Abstract
Background
Helicobacter pylori is a Gram-negative bacterium that colonizes the gastric mucosa in humans. One of the main virulence factors of H. pylori is the cag pathogenicity island (cagPAI), which encodes a type 4-secretion system (T4SS) and the cytotoxin CagA. Translocation of CagA through the T4SS triggers host-signaling pathways. One of the T4SS proteins is CagL, which is necessary for CagA translocation. CagL is a 26-kDa protein that contains a hypervariable motif, which spans residues 58 to 62. Several polymorphisms in this region have been associated with different disease outcomes, e.g. in Mexico, N58 is associated with a higher risk of gastric cancer. The aim of this work is to analyze the sequence of the hypervariable motif (residues 58 to 62) of clinical isolates from Mexican patients with chronic gastritis, and to correlate these polymorphisms with the vacA genotype.
Results
Of the 164 biopsies analyzed, only 30.5% (50/164) were positive for H. pylori. Thirty-six of the 50 clinical isolates (72%) were cagA positive, and 40 (80%) had the most virulent vacA genotype (s1/m1). Of the cagA positive strains, 94.4% were vacA s1/m1. All the cagA+ strains contained the cagL gene. The most prevalent sequence in the polymorphic region (residues 58–62) was DKMGE (75.8%, 25/33), followed by NKMGQ and NEIGQ (6.1%, 2/33), and DEIGQ, NKMGE, DKIGE, and DKIGK (3%, 1/33). Regarding polymorphisms in positions 58 and 59, the most common were D58/K59 (81.8%, 27/33), followed by N58/K59 (9.1%, 3/33), and D58/E59 (3%, 1/33). Only two isolates (6.1%) contained residues N58/E59, which correspond to those found in H. pylori strain ATCC 26695. 92.6% of the clinical isolates having polymorphism D58/K59 had the genotype vacA s1/m1, considered to be the most virulent, while 7.4% had the genotypes vacA s1/m2 and s2/m2.
Conclusions
In Mexican patients, CagL polymorphisms D58, K59, M60, E62, K122, and I134 are more common in patients with chronic gastritis.
Keywords: H. pylori, cagL polymorphisms, Polymorphic region, Chronic gastritis
Background
Helicobacter pylori is a Gram-negative, spiral-shaped bacterium that colonizes the gastric mucosa in humans. In many cases, it can persist without causing symptoms, although in some cases it can produce chronic gastritis. The persistent colonization of the gastric mucosa by H. pylori can lead to the development of gastroduodenal diseases like peptic ulcer, gastric lymphoma, and gastric cancer [1]. H. pylori has two main virulence factors, the cag pathogenicity island (cagPAI) and the pore forming toxin vacA, whose presence or absence identifies highly virulent (type-1) from less virulent (type-2) strains, respectively, thus being strong predictors of severe disease outcome [2, 3]. The cagPAI is a 40-kb chromosomal region that contains 27–31 genes that encode the type IV secretion system (T4SS) and an effector protein [4]. The T4SS forms a needle-like surface appendage called the T4SS pilus, which is induced upon contact with the host cell membrane [5]. The effector protein CagA is encoded by the cagA gene. This protein is translocated into the cytoplasm of the gastric epithelial cells through the T4SS, where it is phosphorylated at the tyrosine residues of the EPIYA motifs, causing multiple cellular alterations [6, 7]. One of the genes that encode the T4SS is cagL, which encodes for the 26-kDa protein CagL. It has been shown that this protein is localized on the surface of the T4SS of H. pylori and in intracellular pools, is necessary for CagA translocation, and interacts with CagI [5, 8]. It also contributes to transient hypochlorhydria by disrupting the interaction between the integrin and metalloprotease ADAM17 and the integrin α5β1, thus activating NF-κB-mediated repression of the gastric H, K-adenosine triphosphatase α-subunit (HKα) [9].
The role of some regions or motifs of CagL in different phenotypes has been assessed. For example, its C-terminal coiled-coil region is involved in IL-8 secretion, host cell elongation, and binding of the T4SS to the host cell [10]. CagL also contains an arginine–glycine–aspartate (RGD) motif at residues 76–78, located within the second large α-helix [11], which is essential for binding to the human integrin α5β1 receptor [5], as well as integrins αvβ5 and αVβ3 [12, 13], although it can also bind to human fibronectin in a RGD independent manner [14]. This motif is also responsible of CagL fibronectin-like effect on host cells, since it is involved in cell spreading triggering, formation of focal adhesions, and activation of several tyrosine kinases [15]. The RGD motif is right next to the sequence LXXL, of which, the two-leucine residues (L79 and L82) contribute to the adhesion to different cell lines through interaction with integrin αVβ6 [16]. CagL also contains a second motif named RHS (RGD helper sequence), which contains residues phenylalanine–glutamic acid–alanine–asparagine–glutamic acid (FEANE). This motif also contributes to CagL binding to integrins [17]. The third important region in CagL is the CagL hypervariable motif (CagLHM), which spans residues 58 to 62 [18]. It is located in an unresolved flexible region between helices α1 and α2 [19]. Although its function is still controversial, it has been shown that certain polymorphisms correlate with diseases in a geographical-dependent manner. For example, polymorphism Y58/E59 is associated with gastric cancer in patients from Taiwan, while in India the polymorphism associated with this disease is D58/K59 [20, 21]. In Mexico, only the polymorphism N58 has been associated with a higher risk of gastric cancer [22]. So far, 33 combinations of polymorphisms in the hypervariable motif have been identified worldwide, the most common being DKMGE, NEIGQ, NKIGQ, and DKIGK. Of these, all are found in North and South American strains, except for DKIGK, which is more prevalent in East/Southeast Asia/Australasia [23]. The aim of this work was to analyze the sequence of the hypervariable motif (residues 58 to 62) of clinical isolates from Mexican patients with chronic gastritis, and to correlate these polymorphisms with the vacA genotype of H. pylori.
Results
Helicobacter pylori prevalence
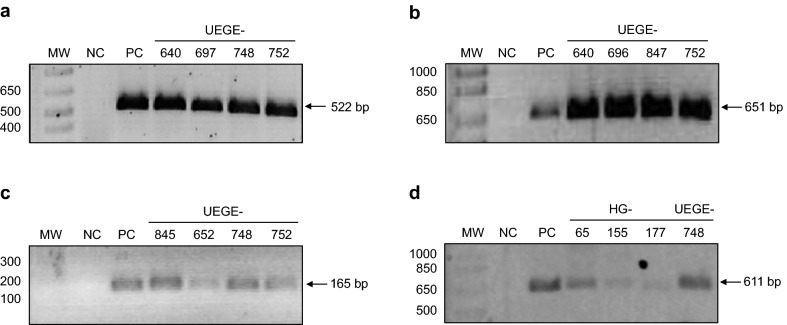
We analyzed 164 patients with histopathological diagnosis of chronic gastritis, of which 61.6% (101/164) were female, and 38.4% (63/164) were male. The mean age of the patients was 48 years old (± 17), ranging between 19 and 89 years old. The frequency of H. pylori isolation was 30.5% (50/164). All strains identified as H. pylori by culture were confirmed by PCR amplification of a fragment of the 16S rRNA gene (Fig. 1a).
Fig. 1.
Representative gels of PCR amplified fragments. a 16S RNA fragment. Lanes: MW—molecular weight marker (bp), NC—negative control, PC—positive control (DNA from strain ATCC 43504), 4 to 7—clinical isolates UEGE-640, UEGE-697, UEGE-748, and UEGE-752. b cagL fragment amplified with the first set of primers (651 bp). Lanes: MW—molecular weight marker (bp), NC—negative control, PC—positive control (DNA from strain ATCC 43504), 4 to 7—clinical isolates UEGE-640, UEGE-696, UEGE-847, and UEGE-752. c cagL fragment amplified with the second set of primers (165 bp). Lanes: MW—molecular weight marker (bp), NC—negative control, PC—positive control (DNA from strain HP26695), 4 to 7—clinical isolates: UEGE-845, UEGE-652, UEGE-748, and UEGE-752. d cagL fragment amplified with the third set of primers (611 bp). Lanes: MW—molecular weight marker (bp), NC—negative control, PC—positive control (DNA from strain HP26695), 4 to 7—clinical isolates: HG-65, HG-155, HG-177, and UEGE-748
Thirty-six of the 50 clinical isolates (72%) were cagA positive, and 40 (80%) had the most virulent vacA genotype (s1/m1). Of the cagA positive strains, 94.4% were vacA s1/m1 (Table 1). In the cagA negative isolates, absence of the cagPAI was confirmed by empty site PCR.
Table 1.
Frequency of cagA and vacA genotypes in clinical isolates of H. pylori from patients with chronic gastritis
| cagA | ||
|---|---|---|
| Positive | Negative | |
| n = 36 (100%) | n = 14 (100%) | |
| vacA | ||
| s1/m1 | 34 (94.4%) | 6 (42.9%) |
| s1/m2 | 1 (2.8%) | 0 |
| s2/m2 | 1 (2.8%) | 8 (57.1%) |
Prevalence of cagL
A PCR product of 651 bp was amplified in 24 of the 36 cagA positive clinical isolates (Fig. 1b) using primers cagL sense and cagL antisense (Table 4). To determine if the 12 remaining strains were cagL negative, we performed a second PCR using primers cagL-Fwd-2 and cagL-16 (Table 4). A product of 165 bp was amplified in the 12 strains (Fig. 1c), indicating that all cagA positive strains contain the cagL gene. In order to obtain sequences suitable for uploading in the GenBank (> 200 bp), the DNA from those strains that were positive with the second set of primers was subjected to a third PCR using primers cagL-Fwd-2 and cagL antisense. With the new combination of primers, a product of 611 bp was amplified in 9 of the 12 strains (Fig. 1d), and the 33 PCR products amplified were sequenced.
Table 4.
Oligonucleotides used in this work
| Gene | Oligonucleotide | Sequence 5′–3′ | Amplicon size (bp) | References |
|---|---|---|---|---|
| 16S rRNA | HP16SF | GCTAAGAGATCAGCCTATGTCC | 522 | [39] |
| HPGR16SR | CAATCAGCGTCAGTAATGTTC | |||
| vacAs1 | VAIF | ATGGAAATACAACAAACACAC | 259 | [40] |
| vacAs2 | VAIR | CTGCTTGAATGCGCCAAAC | 286 | |
| vacAm1 | VAGF | CAATCTGTCCAATCAAGCGAG | 570 | [41] |
| vacAm2 | VAGR | GCGTCTAAATAATTCCAAGG | 645 | |
| cagA | F1 | GATAACAGGCAAGCTTTTGAGG | 349 | [42] |
| B1 | CTGCAAAAGATTGTTTGGCAGA | |||
| Empty site | ESf | ACATTTTGGCTAAATAAACGCTG | 360 | [43] |
| ESr | TCATGCGAGCGGCGATGTG | |||
| cagL | cagL sense | GAAGATATAACAAGCGGTTT | 651 | [44] |
| cagL antisense | TTTAACAATGATCTTACTTGA | |||
| cagL-Fwd-2 | ACVAAGAGACCAACCARCAAG | 165 | This work | |
| cagL-16 | TCGCTTCAAAATTGGCTTTC | [31] | ||
| cagL-Fwd-2 | ACVAAGAGACCAACCARCAAG | 611 | This work | |
| cagL-16 | TTTAACAATGATCTTACTTGA | [44] |
CagL polymorphisms
The sequences of 33 of the 36 cagL+ clinical isolates were aligned with the sequence of strain ATCC 26695, showing a high level of conservation (Fig. 2). Motifs RGD and RHS are 100% identical in all strains.
Fig. 2.

Sequence alignment of CagL from 33 clinical isolates registered in GenBank. Sequence from strain ATCC 26695 was used as reference. CagLHM, RGD, and RHS (FEANE) motifs are indicated in squares, black squares indicate the polymorphic region (residues 58 to 62), and the RDG and RHS motifs in the reference sequence. Polymorphisms are shown in gray. Accession numbers: MG051618-MG051641 and MG214979-MG214987
The most prevalent sequence in the polymorphic region (residues 58–62) was DKMGE (75.8%, 25/33), followed by NKMGQ and NEIGQ (6.1%, 2/33, respectively), DEIGQ, NKMGE, DKIGE, and DKIGK (3%, 1/33, respectively) (Fig. 3). One clinical isolate had four polymorphisms (3%) (UEGE-740), two had three polymorphisms (6.1%) (HG-193 and HG-211), one had only one polymorphism (3%) (HG-02), and the rest (87.9%) had two (Fig. 2). Interestingly, one of the clinical isolates with three polymorphisms (HG-193), and the one with four (UEGE-740) had the sequence DKI, which is more prevalent in East-Asian strains [24].
Fig. 3.

Frequency of sequences in the CagLHM motif (positions 58 and 62). Sequence DKMGE was found in 25 of 33 clinical isolates, sequences NKMGQ and NEIGQ in 2, and sequences DEIGQ, NKMGE, DKIGE, and DKIGK in 1 clinical isolate each. Frequencies are shown in percentages
Regarding polymorphisms in positions 58 and 59, the most common were D58/K59 (81.8%, 27/33), followed by N58/K59 (9.1%, 3/33), and D58/E59 (3%, 1/33) (Fig. 4). Only two isolates (6.1%) contained residues N58/E59 (HG-43 and HG-210), which correspond to those found in H. pylori strain ATCC 26695.
Fig. 4.
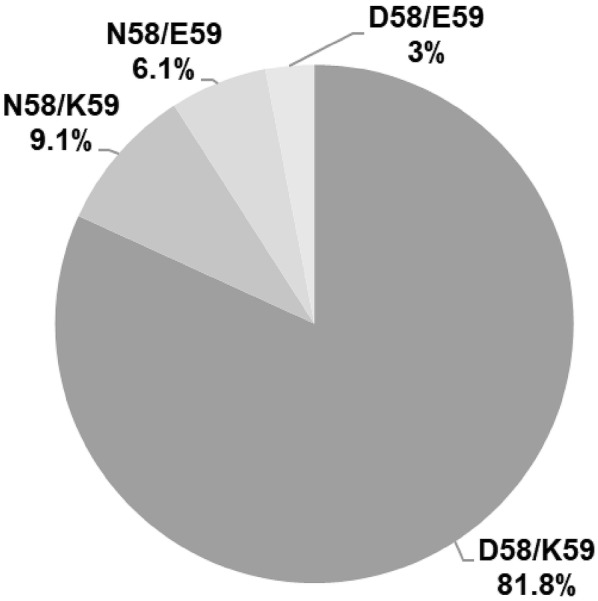
Frequency of CagL polymorphisms in positions 58 and 59. Polymorphism D/K was found in 27 clinical isolates, polymorphism N/K in 3, polymorphism N/E in 2, and polymorphism D/E in 1. Frequencies are shown in percentages
Our sequence alignment (Fig. 2) revealed that, besides the polymorphisms found in the CagLHM motif, there were other 19 polymorphisms both upstream and downstream of this region (Table 2). The most common were A41, M73, K122, I134, and I175, being found in > 9 strains; while the rest (F48, V48, T56, V99, A112, G140, A170, V171, I181, Q200, I203, K210, S216, and N221) were identified in < 6 strains.
Table 2.
CagL polymorphisms in strains isolated from patients with chronic gastritis
| Residue in 26695 | Polymorphism | Frequency n (%) | Strain (s) | CagL region |
|---|---|---|---|---|
| V41 | A | 9/33 (27.3%) | HG-44, -51, -150, -162, -189, -199, -224 UEGE-640, -826 |
α1 |
| I48 | F | 2/33 (6%) | HG-70, UEGE-826 | L1 |
| V | 1/33 (3%) | UEGE-696 | ||
| A56 | T | 2/33 (6%) | HG-66, HG-193 | L1 |
| N58 | D | 28/33 (84.8%) | HG-44, -51, -65, -66, -150, -155, -162, -177, -179, -189, -190, -193, -199, -211, -224, UEGE-640, -666, -696, -740, -748, -751, -752, -753, -843, -845, -846, -847, -852 |
L1 |
| E59 | K | 30/33 (90.9%) | HG-02, -44, -51, -65, -66, -70, -150, -155, -162, -177, -179, -189, -190, -193, -199, -224 UEGE-640, -666, -696, -740, -748, -751, -752, -753, -826, -843, -845, -846, -847, -852 |
L1 |
| M60 | I | 5/33 (15.1%) | HG-43, -193, -210, -211, UEGE-740 |
L1 |
| E62 | Q | 5/33 (15.1%) | HG-43, -70, -210, -211 UEGE-826 |
α2 |
| K | 1/33 (3%) | UEGE-740 | ||
| I73 | M | 20/33 (60.6%) | HG-43, -51, -65, -66, -150, -162, -179, -189, -190, -224, UEGE-640, -666, -696, -748, -751, -752, -753, -843, -846, -852. |
α2 |
| I99 | V | 2/33 (6%) | HG-44, UEGE-696 | α2 |
| T112 | A | 1/33 (3%) | HG-70 | α3 |
| N122 | K | 27/33 (81.8%) | HG-02, -44, -51, -65, -66, -70, -150, -155, -162, -177, -179, -190, -193, -199, -210, -211, -224 UEGE-666, -696, -748, -751, -753, -826, -843, -845, -847, -852 |
α4 |
| V134 | I | 27/33 (81.8%) | HG-02, -44, -51, -65, -66, -70, -150, -155, -162, -177, -179, -190, -193, -199, -210, -211, -224 UEGE-696, -748, -751, -752, -753, -843, -845, -846, -847, -852 |
α5 |
| E140 | G | 6/33 (18.2%) | HG-51, -66, -162 UEGE-740, -753, -852 |
α5 |
| T170 | A | 1/33 (3%) | UEGE-740 | α5 |
| A171 | V | 1/33 (3%) | HG-190 | α5 |
| T175 | I | 14/33 (42.4%) | HG-02, -44, -65, -150, -155, -162, -179, -190, -199 UEGE-748, -752, -826, -843, -846 |
α5 |
| V181 | I | 1/33 (3%) | UEGE-640 | α6 |
| H200 | Q | 6/33 (18.2%) | HG-02, -44, -66, -177, -210 UEGE-753 |
α6 |
| V203 | I | 1/33 (3%) | HG-43 | α6 |
| E210 | K | 2/33 (6%) | UEGE-740, -843 | α6 |
| R216 | S | 2/33 (6%) | HG-224, UEGE-740 | α6 |
| S221 | N | 2/33 (6%) | HG-02, -66 | α6 |
CagL polymorphisms and vacA genotypes of H. pylori
As mentioned before, polymorphisms in residues 58 and 59 have been associated with a higher risk of gastric disease, so we analyzed their relationship with the genotype of vacA. As seen in Table 3, 80.6% (25/31) of the strains carrying the vacA genotype s1/m1 had the polymorphism D58/K59, while of the five isolates with polymorphism N58, 100% had the genotype vacA s1/m1 regardless of the polymorphism in position 59. These results suggest that the polymorphism D58/K59 correlates with genotype vacA s1/m1.
Table 3.
CagL polymorphisms and vacA genotypes of clinical isolates of H. pylori
| CagL polymorphisms | vacA genotype | p value | ||
|---|---|---|---|---|
| s1/m1 n = 31 |
s1/m2 n = 1 |
s2/m2 n = 1 |
||
| N58/E59 | 2 (6.5%) | 0 | 0 | |
| N58/K59 | 3 (9.7%) | 0 | 0 | |
| D58/K59 | 25 (80.6%) | 1 (100%) | 1 (100%) | < 0.05* |
| D58/E59 | 1 (3.2%) | 0 | 0 | |
* Fisher’s exact test
Phylogenetic relationship of CagL sequences
Since we identified 4 polymorphic region sequences that have been found predominantly in Asian strains (NKMGQ, NEIGQ, DEIGQ, and DKIGK), we performed a phylogenetic analysis to determine if they are related. As shown in Fig. 5, at the bottom of the tree is a group formed by strains containing sequences NEIGQ and DEIGQ (HG-43, HG-210, and HG-211), and the reference strain ATCC 26695, which has the sequence NEMGE (found only in Europe). Related to this group is the strain carrying the sequence DKIGK (UEGE-740). Forming part of a second major group, which contains strains with sequences DKMGE and NKMGE, are the strains carrying the sequence NKMGQ. However, these are more related to the strain carrying the DKIGE sequence than to the rest.
Fig. 5.
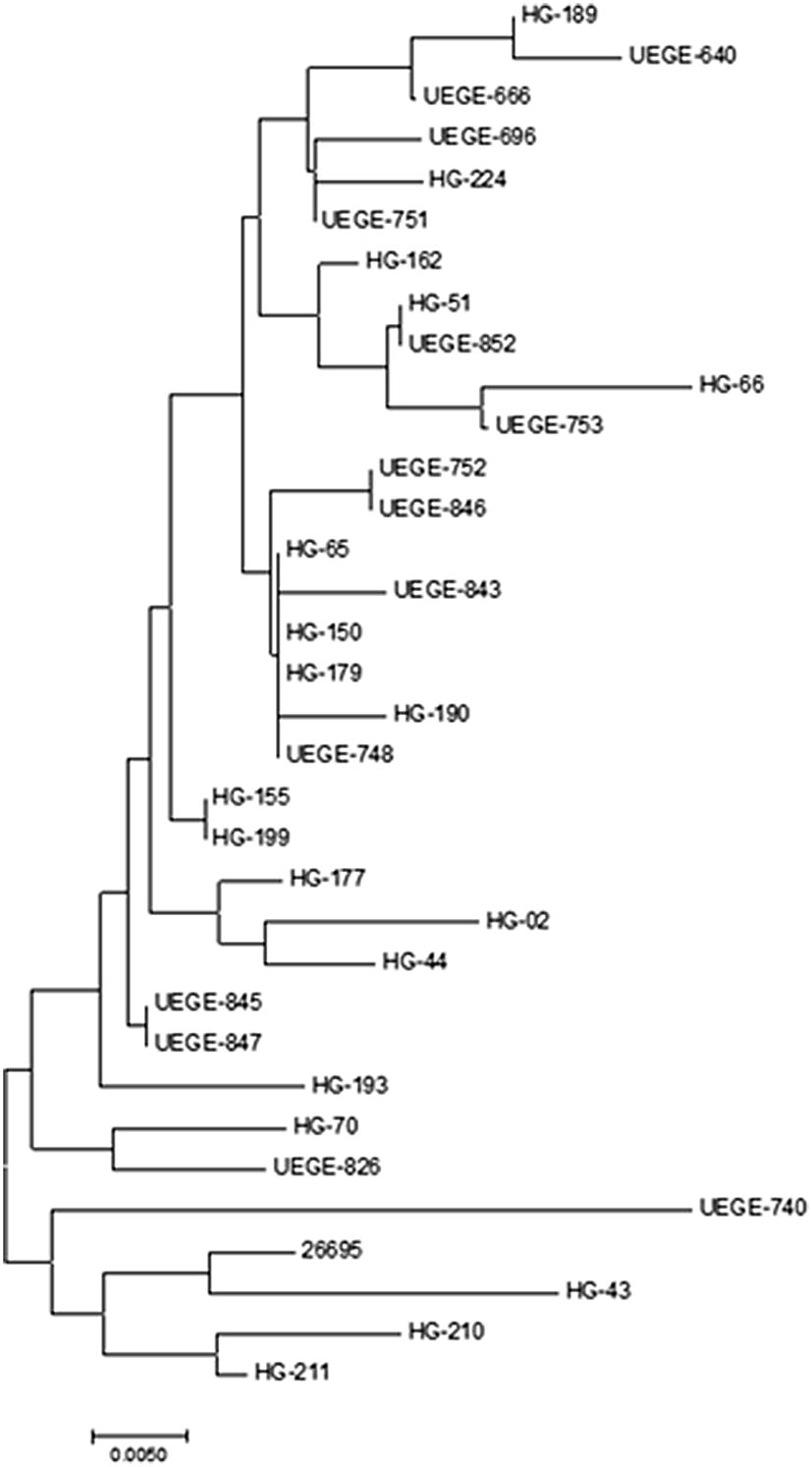
Phylogenetic tree of CagL sequences. Neighbor-Joining tree analysis of the CagL sequences of the 33 clinical isolates and strain ATCC 26695
Discussion
Gastric diseases are of diverse etiology, and although chronic gastritis and more severe gastroduodenal disorders are attributed to H. pylori, Epstein–Barr virus and human Cytomegalovirus can also cause these diseases [25]. In this study we found that the prevalence of H. pylori, determined by bacterial isolation, was 30.5%. This percentage is similar to that reported by Román-Román et al. (47.8%) [26], but lower than that reported by other authors [Paniagua et al. (60.1%); Martínez-Carrillo et al. (77%)] [27, 28]. This variation in frequencies may be due to bias in the information provided by the patients; also, it is impossible to rule out that they have received antimicrobial treatment against H. pylori or any other infection in the recent past. Among the Mexican population self-medication is common and, although the sale of antibiotics without medical prescription is prohibited, in the state of Guerrero the metronidazole, an antiparasitic that is part of the treatment scheme for H. pylori, is available over the counter. Additionally, the probability of finding H. pylori in biopsies decreases as structural and functional changes appear in the infected mucosa. Another factor that may have contributed to the low frequency found in this work, is that the prevalence of H. pylori infection was determined by culture, which is a very specific method (95–100%) but not very sensitive (80–90%), since the results depend on the number of viable bacilli in the biopsy [29].
Among infected patients, the clinical outcome is determined by the genetic background and lifestyle of the host, as well as by H. pylori virulence factors. It has been proposed that the H. pylori virulence factor CagL has an important role in the pathogenesis of gastroduodenal diseases, and that its function can be determined by the amino acid sequence in specific positions [11, 12]. We analyzed the prevalence of cagL in H. pylori clinical isolates from Mexican patients with chronic gastritis, which was 72%. This is a lower percentage than those found in Asian patients, > 85% in Chinese, Indian, Iranian, Taiwanese and Malay patients [20, 21, 30, 31]; however, these patients had more severe diseases, like gastric cancer, peptic ulcer disease, and duodenal ulcer. Nevertheless, our result is higher to that found in Iranian patients with gastritis (64.2%) [32]. Apparently, the prevalence of cagL in clinical isolates from patients with gastritis is lower than that found in isolates from patients with peptic ulcer disease or gastric cancer. All our cagA+ isolates were cagL+, which is in accordance with previous reports that have shown that the prevalence of cagL is higher than, or very close to that of cagA [20, 30, 33].
We also analyzed the polymorphic region (residues 58 to 62) of CagL. We found that the most common polymorphism in position 58 corresponds to aspartic acid (D) (84.8%), followed by asparagine (N) (15.2%). It has been proposed that strains carrying aspartic acid at this position lead to a lower risk of gastric cancer in comparison with the asparagine carrying strains. In agreement with this, the polymorphism N58 was significantly associated with gastric cancer in Mexican patients from Mexico City [22, 32]. Regarding residue combinations in positions 58 and 59, polymorphism D58/K59 has been associated with gastric cancer in India, where patients carrying bacteria with this polymorphism are 3.8 times more likely to develop this disease [20]. However, our results showed that D58/K59 was the most common polymorphism in Mexican patients with chronic gastritis and it is associated with the vacA genotype s1/m1. We also found a high frequency of the polymorphisms M60 (85%), which has been previously shown to increase the chance of developing gastritis [32], and E62 (81.8%), which has been shown to be the most common polymorphism in strains from Iranian patients with chronic gastritis [32]. It is worth noting that in this region the only invariable residue is G61.
Besides the polymorphisms in the polymorphic region, Cherati et al. [32], reported other polymorphisms outside this region associated with different disease outcomes. They found that the polymorphism K122 had frequency of 88.8% in strains from patients with gastritis. Similarly, we found that the frequency of K122 in our isolates was 82%. In fact, 24/33 strains (73%) had the combination M60/K122. They also found that the frequency of polymorphism V134 is significantly higher in patients with gastric cancer than gastritis. In our strains, V134 had a prevalence of only 18.2%, being the most common the polymorphism I134 (81.8%). This result shows that in Mexican patients, polymorphism I134 is more common in chronic gastritis. We also identified two more residues with high variability, I73 and T175 (in H. pylori ATCC 26695). In strains isolated from Mexican patients with chronic gastritis, polymorphisms M73 and I175 have a frequency of 60.6% and 42.4%, respectively, however, in the literature there is no information regarding these residues or their association with clinical outcome, hence, the clinical significance of these results as well as the biological function of these polymorphisms need to be addressed in more detail. With respect to the I73 M mutation, isoleucine (I) and methionine (M) are nonpolar amino acids without charge, therefore, it is likely that this change has no important effects on the structure and function of CagL. The I175 mutation consists of the change of a polar threonine residue by one of nonpolar isoleucine, next to the TASLI motif and it is probable that this change influences the functions of CagL. It has been proposed that the TASLI region binds to integrin in an RGD-independent manner and it is probably that I175 residue contributes also to this function and to other functions of CagL. The TASLI motif is involved in binding to the cell, although only in the absence of another host cell ligands. Deletion of TASLI is related with a moderate reduction in IL-8 secretion and CagA translocation. In addition, it has been shown that the deletion of TASLI reduces de CagL binding to integrin [34].
Interestingly, the most common CagLHM sequence found in our study population was DKMGE, which is the most frequent worldwide, mainly in Africa and the American Continent. The second most frequent sequences were NEIGQ and NKMGQ. NEIGQ is also the second most common worldwide mainly in Europe, Asia, and North America, while NKMGQ has been found in Asia/Australasia but not in the American Continent. Additionally, we also found three sequences that are prevalent in East/Southeast Asia/Australasia (DKIGK), West/Central/South Asia (DEIGQ), and Central/South America (NKMGE) [23], as well as a new sequence not informed before (DKIGE). This sequence was found in only 1 clinical isolate (HG-193), and contains the sequence DKI, which is found mainly in strains from Asia/Australasia [23, 24, 35]. Despite of this, this isolate seems to be more related with those carrying sequence DKMGE, and in a lesser degree to strains carrying sequence NKMGQ (Fig. 5). These results show that the CagLHM sequences found in Mexican strains are diverse, with sequences not only common in our continent, but also in Asia, although all the CagA sequences are Western according to the EPIYA motifs [36].
The sequence diversity of CagL in the locally circulating strains may reflect the mobility and complex human interactions with the bacteria, but it may also be related to differences that modify the pathogenic function of the protein. More studies are needed on the diversity of CagL and the relationship of its polymorphisms with gastric diseases, as well as on the role of variations in the CagL hypervariable motif on the structure and function of the protein, and on the inflammatory process.
In the population of the state of Guerrero, Mexico, a high frequency of gastric diseases related to H. pylori persists, therefore, it is important to identify the virulence factors that confer a higher risk of developing serious diseases. The knowledge about these virulence factors will allow a better understanding of this process, facilitating the identification and characterization of biomarkers helpful in the detection of patients with greater risk of developing gastric cancer. It will also help to determine schemes and priorities of eradication treatments with anti–microbial compounds, as a measure to prevent gastric cancer.
Conclusions
Most of the clinical isolates of H. pylori analyzed in this study were cagL positive, and all of them carried conserved RGD and RHS motifs. In Mexican patients with chronic gastritis, CagL polymorphisms D58, K59, M60, E62, K122, and I134 are the most common. CagLHM polymorphisms D58/K59 are related with the virulent genotype vacA s1m1 of H. pylori.
Methods
Patients
We performed a cross-sectional study among 164 patients that attended to the Gastroenterology Service at the General Hospital Dr. Raymundo Abarca Alarcón, and to the Specialized Unit in Gastroenterology Endoscopy, both in Chilpancingo, Guerrero, Mexico. Patients who attended for an endoscopic study due to dyspepsia symptoms, and that have had no H. pylori eradication treatment during 1 month prior to the endoscopic procedure were selected. None of the patients included in this study were under treatment with proton pump inhibitors or with gastric pH neutralizing agents within 15 days prior to biopsy. Patients receiving non-steroidal anti-inflammatory therapy were excluded from the study. All patients signed a letter of consent. This project was approved by the Bioethics Committee of the Autonomous University of Guerrero, by the Department of Education and Research of the General Hospital Dr. Raymundo Abarca Alarcón, and by the authorized personnel of the Specialized Unit in Gastroenterology Endoscopy.
Biopsies
The endoscopic study was performed after a fasting night with a video processor and video gastroscope (Fujinon, Wayne, NJ USA). Two biopsies were taken from the antrum of patients with chronic gastritis, one was immediately fixed in 10% formalin for histological examination, and the other one was placed in Brain Heart Infusion Broth (BHI) (Becton–Dickinson, North Carolina, USA) with 10% glycerol for the isolation of H. pylori. The biopsies were transported at 4 °C, and those intended for isolation of H. pylori were processed immediately.
Isolation and identification of H. pylori
Each biopsy transported in BHI broth with 10% glycerol was macerated with a sterile wood applicator. Fifty microliters of the homogenates were cultivated on Columbia Agar plates (Becton–Dickinson, North Carolina, USA) supplemented with 10% ram blood, IsoVitaleX Enrichment and Helicobacter pylori selective supplement Dent (10 mg/L of vancomycin, 5 mg/L of trimethoprim, 5 mg/L of cefsulodin, 5 mg/L of amphotericin B) (Oxoid, Basingstoke, UK) at pH 6.8 to 7.0. The homogenates were distributed on the culture medium by isolation strip. The inoculated plates were incubated under microaerophilic conditions with 5% O2 and 5% CO2 at 37 °C in GasPak jars for 3–7 days. H. pylori was identified by colony morphology (small, transparent colonies, 1 mm in diameter), Gram staining and biochemical tests (urease, catalase and oxidase positive). H. pylori strain ATCC 43504 was used as positive control.
Bacterial DNA extraction
Isolates identified as H. pylori were subcultured and incubated for 72 h. A pool of colonies from each isolate was resuspended in extraction solution (10 mM Tris pH 8, 10 mM EDTA, 0.5% SDS) for digestion with proteinase K. Total DNA was obtained by phenol: chloroform: isoamylic alcohol technique [37]. Total DNA concentration was determined using a NanoDrop 2000. DNA samples were stored at − 20 °C until use.
Molecular confirmation and genotyping of vacA and cagA of H. pylori strains
Confirmation of H. pylori strains was done using oligonucleotides HP16SF and HPGR16SR (Table 4), which amplify a fragment of the 16S rRNA gene, according to the method described by Román-Román et al. [38]. vacA and cagA genotyping was assessed by multiple PCR with specific oligonucleotides VAIF and VAIR, VAGF and VAGR, and F1 and B1, respectively (Table 4). The reaction mixture contained 1.5 mM MgCl2; 0.2 mM dNTPs; 2.5 pmol of oligonucleotides F1 and B1, or 5 pmol of VAGF and VAGR, or 2.5 pmol of VAIF and VAIR; 1.5 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 200 ng of DNA, in a total volume of 25 μL. Amplification conditions were: 1 cycle at 94 °C for 10 min; 35 cycles at 94 °C for 1 min, 57 °C for 1 min, 72 °C for 1 min; and a final extension cycle at 72 °C for 10 min. The PCR products were subjected to 2.5% agarose gel electrophoresis, stained with ethidium bromide and visualized with ultraviolet light (UV). In each PCR, DNA from strain ATCC 43504 (vacA s1m1/cagA+) was used as positive control, and as negative control DNA was replaced with sterile deionized water. All reactions were done in a Mastercycler Ep gradient thermal cycler (Eppendorf, Hamburg, Germany).
Confirmation of cagPAI empty site
The absence of cagA and the pathogenicity island cagPAI in the cagA− strains was confirmed by the empty-site assay by conventional PCR, using the ESf and ESr oligonucleotides (Table 4), which bind upstream and downstream, respectively, of the region where the cagPAI is inserted in the genome of the reference strain NCTC 12455 (NCTC: National Collection of Type Culture) [45]. The PCR mixture contained 50 ng of DNA, 0.08 mM dNTPs (Invitrogen, Carlsbad, CA, USA), 1 mM MgCl2, 5 pmol of each oligonucleotide and 1 U of Platinium Taq DNA Polymerase (Invitrogen, Carlsbad, USA), in a final volume of 15 μL. The amplification conditions were: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 61 °C for 30 s and 72 °C for 45 s; and one final extension cycle at 72 °C for 7 min. PCR products were subjected to 2% agarose gel electrophoresis, followed by ethidium bromide staining and UV light observation. As negative control, DNA was replaced with sterile deionized water. DNA from strain ATCC 43504 (cagA+, cagPAI+) was used as a second negative control, and strain UEGE-644 (cagA−, cagPAI−) as positive control. The presence of a 360 bp product was considered indicative of the absence of cagA and cagPAI [43, 45].
CagL amplification by PCR
A fragment of 651 bp from gene cagL (hp0539) was amplified by PCR using primers cagL sense and cagL antisense (Table 4). The reaction mixture had a final volume of 15 μL, and contained 2.5 mM MgCl2, 0.25 mM dNTP’s, 5 pmol of each oligonucleotide, 1 U of Taq recombinant DNA polymerase (Invitrogen, Massachusetts, USA) and 50 ng of DNA. The conditions used were: 1 cycle at 94 °C for 5 min; 45 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min; and 1 cycle at 72 °C for 7 min. Each reaction included a positive (DNA from strain 26695) and a negative (DNA was substituted with water) control. All reactions were performed in a Mastercycler Ep gradient thermocycler (Eppendorf, Hamburg, Germany). PCR products were analyzed by agarose gel electrophoresis at 1.5% and stained with ethidium bromide. A second PCR was performed with DNA from those strains that were negative in the first reaction, using primers cagL-Fwd-2 and cagL-16, which amplified a 165 bp product (Table 4). The reaction mixture had a final volume of 15 μL, and contained 1.5 mM MgCl2, 0.25 mM dNTP’s, 5 pmol of each oligonucleotide, 1 U of Taq recombinant DNA polymerase (Invitrogen, Massachusetts, USA) and 50 ng of DNA. The conditions used were: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 45 s, 54 °C for 30 s, and 72 °C for 1 min; and 1 cycle at 72 °C for 7 min. Each reaction included a positive (DNA from strain 26695) and a negative (DNA was substituted with water) control. PCR products were analyzed by agarose gel electrophoresis at 1.8% and stained with ethidium bromide. A third PCR was performed with DNA from those strains that were negative in the first reaction, using primers cagL-Fwd-2 and cagL antisense (Table 4), in order to amplify a fragment of 611 bp suitable to upload in the GeneBank. The reaction mixture had a final volume of 25 μL and contained 1.5 mM MgCl2, 0.25 mM dNTP’s, 5 pmol of each oligonucleotide, 1 U of Taq recombinant DNA polymerase (Invitrogen, Massachusetts, USA) and 50 ng of DNA. The conditions used were: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 45 s, 56 °C for 30 s, and 72 °C for 45 s; and 1 cycle at 72 °C for 3 min. PCR products were analyzed by agarose gel electrophoresis at 1.8% and stained with ethidium bromide.
Purification and sequencing of PCR products
PCR products were purified by the isopropanol method and sequenced in the Unidad de Síntesis y Secuenciación of the Instituto de Biotecnología, UNAM, using primers cagL sense and primer cagL-Fwd-2 (Table 4). All sequences were deposited in GenBank with accession numbers MG051618-MG051641 and MG214979-MG214987.
Bioinformatic analysis
Nucleotide sequences were translated to aminoacidic sequences using the ExPASy Translate Tool (http://web.expasy.org/translate/). Multiple sequence alignment of translated sequences and phylogenetic analysis were performed using Molecular Evolutionary Genetics Analysis version 7.0 (MEGA7) software [46].
The evolutionary history was inferred using the Neighbor-Joining method [47]. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The analysis involved 34 amino acid sequences of CagL.
Authors’ contributions
Conception and design: GF-T, AR-R. Experiments: AJA-M, PG-M, CAC-S, DGS-F. Data analysis: VIM-S, GF-T, AR-R, DNM-C. Drafting the manuscript: VIM-S, GF-T, AR-R, DNM-C. Sample collection: AJA-M, PG-M. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the endoscopist gastroenterologists who carried out the endoscopic study and obtained the biopsies, and the histopathologists who performed the histological diagnosis of the biopsies.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This project was approved by the Bioethics Committee of the Autonomous University of Guerrero, by the Department of Education and Research of the General Hospital Dr. Raymundo Abarca Alarcón, and by the authorized personnel of the Specialized Unit in Gastroenterology Endoscopy.
Patients signed informed consent statements. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was supported with funding granted by the Ministry of Public Education, through ProDES 2014 and 2015.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adolfo Román-Román, Email: arroman6046@gmail.com.
Verónica I. Martínez-Santos, Email: iranzu23@gmail.com
Carlos A. Castañón-Sánchez, Email: carlos.ctn@gmail.com
Alan J. Albañil-Muñoz, Email: alan_daft17@hotmail.com
Paola González-Mendoza, Email: chica_chivas_don26@hotmail.com.
Diana G. Soto-Flores, Email: hollywoo_london@hotmail.com
Dinorah N. Martínez-Carrillo, Email: dnmartinez@uagro.mx
Gloria Fernández-Tilapa, Phone: +52 1 (747) 1229737, Email: gferti@hotmail.com.
References
- 1.Solnick JV, Tompkins LS. Helicobacter pylori and gastroduodenal disease: pathogenesis and host–parasite interaction. Infect Agents Dis. 1992;1(6):294–309. [PubMed] [Google Scholar]
- 2.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridge DR, Merrell DS. Polymorphism in the Helicobacter pylori CagA and VacA toxins and disease. Gut Microbes. 2013;4(2):101–117. doi: 10.4161/gmic.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backert S, Tegtmeyer N, Fischer W. Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Future Microbiol. 2015;10(6):955–965. doi: 10.2217/fmb.15.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449(7164):862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 6.Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2(2):155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 7.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287(5457):1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 8.Pham KT, Weiss E, Jimenez Soto LF, Breithaupt U, Haas R, et al. CagI is an essential component of the Helicobacter pylori Cag type IV secretion system and forms a complex with CagL. PLoS ONE. 2012;7(4):e35341. doi: 10.1371/journal.pone.0035341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha A, Backert S, Hammond CE, Gooz M, Smolka AJ. Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase alpha subunit. Gastroenterology. 2010;139(1):239–248. doi: 10.1053/j.gastro.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedemann T, Hofbaur S, Loell E, Rieder G. A C-terminal coiled-coil region of CagL is responsible for Helicobacter pylori-induced Il-8 expression. Eur J Microbiol Immunol. 2016;6(3):186–196. doi: 10.1556/1886.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barden S, Lange S, Tegtmeyer N, Conradi J, Sewald N, et al. A helical RGD motif promoting cell adhesion: crystal structures of the Helicobacter pylori type IV secretion system pilus protein CagL. Structure. 2013;21(11):1931–1941. doi: 10.1016/j.str.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Conradi J, Huber S, Gaus K, Mertink F, Royo Gracia S, et al. Cyclic RGD peptides interfere with binding of the Helicobacter pylori protein CagL to integrins alphaV beta3 and alpha5 beta1. Amino Acids. 2012;43(1):219–232. doi: 10.1007/s00726-011-1066-0. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemann T, Hofbaur S, Tegtmeyer N, Huber S, Sewald N, et al. Helicobacter pylori CagL dependent induction of gastrin expression via a novel alphav beta5-integrin-integrin linked kinase signalling complex. Gut. 2012;61(7):986–996. doi: 10.1136/gutjnl-2011-300525. [DOI] [PubMed] [Google Scholar]
- 14.Backert S, Fronzes R, Waksman G. VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol. 2008;16(9):409–413. doi: 10.1016/j.tim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Tegtmeyer N, Hartig R, Delahay RM, Rohde M, Brandt S, et al. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J Biol Chem. 2010;285(30):23515–23526. doi: 10.1074/jbc.M109.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barden S, Niemann HH. Adhesion of several cell lines to Helicobacter pylori CagL is mediated by integrin alphaV beta6 via an RGDLXXL motif. J Mol Biol. 2015;427(6 Pt B):1304–1315. doi: 10.1016/j.jmb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Conradi J, Tegtmeyer N, Wozna M, Wissbrock M, Michalek C, et al. An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA. Front Cell Infect Microbiol. 2012;2:70. doi: 10.3389/fcimb.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tafreshi M, Zwickel N, Gorrell RJ, Kwok T. Preservation of Helicobacter pylori CagA translocation and host cell proinflammatory responses in the face of CagL hypervariability at amino acid residues 58/59. PLoS ONE. 2015;10(7):e0133531. doi: 10.1371/journal.pone.0133531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barden S, Schomburg B, Conradi J, Backert S, Sewald N, et al. Structure of a three-dimensional domain-swapped dimer of the Helicobacter pylori type IV secretion system pilus protein CagL. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 5):1391–1400. doi: 10.1107/S1399004714003150. [DOI] [PubMed] [Google Scholar]
- 20.Shukla SK, Prasad KN, Tripathi A, Jaiswal V, Khatoon J, et al. Helicobacter pylori cagL amino acid polymorphisms and its association with gastroduodenal diseases. Gastric Cancer. 2013;16(3):435–439. doi: 10.1007/s10120-012-0189-7. [DOI] [PubMed] [Google Scholar]
- 21.Yeh YC, Chang WL, Yang HB, Cheng HC, Wu JJ, et al. H. pylori cagL amino acid sequence polymorphism Y58E59 induces a corpus shift of gastric integrin alpha5 beta1 related with gastric carcinogenesis. Mol Carcinog. 2011;50(10):751–759. doi: 10.1002/mc.20753. [DOI] [PubMed] [Google Scholar]
- 22.Rizzato C, Torres J, Plummer M, Munoz N, Franceschi S, et al. Variations in Helicobacter pylori cytotoxin-associated genes and their influence in progression to gastric cancer: implications for prevention. PLoS ONE. 2012;7(1):e29605. doi: 10.1371/journal.pone.0029605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorrell RJ, Zwickel N, Reynolds J, Bulach D, Kwok T. Helicobacter pylori CagL hypervariable motif: a global analysis of geographical diversity and association with gastric cancer. J Infect Dis. 2016;213(12):1927–1931. doi: 10.1093/infdis/jiw060. [DOI] [PubMed] [Google Scholar]
- 24.Choi JM, Choi YH, Sudhanva MS, Devakumar S, Lee KH, et al. Crystal structure of CagL from Helicobacter pylori K74 strain. Biochem Biophys Res Commun. 2015;460(4):964–970. doi: 10.1016/j.bbrc.2015.03.135. [DOI] [PubMed] [Google Scholar]
- 25.Del Moral-Hernandez O, Castanon-Sanchez CA, Reyes-Navarrete S, Martinez-Carrillo DN, Betancourt-Linares R, et al. Multiple infections by EBV, HCMV and Helicobacter pylori are highly frequent in patients with chronic gastritis and gastric cancer from Southwest Mexico: an observational study. Medicine. 2019;98(3):e14124. doi: 10.1097/MD.0000000000014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman-Roman A, Martinez-Carrillo DN, Atrisco-Morales J, Azucar-Heziquio JC, Cuevas-Caballero AS, et al. Helicobacter pylori vacA s1m1 genotype but not cagA or babA2 increase the risk of ulcer and gastric cancer in patients from Southern Mexico. Gut Pathog. 2017;9:18. doi: 10.1186/s13099-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paniagua GL, Monroy E, Rodriguez R, Arroniz S, Rodriguez C, et al. Frequency of vacA, cagA and babA2 virulence markers in Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. Ann Clin Microbiol Antimicrob. 2009;8:14. doi: 10.1186/1476-0711-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Carrillo DN, Garza-Gonzalez E, Betancourt-Linares R, Monico-Manzano T, Antunez-Rivera C, et al. Association of IL1B -511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol. 2010;10:126. doi: 10.1186/1471-230X-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan RP, Walker MM. ABC of the upper gastrointestinal tract: epidemiology and diagnosis of Helicobacter pylori infection. BMJ. 2001;323(7318):920–922. doi: 10.1136/bmj.323.7318.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt HM, Andres S, Nilsson C, Kovach Z, Kaakoush NO, et al. The cag PAI is intact and functional but HP0521 varies significantly in Helicobacter pylori isolates from Malaysia and Singapore. Eur J Clin Microbiol Infect Dis. 2010;29(4):439–451. doi: 10.1007/s10096-010-0881-7. [DOI] [PubMed] [Google Scholar]
- 31.Raei N, Latifi-Navid S, Zahri S. Helicobacter pylori cag pathogenicity island cagL and orf17 genotypes predict risk of peptic ulcerations but not gastric cancer in Iran. Asian Pac J Cancer Prev. 2015;16(15):6645–6650. doi: 10.7314/apjcp.2015.16.15.6645. [DOI] [PubMed] [Google Scholar]
- 32.Cherati MR, Shokri-Shirvani J, Karkhah A, Rajabnia R, Nouri HR. Helicobacter pylori cagL amino acid polymorphism D58E59 pave the way toward peptic ulcer disease while N58E59 is associated with gastric cancer in north of Iran. Microb Pathog. 2017;107:413–418. doi: 10.1016/j.micpath.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Yadegar A, Mobarez AM, Alebouyeh M, Mirzaei T, Kwok T, et al. Clinical relevance of cagL gene and virulence genotypes with disease outcomes in a Helicobacter pylori infected population from Iran. World J Microbiol Biotechnol. 2014;30(9):2481–2490. doi: 10.1007/s11274-014-1673-5. [DOI] [PubMed] [Google Scholar]
- 34.Bonig T, Olbermann P, Bats SH, Fischer W, Josenhans C. Systematic site-directed mutagenesis of the Helicobacter pylori CagL protein of the Cag type IV secretion system identifies novel functional domains. Sci Rep. 2016;6:38101. doi: 10.1038/srep38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa H, Iwamoto A, Tanahashi T, Okada R, Yamamoto K, et al. Genetic variants of Helicobacter pylori type IV secretion system components CagL and CagI and their association with clinical outcomes. Gut Pathog. 2017;9:21. doi: 10.1186/s13099-017-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atrisco-Morales J, Martinez-Santos VI, Roman-Roman A, Alarcon-Millan J, De Sampedro-Reyes J, et al. vacA s1m1 genotype and cagA EPIYA-ABC pattern are predominant among Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. J Med Microbiol. 2018;67(3):314–324. doi: 10.1099/jmm.0.000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Maccallum P, Russel D, editors. Molecular cloning: a laboratory manual. New York: Cold Spring Harbour Press; 2001. [Google Scholar]
- 38.Roman-Roman A, Giono-Cerezo S, Camorlinga-Ponce M, Martinez-Carrillo DN, Loaiza-Loeza S, et al. vacA genotypes of Helicobacter pylori in the oral cavity and stomach of patients with chronic gastritis and gastric ulcer. Enferm Infecc Microbiol Clin. 2013;31(3):130–135. doi: 10.1016/j.eimc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Chang YH, Wang L, Lee MS, Cheng CW, Wu CY, et al. Genotypic characterization of Helicobacter pylori cagA and vacA from biopsy specimens of patients with gastroduodenal diseases. Mt Sinai J Med. 2006;73(3):622–626. [PubMed] [Google Scholar]
- 40.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, et al. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, et al. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3(4):241–253. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, et al. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37(7):2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Huang S, Zhao J, Han J, Guan X, et al. Expression of CagL from Helicobacter pylori and preliminary study of its biological function. Indian J Microbiol. 2013;53(1):36–40. doi: 10.1007/s12088-012-0341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slater E, Owen RJ, Williams M, Pounder RE. Conservation of the cag pathogenicity island of Helicobacter pylori: associations with vacuolating cytotoxin allele and IS605 diversity. Gastroenterology. 1999;117(6):1308–1315. doi: 10.1016/s0016-5085(99)70281-7. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.