Abstract
Alkenes are fundamental functionalities in nature and highly useful intermediates in organic synthesis, medicinal chemistry and material sciences. Transition-metal-catalyzed Heck couplings with organic halides as electrophiles have been established as a powerful protocol for the synthesis of this valuable building block. However, the requirement of organic halides and the generation of stoichiometric hazardous halide wastes may cause significant sustainable concerns. The halide-free oxidative Heck alkenylations involving organometallics or arenes as the coupling partners provide a facile and alternative pathway. Nonetheless, stoichiometric amounts of extra oxidant are essential in most cases. Herein, we present a direct dehydrogenative alkyl Heck-coupling reaction under oxidant-free conditions, liberating hydrogen, nitrogen and water as the side products. Excellent regioselectivity is achieved via neighboring oxygen atom coordination. Broad substrate scope, great functional group (ketone, ester, phenol, free amine, amide etc) tolerance and modification of pharmaceutical candidates and biological molecules exemplified its generality and practicability.
Heck coupling of alkenes with organic halides, arenes or organometallics poses sustainability issues due to hazardous waste formation and use of oxidants. Here, the authors show an oxidant-free alkyl Heck-coupling of vinylarenes with umpolung aldehydes, liberating H2, N2 and H2O as sole side products.
Introduction
Since its first discovery in the 1970s, the palladium-catalyzed Heck (or Mizoroki-Heck) reaction has emerged as the most powerful and straightfoward tool for the cross-couplings of alkenes and aryl (pseudo) halides (Fig. 1a)1,2. Despite its remarkable importance and widespread applications in organic synthesis3–5 over the past decades, this protocol suffers from the inherent disadvantage of the required pre-synthesizing the organic halides and accompanied formation of a stoichiometric amount of hazardous halide salt, which can cause significant environmental concerns. To persuit a more sustainable alkenylation process, the halide-free oxidative Heck coupling reactions via transmetallation or directed C–H activation have been developed6–8. Organometallic reagents are generally moisture/air sensitive and often not commercially available especially when bearing functional groups (Fig. 1b). Besides, preparation of these reagents requires stoichiometric quantities of metals, thus producing extra metal wastes. Alternatively, the alkenylations through direct cleavage of two C–H bonds represent an more environmentally benign and economically attractive strategy (Fig. 1c)9–12. As early as in 1967, Fujiwara and Moritani reported the cross-couplings of electron-rich arenes with activated alkenes13. Inspired by this precedent work, great achievements have been made in this field14,15. However, due to the inherent challenges of selectivity in C–H bond activation, the nucleophiles were mostly restricted to the (hetero) arenes with a directing group. Above all, the oxidative Heck reaction generally requires stoichiometric amounts of oxidant (such as metal salt, peroxide, benzoquinone, K2S2O8, O2, acetone16, or internal oxidant17–19 etc.) to regenerate the active metal species, thus often affording additional side products. Hence, the development of a dehydrogenative Heck-coupling reaction under oxidant-free conditions is promising and highly desirable20,21. In 2016, Jeganmohan and coworkers reported an attractive ruthenium-catalyzed oxidant-free ortho alkenylation of aromatic amides and anilides with acetic acid as an additive22. Two examples of electrocatalytic aromatic dehydrogenative Heck coupling of arenes were disclosed by Jutand and Lei groups23,24. Recently, Lei and coworkers also realized a direct dehydrogenative C–H alkenylation of electron-rich arenes with styrene derivatives via a photo-induced electron transfer process25.
Fig. 1.
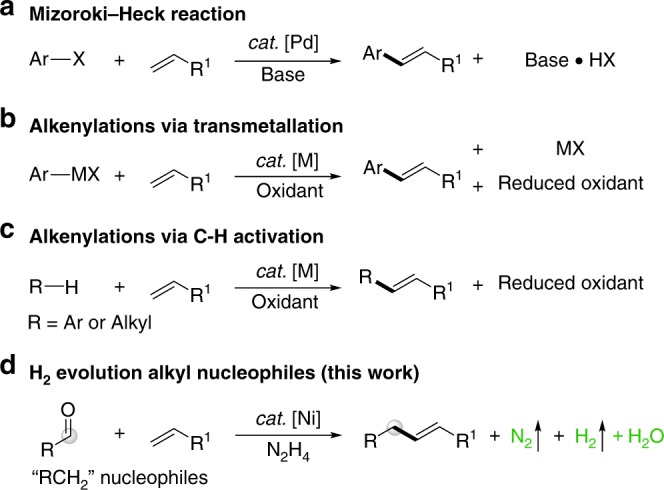
Strategies in the Heck coupling reaction. a Traditional Mizoroki-Heck coupling with hazardous organic halide; b Oxidative Heck coupling with pre-synthesized organometallic reagent; c Stoichiometric oxidant mediated Heck coupling via C–H bond activation; d Oxidant-free direct dehydrogenative alkyl Heck-coupling
In contrast, alkyl-Heck-type reactions were much more challenging and rarely explored until the last decade. Pioneered by Oshima26 and Fu’s seminal works27, effective strategies have been developed to facilitate this alkenylations with simple alkyl halides as electrophiles28–44. Notably, two elegant palladium-catalyzed cases involving alkyl C–H activation were disclosed by the groups of Yu45 and Sanford46, which employed N-arylamide or pyridine as a neighboring directing group with stoichiometric amounts of external oxidant. However, up to now, the oxidant-free, direct generation of H2 from alkyl Heck-type reaction still remains a highly formidable task.
To address these challenges and towards the goal of sustainable transformations, herein, we wish to report the example of direct dehydrogenative alkyl Heck-couplings of vinylarenes with umpolung aldehydes (Fig. 1d). Inspiration of this project stemsfrom our recent studies on hydrazone chemistry, in which umpolung aldehydes act as carbanion equivalents in the catalytic nucleophilic addition47–52 and cross-coupling reactions53–56. Highlighted features of this strategy are (a) no oxidant needed; (b) H2, N2 and H2O as innocuous side products; (c) naturally rich aldehydes as environmentally benign alkyl nucleophiles; (d) excellent regioselectivity achieved via vicinal oxygen atom chelation; (e) first-row abundant nickel as catalyst; (f) broad substrate scope and great functional group compatibility; and (g) modification of pharmaceutical candidates and biological molecules.
Results
Screening of reaction conditions
Our study commenced with a model reaction of styrene (3a) with hydrazone (2a) generated in situ from benzaldehyde (1a) with hydrazine monohydrate (Table 1) [Warning: hydrazine monohydrate is potentially hazardous and should be performed with appropriate personal protection]. We first examined Ni(cod)2 as a catalyst precursor and N,N-diisopropyl ethylamine as the base (DIPEA) for the Heck-type reaction. Among the various phosphine ligands evaluated (entries 1–6, see Supplementary Table 1 for details), only the sterically hindered, strong σ-donor bidentate alkyl phosphine ligand, 1,2-bis(dicyclohexyl phosphanyl)ethane (dcype), favored this transformation, and the product prop-1-ene-1,3-diyldibenzene 4aa was obtained in 78% yield with 87:13 E:Z ratio (entry 6). The liberation of H2 gas was confirmed by gas chromatograph with a TCD detector. The operationally simple Ni(II) precatalysts were then tested (entries 7–10), only Ni(acac)2 afforded the target molecule in 10% yield. Gratifyingly, 91% yield of 4aa was observed with more than 95:5 E:Z selectivity when 0.5 equivalent of NaI was added (entry 11). Notably, the efficiency of this transformation was almost not affected using twofold excess of benzaldehyde hydrazone 2a (entry 12). Equimolar reaction of styrene 3a and 2a still delivered the desired product 4aa in 58% yield (entry 14). When the dosage of Ni(cod)2 and dcype was decreased to 5 mol%, 78% yield of corresponding 4aa was obtained (entry 15). Control experiments were also carried out to understand the role of each component. It is noteworthy that in absence of base, the reaction efficiency was only decreased slightly (entry 16). This result indicated that base was not essential for this transformation, but might partially assist the initial oxidative addition of nickel complex to N–H bond of hydrazone57. In absence of either the nickel catalyst or a ligand, the desired product 4aa was not detected (entries 17 and 18).
Table 1.
Optimization of the reaction conditions
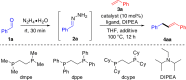
| ||||
|---|---|---|---|---|
| Entry | Catalyst | Ligand | Additive | 4aa (%)a,b |
| 1 | Ni(cod)2 | PMe3 | — | N.D. |
| 2 | Ni(cod)2 | PPh3 | — | N.D. |
| 3 | Ni(cod)2 | PCy3 | — | N.D. |
| 4 | Ni(cod)2 | dmpe | — | N.D. |
| 5 | Ni(cod)2 | dppe | — | N.D. |
| 6 | Ni(cod)2 | dcype | — | 78 (83:17) |
| 7 | NiCl2 | dcype | — | Trace |
| 8 | NiBr2 | dcype | — | Trace |
| 9 | NiBr2•glyme | dcype | — | Trace |
| 10 | Ni(acac)2 | dcype | — | 10 (78:22) |
| 11 | Ni(cod)2 | dcype | NaI | 91 (87)c (>95:5) |
| 12d | Ni(cod)2 | dcype | NaI | 85 (>95:5) |
| 13e | Ni(cod)2 | dcype | NaI | 71 (>95:5) |
| 14f | Ni(cod)2 | dcype | NaI | 58 (>95:5) |
| 15g | Ni(cod)2 | dcype | NaI | 78 (>95:5) |
| 16h | Ni(cod)2 | dcype | NaI | 69 (>95:5) |
| 17 | Ni(cod)2 | — | NaI | N.D. |
| 18 | — | dcype | NaI | N.D. |
Reaction conditions: 3a (0.2 mmol), 1a (0.6 mmol), N2H4•H2O (0.72 mmol), Ni(cod)2 (10 mol%), ligand (20 mol% for monodentate, 10 mol% for bidentate), DIPEA (0.4 mmol), NaI (0.1 mmol), THF (1.0 mL), 100 °C, 12 h under N2 unless otherwise noted
N.D. not detected
aNMR yields were determined by 1H NMR using mesitylene as an internal standard and based on 3a
bThe E:Z ratio in parenthesis was determined by 1H NMR analysis of the crude mixture
cThe isolated yield in parenthesis
d1a (0.4 mmol), N2H4•H2O (0.48 mmol) instead
e1a (0.3 mmol), N2H4•H2O (0.36 mmol) instead
f1a (0.2 mmol), N2H4•H2O (0.24 mmol) instead
gNi(cod)2 (5 mol%), ligand (5 mol%) instead
hWithout adding DIPEA
Scope and limitation of the reaction
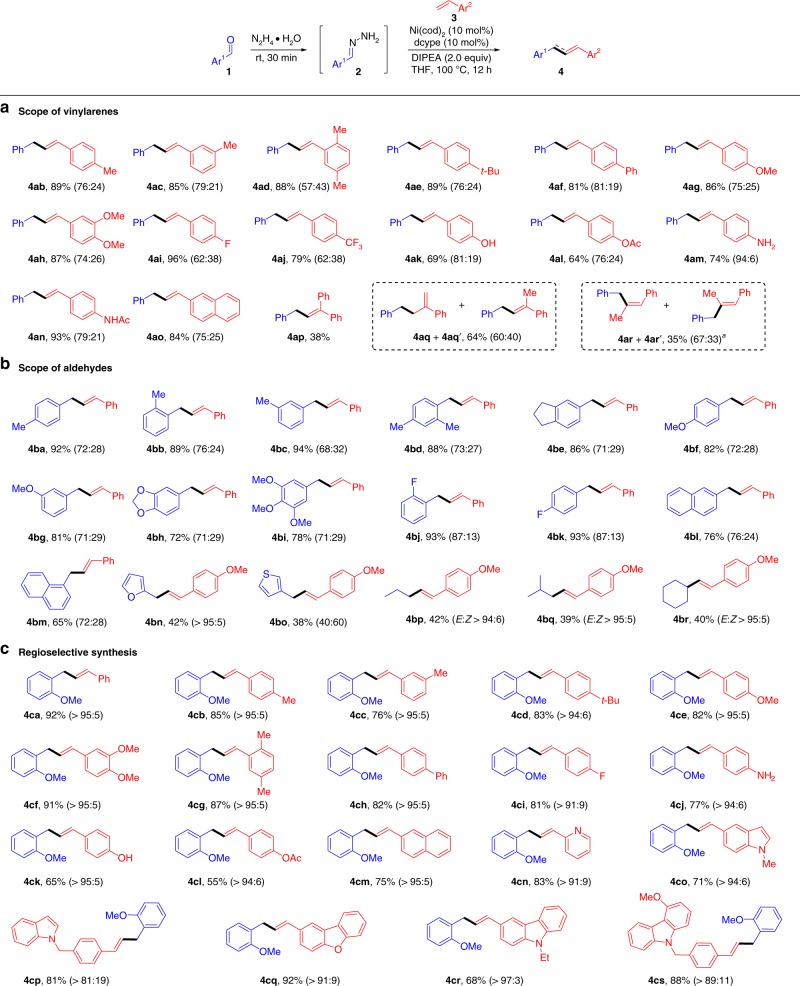
With the optimized conditions identified, the substrate scope of olefins 3 was investigated (Fig. 2a). To our delight, an array of mono-substituted vinylarenes containing both electron-donating and electron-withdrawing groups were all proved to be competent substrates, delivering the desired products 4ab-ao in 64–96% yields. The allylic isomerization of the product was attributed to the presence of Ni–H species in the reaction process. Trace amount of hydrogenation product of styrene was also detected by GC-MS. Various functional groups, including methyl (4ab-ad), tert-butyl (4ae), phenyl (4af), methoxyl (4ag-ah), fluoride (4ai), and trifluoromethyl (4aj), were accommodated under the optimal conditions. Remarkably, the alkenes with sensitive functional groups such as hydroxyl (4ak), ester (4al), amine (4am), and amide (4an), which are typically biased in the presence of organometallic reagents, could efficiently participated in this reaction. Steric hindrance on the double bond of vinylarene diminished the yield. For example, the target product 4ap was obtained in 38% yield when 1,1-diphenylethylene was applied. Treatment of α-methylstyrene under the standard conditions gave the allylic isomers 4aq and 4aq’ in combined 64% yield with 60:40 regioselectivity. With respect to β-methylstyrene, Z:E isomers (4ar and 4ar’) were achieved in combined 35% yield. Unfortunately, vinylsilane, aliphatic and electron-deficient alkenes failed to give the corresponding products under the current catalytic system.
Fig. 2.
Scope of substrates. Reaction conditions: 3 (0.2 mmol), 1 (0.6 mmol), N2H4•H2O (0.72 mmol), Ni(cod)2 (10 mol%), dcype (10 mol%), DIPEA (0.4 mmol), NaI (0.1 mmol), THF (1.0 mL), 100 °C, 12 h under N2. Reported yields are the isolated ones (the ratio of allylic isomerization was in parentheses), the E:Z ratio was more than 20:1 unless otherwise noted. aThe Z:E ratio in parenthesis
Next, we proceeded to examine the substrate scope of aldehydes 1 (Fig. 2b). In general, the electronic effects of the nucleophiles did not influence the efficiency of this transformation. Aldehydes bearing both electron-donating and electron-withdrawing substituents all reacted smoothly with styrene 3a to afford the products 4ba-bm in 65–94% yields. Hetero-aromatic aldehydes, such as furan-2-carbaldehyde and thiophene-3-carbaldehyde, also successfully gave the desired products (4bn and 4bo) in moderate yields. To our delight, aliphatic aldehydes, such as propionaldehyde and iso-butyraldehyde, which are challenging substrates in the previous reports, were also applicable in this Heck-type reaction (4bp and 4bq). Moreover, the attempt to apply ketone as a nucleophile in this transformation also proved feasible (4br).
It is worth noting that an excellent regioselectivity (>95:5) was observed when furan-2-carbaldehyde 1n was examined (Fig. 2b, 4bn). We therefore hypothesized that if the chelation or directing effect of the vicinal oxygen atom inhibited the allylic isomerization58. Thus, the phenyl aldehyde with an ortho-methoxyl group 1s was selected as the electrophile candidate. As shown in Fig. 2c, a variety of vinylarenes attached with different functional groups were re-examined with 2-methoxybenzaldehyde 1s under the optimal conditions, and excellent regioselectivity (mostly >95:5) of the desired products 4ca-cm were obtained also with good yields. Furthermore, the olefins bearing heterocyclic skeletons including pyridine, dibenzo-furan, functionalized indole and carbazole, were all viable substrates, affording the corresponding products (4cn-cs) in 68–92% yields.
Synthetic applications
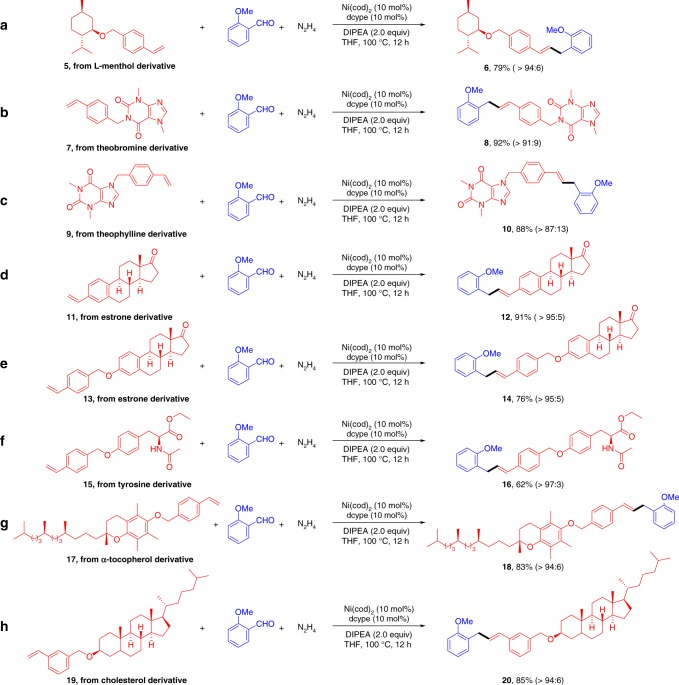
The promising functional group tolerance and high-efficiency of this protocol enabled its application to the modification of pharmaceuticals and natural product derivatives (Fig. 3). For example, introducing ortho-methoxyl benzyl group into L-Menthol (5) was successfully achieved in excellent yield with good regioselectivity (>94:6). Alkaloids such as Theobromine (7) and Theophylline derivatives (9) reacted smoothly under current catalytic system. The estrone derivatives 11 and 13 were also tested as superior candidates, delivering the target molecules 12 and 14 with the ketone moiety untouched. In addition, the structural elaboration of tyrosine derivative (15) was readily accomplished with this nickel-catalyzed oxidant-free Heck-coupling strategy. Moreover, the cross-couplings of 1 s with α-Tocopherol and Cholesterol derivatives were investigated under the optimized conditions, and the expected benzylation products (18 and 20) were all obtained in good yields (>80%). These examples highlighted the wide applicablity and compatiblity of the method, and its enrichment of the tool box for the modification of complex bioactive molecules.
Fig. 3.
Functionalization of naturally and pharmaceutically important derivatives. a Regioselective Heck coupling of L-Menthol derivative; b Regioselective Heck coupling of Theobromine derivative; c Regioselective Heck coupling of Theophylline derivative; d Regioselective Heck coupling of vinyl Estrone derivative; e Regioselective Heck coupling of Estrone derivative; f Regioselective Heck coupling of Tyrosine derivative; g Regioselective Heck coupling of α-Tocopherol derivative; h Regioselective Heck coupling of Cholesterol derivative
Mechanistic investigation
To gain preliminary insights into the reaction mechanism, several control experiments were subsequently carried out. Firstly, the reaction of styrene (3a) with phenyldiazomethane (21) did not afford the desired (E)-prop-1-ene-1,3-diyldibenzene (4aa) (Fig. 4a). When 1,2-diphenylcyclopropane (22) was applied under the standard conditions, the desired product 4aa was also not observed (Fig. 4b). These two results ruled out the possibility that hydrazone played the role of carbene precursor or diazo species to undergo cyclopropanation and subsequent ring opening during the reaction course59. Secondly, when 1-methoxy-4-(3-phenylpropyl)benzene (23) was tested, neither 4ag nor its regioisomer 4bf was detected (Fig. 4c). This observation suggested the unlikely involvement of hydrazone addition to the vinylarene followed by dehydrogenation pathway. Thirdly, the efficiency of the model reaction was almost unaffected in the presence of a radical scavenger, 2,6-di-tert-butyl-4-methylphenol (BHT), indicating the unlikelihood of a radical mechanism in the current reaction (Fig. 4d). When N-Ts hydrazone 24 was used in the place of simple hydrazone 1a, no desired product 4aa was observed, which accounted for the totally diverse characters between the two different types of hydrazones (Fig. 4e)60–62. Finally, isotope experiment was carried out (Fig. 4f). H/D exchanges both in the double bond moiety (73% D) and benzylic position (15% D) revealed that iterative Ni-(H)D species addition/elimination steps existed during the reaction process63,64.
Fig. 4.
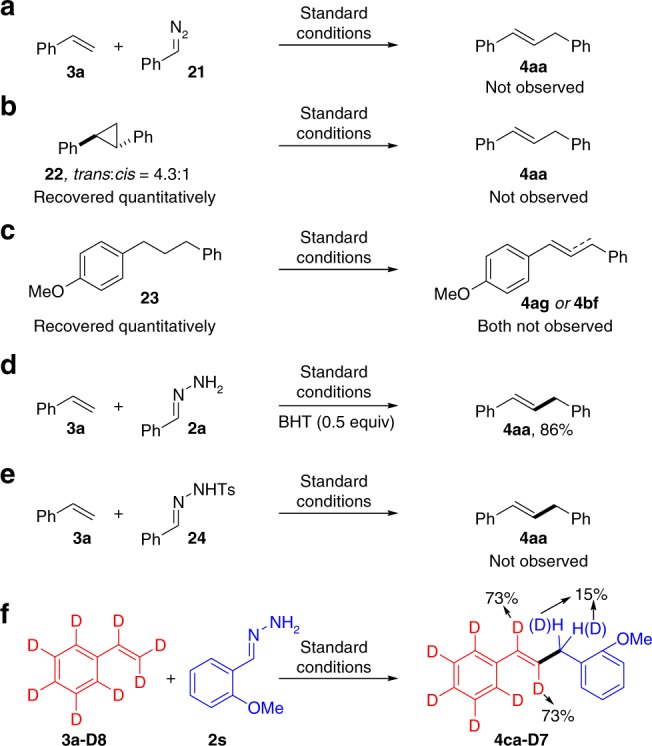
Mechanistic studies. a Reaction of styrene with phenyldiazomethane did not give the desired product; b Reaction of 1,2-diphenylcyclopropane under the standard conditions did not give the desired product; c Reaction of 1-methoxy-4-(3-phenylpropyl)benzene under the standard conditions did not give the desired product; d Radical scavenger, 2,6-di-tert-butyl-4-methylphenol (BHT) added and the reaction was almost unaffected; e N-Ts hydrazone used instead of simple hydrazone did not give the desired product; f H/D exchanges occurred in the isotope experiment
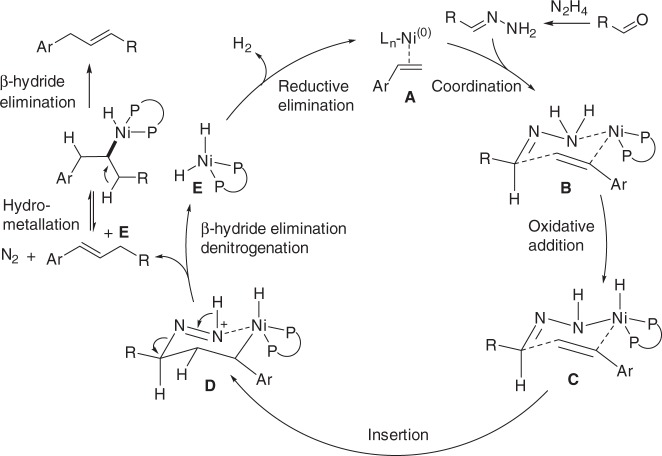
Although, the exact mechanism still remained unclear at this moment, on the basis of literature reports6,47–56 and our findings, a plausible reaction pathway was proposed in Fig. 5. Initially, the active nickel(0) species, which potentially complexed to the olefin, coordinated with hydrazone to form a six-membered intermediate B. Then the nickel catalyst undergoes oxidative addition to the N–H bond likely with the assistance of DIPEA and delivers the key intermediate C. A similar N–H bond activation of simple hydrazone was also observed with a manganese pincer complex by Milstein and coworkers57. At this stage, intramolecular regioselective 1,2-insertion occurs to afford the complex D, which then undergoes concerted β-hydride elimination and releases N2 gas (Wolff-Kishner reductive denitrogenation) to deliver the desired product 4 and Ni–H species E. This metal-hydride releases H2 gas and regenerates the active Ni(0) catalyst to close the catalytic cycle. NaI as the additive enhances both the reactivity and E:Z selectivity, possibly through halide effects that assist the decomposition of intermediate D to produce thermodynamically more favored trans-alkene65. The allylic isomerization was due to the iterative Ni–H addition/elimination.
Fig. 5.
Reaction mechanism. Proposed pathway for the oxidant-free Heck-type reaction
Discussion
In summary, we have documented an efficient protocol for alkyl Heck-type reaction of vinylarenes with umpolung aldehydes under a simple nickel catalyst system. This strategy proceeds smoothly in absence of halides, organometallics or oxidant, liberating hydrogen, nitrogen, and water as innocuous side products. Excellent regioselectivity was achieved with the assistance of heteroatom chelation effect. Broad substrate scope, great functional group compatibility and modification of complex organic molecules containing olefin moieties made this methodology synthetically useful and valuable. Preliminary mechanistic studies revealed that the transformation did not proceed through carbene insertion, cyclopropane opening, nucleophilic addition/dehydrogenation or radical process. Detailed studies aimed at elucidating the mechanism and further applications of aldehydes as alkyl nucleophiles in chemical transformations are ongoing in our lab.
Methods
In situ preparation of hydrazone solution
A mixture of aldehydes 1 (0.6 mmol, 3.0 equiv) and hydrazine monohydrate (36 µL, 0.72 mmol, 64–65 wt%, 3.6 equiv) in THF (0.6 mL) solution was stirred for 30 min at room temperature in air. Before use, a small amount of anhydrous Na2SO4 and 4Ǻ MS was added.
General procedure for dehydrogenative alkyl Heck-couplings
In a glovebox, a flame-dried reaction tube (10 cm3) equipped with a magnetic stir bar was charged with Ni(cod)2 (5.6 mg, 10 mol%), dcype (8.5 mg, 10 mol%) and THF (0.4 mL) before being sealed with a rubber septum. The reaction mixture was stirred at room temperature for 30 min. Then vinylarene 3 (0.2 mmol, 1.0 equiv), hydrazone solution 2 (0.6 mmol in 0.6 mL THF), DIPEA (0.4 mmol, 67 µL) and NaI (0.1 mmol, 15 mg) were added sequentially. After that, the reaction mixture was sealed with aluminum cap, moved out of glovebox and stirred at 100 °C for 12 h. After the mixture was cooled to rt, the resulting solution was directly filtered through a pad of silica by EtOAc (3.0 mL). The crude mixture was analyzed by GC-MS. The solvent was evaporated in vacuo to give the crude products. NMR yields were determined by 1H NMR using mesitylene as an internal standard. The residue was purified by preparative TLC (ethyl acetate/hexane) to give the pure product 4.
Notes
Use of the glovebox is not necessary unless to store and manipulate air-sensitive Ni(cod)2 catalyst. The other operations can be successfully performed outside the glovebox with standard Schlenk line procedure. One pot reaction of vinylarenes, aldehydes and hydrazine without preparation of hydrazones beforehand afforded only trace amount of desired products.
Supplementary information
Acknowledgements
We are grateful to the Canada Research Chair Foundation (to C.-J.L.), the Canadian Foundation for Innovation, FRQNT Centre in Green Chemistry and Catalysis, and the Natural Science and Engineering Research Council of Canada for support of our research. Dr. L.L. is grateful for the support from National Postdoctoral Program for Innovative Talents (No. BX201700110) and China Postdoctoral Science Foundation Funded Project (No. 2017M623270).
Author contributions
L.L. and D.Z. both performed the experiments and analyzed the data. C.-J.L. directed and conceived the project. L.L. wrote the paper with feedback and guidance from C.-J.L. All authors discussed the experimental results and commented on the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Leiyang Lv, Dianhu Zhu.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08631-1.
References
- 1.Tsutomu M, Kunio M, Atsumu O. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971;44:581–581. [Google Scholar]
- 2.Heck RF, Nolley JP. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972;37:2320–2322. [Google Scholar]
- 3.Beletskaya IP, Cheprakov AV. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 4.Dounay AB, Overman LE. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003;103:2945–2964. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- 5.Oestreich M. The Mizoroki-Heck Reaction. Chichester: Wiley & Sons; 2009. [Google Scholar]
- 6.Le Bras J, Muzart J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011;111:1170–1214. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- 7.Yeung CS, Dong VM. Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira, E. M., Zhang, H. & Stoltz, B. M. Oxidative Heck-Type Reactions, vol. 9 (ed Oestreich, M.) 345–382 (Wiley-VCH, Weinheim, 2009).
- 9.Tang S, Liu K, Liu C, Lei A. Olefinic C-H functionalization through radical alkenylation. Chem. Soc. Rev. 2015;44:1070–1082. doi: 10.1039/c4cs00347k. [DOI] [PubMed] [Google Scholar]
- 10.Werner EW, Mei TS, Burckle AJ, Sigman MS. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozhushkov SI, Ackermann L. Ruthenium-catalyzed direct oxidative alkenylation of arenes through twofold C-H bond functionalization. Chem. Sci. 2013;4:886–896. [Google Scholar]
- 12.Li CJ. Cross-Dehydrogenative Coupling (CDC): exploring C-C bond formations beyond functional group transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 13.Moritani I, Fujiwara Y. Aromatic substitution of styrene-palladium chloride complex. Tetrahedron Lett. 1967;8:1119–1122. [Google Scholar]
- 14.Jia C, Kitamura T, Fujiwara Y. Catalytic functionalization of arenes and alkanes via C-H bond activation. Acc. Chem. Res. 2001;34:633–639. doi: 10.1021/ar000209h. [DOI] [PubMed] [Google Scholar]
- 15.Jia C, et al. Efficient activation of aromatic C-H bonds for addition to C-C multiple bonds. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]
- 16.Ruan J, Li X, Saidi O, Xiao J. Oxygen and base-free oxidative Heck reactions of arylboronic acids with olefins. J. Am. Chem. Soc. 2008;130:2424–2425. doi: 10.1021/ja0782955. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Cui X, Chen L, Jiang G, Wu Y. Palladium-catalyzed alkenylation of quinoline-N-oxides via C-H activation under external-oxidant-free conditions. J. Am. Chem. Soc. 2009;131:13888–13889. doi: 10.1021/ja902762a. [DOI] [PubMed] [Google Scholar]
- 18.Guimond N, Gorelsky SI, Fagnou K. Rhodium(III)-catalyzed heterocycle synthesis using an internal oxidant: improved reactivity and mechanistic studies. J. Am. Chem. Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]
- 19.Rakshit S, Grohmann C, Besset T, Glorius F. Rh(III)-catalyzed directed C-H olefination using an oxidizing directing group: mild, efficient, and versatile. J. Am. Chem. Soc. 2011;133:2350–2353. doi: 10.1021/ja109676d. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Zhang H, Shi W, Lei A. Bond formations between two nucleophiles: transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011;111:1780–1824. doi: 10.1021/cr100379j. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, et al. Oxidative coupling between two hydrocarbons: an update of recent C-H functionalizations. Chem. Rev. 2015;115:12138–12204. doi: 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]
- 22.Manikandan R, Madasamy P, Jeganmohan M. Ruthenium-catalyzed ortho alkenylation of aromatics with alkenes at room temperature with hydrogen evolution. ACS. Catalysis. 2016;6:230–234. [Google Scholar]
- 23.Amatore C, Cammoun C, Jutand A. Electrochemical recycling of benzoquinone in the Pd/benzoquinone-catalyzed Heck-type reactions from arenes. Adv. Synth. Catal. 2007;349:292–296. [Google Scholar]
- 24.Tang S, Gao X, Lei A. Electrocatalytic intramolecular oxidative annulation of N-aryl enamines into substituted indoles mediated by iodides. Chem. Commun. 2017;53:3354–3356. doi: 10.1039/c7cc00410a. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, et al. Photoinduced oxidative activation of electron-rich arenes: alkenylation with H2 evolution under external oxidant-free conditions. Chem. Sci. 2018;9:1521–1526. doi: 10.1039/c7sc04634k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Nakamura T, Yorimitsu H, Oshima K. Cobalt-catalyzed Heck-type reaction of alkyl halides with styrenes. J. Am. Chem. Soc. 2002;124:6514–6515. doi: 10.1021/ja026296l. [DOI] [PubMed] [Google Scholar]
- 27.Firmansjah L, Fu GC. Intramolecular Heck reactions of unactivated alkyl halides. J. Am. Chem. Soc. 2007;129:11340–11341. doi: 10.1021/ja075245r. [DOI] [PubMed] [Google Scholar]
- 28.Matsubara R, Gutierrez AC, Jamison TF. Nickel-catalyzed Heck-type reactions of benzyl chlorides and simple olefins. J. Am. Chem. Soc. 2011;133:19020–19023. doi: 10.1021/ja209235d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloome KS, McMahen RL, Alexanian EJ. Palladium-catalyzed Heck-type reactions of alkyl iodides. J. Am. Chem. Soc. 2011;133:20146–20148. doi: 10.1021/ja2091883. [DOI] [PubMed] [Google Scholar]
- 30.Weiss ME, Kreis LM, Lauber A, Carreira EM. Cobalt-catalyzed coupling of alkyl iodides with alkenes: deprotonation of hydridocobalt enables turnover. Angew. Chem. Int. Ed. 2011;50:11125–11128. doi: 10.1002/anie.201105235. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, et al. Nickel-catalyzed Heck-type alkenylation of secondary and tertiary α-carbonyl alkyl bromides. Angew. Chem. Int. Ed. 2012;51:3638–3641. doi: 10.1002/anie.201108350. [DOI] [PubMed] [Google Scholar]
- 32.Nishikata T, Noda Y, Fujimoto R, Sakashita T. An efficient generation of a functionalized tertiary-alkyl radical for copper-catalyzed tertiary-alkylative Mizoroki-Heck type reaction. J. Am. Chem. Soc. 2013;135:16372–16375. doi: 10.1021/ja409661n. [DOI] [PubMed] [Google Scholar]
- 33.Tang S, Liu C, Lei A. Nickel-catalysed novel β,γ-unsaturated nitrile synthesis. Chem. Commun. 2013;49:2442–2444. doi: 10.1039/c3cc00029j. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Liu C, Yuan J, Lei A. Copper-catalyzed oxidative coupling of alkenes with aldehydes: direct access to α,β-unsaturated ketones. Angew. Chem. Int. Ed. 2013;52:2256–2259. doi: 10.1002/anie.201208920. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Wei Y. copper catalyzed direct alkenylation of simple alkanes with styrenes. Chem. Sci. 2014;5:2379–2382. [Google Scholar]
- 36.Tang S, Zeng L, Liu Y, Lei A. Zinc-catalyzed dehydrogenative cross-coupling of terminal alkynes with aldehydes: access to ynones. Angew. Chem. Int. Ed. 2015;54:15850–15853. doi: 10.1002/anie.201509042. [DOI] [PubMed] [Google Scholar]
- 37.Yuan J, et al. Bimetallic zinc complex—active species in coupling of terminal alkynes with aldehydes via nucleophilic addition/Oppenauer oxidation. Chem. Commun. 2015;51:576–579. doi: 10.1039/c4cc08152h. [DOI] [PubMed] [Google Scholar]
- 38.Zou Y, Zhou J. Palladium-catalyzed intermolecular Heck reaction of alkyl halides. Chem. Commun. 2014;50:3725–3728. doi: 10.1039/c4cc00297k. [DOI] [PubMed] [Google Scholar]
- 39.Ye J, et al. Remote C-H alkylation and C-C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 2016;9:361–368. doi: 10.1038/nchem.2631. [DOI] [PubMed] [Google Scholar]
- 40.Wang GZ, Shang R, Cheng WM, Fu Y. Irradiation-induced Heck reaction of unactivated alkyl halides at room temperature. J. Am. Chem. Soc. 2017;139:18307–18312. doi: 10.1021/jacs.7b10009. [DOI] [PubMed] [Google Scholar]
- 41.Kurandina D, Parasram M, Gevorgyan V. Visible light-induced room-temperature Heck reaction of functionalized alkyl halides with vinyl arenes/heteroarenes. Angew. Chem. Int. Ed. 2017;56:14212–14216. doi: 10.1002/anie.201706554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H, et al. HOTf-catalyzed alkyl-Heck-type reaction. iScience. 2018;3:255–263. doi: 10.1016/j.isci.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng SS, Jamison TF. Simple alkenes as substitutes for organometallic reagents: nickel-catalyzed, intermolecular coupling of aldehydes, silyl triflates, and alpha olefins. J. Am. Chem. Soc. 2005;127:14194–14195. doi: 10.1021/ja055363j. [DOI] [PubMed] [Google Scholar]
- 44.Zhang G, et al. External oxidant-free oxidative cross-coupling: a photoredox cobalt-catalyzed aromatic C-H thiolation for constructing C-S bonds. J. Am. Chem. Soc. 2015;137:9273–9280. doi: 10.1021/jacs.5b05665. [DOI] [PubMed] [Google Scholar]
- 45.Wasa M, Engle KM, Yu JQ. Pd(II)-catalyzed olefination of sp3 C-H bonds. J. Am. Chem. Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stowers KJ, Fortner KC, Sanford MS. Aerobic Pd-catalyzed sp3 C-H olefination: a route to both N-heterocyclic scaffolds and alkenes. J. Am. Chem. Soc. 2011;133:6541–6544. doi: 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Dai XJ, Li CJ. Aldehydes as alkyl carbanion equivalents for additions to carbonyl compounds. Nat. Chem. 2017;9:374–378. doi: 10.1038/nchem.2677. [DOI] [PubMed] [Google Scholar]
- 48.Dai XJ, Wang H, Li CJ. Carbonyls as latent alkyl carbanions for conjugate additions. Angew. Chem. Int. Ed. 2017;56:6302–6306. doi: 10.1002/anie.201700059. [DOI] [PubMed] [Google Scholar]
- 49.Chen N, Dai XJ, Wang H, Li CJ. Umpolung addition of aldehydes to aryl imines. Angew. Chem. Int. Ed. 2017;56:6260–6263. doi: 10.1002/anie.201610578. [DOI] [PubMed] [Google Scholar]
- 50.Wei W, et al. Ruthenium(II)-catalyzed olefination via carbonyl reductive cross-coupling. Chem. Sci. 2017;8:8193–8197. doi: 10.1039/c7sc04207h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CC, et al. Iron-catalyzed nucleophilic addition reaction of organic carbanion equivalents via hydrazones. Org. Lett. 2018;20:3801–3805. doi: 10.1021/acs.orglett.8b01391. [DOI] [PubMed] [Google Scholar]
- 52.Yan SS, et al. Ruthenium-catalyzed umpolung carboxylation of hydrazones with CO2. Chem. Sci. 2018;9:4873–4878. doi: 10.1039/c8sc01299g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang J, et al. Nickel-catalyzed cross-coupling of aldehydes with aryl halides via hydrazone intermediates. Chem. Commun. 2018;54:1750–1753. doi: 10.1039/c7cc09290c. [DOI] [PubMed] [Google Scholar]
- 54.Zhu D, et al. Umpolung of carbonyl groups as alkyl organometallic reagent surrogates for palladium-catalyzed allylic alkylation. Angew. Chem. Int. Ed. 2018;57:16520–16524. doi: 10.1002/anie.201809112. [DOI] [PubMed] [Google Scholar]
- 55.Lv L, Qiu Z, Li J, Liu M, Li CJ. N2H4 as traceless mediator for homo- and cross- aryl coupling. Nat. Commun. 2018;9:4739. doi: 10.1038/s41467-018-07198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lv L, et al. Cross-coupling of phenol derivatives with umpolung aldehydes catalyzed by nickel. ACS Catal. 2018;8:4622–4627. [Google Scholar]
- 57.Das UK, Chakraborty S, Diskin-Posner Y, Milstein D. Direct conversion of alcohols into alkenes by dehydrogenative coupling with hydrazine/hydrazone catalyzed by manganese. Angew. Chem. Int. Ed. 2018;57:13444–13448. doi: 10.1002/anie.201807881. [DOI] [PubMed] [Google Scholar]
- 58.Paquette LA, Lobben PC. π-Facial diastereoselection in the 1,2-addition of allylmetal reagents to 2-methoxycyclohexanone and tetrahydrofuranspiro-(2-cyclohexanone) J. Am. Chem. Soc. 1996;118:1917–1930. [Google Scholar]
- 59.Xia Y, Qiu D, Wang J. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem. Rev. 2017;117:13810–13889. doi: 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- 60.Barluenga J, Valdés C. Tosylhydrazones: new uses for classic reagents in palladium-catalyzed cross-coupling and metal-free reactions. Angew. Chem. Int. Ed. 2011;50:7486–7500. doi: 10.1002/anie.201007961. [DOI] [PubMed] [Google Scholar]
- 61.Shao Z, Zhang H. N-Tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012;41:560–572. doi: 10.1039/c1cs15127d. [DOI] [PubMed] [Google Scholar]
- 62.Xia Y, Wang J. N-Tosylhydrazones: versatile synthons in the construction of cyclic compounds. Chem. Soc. Rev. 2017;46:2306–2362. doi: 10.1039/c6cs00737f. [DOI] [PubMed] [Google Scholar]
- 63.Chen F, et al. Remote migratory cross-electrophile coupling and olefin hydroarylation reactions enabled by in situ generation of NiH. J. Am. Chem. Soc. 2017;139:13929–13935. doi: 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- 64.Sommer H, Juliá-Hernández F, Martin R, Marek I. Walking metals for remote functionalization. ACS Cent. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fagnou K, Lautens M. Halide effects in transition metal catalysis. Angew. Chem. Int. Ed. 2002;41:26–47. doi: 10.1002/1521-3773(20020104)41:1<26::aid-anie26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request.