Abstract
Melanoma is an incurable disease for which alternative treatments to chemotherapy alone are sought. Here, using a melanoma model, we investigated the antitumor potential of combining ultrasound (US) with poly(lactic-co-glycolic acid) (PLGA) microspheres loaded with doxorubicin (DOX). The aim was to achieve synergistic tumoricidal activity through direct and indirect US-mediated damage of tumor cells combined with sustained and potentially controllable release (when combined with US) of DOX from microspheres. An in vitro release assay demonstrated an ability of US to affect the release kinetics of DOX from DOX-loaded PLGA microspheres by inducing a 12% increase in rate of release. In vitro viability assays demonstrated that combining US with DOX-loaded PLGA microspheres resulted in synergistic tumor cell (B16-F10 melanoma cells) killing. Melanoma-bearing mice were treated intratumorally with DOX (8 µg)-loaded microspheres and subjected to US treatment at the tumor site. This treatment could significantly extend survival (mean survival (MS) = 22.1 days) compared to untreated mice (MS = 10.4 days) and most other treatments, such as blank microspheres plus US (MS = 11.5 days) and DOX (8 µg)-loaded microspheres alone (MS = 13 days). The findings that immune checkpoint blockade did not significantly extend survival of mice treated with DOX (8 µg)-loaded microspheres plus US, and that tumor-free (“cured”) mice were not protected from subsequent tumor rechallenge suggests minimal involvement of the adaptive immune response in the observed antitumor activity. Nevertheless, the synergistic increase in survival of melanoma-challenged mice treated with the combination of US and DOX-loaded microspheres implicates such a treatment methodology as a promising additional tool for combatting otherwise currently incurable cancers.
Keywords: Ultrasound, PLGA microspheres, doxorubicin, melanoma, controlled drug release, inertial cavitation, cancer therapy
1. Introduction
Cancer is the second leading cause of death behind heart disease in the United States (Prevention, 2016), and many cancer types are largely refractory to current conventional treatments. This is particularly so for patients with advanced melanoma and is at least partially due to a lack of antitumor efficacy of chemotherapeutic drugs imposed by multidrug resistance and the narrow therapeutic window (Fletcher et al., 2010; Goldblatt and Lee, 2010; Liang et al., 2010). Increasing the therapeutic index of chemotherapeutic drugs such as DOX is important if progress is to be made in improving tumor regression rates. DOX has the potential to cause fatal cardiomyopathy once the lifetime cumulative total soluble dose exceeds 450 mg/m2 (Rivankar, 2014). As a result, DOX formulations have been formulated and introduced into the clinic, such as pegylated liposomes, known as Doxil® (Rivankar, 2014), which have exhibited decreased drug toxicity compared to conventional DOX therapy although they have not made significant improvements in antitumor efficacy and long term survival (Ngan and Gupta, 2016; Vici et al., 2011; Wasle et al., 2015). Additionally, Doxil® still has dose-limiting side effects, such as hand-foot syndrome (Rivankar, 2014).
It is becoming increasingly apparent, due at least in part to the heterogeneity of tumors, that a multipronged therapeutic approach is necessary for the treatment of advanced cancers where each therapy can have additive or, preferably, synergistic tumoricidal consequences. In this research, we attempted this by administering US to DOX-loaded microspheres, made from United States Food and Drug Administration-approved, biodegradable and biocompatible PLGA (Anderson and Shive, 1997; Makadia and Siegel, 2011), at the tumor site. Appropriate formulations need to be generated that provide safe and sustained release of the drug payload within the therapeutic window. Techniques such as double emulsion solvent evaporation (water-in-oil-in-water) (Joshi et al., 2013), aqueous-aqueous emulsion (followed by solid-in-oil-in-water) (Kang et al., 2014; Zhao et al., 2013), solid-in-oil-in-hydrophilic oil-in-water (Yuan and Liu, 2012), and solid-in-oil-in-hydrophilic oil-in-ethanol (Yuan et al., 2010) have generated microspheres with abilities to provide sustained release of drugs from periods of weeks to months. The application of US can promote the inertial cavitation of air bubbles (or nucleation sites) (Deng et al., 1996) in close proximity to, or in direct contact with, microspheres or cells, resulting in direct (shock waves from inertial cavitation) or indirect (microsphere collisions) damage of these microspheres or cells. Inertial cavitation is a process where air bubbles expand to 2 – 3 times their resonant size and then implode during a single compression generating large gas pressures and temperatures (Frenkel et al., 1999; Miller and Thomas, 1995). The effects of US-induced inertial cavitation have also been seen to accelerate solid particle velocities which are capable of producing interparticle collisions resulting in morphological and compositional changes (Doktycz and Suslick, 1990; Gâmbuteanu and Alexe, 2013; Suslick, 1997). Although the concept of combining US and non-acoustically active micro-, or nanospheres for the purposes of drug or gene delivery has received some attention there have been no investigations into the application of such a system for the purposes of tumor cell killing (Figueiredo and Esenaliev, 2012; Jang et al., 2017). By combining US and DOX-loaded microspheres there is the increased potential for manifold modes of cytotoxicity, which include physically-mediated direct lethal damage to cells or sufficiently disrupting membrane integrity (non-lethal) so as to increase intracellular uptake of DOX. Also, US may have a role in enhancing the release of DOX from microspheres by damaging microspheres through inertial cavitation of neighboring air bubbles, or indirectly through microsphere collisions. In addition, DOX possesses the ability to not only kill cells by inhibition of DNA synthesis, it also induces an immunogenic form of cell death thereby potentiating tumor-specific immune responses (Casares et al., 2005). Thus local treatment of a tumor with chemotherapeutic agents such as DOX can have systemic ramifications in terms of the generation of tumor specific immune responses (Tongu et al., 2010) as has been more commonly observed with radiation therapy (and is often referred to as “the abscopal effect”)(Demaria and Formenti, 2012). We hypothesized that the combination of US with DOX-loaded microspheres delivered intratumorally could have a synergistic tumoricidal effect through multiple modes of tumor cell killing, thereby enhancing survival in tumor-challenged mice. In this study DOX was encapsulated within microspheres (diameter 4–8 µm) made from PLGA using a double emulsion solvent evaporation technique. PLGA microspheres in this size range often degrade gradually over a period of days to several weeks, releasing the encapsulated drug gradually, however, we hypothesized that with the application of US, a more rapid release rate can be triggered through the damage caused to the surface of drug loaded microspheres as a result of inertial cavitation of neighboring air bubbles. Although increased release rates from drug-loaded echogenic nanoparticles has previously been reported, such findings with solid and henceforth presumably non-echogenic particles have not been well documented (Figueiredo and Esenaliev, 2012). In this study, we evaluated the effects of combining US with DOX-loaded solid microspheres and speculate how this combination may have contributed to the reduced growth of B16-F10 melanoma cells observed in vitro and in vivo.
2. Materials and Methods
2.1.1. Microsphere Fabrication
Microspheres were fabricated using a previously described double emulsion solvent method (Joshi et al., 2013). Briefly, a stock solution of doxorubicin (DOX: Sigma-Aldrich, St. Louis, MO) was made by dissolving 10 mg of DOX in 250 µl of 1% poly(vinyl alcohol) (PVA; Mowiol® 8–88; MW ∼67,000; Sigma-Aldrich). This solution was incubated overnight at 4°C to ensure a complete and homogenous solution. Water phase 1 consisted of either 75 µl of the DOX solution or 75 µl of 1% PVA (for blank particles). An oil phase was created by dissolving 200 mg of PLGA (Resomer® RG 503; Boehringer Ingelheim KG, Germany) in 1.5 mL of dichloromethane (DCM). A primary emulsion was prepared by sonication of 75 µl of the water phase 1 into the oil phase using a sonic dismembrator ultrasonic processor (Fisher Scientific, Pittsburgh, PA) at 40% amplitude for 30 s. This emulsified solution was then added to 30 mL of 1% PVA in ammonium acetate buffer solution (pH:8.4) and homogenized using an Ultra Turrax T-25 basic homogenizer (IKA-WERKE, Inc., Wilmington, NC) at speed 4, 17500 min−1, for 30 s. The emulsion was stirred for 1.5–2.0 h in a fume hood to allow DCM to evaporate. The particles were then centrifuged at 7*g for 5 minutes at room temperature to filter out particles larger than desired size. The supernatant was collected and centrifuged at 180*g for 5 min, washed twice with nanopure water, dispersed in 5 mL of water, and lyophilized using a FreeZone 4.5 freeze dry system (Labconco, Kansas City, MO).
2.1.2. Determining size, DOX-loading and DOX-loading efficiency of PLGA microspheres
Eight batches of DOX-loaded PLGA microspheres were prepared as described above. To determine loading and loading efficiency, samples from each batch were tested. To determine loading, a sample of DOX-loaded microspheres (3–5 mg) post-lyophilization was dissolved in DMSO and the yield of DOX was calculated using a standard curve. Along with the standard curve samples, the test samples were measured for DOX at λex485, λem570 using a SPECTRAmax M5 Microplate Spectrofluorometer (Molecular Devices, San Diego, CA). The yield of DOX was then divided by the weight of DOX-loaded microspheres (in the sample) to determine the loading per mg of microspheres. To determine loading efficiency, the known amount of total DOX-loaded PLGA particles from each batch post-lyophilization was multiplied by the calculated loading (as determined above) and then divided by the original 3 mg of DOX added to fabricate the particles. This value was then multiplied by 100 to achieve a percent value. The size (diameter) of the fabricated microspheres was calculated using scanning electron microscopic (SEM) images (Hitachi High-Technologies, Tokyo, Japan) and analysis using ImageJ software.
2.2. Device Configuration for US Generation
Device configuration and US generation were set-up as previously described(Jang et al., 2017) where a 4040B 20 MHz DDS Function Generator (BK Precision, Yorba Linda, CA) was used to generate the desired waveforms. The function generator was then connected to a radio frequency ENI 310L RF Power Amplifier (ValueTronics Intl Inc., Algin, IL) which helped to produce excitation signals to drive the transfer of the waveforms to a custom designed 1 MHz transducer (L = 2.5”, D = 1”, ID = 0.5”) (Ultrasonic S-Lab, Concord,CA). Refer to Figure 1 for instrument configuration. The function generator parameters were programmed to have continuous sinusoidal waves at a frequency of 1 MHz and an amplitude of 0.2 V in order to output the desired ultrasonic waves necessary for microsphere damage. US application was performed using these settings for a period of 10 seconds at an intensity of 900 mW/cm2 as determined by radiation force balance.
Figure 1:
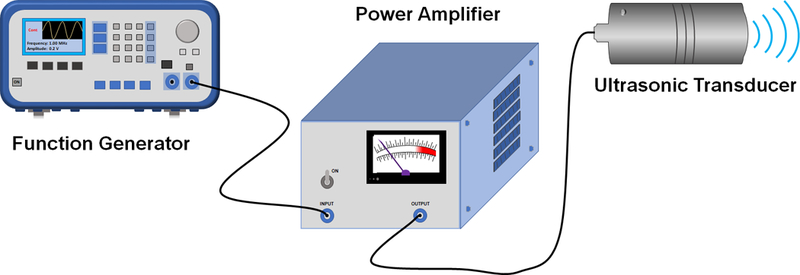
US generation instrument set up.
2.3. Ultra-morphology of Blank Particles after the Application of US
Blank particles were weighed and suspended in nanopure water at a concentration of ~3 mg/mL and added to a 1.5 mL microcentrifuge tube. The suspensions were then divided in two, where one sample was the control, while the other was the test group. To demonstrate the importance of the presence of air bubbles in mediating US-triggered particle damage, particle suspensions were also made using degassed nanopure water. Nanopure water was degassed under vacuum at high stirring for 24 h. Samples were then sealed with paraffin film and fully submerged in a petri dish containing water at room temperature. Underneath this petri dish was a HAM A acoustic absorber (Precision Acoustics, United Kingdom) which absorbed deflected ultrasonic waves and reduced reflected waves. This acoustic absorber was used to mimic the absorption of waves that occurs when US is applied to organs, which is the main contributor to attenuation. The US probe was also submerged into the water, to reduce any attenuation of sounds waves, and then US was directly applied to the microcentrifuge tube for 10 seconds whilst the tube was rotated clockwise at a rate of 0.2 rotations/s. A schematic of the set up can be seen in Figure 2. The ultra-morphology of US treated blank microspheres was examined using Scanning Electron Microscopy (SEM). Briefly, microsphere samples from the US treated and untreated tubes were placed on silicon wafers and mounted on a SEM stub using double sided carbon tape. These samples were then left to dry overnight in ambient air for 24 h prior to being coated with gold-palladium using an argon beam K550 sputter coater (Emitech Ltd., Kent, England). Once coated, samples were imaged using a Hitachi S-4800 SEM (Hitachi High-Technologies). Particle size distributions were measured from SEM micrographs using ImageJ software.
Figure 2:
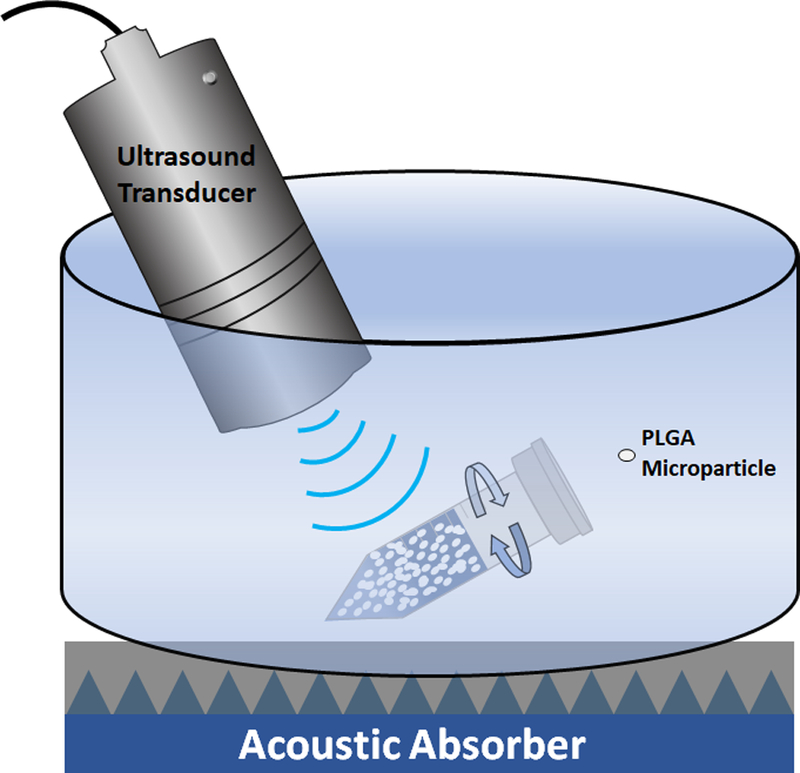
Schematic of the application of US to blank microspheres.
2.4. US Induced Drug Release
DOX-loaded microspheres ( ~3 mg) were added to amber microcentrifuge tubes containing 1 mL of PBS. After US application (as described in Section 2.3), the tubes were then placed in a shaker incubator set at 300 rpm and 37°C. Samples were collected after 1, 3, 6, 12, 24, 48, 72, 96, 168, 336, 672, 840, and 1008 h. Sampling involved centrifuging the microcentrifuge tubes at 180*g for 5 minutes from which 300 µl of supernatant was collected. Then 300 µl of fresh PBS was added back to the tubes and the microsphere pellets were resuspended. The collected samples were measured at λex485, λem570 using a SPECTRAmax M5 Microplate Spectrofluorometer (Molecular Devices). These readings were then compared to a standard curve and normalized for photodegradation (data not shown) and weight to determine the amount of DOX released.
2.5. Evaluating the Effect of Blank and DOX-Loaded Microspheres plus US on Cell Viability
2.5.1. Cell Lines and Cell Culture
The murine melanoma cell line, B16-F10, was acquired from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco®, Life Technologies Corporations, NY) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 10 mM HEPES (Gibco®), 50 μg/mL gentamycin sulfate (Cellgro, Manassas, VA), 1 mM sodium pyruvate (Gibco®), and 1 mM Glutamax (Gibco®). These cells were incubated at 37°C and 5% CO2 in a humidified atmosphere.
2.5.2. Assessing the Effect of Blank and DOX-Loaded Microspheres plus US on Cell Viability In Vitro
B16-F10 cells were initially seeded at a density of 1 × 105 cells in 4 mL of complete DMEM media in 6-well plates and incubated for 24 h. Then the cells were treated with one of the following treatment systems: untreated/control (no microspheres, no US); US (no microspheres); blank microspheres alone; blank microspheres with US; DOX-loaded microspheres alone; DOX-loaded microspheres with US; and soluble DOX alone. The dose of DOX used (0.5 µg/ml) for all treatment groups was the LD50 (for B16-F10 cells as determined from a two day incubation period). The LD50 was determined from a study involving B16-F10 cells seeded into wells of a 96-well tray and was simply used as a guide as to what dose to use that would yield sufficient specific cell killing. The amount of blank particles and DOX-loaded microspheres added were comparable. After treatment, cells were incubated for 48 hours and then washed with 1 mL of PBS before being detached from the plate surface with 0.5 mL of 0.25% trypsin-EDTA (1X) (Gibco) for 2 minutes, followed by 2 mL of complete media to quench the trypsin. Wells were then flushed 5–10 times with a 1 mL pipette to remove additional cells from the plate surface. The cell suspensions were then centrifuged at 230*g for 5 minutes, the supernatant was discarded and the cell pellets were resuspended in 200 µl of media. Cells were then counted on a hemocytometer using a 1:1 mixture of cells to 0.4 % trypan blue to compare the effectiveness of treatments on cell viability. Cell viability was measured based on the number of live cells within each sample and then comparing these results to the control group. In order to determine if the treatment with DOX-loaded microspheres plus US was synergistic, a comparison of the percent cytotoxicity generated by this treatment was compared to the combined cytotoxicities of DOX-loaded microspheres alone and US-treated blank microspheres. Prior to comparison the nonspecific cytotoxicity generated by blank microspheres alone was subtracted from all three cytotoxicity values.
2.6. US/DOX-loaded Microsphere Treatment of Tumor Challenged Mice
For tumor challenge, 7 – 10 week old C57BL/6 female mice (5 per group) purchased from Jackson Laboratories (Bar Harbor, ME) were anesthetized by intraperitoneal injection of a ketamine/xylazine mix (87.5 mg/kg ketamine; 2.5 mg/kg xylazine). All animal care, housing and experimental procedures were performed and carried out in accordance with the requirements of the University of Iowa Animal Care and Use Committee. Mice were then challenged with 1 × 105 B16-F10 cells by subcutaneous (s.c.) injection (in 100 μl of FCS-free media) into the shaved dorsal right flank. Seven days after tumor challenge, US treatments were applied and repeated on days 7, 8, 9 and 13. After mice were anesthetized, intratumoral injections of 100 µL of suspended particles or PBS solution were administered. For blank particles, mice were injected with concentrations (w/v) equivalent to the weight of particles delivering the highest dose of DOX. Injections were split into two doses, where 50 µL of the particle suspension would first be injected using an insulin needle/syringe, followed by the application of US for 10 seconds, while keeping the needle inside the injection site to avoid excessive injections. Before the application of US, a smear (enough to cover the US probe and the targeted area) of US transmission gel (Chattanooga Group, Hixson, TN) was applied to the shaved skin surface above the tumor. US, at the settings described in section 2.2, was directly applied to the tumor containing the US transmission gel, making sure that the tip of the probe was submerged within the transmission gel. These steps were then repeated for the remaining 50 µL in the insulin syringe. The starting dose of DOX (2 µg) was based on a previous study using DOX-loaded microspheres where intratumoral therapy (without US) was administered (Joshi, 2014; Makkouk et al., 2015). Tumor outgrowth, determined by tumor size as a function of time, was measured multiple times per week and tumor volume was calculated by the equation for determining the volume of an ellipsoid: [(Diameter 1 × Diameter 2 × Height) × (π/6)]. Mice were euthanized when tumors reached or exceeded 20 mm in any direction.
2.7. Data Analysis
Data were analyzed and graphed using GraphPad Prism 7. Statistical analyses were performed on cell viability studies using one-way ANOVA followed by a Tukey’s multiple comparison test. The Mantel-Cox test was used to analyze survival curves. Global significance was then followed by a Tukey-Kramer multiple comparison test that compared the means of each group to one another. The cumulative release data (Figure 4a) were analyzed using nonlinear regression with a two-phase exponential decay function. The initial values and parameter constraints were as automatically provided by the program. Least square fitting was used. The best curve that fitted the data set was selected based on the extra sum-of-squares F-test. Data were presented as mean ± SD, unless stated otherwise. The percent DOX release study at t = 3 h (Figure 4b) was analysed using the student t-test (unpaired, two-tailed).
Figure 4: Cumulative release of DOX from DOX-loaded particles with or without US exposure.
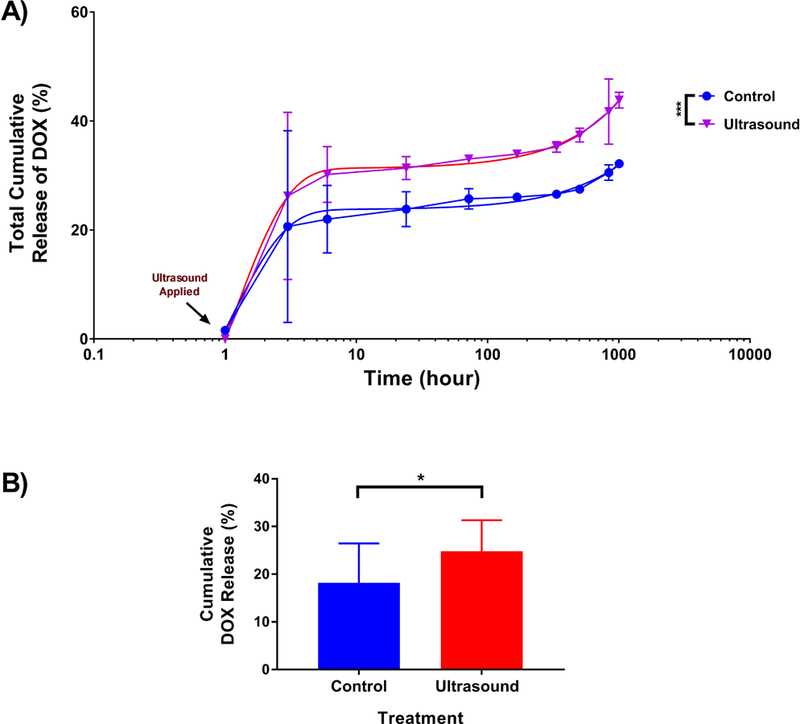
US was either applied or not applied to DOX-loaded microspheres (loading of 1.94 µg DOX/mg PLGA microspheres), as described in the methods section 2.3.1. A) Release was subsequently monitored over the indicated time period and showed an enhance burst release of 6% (3 h) and a total increase in 12% over the span of the release study (1008 h). B) Pooled data from 2 experiments (n = 13) comparing percent release DOX from US treated and non-treated microspheres at t = 3 h. *** p < 0.001. Error bars represent standard deviation of the mean. Also shown are curves of best fit based on the extra sum-of-squares F-test. * p < 0.05.
3. Results
3.1.1. Characterization of DOX-loaded and blank microspheres
SEM images of the fabricated microspheres were analyzed for particle size using ImageJ and blank microspheres were 6.42 ± 1.75 µm (n = 200), while DOX-loaded microspheres were determined to be 6.23± 1.78 µm (n = 200). All microsphere preparations demonstrated smooth surfaced intact spheres. The average loading of a total of 8 batches of DOX-loaded microspheres was 6.76 ± 2.13 µg DOX/mg of PLGA microspheres, while the loading efficiency was approximately 25 ± 8%. The average yield per batch of DOX-loaded microspheres was 113 ± 9.68 mg.
3.1.2. Evaluation of US effects on ultra-morphology of blank microspheres
US was applied to blank microspheres (in water) which were then evaluated, using SEM, for any damaging effects caused by US-induced inertial cavitation. Compared to the untreated microspheres (Figure 3A, 3B), the ultra-morphology of the microspheres that were treated with US displayed noticeable signs of superficial damage (Figure 3C, 3D). A small percentage (approx. 15%) of microspheres treated with US showed signs of surface damage that may have been caused directly or indirectly (particle collisions) by inertial cavitation. This percentage is likely an underestimation since we can only visualize one face of the microspheres using SEM. Multiple exposures (up to 4x) to blank microspheres did not further increase detectable damage (data not shown). That this damage was not readily detected on microspheres not treated with US nor on US-treated microspheres resuspended in degassed water (Figure 3E, 3F) strongly implicates the involvement of air bubbles already present within the medium (water).
Figure 3: US-mediated damage to the surface of PLGA microspheres.
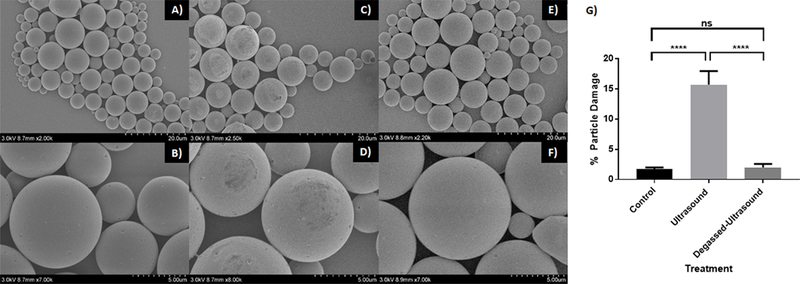
(A–F) SEM images of blank PLGA microspheres either untreated (A,B), or treated with US without degassing (C,D) or with degassing (E,F). (G) Graph illustrating the percent of particles displaying surface damage (E). **** p < 0.0001. Error bars represent standard deviation of the mean.
3.2. Effect of US on the drug release profile of DOX-loaded microspheres
The effect of US on the release kinetics of DOX from DOX-loaded microspheres was investigated. Comparing release profiles of US-treated versus untreated DOX-loaded microspheres revealed an increased rate of release of DOX by the former. Upon exposure to US, there was an approximately 12% increase in the cumulative release profile compared to the control group (Figure 4a). Analysis using nonlinear regression with a two-phase exponential decay function revealed that cumulative release rates of treated versus untreated microspheres were statistically different (p < 0.001) (Figure 4a). The majority of the enhanced release was observable within 3 h of US treatment and shown to be significant upon analysis of two pooled experiments (Figure 4b).
3.3. Effect of the combination of US and DOX-loaded microspheres on killing melanoma cells in vitro.
The effect of US +/− DOX-loaded microspheres on B16-F10 tumor cell viability in vitro was investigated. US alone and DOX-loaded microspheres alone provided 23% and 29% tumor cell killing, respectively. Blank microspheres alone caused the least tumor cell death (15%), while DOX in solution alone provided the greatest tumor cell killing (94%). Blank microspheres + US induced 54% killing, while DOX-loaded microspheres + US caused 76% tumor cell killing (Figure 5). The lower degree of cell death caused by DOX-loaded particles + US compared to DOX in solution can be attributed to the fact that the DOX is encapsulated in the PLGA microspheres and is not completely and immediately available for cells to take up compared to the DOX in solution. With the addition of US to blank microspheres there was a synergistic increase in cell death compared to either treatment alone implicating that inertial cavitation of air bubbles in the presence of microspheres contributed to the cytotoxicity. We speculate that the blank microspheres may have directly damaged cells by physical impact as a result of the forces applied to the microspheres by cavitating air bubbles. When DOX-loaded microspheres were combined with US, synergy was observed (see section 2.5.2 for description of how synergy was calculated) suggesting that US was responsible for enhanced killing of cells by DOX-loaded microspheres due not only to enhanced physical cell damage but also due to enhanced release rate of DOX and/or enhanced permeability of non-lethally damaged cells to soluble/released DOX.
Figure 5: Cell viability of B16-F10 cells treated with particles (w/wo DOX) and/or US.
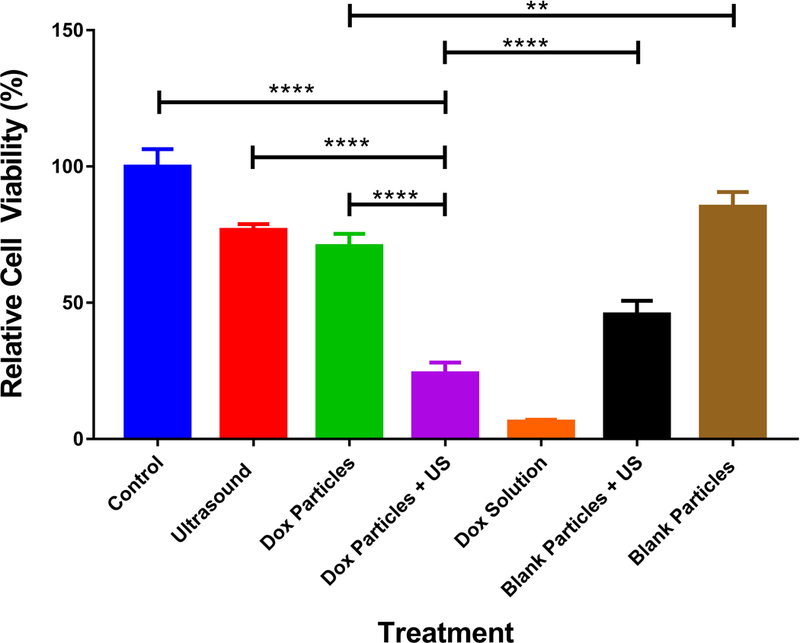
Cells were subjected to treatment and incubated for 48h prior to analysis by counting (see methods section. Statistical analysis was performed using one-way ANOVA with a Tukey’s multiple comparison test and showed statistical significance between all groups (only a selection are indicated) except for US vs. blank microspheres and US vs. DOX-loaded microspheres. (n = 4, ** p < 0.01, **** p < 0.0001). Error bars represent standard deviation of the mean.
3.4. Effect of the combination of US and DOX-loaded microspheres on survival in a mouse melanoma model
Mice challenged with B16-F10 melanoma cells were treated with intratumoral injections of PBS, blank microspheres or DOX-loaded microspheres with or without US on day 7 post tumor challenge (see Figure 6A for regimen). The naïve group treated with PBS had tumors that progressed at a greater rate than other treatment groups (see supplementary Figure 1) and these mice consequently had the lowest mean survival (10.4 days, Table 1). Statistical analysis revealed that DOX-loaded microspheres (containing 8 µg DOX) plus US was the only treatment to have significant therapeutic benefit over most other groups (Figure 6B), having a mean survival of 22.1 days compared to 10.4 days for PBS treated mice (Table 1). This treatment group demonstrated statistically significant extended survival over negative control groups as well as over mice treated with DOX-loaded microspheres (containing 8 µg DOX) alone (p < 0.05) or with DOX-loaded microspheres (containing 2 µg DOX) plus US (p < 0.01). Mice treated with US as a part of the therapy trended toward longer survival times compared to treatment counterparts without US. In addition, the mice treated with US plus DOX-loaded microspheres (8 µg DOX) exhibited a 13% “cure rate” (Figure 6B) where “cured” simply means the mice were tumor-free at the conclusion of the survival study (Day 60). It was also noted that survival rates were dose dependent, where higher concentrations of DOX yielded a trend toward increased survival. Based on a combination of encapsulation efficiency and injectable volume limitations (100 µl was the maximum volume feasible intratumorally), the maximum dose we could administer was 8 µg of DOX. Analysis of hazard ratios (Table 1) revealed the percent chance survival increased synergistically from 6 percent for mice treated with blank microspheres plus US and from 34.1% for mice treated with DOX-loaded (8 µg) microspheres alone, to 74% for mice treated with DOX-loaded (8 µg) microspheres plus US. This synergy may be at least partially explained by the additional role of DOX as an inducer of immunogenic apoptosis where the host’s immune system is stimulated to recognize the B16-F10 melanoma cells as foreign by the induction of tumor specific T cells. Thus, we chose to add an immune checkpoint blocker (anti-PD-1) to the most effective treatment group, DOX-loaded (8 µg) microspheres plus US, to see if this could further improve survival outcome by enhancing any tumor-specific effector T cell response. No significant increase in survival was observed (Figure 6C), suggesting either that the impact of DOX-loaded microspheres/US on the effector arm of the immune response may have been limited or that the immune checkpoint axis of PD-1:PDL1 was not of significant influence in suppressing any induced antitumor immune response. In addition, at day 90 subsequent to initial tumor challenge, all “cured”, or tumor-free, mice were rechallenged by subcutaneous injections of 1 × 105 B16-F10 cells (contralateral to original challenge), in a similar manner to that described in methods section 2.6. All mice developed detectable tumors within 2 weeks of rechallenge demonstrating the lack of a protective adaptive immune response (data not shown).
Figure 6: Survival study of in vivo tumor therapy.
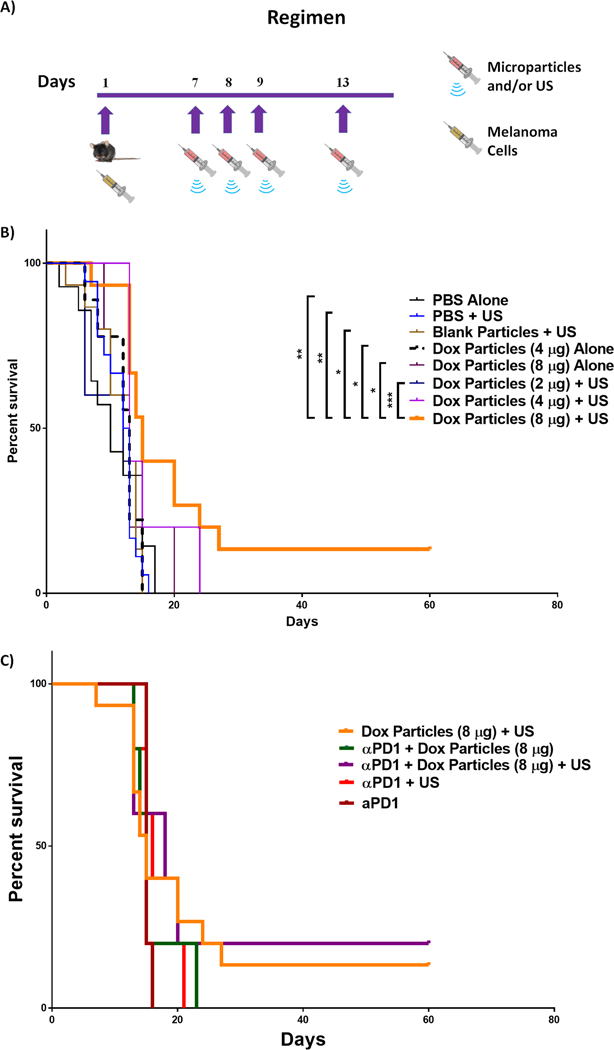
A) Tumor challenge and treatment regimen. B) Survival curve (pooled data) of tumor (B16-F10) challenged mice that received the following treatments on indicated days: PBS only (n = 14), PBS + US (n = 18), blank particles + US (n = 15), DOX-loaded particles (4 µg) alone (n = 9), DOX-loaded particles (2 µg) + US (n = 5), DOX-loaded particles (4 µg) + US (n = 10), and DOX-loaded particles (8 µg) alone (n = 5), DOX-loaded particles (8 µg) + US (n = 15). A Mantel-Cox test was performed to analyze the survival distribution yielding a p-value of 0.0110. This global significance was then followed by a Tukey-Kramer post-test with multiple comparisons * p < 0.05, ** p < 0.01, *** p < 0.001. C) Survival curve comparing mice treated with DOX-loaded particles (8 µg) + US and/or anti-PD1, where all anti-PD1 groups were n = 5.
Table 1:
Mean survival times for tumor-challenged mice receiving indicated treatment.
| Treatment | Mean Survival (Day) | Hazard Ratio (95% CI) |
|---|---|---|
| PBS Alone | 10.4 | 1 |
| PBS + US | 11.3 | 1.093 (0.540,2.214) |
| Blank microspheres + US | 11.5 | 0.939 (0.448,1.968) |
| DOX-loaded microspheres (4 µg) alone | 11.6 | 0.927 (0.399,2.158) |
| DOX-loaded microspheres (8 µg) alone | 13 | 0.659 (0.233,1.864) |
| DOX-loaded microspheres (2 µg) + US | 10 | 1.463 (0.518,4.132) |
| DOX-loaded microspheres (4 µg) + US | 15.3 | 0.447 (0.196,1.021) |
| DOX-loaded microspheres (8 µg) + US | 22.1 | 0.259 (0.113,0.593) |
4. Discussion
In this study, we demonstrated the tumoricidal benefits of combining DOX-loaded microspheres with US. We speculate that the combination of: 1) physically based cell killing/damage mediated directly or indirectly (microsphere collisions) by US, 2) chemically based killing mediated by DOX, and 3) enhanced release rate of DOX from US-treated PLGA microspheres likely contributed to the observed enhanced tumor cell killing in vitro and greater survival in vivo when compared to DOX-loaded microspheres alone or US/blank microspheres alone. Microspheres treated with US in vitro were observed to have undergone discernible and quantifiable superficial damage (see SEM images, Figure 3). Such damage can be visualized as disrupted uniformity at the surface of the particles. Since the surface damage is not observed on US treated microspheres in degassed medium (Figures 3E, 3F, 3G) we speculate that the damage is attributed to the process of inertial cavitation of proximal air bubbles that are capable of damaging the particles directly and/or indirectly by inducing interparticle collisions (Doktycz and Suslick, 1990).
The application of US to DOX-loaded microspheres in vitro was shown to increase the rate of release of DOX from the microspheres (Figure 4) which we propose may have been a direct result of the damage described above (Figure 3). Whether the increase in release rate of DOX was due to the direct impact of air bubbles undergoing inertial cavitation, the impact of particle:particle collisions (caused indirectly by the inertial cavitation of air bubbles) or a combination of the two is unknown. Nevertheless, the application of US provides the opportunity for controllable drug release. In addition to the US-inducible increased rate of drug release from the nonechogenic PLGA microspheres, these microspheres have the added advantage of simultaneously providing sustained drug release since only a fraction of the DOX payload is stimulated to be released upon US treatment (Joshi et al., 2013; Makkouk et al., 2015). This is in contrast to the immediate payload release that results when echogenic particles are used (Bouakaz et al., 2005; Hernot and Klibanov, 2008; Lentacker et al., 2009).
Cell viability studies were performed to assess the effects of combinations of US +/− DOX-loaded particles on B16-F10 melanoma tumor cells in vitro (Figure 5). Although DOX solution exhibited the highest tumor cell death (94%), this is not a viable direction for treating melanoma in the clinic due to: 1) the vesicant properties of soluble DOX, particularly if administered intratumorally, as well as 2) the undesired acute and chronic toxicities that result from soluble DOX (Balazsovits et al., 1989; Kreidieh et al., 2016). Thus, soluble DOX was used in vitro as a positive control. Excluding the soluble DOX-treated group, tumor killing was greatest (76%) when cells were treated with DOX-loaded microspheres plus US. We speculate this to be due to a synergistic effect resulting from the presence of microspheres/US causing physical cell damage (Hwang et al., 2006) plus the cytotoxicity generated by released DOX (Kim et al., 2012) where the rate of release increased upon US treatment (Figure 4). The statistically significant difference in cytotoxicities between DOX-loaded microspheres and blank particle treatments confirm that DOX released from these particles impacted on cell viability since uptake of DOX-loaded microspheres would be unlikely due to their large size. US alone did cause a small amount of cell killing, which we propose was most likely through sonoporation/inertial cavitation, where the intensity of the US causes irreversible damage to the cell membrane (Figueiredo and Esenaliev, 2012). The degree to which the increased release of DOX from the US-treated microspheres contributed to the increased cytotoxicity is difficult to assess since the combination of US and microspheres may have increased cell permeability to DOX, therefore potentially further contributing to the observed cytotoxicity of DOX regardless of the enhanced release (Korosoglou et al., 2006).
An in vivo murine melanoma model was employed to observe the effect of combining US and intratumorally administered DOX-loaded microspheres on survival (Figure 6). Supporting the findings from the in vitro experiments, the combinatorial treatment of tumors with DOX-loaded microspheres (8 µg) plus US was the most effective treatment group, as demonstrated by longer survival times and 13% tumor-free mice (“cured”) at the termination of the survival study (Day 60). US, when combined with DOX (4 µg or 8 µg)-loaded microspheres, enhanced survival times compared to the same treatment groups without US. In particular, when the treatments with DOX (8 µg)-loaded microspheres in the presence and absence of US were compared, a significant difference was observed (p < 0.05). However, US alone or in combination with blank microspheres only had a marginal and non-significant impact on survival. This contrasted with the in vitro data where US alone and the combination of US and blank microspheres were significantly cytotoxic. Such findings suggest that the cell death directly caused by physical damage to melanoma cells in vivo was likely to have been minimal. It has been shown that the impact of US on cell viability is indirectly correlated to cell density (Ellwart et al., 1988), and therefore tumor cell killing in vitro would have been expected to be higher than it would be for a densely packed tumor mass. Thus, we propose that the synergy observed in vivo when US and DOX (4 µg or 8 µg)-loaded microspheres were used in combination may have stemmed from US-mediated enhanced cell permeability (Korosoglou et al., 2006), through the generation of non-lethal cell membrane damage thereby resulting in increased uptake of DOX. Support for this comes from the recent finding that US alone can increase cancer cell uptake of chemotherapeutic agents (Hu et al., 2016). That US could induce an increased release of DOX was demonstrated in vitro (Figure 4), however, whether it occurred in vivo to the same or any extent is uncertain since this was not easily measurable. Nevertheless, it is a possible explanation for how the combinatorial treatment may have at least partially contributed to the observed synergistic effect in vivo.
Another possible interpretation of the observed synergy was that a mode of killing aside from US/microsphere mediated physical damage and direct DOX-mediated cytotoxicity occurred. One possibility that we considered was that some amount of immune based killing was generated through the ability of DOX to induce immunogenic apoptosis of tumor cells (Casares et al., 2005). However, when the mice surviving (“cured”) from treatment with DOX-loaded (8 µg) microspheres plus US were subsequently rechallenged with B16-F10 cells they succumbed to the tumor therefore suggesting that the adaptive immune response may not have played a role in the enhanced survival caused by the combinatorial treatment. This likely lack of immune involvement in tumor cell killing is further supported by the finding that immune checkpoint blockade (anti-PD1) could not significantly extend survival of mice treated with DOX-loaded microspheres plus US (Figure 6c). It would have been desirable to have generated a detectable antitumor immune response as this would have benefits for the treatment of metastatic melanoma due to the generation of a potential abscopal-like response. In order to address this it may be necessary to include additional chemotherapeutic agents capable of promoting antitumor T cell responses when used in combination with DOX, such as cyclophosphamide (Tongu et al., 2010).
To the best of our knowledge this is the first study using drug loaded microspheres in combination with US that go on to test cytotoxic impact in an in vitro and in vivo cancer cell system. The results from the melanoma mouse model, whilst promising, indicate that there is room for generating improved efficacy through formulation modifications and these potential adjustments may stem from findings from independent sources. Other researchers have implemented US as a method of promoting pulsatile drug release, using cross-linked hydrogels containing mitoxantrone for the treatment of breast cancer in preclinical in vivo studies (Huebsch et al., 2014). The researchers found that providing US to gels in vivo marginally, but not significantly, improved the anti-tumor activity of the mitoxantrone compared to gels not treated with US, and suggested that spatial and temporal optimizations can be performed to maximize drug efficacy. Another approach, using US with DOX-liposome-loaded microbubbles, has been shown to kill melanoma cells in vitro, however, this system does not provide a means of sustained drug release since the majority of the payload is released upon US treatment (Lentacker et al., 2010). Our system provides a means of US-triggered enhanced drug release, as well as retaining a sustained release profile since the PLGA microspheres were only partially affected by US treatment when measured in vitro. The formulation may benefit from adjustments that allow for a greater amount of DOX released upon US treatment, which may be achieved through a heterogenous mix of DOX-loaded microspheres/microcapsules with varying susceptibilities to US. It would also be valuable in future approaches to explore the implementation of multiple US treatments of tumors containing DOX-loaded microspheres so as to generate a pulsatile release profile as Huebsch et al. obtained with their treatment system. The application of US on microspheres loaded with anti-cancer agents such as DOX has the potential to be used as a cancer treatment system, possibly in combination with a cancer vaccine and/or intraoperatively upon resection of superficial lesions. The former may boost the possibility of an effective systemic immune response, whilst the latter would reduce the chances of local tumor recurrence. The above system can be considered as a potential treatment for melanoma patients with skin lesions readily accessible to treatment. Further optimization is required to provide a systemically deliverable nanoparticle version capable of targeting metastasized lesions.
Supplementary Material
Supplemental Figure 1: Spyder graph of pooled experiments displaying tumor progression for each mouse throughout the tenure of 60 days or until tumors exceeded the growth restriction (20 mm x 20 mm x 10 mm) in all experimental groups.
Acknowledgements:
The P30 CA086862 Cancer Center support grant and the Lyle and Sharon Bighley Chair to A.K.S. supported this work.
Footnotes
Conflicts of interest:
The authors declare no conflicts of interest
References
- Anderson JM, Shive MS, 1997. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews 28, 5–24. [DOI] [PubMed] [Google Scholar]
- Balazsovits JAE, Mayer LD, Bally MB, Cullis PR, McDonell M, Ginsberg RS, Falk RE, 1989. Analysis of the effect of liposome encapsulation on the vesicant properties, acute and cardiac toxicities, and antitumor efficacy of doxorubicin. Cancer Chemotherapy and Pharmacology 23, 81–86. [DOI] [PubMed] [Google Scholar]
- Bouakaz A, Versluis M, de Jong N, 2005. High-speed optical observations of contrast agent destruction. Ultrasound in Medicine & Biology 31, 391–399. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Métivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G, 2005. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. The Journal of Experimental Medicine 202, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Formenti SC, 2012. Role of T lymphocytes in tumor response to radiotherapy. Frontiers in oncology 2, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CX, Xu Q, Apfel RE, Holland CK, 1996. Inertial cavitation produced by pulsed ultrasound in controlled host media. The Journal of the Acoustical Society of America 100, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Doktycz S, Suslick K, 1990. Interparticle collisions driven by ultrasound. Science 247, 1067–1069. [DOI] [PubMed] [Google Scholar]
- Ellwart JW, Brettel H, Kober LO, 1988. Cell membrane damage by ultrasound at different cell concentrations. Ultrasound in medicine & biology 14, 43–50. [DOI] [PubMed] [Google Scholar]
- Figueiredo M, Esenaliev R, 2012. PLGA Nanoparticles for Ultrasound-Mediated Gene Delivery to Solid Tumors. J Drug Deliv 2012, 767839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD, 2010. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10, 147–156. [DOI] [PubMed] [Google Scholar]
- Frenkel V, Kimmel E, Iger Y, 1999. Ultrasound-induced cavitation damage to external epithelia of fish skin. Ultrasound in Medicine & Biology 25, 1295–1303. [DOI] [PubMed] [Google Scholar]
- Gâmbuteanu C, Alexe P, 2013. PRINCIPLES AND EFFECTS OF ACOUSTIC CAVITATION. The Annals of the University of Dunarea de Jos of Galati. Fascicle VI. Food Technology 37, 9–17. [Google Scholar]
- Goldblatt EM, Lee W-H, 2010. From bench to bedside: the growing use of translational research in cancer medicine. American Journal of Translational Research 2, 1–18. [PMC free article] [PubMed] [Google Scholar]
- Hernot S, Klibanov AL, 2008. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 60, 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, Yang B, Cao W, 2016. Enhancement of anti-tumor effects of 5-fluorouracil on hepatocellular carcinoma by low-intensity ultrasound. Journal of Experimental & Clinical Cancer Research 35, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar CA, Suo Z, Mooney DJ, 2014. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. U. S. A 111, 9762–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA, 2006. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound in Medicine & Biology 32, 1611–1619. [DOI] [PubMed] [Google Scholar]
- Jang KW, Seol D, Ding L, Heo DN, Lee SJ, Martin JA, Kwon IK, 2017. Ultrasound-triggered PLGA microparticle destruction and degradation for controlled delivery of local cytotoxicity and drug release. International journal of biological macromolecules [DOI] [PubMed]
- Joshi VB, 2014. title. Doctoral dissertation, The University of Iowa, The University of Iowa’s Insitutional Repository
- Joshi VB, Geary SM, Salem AK, 2013. Biodegradable particles as vaccine delivery systems: size matters. Aaps j 15, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Wu F, Cai Y, Xu M, He M, Yuan W, 2014. Development of Recombinant Human Growth Hormone (rhGH) sustained-release microspheres by a low temperature aqueous phase/aqueous phase emulsion method. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 62, 141–147. [DOI] [PubMed] [Google Scholar]
- Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT, Shin BS, Lee KC, Youn YS, 2012. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained- release inhalation system for the treatment of metastatic lung cancer. Biomaterials 33, 5574–5583. [DOI] [PubMed] [Google Scholar]
- Korosoglou G, Hardt SE, Bekeredjian R, Jenne J, Konstantin M, Hagenmueller M, Katus HA, Kuecherer H, 2006. Ultrasound exposure can increase the membrane permeability of human neutrophil granulocytes containing microbubbles without causing complete cell destruction. Ultrasound in Medicine & Biology 32, 297–303. [DOI] [PubMed] [Google Scholar]
- Kreidieh FY, Moukadem HA, El Saghir NS, 2016. Overview, prevention and management of chemotherapy extravasation. World Journal of Clinical Oncology 7, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentacker I, De Smedt SC, Sanders NN, 2009. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter 5, 2161–2170. [Google Scholar]
- Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN, 2010. Design and Evaluation of Doxorubicin-containing Microbubbles for Ultrasound-triggered Doxorubicin Delivery: Cytotoxicity and Mechanisms Involved. Molecular Therapy 18, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X-J, Chen C, Zhao Y, Wang PC, 2010. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods in molecular biology (Clifton, N.J.) 596, 467–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ, 2011. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK, Weiner GJ, 2015. Biodegradable Microparticles Loaded with Doxorubicin and CpG ODN for In Situ Immunization Against Cancer. The AAPS journal 17, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, 1995. Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound in Medicine & Biology 21, 1059–1065. [DOI] [PubMed] [Google Scholar]
- Ngan Y, Gupta M, 2016. A comparison between liposomal and nonliposomal formulations of doxorubicin in the treatment of cancer: An updated review. Archives of Pharmacy Practice 7, 1–13. [Google Scholar]
- Prevention C f.D.C.a, 2016. National Center for Health Statistics, in: Services, U.S.D.o.H.a.H (Ed.). Centers for Disease Control and Prevention, CDC/National Center for Health Statistics. [Google Scholar]
- Rivankar S, 2014. An overview of doxorubicin formulations in cancer therapy. Journal of cancer research and therapeutics 10, 853–858. [DOI] [PubMed] [Google Scholar]
- Suslick KS, 1997. Sonoluminescence and sonochemistry, 1997 IEEE Ultrasonics Symposium Proceedings. An International Symposium (Cat. No.97CH36118), pp. 523–532 vol.521. [Google Scholar]
- Tongu M, Harashima N, Yamada T, Harada T, Harada M, 2010. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer immunology, immunotherapy : CII 59, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Colucci G, Giotta F, Sergi D, Filippelli G, Perri P, Botti C, Vizza E, Carpino A, Pizzuti L, Latorre A, Giannarelli D, Lopez M, Di Lauro L, 2011. A multicenter prospective phase II randomized trial of epirubicin/vinorelbine versus pegylated liposomal doxorubicin/vinorelbine as first-line treatment in advanced breast cancer. A GOIM study. Journal of Experimental & Clinical Cancer Research 30, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasle I, Gamerith G, Kocher F, Mondello P, Jaeger T, Walder A, Auberger J, Melchardt T, Linkesch W, Fiegl M, Mian M, 2015. Non-pegylated liposomal doxorubicin in lymphoma: patterns of toxicity and outcome in a large observational trial. Annals of Hematology 94, 593–601. [DOI] [PubMed] [Google Scholar]
- Yuan W, Liu Z, 2012. Controlled-release and preserved bioactivity of proteins from (self-assembled) core-shell double-walled microspheres. International journal of nanomedicine 7, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan W, Zhang Y, Wu F, Li H, Cai Y, Zhang Y, Han M, Jin T, 2010. Preparation of protein-loaded sustained-release microspheres via ‘solid-in-oil-in-hydrophilic oil-in-ethanol (S/O/hO/E)’ emulsification. Colloids and surfaces. B, Biointerfaces 79, 326–333. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wu F, Cai Y, Chen Y, Wei L, Liu Z, Yuan W, 2013. Local antitumor effects of intratumoral delivery of rlL-2 loaded sustained-release dextran/PLGA-PLA core/shell microspheres. International journal of pharmaceutics 450, 235–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Spyder graph of pooled experiments displaying tumor progression for each mouse throughout the tenure of 60 days or until tumors exceeded the growth restriction (20 mm x 20 mm x 10 mm) in all experimental groups.


