Abstract
Use of alcohol, cannabis and opioids is highly prevalent and is associated with global disease burden and high economic costs. The exact pathophysiology of abuse or addiction associated with these sedative substances is not completely understood, but previous research implicates the important role of the striatal dopamine system in the addiction process. Multiple studies investigated changes in the striatal dopamine systems of users of sedative substances, but currently these results are very heterogeneous. Therefore, we conducted a meta-analysis of in vivo neuroimaging studies investigating dopaminergic alterations in the striatum of users of alcohol, opioids or cannabis. Analyses for each substance were conducted separately for the availability of D2/D3 dopamine receptors, dopamine transporters and dopamine synthesis capacity. In total, 723 substance users and 752 healthy controls were included. The results indicated a significant lower striatal D2/D3 receptor availability in alcohol users compared to controls (g = 0.46) but no difference in dopamine transporter availability or dopamine synthesis capacity. Our analysis indicated that changes of dopamine receptors and transporters are moderated by the duration of abstinence. Comparing opioid users with controls revealed a significant lower D2/D3 receptor availability (g = 1.17) and a significantly lower transporter availability (g = 1.55) in opioid users. For cannabis users, there was no significant difference in receptor availability compared to controls and too few studies provided information on dopamine transporter availability or synthesis capacity. Our analysis provides strong evidence for a central role of the striatal dopamine system in use of alcohol or opioids. Further studies are needed to clarify the impact of the dopamine system in cannabis users.
Subject terms: Neurotransmitters, Addiction
Introduction
Drug use is a global major health problem and despite much effort, it apparently remains stable over the last few years [1]. With an estimated global prevalence of 3.8% in 2015 (183 million people), cannabis represents the world’s most widely used illegal drug [1]. Alcohol is another globally prevalent substance which is related to high economic, social and health costs. According to the World Health Organization (WHO), the prevalence of alcohol use disorders in the whole world is 4.1%, with its highest prevalence in the WHO European regions with 7.5% and American regions with 6.0% [2]. While the prevalence of opioid-dependency is less marked (globally 0.22%, Europe 0.35% and North America 0.30%), it comes with the high risk of serious health consequences, especially if considering the high number of drug-related deaths [3]. This marked impact of sedative drugs on global health underscores the importance for a better understanding of the underlying pathophysiological processes in order to prevent the development of addiction and to offer effective therapies. Current in vivo research with positron emission tomography (PET) and single-photon emission computed tomography (SPECT) suggests a crucial role of the dopaminergic system in emergence and maintenance of addiction [4–6]. Especially striatal regions innervate strongly with dopaminergic neurons and thus seem to be key regions in drug addiction [7]. Specifically, evidence for reduced striatal dopamine transporter (DAT) availability in alcohol users was found in some [8–10] but not all studies [11–13]. Similarly, there is evidence for a lower striatal dopamine receptor binding in alcohol-dependent patients relative to healthy controls [13–15] but again, other studies reported conflicting results [16, 17]. Investigation of dopamine synthesis capacity and alcohol use yielded mixed results, including findings of higher presynaptic dopamine function in the putamen of alcohol users compared to controls [18], but also no difference in presynaptic dopamine function between alcohol users and controls [18, 19]. Opioid users have been repeatedly reported to exhibit reductions in D2 [20–22] and DAT availability [23–26]. Interestingly, alterations in D2 receptor availability in cannabis users seem to be less prevalent [27–29], while there is evidence for reduced DAT [30, 31] and dopamine synthesis capacity [32] in cannabis users.
In summary, there are inconsistent results regarding the influence of sedative drugs on the striatal dopaminergic system. Therefore, the current work summarizes findings on striatal dopaminergic functions and sedative substances from human PET and SPECT studies into a systematic review and meta-analysis.
Methods
Literature search and data extraction
We conducted a comprehensive literature search to identify all neuroimaging studies using PET and SPECT to investigate DAT, dopamine D2/D3 receptors and dopamine synthesis capacity in human participants abusing alcohol, cannabis or opioids. Details of the literature search and data extraction are reported in the supplementary methods. Exclusion criteria were non-English language, postmortem studies, no original data, single-case studies, only investigation of extrastriatal regions or only voxel-wise analysis. The first author extracted the relevant data and all extractions were cross-checked by the second and third author. If data were not provided we contacted the authors of respective papers. The main outcome measure was the difference in the availability of D2/D3 receptors, availability of DAT or dopamine synthesis capacity between the control group (healthy non-users) and the user group. Depending on the methodological approach of the individual studies, availability was estimated based on BPND (ratio of specific to nonspecific binding), BPP (ratio of specific binding to total plasma parent), VT (ratio of the concentration of radioligand in a region of tissue to that in plasma), distribution volume ratio (the ratio of the distribution volume in a receptor region to the distribution volume in a nonreceptor-containing region), specific uptake ratio (uptake ratio of binding in a region of interest in comparison to a non-binding reference region) or Fi (mean fractional tracer uptake values).
The specific measurement of this difference in binding potential varied across the studies and is displayed for each study in the supplementary tables (Table S1–S3). To calculate effect sizes, means, standard deviations (SDs), standard errors of the mean (SEM) and confidence intervals were extracted. Additionally, the name of the authors, year of publication, imaging method (PET or SPECT), investigated hemisphere and striatal region, radioligand used and population characteristics (group size, age, number of females and males, smoking status, positive or negative drug status at the time of scan, minimum and mean duration of abstinence of the patient group) were extracted.
Data analysis
Separate meta-analyses were conducted for the different substances and brain regions. For each analysis, the effect size (Hedge’s g) of the difference between the control and user group in binding potential was computed. A random-effects meta-analytic model was employed, as we did not assume homogeneity among studies [33]. The summary effect sizes were computed using a restricted maximum-likelihood estimator [34] by using the Metafor package [35] in R, version 3.3.2 [36]. To assess the impact of heterogeneity on the meta-analysis, we calculated the inconsistency parameter (I2 value). Moderator analyses were conducted with duration of abstinence, age, gender and year of publication as moderator variables. Publication bias was evaluated by the Egger’s regression test [37]. We determined a minimum of three studies to conduct a meta-analysis. The significance level was set at p < 0.05 (two-tailed).
Results
Selected articles
The initial literature search revealed 225 articles. After exclusion of 190 publications, 35 studies including 723 substance users and 752 healthy controls remained for a qualitative analysis (see Figure S1 for a flow chart). Many studies investigated multiple samples [8, 9, 12, 13, 15, 17, 24, 25, 31, 38–41]. Thus, we use k as an index for the number of samples, n for the number of studies and N for the number of participants. Included articles were separated by the investigated substance, resulting in n = 16 studies (k = 23 samples) for a qualitative synthesis of alcohol, n = 12 studies (k = 17) for a qualitative synthesis of opioids and n = 7 studies (k = 8) for a qualitative synthesis of cannabis. Two studies [10, 42] had overlapping samples, wherefore only the study with the larger sample [10] was included. Tomasi et al. [43] presented the relevant data only graphically; therefore, we estimated means and SDs from the respective figure using Web Plot Digitizer 3.10 [44]. Two publications reported other measures of dispersion than SDs: confidence intervals [8] and standard errors of the mean [22] which were transformed into SDs. Several studies reported means and SDs separately for the left and right hemisphere [18, 19, 22, 24, 26–28, 39, 45]. Here, measures for the brain region spanning both hemispheres were summarized as explained in the supplementary methods. Some articles only reported results for striatal subregions; hence, we summarized this information into indicators of the whole striatum [16, 20, 27, 29, 43, 46, 47] or into indicators of the dorsal striatum [15, 18, 21, 22, 24, 28, 31, 39]. Two studies used the same control and patient group for investigating DAT and D2/D3 receptor and were included in both analyses [9, 13]. Guardia et al. [17] reported separated results for the patient group based on a later relapse. In this case, we compared the same control group once with the relapsed patient group and once with the patient group without relapse. Shi et al. [24] used the same control group as a comparison for two different patient groups.
All participants were aged over 18 years and the majority did not have a major psychiatric or neurological illness, except substance abuse or dependency in the patient group. The duration of abstinence in the patient group varied across studies, with some studies also investigating current users. When studies reported the results of two scanning times for the patient group (before and after treatment), results of both scans were included in the analyses in order to gain more samples and additional information on the moderation effect of abstinence [8, 15, 38–41]. Details about characteristics of the participants, imaging methods and analysed regions are reported in the supplementary results and displayed in Tables S1–S3. In sum, there were sufficient studies to conduct separate meta-analyses of striatal and substriatal D2/D3 and striatal DAT availability in alcohol users, striatal and substriatal DAT availability in opioid users and striatal and substriatal D2/D3 in cannabis users.
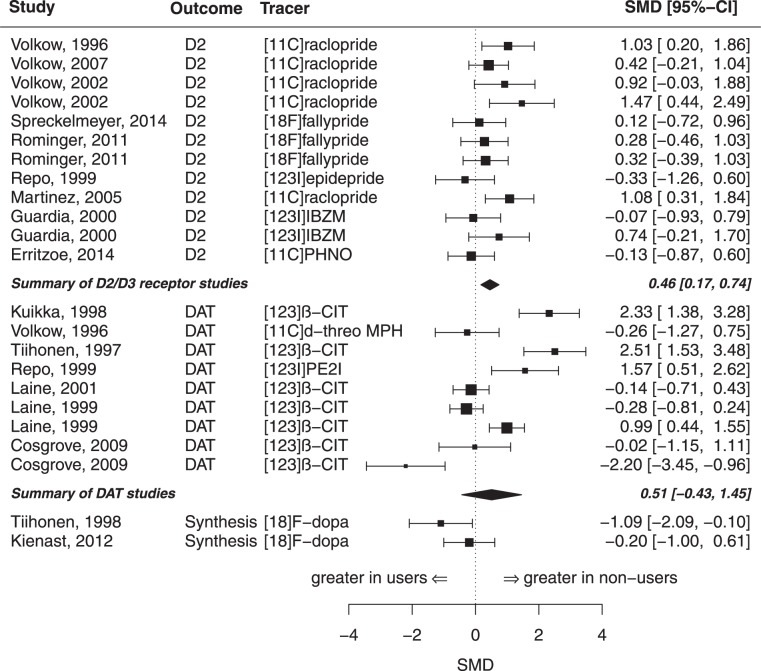
Alcohol
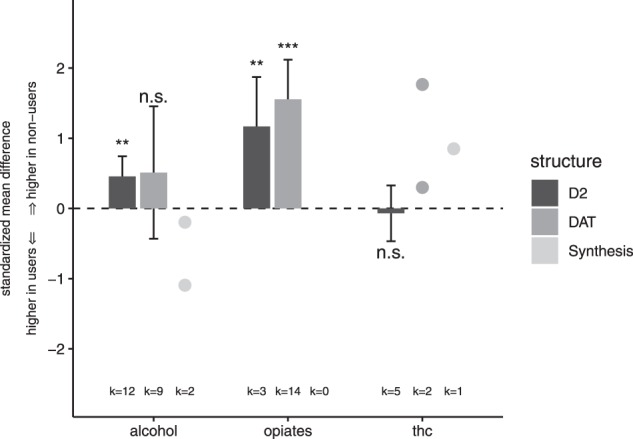
We included k = 12 samples comprising N = 127 alcohol users and N = 119 controls for the analysis of D2/D3 receptor availability in the whole striatum (see table S1 for characteristics of the studies). There was a significant summary effect size of g = 0.46 (z = 3.13, p < 0.01, I2 = 32.36, see Figs. 1 and 2), indicating lower D2/D3 receptor availability in alcohol users compared to controls. There was no evidence for a publications bias in the Egger’s test (p = 0.32). Moderator analyses did not show evidence for an effect of year of publication or age on the estimated summary effect size. However, there was a significant effect of mean days of abstinence (p = 0.02), indicating a decreasing effect with increasing abstinence duration, see Figure S2. In a further analysis, we investigated the D2/D3 receptor availability in the subregions of the striatum (nucleus caudate and putamen). For the analysis of the caudate, we included eight samples (from n = 6 studies) comprising N = 87 alcohol patients and N = 84 controls. There was a significant summary effect size (g = 0.51, see Fig. 3), indicating lower caudate D2/D3 receptor availability in the patient group (k = 8, z = 2.60, p < 0.01, I2 = 48.29). However, the Egger’s test indicated the potential presence of a publication bias (p = 0.03). For the analysis of the putamen, we included the same eight samples with the respective number of participants. There was a significant summary effect size (g = 0.45), indicating lower receptor availability in the user group (k = 8, z = 2.58, p = 0.01, I2 = 34.31). There was no evidence for publication bias (p = 0.13).
Fig. 1.
Barplot overview: changes of striatal dopamine in sedative drug users
Fig. 2.
Forest plot of meta-analysis of striatal dopaminergic function in alcohol users
Fig. 3.
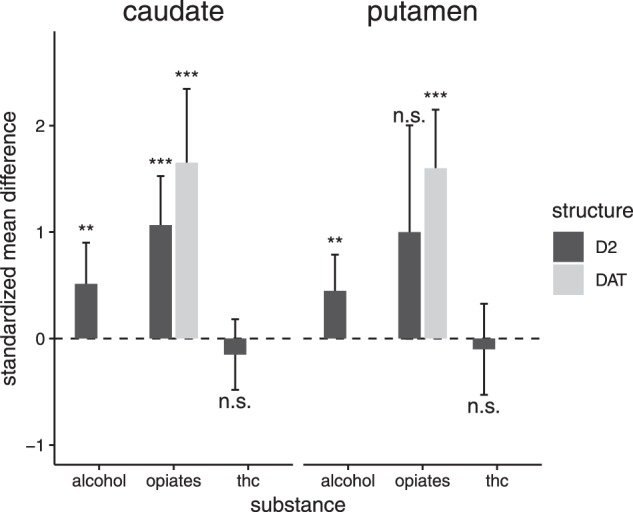
Barplot overview: changes of substriatal dopamine in sedative drug users
In the meta-analysis of DAT availability for the whole striatum, we included k = 9 samples comprising N = 107 alcohol users and N = 136 controls (see Table S1). The summary effect size of g = 0.51 (z = 1.06, I2 = 92.06, see Fig. 1) did not reach significance (p = 0.29). Egger’s test did not show evidence for publication bias (p = 0.81). Moderator analyses did not show evidence for an effect of age (p = 0.33) but for year of publication (p = 0.04) and for mean days of abstinence (p < 0.01), showing an increasing effect with increasing abstinence (see Figure S2). There were no studies investigating the DAT availability in the subregions of caudate and putamen.
There were only two samples investigating the dopamine synthesis capacity in the whole striatum in a total of N = 21 alcoholic patients and N = 21 controls (see Table S1). One of these studies [18] found higher—but not statistically significant—striatal dopamine uptake values in the patient group (g = –1.09; z = –2.15; 95% CI: –2.09 to –0.10; p = 0.28), while the other study [19] did not find evidence for a difference in dopamine uptake between alcohol users and controls (see also Figs. 1, 2). Only Tiihonen et al. [18] investigated the dopamine synthesis capacity in the subregions caudate and putamen for N = 10 alcohol patients and N = 8 controls. Here, a significant effect size of g = –1.06 was detected in the putamen (z = –2.11; 95% CI: –2.06 to –0.07; p = 0.03), showing an increased presynaptic dopamine function in alcoholic patients. The difference in synthesis capacity in the caudate however did not reach significance (g = –0.83; z = –1.68; 95% CI: –1.80 to 0.14, p = 0.09).
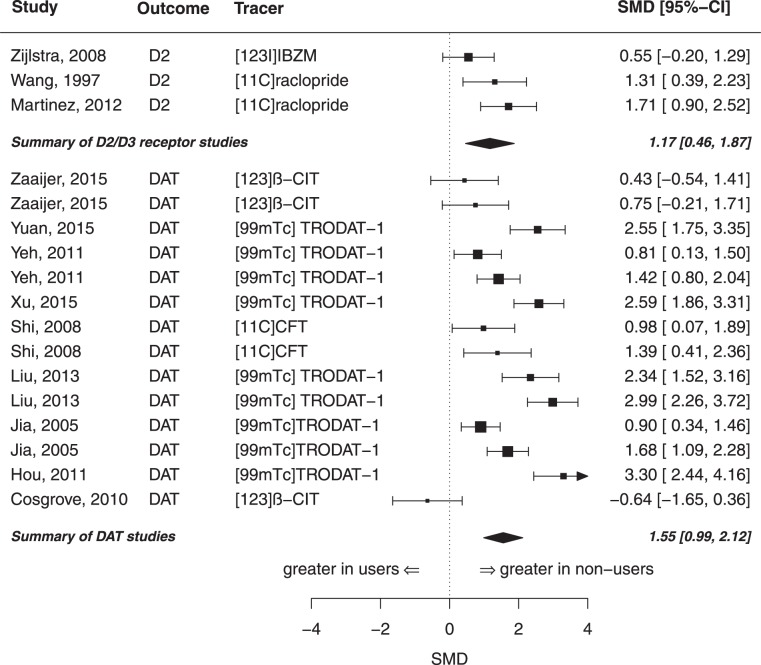
Opioids
Three samples investigated the D2/D3 receptor availability in the whole striatum in a total of N = 39 opioid users and N = 45 controls (see Table S2). The summary effect size for striatal D2/D3 receptor availability between opioid users and controls was highly significant with g = 1.17 (z = 3.24, p < 0.01, I2 = 54.78), indicating a lower receptor availability in opioid users (see Figs. 1 and 4). There was no evidence for publication bias (p = 0.52). The same samples were included in the meta-analysis of the striatal subregions caudate and putamen. There was a significant difference between opioid users and controls in the receptor availability in the caudate (see Fig. 3) with an effect size of g = 1.07 (z = 4.58, p < 0.001, I2 = 0.00). Likewise, opioid users also had a lower D2/D3 receptor availability in the putamen, but this difference slightly failed statistical significance (g = 1.00; z = 1.96, p = 0.05, I2 = 77.86).
Fig. 4.
Forest plot of meta-analysis of striatal dopaminergic function in opioid users
A total of 14 samples comprising N = 253 opioid users and N = 164 controls met the inclusion criteria for the meta-analysis of DAT in the whole striatum (see Table S2). There was a significant difference of striatal DAT availability between opioid users and controls with an estimated effect size of g = 1.55 (z = 5.39, p = < 0.001, I2 = 86.71), indicating lower DAT availability in opioid users (see Figs. 1, 4). There was no evidence for a publication bias (p = 0.29). Moderator analyses showed a decreasing effect size with increasing age (p = 0.03, see Figure S3). The variables year of publication and mean days of abstinence did not have an effect (p > 0.05). There were seven samples comprising N = 116 opioid users and N = 76 controls for the meta-analysis of DAT availability in the subregion of nucleus caudate. The summary effect size for DAT availability in the caudate between opioid users and controls was significant with g = 1.65 (z = 4.67, p < 0.001, I2 = 77.56), see Fig. 3. However, the Egger’s test revealed a potential publication bias (p < 0.001). Meta-analysis of the putamen including the same samples indicated a significantly lower DAT availability in opioid users (g = 1.60, z = 5.71, p < 0.001, I2 = 70.26), see Fig. 3. Again, the Egger’s test yielded evidence for a potential publication bias (p < 0.01). There was no study investigating the dopamine synthesis capacity in opioid users in neither the whole striatum, nor in a striatal subregion.
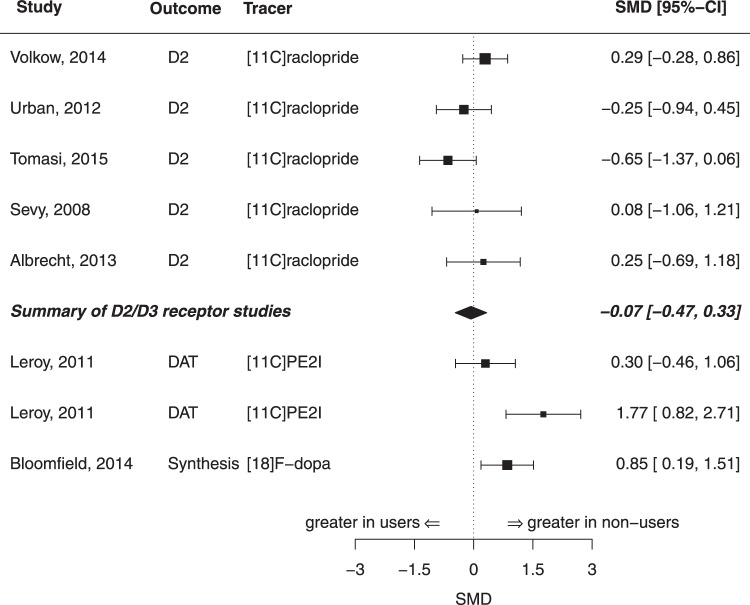
Cannabis
We included five samples comprising N = 67 cannabis users and N = 65 controls in the meta-analysis of D2/D3 receptor availability in the whole striatum (see Table S3). There was no difference in striatal D2/D3 availability between cannabis users and controls (g = 0.07, z = 0.34, p = 0.734, I2 = 26.24, see Figs. 1, 5). There was no evidence for publication bias (p = 0.92). Moderator analyses did not show evidence for an effect of year of publication (p = 0.66) or age (p = 0.86) on the estimated summary effect size. The same five samples were also used for the meta-analysis of D2/D3 receptor availability in the substriatal regions of caudate and putamen (see Fig. 3). There was no significant difference in D2/D3 availability between cannabis users and controls neither in caudate (g = –0.15; z = –0.89, p = 0.37, I2 = 0.00) nor in putamen (g = –0.10; z = –0.46, p = 0.64, I2 = 34.91). In both analyses, there was no evidence for publication bias (p > 0.05).
Fig. 5.
Forest plot of meta-analysis of striatal dopaminergic function in cannabis users
The only sample investigating striatal DAT availability in N = 13 cannabis users had two different control groups: one control group consisted of nonsmokers (N = 14), while in the other group all subjects were smokers (N = 14). The comparison of cannabis users and nonsmoking controls revealed a highly significant effect size of g = 1.77 (z = 3.66; 95% CI: 0.82–2.71, p < 0.001), while the comparison of cannabis users and smokers showed no significant difference (g = 0.30, z = 0.77; 95% CI: –0.46 to 1.06, p = 0.45, see Fig. 5). Similar results were obtained when analysing striatal subregions (caudate and putamen). There was a significant difference between cannabis users and nonsmoking controls in both subregions (caudate: g = 1.76, z = 3.65, 95% CI: 0.81–2.7, p < 0.001; putamen: g = 1.37, z = 3.01, 95% CI: 0.48–2.26, p < 0.01) but no difference between cannabis users and nicotine-smoking controls (caudate: g = 0.27, z = 0.7, 95% CI: –0.49 to 1.03, p = 0.49; putamen: g = 0.24, z = 0.63, 95% CI: –0.51 to 1.0, p= 0.54).
There was one sample studying the dopamine synthesis capacity in the whole striatum in N = 19 cannabis users and N = 19 controls, which revealed a significant difference between users and controls, showing a lower striatal synthesis capacity in cannabis users (g = 0.85, z = –2.51; 95% CI: 0.19–1.51, p < 0.01, see Fig. 5).
Discussion
As far as we know, this is the first meta-analysis on the influence of sedative drugs on dopaminergic functioning in the human brain. Overall, our results indicate marked alterations in sedative drug users and thus provide support for the importance of dopamine in the pathophysiology of drug use and addiction.
The outcome of reduced D2/D3 receptors in opioid and alcohol users relative to non-using controls is in line with previous reports in users of psychostimulants such as cocaine and (meth-) amphetamine [4] and supports the hypothesis of generally lower striatal dopamine receptor availability in substance users [48]. Because most D2/D3 ligands do not differentiate precisely between D2 and D3 receptors or the high- and the low-affinity state of the D2 receptor, our finding of reduced D2/D3 receptor availability in opioid and alcohol users could be attributed to any of those structures. One study investigating alcohol users employed the radioligand [11C]PHNO, a tracer that is to some degree specific for the D3 receptor and for the high-affinity form of the D2 receptor, found no difference between drinkers and controls [16]. Thus, the result of reduced receptor availability in alcohol users predominantly seems to reflect reduced levels of D2 receptors in the low-affinity form. Further studies with different tracers are needed to gain insights regarding the specific receptor changes associated with opioid use.
One potential reason for the reduced receptor availability found in alcohol and opioid users could represent an overexposure of postsynaptic cells with dopamine. Support for this hypothesis comes from animal research demonstrating an increase of extracellular dopamine following the administration of alcohol and opiates [49]. Yet similar results from human studies are rare, but Boileau et al. [50] could prove an alcohol- induced dopamine release in a small sample of healthy humans. Studies examining this effect in humans after opioid intake are lacking. If we follow the results from animal research and the hints from the Boileau [50] study, the increased exposure to dopamine associated with acute alcohol and opioid use can result in a subsequent downregulation of dopamine receptors. This in turn means a reduced activation of reward circuits, which induces an ongoing 'need for activation' and therefore an enhanced motivation for substance use. There are indeed animal and human studies demonstrating a correlation between the availability of dopamine receptors and alcohol intake or craving [51, 52]. Considering the results from our moderator analysis, a reversibility of the downregulated D2 receptors in alcohol users with ongoing abstinence seems to be likely and could therefore protect from further relapse. However, more long-term studies are needed to verify the potential reversibility since the effect of our moderator analysis could be overrated by one study with very long abstinence duration [9]. Nevertheless, evidence for a 'self-protecting mechanism' in the form of increasing D2 receptors also comes from research on non-alcoholic members of an alcoholic family: Volkow et al. [53] found a higher D2 receptor availability in these subjects compared to members of families without a history of alcohol abuse. The author’s interpretation, the idea that high levels of D2 receptors may protect against alcoholism, is therefore in line with our reflections. They also investigated the association between the D2 availability and metabolism in frontal brain regions and suggest that the protective function of a higher D2 availability arises from regulating behavioural and emotional control circuits. This hypothesis also matches results from research about impulsive behaviour (which itself is linked to addiction) and decreased D2 receptor levels [54].
Data about receptor alterations and their behavioural correlates in opioid users are more vague as seen in alcohol studies. Zijlstra et al. [22] found hints for a correlation between D2 availability and acute (but not chronic) craving and between dopamine release and chronic craving. However, Martinez et al. [20] found no correlation between dopamine receptor availability or presynaptic dopamine and the choice to self-administer heroin. Nevertheless, research about dopamine release and long-term use of alcohol or opioids again showed similar results for both substances: Several studies demonstrated a blunted dopamine release after an amphetamine challenge in alcohol-dependent [14, 47] and in heroin-dependent individuals [20] relative to controls. Following this path of reduced extracellular dopamine in permanent users, another downregulation of dopamine transporters would be reasonable as an adaptive process to avoid a loss of dopamine. In fact, we did find a significant difference in DAT availability between opioid users and controls with a large effect size supporting the idea of downregulated DAT. In alcohol users, there was a numerically similar effect size for DAT as for D2/D3 reductions, but due to a larger heterogeneity between studies, the effect size of DAT reduction did not reach significance. Surprisingly, we found a negative moderator effect of abstinence duration on DAT availability in alcohol users, which is in contrast to previous results [55] and to our result of a positive moderator effect of abstinence on receptor availability in alcohol users. Maybe our moderator analysis of DAT and abstinence in alcohol users was blurred by two studies [9, 10] which reported extreme heterogeneous abstinence durations (1 week to 4 years) and from which we only estimated the mean abstinence duration. Concerning alterations in DAT and their clinical implications there are only preliminary results. Shi et al. [24] could not find a correlation between DAT availability and craving in opioid users, and to our knowledge, studies investigating this association in alcohol users are missing. However, Heinz et al. [56] found a negative correlation between dopamine synthesis capacity and alcohol craving but there are conflicting results concerning a potentially altered presynaptic dopamine synthesis capacity in alcohol users. Tiihonen et al. [18] found hints for an elevated dopamine synthesis capacity in alcohol users which could be seen as another compensation for low postsynaptic dopamine function, but Kienast et al. [19] did not find this effect.
Taken together, our results indicate a damping effect of both, chronic alcohol and opioid use on the dopamine system but it remains unclear which specific processes are involved in these alterations. Possible moderating factors we could find are abstinence duration in alcohol users and age in opioid users. Surprisingly, we saw the effect of decreasing DAT with increasing age in opioid and not alcohol users, but since this effect is seen in healthy humans as well [57], it might not be opioid specific. Especially when interpreting the result of lower D2 receptor availability in alcohol and in opioid users, other moderating factors than those we could investigate in our analyses must be kept in mind, since former research on D2 receptor availably in non-users revealed further possible influencing factors as, for example, the influence of social dominance [58], genetic ancestry [59] or sleep deprivation [60].
Considering the clinical implications of the dopaminergic changes, much more research is needed to identify crucial pathways in triggering craving and relapse.
There were only few neuroimaging studies investigating the dopamine system in cannabis users. In the meta-analysis of D2/D3 receptor availability, striatal dopamine receptor availability did not differ between cannabis users and non-users. Volkow et al. [29] suggest that this could result from the fact that cannabis also affects cannabinoid 1 (CB1) receptors, which form heterodimeric receptor complexes with D2 receptors. The stimulation of CB1 receptors could alter the effect of D2 receptor agonists and thus prevent the receptor downregulation seen in other substance users. We could neither see an effect of cannabis on striatal DAT availability, but there were too less studies to conduct a quantitative analysis. It needs further research to identify if the use of cannabis really does not affect striatal DAT availability and if so, which processes differ from that triggered by opioids and alcohol. One potential difference could already represent the missing of a blunted dopamine release in cannabis users [48].
The only study examining the striatal dopamine synthesis capacity in cannabis users did find a significant difference between users and controls with a large effect size. Restrictively, it has to be said that in this study, the cannabis users experienced psychotic-like symptoms when consuming cannabis, so again more research is needed to clarify if this result can be replicated generally in cannabis users. At the current state of research, cannabis seems to have less direct influence on the dopamine system compared with alcohol or opioids.
In sum, this meta-analysis illustrates the importance of future neuroimaging research about associations between drug use and dopaminergic alterations. Future studies can be highly beneficial when including information of possible moderating factors as described earlier, or when comparing different substance users directly (e.g. one study investigating alcohol users, opioid users and non-users) and when investigating long-term effects of drug use abstinence, respectively.
Limitations
In general, the quality of a meta-analysis is to a great degree limited by the quality of the individual studies included. In our present analysis, there was evidence for between-study heterogeneity most likely resulting from differences in the included patient samples as well as differences in the employed methodology. Such heterogeneity might not only represent a limitation but also offers the opportunity for the investigations of moderator effects as we did where possible. For example, we included studies in our meta-analysis where the user group received two scans, because we preferred to use this additional information to gain more insights about the moderation effect of abstinence. We believe that in a meta-analysis, there is not only the risk of bias associated with inclusion criteria that are too lenient but also with criteria that are too restrictive and lead to bias through exclusion of valuable information. Therefore, we decided to include additional information where possible and to analyse them in our moderator analyses. However, we could not analyse the effect of lifetime dose of drug use, the duration of consumption or the pharmacologic treatment status of the patients as very few studies provided sufficient information. Another potentially moderating factor we could not further investigate represents the smoking status. There is evidence that nicotine influences dopaminergic structures as well, especially in interaction with other substances, so these effects need to be further explored [12, 31, 61]. Furthermore, while most studies stated their participants had no 'major' psychiatric comorbidities, this might still play a role in the current analysis as dopaminergic alterations have been reported for, e.g. schizophrenia [62, 63] or bipolar disorder [64]. Finally, as already discussed, many factors affecting especially dopamine receptors could already be seen in nonuser subjects. Considering these influences of impulsivity, social dominance, sleep or genetic ancestry it seems likely that these factors have an even greater impact in substance users. However, most of the included studies did not report these measures, so we were not able to determine their impact on our results.
Apart from differences in patient samples, methodological factors represent another source of heterogeneity. One methodical varying factor lies in the different investigated regions of interest. For example, due to small samples, we had to merge studies with different determination of striatum. Furthermore, the use of different radio ligands and binding potential outcomes can result in heterogeneity, as can factors affecting the measurement of dopaminergic functions in general (e.g., the different reference regions or differences in measures from whole-blood, plasma or tissue).
Finally, drug addiction is a complex neuropsychological process and involves many neurobiological and psychological factors. Other brain regions or different neurotransmitters may also play a role in substance dependency. Especially, the investigation of extrastriatal regions might evolve further insights on interrelated neuronal pathways. Unfortunately, at the present time, there are not enough studies to draw conclusions according to the literature available. Furthermore, it remains unclear if the dopaminergic alterations are really caused by the use of substances or if they already existed before the onset of substance abuse and rather display risk factors.
Conclusion
There is marked evidence for a reduction of D2 receptor availability in alcohol and opioid users, as well as for a reduction of DAT availability in opioid users but no distinct evidence for dopaminergic changes in cannabis users. Insights about the therapeutical relevance of these changes need to be further explored. Abstinence duration seems to influence the dopaminergic alterations caused by chronic alcohol consumption.
Electronic supplementary material
Acknowledgments
Funding and disclosure
FK and LP are employed and funded by the German Federal Ministry of Health (Project ZMVI1-2516DSM216).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0191-9).
References
- 1.United Nations. Office on drugs and crime. World drug report 2017. Vienna: United Nations Publication; 2017. [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 2014;109:1320–33. doi: 10.1111/add.12551. [DOI] [PubMed] [Google Scholar]
- 4.Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:511–9. doi: 10.1001/jamapsychiatry.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–41. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, et al. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999;4:189–91. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- 9.Repo E, Kuikka JT, Bergstrom KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology. 1999;147:314–8. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- 10.Tiihonen J, Kuikka JT, Bergstrom KA, Karhu J, Viinamaki H, Lehtonen J, et al. Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. Eur J Nucl Med. 1997;24:1253–60. doi: 10.1007/s002590050149. [DOI] [PubMed] [Google Scholar]
- 11.Laine TP, Ahonen A, Rasanen P, Tiihonen J. Dopamine transporter density and novelty seeking among alcoholics. J Addict Dis. 2001;20:91–6. doi: 10.1300/J069v20n04_08. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove KP, Krantzler E, Frohlich EB, Stiklus S, Pittman B, Tamagnan GD, et al. Dopamine and serotonin transporter availability during acute alcohol withdrawal: effects of comorbid tobacco smoking. Neuropsychopharmacology. 2009;34:2218–26. doi: 10.1038/npp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, et al. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–72. doi: 10.1016/S0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 16.Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, et al. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–12. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, et al. Striatal dopaminergic D(2) receptor density measured by (123)Iiodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry. 2000;157:127–9. doi: 10.1176/ajp.157.1.127. [DOI] [PubMed] [Google Scholar]
- 18.Tiihonen J, Vilkman H, Rasanen P, Ryynanen OP, Hakko H, Bergman J, et al. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry. 1998;3:156–61. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- 19.Kienast T, Schlagenhauf F, Rapp MA, Wrase J, Daig I, Buchholz HG, et al. Dopamine-modulated aversive emotion processing fails in alcohol-dependent patients. Pharmacopsychiatry. 2013;46:130–6. doi: 10.1055/s-0032-1331747. [DOI] [PubMed] [Google Scholar]
- 20.Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–8. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, et al. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–82. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- 22.Zijlstra F, Booij J, van den Brink W, Franken IHA. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–70. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Hou H, Yin S, Jia S, Hu S, Sun T, Chen Q, et al. Decreased striatal dopamine transporters in codeine-containing cough syrup abusers. Drug Alcohol Depend. 2011;118:148–51. doi: 10.1016/j.drugalcdep.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, Tian J, et al. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008;579:160–6. doi: 10.1016/j.ejphar.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Yeh TL, Chen KC, Lin SH, Lee IH, Chen PS, Yao WJ, et al. Availability of dopamine and serotonin transporters in opioid-dependent users--a two-isotope SPECT study. Psychopharmacology. 2012;220:55–64. doi: 10.1007/s00213-011-2454-6. [DOI] [PubMed] [Google Scholar]
- 26.Yuan J, Liu XD, Han M, Lv RB, Wang YK, Zhang GM, et al. Comparison of striatal dopamine transporter levels in chronic heroin-dependent and methamphetamine-dependent subjects. Addict Biol. 2015;22:229–34. doi: 10.1111/adb.12271. [DOI] [PubMed] [Google Scholar]
- 27.Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, et al. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend. 2013;128:52–7. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology. 2008;197:549–56. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci USA. 2014;111:E3149–56. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuikka JT, Tiihonen J, Bergström KA, Karhu J, Räsänen P, Eronen M. Abnormal structure of human striatal dopamine re-uptake sites in habitually violent alcoholic offenders: a fractal analysis. Neurosci Lett. 1998;253:195–7. doi: 10.1016/S0304-3940(98)00640-5. [DOI] [PubMed] [Google Scholar]
- 31.Leroy C, Karila L, Martinot JL, Lukasiewicz M, Duchesnay E, Comtat C, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict Biol. 2012;17:981–90. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield MAP, Morgan CJA, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–8. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. doi: 10.1037/1082-989X.3.4.486. [DOI] [Google Scholar]
- 34.Raudenbusch S. Analysing effect sizes: random effects models. In: Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 35.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48.
- 36.Core Team R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia SW, Wang W, Liu Y, Wu ZM. Neuroimaging studies of brain corpus striatum changes among heroin-dependent patients treated with herbal medicine, U’finer capsule. Addict Biol. 2005;10:293–7. doi: 10.1080/13556210500222456. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Han M, Liu X, Deng Y, Li Y, Yuan J, et al. Dopamine transporter availability in heroin-dependent subjects and controls: longitudinal changes during abstinence and the effects of Jitai tablets treatment. Psychopharmacology. 2013;230:235–44. doi: 10.1007/s00213-013-3148-z. [DOI] [PubMed] [Google Scholar]
- 40.Rominger A, Cumming P, Xiong G, Koller G, Boning G, Wulff M, et al. 18FFallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol. 2012;17:490–503. doi: 10.1111/j.1369-1600.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- 41.Zaaijer ER, van Dijk L, Bruin K, de, Goudriaan AE, Lammers LA, Koeter MWJ, et al. Effect of extended-release naltrexone on striatal dopamine transporter availability, depression and anhedonia in heroin-dependent patients. Psychopharmacology. 2015;232:2597–607. doi: 10.1007/s00213-015-3891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, et al. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nat Med. 1995;1:654–7. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- 43.Tomasi D, Wang GJ, Volkow ND. Balanced modulation of striatal activation from D2 /D3 receptors in caudate and ventral striatum: disruption in cannabis abusers. Hum Brain Mapp. 2015;36:3154–66. doi: 10.1002/hbm.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohatgi A. WebPlotDigitizer. 2016. http://arohatgi.info/WebPlotDigitizer. Accessed 20 Feb 2017.
- 45.Xu S, Liu Y, Li Y, Deng Y, Huang Y, Yuan J, et al. Longitudinal changes of dopamine transporters in heroin users during abstinence. Psychopharmacology. 2015;232:3391–401. doi: 10.1007/s00213-015-3992-0. [DOI] [PubMed] [Google Scholar]
- 46.Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, Schaffrath S, van Waesberghe J, et al. Opiate-induced dopamine release is modulated by severity of alcohol dependence: an (18)Ffallypride positron emission tomography study. Biol Psychiatry. 2011;70:770–6. doi: 10.1016/j.biopsych.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–12. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 49.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 51.Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–9. doi: 10.1176/ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 52.Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, et al. Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 2005;77:130–9. doi: 10.1016/j.lfs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 53.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 54.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76 Pt B:498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang GJ, Baler R. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: studies with positron emission tomography. Neuropharmacology. 2017;122:175–88. doi: 10.1016/j.neuropharm.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined 18FDOPA and 18FDMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–20. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 57.Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57:36–46. doi: 10.1016/j.neurobiolaging.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–8. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiers CE, Towb PC, Hodgkinson CA, Shen P-H, Freeman C, Miller G, et al. Association of genetic ancestry with striatal dopamine D2/D3 receptor availability. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 60.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, et al. Sleep deprivation decreases binding of 11Craclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiers CE, Cabrera EA, Tomasi D, Wong CT, Demiral ŞB, Kim SW, et al. Striatal dopamine D2/D3 receptor availability varies across smoking status. Neuropsychopharmacology. 2017;42:2325–2332. [DOI] [PMC free article] [PubMed]
- 62.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–9. doi: 10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- 64.Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22:666–79. doi: 10.1038/mp.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cosgrove KP, Tellez-Jacques K, Pittman B, Petrakis I, Baldwin RM, Tamagnan G, et al. Dopamine and serotonin transporter availability in chronic heroin users: a (1)(2)(3)Ibeta-CIT SPECT imaging study. Psychiatry Res. 2010;184:192–5. doi: 10.1016/j.pscychresns.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban NBL, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. Dopamine release in chronic cannabis users: a 11craclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–83. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bortz J, Schuster C. Statistik für human- und Sozialwissenschaftler. 7th ed. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.