Abstract
Inhibins are gonadal hormones that act on pituitary gonadotrope cells to suppress FSH synthesis and secretion. Inhibin A and B are heterodimers of the inhibin ⍺-subunit disulfide-linked to one of two inhibin β-subunits. Homodimers or heterodimers of the inhibin β-subunits form the activins, which stimulate FSH production. Activins signal through complexes of type I and II receptor serine/threonine kinases to increase transcription of the FSHβ subunit gene. According to in vitro observations, inhibins impair FSH synthesis by competitively binding to activin type II receptors, particularly in the presence of the TGFβ type III receptor (TGFBR3, or betaglycan). The role of TGFBR3 in inhibin action in vivo has not been determined. Here, we ablated Tgfbr3 specifically in murine gonadotropes. Conditional knockout females were supra-fertile, exhibiting enhanced folliculogenesis, numbers of ovulated eggs per cycle, and litter sizes relative to control mice. Despite these phenotypes, FSH levels appeared to be unaltered in knockout mice, and the mechanisms underlying their enhanced fertility remain unexplained. Inhibin B is the predominant form of the hormone in males and in females during most stages of the estrous cycle. Remarkably, inhibin A, but not inhibin B, suppression of FSH synthesis was impaired in cultured pituitaries of knockout mice, which may explain the absence of discernible changes in FSH levels in vivo. Collectively, these data challenge current dogma by demonstrating that TGFBR3 (betaglycan) functions as an inhibin A, but not an inhibin B, coreceptor in gonadotrope cells in vivo. Mechanisms of inhibin B action merit further investigation.
The term inhibin was coined in the early 1930s to describe a hypothetical hormone from the testicular seminiferous epithelium that regulates the morphology of cells in the anterior pituitary gland (1). Four decades later, inhibin-like activity was discovered in ovarian follicular fluid and shown to selectively inhibit FSH secretion from pituitary gonadotrope cells while having no effects on the related LH (2). It took another decade before two forms of inhibin (A and B) were finally purified (3–7). Inhibins A and B are dynamically and differentially secreted from granulosa (and luteal) cells of the ovary across female reproductive cycles. In contrast, adult males of most species predominately secrete inhibin B from testicular Sertoli cells (8–11).
Biochemical and molecular characterization of the inhibins revealed that they were heterodimeric TGFβ superfamily ligands composed of the inhibin α-subunit disulfide-linked to one of two inhibin β-subunits (βA in inhibin A or βB in inhibin B). Shortly thereafter, it was discovered that inhibin β-subunits homodimerize or heterodimerize to form what we now call the activins: activin A (βA-βA), activin B (βB-βB), or activin AB (βA-βB) (12–14). In marked contrast to the inhibins, activins potently and selectively stimulate FSH synthesis and secretion without affecting LH. Research over the last 30+ years has unveiled mechanisms of activin and inhibin action in gonadotropes and other cells.
Like other TGFβ ligands, activins signal via complexes of type I and II serine/threonine kinase receptors (15). The type II receptors bind ligand and trans-phosphorylate the type I receptors, which in turn phosphorylate the intracellular signaling protein homolog of Drosophila mothers against decapentaplegic 3 (SMAD3). SMAD3 associates with SMAD4 and accumulates in the nucleus. SMAD3/4 complexes partner with forkhead box L2 (FOXL2) and bind to the proximal promoter of the FSHβ subunit gene (Fshb) (16, 17). Fshb transcription is the rate-limiting step in dimeric FSH synthesis. Mechanisms of inhibin action are less well described, particularly in vivo.
According to in vitro models, inhibins do not generate intracellular signals, but rather antagonize activin signaling by competitively binding to activin type II receptors (18, 19). Because inhibins bind to type II receptors with at least 10-fold lower affinity than activins (20–22), effective antagonism should require inhibins to be in large excess relative to activins. However, inhibins robustly block activin action when present at equimolar or even lower concentrations (23–25). It was subsequently discovered that the TGFβ type III receptor (TGFBR3, also known as betaglycan) markedly increases the affinity of inhibins for activin type II receptors in vitro (21, 26). In fact, in the presence of TGFBR3, inhibin A blocks activin A binding to the activin type IIA receptor (21, 27–29), providing a candidate mechanism for inhibins to potently suppress FSH production by gonadotrope cells.
Nonetheless, prior to the current study, it was unclear whether inhibins act via TGFBR3 to suppress FSH in vivo. Tgfbr3 knockout mice die during embryonic development because of heart and liver defects, precluding their use for studies of inhibin action in adulthood (30). To circumvent this problem, we developed a conditional (floxed) Tgfbr3 mouse model, enabling us to ablate the protein selectively in gonadotropes using the Cre/lox system. The resulting animals were viable and fertile. In fact, knockout females produced larger litters than controls, though FSH levels were surprisingly unaltered. The data further show that TGFBR3 mediates the actions of inhibin A, but not inhibin B, in gonadotropes, which may in part explain the absence of elevated FSH levels in the conditional knockout mice.
Materials and Methods
Generation of floxed Tgfbr3 mice
Mice harboring a floxed Tgfbr3 allele were produced by conventional gene targeting in murine embryonic stem cells using standard techniques. Briefly, a loxP site was introduced ∼450 bp upstream of exon 2 (in the first intron), and a floxed neomycin (Neo) positive selection cassette was introduced ∼490 bp downstream of exon 2 (in intron 2; see Fig. 1A). The targeting construct was electroporated into J1 embryonic stem cells, and correctly targeted clones were injected into C57BL6 blastocysts. Resulting chimeric males were crossed to C57BL6 females, and agouti pups were genotyped. Mice heterozygous for the modified allele (Tgfbr3fx-Neo/+) were crossed with the EIIa-Cre deleter strain to excise the floxed Neo cassette. We identified mice in which Neo was removed, but the floxed exon 2 remained intact. These mice (Tgfbr3tm1.1Hlin; hereafter Tgfbr3fx) were then crossed with wild-type C57BL6 mice to segregate the floxed Tgfbr3 and Cre alleles. Tgfbr3fx/+ males and females were intercrossed to generate Tgfbr3fx/fx homozygotes. The latter are overtly normal and fertile, suggesting that introduction of the loxP sites does not affect gene function.
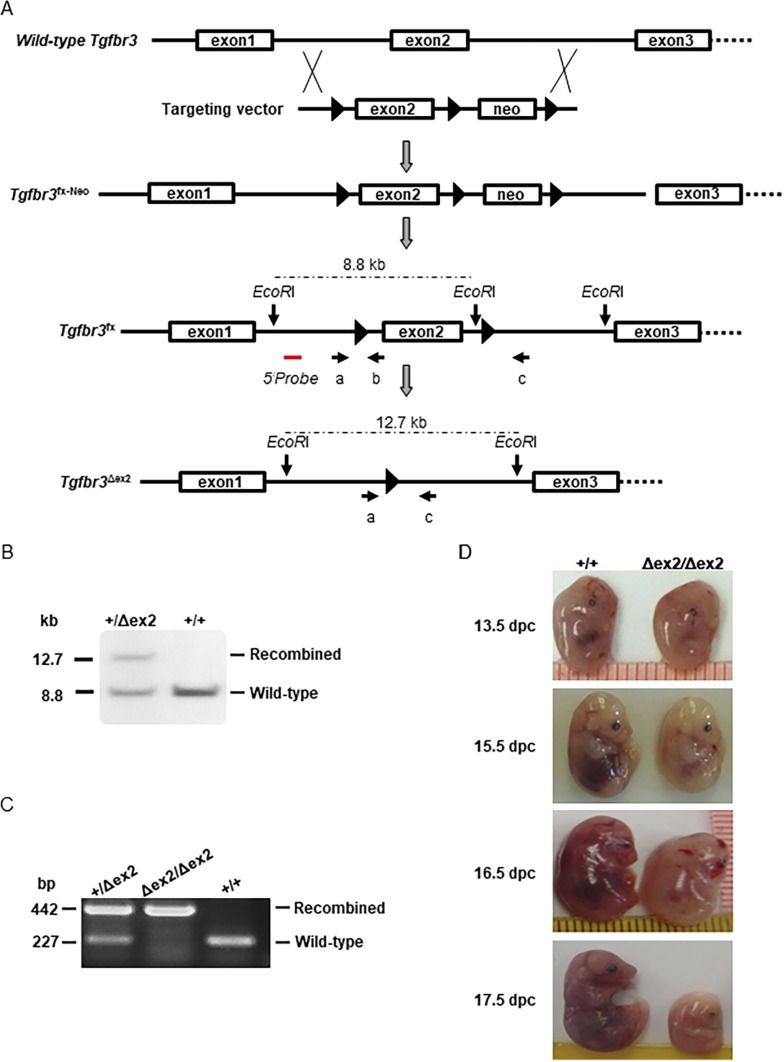
Figure 1.
Generation and validation of floxed Tgfbr3 mice. (A) Schematic representation shows the strategy used to generate the conditional Tgfbr3 allele. LoxP sites are pictured as black triangles, and exons are shown as boxes. The red line indicates the approximate position of the Southern blot probe used in (B). Primers used in PCR in (C) are shown as arrows and labeled a, b, and c. Note that exons, introns, the Southern probe, and primers are not drawn to scale. (B) Southern blot analysis of wild-type (+) and recombined (Δex2) Tgfbr3 alleles is shown. Genomic DNA was digested with EcoRI. The approximate locations of the EcoRI sites and restriction fragment sizes are indicated in (A). (C) Examples show PCR genotyping of heterozygous (Tgfbr3+/∆ex2), homozygous (Tgfbr3∆ex2/∆ex2), and wild-type (Tgfbr3+/+) mice. The wild-type allele was detected with primers a and b (note that the loxP site is absent in this allele but present in the floxed allele). The recombined allele was detected with primers a and c. (D) Examples show wild-type (+/+) and homozygous knockout (∆ex2/∆ex2) embryos at the indicated ages. dpc, day post coitus; neo, neomycin selection cassette.
Global Tgfbr3 knockout mice
To generate mice with a global deletion of Tgfbr3 exon 2, Tgfbr3fx/fx animals were again crossed with EIIa-Cre mice [FVB/N-Tg(EIIa-Cre) C5379Lmgd/J; 003724; The Jackson Laboratory] to obtain heterozygous (Tgfbr3+/Δex2;EIIa-Cre/+) progeny. Tgfbr3+/Δex2;EIIa-Cre/+ mice were then crossed with wild-type C57BL6 mice to obtain Tgfbr3+/Δex2 offspring without the EIIa-Cre transgene. Finally, Tgfbr3+/Δex2 males and females were crossed to generate the following three genotypes: Tgfbr3+/+, Tgfbr3+/Δex2, and Tgfbr3Δex2/Δex2. Genotyping was performed on genomic DNA using the primers in Table 1.
Table 1.
Genotyping and qPCR Primers
|
Genotype
| |
|---|---|
| Tgfbr3 | 5′ – 3′ |
| Forward | TGATCTTAGTGGTAACCTCGCC |
| Reverse | CTAGCATGACAGGAATGTAC |
| Recombined | TTAGGTCGGTGCTGTCCTTGTT |
| Gnrhr GRIC | |
| Forward | GGACATGTTCAGGGATCGCCAGGC |
| Reverse | GCATAACCAGTGAAACAGCATTGCTG |
| Wild-type allele | |
| Forward | GAACTACAGCTGAATCAGTC |
| Reverse | CTAACAACAAACTCTGTACA |
| qPCR | |
|---|---|
| Rpl19 | |
| Forward | CGGGAATCCAAGAAGATTGA |
| Reverse | TTCAGCTTGTGGATGTGCTC |
| Fshb | |
| Forward | GTGCGGGCTACTGCTACACT |
| Reverse | CAGGCAATCTTACGGTCTCG |
| Cga | |
| Forward | TCCCTCAAAAAGTCCAQGAGC |
| Reverse | GAAGAGAATGAAGAATATGCAG |
| Lhb | |
| Forward | AGCAGCCGGCAGTACTCGGA |
| Reverse | ACTGTGCCGGCCTGTCAACG |
| Gnrhr | |
| Forward | CACGGGTTTAGGAAAGCAAA |
| Reverse | TTCGCTACCTCCTTTGTCGT |
Abbreviation: qPCR, quantitative PCR.
Conditional Tgfbr3 knockout mice
Gonadotrope-specific Tgfbr3 knockout mice were generated using Tgfbr3fx/fx and GnrhrGRIC/GRIC (31) mice. The GnrhrGRIC allele was always introduced from the female because of Cre activity in the male germline in this strain (32). First, Tgfbr3fx/fx males were crossed with GnrhrGRIC/GRIC females, generating Tgfbr3fx/+;GnrhrGRIC/+ pups. Because the Tgfbr3 and Gnrhr genes are linked on chromosome 5, the Tgfbr3fx and GnrhrGRIC alleles were in trans (on sister chromatids) in these mice. To generate conditional knockout mice (Tgfbr3fx/fx;GnrhrGRIC/+), the Tgfbr3fx and GnrhrGRIC alleles first had to be positioned in cis (on the same chromatid). This was accomplished via meiotic recombination. Tgfbr3fx/+;GnrhrGRIC/+ (trans) females were crossed to wild-type (Tgfbr3+/+;Gnrhr+/+) males. In offspring that inherited both the Tgfbr3fx and GnrhrGRIC alleles (Tgfbr3fx/+;GnrhrGRIC/+), Tgfbr3fx and GnrhrGRIC were by definition in cis. We observed cosegregation of the modified or wild-type alleles in seven of 92 genotyped pups, indicating that the Tgfbr3 and Gnrhr genes are separated by approximately 7.6 cM (close to the predicted 8.34 cM). Subsequently, Tgfbr3fx/+;GnrhrGRIC/+ (cis) females were crossed with Tgfbr3fx/fx;Gnrhr+/+ males to generate Tgfbr3fx/fx;GnrhrGRIC/+ mice. Tgfbr3fx/fx;GnrhrGRIC/+ females were then bred to Tgfbr3fx/fx;Gnrhr+/+ males to produce Tgfbr3fx/fx;Gnrhr+/+ (control) and Tgfbr3fx/fx;GnrhrGRIC/+ (experimental or T3cKO) littermates in a 1:1 ratio. Genotypes were determined by PCR using the primers listed in Table 1. All animal work was performed in accordance with institutional and federal guidelines and approved by the McGill University and Goodman Cancer Centre Facility Animal Care Committee (protocol 5204).
Southern blotting
Ten micrograms of tail genomic DNA was digested with EcoRI (R6018; Promega, Madison, WI) and separated on 0.8% agarose gel. DNA was denatured with an alkaline solution (pH, 12.0) containing 1.5 M NaCl (SOD001; Bioshop, Burlington, ON, Canada) and 0.5 M NaOH (SHY700; Bioshop) at room temperature for 45 minutes and then neutralized with 1.5 M NaCl in 1 M Tris (TRS001; Bioshop) buffer (pH, 7.4) for 30 minutes to prevent rehybridization. DNA was transferred onto a positively charged nylon membrane (RN303B; Amersham, GE Healthcare, Chicago, IL) for 18 hours by capillary action with 20× concentrated saline sodium citrate (SSC795; Bioshop) buffer. DNA was crosslinked to the membrane with UV light (Ultraviolet Stratalinker 800; Stratagene, San Diego, CA). A double-stranded probe was generated by PCR using primers GGGGCCCTTCTGTAGAAATC and CTGCATGCCTTTTCCTCATT and genomic DNA from a wild-type Tgfbr3 allele as a template. The probe was denatured at 95°C for 2 minutes and immediately cooled on ice; 110 ng of probe was radioactively labeled using the Prime-a-Gene® Labeling system (U115A and M220A; Promega) and [α32P]dATP (BLU012H250UC; PerkinElmer, Woodbridge, ON, Canada), [α32P]dCTP (NEG013100UC; PerkinElmer), dGTP, and dTTP (U1330; Promega) on a 37°C hot plate for 1 hour. The membrane was prehybridized in 50% formamide (47670; Fluka, Fisher Scientific, Toronto, ON, Canada), 1 M NaCl, 1% SDS (SDS001; Bioshop), 10% dextran sulfate (S4030; EMD Millipore, Etobicoke, ON, Canada), and 2 mg of salmon sperm DNA (M3011; Promega) at 65°C for 1 hour. Hybridizaton was performed overnight in the same buffer with 88 ng probe at 42°C overnight. The membrane was washed with 1× saline sodium citrate buffer plus 0.1% SDS at room temperature. The bands were visualized on X-ray film by autoradiography (3.5-day exposure at −80°C with intensifying screen). All radioactive work was performed under McGill Internal Radioisotope Permit (R-00314).
Immunofluorescence
Paraformaldehyde-fixed (158127; Sigma, Markham, ON, Canada) and paraffin-embedded pituitary sections were prepared, and immunofluorescence was performed as previously described (33). Primary antibodies used were anti-FSHβ (NIDDK AFP7798-1289P; 1:500, raised in rabbit) and anti-TGFBR3 [AF-242-PB; R&D; RRID: AB_354417 (34); 5 μg/mL, raised in goat]. Secondary antibodies used were Alexa fluor 594-conjugated anti-rabbit [A21207; Invitrogen; RRID: AB_141637 (35); 1:500 raised in donkey], biotinylated anti-goat [BA-9500; Vector, Burlingame, CA; RRID: AB_2336123 (36); 1:150, raised in horse], and Alexa fluor 488-conjugated Streptavidin [S-11223; Invitrogen; RRID: AB_2336881 (37); 1:500]. Images were acquired on a Zeiss LSM 510 confocal microscope.
Primary pituitary cultures
Primary pituitary cultures were prepared as previously described (33, 38). In the in vitro recombination experiments, pituitary cells from an equal number of male and female Tgfbr3fx/fx mice were seeded at a density of 400,000 cells per well in 48-well plates. Cells were cultured for 24 hours after plating and then transduced with adenoviruses expressing GFP (Ad-GFP) or Cre-IRES-GFP (Ad-Cre) (Baylor College of Medicine Vector Development Laboratory, Houston, TX) at a multiplicity of infection of 600 in 10% fetal bovine serum (FBS)–containing media (10438026; Life Technologies) for 24 hours. In a second set of experiments, pituitaries were collected from control and T3cKO females and seeded at the same density as described previously in culture medium supplemented with 10% FBS. In all culture experiments, the cells were further cultured for 24 hours in medium with 2% FBS containing recombinant inhibin A (generously provided by Dr. Teresa Woodruff, Northwestern University, Chicago, IL) or inhibin B (677-IB/CF; R&D Systems, Minneapolis, MN), as indicated in the figure legends. Cells were harvested with 0.25% trypsin, and RNA was extracted using the Total RNA Mini Kit (FA32808-PS; Geneaid, New Taipei City, Taiwan) following the manufacturer’s instructions. In some experiments, culture medium was collected for measurement of secreted FSH (see the following section).
Inhibin preparation and injection
Recombinant inhibin A was prepared as described (39) and was kept at −80°C before use. Inhibin A was diluted in 50 mM sodium acetate (pH, 5.0) supplemented with 0.1% BSA to make a working dilution of 1 µg/100 µL. Ten-week-old control and T3cKO males were IV (tail vein) injected with 50 µg/kg body weight of inhibin A or equivalent volume of vehicle. Before injection, a small blood sample (∼50 µL) was collected from each mouse via submandibular venipuncture. Six hours after inhibin A injection, terminal blood samples were collected via cardiac puncture. All blood samples were allowed to clot at room temperature, and serum was collected following centrifugation. Serum FSH levels were compared before and after inhibin A administration in the same animals.
Inhibin B was prepared according to the manufacturer’s instructions. Briefly, 100 ng of inhibin B was dissolved in ice-cold PBS supplemented with 0.1% BSA and stored at −80°C. We had enough inhibin B for only in vitro experiments.
Anti-inhibin serum preparation and injection
Anti-inhibin serum (AIS) was purchased from Central Research (Tokyo, Japan; Lot AIG890208L3). Lyophilized AIS was dissolved in sterile H2O and stored at −80°C. One hundred milligrams of AIS (in 0.1 mL) was IP injected in control and T3cKO males and females (diestrus 7 am). A small amount of blood was collected before and 11 hours after AIS injection via submandibular venipuncture.
Blood sampling in females
Estrous cyclicity was monitored via vaginal cytology for at least 2 weeks before blood samples were collected from 8- to 12-week-old females on diestrus (3 pm), proestrus (10 am and 6 pm), and estrus (3, 7, 9, and 11 am and 12 pm) via submandibular venipuncture or the tail vein.
GnRH agonist and antagonist injections
GnRH (L8008; Sigma) was dissolved in saline. Blood samples were collected through the tail vein before and after GnRH injections (subcutaneous). Cetrorelix acetate (C5249; Sigma) was dissolved in sterile water, and 500 µg/kg body weight of Cetrorelix acetate was subcutaneously injected in control and T3cKO males.
Hormone analyses
In both males and females, blood samples were collected via submandibular, tail vein, or cardiac puncture as specified in each experiment. Serum was obtained as described previously (16). Serum FSH and LH levels were assessed by multiplex ELISA [performed at the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia (UVa)] or Luminex assay (FSH only; MPTMAG-49K; Millipore), following manufacturer’s instructions, or by an in-house sandwich LH ELISA as described previously (16). The specific assays used are specified in the figure legends. Pituitary homogenates were prepared as described previously (33) from males and females (on estrus morning at ∼7 am). Pituitary FSH content and secreted FSH levels in culture medium were measured by RIA at the Ligand Assay and Analysis Core at UVa. The reportable ranges were 2.9 to 40.0 ng/mL for pituitary FSH content and 1.7 to 60.0 ng/mL for FSH in culture medium; intra-assay coefficients of variation (CVs) were both <20%.
Reportable ranges for serum FSH levels assessed by multiplex ELISA were 2.4 to 300.0 ng/mL, intra-assay CV <5%; for serum LH levels assessed by multiplex ELISA, ranges were 0.24 to 30.0 ng/mL, intra-assay CV <10%; for serum FSH levels assessed by Luminex, ranges were 24 to 100,000 pg/m, intra-assay CV <10%; and for serum LH levels assessed by in-house ELISA, ranges were 0.117 to 30 ng/mL, intra-assay CV <10%.
Reproductive organ analyses
Reproductive organs were collected from 10-week-old males and females (metestrus/diestrus afternoon) and weighed on a precision balance. Reproductive organ weight was normalized to the body weight assessed after euthanasia.
Quantitative RT-PCR analysis
Pituitaries were isolated from 10-week-old control and T3cKO mice, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. Females were euthanized at different estrous cycle stages and times, as indicated in the figure legends. All pituitaries were homogenized in 500 μL TRIzol reagent (15596026; Life Technologies), and total RNA was extracted following manufacturer’s instructions. RNA concentration was determined by NanoDrop. Next, 200 ng of RNA per sample was reverse transcribed into cDNA using Moloney murine leukemia virus reverse transcription (172807; Promega) and random hexamer primers (184865; Promega) in a final volume of 40 μL. Then 2 μL of cDNA was used as a template for quantitative PCR analysis on a Corbett Rotorgene 600 instrument (Corbett Life Science) using EvaGreen reagent (ABM Mastermix-S; Diamed, Mississauga, ON, Canada) and the primers listed in Table 1. Relative gene expression, normalized to the housekeeping gene ribosomal protein L19 (Rpl19), was determined using the 2−ΔΔCT method (40). All oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA).
Puberty onset, estrous cyclicity, and fertility assessment
Starting at 7 weeks of age, estrous cyclicity was assessed daily in the morning (∼10 am) by collecting vaginal cells with a cotton swab dampened with sterile saline. The cells were smeared on a glass slide, stained with 0.1% methyl blue, and examined by light microscopy. Staging was assessed according to published guidelines (41, 42). One cycle was defined as the sequential appearance of cells characteristic of all estrous cycle stages, regardless of the number of days spent in each stage. To assess fertility, 10-week-old female T3cKO and control mice were paired with C57BL6 male mice (000664; Charles River, Senneville, QC, Canada), for a period of 6 months. Starting from 20 days after pairing, the cages were inspected daily for the presence of newborn mice. As soon as a litter was present, pups were carefully counted and put back into the cage. Pups were separated from the mother at postnatal day 15 to avoid interfering with the following litter.
Natural ovulation
Ten-week-old control and T3cKO females were paired with age-matched wild-type C57BL6 males. Females were inspected daily at 7 am until a vaginal plug was visualized. Females were then euthanized, and cumulus-oocyte complexes (COCs) were harvested in PBS from ampullae of the oviducts on both sides. Cumulus cells were dissociated from the oocytes by incubating with 0.5 mg/mL hyaluronidase (H3884; Sigma) for 10 minutes at 37°C. Total oocyte numbers from each female were counted under an inverted microscope.
Superovulation
Superovulation was performed in juvenile (postnatal days 25 to 28) control and T3cKO females as described previously (16). Briefly, 5 IU of pregnant mare’s serum gonadotropin (eCG; G4877; Sigma) was IP injected in females at 5 pm. Forty-eight hours later, the mice were IP injected with 5 IU of human chorionic gonadotropin (C1063; Sigma). Fourteen to 16 hours later, the mice were euthanized, and COCs were retrieved and counted.
Uterine implantation site assessment
Estrous cyclicity was recorded in both control and T3cKO females for 4 or 5 cycles. On the next proestrus afternoon, control and T3cKO females were paired with wild-type males. The following morning, plug formation was inspected as described previously. The day of plug formation was considered 0.5 day post coitus (dpc). On 5.5 dpc, females were IV injected with 100 µL of 1% Evans blue dye (E2129; Sigma) prepared in saline. Five minutes after injection, females were euthanized, uteri were isolated, and implantation sites were counted.
Histology
Ovarian samples were isolated from 10-week-old control and T3cKO females and fixed in 10% formalin at room temperature. Tissues were then paraffin-embedded and cut at 5-µm thickness. All sections were collected continuously and in order through the ovary. Every fifth section was stained with hematoxylin and eosin for antral follicle and corpora lutea (CL) counting. Both ovaries were analyzed, and the follicle or CL counts were summed for statistical analysis.
Statistics
Female fertility, ovulation, CL and antral follicle counts, serum hormones, pituitary gene and protein levels, estrous cycle frequency, organ weights, and puberty onset were compared using Student t tests. Estrous cycle stages and serum FSH levels before and after inhibin A injection were analyzed using two-way ANOVA followed by Sidak correction. Data in primary culture experiments and serum gonadotropin levels in GnRH agonist or antagonist experiments were analyzed by one-way ANOVA, followed by Holm-Sidak correction. Each treatment group was compared with its own no-treatment or vehicle-treated controls. Data were log transformed when variances were not equal between groups. Statistical analyses were performed using Prism 6 (GraphPad). P < 0.05 was considered statistically significant.
Results
Recombination of floxed Tgfbr3 created a loss of function allele
Previously, homozygous deletion of Tgfbr3 exon 2 created a loss of gene function and embryonic lethality (30). Here, we floxed exon 2 of Tgfbr3 (Tgfbr3fx/fx; Fig. 1A) and crossed the mice with the EIIa-Cre deleter strain to recombine the allele in early development (43, 44). Resulting Tgfbr3+/Δex2 males and females (Fig. 1B) were then intercrossed to generate wild-type (Tgfbr3+/+), heterozygous (Tgfbr3+/Δex2), and homozygous progeny (Tgfbr3Δex2/Δex2). Genotypes were determined by PCR of genomic DNA (Fig. 1C). Among the 134 live-born pups analyzed, 56 (41.8%) were wild-type and 78 (58.2%) were heterozygotes. We did not identify any live-born mice with the homozygous knockout genotype (Tgfbr3Δex2/Δex2). We next repeated the heterozygous crosses and isolated the embryos from pregnant females at 13.5, 15.5, 16.5, or 17.5 dpc (Fig. 1D). Beginning as early as 13.5 dpc, homozygotes exhibited apparent hematopoietic defects relative to wild-type and heterozygous (not pictured) littermates, as reflected by their paler appearance, particularly in the liver. Resorption of homozygous mice was frequently observed by 17.5 dpc. These data indicate that recombination of the floxed Tgfbr3 allele caused a loss of gene function.
Generation of gonadotrope-specific Tgfbr3 knockout mice
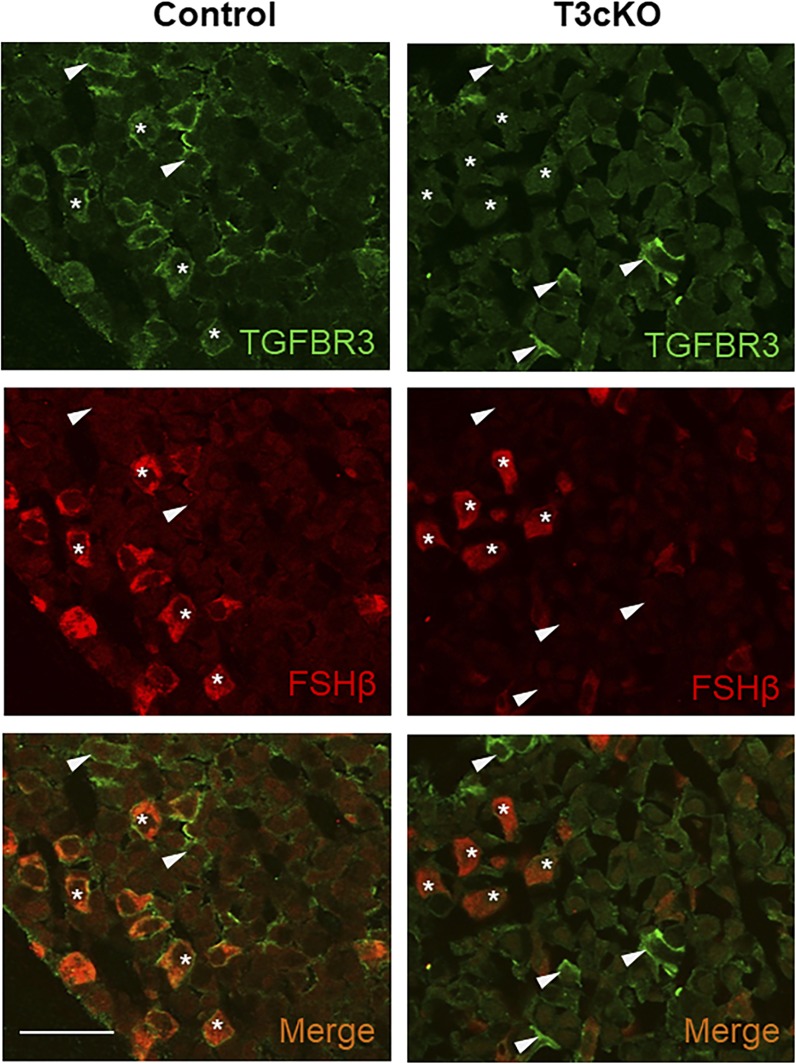
To study TGFBR3 function in canonical inhibin target cells, we used a GnRH receptor-dependent Cre-driver (GRIC) (31) to ablate the gene/protein specifically in pituitary gonadotropes. Tgfbr3fx/fx;GnrhrGRIC/+ females were crossed with Tgfbr3fx/fx males to generate control (Tgfbr3fx/fx) and conditional knockout mice (Tgfbr3fx/fx;GnrhrGRIC/+; hereafter, T3cKO). To confirm the specificity of gene recombination, we collected various tissues for analysis by PCR. Recombination was detected in pituitaries of T3cKO mice of both sexes and in the testes and epididymides of males (data not shown), consistent with previous reports of Cre activity in gonadotropes and in the male germline of GRIC mice (32). Recombination was not observed in the brain, heart, liver, kidney, adrenal, pancreas, lung, stomach, intestine, spleen, ovary, or uterus (data not shown). To determine the efficiency of recombination in gonadotropes, we performed double-label immunofluorescence for TGFBR3 and FSHβ (Fig. 2). TGFBR3 immunoreactivity was detected at the membrane of all FSHβ-immunoreactive cells as well as in FSHβ-negative cells of control pituitaries. In contrast, TGFBR3 staining was lost in FSHβ-positive cells (examples labeled by asterisks in Fig. 2, right panels) but not in the other pituitary cell lineages of T3cKO mice (arrowheads in Fig. 2, right panels). Although we did not quantify the recombination efficiency in this model, Tgfbr3 mRNA levels were reduced by 98% in gonadotropes in a complementary model, Tgfbr3fx/−;GnrhrGRIC/+ (data not shown). Thus, TGFBR3 was selectively and effectively depleted in gonadotropes of T3cKO mice.
Figure 2.
Gonadotrope-specific knockout of TGFBR3. Immunofluorescence of pituitary sections from adult control (left) and T3cKO (right) males using antibodies against TGFBR3 (green, top) and FSHβ (red, middle) are shown. Overlays (merge) of the two images are shown at the bottom. Arrowheads represent examples of cells expressing TGFBR3, but not FSH. Asterisks represent examples of FSH-expressing cells. Scale bar, 50 µm.
Impaired inhibin A antagonism of FSH synthesis and release in the absence of TGFBR3 in vivo and in vitro
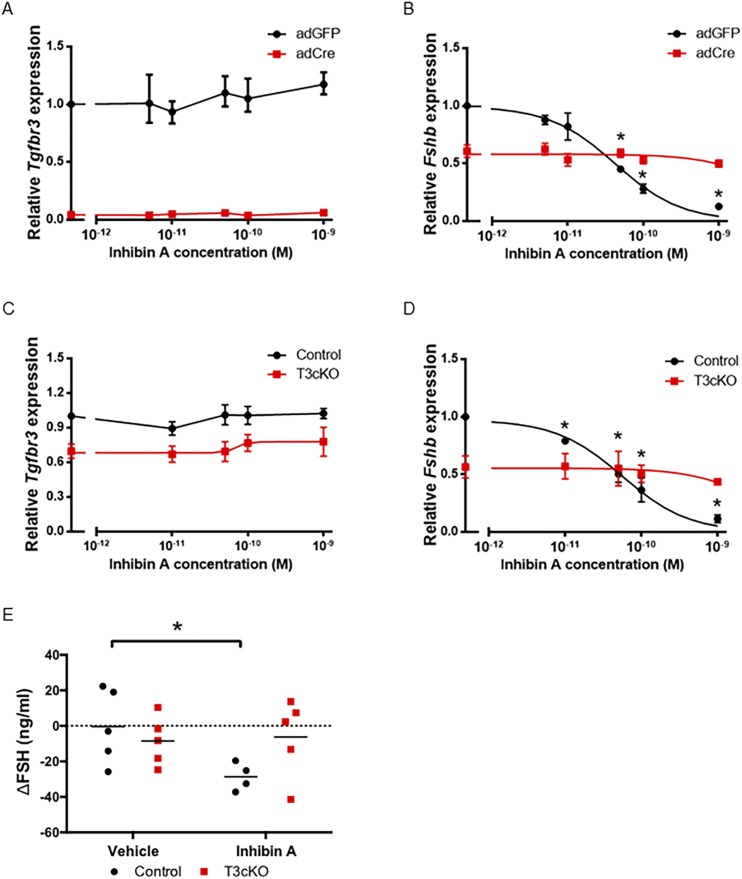
To assess whether TGFBR3 is required for inhibin action in gonadotropes, we first performed two in vitro experiments. In the first, we acutely ablated Tgfbr3 gene function in primary pituitary cultures from Tgfbr3fx/fx mice using a Cre-expressing adenovirus (adCre). Tgfbr3 mRNA expression was profoundly reduced in cells transduced with a Cre-expressing adenovirus relative to a control adenovirus expressing GFP (adGFP) (Fig. 3A). In the second experiment, we cultured pituitaries from control and T3cKO mice. Here, the reduction in Tgfbr3 mRNA expression reflected the deletion specific to gonadotrope cells (Fig. 3C), whereas in the first experiment, the Tgfbr3 allele was recombined in all cells. Inhibin A potently and dose-dependently inhibited Fshb mRNA expression and FSH secretion in cultures from Tgfbr3fx/fx mice transduced with adGFP in the first experiment [Fig. 3B and (45)] and from control mice in the second experiment [Fig. 3D and (45)]. In contrast, inhibin A action was abrogated in Tgfbr3-deficient cells in both experiments [Fig. 3B and 3D and (45)]. Complementing the expression data in Fig. 2, these results demonstrate functional ablation of TGFBR3 in gonadotropes. Notably, we consistently observed a reduction in basal Fshb expression in Tgfbr3-deficient cells. The mechanism underlying this decrease is not yet clear.
Figure 3.
Inhibin A dose dependently antagonized Fshb expression in control but not Tgfbr3-deficient gonadotropes. Pituitary cultures were prepared from (A and B) Tgfbr3fx/fx mice of both sexes or from (C and D) control (black lines) and T3cKO (red lines) females. In (A) and (B), cells were transduced with a Cre-expressing adenovirus (adCre; red lines) or control adenovirus (adGFP; black lines). Cells were treated with the indicated concentrations of inhibin A. (A and C) Tgfbr3 or (B and D) Fshb mRNA expression was measured by quantitative RT-PCR. The housekeeping gene Rpl19 was used for normalization. (A‒D) Data shown reflect mean ± SEM from three independent experiments. (E) Changes in serum FSH levels (∆FSH) 6 h after vehicle or inhibin A injection in control (black) and T3cKO (red) males are shown. Here, the data reflect FSH levels after inhibin A minus FSH levels in the same animals before the injection. Serum FSH levels were assessed by multiplex ELISA. *P < 0.05.
Next, we assessed inhibin action in vivo by injecting inhibin A or vehicle into the tail vein of 10-week-old control or T3cKO mice. Males were used because their FSH levels are relatively stable and are higher than those of females. We measured serum FSH before and 6 hours after injection in all animals. FSH levels were significantly reduced in control but not in T3cKO males after inhibin A injection (Fig. 3E). Serum FSH levels were unaffected by vehicle treatment in the two genotypes. Thus, both in vitro and in vivo, depletion of Tgfbr3 rendered gonadotropes relatively insensitive to exogenous inhibin A.
Enhanced folliculogenesis and fertility in female T3cKO mice
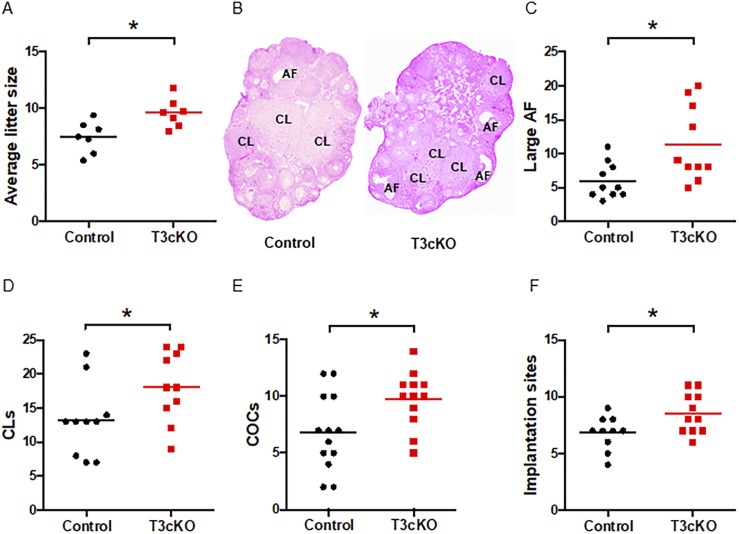
We next examined fertility in T3cKO mice. Although we studied both sexes, our primary focus was on females given that male mice do not require FSH for fertility (46). Puberty onset (assessed by vaginal opening) and estrous cyclicity were comparable in control and T3cKO females (45). Reproductive organ weights were also similar between genotypes in adults of both sexes (45). Over the course of a 6-month breeding trial, T3cKO females reproduced on average two more pups per litter than their control littermates (9.6 ± 0.5 vs 7.5 ± 0.5) (Fig. 4A). This enhanced fertility appeared to derive from increased folliculogenesis, as T3cKO ovaries contained more large antral follicles (11.4 ± 1.8 vs 6.0 ± 0.8) and CL (18.1 ± 1.6 vs 13.2 ± 1.7) than controls (Fig. 4B‒4D). Moreover, T3cKO females ovulated more eggs in natural cycles (9.8 ± 0.7 vs 6.8 ± 0.9) (Fig. 4E) and exhibited a greater number of embryo implantation sites in their uteri compared with controls (8.5 ± 0.5 vs 6.8 ± 0.5) (Fig. 4F). It is interesting that antral follicle counts and numbers of CL were increased, whereas ovarian weight was not statistically elevated in T3cKO mice (45). We do not have an explanation for this apparent discrepancy.
Figure 4.
Increased fertility, ovulation, and implantation in T3cKO females. (A) Average litter sizes in 6-mo breeding trials are shown. (B) Representative ovarian histology (hematoxylin and eosin) in 10-wk-old females is shown. Antral follicles (AFs) and CL are labeled. Total numbers of (C) large AFs and (D) CLs were counted and summed from both ovaries. (E) COCs were counted on the morning (∼7 am) after mating. (F) Implantation sites were counted at 5.5 dpc. Data were analyzed by Student t test in all panels except panel (B). Significance was assessed relative to *P < 0.05.
FSH synthesis and secretion appeared normal in T3cKO mice
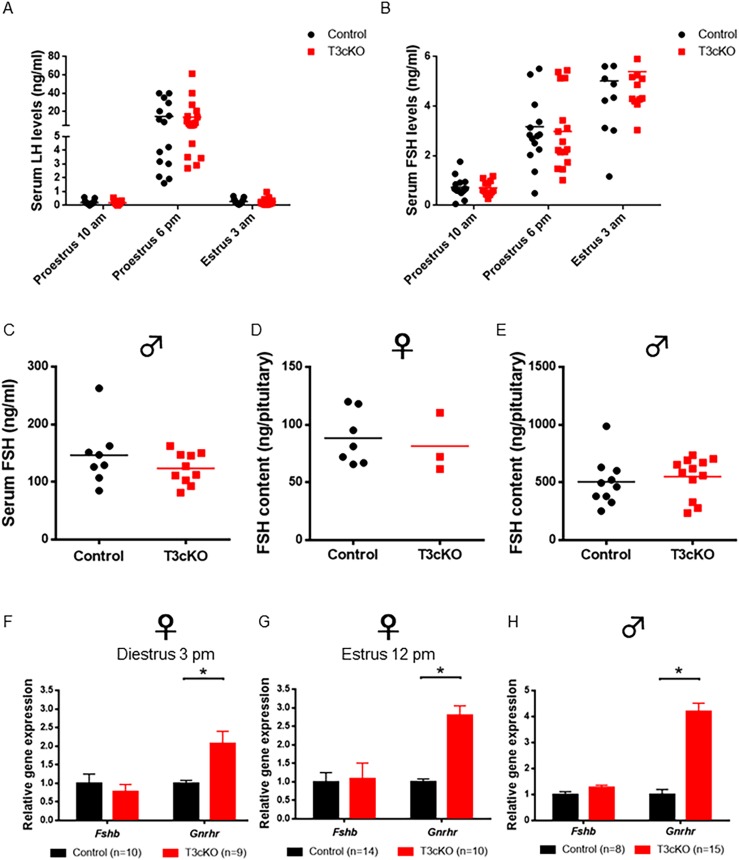
Enhanced folliculogenesis in T3cKO females would be predicted to derive from enhanced FSH secretion, which we anticipated in the context of impaired inhibin action in gonadotrope cells of these mice. Surprisingly, however, serum FSH levels were indistinguishable between littermate control and T3cKO females assessed at multiple stages of the estrous cycle, including on the afternoon of diestrus, morning of proestrus, or at four time points on the morning of estrus (45). We took repeated samples from mice of both genotypes at two time points on proestrus and one on early estrus morning to compare the amplitudes of the primary and secondary FSH surges. Measurements of LH confirmed the proper staging of the mice, as we observed marked surges of LH on the afternoon of proestrus in all mice of both genotypes (Fig. 5A). Primary and secondary FSH surges were observed on the afternoon of proestrus (6 pm, lights out at 7 pm) and the morning of estrus (3 am), respectively, but there were no differences between genotypes (Fig. 5B). Serum FSH levels were also equivalent between genotypes in adult males (Fig. 5C). In the pituitary, FSH content (Fig. 5D and 5E) and Fshb (Fig. 5F‒5H) mRNA levels were equivalent between genotypes in both sexes (pituitary RNA was sampled at two time points in females, noon on estrus and 3 pm of diestrus). LHβ subunit (Lhb) and common gonadotropin α-subunit (Cga) mRNA levels were also largely unaffected by genotype (45). In contrast, Gnrhr mRNA expression was significantly increased in the pituitaries of both male and female T3cKO mice relative to controls (Fig. 5F‒5H). However, these animals did not show enhanced sensitivity to exogenous GnRH (45) or increased dependence on endogenous GnRH (45).
Figure 5.
Normal serum and pituitary FSH levels in control and T3cKO females and males. (A) Serum LH and (B) FSH levels in control (black) and T3cKO females (red) sampled at 10 am and 6 pm on proestrus and 3 am on estrus morning are shown. LH was measured by in-house ELISA and FSH by Luminex (MPTMAG-49K; Millipore). (C) Serum FSH levels in adult males as assessed by multiplex ELISA are shown. Pituitary FSH content in 10-wk-old (D) female and (E) male mice, as assessed by RIA, is shown. Quantitative RT-PCR analysis of relative pituitary gene expression in 10-wk-old females at (F) diestrus (3 pm) and (G) estrus (12 pm), and in (H) males is shown. Data were analyzed by Student t test in each panel. Data in (F)–(H) are means (+SEM). *P < 0.05 was considered statistically significant.
Control and T3cKO females responded similarly to exogenous gonadotropins
Because there were no apparent differences in FSH synthesis and secretion, we examined whether ovaries of T3cKO mice were more sensitive to gonadotropin stimulation than those of controls. We treated juvenile females with pregnant mare’s serum gonadotropin (eCG) and human chorionic gonadotropin and then counted ovulated COCs in the oviduct. The responses of control and T3cKO mice were comparable (45), largely ruling out an ovary-intrinsic phenotype.
Pituitaries of T3cKO mice retained sensitivity to inhibin B
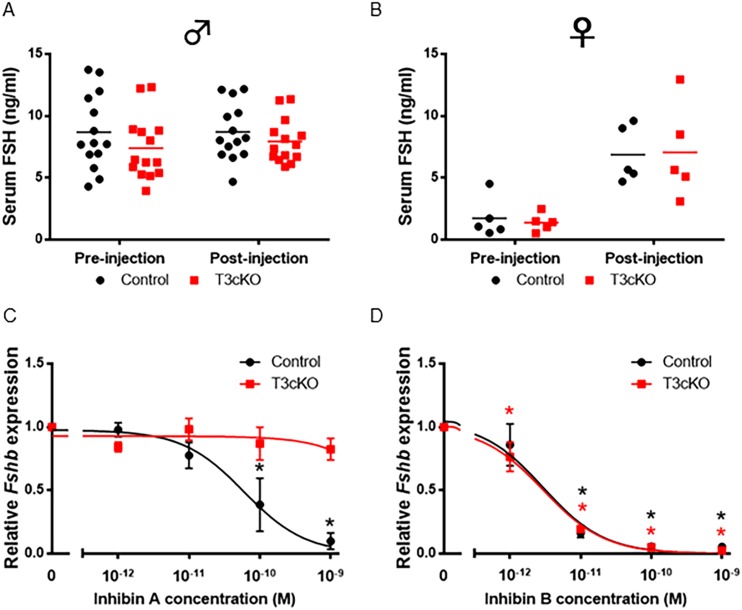
Although we showed previously that T3cKO pituitaries have greatly reduced sensitivity to exogenous inhibin A (Fig. 3), we next asked whether these mice retained sensitivity to endogenous inhibins. We collected blood samples before and after treating males and females of both genotypes with AIS. FSH levels were unaltered in males of either genotype (Fig. 6A), suggesting that adult male mice have little to no inhibin B tone. In contrast, FSH levels increased significantly and to equivalent extents in females of both genotypes after treatment with AIS (Fig. 6B). We tested females on diestrus when inhibin A levels are relatively low (data not shown). These observations, coupled with the fact that gonadotropes of T3cKO mice appeared to be insensitive to inhibin A (Fig. 3), suggested that the AIS was likely exerting its actions on endogenous inhibin B and, by extension, that the pituitaries of these mice may still be sensitive to this form of the hormone. To test this idea, we performed additional experiments on primary pituitary cultures from control and T3cKO mice. As shown previously (Fig. 3D), inhibin A antagonism of Fshb mRNA expression was essentially lost in T3cKO mice at concentrations up to 1 nM (Fig. 6C). In remarkable contrast, inhibin B antagonism of Fshb expression remained intact in T3cKO mice (Fig. 6D). These data demonstrate that TGFBR3 acts as an obligate coreceptor for inhibin A, but not for inhibin B, in murine gonadotrope cells.
Figure 6.
T3cKO females remained sensitive to inhibin B. Serum FSH levels in control (black) and T3cKO (red) (A) males and (B) females on diestrus morning before and 11 h after treatment with AIS are shown. FSH levels were assessed by Luminex assays (MPTMAG-49K; Millipore). (C and D) Pituitaries were cultured as in Fig. 3D and treated with the indicated concentrations of inhibin A or B. Fshb mRNA levels were assessed by quantitative RT-PCR. Note that the drop in basal Fshb mRNA was also observed in T3cKO cultures in this experiment, as in Fig. 3D. However, data were normalized for each genotype to better demonstrate the relative effects of inhibin A vs inhibin B. Data represent the mean (±SEM) of three independent experiments. Significance symbols in black and red correspond to control and T3cKO groups, respectively. *P < 0.05.
Discussion
We developed a “floxed” Tgfbr3 murine model, allowing us to conditionally ablate the Tgfbr3 gene in gonadotropes. A priori, this manipulation was predicted to reduce gonadotrope sensitivity to inhibins, leading to enhanced FSH secretion, folliculogenesis, and fertility in females. Although antral follicle development and litter sizes were in fact augmented, FSH levels were unaltered in both female and male T3cKO mice. This was initially a surprising result in light of both published and our own observations (e.g.,Fig. 3D) that inhibin A requires TGFBR3 for high affinity antagonism of FSH synthesis and secretion (21, 29, 47). This led us to question whether TGFBR3 also functions as an inhibin B coreceptor in gonadotropes. The data show that inhibin B can potently antagonize FSH synthesis in the absence of TGFBR3 (Fig. 6D). To our knowledge this is the most conclusive demonstration that mechanisms of action of inhibin A and inhibin B differ, at least in murine gonadotropes, the canonical inhibin target cell.
Prior reports portended that the two hormones might function differently. First, the two inhibins appeared to bind to similar proteins in Leydig and Sertoli cell lines, but inhibin B bound with lower affinity than inhibin A (48). Second, inhibin A more potently antagonized activin A or B action than did inhibin B in an adrenocortical cell line (49). Third, some, but not all, studies showed that inhibin A bound to TGFBR3 with significantly higher affinity than did inhibin B (28, 39, 50). Fourth, despite its lower (or equivalent) affinity for TGFBR3 (and activin type II receptors), inhibin B more potently suppressed FSH secretion than did inhibin A in rats in vivo and in rat pituitary cultures (50). There are at least two important implications of these and the present observations: (i) the inhibin β-subunits must somehow contribute to differential actions of the inhibins and to their binding to TGFBR3, and (ii) an additional coreceptor (or coreceptors) can selectively or preferentially mediate the actions of inhibin B in gonadotropes in rodents.
The first point is intriguing because, until now, inhibins have been argued to bind TGFBR3 exclusively via the α-subunit, which is identical in inhibin A and B (47, 50). Whether the β-subunits contribute to the coreceptor interaction surface or allosterically modulate α-subunit binding to TGFBR3 is not yet clear, but could be resolved with structural analyses of inhibin/TGFBR3 complexes. With respect to the second point, to our knowledge, no unique inhibin B‒binding proteins have been identified to date. However, inhibin B was shown to bind to a unique protein in a murine gonadotrope cell line, LβT2, and these cells also appeared to be more sensitive to inhibin B than inhibin A (50). Whether this protein functions as an obligate inhibin B coreceptor in gonadotropes or compensates in the absence of TGFBR3 can be determined only once it is identified. The fact that this protein binds inhibin B, but not inhibin A, again argues that the β-subunits must contribute to coreceptor binding.
Although TGFBR3 is dispensable for inhibin B action in murine gonadotropes, our data do not preclude a role for TGFBR3 in inhibin B action in other cells; that is, it is possible that gonadotropes express a unique inhibin B coreceptor that is absent in other cell types. In these other contexts, inhibin B may depend on TGFBR3 or perhaps other unidentified coreceptor proteins. Indeed, both inhibin A and B bind a variety of uncharacterized proteins in testicular and adrenocortical cell lines, pituitary cells in culture, and gonadal tumors (48, 51–53). It is also possible that inhibin B might act via TGFBR3 in the absence of the gonadotrope-specific coreceptor. Indeed, once the latter is identified, it may prove necessary to knock it out in combination with TGFBR3 to fully impair inhibin B action.
Although the data provide a likely explanation for apparently normal FSH levels in T3cKO mice, the enhanced fertility in these females remains unexplained. Because the genetic manipulation was gonadotrope specific, it seems most parsimonious to propose a pituitary-derived mechanism. Moreover, the ovaries of conditional knockout females did not appear to be more sensitive to exogenous gonadotropins. We therefore hypothesize that some aspect of FSH secretion or synthesis may have been altered in ways we failed to detect. First, it is possible that we missed a transient increase in FSH levels at a stage or time of the cycle we did not sample. That said, we examined multiple cycle stages and times and failed to detect even a hint of difference between genotypes. This included the afternoon of proestrus (Fig. 5B), when inhibin A levels peak (data not shown) and the loss of TGFBR3 might be expected to have its most pronounced effect. Second, there may have been subtle changes in the nature of FSH release that we failed to detect. In general, FSH is not considered a pulsatile hormone. However, folliculogenesis may be enhanced in murine ovaries in response to a more pulsatile FSH stimulus or at least to FSH released predominantly via the regulated secretory pathway (54). At present, we lack an assay with sufficient sensitivity to measure FSH in serial blood samples (e.g., every 5 to 10 minutes). Therefore, we were unable to assess whether FSH is released in a more pulsatile fashion in these animals. Nevertheless, measurements every hour or two on estrous morning revealed relatively constant FSH levels. Third, FSH is a glycoprotein, and post-translational modifications of the protein are regulated across the reproductive cycle in women (55–58). According to recent data, hypoglycosylated FSH is more active than hyperglycosylated FSH in ovarian granulosa cells (57). Unfortunately, given the small blood volume of mice, we were unable to directly measure FSH glycoforms and activity in control and T3cKO animals. However, different glycoforms have different serum half-lives in humans (59–63). Here, FSH elimination rates were comparable following GnRH antagonist treatment in male control and T3CKO mice. Therefore, any changes in FSH glycoforms were not obvious, at least not in males. Finally, it is possible that our FSH assay did not detect all forms of the hormone. This seems unlikely, however, as there were also no changes in Fshb subunit expression.
In summary, we ablated the putative inhibin coreceptor, TGFBR3, in pituitary gonadotropes and observed the expected increases in folliculogenesis and fertility in female mice, but not for the predicted reasons (i.e., overtly elevated FSH levels). The findings described here are significant for at least three reasons. First, they describe the development of a mouse model that will enable us and others to interrogate TGFBR3 (betaglycan) function in any cell type of interest. Second, they provide proof of principle that perturbations of inhibin action or inhibin coreceptors could be used to enhance fertility in vivo. Finally, these results will motivate efforts to identify novel coreceptors for inhibin B and perhaps other ligands in the TGFβ family.
Acknowledgments
Dr. Teresa Woodruff (Northwestern University, Chicago, IL) generously provided recombinant human inhibin A. The authors thank Ying Wang for performing some of the FSH assays, Adelina Artenie for providing technical assistance at the beginning of the project, and Emilie Brûlé for providing feedback on the manuscript.
Financial Support: This work was funded by Canadian Institutes of Health Research (CIHR) operating Grant MOP-133394 (to D.J.B.). Y.L. and J.F. received Dr. Samuel Solomon Fellowships in Endocrinology from the McGill Faculty of Medicine. J.F. also received a doctoral research award from CIHR. L.O. was supported by a Ferring Postdoctoral Fellowship in Reproductive Health. The UVa Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health [National Centers for Translational Research in Reproduction and Infertility (NCTRI)] Grant P50-HD28934.
Author Contributions: Y.L., J.F., and D.J.B. designed the experiments. Y.L. and J.F. performed most of the experiments. L.O. participated in the natural ovulation and ovarian histology experiments. X.Z. participated in assessing hormone levels across the mouse estrous cycle. H.Y.L. and A.S. generated the floxed mouse strain. U.B. provided the GRIC mouse strain. Y.L., J.F., and D.J.B. analyzed the data. Y.L. and D.J.B. wrote the original manuscript, which was edited and approved by all coauthors.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AIS
anti-inhibin serum
- CL
corpora lutea
- COC
cumulus-oocyte complex
- CV
coefficient of variation
- dpc
day post coitus
- FBS
fetal bovine serum
- Fshb
FSHβ subunit gene
- GRIC
GnRH receptor-dependent Cre-driver
- Neo
neomycin
- SMAD3
homolog of Drosophila mothers against decapentaplegic 3
- T3cKO
gonadotrope-specific Tgfbr3 knockout mice
- TGFBR3
TGFβ type III receptor
- UVa
University of Virginia
References
- 1. McCullagh DR. Dual endocrine activity of the testes. Science. 1932;76(1957):19–20. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz NB, Channing CP. Evidence for ovarian “inhibin”: suppression of the secondary rise in serum follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc Natl Acad Sci USA. 1977;74(12):5721–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivier J, Spiess J, McClintock R, Vaughan J, Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985;133(1):120–127. [DOI] [PubMed] [Google Scholar]
- 4. Mason AJ, Hayflick JS, Ling N, Esch F, Ueno N, Ying SY, Guillemin R, Niall H, Seeburg PH. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985;318(6047):659–663. [DOI] [PubMed] [Google Scholar]
- 5. Ling N, Ying SY, Ueno N, Esch F, Denoroy L, Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci USA. 1985;82(21):7217–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyamoto K, Hasegawa Y, Fukuda M, Nomura M, Igarashi M, Kangawa K, Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985;129(2):396–403. [DOI] [PubMed] [Google Scholar]
- 7. Robertson DM, Foulds LM, Leversha L, Morgan FJ, Hearn MT, Burger HG, Wettenhall RE, de Kretser DM. Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun. 1985;126(1):220–226. [DOI] [PubMed] [Google Scholar]
- 8. Makanji Y, Zhu J, Mishra R, Holmquist C, Wong WP, Schwartz NB, Mayo KE, Woodruff TK. Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev. 2014;35(5):747–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crofton PM, Illingworth PJ, Groome NP, Stirling HF, Swanston I, Gow S, Wu FC, McNeilly A, Kelnar CJ. Changes in dimeric inhibin A and B during normal early puberty in boys and girls. Clin Endocrinol (Oxf). 1997;46(1):109–114. [DOI] [PubMed] [Google Scholar]
- 10. Groome NP, Illingworth PJ, O’Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81(4):1401–1405. [DOI] [PubMed] [Google Scholar]
- 11. Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137(12):5463–5467. [DOI] [PubMed] [Google Scholar]
- 12. Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. A homodimer of the β-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem Biophys Res Commun. 1986;138(3):1129–1137. [DOI] [PubMed] [Google Scholar]
- 13. Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature. 1986;321(6072):779–782. [DOI] [PubMed] [Google Scholar]
- 14. Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321(6072):776–779. [DOI] [PubMed] [Google Scholar]
- 15. Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6):2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fortin J, Ongaro L, Li Y, Tran S, Lamba P, Wang Y, Zhou X, Bernard DJ. Minireview: activin signaling in gonadotropes: what does the FOX say… to the SMAD? Mol Endocrinol. 2015;29(7):963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vale W, Wiater E, Gray P, Harrison C, Bilezikjian L, Choe S. Activins and inhibins and their signaling. Ann N Y Acad Sci. 2004;1038(1):142–147. [DOI] [PubMed] [Google Scholar]
- 19. Martens JW, de Winter JP, Timmerman MA, McLuskey A, van Schaik RH, Themmen AP, de Jong FH. Inhibin interferes with activin signaling at the level of the activin receptor complex in Chinese hamster ovary cells. Endocrinology. 1997;138(7):2928–2936. [DOI] [PubMed] [Google Scholar]
- 20. Walton KL, Makanji Y, Harrison CA. New insights into the mechanisms of activin action and inhibition. Mol Cell Endocrinol. 2012;359(1-2):2–12. [DOI] [PubMed] [Google Scholar]
- 21. Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404(6776):411–414. [DOI] [PubMed] [Google Scholar]
- 22. Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65(6):973–982. [DOI] [PubMed] [Google Scholar]
- 23. Ying SY. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988;9(2):267–293. [DOI] [PubMed] [Google Scholar]
- 24. Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol. 1989;3(12):1969–1976. [DOI] [PubMed] [Google Scholar]
- 25. Weiss J, Crowley WF Jr, Halvorson LM, Jameson JL. Perifusion of rat pituitary cells with gonadotropin-releasing hormone, activin, and inhibin reveals distinct effects on gonadotropin gene expression and secretion. Endocrinology. 1993;132(6):2307–2311. [DOI] [PubMed] [Google Scholar]
- 26. Bernard DJ, Chapman SC, Woodruff TK. Inhibin binding protein (InhBP/p120), betaglycan, and the continuing search for the inhibin receptor. Mol Endocrinol. 2002;16(2):207–212. [DOI] [PubMed] [Google Scholar]
- 27. Esparza-López J, Montiel JL, Vilchis-Landeros MM, Okadome T, Miyazono K, López-Casillas F. Ligand binding and functional properties of betaglycan, a co-receptor of the transforming growth factor-β superfamily: specialized binding regions for transforming growth factor-β and inhibin A. J Biol Chem. 2001;276(18):14588–14596. [DOI] [PubMed] [Google Scholar]
- 28. Chapman SC, Bernard DJ, Jelen J, Woodruff TK. Properties of inhibin binding to betaglycan, InhBP/p120 and the activin type II receptors. Mol Cell Endocrinol. 2002;196(1-2):79–93. [DOI] [PubMed] [Google Scholar]
- 29. Wiater E, Lewis KA, Donaldson C, Vaughan J, Bilezikjian L, Vale W. Endogenous betaglycan is essential for high-potency inhibin antagonism in gonadotropes. Mol Endocrinol. 2009;23(7):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol Cell Biol. 2003;23(12):4371–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–2711. [DOI] [PubMed] [Google Scholar]
- 32. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107(37):16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB J. 2014;28(8):3396–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RRID: AB_354417.
- 35.RRID: AB_141637.
- 36.RRID: AB_2336123.
- 37.RRID: AB_2336881.
- 38. Fortin J, Boehm U, Weinstein MB, Graff JM, Bernard DJ. Follicle-stimulating hormone synthesis and fertility are intact in mice lacking SMAD3 DNA binding activity and SMAD2 in gonadotrope cells. FASEB J. 2014;28(3):1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makanji Y, Harrison CA, Stanton PG, Krishna R, Robertson DM. Inhibin A and B in vitro bioactivities are modified by their degree of glycosylation and their affinities to betaglycan. Endocrinology. 2007;148(5):2309–2316. [DOI] [PubMed] [Google Scholar]
- 40. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 41. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4a):609–614. [DOI] [PubMed] [Google Scholar]
- 42. Rhodes ME, Balestreire EM, Czambel RK, Rubin RT. Estrous cycle influences on sexual diergism of HPA axis responses to cholinergic stimulation in rats. Brain Res Bull. 2002;59(3):217–225. [DOI] [PubMed] [Google Scholar]
- 43. Dooley TP, Miranda M, Jones NC, DePamphilis ML. Transactivation of the adenovirus EIIa promoter in the absence of adenovirus E1A protein is restricted to mouse oocytes and preimplantation embryos. Development. 1989;107(4):945–956. [DOI] [PubMed] [Google Scholar]
- 44. Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93(12):5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y, Fortin J, Ongaro L, Zhou X, Boehm U, Schneyer A, Bernard DJ. Data from: Betaglycan (TGFBR3) functions as an inhibin A, but not inhibin B, co-receptor in pituitary gonadotrope cells in mice. Figshare Digital Repository 2018. Deposited 30 August 2018. https://figshare.com/s/4358123086338e54587a. [DOI] [PMC free article] [PubMed]
- 46. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 47. Makanji Y, Walton KL, Wilce MC, Chan KL, Robertson DM, Harrison CA. Suppression of inhibin A biological activity by alterations in the binding site for betaglycan. J Biol Chem. 2008;283(24):16743–16751. [DOI] [PubMed] [Google Scholar]
- 48. Harrison CA, Farnworth PG, Chan KL, Stanton PG, Ooi GT, Findlay JK, Robertson DM. Identification of specific inhibin A-binding proteins on mouse Leydig (TM3) and Sertoli (TM4) cell lines. Endocrinology. 2001;142(4):1393–1402. [DOI] [PubMed] [Google Scholar]
- 49. Farnworth PG, Stanton PG, Wang Y, Escalona R, Findlay JK, Ooi GT. Inhibins differentially antagonize activin and bone morphogenetic protein action in a mouse adrenocortical cell line. Endocrinology. 2006;147(7):3462–3471. [DOI] [PubMed] [Google Scholar]
- 50. Makanji Y, Temple-Smith PD, Walton KL, Harrison CA, Robertson DM. Inhibin B is a more potent suppressor of rat follicle-stimulating hormone release than inhibin A in vitro and in vivo. Endocrinology. 2009;150(10):4784–4793. [DOI] [PubMed] [Google Scholar]
- 51. Farnworth PG, Harrison CA, Leembruggen P, Chan KL, Stanton PG, Ooi GT, Rahman NA, Huhtaniemi IT, Findlay JK, Robertson DM. Inhibin binding sites and proteins in pituitary, gonadal, adrenal and bone cells. Mol Cell Endocrinol. 2001;180(1-2):63–71. [DOI] [PubMed] [Google Scholar]
- 52. Farnworth PG, Wang Y, Leembruggen P, Ooi GT, Harrison C, Robertson DM, Findlay JK. Rodent adrenocortical cells display high affinity binding sites and proteins for inhibin A, and express components required for autocrine signalling by activins and bone morphogenetic proteins. J Endocrinol. 2006;188(3):451–465. [DOI] [PubMed] [Google Scholar]
- 53. Draper LB, Matzuk MM, Roberts VJ, Cox E, Weiss J, Mather JP, Woodruff TK. Identification of an inhibin receptor in gonadal tumors from inhibin alpha-subunit knockout mice. J Biol Chem. 1998;273(1):398–403. [DOI] [PubMed] [Google Scholar]
- 54. Wang H, Larson M, Jablonka-Shariff A, Pearl CA, Miller WL, Conn PM, Boime I, Kumar TR. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc Natl Acad Sci USA. 2014;111(15):5735–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davis JS, Kumar TR, May JV, Bousfield GR. Naturally occurring follicle-stimulating hormone glycosylation variants. J Glycomics Lipidomics. 2014;4(1):e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bousfield GR, Butnev VY, Rueda-Santos MA, Brown A, Hall AS, Harvey DJ. Macro- and micro-heterogeneity in pituitary and urinary follicle-stimulating hormone glycosylation. J Glycomics Lipidomics. 2014;4:1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bousfield GR, Butnev VY, Butnev VY, Hiromasa Y, Harvey DJ, May JV. Hypo-glycosylated human follicle-stimulating hormone (hFSH21/18) is much more active in vitro than fully-glycosylated hFSH (hFSH24). Mol Cell Endocrinol. 2014;382(2):989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wide L, Eriksson K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups J Med Sci. 2013;118(3):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bishop LA, Nguyen TV, Schofield PR. Both of the beta-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136(6):2635–2640. [DOI] [PubMed] [Google Scholar]
- 60. Wide L, Eriksson K, Sluss PM, Hall JE. Serum half-life of pituitary gonadotropins is decreased by sulfonation and increased by sialylation in women. J Clin Endocrinol Metab. 2009;94(3):958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perlman S, van den Hazel B, Christiansen J, Gram-Nielsen S, Jeppesen CB, Andersen KV, Halkier T, Okkels S, Schambye HT. Glycosylation of an N-terminal extension prolongs the half-life and increases the in vivo activity of follicle stimulating hormone. J Clin Endocrinol Metab. 2003;88(7):3227–3235. [DOI] [PubMed] [Google Scholar]
- 62. Baenziger JU, Kumar S, Brodbeck RM, Smith PL, Beranek MC. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc Natl Acad Sci USA. 1992;89(1):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Green ED, Boime I, Baenziger JU. Differential processing of Asn-linked oligosaccharides on pituitary glycoprotein hormones: implications for biologic function. Mol Cell Biochem. 1986;72(1-2):81–100. [DOI] [PubMed] [Google Scholar]