Abstract
Despite their high prevalence, lack of understanding of the exact pathophysiology of the functional gastrointestinal disorders has restricted us to symptomatic diagnostic tools and therapies. Complex mechanisms underlying the disturbances in the bidirectional communication between the gastrointestinal tract and the brain have a vital role in the pathogenesis and are key to our understanding of the disease phenomenon. Although we have come a long way in our understanding of these complex disorders with the help of studies on animals especially rodents, there need to be more studies in humans, especially to identify the therapeutic targets. This review study looks at the anatomical features of the gut-brain axis in order to discuss the different factors and underlying molecular mechanisms that may have a role in the pathogenesis of functional gastrointestinal disorders. These molecules and their receptors can be targeted in future for further studies and possible therapeutic interventions. The article also discusses the potential role of artificial intelligence and machine learning and its possible role in our understanding of these scientifically challenging disorders.
Keywords: Functional gastrointestinal disorders, Idiopathic bowel syndrome, Gut-brain axis, Microbiome-gut-brain axis, Machine learning, Artificial intelligence
Core tip: The multifactorial nature of functional gastrointestinal disorders makes the diagnosis challenging. The identification of pathogenic microbiome signatures, combined with demographical, immunologic and neuroimaging findings can be encoded into machine learning algorithms which may help identify trends and patterns that can be studied to further our understanding of these disorders. These patterns can help determine the causality or can guide further research.
INTRODUCTION
Functional gastrointestinal disorders (FGIDs) are a highly prevalent group of disorders diagnosed solely by symptomatology as there is a lack of understanding of the underlying structural or chemical abnormalities. The main symptoms described by patients with FGIDs include abdominal pain, dyspepsia, regurgitation, bloating, constipation, diarrhea, incontinence, problems in the passage of food or stool, or any combination of these symptoms. Different mechanisms have been understood to play a role in pathogenesis including disturbance in motility, altered mucosal and immune function, visceral hypersensitivity, disturbance in gut microbiota, and altered processing of visceral signals in the central nervous system (CNS). Common FGIDs include gastroesophageal reflux disease (GERD), functional dysphagia, functional dyspepsia, gastroparesis, irritable bowel syndrome (IBS), functional constipation, diarrhea, and fecal Incontinence.
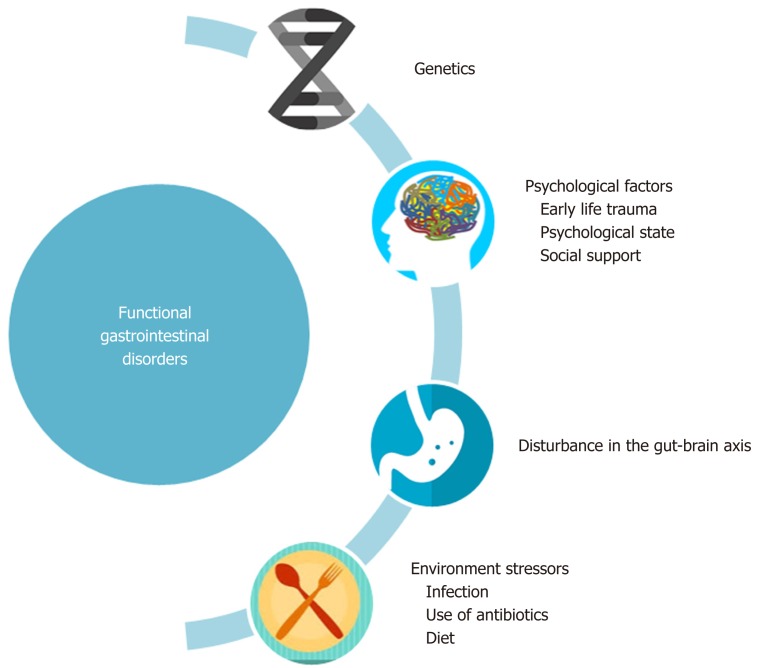
It has been well established that patients with FGIDs, along with having symptoms related to the gastrointestinal tract, have co-existing psychosocial symptoms such as stress, anxiety and depression and thus a biopsychosocial model has been proposed for FGIDs as depicted in Figure 1[1]. The bidirectional communication pathways between the gut and the brain, involved in the pathogenesis of FGIDs, are collectively known as the gut-brain axis[2]. This communication occurs through a number of neuronal pathways and is modified by environmental and anatomical factors such as hypothalamus-pituitary axis, limbic system, autonomic nervous system, and endocrine system. The gut microbiota has recently emerged as a possible influencer of the axis and has seized the much-needed attention of researchers.
Figure 1.
The biopsychosocial model for functional gastrointestinal disorder. The figure illustrates the interaction of psychosocial factors, environmental factors and disturbances in gut-brain axis with functional GI disorders. Early life stress events combined with psychosocial state of an individual determines the symptomatology and quality of life of individuals. Adopted from ROME IV[1].
Although it has been a widely held belief that human cells are outnumbered by microorganisms by a ratio of 1:10 recent literature shows that the ratio is closer to1:1[3]. This, however, does not diminish the important role of microbiota in our bodies. The microbiota, living in harmony with the human tissues, has a number of synergistic roles. Although the exact composition of the microbiota may differ among individuals as each individual has their own microbiome signature, its functional role in homeostasis and development is ubiquitous to all humans. From helping in digestion, to protecting against pathogenic microorganisms, the gut microbiota has played an important role in maintaining immunity and homeostasis. Recently, studies have shown that one of the main inputs to the gut-brain axis comes from microbiota, leading to the coining of the term ‘microbiome-gut-brain axis’[4].
This article reviews the important anatomical aspects of the gut-brain axis in order to discuss the underlying molecular mechanisms that may have a role in the pathogenesis of FGIDs. It further discusses the different factors that may influence the gut-brain axis and can be used in the future as possible therapeutic targets. The article also looks at the potential new approaches such as the use of artificial intelligence and machine learning to help advance our understanding of these complex disorders.
ANATOMICAL CORRELATES OF GUT AND BRAIN CONNECTIONS IN RELATION TO FGIDS
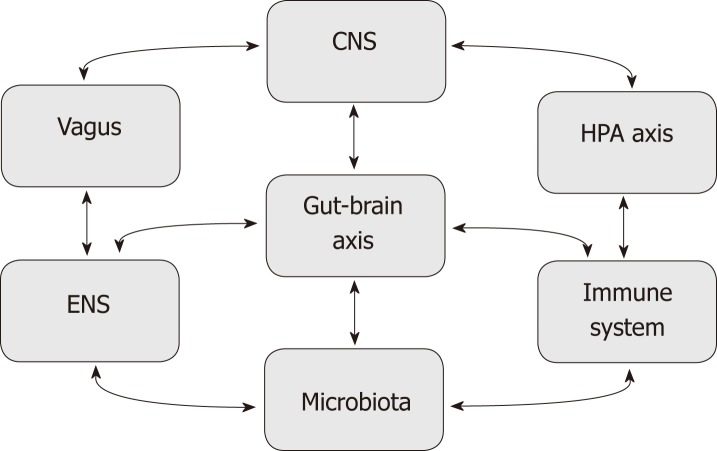
The brain and gut communicate continuously through a number of complex pathways involving the enteric nervous system (ENS), the autonomic nervous system (ANS), the hypothalamus-pituitary axis (HPA), and the central nervous system (CNS) as shown in Figure 2[2]. Each pathway is highly integrated and regulated by interrelational neuronal and neurohumoral factors.
Figure 2.
Schematic representation of different factors modulating the gut-brain axis. The microbiota and central nervous system interact in a bidirectional relationship bridged by the gut-brain axis. This axis is also influenced by Immune system, enteric nervous system, hypothalamic-pituitary axis, and vagus nerve. CNS: Central nervous system; ENS: Enteric nervous system; HPA: Hypothalamic-pituitary.
FGIDS: Enteric nervous system and central nervous system interaction
The ENS is responsible for the intrinsic innervation of the GI tract and consists of two plexuses. The outer plexus, called myenteric plexus, is involved in the regulation of smooth muscles controlling the gut movements such as peristalsis. The inner, submucosal, plexus is responsible for secretion and absorption[5]. The ENS is modulated by the extrinsic innervation coming from the ANS and the CNS. The ANS consists of sympathetic (splanchnic) nerves and parasympathetic (vagal-sacral) nerves. The somatic nervous system controls the striated muscles of the proximal esophagus and the external anal sphincter. In CNS, the highly integrated gut-brain communication axis is mainly controlled by the limbic system which receives input from the ENS through the ANS and is modulated by higher cortical areas[2].
The limbic system consists of the amygdala, hypothalamus, medial thalamus and the anterior cingulate cortex (ACC). It is primarily concerned with the regulation of behavior, emotions, arousal, memory and motivation. It is also partly responsible for the modulation of the visceral organs by providing input through a number of hemostatic mechanisms. The visceral region, also known as ‘visceral brain’, lies specifically in the hypothalamus which is a central component of the limbic system[6]. The amygdala is responsible for emotional and stressful responses and drives other areas such as the prefrontal cortex for execution of complex functions. Functional brain imaging using magnetic resonance imaging (MRI) has shown that damage to the amygdala results in an alteration in stress responses such as flat affect seen in schizophrenia patients[7]. The damage may also lead to inhibition of social interaction and emotional conditioning[7]. The amygdala also consolidates memories with the help of emotional stimuli[6].
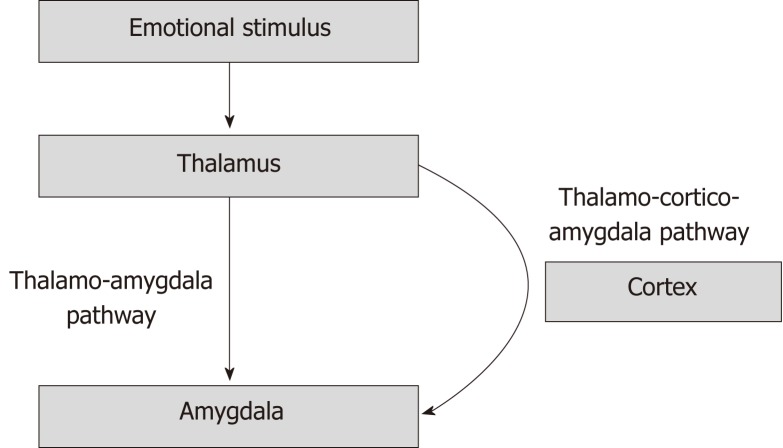
There are two pathways involved in the processing of the emotional input. A direct pathway, which processes crude information, is called the thalamo-amygdala pathway. It is chiefly responsible for rapid, unconditioned fear response without the intervention of the cortex. The thalamo-cortico-amygdala pathway, on the other hand, allows for a slower, conditioned response by more complex processing of the emotional stimuli (Figure 3)[8].
Figure 3.
The pathways involved in emotional response. The thalamo-amygdala pathway is responsible for unconditioned fast response without the input from the cortex. The thalamo-cortico amygdala pathway provides input for a complex, conditioned response due to input from the cerebral cortex.
The anterior cingulate cortex (ACC) has a central role in the thalamo-cortico-amygdala pathway. The ACC detects the conflict between the current emotional state and any new stimulus that can incite a new affective or motivational response. It relays information to the pre-frontal cortex (PFC) where further processing of the input takes place and the decision is made about how to respond to the stimuli. Functional Magnetic Resonance Imaging (fMRI) has highlighted that the perigenual ACC becomes activated in response to an emotional input. In situations of conflict, the PFC cortex further activates different regions of the cortex which helps in the decision-making process for deriving the appropriate response to the presented stimuli. Patients with depression have been often shown to have abnormalities in the left PFC and may also express difficulties in executive functioning.
Interaction between ANS and the Vagus nerve in FGIDs
Disturbance in the ANS has often shown to correlate with flare-up of IBS symptoms[9], however, no definite pattern of ANS activation has emerged. Some studies suggest activation or inhibition of parasympathetic innervation while others suggest increased or decreased sympathetic activity. Some authors suggest that there is a specific pattern in IBS depending on diarrhea or constipation-predominant symptoms but the reproducibility of such patterns has been inconsistent due to methodological limitations or limited power to allow for a specific pattern to emerge[2,10,11]. The role of ANS in psychiatric disorders, acute stress and pain is relatively more evident[2,12].
The Vagus nerve is responsible for relaying visceral information to the brain via parasympathetic pathways. It contains both sensory and motor pathways and provides innervation to the entire gut except the distal colon, rectum and internal anal sphincter which are innervated by the sacral (parasympathetic) ganglion. The afferent fibers carry sensations to the nucleus of solitary tract (NTS) and mediate both noxious stimuli, such as dull pain, non-noxious sensations such as hunger and nausea[13]. The motor fibers travel from nucleus ambiguus (NA) and the dorsal motor nucleus (DMN) in the brain stem to the ENS and regulate physiological functions such as motility and secretion. A number of vasovagal reflexes such as enterogastric and gastrocolic also modulate GI function[14].
The higher cortex influences the vagus nerve as well by modulating the vagus nerve nuclei in the brainstem. The NTS receives sensory information from the abdominal viscera and relays it to the higher brain regions[15]. Projections from NTS also terminate into the hypothalamus and limbic system which may explain why there is an altered perception of visceral pain in individuals with psychiatric symptoms. Vagal motor nuclei receive input from brain regions such as the hypothalamus, area postremia, and inferior-anterior cingulate cortex. These networks assimilate sensory input coming from NTS and formulate appropriate downstream responses via nucleus ambiguus[14].
The HPA axis in FGIDs
The Corticotropin-releasing hormone (CRH) seems to affect the motility and sensitivity of the gut, however, it is not completely understood if it is a primary response or occurs due to another stimulus[16]. Studies have reported increased levels of CRH in IBS patients and increased baseline levels in patients with anxiety disorders, however, the replication of these results has often been inconsistent.
The role of different emotional states in FGID has been well established. For example, volunteers who were subjected to different stressors showed alterations in their GI function[17-19]. Studies on rodents have shown that when these animals are put under stress, their brain undergoes neurochemical changes[20,21] along with augmented visceral sensitivity[22].
In one study, women with IBS showed altered cellular immune response compared to control subjects. This in part is thought to be mediated by adrenergic pathways but does not essentially include the activation of the HPA axis[23]. Dinan et al[24] showed that patients with IBS had increased response to CRH and expressed increased levels of inflammatory hormone Interleukin-6 and Interleukin-8. Another study involving twenty-one IBS patients and 18 controls, showed that IBS patients had increased HPA axis responsiveness, however, this overstimulation was more likely due to traumatic events early in life which are common in patients with IBS[25].
Neuroimmune interactions in FGIDs
The ENS, also called the ‘second brain’, is capable of independent functioning without intermediation from the ANS. It consists of an estimated 108 neurons arranged in two ganglionic plexuses[26]. There is a complex interdependent relationship between the gut immune system and the ENS. Physiological functions of the gut such as motility, absorption and secretion are all very sensitive to subtle changes in this fine balance between the immune system and the nervous system[27]. For example, immune activation due to local inflammation can have a diffuse effect on GI motility[28].
Patients with post-infectious IBS (PI-IBS) have been studied for understanding the role of the immune system in FGIDs. The incidence of PI-IBS is reported between 5% and 32%[29]. The risk of developing PI-IBS increases many times if the presenting illness is predominantly diarrheal and lasts for more than 3 wk[30]. Additionally, hypochondriasis and stressful life event at the initial illness doubles the risk of PI-IBS, providing further evidence for the involvement of the immune system[31].
Ascending and descending pain pathways and FGIDs
Diffuse, visceral pain is the most common symptom and hallmark feature in a number of patients with FGIDs. Significant pain or abdominal disturbance leads to impairment in social functioning which may prompt hospital visits.
While the neurological pathways and underlying molecular mechanisms for somatic pain have been well studied, the understanding of the visceral pain remains a daunting challenge. The altered perception of pain can occur due to an abnormality in the visceral pain pathways and can occur at the level of the nociceptors, neuronal pathways, thalamus, and corticolimbic signaling pathway[32].
Pain receptors in the viscera mainly respond to chemical stimulation, mechanical stimulation such as distension or stretching, and ischemia or infection[33]. Initial insult leads to a release of inflammatory mediators such as prostaglandins, adenosine triphosphate, histamine, serotonin, and bradykinin. These inflammatory mediators cause pain sensitization by acting on a number of receptors, prominent among which are transient receptor potential vallinoid (TRPV) receptors 1 and 4, voltage-gated sodium calcium channels (VGSCs) and protease-activated receptors 2 (PAR(2)).
TRPV1 and 4 receptors mainly sense mechanical stimulus. TRPV1 is particularly implicated in patients with FGIDs as it has been shown to play a role in visceral pain hypersensitization. In rat models, Akbar et al[34] have shown that there was a 3.5-times increase in the concentration of TRPV1 receptors in IBS patients compared to healthy individuals. Reciprocally, TRPV1 antagonists have been shown to decrease the visceral pain sensitivity in rat models and may present a possible therapeutic target[35]. The TRPV4 receptors and PAR (2) receptors work closely and may become activated by serine proteases. These proteases are found in increased concentration in patients with FGIDs. Antagonizing these receptors reduces nerve discharge possibly preventing the sensitization[36].
The afferent pain fibers, which originate in viscera, relay to the dorsal horn of the spinal cord and then ascend through the spinal cord to the midbrain and cortex. Biochemically active agents such as substance P, glutamate, aspartate and vasoactive intestinal peptides are released in nerve terminals of the dorsal column of the spinal cord. These nociceptive signals ascend in two major ascending pathways: the spinothalamic and the spinoparabrachial tracts. Studies using positron emission tomography (PET) have helped in outlining the functioning of these pathways. The mid-cingulate portion of the ACC has often been stimulated in patients with visceral hypersensitivity compared to healthy individuals. This is also the same area which is involved in the perception of fear and obnoxiousness[37].
The afferent signal is processed and modulated by the higher cortex which then transmits it to the perigenual ACC. This signal further travels down to the spinal cord via opioidergic, serotoninergic and noradrenergic systems to dorsal column of the spinal cord and modulates the afferent pain signals[38]. As the same limbic areas control emotional and cognitive signals, it is thought that the downstream inhibitory pain signals may be altered by the attentional and emotional state of an individual.
ROLE OF MICROBIOTA-GUT-BRAIN AXIS IN FGIDs
Microbiota has recently emerged as a key player in the gut-brain axis. This interaction between the brain and the microbiota has led to the recognition of a new term called ‘microbiota-gut-brain axis’[39]. This interaction is bidirectional, meaning that the disturbance in the complex community of microbiota (dysbiosis) can affect the brain and vice-versa. The underlying signaling mechanisms for these communicating networks between gut flora and the gut-brain axis have been of special interest to researchers and pharmacists who are seeking potential therapeutic interventions.
The microbiota-gut-brain axis has been mainly studied in rodent models and two approaches have been used. In the first one, germ-free mice are compared with healthy mice with normal gut-flora to look for changes in desired characteristics or behaviors. Although the germ-free model has certain limitations, it is an excellent tool that has been used over the years to help advance our understanding of the axis. In the other approach, wide-spectrum antibiotics are used to induce changes in the composition of microbiota and then these treated mice are compared with untreated mice to look for the desired characteristics.
The evidence for bidirectional communication comes from studies that have shown that early life stress can alter the composition of gut microbiota, highlighting the role of the brain as an influencer on the gut through the gut-brain axis[22]. Reciprocally, healthy mice showed anxiety-like behaviors after the administration of pathogenic bacteria such campylobacter jejni or Citrobacter rodentium[40,41], suggesting an inverse role of the role microbiome on the brain.
Dysbiosis
Every human has a unique and subject specific community of microorganisms. This fingerprint of microorganisms, which is an ecosystem in homeostasis, develops early in life and may undergo some modifications but by and large, it remains stable throughout life[42]. The modifications may be due to competition from other microorganisms or pressure from the host[43]. Microbiota participates in the modulation of intestinal motility, blood flow, secretions, immunity and perception of visceral signals[44]. Therefore it is speculated that dysbiosis plays an important role in the pathogenesis and symptoms perception of FGID.
Studies demonstrate that the germ-free mice show developmental changes from mice with normal microbiota. These changes may resemble features of functional GI diseases[45,46]. Conversely, recolonization of these mice can restore some functions such as mucosal immunity[47].
On the contrary, when these mice are infected with pathogenic microorganisms, they may produce features that may resemble symptoms of IBS and in some cases IBD[48]. Other mechanisms that have been suggested include gut distention and alterations in secretion and motility of the GI tract. These changes seem to arise from the production of gas and fatty acids by the bacterial flora influencing the microbiota to host signaling[49].
Dysbiosis is seen in different GI diseases including celiac disease, IBS and IBD[50,51]. Although dysbiosis seems to be an important denominator in patients with FGIDs, no specific symbiotic signature has been identified. This could be due, in part, to different sites used for sampling and varying techniques used to analyze the cultures[52-54].
Dysbiosis can also upset the HPA axis; for example, compared to mice with standard microbiota, germ-free mice produce increased levels of the adrenecorticotrophin (ACTH) and other stress hormones[55]. Conversely, on restoring the intestinal flora of germ-free mice, there is decreased production of stress hormones and a partial reversal of anxiety-like behaviors[55]. Germ-free mice also have decreased levels of brain-derived neurotrophic factor (BDNF) due to decreased expression of NMDA receptors[56]. These proteins are involved in the differentiation and growth of new neurons[55,57].
Other studies have also highlighted the role of microbiota in the development of the brain and gut through a number of complex pathways. Thus it is thought that dysbiosis could be one of the causes of behavioral traits associated with anxiety disorders[56]. Table 1 represents the different factors that that modify gut-brain-axis and play a role in the pathophysiology of FGIDs.
Table 1.
Different factors that influence the brain - Gut interaction in functional gastrointestinal disorders
| Nature of link | Evidence | Comments |
| Dysbiosis | Kassinen et al[82]; Tojo et al[83]; Chassard et al[84]; Cryan et al[85] | Disturbance in the complex community of microbiota seems to influence gut-brain axis by modulating neuroendocrine, neuroimmunal and visceral sensory system. |
| Altered mucosal secretions | Mazmanian et al[86]; Xue et al[87] | Secretion is modulated by complex interaction of intrinsic and extrinsic factors acting on gut mucosa. Dysregulation of the epithelial cells due to autonomic reactivity may lead to 5-HT release contributing to altered secretion |
| Disturbance in motility | Randich et al[13]; Dass et al[88]; Barbara et al[89] | Products of metabolism of gut bacteria, such as short-chain fatty acids modulate enteric system and influence the rate of gut transit |
| Visceral hypersensitivity | O'Mahony et al[22]; Akbar et al [34] | Patients with IBS have been found to have an increased concentration of pain-sensing receptors such as TPRV1 compared to the controls. |
| Altered processing of visceral signals | Lemann et al[90]; Mertz et al[91] | There is increased activation of certain cerebral areas in IBS patients compared to the controls. Altered processing of the visceral pain in the central nervous system has been a recurring theme in many studies. |
| Immune dysfunction | Chadwick et al[92]; Dinan et al[24]; Keely et al[93] | Patients with prolonged Infectious diarrhea are much more prone to developing IBS. Also, biopsies of patients with IBS have shown increased immune cells in the mucosa[92]. |
| Psychological disturbances | Creed et al[17]; Gwee et al[94]; Drossman et al[95]; Monnikes et al[2,12] | Patients with FGIDs have co-existing psychosocial symptoms such as stress, anxiety and depression and thus a biopsychosocial model has been proposed for FGIDs |
| Early life stress | O'Mahony et al[22]; Bailey et al[96] | Early life-stress can alter the composition of gut microbiota |
5-HT: 5-hydroxytryptamine; FGID: Functional Gastrointestinal diseases; IBS: Inflammatory bowel disease; TRPV1: Transient receptor potential vallinoid 1.
MODULATION OF THE GUT-BRAIN AXIS IN FGIDS
Our understanding of the pathophysiology of FGIDs is still developing, as currently there is a dearth of studies on human subjects and the challenge is to test whether findings in animals are translatable to humans. Only a few controlled studies have been done in human subjects which have highlighted the modifying role of probiotics, antibiotics, diet and fecal microbiota transplantation.
Role of probiotics in FGIDs
Probiotics contain live microorganisms that, when ingested, can have a beneficial effect on the host. Probiotics have found application in a number of gastrointestinal and immune system disorders[58] and their evaluation as a possible therapeutic target in FGIDs is one trending in the research community. One thing is certain that the role of probiotics cannot be overlooked as has been shown in numerous studies on animals and humans as highlighted below.
Studies on rodents have shown that the administration of probiotics leads to a reduction in visceral pain sensitization[59,60]. Mckernan et al[60] demonstrated that 14 d oral gavage of Bifidobacterium infantis showed reduced colorectal distension, increased pain threshold and delayed first pain behavior. Bercik et al[61] infected mice with Trichuris muris, a noninvasive parasite, and treated with etanercept, budesonide, or specific probiotics. They found that infected mice showed anxiety-like behaviors secondary to colonic inflammation. There was decreased BDNF messenger RNA (mRNA) in the hippocampus. The mice also showed immune system changes with increased Circulating tumor necrosis factor-α and interferon-γ. The administration of Bifidobacterium longum restored the normal behavior to some extent and normalized BDNF mRNA levels but did not affect immune system changes[61]. The study shows that the role of probiotics may be selective in that they may affect the gastrointestinal system but spare other systems.
Studies in human subjects are relatively limited. In a randomized controlled study in patients with IBS, the intake of Bifidobacterium lactis significantly reduced abdominal distension and resulted in an overall improvement of symptoms[62]. O’Mahony et al[63] demonstrated that 8 weeks intake of Bifidobacterium infantis led to an improvement of IBS symptoms and restoration of normal interleukin10:interleukin 12 ratio in blood.
The evidence for the use of probiotics has been mounting but specific strains and molecular targets remain to be determined.
FGIDs and role of antibiotics
Antibiotics can rapidly reduce the diversity of the organisms allowing certain pathogenic bacteria to wreak havoc. Antibiotics have been shown to influence psychiatric behaviors in individuals being treated for different reasons. Mohle et al observed that in comparison to non-treated mice, stressful behavior and poor memory and poor hippocampal functioning was evident in those who were treated with antibiotics. The same mice, when treated with probiotics, showed improvement in these cognitive symptoms[64]. Verdú et al[59] showed that the administration of oral antimicrobials in mice led to mucosal inflammation and visceral hypersensitivity due to increased substance P expression in the enteric nervous system (ENS). This inflammatory response was alleviated by the administration of Lactobacillus paracasei[59].
FGIDs and role of diet
Diet can improve the symptoms of FGID in two ways by modulating the gut-brain axis: improving psychological symptoms and by having a probiotic-like effect[65]. Magnusson et al[66] studied changes in the behavior of mice and their gut flora composition following the administration of different diets. Analysis showed that Erysipelotrichales was increased in the gut of mice which were fed high fat diet and Lactobacillus was increased in the mice which were given high sucrose diet. The diet influenced the cognitive behavior of the mice and these changes correlated with the composition of different bacteria in the gut of these mice[66]. Li et al[67] observed that compared to control mice, the mice which were fed a meat-containing diet showed better microbial diversity, improved memory and less stressful behavior. Similar findings were reported by a number of other studies suggesting that diet can have a profound effect on the composition of microbiota in mice and can improve or worsen anxiety-like behaviors[68,69]. However, due to difficulties in analyzing the gut microbiota, the reproducibility of these results in human subjects requires continued efforts with new research approaches.
POTENTIAL USEFULNESS OF FUNCTIONAL AND STRUCTURAL NEUROIMAGING IN FGIDS
The complex nature of the psychosocial interactions that underlie the pathophysiology of the FGID has always eluded researchers. The plethora of studies highlighting the role of gut-brain axis in the development of symptoms in FGID has not been met with effective strategies that can use this relationship to develop key diagnostic tools and therapeutic agents[70]. Studies have mainly focused on the biochemical interactions that play a role in the gut-brain axis but in vivo studies have been limited so far due to lack of noninvasive neurophysiological techniques. Recently, the utilization of new techniques such as functional brain imaging has allowed an objective assessment of the axis. Table 2 summarises the different techniques that have been used to study the gut-brain axis.
Table 2.
Methods used to study brain - Gut interaction in functional gastrointestinal disorders
| Link to be tested | Name of the test | Evidence | Comments |
| Microbiota-gut-brain axis | |||
| Germ-free mice | Abrams et al[45] | This has been the most widely used technique to study the gut-brain axis. Germ-free mice are compared with healthy to look for changes in desired characteristics or behaviors | |
| Antibiotic-treated mice | Verdú EF et al[59] | Antibiotics are used to induce changes in the composition of microbiota and then these treated mice are compared with untreated mice to look for the desired characteristics. Antibiotics are useful for selectively eliminating certain bacteria from the gut, allowing the growth of other strains. | |
| Mice treated with probiotics | Mohle et al[64] | Once germ-free mice have been studied, they can be injected with probiotics to establish the reciprocity of the relationship that has been studied. | |
| The interactions between visceral, peripheral and central pathways | |||
| Functional MRI (fMRI) | Tillisch et al[72]; Aziz et al[73]; Mayer et al[75], and Labus et al[76] | fMRI measures the changes in oxygenated and deoxygenated hemoglobin where oxygenated hemoglobin denotes the group of neurons that have increased activity. They are useful in studying the complex relationship between visceral stimuli and brain response. | |
| PET imaging | Tillisch[72] | PET imaging has the advantage of probing a particular receptor by developing a radiolabeled ligand. This important feature can be used to assess specific receptor activities during pain and stress response in control and FGID patients. | |
| Structural MRI (sMRI) | Seminowicz et al[77] | Whole and regional brain images using sMRIs have been used to study differences between individuals with FGIDs and control groups | |
FGIDs: Functional gastrointestinal diseases; MRI: Magnetic resonance imaging; PET: Positron emission tomography.
The field of neuroimaging has rapidly evolved recently and provides a promising new approach to the complex interactions of peripheral nerves and the brain. Since pain and discomfort are the main symptoms in people with FGID, studies have been limited to peripheral pain reflex pathways and ANS responses. It is thought that the treatment models that have worked on rodents and failed in human subjects might be due to the increased CNS modulation of the subcortical pathways[71]. Neuroimaging offers a non-invasive method to evaluate the interactions between the visceral and central pathways and the effect of psychological symptoms on these pathways.
Functional brain imaging in FGIDs
Functional brain imaging assesses brain function in response to different visceral stimuli. There are two main modalities that are used for imaging brain function namely, functional magnetic resonance imaging (fMRI) and positron emission tomography (PET)[72].
MRI has been the focus of attention for experts from a number of fields. For example, psychiatrists have used fMRI to gain insight into complex psychological disorders such as obsessive-compulsive disorders and schizophrenia. Functional MRI (fMRI) measures the changes in oxygenated and deoxygenated hemoglobin where oxygenated hemoglobin denotes the group of neurons that have increased activity[73].
Studies that have looked at the complex relationship between visceral stimuli and brain response have frequently produced inconsistent results which are often difficult to interpret. The only consistent finding that has been elicited so far after a meta-analysis of a number of studies has shown that the rectal distension in IBS patients is linked to the stimulation of anterior cingulate gyrus. Compared to the controls, the areas of the brain for emotional arousal in anterior cingulate gyrus were differentially activated in patients with IBS. The same meta-analysis also showed that the history of abuse and stressful life events synergistically correlated with the activation of these same brain areas[74]. The anterior cingulate gyrus has also been implicated in the modulation of the autonomic nervous system, maintenance of homeostasis and has been shown to have been affected by disturbances in emotions and the gut-brain axis[75]. Despite the limited results so far, fMRI remains an important modality and ongoing research promises to understand the functional neural networks. Studies are aiming to examine the entire functioning networks rather than focusing on particular regions. Two main functioning networks that have been the focus of scientists are the emotional arousal network (including amygdala and anterior cingulate cortical subregions) and hemostatic afferent network (anterior mid cingulate cortex, posterior insula, thalamus, dorsal pons) as previous studies have suggested an increased activity in these networks in patients with FGIDs[75,76].
The use of positron emission tomography (PET) imaging in FGIDs has been limited due to high costs, difficulty in engineering relevant ligands and the widespread availability of fMRIs. However, PET imaging has the advantage of probing a particular receptor by developing a radiolabeled ligand. This important feature can be used to assess specific receptor activities during pain and stress response in control and FGID patients. The technique may also attract pharmacists who want to study the distribution and response of a particular therapeutic agent targeted at a receptor and comparisons can be made between the placebo and intervention groups[72].
Structural brain imaging in FGIDs
Whole and regional brain images have been used to study differences between individuals with FGIDs and control groups. It has also been useful in baseline imaging and response to treatment. Structural brain images use high resolution structural MRI (sMRI) to measure cortical thickness and gray matter density which can be compared between different groups. The role of structural brain imaging in FGID has been not explored in great detail compared to psychiatric disorders. Structural changes in the brain have been seen in individuals with a history of childhood trauma. Although the structural changes may seem ‘fixed’, dynamic changes have been seen in patients under conditions such as learning, illness and stress. The few studies that have been carried out on FGID patients using structural images have indicated that there is decreased grey matter density in certain areas of the brain such as the medial prefrontal and ventrolateral prefrontal cortex, striatum and thalamus; while there may be increased density in the anterior cingulate and orbitofrontal cortex. Interestingly, controlling for stressors showed that there was no significant difference in the grey matter density between the patients with FGIDs and controls[77].
WHAT DOES THE FUTURE HOLD?
There is still a long way to go to understand the exact role of the neural, immunologic, biochemical and other pathways in the gut-brain axis and the FGIDs. There is also a need to these translate findings of animal studies to human subjects using multi-population randomized controlled trials. The role of microbiota has opened exciting new avenues and its exact role needs to be explored with the identification of the individual specific strains that may help to tailor the probiotic therapy. The neuroimaging combined with immunologic and biochemical findings can be used to develop patterns of pathogenesis and for guiding further research.
The application of machine learning to medical diagnosis
The multifactorial nature of FGID can make diagnosis a challenging and time-consuming task. Machine learning and artificial intelligence (AI) are important tools that can potentially be employed to help with the diagnosis of FGID and aid healthcare professionals and researchers in combing through the plethora of available patient data to identify trends and investigate causal relationships between the trends. Once causality has been established it could shed light on the mechanisms behind the pathogenesis of FGID and help researchers understand how the gut-brain-microbiome axis functions.
The use of machine learning processes such as inductive learning to define rules that govern diagnostic algorithms in different plant species has been shown to be effective and at times superior to rules obtained by human experts. It has often been proven to outperform rules defined by human experts even as early as 1980[78]. Inductive learning algorithms can learn from examples or prototypes and come up with diagnostic rules for the examples provided[79].
In recent times, the availability of large databases of digitized patient data provides a unique opportunity for machine learning algorithms to analyze and interpret databases to identify correlations between different symptoms, imaging findings and biochemical findings. The algorithms then develop classifiers by pattern recognition which can be used as a framework to develop a diagnostic algorithm or improve existing algorithms by incorporating new classifiers into them or testing the reliability of established classifiers. A machine learning program can be used in such a way to diagnose conditions based on clinical findings and investigations[80]. For example, Kukar et al successfully used a machine learning approach to successfully diagnose patients with ischemic heart disease using an algorithm employing a step-wise diagnostic approach. The algorithms improved the detection of positive and negative cases by 6% compared to manual detection by the clinicians[81].
The advantages of machine learning algorithms
Autonomy and automation: Most machine learning algorithms are autonomous to variable degrees and the process of obtaining knowledge is largely automated which makes these methods of learning and data analyses highly efficient. The algorithms also look for trends in the data that the researchers may not have considered and therefore provides more knowledge than conventional methods of data analysis over a significantly smaller amount of time[80].
Dealing with incomplete or noisy data: Machine learning algorithms are highly adaptable and consequently very good at dealing with and accounting for missing and/or noisy data which is a very common occurrence in electronic patient records[80].
Explanation ability: Most algorithms have the ability to explain the trends and qualifiers that they identify. These explanations can be very valuable for guiding further research[79,80].
Transparency: The knowledge generated and the explanations behind the decisions made by most algorithms is transparent to the physicians and therefore the reasoning of the algorithm is easy to understand.
Future studies on the gut-brain-microbiome axis need to evaluate the feasibility of using machine learning programs to study patient data to identify correlations between the clinical findings, demographic information, neuroimaging findings, lab tests and gut microbiome analysis to develop newer and more reliable diagnostic criteria.
Role of gut microbiota
An aspect of the gut-brain-microbiome axis which requires further investigation is the gut microbiota and its effects on the pathogenesis of FGID. Future studies need to look at both the bacterial and fungal parts of the microbiome to ascertain the extent to which the gut microbiota plays a role (if at all) in FGID as there is a dearth of literature on this topic.
CONCLUDING
The gastrointestinal tract and nervous system are constantly communicating with each other in a bidirectional relationship which is influenced by the autonomic nervous system, immune system, hypothalamic-pituitary axis and gut microbiota. Understanding the molecular and biochemical mechanisms disturbing this complex network of communication is key to our understanding of the pathophysiology of the functional GI diseases. Studies in rodents have provided us with substantial evidence about the underlying mechanisms, however, there is a need to translate these findings in human subjects to successfully identify the therapeutic targets.
The identification of pathogenic microbiome signatures in individuals combined with demographical data, serological findings and neuroimaging findings can be encoded into machine learning algorithm which may help identify trends and patterns that can be potentially overlooked by humans.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Peer-review started: October 14, 2018
First decision: November 1, 2018
Article in press: December 27, 2018
P- Reviewer: Chowdhury P, De Palma GD, Yan SL, Yücel O S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Kashif Mukhtar, Centre of Excellence in Women and Child Health, Aga Khan University, Karachi, Sindh 74800, Pakistan.
Hasham Nawaz, Department of Medicine, Section of Gastroenterology, Aga Khan University, Karachi, Sindh 74800, Pakistan.
Shahab Abid, Department of Medicine, Section of Gastroenterology, Aga Khan University, Karachi, Sindh 74800, Pakistan. shahab.abid@aku.edu.
References
- 1.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Jones MP, Dilley JB, Drossman D, Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. 2006;18:91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 3.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 5.Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb B, Whishaw IQ. Fundamentals of human neuropsychology: Macmillan, 2009 [Google Scholar]
- 7.Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 8.Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. An anatomically constrained neural network model of fear conditioning. Behav Neurosci. 1995;109:246–257. doi: 10.1037//0735-7044.109.2.246. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Sheffield D, Verne GN. Evidence for autonomic dysregulation in the irritable bowel syndrome. Dig Dis Sci. 2002;47:1716–1722. doi: 10.1023/a:1016424007454. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 11.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol. 2001;96:460–466. doi: 10.1111/j.1572-0241.2001.03526.x. [DOI] [PubMed] [Google Scholar]
- 12.Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Mönnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 13.Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 14.Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. Handbook of Physiology The Gastrointestinal System Motility and Circulation. 1989;1:621–683. [Google Scholar]
- 15.Andrews PL. Vagal afferent innervation of the gastrointestinal tract. Prog Brain Res. 1986;67:65–86. doi: 10.1016/s0079-6123(08)62757-0. [DOI] [PubMed] [Google Scholar]
- 16.Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13 Suppl A:18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- 17.Creed F, Guthrie E. Psychological factors in the irritable bowel syndrome. Gut. 1987;28:1307–1318. doi: 10.1136/gut.28.10.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins SM. Stress and the Gastrointestinal Tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- 19.Wu JC. Psychological Co-morbidity in Functional Gastrointestinal Disorders: Epidemiology, Mechanisms and Management. J Neurogastroenterol Motil. 2012;18:13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 21.O'Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2011;214:221–229. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- 22.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Elsenbruch S, Holtmann G, Oezcan D, Lysson A, Janssen O, Goebel MU, Schedlowski M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am J Gastroenterol. 2004;99:703–710. doi: 10.1111/j.1572-0241.2004.04138.x. [DOI] [PubMed] [Google Scholar]
- 24.Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2005;21:176–182. doi: 10.1097/01.mog.0000153315.28327.6e. [DOI] [PubMed] [Google Scholar]
- 27.Langley J. 1921. The autonomic nervous system (Pt. I) [Google Scholar]
- 28.Langworthy OR, Rosenberg SJ. Control by the central nervous system of rectal smooth muscle. J Neurophysiol. 1939;2:356–360. [Google Scholar]
- 29.Thabane M, Marshall JK. Post-infectious irritable bowel syndrome. World J Gastroenterol. 2009;15:3591–3596. doi: 10.3748/wjg.15.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 31.De Groat WC, Krier J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J Physiol. 1978;276:481–500. doi: 10.1113/jphysiol.1978.sp012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rea K, O'Mahony S, Dinan TG, Cryan JF. Visceral pain: role of the microbiome-gut-brain axis. 2017 [Google Scholar]
- 33.Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–136. doi: 10.1093/bmb/ldp026. [DOI] [PubMed] [Google Scholar]
- 34.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- 36.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 38.Vogt BA, Watanabe H, Grootoonk S, Jones AK. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Human brain mapping. 1995;3:1–12. [Google Scholar]
- 39.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Rajilić-Stojanović M, Heilig HG, Tims S, Zoetendal EG, de Vos WM. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 2012 doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 43.Bäckhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58 Suppl 2:44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- 44.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403–413. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 45.Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963;12:355–364. [PubMed] [Google Scholar]
- 46.Gordon HA, Bruckner-Kardoss E. Effect of normal microbial flora on intestinal surface area. Am J Physiol. 1961;201:175–178. doi: 10.1152/ajplegacy.1961.201.1.175. [DOI] [PubMed] [Google Scholar]
- 47.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 48.Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM WEL Investigators. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 49.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009;62:264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 51.Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 52.Lagier JC, Million M, Hugon P, Armougom F, Raoult D. Human gut microbiota: repertoire and variations. Front Cell Infect Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609, 609.e1-609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 54.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 58.Quigley EM. Probiotics in functional gastrointestinal disorders: what are the facts? Curr Opin Pharmacol. 2008;8:704–708. doi: 10.1016/j.coph.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010;22:1029–1035, e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 61.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 62.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 63.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 64.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 65.Oriach CS, Robertson RC, Stanton C, Cryan JF, Dinan TG. Food for thought: The role of nutrition in the microbiota-gut-brain axis. Clin Nutr Exp. 2016;6:25–38. [Google Scholar]
- 66.Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R, Perrone A, Bermudez LE. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–140. doi: 10.1016/j.neuroscience.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 68.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradesi S, Mayer EA. Experimental models of stress and pain: do they help to develop new therapies? Dig Dis. 2009;27 Suppl 1:55–67. doi: 10.1159/000268122. [DOI] [PubMed] [Google Scholar]
- 72.Tillisch K, Labus JS. Advances in imaging the brain-gut axis: functional gastrointestinal disorders. Gastroenterology. 2011;140:407–411.e1. doi: 10.1053/j.gastro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- 74.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 76.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57.e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michalski RS, Chilausky R. Knowledge acquisition by encoding expert rules versus computer induction from examples: a case study involving soybean pathology. 1980 [Google Scholar]
- 79.Stefik MJ. Machine learning: An artificial intelligence approach: RS Michalski, JG Carbonell and TM Mitchell,(Tioga, Palo Alto, CA); 572 pages, Elsevier, 1985 [Google Scholar]
- 80.Kononenko I. Machine learning for medical diagnosis: history, state of the art and perspective. Artif Intell Med. 2001;23:89–109. doi: 10.1016/s0933-3657(01)00077-x. [DOI] [PubMed] [Google Scholar]
- 81.Kukar M, Kononenko I, Groselj C, Kralj K, Fettich J. Analysing and improving the diagnosis of ischaemic heart disease with machine learning. Artif Intell Med. 1999;16:25–50. doi: 10.1016/s0933-3657(98)00063-3. [DOI] [PubMed] [Google Scholar]
- 82.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chassard C, Dapoigny M, Scott KP, Crouzet L, Del'homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, Dubray C, Flint HJ, Bernalier-Donadille A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 85.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 86.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 87.Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci. 2007;133:55–63. doi: 10.1016/j.autneu.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 89.Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 90.Lémann M, Dederding JP, Flourié B, Franchisseur C, Rambaud JC, Jian R. Abnormal perception of visceral pain in response to gastric distension in chronic idiopathic dyspepsia. The irritable stomach syndrome. Dig Dis Sci. 1991;36:1249–1254. doi: 10.1007/BF01307517. [DOI] [PubMed] [Google Scholar]
- 91.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 92.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 93.Keely S, Walker MM, Marks E, Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest. 2015;45:1350–1359. doi: 10.1111/eci.12548. [DOI] [PubMed] [Google Scholar]
- 94.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drossman DA. Presidential address: Gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]