Abstract
Background:
Asthma is the most prevalent chronic respiratory disease worldwide, affecting 358 million people in 2015. Ambient air pollution exacerbates asthma among populations around the world and may also contribute to new-onset asthma.
Objectives:
We aimed to estimate the number of asthma emergency room visits and new onset asthma cases globally attributable to fine particulate matter (), ozone, and nitrogen dioxide () concentrations.
Methods:
We used epidemiological health impact functions combined with data describing population, baseline asthma incidence and prevalence, and pollutant concentrations. We constructed a new dataset of national and regional emergency room visit rates among people with asthma using published survey data.
Results:
We estimated that 9–23 million and 5–10 million annual asthma emergency room visits globally in 2015 could be attributable to ozone and , respectively, representing 8–20% and 4–9% of the annual number of global visits, respectively. The range reflects the application of central risk estimates from different epidemiological meta-analyses. Anthropogenic emissions were responsible for and 73% of ozone and impacts, respectively. Remaining impacts were attributable to naturally occurring ozone precursor emissions (e.g., from vegetation, lightning) and (e.g., dust, sea salt), though several of these sources are also influenced by humans. The largest impacts were estimated in China and India.
Conclusions:
These findings estimate the magnitude of the global asthma burden that could be avoided by reducing ambient air pollution. We also identified key uncertainties and data limitations to be addressed to enable refined estimation. https://doi.org/10.1289/EHP3766
Introduction
Approximately 358 million people worldwide were estimated to have had asthma in 2015 (GBD 2015 Chronic Respiratory Disease Collaborators 2017), including about 14% of the world’s children (Global Asthma Network 2014). Asthma prevalence is considered the fourth leading cause of years lived with disability (YLDs) for children ages 5–14 globally, and the 16th leading cause of YLDs for all ages (GBD 2015 Chronic Respiratory Disease Collaborators 2017). Asthma is among the top causes of YLDs among children ages 5–14 across all sociodemographic index categories, affecting both high- and low-income populations. Economic costs are substantial and include both direct [e.g., inpatient care, emergency room visits (ERVs), physician visits, diagnostic tests, and medication] and indirect costs (e.g., school and work days lost; Bahadori et al. 2009). Epidemiological and clinical experimental studies have shown over decades that exposure to air pollution is a key risk factor for asthma exacerbation and may also contribute to new-onset asthma (Guarnieri and Balmes 2014; Toskala and Kennedy 2015).
Ambient fine particulate matter () exposure, currently considered the leading environmental risk factor globally, is estimated to be associated with 4.2 million premature deaths and 103 million Disability Adjusted Life Years (DALYs, YLDs plus Years of Life Lost) in 2015 (Cohen et al. 2017). Updated for 2016, the disease burden estimate includes 4.1 million deaths and 106 million DALYs (GBD 2016 Risk Factors Collaborators 2017). This global burden attributable to accounts for 27% of all DALYs from chronic obstructive pulmonary disease (COPD; an additional 6% are attributable to ambient ozone); 20% of those from ischemic heart disease; 16% of those from stroke; 17% of those from tracheal, bronchus, and lung cancer; and 31% from child acute lower respiratory infections (GBD 2016 Risk Factors Collaborators 2017). The Global Burden of Disease Study 2016 estimated that smoking and occupational asthmagens were each responsible for 10% of DALYs from asthma in 2016 (GBD 2015 Chronic Respiratory Disease Collaborators 2017).
The contribution of air pollution to asthma exacerbation and new asthma incidence remains unquantified and has not been included in global burden of disease studies (Cohen et al. 2017; GBD 2016 Risk Factors Collaborators 2017; WHO 2016, 2017a). Estimates of air pollution’s impact on asthma in individual countries or sets of cities suggest that the global burden could be substantial (Fann et al. 2012; Perez et al. 2013). Nitrogen dioxide () has not yet been included in global burden of disease assessments but is associated with asthma exacerbation and incidence (Orellano et al. 2017; Zhang et al. 2016; Zheng et al. 2015) and has been considered a key pollutant in country- and city-level air pollution impact estimates (Walton et al. 2015). Here we quantitatively estimate asthma ERVs and, as a secondary analysis, new asthma cases that may be attributable to ambient ozone, , and globally. We use epidemiologically derived health-impact functions combined with datasets on population, baseline asthma incidence and prevalence rates, and pollutant concentrations. As these first estimates of the global asthma burden from ambient air pollution are subject to many important assumptions and uncertainties, we anticipate that future studies will further advance the input data and methods piloted here.
Methods
The analytical process is similar to that typically used to estimate air pollution-related premature deaths (Anenberg et al. 2010), but uses data that are specific to asthma. We first combined national asthma incidence and prevalence rates and a newly constructed dataset of asthma ERV rates from survey data in 54 countries and Hong Kong (Table 1 and Figures S1 and S2) with globally gridded population counts to estimate the baseline number of new asthma cases and asthma ERVs at resolution. We then estimated the pollution-attributable fraction of asthma ERVs and new asthma cases using globally gridded pollution concentrations, derived from satellite remote sensing and chemical transport modeling ( resolution; Figure 1), with concentration–response factors drawn from several multinational meta-analyses of epidemiological studies (Table 2). These epidemiological studies examined associations between air pollutants and both asthma exacerbation and incident asthma in 27 countries spanning North America, Europe, Asia, and Latin America (Table S1). We used best practices in data reporting outlined by the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) (Stevens et al. 2016).
Table 1.
Fraction of asthma group visiting the emergency room per year in each country, with data source.
| Country | Study | Study Surveya | Fraction visiting emergency roomb |
|---|---|---|---|
| Algeria | Nafti et al. 2009 | AIR | 0.379 |
| Argentina | Neffen et al. 2010 | AIR | 0.417 |
| Australia | Thompson et al. 2013 | AIM | 0.150 |
| Austria | Price et al. 2014 | REALISE | 0.239 |
| Belgium | Price et al. 2014 | REALISE | 0.239 |
| Brazil | Neffen et al. 2010 | AIR | 0.417 |
| Bulgaria | Rabe et al. 2004 | AIR | 0.207 |
| Canada | Sastre et al. 2016 | AIR | 0.280 |
| Chile | Neffen et al. 2010 | AIR | 0.417 |
| China | Weightedc | 0.329 | |
| Rabe et al. 2004 | AIR | 0.190 | |
| Zainudin et al. 2005 | AIR | 0.315 | |
| Thompson et al. 2013 | AIM | 0.40 | |
| Su et al. 2013 | AIR | 0.339 | |
| Colombia | Neffen et al. 2010 | AIR | 0.417 |
| Costa Rica | Neffen et al. 2010 | AIR | 0.417 |
| Croatia | Rabe et al. 2004 | AIR | 0.207 |
| Czech Republic | Rabe et al. 2004 | AIR | 0.207 |
| Ecuador | Neffen et al. 2010 | AIR | 0.417 |
| Ethiopia | Zemedkun et al. 2014 | ACT | 0.312 |
| Finland | Price et al. 2014 | REALISE | 0.239 |
| France | Weighted | 0.079 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Germany | Weighted | 0.195 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Sastre et al. 2016 | AIM | 0.180 | |
| Hungary | Rabe et al. 2004 | AIR | 0.207 |
| India | Thompson et al. 2013 | AIM | 0.420 |
| Italy | Weighted | 0.143 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Sastre et al. 2016 | AIM | 0.150 | |
| Allegra et al. 2012 | PRISMA | 0.065 | |
| Corrado et al. 2013 | Survey of patients in 16 Italian pulmonary units | 0.499 | |
| Japan | Rabe et al. 2004 | AIR | 0.130 |
| Jordan | Khadadah et al. 2009 | AIR | 0.520 |
| Kuwait | Khadadah et al. 2009 | AIR | 0.520 |
| Latvia | Rabe et al.2004 | AIR | 0.207 |
| Lebanon | Khadadah et al. 2009 | AIR | 0.520 |
| Lithuania | Rabe et al. 2004 | AIR | 0.207 |
| Malaysia | Weighted | 0.246 | |
| Rabe et al. 2004 | AIR | 0.190 | |
| Zainudin et al. 2005 | AIR | 0.120 | |
| Thompson et al. 2013 | AIM | 0.400 | |
| Neffen et al. 2010 | AIR | 0.417 | |
| Morocco | Nafti et al. 2009 | AIR | 0.210 |
| Netherlands | Weighted | 0.195 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Nigeria | Desalu et al. 2013 | GAPP | 0.427 |
| Norway | Price et al. 2014 | REALISE | 0.239 |
| Oman | Khadadah et al. 2009 | AIR | 0.520 |
| Peru | Neffen et al. 2010 | AIR | 0.417 |
| Philippines | Weighted | 0.211 | |
| Rabe et al. 2004 | AIR | 0.19 | |
| Zainudin et al. 2005 | AIR | 0.252 | |
| Poland | Rabe et al. 2004 | AIR | 0.207 |
| Romania | Rabe et al. 2004 | AIR | 0.207 |
| Russia | Rabe et al. 2004 | AIR | 0.207 |
| Singapore | Weighted | 0.164 | |
| Rabe et al. 2004 | AIR | 0.190 | |
| Zainudin et al. 2005 | AIR | 0.114 | |
| Thompson et al. 2013 | AIM | 0.170 | |
| Slovak Republic | Rabe et al. 2004 | AIR | 0.207 |
| Slovenia | Rabe et al. 2004 | AIR | 0.207 |
| South Korea | Weighted | 0.148 | |
| Zainudin et al. 2005 | AIR | 0.058 | |
| Rabe et al. 2004 | AIR | 0.190 | |
| Thompson et al. 2013 | AIM | 0.160 | |
| Spain | Weighted | 0.235 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Sastre et al. 2016 | AIM | 0.360 | |
| Sweden | Weighted | 0.184 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Thailand | Weighted | 0.350 | |
| Thompson et al. 2013 | AIM | 0.350 | |
| Boonsawat et al. 2015 | AIM | 0.350 | |
| Tunisia | Nafti et al. 2009 | AIR | 0.254 |
| Turkey | Turktas et al. 2010 | AIR | 0.233 |
| Ukraine | Rabe et al. 2004 | AIR | 0.207 |
| United Arab Emirates | Weighted | 0.400 | |
| Khadadah et al. 2009 | AIR | 0.520 | |
| Mahboub et al. 2010 | AIR | 0.280 | |
| United Kingdom | Weighted | 0.175 | |
| Rabe et al. 2004 | AIR | 0.100 | |
| Price et al. 2014 | REALISE | 0.239 | |
| Sastre et al. 2016 | AIM | 0.090 | |
| United States | Rabe et al. 2004 | AIR | 0.230 |
| Uruguay | Neffen et al. 2010 | AIR | 0.417 |
| Venezuela | Neffen et al. 2010 | AIR | 0.417 |
| Vietnam | Weighted | 0.206 | |
| Rabe et al. 2004 | AIR | 0.190 | |
| Zainudin et al. 2005 | AIR | 0.225 | |
| Hong Kong | Weighted | 0.168 | |
| Thompson et al. 2013 | AIM | 0.150 | |
| Zainudin et al. 2005 | AIR | 0.164 | |
| Rabe et al. 2004 | AIR | 0.190 |
Note: ACT, International Asthma Control Test; AIM, Asthma Insights and Management Survey; AIR, Asthma Insights and Reality Survey; GAPP, Global Asthma Physician and Patient survey; PRISMA, Prospective Study on Asthma Control; Realise, Recognise Asthma and Link to Symptoms and Experience Survey.
The studies reported the rate of ERVs and hospital admissions among study participants, which we used as nationwide rates, assuming the survey sample was nationally representative.
For countries where more than one asthma ERV rate were available from the included surveys, we weighted the rates based on study sample size and used this rate for the total countrywide rate.
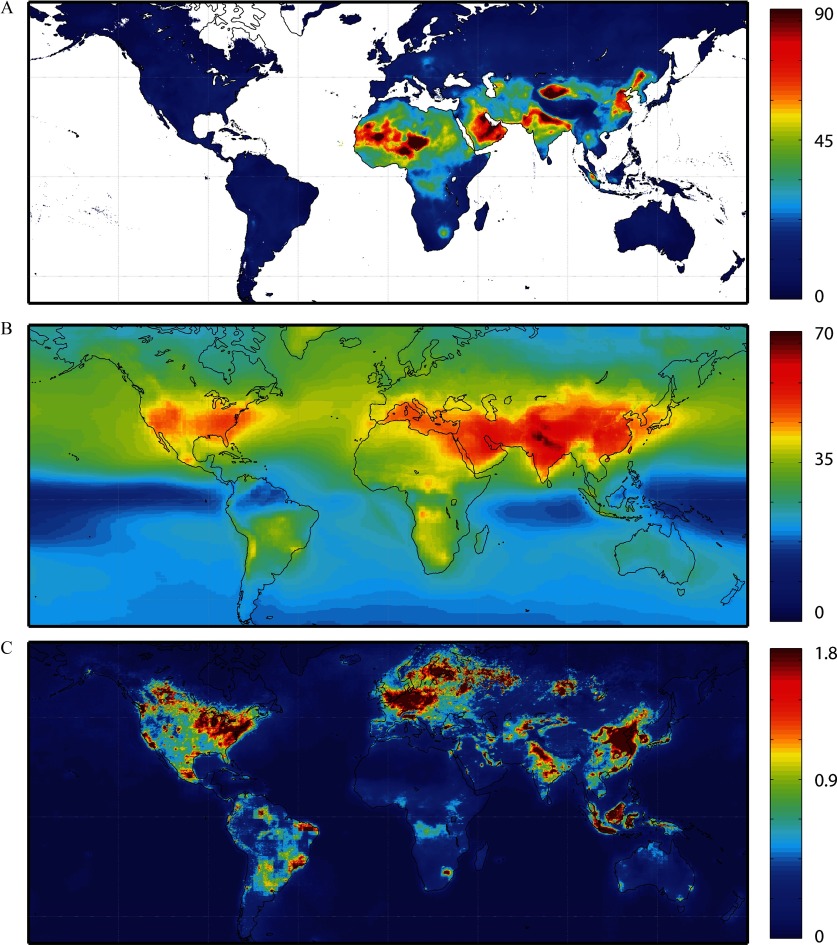
Figure 1.
Pollutant concentrations used to estimate asthma impacts. (A) concentrations (annual average, in ) in 2015, described by van Donkelaar et al. (2016; ). (B) Ozone concentrations in 2015 (annual average of 8hr daily maximum, in ppb), using multi-model average from TF HTAP ensemble (). (C) concentrations in 2015 (annual average, in ppb), using satellite-derived dataset from method described by Lamsal et al. (2008) and adjusted by modeled ratio of daily average to concentration ().
Table 2.
Relative risks (RRs), extracted from meta-analyses of epidemiological studies, which were used for estimating asthma impacts [95% confidence intervals (CI) in parentheses]. RRs are reported per for and per for ozone and (RRs reported per were converted to RRs per assuming ambient pressure of 1 atmosphere and temperature of 25°C).
| Pollutant | Study | Concentration range | Relative Risk – all ages | Relative Risk – pediatric () | Relative Risk – adult (18–64 years) | Relative Risk – elderly (65 years and older) | Used? |
|---|---|---|---|---|---|---|---|
| Short-term exposure and asthma exacerbation | |||||||
| Orellano et al. (2017) | NR | 1.03 (1.01–1.05) | – | – | – | Core | |
| Zheng et al. (2015) | 1.02 (1.02–1.03) | 1.03 (1.01–1.04) | 1.03 (1.01–1.05) | 1.02 (1.01–1.03) | Core | ||
| Zhang et al. (2016) | 1.01 (1.00–1.03) | 1.02 (1.02–1.03) | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) | Core | ||
| Fan et al. (2016) | – | 1.04 (1.02–1.05) | 1.02 (1.01–1.03) | – | SA – Pediatric | ||
| Lim et al. (2016) | – | 1.05 (1.03–1.07) | – | – | SA – Pediatric | ||
| Ozone | Orellano et al. (2017) | NR | 1.03 (1.01–1.06) | – | – | – | Core |
| Zheng et al. (2015) | 1.02 (1.01–1.02) | 1.02 (1.01–1.02) | 1.03 (1.02–1.04) | 1.02 (1.00–1.03) | Core | ||
| Zhang et al. (2016) | 1.05 (1.04–1.07) | 1.06 (1.04–1.07) | 1.05 (1.00–1.11) | 1.05 (1.03–1.06) | Core | ||
| Orellano et al. (2017) | NR | 1.02 (1.01–1.04) | 1.04 (1.00–1.08) | – | – | Core | |
| Zheng et al. (2015) | 1.03 (1.03–1.04) | 1.03 (1.03–1.04) | 1.02 (1.01–1.03) | 1.04 (1.03–1.05) | Core | ||
| Zhang et al. (2016) | 1.03 (1.02–1.05) | 1.07 (1.05–1.09) | 1.02 (0.98–1.07) | 1.05 (1.03–1.07) | Core | ||
| Favarato et al. (2014) | – | 1.12 (1.00–1.22) | – | – | SA – Pediatric | ||
| Weinmayr et al. (2010) | – | 1.06 (1.00–1.12) | – | – | SA – Pediatric | ||
| Long-term exposure and asthma incidence | |||||||
| Anderson et al. (2013) | NR | 1.16 (0.98–1.37) | 1.34 (0.96–1.86) | – | – | Core | |
| Jacquemin et al. (2015) | 1.08 (0.77–1.51) | – | – | – | Core | ||
| Khreis et al. (2017) | NR | – | 1.34 (1.11–1.63) | – | – | Core | |
| Bowatte et al. (2015) | NR | – | 1.93 (1.00–3.71) | – | – | No | |
| Gasana et al. (2012) | NR | – | 1.40 (0.77, 2.56) | – | – | No | |
| Anderson et al. (2013) | NR | 1.14 (1.04–1.26) | 1.10 (1.02–1.20) | 1.93 (1.28–2.96) | – | Core | |
| Jacquemin et al. (2015) | 1.20 (0.98–1.43) | – | 1.08 (0.96–1.21) | 1.02 (0.94–1.12) | Core | ||
| Age | Age | ||||||
| Khreis et al. (2017) | NR | – | 1.26 (1.10–1.37) | – | – | Core | |
| Bowatte et al. (2015) | NR | – | 1.18 (0.93–1.48) | – | – | No | |
| Gasana et al. (2012) | NR | – | 1.28 (1.12–1.50) | – | – | No | |
| Takenoue et al. (2012) | NR | – | 1.14 (1.03–1.25) | – | – | No | |
Note: –, no information was collected at that particular examination point; ; .
Concentration–Response Factors
Concentration–response factors for asthma exacerbation and incidence were taken from meta-analyses of epidemiological studies that combined multiple individual studies from different countries into pooled risk estimates. We first conducted a literature review to identify meta-analyses of epidemiological studies on air pollution and asthma, using MEDLINE, PubMed, and Google Scholar, supplemented with additional reviews of U.S. Environmental Protection Agency Integrated Science Assessments for (U.S. EPA 2009) and ozone (U.S. EPA 2015a). We limited the search to meta-analyses pooling results from epidemiological studies across multiple countries to derive relative risk (RR) estimates that might be most generalizable to the global population. We included only studies that were written in English and reported a numerical concentration–response relationship accompanied by an estimate of precision. We excluded studies that only examined personal exposure rather than ambient concentrations (because the concentration estimates we use do not represent personal exposure) or that only included lung function tests.
Based on these criteria, we found 10 meta-analyses on short-term exposure to air pollution (including ozone, , and ) and asthma exacerbation or prevalence and six meta-analyses on long-term exposure to air pollution (including and ) and asthma incidence. Our search did not identify any multinational meta-analyses reporting a significant relationship between ozone and asthma incidence, though some individual studies report increased risk (Anderson et al. 2013; McConnell et al. 2002). Although some studies examined relationships between asthma and concentrations, we focused on because globally gridded concentration estimates are not available for .
Asthma exacerbation endpoints were either self-reported increase of symptoms (Karakatsani et al. 2012) or more commonly, asthma-related ERVs and/or hospital admissions. We identified three asthma exacerbation studies that addressed ozone, , and for all ages, children, and adults (Orellano et al. 2017; Zhang et al. 2016; Zheng et al. 2015), and used these studies for the core results because they included the broadest range of populations, pollutants, and ages. We found six additional meta-analyses reporting relationships between asthma ERVs and and for children only (Fan et al. 2016; Favarato et al. 2014; Gehring et al. 2015; Lim et al. 2016; Mölter et al. 2015; Weinmayr et al. 2010). We excluded two studies that had the narrowest age ranges (Gehring et al. 2015; Mölter et al. 2015). We also excluded one study that relied on self-reported asthma symptoms rather than official hospital records or medical diagnoses. In total, we used seven of the 10 meta-analyses found in our literature review for asthma exacerbation. From each of these studies, we extracted the concentration–response functions that we judged to be most broadly representative of exposures among the general population (e.g., all year versus warm or cold season only, pooled all individual studies rather than only statistically significant studies, included both men and women, etc.), without regard for the magnitude or significance of the risk estimates. We used RRs for combined ERVs and hospital admissions to take advantage of the largest number of underlying studies in the meta-analyses and the largest number of meta-analyses, as only a subset of studies and meta-analyses reported RRs specifically for ERVs (e.g., Orellano et al. 2017 reported RRs only for ERVs and hospital admissions together). Ideally, a broad category of asthma exacerbations would most comprehensively account for the effect of air pollution on worsening asthma symptoms, as only a portion of exacerbations end in ERVs or hospital admissions (e.g., Pollart et al. 2011). We judged that using the combined ERV and hospital admission RRs would capture more of the exacerbation effect of pollution, in comparison with using only ERV or hospital admission RRs.
For asthma incidence, we identified two meta-analyses of long-term epidemiological studies that included and and asthma development among all ages (Anderson et al. 2013; Jacquemin et al. 2015). Neither study reported significant associations for , but one reported a significant association for (Anderson et al. 2013). We also found three meta-analyses examining traffic-related and associations with asthma development among children (Bowatte et al. 2015; Gasana et al. 2012; Khreis et al. 2017), and one on from any source and children (Takenoue et al. 2012). For children, two of the four analyses (Bowatte et al. 2015; Khreis et al. 2017) examining (both traffic-related studies) and all five analyses examining (Anderson et al. 2013; Bowatte et al. 2015; Gasana et al. 2012; Khreis et al. 2017; Takenoue et al. 2012) reported significant associations with pediatric asthma development. To estimate and -attributable asthma incidence, we applied RR estimates for all ages from Anderson et al. (2013) and Jacquemin et al. (2015). We also estimated and -attributable pediatric asthma incidence using RR estimates from Anderson et al. (2013) and from the most recent of the meta-analyses focusing explicitly on traffic-related pollution, Khreis et al. (2017). All RR estimates considered are listed in Table 2. In total, we used three of the six meta-analyses found in our literature review for asthma incidence. One of these meta-analyses reported ranges of 8 to and 0 to (Jacquemin et al. 2015). Concentration ranges in the other two meta-analyses were unreported but are unlikely to be substantially wider because all three meta-analyses were limited to epidemiological studies conducted in the United States and Europe (Anderson et al. 2013; Khreis et al. 2017). Several meta-analyses indicated that most underlying epidemiological studies focused on within-city variation in exposure levels, and studies comparing exposures between communities did not show significant associations with asthma incidence (Anderson et al. 2012, 2013). To more closely capture asthma incidence from the increment of exposure over background levels within each community, we focused on the health burden due to anthropogenic contributions. This method could still overestimate asthma incidence from air pollution because background concentrations within communities are also influenced by regional anthropogenic emissions.
Central RR estimates for asthma ERVs among all ages ranged from 1.01–1.03 per increase in , 1.02–1.05 per increase in ozone, and 1.02–1.03 per increase in (Table 2). For pediatric incident asthma, central RR estimates ranged from 1.34–1.93 per and 1.10–1.28 per . These relationships were all based on single-pollutant models that did not control for other pollutants; thus, there may be overlap between asthma effect estimates for ozone, , and . As these meta-analyses used different methods, captured different sets of studies (with some overlap), and often examined different models and subgroups, we applied RR estimates from multiple studies separately. Of the 87 studies included by Zheng et al. (2015), 12 studies were overlapping with Zhang et al. (2016) and nine studies were overlapping with Orellano et al. (2017). Zheng et al. (2015) and Zhang et al. (2016) had only two studies in common. Concentrations ranges included in the underlying epidemiological studies were reported by two of the three meta-analyses, and ranged from 6.1 to , 2 to ozone, and 5.4 to (Zhang et al. 2016; Zheng et al. 2015). The third meta-analysis is likely to also include wide concentration ranges because the underlying epidemiological studies were conducted in 12 different countries spanning relatively clean locations (e.g., Australia, Canada) to relatively polluted locations (e.g., China; Orellano et al. 2017).
Health Impact Functions
We used the “attributable fraction” approach to estimate asthma ERVs and new asthma cases attributable to each pollutant (e.g., Miettinen 1974), following other air pollution health impact assessments (e.g., Anenberg et al. 2010; Cohen et al. 2017; Fann et al. 2012). It is unclear based on these meta-analyses whether the concentration–response functions between pollutant and asthma effect are linear or nonlinear. Several of the studies indicate that the results given describe linear relationships (Lim et al. 2016; Orellano et al. 2017; Zhang et al. 2016; Zheng et al. 2015), whereas the others did not specify the functional shape (Anderson et al. 2013; Fan et al. 2016; Jacquemin et al. 2015). Linear functions would imply that the same change in risk per unit concentration change would occur in very polluted and very clean atmospheres. Other diseases caused by appear to have nonlinear relationships that flatten out at high concentrations (Burnett et al. 2014; Cohen et al. 2017). Here, to account for potential flattening of the concentration–response relationship at very high concentrations, we applied log-linear relationships for , ozone, and . The log-linear and linear functions describe similar concentration–response relationships within the range of concentrations found in most developed countries ( ) and begin to diverge at higher concentrations found in many developing countries (). We estimated 3–8% more asthma ERVs globally associated with ozone and using a linear rather than log-linear function. Nevertheless, the lack of information about the shape of the concentration–response function or heterogeneity in risk estimates at different concentration levels is a limitation.
The health impact function for the change in asthma ERVs attributable to , ozone, or () is:
| (1) |
where Pop is the gridded population for age group a, Prev is asthma prevalence for country c and age group a, ERV is the fraction of total number of individuals with asthma visiting the emergency room in the last year for country c, is the concentration–response factor from the meta-analyses of epidemiological studies, and X is the gridded pollutant concentration in 2015.
The health impact function for asthma incidence attributable to or () is:
| (2) |
where Inc is the asthma incidence rate for country c and age group a.
We applied these health impact functions in each grid cell globally, separately for each pollutant and concentration–response factor. For estimates of the asthma burden attributable to anthropogenic-only concentrations, we subtracted the gridded burden from natural concentrations from the gridded burden using total concentrations.
Uncertainty bounds for health impacts were calculated by applying the 2.5 percentile or 97.5 percentile of the RR estimate, following Anenberg et al. (2010). Each input to the health impact function is uncertain, including estimated concentrations, baseline disease rates, asthma ERV rates, the concentration–response factor, and the existence and magnitude of population-level low-concentration thresholds, below which air pollutants would not affect asthma. Uncertainty estimates are available for some of these variables ( concentrations, baseline disease rates, concentration–response factor), but not others (ozone and concentrations, asthma ERV rates, low-concentration thresholds). For ozone and concentrations, output from multiple modeling studies are available at global scales to quantify model variability in concentration estimates. However, this variability reflects differences between models and their parameterizations and not necessarily errors in their concentration estimates, which are more difficult to evaluate globally given the paucity of observational data outside the United States, Europe, and parts of Asia. For this pilot analysis, we simplify the treatment of uncertainty by incorporating only error in the concentration–response functions, as the air pollution–asthma RRs are a key novel aspect of this study and likely to contribute most to differences in results that were calculated separately for each RR. The choice of RR was also found to be the most influential source of uncertainty in estimating the global burden of air pollution on mortality (Ostro et al. 2018). Because our resulting confidence intervals reflect only the error in the concentration–response factor for each epidemiological meta-analysis, reported confidence intervals (CIs) should be interpreted within the context of the larger uncertainty surrounding the entire analysis, as has been cautioned elsewhere (Hubbell et al. 2005). In future work, it should be feasible to generate more comprehensive uncertainty intervals that incorporate error in concentrations and baseline disease rates, though additional advances in estimating uncertainty around global ozone and concentrations, asthma ERV rates, and other health impact function inputs will be needed to fully characterize uncertainties in the results.
, Ozone, and Concentrations
We used globally gridded annual average surface concentration estimates (maximum concentration ) for 2015 at resolution developed using information from satellites, a chemical transport model, and in situ monitors (van Donkelaar et al. 2016). Aerosol optical depth from multiple satellite products was combined with a simulation using the GEOS-Chem chemical transport model, followed by geographic weighted regression to fuse the estimates with in situ monitors. The resultant estimates were highly consistent () with out-of-sample cross-validated concentrations from in situ monitors. Although the asthma exacerbation studies examined short-term daily exposures, we used annual average concentrations, following previous studies applying longer-term average concentrations with RR estimates from acute ozone and exposures (West et al. 2006; Anenberg et al. 2009; Fann et al. 2012). Using annual average concentrations has several advantages: computational efficiency and the ability to use datasets that assimilate satellite observations with chemical transport models. Because the concentration–response functions applied here are near-linear, using annual averages is not expected to introduce a substantial amount of error. This method is further supported by only 3–8% differences in estimated ozone and -attributable asthma ERVs when using a linear health impact function in comparison with the log-linear one used in the main results; using a linear model, the sum of daily impact results estimated using daily concentrations would be equal to annual impacts estimated using annually averaged concentrations.
To estimate asthma ERVs attributable to just the anthropogenic portion of each pollutant, we subtracted the asthma burden from modeled natural background concentrations. Natural background concentrations were simulated using the GEOS-Chem chemical transport model with combustion emissions of all species (including from biomass burning) removed. We considered soil dust concentrations to be natural, although they can be subject to human influence, including from land-use changes, population density, and already-realized influence of anthropogenic climate change.
For ground-level ozone, which is not easily detected using satellites (Martin 2008), we used gridded multimodel mean concentrations for the year 2010 simulated by an ensemble of five chemical transport models organized for Phase 2 of the Task Force on Hemispheric Transboundary Air Pollution (TF HTAP) project (Galmarini et al. 2017): CHASER_re1 (Sudo et al. 2002), CHASER_t106 (Sudo et al. 2002), EMEP_rv48 (Simpson et al. 2012), GEOS-Chem adjoint (Henze et al. 2007), and HadGEM2-ES (Collins et al. 2011). Model configurations and details were described by Galmarini et al. (2017). Concentrations were regridded to a common grid and then averaged. We used annual average daily 8-h maximum ozone concentrations (). Using an annual average is consistent with the RR estimates from the meta-analyses, which reported ozone RR estimates for the full year. One meta-analysis found that ozone RR estimates for asthma were similar between warm and cold seasons (Zheng et al. 2015). Because several of the meta-analyses did not distinguish which metric was used to derive the pooled risk estimates, we present results using the daily 8-h maximum because it is between the 24-h average and 1-h maximum in terms of magnitude. Standard deviations in simulated concentrations across the five TF HTAP models were generally greatest in areas with large biomass burning or biogenic influences, because the TF HTAP models only harmonized anthropogenic emissions and not natural emissions (Figure S3).
We used surface concentrations () for 2015 from a dataset developed from satellite observations, following Lamsal et al. (2008). Column observations from Ozone Monitoring Instrument (OMI) onboard the Aura satellite were gridded to resolution, and satellite-derived ground-level concentrations were estimated using vertical structure from the GMI-Replay chemical transport model. Concentrations are annual average at the Aura satellite sun-synchronous overpass time of local time. These concentrations could underestimate actual exposures because midafternoon concentrations are typically at a low point of the diurnal concentration cycle, resulting from a combination of fewer emissions between the morning and evening rush hours, high boundary layer heights, and photolysis of to NO (Lamsal et al. 2008). We therefore multiplied the satellite-derived exposure estimates by the gridded ratio of 24-h average to concentrations simulated by the GMI-Replay model at resolution for the year 2010 (Figure S3). Resulting concentrations used to estimate health impacts are annual average of the 24-h average concentration in each gridcell. Because our grid is too coarse to resolve near-roadway concentrations, these concentrations are still likely lower than the measured concentrations used in epidemiological studies focusing on traffic-related air pollution in particular. Because the use of satellite-derived concentrations is relatively novel for global health impact assessment, we compared the annual average surface derived from Aura OMI with those derived from the Dutch OMI (DOMINO) data product V2.0 (Boersma et al. 2011), finding only minor differences (Figure S3).
Demographics and Baseline Asthma Rates
We used global gridded population of the world from the Center for International Earth Science Information Network (CIESIN) Gridded Population of the World v4 for the year 2015, regridding the 30 arc-second resolution to (total population 7.34 billion). We obtained country- and age-specific incidence and prevalence rates for asthma (defined by ICD-10 code J45–J45.5, J45.8–J45.9, and ICD-9 code 493–493.4, 493.8–493.9) from the Institute for Health Metrics and Evaluation (IHME) for 2015 (GBD 2015 Chronic Respiratory Disease Collaborators 2017). The definition of asthma was a reported diagnosis by a physician, with wheezing in the past 12 months, though asthma can also have clinical subphenotypes (Anderson et al. 2013). National asthma incidence and prevalence rates were estimated using the DisMod MR 2.1 modeling tool (earlier version described by Barendregt et al. 2003) with input data from the following sources: WHO Study on Global Aging and Adult Health series, the WHO World Health Survey series, the Belgian Health Interview Survey, studies carried out as part of the International Study of Asthma and Allergies in Childhood (ISAAC) collaboration, claims data in the United States, and other sources for individual countries where available (GBD 2015 Chronic Respiratory Disease Collaborators 2017). In the absence of data from a particular geography (country or in limited cases, subnational administrative regions), information from the parent geography was used. The percentage of geographies with data on asthma rates (65%) was relatively high in comparison with many other diseases, including ischemic heart disease (51%) and COPD (32%). The top five countries for both asthma incidence and prevalence were India, China, Brazil, Indonesia, and the United States, driven largely by population size (GBD 2015 Chronic Respiratory Disease Collaborators 2017). We used the IHME datasets to calculate pediatric (under 18 y), adult (18–64 y), and elderly (65 y and older) populations. Country-specific baseline disease rates are uncertain, particularly in countries where rates were modeled rather than derived empirically (GBD 2015 Chronic Respiratory Disease Collaborators 2017), and estimated pollution-attributable asthma impacts would scale linearly with a change in the baseline disease rate.
We used asthma ERVs as a proxy for asthma exacerbation, though many cases of exacerbation may be self-treated with medication and may not manifest in an ERV. To estimate pollution-attributable asthma ERVs, we constructed a dataset of the rate of ERVs among asthmatics around the world. We conducted a literature review and identified 16 studies surveying health care utilization rates among asthmatics in 54 countries and Hong Kong (Table 1; Allegra et al. 2012; Boonsawat et al. 2015; Corrado et al. 2013; Desalu et al. 2013; Mahboub et al. 2010; Khadadah et al. 2009; Nafti et al. 2009; Neffen et al. 2010; Price et al. 2014; Rabe et al. 2004; Sastre et al. 2016; Su et al. 2013; Thompson et al. 2013; Turktas et al. 2010; Zainudin et al. 2005; Zemedkun et al. 2014). Surveys administered included the Asthma Insights and Reality Survey (AIR), Recognise Asthma and Link to Symptoms and Experience (REALISE), Insights and Management Survey (AIM), and International Asthma Control Test (ACT). The studies reported the rate of ERVs and hospital admissions among study participants, which we used to derive a nationwide rate. For countries where more than one estimate were available from the included studies, we weighted the rates based on study sample size and used this rate for the country-wide rate. We categorized the available country rates by World Health Organization (WHO) regions and World Bank income categories (WHO 2017c, 2017b) to generate regional rates (average of available national rates weighted by sample size) and applied these to countries without data. In regions where only one estimate was available for the region, the available estimate was applied to all countries with missing data in that region. For low-income countries where no rates were available in the eastern Mediterranean (4 countries), Europe (3), Americas (1), and Southeast Asia (4), we applied the weighted rate from low-middle income countries in that region.
As the data set is not age-specific, we applied the ERV rate among people with asthma uniformly to each age group. The fraction of the total number of people with asthma visiting an emergency department in the last year ranged from 7.9% in France to 52% in Jordan, Kuwait, Lebanon, Oman, and United Arab Emirates, though approximately 60% of locations for which survey data were available had fractions in the 20–40% range (Figures S1 and S2). Estimated pollution-attributable asthma ERVs would scale linearly with a change in this fraction. We used this survey approach to maximize consistency across countries, but the approach is limited by only including approximately one-quarter of countries globally and by small survey sample size in each country. Future studies could explore the utility of combining this information with medical records of ERVs to create a more comprehensive dataset of global asthma-related ERVs. We were unable to develop fractions for a combined category of asthma ERVs and hospital admissions from these survey results; we therefore used ERV fractions with the RRs for combined ERVs and hospital admissions, judging that ERVs are likely to capture more asthma exacerbations in comparison with hospital admissions. The use of ERV fractions with RRs combining ERVs and hospital admissions may overestimate pollution-attributable asthma ERVs but likely underestimates pollution-attributable asthma exacerbations (because pollution-attributable hospital admissions are not calculated).
Results
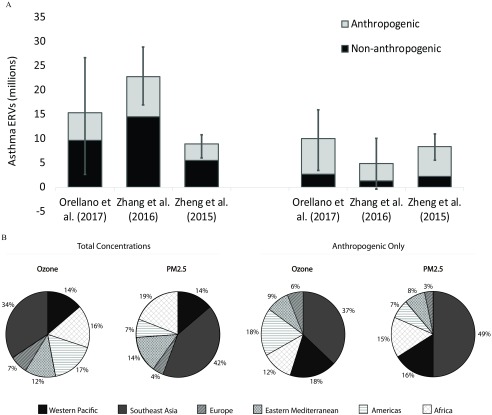
Globally, we estimated that 9–23 million asthma ERVs among all ages in 2015 were attributable to ozone (Figure 2), 5–10 million were attributable to , and 0.4–0.5 million were attributable to (0.4%; Figure S4), considering both anthropogenic and natural sources of pollution. Respectively, ozone, , and -attributable asthma ERVs represented 8–20%, 4–9%, and 0.4% of the 116 million global asthma ERVs in total (Figure 3 and Figure S5). The ranges reflect central RR estimates of three different epidemiological meta-analyses (Orellano et al. 2017; Zhang et al. 2016; Zheng et al. 2015). impacts are likely underestimated because our methods do not fully capture near-roadway exposures, which can be larger than concentrations less than half a kilometer away (Karner et al. 2010), though there is also likely to be some overlap with the -attributable asthma ERV estimates. To evaluate the fraction of the burden associated with pollution sources that are more easily controllable, we also examined the influence of anthropogenic-only concentrations by subtracting “natural concentration” (simulated by setting anthropogenic emissions to zero in the chemical transport model) from total concentrations. Results using total concentrations were 2.7 times and 1.4 times higher than impacts of anthropogenic-only concentrations for ozone (3.4–8.3 million asthma ERVs, 3–7% of global total) and (3.6–7.3 million asthma ERVs, 3–6%), respectively (Figure S6). The higher impact of total versus anthropogenic concentrations is largely due to sea salt and dust, though some airborne dust is attributable to human influences, such as land-use changes, population density, and potentially already-realized effects of anthropogenic climate change.
Figure 2.
Global asthma ERVs associated with total ozone and concentrations among all ages in 2015, using RR central estimates from three epidemiological meta-analyses. (A) Asthma ERVs (millions) attributable to ozone and from anthropogenic and all sources. Confidence intervals (CI) (95%) reflect uncertainty in RR only. (B) Portion of pollution-attributable asthma ERVs occurring in each world region (results identical for all three RR estimates).
Figure 3.
Percent of global and regional asthma ERVs for all ages in 2015 that are attributable to total ozone (top) and (bottom) concentrations, using RR central estimates from three epidemiological meta-analyses.
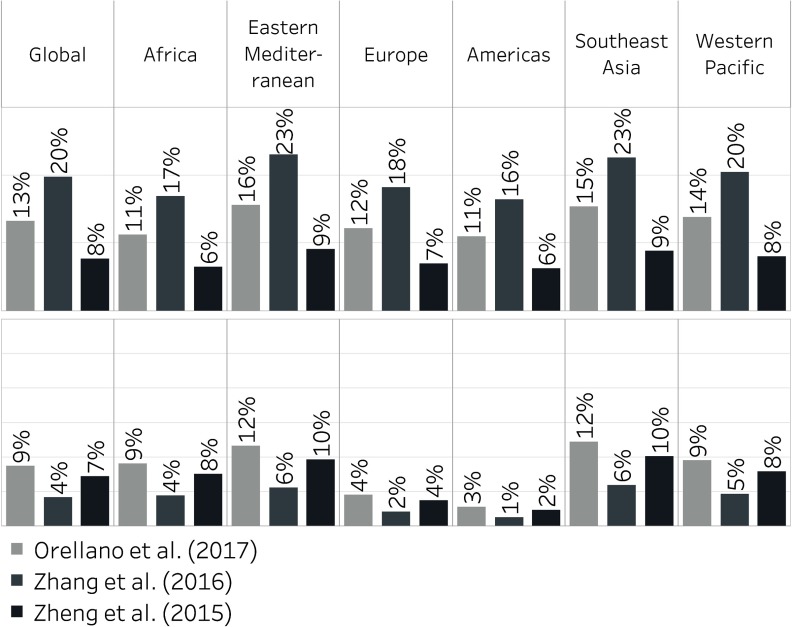
We tabulated gridded asthma ERVs by WHO regions. Nearly half (48%) of estimated ozone-attributable and over half (56%) of -attributable asthma ERVs were estimated in Southeast Asia (includes India), and western Pacific regions (includes China; Figure 2). Larger percentages were estimated in Africa and eastern Mediterranean regions when using total concentrations compared with anthropogenic-only concentrations, driven by high dust concentrations in these regions. Ozone and were estimated to be responsible for 6–23% and 1–12%, respectively, of all asthma ERVs regionally, depending on the region and risk estimate applied (Figure 3). For Europe, we estimated that ozone and were responsible for 7–18% and 2–4% of asthma ERVs, respectively. The percentage for is lower than a previous estimate of 15% attributable to road traffic among children in European cities, which used distance from busy roads as a proxy for pollutant exposure (Perez et al. 2013).
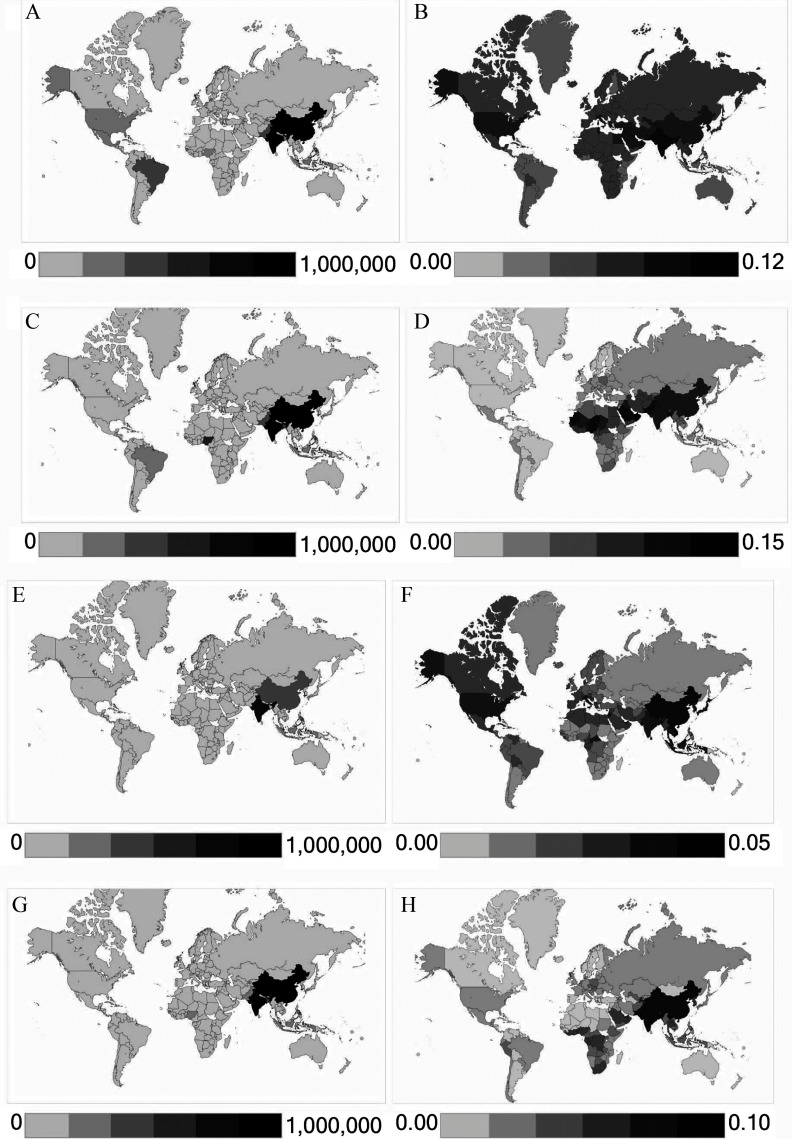
Of all countries globally, India and China had the most estimated asthma ERVs attributable to total air pollution concentrations, respectively contributing 23% and 10% of global asthma ERVs estimated to be associated with ozone, 30% and 12% for , and 15% and 17% for (Figure 4). Comparing -attributable with ozone-attributable asthma ERVs in each country, whether one of these pollutants dominates over the other in terms of impacts on asthma exacerbation is not clear (Figure 4 and Figure S7). However, in the Americas and in most European countries, ozone appears to exert more influence on asthma ERVs, for both total and anthropogenic concentrations, regardless of the RR estimate applied. In Africa, Southeast Asia, western Pacific regions, and eastern Mediterranean regions, we estimated substantial impacts for both and ozone, and which pollutant dominates depends on the RR estimate applied.
Figure 4.
Asthma ERVs attributable to and ozone in 2015, using Zheng et al. (2015) RR central estimates. Panels show asthma ERVs attributable to total: (A) ozone, number of cases; (B) ozone, fraction of national asthma ERVs; (C) , number of cases (D) , fraction of national asthma ERVs. Panels (E–H) show the same results but using anthropogenic concentrations.
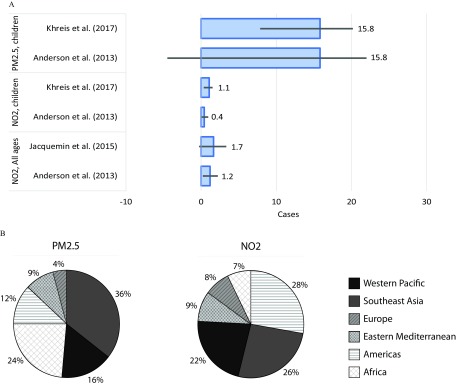
We estimated the impacts of air pollution exposure on incident asthma as a secondary analysis, focusing only on pollutants and age groups for which epidemiological meta-analyses reported significant risk estimates: exposure among all age groups (all ages, children, and adults), and exposure among children (Table 2). Evidence of significant associations between and asthma incidence appears relatively consistent across epidemiological meta-analyses (Anderson et al. 2013; Gasana et al. 2012; Khreis et al. 2017; Takenoue et al. 2012) with only one meta-analysis finding an insignificant association for children (Bowatte et al. 2015). However, the evidence for is mixed, with two of the four studies in our literature review reporting significant associations with pediatric asthma incidence (Bowatte et al. 2015; Khreis et al. 2017) and two others reporting no significant associations (Anderson et al. 2013; Gasana et al. 2012). In contrast to asthma ERVs for which we estimated impacts of total concentrations as main results, our main results for air pollution-attributable asthma incidence used concentrations above “natural background” levels in each gridcell, because the epidemiological studies focused on within-city variation rather than between-city variation. Using central RR estimates from four epidemiological meta-analyses, we estimated that 400,000 (95% CI: 100,000 to 800,000) to 1.1 million (400,000 to 1.4 million) new pediatric asthma cases could occur globally each year due to anthropogenic concentrations (Figure 5). Here, our satellite-derived concentration estimates were too coarsely resolved to fully capture high near-roadway concentrations. Although the epidemiological evidence for associations with is stronger, our estimates for -attributable asthma incidence are 1–2 orders of magnitude higher. Using RR estimates from two epidemiological meta-analyses (Anderson et al. 2013; Bowatte et al. 2015), we estimated that 16 million (95% CIs: 9 to 19 million and to 20 million) new pediatric asthma cases could occur globally each year due to anthropogenic concentrations, translating to 33% of global pediatric asthma incidence (Figure S8). The percentage of national pediatric asthma incidence that may be attributable to anthropogenic was estimated to be 22% in the United States, 57% in India, 51% in China, and over 70% in Bangladesh (Figure S9). Approximately half of estimated - and - attributable new asthma cases were in the Southeast Asia and western Pacific regions (Figure 5). Estimated asthma incidence attributable to and translate to DALYs among children ( of these attributable to ), adding to the most recently estimated DALY burden from ambient air pollution among all ages (Cohen et al. 2017). Results are 1.2 and 1.6 times higher when using total concentrations for and , respectively.
Figure 5.
Asthma incidence attributable to anthropogenic and in 2015. (A) Number of new asthma cases (millions) associated with and for various age groups using RRs from multiple epidemiological meta-analyses. Confidence intervals (CI) (95%) reflect error in the RR estimate only. (B) Percent of pollution-attributable new asthma cases among children occurring in each region (for each pollutant, results are identical for all RR estimates applied).
Discussion and Conclusions
We estimated that 9–23 million and 5–10 million annual asthma emergency room visits globally in 2015 could be attributable to total ozone and concentrations, respectively, corresponding to 8–20% and 4–9%, respectively, of the annual number of global visits. The range reflects the application of central risk estimates from different epidemiological meta-analyses. We estimated that anthropogenic emissions are responsible for approximately 37% of ozone impacts and 73% of impacts. The remaining impacts are attributed to naturally occurring ozone precursor emissions (e.g., from vegetation and lightning) and (e.g., dust, sea salt), though several of these sources are also subject to human influence from land-use changes, anthropogenic climate changes, and other interactions between humans and the environment. India and China were estimated to contribute 23% and 10%, respectively, of all asthma ERVs attributable to ozone globally, and 30% and 12%, respectively, of those ERVs attributable to .
These results are subject to a number of important limitations and uncertainties, and estimated pollution-attributable asthma impacts could be overestimated or underestimated as a result. First, we assumed that reported associations between , ozone, and and the asthma exacerbation and incidence outcomes included in our study are causal associations, following previous health impact analyses by the U.S. EPA (U.S. EPA 2012, 2015b) and in the literature (e.g., Fann et al. 2012). Although the IHME Global Burden of Disease study considers only health effects of and ozone in estimating the ambient air pollution burden of disease, we also considered , because of the strength and significance of its associations with asthma exacerbation and incidence, and because may be a more precise marker of combustion-related pollution, because both and ozone can be created by noncombustion sources (e.g., dust, biogenic precursors). As we used single pollutant epidemiological models that did not control for other pollutants, some overlap among ozone, , and impacts is likely (thus causing our pollutant-specific results to together overestimate the burden from combined pollution components) (Winquist et al. 2014). Similar overlap among health impacts attributed to individual pollutants has been identified in previous assessments focusing on mortality effects, for which multipollutant models are available (WHO 2013). Without direct evaluation, the degree of underestimation or overestimation from the effects of pollution mixtures is unknown. Realistically, each of these pollutants may be an indicator of broader pollution mixtures. The magnitude of overlap in asthma ERVs attributable to individual pollutants is currently unquantifiable, though limited evidence suggests that effects of ozone on asthma ERVs could be independent from traffic-related pollution (Strickland et al. 2010). In addition, our reliance on single-pollutant models, though necessary given the lack of available multipollutant models, leads to uncertainty regarding the existence and magnitude of associations between each pollutant and asthma outcomes, because it is possible that reported associations are sensitive to control for copollutants.
Although we included uncertainty in the RR estimates to compare across epidemiological meta-analyses, we were unable to incorporate important uncertainties in other input variables to the health impact function, including asthma ERV rates and concentrations. The country-specific survey data we used to construct the dataset of asthma ERV rates were often limited to a small sample size, ranging from 100 to 5,063 (across multiple studies in Italy). It is not clear whether these population subsets are representative of the general population living with asthma, and given the small sample sizes, these asthma ERV rates are highly uncertain. Variation in asthma ERV rates around the world could be due to differences in asthma prevalence, health services and how they are used, and degree of asthma management, all of which vary worldwide and would realistically affect the number of estimated cases of asthma health end points attributable to air pollution. We also included only asthma ERVs among the asthma group, though the epidemiological studies did not distinguish between asthma ERVs among undiagnosed and diagnosed individuals. Although the percentage of asthma ERVs among people with undiagnosed wheezing is lower than that for individuals diagnosed with asthma, the prevalence of people with undiagnosed wheezing could be large (Gerald et al. 2009; Yeatts et al. 2003). However, some ERVs occurring among people with asthma may not actually be related to asthma, and if this number is substantial, our results could be overestimates.
We also transferred pooled risk estimates and asthma ERV rates to countries with no data, though the meta-analyses used represented a variety of countries on all populated continents except Africa, and ERV rates were from 55 locations on all populated continents. Although extrapolating beyond these countries is necessary in a global assessment, differences in health systems may exist that we were unable to account for (including acute-care delivery and diagnostic practices, population characteristics and behavior, and other factors), that would influence the representativeness of data from one country on another. These surveys may not be adequately nationally representative and could be biased by the age pattern of individuals in the study. Future studies could examine availability and consistency of coded emergency room records from different countries as another data source for this variable. The direction of influence of these uncertainties on estimated pollution-attributable asthma ERVs is unknown. Relying only on asthma ERVs will underestimate the impact of air pollution on asthma exacerbation more broadly, as it excludes hospital admissions and less severe subclinical symptoms, such as respiratory symptoms and lung function decrements (Guarnieri and Balmes 2014).
Another important limitation is the potential mismatch between the exposure metrics we used versus those used in the epidemiology studies. For example, we used the annual average of the 8-h daily maximum for ozone concentrations, because the meta-analyses did not clarify whether they used 1-h daily maximum, 24-h average, or some other metric. Our results may underestimate ozone asthma impacts if the RR estimates were derived from 1-h daily maximum but may overestimate impacts if the RR estimates were derived from 24-h averages. As has been shown elsewhere, the choice of concentration metric has important implications for health impact assessment (Malley et al. 2017). Additionally, although the asthma-exacerbation epidemiological studies examined daily exposures, following previous studies (e.g., Fann et al. 2012), we used annual average concentrations for computational efficiency and to leverage concentration datasets that assimilate satellite observations with chemical transport models. Using annual averages could lead to error in estimated pollution-attributable asthma ERVs, particularly for locations where daily concentrations are highly variable. Our approach is likely a reasonable approximation in many countries, because the concentration–response functions applied here are near-linear throughout a broad range of concentrations, and our assumption is supported by only 3–8% differences in estimated air pollution-attributable asthma ERVs using a linear model in comparison with the log-linear model used for the core results. However, it is possible that there is an adaptation of individuals to higher-than-average concentrations, such that only short-term fluctuations exacerbate asthma and that constant elevated levels do not. This possibility is not an issue for asthma incidence, which is related to long-term exposure. We used a multimodel ensemble for ozone concentration to leverage multiple model parameterizations, but all models were too coarse to sufficiently capture chemical nonlinearities in ozone formation and loss at urban and suburban scales (e.g., ozone titration). For , our spatial resolution of was too coarse to capture high near-road exposures. We also applied concentration–response relationships regardless of particle type or mixture, as is commonly assumed to assess mortality impacts (Cohen et al. 2017). For asthma incidence, we estimated the impacts of and concentrations above the “natural background” as our main results, because the epidemiological studies focused on effects of within-city concentration variation, rather than between-city variation. This method could still overestimate asthma incidence from air pollution since background concentrations within communities are also influenced by regional anthropogenic emissions.
We have provided first estimates of the global burden of ambient air pollution on asthma exacerbation and incidence, though they are subject to many assumptions and data limitations. We anticipate that future studies will further advance the input data and methods piloted here, for example by considering multipollutant epidemiological models. Improved epidemiological data, exposure estimates, and disease rates would enable refinements to analyses of air pollution’s contribution to the large global asthma burden. Future studies should also strive to capture asthma impacts from high near-roadway and exposures at finer spatial scales (Khreis et al. 2018; Larkin et al. 2017). Nevertheless, these first estimates further demonstrate the range of global public health impacts that may be associated with ambient air pollution, already considered the leading environmental health risk factor globally (Cohen et al. 2017; GBD 2016 Risk Factors Collaborators 2017). These estimates also indicate the magnitude of the asthma burden that could be avoided by addressing air pollution.
Supplementary Material
Acknowledgments
Support was provided by the NASA Health and Air Quality Applied Science Team, NASA Aura ACMAP, the Stockholm Environment Institute Low Emissions Development Pathways Initiative, and the Global Environment Research Fund (S-12) of the Japan Ministry of the Environment. The authors thank the HTAP modelers for access to their simulated ozone concentrations and the Institute for Health Metrics and Evaluation for making their baseline disease rates publicly available. The authors thank A. Cohen for helpful discussions on the methods. The views in this manuscript are those of the authors alone and do not necessarily reflect the policy of their employers. The views expressed in this document are solely those of the authors and their employers do not endorse any products or commercial services mentioned in this publication.
References
- Allegra L, Cremonesi G, Girbino G, Ingrassia E, Marsico S, Nicolini G, et al. 2012. Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med 106(2):205–214, PMID: 22035853, 10.1016/j.rmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Butland BK, van Donkelaar A, Brauer M, Strachan DP, Clayton T, et al. 2012. Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ Health Perspect 120(9):1333–1339, PMID: 22548921, 10.1289/ehp.1104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Favarato G, Atkinson RW. 2013. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health 6(1):47–56, 10.1007/s11869-011-0144-5. [DOI] [Google Scholar]
- Anenberg S, Horowitz LW, Tong DQ, West JJ. 2010. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect 118(9):1189–1195, PMID: 20382579, 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anenberg S, West JJ, Fiore AM, Jaffe DA, Prather MJ, Bergmann D, et al. 2009. Intercontinental impacts of ozone pollution on human mortality. Environ Sci Technol 43(17):6482–6487, PMID: 19764205, 10.1021/es900518z. [DOI] [PubMed] [Google Scholar]
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. 2009. Economic burden of asthma: a systematic review. BMC Pulm Med 9:24, PMID: 19454036, 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt JJ, van Oortmarssen GJ, Vos T, Murray CJ. 2003. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr 1(1):4, PMID: 12773212, 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma KF, Eskes HJ, Dirksen RJ, van der A RJ, Veefkind JP, Stammes P, et al. 2011. An improved tropospheric NO2 column retrieval algorithm for the Ozone Monitoring Instrument. Atmos Meas Tech 4(9):1905–1928, 10.5194/amt-4-1905-2011. [DOI] [Google Scholar]
- Boonsawat W, Thompson PJ, Zaeoui U, Samosorn C, Acar G, Faruqi R, et al. 2015. Survey of asthma management in Thailand - the asthma insight and management study. Asian Pac J Allergy Immunol 33(1):14–20, PMID: 25840629, 10.12932/AP0473.33.1.2015. [DOI] [PubMed] [Google Scholar]
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. 2015. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 70(3):245–256, PMID: 25495759, 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Pope CA 3rd, Ezzati M, Olives C, Lim SS, Sumi Mehta, et al. 2014. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect 122(4):397–403, PMID: 24518036, 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389(10082):1907–1918, PMID: 28408086, 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WJ, Bellouin N, Doutriaux-Boucher M, Gedney N, Halloran P, Hinton T, et al. 2011. Development and evaluation of an Earth-System model – HadGEM2. Geosci Model Dev 4(4):1051–1075, 10.5194/gmd-4-1051-2011. [DOI] [Google Scholar]
- Corrado A, Renda T, Polese G, Rossi A. SERENA (Studio ossERvazionalE per il monitoraggio dell’asma non coNtrollAto)/AIPO Study Group. 2013. Assessment of asthma control: the SERENA study. Respir Med 107(11):1659–1666, PMID: 24045118, 10.1016/j.rmed.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Desalu OO, Onyedum CC, Adeoti AO, Ozoh OB, Fadare JO, Salawu FK, et al. 2013. Unmet needs in asthma treatment in a resource-limited setting: findings from the survey of adult asthma patients and their physicians in Nigeria. Pan Afr Med J 16:20, PMID: 24498469, 10.11604/pamj.2013.16.20.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Li S, Fan C, Bai Z, Yang K. 2016. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res Int 23(1):843–850, PMID: 26347419, 10.1007/s11356-015-5321-x. [DOI] [PubMed] [Google Scholar]
- Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. 2012. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal 32(1):81–95, PMID: 21627672, 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- Favarato G, Anderson HR, Atkinson R, Fuller G, Mills I, Walton H. 2014. Traffic-related pollution and asthma prevalence in children. Quantification of associations with nitrogen dioxide. Air Qual Atmos Health 7(4):459–466, PMID: 25431630, 10.1007/s11869-014-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmarini S, Koffi B, Solazzo E, Keating T, Hogrefe C, Schulz M, et al. 2017. Technical note: coordination and harmonization of the multi-scale, multi-model activities HTAP2, AQMEII3, and MICS-Asia3: simulations, emission inventories, boundary conditions, and model output formats. Atmos Chem Phys 17(2):1543–1555, PMID: 29541091, 10.5194/acp-17-1543-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. 2012. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res 117:36–45, PMID: 22683007, 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- GBD 2015 Chronic Respiratory Disease Collaborators. 2017. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 5(9):691–706, PMID: 28822787, 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Risk Factors Collaborators. 2017. Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390 (10100):1345–1422, PMID: 28919119, 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, et al. 2015. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 3(12):933–942, PMID: 27057569, 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- Gerald JK, Sun Y, Grad R, Gerald LB. 2009. Asthma morbidity among children evaluated by asthma case detection. Pediatrics 124(5):e927–e933, PMID: 19841121, 10.1542/peds.2008-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Asthma Network. 2014. The Global Asthma Report 2014. http://www.globalasthmareport.org/2014/resources/Global_Asthma_Report_2014.pdf [accessed 30 August 2017].
- Guarnieri M, Balmes JR. 2014. Outdoor air pollution and asthma. Lancet 383(9928):1581–1592, PMID: 24792855, 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DK, Hakami A, Seinfeld JH. 2007. Development of the adjoint of GEOS-Chem. Atmos Chem Phys 7(9):2413–2433, 10.5194/acp-7-2413-2007. [DOI] [Google Scholar]
- Hubbell BJ, Hallberg A, McCubbin DR, Post E. 2005. Health-related benefits of attaining the 8-hr ozone standard. Environ Health Perspect 113(1):73–82, PMID: 15626651, 10.1289/ehp.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin B, Siroux V, Sanchez M, Carsin A-E, Schikowski T, Adam M, et al. 2015. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ Health Perspect 123(6):613–621, PMID: 25712593, 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakatsani A, Analitis A, Perifanou D, Ayres JG, Harrison RM, Kotronarou A, et al. 2012. Particulate matter air pollution and respiratory symptoms in individuals having either asthma or chronic obstructive pulmonary disease: a European multicentre panel study. Environ Health Glob Access Sci Source 11:75, PMID: 23039312, 10.1186/1476-069X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA. 2010. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol 44(14):5334–5344, PMID: 20560612, 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Khadadah M, Mahboub B, Al-Busaidi NH, Sliman N, Soriano JB, Bahous J. 2009. Asthma insights and reality in the Gulf and the near East. Int J Tuberc Lung Dis Off Dis 13(8):1015–1022, PMID: 19723383. [PubMed] [Google Scholar]
- Khreis H, de Hoogh K, Nieuwenhuijsen MJ. 2018. Full-chain health impact assessment of traffic-related air pollution and childhood asthma. Environ Int 114:365–375, PMID: 29602620, 10.1016/j.envint.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. 2017. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int 100:1–31, PMID: 27881237, 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Lamsal LN, Martin RV, van Donkelaar A, Steinbacher M, Celarier EA, Bucsela E, et al. 2008. Ground-level nitrogen dioxide concentrations inferred from the satellite-borne Ozone Monitoring Instrument. J Geophys Res 113, 10.1029/2007JD009235. [DOI] [Google Scholar]
- Larkin A, Geddes JA, Martin RV, Xiao Q, Liu Y, Marshall JD, et al. 2017. Global land use regression model for nitrogen dioxide air pollution. Environ Sci Technol 51(12):6957–6964, PMID: 28520422, 10.1021/acs.est.7b01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Kwon HJ, Lim JA, Choi JH, Ha M, Hwang SS, et al. 2016. Short-term effect of fine particulate matter on children’s hospital admissions and emergency department visits for asthma: a systematic review and meta-analysis. J Prev Med Public Health 49(4):205–219, PMID: 27499163, 10.3961/jpmph.16.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboub BHS, Santhakumar S, Soriano JB, Pawankar R. 2010. Asthma insights and reality in the United Arab Emirates. Ann Thorac Med 5(4):217–221, PMID: 20981181, 10.4103/1817-1737.69109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley CS, Henze DK, Kuylenstierna JCI, Vallack HW, Davila Y, Anenberg SC, et al. 2017. Updated global estimates of respiratory mortality in adults ≥ 30 years of age attributable to long-term ozone exposure. Environ Health Perspect 125(8):087021, PMID: 28858826, 10.1289/EHP1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RV. 2008. Satellite remote sensing of surface air quality. Atmos Environ 42(34):7823–7843, 10.1016/j.atmosenv.2008.07.018. [DOI] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. 2002. Asthma in exercising children exposed to ozone: a cohort study. Lancet 359(9304):386–391, PMID: 11844508, 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- Miettinen OS. 1974. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 99(5):325–332, PMID: 4825599, 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- Mölter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, et al. 2015. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J 45(3):610–624, PMID: 25323237, 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- Nafti S, Taright S, El Ftouh M, Yassine N, Benkheder A, Bouacha H, et al. 2009. Prevalence of asthma in North Africa: the Asthma Insights and Reality in the Maghreb (AIRMAG) study. Respir Med 103 (Suppl 2):S2–11, PMID: 20122625, 10.1016/S0954-6111(09)70022-8. [DOI] [PubMed] [Google Scholar]
- Neffen H, Gonzalez SN, Fritscher CC, Dovali C, Williams AE. 2010. The burden of unscheduled health care for asthma in Latin America. J Investig Allergol Clin Immunol 20(7):596–601, PMID: 21314001. [PubMed] [Google Scholar]
- Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. 2017. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PloS One 12(3):e0174050, PMID: 28319180, 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Spadaro JV, Gumy S, Mudu P, Awe Y, Forastiere F, et al. 2018. Assessing the recent estimates of the global burden of disease for ambient air pollution: methodological changes and implications for low- and middle-income countries. Environ Res 166:713–725, PMID: 29880237, 10.1016/j.envres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Perez L, Declercq C, Iñiguez C, Aguilera I, Badaloni C, Ballester F, et al. 2013. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur Respir J 42(3):594–605, PMID: 23520318, 10.1183/09031936.00031112. [DOI] [PubMed] [Google Scholar]
- Pollart SM, Compton RM, Elward KS. 2011. Management of acute asthma exacerbations. Am Fam Physician 84:41–47, PMID: 21766754. [PubMed] [Google Scholar]
- Price D, Fletcher M, van der Molen T. 2014. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 24:14009, PMID: 24921985, 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe KF, Adachi M, Lai CKW, Soriano JB, Vermeire PA, Weiss KB, et al. 2004. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 114(1):40–47, PMID: 15241342, 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Sastre J, Fabbri LM, Price D, Wahn HU, Bousquet J, Fish JE, et al. 2016. Insights, attitudes, and perceptions about asthma and its treatment: a multinational survey of patients from Europe and Canada. World Allergy Organ J 9:13, PMID: 27195057, 10.1186/s40413-016-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D, Benedictow A, Berge H, Bergström R, Emberson LD, Fagerli H, et al. 2012. The EMEP MSC-W chemical transport model – technical description. Atmos Chem Phys 12(16):7825–7865, 10.5194/acp-12-7825-2012. [DOI] [Google Scholar]
- Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. 2016. Correction: Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLOS Med 13(6):e1002056, PMID: 27504831, 10.1371/journal.pmed.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. 2010. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med 182(3):307–316, PMID: 20378732, 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Lin J, Chen P, Li J, Wu C, Yin K, et al. 2013. Evaluation of asthma control and patient’s perception of asthma: findings and analysis of a nationwide questionnaire-based survey in China. J Asthma Off J Assoc Care Asthma 50(8):861–870, 10.3109/02770903.2013.808346. [DOI] [PubMed] [Google Scholar]
- Sudo K, Takahashi M, Kurokawa J, Akimoto H. 2002. CHASER: A global chemical model of the troposphere 1. Model description: CHASER 1. MODEL DESCRIPTION. J Geophys ;Res 107(D17):ACH 7-1–ACH 7-20, 10.1029/2001JD001113. [DOI] [Google Scholar]
- Takenoue Y, Kaneko T, miyamae T, Mori M, Yokota S. 2012. Influence of outdoor NO2 exposure on asthma in childhood: meta-analysis. Pediatr Int 54(6):762–769, PMID: 22640481, 10.1111/j.1442-200X.2012.03674.x. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Salvi S, Lin J, Cho YJ, Eng P, Abdul Manap R, et al. 2013. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology 18(6):957–967, PMID: 23730953, 10.1111/resp.12137. [DOI] [PubMed] [Google Scholar]
- Toskala E, Kennedy DW. 2015. Asthma risk factors. Int Forum Allergy Rhinol 5(Suppl1):S11–S16, PMID: 26335830, 10.1002/alr.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turktas H, Mungan D, Uysal MA, Oguzulgen K. Turkish Asthma Control Survey Study Group, 2010. Determinants of asthma control in tertiary level in Turkey: a cross-sectional multicenter survey. J Asthma 47(5):557–562, PMID: 20560829, 10.3109/02770901003692777. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2015a. Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants. https://www.epa.gov/isa/integrated-science-assessment-isa-ozone [accessed 15 June 2017].
- U.S. EPA. 2009. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009). https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=216546 [accessed 24 July 2018].
- U.S. EPA. 2012. Regulatory Impact Analysis for the Final Revisions to the National Ambient Air Quality Standards for Particulate Matter. https://www3.epa.gov/ttn/ecas/docs/ria/naaqs-pm_ria_final_2012-12.pdf [accessed 22 July 2018].
- U.S. EPA. 2015b. Regulatory Impact Analysis of the Final Revisions to the National Ambient Air Quality Standards for Ground-Level Ozone. https://www3.epa.gov/ttn/ecas/docs/ria/naaqs-o3_ria_final_2015-09.pdf [accessed 22 July 2018].
- van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. 2016. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol 50(7):3762–3772, PMID: 26953851, 10.1021/acs.est.5b05833. [DOI] [PubMed] [Google Scholar]
- Walton H, Dajnak D, Beevers S, Williams M, Watkiss P, Hunt A. 2015. Understanding the Health Impacts of Air Pollution in London. https://www.london.gov.uk/sites/default/files/hiainlondon_kingsreport_14072015_final.pdf [accessed 10 November 2017].
- Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. 2010. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect 118(4):449–457, PMID: 20064785, 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JJ, Fiore AM, Horowitz LW, Mauzerall DL. 2006. Global health benefits of mitigating ozone pollution with methane emission controls. Proc Natl Acad Sci USA 103(11):3988–3993, PMID: 16537473, 10.1073/pnas.0600201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Kirrane E, Klein M, Strickland M, Darrow LA, Sarnat SE, et al. 2014. Joint effects of ambient air pollutants on pediatric asthma emergency department visits in Atlanta, 1998-2004. Epidemiology 25(5):666–673, PMID: 25045931, 10.1097/EDE.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2013. Health risks of air pollution in Europe - HRAPIE project: recommendations for concentration-response functions for cost-benefit analysis of particulate matter, ozone, and nitrogen dioxide. http://www.euro.who.int/__data/assets/pdf_file/0006/238956/Health_risks_air_pollution_HRAPIE_project.pdf?ua=1 [accessed 5 July 2018].
- WHO. 2016. Preventing disease through healthy environments: a global assessment of the burden of disease from environmental risks. http://apps.who.int/iris/bitstream/10665/204585/1/9789241565196_eng.pdf?ua=1 [accessed 9 August 2017].
- WHO. 2017a. Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. http://www.who.int/quantifying_ehimpacts/publications/preventing-ncds/en/ [accessed 24 July 2018].
- WHO. 2017b. WHO definition of regional groupings.. http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/ [accessed 15 June 2017].
- WHO. 2017c. WHO regional offices. http://www.who.int/about/regions/en/ [accessed 15 June 2017].
- Yeatts K, Shy C, Sotir M, Music S, Herget C. 2003. Health Consequences for Children With Undiagnosed Asthma-like Symptoms. Arch Pediatr Adolesc Med 157(6):540, PMID: 12796233, 10.1001/archpedi.157.6.540. [DOI] [PubMed] [Google Scholar]
- Zainudin BMZ, Lai CKW, Soriano JB, Jia-Horng W, De Guia TS. Asthma Insights and Reality in Asia-Pacific (AIRIAP) Steering Committee. 2005. Asthma control in adults in Asia-Pacific. Respirology 10(5):579–586, 10.1111/j.1440-1843.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- Zemedkun K, Woldemichael K, Tefera G. 2014. Assessing control of asthma in Jush, Jimma, South West Ethiopia. Ethiop J Health Sci 24(1):49–58, PMID:24591799, 10.4314/ejhs.v24i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li G, Tian L, Guo Q, Pan X. 2016. Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: A systematic review and meta-analysis. Environ Res 148:15–23, PMID: 26995350, 10.1016/j.envres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Zheng X, Ding H, Jiang L, Chen S, Zheng J, Qiu M, et al. 2015. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PloS One 10(9):e0138146, PMID: 26382947, 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.