Abstract
Objectives
This study was to investigate mean Z-score of BMD of Korean premenopausal women and also to investigate the relationship between BMD and body composition, such as skeletal muscle (SM) mass, body fat mass, and abdominal adiposity among healthy Korean premenopausal women in their forties.
Methods
A total of 2711 premenopausal women in her forties (40–49 years) who had taken dual energy X-ray absorptiometry (DXA) for BMD screening and body composition analyser (InBody J10®) from 2012 to 2013 at health promotion center of Ewha Womans university Mokdong hospital were analyzed retrospectively. Demographic data such as body mass index (BMI), waist circumference (WC), hip circumference (HC), waist hip ratio (WHR), SM mass, body fat mass, and serum lipid profile were included in this study. The Pearson's correlation coefficient (CC) was used to identify co-efficiency between BMD and other parameters.
Results
The mean age was 44.2 ± 4.44 (years) and mean BMI was 22.43 ± 2.99 (kg/m2). Mean Z-score of BMD-lumbar 1–4 (BMD-L) and BMD-femur total hip (BMD-F) was 0.33 ± 1.14 and −0.19 ± 0.85. Mean of BMD-L and BMD-F were 1.18 ± 0.16 (g/cm2) and 0.96 ± 0.12 (g/cm2). Skeletal muscle mass showed a strong significant correlation coefficient (CC) only with BMD-F (CC = 0.13, p-value = 4.78 × 10−11). However serum lipid profile, body fat mass, and WHR did not show significant CC with mean Z-score of BMD-F and BMD-L.
Conclusions
Skeletal muscle mass measured by body composition analyzer of BIA method is a strong correlation factor for BMD especially of hip bone among healthy Korean premenopausal women in their forties.
Keywords: Bone, Hip, Menopause, Skeletal muscle
1. Introduction
Osteopenia and osteoporosis are much more common in postmenopausal women than in premenopausal women. However maintaining optimal bone mineral density (BMD) levels during the premenopausal years is important for reducing the risk of osteoporosis and fractures during the postmenopausal years [1], [2]. The peak bone mass (PBM), which is defined as the amount of bony tissue present at the end of the skeletal maturation [3], is a well known determinant of osteoporotic fracture risk. The greater their peak bone mass, the lower their risk for osteoporosis later in life. The importance of obtaining high PBM is emphasizing in these days because the mean life expectancy is more increasing. It is well known that the PBM usually obtained until in one's early thirties and thereafter demineralization process is continued. Main factors affecting the PBM are genetic factors such as age and race [4]. However, non genetic factors such as nutrition, physical activity, and behavioral factor including smoking also affect the PBM. We can increase the PBM by improving the non-genetic factors, therefore continuous exploration about the way to increase PBM by changing the non-genetic factors is important for improving bony health [5]. Among these, physical activity especially regular weight-bearing exercise which can increase muscle mass is the best way to exercise for the healthy bone. High impact weight bearing exercise is effective on increase of hip BMD and strength [6]. Recent study in premenopausal women also confirmed the impact exercise can improve the BMD [7].
Evidence of the inter-relationship between muscle and bone metabolism is increasing, and the significance of sarcopenia, the age-related loss of skeletal muscle (SM) mass and function, is also more important [8], [9], [10]. Although there are no standard diagnosis criteria, diagnosis is based on low muscle mass, low muscle strength, and/or low physical performance. Sarcopenia and low SM mass increase the risk of physical limitation and subsequent disability; therefore, recent articles report that this condition increases the risk of comorbid conditions [11], [12]. For example, sarcopenia is reported to correlate highly with fragility and increases the risk of falling in the elderly, which is an important risk factor for disability and mortality [13], [14]. Moreover, the loss of SM mass, in particular of its strength, is important based on the evidence that SM mass predicts future mortality in middle-aged, as well as older, adults [14]. What age is reported to have peak SM mass? In terms of total lean body mass, it is reported to begin to decrease early in the third decade of life and shows a sharp decline at 45 years of age and older [15], [16], [17]. The peak SM index occurs in Korean women in their thirties and forties. In Korean females, appendicular skeletal mass (ASM), BMD, and PBM reach peak levels in the 40–49-year-old age group, and then these levels decrease [18], [19]. Therefore, this study aimed to investigate a mean Z-score of the BMD in the lumbar spine L 1–4 (BMD-L) and the femur total hip (BMD-F) in healthy premenopausal women in their forties, as this age was revealed to have peak SM mass. We also aimed to find a relationship between BMD and body composition, such as SM mass, body fat mass, and abdominal adiposity, among healthy Korean women in their forties whose SM index was known to be at the peak level.
2. Materials and methods
This was a retrospective study that analyzed a total of 2711 premenopausal women, aged 40–50 years, who had a dual-energy X-ray (DXA, GE-Lunar Prodigy advance, Lunar software, version 11.20, Madison, WI, USA) at both the lumbar spine and the total hip, from 2012 to 2013 at the Health Promotion Center of Ewha Womans University, Mokdong Hospital. Women who were current smokers and women who drank more than 3 units of alcohol per week were excluded from this study. This study also excluded women who had thyroid disease, rheumatic arthritis, hepatic or renal dysfunction, or malignancy or who were taking medications that could affect BMD. We used demographic data, such as height, weight, BMI, waist circumference (WC), hip circumference (HC), waist-hip ratio (WHR), and serum lipid profile (including total cholesterol, low density lipoprotein [LDL], high-density lipoprotein [HDL], and triglyceride [TG] levels). A body composition analyzer (InBody J10®, Biospace, Seoul, Korea) was used to measure SM mass and total fat mass. All continuous data are presented as mean ± standard deviation. Statistical analyses were performed using R software, version 3.1, and differences were considered significant if p < 0.05. The relationship between BMD, Z-score of BMD-L (L1–L4), BMD-F (femur total hip), and other parameters was analyzed. The Pearson's correlation coefficient (CC) was used to identify the coefficiency between the Z-score of BMD and other parameters. The CC was expressed using conditioning plots-coplots. This study was approved by the Institutional Review Board of EUMC (No. EUMC 2016-03-006).
3. Results
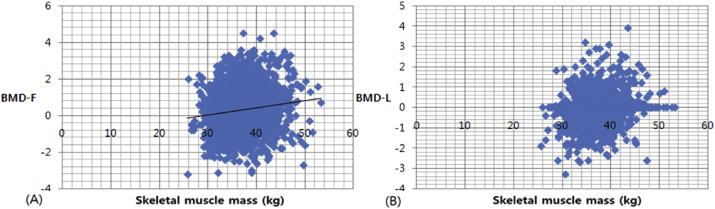
The mean age was 44.20 ± 1.02 years. The mean height, weight, and BMI were 162.82 ± 5.06 cm, 55.28 ± 8.24 kg, and 22.43 ± 2.99 kg/m2, respectively. The mean BMD-L and BMD-F were 1.18 ± 0.16 g/cm2 and 0.96 ± 0.12 g/cm2, respectively. The mean Z-score of BMD-L and BMD-F were 0.33 ± 1.14 and −0.19 ± 0.85, respectively. The mean SM mass and mean body fat mass were 37.17 ± 3.80 kg and 18.12 ± 5.54 kg, respectively. The mean WC and HC were 73.90 ± 8.02 cm and 92.38 ± 5.96 cm, respectively. The mean WHR was 0.80 ± 0.06. The serum lipid profile, including total cholesterol, HDL, LDL, and TG levels, was within the normal limit and did not show a significant CC. In addition, total body fat mass, abdominal obesity, and WHR did not show a significant CC (Table 1). Only the SM mass showed a strong CC with BMD-F (CC = 0.13, p = 4.78 × 10−11). However, SM mass and BMD-L showed only a weak positive correlation (Fig. 1).
Table 1.
Correlation of variables with BMD Femur z-score of Korean premenopausal women in their forties.
| Variables | Values (n = 2711) | CC | p |
|---|---|---|---|
| Age (y) | 44.20 ± 3.02 | 0.002 | 0.301 |
| BMI (kg/m2) | 22.43 ± 2.99 | 0.024 | 0.206 |
| T-Cholesterol (mg/dl) | 88.82 ± 63.23 | −0.031 | 0.097 |
| LDL-C (mg/dl) | 115.58 ± 29.7 | −0.022 | 0.241 |
| HDL-C (mg/dl) | 60.45 ± 12.86 | −0.017 | 0.376 |
| TG (mg/dl) | 106.38 ± 68.74 | 0.003 | 0.891 |
| Skeletal muscle mass (kg) | 37.17 ± 3.8 | 0.126 | 4.781 × 10−11 |
| Body fat mass (kg) | 18.12 ± 5.54 | −0.007 | 0.705 |
| WH ratio | 0.8 ± 0.06 | −0.010 | 0.581 |
Data are mean ± SD. CC, correlation coefficient; BMI, body mass index; TC, total cholesterol; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoproteincholesterol; TG, triglyceride; WH ratio, waist-hip ratio.
Fig. 1.
Scatter plot showing mild correlation between BMD-F(A), BMD-L(B), and skeletal muscle mass. BMD-F: bone mineral density-femur total; BMD-L: bone mineral density-lumbar (Lumbar 1–4).
4. Discussion
We investigated the mean Z-score of the BMD of Korean premenopausal women in their forties. The mean Z-score and BMD of the lumbar spine and total hip were similar to previous reports of Korean women [2], [20], [21]. Usually, the proportion of women with low bone mass is low in premenopausal women, especially young premenopausal women, who are high in estrogenic condition. However, we have to consider the possibility of low bone mass in Korean premenopausal women because of the social trend of women being too thin, and many women are on strict diets. In this study, the results were as expected. There were not many cases that the Z-score of BMD was low enough to fit in the WHO criteria of “below the expected range for age” (i.e., Z-score −2.0 and below). In this study, a small percentage of premenopausal women (28 women in total [1.03%]) was included in these criteria. We also investigated the relationship between BMD and BMI, WHR, SM mass, body fat mass, and serum lipid profile. Our results showed that SM mass was a strong correlation factor for BMD-F among premenopausal Korean women in their forties. This result is concordant with the recent study on Korean premenopausal women demonstrating that low body muscle mass is associated with low BMD [21]. However, BMI, WHR, body fat mass, and serum lipid profile were not correlated with BMD in premenopausal women in this study. This is concordant with a recent report on the relationship between obesity and BMD [22]. Lean mass, not fat mass, was an independent associated factor of aerial BMD in both men and women [22]. Although BMI was not correlated with BMD in our study, low BMI and low vitamin D levels were reported to be correlated with the Z-score of BMD-L, but they were not correlated with the Z-score of BMD-F [21]. In a recent article regarding PBM in Korean mother–daughter pairs, BMI also appeared to be related to BMD [2]. The study population was healthy premenopausal women who were not obese or skinny, considering the mean BMI was 22.43 ± 2.99 kg/m2. In terms of central obesity, when we consider the WHR (0.8 ± 0.20), which is considered one of the most reliable parameters of abdominal adiposity, and normal range is considered below 0.85 for a woman [23], the study population did not have central obesity. We chose the age group of 40–49 years old because Korean women show the peak SM index in their forties [18]. This is concordant with studies reporting that SM mass gradually declines beginning at approximately 45 years of age [15], [16], [17]. The strength of this study is as follows: First, this was a relatively large-scale study with over 2700 premenopausal women in a single institution. Second, in terms of examination conditions, we analyzed data obtained from a single health promotion center where the BMD tests were performed in a constantly stable condition. The same machine comparing National Health and Nutrition Examination Survey (KNHANES) data in which health examination test including DXA was performed in a big health examination bus, a relatively less stable condition [2], [21]. Third, the SM mass was measured by a body composition analyzer, InBody J10®, which is a bioelectrical impedance analysis (BIA) method used to calculate body composition quickly by measuring tissue conductivity. Of course, a body composition analyzer of a BIA method is not considered a standard test for measuring SM mass, and some reported that the limitation of a BIA method for the standard error is approximately 9% [11], [24]. Magnetic resonance imaging (MRI) and DXA are considered precise and reliable measurements of SM [6], [11], [23] and are considered reference methods for measuring SM mass in vivo [16], [21] In addition, these measurements are ideally suited for measuring whole body SM mass [24]. However, there was a report stating that a body composition analyzer of a BIA method is strongly correlated to MRI-measured SM mass; therefore, a BIA method is a valid method for estimating SM mass within Caucasian, Hispanic, and African-American subjects [11], [24]. Additional studies in Asian populations are still needed. The limitations of this study are the lack of information of physical activity and nutritional status and the use of total body muscle mass, as muscle mass was not classified into different body regions. Clinicians can counsel women who have low SM mass, explaining that their BMD-F may be low as well. In turn, the women can make extra efforts to increase their BMD by being informed of the non-genetic factors of BMD and the ways to increase BMD in their daily lives. If we can get the same results with this study from a large-scale study, then we might estimate the BMD-F in healthy premenopausal women by performing a BIA method without undergoing DXA testing, which would eliminate the risk of exposure to radiation. In conclusion, the mean Z-scores of BMD-L and BMD-F adjusted for BMI among Korean premenopausal women in their forties were 0.33 ± 1.14 and −0.19 ± 0.85, respectively. SM mass measured by an easily accessible body composition analyzer with a BIA method is a strong correlation factor for the Z-score of BMD-F among premenopausal Korean women.
Conflicts of interest
The authors have no financial conflicts of interest.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Kelley G.A., Kelley K.S., Kohrt W.M. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2013:741639. doi: 10.1155/2013/741639. Epub 2013 Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K.M., Kim Y.J., Choi S.H., Lim S., Moon J.H., Kim J.H. The effects of body mass index on the hereditary influences that determine peak bone mass in mother-daughter pairs (KNHANES V) Osteoporos Int. 2016;27:2057–2064. doi: 10.1007/s00198-016-3487-9. [DOI] [PubMed] [Google Scholar]
- 3.Bonjour J.P., Theintz G., Law F., Slosman D., Rizzoli R. Peak bone mass. Osteoporos Int. 1994;4(Suppl 1):7–13. doi: 10.1007/BF01623429. [DOI] [PubMed] [Google Scholar]
- 4.Riggs B.L., Melton L.J., III Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 5.Weaver C.M., Gordon C.M., Janz K.F., Kalkwarf H.J., Lappe J.M., Lewis R. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A1 Vainionpää, Korpelainen R., Leppäluoto J., Jämsä T. Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int. 2005;16:191–197. doi: 10.1007/s00198-004-1659-5. [DOI] [PubMed] [Google Scholar]
- 7.Greenway K.G., Walkley J.W., Rich P.A. Impact exercise and bone density in premenopausal women with below average bone density for age. Eur J Appl Physiol. 2015;115:2457–2469. doi: 10.1007/s00421-015-3225-6. [DOI] [PubMed] [Google Scholar]
- 8.Frontera W.R., Hughes V.A., Lutz K.J., Evans W.J. A cross-sectional study of muscle strength and mass in 45- to 78yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 9.Metter E.J., Lynch N., Conwit R., Lindle R., Tobin J., Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–B218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 10.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 11.Delmonico M.J., Harris T.B., Lee J.S., Visser M., Nevitt M., Kritchevsky S.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Cooper R., Kuh D., Cooper C., Gale C.R., Lawlor D.A., Matthews F. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J. Frailty in older adults: evidence for a pheno-type. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 16.Kehayias J.J., Fiatarone M.A., Zhuang H., Roubenoff R. Total body potassium and body fat: relevance to aging. Am J Clin Nutr. 1997;66:904–910. doi: 10.1093/ajcn/66.4.904. [DOI] [PubMed] [Google Scholar]
- 17.Lexell J., Downham D., Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci. 1986;72:211–222. doi: 10.1016/0022-510x(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 18.Kwon H.J., Ha Y.C., Park H.M. The reference value of skeletal muscle mass index for defining the sarcopenia of women in Korea. J Bone Metab. 2015;22:71–75. doi: 10.11005/jbm.2015.22.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon S. Relationship of lean body mass with bone mass and bone mineral density in the general Korean population. Endocrine. 2014;47:234–243. doi: 10.1007/s12020-013-0160-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee D.H., Jung K.Y., Hong A.R., Kim J.H., Kim K.M., Shin C.S. Femoral geometry, bone mineral density, and the risk of hip fracture in premenopausal women a case control study. BMC Musculoskelet Disord. 2016;17:42. doi: 10.1186/s12891-016-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park K.H., Lim J.S., Kim K.M., Rhee Y., Lim S.K. Z-score discordance and contributing factors in healthy premenopausal women with low bone mineral density: the Korean National Health and Nutrition Examination Survey 2008–9. J Bone Miner Metab. 2015 Oct 7 doi: 10.1007/s00774-015-0715-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Maïmoun L., Mura T., Leprieur E., Avignon A., Mariano-Goulart D., Sultan A. Impact of obesity on bone mass throughout adult life: influence of gender and severity of obesity. Bone. 2015;90:23–30. doi: 10.1016/j.bone.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 23.O'Dea K., Walker K., Colagiuri S., Hepburn A., Holt P., Colagiuri R. Diabetes Australia and NHMRC; Canberra: 2002. Evidence based guidelines for type 2 diabetes mellitus. Primary prevention. [Google Scholar]
- 24.Janssen I., Heymsfield S.B., Baumgartner R.N., Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]