Abstract
Over the past century, the dendrochronology technique of crossdating has been widely used to generate a global network of tree-ring chronologies that serves as a leading indicator of environmental variability and change. Only recently, however, has this same approach been applied to growth increments in calcified structures of bivalves, fish and corals in the world's oceans. As in trees, these crossdated marine chronologies are well replicated, annually resolved and absolutely dated, providing uninterrupted multi-decadal to millennial histories of ocean palaeoclimatic and palaeoecological processes. Moreover, they span an extensive geographical range, multiple trophic levels, habitats and functional types, and can be readily integrated with observational physical or biological records. Increment width is the most commonly measured parameter and reflects growth or productivity, though isotopic and elemental composition capture complementary aspects of environmental variability. As such, crossdated marine chronologies constitute powerful observational templates to establish climate–biology relationships, test hypotheses of ecosystem functioning, conduct multi-proxy reconstructions, provide constraints for numerical climate models, and evaluate the precise timing and nature of ocean–atmosphere interactions. These ‘present–past–future’ perspectives provide new insights into the mechanisms and feedbacks between the atmosphere and marine systems while providing indicators relevant to ecosystem-based approaches of fisheries management.
Keywords: sclerochronology, crossdating, proxy, palaeoceanography, dendrochronology, climate change
1. Background
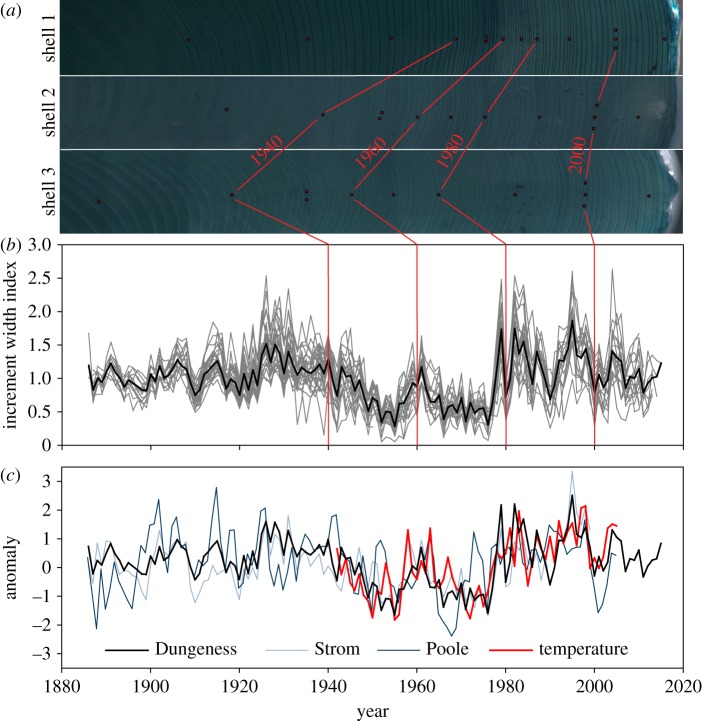
In terrestrial systems, tree-ring data are well replicated from multiple individuals, absolutely dated, and thus constitute the ‘gold standard’ of high-resolution environmental archives. This level of accuracy is possible through crossdating, a technique that assumes some aspect of the environment influences growth, varies over time, and thereby induces a synchronous growth pattern among samples of a given population and location. Starting at the increment formed during the known year of collection, the synchronous pattern is cross-matched among samples backward through time. If an increment has been missed or falsely identified, the pattern will be offset by a year relative to that in other samples, beginning where the error occurred. Errors are then confirmed and corrected by visually re-examining the sample [1] (figure 1). The absence of dating errors ensures high-frequency variability is not smeared, attenuated or blurred, which allows for seamless integration among chronologies, instrumental climate histories and other observational physical or biological records [2] (figure 1). Given the wide application of this approach in forests around the globe, over 4500 tree chronologies are now publicly available through the International Tree-Ring Data Bank (ITRDB; [3]), a rich and diverse resource that has facilitated a number of highly influential, broad-scale reconstructions of climate and disturbance [4–6].
Figure 1.
Crossdating for absolute dating control. (a) Synchronous growth among three Pacific geoduck samples from Dungeness Spit, Washington, USA. Each decade is labelled with a dot; 2000 with three dots; 1950 with two dots. (b) Measurements of 30 Dungeness Spit samples after age-related growth declines have been removed. Also shown is their mean (the chronology). (c) The Dungeness Spit chronology plus two other geoduck chronologies from southern British Columbia, Canada. Superimposed is mean annual sea surface temperature anomaly for the British Columbia coast. Agreement within and among chronologies and instrumental records corroborate absolute dating. (Online version in colour.)
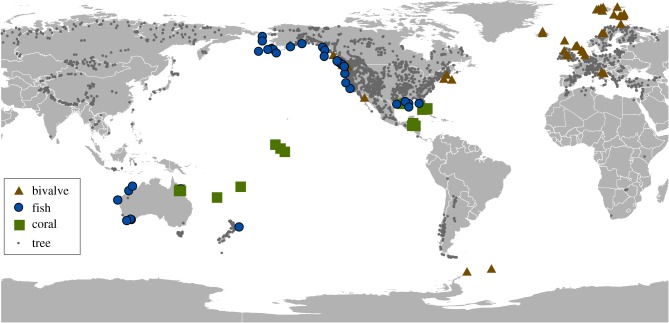
Over the past decade, an increasing number of studies have demonstrated that the same powerful crossdating approach can be applied to marine organisms (figure 2). A wide variety of species spanning tropical to polar latitudes are long-lived, form annual growth increments, and are represented in extensive archival collections in fisheries laboratories and museums around the world [7]. Archaeological and sub-fossil specimens are available to further extend records back in time [8–10]. Resulting crossdated sclerochronologies continuously span multiple decades to centuries, are comparable in quality to tree-ring datasets, and capture signals representing a range of depths, habitats, trophic levels and functional types [8,11]. These time series are of high value in marine systems where instrumental records greater than 50 years or observational biological records greater than 20 years in length are uncommon [12,13]. As such, this approach is unlocking a new, vast, global array of data streams in the marine realm to reveal relationships between biological processes and climate, hind-cast past environmental variability, calibrate climate models and identify key target variables for forecasting into the future.
Figure 2.
Crossdated marine chronologies. Locations of crossdated tree-ring chronologies available through the International Tree-Ring Data bank. Locations of published marine sclerochronologies for which there was replication (generally n > 5) and at least some mention of visual cross-matching of patterns among samples. Note: chronology metadata are provided in electronic supplementary material, table S1. (Online version in colour.)
2. Present
In many marine systems, the fundamental environmental drivers of productivity or functioning remain poorly understood. This is largely due to the scarcity of multidecadal biological time series [12,13]. However, crossdated marine sclerochronologies serve as growth proxies with the accuracy and temporal extension required to quantify long-term variability and establish robust statistical relationships with observational environmental indices. For example, productivity in the California Current along the west coast of North America has long been assumed to be largely driven by spring and summer conditions when coastal upwelling is the strongest and most sustained. However, rockfish (Sebastes spp.) chronologies derived from otolith increment widths strongly relate to wintertime upwelling, the amplitude of which varies greatly from year to year [14]. This wintertime volatility is likely imprinted on biology via some preconditioning the system for high productivity during the upcoming warm season or its effects on growing-season length. Moreover, fish increment-width sclerochronologies have been integrated with other observational biological time series such as seabird reproductive success and plankton community composition to demonstrate climate-induced covariance across taxa and trophic levels, which underscores the importance of winter climate in biology [15,16]. Crossdated sclerochronologies and tree-ring chronologies have also been used to document that broad-scale atmospheric phenomena can simultaneously influence factors limiting growth on land, such as precipitation, as well as factors limiting growth at sea, such as coastal upwelling, to induce covariance between marine and terrestrial ecosystem productivity [11,17].
Patterns of synchrony reveal the extent and magnitude to which environmental variability influences biological processes and afford some degree of predictive power, especially when associated climate drivers can be determined. Indeed, crossdating quantifies the extent to which growth anomalies covary within and among populations, and provides exactly dated and well-replicated biological time series with which to identify this synchrony [9,18,19] (figure 2). Human impacts may also be assessed, such as quantifying reduced resilience of corals in heavily populated areas of the Mesoamerican Reef to bleaching events [20]. Such information is highly relevant to coral reef and fisheries management and aiding the desired transition from single stock assessment to ecosystem-based approaches. Crossdated marine chronologies could inform multiple aspects of Integrated Ecosystem Assessment by quantifying multidecadal ranges of variability, long-term changes in biological reference points, climate drivers and ecosystem indicators [21]. Integrating the growing networks of crossdated sclerochronlogies with existing biological observational records has the potential to provide baseline information on biological synchrony and the interactions between climate and human influence.
3. Past
In the marine realm, sediment cores are the most commonly used archives to provide long-term perspectives on environmental variability prior to the instrumental record. These archives often span multiple millennia, have been broadly sampled across the ocean floor, and in some environments may be sub-decadally resolved. Moreover, they capture a diversity of microorganisms and geochemical proxies to assess long-term environmental variability and biological response [13,22–24]. Although crossdated marine sclerochronologies very rarely span multiple centuries and are generally limited to the continental shelves (figure 2), they are annually resolved, absolutely dated, and can be readily calibrated against instrumental records to hind-cast pre-industrial baselines, rates of change, and the frequency of extreme events [8,25,26]. Relatively long crossdated sclerochronologies allow for the examination of the role that natural external forcing (e.g. total solar irradiance and volcanic aerosols) and internal climate mechanisms and feedbacks (e.g. ocean–atmosphere interactions, ocean circulation and ice-related albedo feedbacks) play in driving past marine variability [27]. For example, a millennial-length oxygen stable isotope series from a crossdated bivalve shell growth chronology demonstrated that oceanic changes near Iceland generally preceded those in the atmosphere prior to the industrial period (CE 1000–1800); however, this relationship reversed after CE 1800 likely reflecting anthropogenic influence on the climate [28].
For some species and locations, increment width is strongly related to a single climate variable. Along the western North America coastline, 70% of the variance in Pacific geoduck (Panopea generosa) chronologies can be explained by regional sea surface temperature variability [29,30]. In other cases, even when there is a high degree of increment-width synchrony among individuals from a given species and site, the environmental drivers of growth rate are complex and less obvious [31–36]. However, other measurement parameters such as isotope signatures, trace and minor elements, or microstructures that are embedded in the precisely dated material [9,25,28,37,38] may better reflect climate variability, can often be mechanistically linked to aspects of the environment, and used to robustly reconstruct past environments. For example, regionally crossdated bivalve series demonstrate highly synchronous Ba/Ca ratios in shell aragonite potentially related to productivity dynamics [39]. Stable carbon (13C) isotope values [40] from exactly dated increments provide constraints on carbon cycling and the so-called Suess effect [41,42] through space and time. Moreover, radiocarbon measurements from exactly dated increments can be used to assess changes in circulation and provide tight constraints for the marine reservoir effect [10,25,37]. One of the factors that hinders more accurate 14C dating in marine sediment cores is the paucity of information about how the marine reservoir age varied back through time. For the late Holocene, crossdated marine sclerochronologies improve this by eliminating dating uncertainty [10,37].
A useful property of sclerochronologies is that they directly target marine environmental variability, including fine-scale processes or those at depth that are not linked to the atmosphere and are thus undetectable by land-based archives [9,10,19,42–44]. Where tree-ring chronologies do capture coupled ocean–atmosphere climate phenomena such as the Pacific Decadal Oscillation, El Niño-Southern Oscillation or Atlantic Multidecadal Oscillation, crossdated marine archives offer complementary perspectives of habitat and life history that provide a more robust estimate of past climate than any single archive could provide individually [29,45–47]. Finally, crossdated marine sclerochronologies identify key climate drivers of marine ecosystem functioning, which may be associated with atmospheric processes that influence tree growth. This information provides novel targets for tree-ring-based reconstructions. For example, rockfish otolith chronologies in the California Current are influenced by winter upwelling, which is driven by anomalies in atmospheric pressure that also drive drought on land. Thus, moisture-sensitive blue oak (Quercus douglasii) tree-ring chronologies can be used to reconstruct a 600-year history of this key indicator of biological functioning and productivity in the California Current marine ecosystem [11].
4. Future
The fundamental knowledge provided by crossdated sclerochronologies on the present and past, as described above, are foundational to accurately predict the future of both the climate system and the marine ecosystems. One such approach is to use these records to compare with, calibrate, test, benchmark or assimilate into general circulation models (GCMs) [48]. Sclerochronological records can also be used to assess longer-term bias, quantify the amplitude and spatial patterns of uncertainties in GCM runs compared to instrumental data products, and to evaluate climate field reconstruction methods [49]. The quantification and characterization of these uncertainties coupled with the general improvement in our understanding of the forcing mechanisms that drive the coupled ocean–atmosphere climate system will ultimately facilitate the continued improvement of the individual GCMs, enhancing the ability of the numerical models to provide robust simulations of likely future climate change. Numerical models can also be used to identify and guide selection of sites where new chronologies likely have maximum palaeoclimatic significance [49,50]. Finally, crossdated marine chronologies can constrain quasi/multi-decadal climate variability over the past few centuries to millennia [9]. Such information can test and improve the skill of numerical climate models, which poorly capture variability in these spectral domains. Once crossdated sclerochronologies have been constructed [2], novel proxies, such as nitrogen [51] and boron isotopes [52], or emerging geochemical proxies, promise to provide essential constraints on marine ecosystems, ocean acidification and climate. The recent metagenomic discovery that bivalve shell carbonate contains environmental DNA [53] heralds the possibility of using crossdated shell series to reconstruct marine biodiversity across major anthropogenic transitions, enabling reconstructions of marine ecosystem baselines and rates of biodiversity loss. Ultimately, the long-term histories of climate variability, its coupling with the atmosphere and impacts on biology will be critical for understanding the future climate change and ecosystem impacts.
5. Conclusion
For many long-lived fish and bivalve species, adequate replicates for crossdatable chronologies can be obtained through archival collections, especially if they are commercially important species [7]. For some species such as tropical corals, the expense of sampling can be high, but where replication is available, crossdating can yield annually resolved, environmentally sensitive chronologies [20,25,54–56]. Crossdating may also be possible with increments (or layers) in coralline algae, deep sea corals, sclerosponges, speleothems, ice cores, varved sediment cores and perhaps in sub-annual (daily or tidal) increments [57–59]. If increment widths are not visually evident or lack adequate interannual variability, crossdating could be attempted using chemical or morphological properties such as trace and minor element concentrations, isotope signatures, shell microstructures, or even the brightness of the internal banding structure [39,43,55]. Crossdating may not be feasible for short-lived species (less than 15-year lifespan) given that time series are insufficiently long to confidently match patterns among individuals, even for sample sets with known collection dates. However, environmentally sensitive, annually resolved chronologies appear to be possible [60,61]. This likely reflects the fact that dating errors are not as impactful in short-lived species as long-lived species for which frameshifts can have effects that extend over decades or centuries. Yet, in the absence of crossdating there will remain some unknown error rate and loss of high-frequency signals, the incidence of which is likely to increase with length of the measurement time series [2].
The main thrust of a growing body of literature shows that crossdating is possible and practical for numerous species and environments in the world's oceans. Indeed, crossdating is the technique that truly defines the dendrochronological approach that has been so successful in terrestrial systems. Given that high- and low-frequency signals are retained, these time series can be readily integrated with one another or instrumental records, and further combined with other archives such as sediment cores to evaluate shared patterns in low-frequency time domains [13,22,24,62]. Thus, crossdating and internal replication can be broadly applied to evaluate linkages across ocean basins, ocean–atmosphere connections, and covariance among marine, terrestrial, and freshwater ecosystems. The application and continued development of this technique is now beginning to revolutionize our understanding of biological and climatic processes in marine systems and their interactions with the atmosphere across a range of temporal and spatial scales.
Supplementary Material
Acknowledgements
The authors wish to thank all members of the sclerochronology workshop held during the European Geophysical Union 2017 Annual Meeting during which the justification and general content of this paper was developed. We also thank David Frank for comments that improved an earlier draft of the manuscript as well as Pheobe Chan for helpful conversations about coralline algae records.
Ethics
There was no data collection for this review article; it is based entirely on previously published research.
Data accessibility
All data used in this study have been published. Citations of these datasets can be found in electronic supplementary material, table S1.
Authors' contributions
B.A.B. led the writing efforts. All authors contributed to writing, literature review and identifying relevant datasets. B.A.B. and P.v.d.S. developed the figures.
Competing interests
We have no competing interests.
Funding
B.A.B. was supported by National Science Foundation grant OCE 1602828. A.D.W. was supported by the National Science Foundation grants OCE 1003438 and OPP 1417766. M.L.C. was supported by the Research Council of Norway on grants 227046 and 228107. C.A. was funded by the Norwegian Research Council project ECHO (240555). D.J.R. and J.D.S. were supported by National Environment Research Council Project NE/N001176/1. P.G.B. was supported by the EU 7th Framework Programme project ARAMACC (604802).
References
- 1.Douglass AE. 1941. Crossdating in dendrochronology. J. For. 39, 825–831. [Google Scholar]
- 2.Black BA, et al. 2016. The value of crossdating to retain high-frequency variability, climate signals, and extreme events in environmental proxies. Global Change Biol. 22, 2582–2595. ( 10.1111/gcb.13256) [DOI] [PubMed] [Google Scholar]
- 3.Grissino-Mayer HD, Fritts HC. 1997. The International Tree-Ring Data Bank: an enhanced global database serving the global scientific community. Holocene 7, 235–238. ( 10.1177/095968369700700212) [DOI] [Google Scholar]
- 4.Briffa KR, Osborn TJ, Schweingruber FH. 2004. Large-scale temperature inferences from tree rings: a review. Global Planet. Change 40, 11–26. [Google Scholar]
- 5.Cook ER, Meko DM, Stahle DW, Cleaveland MK. 1999. Drought reconstructions for the continental United States. J. Clim. 12, 1145–1162. () [DOI] [Google Scholar]
- 6.Trouet V, Esper J, Graham NE, Baker A, Scourse JD, Frank DC. 2009. Persistent positive North Atlantic oscillation mode dominated the medieval climate anomaly. Science 324, 78–80. ( 10.1126/science.1166349) [DOI] [PubMed] [Google Scholar]
- 7.Morrongiello JR, Thresher RE, Smith DC. 2012. Aquatic biochronologies and climate change. Nat. Clim. Change 2, 849–857. ( 10.1038/Nclimate1616) [DOI] [Google Scholar]
- 8.Butler PG, Wanamaker AD, Scourse JD, Richardson CA, Reynolds DJ. 2013. Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 373, 141–151. ( 10.1016/j.palaeo.2012.01.016) [DOI] [Google Scholar]
- 9.Reynolds DJ, Richardson CA, Scourse JD, Butler PG, Hollyman P., Roman-Gonzalez A, Hall IR. 2017. Reconstructing North Atlantic marine climate variability using an absolutely-dated sclerochronological network. Palaeogeogr. Palaeoclimatol. Palaeoecol. 465, 333–346. ( 10.1016/j.palaeo.2016.08.006) [DOI] [Google Scholar]
- 10.Wanamaker AD, Butler PG, Scourse JD, Heinemeier J., Eiriksson J., Knudsen KL, Richardson CA. 2012. Surface changes in the North Atlantic meridional overturning circulation during the last millennium. Nat. Commun. 3, Article number: 899 ( 10.1038/ncomms1901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black BA, Sydeman WJ, Frank DC, Griffin D., Stahle DW, Garcia-Reyes M., Rykaczewski RR, Bograd SJ, Peterson WT. 2014. Six centuries of variability and extremes in a coupled marine–terrestrial ecosystem. Science 345, 1498–1502. ( 10.1126/science.1253209) [DOI] [PubMed] [Google Scholar]
- 12.Richardson AJ, et al. 2012. Climate change and marine life. Biol. Lett. 8, 907–909. ( 10.1098/rsbl.2012.0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuhara M, Doi H, Wei CL, Danovaro R, Myhre SE. 2016. Biodiversity–ecosystem functioning relationships in long-term time series and palaeoecological records: deep sea as a test bed. Phil. Trans. R. Soc. B 371, 20150282 ( 10.1098/rstb.2015.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black BA, Schroeder ID, Sydeman WJ, Bograd SJ, Wells BK, Schwing FB. 2011. Winter and summer upwelling modes and their biological importance in the California current ecosystem. Global Change Biol. 17, 2536–2545. ( 10.1111/j.1365-2486.2011.02422.x) [DOI] [Google Scholar]
- 15.Garcia-Reyes M., Sydeman WJ, Thompson SA, Black BA, Rykaczewski RR, Thayer JA, Bograd SJ. 2013. Integrated assessment of wind effects on Central California's pelagic ecosystem. Ecosystems 16, 722–735. ( 10.1007/s10021-013-9643-6) [DOI] [Google Scholar]
- 16.Thompson SA, Sydeman WJ, Santora JA, Black BA, Suryan RM, Calambokidis J., Peterson WT, Bograd SJ. 2012. Linking predators to seasonality of upwelling: using food web indicators and path analysis to infer trophic connections. Prog. Oceanogr. 101, 106–120. ( 10.1016/j.pocean.2012.02.001). [DOI] [Google Scholar]
- 17.Ong JJL, et al. 2016. Evidence for climate-driven synchrony of marine and terrestrial ecosystems in northwest Australia. Global Change Biol. 22, 2776–2786. ( 10.1111/gcb.13239) [DOI] [PubMed] [Google Scholar]
- 18.Matta ME, Helser TE, Black BA. 2016. Otolith biochronologies reveal latitudinal differences in growth of Bering Sea yellowfin sole Limanda aspera. Polar Biol. 39, 2427–2439. ( 10.1007/s00300-016-1917-y) [DOI] [Google Scholar]
- 19.Ambrose WG, Carroll ML, Greenacre M., Thorrold SR, McMahon KW. 2006. Variation in Serripes groenlandicus (Bivalvia) growth in a Norwegian high-Arctic fjord: evidence for local- and large-scale climatic forcing. Global Change Biol. 12, 1595–1607. ( 10.1111/j.1365-2486.2006.01181.x) [DOI] [Google Scholar]
- 20.Carilli JE, Norris RD, Black B, Walsh SM, McField M. 2010. Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Global Change Biol. 16, 1247–1257. ( 10.1111/j.1365-2486.2009.02043.x) [DOI] [Google Scholar]
- 21.Levin PS, Fogarty MJ, Murawski SA, Fluharty D. 2009. Integrated ecosystem assessments: developing the scientific basis for ecosystem-based management of the ocean. PLoS Biol. 7, 23–28. ( 10.1371/journal.pbio.1000014) [DOI] [Google Scholar]
- 22.Cunningham LK, et al. 2013. Reconstructions of surface ocean conditions from the northeast Atlantic and Nordic seas during the last millennium. Holocene 23, 921–935. ( 10.1177/0959683613479677) [DOI] [Google Scholar]
- 23.Soutar A, Isaacs JD. 1974. Abundance of pelagic fish during 19th and 20th centuries as recorded in anaerobic sediment off the Californias. Fish. Bull. 72, 257–273. [Google Scholar]
- 24.Yasuhara M, Tittensor DP, Hillebrand H, Worm B. 2017. Combining marine macroecology and palaeoecology in understanding biodiversity: microfossils as a model. Biol. Rev. 92, 199–215. ( 10.1111/brv.12223) [DOI] [PubMed] [Google Scholar]
- 25.DeLong KL, Flannery JA, Poore RZ, Quinn TM, Maupin CR, Lin K, Shen CC. 2014. A reconstruction of sea surface temperature variability in the southeastern Gulf of Mexico from 1734 to 2008 CE using cross-dated Sr/Ca records from the coral Siderastrea siderea. Paleoceanography 29, 403–422. ( 10.1002/2013PA002524) [DOI] [Google Scholar]
- 26.Marchitto TM, Jones GA, Goodfriend GA, Weidman CR. 2000. Precise temporal correlation of holocene mollusk shells using sclerochronology. Quatern. Res. 53, 236–246. ( 10.1006/qres.1999.2107) [DOI] [Google Scholar]
- 27.Swingedouw D, Ortega P, Mignot J, Guilyardi E, Masson-Delmotte V, Butler PG, Khodri M, Seferian R. 2015. Bidecadal North Atlantic ocean circulation variability controlled by timing of volcanic eruptions. Nat. Commun. 6, 6545 ( 10.1038/ncomms7545) [DOI] [PubMed] [Google Scholar]
- 28.Reynolds DJ, et al. 2016. Annually resolved North Atlantic marine climate over the last millennium. Nat. Commun. 7, 13502 ( 10.1038/ncomms13502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black BA, Copenheaver CA, Frank DC, Stuckey MJ, Kormanyos RE. 2009. Multi-proxy reconstructions of northeastern Pacific sea surface temperature data from trees and Pacific geoduck. Palaeogeogr. Palaeoclimatol. Palaeoecol. 278, 40–47. ( 10.1016/j.palaeo.2009.04.010) [DOI] [Google Scholar]
- 30.Strom A. 2003. Climate and fisheries in the Pacific Northwest: historical perspectives from geoducks and early explorers. MS thesis University of Washington. [Google Scholar]
- 31.Ansell AD. 1968. The rate of growth of the hard clam Mercenaria mercenaria (L) throughout the geographical range. J. du Conseil/Conseil Permanent Int. pour l'Exploration de la Mer 31, 364–409. ( 10.1093/icesjms/31.3.364) [DOI] [Google Scholar]
- 32.Ballesta-Artero I, Witbaard R, Carroll ML, van der Meer J.. 2017. Environmental factors regulating gaping activity of the bivalve Arctica islandica in Northern Norway. Mar. Biol. 164, 116 ( 10.1007/s00227-017-3144-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler PG, Richardson CA, Scourse JD, Wanamaker AD, Shammon TM, Bennell JD. 2010. Marine climate in the Irish Sea: analysis of a 489-year marine master chronology derived from growth increments in the shell of the clam Arctica islandica. Quat. Sci. Rev. 29, 1614–1632. ( 10.1016/j.quascirev.2009.07.010) [DOI] [Google Scholar]
- 34.Schöne BR, Tanabe K, Dettman DL, Sato S. 2003. Environmental controls on shell growth rates and δ18O of the shallow-marine bivalve mollusk Phacosoma japonicum in Japan. Mar. Biol. 142, 473–485. ( 10.1007/s00227-002-0970-y) [DOI] [Google Scholar]
- 35.Weymouth FW. 1922. The life-history and growth of the Pismo clam (Tivela stultorum Mawe). Fish Bull. Calif. Dep. Fish Game 7, 1–120. [Google Scholar]
- 36.Witbaard R, Jansma E, Klaassen US. 2003. Copepods link quahog growth to climate. J. Sea Res. 50, 77–83. ( 10.1016/S1385-1101(03)00040-6) [DOI] [Google Scholar]
- 37.Butler PG, Scourse JD, Richardson CA, Wanamaker AD, Bryant CL, Bennell JD. 2009. Continuous marine radiocarbon reservoir calibration and the C-13 Suess effect in the Irish Sea: results from the first multi-centennial shell-based marine master chronology. Earth Planet Sci. Lett. 279, 230–241. ( 10.1016/j.epsl.2008.12.043) [DOI] [Google Scholar]
- 38.Schöne BR, Radermacher P, Zhang ZJ, Jacob DE. 2013. Crystal fabrics and element impurities (Sr/Ca, Mg/Ca, and Ba/Ca) in shells of Arctica islandica-Implications for paleoclimate reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 373, 50–59. ( 10.1016/j.palaeo.2011.05.013) [DOI] [Google Scholar]
- 39.Marali S, Schöne BR, Mertz-Kraus R, Griffin SM, Wanamaker AD, Matras U, Butler PG. 2017. Ba/Ca ratios in shells of Arctica islandica—implications environmental proxy and crossdating tool. Palaeogeogr. Palaeoclimatol. Palaeoecol. 465, 347–361. ( 10.1016/j.palaeo.2015.12.018) [DOI] [Google Scholar]
- 40.Beirne EC, Wanamaker AD, Feindel SC. 2012. Experimental validation of environmental controls on the delta C-13 of Arctica islandica (ocean quahog) shell carbonate. Geochim. Cosmochim. Acta 84, 395–409. ( 10.1016/j.gca.2012.01.021) [DOI] [Google Scholar]
- 41.Reynolds DJ, Hall IR, Scourse JD, Richardson CA, Wanamaker AD, Butler PG. 2017. Biological and climate controls on North Atlantic marine carbon dynamics over the last millennium: insights from an absolutely dated shell-based record from the north Icelandic shelf. Global Biogeochem. Cycle 31, 1718–1735. ( 10.1002/2017GB005708) [DOI] [Google Scholar]
- 42.Schöne BR, Wanamaker AD, Fiebig J, Thebault J, Kreutz K. 2011. Annually resolved δ13Cshell chronologies of long-lived bivalve mollusks (Arctica islandica) reveal oceanic carbon dynamics in the temperate North Atlantic during recent centuries. Palaeogeogr. Palaeoclimatol. Palaeoecol. 302, 31–42. ( 10.1016/j.palaeo.2010.02.002) [DOI] [Google Scholar]
- 43.van der Sleen P, et al. 2017. Long-term Bering Sea environmental variability revealed by a centennial-length biochronology of Pacific Ocean perch Sebastes alutus. Clim. Res. 71, 33–45. ( 10.3354/cr01425) [DOI] [Google Scholar]
- 44.Carroll ML, Ambrose WG, Locke VWL, Ryan SK, Johnson BJ. 2014. Bivalve growth rate and isotopic variability across the Barents Sea Polar Front. J. Mar. Syst. 130, 167–180. ( 10.1016/j.jmarsys.2013.10.006) [DOI] [Google Scholar]
- 45.Gedalof Z, Mantua NJ, Peterson DL. 2002. A multi-century perspective of variability in the Pacific decadal oscillation: new insights from tree rings and coral. Geophys. Res. Lett. 29, 2204 ( 10.1029/2002GL015824) [DOI] [Google Scholar]
- 46.Wilson R, Cook E, D'Arrigo R, Riedwyl N, Evans MN, Tudhope A, Allan R. 2010. Reconstructing ENSO: the influence of method, proxy data, climate forcing and teleconnections. J. Quat. Sci. 25, 62–78. ( 10.1002/jqs.1297) [DOI] [Google Scholar]
- 47.Mette MJ, Wanamaker AD, Carroll ML, Ambrose WG, Retelle MJ. 2016. Linking large-scale climate variability with Arctica islandica shell growth and geochemistry in northern Norway. Limnol. Oceanogr. 61, 748–764. ( 10.1002/lno.10252) [DOI] [Google Scholar]
- 48.Pyrina M, Wagner S, Zorita E. 2017. Evaluation of CMIP5 models over the northern North Atlantic in the context of forthcoming paleoclimatic reconstructions. Clim. Dyn. 49, 3673–3691. ( 10.1007/s00382-017-3536-x) [DOI] [Google Scholar]
- 49.Pyrina M, Wagner S, Zorita E. 2017. Pseudo-proxy evaluation of climate field reconstruction methods of North Atlantic climate based on an annually resolved marine proxy network. Clim. Past 13, 1339–1354. ( 10.5194/cp-13-1339-2017) [DOI] [Google Scholar]
- 50.Comboul M, Emile-Geay J, Hakim GJ, Evans MN. 2015. Paleoclimate sampling as a sensor placement problem. J. Clim. 28, 7717–7740. ( 10.1175/JCLI-D-14-00802.1) [DOI] [Google Scholar]
- 51.Gillikin DP, Lorrain A, Jolivet A, Kelemen Z, Chauvaud L, Bouillon S. 2017. High-resolution nitrogen stable isotope sclerochronology of bivalve shell carbonate-bound organics. Geochim. Cosmochim. Acta 200, 55–66. ( 10.1016/j.gca.2016.12.008) [DOI] [Google Scholar]
- 52.Liu YW, Aciego SM, Wanamaker AD. 2015. Environmental controls on the boron and strontium isotopic composition of aragonite shell material of cultured Arctica islandica. Biogeosciences 12, 3351–3368. ( 10.5194/bg-12-3351-2015) [DOI] [Google Scholar]
- 53.Der Sarkissian C, et al. 2017. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Mol. Ecol. Resour. 17, 835–853. ( 10.1111/1755-0998.12679) [DOI] [PubMed] [Google Scholar]
- 54.DeLong KL, Quinn TM, Taylor FW. 2007. Reconstructing twentieth-century sea surface temperature variability in the southwest Pacific: a replication study using multiple coral Sr/Ca records from New Caledonia. Paleoceanography 22 PA4212. ( 10.1029/2007PA001444) [DOI] [Google Scholar]
- 55.Hendy EJ, Gagan MK, Lough JM. 2003. Chronological control of coral records using luminescent lines and evidence for non-stationary ENSO teleconnections in northeast Australia. Holocene 13, 187–199. ( 10.1191/0959683603hl606rp) [DOI] [Google Scholar]
- 56.Hudson JH, Shinn EA, Halley RB, Lidz B. 1976. Sclerochronology: a tool for interpreting past environments. Geology 4, 361–364. () [DOI] [Google Scholar]
- 57.Baker A., Smart PL, Edwards RL, Richards DA. 1993. Annual growth banding in a cave stalagmite. Nature 364, 518–520. ( 10.1038/364518a0) [DOI] [Google Scholar]
- 58.Folkvord A, Gundersen G, Albretsen J, Asplin L, Kaartvedt S, Giske J. 2016. Impact of hatch date on early life growth and survival of Mueller's pearlside (Maurolicus muelleri) larvae and life-history consequences. Can. J. Fish. Aquat. Sci. 73, 163–176. ( 10.1139/cjfas-2015-0040) [DOI] [Google Scholar]
- 59.Chan P, et al. 2017. Multicentennial record of Labrador Sea primary productivity and sea-ice variability archived in coralline algal barium. Nat. Commun. 8, 15543 ( 10.1038/ncomms15543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smolinski S, Mirny Z. 2017. Otolith biochronology as an indicator of marine fish responses to hydroclimatic conditions and ecosystem regime shifts. Ecol. Indicators 79, 286–294. ( 10.1016/j.ecolind.2017.04.028) [DOI] [Google Scholar]
- 61.van der Sleen P, Stransky C, Morrongiello JR, Haslob H, Peharda M, Black BA.. 2018. Otolith increments in European plaice (Pleuronectes platessa) reveal temperature and density-dependent effects on growth. ICES J. Mar. Sci. 75, 1151 ( 10.1093/icesjms/fsy011) [DOI] [Google Scholar]
- 62.Reynolds DJ, Butler PG, Williams SM, Scourse JD, Richardson CA, Wanamaker AD, Austin WEN, Cage AG, Sayer MDJ. 2013. A multiproxy reconstruction of Hebridean (NW Scotland) spring sea surface temperatures between AD 1805 and 2010. Palaeogeogr. Palaeoclimatol. Palaeoecol. 386, 275–285. ( 10.1016/j.palaeo.2013.05.029) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study have been published. Citations of these datasets can be found in electronic supplementary material, table S1.