32. [DOI] [PubMed] [Google Scholar]
- 120.Giap F, Lau SKM, Gannavarapu BS, Iyengar P. Impact of cachexia at diagnosis on radiotherapy utilization and survival in non-small cell lung cancer. J Clin Oncol. 2016;34(26_suppl):133. [Google Scholar]
- 121.Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2017;47(6):558–65. [DOI] [PubMed] [Google Scholar]
- 122.Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia and the Modified Glasgow Prognostic Score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Target Oncol. 2016;11(5):605–17. [DOI] [PubMed] [Google Scholar]
- 123.Kamachi S, Mizuta T, Otsuka T, Nakashita S, Ide Y, Miyoshi A, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. 2016;46(2):201–8. [DOI] [PubMed] [Google Scholar]
- 124.Liu J, Motoyama S, Sato Y, Wakita A, Kawakita Y, Saito H, et al. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Anticancer Res. 2016;36(12):6677–85. [DOI] [PubMed] [Google Scholar]
- 125.Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KC, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263(2):320–5. [DOI] [PubMed] [Google Scholar]
- 126.Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35(5):1103–9. [DOI] [PubMed] [Google Scholar]
- 127.Taguchi S, Akamatsu N, Nakagawa T, Gonoi W, Kanatani A, Miyazaki H, et al. Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer. 2016;14(3):237–43. [DOI] [PubMed] [Google Scholar]
- 128.Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142(2):311–6. [DOI] [PubMed] [Google Scholar]
- 129.Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MC, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15. [DOI] [PubMed] [Google Scholar]
- 130.Sjoblom B, Gronberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35(6):1386–93. [DOI] [PubMed] [Google Scholar]
- 131.Veld J, Vossen JA, De Amorim Bernstein K, Halpern EF, Torriani M, Bredella MA. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol. 2016;26(12):4649–55. [DOI] [PubMed] [Google Scholar]
- 132.Go SI, Park MJ, Song HN, Kang MH, Park HJ, Jeon KN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24(5):2075–84. [DOI] [PubMed] [Google Scholar]
- 133.Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int J Surg. 2017;39:45–51. [DOI] [PubMed] [Google Scholar]
- 134.Xiao DY, Luo S, O’Brian K, Ganti A, Riedell P, Sanfilippo KM, et al. Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am J Hematol. 2016;91(10):1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Srdic D, Plestina S, Sverko-Peternac A, Nikolac N, Simundic AM, Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24(11):4495–502. [DOI] [PubMed] [Google Scholar]
- 136.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin Cancer Res. 2017;23(3):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Owen OE, Trapp VE, Reichard GA Jr., Mozzoli MA, Moctezuma J, Paul P, et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72(5):1821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55(8):863–70. [DOI] [PubMed] [Google Scholar]
- 140.Itoh S, Shirabe K, Yoshizumi T, Takeishi K, Harimoto N, Ikegami T, et al. Skeletal muscle mass assessed by computed tomography correlates to muscle strength and physical performance at a liver-related hospital experience. Hepatol Res. 2016;46(4):292–7. [DOI] [PubMed] [Google Scholar]
- 141.Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016;46(8):743–51. [DOI] [PubMed] [Google Scholar]
- 142.Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, et al. Prognostic significance of low skeletal muscle mass compared with protein-energy malnutrition in liver cirrhosis. Hepatol Res. 2016;47(10):1042–52. [DOI] [PubMed] [Google Scholar]
- 143.Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, et al. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99(6):1369–77. [DOI] [PubMed] [Google Scholar]
- 144.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–7. [DOI] [PubMed] [Google Scholar]
- 145.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–60. [DOI] [PubMed] [Google Scholar]
- 146.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality: Results from the InCHIANTI study. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2009;64(3):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35(1):51–65. [DOI] [PubMed] [Google Scholar]
- 149.Bouchi R, Fukuda T, Takeuchi T, Minami I, Yoshimoto T, Ogawa Y. Sarcopenia is associated with incident albuminuria in patients with type 2 diabetes: A retrospective observational study. J Diabetes Investig. 2017;8(6):783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, et al. Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bai HJ, Sun JQ, Chen M, Xu DF, Xie H, Yu ZW, et al. Age-related decline in skeletal muscle mass and function among elderly men and women in Shanghai, China: a cross sectional study. Asia Pac J Clin Nutr. 2016;25(2):326–32. [DOI] [PubMed] [Google Scholar]
- 152.Chang JS, Kim TH, Kim H, Choi EH, Kim N, Kong ID. Qualitative muscle mass index as a predictor of skeletal muscle function deficit in Asian older adults. Geriatr Gerontol Int. 2017;17(1):99–107. [DOI] [PubMed] [Google Scholar]
- 153.Han DS, Chang KV, Li CM, Lin YH, Kao TW, Tsai KS, et al. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci Rep. 2016;6:19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nishiyama O, Yamazaki R, Sano H, Iwanaga T, Higashimoto Y, Kume H, et al. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology. 2016. [DOI] [PubMed] [Google Scholar]
- 155.Orsatti FL, Nunes PR, Souza AP, Martins FM, de Oliveira AA, Nomelini RS, et al. Predicting functional capacity from measures of muscle mass in postmenopausal women. PM R. 2017;9(6):596–602. [DOI] [PubMed] [Google Scholar]
- 156.Schweitzer L, Geisler C, Johannsen M, Gluer CC, Muller MJ. Associations between body composition, physical capabilities and pulmonary function in healthy older adults. Eur J Clin Nutr. 2017;71(3):389–94. [DOI] [PubMed] [Google Scholar]
- 157.Silva Neto LS, Karnikowski MG, Osorio NB, Pereira LC, Mendes MB, Galato D, et al. Association between sarcopenia and quality of life in quilombola elderly in Brazil. Int J Gen Med. 2016;9:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tramontano A, Veronese N, Sergi G, Manzato E, Rodriguez-Hurtado D, Maggi S, et al. Prevalence of sarcopenia and associated factors in the healthy older adults of the Peruvian Andes. Arch Gerontol Geriatr. 2017;68:49–54. [DOI] [PubMed] [Google Scholar]
- 159.Trombetti A, Reid KF, Hars M, Herrmann FR, Pasha E, Phillips EM, et al. Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int. 2016;27(2):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Graf CE, Herrmann FR, Spoerri A, Makhlouf AM, Sorensen TI, Ho S, et al. Impact of body composition changes on risk of all-cause mortality in older adults. Clin Nutr. 2016;35(6):1499–505. [DOI] [PubMed] [Google Scholar]
- 161.Wu TY, Liaw CK, Chen FC, Kuo KL, Chie WC, Yang RS. Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc. 2016;17(12):1129–35. [DOI] [PubMed] [Google Scholar]
- 162.Zhao Q, Zmuda JM, Kuipers AL, Jonnalagadda P, Bunker CH, Patrick AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45(4):529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodriguez AJ, Scott D, Khan B, Khan N, Hodge A, English DR, et al. Low relative lean mass is associated with increased likelihood of abdominal aortic calcification in community-dwelling older Australians. Calcif Tissue Int. 2016;99(4):340–9. [DOI] [PubMed] [Google Scholar]
- 164.El Maghraoui A, Ebo’o FB, Sadni S, Majjad A, Hamza T, Mounach A. Is there a relation between pre-sarcopenia, sarcopenia, cachexia and osteoporosis in patients with ankylosing spondylitis? BMC Musculoskelet Disord. 2016;17:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25(11):3424–31. [DOI] [PubMed] [Google Scholar]
- 166.Inomoto A, Fukuda R, Deguchi Phn J, Kato G, Kanzaki Rpt R, Hiroshige Rpt K, et al. The association between the body composition and lifestyle affecting pulmonary function in Japanese workers. J Phys Ther Sci. 2016;28(10):2883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang R, Zhang Y, Shen X, Yan S. Sarcopenia associated with renal function in the patients with type 2 diabetes. Diabetes Res Clin Pract. 2016;118:121–9. [DOI] [PubMed] [Google Scholar]
- 168.Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage. 2017. [DOI] [PubMed] [Google Scholar]
- 169.Haider S, Luger E, Kapan A, Titze S, Lackinger C, Schindler KE, et al. Associations between daily physical activity, handgrip strength, muscle mass, physical performance and quality of life in prefrail and frail community-dwelling older adults. Qual Life Res. 2016;25(12):3129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48(4):381–6. [DOI] [PubMed] [Google Scholar]
- 171.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan. 2014;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64. [DOI] [PubMed] [Google Scholar]
- 173.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chuang SY, Hsu YY, Chen RC, Liu WL, Pan WH. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A Biol Sci Med Sci. 2016;71(8):1049–55. [DOI] [PubMed] [Google Scholar]
- 175.Pasco JA, Mohebbi M, Holloway KL, Brennan-Olsen SL, Hyde NK, Kotowicz MA. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle. 2017;8(3):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reinders I, Murphy RA, Brouwer IA, Visser M, Launer L, Siggeirsdottir K, et al. Muscle quality and myosteatosis: Novel associations with mortality risk: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am J Epidemiol. 2016;183(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djousse L, et al. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis. 2016;26(11):1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol. 2016;117(8):1355–60. [DOI] [PubMed] [Google Scholar]
- 179.Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, et al. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: The Concord Health And Ageing in Men Project. J Bone Miner Res. 2017;32(3):575–83. [DOI] [PubMed] [Google Scholar]
- 180.He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–82. [DOI] [PubMed] [Google Scholar]
- 181.Oliveira PD, Wehrmeister FC, Perez-Padilla R, Goncalves H, Assuncao MC, Horta BL, et al. Relationship between body composition and pulmonary function in early adult life: A cross-sectional analysis nested in two birth cohort studies. PLoS One. 2016;11(9):e0163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing. 2017;46(3):413–20. [DOI] [PubMed] [Google Scholar]
- 183.Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, et al. Low lean mass predicts incident fractures independently from frax: a prospective cohort study of recent retirees. J Bone Miner Res. 2016;31(11):2048–56. [DOI] [PubMed] [Google Scholar]
- 184.Sornay-Rendu E, Duboeuf F, Boutroy S, Chapurlat RD. Muscle mass is associated with incident fracture in postmenopausal women: The OFELY study. Bone. 2017;94:108–13. [DOI] [PubMed] [Google Scholar]
- 185.An KO, Kim J. Association of sarcopenia and obesity with multimorbidity in korean adults: A nationwide cross-sectional study. J Am Med Dir Assoc. 2016;17(10):960 e1–7. [DOI] [PubMed] [Google Scholar]
- 186.Cawthon PM, Lui LY, McCulloch CE, Cauley JA, Paudel ML, Taylor B, et al. Sarcopenia and health care utilization in older women. J Gerontol A Biol Sci Med Sci. 2017;72(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bauer JM, Kaiser MJ, Sieber CC. Sarcopenia in nursing home residents. J Am Med Dir Assoc. 2008;9(8):545–51. [DOI] [PubMed] [Google Scholar]
- 188.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121–6. [DOI] [PubMed] [Google Scholar]
- 190.Huang CY, Hwang AC, Liu LK, Lee WJ, Chen LY, Peng LN, et al. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. 2016;19(1):71–8. [DOI] [PubMed] [Google Scholar]
- 191.Moon JH, Moon JH, Kim KM, Choi SH, Lim S, Park KS, et al. Sarcopenia as a predictor of future cognitive impairment in older adults. J Nutr Health Aging. 2016;20(5):496–502. [DOI] [PubMed] [Google Scholar]
- 192.Yamanouchi A, Yoshimura Y, Matsumoto Y, Jeong S. Severely decreased muscle mass among older patients hospitalized in a long-term care ward in Japan. J Nutr Sci Vitaminol (Tokyo). 2016;62(4):229–34. [DOI] [PubMed] [Google Scholar]
- 193.Sugimoto T, Ono R, Murata S, Saji N, Matsui Y, Niida S, et al. Sarcopenia is associated with impairment of activities of daily living in Japanese patients with early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2017;31(3):256–8. [DOI] [PubMed] [Google Scholar]
- 194.Tufan A, Bahat G, Ozkaya H, Tascioglu D, Tufan F, Saka B, et al. Low skeletal muscle mass index is associated with function and nutritional status in residents in a Turkish nursing home. Aging Male. 2016;19(3):182–6. [DOI] [PubMed] [Google Scholar]
- 195.Takagi D, Hirano H, Watanabe Y, Edahiro A, Ohara Y, Yoshida H, et al. Relationship between skeletal muscle mass and swallowing function in patients with Alzheimer’s disease. Geriatr Gerontol Int. 2016. [DOI] [PubMed] [Google Scholar]
- 196.Yalcin A, Aras S, Atmis V, Cengiz OK, Cinar E, Atli T, et al. Sarcopenia and mortality in older people living in a nursing home in Turkey. Geriatr Gerontol Int. 2017;17(7):1118–24. [DOI] [PubMed] [Google Scholar]
- 197.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. [DOI] [PubMed] [Google Scholar]
- 198.Du K, Goates S, Arensberg MB, Pereira S, Gaillard T, Hegazi R. Impact of sarcopenia on falls and hospitalization in community dwelling adults. International Conference on Frailty and Sarcopenia Research 2017. [Google Scholar]
- 199.Goates S, Du K, Arensberg M, Pereira S, Gaillard T, Hegazi R. Cost Burden of Sarcopenia Associated Hospitalization in Adults by Age and Race/Ethnicity. International Society of Pharmacoeconomics and Outcomes Research; 2018. [Google Scholar]
- 200.Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc. 2017;18(6):553 e1–e16. [DOI] [PubMed] [Google Scholar]
- 201.Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30(6):759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Engelen MP, Safar AM, Bartter T, Koeman F, Deutz NE. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann Oncol. 2015;26(9):1960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Engelen M, Klimberg VS, Allasia A, Deutz NE. Presence of early stage cancer does not impair the early protein metabolic response to major surgery. J Cachexia Sarcopenia Muscle. 2017;8(3):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metabolism. 2017;69:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–2. [DOI] [PubMed] [Google Scholar]
- 206.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. Journal of Cachexia, Sarcopenia and Muscle. 2016;7(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Earthman CP. Body Composition Tools for Assessment of Adult Malnutrition at the Bedside: A Tutorial on Research Considerations and Clinical Applications. JPEN J Parenter Enteral Nutr. 2015;39(7):787–822. [DOI] [PubMed] [Google Scholar]
- 208.Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, Koskinen S. Hand-Grip Strength Cut-Points to Screen Older Persons at Risk for Mobility Limitation. J Am Geriatr Soc. 2010;58(9):1721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mauricio SF, Xiao J, Prado CM, Gonzalez MC, Correia M. Different nutritional assessment tools as predictors of postoperative complications in patients undergoing colorectal cancer resection. Clin Nutr. 2017;In Press. [DOI] [PubMed] [Google Scholar]
- 210.Fearon KCH, Argiles JM, Baracos VE, Bernabei R, Coats AJS, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. Journal of Cachexia, Sarcopenia and Muscle. 2015;6(4):272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 212.Laban MM. Atrophy and clinical weakness of the iliopsoas muscle: a manifestation of hip osteoarthritis. Am J Phys Med Rehabil. 2006;85(7):629. [DOI] [PubMed] [Google Scholar]
- 213.Cooper RG, Forbes WSC, Jayson MIV. Radiographic demonstration of paraspinal muscle wasting in patients with chronic low back pain. Rheumatology. 1992;31(6):389–94. [DOI] [PubMed] [Google Scholar]
- 214.Avrutin E, Moisey LL, Zhang R, Khattab J, Todd E, Premji T, et al. Clinically practical approach for screening of low muscularity using electronic linear measures on computed tomography images in critically ill patients. Journal of Parenteral and Enteral Nutrition.In Press:n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 215.Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(4):527–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol. 1997;4(1):S10–4. [PubMed] [Google Scholar]
- 217.Brewster DJ, Strauss BJ, Crozier TM. Measuring visceral fat, subcutaneous fat and skeletal muscle area changes by computed tomography in acute pancreatitis: a retrospective, single-centre study. Crit Care Resusc. 2014;16(1):42–7. [PubMed] [Google Scholar]
- 218.Paris M, Mourtzakis M. Assessment of skeletal muscle mass in critically ill patients: considerations for the utility of computed tomography imaging and ultrasonography. Curr Opin Clin Nutr Metab Care. 2016;19(2):125–30. [DOI] [PubMed] [Google Scholar]
- 219.Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.ClinicalTrials.gov. Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC). NCT02330926 January 2017.
- 221.Clinicaltrials.gov. NCT03021902: Nutrition and Exercise in Critical Illness (NEXIS) Bethesda, MD2017.
- 222.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. [DOI] [PubMed] [Google Scholar]
- 223.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. [DOI] [PubMed] [Google Scholar]
- 224.Delmonico MJ, Harris TB, Lee J-S, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–74. [DOI] [PubMed] [Google Scholar]
- 225.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602–9. [DOI] [PubMed] [Google Scholar]
- 226.Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56(9):1710–5. [DOI] [PubMed] [Google Scholar]
- 227.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.