Summary
To meet the challenge to human health posed by obesity, a better understanding of the regulation of feeding is essential. Medications targeting 5-hydroxytryptamine (5-HT; serotonin) 2C receptors (htr2c; 5-HT2CR) improve obesity. Here we probed the functional significance of 5-HT2CRs specifically within the brainstem nucleus of the solitary tract (5-HT2CRNTS) in feeding behavior. Selective activation of 5-HT2CRNTS decreased feeding and was sufficient to mediate acute food intake reductions elicited by the 5-HT2CR agonist obesity medication lorcaserin. Similar to pro-opiomelanocortin neurons expressed within the hypothalamic arcuate nucleus (POMCARC), a subset of POMCNTS neurons co-expressed 5-HT2CRs and were activated by 5-HT2CR agonists. Knockdown of POMCNTS prevented the acute appetite-suppressive effect of lorcaserin, whereas POMCARC knockdown prevented the full anorectic effect. These data identify 5-HT2CRNTS as a sufficient subpopulation of 5-HT2CRs in reducing food intake when activated and reveal that 5-HT2CR agonist obesity medications require POMC within the NTS and ARC to reduce food intake.
Keywords: serotonin, 5-HT2CR, lorcaserin, food intake, nucleus of the solitary tract, obesity
Graphical Abstract
Highlights
-
•
Selective 5-HT2CRNTS neuron activation significantly reduces food intake
-
•
5-HT2CRNTS are sufficient for obesity medication acute appetite suppression
-
•
5-HT2CRNTS are co-expressed with POMCNTS; lorcaserin activates POMCNTS cells
-
•
POMCNTS and POMCARC are required for 5-HT2CR agonists to reduce feeding
The brain serotonin pathway regulates appetite and body weight. D’Agostino, Heisler et al. show that the anti-obesity 5-HT2CR agonist lorcaserin targets both the POMC circuitry of the hypothalamus and the brainstem nucleus of the solitary tract (5-HT2CRNTS) to mediate appetite suppression.
Introduction
As demonstrated 40 years ago, brain 5-hydroxytryptamine (5-HT; serotonin) is a significant regulator of appetite and body weight (Breisch et al., 1976). Targeting this neurochemical machinery, medications increasing 5-HT bioavailability were developed to treat obesity. However, increasing 5-HT activity at peripheral 5-HT receptors (5-HTRs) contributed to side effects that led to the withdrawal of medications such as d-fenfluramine in the 1990s and sibutramine in the 2000s (Burke and Heisler, 2015). Efforts to delineate 5-HTRs mediating 5-HT's therapeutic effects on food intake revealed the 2C receptor subtype (Htr2cr, 5-HT2CR) as the principal mediator (Tecott et al., 1995, Williams et al., 2011). The clinical significance of this discovery is underscored by a new obesity medication, the 5-HT2CR agonist, lorcaserin, which was recently launched in the United States (Aronne et al., 2014, Smith et al., 2010).
5-HT2CRs are widely distributed within the brain where they act to modulate a diverse array of behaviors and physiological processes (Burke and Heisler, 2015, Julius et al., 1988). Of the neurochemically defined cells expressing 5-HT2CRs, it is a subpopulation of neurons within the arcuate nucleus of the hypothalamus (ARC) that co-express the precursor polypeptide pro-opiomelanocortin (POMC) that have been proposed to be a principal mediator of 5-HT2CR's effects on metabolic functions (Berglund et al., 2013, Burke et al., 2017, Doslikova et al., 2013, Heisler et al., 2002, Xu et al., 2010). However, anorectic doses of 5-HT2CR agonists have been shown to increase markers of neuronal activity in caudal brainstem regions, including the nucleus of the solitary tract (NTS) (Lam et al., 2009, Stark et al., 2006). Although the NTS is a brainstem structure known to be a key regulatory center for the integration of food related signals of both peripheral and central origin (Fan et al., 2004, Grill and Hayes, 2009, Zhan et al., 2013), no direct functional assessment of 5-HT2CRs in this brain region has been undertaken. Here we probed the functional significance of 5-HT2CRs within the NTS, their contribution to the therapeutic effect of 5-HT2CR agonist obesity medications, and the mechanism underpinning their appetite-reducing effect via exclusive action within the NTS.
Results
Selective 5-HT2CRNTS Neuron Activation Decreases Food Intake
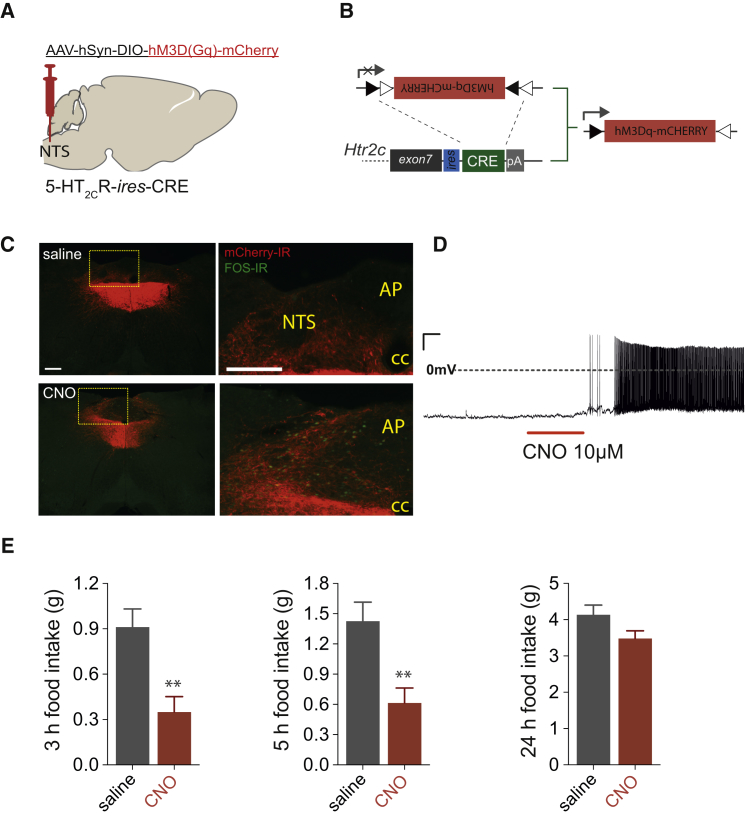
To determine whether 5-HT2CR-expressing NTS (5-HT2CRNTS) neurons play a role in appetite control, we first employed the chemogenetic designer receptors exclusively activated by designer drugs (DREADDs; Armbruster et al., 2007) approach to specifically express Gq-coupled (hM3Dq) or Gi-coupled (hM4Di) designer receptors in 5-HT2CRNTS neurons. To target 5-HT2CRNTS neurons, we stereotactically delivered Cre-dependent adeno-associated viruses (AAVs) encoding designer receptor hM3Dq or hM4Di into the NTS of 5-HT2CRCre mice (Burke et al., 2016, Burke et al., 2017, Marcinkiewcz et al., 2016) to generate 5-HT2CRNTS-hM3Dq (Figures 1A and 1B) and 5-HT2CRNTS-hM4Di cohorts (Figures S1A and S1B). Cre recombinase activity in 5-HT2CRCre mice efficiently recombined the DREADD-mCherry allele in the NTS, as seen by the expression of the mCherry reporter protein in transduced cells (Figure 1C). A degree of spread was observed in adjacent regions following amplification of mCherry signal with immunohistochemistry (Figure 1C). Following treatment with designer drug clozapine-N-oxide (CNO; 1 mg/kg, intraperitoneally [i.p.]), c-fos immunoreactivity (FOS-IR; a surrogate marker of neuronal activation) was observed within the NTS of 5-HT2CRNTS-hM3Dq mice. In 5-HT2CRNTS-hM3Dq mice that had been fasted overnight to decrease baseline FOS-IR, quantification analysis revealed that approximately 76.4% ± 3.7% of mCherry-positive NTS cells expressed FOS-IR following CNO treatment whereas less than 1% expressed FOS-IR following saline treatment (Figure 1C). Consistent with these in vivo data, in ex vivo NTS slices, bath application of CNO (10 μM) increased the firing frequency of 5-HT2CRNTS-hM3Dq-expressing neurons (n = 5/5; Figure 1D). These data indicate that CNO allows remote chemical control of 5-HT2CRNTS neuronal activity in 5-HT2CRNTS-hM3Dq-expressing mice. We next examined the effect of CNO on feeding behavior. CNO (1 mg/kg, i.p.) significantly reduced the first 5 hr of ad libitum dark cycle chow intake compared with saline treatment in 5-HT2CRNTS-hM3Dq mice (Figure 1E).
Figure 1.
Activation of 5-HT2CRNTS-Expressing Neurons Reduces Food Intake
(A) Schematic of the strategy used to selectively activate 5-HT2CRNTS neurons; stereotactic injection of Cre-dependent DREADD vectors into NTS of 5-HT2CRCre mice.
(B) 5-HT2CRCre construct and Cre-mediated recombination of DREADD allele.
(C) Expression of the DREADD-fused mCherry reporter protein within the NTS using immunohistochemistry for mCherry (mCherry-IR; red). In vivo, CNO (1 mg/kg, intraperitoneally [i.p.]) increased FOS-IR (green) expression in the majority of 5-HT2CRNTS-hM3Dq-expressing neurons (red, bottom panels), whereas saline treatment did not (top panels).
(D) Ex vivo, bath application of CNO (10 μM) increased the firing frequency of 5-HT2CRNTS-hM3Dq-expressing neurons.
(E) CNO (1 mg/kg, i.p.) elicited a significant reduction in ad libitum dark cycle food intake compared with saline treatment in 5-HT2CRNTS-hM3Dq mice (n = 6–7; 3 hr: t12 = 3.57, p = 0.004; 5 hr: t12 = 3.379, p = 0.005; 24 hr: t12 = 1.876, p = 0.0874). Data are presented as mean ± SEM. ∗∗p < 0.01.
NTS, nucleus of the solitary tract; AP, area postrema; CC, central canal. Scale bar, 200 μm. See also Figure S1.
Despite the CNO (10 μM)-induced reduction in membrane potential observed in ex vivo NTS slices from 5-HT2CRNTS-hM4Di mice (n = 5/5; Figure S1C), CNO (1 mg/kg, i.p.) did not affect ad libitum food intake in 5-HT2CRNTS-hM4Di mice compared with saline (Figure S1D). The effect of CNO on feeding behavior in 5-HT2CRNTS-hM3Dq but not 5-HT2CRNTS-hM4Di mice illustrates that CNO is not altering food intake via action at endogenous receptors. Rather, the designer drug CNO is reducing food intake via action at the designer 5-HT2CRNTS-hM3Dq receptors. These data reveal that activation of 5-HT2CRNTS neurons significantly suppresses feeding and identifies a novel population of NTS cells that may be targeted for food intake reduction.
Selective 5-HT2CRNTS/DMV Activation Is Sufficient to Promote 5-HT2CR Agonist Hypophagia
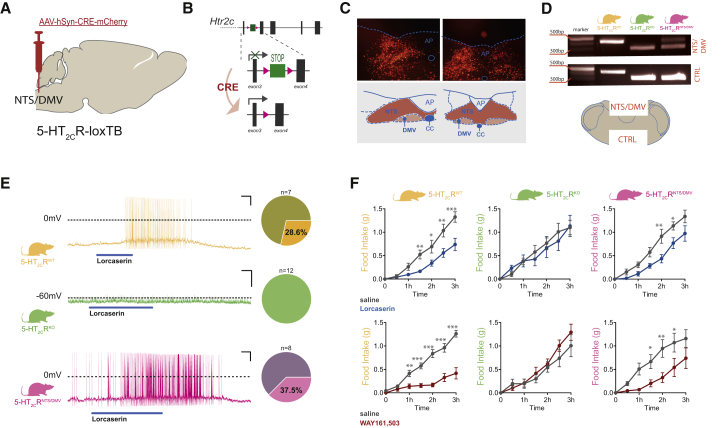
We next evaluated whether an obesity medication currently in human use employs the subset of 5-HT2CRNTS to promote a reduction in feeding behavior. To exclusively activate 5-HT2CRNTS with clinical and preclinical obesity medications, we restricted the expression of the endogenous receptor to the caudal aspects of the dorsal vagal complex (the NTS and dorsal motor nucleus of the vagus [DMV]; 5-HT2CRNTS/DMV). We achieved this using a reversible 5-HT2CR null line, in which expression of Htr2cr is prevented by a loxP-flanked transcriptional blocker (Xu et al., 2008). The expression of Htr2cr was site-specifically reactivated via stereotactic delivery of an AAV-expressing Cre recombinase (AAV-Cre) into the NTS/DMV, which removes the transcriptional blocker (Figures 2A and 2B), allowing 5-HT2CRs expression only in the NTS/DMV (Figures 2C and 2D). To confirm the functional re-expression of Htr2c, we made ex vivo whole-cell electrophysiological recordings from neurons within NTS slices in 5-HT2CRWT (wild-type), 5-HT2CRKO (knockout), and mice with 5-HT2CRs restored exclusively within the NTS/DMV (5-HT2CRNTS/DMV). As expected, NTS neurons from 5-HT2CRKO mice failed to respond to lorcaserin, while similar proportions of NTS neurons from 5-HT2CRWT and 5-HT2CRNTS/DMV mice were excited by lorcaserin (Figure 2E). These data indicate that AAV-Cre restores functional 5-HT2CR expression in 5-HT2CRNTS/DMV mice.
Figure 2.
5-HT2CRNTS Is Sufficient to Mediate the Appetite-Suppressive Effect of 5-HT2CR Agonists
(A) Schematic of the strategy used to selectively restore 5-HT2CRs within the NTS of loxTB5-HT2CR null mice through stereotactic injection of Cre-dependent DREADD vectors into NTS.
(B) 5-HT2CR allele expression is disrupted by a loxP-flanked transcriptional blocker (loxTB) inserted between exons 3 and 4 of the htr2c gene in loxTB5-HT2CR mice (5-HT2CRKO). Stereotactic injection of AAV vector expressing Cre recombinase and mCherry reporter driven by the hSyn promoter removes the TB and allows 5-HT2CR expression only in the NTS (5-HT2CRNTS/DMV).
(C) Representative image of Cre-mCherry expression within the rostrocaudal aspect of the NTS/DMV.
(D) Cre-mediated recombination of the mutant allele and TB removal specifically within the NTS/DMV in 5-HT2CRNTS/DMV mice (wild-type band 437 bp, mutant 310 bp).
(E) Representative current-clamp traces and proportion of neurons responding to bath application of lorcaserin in ex vivo NTS slices prepared from 5-HT2CRWT (n = 7), 5-HT2CRKO (n = 12), and 5-HT2CRNTS/DMV (n = 8) mice.
(F) Sidak's post hoc comparisons revealed that lorcaserin and WAY161,503 (7 mg/kg, i.p.) reduced ad libitum food intake at specific time points within the first 3 hr of the dark cycle (tick marks on x axis represent 20-min intervals) in 5-HT2CRWT (lorcaserin, n = 5; time: F6,24 = 55.19, p < 0.0001; treatment: F1,4 = 16.81, p = 0.0149; interaction: F6,24 = 8.991, p < 0.0001. WAY161,503, n = 6; time: F6,30 = 53.37, p < 0.0001; treatment: F1,5 = 61.55, p = 0.0005; interaction: F6,30 = 26.16, p < 0.0001) and 5-HT2CRNTS/DMV mice (lorcaserin, n = 5; time: F6,24 = 63.53, p < 0.0001; treatment: F1,4 = 23.78, p = 0.0082; interaction: F6,24 = 4.099, p = 0.0057. WAY161,503, n = 6; time: F6,30 = 51.89, p < 0.0001; treatment: F1,5 = 4.028, p = 0.1010; interaction: F6,30 = 1.878, p = 0.1175), but not 5-HT2CRKO mice. Data are presented as mean ± SEM. Sidak's post hoc comparisons ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
NTS, nucleus of the solitary tract; AP, area postrema; CC, central canal; DMV, dorsal motor nucleus of the vagus. See also Figure S2.
Consistent with previous reports, body weight was significantly higher in adult 5-HT2CRKO compared with 5-HT2CRWT mice (Nonogaki et al., 2003, Xu et al., 2008); an effect that failed to normalize following post-developmental re-expression of the 5-HT2CR exclusively within the NTS/DMV (Figure S2A). Further analysis of energy balance revealed no difference in daily, nocturnal, or diurnal energy expenditure between genotypes (Figures S2B and S2C). We were unable to detect the predicted increase in ad libitum chow intake (Figure S2D) or chow intake following an overnight fast in 5-HT2CRKO mice (Figure S2E), which may be related to the previously reported variability in the magnitude of hyperphagia on a chow diet with age in 5-HT2CRKO mice (Wade et al., 2008). These results indicate that restoration of 5-HT2CRs only within the NTS/DMV is not sufficient to correct the increased body weight phenotype of chow-fed 5-HT2CRKO mice.
To test the significance of 5-HT2CRNTS/DMV in a therapeutic context, we treated 5-HT2CRWT, 5-HT2CRKO, and 5-HT2CRNTS/DMV mice with 5-HT2CR agonists lorcaserin and WAY161,503. In line with previous research (Burke et al., 2014, Fletcher et al., 2009), the obesity medication lorcaserin and the preclinical compound WAY161,503 significantly reduced ad libitum food intake in 5-HT2CRWT mice compared with saline treatment (Figure 2F). This effect was absent in 5-HT2CRKO mice but predominantly restored in 5-HT2CRNTS/DMV mice (Figure 2F). Thus, consistent with chemogenetic activation of 5-HT2CRNTS cells, pharmacological activation of this discrete subset of cells has a significant impact on feeding behavior. These data also reveal that 5-HT2CRs expressed within the NTS/DMV are sufficient to mediate acute therapeutic effects of a medication currently in clinical use to treat obesity, thereby highlighting a specific subset of 5-HT2CRs that may be exploited for future obesity medication development.
5-HT2CR Agonists Directly Activate POMCNTS Cells
We next interrogated the neurochemical phenotype of 5-HT2CRNTS cells to probe the mechanism through which 5-HT2CRNTS-mediated effects on appetite are achieved. Though POMC neurons within the ARC have been proposed to be a primary mediator of 5-HT2CR's effects on metabolic functions (Berglund et al., 2013, Burke et al., 2014, Burke et al., 2017, Doslikova et al., 2013, Heisler et al., 2002, Xu et al., 2010), a smaller and less well characterized population of POMC expressing neurons reside within the caudal aspect of the NTS (POMCNTS), a region receiving inputs from vagal afferent fibers that transmit meal-related information from the gastrointestinal system (Appleyard et al., 2005, Fan et al., 2004, Joseph et al., 1983, Zhan et al., 2013). We thus hypothesized that POMCNTS is a functional component of 5-HT2CRNTS-mediated hypophagia.
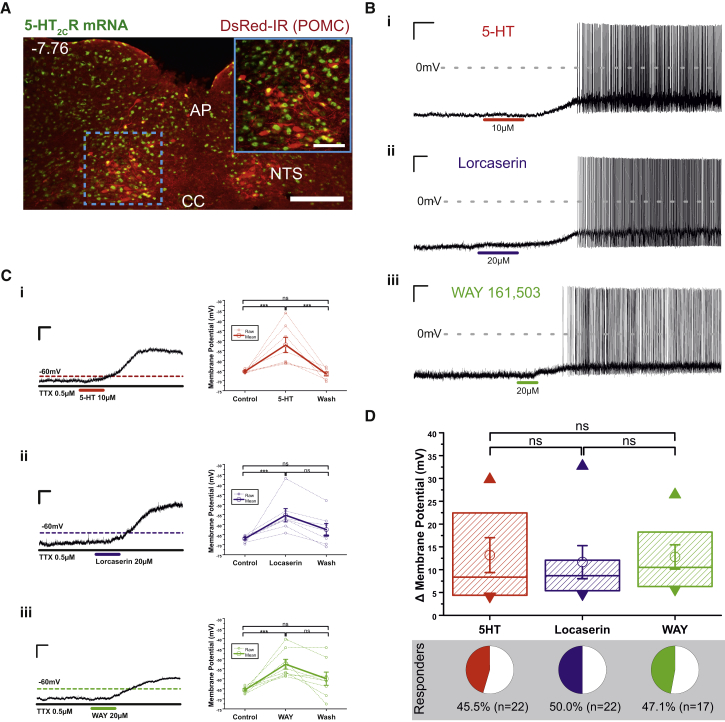
To visualize anatomical expression, we utilized PomcDsRED reporter mice (Hentges et al., 2009) and fluorescent in situ hybridization to label endogenous 5-HT2CR mRNA. Although more than one-third (38.2% ± 1.3%) of POMCNTS cells express 5-HT2CR mRNA, POMCNTS neurons represent only a small (approximately 4%) subset of the total NTS 5-HT2CR mRNA-expressing population (Figure 3A). Next, to evaluate the impact of 5-HT2CR activation on POMCNTS neuronal activity, we performed whole-cell electrophysiological recordings in PomcDsRED NTS slices. Consistent with the anatomical co-expression profile, 45.5% (10/22) of PomcDsRED cells were responsive to 5-HT (10 μM), the endogenous 5-HT2CR agonist (Figures 3B and 3C). A similar proportion of responders was observed following application of 5-HT2CR agonists lorcaserin (20 μM) and WAY161,503 (20 μM), with 47.1% (8/17) and 50% (11/22) of PomcDsRED cells responding, respectively (Figures 3B and 3C). All responses were characterized by depolarization and commencement/enhancement of action potential discharge. These effects were independent of action potential-dependent synaptic transmission, as depolarizations endured in the presence of 500 nM tetrodotoxin (TTX) (5-HT, 13.2 ± 3.8 mV, n = 7; lorcaserin, 11.7 ± 3.6 mV, n = 7; WAY161,503, 12.8 ± 2.7 mV, n = 8; Figure 3C), indicating that 5-HT and 5-HT2CR agonists directly activate POMCNTS cells via a post-synaptic mechanism. 5-HT and 5-HT2CR agonists induced a similar degree of depolarization and activated a similar percentage of POMCNTS cells (Figure 3D). These data reveal that 5-HT2CRs are anatomically positioned to increase the activity of a subset of POMCNTS cells.
Figure 3.
5-HT and 5-HT2CR Agonists Directly Activate POMCNTS Neurons
(A) Representative image illustrating 38% of DsRED-positive POMCNTS neurons (red) express 5-HT2CR mRNA (green; co-expressed, yellow) in PomcDsRED mice. Scale bar, 200 μm; inset scale bar, 50 μm.
(B) Representative current-clamp recordings of POMCNTS neurons in slices from PomcDsRED mice following (i) 10 μM 5-HT, (ii) 20 μM lorcaserin, (iii) and 20 μM WAY161,503.
(C) (i) 5-HT (n = 7; F2,18 = 12.9, p = 0.001), (ii) lorcaserin (n = 7; F2,18 = 8.2, p = 0.006), and (iii) WAY161,503 (n = 8; F2,21 = 10.3, p = 0.002) elicit a significant and reversible change in membrane potential (recorded in 500 nM TTX and from an initial membrane potential of −65 mV, a value achieved through the injection of DC current). Thick lines represent mean ± SEM; dashed lines, raw data. ∗∗∗p < 0.001.
(D) Summary of membrane potential changes induced by 5-HT, lorcaserin, and WAY161,503. Boxes represent 25th, 50th, and 75th percentile with superimposed mean ± SEM and maximum and minimum values.
NTS, nucleus of the solitary tract; AP, area postrema; CC, central canal.
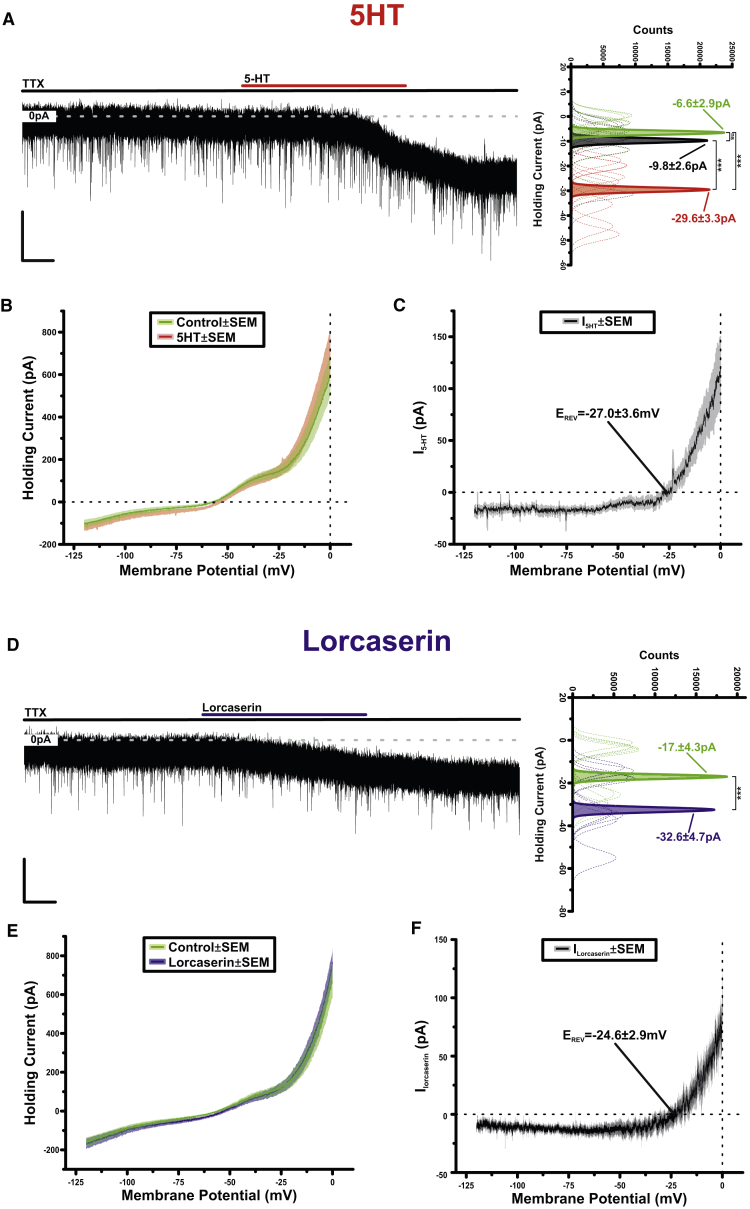
To examine the post-synaptic ionic mechanisms underpinning the excitatory effects of 5-HT and lorcaserin, we recorded POMCNTS neurons in a voltage clamp (VC) and in the presence of TTX. At a holding potential of −60 mV, application of 5-HT (10 μM) induced a reversible inward current of −23.1 ± 2.5 pA (n = 10; Figure 4A). The current-voltage relationship of I5-HT was examined using VC ramps, which drove the membrane potential from −120 mV to 0 mV at a rate of 45.7 mV s−1 (Figure 4B; n = 10). The digital subtraction of the ramp in control from the ramp at the peak of the response revealed I5-HT to be outwardly rectifying and to reverse at −27.0 ± 3.6 mV (Figure 4C; n = 10). Likewise, lorcaserin (20 μM) induced an inward current of −15.5 ± 2.5 pA (n = 12; Figure 4D) that was shown to be outwardly rectifying and to reverse at −24.6 ± 2.9 mV (Figures 4E and 4F). These data illustrate that 5-HT and lorcaserin excite POMCNTS neurons via the activation of a post-synaptic, mixed cationic current. This mechanism is consistent with that observed for 5-HT2CR activation of POMCARC neurons (Sohn et al., 2011).
Figure 4.
5-HT and 5-HT2CR Agonists Activate POMCNTS Neurons Via Post-synaptic, Mixed Cationic Current
(A) Representative voltage-clamp recording of a POMCNTS neuron from PomcDsRED NTS slices in the presence of TTX (500 nM). Application of 5-HT (10 μM) results in an inward current (scale bar, 20 s). To the right, sharing its y axis with the raw trace, are Gaussian fits of averaged (solid lines) holding current frequency distributions in control (green), 5-HT (red), and wash (black). Raw data used to produce averages shown as dashed lines (n = 10, F2,27 = 18.1, p = 1.02 × 10−5; post hoc Tukey test, ∗∗∗p < 0.001).
(B) Averaged voltage-clamp ramps (n = 10) acquired in control (green) and at the peak of response (red). Line denotes mean, shading the SEM.
(C) 5-HT-induced current (I5-HT) obtained by the digital subtraction of the traces displayed in (B). Note the reversal at −27.0 ± 3.6 mV (n = 10). Line denotes mean, shading the SEM.
(D) Representative voltage-clamp recording of a POMCNTS neuron in the presence of TTX. Application of lorcaserin (20 μM) results in an inward current (scale bar, 20 s). To the right, sharing its y axis with the raw trace, are Gaussian fits of averaged (solid lines) holding current frequency distributions in control (green) and lorcaserin (blue). Raw data used to produce averages shown as dashed lines (n = 13, t12 = 6.2, p = 4.5 × 10−5, t-test paired, ∗∗∗p < 0.001).
(E) Averaged voltage-clamp ramps (n = 12) acquired in control (green) and at the peak of response (blue). Line denotes mean, shading the SEM.
(F) Lorcaserin-induced current (ILorcaserin) obtained by the digital subtraction of the traces displayed in (E). Note the reversal at −24.6 ± 2.9 mV (n = 12). Line denotes mean, shading the SEM.
Brain POMC Is Necessary to Mediate 5-HT2CR Agonist Feeding Effects
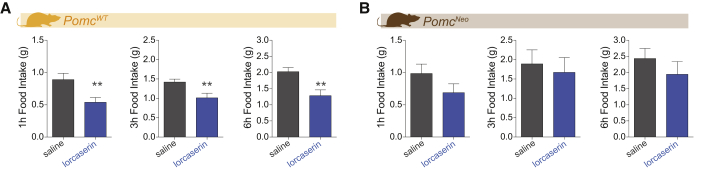
Thus far, we have established that the 5-HT2CRs are anatomically positioned to influence the activity of approximately 40% of POMCNTS neurons and previous reports indicate that 5-HT2CRs are co-expressed with approximately 40% of POMCARC neurons (Heisler et al., 2002, Lam et al., 2008). Earlier transgenic approaches have revealed that the subset of 5-HT2CRs that are necessary and sufficient for 5-HT2CR agonists to reduce food intake are co-expressed with POMC (Berglund et al., 2013, Xu et al., 2008). These approaches manipulate the expression of the receptor, not POMC production. We aimed to determine the function of POMC in lorcaserin's anorectic effect. As a first step toward this goal, we employed a mouse model with full POMCARC knockout and approximately half POMCNTS knockdown (PomcNEO) (Bumaschny et al., 2012). PomcNEO and wild-type (PomcWT) littermates were treated with saline or lorcaserin (7 mg/kg, i.p.) at the onset of the dark cycle and ad libitum home cage chow intake was measured 1, 3, and 6 hr later. As expected, PomcWT mice treated with lorcaserin significantly reduced food intake compared with saline-treated littermates at each time point (Figure 5A). However, lorcaserin did not have a significant effect on food intake at any of these time points in PomcNEO mice compared with saline-treated littermates (Figure 5B). These results were replicated in further cohorts of PomcNEO and PomcWT littermates using a within-subjects experimental design (data not shown). These data reveal that brain POMC is required for lorcaserin to promote its effects on food intake.
Figure 5.
Brain POMC Is a Necessary Neurochemical Mediating 5-HT2CR Agonist Feeding Effects
(A) Male and female wild-type mice (PomcWT) treated with lorcaserin (7 mg/kg, i.p.) significantly reduced 1-, 3-, and 6-hr dark cycle food intake compared with saline-treated PomcWT littermates (n = 11 per group; 1 hr: t20 = 2.884, p = 0.0092; 3 hr: t20 = 2.916, p = 0.0085; 6 hr: t20 = 3.373, p = 0.0030).
(B) In contrast, male and female mice with full POMCARC knockout and partial POMCNTS knockdown (PomcNeo) exhibited a blunted response to lorcaserin treatment as compared with PomcNeo littermates treated with saline (n = 6–7 per group; 1 hr: t11 = 1.3204, p = 0.216; 3 hr: t11 = 0.4098, p = 0.6898; 6 hr: t11 = 0.9486, p = 0.3632).
Data are presented as mean ± SEM. ∗∗p < 0.01.
POMCARC Is Required for the Full Effect of 5-HT2CR Agonist on Food Intake
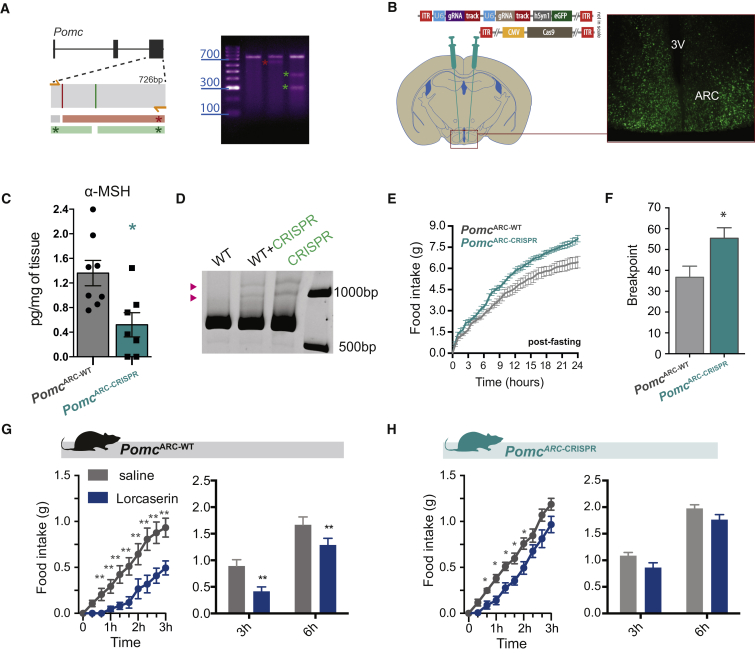
We next sought to determine the functional significance of the discrete subset of POMCNTS versus POMCARC in 5-HT2CR agonist-induced reduction of food intake. We first targeted Pomc in the hypothalamus, since this population of Pomc expressing neurons is larger, better characterized, and projects widely within the hypothalamus, allowing efficient tissue sampling and recovery of peptides that localize at neuron terminals (e.g., POMC-derived peptide α-melanocyte-stimulating hormone [α-MSH] [D'Agostino and Diano, 2010]). To knock down POMCARC, we employed the clustered, regularly interspaced, short palindromic repeats (CRISPR) and associated endonuclease 9 (Cas9) gene-editing technique (Doudna and Charpentier, 2014, Hsu et al., 2014). Specifically targeted by synthetic guide RNA (sgRNA) sequences, Cas9-induced double-stranded breaks typically result in frameshifting insertion/deletions (indels) owing to the action of non-homologous end-joining (NHEJ) DNA repair mechanisms, leading to disruption of the encoded protein. Following an in vitro screening of selected sgRNAs targeting the third exon of the Pomc gene, Cas9-induced indels at the predicted positions were detected using a T7 endonuclease I assay (Figure 6A). Two active sgRNAs were cloned into an AAV vector that also expressed eGFP to enable site-specific brain delivery (Swiech et al., 2015).
Figure 6.
PomcARC Is Required for 5-HT2CR Agonist Hypophagia
(A) Representative screening of sgRNAs targeting the exon 3 of the Pomc gene in Neuro2A cells and indels detection using a T7 endonuclease I assay. (A, left) Sites of potential indels in exon 3 are indicated by red or green bars, and the presence of indels results in the fragments indicated by red or green asterisks, respectively, following endonuclease treatment. (A, right) Bands corresponding to these fragments are indicated with red and green asterisks on the gel.
(B) Schematic of bilateral mediobasal hypothalamic stereotactic delivery of AAVs expressing Pomc-targeting CRISPR/Cas9 and EGFP (PomcARC-CRISPR). Representative image of GFP within the arcuate nucleus of the hypothalamus (ARC) third ventricle (3V).
(C) Hypothalamic content of Pomc peptide product α-melanocyte-stimulating hormone (α-MSH) 6 weeks after AAV injection is significantly reduced as measured by quantitative fluorescent EIA assay in PomcARC-CRISPR mice compared with controls injected with AAV-expressing sgRNA-eGFP alone (PomcARC-WT), illustrating that in vivo CRISPR targeting knocked down Pomc in the ARC (p = 0.0059; Mann-Whitney test).
(D) PAGE analysis identified heteroduplexes (pink arrowheads) from exon 3 of Pomc amplified from the mediobasal hypothalamus of PomcARC-CRISPR mice.
(E) PomcARC-CRISPR mice exhibit 24 hr of hyperphagia for home cage chow following food restriction compared with PomcARC-WT mice (n = 7–8; F1,13 = 11.27, p = 0.005).
(F) Likewise, PomcARC-CRISPR mice show greater motivation to work for palatable food compared with PomcARC-WT mice as assessed by the breakpoint of lever presses for chocolate pellets using a progressive-ratio schedule (n = 7–8; t12 = 2.463, p = 0.029).
(G) (Left) Lorcaserin (7 mg/kg, i.p.) reduced acute food intake within the first 3 hr (tick marks on x axis represent 20-min intervals) of the dark cycle in PomcARC-WT mice (n = 7; time: F9,54 = 38.05, p < 0.0001; treatment: F1,6 = 39.94, p = 0.0007; interaction: F9,54 = 13.91, p < 0.0001) and (right) as analyzed as total 3- and 6-hr cumulative intake (time: F1,6 = 169.7, p < 0.0001; treatment: F1,6 = 11.22, p = 0.0154; interaction: F1,6 = 0.8351, p = 0.3960).
(H) (Left) Lorcaserin (7 mg/kg, i.p.) also reduced acute food intake within the first 3 hr in PomcARC-CRISPR mice (n = 10; time: F9,81 = 168.1, p < 0.0001; treatment: F1,9 = 16.46, p = 0.0029; interaction: F9,81 = 2.108, p = 0.0381). (Right) However, lorcaserin was not effective in reducing cumulative food intake compared with saline treatment in PomcARC-CRISPR mice when analyzed at 3 or 6 hr food intake with Sidak's post hoc comparisons (n = 10; time: F1,9 = 130.8, p < 0.0001; treatment: F1,9 = 110.44, p = 0.0103; interaction: F1,9 = 0.01045, p = 0.9208).
Data are presented as mean ± SEM. Sidak's post hoc comparisons ∗p < 0.05, ∗∗p < 0.01.
AAVs expressing Pomc-targeting Cas9 and sgRNA-eGFP (PomcARC-CRISPR) or AAVs expressing sgRNA-eGFP alone (PomcARC-WT) were then stereotactically injected into the ARC (Figure 6B). Quantitative analysis of hypothalamic α-MSH content revealed PomcARC-CRISPR mice had an approximately 60% reduction in α-MSH (Figure 6C). To control for the occurrence of CRISPR/Cas9-induced indels, we implemented a method allowing ex vivo detection of DNA heteroduplexes. This is a widely used proxy of indel occurrence in vitro. Genomic DNA from PomcARC-CRISPR and PomcARC-WT mice was extracted and the third exon of the Pomc gene was amplified by PCR. Subsequent PAGE analysis revealed that DNA heteroduplexes were exclusively in samples obtained from the mediobasal hypothalamus in PomcARC-CRISPR mice. DNA heteroduplexes were not found in the hypothalamus of PomcARC-WT mice (Figure 6D). Behaviorally, PomcARC-CRISPR mice displayed phenotypes consistent with reduced POMCARC function, including hyperphagia following food deprivation (Figure 6E) and greater operant responding for palatable food (Figure 6F). Next, we assessed the role of POMCARC in lorcaserin's effects on food intake. As expected, lorcaserin significantly reduced ad libitum dark cycle food intake compared with saline treatment in PomcARC-WT mice when analyzed over the first 3 hr of the dark cycle (Figure 6G, left) and as cumulative 3 and 6 hr of food intake (Figure 6G, right). In contrast, PomcARC-CRISPR mice only significantly responded to the acute anorectic effect of lorcaserin (Figure 6H, left). Analysis of 3- and 6-hr cumulative intake revealed that PomcARC-CRISPR mice exhibited an attenuated response to lorcaserin. Specifically, lorcaserin did not significantly alter food intake compared with saline treatment at either time point in PomcARC-CRISPR mice (Figure 6H, right). One interpretation of these data is that POMCARC is not a principal mediator of lorcaserin's acute effects on food intake; rather, it is necessary for the longer-term effect. However, it is possible that the acute response to lorcaserin in PomcARC-CRISPR mice is due to incomplete knockdown of POMCARC and that the subset remaining is sufficient for lorcaserin to reduce feeding at this earlier time point. Nevertheless, these data indicate that lorcaserin requires functional Pomc within the ARC to promote its full effect on food intake.
POMCNTS Is Required for the Acute Effect of 5-HT2CR Agonists on Food Intake
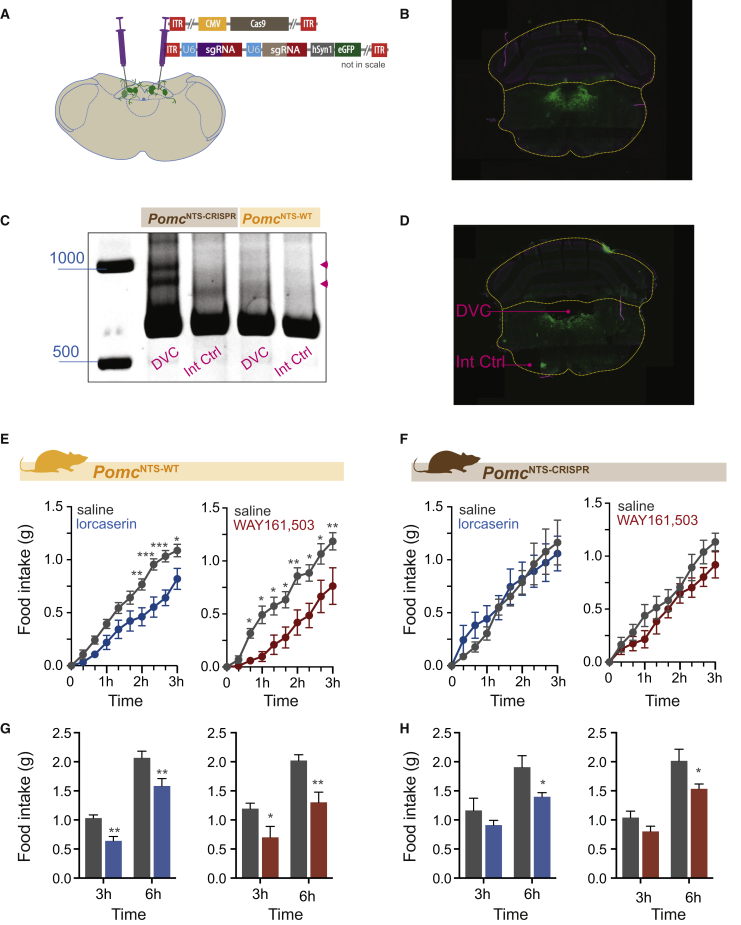
We next examined the functional significance of POMCNTS in the therapeutic effects of 5-HT2CR agonist obesity medications. We created two cohorts of mice by stereotactically delivering AAVs expressing Pomc-targeting Cas9 and sgRNA-eGFP (PomcNTS-CRISPR) or AAVs expressing sgRNA-eGFP alone (PomcNTS-WT) to the NTS (Figures 7A and 7B). Since α-MSH expression from NTS explants is not at a high enough level for reliable measurement, we adopted the method used above for PomcARC-CRISPR validation, allowing ex vivo detection of DNA heteroduplexes to control for the occurrence of CRISPR/Cas9-induced indels. Genomic DNA from PomcNTS-CRISPR and PomcNTS-WT mice was extracted and the third exon of the Pomc gene was amplified by PCR. Subsequent PAGE analysis revealed that DNA heteroduplexes were exclusively in samples obtained from the NTS/DMV and not an internal control region in PomcNTS-CRISPR mice (Figures 7C and 7D). As expected, DNA heteroduplexes were not found in either the NTS/DMV or an internal control region of PomcNTS-WT mice (Figure 7C). These data suggest that Pomc has been successfully targeted in PomcNTS-CRISPR mice, although this method does not provide quantification of the degree of knockdown. As expected, lorcaserin and WAY161,503 significantly reduced ad libitum dark cycle food intake in PomcNTS-WT mice over 6 hr (Figures 7E and 7G). However, following administration of 5-HT2CR agonists, PomcNTS-CRISPR mice exhibited an attenuated anorectic response to both lorcaserin and WAY161,503 during the first 3 hr of the dark cycle (Figure 7F). This attenuated effect to lorcaserin and WAY161,503 did not persist for the full 6 hr in PomcNTS-CRISPR mice. Lorcaserin significantly reduced 6-hr cumulative intake in PomcNTS-CRISPR mice compared with saline treatment (Figure 7H). Taken together with the PomcNEO and PomcARC-CRISPR results, these data suggest that POMCNTS is required for the acute effect of lorcaserin on food intake, whereas POMCARC is required for the longer-term effect on feeding. These findings are generally consistent with previous reports using chemogenetic and optogenetic approaches to examine POMC neuron function, which suggest that POMCARC and POMCNTS neurons affect feeding on different time scales (Aponte et al., 2011, Koch et al., 2015, Zhan et al., 2013).
Figure 7.
PomcNTS Is Required for 5-HT2CR Agonist Hypophagia
(A) AAV-mediated strategy to express in the nucleus of the solitary tract (NTS) Pomc-targeting sgRNAs and Cas9. Schematic of bilateral NTS stereotactic delivery of AAVs expressing Pomc-targeting CRISPR/Cas9 and EGFP (PomcNTS-CRISPR).
(B) Representative image of eGFP expression within the dorsal vagal complex (DVC).
(C) PAGE analysis identified heteroduplexes (pink arrowheads) from exon 3 of Pomc amplified from the DVC of PomcNTS-CRISPR mice.
(D) Representative image of tissue samples taken from the NTS and broader dorsal vagal complex (DVC) and an internal control region (Int Ctrl).
(E and F) 5-HT2CR agonists lorcaserin and WAY161,503 (7 mg/kg, i.p.) reduced acute food intake in control PomcNTS-WT mice (E) within the first 3 hr (tick marks on x axis represent 20-min intervals) of the dark cycle (lorcaserin, n = 8; time: F9,63 = 102.2, p < 0.0001; treatment: F1,7 = 18.55, p = 0.0035; interaction: F9,63 = 6.245, p < 0.001. WAY161,503, n = 8; time: F9,63 = 54.95, p < 0.0001; treatment: F1,7 = 24.28, p = 0.0017; interaction: F9,63 = 3.369, p = 0.0020), but not in PomcNTS-CRISPR mice (F) (lorcaserin, n = 7; time: F9,54 = 38.39, p < 0.0001; treatment: F1,6 = 0.1580, p = 0.7048; interaction: F9,54 = 0.2533, p = 0.9841. WAY161,503, n = 7; time: F9,54 = 62.69, p < 0.0001; treatment: F1,6 = 2.444, p = 0.1690; interaction: F9,54 = 0.7558, p = 0.6567).
(G) Likewise, when analyzed as total cumulative 3- and 6-hr dark cycle intake, lorcaserin and WAY161,503 significantly reduced food intake in control PomcNTS-WT mice (lorcaserin, n = 8; time: F1,7 = 96.71, p < 0.0001; treatment: F1,7 = 70.40, p < 0.0001; interaction: F1,7 = 0.6355, p = 0.4515. WAY161,503, n = 8; time: F1,7 = 62.88, p < 0.0001; treatment: F1,7 = 17.53, p = 0.0041; interaction: F1,7 = 4.238, p = 0.0785).
(H) POMCNTS knockdown in PomcNTS-CRISPR mice prevented the acute 3-hr anorectic effect of 5-HT2CR agonists, but was not sufficient to prevent the full effect 6 hr post treatment as analyzed with Sidak's post hoc comparisons (lorcaserin, n = 7; time: F1,6 = 92.76, p < 0.0001; treatment: F1,6 = 2.823, p = 0.1439; interaction: F1,6 = 5.309, p = 0.0608. WAY161,503, n = 7; time: F1,6 = 66.68, p = 0.0002; treatment: F1,6 = 4.152, p = 0.00877; interaction: F1,6 = 4.005, p = 0.0923).
Data are presented as mean ± SEM. Sidak's post hoc comparisons ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
Obesity negatively affects human health on a global scale. If we are to meet this challenge and design new medications, it is paramount that we gain a more complete understanding of the mechanisms regulating food intake and body weight. The 5-HT2CR agonist lorcaserin is a current obesity medication; however, neither the specific subset of 5-HT2CRs coordinating the therapeutic effect nor the neurochemical mediator targeted by these receptors has been fully defined. Earlier research genetically manipulating the receptor revealed that the subset of 5-HT2CRs co-expressed with brain POMC are both sufficient (Xu et al., 2010) and necessary (Berglund et al., 2013) to modulate the anorectic effect of preclinical 5-HT2CR agonists. Given that 5-HT2CR agonists increase the activity of POMCARC neurons (Burke and Heisler, 2015, Doslikova et al., 2013, Heisler et al., 2002), it was presumed that these neurons were the mediator of this effect. However, in light of recent findings that POMCARC neurons suppress appetite on a time scale of hours (Aponte et al., 2011, Koch et al., 2015, Zhan et al., 2013), we considered whether 5-HT2CRs outside the ARC are necessary for 5-HT2CR's acute effects on feeding. Here we identify the function of a previously uncharacterized subset of 5-HT2CR expressing neurons, localized within the NTS, and reveal that the activation of this subpopulation has a significant and rapid impact on feeding behavior, and furthermore is sufficient to drive lorcaserin's acute reduction in food intake. In addition, we report that a subset of POMCNTS neurons express 5-HT2CRs and that 5-HT and 5-HT2CR agonists directly activate POMCNTS neurons via a post-synaptic, mixed cationic current. We also clarify that brain POMC is necessary for lorcaserin to influence feeding. Although POMC is not abundantly expressed within the NTS, we report that POMC in this region is required for 5-HT2CR agonist obesity medications to acutely reduce food intake. These data thereby reveal a subpopulation of 5-HT2CRs, which when pharmacologically activated significantly reduce food intake, identify that the mechanistic underpinnings of this acute effect is via POMCNTS, and demonstrate that this discrete 5-HT2CRNTS subpopulation is therapeutically relevant to obesity medications targeting the 5-HT2CRs.
Limitations of Study
It is possible that the results obtained are affected by incomplete knockdown of Pomc in the ARC and NTS. Although altering POMCNTS expression significantly attenuated the acute effect of lorcaserin on food intake, at baseline PomcNTS-CRISPR mice displayed food intake comparable with PomcNTS-WT mice during ad libitum feeding (Figures 7E and 7F; and data not shown). These data are consistent with a previous report of diphtheria toxin POMCNTS cell ablation in a PomcCre mouse line, which did not produce an effect on ad libitum food intake (Zhan et al., 2013). However, we cannot exclude the possibility that this absence of baseline phenotype PomcNTS-CRISPR mice is due to incomplete knockdown of PomcNTS. Further studies are required to fully interrogate the physiological role of POMCNTS. Nevertheless, our data reveal that the relatively small source of POMC specifically derived within the NTS is necessary for the obesity medication lorcaserin and the preclinical compound WAY161,503 to promote their full effects on feeding behavior.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti-GFP | AbCam | Cat# ab13970; RRID: AB_300798 |
| Rabbit anti-dsRED | Rockland | Cat# 600-401-379; RRID: RRID: AB_2209751 |
| Rabbit anti-c-FOS | Calbiochem | Cat# PC38; RRID:AB_2106755 |
| Goat mCherry (RFP) | Sicgen | Cat# AB0040-200; RRID:AB_2333092 |
| Donkey polyclonal anti-chicken alexa 488 | Jackson ImmunoResearch | Cat# 703-545-155; RRID: AB_2340375 |
| Goat anti-Rabbit IgG Secondary Antibody, Alexa Fluor 594 | Life Technologies | Cat# A-11012, RRID:AB_141359 |
| Donkey anti-Goat IgG Secondary Antibody, Alexa Fluor 594 | Life Technologies | Cat# A-11057; RRID:AB_142581 |
| Biotin-SP-AffiniPure F(ab')2 Fragment Donkey Anti-Rabbit IgG | Jackson ImmunoResearch | Cat# 711-066-152, RRID:AB_2340594 |
| Streptavidin, Alexa Fluor® 568 conjugate antibody | Life Technologies | Cat# S-11226, RRID:AB_2315774 |
| Bacterial and Virus Strains | ||
| One Shot® Stbl3™ Chemically Competent E. coli | Invitrogen | Cat# C737303 |
| AAV8-hSyn-DIO-hM3Dq-mCherry | University North Carolina Vector Core | N/A |
| AAV8-hSyn-DIO-hM4Di-mCherry | University North Carolina Vector Core | N/A |
| AAV8-hSyn-mCherry-Cre | University North Carolina Vector Core | N/A |
| AAV-U6sgRNA(SapI)_hSyn-GFP-KASH-bGH | Unitat de Producció de Vectors, Universitat Autonoma Barcelona, Spain | N/A |
| AAV/DJ-CMV-spCas9 | Vector Biolabs | Cat# 7120 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Clozapine-N-oxide (CNO) | Tocris Bioscience | Cat. No. 4936; CAS: 34233-69-7 |
| Lorcaserin Hydrochloride | LGM Pharma | Cat# 846589-98-8 Lot# 20130115 |
| WAY 161503 hydrochloride | Tocris Bioscience | Cat. No. 1801; CAS: 276695-22-8 |
| Critical Commercial Assays | ||
| In-Fusion HD cloning Kit | Clonotech Laboratories | Cat No. 638910 |
| Plasmid plus Maxi kit | QIAGEN | Cat No. 12943 |
| QIAquick Gel Extraction Kit | QIAGEN | Cat No. 28704 |
| FuGENE HD Transfection Reagent | Promega | Cat No E2311 |
| PureLink Genomic DNA Mini Kit | Invitrogen | Cat No K182001 |
| Surveyor Mutation Detection Kit | Integrated DNA Technologies | Cat No. 706020 |
| MSH, alpha EIA kit | Phoenix Pharmaceutical Inc. | Cat No FEK-043-01 |
| Experimental Models: Cell Lines | ||
| Neuro-2a (N2a) cells | ATCC | CCL-131 |
| Experimental Models: Organisms/Strains | ||
| Mouse: 5-HT2CR-iresCre | Burke et al., 2016, Marcinkiewcz et al., 2016 | N/A |
| Mouse: POMCdsRed | Hentges et al., 2009 | N/A |
| Mouse: POMCNEO | Bumaschny et al., 2012 | N/A |
| Mouse: loxtb5-HT2CR (B6.129-Htr2ctm1Jke/J) | Jackson Laboratory | JAX: 015821 |
| Mouse: C57BL/6J | Jackson Laboratory | JAX: 000664 |
| Oligonucleotides | ||
| Genomic DNA Pomc PCR primer F: GCTTGCATCCGGGCTTGC |
This paper | N/A |
| genomic DNA Pomc PCR primer R: GACTTTATTTACGCAGTTTTTATTGAAGATCAGAGC |
This paper | N/A |
|
Pomc gRNA1: GGTGGGCAAGAAACGGCGCC |
This paper | N/A |
|
Pomc gRNA2: GTGACCCATGACGTACTTCCG |
This paper | N/A |
| Recombinant DNA | ||
| pX330-U6-Chimeric_BB-CBh-hSpCas9 (PX330) | Addgene | Addgene Plasmid #42230 |
| pAAV-U6sgRNA(SapI)_hSyn-GFP-KASH-bGH (PX552) | Addgene | Addgene Plasmid #60958 |
| cDNA HTR2C cDNA | Julius et al., 1988 | N/A |
| Software and Algorithms | ||
| pClamp10 software | Molecular Devices | |
| CRISPR Design | Zhang Lab, MIT | http://crispr.mit.edu/ |
| PhenoMaster | TSE | https://www.tse-systems.com/product-details/phenomaster |
| MATLAB | Mathworks | https://www.mathworks.com/ |
| Prism6 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to the Lead Contact Lora Heisler (lora.heisler@abdn.ac.uk).
Experimental Model and Subject Details
Mice
5-HT2CR-iresCre (Burke et al., 2016, Marcinkiewcz et al., 2016), loxtb5-HT2CR (B6.129-Htr2ctm1Jke/J; The Jackson Laboratory, Bar Harbor, ME USA), PomcDsRED (Hentges et al., 2009) and PomcNEO (Bumaschny et al., 2012) mice were housed with ad libitum food and water access (unless otherwise stated) in a light- (12 h on/12 h off) and temperature-controlled (21.5°C to 22.5°C) environment. All procedures were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and local ethical approvals.
Method Details
Stereotaxic Surgery and Viral Vectors
Viral constructs were packaged in AAV serotype-8 and were delivered to the NTS via bilateral stereotaxic injections, as previously described (D'Agostino et al., 2016).
AAV vectors
The DREADD viruses used have been described previously: AAV8-hSyn-DIO-hM3Dq-mCherry, AAV8-hSyn-DIO-hM4Di-mCherry and AAV8-hSyn-mCherry-Cre were packaged in AAV serotype-8 at a titer of 1.3 x 1013 vg/ml (University North Carolina Vector Core, Chapel Hill, NC, USA) (Krashes et al., 2011, D'Agostino et al., 2016). AAV/DJ-CMV-spCas9 was obtained from Vector Biolabs (Cat No. 7120; Vector Biolabs, Malvern, PA, USA). High titer pAAV-U6sgRNA(SapI)_hSyn-GFP-KASH-bGH (PX552) were packaged in AAV serotype-8 at a titer of 4.1 x 1012 vg/ml (Unitat de Producció de Vectors, Universitat Autonoma Barcelona, Spain).
Stereotaxic Surgery
NTS delivery of AAVs was achieved through a stereotaxic procedure. Briefly, 5–8 week old male mice were anesthetized with a mixture of ketamine and xylazine dissolved in saline (100 and 10 mg/kg, respectively injected in a volume of 10 ml/kg, i.p.). Mice were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA USA) using ear bars with the head angled at 45°. Under magnification, an incision was made at the level of the cisterna magna and neck muscles carefully retracted. A 33G needle was used for dura incision. The obex served as reference point for injection. Bilateral injections were performed using a glass micropipette (diameter 20–40 μm). NTS coordinates used were A/P, -0.2; M/L, ±0.2; D/V, -0.2 from the obex. Virus was delivered under air pressure using a PLI-100A Pico-Injector (Harvard Apparatus, Cambridge, UK). 150 nl of virus per side was delivered with multiple microinjections over 4–5 minutes. The pipette remained in place for a minimum of 3 minutes after injection. Following all surgery, animals were closely monitored, kept warm and appropriate postsurgical care was taken. Animals were also administered an analgesic (5 mg/kg carprofen, s.c.) daily for 2 days post-operatively. Prior to experimentation, mice were acclimated to handling and i.p. injections.
Mice were given a minimum of 14 days of surgical recovery before experimentation. For chemogenetic experiments, mCherry was visualised by immunohistochemistry. Mice in which the expression of reporter fluorescent protein was bilaterally detected in the caudal aspect of the DVC were included in the analysis, whereas mice without reporter fluorescent expression or expression outside the DVC were excluded from the analysis. Caudal DVC was defined by the presence of the area postrema in the same section of brain tissue.
DNA Constructs
For SpCas9 target selection and generation of single guide RNA (sgRNA), the 20-nt target sequences were selected to precede a 5’-NGG protospacer-adjacent motif (PAM) sequence. To minimize off-targeting effects, the CRISPR design tool was used (http://crispr.mit.edu/). The 20-nt target and complement sequences were synthesized to include BbsI overhangs. Oligos were annealed, phosphorylated and ligated into the pX330-U6-Chimeric_BB-CBh-hSpCas9 (PX330; Addgene plasmid #42230) plasmid after BbsI digestion.
pAAV-U6sgRNA(SapI)_hSyn-GFP-KASH-bGH (PX552; Addgene plasmid # 60958) was used for cloning sgRNAs into AAV the backbone and generate viral particles. The plasmid was first digested with SapI to create sticky ends for ligation. The 20-nt target and complement sequences were synthesized to include SapI overhangs and ligated. A second sgRNA was then inserted into this vector. The second sgRNA was first PCR-amplified from PX330. Primers were designed to include 15bp homology arms to the PspOMI-lineraised PX552 into the PCR clone sgRNA guide. The PCR product was column-purified and then cloned into the PspOMI-lineraised PX552 vector via homologous recombination-assisted cloning (In-Fusion HD cloning Kit®, Cat No. 638910; Clonotech Laboratories). Primers sequences: AGACTGCAGAGGGCCGAGGGCCTATTTCCCATGATTCCT, forward; CTCATACGCAGGGCCCTAAAACAAAAAAGCACCGACTCGG, reverse. All obtained constructs were verified by sequencing. All plasmids were amplified using One Shot® Stbl3™ Chemically Competent E. coli (Cat No. C737303; Invitrogen) and column-purified (Cat No. 270104; Qiagen).
Pomc targeting sgRNAs (5’ to 3’): sgRNA 1- GGTGGGCAAGAAACGGCGCC(cgg); sgRNA 2- GTGACCCATGACGTACTTCCG(ggg).
Cell Line Culture and Transfection
Mouse Neuro-2a (N2a) cells were grown in DMEM containing 10% FBS. Cells were maintained at 37°C in 5% CO2 atmosphere. Transfection were performed using FuGENE ® HD Transfection Reagent (Cat No E2311; Promega) according to the manufacturer’s protocol. Briefly, 1.5x104 cells per well were plated into a 12 well plate the day before transfection. 1 ug of PX330 plasmid was mixed with Fugene HD in Optimem medium (ratio 1:3). Cells were incubated for 48 hours. Genomic DNA was extracted from transfected cells using PureLink® Genomic DNA Mini Kit (Cat No K182001, Invitrogen) following the manufacturer’s instructions.
Detection of Insertions/Deletions (Indels)
The third exon of the mouse Pomc gene was PCR amplified from genomic DNA using the following primers: GCTTGCATCCGGGCTTGC, forward; GACTTTATTTACGCAGTTTTTATTGAAGATCAGAGC, reverse. This PCR reaction generates a single PCR product of the expected 726bp size. PCR product was sequence verified. To detect sgRNA/spCas9-induced indels, genomic DNA from cells was amplified using the same primers and this 726bp product corresponding to third exon of the mouse Pomc gene was used for the endonuclease I assay (Surveyor® Mutation Detection Kit; Cat No. 706020; Integrated DNA Technologies) following the manufacturer’s instructions. Briefly, PCR amplicons were heat-denaturated and re-annealed using a thermocycler to create DNA heteroduplexes. DNA heteroduplexes were incubated with endonuclease I enzyme, which cleaves DNA at the site of a base mismatch with high specificity, for 1 hour at 42°C. Genomic modifications were verified using gel agarose electrophoresis.
Feeding Studies
Dark Cycle Food Intake
Mice were injected with vehicle or drug 30 minutes prior to the onset of the dark cycle and food was removed. At the onset of dark, food was returned and manually (home cage) or automatically (Phenomaster chambers, TSE, Bad Homburg, Germany) weighed using methods previously described (D'Agostino et al., 2016).
Post-fast Refeeding
Food was removed at the onset of the dark cycle and returned 2 hours after the onset of the light cycle the following day. Food intake was measured manually (home cage) or automatically (TSE Phenomaster chambers) using methods previously described (D'Agostino et al., 2016).
Progressive Ratio
Mice were trained in operant chambers housed in sound-attenuating boxes and controlled by a PC Med-PC-IV programming language (MED Associates, Inc., Fairfax, VT USA). Chambers (21.6 cm long × 17.8 cm wide × 12.7 cm high) have a retractable lever, a pellet receptacle and a 3 watt house-light on the opposite wall (Cat No. MED-307W-D1, MED Associates, Inc.). Under food restriction, mice were trained to press the lever for 20 mg chocolate pellet reinforcers (TestDiet, St. Louis, MO USA) under a fixed ratio (FR) 1 schedule for 3 days followed by FR 5 for a further 3 days. Then they were fed ad libitum and underwent daily training under a PR schedule based on an exponential progression derived from the formula (5 × e0.2n)−5, rounded to the nearest integer, where n is the position in the ratio sequence (Richardson and Roberts, 1996). 50 minute sessions took place at the same time each day during the light phase (between 08:00 and 13:00 hours) for 1 week. The breakpoint or alternatively the highest completed ratio (Olarte-Sánchez et al., 2012) was defined as the last ratio completed before 5 minutes elapsed without any responding.
Immunohistochemistry (IHC) and Fluorescent In Situ Hybridization Histochemistry (FISH)
IHC. Tissue was processed for GFP, mCherry, dsRed and/or c-Fos (FOS-IR) IHC as previously described (D’Agostino et al., 2016). Briefly, mice were transcardially perfused with phosphate buffered saline (PBS) followed by 10% neutral buffered formalin (Sigma-Aldrich). Brains were extracted, post-fixed in 10% neutral buffered formalin at 4°C, cryoprotected in 20% sucrose at 4°C and then sectioned coronally on a freezing sliding microtome at 25 μm. Tissue was processed for chicken anti-GFP (1:1000; AbCam, ab13970), rabbit anti-dsRED (1:1000; Rockland, 600-401-379), goat anti-mCherry/RFP (1:1000; Sicgen, AB0040-200) or anti-c-FOS (1:5000, rabbit, Calbiochem, PC38) primary antibodies and a biotinylated donkey anti-rabbit (1:500, Jackson ImmunoResearch Laboratories, Inc.) or Alexa Fluor (1:500, Life Technologies) secondary antibodies using standard protocols previously described (Lam et al., 2009, Heisler et al., 2006). Tissue was then mounted on slides, cover slipped and the NTS visualized using an Axioskop II microscope (Carl Zeiss, Oberkochen, Germany) and Adobe Photoshop CS5 software. Images of single-label immuoreactivity (IR) for GFP or mCherry were used to visualize and analyze NTS injection sites.
Dual-IHC
Dual-IHC was performed to visualize co-expression of mCherry-IR and FOS-IR in 5-HT2CRNTS-hM3Dq mice. Specifically, mice were fasted overnight and then injected with designer drug CNO or saline. Brains were extracted 90 minutes later and dual-label mCherry and FOS-IR performed using method outlined above. Quantitative analysis of single and dual-labelled cells was performed manually using an Axioskop II microscope and Adobe Photoshop CS5 software.
IHC and FISH
IHC and FISH were performed to visualize co-expression of POMC and 5-HT2CR within the NTS in POMCdsRED mice. To label 5-HT2CR expressing neurons, a RNA expression vector (pBluescript SK-) containing the 3-kilobase (kb) coding region of 5-HT2CR cDNA was used to generate single-stranded RNA probes (Julius et al., 1988). Briefly, in vitro transcription was performed using Digoxigenin (DIG)-RNA Labelling Mix (Roche, Mannheim, Germany). Sections were treated with 1% Sodium Borohydride solution and 0.25% Acetic Anhydride in Triethanolamine (TEA) solution. Sections were then incubated in a hybridization buffer containing a DIG-UTP-labelled 5-HT2CR riboprobe overnight at 55°C. Sections were rinsed in a standard sodium citrate/50% formamide solution, rinsed in an RNase (0.02 mg/ml RNase A) solution, incubated in a 3% H2O2 solution for 30 minutes and blocked in a 2% sheep serum (Sigma-Aldrich, Saint Louis, MO USA). Sections were then incubated with an anti-DIG antibody (1:5000, Roche, Mannheim, Germany), treated with TSA PLUS Biotin Kit (PerkinElmer Inc., Waltham, MA USA) and revealed with streptavidin conjugated Alexa Fluor® 568 (1:2000, Life Technologies, Carlsbad, CA USA). To label POMC, sections were blocked again and incubated with rabbit anti-dsRED (1:1000; Rockland, Limerick, PA USA; 600-401-379) overnight at 4°C using the IHC protocol described above. The sections were incubated with Alexa Fluor® 568 donkey anti-rabbit secondary antibody (1:500, Invitrogen™ Life Technologies, Carlsbad, CA USA) for 1 hour. Tissue was then mounted on slides, cover slipped and single- and dual-labelling assessed in the NTS using an Axioskop II microscope and Adobe Photoshop CS5 software. Quantitative analysis of single and dual-labelled dsRed-IR and 5-HT2CR mRNA cells was conducted manually.
Enzyme Immunoassay (EIA) for Alpha-Melanocyte-Stimulating Hormone (Alpha-MSH)
Mice were anesthetized with sodium pentobarbital (Euthatal) and decapitated. The brain was rapidly removed, the hypothalamus was dissected using razor blades and mouse acrylic brain matrices (Stoelting Co.) and frozen in dry ice. Hypothalamic lysates were obtained using a probe sonicator. Lysis consisted of an acid-ethanol solution obtained by combining 1 part of concentrated HCl with 7 parts pure ethanol. 0.1ml of lysis was used per 10 mg of tissue (weight of each hypothalamic explant was about 30 mg). 10 μl of crude homogenate were combined with 50 μl of fresh lysis buffer and centrifuged 3500 g for 30 min. Supernatants were collected and dried using a vacuum centrifuge in low protein binding tubes (Eppendorf® LoBind, Sigma-Aldrich, Cat No. Z666505). Dry fractions were solubilised in 150 μl of assay buffer and alpha-MSH content analysed using a fluorescent EIA ultra-sensitive assay (Phoenix Pharmaceutical Inc.; Cat No FEK-043-01) following the manufacturer’s instructions.
Electrophysiology and Chemogenetic Validation Analysis
For electrophysiological experiments, male and female POMCDS-Red (n=44), male loxtb5-HT2CR (n=9), male 5-HT2CRNTS-hM3Dq (n=2) or male 5-HT2CRNTS-hM4Di (n=2) mice aged 2-6 months were anesthetized with sodium pentobarbital (Euthatal) and decapitated. The brain was rapidly removed and placed in cold (0–4°C), oxygenated (95%O2/5%CO2) ‘slicing’ solution containing (in mM) sucrose (214), KCl (2.5), NaH2PO4 (1.2), NaHCO3 (26), MgSO4 (4), CaCl2 (0.1), D-glucose (10). The brain was fixed to a vibrating microtome (Campden Instruments, Loughborough, UK) and 200-μm thick coronal sections of the brainstem containing the NTS were prepared. Slices were immediately transferred to a ‘recording’ solution containing (in mM) NaCl (127), KCl (2.5), NaH2PO4 (1.2), NaHCO3 (26), MgCl2 (1.3), CaCl2 (2.4) and D-glucose (10) in a continuously oxygenated holding chamber at 35°C for a period of 25 minutes. Slices were remained in the recording solution at room temperature for a minimum of 1 hour before recording. For whole-cell recordings, slices were transferred to a submerged chamber and a Slicescope upright microscope (Scientifica, Uckfield, UK) was used for infrared - differential interference contrast and fluorescence visualization of cells. During recording, slices were continuously perfused at a rate of ca. 2 ml/minute with oxygenated recording solution at room temperature. All pharmacological compounds were bath applied. Whole cell current-clamp recordings were performed with pipettes (3–7 MΩ when filled with intracellular solution) made from borosilicate glass capillaries (World Precision Instruments, Aston, UK) pulled on a Zeitz DMZ micropipette puller (Zeitz Instruments GmBH, Martinsried, Germany). The intracellular recording solution contained (in mM) K-gluconate (140), KCl (10), HEPES (10), EGTA (1), Na2ATP (2), pH 7.3 (with KOH). Recordings were performed using a Multiclamp 700B amplifier and pClamp10 software (Molecular Devices, Sunnyvale, CA USA). Liquid junction potential was 16.4 mV and not compensated. The recorded current was sampled at 10 kHz and filtered at 2 kHz unless otherwise stated.
Drugs
Lorcaserin (LGM Pharma, Erlanger KY USA), WAY161,503 (Tocris Bioscience, Abingdon UK) and clozapine-n-oxide (CNO; Tocris Bioscience) were prepared in double distilled H2O, diluted in sterile phosphate-buffered saline (PBS) and administered in vivo i.p. in a volume of 10 ml/kg. 5-HT (Sigma-Aldrich), lorcaserin, WAY161,503 and CNO were dissolved in artificial cerebrospinal fluid (aCSF) for electrophysiology experiments.
Quantification and Statistical Analysis
Data were analyzed with t-test, Mann Whitney U test, One-way, Two-way or Repeated Measures ANOVA followed by Tukey’s or Sidak’s post hoc tests, where appropriate. For all analyses, significance was assigned at p<0.05. Data are presented as mean±SEM. All statistical analyses were completed with GraphPad Prism 7.0 (GraphPad Software, San Diego, CA USA).
Acknowledgments
The authors wish to thank members of staff of the Medical Research Facility, University of Aberdeen, Ms. Raffaella Chianese and Dr. Susan Jalicy, for technical assistance. PX330 and PX552 plasmids were a gift from Prof. Feng Zhang (Massachusetts Institute of Technology, Massachusetts, USA). DREADD vectors were a gift from Prof. Bryan Roth (University of North Carolina at Chapel Hill, North Carolina, USA). PomcDsRED and PomcNEO mice were a gift from Prof. Malcolm Low (University of Michigan, Michigan, USA). Codes to analyze operant-responding for food were a gift from Dr. Vladimir Orduña Trujillo (National Autonomous University of Mexico, Mexico). This work was supported by the Wellcome Trust (L.K.H.; WT098012), Wellcome Trust and the University of Aberdeen (G.D.; 105625/Z/14/Z), Biotechnology and Biological Sciences Research Council (L.K.H., BB/K001418/1, BB/N017838/1; and J.J.R., BB/K017772/1), Medical Research Council (J.J.R., MR/L002620/1; G.D., MR/P009824/1; L.K.H., J.J.R., G.D., MC/PC/15077), British Society of Neuroendocrinology (G.D.), NIH and the Marilyn H. Vincent Foundation (M.G.M.; DK056731, DK034933).
Author Contributions
G.D. and L.K.H. conceived the study and designed experiments. G.D. performed experiments with help from B.D., C.O.-S., P.B.M.d.M., T.G., and C. Cristiano. M.L. and J.J.R. performed cell-culture experiments. C. Cansell performed fluorescence in situ hybridization experiments. L.K.B. and B.D. performed studies with PomcNEO mice. M.G.-Y. and M.G.M. generated 5-HT2CRCre mice. D.L. performed electrophysiology experiments. G.D., D.L., and L.K.H. wrote the manuscript with input from all other authors.
Declarations of Interests
L.K.H. has consulted for Eisai, Inc.
Published: August 23, 2018
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.cmet.2018.07.017.
Contributor Information
Giuseppe D'Agostino, Email: giuseppe.dagostino@abdn.ac.uk.
Lora K. Heisler, Email: lora.heisler@abdn.ac.uk.
Supplemental Information
References
- Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard S.M., Bailey T.W., Doyle M.W., Jin Y.H., Smart J.L., Low M.J., Andresen M.C. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J. Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster B.N., Li X., Pausch M.H., Herlitze S., Roth B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronne L., Shanahan W., Fain R., Glicklich A., Soliman W., Li Y., Smith S. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad. Med. 2014;126:7–18. doi: 10.3810/pgm.2014.10.2817. [DOI] [PubMed] [Google Scholar]
- Berglund E.D., Liu C., Sohn J.W., Liu T., Kim M.H., Lee C.E., Vianna C.R., Williams K.W., Xu Y., Elmquist J.K. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breisch S.T., Zemlan F.P., Hoebel B.G. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192:382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchon V., de Souza F.S., Rubinstein M., Low M.J. Obesity-programmed mice are rescued by early genetic intervention. J. Clin. Invest. 2012;122:4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke L.K., Doslikova B., D'Agostino G., Garfield A.S., Farooq G., Burdakov D., Low M.J., Rubinstein M., Evans M.L., Billups B. 5-HT obesity medication efficacy via POMC activation is maintained during aging. Endocrinology. 2014;155:3732–3738. doi: 10.1210/en.2014-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke L.K., Doslikova B., D'Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R., Martinez de Morentin P.B., Ogunnowo-Bada E., Cansell C., Valencia-Torres L. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol. Metab. 2016;5:245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke L.K., Heisler L.K. 5-hydroxytryptamine medications for the treatment of obesity. J. Neuroendocrinol. 2015;27:389–398. doi: 10.1111/jne.12287. [DOI] [PubMed] [Google Scholar]
- Burke L.K., Ogunnowo-Bada E., Georgescu T., Cristiano C., de Morentin P.B.M., Valencia Torres L., D'Agostino G., Riches C., Heeley N., Ruan Y. Lorcaserin improves glycemic control via a melanocortin neurocircuit. Mol. Metab. 2017;6:1092–1102. doi: 10.1016/j.molmet.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G., Diano S. Alpha-melanocyte stimulating hormone: production and degradation. J. Mol. Med. (Berl.) 2010;88:1195–1201. doi: 10.1007/s00109-010-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G., Lyons D.J., Cristiano C., Burke L.K., Madara J.C., Campbell J.N., Garcia A.P., Land B.B., Lowell B.B., Dileone R.J. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife. 2016;5 doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doslikova B., Garfield A.S., Shaw J., Evans M.L., Burdakov D., Billups B., Heisler L.K. 5-HT2C receptor agonist anorectic efficacy potentiated by 5-HT1B receptor agonist coapplication: an effect mediated via increased proportion of pro-opiomelanocortin neurons activated. J. Neurosci. 2013;33:9800–9804. doi: 10.1523/JNEUROSCI.4326-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Fan W., Ellacott K.L., Halatchev I.G., Takahashi K., Yu P., Cone R.D. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Fletcher P.J., Tampakeras M., Sinyard J., Slassi A., Isaac M., Higgins G.A. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009;57:259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Grill H.J., Hayes M.R. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int. J. Obes. (Lond.) 2009;33(Suppl 1):S11–S15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- Heisler L.K., Cowley M.A., Tecott L.H., Fan W., Low M.J., Smart J.L., Rubinstein M., Tatro J.B., Marcus J.N., Holstege H. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler L.K., Jobst E.E., Sutton G.M., Zhou L., Borok E., Thornton-Jones Z., Liu H.Y., Zigman J.M., Balthasar N., Kishi T. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hentges S.T., Otero-Corchon V., Pennock R.L., King C.M., Low M.J. Proopiomelanocortin expression in both GABA and glutamate neurons. J. Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.A., Pilcher W.H., Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci. Lett. 1983;38:221–225. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A.B., Axel R., Jessell T.M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Koch M., Varela L., Kim J.G., Kim J.D., Hernandez-Nuno F., Simonds S.E., Castorena C.M., Vianna C.R., Elmquist J.K., Morozov Y.M. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S., Maratos-Flier E., Roth B.L., Lowell B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D.D., Przydzial M.J., Ridley S.H., Yeo G.S., Rochford J.J., O'Rahilly S., Heisler L.K. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D.D., Zhou L., Vegge A., Xiu P.Y., Christensen B.T., Osundiji M.A., Yueh C.Y., Evans M.L., Heisler L.K. Distribution and neurochemical characterization of neurons within the nucleus of the solitary tract responsive to serotonin agonist-induced hypophagia. Behav. Brain Res. 2009;196:139–143. doi: 10.1016/j.bbr.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz C.A., Mazzone C.M., D'Agostino G., Halladay L.R., Hardaway J.A., DiBerto J.F., Navarro M., Burnham N., Cristiano C., Dorrier C.E. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K., Abdallah L., Goulding E.H., Bonasera S.J., Tecott L.H. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- Olarte-Sánchez C.M., Valencia Torres L., Body S., Cassaday H.J., Bradshaw C.M., Szabadi E. Effect of orexin-B saporin-induced lesions of the lateral hypothalamus on performance on a progressive ratio schedule. J. Psychopharmacol. 2012;26:871–886. doi: 10.1177/0269881111409607. [DOI] [PubMed] [Google Scholar]
- Richardson N.R., Roberts D.C. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Smith S.R., Weissman N.J., Anderson C.M., Sanchez M., Chuang E., Stubbe S., Bays H., Shanahan W.R. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Sohn J.W., Xu Y., Jones J.E., Wickman K., Williams K.W., Elmquist J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J.A., Davies K.E., Williams S.R., Luckman S.M. Functional magnetic resonance imaging and c-Fos mapping in rats following an anorectic dose of m-chlorophenylpiperazine. Neuroimage. 2006;31:1228–1237. doi: 10.1016/j.neuroimage.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Swiech L., Heidenreich M., Banerjee A., Habib N., Li Y., Trombetta J., Sur M., Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott L.H., Sun L.M., Akana S.F., Strack A.M., Lowenstein D.H., Dallman M.F., Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Wade J.M., Juneja P., MacKay A.W., Graham J., Havel P.J., Tecott L.H., Goulding E.H. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;149:955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.W., Scott M.M., Elmquist J.K. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. Eur. J. Pharmacol. 2011;660:2–12. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jones J.E., Kohno D., Williams K.W., Lee C.E., Choi M.J., Anderson J.G., Heisler L.K., Zigman J.M., Lowell B.B. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jones J.E., Lauzon D.A., Anderson J.G., Balthasar N., Heisler L.K., Zinn A.R., Lowell B.B., Elmquist J.K. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J. Neurosci. 2010;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C., Zhou J., Feng Q., Zhang J.E., Lin S., Bao J., Wu P., Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.