Abstract
Bacteria and fungi form complex communities (microbiomes) in above- and below-ground organs of plants, contributing to hosts’ growth and survival in various ways. Recent studies have suggested that host plant genotypes control, at least partly, plant-associated microbiome compositions. However, we still have limited knowledge of how microbiome structures are determined in/on grafted crop plants, whose above-ground (scion) and below-ground (rootstock) genotypes are different with each other. By using eight varieties of grafted tomato plants, we examined how rootstock genotypes could determine the assembly of leaf endophytic microbes in field conditions. An Illumina sequencing analysis showed that both bacterial and fungal community structures did not significantly differ among tomato plants with different rootstock genotypes: rather, sampling positions in the farmland contributed to microbiome variation in a major way. Nonetheless, a further analysis targeting respective microbial taxa suggested that some bacteria and fungi could be preferentially associated with particular rootstock treatments. Specifically, a bacterium in the genus Deinococcus was found disproportionately from ungrafted tomato individuals. In addition, yeasts in the genus Hannaella occurred frequently on the tomato individuals whose rootstock genotype was “Ganbarune”. Overall, this study suggests to what extent leaf microbiome structures can be affected/unaffected by rootstock genotypes in grafted crop plants.
Introduction
In both natural and agricultural ecosystems, bacteria and fungi in diverse taxonomic groups are associated with plants, positively and/or negatively influencing the survival and growth of their hosts1–4. An increasing number of studies have shown that plant-associated microbes not only improve nutritional conditions of host plants but also increase plants’ resistance to abiotic stresses (e.g., high temperature, drought, and soil pollution) and that to pathogens and pests5–8. In contrast, bacterial and fungal communities associated with plants can be regarded as serious risk factors in agriculture and forestry because they are occasionally dominated by plant pathogenic species or strains9,10. Therefore, controlling plant-associated microbiomes has been recognized as a major challenge towards the development of stable and sustainable management of crop fields and plantations11–14.
Host plant genotypes, along with external environmental conditions, are important factors determining microbiome structures15–18. Developing disease-resistant crop plant varieties has been one of the major goals in breeding science19–21. Moreover, recent studies have explored genes and mutations influencing whole microbiome structures22,23, providing a basis for optimizing communities of plant-growth-promoting bacteria and/or fungi. Meanwhile, to gain more insights into mechanisms by which plant microbiomes are controlled, studies using plant individuals with complex genetic backgrounds have been awaited. Specifically, by using grafted plants, whose above- and below-ground genotypes are different with each other, we will be able to examine, for instance, how below-ground genetic factors control above-ground microbiome structures. Because root genotypes can control not only uptake of water and nutrients but also transport of phytohormones or signaling molecules24–26, their effects on leaf physiology potentially influence community compositions of endophytic and epiphytic microbes in above-ground plant tissue. Although studies focusing on such mechanisms interlinking above- and below-ground processes can provide essential insights into plants’ microbiome control, few attempts27,28, to our knowledge, have been made to conduct experiments using grafted plants.
Grafting per se is a classic technique but it has been increasingly considered as a promising method for increasing yield, crop quality, abiotic stress resistance, and pathogen resistance of various plants (e.g., tomato, melon, grapevine, apple, and citrus) in agriculture29–33. In general, performance of grafted plants depends greatly on compatibility between scion and rootstock genotypes34–36. However, we still have limited knowledge of how scion–rootstock genotypic combinations determine microbiome structures in leaves, roots, and other plant organs27. Moreover, although some pioneering studies have investigated microbial community compositions of grafted plants37–39, most of them focused on subsets of microbiomes (i.e., either bacteria or fungi but not both). Therefore, new lines of studies examining relationships between scion/rootstock genotypes and whole microbiome structures in roots/leaves have been awaited.
In this study, we evaluated how below-ground genotypes of plants determine bacterial and fungal community structures in/on leaves under field conditions. After growing grafted tomato [Solanum lycopersicum (=Lycopersicon lycopersicum)] individuals in a field experiment, we analyzed the leaf microbial community compositions of the sampled tomatoes based on Illumina sequencing. The contributions of below-ground genotypes on the leaf microbiome structures were then evaluated by comparing the microbial community datasets of eight tomato rootstock varieties. We also performed randomization-based statistical analyses to explore bacterial and fungal taxa that had strong signs of preferences for specific tomato rootstock varieties. Overall, this study suggests to what extent below-ground genotypes of plants influence plant–microbe associations in leaves, providing a basis for managing microbiomes of grafted plants in agriculture and forestry.
Methods
Grafted tomato seedlings
To prepare rootstocks, seeds of eight tomato varieties (“Chibikko”, “Ganbarune”, “M82”, “Micro-Tom”, “Regina”, “Spike”, “Triper”, and “Momotaro-Haruka”; see Supplementary Table 1 for characteristics of respective varieties) were sown on June 7, 2017 for “Momotaro-Haruka” and June 1, 2017 for the others, and then the pots were grown in a greenhouse of Togo Field, Nagoya University, Nagoya, Japan (35.112°N; 137.083°E). To reproduce conventional agricultural conditions for raising seedlings, the seeds were sown in 6-cm pots filled with commercially available potting soil [Hanachan-baiyodo, (Hanagokoro Co., Ltd., Nagoya): Vermiculite GS (NITTAI Co., Ltd., Osaka) = 1:1]. On June 22–23, seedlings for the field experiment detailed below were produced by grafting “Momotaro-Haruka” scions on each of the eight varieties of rootstocks: i.e., above-ground parts of the grafted seedlings were all Momotaro-Haruka, while below-ground parts differed among seedling individuals. Ungrafted “Momotaro-Haruka” seedlings were also prepared as control samples. The grafted (including Momotaro-Haruka/Momotaro-Haruka self-grafted seedlings) and ungrafted seedlings (in total, nine treatments) were grown in a greenhouse of Togo Field and, on July 7, they were transported to Center for Ecological Research, Kyoto University, Kyoto, Japan (34.972°N; 135.958°E). Each seedling was then transferred to a 9-cm pot filled with commercially-available culture soil (Rakuyo Co., Ltd., Kyoto) on the day and they were kept on the field nursery shelf of Center for Ecological Research until the field experiment.
Field transplantation
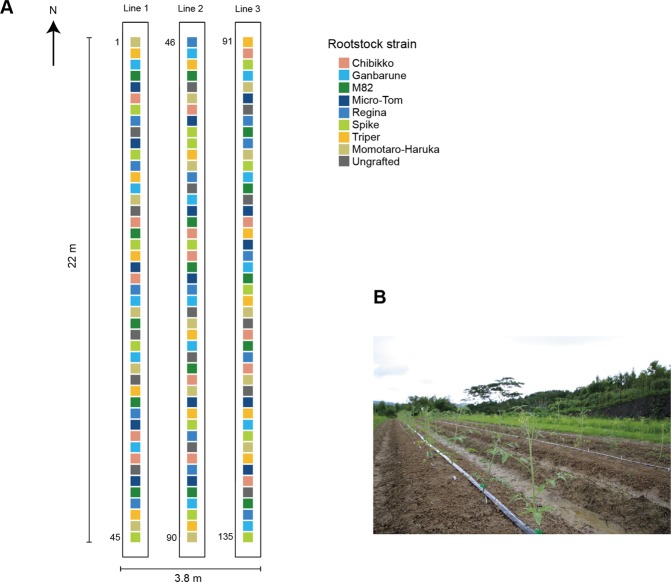
On July 13, base fertilizer was provided to the soil in the experimental field of Center for Ecological Research (N = 13.6 g/m2; P2O5 = 13.6 g/m2; K2O = 13.6 g/m2). On July 25, the abovementioned seedlings (ca. 50 cm high) were transplanted to the open field at 50 cm horizontal intervals in three lines in a randomized order (9 seedling treatment × 5 replicates per line × 3 lines (sets) = 135 individuals; Fig. 1). The tomato individuals were watered twice (morning and evening) every day. On September 13, a ca. 1-cm2 disc of a mature leaf was sampled from each tomato individual and placed in a 2-mL microtube. The leaf samples were transferred to a laboratory of Center for Ecological Research using a cool box and they were then preserved at −80 °C in a freezer until DNA extraction.
Figure 1.
Field site. (A) Nine tomato rootstock varieties (treatments) in the field. For each rootstock variety, 15 replicate samples were transplanted to the field site (15 replicates × 9 varieties = 135 tomato individuals). The above-ground parts of all the 135 tomato individuals had the genotype of the tomato variety “Momotaro-Haruka”. (B) Transplanted tomato individuals.
DNA extraction, PCR, and sequencing
Each leaf disc was immersed in ×1/100 NaClO (Nacalai Tesque; 31518-35) for 1 min and it was subsequently washed in 70% ethanol. DNA extraction was extracted with a cetyltrimethylammonium bromide (CTAB) method after pulverizing the leaves with 4 mm zirconium balls at 25 Hz for 3 min using a TissueLyser II (Qiagen).
For each leaf disc sample, the 16 S rRNA V4 region of the prokaryotes and the internal transcribed spacer 1 (ITS1) region of fungi were PCR-amplified. The PCR of the 16S rRNA region was performed with the forward primer 515f40 fused with 3–6-mer Ns for improved Illumina sequencing quality41 and the forward Illumina sequencing primer (5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG- [3–6-mer Ns] - [515f] -3′) and the reverse primer 806rB42 fused with 3–6-mer Ns and the reverse sequencing primer (5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA G [3–6-mer Ns] - [806rB] -3′) (0.2 μM each). To inhibit the PCR-amplification of mitochondrial and chloroplast 16 S rRNA sequences of host plants, specific peptide nucleic acids [mPNA and pPNA41] (0.25 μM each) were added to the reaction mix of KOD FX Neo (Toyobo). To reduce the proportion of host mitochondrial/chloroplast reads to prokaryote sequencing reads through selective amplification, the number of PCR cycles was set to 35. The temperature profile of the PCR was 94 °C for 2 min, followed by 35 cycles at 98 °C for 10 s, 78 °C for 10 s, 60 °C for 30 s, 68 °C for 50 s, and a final extension at 68 °C for 5 min. To prevent generation of chimeric sequences, the ramp rate through the thermal cycles was set to 1 °C/sec.43. Illumina sequencing adaptors were then added to respective samples in the supplemental PCR using the forward fusion primers consisting of the P5 Illumina adaptor, 8-mer indexes for sample identification44 and a partial sequence of the sequencing primer (5′-AAT GAT ACG GCG ACC ACC GAG ATC TAC AC - [8-mer index] - TCG TCG GCA GCG TC-3′) and the reverse fusion primers consisting of the P7 adaptor, 8-mer indexes, and a partial sequence of the sequencing primer (5′-CAA GCA GAA GAC GGC ATA CGA GAT - [8-mer index] - GTC TCG TGG GCT CGG-3′). KOD FX Neo was used with a temperature profile of 94 °C for 2 min, followed by 8 cycles at 98 °C for 10 s, 55 °C for 30 s, 68 °C for 50 s (ramp rate = 1 °C/s), and a final extension at 68 °C for 5 min.
The PCR amplicons of the 135 tomato individuals (and negative control samples) were then pooled after a purification/equalization process with the AMPureXP Kit (Beckman Coulter). Primer dimers were removed from the pooled library by a supplemental AMpureXp purification process, in which the ratio of AMPureXP reagent to the pooled library was set to 0.6 (v/v).
The PCR of the fungal ITS1 region was performed with the forward primer ITS1F_KYO145 fused with 3–6-mer Ns for improved Illumina sequencing quality41 and the forward Illumina sequencing primer (5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG- [3–6-mer Ns] – [ITS1F_KYO1] -3′) and the reverse primer ITS2_KYO245 fused with 3–6-mer Ns and the reverse sequencing primer (5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA G [3–6-mer Ns] - [ITS2_KYO2] -3′). The PCR was performed based on the buffer and polymerase system of KOD FX Neo with a temperature profile of 94 °C for 2 min, followed by 35 cycles at 98 °C for 10 s, 58 °C for 30 s, 68 °C for 50 s, and a final extension at 68 °C for 5 min. Illumina sequencing adaptors and 8-mer index sequences were added in the additional PCR and then the amplicons were purified and pooled as described above.
The sequencing libraries of the prokaryote 16S and fungal ITS regions were processed in an Illumina MiSeq sequencer (run center: KYOTO-HE; 15% PhiX spike-in). In general, quality of forward sequence data is generally higher than that of reverse sequence data in Illumina sequencing. Therefore, we optimized the settings of the Illumina sequencing run by targeting only forward sequences. Specifically, the numbers of the forward and reverse cycles were set 271 and 31, respectively: the reverse sequences were used only for discriminating between 16S and ITS1 sequences in silico based on the sequences of primer positions. Note that similar results of molecular taxonomic assignment have been obtained for 200 bp 16S/ITS sequences and for longer 16S/ITS sequences in a comprehensive benchmark analysis with the bioinformatic pipeline detailed below46.
Bioinformatics
The raw sequencing data were converted into FASTQ files using the Illumina’s program bcl2fastq 1.8.4. The obtained FASTQ files were demultiplexed with the program Claident v0.2.2018.05.2946,47, by which sequencing reads whose 8-mer index positions included nucleotides with low (<30) quality scores were removed. The sequencing data were deposited to DNA Data Bank of Japan (DDBJ) (DDBJ Sequence Read Archive accession: DRA007061). Only forward sequences were used in the following analyses after trimming low-quality 3′-end sequences using Claident: each sequencing read was trimmed to the point at which the quality values of three consecutive nucleotides were 30 or higher. Noisy reads47 were subsequently discarded and then denoised dataset consisting of 1,201,840 16S and 1,730,457 ITS1 reads were obtained.
For each region (16S or ITS1), filtered reads were clustered with a cut-off sequencing similarity of 97% using the program VSEARCH48 as implemented in Claident. The operational taxonomic units (OTUs) representing less than 10 sequencing reads were discarded and then the molecular identification of the remaining OTUs was performed based on the combination of the query-centric auto-k-nearest neighbor (QCauto) algorithm of reference database search46 and the lowest common ancestor (LCA) algorithm of taxonomic assignment49 as implemented in Claident. Note that taxonomic identification results based on the QCauto-LCA pipeline are comparable to, or sometimes more accurate than, those with alternative approaches46,50,51. In total, 143 prokatyote (bacterial or archaeal) OTUs and 529 fungal OTUs were obtained for the 16S and ITS1 regions, respectively (Supplementary Data 1). The UNIX codes used in the above bioinformatic pipeline are provided as Supplementary Data 2.
For each target region (16S or ITS1), we obtained a sample × OTU matrix, in which a cell entry depicted the number of sequencing reads of an OTU in a sample (Supplementary Data 3). To minimize effects of PCR/sequencing errors, cell entries whose read counts represented less than 0.1% of the total read count of each sample were removed (cf. ref.52). The filtered matrix was then rarefied to 500 reads per sample using the “rrarefy” function of the vegan 2.4–5 package53 of R 3.4.354. Samples with less than 500 reads were discarded in this process. In total, the rarefied matrices of the 16S and ITS1 regions included 125 and 132 samples, respectively: at least 13 replicate samples per treatment were retained in both datasets (Supplementary Data 4).
Microbiome structure
Relationship between the number of sequencing reads and that of prokaryote/fungal OTUs was examined for each dataset (16S or ITS1) with the vegan “rarecurve” function of R. Likewise, relationship between the number of samples and that of OTUs was examined with the vegan “specaccum” function. For each dataset, difference in order- or genus-level community compositions among seedling treatments (rootstock varieties) was examined by the permutational analysis of variance (PERMANOVA55) with the vegan “adonis” function (10,000 permutations). To control spatial effects in the field experiment data, the information of replicate sample sets (Fig. 1) was included as an explanatory variable in the PERMANOVA. The “Raup-Crick” metric56 was used to calculate β-diversity based on the order- or genus-level data matrices (Supplementary Data 5).
To explore prokaryote/fungal taxa whose occurrences on tomato individuals were associated with rootstock varieties, a series of analysis of variance (ANOVA) was performed. Specifically, based on the genus-level matrix of the 16S or ITS1 dataset (Supplementary Data 5), an ANOVA model was constructed for each prokaryote/fungal genus by including the proportion of the sequencing reads of the target genus and the rootstock variety information of host tomatoes as response and explanatory variables, respectively. The information of replicate samples (i.e., location information) was included as an additional explanatory variable. Genera that occurred in less than 30 tomato individuals were excluded from the analysis.
Host-genotype preferences
We further explored prokaryote/fungal taxa showing preferences for specific rootstock varieties based on a randomization analysis. In the sample × genus matrix of the 16S or ITS1 dataset (Supplementary Data 5), the labels of rootstock varieties were shuffled (100,000 permutations) and then preference of a prokaryote/fungal genus (i) for a rootstock variety (j) was evaluated as follows:
where Nobserved (i, j) denoted the mean number of the sequencing reads of genus i across rootstock variety j tomato samples in the original data, and the Mean (Nranodomized (i, j)) and SD (Nranodomized (i, j)) were the mean and standard deviation of the number of sequencing reads for the focal genus–rootstock combination across randomized matrices. Genera that occurred in 30 or more tomato individuals were subjected to the randomization analysis.
For the genera that showed preferences for specific tomato rootstock varieties, we performed an additional analysis to evaluate which bacterial/fungal OTUs in each genus had strong host-variety preferences. Specifically, the randomization analysis of the above preference index (100,000 permutations) was applied to rarefied sample × OTU matrix of the 16S or ITS1 dataset (Supplementary Data 4). OTUs that occurred in less than 30 tomato individuals were excluded from the analysis.
Results
Microbiome properties
On average, 13.6 (SD = 4.2) prokaryote and 26.3 (SD = 9.4) fungal OTUs per sample were observed in the rarefied data matrices (Supplementary Fig. 1). The total numbers of prokaryote and fungal OTUs included in the rarefied datasets were 116 and 413, respectively (Supplementary Data 4). All the prokaryote OTUs belonged to Bacteria: no archaeal OTUs were observed.
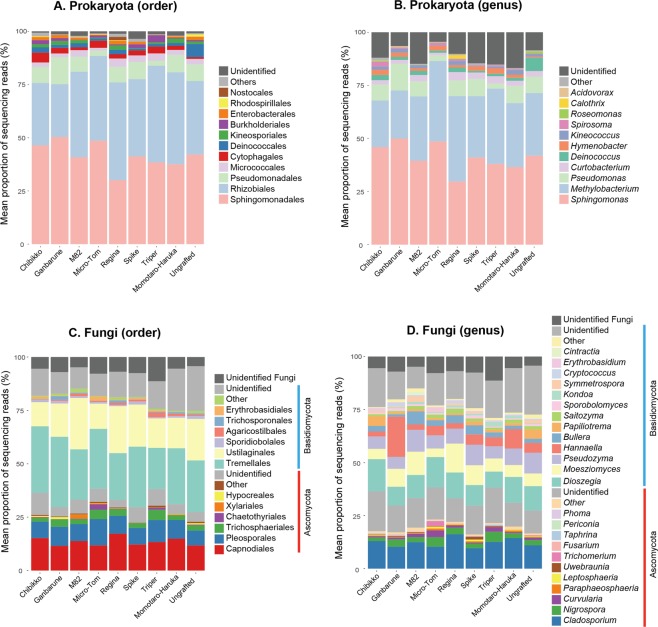
In the bacterial community of the tomato leaves, bacteria in the orders Sphingomonadales and Rhizobiales were dominant (Fig. 2A). Bacteria in the order Pseudomonadales were frequently observed, too, across the tomato varieties examined. Meanwhile, bacteria in the order Deinococcales were abundant only in the ungrafted tomato individuals (Fig. 2A). At the genus-level, the genera Sphingomonas, Methylobacterium, and Pseudomonas were frequently observed across the rootstock varieties examined, while Deinococcus bacteria were abundant only in the ungrafted tomatoes (Fig. 2B).
Figure 2.
Structure of the leaf-associated microbial communities. The leaf-associated microbial community compositions were compared among tomato individuals with different rootstock genotypes. (A) Order-level community structure of prokaryotes. (B) Genus-level community structure of prokaryotes. (C) Order-level community structure of fungi. (D) Genus-level community structure of fungi.
In the leaf-associated fungal community, ascomycete fungi in the orders Capnodiales and Plesporales and the basidiomycete fungi in the orders Tremellales and Ustiaginales were abundant (Fig. 2C). At the genus-level, Cladosporium, Dioszegia, Moesziomyces (anamorph = Pseudozyma), and Hannaella were frequently observed (Fig. 2D). Among them, Hannaella fungi dominated the leaf-associated fungal community of the tomato rootstock variety “Ganbarune” (the proportion of Hannaella reads = 19.0%), while their proportion was relatively low on other host varieties (2.3–9.1%; Fig. 2D).
A statistical test based on PERMANOVA showed that replicate sampling positions, but not tomato rootstock varieties, significantly explained variation in the whole structure of the bacterial/fungal community (Table 1). However, further analyses targeting respective genera (Tables 2 and 3) indicated that the proportion of the fungal genus Hannaella varied among tomato rootstock varieties, although the pattern was non-significant after a Bonferroni correction of P values. Meanwhile, the proportion of some taxa such as the bacterial genus Sphingomonas and the fungal genus Cladosporium varied significantly among replicates (Tables 2 and 3), suggesting that spatial positions in the experimental field affected the formation of the leaf-associated microbiomes of the tomato plants.
Table 1.
Effects of rootstock varieties and spatial positions on the entire microbial community structure.
| Taxon | Taxonomic level | Variable | df | F model | R2 | P |
|---|---|---|---|---|---|---|
| Prokaryotes | Order | Variety | 8 | 1.0 | 0.061 | 0.4731 |
| Location | 14 | 1.6 | 0.173 | 0.0379 | ||
| Genus | Variety | 8 | 1.1 | 0.064 | 0.3733 | |
| Location | 14 | 2.1 | 0.207 | 0.0035 | ||
| Fungi | Order | Variety | 8 | 0.6 | 0.033 | 0.7509 |
| Location | 14 | 2.2 | 0.213 | 0.0119 | ||
| Genus | Variety | 8 | 0.9 | 0.050 | 0.5586 | |
| Location | 14 | 1.9 | 0.185 | 0.0350 |
A PERMANOVA was conducted for each target community (prokaryotes or fungi) at each taxonomic level (order or genus). The rootstock varieties of host tomato and spatial positions in the field (location; Fig. 1A) were considered as explanatory variables.
Table 2.
Effects of rootstock varieties and spatial positions on the proportion of each prokaryote genus in the community data.
| Genus | Variety | Location | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Curtobacterium | 8 | 0.3 | 0.9710 | 14 | 1.1 | 0.3260 |
| Deinococcus | 8 | 1.8 | 0.0944 | 14 | 1.3 | 0.2386 |
| Hymenobacter | 8 | 0.5 | 0.8730 | 14 | 1.1 | 0.3900 |
| Kineococcus | 8 | 0.7 | 0.6710 | 14 | 0.6 | 0.8970 |
| Methylobacterium | 8 | 1.7 | 0.0986 | 14 | 2.0 | 0.0229 |
| Pseudomonas | 8 | 1.7 | 0.1060 | 14 | 0.6 | 0.8490 |
| Sphingomonas | 8 | 2.0 | 0.0538 | 14 | 3.2 | 0.0004 |
| Spirosoma | 8 | 1.0 | 0.4230 | 14 | 1.0 | 0.4310 |
For each prokaryote genus, an ANOVA model of the mean proportion of sequencing reads was constructed by including the rootstock varieties of host tomato and spatial positions in the field (location; Fig. 1A) as explanatory variables. Genera that occurred in 30 or more tomato individuals were subjected to the analysis.
Table 3.
Effects of rootstock varieties and spatial positions on the proportion of each fungal genus in the community data.
| Genus | Variety | Location | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Bullera | 8 | 0.8 | 0.5740 | 14 | 1.0 | 0.4570 |
| Cladosporium | 8 | 0.7 | 0.6752 | 14 | 2.4 | 0.0051 |
| Cryptococcus | 8 | 1.1 | 0.3830 | 14 | 1.0 | 0.4620 |
| Curvularia | 8 | 1.3 | 0.2640 | 14 | 0.8 | 0.6470 |
| Dioszegia | 8 | 0.4 | 0.9390 | 14 | 1.1 | 0.3670 |
| Hannaella | 8 | 2.3 | 0.0281 | 14 | 0.8 | 0.7046 |
| Kondoa | 8 | 1.0 | 0.4730 | 14 | 0.8 | 0.6720 |
| Leptosphaeria | 8 | 1.1 | 0.3660 | 14 | 1.4 | 0.1820 |
| Moesziomyces | 8 | 1.5 | 0.1507 | 14 | 1.6 | 0.0833 |
| Nigrospora | 8 | 0.7 | 0.7050 | 14 | 1.2 | 0.3240 |
| Papiliotrema | 8 | 1.5 | 0.1720 | 14 | 0.7 | 0.7450 |
| Paraphaeosphaeria | 8 | 0.7 | 0.6570 | 14 | 1.0 | 0.4990 |
| Pseudozyma | 8 | 0.5 | 0.8690 | 14 | 0.5 | 0.9500 |
| Saitozyma | 8 | 0.2 | 0.9800 | 14 | 1.1 | 0.3890 |
| Sporobolomyces | 8 | 0.5 | 0.8504 | 14 | 1.8 | 0.0475 |
For each fungal genus, an ANOVA model of the mean proportion of sequencing reads was constructed by including the rootstock varieties of host tomato and spatial positions in the field (location; Fig. 1A) as explanatory variables. Genera that occurred in 30 or more tomato individuals were subjected to the analysis.
Host-genotype preferences
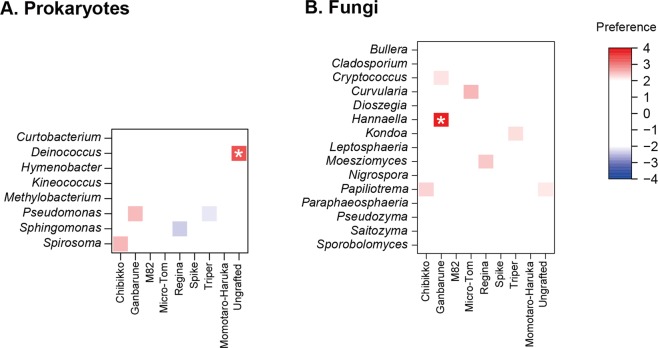
A randomization analysis showed that the bacterial genus Deinococcus occurred preferentially on the ungrafted tomato individuals (Fig. 3A; Table 4). Likewise, the fungal genus Hannaella showed preferences for the rootstock variety “Ganbarune” (Fig. 3B). In an additional randomization analysis, a bacterial OTU phylogenetically allied to Deinococcus citri (P_040) and fungal OTUs allied to Hannaella oryzae (F_427 and F_428) displayed preferences for ungrafted and “Ganbarune” tomato plants, respectively (Table 4).
Figure 3.
Randomization analysis of preferences for rootstock varieties. An asterisk indicates significant preference index score in a combination of a microbial genus and a host rootstock variety (Bonferroni correction for each OTU compared across nine rootstock conditions; α = 0.05). (A) Prokatyote genera. (B) Fungal genera.
Table 4.
Prokaryote and fungal OTUs showing statistically significant preferences for tomato rootstock varieties. An asterisk indicates a significant preference for a rootstock condition (Bonferroni correction for each OTU compared across nine rootstock conditions; α = 0.05).
| OTU | Preferred variety | Phylum | Class | Order | Family | Genus | NCBI Blast top hit | Accession | Cover | Identity |
|---|---|---|---|---|---|---|---|---|---|---|
| Prokaryotes | ||||||||||
| P_040 | Ungrafted (P = 0.00321*) | Deinococcus-Thermus | Deinococci | Deinococcales | Deinococcaceae | Deinococcus | Deinococcus citri | LT602922 | 100% | 100% |
| Fungi | ||||||||||
| F_427 | Ganbarune (P = 0.00078*) | Basidiomycota | Tremellomycetes | Tremellales | Bulleribasidiaceae | Hannaella | Hannaella oryzae | KY103504 | 89% | 99% |
| F_428 | Ganbarune (P = 0.00099*) | Basidiomycota | Tremellomycetes | Tremellales | Bulleribasidiaceae | Hannaella | Hannaella oryzae | KY103504 | 89% | 99% |
Discussion
The field experiment using eight tomato rootstock varieties suggested that below-ground plant genotypes did not significantly affect the entire structures of the leaf-associated microbiomes (Table 1). However, detailed analyses suggested the existence of leaf microbial taxa whose associations with host plants might be affected by below-ground plant genotypes (Figs 2 and 3; Tables 2–4). Thus, along with recent studies on tomato-associated microbiomes28,57, this study provides a starting point for evaluating to what extent leaf-endophytic microbiome structures of grafted crop plants are affected/unaffected by rootstock varieties.
The leaf-associated bacterial communities of the tomato individuals analyzed in this study were dominated by Alphaproteobacteria (e.g., Sphingomonas and Methylobacterium) as well as Gammaproteobacteria (e.g., Pseudomonas) as has been reported in previous studies on crop and non-crop plants1,2,58 (Fig. 2). Among the dominant bacteria, Pseudomonas is recognized mainly as plant pathogenic taxon59,60, although some Pseudomonas species are known to suppress leaf fungal pathogens by producing antibiotics61,62. The genus Sphingomonas is known to involve species that protect host plants against Pseudomonas pathogens63,64 or promote plant growth by producing phytohormones such as gibberellins and indole acetic acid65. Bacteria in the genus Methylobacterium are often localized around stomatal pores on leaves66, using plant-derived methanol as principal carbon source67–70. Genomic studies have shown that Methylobacterium genomes involve genes of metabolic pathways that potentially contribute to host plant growth (e.g., auxin biosysnthesis, cytokine biosynthesis, and vitamin B12 biosynthesis)71. Methylobacterium is also known to induce resistance of plants against fungal pathogens, nominated as prospective a biocontrol agent72. Thus, these dominant bacteria, whose associations with hosts are likely irrespective of host below-ground genotypes (Fig. 2), may affect physiological conditions of tomato plants both positively and negatively.
Our data also indicated that fungi in the ascomycete genus Cladosporium and the basidiomycete genera Dioszegia and Moesziomyces (anamorph = Pseudozyma) were abundant within the leaf-associated microbiome of tomato plants (Fig. 2). Among them, Cladosporium involves a well-characterized pathogenic species, C. fulvum, which causes tomato leaf mold73–76. The basidiomycete taxa listed above are characterized by their anamorphic yeast forms and they have been observed in leaves of various plant species77–80. For example, Dioszegia, a basidiomycete taxon in the order Tremellales, has been reported from cereal and Arabidopsis80,81, potentially playing key roles within microbe–microbe interaction webs in leaf-associated microbiomes11. The genus Moesziomyces is represented by plant-pathogenic smut fungi82. However, a recent phylogenetic study of teleomorphic (Moesziomyces) and anamorphic (Pseudozyma) specimens77 suggested that this Ustilaginaceae taxon could involve not only phytopathogenic species but also species with antifungal properties against the causal agent of cucumber powdery mildew (Podosphaera fuliginea)83 or species that can induce resistance of host plants against fungal pathogens such as Botrytis cinerea84. Thus, the community data, as a whole, suggest that not only dominant bacterial taxa but also various fungal taxa potentially play complex physiological roles in tomato leaves.
While there were bacterial and fungal taxa commonly associated with tomato plants irrespective of host below-ground genotypes, fungi in the genus Hannaella displayed signs of preferences for rootstock genotypes (Fig. 3; Tables 3 and 4). Specifically, Hannaella was the most abundant fungal taxon in the tomato individuals whose rootstock genotype was “Ganbarune” (Fig. 2). Like other yeast taxa in Tremellaceae (e.g., Derxomyces and Dioszegia)85, Hannaella yeasts are frequently observed in the phyllosphere of various plant species86–89. Some Hannaella species are known to produce indol acetic acid87,90, although a study has suggested that the yeasts do not necessarily promote plant growth90. Therefore, it remains a challenge to understand how Hannaella yeasts interact with other yeasts and bacterial/fungal species in/on plant leaves and how they influence plant performance host-genotype specifically.
The randomization analysis performed in this study also implied that a bacterial OTU, which are phylogenetically allied to the Deinococcus species isolated from leaf canker lesions of citrus trees91, was preferentially associated with ungrafted tomato individuals (Fig. 3; Tables 2 and 4). Given that this bacterial OTU was rarely observed in self-grafted tomato individuals (Fig. 2), grafting treatment per se, rather than plant genotypes, could be responsible for the biased distribution of the bacterium. This finding is of particular interest because Deinococcus is famous for its high tolerance to desiccation92,93. Potential physiological effects of this bacteria on host plants deserve some inoculation experiments.
Although this study provides some implications for how leaf-associated microbiomes of grafted plants can be influenced by rootstock genotypes, potential pitfalls of the present results should be taken into account. First, as our data were based on snapshot sampling in the late growing season of tomato, we are unable to infer the timing at which the observed bacteria and fungi colonized the tomato leaves. Therefore, some of the detected bacterial and fungal OTUs might colonize the tomato individuals before they were transplanted into the experimental field. However, given that spatial positions within the field had significant effects on the microbial community structures (Table 1), colonization of indigenous (resident) microbes in the field could be a major factor determining the observed microbiome pattern. For more comprehensive understanding, time-series sampling throughout the growing season of tomato should be conducted in multiple field sites differing in biotic/abiotic environmental conditions. Second, we need to acknowledge that microbiome profiling with high-throughput DNA sequencing per se does not reveal the fine-scale distribution of the detected microbial OTUs in/on plant leaves. Although we tried to surface-sterilize the leaf samples, the microbiome data involved not only possibly endophytic taxa but also bacteria and fungi that have been regarded as epiphytes (e.g., Methylobacterium)66,94 (but see ref.95). Microscopic analyses with taxon-specific fluorescent probes, for example, will provide essential insights into the localization of the observed microbes in/on leaves. Third, while this study was designed to examine effects of below-ground genotypes on above-ground parts of grafted plants, recent studies have shown that genetic materials (i.e., DNA) can be transported between scion and rootstock tissue, at least at graft junction region, in a grafted plant96. Thus, contributions of above-/below-ground genotypes to root/leaf microbiomes may be much more complex than had been assumed in this study.
Overall, this study suggested that majority of leaf-associated microbes can colonize grafted tomato plants irrespective of rootstock genotypes of their hosts. Meanwhile, leaf-associated microbial taxa may display preferences for grafted/ungrafted plants or specific host rootstock varieties. Both grafting and the use of plant-beneficial microbes have been regarded as prospective options for securing agricultural/forestry production in the era of increasing biotic and abiotic environmental stresses12,13,29,34. Further integrative studies will help us explore best conditions in which grafting and microbiome technologies are merged into a solid basis of stable and sustainable agricultural practices.
Supplementary information
Acknowledgements
We thank Center for Ecological Research, Kyoto University for the permission of research, Satomi Yoshinami, Akira Matsumoto, Tomoaki Muranaka, Sarasa Amma, and Hiroki Kawai for their support in field experiment and/or molecular experiments, and Makoto Nakaune for detailed information about tomato cultivars and National BioResource Project (NBRP; http://nbrp.jp/) for a part of plant materials. We are also grateful to anonymous reviewers for their productive comments that improved the manuscript. This work was financially supported by JST PRESTO (JPMJPR16Q6) to H.T. and MAFF science and technology research promotion program for agriculture, forestry, fisheries and food industry grant (16770567) and JST PRESTO (JPMJPR15O3) to M.N.
Author Contributions
H.T. and M.N. designed the work. H.T., K.O., and M.N. performed experiments. H.T. analyzed data. H.T. wrote the paper with M.N. All authors read and approved the manuscript.
Data Availability
The raw DNA sequencing data are deposited on the DDBJ Sequence Read Archive (accession: DRA007061).The data matrices used in the statistical analyses are provided as Supplementary Data 1–5.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38344-2.
References
- 1.Bai Y, et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature. 2015;528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- 2.Vorholt JA. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012;10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 3.Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016;14:434–447. doi: 10.1038/nrmicro.2016.59. [DOI] [PubMed] [Google Scholar]
- 4.Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 5.Busby PE, et al. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLOS Biol. 2017;15:e2001793. doi: 10.1371/journal.pbio.2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold AE, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendes R, et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 8.Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A. The importance of the microbiome of the plant holobiont. New Phytol. 2015;206:1196–1206. doi: 10.1111/nph.13312. [DOI] [PubMed] [Google Scholar]
- 9.Callaway E. Devastating wheat fungus appears in Asia for first time. Nature. 2016;532:421–422. doi: 10.1038/532421a. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PK, et al. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Agler MT, et al. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLOS Biol. 2016;14:e1002352. doi: 10.1371/journal.pbio.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlaeppi K, Bulgarelli D. The plant microbiome at work. Mol. Plant-Microbe Int. 2015;28:212–217. doi: 10.1094/MPMI-10-14-0334-FI. [DOI] [PubMed] [Google Scholar]
- 13.Toju H, et al. Core microbiomes for sustainable agroecosystems. Nat. Plants. 2018;4:247–257. doi: 10.1038/s41477-018-0139-4. [DOI] [PubMed] [Google Scholar]
- 14.Vorholt JA, Vogel C, Carlstrom CI, Muller DB. Establishing Causality: Opportunities of Synthetic Communities for Plant Microbiome Research. Cell Host Microbe. 2017;22:142–155. doi: 10.1016/j.chom.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Bodenhausen N, Bortfeld-Miller M, Ackermann M, Vorholt JA. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLOS Genetics. 2014;10:e1004283. doi: 10.1371/journal.pgen.1004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whipps J, Hand P, Pink D, Bending GD. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008;105:1744–1755. doi: 10.1111/j.1365-2672.2008.03906.x. [DOI] [PubMed] [Google Scholar]
- 17.Bulgarelli D, et al. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe. 2015;17:392–403. doi: 10.1016/j.chom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards J, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA. 2015;112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collard BC, Mackill DJ. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Phil. Trans. R. Soc. B. 2008;363:557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean R, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genetics. 2010;11:539. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 22.Hiruma K, et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell. 2016;165:464–474. doi: 10.1016/j.cell.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castrillo G, et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature. 2017;543:513–518. doi: 10.1038/nature21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt EE. Plant grafting: new mechanisms, evolutionary implications. Front. Plant Sci. 2014;5:727. doi: 10.3389/fpls.2014.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Notaguchi M, Okamoto S. Dynamics of long-distance signaling via plant vascular tissues. Front. Plant. Sci. 2015;6:161. doi: 10.3389/fpls.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi F, et al. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature. 2018;556:235. doi: 10.1038/s41586-018-0009-2. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, et al. Apple endophytic microbiota of different rootstock/scion combinations suggests a genotype-specific influence. Microbiome. 2018;6:18. doi: 10.1186/s40168-018-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poudel, R. et al. Rootstocks shape the rhizobiome: Rhizosphere and endosphere bacterial communities in the grafted tomato system. Appl. Environ. Microbiol., AEM. 01765–01718 (2018). [DOI] [PMC free article] [PubMed]
- 29.Warschefsky EJ, et al. Rootstocks: diversity, domestication, and impacts on shoot phenotypes. Trends Plant. Sci. 2016;21:418–437. doi: 10.1016/j.tplants.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Khah E, Kakava E, Mavromatis A, Chachalis D, Goulas C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hort. 2006;8:3–7. [Google Scholar]
- 31.Flores FB, et al. The effectiveness of grafting to improve tomato fruit quality. Scientia Horticulturae. 2010;125:211–217. doi: 10.1016/j.scienta.2010.03.026. [DOI] [Google Scholar]
- 32.Martinez-Rodriguez MM, et al. The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Env. Exp. Bot. 2008;63:392–401. doi: 10.1016/j.envexpbot.2007.12.007. [DOI] [Google Scholar]
- 33.Rivard CL, O’Connell S, Peet MM, Welker RM, Louws FJ. Grafting tomato to manage bacterial wilt caused by Ralstonia solanacearum in the southeastern United States. Plant Disease. 2012;96:973–978. doi: 10.1094/pdis-12-10-0877. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz D, Rouphael Y, Colla G, Venema JH. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Scientia Hort. 2010;127:162–171. doi: 10.1016/j.scienta.2010.09.016. [DOI] [Google Scholar]
- 35.Ruiz JM, Romero L. Nitrogen efficiency and metabolism in grafted melon plants. Scientia Hort. 1999;81:113–123. doi: 10.1016/s0304-4238(98)00200-3. [DOI] [Google Scholar]
- 36.Martinez-Ballesta MC, Alcaraz-Lopez C, Muries B, Mota-Cadenas C, Carvajal M. Physiological aspects of rootstock-scion interactions. Scientia Hort. 2010;127:112–118. doi: 10.1016/j.scienta.2010.08.002. [DOI] [Google Scholar]
- 37.Song F, et al. The scion/rootstock genotypes and habitats Affect arbuscular mycorrhizal fungal community in citrus. Front. Microbiol. 2015;6:1372. doi: 10.3389/fmicb.2015.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling N, et al. The response of root-associated bacterial community to the grafting of watermelon. Plant Soil. 2015;391:253–264. doi: 10.1007/s11104-015-2399-3. [DOI] [Google Scholar]
- 39.Marasco R, Rolli E, Fusi M, Michoud G, Daffonchio D. Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality. Microbiome. 2018;6:3. doi: 10.1186/s40168-017-0391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundberg DS, Yourstone S, Mieczkowski P, Jones CD, Dangl JL. Practical innovations for high-throughput amplicon sequencing. Nat. Methods. 2013;10:999–1002. doi: 10.1038/nmeth.2634. [DOI] [PubMed] [Google Scholar]
- 42.Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015;75:129–137. doi: 10.3354/ame01753. [DOI] [Google Scholar]
- 43.Stevens JL, Jackson RL, Olson JB. Slowing PCR ramp speed reduces chimera formation from environmental samples. J. Microbiol. Methods. 2013;93:203–205. doi: 10.1016/j.mimet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods. 2008;5:235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toju H, Tanabe AS, Yamamoto S, Sato H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLOS ONE. 2012;7:e40863. doi: 10.1371/journal.pone.0040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanabe AS, Toju H. Two new computational methods for universal DNA barcoding: a benchmark using barcode sequences of bacteria, archaea, animals, fungi, and land plants. PLOS ONE. 2013;8:e76910. doi: 10.1371/journal.pone.0076910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanabe, A. S. Claident v0.2. 2018.05.29, a software distributed by author at, http://www.fifthdimension.jp/ (2018).
- 48.Rognes, T., Mahé, F., Flouri, T., Quince, C. & Nichols, B. Vsearch: program available at, https://github.com/torognes/vsearch (2014). [DOI] [PMC free article] [PubMed]
- 49.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toju H, Tanabe A, Ishii H. Ericaceous plant–fungus network in a harsh alpine–subalpine environment. Mol. Ecol. 2016;25:3242–3257. doi: 10.1111/mec.13680. [DOI] [PubMed] [Google Scholar]
- 51.Toju H, Yamamoto S, Tanabe AS, Hayakawa T, Ishii HS. Network modules and hubs in plant-root fungal biome. J. R. Soc. Interface. 2016;13:20151097. doi: 10.1098/rsif.2015.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peay KG, et al. Lack of host specificity leads to independent assortment of dipterocarps and ectomycorrhizal fungi across a soil fertility gradient. Ecol. Lett. 2015;18:807–816. doi: 10.1111/ele.12459. [DOI] [PubMed] [Google Scholar]
- 53.Oksanen, J. et al. Vegan: community ecology package. v. 2.4–5, a software available at, https://github.com/vegandevs/vegan (2017).
- 54.R 3.4.3: A language and environment for statistical computing available at, http://www.R-project.org/ (R Foundation for Statistical Computing, Vienna, Austri, 2017).
- 55.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 56.Chase JM, Kraft NJ, Smith KG, Vellend M, Inouye BD. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere. 2011;2:1–11. doi: 10.1890/ES10-00117.1. [DOI] [Google Scholar]
- 57.Kwak M-J, et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nature Biotech. 2018;36:1100. doi: 10.1038/nbt.4232. [DOI] [PubMed] [Google Scholar]
- 58.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl. Env. Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buell CR, et al. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA. 2003;100:10181–10186. doi: 10.1073/pnas.1731982100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X, et al. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc. Natl. Acad. Sci. USA. 2013;110:E425–E434. doi: 10.1073/pnas.1221892110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Meyer G, Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 62.Flaishman MA, Eyal Z, Zilberstein A, Voisard C, Haas D. Suppression of Septoria tritici blotch and leaf rust of wheat by recombinant cyanide-producing strains of Pseudomonas putida. Mol. Plant Microb. Int. 1996;9:642–645. doi: 10.1094/Mpmi-9-0642. [DOI] [Google Scholar]
- 63.Innerebner G, Knief C, Vorholt JA. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Env. Microbiol. 2011;77:3202–3210. doi: 10.1128/AEM.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel C, Innerebner G, Zingg J, Guder J, Vorholt JA. Forward genetic in planta screen for identification of plant-protective traits of Sphingomonas sp. strain Fr1 against Pseudomonas syringae DC3000. Appl. Env. Microbiol. 2012;78:5529–5535. doi: 10.1128/AEM.00639-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan AL, et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014;52:689–695. doi: 10.1007/s12275-014-4002-7. [DOI] [PubMed] [Google Scholar]
- 66.Abanda-Nkpwatt D, Müsch M, Tschiersch J, Boettner M, Schwab W. Molecular interaction between Methylobacterium extorquens and seedlings: growth promotion, methanol consumption, and localization of the methanol emission site. J. Exp. Bot. 2006;57:4025–4032. doi: 10.1093/jxb/erl173. [DOI] [PubMed] [Google Scholar]
- 67.Schauer S, Kutschera U. A novel growth-promoting microbe, Methylobacterium funariae sp. nov., isolated from the leaf surface of a common moss. Plant Signal. Behav. 2011;6:510–515. doi: 10.4161/psb.6.4.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryffel F, et al. Metabolic footprint of epiphytic bacteria on Arabidopsis thaliana leaves. ISME J. 2016;10:632–643. doi: 10.1038/ismej.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delmotte N, et al. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA. 2009;106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knief C, et al. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 2012;6:1378–1390. doi: 10.1038/ismej.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwak M-J, et al. Genome information of Methylobacterium oryzae, a plant-probiotic methylotroph in the phyllosphere. PLOS ONE. 2014;9:e106704. doi: 10.1371/journal.pone.0106704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madhaiyan M, et al. Plant growth–promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Current Microbiol. 2006;53:270–276. doi: 10.1007/s00284-005-0452-9. [DOI] [PubMed] [Google Scholar]
- 73.Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones J. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994;266:789–793. doi: 10.1126/science.7973631. [DOI] [PubMed] [Google Scholar]
- 74.De Wit PJ, Spikman G. Evidence for the occurrence of race and cultivar-specific elicitors of necrosis in intercellular fluids of compatible interactions of Cladosporium fulvum and tomato. Physiol. Plant Pathol. 1982;21:1–11. doi: 10.1016/0048-4059(82)90002-9. [DOI] [Google Scholar]
- 75.van Kan JA, Van den Ackerveken G, De Wit P. Cloning and characterization of cDNA of avirulence gene avr9 of the fungal pathogen Cladosporium fulvum, causal agent of tomato leaf mold. Mol. Plant Microb. Int. 1991;4:52–59. doi: 10.1094/MPMI-4-052. [DOI] [PubMed] [Google Scholar]
- 76.Rivas S, Thomas CM. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum. Annu. Rev. Phytopathol. 2005;43:395–436. doi: 10.1146/annurev.phyto.43.040204.140224. [DOI] [PubMed] [Google Scholar]
- 77.Kruse J, Doehlemann G, Kemen E, Thines M. Asexual and sexual morphs of Moesziomyces revisited. IMA Fungus. 2017;8:117–129. doi: 10.5598/imafungus.2017.08.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inácio J, Portugal L, Spencer-Martins I, Fonseca Á. Phylloplane yeasts from Portugal: seven novel anamorphic species in the Tremellales lineage of the Hymenomycetes (Basidiomycota) producing orange-coloured colonies. FEMS Yeast Res. 2005;5:1167–1183. doi: 10.1016/j.femsyr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Karlsson I, Friberg H, Steinberg C, Persson P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLOS ONE. 2014;9:e111786. doi: 10.1371/journal.pone.0111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sapkota R, Knorr K, Jørgensen LN, O’Hanlon KA, Nicolaisen M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015;207:1134–1144. doi: 10.1111/nph.13418. [DOI] [PubMed] [Google Scholar]
- 81.Wang K, Sipilä T, Overmyer K. The isolation and characterization of resident yeasts from the phylloplane of Arabidopsis thaliana. Sci. Rep. 2016;6:39403. doi: 10.1038/srep39403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diagne-Leye G, et al. The life cycle of the smut fungus Moesziomyces penicillariae is adapted to the short-cycle of the host, Pennisetum glaucum. Fungal Biol. 2013;117:311–318. doi: 10.1016/j.funbio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Avis T, Caron S, Boekhout T, Hamelin R, Bélanger R. Molecular and physiological analysis of the powdery mildew antagonist Pseudozyma flocculosa and related fungi. Phytopathology. 2001;91:249–254. doi: 10.1094/PHYTO.2001.91.3.249. [DOI] [PubMed] [Google Scholar]
- 84.Buxdorf K, Rahat I, Gafni A, Levy M. The epiphytic fungus Pseudozyma aphidis induces jasmonic acid-and salicylic acid/nonexpressor of PR1-independent local and systemic resistance. Plant physiology. 2013;161:2014–2022. doi: 10.1104/pp.112.212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang QM, Bai FY. Molecular phylogeny of basidiomycetous yeasts in the Cryptococcus luteolus lineage (Tremellales) based on nuclear rRNA and mitochondrial cytochrome b gene sequence analyses: proposal of Derxomyces gen. nov. and Hannaella gen. nov., and description of eight novel Derxomyces species. FEMS Yeast Res. 2008;8:799–814. doi: 10.1111/j.1567-1364.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 86.Nasanit R, Krataithong K, Tantirungkij M, Limtong S. Assessment of epiphytic yeast diversity in rice (Oryza sativa) phyllosphere in Thailand by a culture-independent approach. Antonie Van Leeuwenhoek. 2015;107:1475–1490. doi: 10.1007/s10482-015-0442-2. [DOI] [PubMed] [Google Scholar]
- 87.Nutaratat P, Srisuk N, Arunrattiyakorn P, Limtong S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014;118:683–694. doi: 10.1016/j.funbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Nasanit R, Jaibangyang S, Tantirungkij M, Limtong S. Yeast diversity and novel yeast D1/D2 sequences from corn phylloplane obtained by a culture-independent approach. Antonie van Leeuwenhoek. 2016;109:1615–1634. doi: 10.1007/s10482-016-0762-x. [DOI] [PubMed] [Google Scholar]
- 89.Kaewwichian R, Jindamorakot S, Am-In S, Sipiczki M, Limtong S. Hannaella siamensis sp. nov. and Hannaella phetchabunensis sp. nov., two new anamorphic basidiomycetous yeast species isolated from plants. Int. J. Syst. Evol. Microbiol. 2015;65:1297–1303. doi: 10.1099/ijs.0.000101. [DOI] [PubMed] [Google Scholar]
- 90.Sun P-F, et al. Indole-3-acetic acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PLOS ONE. 2014;9:e114196. doi: 10.1371/journal.pone.0114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmed I, et al. Deinococcus citri sp. nov., isolated from citrus leaf canker lesions. Int. J. Syst. Evol. Microbiol. 2014;64:4134–4140. doi: 10.1099/ijs.0.066555-0. [DOI] [PubMed] [Google Scholar]
- 92.Mattimore V, Battista JR. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanaka M, et al. Analysis of Deinococcus radiodurans’s transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004;168:21–33. doi: 10.1534/genetics.104.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omer ZS, Tombolini R, Gerhardson B. Plant colonization by pink-pigmented facultative methylotrophic bacteria (PPFMs) FEMS Microbiol. Ecol. 2004;47:319–326. doi: 10.1016/S0168-6496(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 95.Jourand P, et al. Methylobacterium nodulans sp. nov., for a group of aerobic, facultatively methylotrophic, legume root-nodule-forming and nitrogen-fixing bacteria. Int. J. Syst. Evol. Microbiol. 2004;54:2269–2273. doi: 10.1099/ijs.0.02902-0. [DOI] [PubMed] [Google Scholar]
- 96.Stegemann S, Bock R. Exchange of genetic material between cells in plant tissue grafts. Science. 2009;324:649–651. doi: 10.1126/science.1170397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw DNA sequencing data are deposited on the DDBJ Sequence Read Archive (accession: DRA007061).The data matrices used in the statistical analyses are provided as Supplementary Data 1–5.