Abstract
Background
Insufficient consumption of fruits and vegetables in childhood increases the risk of future non‐communicable diseases, including cardiovascular disease. Interventions to increase consumption of fruit and vegetables, such as those focused on specific child‐feeding strategies and parent nutrition education interventions in early childhood may therefore be an effective strategy in reducing this disease burden.
Objectives
To assess the effectiveness, cost effectiveness and associated adverse events of interventions designed to increase the consumption of fruit, vegetables or both amongst children aged five years and under.
Search methods
We searched CENTRAL, MEDLINE, Embase and two clinical trials registries to identify eligible trials on 25 January 2018. We searched Proquest Dissertations and Theses in November 2017. We reviewed reference lists of included trials and handsearched three international nutrition journals. We contacted authors of included studies to identify further potentially relevant trials.
Selection criteria
We included randomised controlled trials, including cluster‐randomised controlled trials and cross‐over trials, of any intervention primarily targeting consumption of fruit, vegetables or both among children aged five years and under, and incorporating a dietary or biochemical assessment of fruit or vegetable consumption. Two review authors independently screened titles and abstracts of identified papers; a third review author resolved disagreements.
Data collection and analysis
Two review authors independently extracted data and assessed the risks of bias of included studies; a third review author resolved disagreements. Due to unexplained heterogeneity, we used random‐effects models in meta‐analyses for the primary review outcomes where we identified sufficient trials. We calculated standardised mean differences (SMDs) to account for the heterogeneity of fruit and vegetable consumption measures. We conducted assessments of risks of bias and evaluated the quality of evidence (GRADE approach) using Cochrane procedures.
Main results
We included 63 trials with 178 trial arms and 11,698 participants. Thirty‐nine trials examined the impact of child‐feeding practices (e.g. repeated food exposure) in increasing child vegetable intake. Fourteen trials examined the impact of parent nutrition education in increasing child fruit and vegetable intake. Nine studies examined the impact of multicomponent interventions (e.g. parent nutrition education and preschool policy changes) in increasing child fruit and vegetable intake. One study examined the effect of a nutrition education intervention delivered to children in increasing child fruit and vegetable intake.
We judged 14 of the 63 included trials as free from high risks of bias across all domains; performance, detection and attrition bias were the most common domains judged at high risk of bias for the remaining studies.
There is very low quality evidence that child‐feeding practices versus no intervention may have a small positive effect on child vegetable consumption equivalent to an increase of 3.50 g as‐desired consumption of vegetables (SMD 0.33, 95% CI 0.13 to 0.54; participants = 1741; studies = 13). Multicomponent interventions versus no intervention may have a very small effect on child consumption of fruit and vegetables (SMD 0.35, 95% CI 0.04 to 0.66; participants = 2009; studies = 5; low‐quality evidence), equivalent to an increase of 0.37 cups of fruit and vegetables per day. It is uncertain whether there are any short‐term differences in child consumption of fruit and vegetables in meta‐analyses of trials examining parent nutrition education versus no intervention (SMD 0.12, 95% CI ‐0.03 to 0.28; participants = 3078; studies = 11; very low‐quality evidence).
Insufficient data were available to assess long‐term effectiveness, cost effectiveness and unintended adverse consequences of interventions. Studies reported receiving governmental or charitable funds, except for four studies reporting industry funding.
Authors' conclusions
Despite identifying 63 eligible trials of various intervention approaches, the evidence for how to increase children's fruit and vegetable consumption remains limited. There was very low‐ and low‐quality evidence respectively that child‐feeding practice and multicomponent interventions may lead to very small increases in fruit and vegetable consumption in children aged five years and younger. It is uncertain whether parent nutrition education interventions are effective in increasing fruit and vegetable consumption in children aged five years and younger. Given that the quality of the evidence is very low or low, future research will likely change estimates and conclusions. Long‐term follow‐up is required and future research should adopt more rigorous methods to advance the field.
This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Keywords: Child, Preschool; Humans; Infant; Eating; Feeding Behavior; Fruit; Vegetables; Conditioning (Psychology); House Calls; Randomized Controlled Trials as Topic; Reward
Interventions for increasing eating of fruit and vegetables in children aged five years and under
Background
Not eating enough fruit and vegetables is a considerable health burden in developed countries. Eating fruit and vegetables is associated with a reduced risk of future non‐communicable diseases (such as cardiovascular disease). Early childhood represents a critical period for the establishment of dietary habits. Interventions to increase consumption of fruit and vegetables in early childhood may therefore be an effective strategy in reducing this disease burden.
Review question
To assess the impact of interventions designed to increase eating of fruit or vegetables or both among children aged five years and under.
Methods
We searched various electronic databases and relevant journals to find trials. We contacted authors of included trials for additional potentially relevant trials. Any randomised trial (participants have the same chance of being assigned to treatment or control) of interventions aiming to increase the intake of fruit or vegetables or both by children aged five years and under that measured intake was eligible. Two review authors independently searched for and extracted information from studies. The evidence is current to January 2018.
Results
We included 63 trials with 11,698 people taking part. Thirty‐nine trials examined child‐feeding practice interventions (e.g. repeated exposure to vegetables), 14 examined parent nutrition education interventions, nine examined multicomponent interventions (e.g. combining preschool policy changes with parent education) and one examined a child nutrition education intervention. Child‐feeding practice and multicomponent interventions may lead to very small increases in children's intake of fruit and vegetable in the short term (less than 12 months). It is uncertain whether parent nutrition education interventions are effective in increasing children's eating of fruit and vegetables. There was not enough information to assess long‐term effectiveness, cost effectiveness and unintended harms. Studies reporting funding support received governmental or charitable funds, except for four studies that received industry funding.
Conclusions
Child‐feeding practice and multicomponent interventions may increase fruit and vegetable intake by children (by 3.50 g and 0.37 cups per day respectively). This conclusion is based on very low‐ and low‐quality evidence and is very likely to change when future research is undertaken. It is uncertain whether parent nutrition education interventions increase children's fruit and vegetable intake.
This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Summary of findings
Summary of findings for the main comparison.
Child feeding interventions compared to no intervention for children aged five years and under
| Child feeding interventions compared to no intervention for children aged five years and under | ||||||
| Patient or population: children aged five years and under Setting: various: preschool (n = 4), school (n = 1), home + lab (n = 2), child health clinic (n = 1), home (n = 4), home + health facility (n = 2) Intervention: child‐feeding interventions Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with child‐feeding interventions | |||||
| Short‐term impact (< 12 months) child vegetable intake | The mean as‐desired vegetable intake was 7.7 grams1 | The mean as‐desired vegetable intake (grams) in the intervention group was 3.50 higher (1.38 higher to 5.73 higher) | ‐ | 1741 (13 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 3, 4 | Scores estimated using a standardised mean difference of 0.33
(0.13 to 0.54) and a standard deviation of
10.61.1 The mean duration of follow‐up post‐intervention for studies included in the meta‐analysis was 6.2 weeks. Harnack 2012 compared ≥ 1 child‐feeding practice interventions to a no‐treatment control and reported a significant increase in intake of fruit but could not be synthesised in meta‐analysis. |
| Short‐term impact (< 12 months) cost effectiveness ‐ not reported | No child‐feeding interventions reported this outcome | ‐ | ‐ | ‐ | ‐ | |
| Short‐term impact (< 12 months) unintended adverse events | One trial (Spill 2011a) reported no adverse effects on amount of meals consumed | ‐ | 39 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5, 6, 7 | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We used the post‐intervention mean and standard deviation of the control group from Wardle 2003a for the risk with no intervention and to re‐express the SMD in terms of grams of intake. 2Downgraded one level for unexplained heterogeneity: Analysis 1.1 (main analysis): I2 = 70%; Analysis 1.5 (subgroup analysis by modality) I2 = 0% (test for subgroup differences); Analysis 1.6 (subgroup analysis by setting) I2 = 62.4% (test for subgroup differences). 3Downgraded one level for risk of bias: fewer than half of the included studies were rated at low risk of bias for 3 of 4 criteria. 4Downgraded one level for high probability of publication bias: most included studies were not combined in meta‐analysis. 5Downgraded one level for risk of bias: due to being assessed as high risk of bias across multiple domains. 6Downgraded one level for imprecision: total sample size was < 400. 7Downgraded one level for high probability of publication bias: no other studies reported assessing adverse events, so selective reporting suspected.
Summary of findings 2.
Parent nutrition education interventions compared to no intervention for children aged five years and under
| Parent nutrition education interventions compared to no intervention for children aged 5 years and under | ||||||
| Patient or population: children aged 5 years and under Setting: various: parenting group (n = 1), home (n = 4), primary care clinic (n = 1), community health centre (n = 1), preschool (n = 2), preschool + home (n = 1), clinic + home (n = 1) Intervention: parent nutrition education interventions Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with parent nutrition education interventions | |||||
| Short‐term impact (< 12 months) child fruit and vegetable intake | The mean servings of vegetables per day was 1.61 | The mean servings of vegetables per day in the intervention group was 0.12 higher (0.03 lower to 0.28 higher) | ‐ | 3078 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 3, 4 | Scores estimated using a standardised mean difference of 0.12
(‐0.03 to 0.28) and a standard deviation of
1.01 The mean duration of follow‐up post‐intervention for studies included in the meta‐analysis was 8.8 weeks. We were unable to pool results of three trials that reported mixed results in the meta‐analysis. One study found a parent‐responsivity and behaviour‐management intervention to be effective in increasing total fruit intake compared to control (Black 2011); one study found a parent health report on fruit and vegetable consumption to be effective in increasing total vegetable intake compared to control, but not fruit (Hunsaker 2017); and the other study found both a parent‐complementary feeding intervention and a parent‐complementary feeding and home‐visit intervention to be effective in increasing both fruit and vegetable intake compared to control (Vazir 2013). |
| Short‐term impact (< 12 months) cost effectiveness | Information regarding intervention costs was reported in 1 trial (Campbell 2013) | ‐ | 389 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5, 6, 7 | ‐ | |
| Short‐term impact (< 12 months) unintended adverse events | One trial (Wyse 2012) reported no adverse effect on family food expenditure | ‐ | 343 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5, 6, 8 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We used the post‐intervention mean and standard deviation of the control group from Skouteris 2015 for the risk with no intervention and to re‐express the SMD in terms of servings of vegetables per day. 2Downgraded one level for unexplained heterogeneity: Analysis 2.1 (main analysis): I2 = 69%; Analysis 2.4 (subgroup analysis by modality): I2 = 16.2% (test for subgroup differences); Analysis 2.5 (subgroups by setting): I2 = 0%. 3Downgraded one level for risk of bias: most studies were at high risk of bias for lack of blinding, and fewer than half were at low risk of bias for other methodological limitations. 4Downgraded one level for imprecision: the confidence intervals contained the null value. 5Downgraded one level for risk of bias: study assessed as high risk of bias for number of domains. 6Downgraded one level for imprecision: total sample size was < 400. 7 Downgraded one level for high probability of publication bias: no other studies reported cost effectiveness, so selective reporting suspected. 8 Downgraded one level for high probability of publication bias: no other studies reported assessing adverse events, so selective reporting suspected.
Summary of findings 3.
Multicomponent interventions compared to no intervention for children aged five years and under
| Multicomponent interventions compared to no intervention for children aged 5 years and under | ||||||
| Patient or population: children aged 5 years and under Setting: various: preschool (n = 2), school (n = 1), preschool + home (n = 2) Intervention: multicomponent interventions Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with multicomponent interventions | |||||
| Short‐term impact (< 12 months) child fruit and vegetable intake | The mean cups of vegetables per day was 1.081 | The mean cups of vegetables per day in the intervention group was 0.37 higher (0.04 higher to 0.69 higher) | ‐ | 2009 (5 RCTs) | ⊕⊕⊝⊝ LOW 2, 3 | Scores estimated using a standardised mean difference of 0.35
(0.04 to 0.66) and a standard deviation of
1.051 The mean duration of follow‐up post‐intervention for studies included in the meta‐analysis was 1.1 weeks 4 studies could not be pooled in meta‐analysis. 3 reported significant increases in both fruit and vegetable consumption, and 1 significantly increased fruit but not vegetable consumption |
| Short‐term impact (< 12 months) cost effectiveness ‐ not reported | No studies reported this outcome | ‐ | ‐ | ‐ | ‐ | |
| Short‐term impact (< 12 months) unintended adverse events ‐ not reported | No studies reported this outcome | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We used the post‐intervention mean and standard deviation of the control group from Williams 2014 for the risk with no intervention and to re‐express the SMD in terms of cups vegetables per day. 2Downgraded one level for unexplained heterogeneity: Analysis 3.1 (main analysis): I2 = 80%; Analysis 3.4 (subgroup analysis by setting): I2 = 94.8% (test for subgroup differences). 3Downgraded one level for risk of bias: fewer than half of the included studies were rated at low risk of bias for 2 of 4 criteria.
Summary of findings 4.
Child nutrition education interventions compared to no intervention for children aged five years and under
| Child nutrition education interventions compared to no intervention for children aged 5 years and under | ||||||
| Patient or population: children aged 5 years and under Setting: preschool Intervention: child nutrition education interventions Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with child nutrition education interventions | |||||
| Short‐term impact (< 12 months) child fruit and vegetable intake | The mean short‐term impact (< 12 months) child vegetable intake frequency score was 4 (a score of 4 corresponds to consumption of vegetables 3 ‐ 4 times per week) | MD 0 | ‐ | 238 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | The only study (Baskale
2011) reported an increase in some of the fruits
and vegetables assessed in the intervention group and no
significant differences in the control group The duration of follow‐up post‐intervention was 8 weeks |
| Cost or cost effectiveness ‐ not reported | No studies reported this outcome | ‐ | ‐ | ‐ | ‐ | |
| Unintended adverse events ‐ not reported | No studies reported this outcome | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for risk of bias: high risk of bias due to lack of blinding and loss to follow‐up. 2Downgraded one level for imprecision: total sample size < 400.
Background
Description of the condition
Insufficient consumption of fruit and vegetables is associated with a range of non‐communicable diseases, such as cancer and cardiovascular disease (Boeing 2012; Hartley 2013; Micha 2015; World Health Organization 2003; World Health Organization 2011). Globally, 2.8% of all deaths and 1.0% of all disability‐adjusted life years (DALYs) each year are attributable to inadequate fruit and vegetable intake (World Health Organization 2017). Low fruit and vegetable consumption is responsible for 14% of gastrointestinal cancer deaths, 11% of all ischaemic heart disease and 9% of all stroke deaths (World Health Organization 2017) and as a result is a public health priority.
The daily amount of fruit and vegetables recommended for children aged five years and younger varies internationally. For example, in the USA 1 and 1 to 1.5 cups each of fruit and vegetables is recommended respectively for children aged two to three years and four to eight years (U.S. Department of Health and Human Services). Whereas in Australia, 0.5 to 1.15 servings of fruit (75 g to 113 g) and 2 to 4.5 servings of vegetables (150 g to 338 g) are recommended for children aged one to two years, two to three years and four to eight years (National Health and Medical Research Council). Population surveys of children indicate that such recommendations are not met and there is a need to increase children's intake of fruit and vegetables (Australian Bureau of Statistics 2014; Inchley 2016; Lock 2005; National Cancer Institute 2015; World Health Organization 2004a; Yngve 2005). For example, just over a third of school‐aged children from European nations report consuming vegetables on a daily basis (Inchley 2016). Data from younger children are similar. A survey conducted in 2007 to 2010 in the USA reported that 33% of children aged one to three years met fruit recommendations and 13% met vegetable recommendations (National Cancer Institute 2015). A national survey in 2011 to 2012 in Australia reported that 90% of children aged two to eight years consumed the recommended number of fruit servings a day, and 49% of children aged two to three years consumed the recommended servings of vegetables (Australian Bureau of Statistics 2014). Globally, the mean intake of fruit and vegetables is below the World Health Organization (WHO) recommendations across all WHO regions. South American, African, and South East Asian nations report the lowest quantities of child fruit and vegetable intake, where school‐aged children typically consume less than 300 g a day (Lock 2005).
There is some evidence from longitudinal studies to suggest that eating behaviours established in childhood are likely to persist into adulthood (Craigie 2011; Lien 2001; Mikkilä 2004). Follow‐up data at 37 years from the Boyd Orr cohort study of British children, for example, found lower rates of all‐cause cardiovascular mortality among children with greater intake of vegetables in childhood (Ness 2005). Additionally, longitudinal studies have shown that fruit and vegetable consumption in childhood is associated with reductions in non‐communicable diseases in adulthood (Maynard 2003; Ness 2005). Encouraging healthy eating among children may therefore represent an effective primary prevention strategy for reducing the risk of non‐communicable diseases (Boeing 2012; Centers for Disease Control and Prevention 2011; Maynard 2003; Ness 2005; World Health Organization 2004b). Adequate fruit and vegetable intake during childhood may also have a number of immediate benefits, including reducing the risk of micronutrient deficiencies and a number of respiratory illnesses (Antova 2003; Boeing 2012; Forastiere 2005; World Health Organization 2003).
Description of the intervention
The aetiology of fruit and vegetable consumption is complex, involving the dynamic interaction of a variety of factors. Given such complexity, a number of frameworks have been produced to guide the development of interventions to increase fruit and vegetable intake (Centers for Disease Control and Prevention 2011; Klepp 2005; Miller 2000; World Health Organization 2004b). For example, the conceptual framework developed for the international Pro Children Project suggests that interventions targeting a variety of cultural, physical and social environment factors, as well as those targeting personal factors, may be effective in positively influencing fruit and vegetable intake among children (Klepp 2005).
Despite the range of potential intervention targets, including primordial prevention interventions that target the risk factors of non‐communicable diseases before they occur (compared to primary prevention interventions that treat risk factors of non‐communicable diseases), previous trials have tended to focus on those determinants more amenable to intervention, such as nutrition knowledge and skills, or the food environment of settings such as schools (Hector 2008). Among school‐aged children, systematic reviews suggest that the strongest evidence exists for the effectiveness of multicomponent interventions with elements such as curriculum, parental engagement, policy and food environment changes (Blanchette 2005; De Sa 2008; Jaime 2009; Knai 2006; Van Cauwenberghe 2010). Previous reviews in children aged five years and younger (Campbell 2007; Hesketh 2010; Tedstone 1998) have similarly found some evidence for multicomponent interventions. For example, an intervention aiming to prevent the onset of cardiovascular disease in preschoolers targeted multiple risk factors, including child fruit and vegetable consumption (Peñalvo 2013a; Peñalvo 2013b). The multicomponent intervention including curriculum, school environment and family components successfully improved preschoolers' fruit and vegetable habits, which were also maintained over time (Peñalvo 2013a; Peñalvo 2013b; Peñalvo 2015).
How the intervention might work
A number of theories have been used to explain the mechanisms by which interventions may influence children's fruit and vegetable consumption (Rasmussen 2006). In most instances, psychosocial theories such as Social Cognitive Theory (Bandura 1986), the Theory of Planned Behaviour (Ajzen 1991), or the Stages of Change Trans‐theoretical Model (Prochaska 1984) have been used to explain possible causal pathways to fruit and vegetable consumption (Rasmussen 2006). Collectively, such theories assert that changes in attitudes, knowledge and skills and perceived norms and expectancies are required for behavioural change. The international Pro Children Project incorporated Social‐Ecological Model in its conceptual theoretical framework of determinants of children's fruit and vegetable consumption (Klepp 2005). Interventions derived from Social‐Ecological Model recognise the importance of more structural influences on children's intake of fruit and vegetable consumption, for example, the availability or accessibility of fruit and vegetables in the home or in settings such as schools which children frequent.
Why it is important to do this review
Previous reviews have identified a number of factors associated with fruit and vegetable consumption among children (Blanchette 2005; Pearson 2008; Rasmussen 2006; Van der Horst 2007). While such reviews provide important information for the development of interventions, only systematic reviews of intervention trials can determine the effectiveness of strategies to increase child fruit and vegetable consumption. A number of such reviews have been published (Burchett 2003; Ciliska 2000; Delgado‐Noguera 2011; De Sa 2008; Evans 2012; French 2003; Hendrie 2017; Howerton 2007; Knai 2006; Savoie‐Roskos 2017; Van Cauwenberghe 2010). However, only a few have focused specifically on children aged five years and under (Campbell 2007; Hesketh 2010; Tedstone 1998), with the most recent of these conducted in 2010. Despite these reviews reporting a positive effect of such interventions (Hesketh 2010; Tedstone 1998), most lacked important information relevant to practice, such as the effectiveness of interventions for various subpopulations (such as minority groups), the cost effectiveness of interventions, or the presence of any unintended adverse effects of the intervention. Similarly, as positive impacts of health behaviour interventions may not be sustained, an examination of the longer‐term effectiveness of interventions (more than 12 months post‐intervention) is important for policy‐makers and practitioners to assess the potential health benefits of fruit and vegetable interventions (Fjeldsoe 2011; Jones 2011). Previous reviews have not specifically examined the impact of interventions based on the length of post‐intervention follow‐up. A comprehensive systematic review on this issue is therefore required to provide guidance for practitioners and policy‐makers interested in implementing strategies to promote the consumption of fruits and vegetables in early childhood.
Following the publication of the 2017 update of this review, we will maintain it as a living systematic review, as a pilot up until the end of March 2018. This means we will be continually running the searches and rapidly incorporating any newly‐identified evidence into the review (for more information about the living systematic review approach being piloted by Cochrane, see Appendix 1). We believe a living systematic review approach is appropriate for this review, for three reasons. First, the review addresses a particularly important public health issue; the growing burden of disease and mortality attributable to low fruit and vegetable intake. Insufficient consumption of fruits and vegetables is associated with a range of non‐communicable diseases such as cancer and cardiovascular disease, and in most regions of the globe current daily consumption of fruits and vegetables is well below the recommended intake to reduce the risk of non‐communicable diseases. Early childhood represents a critical period for the establishment of healthy eating behaviours, such as fruit and vegetable intake, as dietary habits developed early are likely to persist into adulthood. It is therefore important to better understand how to improve intake of fruits and vegetables during childhood. Secondly, there remains uncertainty in the existing evidence; despite searches including the current update (up to 25 January 2018) identifying 63 studies for inclusion in the review, no high‐quality evidence exists of effective interventions to increase the fruit and vegetable consumption of children. Thirdly, we are aware of multiple ongoing trials in this area of research that will be important to incorporate, and we expect that future research will have an impact on the conclusions.
Objectives
To assess the effectiveness, cost effectiveness and unintended adverse events of interventions designed to increase the consumption of fruit or vegetables or both among children aged five years and under.
Methods
Criteria for considering studies for this review
Types of studies
Eligible trials were randomised controlled trials (RCTs), including cluster‐randomised controlled trials (C‐RCTs) and cross‐over trials, that:
compared two or more alternative intervention programmes to increase the consumption of fruit or vegetables or both of children aged five years and under;
compared an intervention programme to increase the consumption of fruit or vegetables or both of children aged five years and under with a standard‐care or no‐intervention control group.
We excluded trials which did not include fruit or vegetable intake as a primary trial outcome, to avoid the potential confounding effects of other interventions, and because publication bias and selective outcome reporting are more predominant among secondary trial outcomes (or outcomes that were not otherwise stated). We included trials that did not state a primary trial outcome but did assess an eligible fruit or vegetable intake outcome. We included eligible cross‐over trials in the review, as we deemed them a suitable and common method for assessing the effect of interventions to increase the fruit and vegetable consumption of children.
Types of participants
Participants could include:
children aged five years and under. Trials including children older than five years were included only if the mean age of the study sample at baseline was five years or less;
parents, guardians and families responsible for the care of children aged five years and under;
professionals responsible for the care of children aged five years and under, including childcare staff and health professionals.
Types of interventions
We considered any educational, experiential, health promotion and/or psychological or family or behavioural therapy or counselling or management or structural or policy or legislative reform interventions, designed to increase consumption of fruit or vegetables or both in children aged five years and under (as defined in types of participants). Interventions could be conducted in any setting including the home, childcare/preschool services, health services, or community settings.
Comparison: Any alternative intervention to encourage fruit and vegetable consumption as described above, or a no‐intervention control, usual care, or attention control or wait‐list control. Attention controls in randomised trials for behavioural interventions are those that include clinical attention and induce the expectation of therapeutic benefit for control for non‐specific effects of the intervention (Freedland 2011). Wait‐list control groups that are also designed to control for non‐specific effects involve participants being allocated to receive an intervention at study conclusion (delayed start) (Whitehead 2004).
Types of outcome measures
We included studies with evaluated outcomes, measuring biomedical or dietary indices, or both, of the review's primary outcome.
Primary outcomes
The primary outcome was children's fruit and vegetable intake. Fruit and vegetable intake could be assessed using a variety of measures, including:
change in the number of portions or serves of daily fruit or vegetable or both at follow‐up, as measured by diet recalls, food diaries, food frequency questionnaires or diet records completed by an adult on behalf of the child. We grouped the interventions by short‐term effects (less than 12 months post‐intervention) and long‐term effects (at least 12 months post‐intervention);
change in grams of fruit or vegetables or both at follow‐up, as measured by diet recalls, food diaries, food frequency questionnaires or diet records completed by an adult on behalf of the child. We grouped them by short‐term effects (less than 12 months post‐intervention) and long‐term effects (at least 12 months post‐intervention);
changes in biomedical markers of consumption of fruit or vegetables or both, such as α‐carotene, β‐carotene, cryptoxanthin, lycopene and lutein. We grouped them by short‐term effects (less than 12 months post‐intervention) and long‐term effects (12 months or more post‐intervention).
Outcomes of fruit or vegetable juice intake alone were not eligible. Outcomes that included child fruit and vegetable juice intake as part of an aggregate measure of child fruit or vegetable intake were eligible.
Secondary outcomes
Estimates of absolute costs and cost effectiveness of interventions to increase the consumption of fruits and vegetables reported in identified studies.
Any reported adverse effects of an intervention to increase the consumption of fruits and vegetables reported in identified studies. This could include any physical, behavioural, psychological or financial impact on the child, parent or family, or the service or facility where an intervention may have been implemented.
Search methods for identification of studies
This review represents the third update of a review first published in 2012 (Wolfenden 2012) and updated in 2017 (Hodder 2017) and January 2018 (Hodder 2018).
Electronic searches
We searched the following electronic databases on 25 October 2017, 25 November 2017, 25 December 2017 and 25 January 2018 to identify any relevant trials added since the last published review (Hodder 2018):
Cochrane Central Register of Studies (CENTRAL, via CRS‐Web);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 25 January 2018);
Embase (Ovid, 1980 to 2018 Week 4).
As a living systematic review, we are conducting monthly searches of these databases, for which we have set up auto‐alerts to deliver monthly search yields, where possible.
We had previously conducted electronic searches of CINAHL (EBSCO, 1937 to 5 July 2016) (searched 5 July 2016) and PsycINFO (Ovid, 1806 to June week 5 2016) (searched 5 July 2016) (Hodder 2017) .
The search strategies are described in Appendix 2. We applied the sensitivity‐maximising version of the Cochrane RCT filter (Lefebvre 2011) to MEDLINE, and adaptations of it to the other databases except for CENTRAL. We imposed no restrictions by date or language of publication.
Searching other resources
We searched the reference lists of included articles and handsearched all articles published between September 2016 and September 2017 in three relevant international peer‐reviewed journals (Journal of Nutrition Education and Behavior, Public Health Nutrition, and Journal of the Academy of Nutrition and Dietetics (previously titled Journal of the American Dietetic Association)).
We are now running monthly trial registry searches of the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/) and ClinicalTrials.gov (www.clinicaltrials.gov), which we last conducted in January 2018. In September 2016 we also searched a third clinical trials register, the metaRegister of clinical trials (www.isrctn.com/page/mrct).
We also searched a database of published dissertations (Proquest Dissertations and Theses) in November 2017 and GoogleScholar in December 2017.
We contacted the authors of included studies to try to obtain other eligible trials published in peer‐reviewed journals, as well as ongoing trials. We describe ongoing studies, where available, detailing the primary author, research question(s), methods and outcome measures (Characteristics of ongoing studies).
As this is a living systematic review, we will continue to handsearch the three journals listed above, the database of published dissertations and 'grey literature' in GoogleScholar manually every six months.
As additional steps to inform the living systematic review, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available, or to share early or unpublished data. We will contact the corresponding authors of any newly‐included studies for advice as to other relevant studies. We will conduct citation tracking of included studies in Web of Science Core Collection on an ongoing basis. For that purpose, we have set up citation alerts in Web of Science Core Collection. We will manually screen the reference lists of any newly‐included studies and systematic reviews.
We will review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
Data collection and analysis
Selection of studies
Pairs of review authors (from RH, KO, RW, FS, SY, NN) independently screened titles and abstracts of identified papers. Review authors were not blinded to the details of the study author or journal. Review authors applied a standardised screening tool to assess eligibility. We screened articles against the eligibility criteria of participants (mean age of children more than five years), outcome (primary outcome was not fruit and vegetable intake), comparator (was not a no‐intervention, usual care, attention or wait‐list control), intervention (did not aim to increase child fruit or vegetable intake) and study type (was not RCT, C‐RCT or cross‐over trial with random allocation to group). Based on the title and abstract, we excluded papers which clearly did not meet the eligibility criteria of the review. Pairs of review authors (from FS, RH, KO, NN, RS, SY) then independently examined the full text of all remaining articles. We documented Information regarding the reason for the ineligibility of any paper for which we reviewed the full text, and present it in the table 'Characteristics of excluded studies'. A third review author with expertise in review methodology (LW) resolved any disagreements between review authors on study eligibility. For those papers which did not provide sufficient information to determine eligibility, we contacted the study authors for clarification.
We will immediately screen any new citations retrieved by the monthly searches. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) (Wallace 2017), available in the Cochrane Register of Studies (CRS‐Web) (Cochrane 2017a). The classifier assigns a probability (from 0 to 100) to each citation of being a true RCT. For citations that are assigned a probability score of less than 10, the machine learning classifier currently has a specificity/recall of 99.987% (Wallace 2017). We will screen in duplicate and independently all citations that have been assigned a score from 10 to 100. Cochrane Crowd will screen citations that score 9 or less (Cochrane 2017b) and will return any citations that they deem to be potential RCTs to the review authors for screening.
Data extraction and management
Pairs of review authors (from EJ, RW, RH, KB, KO, ER, TCM, RS, NN) independently extracted data from each included trial. Review authors were not blinded to the details of the study author or journal. We recorded data on data extraction forms designed and piloted specifically for this review. Consultation with a third review author with expertise in review methodology (LW) resolved discrepancies between review authors about data extraction. We tried to contact authors of included papers in instances where the information required for data extraction was not available from the published report, or was unclear. One review author entered extracted data into the systematic review software Review Manager 5 (RevMan 5) (RH) and another review author checked it (KO). Where available, we extracted the following information from included trials:
Information on the study, research design and methods, such as the study authors; date of publication; date of study initiation; study duration; setting; number of participants; participants' age, gender, ethnicity, and socioeconomic position;
Information on the experimental conditions of the trial, such as the number of experimental conditions; intervention and comparator components; duration; number of contacts; modalities; interventionist; and integrity;
Information on the trial outcomes and results, such as rates of recruitment and attrition; sample size; number of participants per experimental condition; mean and standard deviation of the primary or secondary outcomes described above; any subgroup analyses by gender, population group or intervention characteristics; and analyses (including whether studies appropriately adjusted for clustering).
Assessment of risk of bias in included studies
Working in pairs, review authors FS, FT and TCM independently assessed the risks of bias in the included studies. We consulted a fourth review author (RH) with expertise in review methodology to resolve any disagreements between review authors. Review authors used the tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the risks of bias. The tool requires an explicit judgement by the review authors, based on trial information, about the risks of bias attributable to the generation of the random sequence, the allocation concealment, the blinding of participants, personnel and outcome assessors, the completeness of outcome data, selective reporting, and any other potential threats to validity. We also judged recruitment bias, baseline imbalance, loss of clusters and incorrect analysis for C‐RCTs. Judgements on the risks of bias for each trial are recorded in the ‘Risk of bias’ tables accompanying the review.
Measures of treatment effect
Where meta‐analyses were performed, we expressed the intervention effect as a mean difference (MD) where outcomes were reported using a standard metric (such as grams), and as a standardised mean difference (SMD) where outcomes were reported using different methods or metrics of fruit and vegetable intake (such as grams, grams per kilogram of body weight, and serves per day).
Unit of analysis issues
We assessed cluster‐randomised trials in the review for unit‐of‐analysis errors. Where cluster‐randomised studies did not account for clustering, we contacted study authors to provide intra‐class correlation coefficients (ICCs) to allow calculation of design effects and effective sample sizes to enable individual‐level pooling. Where ICCs were not available, we estimated a mean ICC from reported ICCs of included studies, and used it to calculate effective sample sizes.
Dealing with missing data
Where available, we reported outcomes of trials using an intention‐to‐treat analysis. If studies did not report intention‐to‐treat analyses, we reported as‐treated analysis of trial outcomes. We explored the impact of including as‐treated trial outcomes in meta‐analysis for studies with a high rate of attrition (more than 20% for short‐term outcomes) in sensitivity analyses (see below Sensitivity analysis). We contacted study authors to obtain any missing data (e.g. standard deviations).
Assessment of heterogeneity
We assessed statistical heterogeneity by visual inspection of forest plots of the included trials, and calculation of the I2 statistic where we were able to pool data from included trials (Higgins 2003). Due to the similarity in trial characteristics (e.g. type of participants, intervention or outcomes), we could not conduct subgroup analyses by trial characteristics to identify the source of substantial heterogeneity (defined as I2 greater than 50%).
Assessment of reporting biases
We checked for reporting bias by visual inspection of the funnel plots.
Data synthesis
We assessed trial outcomes using a variety of dietary assessment tools and reported in various metrics, including vitamin C from fruit, fruit or vegetable serves, and grams of fruit and/or vegetable consumption. We calculated standardised mean differences (SMDs; to account for variable outcome measures) for each comparison, using the generic inverse variance method in a fixed‐effect meta‐analysis model (where there was no or low statistical heterogeneity in the primary analysis) or a random‐effects meta‐analysis model (where there was unexplained heterogeneity in the primary analysis), using the RevMan 5 software. We selected post‐intervention values over change‐from‐baseline data for inclusion in meta‐analysis, to reduce the risk of selective reporting and to maximise the number of studies that could be pooled.
We synthesised studies that provided data suitable for pooling in meta‐analyses grouped by intervention type (infant feeding, parent nutrition education, and multicomponent interventions). When studies reported multiple fruit or vegetable outcomes, we selected the stated primary trial outcome for inclusion in our meta‐analyses, or if a primary outcome was not stated we selected the first reported outcome for inclusion. For studies which reported multiple follow‐up points, we extracted data from the longest follow‐up period for inclusion in meta‐analyses.
We selected reported study estimates that adjusted for potential confounding variables for inclusion in meta‐analysis over reported estimates that did not adjust for potential confounding variables. Similarly, for C‐RCTs that reported study estimates that were unadjusted and adjusted for clustering, we preferred estimates that adjusted for clustering for inclusion in meta‐analyses. For C‐RCTs that did not report post‐intervention study estimates (and a relevant measure of variance) that accounted for clustering, we calculated a design effect and effective sample size using study data (number of clusters, number of participants analysed) and a reported ICC from one of the included studies (vegetable intake: ICC 0.014, fruit intake: ICC 0.016; De Bock 2012). For such C‐RCTs (De Coen 2012; Martinez‐Andrade 2014; Namenek Brouwer 2013; Nicklas 2017; O'Connell 2012; Roset‐Salla 2016; Smith 2017; Verbestel 2014; Williams 2014; Zeinstra 2018), we entered the reported post‐intervention outcome data (e.g. mean and standard deviation) and author‐calculated effective sample sizes into RevMan 5 to calculate individual‐level adjusted study estimates to enable inclusion in meta‐analyses. We tried to pool studies separately that compared two or more alternative interventions.
For cross‐over trials, we tried to synthesise results separately from parallel RCTs, by pooling results from paired analyses that adjust for within‐individual comparisons. If such data were not available, we combined results by pooling data from the first cross‐over period (i.e. essentially a parallel RCT) with parallel RCTs.
In all instances where we could not combine data in a meta‐analysis, we have provided a narrative summary of the trial findings according to the review objectives.
Whenever we find new evidence (i.e. studies, data or information) meeting the review inclusion criteria, we will extract the data, assess risks of bias and incorporate it into the synthesis every three months, as appropriate.
We will incorporate any new study data into existing meta‐analyses using the standard approaches outlined in the Data synthesis section.
We will not adjust the meta‐analyses to account for multiple testing, given that the methods related to frequent updating of meta‐analyses are under development (Simmonds (in press)).
Summary of Findings table and GRADE
We created 'Summary of findings' tables using the following outcomes:
Child fruit and vegetable intake. This could include changes in the number of portions or serves or grams of daily fruit or vegetable or both at follow‐up, as measured by diet recalls, food diaries, food frequency questionnaires or diet records completed by an adult on behalf of the child; or changes in biomedical markers of consumption of fruit or vegetables or both, such as α‐carotene, β‐carotene, cryptoxanthin, lycopene and lutein.
Estimates of absolute costs and cost effectiveness of interventions to increase the consumption of fruit and vegetables reported in the included studies;
Any reported adverse events of an intervention to increase the consumption of fruit and vegetables reported in the included studies. This could include any physical, behavioural, psychological or financial impact on the child, parent or family, or the service or facility where an intervention may have been implemented.
We have produced four 'Summary of findings' tables, one for each of the following comparisons:
Child‐feeding interventions compared to no‐intervention control;
Parent nutrition education interventions compared to no‐intervention control;
Multicomponent interventions compared to no‐intervention control;
Child nutrition education interventions compared to no‐intervention control.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (gradepro.org/). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary. For each comparison where we had calculated a SMD, we re‐expressed it based on the instrument used in the lowest risk of bias in that comparison (e.g. grams of vegetable intake or serves of vegetables a day), by multiplying the post‐intervention standard deviation of the control group by the pooled SMD.
Two review authors (RH and NN), working independently, judged the quality of the evidence, with disagreements resolved by discussion or by involving a third review author (LW). We justified, documented and incorporated the judgements into the reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Subgroup analysis and investigation of heterogeneity
Where possible, we conducted subgroup analyses of interventions for the following subgroups, which we had planned a priori:
Interventions targeting boys and girls (not conducted);
Interventions targeting minority groups including indigenous populations (not conducted, described narratively);
Interventions delivered in various settings including health and children’s services (conducted where possible for some comparisons and settings);
Interventions of varying intensities, defined in terms of the number and duration of intervention contacts or components (not conducted);
Interventions delivered in different modes, such as by telephone, the Internet or face‐to‐face (conducted for some comparisons and modalities, otherwise described narratively).
Sensitivity analysis
Where possible, we conducted sensitivity analyses to explore the impact on the overall assessment of treatment effects:
Excluding studies at high risk of bias (defined a priori);
Excluding studies not reporting an intention‐to‐treat analysis, with high rates of participant attrition defined as greater than 20% (defined a priori);
Excluding studies that did not have a primary outcome of child fruit and vegetable, fruit or vegetable consumption (post hoc).
For the sensitivity analysis excluding studies that did not have a primary outcome of child fruit and vegetable, fruit or vegetable consumption, we considered studies to have a primary outcome of children's fruit and vegetable intake even when this was not explicitly stated if: children's fruit and vegetable intake was the only reported outcome, a sample size calculation for children's fruit and vegetable intake was reported, or children's fruit and vegetable intake was the first reported outcome.
Other
We will review our scope and methods if appropriate in the light of potential changes in the topic area, or the evidence being included in the review (e.g. additional comparisons, interventions or outcomes, or new review methods available).
We are piloting this review as a living systematic review up until March 2018.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We ran searches for the previous reviews (Wolfenden 2012; Hodder 2017; Hodder 2018) and this review update, which together generated a total of 25,480 citations (24,661 previous reviews; 819 this review update). Screening of titles and abstracts for the review update identified 91 records (737 in total, including 646 from the previous reviews) for formal inclusion or exclusion (See Figure 1). Of these, 63 trials (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Blissett 2016; Campbell 2013; Caton 2013; Cohen 1995; Cooke 2011; Correia 2014; Cravener 2015; Daniels 2014; De Bock 2012; De Coen 2012; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Duncanson 2013; Fildes 2014; Fildes 2015; Fisher 2012; Forestell 2007; Gerrish 2001; Haire‐Joshu 2008; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Hunsaker 2017; Keller 2012; Kling 2016; Martinez‐Andrade 2014; Mennella 2008; Namenek Brouwer 2013; Natale 2014a; Nicklas 2017; O'Connell 2012; Remington 2012; Remy 2013; Roe 2013; Roset‐Salla 2016; Savage 2012; Sherwood 2015; Skouteris 2015; Smith 2017; Spill 2010; Spill 2011a; Spill 2011b; Staiano 2016; Sullivan 1994; Tabak 2012; Vazir 2013; Verbestel 2014; Vereecken 2009; Wardle 2003a; Watt 2009; Williams 2014; Witt 2012; Wyse 2012; Zeinstra 2018) met the inclusion criteria, eight of which were new studies identified in the most recent update (Cohen 1995; Forestell 2007; Gerrish 2001; Heath 2014; Kling 2016; Sherwood 2015; Smith 2017; Zeinstra 2018). We contacted authors of the included trials for any missing outcome data, to permit meta‐analysis.
Figure 1.
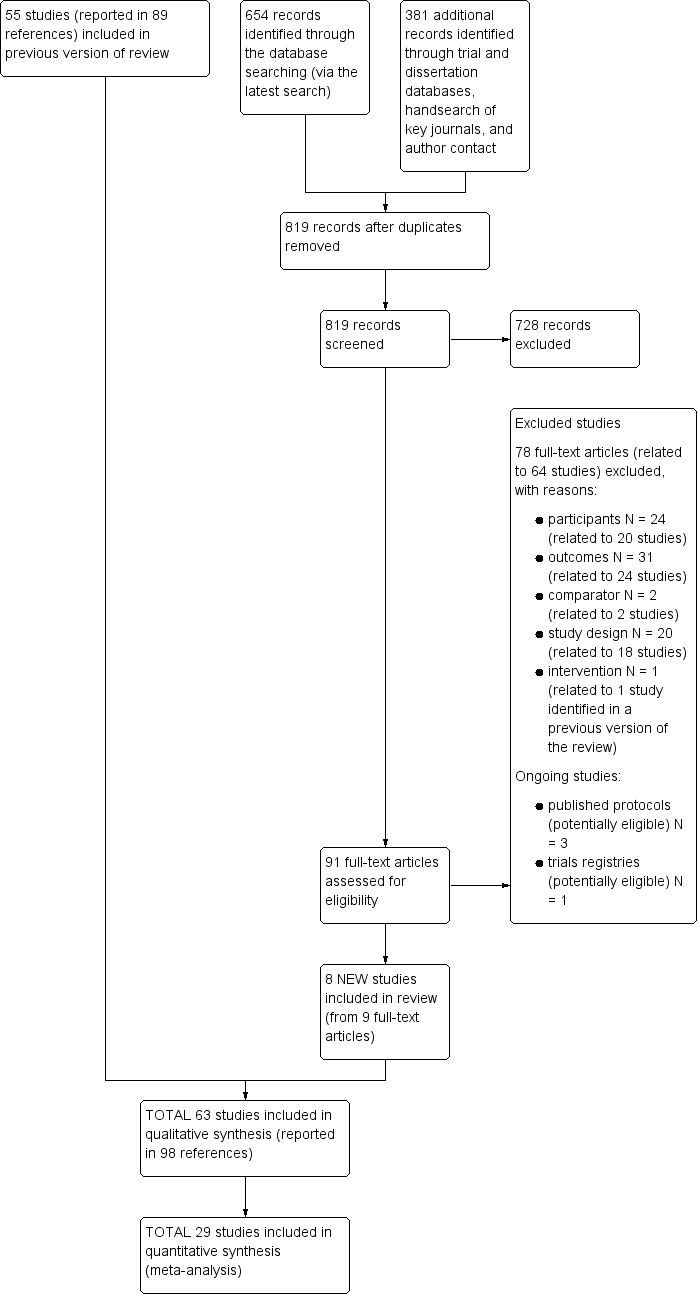
Study flow diagram
Included studies
There were 178 trial arms and 11,698 participants randomised across the 63 included trials. We give full details of the trials in the Characteristics of included studies table. Twenty‐nine trials were undertaken in the USA, nine in the UK, eight in the Netherlands, five in Australia, three in Belgium, and one each in Turkey, Germany, Denmark, Mexico, France, Spain, Honduras and India, and one study that was undertaken in the UK, Greece and Portugal. Thirty‐two of the included studies were RCTs, of which 17 compared an intervention to a no‐treatment control group; 21 were C‐RCTs, of which 18 compared an intervention to a no‐treatment control group; and 10 were cross‐over trials. The unit of randomisation in C‐RCTs included childcare centres or preschools (n = 14), parent groups (n = 2), preschool classrooms (n = 1), primary schools (n = 1), primary school classrooms (n = 1), primary care clinics (n = 1) and villages (n = 1). Twenty‐nine trials were conducted in a preschool or school setting; 14 in a home setting; five in a healthcare setting (e.g. primary care); six in a home and laboratory setting; two in a laboratory setting; three in a preschool and home setting; and four in a home and healthcare setting. Included studies examined the impact of various types of interventions to increase child fruit and vegetable consumption. Fifty‐nine of the included studies assessed intake of vegetables, and 31 assessed intake of fruit. Various objective and subjective measures were used to assess fruit and vegetable intake, such as as‐desired intake and mean daily intake as reported by parents. One trial reported information about intervention costs and two trials reported information on any adverse events or unintended adverse consequences of the intervention. Information on the reliability and validity of selected fruit and vegetable intake outcome measures in children were reported by 11 studies. Post‐intervention follow‐up periods ranged from immediate to 3.5 years. Of the 63 included studies, 13 did not report whether funding support was received to undertake the trial, one study reported no funding support (Baskale 2011), and the remaining 49 studies reported a source of funding. Funding support for such studies were governmental or charitable, with the exception of four studies that reported receiving funding from food industry sources (Fisher 2012; Gerrish 2001; Sullivan 1994; Tabak 2012).
Child‐feeding practice interventions
Thirty‐nine trials tested the impact of specific child feeding‐practice interventions (e.g. repeated exposure) in increasing children's intake of fruit or vegetables (Anzman‐Frasca 2012; Barends 2013; Blissett 2016; Caton 2013; Cohen 1995; Cooke 2011; Correia 2014; Cravener 2015; Daniels 2014; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Forestell 2007; Fildes 2014; Fildes 2015; Fisher 2012; Gerrish 2001; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Keller 2012; Kling 2016; Mennella 2008; O'Connell 2012; Remington 2012; Remy 2013; Roe 2013; Savage 2012; Spill 2010; Spill 2011a; Spill 2011b; Staiano 2016; Sullivan 1994; Wardle 2003a; Zeinstra 2018). Of the trials testing the impact of specific feeding‐practice interventions, 25 compared the effectiveness of two or more interventions and 14 trials compared one or more interventions with a no‐treatment control group; eleven of these were cross‐over trials.
Fourteen trials examined the effect of repeated exposure compared to an alternate or no intervention. Five compared the effect of a repeated exposure intervention to one or more alternative interventions (including associative conditioning, flavour‐flavour learning, flavour‐nutrient learning, choice of vegetable versus no choice) (Anzman‐Frasca 2012; Barends 2013; Caton 2013; Hausner 2012; Remy 2013), one compared the effect of repeated exposure choice offering of vegetable to no choice (de Wild 2015a), one study compared the effect of repeated exposures and variety (Mennella 2008), and one study compared the effect of repeated exposure to a target vegetable using different preparation methods compared to a control vegetable (de Wild 2017). The other six trials examined the effect of a repeated exposure intervention compared to no‐treatment control, of which one trial each examined the effect of repeated exposure alone (O'Connell 2012), taste exposure plus rewards (Fildes 2014), exposure plus social reward and exposure plus tangible reward (Remington 2012), exposure and nutrition information (Wardle 2003a), exposure plus tangible reward, exposure plus social reward and exposure alone (Cooke 2011), and repeated exposure over five months within a childcare setting (Zeinstra 2018).
Two trials examined the effect of flavour nutrient learning, of which one trial compared the effects of low‐energy vegetable soup versus high‐energy vegetable soup (de Wild 2013), and the other trial compared incorporation of vegetable puree into meals at three different levels of energy density (Spill 2011a). A further trial examined the effect of six different levels of portion size and energy density on vegetable intake (Kling 2016).
Five trials examined the effect of parent‐feeding interventions. One trial compared the effects of advice to the parent about introducing vegetables to no‐treatment control (Fildes 2015), one trial compared the effects of an early feeding intervention targeting complementary feeding practices to a no‐treatment control (Daniels 2014), one trial compared the effects of early and repeated exposure to vegetables during complementary feeding to a no‐treatment control (Hetherington 2015), one trial compared parent prompting and modelling, parent prompting alone and modelling alone (Blissett 2016), and the other trial compared exclusive breastfeeding, complementary feeding with breastfeeding, and complementary feeding with breastfeeding on demand (Cohen 1995).
Five trials examined the effect of pairing fruit and vegetables with positive stimuli. One trial compared pairing vegetables with stimuli such as stickers and cartoon packaging to a no‐treatment control (Cravener 2015), one trial compared pairing fruit and vegetables with character branding to a no‐treatment control (Keller 2012), one trial compared pairing of vegetables with a modelling DVD to a non‐food DVD and a no‐DVD control group (Staiano 2016), one trial compared the effect of pairing passive and interactive story‐telling (about a character that eats carrots) featuring either a product‐congruent (a rabbit) or product‐incongruent (a turtle) character across four experimental groups compared to a control group (de Droog 2014), and the fifth trial compared the effects of passive and interactive story‐telling (about a rabbit that eats carrots) with or without the use of a hand puppet (de Droog 2017).
Four trials examined the effect of pairing target vegetables with liked foods (Correia 2014; de Wild 2015b; Fisher 2012; Forestell 2007). Two trials examined the effect of varying serving sizes (Savage 2012; Spill 2011b). One trial examined the effects of dietary experience (salted or unsalted vegetables) (Sullivan 1994). The remaining three trials examined the effect of different serving methods; one trial compared serving fruit and vegetables first before other menu items to a specific plate of prepared food (Harnack 2012), one trial compared three different portion sizes of vegetables served at the beginning of a meal to a control meal (Spill 2010), and the third trial of eight arms compared the impact of a single type of vegetable, a variety of vegetables, a single type of fruit, and a variety of fruits on consumption (Roe 2013). One trial compared the effect of repeated exposure to pureed green beans alone to pureed green beans and peaches on green bean consumption (Forestell 2007).
One trial examined the effect of introducing a variety of flavours when introducing vegetables, which compared exposure to target vegetable (carrot), an alternate vegetable (potato), and a variety of vegetables that did not include the target vegetable (Gerrish 2001). One trial compared exposure to a picture book of a liked, disliked and unfamiliar vegetable on vegetable consumption (Heath 2014).
Parent nutrition education interventions
Fourteen studies tested the impact of parent nutrition education interventions in increasing children's intake of fruit or vegetables (Black 2011; Campbell 2013; Duncanson 2013; Haire‐Joshu 2008; Hunsaker 2017; Martinez‐Andrade 2014; Roset‐Salla 2016; Sherwood 2015; Skouteris 2015; Tabak 2012; Vazir 2013; Verbestel 2014; Watt 2009; Wyse 2012). Four trials were conducted in a healthcare setting: one trial compared a parenting practices intervention to a maternal diet and physical activity intervention to control (Black 2011), one trial compared a dietitian‐delivered intervention in a first‐time parents' group regarding infant feeding, physical activity and sedentary behaviours to control (Campbell 2013), one trial compared a six‐week parent intervention on obesity awareness and prevention to control (Martinez‐Andrade 2014), and the fourth trial compared a multistrategy parent intervention including health snack exposure to control (Skouteris 2015). Five trials were conducted within a home setting: one trial compared the provision of an interactive nutrition education CD and parenting DVD to parents to wait‐list control (Duncanson 2013), one trial compared a parent intervention inclusive of a tailored newsletter, home visits and materials to usual care (Haire‐Joshu 2008), one trial compared a dietitian‐delivered parent intervention on vegetable availability, picky eating, modelling and family meals to control (Tabak 2012); one trial compared a parent health report on fruit and vegetable consumption compared to control (Hunsaker 2017) and the fifth compared a parent intervention on infant‐feeding practices to usual care (Watt 2009). Three trials were conducted in a preschool setting; one trial compared a parent education intervention on dietary knowledge and changing habits to control (Roset‐Salla 2016), one trial compared a parent intervention including a poster with guidelines and tips, and tailored feedback about child dietary behaviours versus control (Verbestel 2014), and the third trial compared a parent intervention including a resource kit and telephone calls to improve parent knowledge and skills about the home food environment versus control (Wyse 2012). One trial conducted in both a home and health setting compared a parent complementary feeding intervention to parent complementary feeding and home visit intervention to control (Vazir 2013). One trial compared a paediatrician counselling and home‐based programme delivered to parents of children at risk of obesity compared to a safety and injury prevention control (Sherwood 2015).
Multicomponent interventions
Nine studies tested the impact of multicomponent interventions (e.g. teacher and parent education, preschool policy changes) in increasing children's intake of fruit or vegetables (De Bock 2012; De Coen 2012; Namenek Brouwer 2013; Natale 2014a; Nicklas 2017; Smith 2017; Vereecken 2009; Williams 2014; Witt 2012). Four trials were conducted in a preschool setting; one trial compared an intervention combining familiarisation, preparation and cooking of meals with children, teachers and parents and parent education regarding modelling and nutrition needs of children to control (De Bock 2012); one trial compared a garden‐based intervention and curriculum materials about targeted fruits or vegetables to control (Namenek Brouwer 2013); one trial compared a teacher curriculum, parent curriculum, and preschool policy intervention to control (Natale 2014a); and the fourth trial compared a nutrition education intervention targeting children, parents and preschool staff to control (Williams 2014). Two trials were conducted in a school setting; one trial compared a community, school and parent intervention for nutrition and physical activity health targets to control (De Coen 2012); and the other trial compared a preschool environment, child, parent and teacher intervention to control (Vereecken 2009). One trial, conducted in both a school and a home setting, compared an interactive education intervention about physical activity and healthful eating inclusive of teacher guides and parent newsletters to control (Witt 2012). An additional trial, conducted in both a preschool and a home setting, compared a motivational theatre intervention which included the screening of four DVDs of a puppet show aimed at persuading children to increase vegetable consumption, and provision of resources to parents including ingredients for a vegetable snack, to a no‐intervention control (Nicklas 2017). One trial conducted in both a preschool and home setting compared provision of fruit and vegetables for consumption at home to a parent and child nutrition education with fruit and vegetable provision and a no‐intervention control (Smith 2017).
Child nutrition education interventions
One study tested the impact of an intervention involving the delivery of nutrition education to children within nursery classrooms in increasing child fruit and vegetable intake (Baskale 2011).
Excluded studies
Following an assessment of study titles and abstracts for the update, we sought the full texts of 92 records for further review for study eligibility (738 in total, when combined with 646 from previous reviews) (Figure 1). We were able to locate the full texts of 90 articles (708 in total, when combined with 618 from previous reviews). We considered 78 records from 64 studies (594 records from 507 studies in total) to be ineligible in this review update following the trial screening process (reasons for exclusion of records included participants n = 24; outcomes n = 31; comparator n = 2; study design n = 20; intervention n = 1). See Characteristics of excluded studies for further details.
Studies awaiting classification
We did not identify any new studies that we were unable to classify (we had identified two trials in previous reviews, as no full text was available). See Characteristics of studies awaiting classification.
Ongoing studies
We identified 11 ongoing trials with a published protocol (Characteristics of ongoing studies), for which neither published nor unpublished data were available (eight from the previous reviews and three new ongoing trials). These include: a C‐RCT (Belanger 2016) testing the effect of a multicomponent intervention involving community partnerships and healthy eating training for staff in early childcare centres compared to a no‐intervention control; a RCT (Horodynski 2011) testing the effect of a child‐feeding intervention focused on maternal self‐efficacy during feeding and appropriate feeding styles compared to usual care; a C‐RCT (Østbye 2015) testing the effect of a multicomponent home and childcare intervention compared to a no‐intervention control; a RCT (Sobko 2016) testing the effect of a multicomponent healthy lifestyle programme delivered to parent‐child dyads compared to a wait list or a no‐intervention control; a RCT(Watt 2014) testing the effect of a multicomponent intervention involving parents and childcare staff compared to a no‐intervention control; a RCT (Helle 2017) testing the effect of an eHealth intervention delivered to parents to promote healthy food habits to a no‐intervention control; a C‐RCT (Kobel 2017) testing the effect of a kindergarten‐based healthy lifestyle intervention delivered to parents and children to a no‐intervention control; a RCT (Seguin 2017) testing the effect of a community‐based and cost‐offset community‐supported agricultural intervention to a no‐intervention control; a factorial RCT (Brophy‐Herb 2017) testing the effect of 65 differing levels of support for family meals delivered to families recruited from disadvantaged preschools to a no‐intervention control; a C‐RCT (Hennink‐Kaminski 2017) testing the effect of a multicomponent intervention (including social marketing, child healthy eating and physical activity education, and home components) delivered to preschool teachers and parents to a wait‐list control; and a RCT (Mennella 2017) testing the effect of an intervention involving consumption of vegetables during breastfeeding delivered to mothers to a no‐intervention control.
We identified a further five new ongoing trials in trials registries (four from the previous review and one new ongoing trial), however no published protocol, nor published or unpublished data were available (Characteristics of ongoing studies). These include a RCT testing the effect of a repeated‐exposure intervention to an infant feeding‐schedule intervention to a repeated‐exposure and infant‐feeding intervention to attention‐control (NTR6572); a C‐RCT testing the effect of a taste‐exposure intervention to a nutritional‐education intervention to a taste‐exposure and nutritional‐education intervention to a no‐intervention control (NCT03003923); a RCT testing the effect of a parental‐cooking intervention to a no‐intervention control (ISRCTN45864056); a C‐RCT testing the effect of a warm lunch with a variety of vegetables to a sensory lesson, meal practice and feeding‐style intervention to a no‐intervention control (ISRCTN98064772); and a factorial RCT testing the effect of five interventions to increase complementary feeding behaviour by mothers to a no‐intervention control (NCT03229629).
Risk of bias in included studies
None of the 63 included studies were at low risk in all risk‐of‐bias domains (Figure 2; Figure 3).
Figure 2.
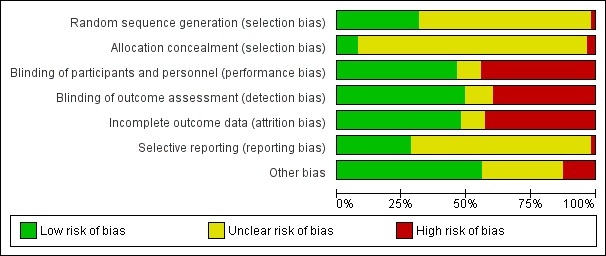
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Figure 3.
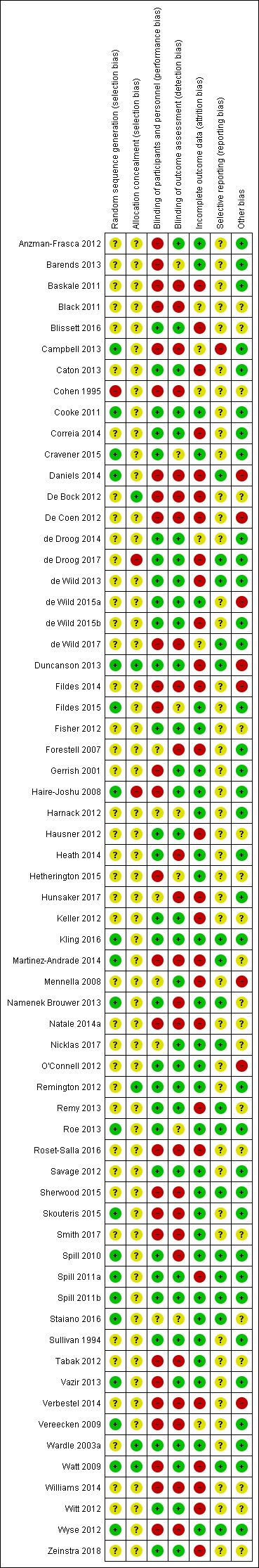
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Random sequence generation
We rated 20 of the 63 studies at low risk of bias for random sequence generation, with all random‐number sequences created using various computer‐based software (Campbell 2013; Cooke 2011; Cravener 2015; Daniels 2014; Duncanson 2013; Fildes 2015; Haire‐Joshu 2008; Kling 2016; Martinez‐Andrade 2014; Namenek Brouwer 2013; Roe 2013; Skouteris 2015; Spill 2010; Spill 2011a; Spill 2011b; Staiano 2016; Vazir 2013; Vereecken 2009; Watt 2009; Wyse 2012). We rated one study (Cohen 1995) at high risk of bias for random sequence generation due to allocation being conducted according to infant's week of birth. The method of sequence generation in the remaining 42 studies was unclear (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Blissett 2016; Caton 2013; Correia 2014; De Bock 2012; De Coen 2012; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Fildes 2014; Fisher 2012; Forestell 2007; Gerrish 2001; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Hunsaker 2017; Keller 2012; Mennella 2008; Natale 2014a; Nicklas 2017; O'Connell 2012; Remington 2012; Remy 2013; Roset‐Salla 2016; Savage 2012; Sherwood 2015; Smith 2017; Sullivan 1994; Tabak 2012; Verbestel 2014; Wardle 2003a; Williams 2014; Witt 2012; Zeinstra 2018).
Allocation
Only five of the 63 studies reported that participant allocation to the experimental group was concealed from those conducting the research (De Bock 2012; Duncanson 2013; Remington 2012; Wardle 2003a; Watt 2009). We judged two studies to have a high risk of selection bias; in one study (de Droog 2017) those responsible for delivering the intervention conducted the allocation and in the other study (Haire‐Joshu 2008), as educators were aware of site allocation when they were enrolling participants to the trial. The remaining 56 studies had an unclear risk of selection bias (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Blissett 2016; Campbell 2013; Caton 2013; Cohen 1995; Cooke 2011; Correia 2014; Cravener 2015; Daniels 2014; De Coen 2012; de Droog 2014; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Fildes 2014; Fildes 2015; Fisher 2012; Forestell 2007; Gerrish 2001; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Hunsaker 2017; Keller 2012; Kling 2016; Martinez‐Andrade 2014; Mennella 2008; Namenek Brouwer 2013; Natale 2014a; Nicklas 2017; O'Connell 2012; Remy 2013; Roe 2013; Roset‐Salla 2016; Savage 2012; Sherwood 2015; Skouteris 2015; Smith 2017; Spill 2010; Spill 2011a; Spill 2011b; Staiano 2016; Sullivan 1994; Tabak 2012; Vazir 2013; Verbestel 2014; Vereecken 2009; Williams 2014; Witt 2012; Wyse 2012; Zeinstra 2018).
Blinding
Performance bias
In 28 of the studies, we judged the potential for trial outcomes to be influenced by participants or personnel delivering the intervention to be high, due to the lack of blinding and the method used for outcome assessment (e.g. self‐report) (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Campbell 2013; Cohen 1995; Daniels 2014; De Bock 2012; De Coen 2012; de Wild 2017; Fildes 2014; Fildes 2015; Gerrish 2001; Haire‐Joshu 2008; Hetherington 2015; Martinez‐Andrade 2014; Natale 2014a; Roset‐Salla 2016; Sherwood 2015; Skouteris 2015; Smith 2017; Tabak 2012; Vazir 2013; Verbestel 2014; Vereecken 2009; Watt 2009; Williams 2014; Wyse 2012). We rated 29 studies at low risk of performance bias, due to blinding or the use of objective outcome assessments, which were unlikely to be influenced by awareness of group allocation (e.g. weighing food on electronic scales) (Blissett 2016; Caton 2013; Cooke 2011; Correia 2014; Cravener 2015; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; Duncanson 2013; Fisher 2012; Hausner 2012; Heath 2014; Keller 2012; Kling 2016; Namenek Brouwer 2013; O'Connell 2012; Remington 2012; Remy 2013; Roe 2013; Savage 2012; Spill 2010; Spill 2011a; Spill 2011b; Sullivan 1994; Wardle 2003a; Witt 2012; Zeinstra 2018). For the six remaining studies the risk of performance bias was unclear (Forestell 2007; Harnack 2012; Hunsaker 2017; Mennella 2008; Nicklas 2017; Staiano 2016).
Detection bias
We rated 25 studies at high risk of detection bias, due to participants or assessors not being blind to group allocation and the use of self‐report measures (Baskale 2011; Black 2011; Campbell 2013; Cohen 1995; Daniels 2014; De Bock 2012; De Coen 2012; de Wild 2017; Fildes 2014; Forestell 2007; Heath 2014; Hunsaker 2017; Martinez‐Andrade 2014; Namenek Brouwer 2013; Natale 2014a; Roset‐Salla 2016; Sherwood 2015; Skouteris 2015; Smith 2017; Spill 2010; Tabak 2012; Verbestel 2014; Vereecken 2009; Williams 2014; Wyse 2012). Blinding of assessors, or the objective measurement of child's fruit and vegetable intake which is unlikely to be impacted by lack of blinding (e.g. the food was weighed or counted), meant that 31 studies had a low risk of detection bias (Anzman‐Frasca 2012; Blissett 2016; Caton 2013; Cooke 2011; Correia 2014; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; Duncanson 2013; Fisher 2012; Gerrish 2001; Haire‐Joshu 2008; Hausner 2012; Keller 2012; Kling 2016; Mennella 2008; Nicklas 2017; O'Connell 2012; Remy 2013; Remington 2012; Savage 2012; Spill 2011a; Spill 2011b; Sullivan 1994; Vazir 2013; Wardle 2003a; Watt 2009; Witt 2012; Zeinstra 2018). The remaining seven studies had an unclear risk of detection bias (Barends 2013; Cravener 2015; Fildes 2015; Harnack 2012; Hetherington 2015; Roe 2013; Staiano 2016).
Incomplete outcome data
Eight studies reported no attrition, and therefore had a very low risk of bias (Anzman‐Frasca 2012; Cravener 2015; Gerrish 2001; Nicklas 2017; O'Connell 2012; Savage 2012; Spill 2010; Staiano 2016). A further 22 studies reported a low loss of participants (usually less than 10%) and similar losses across arms and we considered them to be at low risk, too (Barends 2013; Cooke 2011; de Wild 2015a; Fildes 2015; Fisher 2012; Haire‐Joshu 2008; Hausner 2012; Heath 2014; Hetherington 2015; Kling 2016; Namenek Brouwer 2013; Remington 2012; Roe 2013; Sherwood 2015; Skouteris 2015; Smith 2017; Spill 2011b; Sullivan 1994; Tabak 2012; Vazir 2013; Wardle 2003a; Wyse 2012). Twenty‐seven studies had a high risk of bias due to high attrition rates, unequal attrition across experimental arms, or an intention‐to‐treat analysis not being used (Baskale 2011; Blissett 2016; Caton 2013; Correia 2014; Daniels 2014; De Bock 2012; De Coen 2012; de Droog 2017; de Wild 2013; de Wild 2015b; Duncanson 2013; Fildes 2014; Forestell 2007; Hausner 2012; Hunsaker 2017; Keller 2012; Martinez‐Andrade 2014; Mennella 2008; Natale 2014a; Remy 2013; Roset‐Salla 2016; Spill 2011a; Verbestel 2014; Watt 2009; Williams 2014; Witt 2012; Zeinstra 2018). Six studies had an unclear risk of attrition bias (Black 2011; Campbell 2013; Cohen 1995; de Droog 2014; de Wild 2017; Vereecken 2009).
Selective reporting
Most studies had an unclear risk of selective reporting (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Blissett 2016; Caton 2013; Cohen 1995; Cooke 2011; Correia 2014; Cravener 2015; De Bock 2012; De Coen 2012; de Droog 2014; de Wild 2015a; de Wild 2015b; Fildes 2014; Fildes 2015; Fisher 2012; Forestell 2007; Gerrish 2001; Haire‐Joshu 2008; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Hunsaker 2017; Keller 2012; Mennella 2008; Natale 2014a; O'Connell 2012; Remington 2012; Roset‐Salla 2016; Savage 2012; Skouteris 2015; Smith 2017; Staiano 2016; Sullivan 1994; Tabak 2012; Vazir 2013; Verbestel 2014; Vereecken 2009; Wardle 2003a; Williams 2014; Witt 2012; Zeinstra 2018). We judged one trial (Campbell 2013) to be at high risk of bias due to outcomes referred to in the protocol not being reported. The remaining 17 studies reported all expected outcomes and we rated them at low risk of bias (Daniels 2014; de Droog 2017; de Wild 2013; de Wild 2017; Duncanson 2013; Kling 2016; Martinez‐Andrade 2014; Namenek Brouwer 2013; Nicklas 2017; Remy 2013; Roe 2013; Sherwood 2015; Spill 2010; Spill 2011a; Spill 2011b,Watt 2009; Wyse 2012).
Other potential sources of bias
Of the 32 RCTs, 19 had a low risk of bias (Anzman‐Frasca 2012; Barends 2013; Caton 2013; Cravener 2015; de Droog 2014; de Droog 2017; de Wild 2017; Fildes 2015; Forestell 2007; Gerrish 2001; Heath 2014; Hunsaker 2017; Remington 2012; Savage 2012; Sherwood 2015; Skouteris 2015; Sullivan 1994; Wardle 2003a; Watt 2009), eight had an unclear risk of bias (Black 2011; Blissett 2016; Cohen 1995; Hetherington 2015; Keller 2012; Remy 2013; Staiano 2016; Tabak 2012) and five had a high risk of bias (Daniels 2014; de Wild 2015a; Duncanson 2013; Fildes 2014; Mennella 2008) for other potential sources of bias. One trial did not account for clustering in the analysis, even though the trial protocol said clustering would be accounted for (Daniels 2014). Four trials had a high risk of bias, as they reported baseline imbalances between study groups that were not accounted for in the analysis (de Wild 2015a; Duncanson 2013; Fildes 2014; Mennella 2008).
Of the 21 C‐RCTs, seven had a low risk of bias (Baskale 2011; Campbell 2013; Cooke 2011; Haire‐Joshu 2008; Vazir 2013; Vereecken 2009; Wyse 2012), 12 had unclear risk of bias (De Bock 2012; Fisher 2012; Hausner 2012; Martinez‐Andrade 2014; Namenek Brouwer 2013; Natale 2014a; Nicklas 2017; Roset‐Salla 2016; Smith 2017; Williams 2014; Witt 2012; Zeinstra 2018) and two had high risk of bias (De Coen 2012; Verbestel 2014). Both De Coen 2012 and Verbestel 2014 had high risk of bias due to recruitment bias, as communities were randomised first before schools, childcare centres and participants were invited to participate.
Of the 10 cross‐over trials, nine had a low risk of bias (Correia 2014; de Wild 2013; de Wild 2015b; Harnack 2012; Kling 2016; Roe 2013; Spill 2010; Spill 2011a; Spill 2011b), and one study had high risk of bias (O'Connell 2012), due to differences in baseline vegetable consumption that were not adjusted for in the analysis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Primary outcome. Effectiveness of interventions in increasing the consumption of fruit and/or vegetables
All the included trials reported the impact of the effectiveness of the intervention on a measure of children's fruit or vegetable intake. Variability in the measurement and reporting of intervention effects as change from baseline or final value scores precluded statistical examination of heterogeneity. Nonetheless, examination of the interventions tested, trial settings and study populations suggested that the included trials were heterogeneous and we conducted meta‐analyses pooling data from trials where we considered interventions to be similar. Otherwise, we have provided a narrative synthesis of trial findings.
Child‐feeding practice interventions
Short‐term impact (less than 12 months)
The effects of interventions targeting child‐feeding practices were mixed. Meta‐analysis pooling post‐intervention data (follow‐up period range: immediate to six months) from trials comparing child‐feeding practices to no treatment (Cohen 1995; Cooke 2011; Cravener 2015; Daniels 2014; Fildes 2014; Fildes 2015; Hetherington 2015; Keller 2012; O'Connell 2012; Remington 2012; Staiano 2016; Wardle 2003a; Zeinstra 2018) revealed an overall small positive effect on vegetable consumption (SMD 0.33, 95% CI 0.13 to 0.54; participants = 1741; studies = 13; I2 = 70%; very low‐quality evidence; Analysis 1.1), which was equivalent to an increase of 3.50 g as‐desired consumption of vegetables. Results were similar in sensitivity analyses of studies at low risk of bias (SMD 0.23, 95% CI 0.03 to 0.44; participants = 487; studies = 5; I2 = 14%; Analysis 1.2), of studies with a primary outcome of child fruit or vegetable consumption (SMD 0.45, 95% CI 0.19 to 0.70; participants = 1331; studies = 10; I2 = 73%; Analysis 1.3), and of studies with no or low attrition and studies with high attrition that undertook intention‐to‐treat analyses (SMD 0.29, 95% CI 0.10 to 0.48; participants = 757; studies = 8; I2 = 27%; Analysis 1.4).
Analysis 1.1.
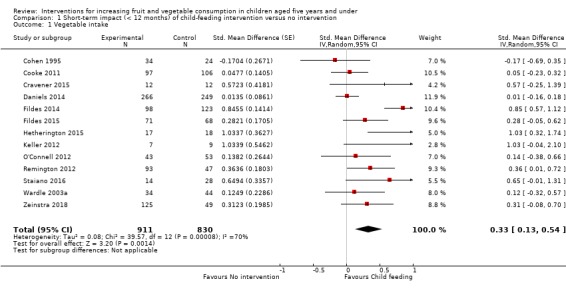
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 1 Vegetable intake.
Analysis 1.2.
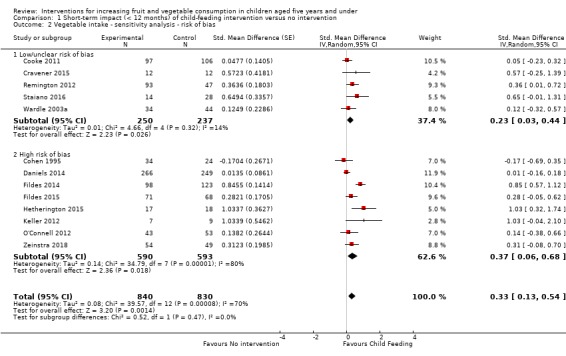
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 2 Vegetable intake ‐ sensitivity analysis ‐ risk of bias.
Analysis 1.3.
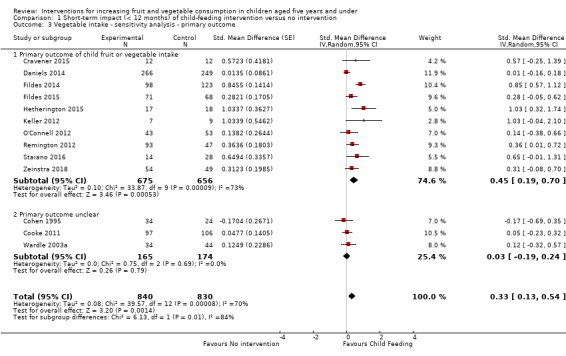
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 3 Vegetable intake ‐ sensitivity analysis ‐ primary outcome.
Analysis 1.4.
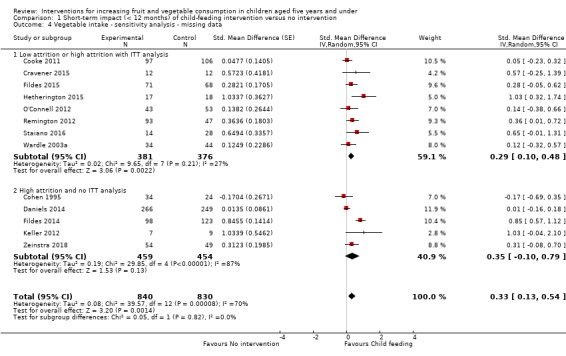
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 4 Vegetable intake ‐ sensitivity analysis ‐ missing data.
One study that compared one or more child‐feeding practice interventions to a no‐treatment control did not report sufficient data to enable pooling. Harnack 2012 reported a significant increase in intake of fruit compared to a control group for an intervention where fruit and vegetables were served prior to a meal.
Twenty‐five trials compared the effectiveness of two or more child‐feeding interventions but we could not synthesise them in meta‐analyses due to variability in the compared interventions (Anzman‐Frasca 2012; Barends 2013; Blissett 2016; Caton 2013; Correia 2014; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Fisher 2012; Forestell 2007; Gerrish 2001; Hausner 2012; Heath 2014; Kling 2016; Mennella 2008; Remy 2013; Roe 2013; Savage 2012; Spill 2010; Spill 2011a; Spill 2011b; Sullivan 1994). The interventions compared in these trials varied greatly; 10 of the 25 trials reported evidence of an increase in fruit or vegetable consumption for one intervention compared to another (de Droog 2014; de Droog 2017; de Wild 2013; Forestell 2007; Gerrish 2001; Heath 2014; Roe 2013; Spill 2010; Spill 2011a; Spill 2011b).
Long‐term impact (12 months or longer)
Two studies tested the long term effect of a child‐feeding practice intervention. One study reported no long‐term effect on either fruit or vegetable intake as measured by 24‐hour recall 3½ years after a complementary feeding intervention compared to usual care (Daniels 2014). The other study (Cohen 1995), which compared exclusive breastfeeding, complementary feeding with breastfeeding, and complementary feeding with breastfeeding on demand reported no difference between groups at 12 months' follow‐up compared to the positive effect that was reported at nine months' follow‐up.
Parent nutrition education interventions
Short‐term impact (less than 12 months)
Interventions targeting parent nutrition education were generally not effective. Meta‐analysis pooling post‐intervention data (follow‐up period range: immediate to six months) from trials comparing parent nutrition education interventions to no treatment (Campbell 2013; Duncanson 2013; Haire‐Joshu 2008; Martinez‐Andrade 2014; Roset‐Salla 2016; Sherwood 2015; Skouteris 2015; Tabak 2012; Verbestel 2014; Watt 2009; Wyse 2012) revealed no overall effect on child consumption of fruit and vegetables (SMD 0.12, 95% CI ‐0.03 to 0.28; participants = 3078; studies = 11; I2 = 69%; very low‐quality evidence; Analysis 2.1). Results were similar in sensitivity analyses of studies with a primary outcome of children's fruit or vegetable consumption (SMD 0.04, 95% CI ‐0.08 to 0.16; participants = 2792; studies = 8; I2 = 46%; Analysis 2.2), and of studies with no or low attrition and studies with high attrition that undertook intention‐to‐treat analyses (SMD 0.12, 95% CI ‐0.00 to 0.24; participants = 2518; studies = 7; I2 = 40%; Analysis 2.3). We did not conduct sensitivity analyses by risk of bias, as we judged all studies to be at high risk of bias in at least one domain.
Analysis 2.1.
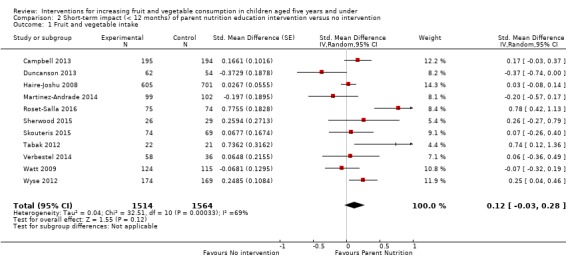
Comparison 2 Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention, Outcome 1 Fruit and vegetable intake.
Analysis 2.2.
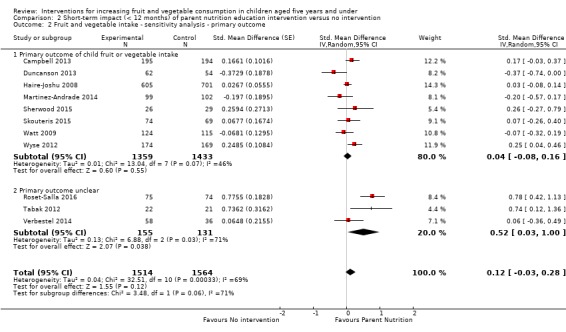
Comparison 2 Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention, Outcome 2 Fruit and vegetable intake ‐ sensitivity analysis ‐ primary outcome.
Analysis 2.3.
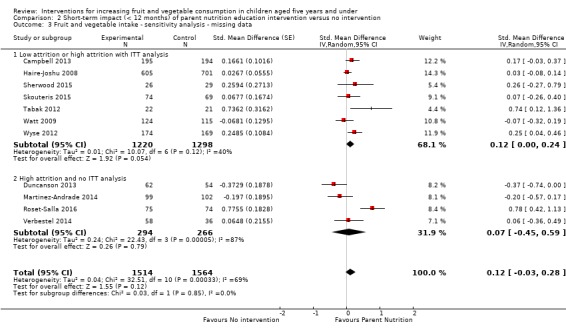
Comparison 2 Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention, Outcome 3 Fruit and vegetable intake ‐ sensitivity analysis ‐ missing data.
We were unable to pool three trials in the meta‐analysis. Black 2011 found an intervention targeting parent responsivity and behaviour management to be effective in increasing total fruit intake compared to control. Hunsaker 2017 found a parent health report on fruit and vegetable consumption to be effective in increasing total vegetable intake (but not fruit intake) compared to control. Vazir 2013 reported both the parent complementary‐feeding intervention and a parent complementary‐feeding and home‐visit intervention to be effective in increasing both fruit and vegetable intake compared to control.
Long‐term impact (12 months or longer)
Four studies reported the long‐term impact of a parent nutrition education intervention (Duncanson 2013; Skouteris 2015; Watt 2009; Wyse 2012). Of these, only one trial reported a significant long‐term effect on children's fruit and vegetable consumption (Watt 2009). The trial examining the impact of a parent intervention targeting infant‐feeding practice found a short‐term effect at nine months and long‐term effect at 15‐month follow‐up on fruit and vegetable consumption compared to usual care (Watt 2009). The other three trials reporting long‐term impacts of parent interventions either reported a short‐term effect that was not sustained at long‐term follow‐up (Skouteris 2015; Wyse 2012), or no effect at either short‐ or long‐term follow‐up on children's fruit or vegetable consumption (Duncanson 2013).
Multicomponent interventions
Short‐term impact (less than 12 months)
The effects of multicomponent interventions were mixed. Meta‐analysis pooling post‐intervention data (follow‐up period range: immediate to one month) from multicomponent intervention trials (De Coen 2012; Namenek Brouwer 2013; Nicklas 2017; Smith 2017; Williams 2014) revealed an overall small positive effect on child consumption of fruit and vegetables (SMD 0.35, 95% CI 0.04 to 0.66; participants = 2009; studies = 5; I2 = 80%; low‐quality evidence; Analysis 3.1). This was equivalent to an increase of 0.37 cups of fruit and vegetables per day. Results were similar in sensitivity analyses of studies with no or low attrition or high attrition that undertook intention‐to‐treat analyses (SMD 0.65, 95% CI 0.43 to 0.88; participants = 413; studies = 3; I2 = 0%; Analysis 3.3). There was, however, no overall effect on child consumption of fruit and vegetables for studies with a primary outcome of children's fruit or vegetable consumption (SMD 0.44, 95% CI ‐0.00 to 0.87; participants = 1315; studies = 4; I2 = 85%; Analysis 3.2). We did not conduct a sensitivity analysis to examine the impact of high risk of bias, as all but one study had a high risk of bias in at least one domain.
Analysis 3.1.
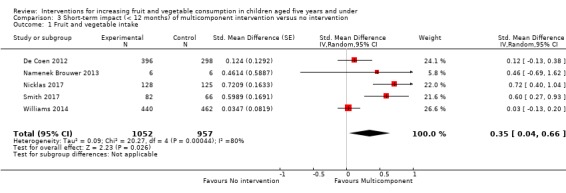
Comparison 3 Short‐term impact (< 12 months) of multicomponent intervention versus no intervention, Outcome 1 Fruit and vegetable intake.
Analysis 3.3.
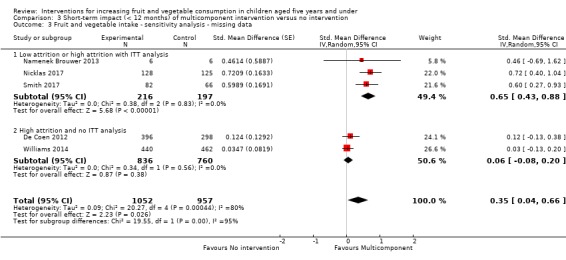
Comparison 3 Short‐term impact (< 12 months) of multicomponent intervention versus no intervention, Outcome 3 Fruit and vegetable intake ‐ sensitivity analysis ‐ missing data.
Analysis 3.2.
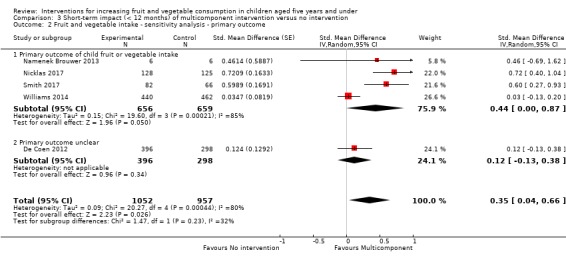
Comparison 3 Short‐term impact (< 12 months) of multicomponent intervention versus no intervention, Outcome 2 Fruit and vegetable intake ‐ sensitivity analysis ‐ primary outcome.
We were unable to pool four studies in meta‐analysis, due to insufficient data (De Bock 2012; Natale 2014a; Vereecken 2009; Witt 2012). Three trials (De Bock 2012; Natale 2014a; Witt 2012) reported significant effects of the interventions tested on both fruit and vegetable consumption, and one trial reported significant effects of the intervention on fruit but not vegetable consumption (Vereecken 2009).
One of the studies (Smith 2017) that was pooled in the multicomponent intervention meta‐analysis additionally compared an intervention of provision of fruit and vegetables to a no‐intervention control group. The study reported a significant effect of the intervention on vegetable consumption.
Long‐term impact (12 months or longer)
No trials testing the multicomponent interventions reported long‐term impact.
Child nutrition education interventions
Short‐term impact (less than 12 months)
The one study that tested the effect of a nutrition education intervention targeting children (Baskale 2011) reported an increase in some of the fruits and vegetables assessed in the intervention group and no significant differences in the control group, but did not report analyses comparing treatment groups (low‐quality evidence).
Long‐term impact (12 months or longer)
The one study that tested the effect of a nutrition education intervention did not report long‐term impact.
Subgroup analyses
Interventions targeting boys and girls
All the included studies in this review covered both boys and girls. The impact of intervention on gender subgroups was not reported in any of the included trials, so subgroup analyses on this basis was not possible.
Interventions targeting minority groups and indigenous populations
Subgroup analysis of trials that targeted minority groups and indigenous populations was not possible, due to the limited number of included studies for each comparison; we therefore present them narratively. Nine of the 63 included trials examined the impact of interventions on predominantly disadvantaged populations (Black 2011; Cohen 1995; Cooke 2011; de Droog 2017; Haire‐Joshu 2008; Natale 2014a; Nicklas 2017; Smith 2017; Watt 2009). Three trials of child‐feeding interventions recruited predominantly disadvantaged populations (Cohen 1995; Cooke 2011; de Droog 2017). One trial recruited participants from low‐income neighbourhoods (Cohen 1995). The trial found that a complementary feeding with breastfeeding on demand intervention increased the consumption of vegetables compared to exclusive breastfeeding at short‐term follow‐up (nine months), but found no effect at long‐term follow‐up (12 months). One trial recruited participants through schools where the proportion of children who had English as a second language, came from minority ethnic backgrounds or were eligible for free school meals was above average (Cooke 2011). The study demonstrated that repeated food exposure coupled with reward significantly increased the consumption of a target vegetable. The third trial recruited participants predominantly from low socioeconomic status households (de Droog 2017). The study found an interactive‐reading intervention significantly increased the consumption of a target vegetable. Three trials of parent interventions recruited participants from disadvantaged communities including underserved families, single or minority parent homes, those living in poverty or low‐income families (Black 2011; Haire‐Joshu 2008; Watt 2009). Two trials found no improvement in overall child fruit or vegetable intake based on the primary trial outcome measures (Haire‐Joshu 2008; Watt 2009); the other trial found the intervention targeting parent responsivity and behaviour management to be effective in increasing total fruit intake (Black 2011). Three trials of multicomponent interventions recruited participants from subsidised childcare centres (Natale 2014a; Nicklas 2017; Smith 2017). One trial found an intervention targeting teachers, parents and childcare policies to increase both fruit and vegetable consumption (Natale 2014a), one trial found a theatre performance intervention involving both parents and teachers increased vegetable consumption (Nicklas 2017), and the other trial found both a fruit and vegetable provision intervention and an intervention involving parent and child nutrition education plus fruit and vegetable provision increased fruit and vegetable consumption (as assessed via skin carotenoid levels compared to a no intervention control (Smith 2017).
Interventions delivered in various settings
Subgroup analyses of child‐feeding practice interventions by setting revealed an overall positive effect on children's vegetable consumption for those interventions delivered in home settings (SMD 0.56, 95% CI 0.18 to 0.95; participants = 474; studies = 4) and in both home and laboratory settings (SMD 0.74, 95% CI 0.09 to 1.39; participants = 40; studies = 2), but no overall effect for those interventions delivered in school or preschool settings (SMD 0.20, 95% CI ‐0.01 to 0.41; participants = 591; studies = 4). Subgroup analyses for other settings (including one set in child health clinics, and one in home or health facilities) was not possible due to the limited number of studies for each setting.
Similar to the main analysis, subgroup analyses of parent nutrition education interventions by setting revealed no overall effect for those interventions delivered in a home setting (SMD 0.06, 95% CI ‐0.16 to 0.27; participants = 2047; studies = 5) or a preschool setting (SMD 0.43, 95% CI ‐0.27 to 1.13; participants = 243; studies = 2). Subgroup analyses for other settings (one each in parenting groups, primary care clinics or community health centres) was not possible, due to the limited number of studies for each setting.
In contrast to the main analysis, subgroup analyses of multicomponent interventions by setting revealed no overall effect for those interventions delivered in school or preschool settings (SMD 0.07, 95% CI ‐0.07 to 0.20; participants = 1608; studies = 3). Subgroup analyses for interventions delivered in either a preschool or a home setting, or clinic and home setting were not possible, due to the limited number of studies for each setting.
Interventions of varying intensities
We did not conduct subgroup analyses of trials based on interventions of varying intensities, due to the limited information across included studies about the number and duration of intervention contacts or components.
Interventions delivered in different modalities
Forty‐eight of the 63 trials used face‐to‐face intervention delivery only (Anzman‐Frasca 2012; Barends 2013; Baskale 2011; Black 2011; Blissett 2016; Caton 2013; Cohen 1995; Cooke 2011; Correia 2014; Cravener 2015; Daniels 2014; De Bock 2012; de Droog 2014; de Droog 2017; de Wild 2013; de Wild 2015a; de Wild 2015b; de Wild 2017; Fildes 2014; Fisher 2012; Forestell 2007; Gerrish 2001; Harnack 2012; Hausner 2012; Heath 2014; Hetherington 2015; Keller 2012; Kling 2016; Martinez‐Andrade 2014; Mennella 2008; Namenek Brouwer 2013; O'Connell 2012; Remington 2012; Remy 2013; Roe 2013; Roset‐Salla 2016; Savage 2012; Skouteris 2015; Spill 2010; Spill 2011a; Spill 2011b; Sullivan 1994; Vazir 2013; Verbestel 2014; Wardle 2003a; Watt 2009; Witt 2012; Zeinstra 2018), reporting mixed findings. Similar to the overall analyses, subgroup analysis of face‐to‐face‐delivered child‐feeding practice interventions versus control revealed an overall positive intervention effect on vegetable consumption (SMD 0.32, 95% CI 0.09 to 0.56; participants = 1489; studies = 11; Analysis 1.5).
Analysis 1.5.
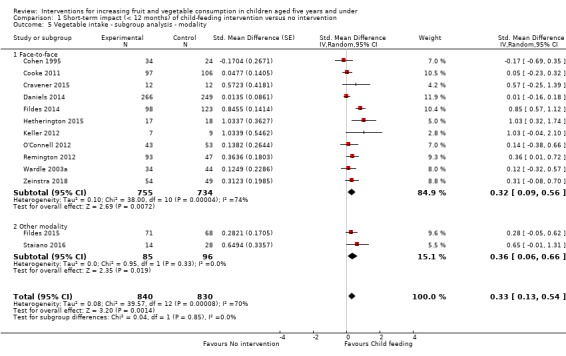
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 5 Vegetable intake ‐ subgroup analysis ‐ modality.
Face‐to‐face‐delivered parent nutrition education interventions versus control revealed no overall intervention effect on children's fruit and vegetable consumption (SMD 0.12, 95% CI ‐0.20 to 0.45; participants = 826; studies = 5; Analysis 2.4). Face‐to‐face intervention delivery alone was used in only one multicomponent intervention (Namenek Brouwer 2013) and the only child nutrition education intervention (Baskale 2011), for which mixed results were reported.
Analysis 2.4.
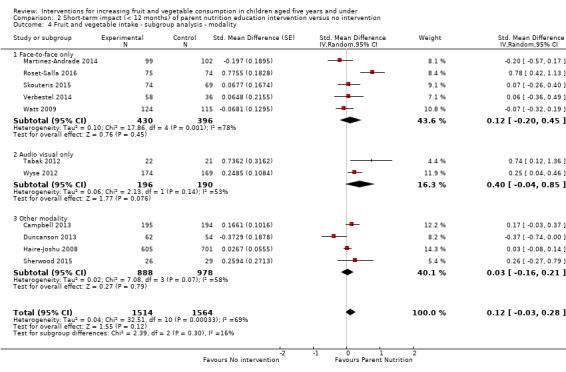
Comparison 2 Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention, Outcome 4 Fruit and vegetable intake ‐ subgroup analysis ‐ modality.
Subgroup analysis for other modalities was not possible due to the limited number of included studies for each comparison. Ten trials used face‐to‐face in combination with other strategies: computer‐tailored newsletters and storybooks (Haire‐Joshu 2008); school‐based education, training, policy and environment change (Vereecken 2009); visual and written materials (Campbell 2013); educational materials, resources (posters, brochures) and letters (De Coen 2012); a leaflet (Fildes 2015); newsletters and menu modification (Natale 2014a); printed materials and resources (Williams 2014); telephone and written materials (Sherwood 2015), written materials and provision of fruit and vegetables (Smith 2017), and DVD (Nicklas 2017). Two trials used audio/visual only: DVDs (Staiano 2016) and DVD and CD (Duncanson 2013). A further two trials used telephone and mail (Tabak 2012; Wyse 2012). One trial used written materials only (Hunsaker 2017). Trials that incorporated other intervention modalities reported mixed findings.
Secondary outcome 1. Cost or cost effectiveness of interventions to increase the consumption of fruit or vegetables or both
Information about intervention costs was reported in one trial (Campbell 2013; very low‐quality evidence). The parent nutrition education trial reported the total estimated cost of delivering a parent intervention for infant feeding, physical activity and sedentary behaviours delivered by a dietitian as approximately AUD 500 per family.
Secondary outcome 2. Adverse effects of interventions to increase the consumption of fruit or vegetables or both
Two trials reported information on any adverse events or unintended adverse consequences of the intervention. One child‐feeding practice intervention trial (Spill 2011a; very low‐quality evidence) reported no adverse effects on the amount of the meal consumed following implementation of an intervention involving incorporation of vegetable puree into meals at three different levels of energy density. The other trial, on parent nutrition education, (Wyse 2012; very low‐quality evidence) reported no adverse effect on family food expenditure following implementation of a multicomponent intervention delivered over the telephone to improve parental knowledge and skills about the home food environment.
Discussion
Summary of main results
In line with the importance of encouraging fruit and vegetable consumption among children in early childhood, this updated review has identified a number of new RCTs of interventions investigating this health behaviour. The findings suggest that child‐feeding practice and multicomponent interventions targeting fruit and vegetable consumption by children aged five and younger are effective. Most of the included studies examined specific child‐feeding practices; whilst meta‐analysis of 13 of the 39 trials suggested these interventions were effective, collectively the findings for these interventions were equivocal. The second and third most common interventions were parent nutrition education and multicomponent interventions, for which we found evidence of effect in the short term in meta‐analyses for multicomponent interventions but not parent nutrition interventions. Only one trial assessed the effect of a child nutrition education intervention. Subgroup analyses on the basis of setting and modality were generally consistent with the main analyses for child‐feeding practices, parent nutrition education and multicomponent interventions. Insufficient evidence was available to determine the long‐term effectiveness of all approaches, or the cost effectiveness or any adverse consequences of the interventions tested.
Overall completeness and applicability of evidence
The review update identified a number of newly published RCTs, in line with efforts globally to increase fruit and vegetable intake (World Health Organization 2003). Such studies predominantly focused on fruit and vegetable consumption determinants such as nutrition knowledge and skills, and food environments. Only one of the included trials in this review reported cost analyses and only two reported any unintended adverse effects. These factors are important considerations for health practitioners and policy makers but are often not reported in randomised trials (Waters 2011) or examined in systematic reviews (Hopewell 2008; Wolfenden 2010b).
Furthermore, the limited number of relevant trials identified for inclusion also prevented thorough examination of the impact of the interventions by gender, indigenous or disadvantaged populations, setting, varying intensity and modality. We found a number of trial protocols (see Characteristics of ongoing studies) which may address some of these gaps in the literature, and are likely to be eligible for inclusion in future updates of the review, including a randomised controlled trial of an eight‐lesson in‐home intervention in economically and educationally disadvantaged parents of children aged one to three years (Horodynski 2011).
The external validity of the review findings are limited. Most of the trials were conducted in the USA, Western Europe or the United Kingdom. Study attrition varied between studies, ranging from 0% to 68%.
Quality of the evidence
We used the GRADE approach to assess the quality of the evidence for the primary outcome of fruit and vegetable intake, which was conducted separately for each intervention type. See Table 1; Table 2; Table 3; Table 4. The quality of the evidence for fruit and vegetable intake across intervention types varied from very low to low. We rated the quality of evidence for specific child‐feeding interventions as very low, downgraded for unexplained heterogeneity, methodological limitations and a high probability of publication bias (Table 1). Methodological limitations related to allocation concealment and selective reporting being at unclear or high risk for most of the trials. A high probability of publication bias related to the relatively few trials being included in the meta‐analysis (12 of 39 trials) and inspection of funnels plots (Figure 4). We assessed the quality of evidence for parent nutrition education interventions as very low, downgraded for unexplained heterogeneity, methodological limitations and imprecision (Table 2; Figure 5). The methodological limitations related to most of the trials being at high risk of bias for lack of blinding, and at unclear or high risk for allocation concealment, loss to follow‐up, and selective reporting. Imprecision related to the confidence intervals crossing the null value of zero. We rated the quality of evidence for multicomponent interventions as low, downgraded for unexplained heterogeneity, and methodological limitations (Table 3; Figure 6). The methodological limitations related to most of the trials being at high risk of bias for lack of blinding, and at unclear or high risk for allocation concealment, loss to follow‐up, and selective reporting. Such assessments suggest that the true effect may be substantially different from the intervention effects reported in the review, with future research very likely to change the estimate for specific infant feeding and parent nutrition education, and likely to change the estimate for multicomponent interventions. We rated the quality of the evidence for child nutrition interventions for the single included study as low, downgraded for methodological limitations and imprecision (Table 4). The methodological limitations related to a high risk of bias due to lack of blinding and loss to follow‐up, and imprecision related to a sample size of fewer than 400 participants. Future research is likely to change the estimate for child nutrition interventions.
Figure 4.
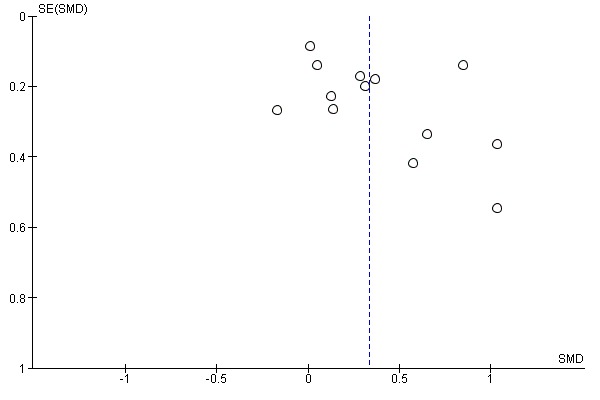
Funnel plot of comparison 1. Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention on child consumption of target fruit or vegetable, outcome 1.1, fruit and/or vegetable intake
Figure 5.
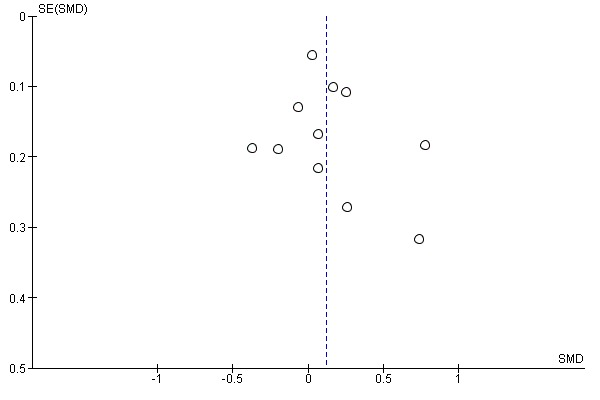
Funnel plot of comparison 3. Short‐term impact (< 12 months) of parent nutrition education intervention versus usual care, outcome 3.1, fruit and/or vegetable intake
Figure 6.
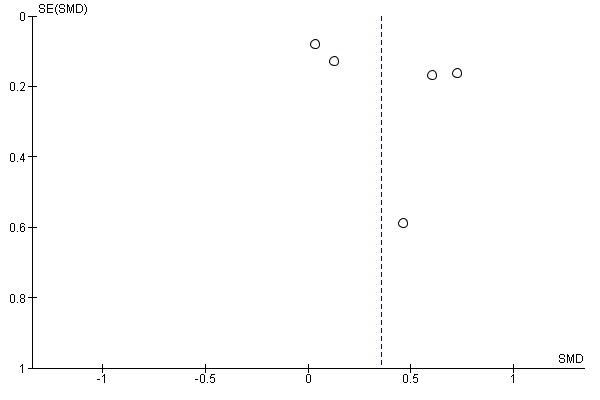
Funnel plot of comparison 4. Short‐term impact (< 12 months) of multicomponent intervention versus usual care, outcome 4.1, fruit and/or vegetable intake
Potential biases in the review process
The review used a comprehensive and rigorous methodology, including a broad search strategy, the screening of trials and extraction of data by two independent review authors, and the appraisal of risks of bias within the included studies. Furthermore, the review did not restrict publications by language. Three aspects of selection bias, however, are worth noting. First, we excluded trials where fruit and vegetable intake was not considered to be a primary trial outcome, to avoid any potential confounding effects of other behavioural interventions (such as physical activity). This restriction may lead to overestimates of intervention effects if in practice they are delivered in the context of other health initiatives. Second, the inclusion of trials that did not explicitly state a primary outcome but did assess fruit or vegetable intake in the review may have biased the results. However sensitivity analyses excluding studies that did not state fruit and vegetable intake as a primary outcome suggested this was limited, as results were similar. Third, studies that were conducted predominantly in disadvantaged populations were included within the overall synthesis. It is possible that effects of the interventions tested may differ between disadvantaged and general populations, which may limit the generalisability of the review findings. Finally, the review was restricted to RCTs and cross‐over trials, with trials included in the review tending to focus on interventions targeting fruit and vegetable consumption determinants, such as nutrition knowledge and skills, or the food environment of settings. Other trials targeting fruit and vegetable intake that may be less amenable to evaluation using randomised controlled designs, such as those requiring macro‐environmental changes, may have been overlooked.
Agreements and disagreements with other studies or reviews
The equivocal findings of the infant‐feeding interventions, such as repeated food exposure, are similar in part to previous reviews. An early systematic review of healthy eating interventions for children aged under five years (Tedstone 1998), published by the Health Education Authority, concluded that repeated food exposure is effective in enhancing children's willingness to consume novel foods provided tasting was included as a part of the exposure. Enhanced food acceptance following repeated food exposure has also been reported in other reviews and controlled trials (Contento 1995). As Cooke 2011 points out in the Background review of research for their randomised trial, evidence about the use of rewards to encourage children's consumption of targeted foods appears more equivocal. The positive impact of both social and non‐tangible rewards reported in Cooke 2011, were however consistent with previous trials in community settings using tangible non‐food rewards and social rewards targeting the fruit or vegetable intake of school‐aged children (Hendy 1999). The large number of trials comparing alternative and heterogenous child‐feeding practice interventions are difficult to interpret, given that they did not include a no‐treatment control group, and few reported one intervention to be more effective than another.
The largely null findings of this review for the impact of parent interventions are consistent with those reported in previous reviews of dietary interventions. For example, a comprehensive review of the impact of home‐visiting programmes delivered to parents concluded that there was little evidence to recommend such interventions as means of improving children's diet, given the mixed findings of the reviewed studies (Elkan 2000). Among the trials with a positive intervention effect included in the Elkan 2000 review was a pre‐post study of an intensive intervention provided to low‐income mothers of children aged one to four years (James 1992). In this study, dietician‐trained general practitioners and health visitors provided advice and support as part of a primary‐care home‐visiting intervention lasting up to 20 weeks. Post‐intervention improvements in diet were reported, including the consumption of fruits and vegetables. Similarly, a systematic review that examined the effectiveness of parental interventions on the diets of children aged two to five found mixed results for children’s diets or feeding practices or both (Peters 2012).
The positive findings for multicomponent interventions are consistent with some previous reviews of interventions. For example, a systematic review of interventions to improve diet, physical activity or to prevent weight gain for children of five years or under, and which included both randomised and non‐randomised designs, identified nine studies of interventions implemented in preschool or childcare settings (Hesketh 2010). Three studies included some assessment of dietary outcome. In the first, Head Start preschools were assigned to either a menu intervention to reduce the fat content of meals provided to children in care; the same menu intervention plus nutrition education; or a third usual‐care control condition (Williams 2004). Both intervention arms of the trial reduced the fat content of foods served to children compared with the preschools in the control condition. The remaining two trials assessed the impact of a healthy eating and physical activity obesity‐prevention programme ‘Hip‐Hop to Health Jr’, implemented in two different populations attending Head Start preschools (Fitzgibbon 2005; Fitzgibbon 2006). In Fitzgibbon 2005, intervention children reported less saturated fat intake at the one‐year follow‐up, but not total fat or dietary fibre. No improvements in dietary intake were reported in the second trial (Fitzgibbon 2006). Similarly, systematic reviews of school‐based fruit and vegetable interventions have frequently concluded that multicomponent initiatives are the most effective in increasing fruit and vegetable consumption in older children, but such effects are only modest and reported to be driven largely by increased fruit intake (Burchett 2003; Ciliska 2000; French 2003; Knai 2006). A systematic review of European school‐based interventions also concluded that multicomponent interventions are effective for improving children’s fruit and vegetable intakes (Van Cauwenberghe 2010).
In contrast to the findings of this review, a number of other reviews have found multicomponent interventions to not be effective. A recent meta‐analysis showed no significant differences between multicomponent interventions that promoted fruit and vegetable consumption and control conditions in a primary school setting (Delgado‐Noguera 2011). Another systematic review that focused on the fruit and vegetable intake of children aged five to 12 found that school‐based interventions had only a minimal effect on vegetable consumption, whereas they found a moderate impact on children’s fruit intake (Evans 2012). A recent systematic review that examined interventions aimed at increasing children’s (aged two to 12 years) vegetable intake in home and community settings found that only a minority of interventions that targeted children’s vegetable intake alone were effective in the short term (Hendrie 2017). In contrast, when vegetable intake was addressed as part of a healthy diet or lifestyle intervention, most interventions showed short‐term effectiveness (Hendrie 2017). The comparison of the findings of this review to each of these previous reviews of multicomponent interventions is limited by their inclusion of older children, which may explain the contrasting findings.
Authors' conclusions
We found little evidence of effect for interventions to increase the fruit and vegetable consumption of children aged five years and under, to provide direction for health policy makers and practitioners. The effect of parent nutrition education is uncertain. Very low‐quality evidence for specific child‐feeding interventions (such as repeated exposure and rewards) and low‐quality evidence for multicomponent interventions suggests such interventions may be effective, but such findings should be interpreted with caution, given that fewer than half of the identified child‐feeding intervention studies could be pooled in meta‐analysis, and that no data were reported for important outcomes such as costs and unintended consequences in child‐feeding or multicomponent interventions. Additionally, the effect size for both child‐feeding and multicomponent interventions was small (equivalent to an increase in as‐desired vegetable intake of 3.50 g and 0.37 cups of fruit and vegetables consumed per day respectively), which may limit the potential public health benefits of implementing these types of interventions.
Despite the large number of trials, the lack of high‐quality research in this area demonstrates the continuing considerable scope for policy makers, researchers and practitioners to develop and evaluate the impact of a variety of initiatives to improve fruit and vegetable intake in children aged five years and under. Behavioural interventions delivered via health professionals, telephone or computer‐based programmes, interventions delivered through preschools, play‐groups, sports clubs, or co‐operatives, and those that address access issues through subsidies or other incentives all have merit, and rigorous evaluation of such interventions for children aged five years and under would contribute greatly to the available evidence base to inform practice. In particular, trials should seek to test interventions that are based on logic models of change, appropriate theoretical frameworks and evidence, and using high‐quality evaluation methods. As the aetiology of child diet is complex, interventions that target multiple determinants across a number of settings may be most likely to be effective.
This review identified a number of opportunities for future or continued intervention research targeting the fruit and vegetable consumption of children aged five years and under, including:
the exploration and development of intervention strategies that can achieve larger effect sizes;
the investigation of potential adverse effects of interventions (e.g. increased family grocery costs, or adverse effects on parent self‐esteem or sense of competence) as a routine part of intervention trials;
examination of the cost effectiveness of interventions found to be effective;
interventions with extended periods of follow‐up;
interventions delivered using electronic modalities such as the web or mobile phones;
interventions implemented across a broader range of settings including health services and sports clubs.
Acknowledgements
We would like to acknowledge the assistance of Charlene Bridges from Cochrane Heart for executing the search for all review updates, and Sarah Haley, Juan Adriano Moran and Filipe Oliveira Dos Santos, who provided translation services.
We would thank and acknowledgement the support of Anneliese Synnott, Anna Noel‐Storr, James Thomas and Julian Elliott in conducting the Living Systematic Review Pilot. We would also like to acknowledge the following Cochrane Crowd members who contributed to the title and abstract screening: Therese Dalsbø, Nikolaos Sideris, Susanna Wisniewski, Riccardo Guarise, Ghaleb Muhammad Mehyar, Stefanie Rosumeck, Donald Bourne, Karen Ma, Tina Jurén, Julia G Lavenberg, Anna Maria Paloma Lohikko, Bernardo Costa, Sarah Robinson and Siddhant Parekh.
We would like to thank the authors who contributed to the 2010 original review: Ben Britton, Karen Campbell and Patrick McElduff. We would also like to acknowledge the contribution of health promotion practitioners, community dieticians, Children's Services staff who provided comment on the scope and focus of the review protocol, and authors of trials who provided further information to the review team to facilitate assessments of trial eligibility and analysis.
Appendices
Appendix 1. Cochrane's living systematic review pilots
Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence as it becomes available (Elliott (in press)). Cochrane is exploring the feasibility of preparing and publishing living systematic reviews in a series of pilots, which includes this review. For the Cochrane pilots, searching is being conducted monthly, and we will incorporate new relevant evidence (studies, data or other information) into the review in a timely manner, so that the findings of the review remain current.
For the most up‐to‐date information about the review, the results of the searches and any new evidence being incorporated, we encourage readers to check the update status information. We will revise the update status information whenever the searches are re‐run. We will update the review with a new citation whenever we find a new trial, or relevant information about already‐included trials (e.g. new outcome data).
Appendix 2. Search strategies
CENTRAL
#1 MeSH descriptor Fruit explode all trees
#2 MeSH descriptor Citrus explode all trees
#3 MeSH descriptor Vegetables explode all trees
#4 fruit*
#5 vegetable*
#6 orange*
#7 apple*
#8 (pear or pears)
#9 (grape or grapes)
#10 banana*
#11 (berry or berries):ti,ab,kw
#12 citrus
#13 carrot*
#14 "greens"
#15 cabbage*
#16 brassica*
#17 blackberr*
#18 blueberr*
#19 cranberr*
#20 kiwi*
#21 guava*
#22 lingonberr*
#23 mango*
#24 melon*
#25 papaya*
#26 pineapple*
#27 raspberr*
#28 strawberr*
#29 tomato*
#30 grapefruit*
#31 mandarin*
#32 satsuma*
#33 tangerine*
#34 (plum or plums)
#35 apricot*
#36 (cherry or cherries)
#37 nectarine*
#38 (peach or peaches)
#39 celery
#40 spinach*
#41 (salad or salads)
#42 (pea or peas)
#43 (bean or beans)
#44 broccoli
#45 cauliflower*
#46 beetroot*
#47 (turnip* or potato* or onion*)
#48 rhubarb
#49 MeSH descriptor Food Habits, this term only
#50 MeSH descriptor Food Preferences, this term only
#51 (health* next eating) or (food next habit*) or (food next preference*) or (eating next habit*) or (eating next preference*) or (eating next behavi*)
#52 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#53 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20)
#54 (#21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30)
#55 (#31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40)
#56 (#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51)
#57 (#52 OR #53 OR #54 OR #55 OR #56)
#58 MeSH descriptor Health Education explode all trees
#59 MeSH descriptor Health Promotion explode all trees
#60 MeSH descriptor Behavior Therapy explode all trees
#61 MeSH descriptor Counseling explode all trees
#62 MeSH descriptor Organizational Policy, this term only
#63 MeSH descriptor Public Policy, this term only
#64 MeSH descriptor Health Policy explode all trees
#65 MeSH descriptor Inservice Training explode all trees
#66 promot*
#67 educat*
#68 program*
#69 (policy or policies)
#70 train*
#71 (diet* near/6 intervention*)
#72 (behavi* near/6 intervention*)
#73 (#58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66)
#74 (#67 OR #68 OR #69 OR #70 OR #71 OR #72)
#75 (#73 OR #74)
#76 MeSH descriptor Infant explode all trees
#77 MeSH descriptor Child, Preschool, this term only
#78 (child or children)
#79 (pre‐school* or preschool*)
#80 (infant or infants or infancy)
#81 (nursery or nurseries or kindergarten)
#82 MeSH descriptor Parents explode all trees
#83 (parent or parents)
#84 (toddler* or baby or babies)
#85 MeSH descriptor Nurseries, this term only
#86 (#76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85)
#87 (#57 AND #75 AND #86)
MEDLINE (Ovid)
1. exp Fruit/
2. exp Citrus/
3. exp Vegetables/
4. fruit*.tw.
5. vegetable*.tw.
6. orange*.tw.
7. apple*.tw.
8. (pear or pears).tw.
9. (grape or grapes).tw.
10. banana*.tw.
11. (berry or berries).tw.
12. citrus.tw.
13. carrot*.tw.
14. greens.tw.
15. cabbage*.tw.
16. brassica*.tw.
17. blackberr*.tw.
18. blueberr*.tw.
19. cranberr*.tw.
20. guava*.tw.
21. kiwi*.tw.
22. lingonberr*.tw.
23. mango*.tw.
24. melon*.tw.
25. papaya*.tw.
26. pineapple*.tw.
27. raspberr*.tw.
28. strawberr*.tw.
29. tomato*.tw.
30. potato*.tw.
31. onion*.tw.
32. grapefruit*.tw.
33. mandarin*.tw.
34. satsuma*.tw.
35. tangerine*.tw.
36. (plum or plums).tw.
37. apricot*.tw.
38. (cherry or cherries).tw.
39. nectarine*.tw.
40. (peach or peaches).tw.
41. celery.tw.
42. spinach*.tw.
43. (salad or salads).tw.
44. (pea or peas).tw.
45. (bean or beans).tw.
46. broccoli.tw.
47. cauliflower*.tw.
48. beetroot*.tw.
49. turnip*.tw.
50. rhubarb.tw.
51. Food Habits/
52. Food Preferences/
53. ((food or eating) adj (habit* or preference*)).tw.
54. eating behavi*.tw.
55. (health* adj eating).tw.
56. or/1‐55
57. exp Health Education/
58. exp Health Promotion/
59. exp Behavior Therapy/
60. exp Counseling/
61. organizational policy/
62. Public Policy/
63. exp Health Policy/
64. exp Inservice Training/
65. promot*.tw.
66. educat*.tw.
67. program*.tw.
68. (policy or policies).tw.
69. train*.tw.
70. (diet* adj6 intervention*).tw.
71. (behavi* adj6 intervention*).tw.
72. or/57‐71
73. exp Infant/
74. Child, Preschool/
75. (child or children).tw.
76. (pre‐school* or preschool*).tw.
77. (infant or infants).tw.
78. infancy.tw.
79. (nursery or nurseries).tw.
80. exp Parents/
81. (parent or parents).tw.
82. toddler*.tw.
83. Nurseries/
84. (baby or babies).tw.
85. or/73‐84
86. 56 and 72 and 85
87. randomized controlled trial.pt.
88. controlled clinical trial.pt.
89. randomized.ab.
90. placebo.ab.
91. drug therapy.fs.
92. randomly.ab.
93. trial.ab.
94. groups.ab.
95. 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94
96. exp animals/ not humans.sh.
97. 95 not 96
98. 86 and 97
Embase Classic and Embase (Ovid)
1. exp Fruit/
2. exp Vegetables/
3. fruit*.tw.
4. vegetable*.tw.
5. orange*.tw.
6. apple*.tw.
7. (pear or pears).tw.
8. (grape or grapes).tw.
9. banana*.tw.
10. (berry or berries).tw.
11. citrus.tw.
12. carrot*.tw.
13. greens.tw.
14. cabbage*.tw.
15. brassica*.tw.
16. blackberr*.tw.
17. blueberr*.tw.
18. cranberr*.tw.
19. guava*.tw.
20. kiwi*.tw.
21. lingonberr*.tw.
22. mango*.tw.
23. melon*.tw.
24. papaya*.tw.
25. pineapple*.tw.
26. raspberr*.tw.
27. strawberr*.tw.
28. tomato*.tw.
29. grapefruit*.tw.
30. mandarin*.tw.
31. satsuma*.tw.
32. tangerine*.tw.
33. (plum or plums).tw.
34. apricot*.tw.
35. (cherry or cherries).tw.
36. nectarine*.tw.
37. (peach or peaches).tw.
38. celery.tw.
39. spinach*.tw.
40. (salad or salads).tw.
41. (pea or peas).tw.
42. (bean or beans).tw.
43. onion*.tw.
44. broccoli.tw.
45. cauliflower*.tw.
46. beetroot*.tw.
47. turnip*.tw.
48. rhubarb.tw.
49. potato*.tw.
50. exp feeding behavior/
51. ((food or eating) adj (habit* or preference*)).tw.
52. eating behavi*.tw.
53. (health* adj eating).tw.
54. or/1‐53
55. exp health education/
56. consumer health information/
57. behavior therapy/
58. exp counseling/
59. policy/
60. health care policy/
61. in service training/
62. promot*.tw.
63. educat*.tw.
64. program*.tw.
65. (policy or policies).tw.
66. train*.tw.
67. (diet* adj6 intervention*).tw.
68. (behavi* adj6 intervention*).tw.
69. lifestyle modification/
70. or/55‐69
71. exp infant/
72. preschool child/
73. (child or children).tw.
74. (pre‐school* or preschool*).tw.
75. (infant or infants).tw.
76. infancy.tw.
77. (nursery or nurseries).tw.
78. exp parent/
79. (parent or parents).tw.
80. toddler/
81. toddler*.tw.
82. nursery/
83. kindergarten/
84. (baby or babies).tw.
85. or/71‐84
86. 54 and 70 and 85
87. random$.tw.
88. factorial$.tw.
89. crossover$.tw.
90. cross over$.tw.
91. cross‐over$.tw.
92. placebo$.tw.
93. (doubl$ adj blind$).tw.
94. (singl$ adj blind$).tw.
95. assign$.tw.
96. allocat$.tw.
97. volunteer$.tw.
98. crossover procedure/
99. double blind procedure/
100. randomized controlled trial/
101. single blind procedure/
102. 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101
103. (animal/ or nonhuman/) not human/
104. 102 not 103
105. 86 and 104
PsycINFO (Ovid)
1. fruit*.tw.
2. vegetable*.tw.
3. orange*.tw.
4. apple*.tw.
5. (pear or pears).tw.
6. (grape or grapes).tw.
7. banana*.tw.
8. (berry or berries).tw.
9. citrus.tw.
10. carrot*.tw.
11. greens.tw.
12. cabbage*.tw.
13. brassica*.tw.
14. blackberr*.tw.
15. blueberr*.tw.
16. cranberr*.tw.
17. guava*.tw.
18. kiwi*.tw.
19. lingonberr*.tw.
20. mango*.tw.
21. melon*.tw.
22. papaya*.tw.
23. pineapple*.tw.
24. raspberr*.tw.
25. strawberr*.tw.
26. tomato*.tw.
27. grapefruit*.tw.
28. mandarin*.tw.
29. satsuma*.tw.
30. tangerine*.tw.
31. (plum or plums).tw.
32. apricot*.tw.
33. (cherry or cherries).tw.
34. nectarine*.tw.
35. (peach or peaches).tw.
36. celery.tw.
37. spinach*.tw.
38. (salad or salads).tw.
39. (pea or peas).tw.
40. (bean or beans).tw.
41. broccoli.tw.
42. cauliflower*.tw.
43. beetroot*.tw.
44. turnip*.tw.
45. rhubarb.tw.
46. onion*.tw.
47. potato*.tw.
48. eating behavior/
49. food preferences/
50. eating attitudes/
51. (health* adj eating).tw.
52. eating behavi*.tw.
53. ((food or eating) adj (habit* or preference*)).tw.
54. or/1‐53
55. health education/
56. health promotion/
57. health literacy/
58. lifestyle changes/
59. exp behavior therapy/
60. exp counseling/
61. organizational policy/
62. exp policy making/
63. exp inservice training/
64. promot*.tw.
65. educat*.tw.
66. program*.tw.
67. (policy or policies).tw.
68. train*.tw.
69. (diet* adj6 intervention*).tw.
70. (behavi* adj6 intervention*).tw.
71. or/55‐70
72. (child or children).tw.
73. (pre‐school* or preschool*).tw.
74. (infant or infants).tw.
75. (nursery or nurseries or kindergarten*).tw.
76. (parent or parents).tw.
77. toddler*.tw.
78. (baby or babies).tw.
79. exp parents/
80. exp nursery school students/
81. kindergarten students/
82. infancy.tw.
83. (“120” or “140” or “160”).ag.
84. or/72‐83
85. 54 and 71 and 84
86. random$.tw.
87. factorial$.tw.
88. crossover$.tw.
89. cross‐over$.tw.
90. placebo$.tw.
91. (doubl$ adj blind$).tw.
92. (singl$ adj blind$).tw.
93. assign$.tw.
94. allocat$.tw.
95. volunteer$.tw.
96. control*.tw.
97. “2000”.md.
98. or/86‐97
99. 85 and 98
CINAHL Plus with Full Text
S102 S83 and S101
S101 S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 or S92 or S93 or S94 or S95 or S96 or S97 or S98 or S99 or S100
S100 TX cross‐over*
S99 TX crossover*
S98 TX volunteer*
S97 (MH “Crossover Design”)
S96 TX allocat*
S95 TX control*
S94 TX assign*
S93 TX placebo*
S92 (MH “Placebos”)
S91 TX random*
S90 TX (doubl* N1 mask*)
S89 TX (singl* N1 mask*)
S88 TX (doubl* N1 blind*)
S87 TX (singl* N1 blind*)
S86 TX (clinic* N1 trial?)
S85 PT clinical trial
S84 (MH “Clinical Trials+”)
S83 S55 and S69 and S82
S82 S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78 or S79 or S80 or S81
S81 TI kindergarten or AB kindergarten
S80 (MH “Schools, Nursery”)
S79 TI (baby or babies) or AB (baby or babies)
S78 TI toddler* or AB toddler*
S77 TI (parent or parents) or AB (parent or parents)
S76 (MH “Parents+”)
S75 TI (nursery or nurseries) or AB (nursery or nurseries)
S74 TI (infant or infants or infancy) or AB (infant or infants or infancy)
S73 TI (pre‐school* or preschool* or “pre school*”) or AB (pre‐school* or preschool* or “pre school*”)
S72 TI (child or children) or AB (child or children)
S71 (MH “Child, Preschool”)
S70 (MH “Infant+”)
S69 S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68
S68 TI (behavi* N5 intervention*) or AB (behavi* N5 intervention*)
S67 TI (diet* N5 intervention*) or AB (diet* N5 intervention*)
S66 TI train* or AB train*
S65 TI (policy or policies) or AB (policy or policies)
S64 TI program* or AB program*
S63 TI educat* or AB educat*
S62 TI promot* or AB promot*
S61 (MH “Public Policy+”)
S60 (MH “Organizational Policies+”)
S59 (MH “Counseling+”)
S58 (MH “Behavior Therapy+”)
S57 (MH “Health Promotion+”)
S56 (MH “Health Education+”)
S55 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or
S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35
or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or
S54
S54 TI (“food habit*” or “food preference*” or “eating habit*” or “eating preference*”) or AB (“food habit*” or “food preference*” or
“eating habit*” or “eating preference*”)
S53 TI “health* eating” or AB “health* eating”
S52 (MH “Food Preferences”)
S51 (MH “Food Habits”)
S50 TI rhubarb or AB rhubarb
S49 TI onion* or AB onion*
S48 TI potato* or AB potato*
S47 TI turnip* or AB turnip*
S46 TI beetroot* or AB beetroot*
S45 TI cauliflower* or AB cauliflower*
S44 TI broccoli or AB broccoli
S43 TI (bean or beans) or AB (bean or beans)
S42 TI (pea or peas) or AB (pea or peas)
S41 TI (salad or salads) or AB (salad or salads)
S40 TI spinach* or AB spinach*
S39 TI celery or AB celery
S38 TI (peach or peaches) or AB (peach or peaches)
S37 TI nectarine* or AB nectarine*
S36 TI (cherry or cherries) or AB (cherry or cherries)
S35 TI apricot* or AB apricot*
S34 TI (plum or plums) or AB (plum or plums)
S33 TI tangerine* or AB tangerine*
S32 TI satsuma* or AB satsuma*
S31 TI mandarin* or AB mandarin*
S30 TI grapefruit* or AB grapefruit*
S29 TI tomato* or AB tomato*
S28 TI strawberr* or AB strawberr*
S27 TI raspberr* or AB raspberr*
S26 TI pineapple* or AB pineapple*
S25 TI papaya* or AB papaya*
S24 TI melon* or AB melon*
S23 TI mango* or AB mango*
S22 TI lingonberr* or AB lingonberr*
S21 TI guava* or AB guava*
S20 TI kiwi* or AB kiwi*
S19 TI cranberr* or AB cranberr*
S18 TI blueberr* or AB blueberr*
S17 TI blackberr* or AB blackberr*
S16 TI brassica* or AB brassica*
S15 TI cabbage* or AB cabbage*
S14 TI “greens” or AB “greens”
S13 TI carrot* or AB carrot*
S12 TI citrus or AB citrus
S11 TI (berry or berries) or AB (berry or berries)
S10 TI banana* or AB banana*
S9 TI (grape or grapes) or AB (grape or grapes)
S8 TI (pear or pears) or AB (pear or pears)
S7 TI apple* or AB apple*
S6 TI orange* or AB orange*
S5 TI vegetable* or AB vegetable*
S4 TI fruit* or AB fruit*
S3 (MH “Vegetables+”)
S2 (MH “Citrus+”)
S1 (MH “Fruit+”)
WHO International Clinical Trials Registry Platform
fruit* or citrus or vegetable* or food habits or food preference* AND infant or child* or preschool or pre‐school or parents or nurser*
ClinicalTrials.gov
child* or preschool or infant
Proquest Dissertations & Theses
(fruit or citrus or vegetable or food habits or food preferences) AND (infant or child, preschool or parents or nurser*)
GoogleScholar
(infant or child* or preschool or pre‐school) AND (fruit* or vegetable* or food habit or food preference)
Appendix 3. Living systematic review protocol
The methods outlined below are specific to maintaining the review as a living systematic review on the Cochrane Library (1). They will be used immediately upon publication of this update. Core review methods, such as the criteria for considering studies in the review and assessment of risk of bias, are unchanged. As such, below we outline only those areas of the Methods for which additional activities or rules apply.
Search methods for identification of studies
We will re‐run electronic database and trial registry searches monthly. For the electronic databases (CENTRAL, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE and Embase) and other electronic sources (WHO International Clinical Trials Registry Platform and clinicaltrials.gov), we will set up auto‐alerts (where possible) to deliver a monthly search yield by email.
We will search other resources (articles published in three relevant international peer reviewed journals: Journal of Nutrition Education and Behavior, Public Health Nutrition, and Journal of the Academy of Nutrition and Dietetics; database of published dissertations; and grey literature in GoogleScholar) manually every six months.
As additional steps to inform the living systematic review, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available, or to share early or unpublished data. We will contact the corresponding authors of any newly‐included studies for advice about other relevant studies. We will conduct citation tracking of included studies in Web of Science Core Collection on an ongoing basis. For that purpose, we have set up citation alerts in Web of Science Core Collection. We will manually screen the reference list of any newly‐included studies and systematic reviews. Also, we will use the 'related citation' feature in PubMed to identify additional articles.
We will review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
Selection of studies
We will immediately screen any new citations retrieved by the monthly searches. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) (Wallace 2017) available in the Cochrane Register of Studies (CRS‐Web) (Cochrane 2017a). The classifier assigns a probability (from 0 to 100) to each citation for being a true randomised controlled trial (RCT). For citations that are assigned a probability score of less than 10, the machine learning classifier currently has a specificity/recall of 99.987% (Wallace 2017). We will screen citations assigned a score from 10 to 100 in duplicate and independently. Cochrane Crowd (Cochrane 2017b) will screen citations that score 9 or less. Any citations that are deemed to be potential RCTs by Cochrane Crowd will be returned to the authors for screening.
Data synthesis
Whenever we find new evidence (i.e. studies, data or information) meeting the review inclusion criteria, we will extract the data, assess risk of bias and incorporate it in the synthesis every three months, as appropriate.
We will incorporate any new study data into existing meta‐analyses using the standard approaches outlined in the Data synthesis section.
Sensitivity analysis
We will not adjust the meta‐analyses to account for multiple testing, given that the methods related to frequent updating of meta‐analyses are under development (Simmonds (in press)).
Other
We will consider the review scope and methods if appropriate in light of potential changes in the topic area, or the evidence being included in the review (e.g. additional comparisons, interventions or outcomes, or new review methods available).
The review is being piloted as a living systematic review up until March 2018.
Data and analyses
Comparison 1.
Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vegetable intake | 13 | 1741 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 2 Vegetable intake ‐ sensitivity analysis ‐ risk of bias | 13 | 1670 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 2.1 Low/unclear risk of bias | 5 | 487 | Std. Mean Difference (Random, 95% CI) | 0.23 [0.03, 0.44] |
| 2.2 High risk of bias | 8 | 1183 | Std. Mean Difference (Random, 95% CI) | 0.37 [0.06, 0.68] |
| 3 Vegetable intake ‐ sensitivity analysis ‐ primary outcome | 13 | 1670 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 3.1 Primary outcome of child fruit or vegetable intake | 10 | 1331 | Std. Mean Difference (Random, 95% CI) | 0.45 [0.19, 0.70] |
| 3.2 Primary outcome unclear | 3 | 339 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.19, 0.24] |
| 4 Vegetable intake ‐ sensitivity analysis ‐ missing data | 13 | 1670 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 4.1 Low attrition or high attrition with ITT analysis | 8 | 757 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.10, 0.48] |
| 4.2 High attrition and no ITT analysis | 5 | 913 | Std. Mean Difference (Random, 95% CI) | 0.35 [‐0.10, 0.79] |
| 5 Vegetable intake ‐ subgroup analysis ‐ modality | 13 | 1670 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 5.1 Face‐to‐face | 11 | 1489 | Std. Mean Difference (Random, 95% CI) | 0.32 [0.09, 0.56] |
| 5.2 Other modality | 2 | 181 | Std. Mean Difference (Random, 95% CI) | 0.36 [0.06, 0.66] |
| 6 Vegetable intake ‐ subgroup analysis ‐ setting | 13 | 1670 | Std. Mean Difference (Random, 95% CI) | 0.33 [0.13, 0.54] |
| 6.1 School or preschool | 4 | 444 | Std. Mean Difference (Random, 95% CI) | 0.19 [‐0.02, 0.40] |
| 6.2 Home | 4 | 474 | Std. Mean Difference (Random, 95% CI) | 0.56 [0.18, 0.95] |
| 6.3 Home + Lab | 2 | 40 | Std. Mean Difference (Random, 95% CI) | 0.74 [0.09, 1.39] |
| 6.4 Other settings | 3 | 712 | Std. Mean Difference (Random, 95% CI) | 0.06 [‐0.14, 0.26] |
Analysis 1.6.
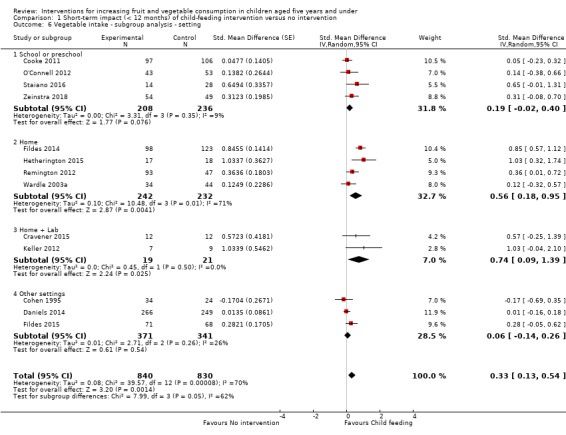
Comparison 1 Short‐term impact (< 12 months) of child‐feeding intervention versus no intervention, Outcome 6 Vegetable intake ‐ subgroup analysis ‐ setting.
Comparison 2.
Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fruit and vegetable intake | 11 | 3078 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.03, 0.28] |
| 2 Fruit and vegetable intake ‐ sensitivity analysis ‐ primary outcome | 11 | 3078 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.03, 0.28] |
| 2.1 Primary outcome of child fruit or vegetable intake | 8 | 2792 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.08, 0.16] |
| 2.2 Primary outcome unclear | 3 | 286 | Std. Mean Difference (Random, 95% CI) | 0.52 [0.03, 1.00] |
| 3 Fruit and vegetable intake ‐ sensitivity analysis ‐ missing data | 11 | 3078 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.03, 0.28] |
| 3.1 Low attrition or high attrition with ITT analysis | 7 | 2518 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.00, 0.24] |
| 3.2 High attrition and no ITT analysis | 4 | 560 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.45, 0.59] |
| 4 Fruit and vegetable intake ‐ subgroup analysis ‐ modality | 11 | 3078 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.03, 0.28] |
| 4.1 Face‐to‐face only | 5 | 826 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.20, 0.45] |
| 4.2 Audio visual only | 2 | 386 | Std. Mean Difference (Random, 95% CI) | 0.40 [‐0.04, 0.85] |
| 4.3 Other modality | 4 | 1866 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.16, 0.21] |
| 5 Fruit and vegetable intake ‐ subgroup analysis ‐ setting | 11 | 3078 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.03, 0.28] |
| 5.1 Home | 5 | 2047 | Std. Mean Difference (Random, 95% CI) | 0.06 [‐0.16, 0.27] |
| 5.2 Preschool | 2 | 243 | Std. Mean Difference (Random, 95% CI) | 0.43 [‐0.27, 1.13] |
| 5.3 Other settings | 4 | 788 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.07, 0.25] |
Analysis 2.5.
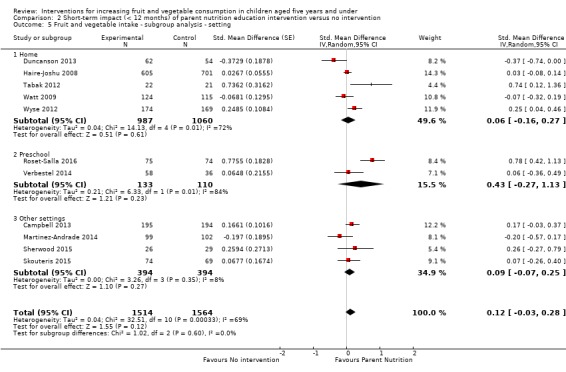
Comparison 2 Short‐term impact (< 12 months) of parent nutrition education intervention versus no intervention, Outcome 5 Fruit and vegetable intake ‐ subgroup analysis ‐ setting.
Comparison 3.
Short‐term impact (< 12 months) of multicomponent intervention versus no intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fruit and vegetable intake | 5 | 2009 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.04, 0.66] |
| 2 Fruit and vegetable intake ‐ sensitivity analysis ‐ primary outcome | 5 | 2009 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.04, 0.66] |
| 2.1 Primary outcome of child fruit or vegetable intake | 4 | 1315 | Std. Mean Difference (Random, 95% CI) | 0.44 [‐0.00, 0.87] |
| 2.2 Primary outcome unclear | 1 | 694 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.13, 0.38] |
| 3 Fruit and vegetable intake ‐ sensitivity analysis ‐ missing data | 5 | 2009 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.04, 0.66] |
| 3.1 Low attrition or high attrition with ITT analysis | 3 | 413 | Std. Mean Difference (Random, 95% CI) | 0.65 [0.43, 0.88] |
| 3.2 High attrition and no ITT analysis | 2 | 1596 | Std. Mean Difference (Random, 95% CI) | 0.06 [‐0.08, 0.20] |
| 4 Fruit and vegetable intake ‐ subgroup analysis ‐ setting | 5 | 2009 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.04, 0.66] |
| 4.1 School or preschool | 3 | 1608 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.07, 0.20] |
| 4.2 Other settings | 2 | 401 | Std. Mean Difference (Random, 95% CI) | 0.66 [0.43, 0.89] |
Analysis 3.4.
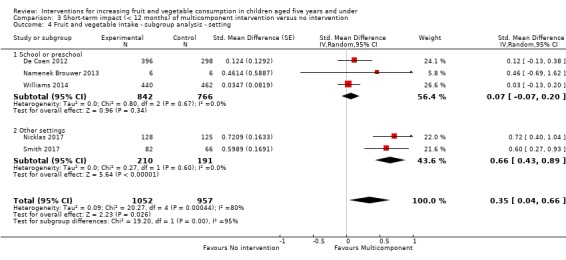
Comparison 3 Short‐term impact (< 12 months) of multicomponent intervention versus no intervention, Outcome 4 Fruit and vegetable intake ‐ subgroup analysis ‐ setting.
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2019 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 25 January 2018 are included in the current update (published May 2018). In addition, the team continues with the monthly screening (last search date 25 December 2018) and has found a further 10 new studies and 5 new ongoing studies that will be included in a future update. |
History
Protocol first published: Issue 6, 2010 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 15 March 2018 | New search has been performed | We conducted an update of the review, which includes
eight new trials based on a search from 25 January 2018
(Cohen
1995; Forestell
2007; Gerrish 2001; Heath 2014; Kling 2016; Sherwood 2015;
Smith 2017;
Zeinstra
2018). This is a Living Systematic Review. Searches are run and screened monthly. The last search for the regular monthly screenings was 25 March 2018 and we found an additional four new studies and one ongoing study that will be included after the May 2018 update. |
| 15 March 2018 | New citation required and conclusions have changed | There is low‐quality and very low‐quality evidence respectively that multicomponent and specific child‐feeding practice interventions increase the consumption of fruit and vegetable amongst children aged five years and under. There is very low‐quality evidence that parent nutrition education interventions may not be effective in increasing fruit and vegetable consumption of children aged five and under. |
| 25 February 2018 | New search has been performed | This is a Living Systematic Review. Searches are run and screened monthly. Search results up to 25 September 2017 are included in the current update (published January 2018). In addition, the team continues with the monthly screening (last search date 25 January 2018) and has found a further 8 new studies and 4 new ongoing studies that will be included in the next update (expected in May 2018). |
| 25 September 2017 | New search has been performed | We conducted an update of the review, which includes five
new trials based on a search from 25 September 2017. This is a Living Systematic Review. Searches are run and screened monthly. The last search for the regular monthly screenings was 25 November 2017 and we found an additional seven new studies and four new ongoing studies that will be included after the January 2018 update. |
| 25 September 2017 | New citation required but conclusions have not changed | There remains very low‐quality evidence that specific child‐feeding practice interventions increase the consumption of vegetables amongst children aged five years and under. There is very low‐quality evidence that parent nutrition education interventions and multicomponent interventions respectively may not be effective in increasing fruit and vegetable consumption of children aged five and under. |
| 30 September 2016 | New search has been performed | We conducted an update of the review which identified 45 new trials eligible for inclusion. |
| 30 September 2016 | New citation required and conclusions have changed | There is very low‐quality evidence that specific child‐feeding practice interventions increase the consumption of vegetables amongst children aged five years and under. There is very low‐quality evidence that parent nutrition education interventions and multicomponent interventions respectively may not be effective in increasing fruit and vegetable consumption of children aged five and under. |
Differences between protocol and review
Consistent with the original review (Wolfenden 2012), we excluded trials if fruit or vegetable intake was not the primary trial outcome, to avoid potential confounding effects of other interventions and reduce the risk of publication bias and selective outcome reporting which is more predominate among secondary trial outcomes (or outcomes that were not otherwise stated). This included trials where fruit and vegetable outcomes were assessed within broader targeted interventions. The protocol stated that trials listing fruit and vegetable intake as a secondary trial outcome would also be included. We included trials that did not state a primary outcome, but did report intake of fruit or vegetables or both. We conducted sensitivity analyses to explore the impact on the overall assessment of treatment effects, excluding studies that did not state a primary outcome of children's fruit and vegetable consumption.
Consistent with the original review (Wolfenden 2012), we amended classification of intervention effects as 'short‐term' from 'three to less than 12 months' in the protocol to less than 12 months in the review.
Consistent with the original review (Wolfenden 2012), we did not contact professional associations as part of the review search strategy, nor did we search the National Institute of Health Randomized Trial Records Database.
Consistent with the original review (Wolfenden 2012), we amended the title and text throughout the review to ensure consistent terminology for the description of age. Specifically, we replaced the age description of children as 'preschool' with a more precise description of 'children aged five years and under', to more accurately reflect the scope of the review. We refer only to preschools when discussing the findings of trials conducted in that setting.
Consistent with the original review (Wolfenden 2012), as some trials included children across a range of ages, we included any trial where the mean age of the sample at baseline was five years or under.
For the review update, while two independent reviewers extracted data from each study, the extraction was undertaken by pairs of reviewers.
For the review update, risk of bias was assessed on published study information and authors of included studies were not contacted to clarify any aspects.
For the review update, we did not conduct planned subgroup analyses by interventions of varying intensities, due to insufficient information being reported across the included studies about the number and duration of intervention contacts or components.
For the review update, pairs of review authors independently screened articles against all pre‐specified eligibility criteria and assessed risk of bias. The sequential method of screening adopted in the original review (that is by order: participants, outcome, comparator, intervention, study type) was not adopted in the review update.
Whilst not explicitly excluded from the original review, for the review update we specifically considered cross‐over trials to be an eligible study design. This was due to the many trials that adopt this design to investigate the effectiveness of interventions to increase the fruit and vegetable consumption of children aged five years and under, and the review authors deeming the study design to be appropriate in this context.
This update includes some new methods relevant for living systematic reviews, which are included in the Methods and also described in Appendix 3.
We did not adopt the planned use of the 'Related citation' feature in PubMed to identify additional articles as a component of the living systematic review methods for the current version of the review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 3 to 6 years attending an independent childcare facility in Central Pennsylvania, USA N (Randomised): 47 children Age: 3 to 6 years (mean = 4.7 years) % Female: 51% SES and ethnicity: Children: White = 83%, Asian = 10% Parents: “Most parents were well‐educated (median education = bachelor’s degree) and were currently employed. The majority of parents reported being married (88%), and the majority of the families reported annual combined family incomes greater than $60,000 (89%).” Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial Exclusion criteria: “Children were excluded if they had intolerance to study foods, a chronic illness affecting food intake, or if they were non‐English speaking. Additionally, individuals with extended absences were excluded from the results.” Recruitment: Not specified Recruitment rate: Unknown Region: Central Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): 41 (not specified by group) Description of intervention: “All children in each classroom received the same vegetable throughout the study”. “children were asked twice weekly over a period of 4 weeks to take of taste of a very small portion (˜4 g) of the vegetable in its assigned condition.” Repeated exposure: Vegetable intake without dip Flavor‐flavor associative conditioning: Vegetable intake with dip. “Dips served in this experiment included two savory dips (ketchup and ranch‐flavored) and one sweet‐tasting dip (cinnamon sugar)” Duration: 4 weeks Number of contacts: 8 exposure sessions (2 exposures/week) Setting: Preschool Modality: Face‐to‐face Interventionist: Research staff Integrity: No information provided Date of study: Unknown Description of control: NA |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of target vegetable (grams). “Children were served a bowl containing 60 g of the vegetable, and children in the AC condition were also served ˜60 g of dip in 3.25 oz soufflé cups, which accompanied the vegetable…. Instructions to children prior to the meal were to eat as much as they wanted, not to share food with others, and to remain in their seats…. When children finished snack, spilled or dropped foods were returned to the correct dish and snack trays were cleared. Vegetables were weighed before serving and were weighed after the intake session was complete, and the difference was recorded as vegetable intake.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 9 weeks Length of follow‐up post‐intervention: 2 weeks Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Unknown if sample size calculation was performed. |
|
| Notes | Sensitivity analysis ‐ primary outcome: Primary outcome not stated. Child fruit and vegetable intake 2nd listed outcome measure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded and it seems likely that children may have been influenced by those children around them and whether or not other children had a flavoured dip |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Food was weighed and it is unlikely to be influenced by whether the researchers were blinded to condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any attrition and therefore low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol so it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “This project was funded by Wageningen University and Research Centre.” |
|
| Participants |
Description: Healthy infants between 4 and 7 months (not being weaned yet) and their parent N (Randomised): 101 parent‐infant pairs Age: Child (mean): Green beans group = 162 days, Artichoke group = 160 days, Apple group = 165 days, Plum group = 162 days Mother (mean): Green beans group = 31 years, Artichoke group = 30 years, Apple group = 31 years, Plum group = 32 years % Female: Child: Green beans group = 54%, Artichoke group = 41%, Apple group = 56%, Plum group = 44% Parent: 96% SES and ethnicity: Parents education: Low = 17%, middle = 32%, high = 50% Inclusion/exclusion criteria: Inclusion criteria: “Only healthy Children between 4 and 7 months old, who were not being weaned yet, were included in the study.” Exclusion criteria: “Children with known food allergies, swallowing or digestion problems, or other medical problems that could influence the ability to eat, were excluded.” Recruitment: “The participants were recruited from the area of Wageningen and Almere in the Netherlands where both the research locations were. They were recruited via local newspapers, maternity or children welfare centers, postnatal care groups, and a mailing to subscribers of babyinfo.nl (a Dutch advertisement website that gives a box with free products for subscribers expecting a baby).” Recruitment rate: Unknown Region: Wageningen and Almere (The Netherlands) |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Green beans group = 24 Artichoke group = 27 Apple group = 24 Plum group = 24 Description of intervention: At the lab (days 1,2,17,18 and 19): “A bowl with two jars of vegetable purée was handed to the mother and the mother fed the infant at their usual rate until the end of the feeding was indicated by the infant (i.e. when it rejected the spoon more than three successive times).” At the home (days 3 ‐ 16): “At the end of the 2nd test‐day at the lab, the mothers received the jars of puréed vegetables or fruits for the home exposure period. Each jar was labelled with the date on which it had to be fed to the infant and numbered from 3 to 16 corresponding to the respective days of the intervention period. The feeding was carried out every day at about the same time and in the same way as during days 1 and 2 in the lab.” Duration: 19 days Number of contacts: 9 exposure sessions Setting: Lab and home Modality: Face‐to‐face Interventionist: Researchers trained parents to offer the target vegetable or fruit puree to their child Integrity: No information provided Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of target vegetable and fruit purees (grams). At the lab: “The pre‐ and post‐weight of the bowl including the spoon and bib was weighted to measure the actual intake.” At the home: “The mother was instructed to empty both jars completely on a plate and to put all what was left over after the feeding, including the vegetable purée that was spilled on the table, floor, bib, child’s face, etc., back in the jar and to seal the jar with the lid and put it in the refrigerator…. In order to have a standardized measure of home intake, the jars had been pre‐weighted in the lab before the home exposure period, and after they were collected and were post‐weighted again in the lab.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 19 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 2% (not specified by group) Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no indication whether the mother who fed the child was blind to group allocation. Given the mother fed the child, at high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no indication whether the mother who fed the child and weighed the food was blinded to group allocation. Given the food was weighed by the mother the risk of detection bias is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% retention and therefore risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol, therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “No external or intramural funding was received.” |
|
| Participants |
Description: Children 5 years of age in 12 nursery schools connected to the Izmir Provincial Directorate of National Education N (Randomised): 6 preschools, 238 children Age: Child: 5 years of age Mother (mean): Intervention = 33.4 years, Control = 33.4 years Father (mean): Intervention = 36.9 years, Control = 36.8 years % Female: Child: Intervention = 60%, Control = 48% SES and ethnicity: Education: Mother: Primary = 9%, Secondary school = 15%, High school = 38%, University = 38% Father: Primary = 10%, Secondary school = 14%, High school = 37%, University = 40% Family SES: Low = 16%, Medium = 73%, Upper = 11% Inclusion/exclusion criteria: Not specified Recruitment: Not specified Recruitment rate: Unknown Region: Izmir (Turkey) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 141, Control = 97 Description of intervention: “The content of the education guided by Piaget’s theory included play and visual materials. Thus, healthy food choices were created by means of play/games. Following age‐appropriate education carried out using Piaget’s theory, improvements are observed in food selection and consumption” Duration: Initial intervention = 6 weeks + at 1 year follow‐up a 3 week refresher intervention (20 ‐ 30 minutes per session) Number of contacts: 9 sessions (1 per week) Setting: Preschool Modality: Face‐to‐face Interventionist: “The researcher (H.B.), who is a nurse educator, was the interventionist for all sessions.” Integrity: No information provided Date of study: February 2007 to June 2008 Description of control: “The children in the control group had not received nutrition education but they had received a general program of education (the nutrition education prescribed by the Ministry of National Education preschool). The yearly syllabus of the Ministry includes subjects on nutrition every 2 months. This time frame, however, may be insufficient for nutrition education.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables assessed using food frequency questionnaire (FFQ) completed by parents Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Post‐test: 4 months (pre‐test February 2007 – post‐test June 2007) Post‐test 2: 16 months (post‐test 2 June 2008) Length of follow‐up post‐intervention: Post‐test: 2 months Post‐test 2: 14 months Subgroup analyses: None Loss to follow‐up (at 2 and 14 months) Intervention: 1%, 52% Control: 9%, 51% Analysis: Unclear Sample size calculation was performed. |
|
| Notes | Sensitivity analysis ‐ primary outcome: Primary outcome not stated, power calculation conducted on knowledge only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nutrition knowledge & food frequency
(self‐reported) There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nutrition knowledge & food frequency There is no mention that participants were blinded to group allocation and therefore the risk of detection bias is high |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 67/141 (48%) in experimental group and 48/97 (49%) in control group completed post‐test 2 and therefore risk of attrition bias is high |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity do not appear to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Low‐income mother/toddler (12 ‐ 30 months) dyads N (Randomised): Unknown Age: Child: mean = 20 months Mother: mean = 27.4 years % Female: Child: 59% SES and ethnicity: “67.3% below poverty index, 34% married, 68% black” Inclusion/exclusion criteria: Low‐income mother (criteria not stated) with toddler 12 ‐ 30 months Recruitment: Recruited from WIC (Women, Infants and Children) Clinics Recruitment rate: Unknown Region: USA |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Preliminary = 151 Description of intervention: “Interventions (5 group & 3 individual sessions) used goal setting to promote: 1) parenting practices or 2) maternal diet and physical activity (PA)" Duration: Not specified Number of contacts: Not specified Setting: WIC Clinic Modality: Face‐to‐face Interventionist: Unclear Integrity: No information provided Date of study: Unknown Description of control: Placebo group, sessions provided on toddler safety. |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Change in vegetable and fruit intake (mypyramid equivalent per 1000 kcal) assessed using 24‐hour diet recall completed by parents Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 and 12 months Length of follow‐up post‐intervention: Unclear Subgroup analyses: None Loss to follow‐up: Unknown Analysis: Unknown if sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 24‐hour diet recall There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 24‐hour diet recall There is no mention that participants were blinded to group allocation and therefore the risk of detection bias is high |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no information provided about attrition rates at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | There is insufficient information to determine the risk of other bias |
| Methods |
Study design: Randomised controlled trial Funding: “Funded by the Feeding For Life Foundation (grant reference number 11‐1170). ” |
|
| Participants |
Description: Children aged 2 to 4 years and their principle caregiver (parent) N (Randomised): 120 parent‐child dyads Age: Child (mean): Prompting no modelling = 27 months, Prompting and modelling = 29 months, Modelling ‘control’ group = 31 months Mothers (mean): Prompting no modelling = 34 years, Prompting and modelling = 26 years, Modelling ‘control’ group = 35 years % Female: Child: 45% Parent: 98% SES and ethnicity: Not specified Inclusion/exclusion criteria: “Inclusion criteria for children included the absence of known food allergies or disorders affecting eating, current or recent major illness or diagnosed intellectual disabilities.” Recruitment: “Caregivers and their children were recruited through the Children and Child Laboratory database, which contains information on families in which caregivers have indicated an interest in research participation at the University of Birmingham.” Recruitment rate: Unknown Region: UK |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Prompting no modelling = 35 dyads Prompting and modelling = 37 dyads Modelling ‘control’ group = 27 dyads Description of intervention: Prompting no modelling: “Caregivers were asked to use physical prompts to eat the novel fruit (NF) (including passing the food to the child, moving the food towards the child, holding the NF up to the child’s face, encouraging the child to touch the NF).” Prompting and modelling: As well as using physical prompts as in PNM, “The caregivers assigned to this condition were also asked to try the NF themselves.” Modelling ‘control’ group: “Caregivers in this condition were not given any information about prompting, but were simply asked to taste the NF themselves.” Duration: 1 day Number of contacts: 1 Setting: Lab Modality: Face‐to‐face Interventionist: Parents Integrity: Prompting no modelling: “Of an original sample of fifty, fifteen were classed as non‐compliant: ten caregivers failed to prompt a minimum of three times, and five caregivers were removed from the group because they ate the NF. This left a sample of thirty‐five parents who physically prompted but did not model eating the fruit.” Prompting and modelling: “Of an original sample of forty‐three dyads, six were non‐compliant because the parent failed to prompt three times or more, leaving a sample of thirty‐seven parents who prompted and modelled eating the fruit.” Modelling ‘control’ group: “There were twenty‐seven dyads in this condition, in which the parent modelled eating of the fruit; all were compliant with this request.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of novel fruit (grams): “All meal items were weighed on scientific scales before and after consumption.” “Owing to differences in weights of the different NF offered, it was not possible to compare conditions based on simple weight of consumption. Therefore, we calculated consumption of the NF based on the percentage consumed of the whole portion offered.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: < 1 day Length of follow‐up post‐intervention: Same day Subgroup analyses: None Loss to follow‐up: Prompting no modelling: 30% Prompting and modelling: 14% Modelling ‘control’ group: No loss to follow‐up Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random sequence generation procedure is unclear. The authors indicate that block randomisation was used to allocate to groups in blocks of 10 participants with conditions changing each week, allocated in order of recruitment |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Fruit intake is an objective measure of child’s fruit intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Fruit intake All meals were weighed on scientific scales before and after consumption therefore at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Used a per‐protocol analysis rather than an intention‐to‐treat analysis and therefore at high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | There was a significant difference in children’s ages and child’s age was controlled for in analyses. Therefore the risk of other bias is unclear |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “National Health and Medical Research Council Grant No. 425801" |
|
| Participants |
Description: First‐time mothers and their infants N (Randomised): 62 parent groups, 542 parent‐child pairs Age: Child (mean): Intervention = 3.9 months, Control = 3.9 months Parent (mean): Intervention = 32.5 years, Control = 32.1 years % Female: Intervention = 48%, Control = 47% SES and ethnicity: Parent: Education level (Completed university degree or beyond): Intervention = 52%, Control = 57% Born in Australia: Intervention = 78%, Control = 78% Inclusion/exclusion criteria: Parent groups: Inclusion criteria: “Parent groups were eligible if ≥8 parents enrolled or ≥6 parents enrolled in areas of low socioeconomic position (SEP) because mothers in areas of low SEP are less likely to attend first‐time parent groups.” No explicit exclusion criteria stated for this trial Parents: Inclusion criteria: “Parents will be eligible to participate if they are able to freely give informed consent, are first‐time parents, members of a participating 'first‐time parents group' and are able to communicate in English.” Exclusion criteria: “Parents will be excluded from the study if they are unable to give informed consent or are unable to communicate in English. Infants with chronic health problems that are likely to influence height, weight, levels of physical activity or eating habits will be excluded from analyses but will be permitted to participate in the study.” Recruitment: “A two‐stage random sampling process will be used to select first‐time parent groups. At the first stage, twelve local government areas within a 60 km radius of the research centre (Deakin University in Burwood, Victoria, Australia) will be randomly selected.” “At the second stage, first‐time parent groups within selected local government areas will be randomly selected, proportional to the total number of first‐time parent groups within each area. The first‐time parents group currently underway will then be invited to participate.” Recruitment rate: Parent: 86% (542/630) Region: Melbourne (Australia) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 195, Control = 194 Description of intervention: “The dietitian‐delivered intervention comprised six 2‐hour sessions delivered quarterly during the first‐time parents’ group regular meeting.” The intervention “sought to build knowledge, skills, and social support regarding infant feeding, physical activity, and sedentary behaviors. Messages were anticipatory in nature, such that concepts were presented before the associated child developmental phase.” “Intervention materials incorporated 6 purpose‐designed key messages (for example, “Color Every Meal With Fruit and Veg,” “Eat Together, Play Together,” “Off and Running”) within a purpose‐designed DVD and written materials. A newsletter reinforcing key messages was sent to participants between sessions.” Duration: 15 months Number of contacts: 6 sessions at 3‐monthly intervals (2 hours per session) Setting: Parenting group Modality: Multiple (face‐to‐face, visual and written materials) Interventionist: Experienced Dietitian Integrity: “Program fidelity was audited via checklists by researchers attending but not delivering the intervention.” No further information reported Date of study: June 2008 to February 2010 Description of control: “Control parents received usual care from their MCH nurse, who may have provided lifestyle advice.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetable (grams) assessed using 3 x 24hr recalls (3 days, including 1 weekend day) conducted by trained nutritionists via telephone interview with parents Outcome relating to absolute costs/cost effectiveness of interventions: Intervention cost per family reported that adjusted “for the fact that a trial setting sees an artificially small number of families included relative to the workforce employed” Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 (mid‐intervention) and 15 months (post‐intervention) Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up (Immediately post‐intervention): Intervention = 28% Control = 28% Analysis: Adjusted for clustering. Sample size calculation was performed. |
|
| Notes | First reported outcome (grams fruit/day) was
extracted for inclusion in the meta‐analysis.
Sample size per group was not reported and instead
calculated based on assumption of equal loss to
follow‐up per group, and reported baseline
sample per group and total sample for diet outcomes
at follow‐up. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, however power calculation was conducted on fruit or vegetable intake |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to condition using a computer‐generated random number schedule developed by a statistician with no contact with the centres |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 24‐hour dietary recall (parent reported) Parents were not blinded to group allocation and therefore the risk of performance bias is high |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 24‐hour dietary recall (parent reported) Parents were not blinded to group allocation and because this is a self‐reported measure the risk of detection bias is high, even though the dietary recalls were administered by telephone by staff blinded to participant’s group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 389/542 (72%) completed the diet outcomes during this long‐term assessment. However the number and reasons for dropout is not reported by study group and so cannot establish if reasons for dropouts are similar across groups |
| Selective reporting (reporting bias) | High risk | There are physical activity outcomes referred to in the protocol that are not reported |
| Other bias | Low risk | There are no differences in baseline characteristics between trial arms & contamination and other bias unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: "This research has received funding from the European Community’s Seventh Framework Programme (FP7/2007‐3) under grant agreement no. 245012‐HabEat coordinated by Dr Sylvie Issanchou. (INRA, UMR 1324, Centre de Sciences du Gouˆt et de l’Alimentation, F‐21000 Dijon France)." |
|
| Participants |
Description: Children aged 6 to 36 months in private daycare nurseries in West and South Yorkshire, UK N (Randomised): Unclear “Of the 108 recruited, fourteen children were excluded due to food allergies (n 3) and for being older than 40 months (n 11). Of the ninety‐four children, six children refused to take part in the study, fifteen were excluded due to lack of attendance at nursery and one was removed for incomplete exposures. Table 2 provides characteristics of the children who took part in the intervention. Out of the potential sample, seventy‐two completed the Study.” Age: Mean: Repeated exposure = 24 months, Flavour‐flavour learning = 23 months, Flavour‐nutrient learning = 24 months % Female: Repeated exposure = 55%, Flavour‐flavour learning = 48%, Flavour‐nutrient learning = 68% SES and ethnicity: Unclear, “to ensure good representation of ethnic background and SES we selected nurseries in a variety of different locations in West and South Yorkshire, UK” Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial “All children reported to have any food allergies were excluded from taking part in the investigation.” Recruitment: “In the first instance, nursery managers were given details of the study to check their interest in the study. If the nursery managers expressed an interest, then the participant information sheets and consent forms were distributed to parents.” Recruitment rate: Unknown Region: West and South Yorkshire (UK) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Repeated exposure = 22 Flavour‐flavour learning = 25 Flavour‐nutrient learning = 25 Description of intervention: “Around 2–4 d after the pre‐intervention period, each child was offered one pot (100 g) of artichoke for ten exposures.” Repeated exposure: “The RE recipe was a basic vegetable puree.” Flavour‐flavour learning: “For the FFL puree, the chosen unconditioned stimulus was sweetness. The selected sweet ingredient was sucrose.” Flavour‐nutrient learning: “For the FNL puree, the chosen unconditioned stimulus was a higher energy density. The selected energy‐dense ingredient was sunflower oil, because of its relatively neutral taste.” Duration: 10 days Number of contacts: 10 Setting: Preschool Modality: Face‐to‐face Interventionist: Nursery staff Integrity: No information provided Date of study: Recruitment took place February – May 2011 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of novel vegetable (artichoke) (grams) and changes in intake (grams) between a familiar (carrot) and novel vegetable (artichoke) “All pots were weighed before and after to determine intake (g) throughout the experiment. Any spillage on tables and bibs were collected after the session and were added back in to the pots before re‐weighing.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: 5 weeks Subgroup analyses: None Loss to follow‐up: Repeated exposure = 27% Flavour‐flavour learning = 40% Flavour‐nutrient learning = 46% Analysis: Unknown if sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake (objective) Objective measure of child’s vegetable intake and staff were blinded to the target vegetable being offered to the children |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake (objective) Food was weighed to determine intake and staff were blinded to the target vegetable being offered to the children |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 72 children taking part in the study 45 (63%) completed the follow‐up and so the risk of attrition bias is high |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “Supported jointly by the Thrasher Research Fund; the World Health Organization; UNICEF/Honduras and the Institute for Reproductive Health (formerly the Institute for International Studies in Natural Family Planning), Georgetown University, under a Co‐operative Agreement with the U.S. Agency for International Development (A.I.D.) (DPE‐3040‐A‐00‐5064‐00 and DPE‐3061‐A‐00‐1029‐00).”s |
|
| Participants |
Description: Low income, first time mothers and their infants N (Randomised): 152 children Age: Infants: Infants were randomised at 16 weeks of age Mother (mean): 20.2 years % Female: 55% SES and ethnicity: “Subjects came from low income neighborhoods in which environmental sanitation was poor (only 60% of the households had indoor piped water). Mean household income was $120/mo.” Inclusion/exclusion criteria: Inclusion criteria: “Selection criteria were that mothers be primiparous, willing to exclusively breast‐feed for 26 wk, not employed outside the home prior to 6 mo postpartum, low income (less than $150/mo), at least 16 years old and healthy (not taking medication on a regular basis), and that infants be healthy, term, and weigh at least 2000g at birth.” Exclusion criteria: “Multiparous and working mothers were excluded because the intervention required a 3‐d stay in the La Leche League unit on three occasions to measure breast milk intake.” Recruitment: “Subjects were recruited from two public hospitals in San Pedro Sula, Honduras” Recruitment rate: 86% (152/176) Region: San Pedro Sula, Honduras |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Solid foods: 42 Solid foods + maintenance: 39 Exclusive breast‐feeding: 42 Description of intervention: Solid foods: introduction of solid foods at 4 months, with breast‐feeding as required 4‐6 months. Solids foods + maintenance: introduction of solid foods at 4 months, with mothers told to continue breast‐feeding as often as they had prior to the intervention. Exclusive breast‐feeding: exclusive breast‐feeding to 6 months; no other liquids (water, milk, formula) or solids In addition all mothers: 1. Stayed at the la Leche League unit at 16 weeks for 3‐days and returned to the unit at weeks 21 and 26 weeks for repeated measurements. 2. Received weekly home visits during the intervention period to collect data on breast‐feeding and infant morbidity. In the solid food groups these weekly visits also were used to monitor use of the foods provided and encourage mothers in the maintenance group to maintain their re‐intervention breast‐feeding frequency. Duration: 2 months Number of contacts: 13 (10 weekly home visits + 5 hospital visits) Setting: Home + hospital Modality: Face‐to‐face Interventionist: Mothers Integrity: “To encourage compliance with study procedures, mothers recorded the number of breastfeeds each day from 16 to 26 weeks on a simple form provided weekly. This was especially important for the SF‐M mothers, who were asked to maintain breastfeeding frequency. At 19 and 24 weeks, 12‐hour in‐home observations were conducted to record breastfeeding frequency and duration, and adherence to the feeding instructions.” Date of study: Recruited from October 1991‐January 1993 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of fruit (grams): “the amount of food offered and consumed at the midday meal was measured (using an electronic scale, to the nearest gram)” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 9 and 12 months Length of follow‐up post‐intervention: 2.5 and 5.5 months Subgroup analyses: None Loss to follow‐up (at 9 and 12 months): Unclear ‐ states “for a subsample of infants, n=60 at 9 months and n= 123 at 12 months” Analysis: Unknown if sample size calculation was performed |
|
| Notes | First reported outcome (frequency of consuming fruit)
at 9 months for the < 12 months was extracted for
inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: primary outcome not stated, fruit or vegetable consumption was not first reported outcome (first reported outcome was dairy). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "At 16 wk, subjects were randomly assigned, by
week of infant's birth, to one of three groups:
1) Control: Exclusive breast‐feeding to 26 wk
(EBF); 2) Solid Foods: Introduction of solid foods
at 16 wk (SF), with ad libitum
breast‐feeding; or 3) Solid Foods‐M:
Introduction of solid foods at 16 wk (SF‐M),
with mothers told to continue breast‐feeding
as often as they had prior to the
intervention.” Allocated to group by week of birth. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed from those conducting the research. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | ”Subjects were not informed of
their assignment until they had completed the first
16 wk of the study.” “All women were visited weekly during the first 4 mo postpartum to assist them in maintaining exclusive breast‐feeding.” Due to the nature of the intervention, both participants and personnel were aware of group allocation after 16 weeks. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “During the 9‐ and
12‐mo visits, the amount of food offered and
consumed at the midday meal was measured (using an
electronic scale, to the nearest gram) for a
subsample of infants (n = 60 at 9 mo, n = 123 at 12
mo), and their mothers were interviewed regarding
the infants' usual daily food intake and
acceptance and frequency of consumption of a variety
of common foods.” It is unclear whether outcome assessors visiting the home were aware of group allocation. Mothers self‐reported food intake and acceptance. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Home visits were conducted for
a subsample only (total n=141). 9mth n=60; 12 mth
n=123.” Unclear if this is
actual subsample or if this reflects
attrition/non‐response It is unclear whether the n value for the subsample represents everyone who was eligible (i.e. had infants younger than 12 months prior to May 1993) with 100% consent rate, or if there were refusals. |
| Selective reporting (reporting bias) | Unclear risk | There is no trial registration or protocol. |
| Other bias | Unclear risk | It is unclear how the women were recruited, what the consent rate was, or how representative the sample was of the target population. |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "This research was supported by a grant from the Medical Research Council National Prevention Research Initiative." |
|
| Participants |
Description: 422 children in reception (4 to 5 years) and Year 1 (5 to 6 years) from 16 classes in 8 schools. N (Randomised): 16 classes, 472 children % Female: 47% female Age: Reception: 4 to 5 years (N = 216) Year 1: 5 to 6 years (N = 206) SES and ethnicity: “To ensure adequate representation of children from families of low socioeconomic status, we selected schools in which the proportions of pupils who were eligible for free school meals, who spoke English as a second language, and who came from minority ethnic backgrounds were above the national average." No individual child data on these variables were reported. Inclusion/exclusion criteria: Not stated Recruitment: Recruited from 16 classes in 8 schools (492 children, 472 consented) Recruitment rate: Children: 96% (472/492) Schools: unknown Region: United Kingdom |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Exposure + tangible non‐food reward (sticker) = 99 Exposure + social reward (praise) = 106 Exposure alone = 105 Control = 112 Description of interventions: “Children in the intervention conditions (ETR, EP, EA)* were seen individually from Day 3 to Day 14 and offered a small piece of their target vegetable.” Exposure + tangible non‐food reward: “Children in the ETR condition were told that if they tasted the vegetable, they could choose a sticker as a reward.” Exposure + social reward: “Children in the EP condition were praised if they tasted the vegetable (e.g. “Brilliant, you're a great taster”) Exposure alone: “Children in the EA condition were invited to taste the target vegetable but received minimal social interaction.” Duration: 3 weeks Number of contacts: 12 exposure sessions Setting: School Modality: Face‐to‐face, exposure Interventionist: Trained researchers Integrity: “Children in the three intervention groups agreed to taste their target vegetable in most sessions" Exposure + tangible non‐food reward (sticker): M = 11.34 sessions, SD = 1.45 Exposure + social reward (praise): M = 10.45 sessions, SD = 1.94; Exposure alone: M = 9.97 sessions,SD = 2.87. “Post hoc analyses showed higher compliance in the ETR condition than in the EP or EA conditions (p < 0.05), and compliance in the latter two conditions did not differ.” Date of study: Unknown Description of control: No‐treatment control: “Children in the control group did not receive taste exposure to the target vegetable during the intervention period.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: As‐desired consumption of target vegetable (grams). “The child was then invited to eat as much of the vegetable as he or she wanted, with intake (in grams) assessed by weighing the dish before and after consumption using a digital scale” (NB. “Care was taken to ensure that children in the ETR condition understood that the sticker reward was no longer available.”) Length of follow‐up from baseline: Acquisition data: day 15 Maintenance data: 1 month and 3 months later Subgroup analyses: None Loss to follow‐up (at 1 month and 3 months follow‐up): Exposure + tangible non‐food reward (sticker): 7%, 9% Exposure + social reward (praise): 8%, 5% Exposure alone: 8%, 8% Control: 11%, 6% Analysis: Analysis adjusted for clustering“Clustering by school was minimal; therefore, the final analyses adjusted only for clustering by class." Sample size calculation was performed "On the basis of evidence that 10 exposures are needed to alter preferences, we decided to repeat all analyses for a restricted subset of children who tasted their target vegetable on at least 10 days (n=365). Because there were no significant differences between the restricted and the full samples, results are reported for the full sample." |
|
| Notes | Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit and vegetable intake 2nd listed outcome after liking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Contact with the author indicated that the study used blocked randomisation performed using an online randomiser programme |
| Allocation concealment (selection bias) | Unclear risk | Randomisation occurred prior to consent. Head teachers were not aware of group allocation. It is unclear if study personnel knew of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Contact with the author indicated that personnel were not blind to group allocations and that there was the potential that participants became aware of group allocation. However, given the objective outcome measure, review authors judged that the outcome would not be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Contact with the author indicated that some, but not all of the outcome assessors were blind to group allocation. The outcome measurement (grams of target vegetable consumed, as measured by a digital scale) was objective and unlikely to have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although reasons for missing data were not provided by group, rates of loss to follow‐up were low and similar across all experimental arms of the trial at both follow‐up points (Exposure+sticker = 6.5%, 8.8%; Exposure+praise = 8.2%, 5.0%; Exposure alone = 8.2%, 8.2%; Control = 10.9%, 5.7%, provided by the author). No reasons were reported for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement Trial was registered, but not prospectively (ISRCTN42922680) |
| Other bias | Low risk | No further risks of bias identified |
| Methods |
Study design: Randomised controlled trial – cross‐over Funding: "This project was part of a larger study funded by the Robert Wood Johnson Foundation Healthy Eating Research program." |
|
| Participants |
Description: Preschoolers enrolled in a Child and Adult Care Food Programme‐participating childcare centre N (Randomised): 57 children Age: Mean = 4.4 years % Female: 35% SES and ethnicity: “Among the children’s racial and ethnic backgrounds, 41.1% were non‐Hispanic black, 37.5% were non‐Hispanic white, 14.3% were Hispanic, and 7.1% were Asian. The median total family income was $33,600 (interquartile range, $19,337–$57,000).” Inclusion/exclusion criteria: “Preschool children enrolled full time were eligible for participation in the study." No explicit exclusion criteria stated for this trial Recruitment: “One large, racially diverse child care center in Connecticut was recruited for participation in the study in 2011.” Recruitment rate: 79% (57/72) Region: Connecticut (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Condition 1: the pairing of a vegetable with a familiar, well‐liked food (lunch) = 43 Condition 2: enhancing the visual appeal of a vegetable (snack) = 42 Description of intervention: “Classrooms were randomly assigned to first participate in either the intervention or control condition for lunch (condition 1) and snack (condition 2).” “The children participated in the second condition one week after the first condition for each meal.” Condition 1: “Steamed broccoli on top of the pizza” Condition 2: “Raw cucumbers arranged as a caterpillar with chive antennae and an olive eye.” Duration: 2 days (1 day per condition) Number of contacts: 2 (1 per condition) Setting: Preschool Modality: Face‐to‐face Interventionist: Teachers and researchers Integrity: No information provided Date of study: 2011 Description of control: Condition 1: “Steamed broccoli on the side of the pizza” Condition 2: “Raw cucumbers as semicircular half‐slices with chive and an olive on the side.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: The two primary outcome measures were: 1. Willingness to taste (defined as consumption of 3 grams or more of the test vegetable) and 2. Total consumption of the test vegetable (grams) “Researchers weighed the children’s meals in the center’s cafeteria in accordance with the CACFP‐recommended preschool serving sizes for all meal components before delivering them to the classrooms. After the meal was completed, researchers weighed the plate waste of meal components in the cafeteria. All weights were recorded to the nearest 0.1 g on a digital electronic balance.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: <1 day Length of follow‐up post‐intervention: Same day Subgroup analyses: None Loss to follow‐up: Condition 1 = 25% Condition 2 = 26% Analysis: Sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake (objective) Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake (objective) Food was weighed to determine intake, but it is unlikely to be influenced by whether the researchers were blinded to condition |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 57 participants 43 (75%) and 42 (74%) were present for both days of lunch and/or snack data collection respectively. Attrition > 20% for short‐term assessments |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: "College of Health and Human Development (Pennsylvania State University)" |
|
| Participants |
Description: Children aged 3 to 5 years with low vegetable intake N (Randomised): 24 children Age: Mean: Intervention = 3.8 years, Control = 4.0 years % Female: Intervention = 50%, Control = 50% SES and ethnicity: “The majority of the participants were white (92%) and 83.3% of mothers and 82.6% of fathers reported graduating from college and/or graduate school.” Inclusion/exclusion criteria: Inclusion criteria: children aged 3 ‐ 5 years, categorised as “at risk for obesity” based on family history, defined as having at least one parent with a body mass index > 25 and consuming 2 or fewer servings of vegetables per day (according to parent report) Exclusion criteria: pre‐existing medical conditions (including relevant food allergies) Recruitment: “recruited via flyers posted around the university community and in local newspapers and websites (e.g. Craigslist).” Recruitment rate: Unknown Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 12, Control = 12 Description of intervention: “children in the treatment group (n=12) received vegetables packaged in containers decorated with their four favorite cartoon characters (selected on the first visit) and granola bars in generic packaging. All vegetable packages contained sticker incentives and children could collect stickers on a special game board and trade them for small prizes at the end of the study. This was done to simulate the concept of promotions that often come with packaged foods. Parents were in charge of deciding when children had eaten enough of a vegetable to be awarded the sticker for their game boards.” Duration: 2 weeks Number of contacts: Parents were instructed “to offer children a choice between either a vegetable or granola bar for at least three snacks and/or meals per day.” Setting: Home + lab Modality: Face‐to‐face Interventionist: Parents Integrity: “To assess compliance, parents completed daily checklists across the intervention to report when vegetables and granola bars were offered and record what children selected. In addition, parents could also report additional comments on these checklists to report other concerns or deviations. Parents were also responsible for keeping daily food diaries for children (data to be reported elsewhere). These logs were reviewed with parents during weekly home visits to assess progress.” Date of study: Recruitment August 2012 to June 2013 Description of control: “children in the control group (n=12) received weekly supplies of generic‐packaged vegetables and granola bars presented as part of a free choice at meals and snacks..” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Children’s intake of vegetables (grams), “Intake was measured as the difference between pre‐ and post‐weights of the foods provided.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 4 weeks Length of follow‐up post‐intervention: 1 week Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Sample size calculation was performed. |
|
| Notes | First reported outcome (broccoli intake grams/day) at
the longest follow‐up (4‐week
follow‐up) was extracted for inclusion in
meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is primary outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to condition using a random‐number generator. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome group: All/ Children’s
vegetable and granola bar intake Families and researchers were not blinded to condition but it is unlikely that this influenced child consumption |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome group: All/ Children’s
vegetable and granola bar intake Families and researchers were not blinded to condition and it is unclear if this had an impact on the weighing of food. The extent to which parents were compliant with instructions to return all leftovers is unknown |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: All/ 100% retention rate and so risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: "Research relating to this article was funded 2008‐2014 by two consecutive grants from the Australian National Health and Medical Research Council (426704, APP1021065); HJ Heinz (to KM); Meat and Livestock Australia; Department of Health South Australia; Food Standards Australia New Zealand; and Queensland University of Technology." |
|
| Participants |
Description: First‐time mothers with healthy term infants N (Randomised): 698 mother‐infant dyads Age: Child (mean): Intervention = 4.3 months, Control = 4.3 months Mother (mean): Intervention = 30.2 years, Control = 29.9 years % Female: Child: Intervention = 51%, Control = 50% SES and ethnicity: Mother: Education (university degree) = 59% Origin (born in Australia) = 79% SEIFA Index of Relative Advantage and Disadvantage (relative disadvantage ≤ 7th decile) = 33% Inclusion/exclusion criteria: Inclusion criteria: “Inclusion criteria were ≥18 years of age, infants >35 weeks gestation, and birth weight ≥2500 g, living in the study cities, facility with written and spoken English” Exclusion criteria: “Mother‐infant dyads will be excluded if the infant has any diagnosed congenital abnormality or chronic condition likely to influence normal development (including feeding behaviour) or the mother has a documented history of domestic violence or intravenous substance abuse or self‐reports eating, psychiatric disorders or mental health problems.” Recruitment: “A consecutive sample of first‐time mothers with healthy term infants was approached at seven maternity hospitals” “Consenting mothers were recontacted for full enrolment when their infant was four (range 2‐7) months old.” Recruitment rate: 16% (698/4376) Region: Brisbane and Adelaide (Australia) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 291, Control = 307 Description of intervention: “The first intervention module started immediately after baseline (children aged 4‐7 months) with the second module commencing 6 months after completion of the first (children aged 13‐16 months). Each module comprised six interactive group sessions (10‐15 mothers per group, total 40 groups) of 1‐1.5 hours duration, co‐facilitated by a dietitian (n=13) and psychologist (n=13). Developmentally appropriate content addressed: (i) repeated neutral exposure to unfamiliar foods combined with limiting exposure to unhealthy foods to promote healthy food preferences and (ii) responsive feeding that recognizes and responds appropriately to cues of hunger and satiety to promote self‐regulation of energy intake to need. A third theme was “feeding is parenting” and positive parenting (encouragement of autonomy, warmth, self‐efficacy).” Duration: 12 months (12 weeks duration for Modules 1 and 2 respectively, with 6‐month gap between Module 1 and 2) Number of contacts: 12 group sessions Setting: Child health clinics Modality: Face‐to‐face, group sessions Interventionist: Co‐facilitated by a dietitian and psychologists Integrity: No information provided Date of study: 2008 to 2011 Description of control: “The control group had access to universal community child health services, which, at the mother’s initiative, could include child weighing and web‐ or telephone‐based information. An important distinction was that controls did not receive anticipatory guidance but sought advice on a specific problem.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables, “assessed using a three‐pass 24‐hour dietary recall conducted via telephone by a dietitian trained” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 20 months and 4.5 years Length of follow‐up post‐intervention: 6 months and 3.5 years Subgroup analyses: None Loss to follow‐up: Intervention = 26% Control = 19% Analysis: Sample size calculation was performed. |
|
| Notes | First reported outcome (vegetable intake g/kg body
weight) at the longest follow‐up < 12
months (6 months after intervention completion) and
≥ 12 months (3.5 years after
intervention completion) was extracted for inclusion
in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, however power calculation was conducted on fruit or vegetable consumption |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to condition using permuted‐blocks randomisation schedule generated by the Institute’s Research Methods Group, which includes this study’s statistician, all of whom will otherwise not be involved in data collection or intervention delivery |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: All/ Food intake records, food
preference, feeding behaviour
(self‐reported) There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There is no blinding to group allocation of participants described, and because self‐reported measures at high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was 22% attrition at short‐term follow‐up and dropout was significantly higher in the intervention than the control group |
| Selective reporting (reporting bias) | Low risk | The measures reported in the protocol paper align with those reported in the outcome papers |
| Other bias | High risk | There were no differences according to group allocation at baseline. However at high risk of incorrect analysis as the protocol specifies that clustering within assessment clinics will be accounted for but this does not appear to have been done in any of the outcome papers |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "This work was supported by a grant from the Baden‐Württemberg Stiftung.” “F.D.B. is supported by the European Social Fund and by the Ministry of Science, Research and the Arts Baden‐Württemberg.” |
|
| Participants |
Description: Children aged 3 to 6 years in 18 preschools from 3 south German regions N (Randomised): 18 preschools, 377 children Age: Mean = 4.26 years % Female: 47% SES and ethnicity: Child: 32.4% came from an immigrant background Education levels (mother): Low = 16%, Middle = 56%, High = 21% Inclusion/exclusion criteria: “Pre‐schools were eligible to participate in the study if they were located in one of three predefined regions and had applied to participate in the nutritional intervention module of a state‐sponsored health promotion programme ‘Komm mit in das gesunde Boot’ (‘Come aboard the health boat’), with at least fifteen children participating.” “Children between 3 and 6 years of age attending one of the participating pre‐schools and participating in the programme were considered eligible for our study.” No explicit exclusion criteria stated for this trial Recruitment: Preschools: Selected from a group of preschools who had already “applied to participate in the nutritional intervention module of a state‐sponsored health promotion programme.” Recruitment rate: Preschool: 64% (18/28) Child: 80% (377/473) Region: 3 regions in Baden‐Württemberg (Germany) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): 202 children (not specified by group) Description of intervention: “Intervention activities consisted of familiarizing with different food types and preparation methods as well as cooking and eating meals together in groups of children, teachers and parents. One session additionally focused on healthy drinking behaviours.” Of the 15 sessions, five actively involved “parents by targeting them alone (discussions on parents’ modelling role and nutritional needs of children) or together with their children.” “Models for healthy eating within the intervention included: (i) use of nutrition experts; (ii) play acting with ‘pirate dolls’ used as props enjoying fruit and vegetables; (iii) active parental involvement; and (iv) involvement of other pre‐school peers. The exposure effect was taken into account by repeatedly offering healthy snacks like fruit and vegetables and water to the children every week.” Duration: 6 months Number of contacts: 15 sessions (1/week, 2hr per session) Setting: Preschool Modality: Face‐to‐face Interventionist: “The intervention was delivered by external nutrition experts” “Pre‐school group teachers assisted the external nutrition expert during each session to enable them to sustain intervention‐related activities after the study end.” Integrity: “Implementation rate was high with all modules delivered completely (5.0/5); no session was cancelled.” “Intervention fidelity was high with the majority of interventions delivered as planned.” Date of study: 2008 to 2009 Description of control: Waiting‐list control, “received the same intervention 6 months later than the intervention arm” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Change in child’s consumption of fruits and vegetables (portions/day) assessed using a questionnaire by parent self‐report Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 and 12 months Length of follow‐up post‐intervention: Immediately and 6 months Subgroup analyses: None Loss to follow‐up: “Of 348 pre‐school children, 29.6% completed all three measurements, 51.4% two measurements and 19% one measurement with 58% providing both pre‐ and post‐intervention measurements.” Individual loss to follow‐up data not reported. Analysis: Sample size calculation was performed. Analysis was not adjusted for clustering, but justification was provided. “As our data stemmed from natural pre‐school‐bound clusters of children, we first determined the extent of clustering. Intraclass correlation coefficients (ICC) on the level of pre‐schools were 0.016 and 0.014 for the primary outcomes of fruit intake and vegetable intake, respectively. With an average cluster size of 19.5 children per pre‐school, the design effect (d = 1 + (average cluster size ‐1) x ICC) did not exceed 2, allowing us to ignore the issue of clustering in our analyses.” |
|
| Notes | Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is primary outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Low risk | Preschool assignment was concealed through the use of sequentially‐numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: All/ Fruit & vegetable intake
(parent self‐reported survey) Due to the nature of the intervention, it was not possible to blind participants or intervention providers and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fruit & vegetable intake (parent
self‐reported survey) Parents were not blinded to group allocation and therefore the risk of detection bias is high |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 348 preschool children, 29.6% completed all 3 measurements, 51.4% 2 measurements and 19% 1 measurement, with 58% providing both pre‐ and post‐intervention measurements |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | The design effect did not exceed 2 and so the authors ignored clustering in the analyses. The impact of this on the analyses is unclear |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “The study was commissioned, financed and steered by the Ministry of the Flemish Community (Department of Economics, Science and Innovation; Department of Welfare, Public Health and Family).” |
|
| Participants |
Description: Children attending pre‐primary and primary schools from 6 communities in Flanders, Belgium N (Randomised): 31 schools, 1589 children Age: Mean: Intervention = 4.86 years, Control = 5.04 years % Female: Intervention = 47%, Control = 55% SES and ethnicity: % Of lower SES children: Intervention = 34%, Control = 29% Inclusion/exclusion criteria: Not specified Recruitment: “All pre‐primary and primary schools in the six communities were invited to participate in the study.” Recruitment rate: Child: 49% (1589/3242) School: 64% (31/49) Region: Flanders (Belgium) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 396, Control = 298 Description of intervention: “The intervention was based on the ‘Nutrition and Physical Activity Health Targets’ of the Flemish Community clustered into: (i) increasing daily consumption of water and decreasing soft drinks consumption; (ii) increasing daily milk consumption; (iii) increasing daily consumption of vegetables and fruit; (iv) decreasing daily consumption of sweets and savoury snacks; and (v) increasing daily PA and decreasing screen‐time behaviour.” The community “Each intervention year, information brochures and posters regarding the five topics of the project were distributed through general practitioners, pharmacists, social services and at relevant community events by the regional health boards and the research team.” The schools “All intervention schools were requested to (i) implement five Healthy Weeks per intervention year (one for each cluster of topics) with a minimum 1 h of classroom time dedicated to the topic together with extracurricular activities (e.g. during the vegetables and fruits week only fruits could be brought to school as a snack; schools organized fruit and vegetable tastings), (ii) evaluate and improve their playground and snack and beverage policy, and (iii) communicate with the parents on the programme and distribute materials to the parents. The intervention started with a meeting with the teachers during which they received manuals and guidelines and an implementation plan was discussed.” The parents “The intervention materials for the parents were newly developed for the project. The parents received a poster visualizing the target messages and containing short tips regarding parenting practices and styles to encourage children to stick to the healthy eating and PA targets. Parents also received five letters, containing detailed information on the intervention topics and a website link with practical information such as tips and recipes. Based on the FFQ in the parental questionnaire, parents received a written, normative individual tailored advice on their child’s consumption of water, milk, fruits, vegetables, soft drinks and sweet and savoury snacks, and their PA and screen‐time behaviour.” The regional health boards “They contacted each school at least twice per year assisting them in selecting relevant intervention materials and supervising the implementation progress.” Duration: “The intervention was implemented over two school years (2008–2009 and 2009–2010) on different levels.” Number of contacts: Unclear (multi‐component) Setting: School Modality: Multiple (face‐to‐face, educational materials, resources (posters, brochures), letters) Interventionist: Multiple Integrity: “Process evaluation data revealed that all schools implemented the requested classroom hour. Regarding the snack and playground policy, it was clear that the requested adjustments asked for more time investment and at the time of observation, most schools did not yet meet up to the standard.” Date of study: 2008 to 2010 Description of control: No information provided |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables (grams/day) assessed using a validated 24‐item semi‐quantitative food frequency questionnaire (FFQ) completed by parents Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 2 years Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 56% (not specified by group) Analysis: Did not adjust for clustering Sample size calculation was performed |
|
| Notes | First reported outcome (fruit consumption grams/day)
was extracted for inclusion in meta‐analysis.
The reported estimate did not account for
clustering, therefore we used
post‐intervention data and calculated an
effective sample size using ICC of 0.016 to enable
inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 2nd listed outcome after BMI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: All/ Fruit and vegetable intake
(self‐reported) There is no blinding to group allocation of participants described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fruit and vegetable intake (self‐reported) There is no mention that participants were blinded to group allocation and therefore the risk of detection bias is high |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 694/1589 (44%) completed 2‐year assessment. Long‐term attrition > 30% therefore at high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | High risk | High risk of recruitment bias as communities were
randomised and then schools within each community
were invited to participate Unclear baseline imbalance as communities differed on nutrition and PA policy, raising awareness for these topics and health promotion expertise |
| Methods |
Study design: Randomised controlled trial (as confirmed by the study author) Funding: "Grant from The Netherlands Organisation for Scientific Research (NWO)." |
|
| Participants |
Description: Children aged 4‐6 years from 6 primary schools in both urban and suburban districts in the Netherlands N (Randomised): 160 children Age: 4‐6 years (no mean provided) % Female: 49% SES and ethnicity: No explicit data: “The sample consisted of various socioeconomic and cultural backgrounds.” Inclusion/exclusion criteria: “Only schools without formal fruit and vegetable programs were selected.” Recruitment: Not specified Recruitment rate: Unknown Region: Urban and suburban districts of the Netherlands |
|
| Interventions |
Number of experimental conditions: 5 Number of participants (analysed): Interactive + congruent = 26 Interactive + incongruent = 26 Passive + congruent = 26 Passive + incongruent = 26 Baseline group = 56 Description of intervention: Children were read a picture book in a quiet room near their class. The picture book story described a main character rescuing his friend. The main character in this story is able to rescue his friend only after eating carrots to make him fit and strong. Passive vs interactive In the interactive sessions, the storyteller used a reading manual to ask children questions about the story and its characters before, during, and after the session. In the passive sessions, children were not asked any questions, but encouraged to sit quietly and listen. Congruent vs incongruent 1 book featured a product–congruent character (a rabbit), and the other featured a product–incongruent character (a turtle) Duration: 5 days Number of contacts: 5 sessions Setting: School Modality: Face‐to‐face Interventionist: Female daycare worker Integrity: No information provided Date of study: October‐December 2011 Description of control: Baseline ‘control’ group “not exposed to the book” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s proportional consumption of vegetables. “Children’s proportional product consumption was measured by dividing the number of pieces of each food eaten by the total number of pieces of foods eaten, for example: number of carrots eaten/total number of foods eaten.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 5 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Unknown if sample size calculation was performed |
|
| Notes | "Children in the experimental groups were randomly assigned to the four experimental conditions (n = 26 per cell)" whereas the children in the baseline control group were not randomised. Therefore the study was classified as a comparative effectiveness trial and we did not consider the data from the baseline control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake: Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake The experimenter counted the number of pieces of each snack eaten and therefore given it is an objective measure unlikely to be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no information about attrition provided |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “This work was supported by a grant from the Dutch Ministry of Health, Welfare and Sport (grant number: 201400117.014.013). The Ministry's sole role was funding, and, thus, was not involved in the design, data collection, data analyses, data interpretation, and writing of the report. None of the authors had a potential conflict of interest.” |
|
| Participants |
Description: Children aged 2‐3 years in nursery schools in Rotterdam, the Netherlands N (Randomised): 163 children Age: Mean = 2.63 years % Female: 48% SES and ethnicity: “The sample consisted of toddlers from mostly low‐SES households with various cultural backgrounds.” Inclusion/exclusion criteria: “Only schools without formal fruit and vegetables programs were selected” Recruitment: Not specified Recruitment rate: 99% (197/199) Region: The Netherlands |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Passive with puppet: 36 Passive without puppet: 40 Interactive with puppet: 41 Interactive without puppet: 37 Description of intervention: Children were read a picture book “Rabbit’s brave rescue”. The embedded message in the book was that “eating carrots makes you strong”. Reading sessions were conducted in a quiet room within the nursery school during one workweek. The reading sessions were being held in small groups of 3‐5 toddlers, and took about 10 minutes. Reading was performed either with or without a hand puppet (hand puppets were developed that resembled the physical appearance of the main character in the picture book, ‘Rabbit’). Children allocated to the passive groups (with or without a puppet) were not asked questions during reading time and children allocated to the interactive groups (with or without a puppet) were asked questions during reading time. Duration: 4 days Number of contacts: 4 reading sessions (1 per day) Setting: Preschool Modality: Face‐to‐face Interventionist: Women with pedagogical education Integrity: The reading sessions were monitored. Date of study: Recruited in February and March 2015 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of carrots (proportion): “The proportion of consumed carrots was calculated by dividing the pieces of carrots the child had eaten by the total number of pieces of foods the child had eaten.” “Proportional scores were used, rather than absolute scores, because the proportional scores take into account the total amount of foods eaten.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 4 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: “Children who were absent on the last reading day (n = 34), were excluded from the analyses.” “The total drop‐out was evenly spread across conditions.” Overall: 17% (not specified by group) Analysis: Unknown if sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “On the first day, the
storytellers picked up the children from class in
order of the name list provided by the school, and
randomly assigned them to one of the four reading
conditions, ensuring balance in
gender.” No mention of how the randomization sequence was generated. |
| Allocation concealment (selection bias) | High risk | The allocation was done by the person delivering the intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “For the reading sessions, four
women with a pedagogical education were recruited
and trained to perform all the different reading
styles and puppetry conditions. These storytellers
were teamed up with four female experimenters who
observed the toddlers during the readings. With each
team being allocated to a specific day of the week,
all the toddlers in the study were exposed to all
the storytellers and
observers.” Those delivering the intervention were aware of group allocation, however this is unlikely to have impacted the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The experimenter conducting the eating task was blinded to group assignment, because the reading sessions and eating tasks took place in different rooms.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were 23% at short‐term follow‐up (in text). However in Consort flowchart, it appears that people were excluded prior to randomization. In the text it says that most were excluded due to not attending on the final measurement day. This sounds like the dropouts should be removed at the analysis/data collection stage. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported as pre‐specified in the trial registration. |
| Other bias | Low risk | No other sources of bias identified |
| Methods |
Study design: Randomised controlled trial – cross‐over Funding: "European Community’s Seventh Framework Programme (FP7/2007‐2013) under the Grant agreement No. 245012‐HabEat." |
|
| Participants |
Description: Preschool‐aged children recruited from 3 daycare centres in Wageningen, the Netherland N (Randomised): 40 children Age: 21 to 46 months (mean = 36 months) % Female: 50% SES and ethnicity: Not specified Inclusion/exclusion criteria: Inclusion criteria: “Inclusion into the study required presence of the child at the day care‐centre for at least 2 days per week.” Exclusion criteria. “Participants were screened for food allergies and health problems (as reported by the parents)” Recruitment: “A total of 40 healthy children aged 2–4 years were recruited from 2 day care‐centres in Wageningen, The Netherlands. Participation was voluntary and parents and day care‐centres were thoroughly informed about the study. Written parental consent was given for the participating children.” Recruitment rate: Unknown Region: Wageningen (The Netherlands) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Spinach high‐energy/endive low‐energy = 15 Endive high‐energy/spinach low‐energy = 13 Description of intervention: “During the intervention period, half of the participants (n = 20) received vegetable soup flavour A low in energy content (LE) consistently paired with vegetable soup flavour B high in energy content (HE), whereas the other half of the participants received the reverse (i.e. flavour A HE + flavour B LE).” Duration: 7 weeks Number of contacts: 14 exposures (twice/week) Setting: Preschool Modality: Face‐to‐face Interventionist: Daycare leaders Integrity: No information provided Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: As‐desired consumption of vegetable soup (grams). “Consumption was measured by pre‐ and post‐weighing on a digital scale with a precision of 0.1 g.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 8 weeks and 4 and 8 months Length of follow‐up post‐intervention: 1 week and at 2 and 6 months Subgroup analyses: None Loss to follow‐up (at 2 and 6 months): Overall: 32%, 39% (not specified by group) Analysis: Sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake (objective): The children and the daycare leaders were blinded to the treatment, i.e. they were unaware which product was high or low in energy and therefore low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake (objective): Outcome was pre‐post weight of soup bowl assessed by researcher. Researchers were not blinded to group allocation (as they served the soup (2 x green soups varying in energy intake)) and researcher was not present in room during consumption of soup |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 40 eligible children, 12 were excluded from data analysis due to low intake levels during the conditioning period. Of 28 children 17 (61%) completed the 6‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: "European Community’s Seventh Framework Programme (FP7/2007‐2013) under the Grant agreement No. 245012‐HabEat." |
|
| Participants |
Description: Preschool‐aged children recruited from 3 daycare centres in Wageningen, the Netherlands N (Randomised): 75 children Age: 1.9‐5.9 years (mean = 3.7 years) % Female: 50% SES and ethnicity: Not specified Inclusion/exclusion criteria: No explicit inclusion/exclusion criteria. “Participants were screened for food allergies and health problems (as reported by the parents)” Recruitment: “Parents with children in the targeted age range received an information letter and an invitation to register their child(ren) for participation via the day‐cares. Participation was voluntary and parents and day care‐centres were thoroughly informed about the study.” Recruitment rate: Unknown Region: Wageningen (The Netherlands) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Choice condition = 34 No‐choice condition = 36 Description of intervention: “Each child was exposed 12 times to six familiar target vegetables at home during dinner, which is the traditional hot meal including vegetables in The Netherlands….the choice group received two types of vegetables from which to choose, or they could choose to eat both vegetables during the meal.” Duration: 12 days Number of contacts: 12 Setting: Home Modality: Face‐to‐face Interventionist: Parents Integrity: No information provided Date of study: Unknown Description of control: “The no‐choice group received only one type of vegetable per dinner session” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: “The main outcome of the study was the children’s intake (in gram) of the vegetables. Vegetable intake was measured by weighing their plates before and after dinner (left overs).” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 6% (not specified by group) Analysis: Sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake (objective measure): Children’s vegetable intake was measured by weighing their plates before and after dinner (left‐overs). There is a low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake (objective measure): Children’s vegetable intake was measured by weighing their plates before and after dinner (left‐overs). There is a low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70/75 (93%) children completed the study and therefore risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | The primary outcomes reported in the paper align with those specified in the trial registration. However in the trial registration the food diary is listed as a secondary outcome but the results are not reported in the outcome paper |
| Other bias | High risk | Despite random assignment, children in the no‐choice group on average liked vegetables better than children in the choice group (P < 0.01) and therefore baseline imbalance between groups |
| Methods |
Study design: Randomised controlled trial – semi‐cross‐over Funding: "European Community’s Seventh Framework Programme (FP7/2007‐2013) under the Grant agreement No. 245012‐HabEat." |
|
| Participants |
Description: Preschool‐aged children recruited from 2 daycare centres in Wageningen, the Netherland N (Randomised): 45 children Age: 18‐45 months (mean = 32.6 months) % Female: 49% SES and ethnicity: Not specified Inclusion/exclusion criteria: No explicit inclusion/exclusion criteria. “Participants were screened for food allergies and health problems (as reported by the parents)” Recruitment: “recruited from two day‐care centres in Wageningen, the Netherlands. Parents signed an informed consent for their child’s participation.” Recruitment rate: Unknown Region: Wageningen (The Netherlands) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Parsnip crisps‐tomato ketchup/red beet crisps‐white sauce = 19 Red beets crisps‐tomato ketchup/parsnip crisps‐white sauce = 20 Description of intervention: “Half of the participants received red beet crisps combined with tomato ketchup (TK [C]) consistently paired with parsnip crisps combined with white sauce (WS [UC]). The other half of the participants received the reverse, i.e. red beet crisps + WS(UC) and parsnip crisps + TK(C).” Duration: 7 weeks Number of contacts: 14 exposures (twice/week) Setting: Preschool Modality: Face‐to‐face Interventionist: Daycare leaders Integrity: No information provided Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: As‐desired consumption of vegetable crisps (grams). “Consumption of crisps and dip sauces were measured by pre‐ and post‐weighing on a digital scale with a precision of 0.1 g.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Post‐test 1: 9 weeks Post‐test 2: 4 months (2 months after conditioning) Post‐test 3: 8 months (6 months after conditioning) Length of follow‐up post‐intervention: Post‐test 1: immediate Post‐test 2: 2 months Post‐test 3: 6 months after conditioning Subgroup analyses: None Loss to follow‐up (at 2 and 6 months): Overall: 5%, 33% (not specified by group) Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable crisps intake (objective): The children were not aware that their intake was measured or which condition they participated in and so the risk of performance bias is low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable crisps intake (objective): The outcome was vegetable chip and dip intake (each assessed separately) by weighing amount before and after consumption. It is not clear who (i.e. researchers or daycare centre staff) weighed the chips & dip, and whether or not they were blinded. Blinding of outcome assessors unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 45 children, 6 were excluded because they had no intake at all of the dip sauces. Of the remaining 39 children, 26 (67%) completed the 6‐month follow‐up. The risk of attrition bias is high |
| Selective reporting (reporting bias) | Unclear risk | The trial registration reports a secondary outcome that is not reported in the outcome paper |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “The research leading to the results presented here received funding from the European Community’s Seventh Framework Programme (FP7/2007‐2013) under grant agreement no. 245012‐HabEat.” |
|
| Participants |
Description: Children aged 2‐4 years in 6 day‐care centres in Wageningen, the Netherlands N (Randomised): 103 children Age: Plain spinach (mean): 34.5 months Creamed spinach (mean): 36.1 months Spinach ravioli (mean): 35.4 months Green beans (mean): 35.8 months % Female: Plain spinach: 50% Creamed spinach: 52% Spinach ravioli: 46% Green beans: 42% SES and ethnicity: Not specified Inclusion/exclusion criteria: No explicit inclusion/exclusion criteria stated for this trial, “Participants were screened for food allergies and health problems (as reported by the parents).” Recruitment: Not specified, recruited from 6 child care centres Recruitment rate: 99% (103/104) Region: Wageningen (the Netherlands) |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Plain spinach: 26 Creamed spinach: 25 Spinach ravioli: 26 Green beans: 26 Description of intervention: “Families received a weekly vegetable parcel, including their vegetable product for one meal (main meal), cooking instructions, and a food diary. A standardized weighing scale with a precision of 1 g (Fiesta; Soehnle) was supplied to all participating families together with the first delivery of the vegetable parcel.” Duration: 6 weeks Number of contacts: 6 (once per week) Setting: Home Modality: Face‐to‐face Interventionist: Parents Integrity: No information provided Date of study: The study was conducted between September 2014 and January 2015 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: As‐desired intake of plain cooked spinach (grams): “Spinach intake was measured by weighing the bowls before and after lunch (leftovers) on a digital scale with a precision of 0.1 g.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 7 weeks Length of follow‐up post‐intervention: 1 week Subgroup analyses: None Loss to follow‐up: “There were no lost to follow up or withdrawals” Analysis: Sample size calculations performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Children were randomly assigned
to one of the four groups using a four‐block design: green beans (control), plain spinach (pure spinach), creamed spinach (diluted), and spinach ravioli (hidden). Randomization was done by a person who was not involved in study recruitment, enrollment, or assignment of participants.” No mention of how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group primary outcomes – preference and
intake “Day‐care center staff members were instructed to behave as they usually did and not to alter their daily routine. The researchers were absent while children ate their spinach at lunch, to not disturb the normal daily lunch routine.” It is unclear whether the day‐care centre staff or researchers were blind to experimental group allocation. Outcome group: secondary outcomes – intake and liking "The products in the plain spinach, creamed spinach, and green beans groups were commercially available (frozen green beans [2.5 kg], frozen chopped spinach [2.5 kg], and frozen spinach a la crème [1 kg]) and were repacked in family portions and delivered frozen via the day‐care centers on a weekly basis.” It is likely parents knew their experimental group allocation and this could have affected the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome group primary outcomes – preference and
intake “Spinach intake was measured by weighing the bowls before and after lunch (leftovers) on a digital scale with a precision of 0.1 g (model S‐4001; Denver Instruments, and model Kern‐572; Kern & Sohn).” It is unclear whether the researchers were blind to group allocation, how the outcome assessment procedure is unlikely to have been impacted. Outcome group: secondary outcomes – intake and liking “Parents weighed the child’s vegetable portion before and after the meal to determine vegetable intake.” “After the main meal, parents completed a food diary, in which information was collected; for example, on deviations from the described procedures, dinnertime, consumption of other meal components, the child’s health status, and the child’s liking of the vegetables (parent’s perception and rated on a 9‐point scale (where 1= extremely disgusting and 9= extremely delicious).” All outcome data was collected by the parents themselves – self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 10 children who had only 1 or 2 data
points for intake of the 6 meals, with no reasons
reported. Not enough information reported about the reasons for missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported as pre‐specified in the trial registration. |
| Other bias | Low risk | No other sources of bias were identified. |
| Methods |
Study design: Randomised controlled trial Funding: “C Collins is supported by a National Health and Medical Research Council Australian Career Development Research Fellowship (#6315005). K Duncanson is supported by a Clinical Education and Training Institute Rural Research Capacity Building Program Grant and New Staff Research Grant (University of Newcastle).” |
|
| Participants |
Description: Parents of children aged 2 to 5 years living in a rural area of New South Wales, Australia N (Randomised): 146 parents Age: Children (mean): Intervention = 4.0 years, Control = 4.0 years Parents: Younger than 30 years: Intervention = 34%, Control = 17% 30 years or older: Intervention = 66%, Control = 83% % Female: Child: Intervention = 47%, Control = 48% Parent: Intervention = 100%, Control = 99% SES and ethnicity: Parent education: Secondary = 46%, Tertiary = 55% Aboriginal: Child = 4%, Parent = 2% Inclusion/exclusion criteria: Inclusion criteria: “Inclusion criteria were eldest child in family ages 2 to 5 years, without a chronic health condition that affected dietary intake.” Exclusion criteria: “A child was excluded if he or she had a chronic disease, such as coeliac disease or a food allergy that has a significant effect on dietary intake. The eldest child within the eligible age range was selected as the study child for consistency and simplicity.” Kids were also excluded if they began primary school Recruitment: “parents of young children were recruited from child care facilities in 5 rural, low socioeconomic localities in NSW, Australia.” Recruitment rate: Parent: 81% (146/180) Region: New South Wales (Australia) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 45, Control = 43 Description of intervention: “The intervention involved dissemination of the Tummy Rumbles interactive CD (16) and the Raising Children DVD (17) at baseline in September 2009, accompanied by written instructions for optimal use. The only prompt provided to parents to use the resources was a reminder note delivered by post with the 3‐month follow‐up surveys. To simulate population‐level resource dissemination, further prompting of parents was not conducted.” “The tummy rumbles interactive nutrition education CD is a self‐directed resource for childcare staff and parents, Raising children is a guide to parenting from birth to 5” Duration: 12 months Number of contacts: DVD and CD played at parents' leisure, 1 contact from researchers at 3 months by phone Setting: Home Modality: DVD/CD Interventionist: N/A (provision of DVD) Integrity: “Intervention group participants were considered to have adhered to the study protocol if they reported using both Tummy Rumbles and Raising Children for at least 1 hour each during the intervention period.” Date of study: September 2009 to September 2010 Description of control: Wait‐list control,“ A generic nutrition brochure and the Active Alphabet physical activity resource were distributed to the control group to simulate real‐life exposure to control resources and facilitate retention and blinding of the control group. Tummy Rumbles and Raising Children were provided to the control group at trial completion.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables (servings) assessed using a semi‐quantitative food frequency questionnaire (FFQ), the Australian Toddler Eating Survey (ATES) completed by parents Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 3 and 12 months Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up (at 3 and 12 months): Intervention = 17%, 40% Control = 24%, 39% Analysis: Sample size calculation was performed. |
|
| Notes | First reported outcome (serves fruit/day) at
3‐month follow‐up was for inclusion in
the short‐term meta‐analysis and 12
month follow‐up for the ≥ 12
months meta‐analysis. Additional data were
provided by the author to allow pooling in
meta‐analysis Sensitivity analysis ‐ primary outcome: Primary outcome not stated, power calculation conducted fruit or vegetable intake |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was created by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed given that sequentially‐numbered unopened returned baseline survey envelopes were matched with computer‐generated random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to group allocation throughout the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were blinded to group allocation throughout the trial. The protocol indicates that assessors of the main outcome measures were blinded to participant group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Short‐term attrition was 21% and long‐term attrition was 40%. No imputation of missing data was carried out |
| Selective reporting (reporting bias) | Low risk | The primary outcomes published in the protocol align with the results reported in the outcomes paper |
| Other bias | High risk | There were no differences at baseline in parent and child characteristics except for % of parents older than 30 years. There is no mention that this was adjusted for in the analysis |
| Methods |
Study design: Randomised controlled trial Funding: "The recruitment of the Gemini cohort was funded by a grant from Cancer Research UK (no. C1418/A7974), and the design and production of the packs used in this study was funded by Weight Concern (registered charity no. 1059686)." |
|
| Participants |
Description: Families with 3‐ to 4‐year‐old children from a larger cohort study (the Gemini study) N (Randomised): 1006 families Age: Child (mean): Intervention = 3.9 years, Control = 3.8 years Parent (mean): Intervention = 38.0 years, Control = 37.3 years % Female: Child: Intervention = 49%, Control = 50% Parent: not specified SES and ethnicity: Maternal education (below university level): intervention 49%, control = 49% Inclusion/exclusion criteria: Not specified Recruitment: “Participants were families with 3‐ to 4‐year‐old children from the Gemini study, a cohort of 2,402 families with twins born during 2007 in England and Wales. Currently active families (n=2,321) were sent information about a study to test a method of increasing children’s acceptance of vegetables” Recruitment rate: Families: 43% (1006/2321) Region: England and Wales |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 98, Control = 123 Description of intervention: “The intervention pack contained an exposure instruction leaflet, progress charts, and stickers. The exposure instructions asked parents to offer the child a single very small piece of their target vegetable every day for 14 days, allowing the child to choose a sticker as a reward if they tried it. They were asked to do this separately with each child and outside mealtimes.” Duration: 14 days Number of contacts: 14 Setting: Home Modality: Face‐to‐face Interventionist: Parents Integrity: “Among the 175 returned (89%), the mean number of exposure sessions was 13.8 (range=11 to 14), and children tasted their target vegetables a mean of 12.4 times (range=0 to 14). Children complied with the intervention by trying their target vegetable on an average of 90% (range 0% to 100%) of the exposure days during the experiment phase.” Date of study: Unknown Description of control: Received no intervention, “Control families were sent the intervention materials on completion of the study.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s intake of the target vegetable (number of pieces). Parents “recorded the number of pieces (including half‐pieces) of vegetable the child ate; this comprised the intake measure.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 14 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Intervention = 68% Control = 68% Analysis: Unknown if sample size calculation was performed |
|
| Notes | Mean and SEM were estimated from a study figure using
an online resource (Plot Digitizer: plotdigitizer.sourceforge.net) for
intervention and control groups at the end of the
experimental phase (T3). Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is listed as primary outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Vegetable intake: There is no mention that the parents were blinded and they were cutting and offering the pieces to the child and this could have influenced performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Vegetable intake: There is no mention that the parents were blinded and they were cutting and offering the pieces to the child and so at high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 472 (47%) out of the 1006 randomised returned the outcome data sheets and therefore high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There are secondary outcomes reported in the trial registration that are not presented in the outcomes paper |
| Other bias | High risk | Children in the intervention group had significantly lower intake and liking than the control group at baseline (i.e. baseline imbalances) |
| Methods |
Study design: Randomised controlled trial Funding: "This research is supported by European Community’s Seventh Framework Programme (FP7/2007‐2013) under the grant agreement no. 245012‐HabEat. The purees offered to participants in this study and the artichoke and peach purees used as a test food were donated by Danone Nutricia Research." |
|
| Participants |
Description: Mothers and their 4‐ to 6‐month‐old infants in the UK, Greece and Portugal N (Randomised): 146 parent‐infant dyads Age: Infant (mean): Intervention = 39.0 weeks, Control = 38.9 weeks Mother (mean, at child’s birth): Intervention = 33.0 years, Control = 32.7 years % Female: Infant: 52% SES and ethnicity: Education (below university) = 27% Inclusion/exclusion criteria: “Mothers were eligible to participate if they were over 18 years old at recruitment, they were sufficiently proficient in each country’s respective native language to understand the study materials and their infant was born after 37 weeks’ gestation, without diagnosed feeding problems.” Recruitment: “Women in the final trimester of their pregnancy and mothers of infants aged less than 6 months were recruited from antenatal clinics (n 327), primary care, paediatricians and hospitals in London (UK), Athens (Greece) and Porto (Portugal) to a larger study exploring children’s fruit and vegetable acceptance during weaning.” Recruitment rate: Mothers: 45% (146/327) Region: London (UK), Athens (Greece) and Porto (Portugal) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 71, Control = 68 Description of intervention: “In the intervention group, a researcher or health professional explained to the participant: (1) the importance of introducing vegetables early in the weaning process, (2) the beneficial effects of offering different single vegetables each day, (3) the techniques of exposure feeding, (4) interpreting infants’ facial reactions to food and (5) the need for persistence when an infant initially rejects a food. “five vegetables were selected as the first foods to be introduced. They were asked to offer the five vegetables in a sequence over 15 d as follows: A,B,C,D,E, A,B,C,D,E, A,B,C,D,E and to record progress on a chart provided. For a further 5 d, participants were told to continue to offer vegetables, but in addition, to start to introduce additional age‐appropriate foods.” Duration: 20 days (15 days exposure, 5 days veg plus other foods) Number of contacts: 20 (15 veg feeding exposures, 5 veg plus other food exposures) Setting: Home or health facility Modality: Face‐to‐face + leaflet Interventionist: Parent Integrity: “Completed intervention charts were returned by 86% of intervention families (UK; 100 % (28/28), Greece; 100 % (16/16), Portugal; 63% (17/27)). Completed charts revealed that over the 15‐d intervention period, parents recorded their infants consuming vegetables on 89% (mean 13·3 (SD 3·0)) of the fifteen possible eating occasions.” Date of study: February 2011 and July 2012 Description of control: Received no intervention, ‘usual care’ |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Infant consumption of fruits and vegetables (serves/day). “Mothers reported separately on the frequency of fruit and vegetable servings they had consumed in the past week and the data were recoded to provide an estimation of the total number each of fruit and vegetable portions consumed daily.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 1 month Length of follow‐up post‐intervention: 2 weeks Subgroup analyses: None Loss to follow‐up: Intervention = 5% Control = 4% Analysis: Sample size calculation was performed. |
|
| Notes | First reported outcome (vegetable intake) was
extracted for inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit and vegetable intake 1st listed outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to experimental group using a block randomisation matrix created by an independent statistician |
| Allocation concealment (selection bias) | Unclear risk | Allocation was revealed to the researcher, but unclear how or when |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Infant’s consumption of novel
vegetable: Mothers offered and fed the vegetable to infants. Given the nature of the intervention, parents in the intervention arm were not blinded and therefore this could have influenced performance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Infant’s consumption of novel
vegetable: The outcome was weighed, but it is not clear who weighed the food (mother who fed the child, or researcher who observed the mother feeding the child). The researcher who was present during outcome assessment was the same researcher who delivered the intervention to the mother. The impact on detection bias is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 139/146 (95%) completed the follow‐up and therefore low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “This work was funded by an investigator‐initiated grant to J.O.F. from the Clorox Company, which owns the Hidden Valley, The Original Ranch brand of dressing used in this research. The authors attest to having full scholarly authority over this work and responsibility for the research design and methods, the integrity of the data, the analyses, and the interpretation of the findings.” |
|
| Participants |
Description: Preschool‐aged children in Head Start classrooms and their parent N (Randomised): 155 parent‐child dyads Age: Child: 3 to 5 years (mean = 4 years) Parent: not specified % Female: Child: 48% Parent: not specified SES and ethnicity: “predominately Hispanic (88%) children” “Of participating parents, close to a majority (n=89) reported being married and slightly greater than one‐third (n=51) reported schooling beyond high school.” Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial Exclusion criteria: “Exclusion criteria included severe food allergies and/or other medical conditions (e.g., diabetes) that might influence the ability to participate in an as‐desired snack and absences at 75% or more of the vegetable exposure trials.” Recruitment: “To achieve a target sample size of 37 children per experimental dip condition, eight preschool classrooms within three Head Start Centers were approached to participate. Parents of 166 children were sent letters to request written consent for their own and their child’s participation in the study.” Recruitment rate: Parent‐child dyads = 93% (155/166) Region: Houston, TX (USA) |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Plain = 39, Regular = 39, Light = 36, Sauce = 38 142 parents (not specified by group) Description of intervention: “At each trial, raw broccoli was presented with 2% milk (8 oz [246 g]) to children in the condition to which they were assigned. Children were instructed to eat as much or as little as desired.” Plain: “broccoli was served without dressing.” Regular: “broccoli was served with 2.5 oz of a regular ranch‐flavored salad dressing.” Light: “broccoli was served with 2.5 oz of a reduced‐energy/fat ranch‐flavored salad dressing.” Sauce: “2.5 oz of the regular dressing was mixed together with broccoli as a sauce” Duration: 7 weeks Number of contacts: “Thirteen exposure trials (twice per week) took place in children’s classrooms across a 7‐week period.” Setting: Preschool Modality: Face‐to‐face Interventionist: Trained research staff Integrity: No information provided Date of study: 2008 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of target vegetables (broccoli) (grams) with/without dressing/sauce. “Weights of broccoli, milk, and the salad dressing (except in the plain condition) were recorded to the nearest 0.1 g once a stable reading was indicated using a calibrated, research grade digital electronic balance before and following the snacks. In the sauce condition, broccoli and the dressing intakes were estimated from the amount of the mixture consumed based on the proportionate contributions of each to the total pre‐weight.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 7 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 2% (not specified by group) Analysis: Adjusted for clustering Sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | (Authors describe as a quasi‐experimental
design although appear to have randomised
classrooms). Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | (Authors describe as a quasi‐experimental
design although appear to have randomised
classrooms). There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake (objective): Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake (objective): Objective measure of child’s vegetable intake and whether those who weighed the food were blinded is unlikely to have an impact on detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 152/155 (98%) completed the study and therefore risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | There is insufficient information about baseline imbalances and whether clustering was adjusted for in the analyses |
| Methods |
Study design: Randomised controlled trial Funding: “This work was supported by National Institutes of Health grant HD37119. Dr Forestell was the recipient of a Canadian Institutes of Health research postdoctoral fellowship.” |
|
| Participants |
Description: Children aged 4‐8 months and their mother N (randomised): 45 mother‐infant dyads Age: Infant (mean): green bean group = 5.6 months, green bean/peaches group = 5.9 months Mother (mean): green bean group = 32.2 years, green bean/peaches group = 31.6 years % Female: Infant: green bean group = 38%, green bean/peaches group = 52% SES and ethnicity: Years of schooling (mean): green bean group = 14.7 years, green bean/peaches group = 14.8 years Inclusion/exclusion criteria: Inclusion criteria: infants had to be born at term, healthy, currently aged between 4 and 8 months and had been weaned to cereal with very little experience with fruits and vegetables. Recruitment: “….recruited through advertisements in local newspapers, breastfeeding support groups, and the Supplemental Nutrition Program for Women, Infants, and Children in Philadelphia, Pennsylvania.” Recruitment rate: Unknown Region: Pennsylvania, USA |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Green bean group: 12 Green bean/peaches group: 26 Description of intervention: Green bean group: fed greens beans throughout the 8‐day home exposure period. Green bean/peaches group: fed greens beans and then within 1 h peaches throughout the 8‐day home exposure period. Both groups were fed green beans in the lab on days 1 and 2 and peaches on days 11 and 12. Duration: 12 days Number of contacts: 12 exposures (8‐day home exposure + 3 lab exposures/test days) Setting: Home + lab Modality: Face‐to‐face Interventionist: Mother Integrity: “To increase compliance, telephone contact was made with the mothers, who recorded the time of day and types and quantities of foods and liquids they fed their infants throughout the study. All of the mothers complied with these instructions.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of green beans and peaches (grams) assessed by weighing the amount of the food in the jar before and after consumption Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Green bean group: 25% Green bean/peaches group: 10% Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Infants were assigned randomly
to 1 of 2 treatment
groups.” It is unclear how randomisation occurred. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed from those conducting the research. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “One group (group GB) was fed
green beans, whereas the other (group GB‐P)
was fed green beans and then (within 1 hour) peaches
throughout the 8‐day home‐exposure
period (days 3–10).” “To increase compliance, telephone contact was made with the mothers, who recorded the time of day and types and quantities of foods and liquids they fed their infants throughout the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Mothers fed at their customary
pace until the child rejected the food
≥ 3 consecutive times or
finished 2 jars of food.” Due to the nature of the intervention, mothers would have been aware of the infant’s group allocation, and this may have impacted the results. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Four infants were excluded from the analyses of green bean acceptance (4/16)and 3 from those of peach acceptance (3/29) because mothers were non‐compliant with test procedures (n=2), infants were sick during testings or exposure (n=2), or infants ate the maximum amount of food offered during their initial exposure (n=3)” |
| Selective reporting (reporting bias) | Unclear risk | There is no trial registration or protocol. |
| Other bias | Low risk | None identified |
| Methods |
Study design: Randomised controlled trial Funding: “Supported by grants HD37119 and HD08428 from the National Institutes of Health and by a grant from the Gerber Companies Foundation. The Gerber Products Company supplied the baby foods used in this study.” |
|
| Participants |
Description: Mothers with healthy, term infants N (Randomised): 48 mother‐infant dyads Age: Infant (mean): carrot group = 4.6 months, potato group = 4.5 months, variety group = 4.8 months Mother (mean): carrot group = 27.4 years, potato group = 25.4 years, variety group = 29.9 years % Female: Infant: carrot group = 50%, potato group = 50%, variety group = 50% SES and ethnicity: “The racial background of the mothers and their infants was 45.8% African American, 39.6% white, 2.1% Hispanic, and 12.5% other ethnic groups.” Inclusion/exclusion criteria: Inclusion criteria: non‐smoking mothers, began feeding cereal to their infants in the past month and planned on introducing other solid foods during the next few weeks, and only mothers of formula‐fed infants. Recruitment: “recruited from advertisements in local newspapers and from Women, Infant and Children programs in Philadelphia.” Recruitment rate: Unknown Region: Philadelphia, USA |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Carrot group: 16 Potato group: 16 Variety group: 16 Description of intervention: Carrot group: during the home exposure period infants were fed pureed carrots only (the target vegetable). Potato group: during the home exposure period infants were fed pureed potatoes only. Variety group: during the home exposure period infants were fed a variety of vegetables that did not include carrots (potato, squash, peas). All groups were fed pureed carrots in the lab on days 1 and 11. Duration: 11 days Number of contacts: 11 exposures (9 day home exposure + 2 lab exposures/test days) Setting: Home + lab Modality: Face‐to‐face Interventionist: Mothers Integrity: “To encourage compliance, each mother kept a daily record of what they fed their infants, and daily phone contact was made with each mother during the exposure period.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of pureed carrots (grams): assessed by weighing the amount of the food in the jar before and after consumption using a top‐loading balance. Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 11 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: No loss to follow‐up Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly assigned to one of 3
experimental groups” not
enough information reported. Randomly allocated to experimental group but the random sequence generation procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | No information reported There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Mothers fed their infants and there is no mention of blinding and so high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake was determined by weighing vegetables and therefore low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up – 16 dyads per
group All participants recruited completed the study and therefore at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol is available |
| Other bias | Low risk | Low risk of other bias |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "Funding for this work was provided by National Cancer Institute (R01 CA68398)." |
|
| Participants |
Description: Parents and their children participating in the 'Parents as Teachers' (PAT) programme sites in rural Missouri (USA) N (Randomised): 16 PAT sites, 1658 families Age: Children: 1 to 3 y: intervention = 67%, control = 61% 4 to 6 y: intervention = 33%, control = 40% Parents: < 25 y: intervention = 28%, control = 21% 25 to 29 y: intervention = 35%, control = 33% 30 to 34 y: intervention = 21%, control = 24% 35+ y: intervention = 17%, control = 23% % Female: Children: intervention = 47%, control = 49% Parents: intervention = 99%, control = 98% SES and ethnicity: Parent ‐ Not high school graduate: intervention = 16%, control = 11% Parent ‐ College graduate: intervention = 20%, control = 25% Household income: < USD 20K: intervention = 30%, control = 25% USD 20K to 35K: intervention = 30%, control = 25% USD 35K to 50K: intervention = 13%, control = 18% USD 50+K: intervention = 28%, control = 32% Ethnicity ‐ White: intervention = 86%, control = 80% Inclusion/exclusion criteria: Not specified Recruitment: "16 PAT programs from rural, southeast Missouri were recruited into the study. Within these sites 2012 families enrolled were assessed for eligibility and willingness to participate by parent educators." PAT is a "parenting and child development program with over 3000 sites across all 50 states and 8 US territories." PAT provides free services on "an annual basis to parents at the time of pregnancy until the youngest child is 3 years of age. However, PAT extends services until the youngest child is 5 years of age in the case of underserved families, defined as single or minority parent homes, those living in poverty or low parent education. In addition, underserved families may receive additional home visits as a means of ensuring complete delivery of the curriculum." Recruitment rate: Families: 79% families PAT sites: unknown Region: Rural southeast Missouri (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 605, Control = 701 Description of intervention: Intervention families received the standard PAT program plus the 'Hi 5 for Kids' (H5‐KIDS) protocol. "H5‐KIDS was comprised of three components: a tailored newsletter, a series of home visits, and materials for the parent and child, including storybooks." Computer‐tailored nutrition newsletter "To develop the tailored newsletter, parents were first formally enrolled in H5‐KIDS and completed a pretest interview. Relevant data was then imported into an in‐house computer‐based tailoring program. Scores were calculated based on FV knowledge and intake, frequency of parental modeling, style of parenting (coercive or non‐coercive), and quality of the home food environment (FV availability). Each newsletter began with a bulleted tailored statement that included the self reported servings of FVs the parent and the child consumed per day. Additional parent data (e.g. FV knowledge, parental role modeling, non‐coercive parenting skills, FV availability) were each uniquely used to individualize messages and describe the themes of each of the four storybook sets the family would receive at their home visits. For example, if participant data indicated a parent did not eat FV in front of their child very often (< 7/week), the tailored messages would emphasize the importance of modeling FV intake in front of the child as a means of improving consumption, and provide relevant examples of how this could be accomplished. The parent was then referred to H5‐KIDS storybooks that provided examples of modeling for the child. In contrast, parents who scored appropriately in each individual area received messages of praise encouraging them to continue their behaviors. Newsletters were mailed to the parent's home at the beginning of the program." Home visits "Parent educators delivered four H5‐KIDS home visits, each of which addressed the core program areas (knowledge, parental modeling of FV intake, non‐coercive feeding practices, FV availability). Parent educators then reinforced the core content in subsequent visits. Consistent with the philosophy of the PAT program, each visit provided examples of parent–child activities designed around healthy nutrition, that the parent could use to promote the child's language and cognitive ability, and fine and gross motor skill development (e.g. having the child learn the names and colors of various FV; child assists with selecting a variety of FV for breakfast). As part of each visit, parents also received materials and informational handouts with suggestions for improving feeding practices and the food environment in the home. Consistent with the standard PAT program, each home visit was designed to allow for 60 min of contact." Sing‐a‐long storybooks with audio cassette "At each home visit children received a H5‐KIDS sing‐a‐long storybook with audio cassette tape and a coloring book. Each storybook reinforced one of the core areas of the H5‐KIDS program through the use of child friendly characters and appealing storylines presented through songs." Duration: 60 minutes per home visit Number of contacts: 4 H5‐KIDS home visits plus 5 standard PAT home visits Setting: Home Modality: Face‐to‐face via home visits Interventionist: Parent educators who received 4 hours of training on nutrition content and overview of materials Integrity: "The H5‐KIDS program was delivered in its entirety to 78% of intervention families." Date of study: 2001 to 2006 Description of control: "Parent educators deliver a standardized curriculum via at least five home visits, on‐site group activities and newsletters." ("PAT ... empowers parents ... by encouraging positive parent‐child communication and increasing parents' knowledge of ways to stimulate children's social and physical development.") |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child's daily servings of fruits and of vegetables assessed using the Saint Louis University for Kids Food Frequency Questionnaire (SLU4Kids FFQ) administered by parent telephone survey Length of follow‐up from baseline: Average time to follow‐up was 7 months (range 6 to 11 months) Subgroup analyses: Normal weight vs overweight children Loss to follow‐up: Intervention: 15% (+ 5% missing or inconsistent data) Control: 17% (+ 5% missing or inconsistent data) Analysis: Analysis was not adjusted, but justification was provided. "There was minimal impact of grouping by site on the principle measures of impact in this study (ICC child fruit and vegetable servings = 0.00095 and ICC parent fruit and vegetable servings = 0.01). Therefore, the analyses did not adjust for group." Sample size calculation was performed. |
|
| Notes | The proportion of normal weight vs overweight
children not reported, making it difficult to
interpret the subgroup analysis. First reported
outcome (fruit intake) was extracted for inclusion
in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake only reported outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated number table was used for random assignment to intervention or control." |
| Allocation concealment (selection bias) | High risk | "Families enrolled in PAT were assessed for eligibility and willingness to participate by parent educators." Contact with the author indicated that parent educators were aware of site allocation when they were enrolling participants to the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study personnel were aware of allocation ‐ "Sites were not blind to assignment." Contact with the author indicated that parent participants completed a consent form which described the activities of their experimental condition, and were therefore unlikely to be blind to allocation. Given the trial outcomes were based on parental report, the review authors judged there was potential for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Contact with the author indicated that outcome assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates of loss to follow‐up (intervention = 15%, control = 17%) and missing/ inconsistent data (intervention = 5%, control = 5%) were similar across groups. No information was provided about reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | A subgroup analysis was conducted based on child's weight status (normal vs overweight). "A final limitation of the study is the limited power to definitely assess the impact of the intervention of children within weight status subgroups." It is unclear whether the subgroup analysis was pre‐specified. |
| Other bias | Low risk | Rationale provided for not adjusting analysis for
clustering. "There was minimal impact of
grouping by site on the principle measures of impact
in this study (ICC child fruit and vegetable
servings = 0.00095 and ICC parent fruit and
vegetable servings = 0.01). Therefore, the analyses
did not adjust for group." No further risks of bias identified. |
| Methods |
Study design: Randomised controlled trial – cross‐over Funding: "Funded by a grant from the Robert Wood Johnson Foundation Healthy Eating Research program." |
|
| Participants |
Description: Preschool‐aged children attending a Head Start centre in Minneapolis, Minnesota, USA N (Randomised): 57 children Age: 2 to 3 years = 51% 4 to 5 years = 49% % Female: Not specified SES and ethnicity: Child: Non‐Hispanic African‐American = 76%, Hispanic or Latina/Latino = 6%, Multi‐racial = 13%, American Indian = 4%, Non‐Hispanic White = 2% Parent education: Less than high school = 9%, High school graduate = 42%, Some college = 49% Inclusion/exclusion criteria: Not specified Recruitment: “Children in three preschool classrooms were recruited. A consent form and letter explaining the study was sent to parents.” Recruitment rate: 98% (57/58) Region: Minneapolis, Minnesota (USA) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Overall = 53 Description of intervention: Fruit and vegetable first: “During the fruit and vegetable first experimental weeks all fruits and non‐starchy vegetables on the lunch menu were served traditional family style five minutes in advance of other menu items. Children were allowed to begin eating the fruit and vegetable items served first, with the remaining menu items (e.g. milk, entrée, side dishes) placed on the tables for traditional family style meal service five minutes following distribution of the first course. All other usual meal service practices remained the same during the fruit and vegetable first experimental condition.” Provider portioned: “During the provider portioned experimental condition, a plate was prepared for each child that contained a specific quantity of each menu item.” Duration: “Each condition was implemented for two one‐week periods over the six week period, for a total of two weeks per condition” Number of contacts: Unclear, each day of the 6‐week period (dependent on how many days children attend) Setting: Preschool Modality: Face‐to‐face Interventionist: Classroom teachers Integrity: No information provided Date of study: Unknown Description of control: Usual ‘control’ meal service: “ "During each day of the control weeks, the usual traditional family style meal service approach to serving lunch meals at the center was followed. During usual lunch meals at the center children are seated around tables, and each food item on the menu is passed around the table from child to child in serving bowls for self‐service.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetable serves (1 cup equivalents). Study staff trained and certified in conducting lunch observations recorded food intake on a meal observation form. “The lunch observation data were entered into Nutrition Data System for Research (NDSR), a dietary analysis software program.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 7% Analysis: Unknown if sample size calculation was performed |
|
| Notes | Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit and vegetable intake is the only outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intake: There is no mention if children were blinded and so it is unclear how this may impact children’s vegetable intake |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Intake: Observers made visual estimations of food amounts to determine the amount taken but it is unclear if observers were blinded to condition. Food amounts may not be accurately estimated by observers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/57 (93%) completed the study and therefore the risk of attrition bias is low |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007‐2013) under the Grant Agreement No. FP7‐245012‐HabEat.” |
|
| Participants |
Description: Children aged 2 to 3 years from 5 nurseries in the Copenhagen area and suburbs N (Randomised): 104 children (“from 5 nurseries, involving 17 groups”) Age: Mean: Mere exposure group = 27.8 months, Flavour‐flavour learning group = 27.5 months, Flavour‐nutrient learning group = 30.8 months % Female: Mere exposure group = 63%, Flavour‐flavour learning group = 42%, Flavour‐nutrient learning group = 54% SES and ethnicity: Not specified Inclusion/exclusion criteria: Not specified Recruitment: “Children aged 2–3 years were recruited for the experiment from five nurseries, involving 17 groups, in the Copenhagen area and suburbs.” Recruitment rate: Unknown Region: Denmark |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Mere exposure group = 20 Flavour‐flavour learning group = 30 Flavour‐nutrient learning group = 21 Description of intervention: Mere exposure group, exposed to unmodified artichoke puree 10 times Flavour‐flavour learning group, exposed to a sweetened artichoke puree 10 times Flavour‐nutrient learning group, exposed 10 times to an energy dense artichoke puree with added fat Duration: 4 weeks Number of contacts: 10 exposures Setting: Preschool Modality: Face‐to‐face Interventionist: Nursery staff Integrity: No information provided Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of unmodified artichoke puree (grams). “Testing took part in group rooms. The children were seated at tables where they would normally eat their lunch to mimic the natural eating environment. The purées were served in preweighted plastic cups at room temperature. The standard serving size was 100 g for artichoke and 130 g carrot. Intake was measured individually and recorded for all sessions with a precision of 1 g.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 5 and 8 months Length of follow‐up post‐intervention: 3 and 6 months Subgroup analyses: None Loss to follow‐up (at 3 and 6 months): Mere exposure group = 9%, 38% Flavour‐flavour learning group = 21%, 9% Flavour‐nutrient learning group = 23%, 46% Analysis: Adjusted for clustering (ANOVA proc mixed models). Unknown if sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake: Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake: Intake was weighed and therefore it is unlikely that this would be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 104 children, 71 (68%) completed the 6‐month follow‐up and therefore at high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | The groups differed in age, but age was included as a covariate to correct for the possible influence on intake. Therefore the risk of other bias is unclear |
| Methods |
Study design: Randomised controlled trial ‐ within subject Funding: “This work was supported by a University of Reading Life Sciences Studentship to the first author.” |
|
| Participants |
Description: Families with children aged between 20 and 24 months N (Randomised): 60 parent‐child dyad Age: Child (mean): 22 months (range 20‐24 months) Parent: NR % Female: Child = 48% Parent: NR SES and ethnicity: “78% came from a household where at least one parent was educated to graduate level.” “88% of families were white" Inclusion/exclusion criteria: Not specified Recruitment: “families with children aged between 20 and 24 months were recruited from the University of Reading’s Child Development Group database” “Parents were contacted by telephone and given a brief overview of the experiment. If a parent gave consent to their participation, the child was randomly allocated to one of three initial status” Recruitment rate: 100% Region: UK |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): 57 Description of intervention: Parents were asked whether their child liked, disliked or had not tried each vegetable listed in the Vegetable Liking and Familiarity Questionnaire. For each child, two vegetables were randomly selected from those for which the parent’s responses matched the initial status set to which the child had been assigned; these became the target (exposed) and control (non‐exposed) foods for that child. Parents were sent a picture book about their child’s target vegetable ‐ the books consisted of pictures and information about the target vegetable. Duration: 2 weeks Number of contacts: 14 readings (5 min/d, 2 weeks) Setting: Home + lab Modality: Face‐to‐face Interventionist: Parents Integrity: “the last page contained a tick‐sheet reading record upon which parents were asked to note how many times they looked at the book with their child.” “According to the reading records provided by parents, children saw their book an average of 14.9 times (SD = 9.9) during the exposure phase” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of target vegetable they had seen in their book and a non‐exposed control vegetable of the same initial status (proportion): “Amount consumed” was coded as a proportion of the portion provided, again using a 5‐point scale (0 = none, 1 = nibble, 2 = less than ½ tsp, 3 = ½ tsp, 4 = whole portion).” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 14 days (unless rescheduled) Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: 5% Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The likelihood of performance bias in relation to vegetable consumption is low, given the children’s age. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. The coder was not blind to the liked/disliked or target/control food on each trial and so high risk of detection bias even though a second blind coder independently coded 20% of the recorded test sessions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 57/60 infants who completed the study. Attrition rate < 20% and therefore low risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol |
| Other bias | Low risk | Low risk of other bias |
| Methods |
Study design: Randomised controlled trial Funding: “Funding received through the EC Seventh Framework Programme (FP7/2007‐2013) under the IAPP 230637 “VIVA: V is for Vegetable – Applying Learning theory to increase liking and intake of vegetables” |
|
| Participants |
Description: Mothers with infants under 12 weeks old N (Randomised): 40 mother‐infant dyads (20 intervention, 20 control) Age: Infant (mean): Intervention = 4.78 months, Control = 4.88 months Mother (mean): Intervention = 33.7 years, Control = 30.9 years % Female: Infant: 57% SES and ethnicity: Not specified Inclusion/exclusion criteria: Not specified Recruitment: “Mothers were recruited from the local community using widespread advertising within mother and baby groups and a recruitment agency.” Recruitment rate: 83% (40/48) Region: UK |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 17, Control = 18 Description of intervention: “IG infants received 12 daily exposures to vegetable puree added to milk (days 1–12), then 12 x 2 daily exposures to vegetable puree added to baby rice at home (days 13–24). Then both groups received 11 daily exposures to vegetable puree (days 25‐35). They were each given a pack containing a 35 day diary and all of the equipment and foodstuffs they would need to complete the study. They were informed that breast or formula feeding should continue as normal.” Duration: 24 days Number of contacts: 24 exposures (daily) Setting: Home + lab Modality: Face‐to‐face Interventionist: Parents Integrity: “Another possible limitation of the study was that most of the intervention was conducted at home. It is then difficult to ensure that instructions were strictly followed.” Date of study: Recruitment took place between September 2011 and May 2012. Description of control: “Plain milk and cereal were given to the control group (days 1‐24)”. |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of vegetables (grams) measured by “a small set of portable digital pocket scales (MYCO MZ‐100, Dalman) to weigh accurately intakes (i.e. by weighing bottles or bowls before and after each feed) of all feeds consumed across the day.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 35 days, 6 months and 18 months Length of follow‐up post‐intervention: Immediate Subgroup analyses: None Loss to follow‐up (immediate, 6 months, 18 months): Intervention = 15%, 25%, 45% Control = 10%, 20%, 15% Analysis: Unknown if sample size calculation was performed. |
|
| Notes | First reported outcome (vegetable intake grams during
laboratory session) at immediate follow‐up
was extracted for inclusion in
meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit and vegetable intake 1st listed outcome in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Mothers were randomised to
either the intervention (n = 20) or control group (n
= 20) after they had consented to the study and
before they had completed any
questionnaires.” No information provided about the randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No information provided about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants were aware of whether or not they
were adding vegetable puree to milk and rice
cereal No blinding, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Food intake was weighed which would be low risk. However, "the researcher and mother made a joint decision on when 3 refusals were reached". This may have impacted on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Forty parents provided informed
consent for their infants to take part in the study;
however, complete data were collected on 36
mother–infant
dyads.” For outcome of vegetable intake grams during laboratory session 17 mothers in the intervention group and 18 mothers in the control group provided data. “At 6 months follow‐up, 15 mothers in the IG completed the two feeding sessions, while 16 mothers completed them in the CG (86% return rate).” |
| Selective reporting (reporting bias) | Unclear risk | No protocol listing prespecified outcomes |
| Other bias | Unclear risk | Recruitment bias may be an issue due to the method
used. Baseline table showed that groups appeared
similar, so there does not appear to be a high risk
of bias. However there is not enough info to
determine the level of risk. “Mothers were recruited from the local community using widespread advertising within mother and baby groups and a recruitment agency between September 2011 and May 2012.” “In total, the research team made contact with 48 mothers and from this initial contact 40 mothers were screened and accepted into the study.” |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children enrolled in the university‐based preschool during the 2013‐2014 academic year and their parents N (Randomised): 65 parent‐child dyads Age: Intervention (mean): 5 years Control (mean): 5 years % Female: Intervention: 38% Control: 64% SES and ethnicity: Monthly income (mean): Intervention = USD 6100, Control = USD 5336 Parent education High school: intervention = 0%, control = 3%; some college: intervention = 0%, control = 6%; Bachelor’s degree: intervention = 45%, control = 55%; Graduate degree: intervention = 45%, control 30% Ethnicity Non‐Hispanic white: intervention = 84%, control = 94%; Hispanic: intervention = 3%, control = 0%; Asian: intervention = 6%, control = 0%; Biracial: intervention = 6%, control = 6% Inclusion/exclusion criteria: No explicit inclusion/exclusion criteria stated for this trial, however the children had to be enrolled in the university‐based preschool during academic year 2013‐14 and were excluded if they participated in the 2012‐2013 academic year. Recruitment: “The parent who self‐identified as most responsible for preparing the child's meals was invited to complete the surveys. Preschool personnel sent an email inviting parents to consent to participate. Consent was obtained through an online survey.” Recruitment rate: 65% (65/100) Region: USA |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 32 parent‐child dyads, control = 33 parent‐child dyads Description of intervention: Parents received a health report describing their child’s average daily fruit and vegetable consumption along with the guidelines that children should consume 5 fruits and vegetables per day. Parents were also given a standardized set of recommendations for increasing fruit and vegetable intake as well as more comprehensive recommendations for how to increase their child’s fruit and vegetable intake (i.e. a more detailed list of parent behaviours to increase consumption). Duration: 4 weeks Number of contacts: Parents received one health report Setting: Home Modality: Written materials Interventionist: Preschool personnel provided the report Integrity: No information provided Date of study: 2013‐2014 academic year Description of control: “A delayed intervention group completed the initial baseline assessment but received no intervention until after the completion of the week 4 assessment.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Children’s consumption of fruit and vegetables (servings per day): “Parents of both groups completed the NCI Fruit and Vegetable Screener Questionnaire…. This measure was adapted to ascertain fruit and vegetable consumption over the previous week to allow for more frequent measurement of intake.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 4 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were randomly
assigned to either an intervention (n=32) or a
control (n=33) group using a random number
generator.” Unclear how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear whether those delivering the intervention, or the parents receiving the intervention were aware of their experimental group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Parents completed the NCI Fruit
and Vegetable Screener Questionnaire as an online
survey.” Child fruit and vegetable consumption assessed via parent self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “In study 2, 22.6%, 44.4%, and
14% of combined fruit and vegetable data were
missing at times 1, 2, and 3, respectively. Missing
values analysis determined that data were missing at
random; thus the researchers used full information
maximum likelihood
estimation.” Greater than 20% missing data at two time points, with over 40% of data missing at Time 2 |
| Selective reporting (reporting bias) | Unclear risk | There is no trial registration or protocol paper. |
| Other bias | Low risk | No other source of bias was identified. |
| Methods |
Study design: Randomised controlled trial Funding: "Funding for this study came from NIH grant K01DK068008 and a St. Luke's Roosevelt Hospital Pilot Award. Additional support came from the Obesity Research Center Grant" |
|
| Participants |
Description: Healthy children aged 4 to 5 years from diverse ethnic backgrounds N (Randomised): 19 children Age: 4 to 5 years % Female: Not specified SES and ethnicity: “from diverse ethnic backgrounds.” Inclusion/exclusion criteria: “All the children were “at risk for obesity,” based on having at least one parent with a BMI≥25 kg/m2, and they had to consume fewer than two servings of F&V per day, based on parental report during a screening phone call.” Recruitment: Not specified Recruitment rate: Unknown Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 7, Control = 9 Description of intervention: “Families in both groups attended weekly, small‐group sessions with the researchers where baseline measures were taken and family‐based nutrition education was delivered.” Children in the intervention group were “given F&V in containers decorated with their favorite cartoon characters. In addition, a sticker was included inside each decorated container to simulate the practice of premiums used by the food industry; children were allowed to collect these stickers on a game board to cash in for a prize the following week.” Duration: 7 weeks Number of contacts: Weekly group sessions and offered F&V containers 3 times a day Setting: Home + Lab Modality: Face‐to‐face Interventionist: Parents and researchers Integrity: No information provided Date of study: Unknown Description of control: “Children who were in the control group received F&V in plain plastic containers throughout the study” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables (grams, servings per day). F&V containers were stored by parents throughout the study period and taken back to the lab to be weighed Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 7 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 16% (not specified by group) Analysis: Unknown if sample size calculations performed. |
|
| Notes | First reported outcome (grams vegetables/week) was
extracted for inclusion in the
meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake only outcome reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There is not enough information to determine the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | There is not enough information to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The outcome is objective consumption of fruit & veg which is unlikely to be influenced by lack of participant & personnel blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective assessment (weight) of fruit and vegetable consumption therefore low risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/19 (84%) children completed the 7‐week study, however 3 children were excluded from the analysis. Intention‐to‐treat analysis was not used, therefore high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | There is not enough information to determine if there is any reporting bias |
| Other bias | Unclear risk | There is baseline imbalance between the study groups. Children in the intervention group consumed more servings of fruit & veg at baseline. Not clear of the impact this may have had on the results |
| Methods |
Study design: Cluster‐randomised controlled trial – cross‐over Funding: “Supported by NIH Grant R01‐DK082580 and USDA National Institute for Food and Agriculture Grant 2011‐67001‐30117 Program A2121‐Childhood Obesity Prevention: Transdisciplinary Graduate Education and Training in Nutrition and Family Sciences” |
|
| Participants |
Description: Children aged 3‐6 years enrolled in 3 childcare centres near University Park, Pennsylvania N (Randomised): 11 classrooms, 31 children Age: Overall mean = 4.4 years % Female: 49% SES and ethnicity: “Based on the 106 parents (88%) who provided family information, household incomes and education levels were above average: 69% of households had an annual income of above $50,000 and 92% of mothers and 90% of fathers had a Bachelor's degree or higher.” “The sample of children was 69% white, 21% Asian, 3% black or African American, and 7% of mixed or another race; 4% were of Hispanic or Latino origin.” Inclusion/exclusion criteria: Inclusion criteria: children had to be enrolled in participating childcare centres Exclusion criteria: children with an allergy or intolerance to the foods or milk being served Recruitment: “Children were recruited by giving letters to parents with 3‐ to 6‐year‐old children enrolled at three childcare centers near University Park, PA.” Recruitment rate: Unknown Region: Pennsylvania, USA |
|
| Interventions |
Number of experimental conditions: 6 Number of participants (analysed): Overall = 120 Description of intervention: Across the 6 meals (groups), all foods and milk were served at 3 levels of portion size (100%, 150%, or 200% of reference amounts) and 2 levels of energy density (100% or 142%) and were consumed ad libitum” “The experimental meal consisted of chicken (grilled breast or breaded nuggets), macaroni and cheese, a green vegetable (broccoli or peas), applesauce, ketchup, and milk.” Duration: 6 weeks Number of contacts: 6 (1 meal/week) Setting: Preschool Modality: Face‐to‐face Interventionist: Teachers and undergraduate research assistants Integrity: No information provided Date of study: “enrolled in the study from May 2013 to July 2014.” Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of vegetables (grams): “To determine the amount consumed, all foods and beverages were weighed before and after the meal in a separate room out of the children's view. Food weights were recorded to the nearest 0.1 g using digital scales” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall: 8% (11/131) Analysis: Unknown if adjusted for clustering Sample size calculations performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The order of the six conditions was counterbalanced across classrooms using Latin squares, and classrooms were randomly assigned one of the condition sequences using a random number generator.” |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed from those conducting the research. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Neither parents nor children
were informed about the purpose of the
study.” “During each meal, adults, including teachers and undergraduate research assistants who did not know the purpose of the study, were instructed to redirect conversations about food‐related topics to minimize peer influence on children's lunch intake.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “During each meal, adults,
including teachers and undergraduate research
assistants who did not know the purpose of the
study” low risk. Researchers who weighed all food and drink before and after the meal. Researchers were blinded to the purpose of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ”A total of 131 children from
11 classrooms at the 3 childcare centers were
enrolled in the study from May 2013 to July 2014.
Eleven children were excluded from the analysis
because they were absent for 3 or more of the 6
experimental meals. Thus, intake data was analyzed
for 120 children (61 boys and 59
girls).” No attrition is reported. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes in trial registry are reported. |
| Other bias | Low risk | No other bias was identified |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 2 to 5 years at 4 primary care clinics and their parent N (Randomised): 4 primary care clinics, 306 children Age: Child (mean): Intervention = 40.1 months, Control = 41.1 months Parent (mean): Intervention = 29.3 years, Control = 29.5 years % Female: Child: 47% Parent: not specified SES and ethnicity: Education: no schooling = 0.3%, Primary school = 8.9%, Junior high = 33.7%, High school = 39.3%, Professional school – 12.5%, Postgraduate = 1.7% Inclusion/exclusion criteria: Inclusion criteria: “Participants comprised children aged 2 ‐ <5 years of age whose BMI (calculated as weight in kilograms divided by height in meters squared) was above the median for age and sex (BMI z‐score 0 ‐ 3); who attended one of the participating IMSS clinics during the recruitment period for pediatric care, vaccination, or accompanying a family member; and whose parent or caregiver gave written consent to participate.” Exclusion criteria: “Families were excluded if they planned to move residences or change primary care clinics during the study period; the child had motor limitations (e.g., physical disability or delay); or required a special diet by medical indication.” Recruitment: “The project manager approached the directors of the 6 primary care clinics in Mexico City with the greatest proportion of preschoolers (approximately 5% children <5 years) to request their support for the project.” Recruitment rate: Primary care clinic = 67% (4/6) Child = 10% (306/3095) (using number of participants approached as denominator) Region: Mexico City |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 168, control = 138 Description of intervention: Intervention participants received a 6‐week curriculum focused on obesity awareness and prevention. 5 aspects dealt with throughout the 6 sessions: 1) Dietary culture, risk‐benefit practices; 2) The process of feeding acquisition/preparation/service/eating behaviours; 3) Physical activity habits; 4) Importance of weighing/measuring oneself and its meaning; 5) feedback and evaluations Duration: 6 weeks Number of contacts: 6 sessions (2 hrs a session) Setting: Primary care clinics Modality: Face‐to‐face, group sessions Interventionist: Nutritionist, nurse and health educator Integrity: Delivery of intervention: “To ensure fidelity, a small group of study staff (nutritionist, nurse and health educator) administered all intervention sessions and completed all screening, baseline and follow‐up assessments. No quantitative measure of delivery of intervention components” Attendance: “Only 52% (88 of the 168 who agreed to participate) attended ≥ 1 educational session (405 sessions attended in total). The total number of expected attendances at educational sessions was 1008 (168 participants attending 6 sessions each). Thus, compliance in the intervention group was 40% (405/1008) of total expected attendances. However, of the 88 receiving any intervention content, 67% (59/88) attended 5‐6 of the intended 6 workshops” Date of study: March 2012 to April 2013 Description of control: Usual‐care control ‐ received no intervention |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables (servings per week), “staff assisted parents in completing a child Food Frequency Questionnaire (FFQ) adapted from the FFQ used to assess dietary intake among 1‐4 year old children in the 2006 Mexican National Nutrition Survey.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 3 and 6 months Length of follow‐up post‐intervention: 1½ and 4½ months Subgroup analyses: None Loss to follow‐up (at 1 ½ and 4 ½ months): Intervention = 41%, 35% Control = 26%, 26% Analysis: Adjusted for clustering Unknown if sample size calculation was performed |
|
| Notes | First reported outcome (fruit servings/week) at the
longest follow‐up < 12 months (3 months
after intervention completion ‐ as
6‐months follow‐up did not report
retention values by group) was extracted for
inclusion in meta‐analysis The reported estimate which adjusted for clustering assessed change from baseline, we therefore used post‐intervention data and calculated an effective sample size using ICC of 0.016 to enable inclusion in meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake listed as primary outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation list designed by a statistician with no connection to the intervention was used for random allocation to experimental group |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Child dietary intake (parent‐reported): “Only after informed consent did participants learn of their treatment assignment”. There is no blinding to group allocation of participants at follow‐up described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Child dietary intake (parent reported): “Only after informed consent did participants learn of their treatment assignment”. There is no blinding to group allocation of participants at follow‐up described and because self‐reported measures were used this is likely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Non‐participation was
greater in the intervention (75 (45%) of 168
participants) than in the usual care (42 (30%) of
138 participants) arm (Figure 1).” Attrition rate was high with >35% of families not completing follow‐up at 3 months. Multiple imputations were performed to address missing data however non‐participation was greater in the intervention than in the usual care condition |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Unclear risk | There were baseline imbalances between the groups,
but results were adjusted. Unclear risk of recruitment bias as individuals were recruited to the trial after clusters have been randomised |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 4 to 9 months and their mother N (Randomised): 88 parent‐children dyads Age: Child (mean): Study 1 fruits = 6.7 months, Study 2 vegetables = 6.3 months Mother (mean): Study 1 fruits = 29 years, Study 2 vegetables = 28 years % Female: Child: Study 1 fruits = 49%, Study 2 vegetables = 43% Parent: 100% SES and ethnicity: Parent: “Their ethnic background was 55.4% (N =41) Black; 29.7% (N =22) White; 2.7% (N =2) Hispanic and 12.2% (N =9) Other/Mixed Ethnicity.” SES not specified Inclusion/exclusion criteria: “To qualify the Children had to have at least two weeks of experience eating cereal or fruit from a spoon and little experience with the target fruits and vegetables.” Recruitment: “Seventy‐four mothers whose Children were between the ages of 4 and 9 months were recruited from advertisements in local newspapers and from Women, Children and Children Programs in Philadelphia, PA.” Recruitment rate: Not specified Region: Philadelphia (USA) |
|
| Interventions |
Number of experimental conditions: 5 Number of participants (analysed): Study 1: fruits Pear group = 20 dyads, between‐meal (BM) group = 19 dyads Study 2: vegetables Green bean group = 11 dyads, between‐meal (BM) group = 12 dyads, between‐meal and within‐meal (BM‐WM) group = 12 dyads Description of intervention: Study 1: fruits “During the home exposure period, one group fed only pears at the target meal (Pear Group, N=20) whereas the other group fed a fruit which was different than the one experienced during the previous 2 days (Between‐Meal (BM) Fruit Variety Group, N=19).” Study 2: vegetables “The three groups differed in the type, amount and variety of foods that infants were fed during the target meal during the 8‐day home exposure period. The infants in the Green Bean Group (N=11) were fed only the target vegetable, green beans, whereas those in the Between‐Meal variety group (BM Vegetable Variety Group, N=12) and the Between‐Meal and Within‐Meal Variety Group (BM–WM Vegetable Variety Group, N=12) were fed a variety of vegetables. The BM Variety Group was fed only one vegetable each day and green and orange vegetables were alternated daily, whereas the BM–WM Variety Group was fed two vegetables each day (one green, one orange). In the latter group, the pair of vegetables varied from day‐to‐day but one of the pair was experienced the prior day.” Duration: 8 days Number of contacts: 8 exposures Setting: Home Modality: Face‐to‐face Interventionist: Mothers Integrity: “All of the mothers complied with these instructions.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetable purees (grams). Mother resealed jars and returned them after the exposure period to be weighed Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 days (4 days of test food(s)) Length of follow‐up post‐intervention: 2 days Subgroup analyses: None Loss to follow‐up: Condition 1: fruits Overall = 15% (no specified by group) Condition 2: vegetables Overall = 17% (no specified by group) Analysis: Unknown if sample size calculation was performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Fruit & vegetable intake: The mother fed the child and there is no mention of blinding, therefore at unclear risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The mother fed the child and there is no mention of blinding. However, this is an objective measure of intake, and therefore low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mother‐infant pairs were excluded from the study because they did not comply with experimental procedures or ate less than 5 grams on the testing days. An intention‐to‐treat approach was not adopted and therefore at high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | High risk | The groups differed significantly in the fruit study (Study 1) in terms of approachability and there is no mention that this difference was adjusted for in the analysis |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children and centre directors from 4 licensed childcare centres in North Carolina N (Randomised): 4 childcare centres Age: < 3 years = 27% 3 to 5 years = 73% % Female: Child: not specified Directors: 100% SES and ethnicity: “All centers had at least some subsidized children enrolled.” Directors: “75% were African American, and 50% had a college degree.” Inclusion/exclusion criteria: “To participate in the study, centers had to provide all foods and beverages to children in care (i.e., parents could not send food from home), not have an open case of abuse or neglect with the state licensing agency, and have at least three children between the ages of three and five years in care on a regular basis.” Recruitment: “We mailed a letter of invitation to every licensed center (n = 6) in the city limits of a small community near our research offices. The letter was followed by a telephone call from the study team. We enrolled the first four centers that agreed to participate. Center directors provided written informed consent to participate in the study; parents were provided a fact sheet describing the study and were asked to contact the project director if they did not want their children observed during the dietary assessment.” Recruitment rate: 100% of centres; recruitment rate for children not reported Region: Central North Carolina (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): 4 childcare centres, “An average of 19.0 (7.9) children were enrolled per center” Description of intervention: “The Watch Me Grow program is a garden‐based intervention aimed to increase the number of vegetables and fruits provided to and consumed by children in child care. The intervention took place in spring 2011. The program includes a “crop‐a‐month” structured curriculum for child‐care providers, consultation by a gardener, and technical assistance from a health educator. Over the course of the four‐month‐long intervention, providers and children in the intervention centers grew (1) lettuce, (2) strawberries, (3) spinach, and (4) broccoli. We designed the garden to yield one crop per month, and provided classrooms in the intervention centers with corresponding curriculum materials highlighting the target fruit or vegetable of the month.” Duration: 4 months Number of contacts: Health educators (technical assistance): monthly Visits from study gardener: at least monthly Centre staff provided curriculum activities: 1 activity per week Setting: Preschool Modality: Face‐to‐face Interventionist: Health educator/Gardener provided intervention to childcare centres Centre Staff provided curriculum/activities to children Integrity: No information provided Date of study: 2011 Description of control: Received no intervention |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables (mean servings, consumed by 3 children in each centre). Registered dietitians observed all meals and snacks over 2 full days and recorded all foods consumed for each of the 3 target children Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: ˜ 5 months Length of follow‐up post‐intervention: 1 month Subgroup analyses: None Loss to follow‐up: N/A: “the same three children may not have been observed pre‐ to post‐intervention.” Analysis: Did not adjust for clustering Unknown if sample size calculation was performed |
|
| Notes | First reported outcome (daily vegetable servings
consumed) was extracted for inclusion in
meta‐analysis. No adjustment was made for clustering; we therefore used post‐intervention data and calculated an effective sample size using ICC of 0.014 to enable inclusion in meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is primary outcome as in trial registry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “either the intervention or control condition on a 1:1 ratio, using the Research Randomizer (www.randomizer.org/form.htm)” The research randomiser was used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dietary observation: A trained registered dietitian blinded to treatment group conducted the dietary assessments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Dietary observation: The outcome is observation of foods served and consumed at mealtimes at the childcare centre undertaken by blinded dietitians. However, there is no blinding of childcare centre staff, cooks, children etc., because they were provided with a garden at their centre, curriculum materials and lessons, and staff met with research team about the garden and how to incorporate it into all aspects of the centre |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomly selected a classroom and then 3 children within classroom at centres to observe pre‐ and post‐intervention; it did not need to be the same 3 children observed pre‐ and post‐intervention |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Unclear risk | “Due to sample size limitations,
we did not conduct formal statistical analysis
beyond comparing crude differences in mean servings
of vegetables and fruits.” Insufficient information was reported to determine whether childcare centres were similar at baseline or recruitment bias. No statistical method to account for clustering, but we calculated an effective sample size prior to inclusion in meta‐analysis to account for this |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "This research was funded by the Miami‐Dade County Children’s Trust (grant number 764‐287)." |
|
| Participants |
Description: Children aged 2 to 5 years enrolled in 8 subsidised childcare centres in Miami‐Dade County, Florida N (Randomised): 8 childcare centres, 307 children Age: “the average age for boys was 3.82 years, the average age for girls was 3.91 years” % Female: Intervention = 49%, Control = 48% SES and ethnicity: “Thirty‐six percent identified their child as black, 34% identified their child as white, 18% chose other, and 14% were unknown. The ethnicity of the sample mirrors that of Miami‐Dade County, with 32% of the parents identifying their child as Hispanic/other, 25% as Hispanic/Cuban, 22% as African American, and 2% as Caucasian. Thirty‐five percent of the sample were primarily Spanish speaking and completed the measures in Spanish, and 65% of the sample were primarily English speaking and completed the measures in English” Inclusion/exclusion criteria: “Center study inclusion criteria consisted of (a) serve >30 children, (b) serve low‐income children, and (c) ethnic makeup had to be reflective of the county as a whole (minority majority). Low income was determined based on whether or not the child received subsidized child care.” No inclusion/exclusion criteria specified for children. Recruitment: “All participants were recruited at the child care center. Parents were approached during drop‐off or pickup times. Consent forms were attached to the interview packets, and parent data were collected during the initial visit.” Recruitment rate: 98% Region: Miami‐Dade County, Florida (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 238, Control = 69 Description of intervention: Teacher curriculum: Modeled after a modified version of Hip‐Hop to Health Jr., included implementation of lessons and a low‐fat, high fibre diet that included more fruits and vegetables with an emphasis on cultural barriers. Parent curriculum: Modeled after a modified version of the Eating Right Is Basic and Hip‐Hop to Health Jr., included a monthly educational dinner (run by dietitians) in which nutrition and physical activity were discussed, monthly newsletters, and at‐home activities, also information on how to introduce new foods and how to encourage eating more fruits and vegetables. Parents were encouraged to reduce TV viewing, increase physical activity, and model healthy eating behaviours for their child at home. Centre‐based modifications: These included: the development of policies to increase physical activity and healthy eating; modifying menus to make them compliant with the policies and also to ensure that the U.S. Department of Agriculture (USDA) nutritional requirements were met; agreeing on a drink policy that included providing water as the primary beverage, not allowing juice or sweetened beverages more than one time per week; changing from whole milk to 1% milk; having a snack policy which consisted of substituting healthy snacks, such as fresh fruit and/or vegetables, for cookies and other high‐lipid snacks; having a physical activity policy to increase physical activity to more than one hour per day and to decrease TV viewing to less than 60 minutes two times a week. Duration: 6 months Number of contacts: Unclear, multiple contacts Setting: Preschool, home Modality: Multiple (face‐to‐face, newsletters) Interventionist: Teachers, Parents and Registered Dieticians Integrity: No information provided Date of study: Unknown Description of control: “The Attention control group centers received a visit from an injury prevention education mobile. The mobile provided parents and teachers with hands‐on safety education and information, as part of an ongoing injury prevention program at the University of Miami.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables assessed using a 16‐item food frequency questionnaire (FFQ) completed by parents and teachers Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 3, 6 and 12 months Length of follow‐up post‐intervention: Immediately and 6 months Subgroup analyses: None Loss to follow‐up (Immediately post‐intervention and 12 months): Overall = 25%, 42% Analysis: Unclear if adjusted for clustering Unknown if sample size calculation performed |
|
| Notes | Sensitivity analysis ‐ primary outcome: Primary outcome not stated, BMI 1st listed outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Food intake: There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Food intake (parent and teacher reported): There is no blinding to group allocation of participants or personnel described and this is likely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 318 child‐parent dyads at baseline, there were 185 (58%) at the 1‐year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | Some evidence of baseline imbalance (e.g.
ethnicity) Unclear recruitment bias Unclear whether potential clustering within childcare centres accounted for |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “This study was sponsored by the National Institutes of Health (NIH)/National Institute of Child Health and Human Development through grant number R21‐HD073608. Partial support was received from the USDA Agriculture Research Service through specific cooperative agreement 58‐6250‐0‐008.” |
|
| Participants |
Description: Preschool‐aged children who were predominantly low‐income African‐American and Hispanics N (Randomised): 6 Head Start centres, 253 children Age: Mean: Intervention = 4.47 years, Control = 4.38 years % Female: Intervention = 49%, Control = 52% SES and ethnicity: Hispanics: Intervention = 46%, Control = 54% African‐American: Intervention = 59%, Control = 41% Inclusion/exclusion criteria: Not specified Recruitment: “Recruitment strategies included flyers that were sent to the home with the children, presentations at parent meetings, face‐to‐face recruitment during child drop‐off and pickup at Head Start, and active involvement of the Head Start manager and staff in the recruitment process” Recruitment rate: Children: 65% (253/391) Region: Houston, TX (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 128, Control = 125 Description of intervention: The intervention included 4 DVDs (videos) theatre‐based puppet shows that aimed at persuading children to increase vegetable consumption through encouragement, rationale/reason, reinforcement, and role modelling that were delivered over 4 consecutive weeks at preschools. Additionally, "each intervention child took home a bag including the DVD video for that week, a pamphlet, main ingredients to prepare a simple vegetable snack, crayons, and a disposable camera (if parents did not have a smart phone) to use as instructed in the booklets." The intervention was “based on the theoretical framework “transportation into a narrative world”, three professionally developed characters, unique storylines and an engaging, repetitious song were incorporated in four 20‐min videotaped puppet shows.” Duration: 4 weeks Number of contacts: 6 contacts per week Setting: Preschool, home Modality: Multiple (face‐to‐face, visual/audio – DVD) Interventionist: Teachers and parents Integrity: No information provided Date of study: Unknown Description of control: “During the 4‐week intervention period the control group did not receive any alternate intervention.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetables assessed using digital photography and plate weight before and after consumption (grams). Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 4 weeks + 2 days Length of follow‐up post‐intervention: 2 days Subgroup analyses: None Loss to follow‐up: No loss to follow‐up Analysis: Adjusted for clustering. Unknown if sample size calculation performed. |
|
| Notes | Reported estimates accounted for clustering, but
confidence intervals or other measures of variance
were not available. We therefore estimated means and
SDs by groups at follow‐up from a study
figure using an online resource (Plot Digitizer:
plotdigitizer.sourceforge.net) and
calculated an effective sample size using ICC of
0.014 to enable inclusion in
meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome was vegetable consumption |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment is provided and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and teachers in intervention preschools were not blinded to the intervention, as children viewed a DVD, and teachers were asked to identify the vegetable components served in the lunch. It is unclear whether this resulted in performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children’s vegetable intake was assessed using the digital photography method and plates were weighed and therefore unlikely to be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 253 children were enrolled and all of them completed the follow‐up assessment, so risk of attrition bias is low |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Unclear risk | There is potential recruitment bias, as it is not clear when or how clusters were randomised, and whether recruitment occurred before or after |
| Methods |
Study design: Cluster‐randomised controlled trial – cross over Funding: “Financial support was provided by the Rudd Foundation.” |
|
| Participants |
Description: Children aged 3 to 6 years attending 2 private preschools in a small north‐eastern city N (Randomised): 2 preschools (number of children not specified, 96 children recruited) Age: “Age ranged from 3 to 6 years old, but most (85%) children were 4 or 5 years old.” % Female: 44% SES and ethnicity: “These preschools primarily serve highly educated households; nearly all (93%) of the children had at least one parent with a bachelor’s degree and 75% had at least one parent with a graduate or professional degree.” “Race/ethnicity was white (69%), Asian (8%), African American (5%), Hispanic (6%), and other (12%).” Inclusion/exclusion criteria: Not specified Recruitment: Not specified Recruitment rate: Unknown Region: New Haven (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 43, control = 53 Description of intervention: “During the intervention, the children at Preschool A were served one of the new vegetables every day for 30 days in a 3‐day cycle (e.g., Monday, cauliflower; Tuesday, snow peas; Wednesday, green pepper) until they had received each vegetable a total of 10 times.” Duration: 6 weeks Number of contacts: 30 (1 per day for 30 days) Setting: Preschool Modality: Face‐to‐face Interventionist: Teachers Integrity: No information provided Date of study: 2007 Description of control: Control/delayed intervention (Preschool B). “ "Preschool B continued routine practices during the first 6 weeks of the study, and then switched conditions with Preschool A for the second 6 weeks” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of new vegetables (grams). “Researchers picked up the bags of vegetables later from the schools, weighed them, and calculated intake to the nearest gram.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: No loss to follow‐up Analysis: Adjusted for clustering (multilevel modelling) Sample size calculations performed |
|
| Notes | Post‐intervention data were extracted
following the first phase of the trial (Time 2)
prior to cross‐over. As an estimate was not
reported for the Time 2 follow‐up that
adjusted for clustering, we used
post‐intervention data and calculated an
effective sample size using ICC of 0.014 to enable
inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: Fruit or vegetable only outcome reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable consumption: Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable consumption: Objective measure of child’s vegetable intake and unlikely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no reported attrition. Data from 96 children were analysed |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | High risk | Baseline imbalances were reported. There were differences in vegetable consumption at baseline |
| Methods |
Study design: Randomised controlled trial Funding: "Supported by Medical Research Council/National Preventive Research Initiative grant G0701864" |
|
| Participants |
Description: Children aged 3 to 4 years attending nursery school and their primary caregiver N (Randomised): 173 parent‐child dyads Age: Child (mean): tangible reward = 3.96 years, social reward = 3.99 years, control = 3.90 years Primary caregiver (mean): tangible reward = 37.44 years, social reward = 37.35 years, control = 37.52 years % Female: Child: tangible reward = 48%, social reward = 54%, control = 55% Primary caregiver (mother): tangible reward = 85%, social reward = 88%, control = 77% SES and ethnicity: Primary caregiver: Ethnicity: White = 66%, Black = 2.9%, South Asian = 6% Education level: Nongraduate = 24%, Degree level of higher = 62% Inclusion/exclusion criteria: Not specified Recruitment: “Children aged 3–4 years and their primary caregivers were recruited through nursery schools in North London, United Kingdom.” “Recruitment was done in 3 waves in 2010. At each wave, teachers distributed consent forms and information letters about the “Tiny Tastes” study, and families were asked to return their contact details in a prepaid envelope if they were interested in taking part. Potential participants were then contacted by telephone.” Recruitment rate: Parent‐child dyads: 82% (173/212) Region: North London (UK) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Taste exposure + tangible reward = 47 Taste exposure + social reward = 46 No treatment control = 47 Description of intervention: Taste exposure + tangible reward: “The parents were asked to offer their child a small piece (˜2.5g) of their target vegetable every day for 12 weekdays and to tell them that they could choose a sticker if they tried it. No tastings were done over the weekends.” Taste exposure + social reward: “Parents were asked to offer the vegetable as described above and to praise their child with phrases such as “brilliant, you're a great vegetable taster” if they tasted it. The parents were to emphasize that the praise was being given for tasting the vegetable” Duration: 3 weeks Number of contacts: 12 taste exposures Setting: Home Modality: Face‐to‐face Interventionist: Primary caregiver Integrity: “The parents were also given a diary to record whether each day’s trial was performed, whether the child tried the vegetable, and whether the reward was given; space was allowed for comment.” “No differences in the number of days that the child was offered or tried the target vegetable were found between the intervention groups” Date of study: 2010 Description of control: “Families assigned to the control group did not perform any daily tastings and were given no instructions or materials for the intervention period, but were told that they would be taught a special technique to help their child to eat more vegetables after the last visit.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of target vegetable (grams). “Intake (in g) was recorded by weighing the bowl containing pieces of the target vegetable before and after consumption with a digital scale (Mettler Toledo).” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 3 weeks, ˜ 2 months and ˜ 4 months Length of follow‐up post‐intervention: Immediately and at 1 and 3 months Subgroup analyses: None Loss to follow‐up (Immediately post‐intervention, and at 1 and 3 months): Taste exposure + tangible reward = 0%, 0%, 3% Taste exposure + social reward = 0%, 3%, 2% No treatment control = 0%, 5%, 2% Analysis: Sample size calculations performed. |
|
| Notes | Data from the longest follow‐up < 12 months
(3 month follow‐up) were extracted for
inclusion in meta‐analysis. Estimates were
reported comparing the tangible reward and control
conditions, but not social reward condition. We
estimated mean and SEM from a study figure using an
online resource (Plot Digitizer: plotdigitizer.sourceforge.net) for all 3
groups. The tangible reward and social reward
conditions were combined into a single intervention
group for inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is primary outcome as per trial registry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Low risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Consumption of target vegetable: There is insufficient information to determine the likelihood of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Consumption of target vegetable: There is insufficient information to determine the likelihood of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion that completed the follow‐up assessments is not reported and therefore the risk of attrition bias is unclear |
| Selective reporting (reporting bias) | Unclear risk | The primary outcomes reported align with those specified in the trial registration. However the secondary outcomes specified on trial registry do not appear to be reported in the abstract |
| Other bias | Low risk | There is insufficient information to determine the risk of other bias |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 4 to 8 months old and their parent N (Randomised): 100 parent‐child dyads Age: Mean: Repeated exposure = 6.3 months, Flavour‐flavour learning = 6.6 months, Flavour‐nutrient learning = 6.2 months Parent: not specified % Female: Child: Repeated exposure = 47%, Flavour‐flavour learning = 35%, Flavour‐nutrient learning = 38% Parent: mostly mothers (exact % not reported) SES and ethnicity: Not specified Inclusion/exclusion criteria: “The criteria for children inclusion were as follows: age between 4 and 8 mo, introduction of complementary foods was started at >2 wk and <2 mo before the start of the study, no health problems or food allergies at the beginning of the study, and gestational age ≥36 wk.” Recruitment: “Parents in the Dijon area of France were recruited using leaflets or posters distributed in health professionals consulting rooms, pharmacies, and day‐care centers.” Recruitment rate: Parent‐child dyads = 81% (100/123) Region: Dijon (France) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Repeated exposure = 32 Flavour‐flavour learning = 30 Flavour‐nutrient learning = 30 Description of intervention: “During the exposure period, infants were exposed 10 times to a basic (RE group), a sweet (FFL group), or an energy‐dense (FNL group) artichoke puree according to their group.” Duration: Approx. 41 days Number of contacts: 2 ‐ 3 times per week Setting: Home Modality: Face‐to‐face Interventionist: Parents Integrity: “parents were given precise instructions, and data collected in the notebook revealed that they complied with the instructions.” Date of study: October 2010 and May 2011 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of varied artichoke purees (grams). “To measure intake, parents were asked to weigh each jar before and after consumption, using a digital kitchen scale (61 g, Soehnle) that we provided them with, and to record the weight in a notebook. After each observation, parents were required to reseal the jar(s) of food, freeze them, and bring the used jars back to the laboratory to check compliance with the study procedure and data accuracy.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: 2 weeks, 3 months and 6 months Subgroup analyses: None Loss to follow‐up (at 2 weeks, 3 and 6 months): Overall = 5%, 7%, 8% Analysis: Sample size calculations performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake: The interventions are all artichoke puree with different nutrient content. Parents would be unable to determine study group from feeding the child, and therefore this would be unlikely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake: This is objective assessment. Parents would be unable to determine study group from feeding the child, and therefore this would be unlikely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 families dropped out during the exposure period and were excluded. An intention‐to‐treat approach was not used and therefore at high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | The outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Unclear risk | The groups differed significantly in relation to weaning, but this was adjusted for in analyses. Therefore the risk of other bias is unclear |
| Methods |
Study design: Cluster‐randomised controlled trial – cross‐over Funding: "Supported by NIH grant R01 DK082580" |
|
| Participants |
Description: Children 3 to 5 years attending the Bennett Family Center on campus at The Pennsylvania State University Age: Mean: 4.4 years % Female: 52% SES and ethnicity: “The children were racially diverse: 56% were white, 29% Asian, 11% black or African American, and 4% Pacific Islander.” Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial Exclusion criteria: “Children who were allergic to any of the foods to be served at the snack were not included in the study.” Recruitment: “Participants in the study were recruited by distributing letters to parents of children in 4 classrooms of the childcare facility that included children aged 3–5 y; these classrooms had a total of ˜75 children present at snack time.” Recruitment rate: Unknown Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 8 Number of participants (analysed): Overall = 61 Description of intervention: Variety type serve: 1 x occasion: a variety of all 3 vegetables offered (cucumber, sweet pepper, tomato) 1 x occasion: a variety of all 3 fruits offered (apple, peach, pineapple) Single‐type serve: 3 x occasions: a single type of vegetable offered (cucumber, sweet pepper, tomato) 3 x occasions: a single type of fruit offered (apple, peach, pineapple) Duration: 4 weeks Number of contacts: 8 Setting: Preschool Modality: Face‐to‐face Interventionist: Childcare helper Integrity: No information provided Date of study: February to April 2011 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables (number of pieces). “The number of pieces of vegetables or fruit selected by each child in the study was recorded independently by 2 observers seated near each table.” “After the meal, the number of uneaten pieces on each child’s plate was recorded as well as any dropped pieces. All uneaten food and beverage items were weighed after the meal with digital scales (models PR5001 and XS4001S; Mettler‐Toledo Inc).” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: No loss to follow‐up Analysis: Unclear if adjusted for clustering Unclear if sample size calculations performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence created using a computerised random‐number generator. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable and fruit intake Child’s vegetable and fruit intake unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Vegetable and fruit intake: 2 observers independently recorded the number of pieces of vegetables or fruit selected by each child. However it is unclear whether these observers were blinded to condition and whether this influenced detection bias. This was observation of the number of pieces of fruit or veg selected and eaten by each child, and weight of any uneaten pieces of fruit/veg on the plate at end of meal. It was assessed by 2 independent observers, but it is not clear if they were blinded or not. Childcare staff sat at table with children and passed around fruit & veg bowls but were unaware of the study hypotheses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 (89%) of the 61 children completed the liking ratings and therefore the risk of attrition bias is low |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "This work was supported by a grant for investigation in nursing from Collegi Oficial d’ Infermeria de Barcelona, 2009 (grant number PR‐5001/09); Primer Premio Nacional de Investigación en Enfermería, 2009, from Hospital Universitario Marqués de Valdecilla; and a grant for investigation in nursing from Acadèmia de Ciències Mèdiques de Catalunya i Balears, filial Maresme, 2010. The funders had no role in the design, analysis or writing of this article." |
|
| Participants |
Description: Children aged 1 to 2 years attending 12 daycare centres and their parent N (Randomised): 12 day‐care centres, 206 children, 195 parents Age: Child (mean): Intervention = 1.3 years, Control = 1.4 years Parent (mean): Intervention = 35 years, Control = 35 years % Female: Child: Intervention = 37%, Control = 49% Parent: Intervention = 93%, Control = 85% SES and ethnicity: Educational level: Primary = 10%, Secondary = 35%, University = 55% Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial Exclusion criteria: “Children still exclusively breast‐feeding at the time of the study, children whose parents were not responsible for their alimentation, children with special diets due to chronic diseases (such as coeliac disease, food intolerances or allergies, inflammatory bowel disease), parents with language difficulties, parents unable to attend the educational workshops and those who did not sign the informed consent.” Recruitment: “At the beginning of the school term, all parents of the children attending the participating day‐care centres were invited to informative meetings regarding the study with the use of pamphlets and posters.” Recruitment rate: Child: 35% (206/581) Region: The city of Mataró (north of Barcelona), Spain |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Child: Intervention = 75, Control = 67 Parent: Intervention = 74, Control 72 Description of intervention: “All parents from the day‐care centres in the intervention group (IG) were invited to attend four educational workshops on alimentation at the beginning of the study and one reminder at 4 months. A model of participatory‐active education was used, in order to achieve practical skills in addition to nutritional knowledge. Cognitive (teaching how to improve diet), emotional (addressing beliefs and attitudes of the participants through discussion and analysis techniques) and skill areas (developing dietary skills) were included. The aim was to incorporate new and better dietary knowledge and to change the habits of the participants.” Duration: 6 months (workshops in October ‐ November and a reminder in March) Number of contacts: 5 workshops Setting: Preschool Modality: Face‐to‐face Interventionist: Nurses trained in nutrition Integrity: No information provided Date of study: October 2010 to May 2011 Description of control: “The parents included in the control group (CG) did not receive any education related to nutrition. In order to avoid drop outs, the participants of the CG were invited to a workshop on a subject not related to the study or nutritional education (manipulation and conservation.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruits and vegetables (servings per day) assessed using a 78‐item food frequency questionnaire (FFQ) completed by parents Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 8 months Length of follow‐up post‐intervention: 2 months Subgroup analyses: None Loss to follow‐up: Child: Intervention = 32%, Control = 35% Parent: Intervention = 9%, Control = 8% Analysis: Did not adjust for clustering. Unknown if sample size calculation performed. |
|
| Notes | First reported outcome (changes in vegetable and
garden produce servings per day) was extracted for
inclusion in the meta‐analysis. To enable
inclusion in meta‐analysis, we calculated
post‐intervention means by group by summing
baseline and change from baseline means, assuming
baseline SDs for post‐intervention SDs, and
we calculated an effective sample size using ICC of
0.014 to account for clustering Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 2nd listed outcome after adherence to Mediterranean diet |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Dietary intake (self‐reported): There is no blinding to group allocation of participants and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Dietary intake (self‐reported): There is no blinding to group allocation of participants and because this is a self‐reported measure this is likely to introduce detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Of the parents randomized to
the IG only sixty‐seven (65 %) attended three
or more workshops, with the remaining parents
considered drop outs. The reasons for not attending
the workshops were mainly difficulties in family
timetables and illness of the children”. 35% of the intervention group did not attend the minimum of 3 workshops and were considered dropouts. Therefore analysis was not undertaken according to intention‐to‐treat principles and risk of attrition bias is high |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | There were baseline imbalances for certain
characteristics between the conditions (e.g.
servings of legumes), although adjusted for in the
analysis and so the impact of this is unclear. Analysis did not accounted for effect of clustering, but we calculated an effective sample size prior to pooling in meta‐analysis to account for this |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 3 to 5 years attending full‐day childcare at the Child Development Laboratory located at The Pennsylvania State University N (Randomised): 21 children Age: Mean = 4.3 years % Female: 59% SES and ethnicity: “most of the families (60%) reported combined family incomes of US>$50,000.” Inclusion/exclusion criteria: “Exclusion criteria were the presence of food intolerance to study foods, chronic illness affecting food intake, consuming <22 g of the entree (<10% of the 220‐g entree portion), dislike of the main entree, uncooperative behavior during lunch, non‐English speaking, or extended absences.” Recruitment: Not specified Recruitment rate: Unknown Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 6 Number of participants (analysed): Overall = 17 (not specified by group) Description of intervention: “Children were served a series of 6 lunches in a random order, once per week, which varied only in entrée portion size (entree portion size order: 100, 160, 220, 280, 320, and 400 g). Children were served lunch on the same day of the week at their regularly scheduled time in an eating laboratory dining room facility near their classroom.” “The menu at all lunches included the portion‐manipulated macaroni and cheese entree and fixed portions of 2% milk and other foods served with the entree (eg, green beans with butter, whole‐wheat roll, and unsweetened applesauce).” Duration: 6 days Number of contacts: 6 (1 lunch per day) Setting: Preschool Modality: Face‐to‐face Interventionist: Research staff Integrity: No information provided Date of study: 2007 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetable for different entree portion sizes (grams). “Food and milk weights were recorded before and after consumption to the nearest 0.1 g by using digital scales (Mettler‐Toledo PR5001 and Mettler‐Toledo XS4001S; Mettler‐Toledo Inc). The amount of each food item consumed (g) was determined by subtracting postmeal weights from premeal weights.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall = 19% (not specified by group) Analysis: Unknown if sample size calculations performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Food and milk intake: Objective measure of child’s food intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Food and milk intake (weighed before and after
consumption): Objective measure of child’s food intake because food was weighed before and after consumption. Low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no reported attrition. Data are reported for all of the 17 children who met predetermined inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “The project described was supported by grant numbers A1R21DK078239 (principal investigator [PI]: Sherwood), P30DK050456 (PI: Levine), and P30DK092924 (PI: Schmittdiel) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).” |
|
| Participants |
Description: Parents with children aged 2‐4 years N (Randomised): 60 parent‐child dyads Age: Children (mean): Busy Bodies/Better Bites = 2.60 years, Healthy Tots/Safe Spots: 2.90 years Parent (mean): Bodies/Better Bites = 34.4 years, Healthy Totes/Safe Spots = 33.4 years % Female: Children: Busy Bodies/Better Bites = 50%, Healthy Totes/Safe Spots = 40% Parents: Busy Bodies/Better Bites = 97%, Healthy Totes/Safe Spots = 90% SES and ethnicity: Busy Bodies/Better Bites: white = 77%, Hispanic = 7%, Healthy Totes/Safe Spots: white = 83%, Hispanic = 7% Inclusion/exclusion criteria: Inclusion criteria: BMI between 85th and 95th percentile for age and gender OR BMI between 50th and 85th percentile and at least 1 overweight parent (BMI ≥ 25kg/m2) and receives care at a HealthPartners Clinic in the Twin Cities Metropolitan Area. Exclusion criteria: children with chronic disease, children who within the last 6 months or currently taking Prednisone, Prednisolone, Decadron, families who have limited English skills, and families who plan to move out of the Metropolitan area within the next 6 months Recruitment: “Parent‐child dyads were recruited through 20 clinics in the greater Minneapolis–St. Paul area” “…a study invitation letter was sent to parents. A subsequent phone call assessed interest and preliminary eligibility, confirmed in a home visit.” Recruitment rate: 94% (60/64) Region: USA |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Busy Bodies/Better Bites: 30 Healthy Totes/Safe Spots: 30 Description of intervention: All participants received pediatric primary care provider counselling during their well‐child visit to raise parental awareness of their child’s obesity risk and provide messaging regarding obesity and injury prevention behaviours. Busy Bodies/Better Bites: participants received an 8‐session phone‐coaching programme focused on healthy eating and PA and an associated workbook and busy bag, which included “a child focused book on television (TV) habits, activity and dinner table conversation idea cards, portion placement and plate, a kid‐friendly, healthy recipe pamphlet, small plastic cones, sidewalk chalk, stickers, a child‐focused dance music CD, and an inflatable beach ball.” Healthy Totes/Safe Spots: participants received an 8‐session phone‐coaching programme focused on safety and injury prevention and an associated workbook and safety tote, which included “a similar number of items [to the busy bag] relevant to the safety and injury prevention topics (e.g., travel‐size sunscreen or fire safety book).” Duration: 6 months Number of contacts: 9 (1 primary care component + 8 phone coaching sessions) Setting: Clinic + home Modality: Multiple (face‐to‐face, telephone, written materials) Interventionist: PCP (face‐to‐face) and experienced interventionists (telephone) Integrity: Provider adherence: “Well‐child visit protocol adherence was assessed by phone survey with parents 1–2 weeks post‐well‐child visit. Parents reported whether their provider talked about BMI percentile, whether they received the HHHK pamphlet, and whether the provider addressed specific PA, sedentary behavior, healthy eating, and safety/injury prevention issues.” Phone coaches: “Phone coaches completed a self‐assessment of session fidelity (e.g., use of behavioral adherence strategies and time spent discussing specific target areas) after each session. Phone sessions were audio recorded, and recordings were utilized during supervision sessions and subsequently coded by independent raters to provide a more in‐depth examination of fidelity.” Well‐child visit intervention component: “Parents reported that 78% of providers discussed BMI percentile. The majority of parents (87%) received the HHHK pamphlet, but less than half (44%) reported that their provider used the HHHK flipchart. The most frequently discussed obesity prevention topics included fruit and vegetable intake (27%), PA (24%), junk food, including sweetened beverages (11%), and media use (7%). Fewer parents reported that the provider discussed family meals (5%), eating breakfast (4%), and eating out at restaurants (0%).” “80% of participants in both arms completed the eight‐session intervention.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of fruits and vegetables (servings) using a multipass 24‐hour recall completed by parents. Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 6 months Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Busy Bodies/Better Bites: 13% Healthy Totes/Safe Spots: 3% Analysis: Unknown if sample size calculation was performed. |
|
| Notes | Sensitivity analysis ‐ primary outcome: primary outcome as per trial registry included fruit and vegetable intake | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Sixty parent‐child dyads
were randomized equally to the Busy Bodies/Better
Bites Obesity Prevention and the Healthy Tots/Safe
Spots Contact control
arms.” It is unclear how the randomisation occurred |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed from those conducting the research. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “After the well‐child
visit, parents received a randomized group
assignment notification
letter…” “Coaches worked with parents to address behavior change areas in order of parent preference, setting goals and discussing challenges and successes at subsequent sessions.” Participants were aware of their group allocation. Due to the nature of the intervention, staff would also have been aware of participant group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “A multipass 24‐hour
dietary recall was administered by staff trained and
certified to use the Nutrition Data System for
Research software versions 2009, 2010, and
2011” It is unclear whether outcome assessors visiting the home were aware of group allocation. Parents self‐reported child dietary intake. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of parents who completed the follow‐up assessments is reported and there was only a small loss to follow‐up that was similar across experimental arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per protocol, except for “Paediatrician participation and satisfaction at 6 months” This was reported after 3 HHHK visits, not at 6 months. |
| Other bias | Low risk | No other bias was identified. |
| Methods |
Study design: Randomised controlled trial Funding: "Australian Research Council Linkage Grant (ARC LP100100049)" |
|
| Participants |
Description: Children aged 20 to 42 months and their parent N (Randomised): 201 parent‐child dyads Age: Child (mean): Intervention = 2.7 years, Control = 2.8 years Parent (mean): Intervention = 35 years, Control = 35 years % Female: Child: Intervention = 49%, Control = 37% Parent: not specified SES and ethnicity: Parent highest level of education (Bachelor degree or higher): Intervention = 57%, Control = 60% Annual family income (AUD): AUD < 450,000: Intervention = 14%, Control = 21% AUD 45,001 – 85,000: Intervention = 41%, Control = 33% AUD 85,001 – 125,000: Intervention = 27%, Control = 27% AUD > 125,000: Intervention = 17%, Control = 19% Location of parents' birth: Australia or New Zealand: Intervention = 77%, Control = 74% Europe: Intervention = 3%, Control = 4% Asia: Intervention = 11%, Control = 9% Inclusion/exclusion criteria: Inclusion criteria: “Families were eligible if their child was aged 20–42 months at baseline (waitlist children would still be ≤4 years when receiving the programme), and if parents were aged ≥ 18 years and could read and write English (with the assistance of an interpreter if required). There were no other qualifying or exclusion criteria.” Recruitment: “We sourced participants through community events, local newspaper and magazine advertisements, flyers distributed through kindergartens/pre‐schools/childcares, maternal and child health centres, and medical centres.” Recruitment rate: Parent‐child dyads = 97% (201/207) Region: Victoria (Australia) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Time 2: Intervention = 80, Control = 72 Time 3: Intervention = 74, Control = 69 Time 4: Intervention = 73, Control = 63 Description of intervention: MEND (Mind, Exercise, Nutrition…Do it! 2 – 4 intervention: “Each session included three sections: (i) 30 min of guided active play; (ii) 15 min of healthy snack time based on an evidence‐based, exposure technique to promote acceptance of fruit and vegetables and (iii) 45 min of supervised creative play activities for the children while parents attended an interactive education and skill development session. Guided active play involved games played with children and parents together that could be easily replicated at home. Healthy snack time centred on a role model (puppet called ‘Max Moon’) who encouraged children to sniff, touch, lick and taste fresh fruit and vegetables. Parents received weekly handouts.” Duration: 10 weeks Number of contacts: 10 (1 per week, 90 minutes a session) Setting: Community health centres Modality: Face‐to‐face Interventionist: Trained program leader Integrity: “Programme leaders were monitored regularly to ensure their practice was in accordance with guidelines.” Date of study: Between May 2010 and December 2012 Description of control: Wait‐list control: "The WLC group did not receive any intervention, but were offered the programme at study completion.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables (usual servings) assessed by the Eating and Physical Activity Questionnaire completed by parents. Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Post‐intervention: 10 weeks Time 2: ˜ 8 ‐ 9 months Time 3: ˜ 15 months Length of follow‐up post‐intervention: Immediately Time 2: 6 months Time 3: 12 months Subgroup analyses: None Loss to follow‐up (Immediately post‐intervention and at 6 and 12 months): Intervention = 12%, 4%, 4% Control = 5%, 6%, 6% Analysis: Sample size calculations performed |
|
| Notes | First reported outcome (usual servings a day of
vegetables) at the longest follow‐up < 12
months (6 months) and ≥ 12 months (12
months) was extracted for inclusion in
meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake listed as primary outcome in trial registry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ”conducted by a researcher not
involved in data management using a randomized
treatment allocation schedule produced by computer
algorithm.” The random sequence was produced by computer algorithm |
| Allocation concealment (selection bias) | Unclear risk | Although the authors indicate that participants were informed of group allocation by opaque envelopes, there is no indication if these envelopes were sealed and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Dietary intake (includes fruit and vegetables): There is no blinding to group allocation of participants described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Dietary intake (includes fruit and vegetables)
(self‐report): There is no blinding to group allocation of participants described and because of the self‐report measure this is likely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was < 20% at follow‐up T4 and missing values of baseline measurements were imputed using mean imputation |
| Selective reporting (reporting bias) | Unclear risk | “Outcomes not addressed here
will be presented in future
papers.” Insufficient evidence to determine, as it appears that future papers with additional outcomes are planned |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Low socio‐economic children aged 3‐5 years attending Head Start preschools in Marion County, Ohio N (Randomised): 4 Head Start centres, 240 children Age: “All clusters combined had a total of 80 (38.3%) three year old children, 116 (55.5%) four year old children, and 13 (6.2%) five year old children in the study sample.” % Female: Access‐only cluster = 54%, access + education = 45%, control = 55% SES and ethnicity: Low socio‐economic “There were 9 (4.3%) Hispanic children, 152 (72.7%) white children, 36 (17.2%) multi‐racial, and 12 (5.7%) black children in the study sample” Inclusion/exclusion criteria: No explicit inclusion criteria Exclusion criteria: “Children or parents were excluded if a medical issue prohibited them from participating in the study. Children who were unable to eat solid foods were asked not to participate in this study. Children with chronic diseases, such as diabetes, were excluded from the study, as children with chronic diseases are known to have reduced carotenoid concentrations” Recruitment: “Purposive sampling was the method chosen for this study”. Parents were approached about consenting to the study at various meetings or when parents were dropping off or picking up their children. Recruitment rate: 83% (240/290) Region: Marion County, Ohio |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Access only: 61 Access + education: 82 Control: 66 Description of intervention: Access only: “received the take home weekly fruits and vegetables, without the educational intervention.” Access + education: “received weekly take home fruits and vegetables, education for the children, and supplemental materials, such as newsletters and recipes, for the families about the produce being provided.” The Supplemental Nutrition Assistance Program Education (SNAP‐Ed) was provided each week. “The Harvest for Healthy Kids curriculum was used and each week the focus was on a high carotenoid fruit or vegetable. Storybooks, activities such as making pumpkin pudding in a bag, and tastings were the foundation of the class sessions.” Duration: 8 weeks Number of contacts: 8 Setting: Preschool + home Modality: Access only: provision of fruit and vegetable Access + education: multiple (provision of fruit and vegetable, face‐to‐face education, written materials) Interventionist: Access only: unclear Access + education: Supplemental Nutrition Assistance Program Education (SNAP‐Ed) programme staff member delivered education Integrity: No information provided Date of study: October‐December 2016 Description of control: “the control group did not receive either the produce or education during the eight weeks.” “The group received education following the study.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of fruit and vegetable consumption measured by carotenoid levels in the skin Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 8 weeks Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Access only: 18% Access + education: 10% Control: 12% Analysis: Unclear if adjusted for clustering Sample size calculations performed |
|
| Notes | We pooled the access + education intervention arm
compared to the no‐intervention control group
in meta‐analysis of multicomponent
interventions. We described the access‐only intervention compared to the no‐intervention control group narratively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Site clusters were randomly
assigned to one of the treatment or control
groups”. Randomly allocated to experimental group but the random sequence generation procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and study team were not blinded. Parent self‐reported survey on fruit and vegetable consumption and therefore at high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Parents were not blinded which may have affected how
they responded to the survey. Parent self‐reported survey on fruit and vegetable consumption and therefore at high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 31/240 withdrawn (27 from intervention, 4 from control). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol is available |
| Other bias | Unclear risk | There appears to be baseline imbalance between groups
with differences between groups on child age and
race. Analysis does not appear to account for clustering. |
| Methods |
Study design: Cluster‐randomised controlled trial – cross‐over Funding: "Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK082580) and the Robert Wood Johnson Foundation" |
|
| Participants |
Description: Children aged 3 to 6 years enrolled in daycare at the Bennett Family Center on campus at The Pennsylvania State University N (Randomised): 5 classrooms, 51 children Age: Mean = 4.4 years % Female: 57% SES and ethnicity: “Of the 51 children in the study, 46 parents provided demographic information for their children. Of these 46 children, 28 (61%) were white, 14 (30%) were Asian, 3 (7%) were black or African American, and 1 (2%) was American Indian or Alaska Native. Parents of the children had above‐average educational levels and household incomes; 90% of mothers and 85% of fathers had a college degree, and 79% of households had an annual income >$50,000.” Inclusion/exclusion criteria: Provided by study author: "Children with an allergy to the foods being served were not eligible to participate in the study. Parents and guardians provided informed written consent for both their own participation and that of their child." Recruitment: “Recruitment began in April 2008 by distributing letters to parents who had children aged 3–6 years enrolled in daycare at the Bennett Family Center at the University Park campus of The Pennsylvania State University.” Recruitment rate: Provided by study author: "100% of children whose parents signed consent form were included in the study" Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Overall = 51 Description of intervention: One day a week for 4 weeks, children were provided with a first course and main course at lunch. Across the weeks the portion size of raw carrots and dip served as the first course of lunch was varied (30 g, 60 g, or 90 g) and during 1 week no first course was provided. Cooked broccoli was served as the vegetable with the main lunch course Duration: 4 weeks Number of contacts: 4 (1 day a week) Setting: Preschool Modality: Face‐to‐face Interventionist Preschool teacher Integrity: Provided by study author: "All children were served the food assigned in the experimental condition. There was no deviation from study protocol. No unplanned or unintended interventions." Date of study: Recruitment began in April 2008 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetables for different first course portion sizes (grams). “Uneaten items were removed, and weights were recorded to the nearest 0.1 g with digital scales. Consumption of the foods and milk was determined by subtracting postmeal weights from premeal weights.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: Immediately Subgroup analyses: Provided by study author: "Differences between girls and boys in age, body weight, height, BMI percentile, and BMI z score were analyzed by using t tests. Analysis of covariance was used to assess the influence of continuous variables (age, body weight, height, BMI percentile, and BMI z score) on the relation between carrot portion size and the main study outcomes. Children who consumed all of the carrots (95% of the weight served) at any meal were identified, and data were analyzed both with and without these children to determine whether they influenced the results. The effect of individual children who were influential on the main study outcomes was assessed." Loss to follow‐up: There was no loss to follow‐up Analysis: Unclear if adjusted for clustering Sample size calculations performed. |
|
| Notes | Sensitivity analysis ‐ primary outcome: Vegetable intake listed as primary outcome in trial registry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Children were enrolled from 5
classrooms; the order of the experimental conditions
across study weeks was assigned to classrooms by
using a Latin square
design.” Provided by study authors: "The orders of the experimental conditions across study weeks were created using Latin squares and then assigned to classrooms using a random number generator." |
| Allocation concealment (selection bias) | Unclear risk | It is not clear who undertook randomisation of
classrooms. Provided by study authors: "Classrooms (and the associated condition order) were assigned a color coding so that participants and teachers were uninformed of the experimental condition." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Incidents of food and drink
spillage were recorded by researchers. Teachers were
instructed to redirect conversations pertaining to
food to nonfood‐related topics to minimize
the influence on lunch
intake.” Objective outcome measurement. Children were not blinded and it seems unlikely that this would influence their intake. Staff present during the meal and staff who served the food to children were not blinded and it seems unlikely this would influence child intake |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Uneaten items were removed, and
weights were recorded to the nearest 0.1 g with
digital scales”.
“Incidents of food and drink
spillage were recorded by
researchers.” Appears that researchers who weighed the food were the same researchers who recorded incidents of food and drink spillage. Researchers were not blinded and this may have had an impact on how the outcome was recorded in different classrooms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “A total of 51 children were
enrolled, and all of them completed the
study” There were no children who dropped out over the study |
| Selective reporting (reporting bias) | Low risk | There is no study protocol and unable to determine if
all prespecified outcomes have been reported as
described Provided by study authors: "All outcomes collected were reported in the paper (vegetable and food intake)" |
| Other bias | Low risk | There are no other sources of potential bias |
| Methods |
Study design: Randomised controlled trial – cross‐over Funding: Provided by study author: "Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK082580)." |
|
| Participants |
Description: Children aged 3‐6 years attending 2 daycare centres at the University Park campus of The Pennsylvania State University N (Randomised): 49 children Age: Mean = 4.7 years % Female: 54% SES and ethnicity: “Of the 39 children, 28 children (72%) were white, 9 children (23%) were Asian, and 2 children (5%) were black or African American. Parents of the children had above average education levels and household incomes; ˜90% of mothers and 80% of fathers had a college degree, and 76% of households had an annual income >$50,000.” Inclusion/exclusion criteria: Provided by study author: "Children with an allergy to the foods being served were not eligible to participate in the study. Parents and guardians provided informed written consent for both their own participation and that of their child." Recruitment: “Recruitment began by distributing letters to parents with children aged 3–6 years who were enrolled in daycare at the Bennett Family Center or the Child Development Laboratory at the University Park campus of The Pennsylvania State University.” Recruitment rate: Provided by study author: "100% of children whose parents signed consent form were included in the study" Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Overall = 39 Description of intervention: “The 3 experimental entrees were manipulated by adding pureed vegetables to a standard recipe (100% energy dense (ED) condition) to reduce the ED by either 15% (85% ED condition) or 25% (75% ED condition). Manipulated entrees were zucchini bread at breakfast, pasta with tomato‐based sauce at lunch, and chicken noodle casserole at dinner and evening snack.” In addition unmanipulated side dishes and snacks were served, including fruit, vegetables, milk and cheese and crackers Duration: 3 weeks Number of contacts: 3 (1 day a week) Setting: Preschool Modality: Face‐to‐face Interventionist Provided by study author: "Preschool teacher" Integrity: Provided by study author: "All children were served the food assigned in the experimental condition. There was no deviation from study protocol. No unplanned or unintended interventions." Date of study: Between January and May 2010 Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetable for difference energy density entrees (grams). “Food and beverage weights were recorded to the nearest 0.1 g with digital scales (PR5001 and XS4001S; Mettler‐Toledo Inc). The consumption of foods and beverages was determined by subtracting postmeal weights from premeal weights.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Effect of intervention on amount of meal consumed Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: Immediately Subgroup analyses: Provided by study author: "ANCOVA was used to assess the influence of continuous subject variables (age, body weight, height, and BMI percentile) on the relation between entree energy dense (ED) and the main study outcomes. t tests were used to test differences between girls and boys in ages, body weights, heights, BMI percentiles, and BMI z scores." Loss to follow‐up: Overall = 18% Analysis: Sample size calculations performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated with computer software |
| Allocation concealment (selection bias) | Unclear risk | ”Random orders were generated
with computer software and assigned to a list of
participant identification
numbers” The random sequence was assigned to a list of participant identification number, but it is unclear if allocation was concealed. Provided by study author: "Allocation was concealed to participants and teachers by assigning each child an ID number that was associated with their random order." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake: Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake: Objective measure of child’s vegetable intake and unlikely to be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49 children were enrolled, but 9 were excluded because they had difficulty following the protocol. Given an intention‐to‐treat approach to analysis was not used, the risk of attrition bias is high |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the paper align with those specified in the trial registration |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Cluster‐randomised controlled trial – cross‐over Funding: Provided by study author: "Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK082580)." |
|
| Participants |
Description: Children aged 3‐5 years attending 2 daycare centres at the University Park campus of The Pennsylvania State University N (Randomised): 5 classrooms, 73 children Age: Range 3.3 to 5.7 years (mean = 4.7 years) % Female: 57% SES and ethnicity: “Parents of the children had above average education levels and household incomes; approximately 95% of mothers and 88% of fathers had a college degree and 70% of households had an annual income above $50,000.” “Parents provided demographic information for 66 of the 72 children; of these, 42 (67%) were white, 17 (27%) were Asian, and 4 (6%) were black or African American” Inclusion/exclusion criteria: Provided by study author: "Children with an allergy to the foods being served were not eligible to participate in the study. Parents and guardians provided informed written consent for both their own participation and that of their child." Recruitment: “Recruitment began by distributing letters to parents who had children within the age range of three to six years enrolled in two daycare centers on the University Park campus of The Pennsylvania State University.” Recruitment rate: Provided by study author: "100% of children whose parents signed consent form were included in the study" Region: Pennsylvania (USA) |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Overall = 72 Description of intervention: “On one day a week for four weeks, children in a daycare setting were provided with breakfast, lunch, and afternoon snack. Across the weeks, the portion size of soup (tomato soup) served in the first course of lunch was varied (150, 225, or 300 g) and during one week no first course was provided. The foods and beverages served in the main course of lunch, as well as the foods and beverages served at breakfast and snack, were not varied in portion size.” Duration: 4 weeks Number of contacts: 4 (1 day per week) Setting: Preschool Modality: Face‐to‐face Interventionist: Teachers Integrity: No information provided. Date of study: Provided by study author: "Data was collected from Dec. 2008 to Mar. 2009." Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetable (grams): tomato consumed from soup + broccoli from main course, Broccoli only, Afternoon snack, Total (soup, broccoli and afternoon snack). Portion sizes of foods were provided and researchers recorded the amount consumed Outcome relating to absolute costs/cost effectiveness of interventions: Provided by study author: "Outside scope of this study; data not collected" Outcome relating to reported adverse events: Provided by study author: "Outside scope of this study; data not collected" Length of follow‐up from baseline: Unclear Length of follow‐up post‐intervention: Immediately Subgroup analyses: Provided by study author: "Analysis of covariance was used to assess the influence of continuous subject variables (age, body weight, height, and BMI percentile) on the relationship between soup portion size and the main study outcomes. T‐tests were used to test differences between girls and boys in age, body weight, height, and BMI percentile." Loss to follow‐up: Overall = 1% Analysis: Provided by study author: "Classroom was tested as a factor in the model, but it was not significant and was removed." Sample size calculations performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Provided by study author: "The orders of the experimental conditions across study weeks were created using Latin squares and then assigned to classrooms using a random number generator." |
| Allocation concealment (selection bias) | Unclear risk | Provided by study author: "Classrooms (and the associated condition order) were assigned a color coding so that participants and teachers were uninformed of the experimental condition." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vegetable intake: Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vegetable intake: Researchers recorded the number of pieces of each food item taken by the child and it is unlikely that this would be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 out of 73 children were included in the vegetable intake analysis and therefore the risk of attrition bias is low |
| Selective reporting (reporting bias) | Low risk | Provided by study author: "All outcomes collected were reported in the paper (soup and food intake)" |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue |
| Methods |
Study design: Randomised controlled trial Funding: “AES is supported, in part, by the 1 U54 GM104940 grant from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center (July, 2015 to June, 2017).” |
|
| Participants |
Description: Children aged 3 to 5 years attending at 2 full‐day preschools N (Randomised): 42 children Age: Mean: Food modelling DVD = 4.5 years, Non‐food DVD = 4.1 years, No DVD (Control) = 4.3 years % Female: 50% SES and ethnicity: Child: White = 74%, African American = 5%, Asian = 10%, Hispanic = 10% Inclusion/exclusion criteria: Not specified Recruitment: Not specified Recruitment rate: 39% (42/108) Region: LA (USA) |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Food modelling DVD = 14 Non‐food DVD = 14 No DVD (Control) = 14 Description of intervention: Food modelling group = Copy‐Kids Eat Fruits and Vegetables DVD Non‐food DVD group = Copy‐Kids Brush Teeth. Day 1: “Depending on the condition, on day 1 the child viewed 1 of 2 video clips or sat quietly for 7.5 minutes. Two plates of snacks (the modelled vegetable and a comparison food) were placed in front of the participant in a standardized format (green bell peppers on the right and dry cereal on the left) on separate, identical white Styrofoam plates. Children were instructed to eat as much or as little as they wished during this time. The video segments were played concurrently during the food presentation” Day 2 and 7: “food items were presented for 7.5 minutes without the concurrent video presentation”. Duration: 1 week ± 2 days Number of contacts: 3 Setting: Preschool Modality: Visual/audio ‐ DVD Interventionist: Unclear Integrity: No information provided Date of study: Unknown Description of control: No DVD Control: food items were presented the same way as in the intervention but no DVD was played on any of the 3 exposure days |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetable (grams). “Study staff weighed 0.5 cups of the modeled vegetable (ie, approximately 80 g of raw, sliced green bell pepper) and 0.5 cups of the comparison food (ie, approximately 16 g of Multi Grain Cheerios; General Mills, Minneapolis, MN) using a transportable scale before and after snack presentation on days 1, 2, and 7.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 1 week ± 2 days Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Unknown if sample size calculations performed. |
|
| Notes | Outcome data from the longest follow‐up <
12 months (day 7). We estimated the mean and SEM
from a study figure using an online resource (Plot
Digitizer: plotdigitizer.sourceforge.net) for all 3
groups. We combined the control DVD and control
conditions into a single control group for inclusion
in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 1st listed outcome in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “used block randomization to
distribute age and sex evenly across conditions
using a randomization schedule generated with SAS
programming” The random sequence was generated using statistical software, SAS |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Vegetable intake (weighed): Objective measure of child’s vegetable intake and unlikely to be influenced by performance bias Parent reported fruit and vegetable consumption: There is no blinding to group allocation of participants or personnel described and this is likely to influence performance. However, it does appear that parents were blinded to the food provided to their children. “Researchers did not inform parents regarding which foods were presented to the children.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Vegetable intake (weighed): Objective measure of child’s vegetable intake and unlikely to be influenced by detection bias Parent reported fruit and vegetable consumption: There is no blinding to group allocation of participants or personnel described and these are self‐reported measures. However, “Researchers did not inform parents regarding which foods were presented to the children.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised completed the study. Therefore low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | The authors state that limitations included potential for within‐school contamination across conditions. No other evidence presented about this potential bias |
| Methods |
Study design: Randomised controlled trial Funding: Supported by the Gerber Products company and National Institutes of Health Grant 2RO0HD197S2‐07 |
|
| Participants |
Description: Mothers and their 4 to 6‐month old infants N (Randomised): 36 children Age: Child (mean): 22 weeks (17‐27 weeks) % Female: 56% SES and ethnicity: Not reported Inclusion/exclusion criteria: “The 36 infants and their mothers who participated met the following criteria: 1. Infants were between 4 and 6 months of age at the beginning of the study; 2. Parents had just begun feeding solid foods and had only given cereals or cereals and fruits; 3. Parents indicated readiness to begin or continue introducing solid foods to the infant; and 4. Absence of medical complications or physical problems.” Recruitment: “Subjects were solicited through birth records and advertisements in local newspapers.” “Parents were contacts and informed of the study before the time their infants would be expected to be introduced to solid foods and contact was reestablished when they were ready to participate.” Recruitment rate: Unknown Region: USA |
|
| Interventions |
Number of experimental conditions: 4 Number of participants (analysed): Peas salted: 9 Peas unsalted: 10 Green beans salted: 8 Green beans unsalted: 9 Description of intervention: “Foods used throughout the study, pureed peas and green beans, were prepared especially for the study by the Gerber Products Company. Salted and unsalted versions of the two vegetables were prepared. The salted version of each food contained 0.3g NaCI/100g. The foods were presented to the mothers in jars, containing 71g of food and labels did not indicate the presence or absence of salt.” Duration: 10 days Number of contacts: 10 (once per day) Setting: Home Modality: Face‐to‐face Interventionist: Parents Integrity: “On each feeding occasion, parents completed a brief form noting information on the number of the jar used (1through 10), date of feeding, time at the start and end of the feed, infant state of alertness at the beginning of the feed, health of the infant, and the overall quality of the interaction during the feeding.” Date of study: Unknown Description of control: N/A |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Children’s consumption of vegetable (grams): Weighed jars of off before feeding session, resealed and frozen once feeding was finished. Jars collected and weighed by research team to determine grams of intake. Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: No adverse reactions were observed Length of follow‐up from baseline: 25 days Length of follow‐up post‐intervention: Immediately and at 1 week Subgroup analyses: None Loss to follow‐up: There was no loss to follow‐up Analysis: Unknown if sample size calculation was performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The 36 infants were randomly
assigned to receive either salted or unsalted peas
or green beans; thus forming a total of four
treatment groups.” No mention of how the randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The foods were presented to the mothers in jars, containing 71 g of food, and labels did not indicate the presence or absence of salt.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All ratings were made while mothers and the research assistant were blind to whether infants were fed peas or beans, whether the feedings observed occurred before or after the repeated exposures, and whether or not the infants were being fed salted or unsalted vegetables.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no attrition reported. |
| Selective reporting (reporting bias) | Unclear risk | There is no trial registration or protocol paper. |
| Other bias | Low risk | No other sources of bias were identified. |
| Methods |
Study design: Randomised controlled trial Funding: “Funding for this research was provided by an unrestricted grant from ‘‘Get Kids in Action,’’ a partnership between the Gatorade Corporation and the University of North Carolina.” |
|
| Participants |
Description: Children aged 2 to 5 years and their parent N (Randomised): 50 parent‐child dyads Age: Child (mean): Intervention = 3.9 years, Control = 3.3 years Parent (mean): Intervention = 36.6 years, Control = 36.2 years % Female: Child: Intervention = 59%, Control = 67% Parent: Intervention = 86%, Control 90% SES and ethnicity: Parent (non‐white): Intervention = 18%, Control = 10% Income (USD): < 50,000: Intervention = 18%, Control = 81% ≥ 50,000: Intervention = 77%, Control = 19% Education: College or less: Intervention = 36%, Control = 43% Inclusion/exclusion criteria: At least 1 child 2 ‐ 5 years old, “Additional eligibility criteria included having lived in their current residence and planning to stay in that residence for at least 6 months. If the family had more than 1 eligible child, the eldest was selected as the reference child” Recruitment: “A convenience sample of 50 parent‐child dyads, with at least 1 child 2‐5 years old, was recruited through child care centers, listservs, and community postings. Interested parents responded to recruitment materials and were screened by phone.” Recruitment rate: Unknown Region: USA |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 22, control = 21 Description of intervention: “addressed vegetable and food issues based on the baseline surveys, and the dietitian helped parents select 1 primary target area for improvement during the intervention from 4 possible options (vegetable availability; picky eating; modeling; family meals). These areas were selected based on Social Cognitive Theory, which posits that there is reciprocal interaction between an individual and his/her environment. This theory also highlights the importance of self‐efficacy, which was thus a target of the intervention as well.” Duration: 4 months Number of contacts: 6 (2 phone calls, 4 newsletters) Setting: Home Modality: Multiple (telephone, newsletters) Interventionist: A registered dietitian Integrity: No information provided Date of study: April and December 2009 Description of control: “Control group families received 4 non‐health/nutrition related children's books, 1 per month.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of vegetables (servings per day) assessed using a Block Kids food frequency questionnaire (FFQ) completed by parents. Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 5 months Length of follow‐up post‐intervention: Immediate Subgroup analyses: None Loss to follow‐up: Intervention = 12% Control = 16% Analysis: Unknown if sample size calculations performed |
|
| Notes | To enable inclusion in meta‐analysis, we
calculated post‐intervention means by group
by summing baseline and change from baseline means,
and assumed baseline SDs for
post‐intervention SDs. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 2nd listed outcome after height and weight |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Child vegetable intake (parent reported): There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Child vegetable intake (parent reported): There is no blinding to group allocation of participants or personnel described and because this is a parent‐reported measure at high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 (86%) of the 50 parent‐child dyads recruited completed the study. Therefore at low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | Participants differed on child age by condition. However although this was adjusted for in the analysis the impact of this imbalance is unclear |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “Indian Council of Medical Research, India and the NIH/NICHD (5 R01 HD042219‐S1); additional funding from UNICEF, New York.” |
|
| Participants |
Description: Mothers and their infants from 60 villages in India N (Randomised): 60 villages (clusters), 607 mother‐infant dyads Age: Child: “The intervention began with infants are about 3 months old” Mother (mean): Complementary feeding group: 22.3 years, Responsive complementary feeding and play group: 22.3 years, Control group: 21.9 years % Female: Child: Complementary feeding group = 52%, Responsive complementary feeding and play group = 51%, Control group = 49% Parent: 100% SES and ethnicity: Percentage mothers finished secondary or high school: Complementary feeding group = 25%, Responsive complementary feeding and play group = 32%, Control group = 27%. Mean standard of living index score: Complementary feeding group = 25.6, Responsive complementary feeding and play group = 25.3, Control group = 26.3 Inclusion/exclusion criteria: Inclusion: had to be part of the ‘Integrated Child Development Services’ project areas, be pregnant in their third trimester No exclusion criteria mentioned in text but in figure states “excluded as per criteria: microcephaly, physical handicap, mother mentally handicapped, cerebral palsy, thalassemia, child passes away.” Recruitment: “We explained the study objectives to all the pregnant women in the villages and asked if they would like to participate in the study. There were no refusals.” Recruitment rate: 100% Region: India |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): Complementary feeding group = 170 Responsive complementary feeding and play group = 145 Control group = 168 Description of intervention: Complementary feeding group: “In addition to the ‘Integrated Child Development Services’, mothers in this group received 11 nutrition education messages on sustained breastfeeding and complementary feeding through twice‐a‐month or four times a month (depending on the age of the infant) home‐visits over 12 months by the trained village women using flip charts, other visual material, demonstrations and counselling sessions.” Responsive complementary feeding and play group: “In addition to the ‘Integrated Child Development Services’, mothers in this group received education on complementary feeding as in the complementary feeding group (11 messages), eight messages and skills on responsive feeding, and eight developmental stimulation messages using five simple toys. This group of mothers also received developmentally appropriate toys five times during the intervention with instructions on how to use them to engage and play with their children.” Duration: 12 months Number of contacts: 30 planned visits “The first visits were in the fourth month, after the baseline when infants were 3 months old. From 4 to 6 months, mothers were visited twice per month, or 6 visits; from 7 to 9 months, they were visited 4 times a month, or 12 visits; and from 10 to 14 months, they were visited twice a month, or 12 visits,” Setting: Home + centre‐based supplemental food Modality: Face‐to‐face Interventionist: The trained village women Integrity: “Trained graduates in nutrition supervised the village women, examined their records of visits and asked mothers independently what they were told in the village woman’s’ last visit. They also held periodic reinforcement training sessions with the village women.” Date of study: Unknown Description of control: “Control group (CG): Mothers and infants in this group received only the routine ‘Integrated Child Development Services’, which were operating across all study groups. These services consist mainly of centre‐based supplemental food provided to 1–6‐year‐olds, pregnant and nursing mothers, home‐visit counselling on breastfeeding and complementary feeding, monthly growth monitoring, and non‐formal preschool education for children 3–5 years of age.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of banana, spinach, pulses (legumes): “Dietary intake was evaluated by the 24‐h recall method using standard cups with specified volume to help recall the food serving amounts.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 months Length of follow‐up post‐intervention: Immediately Subgroup analyses: None Loss to follow‐up: Overall: 15% Analysis: Adjusted for clustering Sample size calculations performed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The random allocation using a random number generator (facilitated through a tailor‐made syntax programme in the Statistical Package for the Social Sciences (SPSS), which uses the select cases function) was undertaken by a researcher who was not familiar with the villages or their characteristics other than what could be derived from the 2001 census data.” |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the village women (VW) delivering the intervention, and mothers receiving the intervention were likely to be aware of their experimental group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The assessment teams (psychologists and nutritionists) were blinded to the intervention and had no interaction with the VWs. They did not meet as they used different transport and timetable of activities. The villages had no identification mark to indicate the group to which they had been randomized.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “After 12 months of intervention
and consequent attrition (15%), the sample comprised
511 mothers and children with 182 in CG, 176 in CFG
and 153 in the RCF&PG. All 60 clusters remained
in the study. Loss to follow‐up was greater
in the RCF&PG (22%) compared with the CG (9%)
and CFG (16%) although this difference was not
statistically
significant.” “Reasons for follow‐up losses during the study were migration (9.2%), house found locked on repeated visits (4.7%) and death of the child (1%). The demographic characteristics of those lost to follow‐up and those who remained were not different.” Loss to follow‐up was uneven across the study arms (not stat significant), but were not due to the trial. No loss of clusters |
| Selective reporting (reporting bias) | Unclear risk | There is no trial registration or protocol paper. |
| Other bias | Low risk | Recruitment bias: (low) “We
explained the study objectives to all the pregnant
women in the villages and asked if they would like
to participate in the study. There were no
refusals.” Baseline imbalance: (low) “There were no significant differences among the three groups in any of the baseline characteristics" Loss of clusters: (low) “All 60 clusters remained in the study.” Incorrect analysis: (low) “Values presented in the text and tables are means & standard deviations at the individual level and ICCs are presented to quantify the clustering effects” |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "The work was supported by the Ministry of the Flemish Community (Department of Economics, Science and Innovation; Department of Welfare, Public Health and Family)." |
|
| Participants |
Description: Children aged 9 to 24 months enrolled at daycare centres in 6 different communities in Flanders (Belgium) N (Randomised): 70 day care centres, 203 children Age: Mean: Intervention = 15.8 months, Control = 14.9 months % Female: Intervention = 47%, Control = 44% SES and ethnicity: Low SES: Intervention = 13%, Control = 24% Inclusion/exclusion criteria: No explicit inclusion criteria stated for this trial Children were excluded if they were not present in daycare on the measurement day for objective height and weight at baseline (i.e. not fulfilling the minimum criteria to be included in the study) Recruitment: “Within each day‐care centre, parents of all children aged 9–24 months were invited to enrol their child in the study.” Recruitment rate: 50% (203/404) Region: Flanders (Belgium) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 100, control = 56 Description of intervention: “The intervention aimed at increasing daily consumption of water (instead of soft drinks), milk, fruit and vegetables, increasing daily physical activity and decreasing daily consumption of sweets and savoury snacks and daily screen‐time behaviour.” “programme that consisted of two components: (i) guidelines and tips presented on a poster and (ii) a tailored feedback form for parents about their children’s activity‐ and dietary related behaviours.” Duration: 12 months Number of contacts: Unclear Setting: Preschool Modality: Face‐to‐face Interventionist: Researchers Integrity: No information provided Date of study: 2008 to 2009 Description of control: No information provided |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables assessed using a 24‐item semi‐quantitative food frequency questionnaire (FFQ) completed by parents. Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 12 months Length of follow‐up post‐intervention: Immediate Subgroup analyses: None Loss to follow‐up: Intervention = 21% Control = 14% Analysis: Did not adjust for clustering Unknown if sample size calculations performed |
|
| Notes | First reported outcome (grams fruit/day) was
extracted for inclusion in the meta‐analysis.
The reported estimate that adjusted for clustering
did not report 95% CI or SEM. Therefore we used
final values and calculated an effective sample size
using ICC of 0.016 to enable inclusion in
meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 2nd listed outcome after BMI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Fruit and vegetable intake (parent reported): Parents were not blinded to group allocation and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fruit and vegetable intake (parent reported): Parents were not blinded to group allocation and this is likely to influence performance |
| Incomplete outcome data (attrition bias) All outcomes | High risk | FT: Of 203 children, 156 (77%) were re‐examined 12 months later at follow‐up (this is the first follow‐up post‐intervention). If we define this as short‐term follow‐up, this is high risk of bias as > 20% dropout |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | High risk | Baseline imbalance: Baseline differences were
observed between the control and intervention groups
in sociodemographic characteristics and body
composition. However although this was adjusted for
in the analysis the impact of this imbalance is
unclear. “The analyses were adjusted for SES, age of the child and BMI Z‐score at baseline to control for the observed baseline imbalance in these variables between intervention and control groups.” Recruitment bias: Appears that parents and childcare centres were recruited after communities had been matched and randomised ‐ high risk Incorrect analyses: Linear mixed models adjusted for clustering within daycare centres, but standard errors were not reported. Reported mean (SD) by group at follow‐up and calculation of effective sample sizes prior to inclusion in meta‐analyses accounted for this, therefore low risk. |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "The development of the intervention was funded by the PWO(Project‐related Scientific Research)‐funding of University College Arteveldehogeschool. Funds for the evaluation were provided by the Provincial Government East‐Flanders." |
|
| Participants |
Description: Children attending 16 preschools in East Flanders (Belgium) N (Randomised) 16 preschools, 1432 preschoolers Age: (DOB) < 2002: intervention = 41%, control = 51% 2002: intervention = 28%, control = 24% 2003: intervention = 31%, control = 26% % Female: Intervention = 53%, control = 44% SES and ethnicity: Predominantly low parental education Low education (mother): intervention = 49%, control = 49% Low education (father): intervention = 60%, control = 57% Ethnicity: No information provided Inclusion/exclusion criteria: Not specified Recruitment: Schools were approached by mail for consent. All parents of preschoolers attending the consenting schools were asked to fill in a food frequency questionnaire Recruitment rate: Parents: 54% Schools: 10% (40 out of 403 schools consented, although only 8 were selected in the end) Region: East Flanders (Belgium) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 308, Control = 168 Description of intervention: 8 preschools received a multi‐component intervention to assist schools to implement a healthy school food policy. "The main objectives were to increase the consumption of fruit, vegetables and water and to decrease the consumption of sugared milk drinks and fruit juice." The main strategies to influence the child and the different environmental factors included: "Child: Guided and self‐guided activities based on experiential education (e.g. tasting) and developmental education (e.g. explanation of concepts of food triangle); Role model, feed back and reinforcement by teachers; Educational role‐model story and characters; Availability of healthy foods; Availability of cooking equipment. Parents: Newsletters; Suggestions for the back and forth diary; Work sheets and creations by children; Parent evenings and other school activities with parents Teacher: Training sessions; Manual including didactic and policy aspects; Digital learning environment; Newsletters; Group discussions with teachers; Examples of good practices School environment: Newsletters; Training sessions for principals and cafeteria staff; Help on demand via e‐mail; Examples of good practices; Policy aspects in the teachers’ manual; Feedback to schools." Duration: 6 months Number of contacts: Unclear (multicomponent) Setting: Preschool Modality: Multiple (staff training, experiential education, newsletters, email support, resources) Interventionist: Not specified Integrity: No information provided Date of study: Sept 2006 ‐ April 2007 Description of control: 8 preschools received the control: no information provided |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Daily consumption of fresh fruit and vegetables (grams) as reported by parents in a written food frequency questionnaire Length of follow‐up from baseline: 6 months (March/April 2007) Subgroup analyses: None Loss to follow‐up Intervention: 47% Control: 45% Analysis: Contact with the author indicated that the analysis was adjusted for clustering by school Unknown if sample size calculation was performed |
|
| Notes | Trial results are reported as change from baseline in
mean daily consumption of fruit and vegetables and
post‐intervention values. No standard
deviations were reported for
post‐intervention data to enable inclusion in
meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake is primary outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Contact with the author indicated that a computerised random‐number generator was used |
| Allocation concealment (selection bias) | Unclear risk | Contact with the author indicated that schools did not know their allocation prior to consenting to the study. It is unclear if study personnel responsible for recruitment were aware of group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Contact with the author indicated that parents and school staff were not blind to group allocation and that parents could have attended information sessions organised by the researchers, or observed posters, newsletters or intervention materials in intervention schools. Given that the relevant trial outcomes were based on parental reports, the review authors judged that there was a risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Contact with the author indicated that parents and school staff were not blind to group allocation and that parents could have attended information sessions organised by the researchers, or observed posters newsletters or intervention materials in intervention schools. Given that the relevant trial outcomes were based on parental reports, the review authors judged that there was a risk of bias. (NB. There were no independent outcome assessors in this trial; the parents completed and returned a food frequency questionnaire about their child's food intake) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although similar across groups (intervention = 47%, control = 45%), rates of loss to follow‐up were high. Contact with the author indicated that no information was collected on reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Contact with the author indicated that analysis was
adjusted for clustering No further risk of bias identified |
| Methods |
Study design: Randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 2 to 6 years and their principal caregiver (parent) who were recruited from a larger study N (Randomised): 156 children Age: Child: 34 to 82 months (mean = 53 months) Parent: mean = 36 years % female: Children (by group): Exposure = 34%, Nutrition Information = 58%, Control = 51% Parent (overall): 95% SES and ethnicity: "68% of parents had left full‐time education at the age of 21 or over" and "the majority of parents held further education qualifications." Ethnicity = 74% white Inclusion/exclusion criteria: No explicit inclusion/exclusion criteria stated for this trial, or for the trial from which participants were recruited. 13 children (1 girl, 12 boys) were excluded when they did not comply with the experimental procedures during the pre‐experimental taste test Recruitment: Participants were recruited from a larger study on the predictors of children's fruit and vegetable intake and expressed an interest in participating in further research to modify their children’s acceptance of vegetables Recruitment rate: Parents: 28% Region: United Kingdom |
|
| Interventions |
Number of experimental conditions: 3 Number of participants (analysed): i) Restricted to at least 10 out of 14 exposures: Exposure = 34, Nutrition Information = 48, Control = 44 ii) All available data: Exposure = 48, Nutrition Information = 48, Control = 44 Description of intervention: Exposure: Taste exposure intervention carried out in the home where parents were asked to offer their child a taste of a target vegetable daily for 14 consecutive days. Parents were given suggestions to encourage the child to taste the vegetable. Parents were given a vegetable diary to record their experiences, and children could record their liking for the vegetable after each session using 'face' stickers. Nutrition Information: Parents were informed about the ‘5 a day’ recommendations and given a leaflet with advice and suggestions for increasing children’s fruit and vegetable consumption Duration: 14 days Number of contacts: 14 (daily for 14 consecutive days) Setting: The home Modality: Face‐to‐face, exposure Interventionist: Researchers trained parents to offer the target vegetable to their child Integrity: 14 participants in the exposure group failed to complete a minimum of 10 out of 14 tasting sessions. ‐ 4 children completed 9 sessions, 2 completed 8 sessions, 2 completed 7 sessions, 1 completed 6 sessions, 4 completed 5 or less sessions Date of study: Not provided Description of control: "No treatment" control ‐ parents received no further intervention |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: As‐desired consumption of target vegetable (grams) assessed by weighing the amount of the vegetable on the plate before and after consumption using a professional digital scale (Tanita Corporation, Japan) Length of follow‐up from baseline: Approximately 2 weeks Subgroup analyses: Restricted sample to only those in the taste exposure group who received 10 or more exposures. This restricted the Exposure group from 48 to 34 children. Loss to follow‐up: 2% (140 provided follow‐up data of 143 who were eligible and provided data at baseline). Exposure: 4% (children withdrawn from their study by their parents following collection of baseline data). Nutrition Information: 0% Control: 2% (children withdrawn from their study by their parents following collection of baseline data). Analysis: Adjustment for clustering not applicable Unknown if sample size calculation was performed |
|
| Notes | "Two sets of analyses were carried out: (a) on a
restricted sample which excluded those in the
Exposure group who completed less than 10 tasting
sessions (n=126) and (b) on the whole sample
(n=140). Results below refer to the reduced sample
size ... results for the whole sample are only
included where they differed from these." Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake 3rd listed outcome after rated and ranked liking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned to one of three experimental treatment groups". No further information provided regarding sequence generation |
| Allocation concealment (selection bias) | Low risk | Contact with the author indicated that allocation was concealed in an opaque envelope opened at participant's homes after baseline data collection |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Contact with the author indicated that personnel delivering the intervention were not blind to group allocation and that parents may not have been blind to group allocation. However, given the objective assessment of outcome (electronic scales), the review authors judged that the study outcome was unlikely to be affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Contact with the author indicated that the outcome assessors were not blind to group allocation. Given the objective measure of outcome (electronic scales), assessment is unlikely to have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates of loss to follow‐up were similar and low across the exposure (4%), nutrition information (0%) and the control conditions (2%). Reasons for loss to follow‐up were provided and were similar |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | No further risk of bias identified |
| Methods |
Study design: Randomised controlled trial Funding: "This work was commissioned by the Food Standards Agency in 2009 and supported by the Department of Health (UK) from 2010." |
|
| Participants |
Description: New mothers attending baby clinics in disadvantaged London neighbourhoods N (Randomised): 312 mothers Age: Children: mean = 10 weeks Parents: mean = 30 years % Female: Children = not stated Parents = 100% SES and ethnicity: 28% lone parents 57% living in social housing 33% receiving income support/job seeker's allowance Ethnicity: 50% from an ethnic minority Inclusion/exclusion criteria: Inclusion criteria: "Women from Registrar General occupational classes II‐V (non‐professional); babies born >/= 37 weeks; babies' birth weight above 2500g; singletons; women able to understand written and spoken English; and resident in the study area." Exclusion criteria: "Women aged under 17 years; infants were diagnosed with a serious medical condition or were on special diets; infants aged over 12 weeks; women or their partners were from social class I (professional). Originally their intention was to restrict the sample to first‐time mothers over the initial 12 week recruitment period. The inclusion criteria was therefore changed to include all new‐mothers." Recruitment: "Women were recruited from December 2002 to February 2004 at baby clinics located in the more disadvantaged neighbourhoods across Camden and Islington where Surestart (a national social welfare initiative targeting families with young children) programmes existed. A standardised technique was used to approach new mothers attending the baby clinics. An overview of the study was given and randomisation explained. If the women were interested, a short screening questionnaire was then used to assess their eligibility." Recruitment rate: Mothers: 82% Region: London, UK |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 124, Control = 115 (12 months) Intervention = 108, Control = 104 (18 months) Description of intervention: A monthly home visiting programme (from 3 to 12 months) delivered by trained local mothers, providing practical support on infant‐feeding practices. Duration: 9 months (duration of each visit = 60 min) Number of contacts: Monthly from 3 to 12 months (maximum = 10 contacts) Setting: The home Modality: Face‐to‐face, via home‐visiting Interventionist: Trained local volunteers "A group of local mothers were recruited and trained to provide the support in a 12‐session programme delivered over a 4‐week period." Integrity: "On average each woman in the intervention group received five volunteer home visits (range 1‐10). A small number of women were also contacted by telephone when home visits were not possible." Date of study: Recruited from Dec 2002 to Feb 2004 Description of control: Usual care. "Women in the control group only received standard professional support from health visitors and GPs." |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Children's intake of vitamin C from fruit Secondary outcome: Proportion of children who consumed specific fruits and vegetables more than once a week Length of follow‐up from baseline: 9 months and 15 months (when children aged 12 months and 18 months, respectively) Subgroup analyses: None Loss to follow‐up: (at 9 and 15 months) Intervention: 27%, 34% Control: 20%, 30% Analysis: Adjustment for clustering not applicable Sample size calculation was performed |
|
| Notes | Vitamin C (mg) from fruit at the longest
follow‐up < 12 months (9 months ‐
children aged 12 months) and ≥ 12
months (15 months ‐ children aged 18 months
old) was extracted for inclusion in
meta‐analysis. Sensitivity analysis ‐ primary outcome: Vitamin C intake from fruit listed as primary outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random allocation schedule was prepared in advance using random digit computer tables." |
| Allocation concealment (selection bias) | Low risk | "Those responsible for recruiting ... were all masked to group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Contact with the author indicated that parent participants and intervention personnel were not blind to group allocation. Given that the trial outcome was based on parental reports of children's fruit intake, the review authors judged that there was a risk of performance bias in this study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those responsible for ... assessing outcomes were all masked to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rates of loss to follow‐up were similar across intervention (27%, 34%) and control (20%, 30%) groups at both time points and were moderate. There were no substantial differences in the reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All primary or secondary outcomes of interest were reported according to the information provided in the trial register (ISRCTN 55500035) |
| Other bias | Low risk | Small deviation in protocol: The original sample was
restricted to first‐time mothers but after 12
weeks of the 14‐month recruit this was
broadened to all new mothers No further risks of bias identified |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "This research was supported by US Department of Agriculture’s (USDA) Food and Nutrition Service (FNS)." |
|
| Participants |
Description: Children attending childcare centres participating in the Child and Adult Care Food Program and their parent N (Randomised): 24 childcare centres, 1143 parent‐child dyads Age: Child: mean = 4.4 years Parent: “Overall, 67% of respondents were between the ages of 18 and 34” % Female: Child = 48% Parent: not specified SES and ethnicity: Parent: “40% were Hispanic or Latino; 24% were white, non‐Hispanic; 27% were black, non‐Hispanic; and 9% were another race or more than one race” Inclusion/exclusion criteria: Not specified Recruitment: “The study sampled child‐care centers participating in the Child and Adult Care Food Program in New York” “Approximately 5 to 6 weeks before the start of the intervention in spring 2010, teachers sent children home with a study invitation and the baseline survey. Parents who agreed to participate in the study were asked to return a contact information card and the completed questionnaire in a separate envelope to preserve confidentiality.” Recruitment rate: Parent: 75% (1143/1518) Region: New York (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 440, control = 462 Description of intervention: Eat Well Play Hard in Child Care Settings program “is a Supplemental Nutrition Assistance Program (SNAP) Education program that allows states to receive funding for nutrition education to improve the likelihood that SNAP participants will make healthy food choices.” “The program includes multilevel messaging targeted to preschool children, their parents, and the childcare center staff who shape the policies and practices in their child‐care environment.” “Some of the most frequently taught modules used for this intervention included trying new foods (Food Mood); eating a variety of vegetables (Vary Your Veggies); eating a variety of fruits (Flavorful Fruit); incorporating more healthy dairy products into the diet (Dairylicious); eating healthier snacks (Smart Snacking); and engaging in physical activity (Fitness Is Fun).” Duration: 6 ‐ 10 weeks Number of contacts: 6 classes for children and parents separately (30‐60 minutes per session) 2 classes for centre’s staff “Finally, the RDN works with each centre director to identify areas of policy improvement that can enhance nutrition at the centre and teaches at least two classes to the centre’s staff to help them integrate the program’s messages into their classroom activities” Setting: Preschool Modality: Multiple (face‐to‐face, printed materials/resources) Interventionist: Registered dietitian nutritionist Integrity: No information provided Date of study: March and June 2010 Description of control: Wait‐list control: “control centers received the intervention after the evaluation was completed, but within the same calendar year.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables (cups per day) by parent self‐report via mail or telephone survey using modified questions from the University of California Cooperative Extension Food and Behaviour Checklist. Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: Unclear, ˜ 7 to 10 weeks Length of follow‐up post‐intervention: 1 week Subgroup analyses: None Loss to follow‐up: Intervention = 20% Control = 22% Analysis: Adjusted for clustering Sample size calculations performed |
|
| Notes | First reported outcome (cups of vegetables child
consumed at home a day) was extracted for inclusion
in the meta‐analysis. We selected
post‐intervention values over change from
baseline estimates, and calculated effective sample
size at follow‐up using an ICC of 0.014 to
enable inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: Primary outcome not stated, power calculation conducted on fruit or vegetable intake |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Child’s fruit and vegetable intake
(parent survey): There is no blinding to group allocation of participants or personnel described and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Child’s fruit and vegetable intake
(parent survey): There is no blinding to group allocation of participants or personnel described and because this is a parent‐reported survey this is likely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 902 (79%) out of 1143 parents completed the follow‐up. Given this was a short‐term follow‐up, the risk of attrition bias is high |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | At baseline, children in the intervention group were
statistically significantly older than children in
the control group, but unclear what impact this may
have had. “At baseline, children in the intervention group were statistically significantly older than children in the control group (difference=0.2 years; 95% CI 0.1 to 0.3). Otherwise, there were no statistically significant differences in the characteristics of respondents and their households or in outcome measures between the intervention and control groups at baseline”. Analyses accounted for clustering |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: Not reported |
|
| Participants |
Description: Children aged 4 or 5 years at 17 childcare centres N (Randomised): 17 childcare centres, 263 children Age: “The researchers were not permitted to obtain specific ages of each child but were informed by the centers’ directors that the majority of the children were 4 or 5 years old.” % Female: 47% SES and ethnicity: Not specified Inclusion/exclusion criteria: Not specified Recruitment: Not specified Recruitment rate: Unknown Region: Boise Idaho (USA) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention: fruit = 83, vegetable = 70 Control: fruit = 70, vegetable = 52 Description of intervention: “Color Me Healthy comes in a ‘‘toolkit’’ that includes a teacher’s guide, 4 sets of picture cards, classroom posters, a music CD that contains 7 original songs, a hand stamp, and reproducible parent newsletters. Color Me Healthy is composed of 12 circle‐time lessons and 6 imaginary trips. The majority of the CMH circle‐time lessons focus on fruits and vegetables of different colors. Several of the lessons provide opportunities for children to try fruits and vegetables. The 6 imaginary trips included in CMH encourage children to use their imagination to explore places, be physically active, and eat fruits and vegetables. Six interactive take home activities were developed for the current evaluation. These interactive activities coincided with the circle‐time lessons.” Duration: 6 weeks Number of contacts: 24 (preschool = 2 circle‐time + 1 imaginary trip per week, each 15 ‐ 30 minutes, home = 6 interactive take home activities) Setting: Preschool + home Modality: Face‐to‐face Interventionist: Lead teachers Integrity: No information provided Date of study: Unknown Description of control: No treatment control: “During the study, comparison classrooms did not incorporate nutrition curriculum into their lesson plans.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetable snacks (grams). “To determine the amount of fruit and vegetable snack consumed, the fruit and vegetable snacks were weighed (in grams) before they were served to children and then weighed again after children had had an opportunity to consume the snack. Percentage of fruit and vegetable snack consumed was calculated for each child.” Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 7 weeks (1 week post‐intervention) and ˜ 5 months (3 months post‐intervention) Length of follow‐up post‐intervention: 1 week and 3 months Subgroup analyses: None Loss to follow‐up (at 3 months): Intervention: fruit = 50%, vegetable = 58% Control: fruit = 29%, vegetable = 47% Analysis: Adjusted for clustering Unknown sample size calculations performed |
|
| Notes | First reported outcome (mean number of pineapple
snacks remaining) at the longest follow‐up (3
month follow‐up) was extracted for inclusion
in meta‐analysis. Insufficient data available
to enable inclusion in meta‐analysis
(standard deviation not reported, nor available from
authors) Sensitivity analysis ‐ primary outcome: Primary outcome not stated, fruit or vegetable intake is only reported outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated to experimental group but the random sequence generation procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Fruit and vegetable snacks (weighed): Objective measure of child’s fruit and vegetable intake and unlikely to be influenced by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Fruit and vegetable snacks (weighed): Objective measure of child’s fruit and vegetable intake and unlikely to be influenced by detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate > 20% for short‐term follow‐up. Only 58% of consenting children received fruit snacks at all 3 time points |
| Selective reporting (reporting bias) | Unclear risk | There is no study protocol therefore it is unclear if there was selective outcome reporting |
| Other bias | Unclear risk | Recruitment bias: it appears that parents were
invited to participate after centres had been
randomised, so unclear risk of bias Baseline imbalance: there are no baseline data comparing study groups, so we cannot tell if groups were balanced at baseline, so unclear risk of bias Incorrect analysis: “The current evaluation was a nested design; children were nested within classrooms. The classrooms were the units of assignment, but the outcome data were collected among the children.” HLM modelling accounted for clustering, therefore low risk of bias |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: "The trial is funded by the Cancer Institute New South Wales (Ref no. 08/ECF/1‐18)." |
|
| Participants |
Description: Children aged 3 to 5 years attending selected preschools, and their parent N (Randomised): 30 preschools, 394 parent‐child dyads Age: Child (mean): Intervention = 4.3 years, Control = 4.3 years Parent (mean): Intervention = 35.7 years, Control = 35.7 years % Female: Child: Intervention = 51%, Control = 46% Parent: Intervention = 95%, Control = 97% SES and ethnicity: Household income AUD ≥ 100K: Intervention = 42%, Control = 40% University education: Intervention = 45%, Control = 50% Aboriginal and/or Torres Strait Islander: Child: Intervention = 1%, Control = 5% Parent: Intervention = 1%, Control = 3% Inclusion/exclusion criteria: Preschool: Inclusion criteria: licensed in NSW Exclusion criteria: “Preschools will be excluded from the trial if they provide meals to children in their care (as this limits parents' capacity to influence the foods their children consume), cater exclusively for children with special needs (given the specialist care required for such children), are Government preschools (as conduct of the research has not been approved by the New South Wales Government Department of Education and Training) or have participated child healthy eating research projects within six months of the commencement of recruitment.” Parent: Inclusion criteria: “participant must be a parent of a child aged 3 to 5 years attending a participating preschool, must reside with that child for at least four days a week (in order for the child to be sufficiently exposed to the intervention strategies that the parent may implement), must have some responsibility for providing meals and snacks to that child, and must be able to understand spoken and written English.” Exclusion criteria: “Parents will be excluded from the trial if their children have special dietary requirements or allergies that would necessitate specialised tailoring of the intervention or that may be adversely affected by the intervention. Such exclusions will be determined by an Accredited Practising Dietitian who is independent of the research team.” Recruitment: Preschools randomly selected “The supervisors of the selected preschools will be sent letters and consent forms informing them of the study and requesting permission to recruit parents through their services.” Recruitment packs will be delivered to each participating preschool Distribution of these packs to parents will occur via methods considered by the preschool supervisor to be most effective and appropriate in engaging parents Where possible, research staff will attend the preschool, hand out recruitment packs to parents and be available to answer parent questions Recruitment rate: Preschool = 51% (30/59) Region: New South Wales (Australia) |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention = 174, Control = 169 Description of intervention: The intervention group will receive a resource kit and weekly scripted telephone contacts. “The kit comprises a participant workbook containing information and activities, a pad of meal planners, and a cookbook including recipes high in fruit and vegetables.” “Each telephone contact aims to provide parents with appropriate knowledge and skills to modify three key domains within the home food environment: availability and accessibility of fruit and vegetables; supportive family eating routines, and parental role‐modelling.” Duration: 4 weeks Number of contacts: 4 (one a week) Setting: Home Modality: Telephone and mailed resources Interventionist: Trained telephone interviewers Integrity: “During each four‐week batch of telephone calls, members of the research team will monitor at least two completed calls made by each interviewer to assess adherence with the intervention protocol.” “In total, 44 intervention calls were monitored, representing 6% of all completed calls and an average of 9 calls per interventionist. Across all monitored calls, interventionists covered 97% of key content areas, and in .80% of calls they “rarely” deviated from the script. In instances in which calls deviated from the script, interventionists were provided with feedback immediately after the call, and the issue was raised during biweekly supervision.” Date of study: April to December 2010 Description of control: “Parents allocated to the control group were mailed the Australian Guide to Healthy Eating—a 22‐page booklet outlining the dietary guidelines and ways to meet them.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Child’s consumption of fruit and vegetables assessed by parent self‐report by telephone survey using items from the Children’s Dietary Questionnaire. Outcome relating to absolute costs/cost effectiveness of interventions: Not reported Outcome relating to reported adverse events: Effect of intervention on family food expenditure Length of follow‐up from baseline: 2 and 6 months Length of follow‐up post‐intervention: 1 and 5 months Subgroup analyses: None Loss to follow‐up (at 1 and 5 months): Intervention = 14%, 16% Control = 4%, 9% Analysis: Adjusted for clustering Sample size calculations performed |
|
| Notes | The fruit and vegetable score outcome at the longest
follow‐up < 12 months (6 months) was
extracted for inclusion in meta‐analysis. The
reported estimate and 95% CI which adjusted for
baseline and clustering were included in
meta‐analysis Sensitivity analysis ‐ primary outcome: Fruit or vegetable intake listed as primary outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated using a random‐number function in Microsoft Excel |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Fruit and vegetable intake
(self‐reported): Participants were unblinded and this is likely to influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fruit and vegetable intake
(self‐reported): Participants were unblinded and because self‐reported measure this is likely to influence detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 394 parents, 343 (87%) completed the 6‐month follow‐up. Sensitivity analyses were also conducted where missing follow‐up data were imputed by using baseline observation carried forward |
| Selective reporting (reporting bias) | Low risk | The primary outcomes reported in the outcomes paper align with those specified in the protocol. The 12‐ and 18‐month fruit and vegetable outcomes are reported in Wolfenden 2014 |
| Other bias | Low risk | Contamination, baseline imbalance, & other bias that could threaten the internal validity are unlikely to be an issue. Analyses adjusted for clustering |
| Methods |
Study design: Cluster‐randomised controlled trial Funding: “This project received financial support from the Fresh Produce Centre and the Ministry of Economic Affairs (grant number TU 1310‐086). Neither organization had any role in the design, analyses, or writing of this article.” |
|
| Participants |
Description: Infants aged 0‐4 years in 4 childcare centres in Utrecht, Netherlands N (Randomised): 4 childcare centres Age: Mean: intervention = 25.6 months, control = 25.0 months % Female: Intervention = 44%, control = 42% SES and ethnicity: Parent education level* ‐ intervention: low (0%), middle (5%), high (95%), control: low (0%), middle (10%), high (90%) *low = primary and/or secondary school, middle = vocational education, high = higher vocational education and/or university degree Inclusion/exclusion criteria: “Healthy children without any allergies to the study products could participate.” Recruitment: Recruited via 4 childcare centres in Utrecht, Netherlands “Information packs were distributed to 526 parents to inform them about the study aims and procedures.” Recruitment rate: Unknown Region: The Netherlands |
|
| Interventions |
Number of experimental conditions: 2 Number of participants (analysed): Intervention (2 centres): 101 children Control (2 centres): 91 children Description of intervention: “To prevent boredom and encourage tasting, each vegetable was presented in two different preparations: pumpkin blanched and as a cracker spread; courgette blanched and as soup; white radish raw and as a cracker spread.” “The study vegetables were offered during the habitual vegetable snack moment in the afternoon, between 15h00 and16h00.” “A vegetable song ‐ developed specifically for this study was played to make the vegetable eating occasion recognizable and fun for the children.” Duration: 21 weeks Number of contacts: Unclear, 21 weeks “was chosen to ensure that each child was exposed to each vegetable at least 10 times” Setting: Preschool Modality: Face‐to‐face Interventionist: Childcare employees Integrity: “Intervention children received on average six exposures to each vegetable product” Date of study: Unknown Description of control: “The control group kept their regular eating routines during this period.” |
|
| Outcomes |
Outcome relating to children's fruit and
vegetable consumption: Consumption of vegetables (grams) as desired, assessed by weighing the vegetable cups before and after consumption. “Vegetable intake was calculated by subtracting the leftovers from the pre‐weight.” Outcome relating to absolute costs/cost‐effectiveness of interventions: Not reported Outcome relating to reported adverse events: Not reported Length of follow‐up from baseline: 21 weeks Length of follow‐up post‐intervention: 4 weeks Subgroup analyses: None Loss to follow‐up: Unclear Analysis: Unclear if adjusted for clustering Sample size calculations performed |
|
| Notes | We extracted first reported outcome (mean g of
pumpkin intake) for inclusion in
meta‐analysis. We estimated mean and SD from a study figure using an online resource (Plot Digitizer: plotdigitizer.sourceforge.net) for intervention and control groups at post‐test. As an estimate at that adjusted for clustering was not reported, we used post‐intervention data and calculated an effective sample size using ICC of 0.014 to enable inclusion in meta‐analysis. Sensitivity analysis ‐ primary outcome: primary outcome not stated, sample size was based on vegetable intake outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Two childcare centres were
randomly assigned to the intervention
condition” Randomly allocated to experimental group but the random sequence generation procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | There is no information provided about allocation concealment and therefore it is unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents and child care staff were blinded to the aims
of the study. The likelihood of performance bias in relation to vegetable consumption is low, given the children’s age. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants present to observe process of
weighing food and eating – however this seems
unlikely to impact child consumption. Vegetable cups were weighed before and after consumption and therefore low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate > 20% (see Table 3) |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol is not available |
| Other bias | Unclear risk | There may be potential recruitment bias as
intervention and control parents were told different
aims of the study (pg 318), which meant that
researchers were aware of study group allocation
before recruiting parents to study. Also unclear if clustering was accounted for during the analysis |
BMI: body mass index EA: exposure alone EP: exposure plus praise ETR: exposure plus tangible non‐food reward DOB: date of birth FV: fruit and vegetables ICC: intra‐class correlation N/A: not applicable PA: physical activity SD: standard deviation SEM: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboud 2008 | This responsive feeding trial was ineligible as its primary outcome was not to increase fruit and vegetable consumption and the study only assessed children's fruit and vegetable consumption post‐hoc in order to describe the mechanism behind a change in weight status among participants in the sample |
| Adams 2011 | No fruit or vegetable intake outcome |
| Adams 2015 | Not RCT: editorial |
| Agrawal 2012 | No fruit or vegetable intake outcome |
| Ahearn 2001 | Not RCT |
| Ahern 2014 | Not RCT |
| Ajie 2016 | Study design: not RCT |
| Al Bashabsheh 2016 | No fruit or vegetable intake outcome |
| Alford 1971 | Children aged 6‐17 years |
| Amin 2016 | Participants were Grade 3‐5 children |
| Anderson 2014 | Mean age of children 5.3 years |
| Anez 2013 | Participant mean age 5.01 years |
| Ang 2016 | Participants were 2nd and 3rd grade children |
| Anliker 1993 | Children aged 14‐17 years |
| Anonymous 2001 | Not RCT: Editorial |
| Anonymous 2002 | Not RCT: editorial |
| Anonymous 2009 | Not RCT: editorial |
| Anonymous 2011a | Not RCT: editorial |
| Anonymous 2011b | Children aged 5‐9 years |
| Anonymous 2012 | Participants were 4th grade children |
| Apatu 2016 | Participants were adult, no participants aged 0‐5 years |
| Aranceta‐Bartrina 2016 | Not RCT |
| Arrow 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was dental caries incidence and prevalence of obesity |
| Au 2015a | No fruit or vegetable intake outcome, only assessed intake of fruit juice |
| Au 2015b | No fruit or vegetable intake outcome |
| Au 2016 | Mean age of participants was 9.8 years |
| Bai 2012 | Participants were elementary school children |
| Bannon 2006 | Outcome is food choice (apple or crackers) |
| Bante 2008 | Not RCT |
| Baranowski 2002 | Children aged 9‐18 years |
| Barkin 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was weight and BMI |
| Baxter 1998 | Not RCT: Editorial |
| Bayer 2009 | Child mean age 6 years |
| Beasley 2012 | Children aged 8‐12 years |
| Beets 2016 | Participants were aged 6‐12 years |
| Bellows 2013 | Intervention was not designed to increase fruit and/or vegetable consumption, intervention aimed to explore individual, family and environmental factors and their relationship to child weight status |
| Benjamin 2008 | Outcome is quality of meals |
| Benjamin Neelon 2016 | No fruit or vegetable intake outcome, only amount served |
| Bensley 2011 | Quasi‐experiemental design |
| Bere 2015 | Participants were 6th and 7th grade children |
| Berg 2016 | Not RCT: book review |
| Bergman 2016 | Participants were 3rd, 4th and 5th grade children |
| Berhe 1997 | No comparison group |
| Berry 2013 | No fruit or vegetable intake outcome |
| Bessems 2012 | Children aged 12‐14 years |
| Best 2016 | Children aged 7‐12 years |
| Bibiloni 2017 | Study design: allocation to conditions not random |
| Birch 1980 | Not randomised |
| Birch 1982 | No control group |
| Birch 1998 | Not RCT |
| Black 2013 | Child mean age of subgroups ranged from 5.8‐11 years |
| Blissett 2012 | No comparison group |
| Blom‐Hoffman 2008 | Child mean age 6.2 years |
| Boaz 1998 | Children aged 7‐9 years |
| Bollella 1999 | Outcome is vitamins and minerals, not fruit and vegetable consumption |
| Bonvecchio‐Arenas 2010 | Participants were primary school children |
| Borys 2016 | Participants were aged 6‐8 years |
| Bouhlal 2014 | Allocation of groups to condition was not randomised |
| Bradley 2014 | No fruit or vegetable intake outcome, outcome is preference |
| Brambilla 2010 | No fruit and vegetable consumption outcome |
| Branscum 2013 | Children aged 8‐11 years |
| Briefel 2006 | No comparison group |
| Briefel 2009 | Children aged 6‐18 years |
| Briefel 2010 | No comparison group |
| Briley 1999 | No comparison group |
| Briley 2011 | Not RCT: Editorial |
| Briley 2016 | Primary outcome was not fruit or vegetable intake; primary outcome was observed servings in packed lunch |
| Britt‐Rankin 2016 | Not RCT ‐ review of resource |
| Brotman 2012 | No fruit and vegetable consumption outcome |
| Bruening 1999 | Non‐equivalent control group design |
| Brunt 2012 | Participants were 4th grade school children |
| Bryant 2017 | Primary outcome not F&V consumption, primary outcomes was parent engagement |
| Burgermaster 2017 | Participants were 5th grade students |
| Burgi 2011 | Child mean age 5.2 years |
| Buttriss 2004 | Not RCT: descriptive review |
| Byrd‐Bredbenner 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI and audits of home environment characteristics/lifestyle practice |
| Byrne 2002 | Outcome was willingness to taste kohlrabi |
| Camelo 2016 | Participants were children aged 6‐13 years |
| Campbell 2016a | Primary outcome was not fruit or vegetable intake; primary outcome was body weight and waist circumference |
| Campbell 2016b | Primary outcomes were length for age score and rates of stunting |
| Campbell 2017 | No fruit and vegetable consumption outcome reported |
| Candido 2013 | No fruit or vegetable intake outcome |
| Capaldi‐Phillips 2014 | Allocation of groups to condition was not randomised |
| Carney 2017 | Not RCT |
| Carter 2005 | Children aged 9‐12 years |
| Cason 2001 | No comparison group |
| Castro 2013 | Child mean age 6 years |
| Cates 2014 | Not RCT |
| Caton 2014 | Study design: results are not reported by study group. Additionally the paper reports data from 3 other included studies: Caton 2013; Hausner 2012; Remy 2013 |
| Chatham 2016 | Participants mean age 6.15 years |
| Chen 2015 | Participants were aged 5‐8 years old |
| Ciampolini 1991 | No comparison group |
| Clason 2016 | No fruit or vegetable intake outcome, only number of days per week child consumes |
| Coelho 2012 | Children aged 8‐12 years |
| Cohen 2014 | Child mean age 8.6 years |
| Coleman 2005 | No fruit and vegetable outcomes |
| Collins 2011 | Child mean age 8 years |
| Condrasky 2006 | Quasi‐experimental: intervention sample randomly selected from 1 church. Control randomly selected from a separate church |
| Cooper 2011 | Children aged 5‐11 years |
| Cooperberg 2014 | No fruit or vegetable intake outcome |
| Copeland 2010 | Child mean age 9 years |
| Coppinger 2016 | Children aged 5‐11 years |
| Corsini 2013 | Participants were children with mean age 5.16 years |
| Cotwright 2015 | No comparison group |
| Cotwright 2017 | No comparison group ‐ pretest‐post‐test design |
| Coulthard 2018 | No fruit and vegetable consumption outcome |
| Court 1977 | No participants, these are guidelines, not research trial |
| Crespo 2012 | Child mean age 5.9 years |
| Croker 2012 | Child mean age 8.3 years |
| Cruz 2014 | As per trial registry, fruit and vegetable consumption was not the primary outcome |
| Cullen 2013 | Participants were kindergarten‐grade 5 and grade 6‐8 children |
| Cullen 2015 | Participants were kindergarten‐grade 5 |
| Curtis 2012 | No child fruit or vegetable intake outcome |
| Céspedes 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was knowledge, attitudes and physical activity habits |
| Dai 2015 | Child mean age 6 years |
| Dalton 2011 | No child fruit or vegetable intake outcome |
| Daniels 2012 | Related to Daniels 2014 ‐ No fruit and vegetable consumption outcome |
| Dannefer 2017 | Not RCT |
| Davis 2013 | Primary outcome was not fruit or vegetable intake as per trial registry |
| Davoli 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Day 2008 | Child mean age 9‐10 years |
| Dazeley 2015 | No fruit or vegetable intake outcome, only assessed foods touched and tasted |
| De Bourdeaudhuij 2015 | Child mean age in intervention group 6.05 year and in control group 5.98 years |
| De Droog 2011 | No fruit or vegetable intake outcome, only assessed liking and purchase request intent |
| De Droog 2012 | No fruit and vegetable consumption outcome |
| De Pee 1998 | No comparison group |
| De Silva‐Sanigorski 2010 | Quasi‐experimental, repeat cross‐sectional design |
| Delgado 2014 | Intervention was not designed to increase fruit and/or vegetable consumption |
| Dick 2016 | Not RCT: Editorial |
| Dollahite 2014 | No child fruit or vegetable intake outcome |
| Dorado 2015 | Children aged 9‐10 years |
| Draper 2010 | Participants were 4, 5 and 6 grade children |
| Duke 2011 | Not RCT: descriptive review |
| Duncanson 2017 | Related to Duncanson 2013 ‐ does not report RCT results |
| Dunn 2004 | No fruit and vegetable consumption outcome |
| Eicholzer‐Helbling 1986 | Outcome no consumption measure |
| Elder 2014 | Child mean age 6.6 years |
| Elizondo‐Montemayor 2014 | Children aged 6‐12 years |
| Epstein 2001 | Children aged 6‐11 years |
| Esfarjani 2013 | Children aged 7 years |
| Esquivel 2016 | Not RCT |
| Estabrooks 2009 | Children aged 8‐12 years |
| Evans 2006 | Children in 4th, 5th grade school |
| Evans 2011 | No child fruit or vegetable intake outcome |
| Evans 2016 | Participants were 3rd grade children |
| Evenson 2016 | No fruit and vegetable consumption outcome |
| Faber 2002 | Cross‐sectional survey |
| Faith 2006 | The intervention programme was not specifically designed to increase consumption of fruit and vegetables; instead primary aim is to illustrate a methodological concept. “This methodological note illustrates the use of co‐twin design for testing substitution, phenomenon, a prominent behavioural economics concept. We test whether fruits and vegetables can substitute for high‐fat snack foods in young children in a single meal laboratory setting.” |
| Fangupo 2015 | Primary outcome as reported in trial registry was not fruit or vegetable intake |
| Fernandes 2011 | Not RCT: measurement tool |
| Fernández‐Alvira 2013 | Child mean age 11 years |
| Fialkowski 2013 | Intervention was not designed to increase fruit and/or vegetable consumption |
| Fisher 2007 | No fruit and vegetable consumption outcome |
| Fisher 2013 | No fruit and vegetable consumption outcome |
| Fisher 2014 | No child fruit or vegetable intake outcome |
| Fishman 2016 | Not RCT: Editorial |
| Fitzgibbon 2002 | Outcome is weight change |
| Fitzpatrick 1997 | Not RCT |
| Fletcher 2009 | Children aged 13‐19 years |
| Foerster 1998 | Children in 4th, 5th grade school |
| Folta 2006 | Children in grades 1‐3 school |
| Fournet 2014 | Children aged 6‐13 years |
| Freedman 2010 | Outcome is child feeding attitudes and practices |
| French 2012 | Intervention was not designed to increase fruit and/or vegetable consumption |
| Frenn 2013 | Participants were 5th, 7th and 8th grade students |
| Friedl 2014 | Not RCT: task force report |
| Friend 2015a | Participants were parents of 8‐12 year‐old children |
| Friend 2015b | No fruit and vegetable consumption outcome reported |
| Gaglianone 2006 | Participants were 1st and 2nd grade children |
| Gallo 2017 | Participants were aged 6‐11 years |
| Gallotta 2016 | Children aged 8‐11 years |
| Garcia‐Lascurain 2006 | Participants were aged 9‐12 years |
| Gardiner 2017 | Participants were at least 18 years of age |
| Gaughan 2016 | No comparison group |
| Gelli 2016 | Child mean age 7.5 years |
| Gentile 2009 | Children in 3rd, 4th, 5th grade school |
| Gittelsohn 2010 | Children aged 8‐12 years |
| Glanz 2012 | No child fruit or vegetable intake outcome |
| Glasper 2011 | Not RCT: Editorial |
| Glasson 2012 | Participants were parents of primary school‐aged children |
| Glasson 2013 | Not RCT |
| Golley 2012 | Child mean age 8.3 years |
| Gordon 2016 | Fruit and vegetable intake not primary outcome as per contact with author very low food security is primary outcome |
| Gorham 2015 | No comparison group |
| Gosliner 2010 | Quasi‐experimental: childcare centres in existing study matched to other childcare centres, then randomised |
| Goto 2012 | No child fruit or vegetable intake outcome |
| Gottesman 2003 | No participants, not research trial |
| Graham 2008 | Outcome not fruit and vegetable consumption |
| Gratton 2007 | Children aged 11‐16 years |
| Gregori 2014 | No comparison group |
| Gripshover 2013 | Intervention was not designed to increase fruit and/or vegetable consumption |
| Gross 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was obesity |
| Guenther 2014 | No participants aged 0‐5 years |
| Guldan 2000 | Not RCT |
| Guo 2015 | Participants were 3rd to 5th grade students |
| Haines 2016 | No child fruit or vegetable intake outcome |
| Hambleton 2004 | Children aged 9‐10 years |
| Hammersley 2017 | Primary outcome not fruit and vegetable intake, primary outcome is BMI |
| Hammons 2013 | Children aged 5‐13 years |
| Hancocks 2011 | Not RCT: Editorial |
| Hanks 2016 | No fruit and vegetable consumption outcome |
| Hansen 2016 | Participants were children aged 6‐14 years |
| Hanson 2017 | Not a randomised study design |
| Hardy 2010a | No fruit or vegetable intake outcome, only assessed lunchbox contents |
| Hardy 2010b | No child fruit or vegetable intake outcome |
| Hare 2012 | Child mean age 6.3 years |
| Haroun 2011 | Participants were primary school children ‐ aged 4‐12 years old |
| Harris 2011 | Children aged 5‐12 years |
| Hart 2016 | No child fruit or vegetable intake outcome |
| Harvey‐Berino 2003 | No fruit and vegetable consumption outcome |
| Havas 1997 | No assessments of children included in study |
| Havermans 2007 | Participants had mean age of 5.2 years |
| Heath 2010 | No fruit and vegetable consumption outcome |
| Heim 2009 | Children in 4th and 6th grade school |
| Helland 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was food neophobia and staff feeding practices |
| Helland 2016 | Primary outcome was not fruit or vegetable intake; primary outcome was food neophobia and staff feeding practices |
| Helland 2017 | No comparison group |
| Hendy 2002 | No comparison group |
| Hendy 2011 | Participants were 1st, 2nd and 4th grade children |
| Herbold 2001 | Participants were 1st and 6th grade children |
| Herring 2016 | Not RCT: Editorial |
| Hildebrand 2010 | No comparison group |
| Hoddinott 2017 | Primary outcome not fruit and vegetable intake as per trial registry |
| Hoffman 2011 | Child mean age 6.2 years |
| Hoffman 2015 | Participants were 6th‐12th grade children |
| Hohman 2017 | F&V intake not primary outcome as per trial registry BMI is primary outcome |
| Hollar 2013 | Participants were Kindergarten‐5th Grade children |
| Holley 2015 | Not RCT ‐ allocation was not randomised |
| Hooft 2013 | No child fruit or vegetable intake outcome |
| Horne 2009 | Child mean age 7 years |
| Horodynski 2004 | Non‐equivalent control group study design |
| Hotz 2012a | Intervention was not designed to increase fruit and/or vegetable consumption, intervention aimed to increase the consumption of orange sweet potato over consumption of white and yellow sweet potato |
| Hotz 2012b | Intervention was not designed to increase fruit and/or vegetable consumption, intervention aimed to increase the consumption of orange sweet potato over consumption of white and yellow sweet potato |
| Howarth 2011 | No comparison group |
| Hu 2010 | Outcome is eating behaviours and weight, not fruit and vegetables |
| Hughes 2007 | Outcome is feeding styles and behaviour |
| Hughes 2016 | No fruit and vegetable consumption outcome |
| Iaia 2017 | Fruit and vegetable intake not primary outcome, primary outcome combined health behaviour score |
| IFIC 2002 | Children aged 9‐12 years |
| Israelashvili 2005 | No fruit and vegetable consumption outcome |
| Issanchou 2017 | Not RCT |
| Izumi 2013 | No child fruit or vegetable intake outcome |
| James 1992 | No comparison group |
| Jancey 2014 | No child fruit or vegetable intake outcome |
| Janicke 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Jansen 2010 | Participants were children with mean age 5.8 years |
| Jansen 2017 | Fruit and vegetable intake not primary outcome |
| Jayne 2009 | Outcome is food choice |
| Johnson 1993 | This study was excluded as fruit and vegetable consumption was measured in terms of dietitian‐classified 'appropriate' versus 'inappropriate' consumption levels, and as such, it failed to meet the inclusion criteria relating to the primary outcome |
| Johnson 2007 | Outcome is food preference and ranking |
| Jordan 2010 | No child fruit or vegetable intake outcome |
| Joseph 2015a | No child fruit or vegetable intake outcome |
| Joseph 2015b | No comparison group |
| Just 2013 | Participants were elementary school children |
| Kabahenda 2011 | No child fruit or vegetable intake outcome |
| Kain 2012 | Participants aged 6‐12 years |
| Kalb 2005 | No participants, not research trial |
| Kang 2017 | Fruit and vegetable intake not primary outcome |
| Kannan 2016 | Not RCT |
| Karanja 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Kashani 1991 | Child mean age 10 years |
| Kaufman‐Shriqui 2016 | Participants mean age 5.28 years |
| Kelder 1995 | Children in 6th grade school |
| Keller 2014 | Not RCT: Editorial |
| Kennedy 2011 | Participants were adults |
| Kessler 2016 | Not RCT: review |
| Khoshnevisan 2004 | Dietary outcomes are not reported for the control group and no comparison is made between experimental conditions |
| Kidala 2000 | Quasi‐experimental: 2 areas, 1 intervention, 1 control, not randomly selected |
| Kilaru 2005 | Outcome is proportion being fed bananas |
| Kilicarslan 2010 | Child mean age 9.3 years |
| Kimani‐Murage 2013 | Primary outcome was exclusive breastfeeding |
| Kipping 2014 | Participants aged 8‐9 years |
| Kipping 2016 | Primary outcome was not fruit or vegetable intake |
| Knoblock‐Hahn 2016 | No fruit and vegetable consumption outcome |
| Knowlden 2015 | Child mean age 5.18 years |
| Ko Linda 2016 | No participants aged < 5 |
| Koehler 2007 | No fruit and vegetable consumption outcome |
| Koff 2011 | No comparison group |
| Kolodinsky 2017 | No outcome data reported ‐ related to ongoing study Seguin 2017 |
| Korwanich 2008 | Quasi‐experimental: 8 intervention schools; 8 matched control schools |
| Kotler 2012 | No fruit or vegetable intake outcome, only number of pieces of food consumed |
| Kotz 2010 | Not RCT: Editorial |
| Kral 2010 | Participants were children with mean age 5.9 years |
| Lanigan 2010 | Not RCT: review |
| Laramy 2017 | No comparison group |
| LaRowe 2010 | No comparison group |
| Larson 2011 | No child fruit or vegetable intake outcome |
| Laureati 2014 | Child mean age 7.9 years |
| Leahy 2008a | No fruit and vegetable outcome |
| Leahy 2008b | No fruit and vegetable consumption outcome |
| Leahy 2008c | Fruit and vegetable consumption was secondary outcome |
| Ledoux 2017 | No comparison group ‐ pretest‐posttest design |
| Leme 2015 | Participants were adolescents |
| Lin 2017 | No fruit and vegetable outcome |
| Ling 2016a | No child fruit or vegetable intake outcome |
| Ling 2016b | Not RCT |
| Lioret 2012 | No fruit and vegetable consumption outcome |
| Lioret 2015 | Not RCT |
| Llargues 2011 | Child mean age 6 years |
| Lloyd 2011 | Participants were fathers of children aged 5‐12 years |
| Locard 1987 | No comparison group |
| Lohse 2017 | Not RCT ‐ Editorial |
| Longacre 2015 | No child fruit or vegetable intake outcome |
| Longley 2013 | Not RCT: Editorial |
| Low 2007 | Quasi‐experimental, 2 intervention areas, and 1 control area selected, in prospective longitudinal study |
| Luepker 1996 | Child mean age 8.8 years |
| Lumeng 2012 | Intervention was not designed to increase fruit and/or vegetable consumption, intervention aimed to improve children's emotional and behavioural self regulation on preventing obesity |
| Maier 2007 | Not RCT ‐ treatment group not randomised |
| Maier 2008 | Not RCT |
| Maier‐Noth 2016 | Not RCT |
| Maier‐Noth 2017 | Not RCT |
| Malekafzali 2000 | No fruit and vegetable consumption data |
| Mallan 2017 | Related to Daniels 2014 ‐ only reports data from the control group |
| Manger 2012 | Child mean age 5.7 years |
| Manios 1999 | Not RCT |
| Manios 2009 | No comparison group |
| Mann 2015 | No outcome data ‐ related to ongoing study Østbye 2015 |
| Mann 2017 | Not RCT ‐ resource review |
| Markert 2014 | Child mean age 9 years |
| Marquard 2011 | No child fruit or vegetable intake outcome |
| Martens 2008 | Children aged 12‐14 years |
| Mathias 2012 | Participants were children with mean age 5.4 years |
| Mbogori 2016 | No comparison group |
| McGowan 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was parental habit strength |
| McKenzie 1996 | Child mean age 6.3‐6.8 years |
| McSweeney 2017 | F&V not primary outcome, primary outcomes were related to feasibility |
| Mehta 2014 | No comparison group |
| Meinen 2012 | Child mean age 9.9 years |
| Mennella 2017a | No fruit and vegetable consumption outcome |
| Metcalfe 2016 | Participants were children aged 8‐13 years |
| Metcalfe 2017 | Participants aged 8‐14 years |
| Mok 2017 | Fruit and vegetable not primary outcome, primary outcome Vitamin D plasma concentrations |
| Molitor 2016 | No comparison group ‐ cross‐sectional study |
| Monterrosa 2013 | Not RCT ‐ quasi‐experimental |
| Morgan 2016 | Not RCT |
| Morgan 2017 | Participants were aged 5‐12 years old |
| Morrill 2016 | Participants were Grade 1‐5 students |
| Murimi 2017 | No fruit and vegetable outcome |
| Nabors 2015 | Participants mean age 6.12 years |
| Nansel 2016 | Participants aged 8.0‐16.9 years |
| NAPNAP 2006 | Guidelines not trial, so no participants |
| Natale 2014 | Primary outcome was not fruit or vegetable intake as per trial registry |
| Nederkoorn 2018 | Mean age of participants 5.85 years |
| Nemet 2007 | Child mean age 5.5 years |
| Nemet 2008 | Children aged 8‐11 years |
| Nemet 2011 | No fruit and vegetable consumption outcome |
| Nerud 2017 | No fruit and vegetable intake outcome |
| Nicklas 2011 | Not fruit and vegetable intake outcome reported, only preference. |
| Noller 2006 | No child fruit or vegetable intake outcome |
| Novotny 2011 | Not RCT |
| Nunes 2017 | Primary outcome is frequency of exclusive and total breastfeeding as per trial registry |
| Nystrom 2017 | Fruit and vegetable not primary outcome, primary outcome was BMI |
| O'Connor 2010 | No comparison group |
| O'Sullivan 2017 | Fruit and vegetable not primary outcome ‐ primary outcomes relate‐school readiness, physical health etc |
| Ogle 2016 | Participants aged 6‐9 years |
| Olvera 2010 | Children aged 7‐13 years |
| Onnerfält 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Paineau 2010 | Participants were children in 2nd and 3rd grade |
| Panunzio 2007 | Children in 4th grade school |
| Parcel 1989 | Children in 3rd, 4th grade school |
| Passehl 2004 | Outcome is process evaluation |
| Peracchio 2016 | No fruit and vegetable consumption outcome |
| Perry 1985 | Children in 3rd, 4th grade school |
| Persson 2018 | Primary outcomes are children's body mass index and waist circumference at four years |
| Peters 2012a | No child fruit or vegetable intake outcome |
| Poelman 2016a | The average age was 5.1 years (SD 0.8, range 4‐6.8 years) |
| Poelman 2016b | The average age was 5.1 years (SD 0.8, range 4‐6.8 years) |
| Polacsek 2017 | No fruit and vegetable consumption outcome |
| Prelip 2011 | Participants were 3rd‐5th grade children |
| Presti 2015 | Participants aged 5‐11 years |
| Prosper 2009 | Child mean age 11.7 years |
| Puia 2017 | Participants aged 5‐15 years |
| Quandt 2013 | No child fruit or vegetable intake outcome |
| Quizan‐Plata 2012 | Participants were primary school children |
| Rackliffe 2016 | Not RCT ‐ resource review |
| Rahman 1994 | Outcome asks if vegetables eaten today (Yes/No). No amount provided |
| Ransley 2007 | Non‐RCT. 1 intervention sample and 1 matched control sample |
| Raynor 2012 | Child mean age 6.7 years |
| Reicks 2012 | Children aged 9‐12 years |
| Reifsnider 2012 | No child fruit or vegetable intake outcome |
| Reinaerts 2007 | Quasi‐experimental: consenting schools paired then randomised to 1 of 2 interventions. Control schools in different area identified and then matched |
| Reinbott 2016 | Primary aim (as per trial registry) is mean height for age z‐scores |
| Reinehr 2011 | Primary outcome was not fruit or vegetable intake, primary outcome was weight |
| Reverdy 2008 | Children aged 8‐10 years |
| Reynolds 1998 | Participants were 4th grade children |
| Reznar 2013 | No fruit or vegetable intake outcome, only assessed diet quality |
| Ribeiro 2014 | Children aged 6‐11 years |
| Rioux 2018 | No fruit and vegetable intake outcome |
| Ritchie 2010 | Children aged 9‐10 years |
| Rito 2013 | Child mean age 8.6 years |
| Robertson 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was waist circumference and self‐esteem |
| Roche 2016 | Not RCT ‐ quasi‐experimental non‐randomized study |
| Rogers 2013 | Child mean age 11 years |
| Rohde 2017 | As per trial registry, fruit and vegetable not primary outcome, anthropometry is primary outcome |
| Rohlfs 2013 | Not RCT |
| Romo‐Palafox 2017 | No comparison group |
| Rubenstein 2010 | No fruit or vegetable intake outcome, only assessed child‐feeding practices |
| Ruottinen 2008 | The intervention programme was not specifically
designed to increase consumption of fruit and
vegetables. The aim of intervention, as reported in a separate paper (Lapinleimu 1995) is“to investigate the effects of an individually supervised, eucaloric, diet with low content of fat, saturated fat and cholesterol in healthy children” |
| Salminen 2005 | Children aged 6‐17 years |
| Sanders 2014 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Sanigorski 2008 | Child mean age 8 years |
| Sanjur 1990 | No fruit and vegetable outcome |
| Sanna 2011 | Intervention was not designed to increase fruit and/or vegetable consumption, intervention focused on dietary fat quality |
| Savage 2010 | Comparison between treatment groups not reported for fruit and vegetable consumption |
| Scherr 2017 | Participants were 4th grade students |
| Schmied 2015 | Participants were parents of children with mean age of 10 years |
| Schumacher 2015 | Child participants had median age of 12.9 years |
| Schwartz 2007a | Study design used convenience sample |
| Schwartz 2007b | Quasi‐experimental ‐ 2 elementary schools randomly allocated to 1 intervention and 1 control |
| Schwartz 2015 | Not RCT |
| Sharafi 2016 | Intervention did not aim to increase consumption of fruit or vegetables |
| Sharma 2016 | Participants were 1st grade children |
| Sharps 2016 | Participants were children aged 6‐11 years |
| Sherwood 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Shilts 2014 | Not RCT as confirmed by author |
| Shim 2011 | No child fruit or vegetable intake outcome |
| Shin 2014 | Participants were 4th‐6th grade children |
| Siega‐Riz 2004 | No comparison group |
| Singh 2018 | Not RCT |
| Skouteris 2014 | No child fruit or vegetable intake outcome |
| Slusser 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Smith 2013 | No fruit and vegetable intake outcome |
| Smith 2015 | No comparison group |
| Sobko 2011 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Sobko 2017 | Not RCT |
| Sojkowski 2012 | No comparison group |
| Solomons 1999 | Review, not trial, no participants |
| Song 2016 | Participants were 4th and 5th grade students |
| Sotos‐Prieto 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was change in overall knowledge, attitudes and habits |
| Speirs 2013 | Participants were parents of elementary school children |
| Stark 1986 | No fruit and vegetable consumption outcome |
| Stark 2011 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Steenbock 2017 | Not RCT ‐ allocation not randomised |
| Stern 2018 | Participants were parents of children aged 5‐13 years |
| Story 2012 | Participants mean age 5.84 years |
| Suarez‐Balcazar 2014 | Participants were Kindergarten and 1st grade children |
| Sun 2017 | No fruit and vegetable intake outcome |
| Sweitzer 2010 | Primary outcome was not fruit or vegetable intake; primary outcome was observed servings in packed lunch |
| Tande 2013 | No comparison group |
| Taylor 2007 | Child mean age 7.7 years |
| Taylor 2010 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Taylor 2013a | Participants were primary school‐aged children 4‐11 years old |
| Taylor 2013b | No child fruit or vegetable intake outcome |
| Taylor 2013c | Primary outcome, as per trial registry, was not fruit or vegetable intake |
| Taylor 2015a | Not RCT: review |
| Taylor 2015b | Participants' mean age 6.5 years |
| Taylor 2016 | Fruit and vegetable intake not primary outcome, primary outcome was anthropometric measures as per trial registry |
| Te Velde 2008 | Children aged 10‐13 years |
| Tharrey 2017 | Primary outcome was not fruit and vegetable intake |
| Thomson 2014 | Fruit and vegetable intake not primary outcome, primary outcome was weight‐for‐length |
| Timms 2011 | Not RCT: Editorial |
| Tobey 2016 | Not RCT ‐ allocation not random |
| Tomayko 2016 | Fruit and vegetable intake not primary outcome, primary outcome was BMI |
| Tomayko 2017 | Not RCT ‐ allocation not random |
| Tovar 2017 | Not RCT ‐ uses baseline data from an ongoing study ‐ Østbye 2015 |
| Tran 2017 | Not RCT |
| Trees 2012 | No comparison group ‐ cross‐sectional survey |
| Tucker 2011 | Participants were 4th and 5th grade school children |
| Tyler 2016 | Participants were aged 8‐12 years |
| Uicab‐Pool 2009 | Outcome was eating habits |
| Upton 2013 | Participants were primary school children aged 4‐11 years |
| Upton 2014 | Not RCT |
| Urrutia 2017 | Not RCT |
| Utter 2017 | Not a RCT |
| Van Horn 2005 | Children aged 8‐10 years |
| Van Horn 2011 | Not RCT: Editorial |
| Van Nassau 2015 | Not RCT: commentary |
| Vandeweghe 2016 | No fruit and vegetable intake outcome |
| Vaughn 2017 | No fruit and vegetable outcome |
| Vecchiarelli 2005 | Children school‐aged |
| Veldhuis 2009 | Outcome was weight, not fruit and vegetable consumption |
| Viggiano 2012 | Children aged 9‐19 years |
| Vio 2014 | Not RCT |
| Vitolo 2010 | Primary outcome was not fruit or vegetable intake; primary outcome was Healthy Eating Index |
| Vitolo 2014 | Fruit and vegetable intake not primary outcome, as per trial registry primary outcome was exclusive breastfeeding |
| Wald 2017 | Participants had mean age of 5.5 years (intervention) or 5.4 years (control) |
| Walton 2015 | Primary outcome, as per trial registry, was not fruit or vegetable intake; primary outcome was BMI |
| Wansink 2013 | Participants were middle school children |
| Wansink 2014 | Participants were middle school children |
| Ward 2011 | Primary outcome was not fruit or vegetable intake; primary outcome was percent body fat |
| Ward 2017 | Primary outcome is change in centre's nutrition environments |
| Wardle 2003b | Child mean age 6 years |
| Warschburger 2018 | Participants were children aged 8‐16 years |
| Wells 2005 | Not RCT ‐ cross‐sectional |
| Wen 2007 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Wen 2011 | Primary outcome: duration of breastfeeding and timing of introduction of solids, as described in the published research protocol |
| Wen 2013 | Primary outcome was not fruit or vegetable intake; primary outcome was good eating behaviour |
| Wen 2017 | Fruit and vegetable intake was secondary outcome |
| Wengreen 2013 | Participants were elementary school children |
| Whaley 2010 | Study design in intervention and matched control site |
| Whiteside‐Mansell 2017 | No fruit and vegetable intake outcome |
| Wijesinha‐Bettoni 2013 | Children aged 6‐12 years |
| Williamson 2013 | Participants were primary school children |
| Wilson 2016 | No fruit and vegetable consumption outcome |
| Wyatt 2013 | Children aged 9‐10 years |
| Wyse 2014 | No child fruit or vegetable intake outcome |
| Yeh 2017 | No fruit and vegetable intake outcome |
| Yin 2012 | Intervention was not designed to increase fruit and/or vegetable consumption |
| Yoong 2017 | Fruit and vegetable intake was not primary outcome, primary outcome was children's service compliance with dietary guidelines |
| Young 2017 | No fruit and vegetable intake outcome |
| Zask 2012 | Primary outcome was not fruit or vegetable intake; primary outcome was BMI |
| Zeinstra 2010 | Participants were children with mean age 5.1‐5.2 years |
| Zhou 2016 | Participants were young adults |
| Zhou 2017 | Not RCT |
| Zota 2016 | Child mean age as reported by author 8.6 years |
| Zotor 2008 | Children aged 11‐15 years |
| Østbye 2012 | Primary outcome was not fruit or vegetable intake; primary outcome as per trial registry was BMI |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No full text available to determine eligibility. Contact with author reported chapter describing study currently underway |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full text only available in Persian. Translation has been sought. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Healthy start‐Départ santé |
| Methods | Cluster‐randomised controlled trial |
| Participants | Approximately 735 children aged 3‐5 years from 62 Early Childcare Centres |
| Interventions | Intervention: “The intervention
is composed of six interlinked components which are
presented in more detail in Fig. 1. These components
include: 1) intersectoral partnerships conducive to
participatory action that leads to promoting healthy
weights in communities and ECC; 2) the Healthy
Start‐Départ Santé
implementation manual for educators on how to
integrate healthy eating and physical activity in
their centre; 3) customized training, role modelling
and monitoring of Healthy Start‐Départ
Santé in ECC; 4) the evidence‐based
resource, LEAP‐GRANDIR [16], which contains
material for both families and educators; 5)
supplementary resources from governmental partners;
and 6) a knowledge development and exchange (KDE),
and communication strategy involving social media
and web‐resources to raise awareness and
mobilize grassroots organizations and
communities. Healthy Start‐Départ Santé is delivered over 6‐8 months and includes a partnership agreement, an initial training session which orients ECC staff to the concepts, the implementation manual and the use of resources, on‐going support and monitoring over time, one tailored booster session, and a family day to celebrate the ECC’ success at the end of the intervention.” Control: “Usual practice controls” “Control sites are given the option of receiving the intervention once their participation in the evaluation has been completed” |
| Outcomes | Usual intake of fruits and vegetables assessed via parent‐reported semi‐quantitative food frequency questionnaire |
| Starting date | Participant recruitment began in Autumn 2013 |
| Contact information | Anne Leis: Anne.Leis@usask.ca |
| Notes |
| Trial name or title | Simply Dinner Study |
| Methods | Multiphase Optimization Strategy (MOST), where the
main, additive and interactive effects of 6 support
strategies are first tested in a screening phase to
identify the intervention components most robustly
associated with increased family meals and
improvements in dietary quality. The MOST factorial design includes 6 intervention components with a Usual Head Start Exposure condition (usual‐care control); thus, individual participants are randomised to one of 64 experimental conditions. The 64 experimental conditions result from the crossing of 6 Simply Dinner intervention components, each of which has 2 conditions (present vs. not), and reflect all possible pairings of the intervention components, including a no‐intervention condition. These components are then tested in the confirming phase via RCT. |
| Participants | Families from Head Start preschools (disadvantaged families) |
| Interventions | 6 intervention components ranging from the most to
least intense forms of support
|
| Outcomes | Children’s diet quality over the previous week assessed using the Block Dietary Data Systems Kids Food Screener—Last Week (Version 2) |
| Starting date | Screening design: "planned completion is Dec
2017” Confirming RCT planned to commence in September 2018 |
| Contact information | Holly‐E Brophy‐Herb: hbrophy@hdfs.msu.edu |
| Notes | Clincaltrials.gov Identifier NCT02487251; Registered 26 June 2015 |
| Trial name or title | Early food for future health: a randomized controlled trial evaluating the effect of an eHealth intervention aiming to promote healthy food habits from early childhood |
| Methods | Randomised controlled trial of parents with children
aged between 3 and 5 months recruited through
Norwegian child health centres and announcements on
Facebook Baseline questionnaires assessed eating behaviour and feeding practices, food variety and diet quality. All participants will be followed up at ages 12 and possibly 24 and 48 months, with questionnaires relating to eating behaviour and feeding practices, food variety and diet quality. |
| Participants | Parents of children aged between 3 and 5 months |
| Interventions | The intervention group received monthly emails with links to an age‐appropriate website when their child was between 6 and 12 months |
| Outcomes | Eating behaviour and feeding practices, food variety and diet quality |
| Starting date | Participant recruitment began in March 2016 |
| Contact information | Christine Helle: christine.helle@uia.no |
| Notes | ISRCTN13601567 |
| Trial name or title | Healthy Me, Healthy We (HMHW) trial |
| Methods | 2‐arm, cluster‐randomised controlled trial, where childcare centres are randomly assigned to an intervention or waitlist control group |
| Participants | 96 childcare centres located in central North
Carolina (NC), USA Classroom teachers of children aged 3‐4 years 768 parents and children dyads (aged 3‐4 years) |
| Interventions | 8‐month social marketing campaign delivered
over the year targeting childcare teachers and
parents Childcare component involves a kick‐off event including hanging of study (HMHW) banner, inviting parents to attend, hanging of classroom poster, signing the Fit Family Promise and engaging in classroom activity. Kick‐off event followed by four 6‐week classroom units targeting healthy eating and physical activity goals through both classroom and home components. Home components include a family guide (targeted at achieving unit goals) and activity tracker (to track completion of at‐home activities), and aim to help parents partner with the childcare centre. Control: waitlist control group |
| Outcomes | Primary: children's dietary intakes will be assessed using a combination of direct observation of foods and beverages consumed while at the centre and parent‐completed food diaries. Dietary intake at child care (outside of parent's supervision) will be assessed by trained data collectors during observations of participating children during breakfast/morning snack, lunch, and afternoon snack using the Diet Observation in Child Care protocol. Children’s diet quality will be assessed with Healthy Eating Index (HEI) scores |
| Starting date | October (year unclear) |
| Contact information | Heidi Hennink‐Kaminski: h2kamins@unc.edu |
| Notes | Registered at ClinicalTrials.gov (NCT0233‐345, 23 December 2014) |
| Trial name or title | The healthy toddlers trial |
| Methods | Randomised controlled trial |
| Participants | Approximately 600 children aged 12 to 26 months recruited from community programmes, immunisation clinics and food pantries |
| Interventions | Intervention: “HT addresses core
nutrition concepts but moves well beyond basic
nutrition to address maternal self‐efficacy
during feeding, appropriate feeding styles, and
practices, including skill development to increase
success in making these behavioural
changes.” “The HT intervention consists of eight in‐home visits by a specially trained paraprofessional instructor plus four weekly telephone follow‐up reinforcement contacts. Particularly for high‐risk families with young children, providing services within the context of the family’s home environment appears to be a useful and effective strategy to provide parents with information, emotional support, access to other services and direct education [19]. The home‐visitation model also engages families who lack transportation or child care, a challenge frequently reported by families with low incomes. Paraprofessional instructors are peer educators who can relate to the target audience. Research shows that people learn best from their peers (people like themselves). Eight home visit sessions have been found to produce behavioral change [20]. At each visit, the paraprofessional spends approximately 1 hour with the mother and toddler dyad. The HT lessons use a variety of techniques and materials to enhance each mother’s learning experience and help reinforce knowledge. Each lesson includes opportunities for discussion, hands‐on activities, and an opportunity for mothers to practice skills covered in the lesson. The eight lessons include a lesson plan, handouts, and recipes. Mothers receive a notebook binder at the beginning of Lesson 1.” Control: “The control group families receive the usual services provided by Building strong families (BSF) or Expanded Food and Nutrition Education Program (EFNEP) in respective states. These families are newly enrolled into BSF or EFNEP as part of the HT study and have not received home visitation previously. The control lessons are similarly delivered as the HT lessons, such that, a paraprofessional instructor provides eight lessons during an in‐home visit, which last approximately 60 minutes. However, the control lessons focus on parenting (BSF) or nutrition (EFNEP) and do not include extensive content on feeding toddlers. Paraprofessionals who provide the lessons for the control group families are different to prevent cross contamination between the two groups.” |
| Outcomes | Child fruit and vegetable intake will be assessed via 3‐day dietary record of child’s intake |
| Starting date | Unknown |
| Contact information | Mildred Horodynski: millie@msu.edu |
| Notes |
| Trial name or title | First food for infants (Randomized controlled trial evaluating a cooking intervention to improve parental cooking skills and thereby improve dietary intake in infants aged 6‐12 months) |
| Methods | Randomised controlled trial |
| Participants | Approximately 160 children aged 5‐6 months attending selected public health clinics and their parent(s) |
| Interventions | Intervention: the intervention group is invited to a
2‐day course including some theory of infant
nutrition, and a main focus on increasing practical
food cooking skills (i.e. how to prepare and cook
the first food for infants). They are also taught
how to store food and how to be confident in making
infants’ food themselves. 5 groups
of participants attend the course on two different
days. Each of the 2 course days lasts 4 hours, and
parents are given theoretical knowledge about the
infant's first food as well as practical
knowledge on how to make nutritious and varied
food. Control: parents receive a booklet containing recipes for homemade foods for infants. |
| Outcomes | Food intake, measured using food frequency questionnaire |
| Starting date | The trial started in June 2012 |
| Contact information | Nina Cecilie Øverby: nina.c.overby@uia.no |
| Notes | ISRCTN45864056 |
| Trial name or title | A cluster randomized web‐based intervention trial among one‐year‐old‐children in kindergarten to reduce food neophobia and promote healthy diets |
| Methods | Cluster‐randomised controlled trial |
| Participants | Approximately 306 children born in 2016, attending kindergartens in the counties of Oppland and Telemark in Norway |
| Interventions | Intervention group 1: kindergartens will be asked to
serve a warm lunch meal with a variety of vegetables
3 days a week during the intervention period which
will last for 3 months. Intervention group 2: kindergartens will be asked to use given pedagogical tools including sensory lessons (the Sapere method) and advice on meal practice and feeding styles, in addition to serving the same meals as intervention group 1. Control: control kindergartens will continue their usual practices. |
| Outcomes | Child vegetable intake, dietary habits and food variety using detailed questionnaires developed for this specific study |
| Starting date | The trial started in August 2017 |
| Contact information | Nina Cecilie Øverby: nina.c.overby@uia.no |
| Notes | ISRCTN98064772 |
| Trial name or title | Happy child study |
| Methods | Two‐arm, cluster‐randomised controlled trial with outcomes assessed at baseline and after 9 months, via a self‐constructed questionnaire |
| Participants | Male and female children aged 3‐6 years attending kindergartens |
| Interventions | Programme in kindergarten setting that aims for the
development of a healthy lifestyle of
kindergartners, offering alternatives for their
diet, exercise behaviour and leisure‐time
activities delivered by the child‐care
workers. The programme is carried out in the course
of a kindergarten year supported by especially
developed pre‐structured lessons to be
integrated into the daily routine as well as the
involvement of parents. The programme’s goal is the increase of physical activity of kindergartners, the reduction of consumption of sweetened drinks as well as the reduction of time spent in front of computers and TVs. |
| Outcomes | Changes in dietary intake of fruit and vegetables |
| Starting date | September/October 2016 |
| Contact information | Susanne Kobel: susanne.kobel at uni‐ulm.de |
| Notes | DRKS00010089 |
| Trial name or title | Learning to like vegetables during breastfeeding |
| Methods | Dyads were randomly assigned into the following groups who differed in the timing and duration of when lactating mothers drank vegetable juices during their infants’ first 4 months postpartum as follows: exposure from 0.5‐1.5 months (1M0.5), exposure from 1.5‐2.5 months (1M1.5), exposure from 2.5‐3.5 months (1M2.5), exposure from 0.5‐3.5 months, or no exposure (control group) |
| Participants | 97 mother‐infant dyads |
| Interventions | Lactating mothers drank vegetable, beet, celery, and
carrot juices for 1 month beginning at 0.5, 1.5, or
2.5 months postpartum or for 3 months beginning at
0.5 months postpartum. Mothers in all groups were
given 118‐mL cups from which to drink the
juice (exposure group) or water (control group).
Women in the experimental groups were provided with
monthly supplies of the juices gratis at the
beginning of the exposure month (i.e. 0.5, 1.5, or
2.5 months) or months (0.5–2.5 months). The
women were instructed to drink a cupful (118 mL) of
the vegetable juices per occasion during their
particular month or months of exposure (resulting in
24 total exposures/month; 8 carrot juice exposures;
and 8 vegetable juice exposures, 4 beet juice
exposures, and 4 celery juice exposures). Control group drank equal volumes of water and avoided drinking the juices |
| Outcomes | The number of minutes that elapsed from the start to end of feeding was the duration of the feed, and the amount consumed was determined by weighing the bowl immediately before and after each feed on a Mettler balance (model SG1600; Mettler‐Toledo). All food that was spilled onto the tray or bib during the feed was placed in the bowl before weighing |
| Starting date | Unclear |
| Contact information | Julie A Mennella: menella@monell.org |
| Notes | This trial was registered at clinicaltrials.gov as NCT01667549 |
| Trial name or title | A randomised control trial of an educational and taste‐exposure intervention to promote vegetable intake in preschool aged children |
| Methods | Cluster‐randomised control trial |
| Participants | 160 children aged 2‐5 years |
| Interventions | Taste exposure: children will be repeatedly offered
the single vegetable, which is unfamiliar to them
over the 12‐week period. Children will be
offered 40 g of the vegetable by their usual nursery
staff. Nutritional education: nursery staff will be trained by the PhunkyFoods team to deliver the nutritional education programme. Children will be taught Eat Well (learning about different food groups) and Strive for Five (learning about eating fruits and vegetables) components of the PhunkyFoods education programme by their usual nursery staff. Nurseries will be advised to deliver as much as possible of the two components over the 12‐week period. Taste exposure and nutritional education: children will be repeatedly offered a single unfamiliar vegetable over the 12‐week period as well as receive the PhunkyFoods educational programme (Eat Well and Strive for Five components). Children will be offered the vegetable and nutritional education by their usual nursery staff. No intervention control: children will be offered a single unfamiliar vegetable at the beginning, end and at the follow‐up. They will be offered the nutritional education after the completion of the study. |
| Outcomes | Intake of an unfamiliar vegetable measured using weight in grams |
| Starting date | The trial started in September 2016 |
| Contact information | Chandani Nekitsing: C.Nekitsing1@leeds.ac.uk |
| Notes | NCT03003923 |
| Trial name or title | What promotes healthy eating? |
| Methods | Factorial, randomised controlled trial |
| Participants | 7200 mother‐father‐child pairs |
| Interventions | Group 1: weekly maternal nutrition
behaviour‐change communication (BCC) sessions
for 4 months Group 2: weekly maternal nutrition BCC sessions for 4 months and weekly paternal nutrition BCC sessions for 3 months Group 3: receipt of a food voucher for 6 months (randomly select 1 parent) Group 4: weekly maternal nutrition BCC sessions for 4 months and receipt of a food voucher for 6 months Group 5 weekly maternal nutrition BCC sessions for 4 months and weekly paternal nutrition BCC sessions for 3 months and receipt of a food voucher for 6 months Control group: unspecified |
| Outcomes | Child dietary diversity score Mean difference in child dietary diversity score defined by consumption of number of food group consumed by a child Food consumption score Mean difference in food consumption score calculated using the frequency of consumption of different food groups consumed by a child. |
| Starting date | Registration date: 25 July 2017 – status was recruiting |
| Contact information | Hyuncheol Kim : hk788@cornell.edu |
| Notes | This trial was registered at clinicaltrials.gov as NCT03229629 |
| Trial name or title | Baby's first bites (The what and how in weaning: a randomised controlled trial to assess the effects of vegetable‐exposure and responsive feeding on vegetable acceptance in infants and toddlers) |
| Methods | Randomised controlled trial |
| Participants | 240 first‐time mothers of healthy term infants |
| Interventions | Intervention A: this intervention repeatedly exposed
infants and toddlers to vegetables and involved 2
days of pre‐test, a 15‐day feeding
schedule and 2 days of post‐test. During 15
consecutive days, children are exposed to one of two
target vegetables according to a set scheme where
one target vegetable is offered to the infant every
other day. On the days in between, infants receive
other vegetables for variety. During the
feeding‐schedule on day 5 and 12 mothers will
receive a phone call to motivate them to continue
exposing their infant to vegetables. When the
children are 8, 13 and 16 months of age, mothers
will receive a booster phone call to reinforce daily
vegetable intake. Mothers are asked to
keep serving their infant vegetables on a daily
basis and receive a folder that emphasises the
importance of repeated exposure to vegetables.
Mothers also receive 20 vegetable purées a
month, until 5 months after the feeding schedule to
reinforce exposure to vegetables. Intervention B: receives an intervention on how to feed their infant, in addition to a 15‐day feeding schedule consisting of mostly fruit. The intervention mothers receive purely focuses on the promotion of responsive feeding practices. The intervention mothers will receive the Video‐feedback Intervention to promote Positive Parenting ‐Feeding Infants (VIPP‐FI) and will be delivered during home visits. VIPP‐FI focuses on improving responsive feeding and sensitive ways of dealing with unwilling infants during the feeding process. Mothers are shown videotapes of their own feeding‐interaction with their infant, and receive feedback on these tapes by a trained intervener. Intervention C: will receive a combination of Intervention A and Intervention B. Mothers will be asked to feed the infant according to the schedule for the vegetable‐exposure intervention and will also receive feedback on how they should go about feeding their infant according to the VIPP‐FI intervention. Attention‐Control Condition D: receive the same feeding schedule as Intervention B and receive phone calls at the same time‐points as the intervention groups in which they will not receive any specific advice, but will be asked about topics such as the general development of the child. If mothers have questions about weaning or feeding, they are referred to “Het Voedingscentrum” or their infant welfare centre. |
| Outcomes | Infants' and toddlers' vegetable consumption |
| Starting date | The trial started in April 2016 |
| Contact information | Judi Mesman: mesmanj@fsw.leidenuniv.nl |
| Notes | NTR6572 and NCT03348176 |
| Trial name or title | Farm Fresh Foods for Healthy Kids (F3HK) |
| Methods | The Farm Fresh Foods for Healthy Kids community‐based, randomised intervention trial will build on formative and longitudinal research to examine the impact of cost‐offset community supported agriculture on diet and other health behaviours as well as the economic impacts on local economies. In each program, families will be recruited to join existing community supported agriculture programs in New York, North Carolina, Vermont, and Washington, and families will be randomised 1:1 to intervention or delayed intervention groups. Data will be collected at baseline, and in the fall and spring for 3 years. |
| Participants | Low‐income families with at least 1 child aged 2‐12 years. Target is 240 families (120 per arm) |
| Interventions | The intervention will involve reduced‐price community supported agriculture shares, which can be paid for on a weekly basis, nine skill‐based and seasonally tailored healthy eating classes, and the provision of basic kitchen tools. |
| Outcomes | Children’s intake of fruits and vegetables |
| Starting date | Unknown |
| Contact information | rs946@cornell.edu |
| Notes | NCT02770196 |
| Trial name or title | Play and grow |
| Methods | Randomised controlled trial |
| Participants | Approximately 240 families with children aged 2‐4 years |
| Interventions | Intervention: “Play & Grow
is a 10‐week family‐based,
multi‐component healthy lifestyle
programme” "The Play & Grow will have educational strategies including instructions, parental peer support and group discussions, and homework tasks, in accordance with the elements developed in our Play & Grow pilot study. Each session will comprise: (i) 15 min of guided active play involving both children and parents; (ii) 15 min of interactive education and skill development for parents; simultaneous supervised active play with foods for children, to promote acceptance of vegetables, and (iii) 15 min of guided active nature games outdoors, involving both children and parents. The sessions will incorporate a lifestyle component, for example: eating, active play and connectedness to nature). These will target the parents’ knowledge and skills on how to introduce and maintain their child’s correct lifestyle routines. A group leader and co‐leader with healthcare backgrounds (and trained by the PI during the Play & Grow pilot study) will facilitate the sessions involving 4 to 5 parent‐child dyads. The proposed intervention, we will employ environmental education and nature‐related activities to help participating families develop skills conducive to improving playtime and eating habits in children." Control: “The (waiting list or control group) WLCG children will be offered the Play & Grow programme at study completion” |
| Outcomes | Child fruit and vegetable intake will be assessed using the Eating and Physical Activity Questionnaire (EPAQ) and The Children’s Eating Behaviour Questionnaire (CEBQ) |
| Starting date | Unknown |
| Contact information | Tanja Sobko: tsobko@hku.hk |
| Notes |
| Trial name or title | Choosing Healthy Eating when Really Young (CHERRY) |
| Methods | Randomised controlled trial |
| Participants | Approximately 288 parents of children aged 18 months to 5 years from children's centres |
| Interventions | Intervention: “The intervention
group participants attended four sessions (one each
week) over 4 weeks. Each session lasted 2 h. The
first hour of each session involved parents
discussing and learning about a variety of aspects
of healthy eating while the children attended a free
crèche in the adjacent room (the
crèche activities were not considered part of
CHERRY and were not monitored). The second hour
involved parents. and children together for a more
practical, ‘hands on’
cook and eat session involving basic food
preparation and tasting. Each session began with a
recap from the previous week and finished with
parents being given a ‘CHERRY at
home’ activity to complete before
the following week’s session; these
were both designed to consolidate
parents’ learning. The intervention group also received SMS reminders via mobile phones between sessions; SMSs included the main messages of the CHERRY programme, as well as reminders to attend each session. The intervention comprised not only individually focused nutrition support, but also encompassed activities directed at developing the capacity of the children’s centre to promote and maintain healthy nutritional practices. In the intervention centres, a staff training session was offered to all staff working in the centres. The training session covered various aspects of healthy eating and nutrition for early years and included an introduction and overview of the CHERRY programme. Each training session was tailored to the needs of the staff, as identified by heads of each intervention centre. Intervention centres were also given support and advice to revise and develop their centre’s food policies in order to support healthy eating practices and procedures.” Control: “The children’s centres randomised to the control group did not receive any of the components of the CHERRY programme. During the study period, the control centres agreed not to implement any new nutritional interventions but continued with existing support. On final completion of the study, the CHERRY resources were disseminated to control centres and other early years settings interested in nutrition.” |
| Outcomes | "Child’s fruit and vegetable consumption at home (portions per day). This was defined as the total weight (grams) of fruit and vegetables consumed the number of different types of fruit and vegetables consumed, and the actual types of fruit and vegetables consumed. The child’s diet was assessed using the multiple‐pass 24‐h recall method. As the children concerned were under 5 years of age, the parents completed the interviews on their behalf.” |
| Starting date | Parents were recruited into the study over 5 recruitment waves between September 2010 and November 2011 |
| Contact information | Richard Geddie Watt: r.watt@ucl.ac.uk |
| Notes |
| Trial name or title | Keys to Healthy Family Child Care Homes (KEYS) |
| Methods | Cluster‐randomised controlled trial |
| Participants | Approximately 450 children aged 18 months to 4 years from 150 Family Child Care Homes |
| Interventions | Intervention: “The Keys
intervention is delivered over nine months, spending
approximately three months on each of three modules.
These modules are designed to help providers (1).
Modify their own weight‐related behaviors so
that they can become role models for children
(Module 1: Healthy You), (2) create environments
that encourage and support
children’s physical activity and
healthy eating habits (Module 2: Healthy Home), and
(3) adopt sound business practices that will help
them sustain the changes introduced (Module 3:
Healthy Business). "The intervention is delivered through workshops, home visits, tailored coaching calls, and educational toolkits." Control: “Participants in the control arm receive the Healthy Business" only |
| Outcomes | Child intake collected using direct observation at the Family Child Care Homes |
| Starting date | Unknown |
| Contact information | Courtney Mann: courtney.mann@dm.duke.edu |
| Notes |
LGA: Local Government Area
Contributions of authors
All authors contributed to the conception of the research and were involved in the preparation of the review including providing critical comment on drafts. RH led the review update and manuscript drafting. RH, KO and FS conducted searches of other sources. RH, KO, FS, RW, NN, SY, EJ and KB screened titles and abstracts. RH, KO, FS, NN, RS, RW, SY and LW screened full texts to determine study eligibility. EJ, TCM, RW, KB, KO, ER, RH, RS and NN extracted data from eligible trials. FS, FT and TCM assessed risk of bias. RH, NN and LW assessed quality of studies (GRADE).
Sources of support
Internal sources
-
Hunter Medical Research Institute, Australia.
Infrastructure support
-
The University of Newcastle, Australia.
Salary Support
-
Deakin University, Australia.
Salary Support
-
Hunter New England Area Health Service, Australia.
Salary Support
-
Cancer Council NSW, Australia.
Salary Support
-
Cancer Institute NSW, Australia.
Salary support
External sources
No sources of support supplied
Declarations of interest
Rebecca K Hodder: none known
Fiona G Stacey: none known
Kate M O'Brien: none known
Rebecca J Wyse: is an author on an included randomised trial of an intervention to increase fruit and vegetable consumption (Wyse 2012); she was not involved in the determination of study eligibility, data extraction or risk of bias assessment for this review. She has not received any benefit, in cash or kind, any hospitality, or any subsidy derived from the food industry or any other source perceived to have an interest in the outcome of the review.
Tara Clinton‐McHarg: none known
Flora Tzelepis: none known
Erica L James: none known
Kate M Bartlem: none known
Nicole K Nathan: none known
Rachel Sutherland: none known
Emma Robson: none known
Sze Lin Yoong: none known
Luke Wolfenden: is an author on an included randomised trial of an intervention to increase fruit and vegetable consumption (Wyse 2012); he was not involved in the determination of study eligibility, data extraction or risk of bias assessment for this review. He has not received any benefit, in cash or kind, any hospitality, or any subsidy derived from the food industry or any other source perceived to have an interest in the outcome of the review.
Edited (no change to conclusions)
References
References to studies included in this review
- Anzman‐Frasca S, Savage JS, Marini ME, Fisher JO, Birch LL. Repeated exposure and associative conditioning promote preschool children's liking of vegetables. Appetite 2012;58(2):543‐53. [DOI] [PubMed] [Google Scholar]; Savage JS, Paul IM, Marini ME, Birch LL. Pilot intervention promoting responsive feeding, the division of feeding responsibility, and healthy dietary choices during infancy. Appetite 2010;54(3):673. [Google Scholar]
- Barends C, Vries J H, Mojet J, Graaf C. Effects of starting weaning exclusively with vegetables on vegetable intake at the age of 12 and 23 months. Appetite 2014;81:193‐9. [DOI] [PubMed] [Google Scholar]; Barends C, Vries J, Mojet J, Graaf C. Effects of repeated exposure to either vegetables or fruits on infant's vegetable and fruit acceptance at the beginning of weaning. Food Quality and Preference 2013;29:157‐65. [Google Scholar]; Barends C, Mojet J, Vries J, Graaf K. Effects of repeated exposure to either fruits or vegetables during the first 18 days of weaning on infant's fruit and vegetable acceptance. Appetite 2011;57(2):553. [Google Scholar]
- Baskale H, Bahar Z. Outcomes of nutrition knowledge and healthy food choices in 5‐ to 6‐year‐old children who received a nutrition intervention based on Piaget's theory. Journal for Specialists in Pediatric Nursing: JSPN 2011;16(4):263‐79. [DOI] [PubMed] [Google Scholar]
- Black MM, Hurley K, Wang Y, Candelaria M, Latta L, Caulfield L, et al. Toddler obesity prevention study (TOPS) increases toddler health‐promoting behaviors. FASEB Journal 2013;27(1 Suppl):37.4. [Google Scholar]; Black MM, Hurley KM, Hager ER, Wang Y, Latta LW, Candelaria M, et al. Toddler obesity prevention: effects of parenting and maternal lifestyles interventions. Obesity 2011;19:S109. [Google Scholar]
- Blissett J, Bennett C, Fogel A, Harris G, Higgs S. Parental modelling and prompting effects on acceptance of a novel fruit in 2‐4‐year‐old children are dependent on children's food responsiveness. British Journal of Nutrition 2016;115(3):554‐64. [DOI] [PubMed] [Google Scholar]
- Cameron AJ, Ball K, Hesketh KD, McNaughton SA, Salmon J, Crawford DA, et al. Variation in outcomes of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT) Program according to maternal education and age. Preventive Medicine 2014;58:58‐63. [DOI] [PubMed] [Google Scholar]; Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The Infant Feeding Activity and Nutrition Trial (INFANT) an early intervention to prevent childhood obesity: cluster‐randomised controlled trial. BMC Public Health 2008;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Campbell KJ, Lioret S, McNaughton SA, Crawford DA, Salmon J, Ball K, et al. A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics 2013;131(4):652‐60. [DOI] [PubMed] [Google Scholar]; Hesketh KD, Campbell K, Salmon J, McNaughton SA, McCallum Z, Cameron A, et al. The Melbourne Infant Feeding, Activity and Nutrition Trial (InFANT) Program follow‐up. Contemporary Clinical Trials 2013;34(1):145‐51. [DOI] [PubMed] [Google Scholar]; Lioret S, Campbell K, McNaughton S, Crawford D, Salmon J, Ball K, et al. Parent focused intervention impacts obesity risk behaviours in infants: results of the Melbourne infant program cluster‐randomised controlled trial. Obesity Facts 2012;5:33. [Google Scholar]; Spence AC, Campbell KJ, Crawford DA, McNaughton SA, Hesketh KD. Mediators of improved child diet quality following a health promotion intervention: The Melbourne inFANT Program. International Journal of Behavioral Nutrition and Physical Activity 2014;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]; Spence AC, McNaughton SA, Lioret S, Hesketh KD, Crawford DA, Campbell KJ, et al. A health promotion intervention can affect diet quality in early childhood. Journal of Nutrition 2013;143(10):1672‐8. [DOI] [PubMed] [Google Scholar]; Walsh AD, Lioret S, Cameron AJ, Hesketh KD, McNaughton SA, Crawford D, et al. The effect of an early childhood obesity intervention on father's obesity risk behaviors: the Melbourne InFANT Program. International Journal of Behavioral Nutrition & Physical Activity 2014;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton SJ, Ahern SM, Remy E, Nicklaus S, Blundell P, Hetherington MM. Repetition counts: repeated exposure increases intake of a novel vegetable in UK pre‐school children compared to flavour‐flavour and flavour‐nutrient learning. British Journal of Nutrition 2013;109(11):2089‐97. [DOI] [PubMed] [Google Scholar]
- Cohen RJ, Rivera LL, Canahuati J, Brown KH, Dewey KG. Delaying the introduction of complementary food until 6 months does not affect appetite or mother's report of food acceptance of breast‐fed infants from 6 to 12 months in a low income, Honduran population. Journal of Nutrition 1995;125(11):2787‐92. [DOI] [PubMed] [Google Scholar]
- Cooke LJ, Chambers LC, Anez EV, Croker HA, Boniface D, Yeomans MR, et al. Eating for pleasure or profit: the effect of incentives on children's enjoyment of vegetables. Psychological Science 2011;22(2):190‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia DC, O'Connell M, Irwin ML, Henderson KE. Pairing vegetables with a liked food and visually appealing presentation: promising strategies for increasing vegetable consumption among preschoolers. Childhood Obesity 2014;10(1):72‐6. [DOI] [PubMed] [Google Scholar]
- Cravener TL, Schlechter H, Loeb KL, Radnitz c, Schwartz M, Zucker N, et al. Feeding strategies derived from behavioral economics and psychology can increase vegetable intake in children as part of a home‐based intervention: results of a pilot study. Journal of the Academy of Nutrition and Dietetics2015; Vol. 115, issue 11:1798‐807. [DOI] [PubMed]
- Daniels L, Mallan K, Nicholson J, Meedeniya J, Magarey A. Child behaviour and weight outcomes of NOURISH RCT. Obesity Facts 2013;6:16. [Google Scholar]; Daniels L, Mallan K, Nicholson J, Thorpe K, Magarey A. Longer term child growth and maternal feeding practices outcomes of the NOURISH obesity prevention trial. Obesity Facts 2014;7:39. [Google Scholar]; Daniels LA, Magarey A, Battistutta D, Nicholson JM, Farrell A, Davidson G, et al. The NOURISH randomised control trial: positive feeding practices and food preferences in early childhood ‐ a primary prevention program for childhood obesity. BMC Public Health 2009;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]; Daniels LA, Magarey AM, Nicholson JM. The NOURISH early feeding trial: an innovative approach to child obesity prevention. Obesity Research and Clinical Practice 2011;5:S5. [Google Scholar]; Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Meedeniya JE, Bayer JK, et al. Child eating behavior outcomes of an early feeding intervention to reduce risk indicators for child obesity: the NOURISH RCT. Obesity (Silver Spring, Md.) 2014;22(5):E104‐11. [DOI] [PubMed] [Google Scholar]; Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics 2013;132(1):e109‐18. [DOI] [PubMed] [Google Scholar]; Magarey A, Mauch C, Mallan K, Perry R, Elovaris R, Meedeniya J, et al. Child dietary and eating behavior outcomes up to 3.5 years after an early feeding intervention: the NOURISH RCT. Obesity 2016;24(7):1537‐45. [DOI] [PubMed] [Google Scholar]
- Bock F, Breitenstein L, Fischer JE. Positive impact of a pre‐school‐based nutritional intervention on children's fruit and vegetable intake: results of a cluster‐randomized trial. Public Health Nutrition 2012;15(3):466‐75. [DOI] [PubMed] [Google Scholar]; Bock F, Fischer JE, Hoffmann K, Renz‐Polster H. A participatory parent‐focused intervention promoting physical activity in preschools: design of a cluster‐randomized trial. BMC Public Health 2010;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen V, Bourdeaudhuij I, Vereecken C, Verbestel V, Haerens L, Huybrechts I, et al. Effects of a 2‐year healthy eating and physical activity intervention for 3‐6‐year‐olds in communities of high and low socio‐economic status: the POP (Prevention of Overweight among Pre‐school and school children) project. Public Health and Nutrition 2012;15(9):1737‐45. [DOI] [PubMed] [Google Scholar]
- Droog SM. Using picture books to stimulate the appeal of healthy food products among pre‐schoolers. Appetite 2012;59(2):624. [Google Scholar]; Droog SM, Buijzen M, Valkenburg PM. Enhancing children's vegetable consumption using vegetable‐promoting picture books. The impact of interactive shared reading and character‐product congruence. Appetite 2014;73:73‐80. [DOI] [PubMed] [Google Scholar]
- Droog SM, Nee R, Govers M, Buijzen M. Promoting toddlers' vegetable consumption through interactive reading and puppetry. Appetite 2017;116:75‐81. [DOI] [PubMed] [Google Scholar]
- Wild VW, Graaf C, Jager G. Effectiveness of flavour nutrient learning and mere exposure as mechanisms to increase toddler’s intake and preference for green vegetables. Appetite 2013;64:89‐96. [DOI] [PubMed] [Google Scholar]
- Wild VW, Graaf C, Boshuizen HC, Jager G. Influence of choice on vegetable intake in children: an in‐home study. Appetite 2015;91:1‐6. [DOI] [PubMed] [Google Scholar]
- Wild V, Graaf C, Jager G. Efficacy of repeated exposure and flavour–flavour learning as mechanisms to increase preschooler's vegetable intake and acceptance. Pediatric Obesity 2015;10(3):205‐12. [DOI] [PubMed] [Google Scholar]
- Wild VWT, Graaf C, Jager G. Use of different vegetable products to increase preschool‐aged children's preference for and intake of a target vegetable: a randomized controlled trial. Journal of the Academy of Nutrition and Dietetics 2017;117(6):859‐66. [DOI] [PubMed] [Google Scholar]
- Duncanson K, Burrows T, Collins C. Effect of a low‐intensity parent‐focused nutrition intervention on dietary intake of 2‐ to 5‐year olds. Journal of Pediatric Gastroenterology and Nutrition 2013;57(6):728‐34. [DOI] [PubMed] [Google Scholar]; Duncanson K, Burrows T, Collins C. Study protocol of a parent‐focused child feeding and dietary intake intervention: the feeding healthy food to kids randomised controlled trial. BMC Public Health 2012;12:564. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duncanson K, Burrows T, Collins C. Twelve month outcomes of the Feeding Healthy Food to Kids Randomised Controlled Trial. Journal of the American Dietetic Association 2011;111(9 Supplement):A105. [Google Scholar]; Duncanson K, Burrows T, Holman B, Collins C. Parents' perceptions of child feeding: a qualitative study based on the theory of planned behavior. Journal of Developmental and Behavioral Pediatrics 2013;34(4):227‐36. [DOI] [PubMed] [Google Scholar]
- Fildes A, Jaarsveld CH, Wardle J, Cooke L. Parent‐administered exposure to increase children's vegetable acceptance: a randomized controlled trial. Journal of the Academy of Nutrition & Dietetics 2014;114:881‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fildes A, Lopes C, Moreira P, Moschonis G, Oliveira A, Mavrogianniet C, et al. An exploratory trial of parental advice for increasing vegetable acceptance in infancy. British Journal of Nutrition 2015;114(2):328‐36. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Mennella JA, Hughes SO, Liu Y, Mendoza PM, Patrick H. Offering "dip" promotes intake of a moderately‐liked raw vegetable among preschoolers with genetic sensitivity to bitterness. Journal of the Academy of Nutrition & Dietetics 2012;112(2):235‐45. [DOI] [PubMed] [Google Scholar]
- Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics 2007;120(6):1247‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrish CJ, Mennella JA. Flavor variety enhances food acceptance in formula‐fed infants. American Journal of Clinical Nutrition 2001;73(6):1080‐5. [DOI] [PubMed] [Google Scholar]
- Haire‐Joshu D, Elliott MB, Caito NM, Hessler K, Nanney MS, Hale N, et al. High 5 for Kids: the impact of a home visiting program on fruit and vegetable intake of parents and their preschool children. Preventive Medicine 2008;47(1):77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnack LJ, Oakes JM, French SA, Rydell SA, Farah FM, Taylor GL. Results from an experimental trial at a Head Start center to evaluate two meal service approaches to increase fruit and vegetable intake of preschool aged children. International Journal of Behavioural Nutrition and Physical Activity2012; Vol. 9:51. [DOI] [PMC free article] [PubMed]
- Hausner H, Olsen A, Moller P. Mere exposure and flavour‐flavour learning increase 2‐3 year‐old children's acceptance of a novel vegetable. Appetite 2012;58(3):1152‐9. [DOI] [PubMed] [Google Scholar]
- Heath P, Houston‐Price C, Kennedy OB. Let's look at leeks! Picture books increase toddlers' willingness to look at, taste and consume unfamiliar vegetables. Frontiers in Psychology 2014;5:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington MM, Schwartz C, Madrelle J, Croden F, Nekitsing C, Vereijken CM, et al. A step‐by‐step introduction to vegetables at the beginning of complementary feeding. The effects of early and repeated exposure. Appetite 2015;84:280‐90. [DOI] [PubMed] [Google Scholar]
- Hunsaker SL, Jensen CD. Effectiveness of a parent health report in increasing fruit and vegetable consumption among preschoolers and kindergarteners. Journal of Nutrition Education and Behavior 2017;49(5):380‐6. [DOI] [PubMed] [Google Scholar]
- Keller K, Forman J, Lee NM, Kuilema LG, Haldford JC. Use of license spokes‐characters to increase intake of fruits and vegetables as part of a childhood obesity prevention program: pilot study results. Obesity 2011;19:S109. [Google Scholar]; Keller KL, Kuilema LG, Lee N, Yoon J, Mascaro B, Combes AL, et al. The impact of food branding on children's eating behavior and obesity. Physiology and Behavior 2012;106(3):379‐86. [DOI] [PubMed] [Google Scholar]
- Kling SM, Roe LS, Keller KL, Rolls BJ. Double trouble: portion size and energy density combine to increase preschool children's lunch intake. Physiology & Behavior 2016;162:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Andrade GO, Cespedes EM, Rifas‐Shiman SL, Romero‐Quechol G, Gonzalez‐Unzaga MA, Benitez‐Trejo MA, et al. Feasibility and impact of Creciendo Sanos, a clinic‐based pilot intervention to prevent obesity among preschool children in Mexico City. BMC Pediatrics 2014;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Nicklaus S, Jagolino AL, Yourshaw LM. Variety is the spice of life: strategies for promoting fruit and vegetable acceptance during infancy. Physiology & Behavior 2008;94(1):29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namenek Brouwer RJ, Benjamin Neelon SE. Watch me grow: a garden‐based pilot intervention to increase vegetable and fruit intake in preschoolers. BMC Public Health 2013;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale RA, Lopez‐Mitnik G, Uhlhorn SB, Asfour L, Messiah SE. Effect of a child care center‐based obesity prevention program on body mass index and nutrition practices among preschool‐aged children. Health Promotion Practice 2014;15(5):695‐705. [DOI] [PubMed] [Google Scholar]
- Nicklas T, Lopez S, Liu Y, Reiher R. Using motivational theatre to increase vegetable consumption by preschool children. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A35. [Google Scholar]; Nicklas T, Lopez S, Liu Y, Saab R, Reiher R. Motivational theater to increase consumption of vegetable dishes by preschool children. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell ML, Henderson KE, Luedicke J, Schwartz MB. Repeated exposure in a natural setting: a preschool intervention to increase vegetable consumption. Journal of the Academy of Nutrition & Dietetics 2012;112(2):230‐4. [DOI] [PubMed] [Google Scholar]
- Remington A, Añez E, Croker H, Wardle J, Cooke L. Increasing food acceptance in the home setting: a randomized controlled trial of parent‐administered taste exposure with incentives. American Journal of Clinical Nutrition 2012;95(1):72‐7. [DOI] [PubMed] [Google Scholar]; Remington AM, Anez EV, Cooke LJ, Wardle J. Tiny tastes. A home based intervention promoting acceptance of disliked vegetables. Appetite 2011;57:S35‐6. [Google Scholar]
- Remy E, Issanchou S, Chabanet C, Nicklaus S. Repeated exposure of infants at complementary feeding to a vegetable puree increases acceptance as effectively as flavor‐flavor learning and more effectively than flavor‐nutrient learning. Journal of Nutrition 2013;143(7):1194‐200. [DOI] [PubMed] [Google Scholar]
- Roe LS, Meengs JS, Birch LL, Rolls BJ. Serving a variety of vegetables and fruit as a snack increased intake in preschool children. American Journal of Clinical Nutrition 2013;98(3):693‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roset‐Salla M, Ramon‐Cabot J, Salabarnada‐Torras J, Pera Guillem Dalmau A. Educational intervention to improve adherence to the Mediterranean diet among parents and their children aged 1–2 years. EniM clinical trial. Public Health Nutrition 2016;19(06):1131‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JS, Fisher JO, Marini M, Birch LL. Serving smaller age‐appropriate entree portions to children aged 3‐5 y increases fruit and vegetable intake and reduces energy density and energy intake at lunch. American Journal of Clinical Nutrition 2012;95:335‐41. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, JaKa MM, Crain AL, Martinson BC, Hayes MG, Anderson JD. Pediatric primary care‐based obesity prevention for parents of preschool children: a pilot study. Childhood Obesity (Print) 2015;11(6):674‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouteris H, Hill B, McCabe M, Swinburn B, Busija L. A parent‐based intervention to promote healthy eating and active behaviours in pre‐school children: evaluation of the MEND 2‐4 randomized controlled trial. Pediatric Obesity 2015;11(1):4‐10. [DOI] [PubMed] [Google Scholar]; Skouteris H, McCabe M, Swinburn B, Hill B. Healthy eating and obesity prevention for preschoolers: a randomised controlled trial. BMC Public Health 2010;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. The Effects of Access and Education on Preschool Children's Fruit and Vegetable Intake [Dissertation]. Vol. 10626889, Ann Arbor: The Ohio State University, 2017. [Google Scholar]
- Spill MK, Birch LL, Roe LS, Rolls BJ. Eating vegetables first: the use of portion size to increase vegetable intake in preschool children. American Journal of Clinical Nutrition 2010;91(5):1237‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spill MK, Birch LL, Roe LS, Rolls BJ. Hiding vegetables to reduce energy density: an effective strategy to increase children's vegetable intake and reduce energy intake. American Journal of Clinical Nutrition 2011;94(3):735‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spill MK, Birch LL, Roe LS, Rolls BJ. Serving large portions of vegetable soup at the start of a meal affected children's energy and vegetable intake. Appetite 2011;57(1):213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiano AE, Marker AM, Frelier JM, Hsia DS, Martin CK. Influence of screen‐based peer modeling on preschool children's vegetable consumption and preferences. Journal of Nutrition Education and Behavior 2016;48(5):331‐5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SA, Birch LL. Infant dietary experience and acceptance of solid foods. Pediatrics 1994;93(2):271‐7. [PubMed] [Google Scholar]
- Anonymous. Erratum... Tabak et al. Family ties to health program: a randomized intervention to improve vegetable intake in children. Journal of Nutrition Education & Behaviour, 2012 Mar/Apr 44(2):166‐71. Journal of Nutrition Education & Behavior 2014;46:202. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tabak RG, Tate DF, Stevens J, Siega‐Riz AM, Ward DS. Family ties to health program: a randomized intervention to improve vegetable intake in children. Journal of Nutrition Education & Behavior 2012;44(2):166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tabak RG, Tate DF, Stevens J, Siega‐Riz AM, Ward DS. Family ties to health study: a randomized intervention to improve vegetable intake in children. Obesity 2011;19:S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazir S, Engle P, Balakrishna N, Griffiths PL, Johnson SL, Creed‐Kanashiro H, et al. Cluster‐randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal & Child Nutrition 2013;9(1):99‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbestel V, Coen V, Winckel M, Huybrechts I, Maes L, Bourdeaudhuij I. Prevention of overweight in children younger than 2 years old: a pilot cluster‐randomized controlled trial. Public Health Nutrition 2014;17(6):1384‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken C, Huybrechts I, Houte H, Martens V, Wittebroodt I, Maes L. Results from a dietary intervention study in preschools "Beastly Healthy at School". International Journal of Public Health 2009;54(3):142‐9. [DOI] [PubMed] [Google Scholar]
- Wardle J, Cooke LJ, Gibson EL, Sapochnik M, Sheiman A, Lawson M. Increasing children's acceptance of vegetables; a randomized trial of parent‐led exposure. Appetite 2003;40(2):155‐62. [DOI] [PubMed] [Google Scholar]
- Scheiwe A, Hardy R, Watt RG. Four‐year follow‐up of a randomized controlled trial of a social support intervention on infant feeding practices. Maternal & Child Nutrition 2010;6(4):328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]; Watt RG, Tull KI, Wiggins M, Kelly Y, Molloy B, Dowler E, et al. Effectiveness of a social support intervention of infant feeding practices: randomised controlled trial. Journal of Epidemiology & Community Health 2009;63(2):156‐62. [DOI] [PubMed] [Google Scholar]
- Williams PA, Cates SC, Blitstein JL, Hersey J, Gabor V, Ball M, et al. Nutrition‐education program improves preschoolers' at‐home diet: a group randomized trial. Journal of the Academy of Nutrition & Dietetics 2014;114(7):1001‐8. [DOI] [PubMed] [Google Scholar]
- Witt KE, Dunn C. Increasing fruit and vegetable consumption among preschoolers: evaluation of color me healthy. Journal of Nutrition Education & Behavior 2012;44(2):107‐13. [DOI] [PubMed] [Google Scholar]
- Wolfenden L, Wyse R, Campbell E, Brennan L, Campbell KJ, Fletcher A, et al. Randomized controlled trial of a telephone‐based intervention for child fruit and vegetable intake: long‐term follow‐up. American Journal of Clinical Nutrition 2014;99(3):543‐50. [DOI] [PubMed] [Google Scholar]; Wyse R, Wolfenden L, Bisquera A. Characteristics of the home food environment that mediate immediate and sustained increases in child fruit and vegetable consumption: mediation analysis from the Healthy Habits cluster randomised controlled trial. International Journal of Behavioral Nutrition & Physical Activity 2015;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wyse R, Wolfenden L, Campbell E, Campbell K, Brennan L, Fletcher A, et al. Increasing fruit and vegetable consumption in 3‐ 5 year old children: results from a cluster randomised controlled trial of a telephone‐based parent intervention, Hunter region, NSW, Australia. Obesity Reviews 2011;12:68. 22008561 [Google Scholar]; Wyse R, Wolfenden L, Campbell E, Campbell KJ, Wiggers J, Brennan L, et al. A cluster randomized controlled trial of a telephone‐based parent intervention to increase preschoolers' fruit and vegetable consumption. American Journal of Clinical Nutrition 2012;96(1):102‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wyse RJ, Wolfenden L, Campbell E, Brennan L, Campbell KJ, Fletcher A, et al. A cluster randomised trial of a telephone‐based intervention for parents to increase fruit and vegetable consumption in their 3‐ to 5‐year‐old children: study protocol. BMC Public Health 2010;10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinstra GG, Vrijhof M, Kremer S. Is repeated exposure the holy grail for increasing children's vegetable intake? Lessons learned from a Dutch childcare intervention using various vegetable preparations. Appetite 2018;121:316‐25. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aboud FE, Moore AC, Akhter S. Effectiveness of a community‐based responsive feeding programme in rural Bangladesh: a cluster randomized field trial. Maternal and Child Nutrition 2008;4(4):275‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A, LaRowe T, Cronin KA, Prince RJ, Jobe JB. Healthy children, strong families: results of a randomized trial of obesity prevention for preschool American Indian children and their families. Obesity 2011;19:S110. [Google Scholar]; Adams AK, LaRowe TL, Cronin KA, Prince RJ, Wubben DP, Parker T, et al. The healthy children, strong families intervention: design and community participation. Journal of Primary Prevention 2012;33(4):175‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MA, Bruening M, Ohri‐Vachaspati P. Use of salad bars in schools to increase fruit and vegetable consumption: where's the evidence?. Journal of the Academy of Nutrition and Dietetics 2015;115(8):1233‐6. [DOI] [PubMed] [Google Scholar]
- Agrawal T, Hoffman JA, Ahl M, Bhaumik U, Healey C, Carter S, et al. Collaborating for impact: a multilevel early childhood obesity prevention initiative. Family & Community Health 2012;35:192‐202. [DOI] [PubMed] [Google Scholar]
- Ahearn WH, Kerwin ME, Eicher PS, Lukens CT. An ABAC comparison of two intensive interventions for food refusal. Behavior Modification 2001;25(3):385‐405. [DOI] [PubMed] [Google Scholar]
- Ahern SM, Caton SJ, Blundell P, Hetherington MM. The root of the problem: increasing root vegetable intake in preschool children by repeated exposure and flavour flavour learning. Appetite 2014;80:154‐60. [DOI] [PubMed] [Google Scholar]
- Ajie W. Totally veggies. Journal of Nutrition, Education and Behavior2016; Vol. 48, issue 10:753.
- Al Bashabsheh Z, Al Bashabsheh Z, Kidd T. Evaluating the effectiveness of nutrition education for WIC service clients in Manhattan, Kansas. Journal of Nutrition Education and Behavior 2016;48(7):S18. [Google Scholar]
- Alford BB, Tibbets MH. Education increases consumption of vegetables by children. Journal of Nutrition Education 1971;3(7):12‐4. [Google Scholar]
- Amin S, Stickle T, Eriksen H, Johnson RK. Nudging pre‐school children's fruit and vegetable consumption during afternoon snack time using older child mentors from the Live Y'ers Afterschool Program. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A25. [Google Scholar]
- Anderson LM, Symoniak ED, Epstein LH. A randomized pilot trial of an integrated school‐worksite weight control program. Health Psychology 2014;33(11):1421‐5. [DOI] [PubMed] [Google Scholar]
- Anez E, Remington A, Wardle J, Cooke L. The impact of instrumental feeding on children's responses to taste exposure. Journal of Human Nutrition and Dietetics 2013;26(5):415‐20. [DOI] [PubMed] [Google Scholar]
- Ang I, Trent R, Gray HL, Wolf R, Koch P, Contento I. Comparison of school lunch cut fruit and whole fruit consumption in a naturalistic elementary school cafeteria setting. Journal of Nutrition Education and Behavior 2016;48(7):S14. [Google Scholar]
- Anliker JA, Drake LT, Pacholski J, Little W. Impacts of a multi‐layered nutrition education program: teenagers teaching children. Journal of Nutrition Education 1993;25(3):140‐3. [Google Scholar]
- Anonymous. Some children baffled by satsumas. Nursing Times 2001;97:5‐5. [Google Scholar]
- Anonymous. Welfare food scheme to be extended. RCM Midwives2002; Vol. 5:404.
- Anonymous. Web campaign invites children to get involved with healthy eating. Paediatric Nursing 2009;21:5‐5. [Google Scholar]
- Anonymous. Postscripts. Nutrition Health Review: The Consumer's Medical Journal2011:20.
- Anonymous. Target parents to prevent obesity. Australian Nursing Journal 2011;18:35. [PubMed] [Google Scholar]
- Anonymous. European Congress on Obesity, ECO2012. Obesity Facts2012; Vol. 5.
- Apatu E, Sealey‐Potts C, Diersing J. Cooking classes: are they effective nutrition interventions in low‐income settings?. Journal of Nutrition Education and Behavior 2016;48(7):S9. [Google Scholar]
- Aranceta‐Bartrina J, Perez‐Rodrigo C. Determinants of childhood obesity: ANIBES study. Nutricion Hospitalaria 2016;33(Suppl 4):339. [DOI] [PubMed] [Google Scholar]
- Arrow P, Raheb J, Miller M. Brief oral health promotion intervention among parents of young children to reduce early childhood dental decay. BMC Public Health 2013;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au LE, Rosen NJ, Ritchie LD. Does eating school meals make a difference in overall diet quality? a comparison study of elementary school students. Journal of the Academy of Nutrition and Dietetics 2015;115(9):A16. [Google Scholar]
- Au L, Whaley S, Rosen N, Meza M, Ritchie L. A randomized controlled trial evaluating online to in person education to improve breakfast behaviors, beliefs and knowledge in WIC participants. FASEB Journal. Conference: Experimental Biology Meeting Abstracts 2015;29(1):264.3. [Google Scholar]; Au LE, Whaley S, Rosen NJ, Meza M, Ritchie LD. Online and in‐person nutrition education improves breakfast knowledge, attitudes, and behaviors: a randomized trial of participants in the special supplemental nutrition program for women, infants, and children. Journal of the Academy of Nutrition and Dietetics 2016;116(3):490‐500. [DOI] [PubMed] [Google Scholar]
- Au LE, Rosen NJ, Fenton K, et al. Eating school lunch Is associated with higher diet quality among elementary school students. Journal of the Academy of Nutrition and Dietetics 2016;116(11):1817‐24. [DOI] [PubMed] [Google Scholar]
- Bai Y, Suriano L, Wunderlich S. Veggiecation for the love of vegetables. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S23‐4. [DOI] [PubMed] [Google Scholar]
- Bannon K, Schwartz MB. Impact of nutrition messages on children's food choice: pilot study. Appetite 2006;46:124‐9. [DOI] [PubMed] [Google Scholar]
- Bante H, Elliott M, Harrod A, Haire‐Joshu D. The use of inappropriate feeding practices by rural parents and their effect on preschoolers' fruit and vegetable preferences and intake. Journal of Nutrition Education and Behavior 2008;40(1):28‐33. [DOI] [PubMed] [Google Scholar]
- Baranowski T, Baranowski J, Cullen KW, DeMoor C, Rittenberry L, Hebert D, et al. 5 a day achievement badge for African‐American boy scouts: pilot outcome results. Preventive Medicine 2002;34(3):353‐63. [DOI] [PubMed] [Google Scholar]
- Barkin SL, Gesell SB, Po'e EK, Escarfuller J, Tempesti T. Culturally tailored, family‐centered, behavioral obesity intervention for Latino‐American preschool‐aged children. Pediatrics 2012;130(3):445‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter SD. Are elementary schools teaching children to prefer candy but not vegetables?. Journal of School Health 1998;68(3):111‐3. [DOI] [PubMed] [Google Scholar]
- Bayer O, Kries R, Strauss A, Mitschek C, Toschke AM, Hose A, et al. Short‐ and mid‐term effects of a setting based prevention program to reduce obesity risk factors in children: a cluster‐randomized trial. Clinical Nutrition 2009;28:122‐8. [DOI] [PubMed] [Google Scholar]; Strauss A, Herbert B, Mitschek C, Duvinage K, Koletzko B. TigerKids. Successful health promotion in preschool settings. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2011;54(3):322‐9. [DOI] [PubMed] [Google Scholar]
- Beasley N, Sharma S, Shegog R, Huber R, Abernathy P, Smith C, et al. The Quest to Lava Mountain: using video games for dietary change in children. Journal of the Academy of Nutrition and Dietetics 2012;112(9):1334‐6. [DOI] [PubMed] [Google Scholar]
- Beets MW, Brazendale K, Weaver RG, Turner‐McGrievy GM, Huberty J, Moore JB, et al. Economic evaluation of a group randomized controlled trial on healthy eating and physical activity in afterschool programs. Preventive Medicine 2017;106:60‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Beets MW, Turner‐McGrievy B, Weaver RG, Huberty J, Moore JB, Ward DS, et al. Intervention leads to improvements in the nutrient profile of snacks served in afterschool programs: a group randomized controlled trial. Translational Behavioral Medicine 2016;6(3):329‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]; Beets MW, Weaver RG, Turner‐McGrievy G, Huberty J, Ward DS, Freedman D, et al. Making healthy eating policy practice: a group randomized controlled trial on changes in snack quality, costs, and consumption in after‐school programs. American Journal of Health Promotion 2016;30(7):521‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows L, Johnson SL, Davies PL, Anderson J, Gavin W, Boles RE. The Colorado LEAP Study: a longitudinal study for obesity prevention in early childhood. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S78. [Google Scholar]; Bellows L, Johnson SL, Davies PL, Gavin W, Boles RE. Findings from the Colorado LEAP Study: a longitudinal study for obesity prevention in early childhood. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S189. [Google Scholar]
- Benjamin SE, Haines J, Ball SC, Ward DS. Improving nutrition and physical activity in child care: what parents recommend. Journal of the American Dietetic Association 2008;108(11):1907‐11. [DOI] [PubMed] [Google Scholar]
- Benjamin Neelon SE, Mayhew M, O’Neill JR, Neelon B, Li F, Pate RR. Comparative evaluation of a South Carolina policy to improve nutrition in child care. Journal of the Academy of Nutrition and Dietetics 2016;116(6):949‐56. [DOI] [PubMed] [Google Scholar]
- Bensley RJ, Anderson JV, Brusk JJ, Mercer N, Rivas J. Impact of internet vs traditional special supplemental nutrition program for women, infants, and children nutrition education on fruit and vegetable intake. Journal of the American Dietetic Association 2011;111(5):749‐55. [DOI] [PubMed] [Google Scholar]
- Bere E, Velde SJ, Smastuen MC, Twisk J, Klepp KI. One year of free school fruit in Norway‐‐7 years of follow‐up. International Journal of Behavioral Nutrition & Physical Activity 2015;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L. Raising a healthy, happy eater. Journal of Nutrition Education and Behavior 2016;48(5):356. [Google Scholar]
- Bergman D, Barry C. This is way better than cheetos; changing children's eating behavior through garden and kitchen‐based nutrition education. Journal of Nutrition Education and Behavior 2016;48(7):S9‐S10. [Google Scholar]
- Berhe G. Tulimbe Nutrition Project: a community‐based dietary intervention to combat micronutrient malnutrition in rural southern Malawi. SCN news 1997;Dec(15):25‐6. [PubMed] [Google Scholar]
- Berry DC, Neal M, Hall EG, Schwartz TA, Verbiest S, Bonuck K, et al. Rationale, design, and methodology for the optimizing outcomes in women with gestational diabetes mellitus and their infants study. BMC Pregnancy and Childbirth 2013;13:No pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessems KM, Assema P, Martens MK, Paulussen TG, Raaijmakers LG, Rooij M, et al. Healthier food choices as a result of the revised healthy diet programme Krachtvoer for students of prevocational schools. International Journal of Behavioral Nutrition and Physical Activity2012; Vol. 9:60. [DOI] [PMC free article] [PubMed]
- Best JR, Goldschmidt AB, Mockus‐Valenzuela DS, Stein RI, Epstein LH, Wilfley DE. Shared weight and dietary changes in parent‐child dyads following family‐based obesity treatment. Health Psychology 2016;35(1):92‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibiloni MDM, Fernandez‐Blanco J, Pujol‐Plana N, Martin‐Galindo N, Fernandez‐Vallejo MM, Roca‐Domingo M, et al. Improving diet quality in children through a new nutritional education programme: INFADIMED. Gaceta Sanitaria2017; Vol. 11:11. [DOI] [PubMed]
- Birch LL. Effects of peer models' food choices and eating behaviors on preschoolers' food preferences. Child Development1980; Vol. 51, issue 2:489‐96.
- Birch LL, Marlin DW. I don't like it; never tried it: effects of exposure on two‐year‐old children's food preferences. Appetite1982; Vol. 3:353‐60. [DOI] [PubMed]
- Birch LL. Development of food acceptance patterns in the first years of life. Proceedings of the Nutrition Society1998; Vol. 57, issue 4:617‐24. [DOI] [PubMed]
- Black AP, Vally H, Morris P, Daniel M, Esterman A, Karschimkus CS, et al. Nutritional impacts of a fruit and vegetable subsidy programme for disadvantaged Australian Aboriginal children. British Journal of Nutrition 2013;110(12):2309‐17. [DOI] [PubMed] [Google Scholar]
- Blissett J, Bennett C, Donohoe J, Rogers S, Higgs S. Predicting successful introduction of novel fruit to preschool children. Journal of the Academy of Nutrition and Dietetics 2012;112(12):1959‐67. [DOI] [PubMed] [Google Scholar]
- Blom‐Hoffman J, Wilcox KR, Dunn L, Leff SS, Power TJ. Family involvement in school‐based health promotion: bringing nutrition information home. School Psychology Review 2008;37(4):567‐77. [PMC free article] [PubMed] [Google Scholar]
- Boaz A, Ziebland S, Wyke S, Walker J. A 'five‐a‐day' fruit and vegetable pack for primary school children. Part II: controlled evaluation in two Scottish schools. Health Education Journal 1998;57:105‐16. [Google Scholar]
- Bollella MC, Spark A, Boccia LA, Nicklas TA, Pittman BP, Williams CL. Nutrient intake of Head Start children: home vs school. Journal of the American College of Nutrition 1999;18(2):108‐14. [DOI] [PubMed] [Google Scholar]
- Bonvecchio‐Arenas A, Theodore FL, Hernandez‐Cordero S, Campirano‐Nunez F, Islas AL, Safdie M, et al. The school as an opportunity for obesity prevention: an experience from the Mexican school system [La escuela como alternativa en la prevencion de la obesidad: La experiencia en el sistema escolar Mexicano]. Revista Española de Nutricion Comunitaria 2010;16:13‐6. [Google Scholar]
- Borys JM, Richard P, Ruault du Plessis H, Harper P, Levy E. Tackling health inequities and reducing obesity prevalence: the EPODE community‐based approach. Annals of Nutrition & Metabolism 2016;68(Suppl 2):35‐8. [DOI] [PubMed] [Google Scholar]
- Bouhlal S, Issanchou S, Chabanet C, Nicklaus S. 'Just a pinch of salt'. An experimental comparison of the effect of repeated exposure and flavor‐flavor learning with salt or spice on vegetable acceptance in toddlers. Appetite 2014;83:209‐17. [DOI] [PubMed] [Google Scholar]
- Bradley CL. The effect of a classroom intervention on fruit and vegetable intake in preschoolers in a public school setting. Dissertation Abstracts International Section A: Humanities and Social Sciences2014; Vol. 75.
- Brambilla P, Bedogni G, Buongiovanni C, Brusoni G, Mauro G, Pietro M, et al. "Mi voglio bene": a pediatrician‐based randomized controlled trial for the prevention of obesity in Italian preschool children. Italian Journal of Pediatrics 2010;36:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branscum P, Sharma M, Wang LL, Wilson BR, Rojas‐Guyler L. A true challenge for any superhero: an evaluation of a comic book obesity prevention program. Family & Community Health 2013;36:63‐76. [DOI] [PubMed] [Google Scholar]; Branscum PW. Designing and evaluating an after‐school social cognitive theory based comic book intervention for the prevention of childhood obesity among elementary aged school children. Dissertation Abstracts International Section A: Humanities and Social Sciences 2012;73:87. [Google Scholar]
- Briefel R, Hanson C, Fox MK, Novak T, Ziegler P. Feeding infants and toddlers study: do vitamin and mineral supplements contribute to nutrient adequacy or excess among US infants and toddlers?. Journal of the American Dietetic Association 2006;106:S52‐S65. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Crepinsek MK, Cabili C, Wilson A, Gleason PM. School food environments and practices affect dietary behaviors of US public school children. Journal of the American Dietetic Association 2009;109:S91‐S107. [DOI] [PubMed] [Google Scholar]
- Briefel RR. New findings from the Feeding Infants and Toddlers Study: data to inform action. Journal of the American Dietetic Association 2010;110(12, Supplement):S5‐7. [DOI] [PubMed] [Google Scholar]; Dwyer JT, Butte NF, Deming DM, Siega‐Riz AM, Reidy KC. Feeding Infants and Toddlers Study 2008: progress, continuing concerns, and implications. Journal of the American Dietetic Association 2010;110(12 Supplement):S60‐7. [DOI] [PubMed] [Google Scholar]; May AL, Dietz WH. The Feeding Infants and Toddlers Study 2008: opportunities to assess parental, cultural, and environmental influences on dietary behaviors and obesity prevention among young children. Journal of the American Dietetic Association 2010;110(12 Supplement):S11‐5. [DOI] [PubMed] [Google Scholar]
- Briley ME, Jastrow S, Vickers J, Roberts‐Gray C. Dietary intake at child‐care centers and away: are parents and child care providers working as partners or at cross‐purposes?. Journal of the American Dietetic Association 1999;99(8):950‐4. [DOI] [PubMed] [Google Scholar]
- Briley M, McAllaster M. Nutrition and the child‐care setting. Journal of the American Dietetic Association 2011;111(9):1298‐300. [DOI] [PubMed] [Google Scholar]
- Briley ME, Romo‐Palfox MJ, Sweitzer SJ, Roberts‐Gray C, Hoelscher DM, Nanjit N. Percent of energy consumed by preschool children vary by type of food offered. Obesity Reviews 2016;17:127‐8. [Google Scholar]
- Britt‐Rankin J. Healthy eating from head to toe. Journal of Nutrition Education and Behavior2016; Vol. 49, issue 1:83.
- Brotman LM, Dawson‐McClure S, Huang K‐Y, Theise R, Kamboukos D, Wang J, et al. Early childhood family intervention and long‐term obesity prevention among high‐risk minority youth. Pediatrics 2012;129(3):e621‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening KS, Gilbride JA, Passannante MR, McClowry S. Dietary intake and health outcomes among young children attending 2 urban day‐care centers. Journal of the American Dietetic Association 1999;99(12):1529‐35. [DOI] [PubMed] [Google Scholar]
- Brunt A. P136 Do spokes‐characters improve consumption of vegetables among children?. Journal of Nutrition Education & Behavior 2012;44:S77‐8. [Google Scholar]
- Bryant M, Burton W, Cundill B, Farrin AJ, Nixon J, Stevens J, et al. Effectiveness of an implementation optimisation intervention aimed at increasing parent engagement in HENRY, a childhood obesity prevention programme ‐ the Optimising Family Engagement in HENRY (OFTEN) trial: study protocol for a randomised controlled trial. Trials2017; Vol. 3, issue 18:40. [DOI] [PMC free article] [PubMed]
- Burgermaster M, Koroly J, Contento I, Koch P, Gray HL. A mixed‐methods comparison of classroom context during food, health & choices, a childhood obesity prevention intervention. Journal of School Health 2017;87(11):811‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer I, Burgi F, Ebenegger V, Schindler C, Marques‐Vidal P, Kriemler S, et al. Effect of a lifestyle intervention on adiposity and fitness in high‐risk subgroups of preschoolers (Ballabeina): a cluster‐randomized trial. Endocrine Reviews2011; Vol. 32. ; Niederer I, Kriemler S, Zahner L, Burgi F, Ebenegger V, Hartmann T, et al. Influence of a lifestyle intervention in preschool children on physiological and psychological parameters (Ballabeina): study design of a cluster randomized controlled trial. BMC Public Health 2009;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]; Puder JJ, Marques‐Vidal P, Schindler C, Zahner L, Niederer I, Bürgi F, et al. Effect of multidimensional lifestyle intervention on fitness and adiposity in predominantly migrant preschool children (Ballabeina): cluster randomised controlled trial. BMJ 2011;343:d6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttriss J. Food promotion to children: the facts. Nutrition Bulletin 2004;29:3‐5. [Google Scholar]
- Byrd‐Bredbenner C, Worobey J, Martin‐Biggers J, Berhaupt‐Glickstein A, Hongu N, Hernandez G. HomeStyles: shaping home environments and lifestyle practices to prevent childhood obesity: a randomized controlled trial. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S190. [Google Scholar]; Byrd‐Bredbenner C, Worobey J, Martin‐Biggers J, Berhaupt‐Glickstein A, Hongu N, Hernandez G, et al. HomeStyles: shaping home environments and lifestyle practices to prevent childhood obesity: a randomized controlled trial. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S81. [Google Scholar]
- Byrne E, Nitzke S. Preschool children’s acceptance of a novel vegetable following exposure to messages in a storybook. Journal of Nutrition Education and Behavior 2002;34:211‐4. [DOI] [PubMed] [Google Scholar]
- Camelo R. Ludotecas Saludables: towards healthier lifestyles. Journal of Nutrition Education and Behavior 2016;48(7):S21. [Google Scholar]
- Campbell KJ, Hesketh KD, McNaughton SA, Ball K, McCallum Z, Lynch J, et al. The extended Infant Feeding, Activity and Nutrition Trial (InFANT Extend) Program: a cluster‐randomized controlled trial of an early intervention to prevent childhood obesity. BMC Public Health 2016;16(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]; Downing KL, Campbell KJ, Pligt P, Hesketh KD. Facilitator and participant use of Facebook in a community‐based intervention for parents: the InFANT Extend Program. Childhood Obesity 2017;13(6):443‐54. [DOI] [PubMed] [Google Scholar]
- Campbell RK, Hurley KM, Shamim AA, Shaikh S, Chowdhury ZT, Mehra S, et al. Effect of complementary food supplementation on breastfeeding and home diet in rural Bangladeshi children. American Journal of Clinical Nutrition2016; Vol. 104, issue 5:1450‐8. [DOI] [PMC free article] [PubMed]
- Campbell KJ, Abbott G, Zheng M, , McNaughton SA. Early life protein intake: food sources, correlates, and tracking across the first 5 years of life. Journal of the Academy of Nutrition and Dietetics2017; Vol. 117, issue 8:1188‐97. [DOI] [PubMed]
- Candido A, Godinho C, Amendoeira J. Health promoting school project as a vehicle for the promotion of healthy lifestyles: the importance of food. Atencion Primaria 2013;45:21. 22981282 [Google Scholar]
- Capaldi‐Phillips ED, Wadhera D. Associative conditioning can increase liking for and consumption of brussels sprouts in children aged 3 to 5 Years. Journal of the Academy of Nutrition and Dietetics 2014;114(8):1236‐41. [DOI] [PubMed] [Google Scholar]
- Carney E. Children's Response to Flavor Variety In Herb and Spice Seasoned Vegetables Served Within a Meal [Masters Thesis]. Pennsylvania: Pennsylvania State University, 2017. [Google Scholar]
- Carter BJ, Birnbaum AS, Hark L, Vickery B, Potter C, Osborne MP. Gem no. 392. Using media messaging to promote healthful eating and physical activity among urban youth. Journal of Nutrition Education & Behavior 2005;37:98‐9. [DOI] [PubMed] [Google Scholar]
- Cason KL. Evaluation of a preschool nutrition education program based on the theory of multiple intelligences. Journal of Nutrition Education 2001;33:161‐4. [DOI] [PubMed] [Google Scholar]
- Castro DC, Samuels M, Harman AE. Growing healthy kids: a community garden‐based obesity prevention program. American Journal of Preventive Medicine 2013;44(3 Suppl 3):S193‐9. [DOI] [PubMed] [Google Scholar]
- Cates S, Williams P, Hersey J, Blitstein J, Kosa K, Singh A, et al. SNAP‐Ed interventions can increase children's at‐home fruit and vegetable consumption and use of fat‐free/low‐fat milk. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S181. [Google Scholar]; Williams PA, Cates SC, Blitstein JL, Hersey JC, Kosa KM, Long VA, et al. Evaluating the impact of six supplemental nutrition assistance program education interventions on children's at‐home diets. Health Education & Behavior 2015;42(3):329‐38. [DOI] [PubMed] [Google Scholar]
- Caton SJ, Blundell P, Ahern SM, Nekitsing C, Olsen A, Moller P, et al. Learning to eat vegetables in early life: the role of timing, age and individual eating traits. PLoS One 2014;9:e97609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Céspedes J, Briceño G, Farkouh M, Vedanthan R, Leal M, Dennis R, et al. A randomized preschool trial to promote cardiovascular health in Colombia: 12 month follow up. Circulation 2012;125(19):e703. [Google Scholar]; Céspedes J, Briceño G, Farkouh ME, Vedanthan R, Baxter J, Leal M, et al. Promotion of cardiovascular health in preschool children: 36‐month cohort follow‐up. American Journal of Medicine 2013;126(12):1122‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Céspedes J, Briceño G, Farkouh ME, Vedanthan R, Baxter J, Leal M, et al. Targeting preschool children to promote cardiovascular health: cluster randomized trial. American Journal of Medicine 2013;126(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham C, Huye HF, Landry AS. Impact of packaging on children's food choices. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A22. [Google Scholar]
- Chen Q, Goto K, Wolff C, Zhao Y. Relationships between children's exposure to ethnic produce and their dietary behaviors. Journal of Immigrant & Minority Health 2015;17(2):383‐8. [DOI] [PubMed] [Google Scholar]
- Ciampolini M, Vicarelli D, Bini S. Choices at weaning: main factor in ingestive behavior. Nutrition 1991;7(1):51‐4. [PubMed] [Google Scholar]
- Clason ER, Meijer D. "Eat your greens": increasing the number of days that picky toddlers eat vegetables. Social Marketing Quarterly 2016;22(2):119‐37. [Google Scholar]
- Coelho JS, Akker K, Nederkoorn C, Jansen A. Pre‐exposure to high‐ versus low‐caloric foods: effects on children's subsequent fruit intake. Eating Behaviors2012; Vol. 13, issue 1:71‐3. [DOI] [PubMed]
- Cohen JFW, Kraak VI, Choumenkovitch SF, Hyatt RR, Economos CD. The CHANGE Study: a healthy‐lifestyles intervention to improve rural children's diet quality. Journal of the Academy of Nutrition and Dietetics 2014;114(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman G, Horodynski MA, Contreras D, Hoerr SM. Nutrition education aimed at toddlers (NEAT) curriculum. Journal of Nutrition Education and Behavior 2005;37(2):96‐7. [DOI] [PubMed] [Google Scholar]; Horodynski MA, Stommel M. Nutrition education aimed at toddlers: an intervention study. Pediatric Nursing 2005;31(5):364‐72. [PubMed] [Google Scholar]
- Burrows T, Janet WM, Collins CE. Long‐term changes in food consumption trends in overweight children in the HIKCUPS intervention. Journal of Pediatric Gastroenterology & Nutrition 2011;53(5):543‐7. [DOI] [PubMed] [Google Scholar]; Burrows T, Warren J, Bau L, Collins C. Impact of a child obesity intervention on dietary intake and behaviors. International Journal of Obesity 2008;32(10):1481‐8. [DOI] [PubMed] [Google Scholar]; Collins CE, Okely AD, Morgan PJ, Jones RA, Burrows TL, Cliff DP, et al. Parent diet modification, child activity, or both in obese children: an RCT. Pediatrics 2011;127(4):619‐27. [DOI] [PubMed] [Google Scholar]
- Condrasky M, Graham K, Kamp J. Cooking with a chef: an innovative program to improve mealtime practices and eating behaviors of caregivers of preschool children. Journal of Nutrition Education and Behavior 2006;38(5):324‐5. [DOI] [PubMed] [Google Scholar]
- Cooper N, Jones C. Improving the quality of packed lunches in primary school children. Journal of Human Nutrition & Dietetics 2011;24:384‐5. [Google Scholar]
- Cooperberg J. Food for Thought: a parental internet‐based intervention to treat childhood obesity in preschool‐aged children. Dissertation Abstracts International: Section B: The Sciences and Engineering2014; Vol. 74.
- Copeland AL, Williamson DA, Kendzor DE, Businelle MS, Rash CJ, Kulesza M, et al. A school‐based alcohol, tobacco, and drug prevention program for children: The Wise Mind study. Cognitive Therapy and Research 2010;34:522‐32. [Google Scholar]
- Coppinger T, Lacey S, O'Neill C, Burns C. 'Project Spraoi': a randomized control trial to improve nutrition and physical activity in school children. Contemporary Clinical Trials Communications 2016;3:94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini N, Slater A, Harrison A, Cooke L, Cox DN. Rewards can be used effectively with repeated exposure to increase liking of vegetables in 4‐6‐year‐old children. Public Health Nutrition 2013;16(5):942‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotwright CJ, Bales DW, Lee JS, Parrott K, Celestin N, Olubajo B. Like peas and carrots: combining wellness policy implementation with classroom education for obesity prevention in the childcare setting. Public Health Reports 2015;132(Suppl 2):74S‐80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotwright C, Bales D, Lee JS, Akin J. Taste & See: improving willingness to try fruit and vegetables among low‐income preschool children. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 7:S2.
- Coulthard H, Williamson I, Palfreyman Z, Lyttle S. Evaluation of a pilot sensory play intervention to increase fruit acceptance in preschool children. Appetite 2018;120:609‐15. [DOI] [PubMed] [Google Scholar]
- Court JM. Obesity in childhood. The Medical Journal of Australia 1977;1(24):888‐91. [DOI] [PubMed] [Google Scholar]
- Crespo NC, Elder JP, Ayala GX, Slymen DJ, Campbell NR, Sallis JF, et al. Results of a multi‐level intervention to prevent and control childhood obesity among Latino children: the Aventuras Para Niños Study. Annals of Behavioral Medicine 2012;43(1):84‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker H, Lucas R, Wardle J. Cluster‐randomised trial to evaluate the 'Change for Life' mass media/ social marketing campaign in the UK. BMC Public Health 2012;12:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TH, Davis SM, FitzGerald CA, Canaca GF, Keane PC. Engagement, recruitment, and retention in a trans‐community, randomized controlled trial for the prevention of obesity in rural American Indian and Hispanic children. Journal of Primary Prevention 2014;35(3):135‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KW, Dave JM, Chen A, Elliott L, Walker F, Jensen H. Implementing the new school meal regulations: do elementary school children select and eat 1 fruit and 2 vegetable servings when allowed?. Journal of the Academy of Nutrition and Dietetics 2013;113(9):A11. [Google Scholar]
- Cullen KW, Chen TA, Dave JM, Jensen H. Differential improvements in student fruit and vegetable selection and consumption in response to the new national school lunch program regulations: a pilot study. Journal of the Academy of Nutrition and Dietetics2015; Vol. 115, issue 5:743‐50. [DOI] [PMC free article] [PubMed]
- Curtis PJ, Adamson AJ, Mathers JC. Effects on nutrient intake of a family‐based intervention to promote increased consumption of low‐fat starchy foods through education, cooking skills and personalised goal setting: the Family Food and Health Project. British Journal of Nutrition 2012;107(12):1833‐44. [DOI] [PubMed] [Google Scholar]
- Dai C‐L. Evaluation of an afterschool obesity prevention program: Children's Healthy Eating and Exercise Program. Dissertation Abstracts International Section A: Humanities and Social Sciences2015; Vol. 76, issue 2‐A(E).
- Dalton WT, Schetzina KE, Holt N, Fulton‐Robinson H, Ho AL, Tudiver F, et al. Parent‐led activity and nutrition (plan) for healthy living: design and methods. Contemporary Clinical Trials 2011;32(6):882‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R, Yeo MEJ, Mallan K, Magarey A, Daniels L. Is higher formula intake and limited dietary diversity in Australian children at 14 months of age associated with dietary quality at 24 months?. Appetite 2018;120:240‐5. [DOI] [PubMed] [Google Scholar]; Daniels LA. Complementary feeding in an obesogenic environment: behavioral and dietary quality outcomes and interventions. Nestle Nutrition Institute Workshop Series 2017;87:167‐81. [DOI] [PubMed] [Google Scholar]; Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. International Journal of Obesity2012; Vol. 36, issue 10:1292‐8. [DOI] [PubMed]; Daniels LA, Mallan KM, Nicholson JM, Thorpe K, Nambiar S, Mauch CE, et al. An early feeding practices intervention for obesity prevention. Pediatrics2015; Vol. 136, issue 1:e40‐9. [DOI] [PubMed]; Daniels LA, Wilson JL, Mallan KM, Mihrshahi S, Perry R, Nicholson JM, et al. Recruiting and engaging new mothers in nutrition research studies: lessons from the Australian NOURISH randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity2012; Vol. 9:129. [DOI] [PMC free article] [PubMed]
- Dannefer R, Power L, Berger R, Sacks R, Roberts C, Bikoff R, et al. Process evaluation of a farm‐to‐preschool program in New York City. Journal of Hunger & Environmental Nutrition 2017;Epub ahead of print:1‐19. [Google Scholar]
- Davis SM, Sanders SG, FitzGerald CA, Keane PC, Canaca GF, Volker‐Rector R. CHILE: an evidence‐based preschool intervention for obesity prevention in Head Start. Journal of School Health 2013;83(3):223‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli AM, Broccoli S, Bonvicini L, Fabbri A, Ferrari E, D'Angelo S, et al. Pediatrician‐led motivational interviewing to treat overweight children: an RCT. Pediatrics2013; Vol. 132, issue 5:e1236‐46. [DOI] [PubMed]
- Day ME, Strange KS, McKay HA, Naylor P. Action schools! BC‐‐Healthy Eating: effects of a whole‐school model to modifying eating behaviours of elementary school children. Canadian Journal of Public Health 2008;99(4):328‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazeley P, Houston‐Price C. Exposure to foods' non‐taste sensory properties. A nursery intervention to increase children's willingness to try fruit and vegetables. Appetite 2015;84:1‐6. [DOI] [PubMed] [Google Scholar]
- Arvidsson L, Bogl LH, Eiben G, Hebestreit A, Nagy P, Tornaritis M, et al. Fat, sugar and water intakes among families from the IDEFICS intervention and control groups: first observations from i.family. Obesity Reviews2015; Vol. 16:127‐37. [DOI] [PubMed]; Bammann K, Peplies J, Sjostrom M, Lissner L, Henauw S, Galli C, et al. Assessment of diet, physical activity and biological, social and environmental factors in a multi‐centre European project on diet‐ and lifestyle‐related disorders in children (IDEFICS). Journal of Public Health 2006;14:279‐89. [Google Scholar]; Bourdeaudhuij I, Verbestel V, Henauw S, Maes L, Huybrechts I, Marild S, et al. Behavioural effects of a community‐oriented setting‐based intervention for prevention of childhood obesity in eight European countries. Main results from the IDEFICS study. Obesity Reviews 2015;16 Suppl 2:30‐40. [DOI] [PubMed] [Google Scholar]; Verbestel V, Henauw S, Maes L, Haerens L, Mårild S, Eiben G, et al. Using the intervention mapping protocol to develop a community‐based intervention for the prevention of childhood obesity in a multi‐centre European project: the IDEFICS intervention. International Journal of Behavioral Nutrition & Physical Activity 2011;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droog SM, Valkenburg PM, Buijzen M. Using brand characters to promote young children's liking of and purchase requests for fruit. Journal of Health Communication 2011;16(1):79‐89. [DOI] [PubMed] [Google Scholar]
- Droog SM, Buijzen M, Valkenburg PM. Use a rabbit or a rhino to sell a carrot? The effect of character‐product congruence on children's liking of healthy foods. Journal of Health Communication 2012;17(9):1068‐80. [DOI] [PubMed] [Google Scholar]
- Delgado EG, Cosso TG, Aragons AC, Pelletier D, Quezada AD, Ramrez SR. Effect of a food aid program on BMI/A of Mexican children, mediated by diet. FASEB Journal2014; Vol. 1.
- Pee S, Bloem MW, Satoto, Yip R, Sukaton A, Tjiong R, et al. Impact of a social marketing campaign promoting dark‐green leafy vegetables and eggs in Central Java, Indonesia. International Journal of Vitamin and Nutrient Research 1998;68(6):389‐98. [PubMed] [Google Scholar]
- Silva‐Sanigorski AM, Bell AC, Kremer P, Nichols M, Crellin M, Smith M, et al. Reducing obesity in early childhood: results from Romp & Chomp, an Australian community‐wide intervention program. American Journal of Clinical Nutrition 2010;91:831‐40. [DOI] [PubMed] [Google Scholar]
- Dick L. Sowing seeds for healthy kids. Journal of Nutrition Education and Behavior 2016;48(5):358. [Google Scholar]
- Dollahite JS, Pijai EI, Scott‐Pierce M, Parker C, Trochim W. A randomized controlled trial of a community‐based nutrition education program for low‐income parents. Journal of Nutrition Education and Behavior 2014;46(2):102‐9. [DOI] [PubMed] [Google Scholar]
- Dorado J, Azana G, Viajar R, Capanzana M. Improving nutrition knowledge, attitude and behavior of selected Filipino schoolchildren in the Healthy Kids Program. Journal of Nutrition Education and Behavior 2015;47(4):S6‐7. [Google Scholar]
- Draper CE, Villiers A, Lambert EV, Fourie J, Hill J, Dalais L, et al. HealthKick: a nutrition and physical activity intervention for primary schools in low‐income settings. BMC Public Health 2010;10:398. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uys M, Draper CE, Hendricks S, Villiers A, Fourie J, Steyn NP, et al. Impact of a South African school‐based Intervention, HealthKick, on fitness correlates. American Journal of Health Behavior 2016;40(1):55‐66. [DOI] [PubMed] [Google Scholar]
- Duke T. Randomised trials in child health in developing countries 2011. Annals of Tropical Paediatrics 2011;31(4):283‐5. [DOI] [PubMed] [Google Scholar]
- Duncanson K, Lee YQ, Burrows T, Collins C. Utility of a brief index to measure diet quality of Australian preschoolers in the Feeding Healthy Food to Kids Randomised Controlled Trial. Nutrition & Dietetics2017; Vol. 74, issue 2:158‐66. [DOI] [PubMed]
- Dunn C, Thomas C, Pegram L, Ward D, Schmal S. Color me healthy, preschoolers moving and eating healthfully. Journal of Nutrition Education and Behavior 2004;36(6):327‐8. [DOI] [PubMed] [Google Scholar]; Dunn C, Thomas C, Ward D, Pegram L, Webber K, Cullitan C. Design and implementation of a nutrition and physical activity curriculum for child care settings. Preventing Chronic Disease 2006;3:A58. [PMC free article] [PubMed] [Google Scholar]
- Eicholzer‐Helbling M, Ritzel G, Ackermann‐Liebrich U, Bachlin A, Muhlemann R. Nutrition education in kindergarten: results of an intervention study [Ernahrungserziehung im kindergarten: resultate einer interventionsstudie]. Sozial‐ und Praventivmedizin 1986;31(4‐5):233‐5. [DOI] [PubMed] [Google Scholar]
- Elder JP, Crespo NC, Corder K, Ayala GX, Slymen DJ, Lopez NV, et al. Childhood obesity prevention and control in city recreation centres and family homes: the MOVE/me Muevo Project. Pediatric Obesity 2014;9(3):218‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo‐Montemayor L, Moreno‐Sanchez D, Gutierrez NG, Monsivais‐Rodriguez F, Martinez U, Lamadrid‐Zertuche AC, et al. Individualized tailor‐made dietetic intervention program at schools enhances eating behaviors and dietary habits in obese Hispanic children of low socioeconomic status. Scientific World Journal 2014;2014:484905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obesity Research 2001;9(3):171‐8. [DOI] [PubMed] [Google Scholar]
- Esfarjani F, Khalafi M, Mohammadi F, Mansour A, Roustaee R, Zamani‐Nour N, et al. Family‐based intervention for controlling childhood obesity: an experience among Iranian children. International Journal of Preventive Medicine 2013;4(3):358‐65. [PMC free article] [PubMed] [Google Scholar]
- Esquivel M, Nigg CR, Fialkowski MK, Braun KL, Li F, Novotny R. Head Start wellness policy intervention in Hawaii: a project of the Children's Healthy Living Program. Childhood Obesity 2016;12(1):26‐32. [DOI] [PubMed] [Google Scholar]
- Estabrooks PA, Shoup JA, Gattshall M, Dandamudi P, Shetterly S, Xu S. Automated telephone counseling for parents of overweight children. A randomized controlled trial. American Journal of Preventive Medicine 2009;36(1):35‐42. [DOI] [PubMed] [Google Scholar]
- Evans AE, Dave J, Tanner A, Duhe S, Condrasky M, Wilson D, et al. Changing the home nutrition environment. Effects of a nutrition and media literacy pilot intervention. Family and Community Health 2005;29(1):43‐54. [DOI] [PubMed] [Google Scholar]
- Evans WD, Christoffel KK, Necheles J, Becker AB, Snider J. Outcomes of the 5‐4‐3‐2‐1 Go! Childhood obesity community trial. American Journal of Health Behavior 2011;35(2):189‐98. [DOI] [PubMed] [Google Scholar]; Evans WD, Wallace J, Snider J. The 5‐4‐3‐2‐1 go! Brand to promote nutrition and physical activity: a case of positive behavior change but negative change in beliefs. Journal of Health Communication 2015;20(5):512‐20. [DOI] [PubMed] [Google Scholar]
- Evans A, Ranjit N, Hoelscher D, Jovanovic C, Lopez M, McIntosh A, et al. Impact of school‐based vegetable garden and physical activity coordinated health interventions on weight status and weight‐related behaviors of ethnically diverse, low‐income students: study design and baseline data of the Texas, Grow! Eat! Go! (TGEG) cluster‐randomized controlled trial. BMC Public Health2016; Vol. 16:973. [DOI] [PMC free article] [PubMed]
- Evenson A, Pulvermacher A, Anderson M. Acceptability of different squash variety recipes to increase red‐orange vegetable consumption. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A42. [Google Scholar]
- Faber M, Phungula MAS, Venter SL, Dhansay MA, Spinnler Benade AJ. Home gardens focusing on the production of yellow and dark‐green leafy vegetables increases the serum retinol concentrations of 2‐5‐y‐old children in South Africa. American Journal of Clinical Nutrition 2002;76(5):1048‐54. [DOI] [PubMed] [Google Scholar]
- Faith MS, Rose E, Matz PE, Pietrobelli A, Epstein LH. Co‐twin control designs for testing behavioral economic theories of child nutrition: methodological note. International Journal of Obesity 2006;30(10):1501‐5. [DOI] [PubMed] [Google Scholar]
- Fangupo LJ, Heath AL, Williams SM, Somerville MR, Lawrence JA, Gray AR, et al. Impact of an early‐life intervention on the nutrition behaviors of 2‐y‐old children: a randomized controlled trial. American Journal of Clinical Nutrition 2015;102(3):704‐12. [DOI] [PubMed] [Google Scholar]
- Fernandes T. Healthy eating. Nursing Standard 2011;25:58. 21661653 [Google Scholar]
- Fernández‐Alvira JM, Bourdeaudhuij I, Singh AS, Vik FN, Manios Y, Kovacs E, et al. Clustering of energy balance‐related behaviors and parental education in European children: the ENERGY‐project. International Journal of Behavioral Nutrition & Physical Activity 2013;10:5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkowski MK, DeBaryshe B, Bersamin A, Nigg C, Leon Guerrero R, Rojas G, et al. A community engagement process identifies environmental priorities to prevent early childhood obesity: The Children's Healthy Living (CHL) program for remote underserved populations in the US Affiliated Pacific Islands, Hawaii and Alaska. Maternal and Child Health Journal 2013;18(10):2261‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Novotny R, Areta A, Bersamin A, Deenik J, Kim JH, Leon‐Guerrero R. Children's Healthy Living Program (CHL) for remote underserved minority populations of the Pacific region. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S196‐7. [Google Scholar]
- Fisher JO, Arreola A, Birch LL, Rolls BJ. Portion size effects on daily energy intake in low‐income Hispanic and African American children and their mothers. American Journal of Clinical Nutrition 2007;86(6):1709‐16. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL, Zhang J, Grusak MA, Hughes SO. External influences on children's self‐served portions at meals. International Journal of Obesity 2013;37(7):954‐60. [DOI] [PubMed] [Google Scholar]
- Fisher M, Fiese B. Implementation of the Sprouts Growing Healthy Habits Curriculum in preschool and kindergarten classrooms: is it feasible?. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S143. [Google Scholar]
- Fishman L. Don't be duped by these fruit & veggie fakes. Journal of Nutrition Education and Behavior 2016;48(2):158.e5. [Google Scholar]
- Fitzgibbon ML, Stolley MR, Dyer AR, VanHorn L, Kaufer Christoffel K. A community‐based obesity prevention program for minority children: rationale and study design for Hip‐Hop to Health Jr. Preventive Medicine 2002;34:289‐97. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P, Molloy B, Johnson Z. Community mothers' programme: extension to the travelling community in Ireland. Journal of Epidemiology & Community Health 1997;51(3):299‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Cooper JR, Helms P, Northington L, Winter K. Stemming the tide of childhood obesity in an underserved urban African American population: a pilot study. The ABNF Journal 2009;20(2):44. [PubMed] [Google Scholar]
- Foerster SB, Gregson J, Beall DL, Hudes M, Magnuson H, Livingston S, et al. The California Children's 5 a day‐ power play! campaign: evaluation of a large‐scale social marketing initiative. Family and Community Health 1998;21(1):46‐64. [Google Scholar]
- Folta SC, Goldberg JP, Economos C, Bell R, Landers S, Hyatt R. Assessing the use of school public address systems to deliver nutrition messages to children: Shape Up Somerville ‐ audio adventures. Journal of School Health 2006;76(9):459‐64. [DOI] [PubMed] [Google Scholar]; Goldberg JP, Collins JJ, Folta SC, McLarney MJ, Kozower C, Kuder J, et al. Retooling food service for early elementary school students in Somerville, Massachusetts: The Shape Up Somerville experience. Preventing Chronic Disease 2009;6(3):1‐8. [PMC free article] [PubMed] [Google Scholar]
- Fournet RM. Teaching gardening and food choices to children living in a food desert... 2014 Food & Nutrition Conference & Expo, October 18‐21, 2014, Atlanta, GA. Journal of the Academy of Nutrition & Dietetics 2014;114:A88. [Google Scholar]
- Freedman MR, Alvarez KP. Early childhood feeding: assessing knowledge, attitude, and practices of multi‐ethnic child‐care providers. Journal of the American Dietetic Association 2010;110(3):447‐51. [DOI] [PubMed] [Google Scholar]
- French GM, Nicholson L, Skybo T, Klein EG, Schwirian PM, Murray‐Johnson L, et al. An evaluation of mother‐centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics 2012;130(3):e507‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Groner JA, Skybo T, Murray‐Johnson L, Schwirian P, Eneli I, Sternstein A, et al. Anticipatory guidance for prevention of childhood obesity: design of the MOMS Project. Clinical Pediatrics 2009;48(5):483‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenn M, Pruszynski JE, Felzer H, Zhang J. Authoritative feeding behaviors to reduce child BMI through online interventions. Journal for Specialists in Pediatric Nursing 2013;18(1):65‐77. [DOI] [PubMed] [Google Scholar]
- Friedl KE, Rowe S, Bellows LL, Johnson SL, Hetherington MM, Froidmont‐Görtz I, et al. Report of an EU‐US Symposium on understanding nutrition‐related consumer behavior: strategies to promote a lifetime of healthy food choices. Journal of Nutrition Education & Behavior 2014;46(5):445‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S, Fulkerson J, Flattum C, Horning M, Olson C, Barlow T, et al. Cooking with kids in rural Minnesota: family meals and interest in family‐focused, community‐based, healthful‐eating programs. Journal of Nutrition Education and Behavior 2015;47(4):S9. [Google Scholar]
- Draxten M, Fulkerson JA, Friend S, Flattum CF, Schow R. Parental role modeling of fruits and vegetables at meals and snacks is associated with children's adequate consumption. Appetite 2014;78:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Friend S, Fulkerson JA, Neumark‐Sztainer D, Garwick A, Flattum CF, Draxten M. Comparing childhood meal frequency to current meal frequency, routines, and expectations among parents. Journal of Family Psychology2015; Vol. 29, issue 1:136‐40. [DOI] [PMC free article] [PubMed]
- Gaglianone CP, Aguiar Carrazedo Taddei JA, Colugnati Fernando AB, Góes Magalhães C, Mochi Davanço G, Macedo L, et al. Nutrition education in public elementary schools of São Paulo, Brazil: the Reducing Risks of Illness and Death in Adulthood project. Revista de Nutrição 2006;19:309‐20. [Google Scholar]
- Gallo S, Kohn Rhoades S, Jonge L, Canales J, Sanchez K. Childhood Health, Education, & Wellness (CHEW): a pilot trial for an individualized, family‐centered and culturally adapted program targeting childhood obesity among Latino children. Journal of the Academy of Nutrition and Dietetics2017; Vol. 117, issue 9:A19.
- Gallotta MC, Iazzoni S, Emerenziani GP, Meucci M, Migliaccio S, Guidetti L, et al. Effects of combined physical education and nutritional programs on schoolchildren's healthy habits. PeerJ 2016;4:e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Lascurain MC, Kicklighter JR, Jonnalagadda SS, Boudolf EA, Duchon D. Effect of a nutrition education program on nutrition‐related knowledge of English‐as‐second‐language elementary school students: a pilot study. Journal of Immigrant & Minority Health 2006;8(1):57‐65. [DOI] [PubMed] [Google Scholar]
- Gardiner CK, Bryan AD. Monetary incentive interventions can enhance psychological factors related to fruit and vegetable consumption. Annals of Behavioral Medicine 2017;51(4):599‐609. [DOI] [PubMed] [Google Scholar]
- Gaughan M, Brinckman D. Telephonic health coaching: an innovative method to promote health behavior change among participants in Supplemental Nutrition Assistance Program‐Education (SNAP‐Ed). Journal of the Academy of Nutrition and Dietetics 2016;116(9):A65. [Google Scholar]
- Gelli A, Masset E, Folson G, Kusi A, Arhinful DK, Asante F, et al. Evaluation of alternative school feeding models on nutrition, education, agriculture and other social outcomes in Ghana: rationale, randomised design and baseline data. Trials 2016;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile DA, Welk G, Eisenmann JC, Reimer RA, Walsh DA, Russell DW, et al. Evaluation of a multiple ecological level child obesity prevention program: Switch® what you Do, View, and Chew. BMC Medicine 2009;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelsohn J, Vijayadeva V, Davison N, Ramirez V, Cheung LWK, Murphy S, et al. A food store intervention trial improves caregiver psychosocial factors and children's dietary intake in Hawaii. Obesity 2010;18(1):S84‐S90. [DOI] [PubMed] [Google Scholar]
- Glanz K, Hersey J, Cates S, Muth M, Creel D, Nicholls J, et al. Effect of a nutrient rich foods consumer education program: results from the nutrition advice study. Journal of the Academy of Nutrition & Dietetics 2012;112(1):56‐63. [DOI] [PubMed] [Google Scholar]
- Glasper A. Does the media promote healthy nutrition for children?. British Journal of Nursing 2011;20(15):940‐1. [DOI] [PubMed] [Google Scholar]
- Glasson C, Chapman K, Gander K, Wilson T, James E. The efficacy of a brief, peer‐led nutrition education intervention in increasing fruit and vegetable consumption: a wait‐list, community‐based randomised controlled trial. Public Health Nutrition 2012;15(7):1318‐26. [DOI] [PubMed] [Google Scholar]
- Glasson C, Chapman K, Wilson T, Gander K, Hughes C, Hudson N, et al. Increased exposure to community‐based education and 'below the line' social marketing results in increased fruit and vegetable consumption. Public Health Nutrition2013; Vol. 16, issue 11:1961‐70. [DOI] [PMC free article] [PubMed]
- Golley RK, Hendrie GA. The impact of replacing regular‐ with reduced‐fat dairy foods on children's wider food intake: secondary analysis of a cluster RCT. European Journal of Clinical Nutrition 2012;66(10):1130‐4. [DOI] [PubMed] [Google Scholar]
- Gordon AR, Briefel RR, Collins AM, Rowe GM, Klerman JA. Delivering summer electronic benefit transfers for children through the supplemental nutrition assistance program or the special supplemental nutrition program for women, infants, and children: benefit use and impacts on food security and foods consumed. Journal of the Academy of Nutrition and Dietetics2016; Vol. 117, issue 3:367‐75. [DOI] [PubMed]
- Gorham G, Dulin‐Keita A, Risica PM, Mello J, Papandonatos G, Nunn A, et al. Effectiveness of Fresh to You, a discount fresh fruit and vegetable market in low‐income neighborhoods, on children's fruit and vegetable consumption, Rhode Island, 2010‐2011.[Erratum appears in Prev Chronic Dis. 2015;12:E188; PMID: 26542140]. Preventing Chronic Disease 2015;12:E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosliner WA, James P, Yancey AK, Ritchie L, Studer N, Crawford PB. Impact of a worksite wellness program on the nutrition and physical activity environment of child care centers. American Journal of Health Promotion 2010;24(3):186‐9. [DOI] [PubMed] [Google Scholar]
- Goto K. UP23 connecting communities and families through locally grown cultural foods for childhood obesity prevention. Journal of Nutrition Education & Behavior 2012;44:S87. [Google Scholar]
- Gottesman MM. HEAT: Healthy Eating and Activity Together. American Journal of Nursing 2007;107(2):49‐50. [DOI] [PubMed] [Google Scholar]; Gottesman MM. Healthy eating and activity together (HEAT): weapons against obesity. Journal of Pediatric Health Care 2003;17(4):210‐5. [DOI] [PubMed] [Google Scholar]
- Graham D, Appleton S, Rush E, McLennan S, Reed P, Simmons D. Increasing activity and improving nutrition through a schools‐based programme: Project Energize. 1. Design, programme, randomisation and evaluation methodology. Public Health Nutrition 2008;11(10):1076‐84. [DOI] [PubMed] [Google Scholar]
- Gratton L, Povey R, Clark‐Carter D. Promoting children’s fruit and vegetable consumption: interventions using the Theory of Planned Behaviour as a framework. British Journal of Health Psychology 2007;12(Pt 4):639‐50. [DOI] [PubMed] [Google Scholar]
- Gregori D, Vecchio MG, Nikolakis A, Galasso F. Even a very intense advertising promoting fruit consumption is not enough to have children eating more fruit: results from an experimental study in Italy. Obesity Facts 2014;7:176. [Google Scholar]
- Gripshover SJ, Markman EM. Teaching young children a theory of nutrition: conceptual change and the potential for increased vegetable consumption. Psychological Science 2013;24:1541‐53. [DOI] [PubMed] [Google Scholar]
- Gross RS, Mendelsohn AL, Gross M, Taylor Lucas C, Fierman AH, Dreyer BP, et al. Starting Early/Empezando Temprano: randomized control trial (RCT) to test the effectiveness of an early obesity prevention program. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S82. [Google Scholar]; Messito MJ, Mendelsohn AL, Gross M, Diaz K, Scheinmann R, Chiasson MA, et al. Starting Early/Empezando Temprano: randomized control trial (RCT) to test the effectiveness of an early obesity prevention program. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S196. [Google Scholar]; Messito MJ, Mendelsohn AL, Gross M, Diaz K, Scheinmann R, Lucas CT, et al. Starting Early/Empezando Temprano: randomized control trial (RCT) to test the effectiveness of an early obesity prevention program. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S86. [Google Scholar]; Messito MJ, Mendelsohn AL, Lucas CT, Gross M, Gross R. Starting Early: primary care‐based obesity prevention beginning in pregnancy. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S10‐1. [Google Scholar]
- Guenther DC. Nutrition education and scratch cooking changes in schools: a mixed methods study of interventions in Aurora public schools. Dissertation Abstracts International: Section B: The Sciences and Engineering2014; Vol. 74.
- Guldan GS, Fan HC, Ma X, Ni ZZ, Xiang X, Tang MZ. Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. The Journal of Nutrition2000; Vol. 130, issue 5:1204‐11. [DOI] [PubMed]
- Guo H, Zeng X, Zhuang Q, Zheng Y, Chen S. Intervention of childhood and adolescents obesity in Shantou city. Obesity Research & Clinical Practice 2015;9(4):357‐64. [DOI] [PubMed] [Google Scholar]
- Haines J, Rifas‐Shiman SL, Gross D, McDonald J, Kleinman K, Gillman MW. Randomized trial of a prevention intervention that embeds weight‐related messages within a general parenting program. Obesity 2016;24(1):191‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton H. Fit 4 Fun. Community Practitioner 2004;77(10):367‐8. [Google Scholar]
- Hammersley ML, Jones RA, Okely AD. Time2bHealthy ‐ an online childhood obesity prevention program for preschool‐aged children: a randomised controlled trial protocol. Contemporary Clinical Trials2017; Vol. 61:73‐80. [DOI] [PubMed]
- Hammons AJ, Wiley AR, Fiese BH, Teran‐Garcia M. Six‐week Latino family prevention pilot program effectively promotes healthy behaviors and reduces obesogenic behaviors. Journal of Nutrition Education & Behavior 2013;45(6):745‐50. [DOI] [PubMed] [Google Scholar]
- Hancocks S. Suffer the little children. British Dental Journal 2011;210(8):341. [DOI] [PubMed] [Google Scholar]
- Hanks AS, Just DR, Brumberg A. Marketing vegetables in elementary school cafeterias to increase uptake. Pediatrics 2016;138(2):e20151720. [DOI] [PubMed] [Google Scholar]
- Hansen A, King M, Cabe J, Pleasant A, Lucero‐Liu A, Schultz J, et al. Using health literacy and hands‐on cooking to improve healthy nutrition behaviors. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A35. [Google Scholar]
- Hanson KL, Kolodinsky J, Wang W, Morgan EH, Jilcott P, Ammerman SB, et al. Adults and children in low‐income households that participate in cost‐offset community supported agriculture have high fruit and vegetable consumption. Nutrients2017; Vol. 9, issue 7:726. [DOI] [PMC free article] [PubMed]
- Hardy LL, King L, Kelly B, Farrell L, Howlett S. Munch and Move: evaluation of a preschool healthy eating and movement skill program. International Journal of Behavioral Nutrition and Physical Activity 2010;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Lowe A, Unadkat A, Thurtle V. Mini‐MEND: an obesity prevention initiative in a children's centre. Community Practitioner 2010;83(6):26‐9. [PubMed] [Google Scholar]
- Hare ME, Coday M, Williams NA, Richey PA, Tylavsky FA, Bush AJ. Methods and baseline characteristics of a randomized trial treating early childhood obesity: the Positive Lifestyles for Active Youngsters (Team PLAY) trial. Contemporary Clinical Trials 2012;33(3):534‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun D, Wood L, Harper C, Nelson M. Nutrient‐based standards for school lunches complement food‐based standards and improve pupils' nutrient intake profile. British Journal of Nutrition 2011;106(4):472‐4. [DOI] [PubMed] [Google Scholar]
- Harris JL, Schwartz MB, Ustjanauskas A, Ohri‐Vachaspati P, Brownell KD. Effects of serving high‐sugar cereals on children's breakfast‐eating behavior. Pediatrics2011; Vol. 127, issue 1:71‐6. [DOI] [PubMed]
- Hart LM, Damiano SR, Paxton SJ. Confident body, confident child: a randomized controlled trial evaluation of a parenting resource for promoting healthy body image and eating patterns in 2‐ to 6‐year old children. International Journal of Eating Disorders 2016;49(5):458‐72. [DOI] [PubMed] [Google Scholar]
- Harvey‐Berino J, Rourke J. Obesity prevention in preschool native‐American children: a pilot study using home visiting. Obesity Research 2003;11(5):606‐11. [DOI] [PubMed] [Google Scholar]
- Havas S, Damron D, Treiman K, Anliker J, Langenberg P, Hammad TA, et al. The Maryland WIC 5 A Day Promotion Program Pilot Study: rationale, results, and lessons learned. Journal of Nutrition Education 1997;29(6):343‐50. [Google Scholar]
- Havermans RC, Jansen A. Increasing children's liking of vegetables through flavour‐flavour learning. Appetite 2007;48(2):259‐62. [DOI] [PubMed] [Google Scholar]
- Heath PM, Houston‐Price C, Kennedy OB. Can visual exposure impact on children's visual preferences for fruit and vegetables?. Proceedings of the Nutrition Society2010; Vol. 69.
- Heim S, Stang J, Ireland M. A garden pilot project enhances fruit and vegetable consumption among children. Journal of the American Dietetic Association 2009;109(7):1220‐6. [DOI] [PubMed] [Google Scholar]
- Helland S, Bere E, Øverby N. Food for preschoolers. Annals of Nutrition and Metabolism 2013;63:621. [Google Scholar]
- Helland SH, Bere E, Øverby NC. Study protocol for a multi‐component kindergarten‐based intervention to promote healthy diets in toddlers: a cluster randomized trial. BMC Public Health 2016;16(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland SH, Bere E, Bjørnarå HB, Øverby NC. Food neophobia and its association with intake of fish and other selected foods in a Norwegian sample of toddlers: a cross‐sectional study. Appetite2017; Vol. 114:110‐7. [DOI] [PubMed]
- Hendy HM. Effectiveness of trained peer models to encourage food acceptance in preschool children. Appetite 2002;39(3):217‐25. [DOI] [PubMed] [Google Scholar]
- Hendy HM, Williams KE, Camise TS. Kid's Choice Program improves weight management behaviors and weight status in school children. Appetite 2011;56(2):484‐94. [DOI] [PubMed] [Google Scholar]; Hendy HM, Williams KE, Camise TS, Alderman S, Ivy J, Reed J. Overweight and average‐weight children equally responsive to "Kids Choice Program" to increase fruit and vegetable consumption. Appetite 2007;49(3):683‐6. [DOI] [PubMed] [Google Scholar]
- Herbold NH, Dennis JD. Gem no. 339. Food for thought: a nutrition monitoring project for elementary school children using the Internet. Journal of Nutrition Education 2001;33(5):299‐300. [DOI] [PubMed] [Google Scholar]
- Herring D, Chang S, Bard S, Gavey E. Five years of MyPlate; looking back and what's ahead. Journal of the Academy of Nutrition and Dietetics 2016;116(7):1069‐71. [DOI] [PubMed] [Google Scholar]
- Hildebrand DA, Shriver LH. A quantitative and qualitative approach to understanding fruit and vegetable availability in low‐income African‐American families with children enrolled in an urban Head Start program. Journal of the American Dietetic Association 2010;110:710‐8. [DOI] [PubMed] [Google Scholar]
- Hoddinott J, Ahmed I, Ahmed A, Roy S. Behavior change communication activities improve infant and young child nutrition knowledge and practice of neighboring non‐participants in a cluster‐randomized trial in rural Bangladesh. Plos ONE2017; Vol. 12, issue 6:e0179866. [DOI] [PMC free article] [PubMed]
- Hoffman JA, Thompson DR, Franko DL, Power TJ, Leff SS, Stallings VA. Decaying behavioral effects in a randomized, multi‐year fruit and vegetable intake intervention. Preventive Medicine 2011;52(5):370‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JA, Rosenfeld L, Schmidt N, Cohen JFW, Gorski M, Chaffee R, et al. Implementation of competitive food and beverage standards in a sample of Massachusetts schools: The NOURISH Study (Nutrition Opportunities to Understand Reforms Involving Student Health). Journal of the Academy of Nutrition and Dietetics 2015;115(8):1299‐1307.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman EE, Paul IM, Birch LL, Savage JS. INSIGHT responsive parenting intervention is associated with healthier patterns of dietary exposures in infants. Obesity2017; Vol. 25, issue 1:185‐91. [DOI] [PMC free article] [PubMed]; Hohman EE, Savage JS, Paul IM, Birch LL. INSIGHT study parenting intervention to prevent childhood obesity improves patterns of dietary exposures in infants. FASEB Journal. Conference: Experimental Biology2016; Vol. 30. [DOI] [PMC free article] [PubMed]
- Hollar D, Lombardo M, Heitz C, Hollar L. HOPE2 nutrition‐focused policy/curricula improve consumption of nutritious foods and dietetic practices in elementary schools. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S63‐4. [Google Scholar]; Hollar D, Lombardo M, Heitz C, Hollar L. Making a significant impact on weight management among elementary‐age children: school‐based dietetic and wellness environmental policies and programs successfully promote lifestyle change. Journal of the Academy of Nutrition and Dietetics 2012;112(9):A15. [Google Scholar]
- Holley CE. Increasing vegetable consumption in early childhood: parents as facilitators [Thesis]. Loughborough University (UK), 2016. [Google Scholar]; Holley CE, Farrow C, Haycraft E. Investigating the role of parent and child characteristics in healthy eating intervention outcomes. Appetite 2016;105:291‐7. [DOI] [PubMed] [Google Scholar]; Holley CE, Haycraft E, Farrow C. 'Why don't you try it again?' A comparison of parent led, home based interventions aimed at increasing children's consumption of a disliked vegetable. Appetite 2015;87:215‐22. [DOI] [PubMed] [Google Scholar]
- Hooft van Huysduynen E, Lee L, Geelen A, Feskens E, T Veer P, Woerkum C, et al. The effect of an individually tailored nutrition intervention for Dutch parents on dietary intake and physical activity of their children. Annals of Nutrition and Metabolism 2013;63:898. [Google Scholar]
- Horne PJ, Hardman CA, Lowe CF, Tapper K, Noury J, Patel P, et al. Increasing parental provision and children’s consumption of lunchbox fruit and vegetables in Ireland: the Food Dudes intervention. European Journal of Clinical Nutrition 2009;63(5):613‐8. [DOI] [PubMed] [Google Scholar]
- Horodynski MAO, Hoerr S, Coleman G. Nutrition education aimed at toddlers. A pilot program for rural, low‐income families. Family and Community Health 2004;27(2):103‐13. [DOI] [PubMed] [Google Scholar]
- Hotz C, Loechl C, Brauw A, Eozenou P, Gilligan D, Moursi M, et al. A large‐scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. British Journal of Nutrition 2012;108(1):163‐76. [DOI] [PubMed] [Google Scholar]
- Hotz C, Loechl C, Lubowa A, Tumwine J K, Ndeezi G, Nandutu Masawi A, et al. Introduction of beta‐carotene‐rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. Journal of Nutrition 2012;142(10):1871‐80. [DOI] [PubMed] [Google Scholar]
- Howarth P, James K. Childhood obesity. Communicating Nursing Research 2011;44:489. [Google Scholar]
- Hu C, Ye D, Li Y, Huang Y, Li L, Gao Y, et al. Evaluation of a kindergarten‐based nutrition education intervention for pre‐school children in China. Public Health Nutrition 2010;13(2):253‐60. [DOI] [PubMed] [Google Scholar]
- Hughes SO, Patrick H, Power TG, Fisher JO, Anderson CB, Nicklas TA. The impact of child care providers’ feeding on children’s food consumption. Journal of Developmental and Behavioral Pediatrics 2007;28(2):100‐7. [DOI] [PubMed] [Google Scholar]
- Hughes L, Cirignano S, Fitzgerald N. Fruit and vegetable tastings in schools offer potential for increasing consumption among kindergarten through sixth grade children. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A19. [Google Scholar]
- Iaia M, Pasini M, Burnazzi A, Vitali P, Allara E, Farneti M. An educational intervention to promote healthy lifestyles in preschool children: a cluster‐RCT. International Journal of Obesity2017; Vol. 41, issue 4:582‐90. [DOI] [PubMed]
- International Food Information Council. Kidnetic.com: tap into the energy: healthful eating and physical activity tips for kids and parents just a click away. Food Insight 2002;1:4‐5. [Google Scholar]
- Israelashvili M, Wegman‐Rozi O. Mentoring at‐risk preschoolers: lessons from the A.R.Y.A. project. Journal of Primary Prevention 2005;26(2):189‐201. [DOI] [PubMed] [Google Scholar]
- Issanchou S. Determining factors and critical periods in the formation of eating habits: results from the Habeat project. Annals of Nutrition and Metabolism 2017;70(3):251‐6. [DOI] [PubMed] [Google Scholar]
- Izumi B, Hoffman J, Hallman J, Eckhardt C, Barberis D, Stott B. Harvest for Healthy KIds: what factors influence implementation of farm‐to‐preschool in head start classrooms?. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S49‐50. [Google Scholar]
- James J, Brown J, Douglas M, Cox J, Stocker S. Improving the diet of under fives in a deprived inner city practice. Health Trends 1992;24(4):160‐4. [PubMed] [Google Scholar]
- Jancey JM, Dos Remedios Monteiro SM, Dhaliwal SS, Howat PA, Burns S, Hills AP, et al. Dietary outcomes of a community based intervention for mothers of young children: a randomised controlled trial. International Journal of Behavioral Nutrition & Physical Activity 2014;11:182‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke DM, Lim CS, Mathews AE, Shelnutt KP, Boggs SR, Silverstein JH, et al. The community‐based healthy‐lifestyle intervention for rural preschools (CHIRP) study: design and methods. Contemporary Clinical Trials 2013;34(2):187‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E, Mulkens S, Jansen A. How to promote fruit consumption in children. Visual appeal versus restriction. Appetite 2010;54(3):599‐602. [DOI] [PubMed] [Google Scholar]
- Jansen EC, Kasper N, Lumeng JC, Brophy Herb HE, Horodynski MA, Miller AL, et al. Changes in household food insecurity are related to changes in BMI and diet quality among Michigan Head Start preschoolers in a sex‐specific manner. Social Science & Medicine2017; Vol. 181:168‐76. [DOI] [PMC free article] [PubMed]
- Jayne CL. Elmo Eats Broccoli: a Look at the Influence of Popular Characters on Children's Food Choices [Thesis]. The University of Mississippi, 2008. [Google Scholar]
- Johnson Z, Howell F, Molloy B. Community mothers' programme: randomised controlled trial of non‐professional intervention in parenting. BMJ 1993;306(6890):1449‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Bellows L, Beckstrom L, Anderson J. Evaluation of a social marketing campaign targeting preschool children. American Journal of Health Behavior 2007;31(1):44‐55. [DOI] [PubMed] [Google Scholar]
- Jordan AB. Children's television viewing and childhood obesity. Pediatric Annals 2010;39(9):569‐73. [DOI] [PubMed] [Google Scholar]
- Joseph S, Stevens AM, Ledoux T, O'Connor TM, O'Connor DP, Thompson D. Rationale, design, and methods for process evaluation in the Childhood Obesity Research Demonstration Project. Journal of Nutrition Education and Behavior 2015;47(6):560‐5.e1. [DOI] [PubMed] [Google Scholar]
- Joseph LS, Gorin AA, Mobley SL, Mobley AR. Impact of a short‐term nutrition education child care pilot intervention on preschool children's intention to choose healthy snacks and actual snack choices. Childhood Obesity 2015;11(5):513‐20. [DOI] [PubMed] [Google Scholar]
- Just D, Price J. Default options, incentives and food choices: evidence from elementary‐school children. Public Health Nutrition 2013;16(12):2281‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabahenda M, Mullis RM, Erhardt JG, Northrop‐Clewes C, Nickols SY. Nutrition education to improve dietary intake and micronutrient nutriture among children in less‐resourced areas: a randomized controlled intervention in Kabarole district, Western Uganda. South African Journal of Clinical Nutrition 2011;24:83‐8. [Google Scholar]
- Kain J, Uauy R, Concha F, Leyton B, Bustos N, Salazar G, et al. School‐based obesity prevention interventions for Chilean children during the past decades: lessons learned. Advances in Nutrition 2012;3(4):616S‐21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb C, Springen K. Pump up the family. Newsweek 2005;145(17):62. [PubMed] [Google Scholar]
- Kang Y, Suh Youn K, Debele L, Juon HS, Christian P. Effects of a community‐based nutrition promotion programme on child feeding and hygiene practices among caregivers in rural Eastern Ethiopia. Public Health Nutrition2017; Vol. 20, issue 8:1461‐72. [DOI] [PMC free article] [PubMed]
- Kannan S, Ganguri HB, Qamar Z, Lakshmanan U, Wittcopp C. From carrots to peas and parsnips: programming flexibility through guided multisensory exploration in an early childhood environment. FASEB Journal. Conference: Experimental Biology2016; Vol. 30.
- Karanja N, Aickin M, Lutz T, Mist S, Jobe JB, Maupome G, et al. A community‐based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study. Journal of Primary Prevention 2012;33(4):161‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani IA, Langer RD, Criqui MH, Nader PR, Rupp J, Sallis JF, et al. Effects of parental behavior modification on children’s cardiovascular risks. Annals New York Academy of Sciences 1991;623:447‐9. [DOI] [PubMed] [Google Scholar]
- Kaufman‐Shriqui V, Fraser D, Friger M, Geva D, Bilenko N, Vardi H, et al. Effect of a school‐based intervention on nutritional knowledge and habits of low‐socioeconomic school children in Israel: a cluster‐randomized controlled trial. Nutrients 2016;8(4):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelder SH, Perry CL, Lytle LA, Klepp K‐I. Community‐wide youth nutrition education: long‐term outcomes of the Minnesota Heart Health program. Health Education Research 1995;10(2):119‐31. [DOI] [PubMed] [Google Scholar]
- Keller KL. The use of repeated exposure and associative conditioning to increase vegetable acceptance in children: explaining the variability across studies. Journal of the Academy of Nutrition and Dietetics 2014;114:1169‐73. [DOI] [PubMed] [Google Scholar]
- Kennedy BM, Harsha DW, Bookman EB, Hill YR, Rankinen T, Rodarte RQ, et al. Challenges to recruitment and retention of African Americans in the gene‐environment trial of response to dietary interventions (GET READI) for heart health. Health Education Research 2011;26(5):923‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler HS. Simple interventions to improve healthy eating behaviors in the school cafeteria. Nutrition Reviews 2016;74(3):198‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnevisan F, Kimiagar M, Kalantaree N, Valaee N, Shaheedee N. Effect of nutrition education and diet modification in iron depleted preschool children in nurseries in Tehran: a pilot study. International Journal for Vitamin and Nutrition Research 2004;74(4):264‐8. [DOI] [PubMed] [Google Scholar]
- Kidala D, Greiner T, Gebre‐Medhin M. Five‐year follow‐up of a food‐based vitamin A intervention in Tanzania. Public Health Nutrition 2000;3(4):425‐31. [DOI] [PubMed] [Google Scholar]
- Kilaru A, Griffiths PL, Ganapathy S, Shanti G. Community‐based nutrition education for improving infant growth in rural Karnataka. Indian Pediatrics 2005;42:425‐32. [PubMed] [Google Scholar]
- Kilicarslan Toruner E, Savaser S. A controlled evaluation of a school‐based obesity prevention in Turkish school children. Journal of School Nursing 2010;26:473‐82. [DOI] [PubMed] [Google Scholar]
- Kimani‐Murage EW, Kyobutungi C, Ezeh AC, Wekesah F, Wanjohi M, Muriuki P, et al. Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Trials 2013;14:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipping RR, Howe LD, Jago R, Campbell R, Wells S, Chittleborough CR, et al. Effect of intervention aimed at increasing physical activity, reducing sedentary behaviour, and increasing fruit and vegetable consumption in children: Active for Life Year 5 (AFLY5) school based cluster randomised controlled trial. BMJ 2014;348(7960):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipping R, Jago R, Metcalfe C, White J, Papadaki A, Campbell R, et al. NAP SACC UK: protocol for a feasibility cluster randomised controlled trial in nurseries and at home to increase physical activity and healthy eating in children aged 2‐4 years. BMJ Open 2016;6(4):e010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblock‐Hahn A, Hand R, Medrow L. Improving food security, nutrition, and healthy family behaviors through the Registered Dietitian Parent Empowerment and Supplemental Food Pilot Program. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A22. [DOI] [PubMed] [Google Scholar]
- Knowlden A, Sharma M. A feasibility and efficacy randomized controlled trial of an online preventative program for childhood obesity: protocol for the EMPOWER Intervention. JMIR Research Protocols 2012;1(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Knowlden A, Sharma M. One‐year efficacy testing of Enabling others to Prevent Pediatric Obesity Through Web‐Based Education and Reciprocal Determinism (EMPOWER) randomized control trial. Health Education & Behavior 2016;43(1):94‐106. [DOI] [PubMed] [Google Scholar]; Knowlden AP. Feasibility and efficacy of the Enabling Mothers to Prevent Pediatric Obesity Through Web‐Based Education and Reciprocal Determinism (EMPOWER) randomized control trial. Dissertation Abstracts International Section A: Humanities and Social Sciences2014; Vol. 75. [DOI] [PubMed]; Knowlden AP, Sharma M, Cottrell RR, Wilson BR, Johnson ML. Impact evaluation of Enabling Mothers to Prevent Pediatric Obesity through Web‐Based Education and Reciprocal Determinism (EMPOWER) randomized control trial. Health Education & Behavior 2015;42(2):171‐84. [DOI] [PubMed] [Google Scholar]
- Koehler S, Sichert‐Hellert W, Kersting M. Measuring the effects of nutritional counseling on total infant diet in a randomized controlled intervention trial. Journal of Pediatric Gastroenterology and Nutrition 2007;45:106‐13. [DOI] [PubMed] [Google Scholar]
- Koff L, Mullis R. Nutrition education and technology: can delivering messages via new media technology effectively modify nutrition behaviors of preschoolers and their families?. Journal of Nutrition Education and Behavior 2011;43(4 Supplement 1):S40. [Google Scholar]
- Ko LK, Rodriguez E, Yoon J, Ravindran R, Copeland WK. A brief community‐based nutrition education intervention combined with food baskets can increase fruit and vegetable consumption among low‐income Latinos. Journal of Nutrition Education and Behavior2016; Vol. 48, issue 9:609‐17. [DOI] [PubMed]
- Kolodinsky JM, Sitaker M, Morgan EH, Connor LM, Hanson KL, Becot F, et al. Can CSA cost‐offset programs improve diet quality for limited resource families?. Choices2017; Vol. 32, issue 1:No pagination.
- Korwanich K, Sheiham A, Srisuphan W, Srisilapanan P. Promoting healthy eating in nursery schoolchildren: a quasi‐experimental intervention study. Health Education Journal 2008;67(1):16‐30. [Google Scholar]
- Kotler JA, Schiffman JM, Hanson KG. The influence of media characters on children's food choices. Journal of Health Communication 2012;17(8):886‐98. [DOI] [PubMed] [Google Scholar]
- Kotz D. Can she end obesity? 5 key steps. Pursuing the first lady's goal may seem pretty straightforward. But it's not. U.S. News & World Report 2010;147:32. [PubMed] [Google Scholar]
- Kral TV, Kabay AC, Roe LS, Rolls BJ. Effects of doubling the portion size of fruit and vegetable side dishes on children's intake at a meal. Obesity (Silver Spring, Md.) 2010;18(3):521‐7. [DOI] [PubMed] [Google Scholar]
- Lanigan J, Barber S, Singhal A. Prevention of obesity in preschool children. Proceedings of the Nutrition Society 2010;69(2):204‐10. [DOI] [PubMed] [Google Scholar]
- Laramy K. A digital approach to behavior change ‐ helping low‐income moms to shop, cook, and eat healthy on a budget. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 7:S4.
- LaRowe TL, Adams AK, Jobe JB, Cronin KA, Vannatter SM, Prince RJ. Dietary intakes and physical activity among preschool‐aged children living in rural American Indian communities before a family‐based healthy lifestyle intervention. Journal of the American Dietetic Association 2010;110(7):1049‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson N, Ward DS, Neelon SB, Story M. What role can child‐care settings play in obesity prevention? A review of the evidence and call for research efforts. Journal of the American Dietetic Association 2011;111(9):1343‐62. [DOI] [PubMed] [Google Scholar]
- Laureati M, Bergamaschi V, Pagliarini E. School‐based intervention with children. Peer‐modeling, reward and repeated exposure reduce food neophobia and increase liking of fruits and vegetables. Appetite 2014;83:26‐32. [DOI] [PubMed] [Google Scholar]
- Leahy KE, Birch LL, Fisher JO, Rolls BJ. Reductions in entree energy density increase children's vegetable intake and reduce energy intake. Obesity 2008;16(7):1559‐65. [DOI] [PubMed] [Google Scholar]
- Leahy KE, Birch LL, Rolls BJ. Reducing the energy density of an entree decreases children's energy intake at lunch. Journal of the American Dietetic Association 2008;108(1):41‐8. [DOI] [PubMed] [Google Scholar]
- Leahy KE, Birch LL, Rolls BJ. Reducing the energy density of multiple meals decreases the energy intake of preschool‐age children. American Journal of Clinical Nutrition 2008;88(6):1459‐68. [DOI] [PubMed] [Google Scholar]
- Ledoux T, Silveira S, Le J, Kamal H, Kung S. Investigating the preliminary effects of little foodies: a health promotion program for parents of toddlers. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 7:S3.
- Leme AC, Philippi ST. The "Healthy Habits, Healthy Girls" randomized controlled trial for girls: study design, protocol, and baseline results. Cadernos de Saude Publica 2015;31(7):1381‐94. [DOI] [PubMed] [Google Scholar]
- Lin S, Gray V, Singh‐Carlson S, Cheffer N, Chery S. Community‐based study of food, feeding, and opportunity in rural Haiti. Journal of the Academy of Nutrition and Dietetics2017; Vol. 117, issue 9:A20.
- Ling J, Robbins LB, Wen F. Interventions to prevent and manage overweight or obesity in preschool children: a systematic review. International Journal of Nursing Studies 2016;53:270‐89. [DOI] [PubMed] [Google Scholar]
- Ling J, Robbins LB, Hines‐Martin V. Perceived parental barriers to and strategies for supporting physical activity and healthy eating among head start children. Journal of Community Health 2016;41(3):593‐602. [DOI] [PubMed] [Google Scholar]
- Lioret S, Campbell KJ, Crawford D, Spence AC, Hesketh K, McNaughton SA. A parent focused child obesity prevention intervention improves some mother obesity risk behaviors: the Melbourne inFANT program. International Journal of Behavioral Nutrition and Physical Activity 2012;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioret S, Cameron AJ, McNaughton SA, Crawford D, Spence AC, Hesketh K, et al. Association between maternal education and diet of children at 9 months is partially explained by mothers' diet. Maternal & Child Nutrition 2015;11(4):936‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llargues E, Franco R, Recasens A, Nadal A, Vila M, Perez M J, et al. Assessment of a school‐based intervention in eating habits and physical activity in school children: the AVall study. Journal of Epidemiology & Community Health 2011;65(10):896‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]; Llargues E, Recasens A, Franco R, Nadal A, Vila M, Perez M J, et al. Medium‐term evaluation of an educational intervention on dietary and physical exercise habits in schoolchildren: the Avall 2 study. Endocrinologia y Nutricion 2012;59:288‐95. [DOI] [PubMed] [Google Scholar]
- Lloyd AB, Morgan PJ, Lubans DR, Plotnikoff RC. Investigating the measurement and operationalisation of obesity‐related parenting variables of overweight fathers in the Healthy Dads, Healthy Kids community program. Obesity Research and Clinical Practice 2011;5:S72. [Google Scholar]
- Locard E, Boyer M, Beroujon M. Evaluation of an educational campaign on nutrition among five years old children. Archives Francaises de Pediatrie 1987;44:205‐9. [PubMed] [Google Scholar]
- Lohse B. Nutrition education does not stop at the borders. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 3:185. [DOI] [PubMed]
- Longacre MR, Roback J, Langeloh G, Drake K, Dalton MA. An entertainment‐based approach to promote fruits and vegetables to young children. Journal of Nutrition Education and Behavior 2015;47(5):480‐3.e1. [DOI] [PubMed] [Google Scholar]
- Longley C. LANA Learning about Nutrition through Activities Deluxe Kit. Journal of Nutrition Education and Behavior 2013;45(6):807.e5. [Google Scholar]
- Low JW, Arimond M, Osman N, Cunguara B, Zano F, Tschirley D. Ensuring the supply of and creating demand for a biofortified crop with a visible trait: lessons learned from the introduction of orange‐fleshed sweet potato in drought‐prone areas of Mozambique. Food and Nutrition Bulletin 2007;28(2):S258‐S270. [DOI] [PubMed] [Google Scholar]
- Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, et al. Outcomes of a field trial to improve children's dietary patterns and physical activity. JAMA 1996;275(10):768‐76. [DOI] [PubMed] [Google Scholar]; Perry CL, Lytle LA, Feldman H, Nicklas T, Stone E, Zive M, et al. Effects of the child and adolescent trial for cardiovascular health (CATCH) on fruit and vegetable intake. Journal of Nutrition Education 1998;30:354‐60. [Google Scholar]
- Lumeng JC, Miller A, Brophy‐Herb H, Horodynski M, Contreras D, Davis R, et al. Enhancing self‐regulation as a strategy for obesity prevention in head start preschoolers. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S89. [Google Scholar]; Lumeng JC, Miller A, Brophy‐Herb H, Horodynski MA, Contreras D, Davis R, et al. Enhancing self regulation as a strategy for obesity prevention in head start preschooler. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S86. [Google Scholar]; Lumeng JC, Miller A, Brophy‐Herb H, Horodynski MA, Contreras D, Peterson KE. Enhancing self‐regulation as a strategy for obesity prevention in head start preschoolers. Journal of Nutrition Education and Behavior 2014;46(4, Supplement):S195. [Google Scholar]
- Maier A, Chabanet C, Schaal B, Issanchou S, Leathwood P. Effects of repeated exposure on acceptance of initially disliked vegetables in 7‐month old infants. Food Quality and Preference 2007;18:1023‐32. [Google Scholar]
- Maier AS, Chabanet C, Schaal B, Leathwood PD, Issanchou SN. Breastfeeding and experience with variety early in weaning increase infants' acceptance of new foods for up to two months. Clinical Nutrition 2008;27(6):849‐57. [DOI] [PubMed] [Google Scholar]
- Maier‐Noth A, Schaal B, Leathwood P, Issanchou S. The lasting influences of early food‐related variety experience: a longitudinal study of vegetable acceptance from 5 months to 6 years in two populations. PLoS ONE 2016;11(3):e0151356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier‐Noth A. Nutrition gourmet or soup kasper? What factors affect the dietary behavior in children, and can we steer it in a positive direction early on?. Padiatrie Und Padologie 2017;52(6):280‐2. [Google Scholar]
- Malekafzali H, Abdollahi Z, Mafi A, Naghavi M. Community‐based nutritional intervention for reducing malnutrition among children under 5 years of age in the Islamic Republic of Iran. Eastern Mediterranean Health Journal 2000;6(2/3):238‐45. [PubMed] [Google Scholar]
- Mallan KM, Daniels LA, Nicholson JM. Obesogenic eating behaviors mediate the relationships between psychological problems and BMI in children. Obesity2017; Vol. 25, issue 5:928‐34. [DOI] [PMC free article] [PubMed]
- Manger WM, Manger LS, Minno AM, Killmeyer M, Holzman RS, Schullinger JN, et al. Obesity prevention in young schoolchildren: results of a pilot study. Journal of School Health 2012;82(10):462‐8. [DOI] [PubMed] [Google Scholar]
- Manios Y, Moschandreas J, Hatzis C, Kafatos A. Evaluation of a health and nutrition education program in primary school children of Crete over a three‐year period. Preventive Medicine 1999;28(2):149‐59. [DOI] [PubMed] [Google Scholar]
- Manios Y, Kourlaba G, Kondaki K, Grammatikaki E, Birbilis M, Oikonomou E, et al. Diet quality of preschoolers in Greece based on the healthy eating index: the GENESIS study. Journal of the American Dietetic Association 2009;109(4):616‐23. [DOI] [PubMed] [Google Scholar]
- Mann CM, Ward DS, Vaughn A, Benjamin Neelon SE, Long Vidal LJ, Omar S, et al. Application of the Intervention Mapping protocol to develop Keys, a family child care home intervention to prevent early childhood obesity. BMC Public Health2015; Vol. 15:1227. [DOI] [PMC free article] [PubMed]
- Mann G. Let's make Spring & Summer healthy!. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 6:529.
- Markert J, Herget S, Petroff D, Gausche R, Grimm A, Kiess W, et al. Telephone‐based adiposity prevention for families with overweight children (T.A.F.F.‐Study): one year outcome of a randomized, controlled trial. International Journal of Environmental Research and Public Health 2014;11(10):10327‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquard J, Stahl A, Lerch C, Wolters M, Grotzke‐Leweling M, Mayatepek E, et al. A prospective clinical pilot‐trial comparing the effect of an optimized mixed diet versus a flexible low‐glycemic index diet on nutrient intake and HbA(1c) levels in children with type 1 diabetes. Journal of Pediatric Endocrinology & Metabolism2011; Vol. 24, issue 7‐8:441‐7. [DOI] [PubMed]
- Martens MK, Assema P, Paulussen TGWM, Breukelen G, Brug J. Krachtvoer‐: effect evaluation of a Dutch healthful diet promotion curriculum for lower vocational schools. Public Health Nutrition 2008;11(3):271‐8. [DOI] [PubMed] [Google Scholar]
- Mathias KC, Rolls BJ, Birch LL, Kral TV, Hanna EL, Davey A, et al. Serving larger portions of fruits and vegetables together at dinner promotes intake of both foods among young children. Journal of the Acadamy of Nutrition and Dietetics 2012;112(2):266‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mathias KC, Rolls BJ, Birch LL, Kral TVE, Fisher JO. Does serving children larger portions of fruit affect vegetable intake?. Obesity (Silver Spring, Md.) 2009;17:S90. [Google Scholar]
- Mbogori T, Murimi M. Effects of a nutrition education intervention on maternal nutrition knowledge, child care practices and nutrition status. Journal of Nutrition Education and Behavior 2016;48(7):S3. [Google Scholar]
- Gardner B, Sheals K, Wardle J, McGowan L. Putting habit into practice, and practice into habit: a process evaluation and exploration of the acceptability of a habit‐based dietary behaviour change intervention. International Journal of Behavioral Nutrition and Physical Activity 2014;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]; McGowan L, Cooke LJ, Gardner B, Beeken RJ, Croker H, Wardle J. Healthy feeding habits: efficacy results from a cluster‐randomized, controlled exploratory trial of a novel, habit‐based intervention with parents. American Journal of Clinical Nutrition 2013;98(3):769‐77. [DOI] [PubMed] [Google Scholar]
- Dixon LB, McKenzie J, Shannon BM, Mitchell DC, Smiciklas‐Wright H, Tershakovec AM. The effect of changes in dietary fat on the food group and nutrient intake of 4‐ to 10‐year‐old children. Pediatrics 1997;100(5):863‐72. [DOI] [PubMed] [Google Scholar]; Dixon LB, Tershakovec AM, McKenzie J, Shannon B. Diet quality of young children who received nutrition education promoting lower dietary fat. Public Health Nutrition 2000;3(4):411‐6. [DOI] [PubMed] [Google Scholar]; McKenzie J, Dixon LB, Smiciklas‐Wright H, Mitchell D, Shannon B, Tershakovec A. Change in nutrient intakes, number of servings, and contributions of total fat from food groups in 4‐ to 10‐year‐old children enrolled in a nutrition education study. Journal of the American Dietetic Association 1996;96(9):865‐72. [DOI] [PubMed] [Google Scholar]
- McSweeney L, Araujo‐Soares V, Rapley T, Adamson A. A feasibility study with process evaluation of a preschool intervention to improve child and family lifestyle behaviours. BMC Public Health2017; Vol. 17, issue 1:248. [DOI] [PMC free article] [PubMed]
- Mehta M, Ashburn L, Mehta M, Sankavaram K. Family‐based behavioral nutrition intervention improves nutrition knowledge, food choices, and BMI in Latino children. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S136. [Google Scholar]
- Meinen A, Friese B, Wright W, Carrel A. Youth gardens increase healthy behaviors in young children. Journal of Hunger and Environmental Nutrition 2012;7:192‐204. [Google Scholar]
- Mennella JA, Daniels LM, Reiter AR. Learning to like vegetables during breastfeeding: a randomized clinical trial of lactating mothers and infants. The American Journal of Clinical Nutrition 2017;106(1):67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe JJ, McCaffrey J. Pre‐testing and refinement of an after school cooking program for children: a pilot study of the Kids in the Kitchen Program. Journal of Nutrition Education and Behavior 2016;48(7):S11. [Google Scholar]
- Metcalfe JJ, Fiese B, Liu R, Emberton E, McCaffrey J. When kids learn to cook: findings from the Illinois Junior Chefs Effectiveness Trial. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 7:S3‐4.
- Mok E, Vanstone CA, Gallo S, Li P, Constantin E, Weiler HA. Diet diversity, growth and adiposity in healthy breastfed infants fed homemade complementary foods. International Journal of Obesity2017; Vol. 41, issue 5:776‐82. [DOI] [PubMed]
- Molitor F, Sugerman SB, Sciortino S. Fruit and vegetable, fat, and sugar‐sweetened beverage intake among low‐income mothers living in neighborhoods with Supplemental Nutrition Assistance Program. Journal of Nutrition Education and Behavior 2016;48(10):683‐90. [DOI] [PubMed] [Google Scholar]
- Monterrosa EC, Frongillo EA, Gonzalez de Cossio T, Bonvecchio A, Villanueva MA, Thrasher JF, et al. Scripted messages delivered by nurses and radio changed beliefs, attitudes, intentions, and behaviors regarding infant and young child feeding in Mexico. Journal of Nutrition 2013;143:915‐22. [DOI] [PubMed] [Google Scholar]
- Morgan R, Edwards Hall LA. Long‐term impact of nutrition education on dietary patterns. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A55. [Google Scholar]
- Morgan PJ, Young MD. The influence of fathers on children's physical activity and dietary behaviors: insights, recommendations and future directions. Current Obesity Reports 2017;6(3):324‐33. [DOI] [PubMed] [Google Scholar]
- Jones Brooke A. Incentivizing children's fruit and vegetable consumption: evaluation and modification of the food dudes program for sustainable use in U.S. elementary schools. Dissertation Abstracts International Section A: Humanities and Social Sciences2016; Vol. 76, issue 7‐A(E). ; Morrill BA, Madden GJ, Wengreen HJ, Fargo JD, Aguilar SS. A randomized controlled trial of the Food Dudes program: tangible rewards are more effective than social rewards for increasing short‐ and long‐term fruit and vegetable consumption. Journal of the Academy of Nutrition & Dietetics 2016;116(4):618‐29. [DOI] [PubMed] [Google Scholar]
- Murimi M, Moyeda Carabaza AF. Effective nutrition interventions for sustainable maternal and child health: lessons from the countries that achieved their MDG 4 and 5 targets. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 7:S20.
- Nabors L, Burbage M, Woodson KD, Swoboda C. Implementation of an after‐school obesity prevention program: helping young children toward improved health. Issues in Comprehensive Pediatric Nursing 2015;38(1):22‐38. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Lipsky LM, Eisenberg MH, Liu A, Mehta SN, Laffel LM. Can families eat better without spending more? Improving diet quality does not increase diet cost in a randomized clinical trial among youth with type 1 diabetes and their parents. Journal of the Academy of Nutrition and Dietetics2016; Vol. 116, issue 11:1751‐9. [DOI] [PMC free article] [PubMed]
- National Association of Pediatric Nurse Practitioners. Healthy Eating and Activity Together (HEAT) Clinical Practice Guideline: identifying and preventing overweight in childhood. Journal of Pediatric Health Care 2006;20(2):1‐64. [Google Scholar]
- Natale R, Messiah S, Asfor L, Uhlhorn S, Arheart K, Delamater A. Healthy Caregivers‐Healthy Children (HC2): a childcare center based obesity prevention. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S86‐7. [Google Scholar]; Natale R, Messiah S, Lopez‐Mitnik G, Uhlhorn S, Scott S, Delamater A. Healthy Caregivers–Healthy Children (HC2): a childcare center–based obesity prevention program. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S82. [Google Scholar]; Natale R, Scott SH, Messiah SE, Schrack MM, Uhlhorn SB, Delamater A. Design and methods for evaluating an early childhood obesity prevention program in the childcare center setting. BMC Public Health 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]; Natale RA, Messiah SE, Asfour L, Uhlhorn SB, Delamater A, Arheart KL. Role modeling as an early childhood obesity prevention strategy: effect of parents and teachers on preschool children's healthy lifestyle habits. Journal of Developmental and Behavioral Pediatrics 2014;35(6):378‐87. [DOI] [PubMed] [Google Scholar]; Natale RA, Messiah SE, Asfour LS, Uhlhorn SB, Englebert NE, Arheart KL. Obesity prevention program in childcare centers: two‐year follow‐up. American Journal of Health Promotion2016; Vol. 13:13. [DOI] [PubMed]
- Nederkoorn C, Theibetaen J, Tummers M, Roefs A. Taste the feeling or feel the tasting: tactile exposure to food texture promotes food acceptance. Appetite 2018;120:297‐301. [DOI] [PubMed] [Google Scholar]
- Nemet D, Perez S, Reges O, Eliakim A. Physical activity and nutrition knowledge and preferences in kindergarten children. International Journal of Sports Medicine 2007;28(10):887‐90. [DOI] [PubMed] [Google Scholar]
- Nemet D, Barzilay‐Teeni N, Eliakim A. Treatment of childhood obesity in obese families. Journal of Pediatric Endocrinology & Metabolism 2008;21(5):461‐7. [DOI] [PubMed] [Google Scholar]
- Nemet D, Geva D, Eliakim A. Health promotion intervention in low socioeconomic kindergarten children. Journal of Pediatrics 2011;158(5):796‐801. [DOI] [PubMed] [Google Scholar]
- Nerud K, Samra HA. Make a move. Intervention to reduce childhood obesity. Journal of School Nursing2017; Vol. 33, issue 3:205‐13. [DOI] [PubMed]
- Nicklas TA, Goh ET, Goodell LS, Acuff DS, Reiher R, Buday R, et al. Impact of commercials on food preferences of low‐income minority preschoolers. Journal of Nutrition Education and Behavior2011; Vol. 43, issue 1:35‐41. [DOI] [PMC free article] [PubMed]
- Noller B, Winkler G, Rummel C. BeKi ‐ an initiative for nutrition education in children: program description and evaluation. Gesundheitswesen 2006;68(3):165‐70. [DOI] [PubMed] [Google Scholar]; Winkler G, Noller B, Waibel S, Wiest M. BeKi ‐ an initiative for nutrition education in children in the federal state of Baden‐Wurttemberg: description, experiences, and considerations for an evaluation framework. Praventivmed 2005;50(3):151‐60. [DOI] [PubMed] [Google Scholar]
- Novotny R, Vijayadeva V, Ramirez V, Lee SK, Davison N, Gittelsohn J. Development and implementation of a food system intervention to prevent childhood obesity in rural Hawai'i. Hawaii Medical Journal 2011;70(7 Suppl 1):42‐6. [PMC free article] [PubMed] [Google Scholar]
- Nunes LM, Vigo A, Oliveira LD, Giugliani ERJ. Effect of a healthy eating intervention on compliance with dietary recommendations in the first year of life: a randomized clinical trial with adolescent mothers and maternal grandmothers. Journal of School Nursing2017; Vol. 33, issue 6:e00205615. [DOI] [PubMed]; Soldateli B, Vigo A, Giugliani ER. Adherence to dietary recommendations for preschoolers: clinical trial with teenage mothers. Revista de Saude Publica2016; Vol. 50:83. [DOI] [PMC free article] [PubMed]
- Nystrom CD, Sandin S, Henriksson P, Henriksson H, Trolle‐Lagerros Y, Larsson C, et al. Mobile‐based intervention intended to stop obesity in preschool‐aged children: the MINISTOP randomized controlled trial. American Journal of Clinical Nutrition2017; Vol. 105, issue 6:1327‐35. [DOI] [PubMed]
- O'Connor TM, Hughes SO, Watson KB, Baranowski T, Nicklas TA, Fisher JO, et al. Parenting practices are associated with fruit and vegetable consumption in pre‐school children. Public Health Nutrition 2010;13(1):91‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A, Fitzpatrick N, Doyle O. Effects of early intervention on dietary intake and its mediating role on cognitive functioning: a randomised controlled trial. Public Health Nutrition2017; Vol. 20, issue 1:154‐64. [DOI] [PMC free article] [PubMed]
- Ogle AD, Graham DJ, Lucas‐Thompson RG, Christina RA. Influence of cartoon media characters on children's attention to and preference for food and beverage products. Journal of the Academy of Nutrition and Dietetics2016; Vol. 117, issue 2:265‐70. [DOI] [PMC free article] [PubMed]
- Olvera N, Bush JA, Sharma SV, Knox BB, Scherer RL, Butte NF. BOUNCE: a community‐based mother‐daughter healthy lifestyle intervention for low‐income Latino families. Obesity 2010;18(1):S102‐S104. [DOI] [PubMed] [Google Scholar]
- Onnerfält J, Erlandsson LK, Orban K, Broberg M, Helgason C, Thorngren‐Jerneck K. A family‐based intervention targeting parents of preschool children with overweight and obesity: conceptual framework and study design of LOOPS‐ Lund overweight and obesity preschool study. BMC Public Health 2012;12:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paineau D, Beaufils F, Boulier A, Cassuto DA, Chwalow J, Combris P, et al. The cumulative effect of small dietary changes may significantly improve nutritional intakes in free‐living children and adults. European Journal of Clinical Nutrition 2010;64(8):782‐91. [DOI] [PubMed] [Google Scholar]
- Panunzio MF, Antoniciello A, Pisano A, Dalton S. Nutrition education intervention by teachers may promote fruit and vegetable consumption in Italian students. Nutrition Research 2007;27:524‐8. [Google Scholar]
- Parcel GS, Simons‐Morton B, O’Hara NM, Baranowski T, Wilson B. School promotion of healthful diet and physical activity: impact on learning outcomes and self reported behavior. Health Education & Behavior 1989;16(1):191‐9. [DOI] [PubMed] [Google Scholar]; Simons‐Morton BG, Parcel GS, O’Hara NM. Implementing organizational changes to promote healthful diet and physical activity at school. Health Education & Behavior 1988;15(1):115‐30. [DOI] [PubMed] [Google Scholar]
- Passehl B, McCarroll C, Buechner J, Gearring C, Smith AE, Trowbridge F. Preventing childhood obesity: establishing healthy lifestyle habits in the preschool years. Journal of Pediatric Health Care 2004;18(6):315‐9. [DOI] [PubMed] [Google Scholar]
- Peracchio H, Jaronko S, Argondezzi T, Latham K, Viteretto C. Growing Gardens, Growing Health Program: a novel approach to nutrition education in a garden setting. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A65. [Google Scholar]
- Perry CL, Mullis RM, Maile MC. Modifying the eating behaviour of young children. Journal of School Health 1985;55(10):399‐402. [DOI] [PubMed] [Google Scholar]
- Persson JE, Bohman B, Tynelius P, Rasmussen F, Ghaderi A. Prevention of childhood obesity in child health services: follow‐up of the PRIMROSE trial. Childhood Obesity 2018;14(2):7. [DOI] [PubMed] [Google Scholar]
- Peters P, Mobley AR, Procter S, Contreras D, Gold AL, Bruns K, et al. Mobilizing rural low‐income communities to assess and improve the ecological environment to prevent childhood obesity. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S86. [Google Scholar]
- Poelman A. Understanding and changing children's sensory acceptance for vegetables [PhD Dissertation]. Wageningen: Wageningen University, 2016. [Google Scholar]
- Poelman AAM, Delahunty CM, Cochet‐Broch M, Zwinkels M, Graaf CZ. The effect of multiple target versus single target vegetable exposure to increase vegetable intake in children. Appetite2016; Vol. 101, issue 223:no pagination.
- Polacsek M, Moran A, Thorndike AN, Boulos R, Franckle RL, Greene JC, et al. A supermarket double‐dollar incentive program increases purchases of fresh fruits and vegetables among low‐income families with children: the Healthy Double Study. Journal of Nutrition Education and Behavior 2017;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelip M, Slusser W, Thai CL, Kinsler J, Erausquin JT. Effects of a school‐based nutrition program diffused throughout a large urban community on attitudes, beliefs, and behaviors related to fruit and vegetable consumption. Journal of School Health 2011;81(9):520‐9. [DOI] [PubMed] [Google Scholar]
- Presti G, Cau S, Oppo A, Moderato P. Increased classroom consumption of home‐provided fruits and vegetables for normal and overweight children: results of the Food Dudes program in Italy. Journal of Nutrition Education & Behavior 2015;47(4):338‐344 7p. [DOI] [PubMed] [Google Scholar]
- Prosper M, Moczulski VL, Qureshi A, Weiss M, Bryars T. Healthy for Life/PE4ME: assessing an intervention targeting childhood obesity. Californian Journal of Health Promotion 2009;7:1‐10. [Google Scholar]
- Puia A, Leucuta DC. Children's lifestyle behaviors in relation to anthropometric indices: a family practice study. Clujul Medical 2017;90(4):385‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt SA, Dupuis J, Fish C, D'Agostino RB Jr. Feasibility of using a community‐supported agriculture program to improve fruit and vegetable inventories and consumption in an underresourced urban community. Preventing Chronic Disease 2013;10:E136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quizan‐Plata T, Villarreal Meneses L, Esparza Romero J, Anaya Barragan C, Galaviz Moreno S, Orozco Garcia ME, et al. Intervention to promote physical activity and dietary lifestyle changes in students attending public primary schools of Sonora Mexico. FASEB Journal2012; Vol. 26. ; Quizan‐Plata T, Villarreal Meneses L, Esparza Romero J, Bolanos Villar AV, Diaz Zavala RG. Educational program had a positive effect on the intake of fat, fruits and vegetables and physical activity in students attending public elementary schools of Mexico. Nutricion Hospitalaria 2014;30(3):552‐61. [DOI] [PubMed] [Google Scholar]
- Rackliffe LJ. Kid approved healthy snacks. Journal of Nutrition Education and Behavior2016; Vol. 49, issue 3:268.
- Rahman MM, Islam MA, Mahalanabis D, Chowdhury S, Biswas E. Impact of health education on the feeding of green leafy vegetables at home to children of the urban poor mothers of Bangladesh. Public Health 1994;108(3):211‐8. [DOI] [PubMed] [Google Scholar]
- Ransley JK, Greenwood DC, Cade JE, Blenkinsop S, Schagen I, Teeman D, et al. Does the school fruit and vegetable scheme improve children's diet? A non‐randomised controlled trial. Journal of Epidemiology and Community Health 2007;61:699‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of U.S. paediatric obesity primary care guidelines: two randomized trials. Pediatric Obesity 2012;7(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicks M, Vickers Z, Mykerezi E, Mann T, Redden J. Using in‐home behavioral economic strategies and enhanced food preparation skills to increase vegetable intake and variety among children. Journal of Nutrition Education and Behavior 2012;44(4 Supplement):S90. [DOI] [PubMed] [Google Scholar]
- Reifsnider EA, Militello L. Reducing childhood obesity among WIC recipients. Communicating Nursing Research 2012;45:442. [Google Scholar]
- Reinaerts E, Crutzen R, Candel M, Vries NK, Nooijer J. Increasing fruit and vegetable intake among children: comparing long‐term effects of a free distribution and multicomponent program. Health Education Research 2008;23(3):987‐96. [DOI] [PubMed] [Google Scholar]; Reinaerts E, Nooijer J, Candel M, Vries N. Increasing children's fruit and vegetable consumption: distribution or a multicomponent programme?. Public Health Nutrition 2007;10(9):939‐47. [DOI] [PubMed] [Google Scholar]
- Reinbott A, Schelling A, Kuchenbecker J, Jeremias T, Russell I, Kevanna O, et al. Nutrition education linked to agricultural interventions improved child dietary diversity in rural Cambodia. British Journal of Nutrition2016; Vol. 116, issue 8:1457‐68. [DOI] [PMC free article] [PubMed]
- Reinehr T, Schaefer A, Winkel K, Finne E, Kolip P. Development and evaluation of the lifestyle intervention "obeldicks light" for overweight children and adolescents. Journal of Public Health 2011;19:377‐84. [Google Scholar]
- Reverdy C, Chesnel F, Schlich P, Koster EP, Lange C. Effect of sensory education on willingness to taste novel food in children. Appetite 2008;51(1):156‐65. [DOI] [PubMed] [Google Scholar]
- Reynolds KD, Raczynski JM, Binkley D, Franklin FA, Duvall RC, Devane‐Hart K, et al. Design of 'High 5': a school‐based study to promote fruit and vegetable consumption for reduction of cancer risk. Journal of Cancer Education 1998;13(3):169‐77. [DOI] [PubMed] [Google Scholar]
- Reznar MM. Application of behavior change and persuasion theories to a multi‐media intervention designed to improve the home food environment and diet quality of resource‐limited parents with young children. Dissertation Abstracts International: Section B: The Sciences and Engineering2013; Vol. 74.
- Ribeiro RQ, Alves L. Comparison of two school‐based programmes for health behaviour change: the Belo Horizonte Heart Study randomized trial. Public Health Nutrition 2014;17:1195‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux C, Lafraire J, Picard D. Visual exposure and categorization performance positively influence 3‐to 6‐year‐old children's willingness to taste unfamiliar vegetables. Appetite 2018;120:32‐42. [DOI] [PubMed] [Google Scholar]
- Ritchie LD, Sharma S, Ikeda JP, Mitchell RA, Raman A, Green BS, et al. Taking Action Together: a YMCA‐based protocol to prevent type‐2 diabetes in high‐BMI inner‐city African American children. Trials 2010;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rito AI, Carvalho MA, Ramos C, Breda J. Program Obesity Zero (POZ) ‐ a community‐based intervention to address overweight primary‐school children from five Portuguese municipalities. Public Health Nutrition 2013;16(6):1043‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson PB. MEND: A family‐based community intervention for childhood obesity and its effectiveness. Dissertation Abstracts International: Section B: The Sciences and Engineering2013; Vol. 73.
- Roche ML, Marquis GS, Gyorkos TW, Blouin B, Sarsoza J, Kuhnlein HV. A community‐based infant and young child nutrition intervention in Ecuador improved diet and reduced underweight. Journal of Nutrition Education and Behavior2016; Vol. 49, issue 3:196‐203. [DOI] [PubMed]
- Rogers VW, Hart PH, Motyka E, Rines EN, Vine J, Deatrick DA. Impact of Let's Go! 5‐2‐1‐0: a community‐based, multisetting childhood obesity prevention program. Journal of Pediatric Psychology 2013;38:1010‐20. [DOI] [PubMed] [Google Scholar]
- Rohde JF, Larsen SC, Angquist L, Olsen NJ, Stougaard M, Mortensen EL, et al. Effects of the Healthy Start randomized intervention on dietary intake among obesity‐prone normal‐weight children. Public Health Nutrition2017; Vol. 20, issue 16:1‐10. [DOI] [PMC free article] [PubMed]
- Rohlfs DP, Gámiz F, Gil M, Moreno H, Márquez Zamora R, Gallo M, et al. Providing choice increases children’s vegetable intake. Food Quality and Preference 2013;30:108‐13. [Google Scholar]
- Romo‐Palafox MJ, Ranjit N, Sweitzer SJ, Roberts‐Gray C, Byrd‐Williams CE, Briley ME, et al. Adequacy of parent‐packed lunches and preschooler's consumption compared to dietary reference intake recommendations. Journal of the American College of Nutrition2017; Vol. 36, issue 3:169‐76. [DOI] [PubMed]
- Rubenstein Cynthia. Assessing and Improving Child Feeding Practices through "Take Charge of Your Family's Health" [DPhil thesis]. Villanova University, 2010. [Google Scholar]
- Lagstrom H, Seppanen R, Jokinen E, Ronnemaa T, Salminen M, Tuominen J, et al. Nutrient intakes and cholesterol values of the parents in a prospective randomized child‐targeted coronary heart disease risk factor intervention trial‐‐the STRIP project. European Journal of Clinical Nutrition 1999;53(8):654‐61. [DOI] [PubMed] [Google Scholar]; Ruottinen S, Niinikoski H, Lagström H, Rönnemaa T, Hakanen M, Viikari J, et al. High sucrose intake is associated with poor quality of diet and growth between 13 months and 9 years of age: The Special Turku Coronary Risk Factor Intervention Project. Pediatrics 2008;121(6):e1676‐e1685. [DOI] [PubMed] [Google Scholar]; Talvia S, Räsänen L, Lagström H, Pahkala K, Viikari J, Rönnemaa T, et al. Longitudinal trends in consumption of vegetables and fruit in Finnish children in an atherosclerosis prevention study (STRIP). European Journal of Clinical Nutrition 2006;60(2):172‐80. [DOI] [PubMed] [Google Scholar]
- Salminen M, Vahlberg T, Ojanlatva A, Kivela SL. Effects of a controlled family‐based health education/counseling intervention. American Journal of Health Behavior 2005;29(5):395‐406. [DOI] [PubMed] [Google Scholar]
- Sanders LM, Perrin EM, Yin HS, Bronaugh A, Rothman RL, Greenlight Study Team. "Greenlight study": a controlled trial of low‐literacy, early childhood obesity prevention. Pediatrics 2014;133(6):e1724‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanigorski AM, Bell AC, Kremer PJ, Cuttler R, Swinburn BA. Reducing unhealthy weight gain in children through community capacity‐building: results of a quasi‐experimental intervention program, Be Active Eat Well. International Journal of Obesity 2008;32(7):1060‐7. [DOI] [PubMed] [Google Scholar]
- Sanjur D, Garcia A, Aguilar R, Furumoto R, Mort M. Dietary patterns and nutrient intakes of toddlers from low‐income families in Denver, Colorado. Journal of the American Dietetic Association 1990;90(6):823‐9. [PubMed] [Google Scholar]
- Sanna T, Saarinen M, Lagstrom H. Tracking and clustering of dietary factors in the prospective dietary intervention trial in childhood and adolescence. Annals of Nutrition and Metabolism 2011;58:326. [Google Scholar]
- Savage JS, Paul IM, Marini ME, Birch LL. Pilot intervention promoting responsive feeding, the division of feeding responsibility, and healthy dietary choices during infancy. Appetite 2010;54(3):673. [Google Scholar]
- Scherr RE, Linnell JD, Dharmar M, Beccarelli L, Bergman JM, Briggs J, et al. A multicomponent, school‐based intervention, the Shaping Healthy Choices Program, improves nutrition‐related outcomes. Journal of Nutrition Education and Behavior2017; Vol. 49, issue 5:368‐79. [DOI] [PubMed]
- Schmied E, Parada H, Horton L, Ibarra L, Ayala G. A process evaluation of an efficacious family‐based intervention to promote healthy eating: the Entre Familia: Reflejos de Salud Study. Health Education & Behavior 2015;42(5):583‐92. [DOI] [PubMed] [Google Scholar]
- Schumacher TL, Burrows TL, Thompson DI, Spratt NJ, Callister R, Collins CE. Feasibility of recruiting families into a heart disease prevention program based on dietary patterns. Nutrients2015; Vol. 7, issue 8:7042‐57. [DOI] [PMC free article] [PubMed]
- Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office‐based motivational interviewing to prevent childhood obesity. Archives of Pediatrics and Adolescent Medicine 2007;161(5):495‐501. [DOI] [PubMed] [Google Scholar]
- Schwartz MB. The influence of a verbal prompt on school lunch fruit consumption: a pilot study. International Journal of Behavioral Nutrition and Physical Activity 2007;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MB, O'Connell M, Henderson KE, Middleton AE, Scarmo S. Testing variations on family‐style feeding to increase whole fruit and vegetable consumption among preschoolers in child care. Childhood Obesity (Print) 2015;11(5):499‐505. [DOI] [PubMed] [Google Scholar]
- Sharafi M, Peracchio H, Dugdale T, Scarmo S, Huedo‐Medina T, Duffy V. Measuring vegetable intake and dietary quality in response to a preschool‐based education program. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A86. [Google Scholar]
- Sharma SV, Markham C, Chow J, Ranjit N, Pomeroy M, Raber M. Evaluating a school‐based fruit and vegetable co‐op in low‐income children: a quasi‐experimental study. Preventive Medicine 2016;91:8‐17. [DOI] [PubMed] [Google Scholar]
- Sharps M, Robinson E. Encouraging children to eat more fruit and vegetables: health vs. descriptive social norm‐based messages. Appetite2016; Vol. 100:18‐25. [DOI] [PMC free article] [PubMed]
- Sherwood NE, French SA, Veblen‐Mortenson S, Crain AL, Berge J, Kunin‐Batson A, et al. NET‐Works: linking families, communities and primary care to prevent obesity in preschool‐age children. Contemporary Clinical Trials 2013;36(2):544‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilts M, Ontai L, Townsend M. Efficacy of a guided goal‐setting intervention for low‐income parents to reduce risk of pediatric obesity: preliminary results. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S118. [Google Scholar]
- Shim JE, Kim J, Mathai RA. Associations of infant feeding practices and picky eating behaviors of preschool children. Journal of the American Dietetic Association 2011;111(9):1363‐8. [DOI] [PubMed] [Google Scholar]
- Shin HS, Valente TW, Riggs NR, Huh J, Spruijt‐Metz D, Chou CP, et al. The interaction of social networks and child obesity prevention program effects: the pathways trial. Obesity 2014;22:1520‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siega‐Riz AM, Kranz S, Blanchette D, Haines PS, Guilkey DK, Popkin BM. The effect of participation in the WIC program on preschoolers' diets. The Journal of Pediatrics 2004;144(2):229‐34. [DOI] [PubMed] [Google Scholar]
- Singh A, Klemm RD, Mundy G, Pandey Rana P, Pun B, Cunningham K. Improving maternal, infant and young child nutrition in Nepal via peer mobilization. Public Health Nutrition 2018;21(4):796‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouteris H, Edwards S, Rutherford L, Cutter‐MacKenzie A, Huang T, O'Connor A. Promoting healthy eating, active play and sustainability consciousness in early childhood curricula, addressing the Ben10™ problem: a randomised control trial. BMC Public Health 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusser W, Frankel F, Robison K, Fischer H, Cumberland WG, Neumann C. Pediatric overweight prevention through a parent training program for 2‐4 year old Latino children. Childhood Obesity 2012;8(1):52‐9. [DOI] [PubMed] [Google Scholar]
- Smith L, Conroy K, Wen H, Rui L, Humphries D. Portion size variably affects food intake of 6‐year‐old and 4‐year‐old children in Kunming, China. Appetite 2013;69:31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Wells K, Stluka S, McCormack LA. The impact of a fruit and vegetable intervention on children and caregivers. American Journal of Health Education 2015;46(6):316‐22. [Google Scholar]
- Sobko T, Svensson V, Ek A, Ekstedt M, Karlsson H, Johansson E, et al. A randomised controlled trial for overweight and obese parents to prevent childhood obesity‐‐Early STOPP (STockholm Obesity Prevention Program). BMC Public Health 2011;11:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobko T, Jia Z, Kaplan M, Lee A, Tseng CH. Promoting healthy eating and active playtime by connecting to nature families with preschool children: evaluation of pilot study "Play&Grow". Pediatric Research 2017;81(4):572‐81. [DOI] [PubMed] [Google Scholar]
- Sojkowski S, Severin S, Kannan S. Sensory exploration of seasonally and locally available vegetables and their effects on vegetable consumption of Western Massachusetts Head Start preschool children. FASEB Journal2012; Vol. 26.
- Solomons NW. Plant sources of vitamin A and human nutrition: how much is still too little?. Nutrition Reviews 1999;57(11):350‐61. [DOI] [PubMed] [Google Scholar]
- Song HJ, Grutzmacher S, Munger AL. Project ReFresh: testing the efficacy of a school‐based classroom and cafeteria intervention in elementary school children. Journal of School Health 2016;86(7):543‐51. [DOI] [PubMed] [Google Scholar]
- Sotos‐Prieto M, Santos‐Beneit G, Penalvo JL, Pocock S, Redondo J, Fuster V. Mediterranean dietary patterns in 3‐5 year old children and their parents: the Program Si! study. Annals of Nutrition and Metabolism 2013;63:921‐2. [Google Scholar]; Sotos‐Prieto M, Santos‐Beneit G, Pocock S, Redondo J, Fuster V, Penalvo JL. Parental and self‐reported dietary and physical activity habits in pre‐school children and their socio‐economic determinants. Public Health Nutrition 2015;18(2):275‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs K, Grutzmacher SK. Lessons learned for enrolling parents in a text message‐based nutrition education program. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S1. [Google Scholar]
- Stark LJ, Collins FL Jr, Osnes PG, Stokes TF. Using reinforcement and cueing to increase healthy snack food choices in preschoolers. Journal of Applied Behavior Analysis 1986;19(4):367‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, et al. A pilot randomized controlled trial of a clinic and home‐based behavioral intervention to decrease obesity in preschoolers. Obesity 2011;19:134‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbock B, Zeeb H, Rach S, Pohlabeln H, Pischke CR. Design and methods for a cluster‐controlled trial conducted at sixty‐eight daycare facilities evaluating the impact of "JolinchenKids ‐ Fit and Healthy in Daycare", a program for health promotion in 3‐ to 6‐year‐old children. BMC Public Health2017; Vol. 18, issue 1:6. [DOI] [PMC free article] [PubMed]
- Stern M, Bleck J, Ewing LJ, Davila E, Lynn C, Hale G, et al. NOURISH‐T: targeting caregivers to improve health behaviors in pediatric cancer survivors with obesity. Pediatric Blood & Cancer 2018;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story M, Hannan PJ, Fulkerson JA, Rock BH, Smyth M, Arcan C, et al. Bright Start: description and main outcomes from a group‐randomized obesity prevention trial in American Indian children. Obesity 2012;20(11):2241‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez‐Balcazar Y, Kouba J, Jones LM, Lukyanova VV. A university‐school collaboration to enhance healthy choices among children. Journal of Prevention & Intervention in the Community 2014;42(2):140‐51. [DOI] [PubMed] [Google Scholar]
- Sun A, Cheng J, Bui Q, Liang Y, Ng T, Chen JL. Home‐based and technology‐centered childhood obesity prevention for Chinese mothers with preschool‐aged children. Journal of Transcultural Nursing2017; Vol. 28, issue 6:616‐24. [DOI] [PubMed]
- Briley ME, Ranjit N, Holescher DM, Sweitzer SJ, Almansour F, Roberts‐Gray C. Unbundling outcomes of a multilevel intervention to increase fruit, vegetables and whole grains parents pack for their preschool children in sack lunches. American Journal of Health Education 2012;43:135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roberts‐Gray C, Briley ME, Ranjit N, Byrd‐Williams CE, Sweitzer SJ, Sharma SV, et al. Efficacy of the Lunch is in the Bag intervention to increase parents' packing of healthy bag lunches for young children: a cluster‐randomized trial in early care and education centers. International Journal of Behavioral Nutrition & Physical Activity 2016;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roberts‐Gray C, Ranjit N, Sweitzer SJ, Byrd‐Williams CE, Romo‐Palafox MJ, Briley ME, et al. Parent packs, child eats: surprising results of Lunch is in the Bag's efficacy trial. Appetite 2017;24:24. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sharma SV, Rashid T, Ranjit N, Byrd‐Williams C, Chuang RJ, Roberts‐Gray C, et al. Effectiveness of the Lunch is in the Bag program on communication between the parent, child and child‐care provider around fruits, vegetables and whole grain foods: a group‐randomized controlled trial. Preventive Medicine 2015;81:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sweitzer SJ, Briley ME, Roberts‐Gray C, Hoelscher DM, Harrist RB, Staskel DM, et al. Lunch is in the Bag: increasing fruits, vegetables, and whole grains in sack lunches of preschool‐aged children. Journal of the American Dietetic Association 2010;110(7):1058‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sweitzer SJ, Briley ME, Roberts‐Gray C, Hoelscher DM, Harrist RB, Staskel DM, et al. Psychosocial outcomes of Lunch is in the Bag, a parent program for packing healthful lunches for preschool children. Journal of Nutrition Education & Behavior 2011;43(6):536‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande D, Niemeier BS, Hwang H, Stastny S, Hektner JM. Intervention changes fruit and vegetable intake among preschoolers in pilot study. Journal of Nutrition Education & Behavior 2013;45:S58‐9. [Google Scholar]
- McAuley KA, Taylor RW, Farmer VL, Hansen P, Williams SM, Booker CS. Economic evaluation of a community‐based obesity prevention program in children: the APPLE project. Obesity2009; Vol. 18, issue 1:131‐6. [DOI] [PubMed]; Taylor RW, McAuley KA, Barbezat W, Farmer VL, Williams SM, Mann JI. Two‐year follow‐up of an obesity prevention initiative in children: the APPLE project. American Journal of Clinical Nutrition 2008;88(5):1371‐7. [DOI] [PubMed] [Google Scholar]; Taylor RW, McAuley KA, Barbezat W, Strong A, Williams SM, Mann JI. APPLE project: 2‐y findings of a community‐based obesity prevention program in primary school‐age children. American Journal of Clinical Nutrition 2007;86(3):735‐42. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Brown D, Dawson AM, Haszard J, Cox A, Rose EA, et al. Motivational interviewing for screening and feedback and encouraging lifestyle changes to reduce relative weight in 4‐8 year old children: design of the MInT study. BMC Public Health 2010;10:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Darby H, Upton P, Upton D. Can a school‐based intervention increase children's fruit and vegetable consumption in the home setting?. Perspectives in Public Health 2013;133(6):330‐6. [DOI] [PubMed] [Google Scholar]
- Taylor JC, Johnson RK. Farm to School as a strategy to increase children's fruit and vegetable consumption in the United States: research and recommendations. Nutrition Bulletin 2013;38:70‐9. [Google Scholar]
- Taylor NJ, Sahota P, Sargent J, Barber S, Loach J, Louch G, et al. Using intervention mapping to develop a culturally appropriate intervention to prevent childhood obesity: the HAPPY (Healthy and Active Parenting Programme for Early Years) study. International Journal of Behavioral Nutrition & Physical Activity 2013;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Upton P, Upton D. Increasing primary school children's fruit and vegetable consumption: a review of the Food Dudes programme. Health Education 2015;115(2):178‐96. [Google Scholar]
- Taylor RW, Cox A, Knight L, Brown DA, Meredith‐Jones K, Haszard JJ, et al. A tailored family‐based obesity intervention: a randomized trial. Pediatrics 2015;136(2):282‐9. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Heath AL, Galland BC, Cameron SL, Lawrence JA, Gray AR, et al. Three‐year follow‐up of a randomised controlled trial to reduce excessive weight gain in the first two years of life: protocol for the POI follow‐up study. BMC Public Health2016; Vol. 16, issue 1:771. [DOI] [PMC free article] [PubMed]
- Velde SJ, Brug J, Wind M, Hildonen C, Bjelland M, Pérez‐Rodrigo C, et al. Effects of a comprehensive fruit‐ and vegetable‐promoting school‐based intervention in three European countries: the Pro Children Study. British Journal of Nutrition 2008;99(4):893‐903. [DOI] [PubMed] [Google Scholar]; Velde SJ, Wind M, Perez‐Rodrigo C, Klepp KI, Brug J. Mothers' involvement in a school‐based fruit and vegetable promotion intervention is associated with increased fruit and vegetable intakes ‐ the Pro Children study. International Journal of Behavioral Nutrition and Physical Activity2008; Vol. 5, issue 48:15. [DOI] [PMC free article] [PubMed]
- Tharrey M, Olaya GA, Fewtrell M, Ferguson E. Adaptation of new Colombian food‐based complementary feeding recommendations using linear programming. Journal of Pediatric Gastroenterology and Nutrition 2017;65(6):667‐72. [DOI] [PubMed] [Google Scholar]
- Thomson JL, Tussing‐Humphreys LM, Goodman MH. Delta Healthy Sprouts: a randomized comparative effectiveness trial to promote maternal weight control and reduce childhood obesity in the Mississippi Delta. Contemporary Clinical Trials2014; Vol. 38, issue 1:82‐91. [DOI] [PubMed]
- Timms V. Early intervention and good feeding advice support healthy eating. Nursing Children & Young People 2011;23:9. [Google Scholar]
- Tobey LN, Koenig HF, Brown NA, Manore MM. Reaching low‐income mothers to improve family fruit and vegetable intake: food hero social marketing campaign‐research steps, development and testing. Nutrients2016; Vol. 8, issue 9:13. [DOI] [PMC free article] [PubMed]
- Tomayko EJ, Prince RJ, Cronin KA, Adams AK. The Healthy Children, Strong Families intervention promotes improvements in nutrition, activity and body weight in American Indian families with young children. Public Health Nutrition2016; Vol. 19, issue 15:2850‐9. [DOI] [PMC free article] [PubMed]; Tomayko EJ, Prince RJ, Cronin KA, Adams AK. The Healthy Children, Strong Families intervention promotes improvements in nutrition, activity, and body weight in American Indian families with young children – ERRATUM. Public Health Nutrition2016; Vol. 20, issue 2:380. [DOI] [PMC free article] [PubMed]
- Tomayko EJ, Mosso KL, Cronin KA, Carmichael L, Kim K, Parker T, et al. Household food insecurity and dietary patterns in rural and urban American Indian families with young children. BMC Public Health2017; Vol. 17, issue 1:611. [DOI] [PMC free article] [PubMed]
- Tovar A, Vaughn AE, Grummon A, Burney R, Erinosho T, Ostbye T, et al. Family child care home providers as role models for children: cause for concern?. Preventive Medicine Reports2017; Vol. 5:308‐13. [DOI] [PMC free article] [PubMed]
- Tran MTN. Parents and School’s Collaboration for Improving Children Food Well‐Being by Knowledge Perspective [Thesis]. Nomi: Japan Advanced Institute of Science and Technology, 2017. [Google Scholar]
- Trees N, Dwyer J. Feeding the next generation. Nutrition Today 2012;47:281‐97. [Google Scholar]
- Tucker S, Lanningham‐Foster L, Murphy J, Olsen G, Orth K, Voss J, et al. A school based community partnership for promoting healthy habits for life. Journal of Community Health 2011;36(3):414‐22. [DOI] [PubMed] [Google Scholar]
- Tyler DO, Horner SD. A primary care intervention to improve weight in obese children: a feasibility study. Journal of the American Association of Nurse Practitioners2016; Vol. 28, issue 2:98‐106. [DOI] [PubMed]
- Uicab‐Pool GA, Ferriani MGC, Gomes R, Pelcastre‐Villafuerte B. Representations of eating and of a nutrition program among female caregivers of children under 5 years old in Tizimin, Yucatan, Mexico. Revista Latino‐Americana de Enfermagem 2009;17(6):940‐6. [DOI] [PubMed] [Google Scholar]
- Upton D, Upton P, Taylor C. Increasing children's lunchtime consumption of fruit and vegetables: an evaluation of the Food Dudes program. Public Health Nutrition 2013;16(6):1066‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]; Upton P, Taylor C, Upton D. The effects of the Food Dudes Programme on children's intake of unhealthy foods at lunchtime. Perspectives in Public Health 2015;135(3):152‐9. [DOI] [PubMed] [Google Scholar]
- Upton D, Taylor C, Upton P. Parental provision and children's consumption of fruit and vegetables did not increase following the Food Dudes programme. Health Education (0965‐4283) 2014;114:58‐66. [Google Scholar]
- Urrutia CN. Evaluation of a Nutrition Education Program Targeting Home‐Based Child Care Centers [Thesis]. El Paso: University of Texas, 2017. [Google Scholar]
- Utter J, Fay AP, Denny S. Child and youth cooking programs: more than good nutrition?. Journal of Hunger & Environmental Nutrition 2017;12(4):554‐80. [Google Scholar]
- Vandeweghe L, Verbeken S, Moens E, Vervoort L, Braet C. Strategies to improve the willingness to taste: the moderating role of children's reward sensitivity. Appetite 2016;103:344‐52. [DOI] [PubMed] [Google Scholar]
- Horn L, Obarzanek E, Aronson Friedman L, Gernhofer N, Barton B. Children's adaptations to a fat‐reduced diet: The Dietary Intervention Study in Children (DISC). Pediatrics 2005;115(6):1723‐33. [DOI] [PubMed] [Google Scholar]
- Horn L. Nutrition and child care: a healthy head start. Journal of the American Dietetic Association 2011;111(9):1282. [DOI] [PubMed] [Google Scholar]
- Nassau F, Singh AS, Mechelen W, Brug J, Chinapaw MJM. Implementation evaluation of school‐based obesity prevention programmes in youth; how, what and why?. Public Health Nutrition 2015;18(09):1531‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AE, Mazzucca S, Burney R, Ostbye T, Neelon B, Tovar SE, et al. Assessment of nutrition and physical activity environments in family child care homes: modification and psychometric testing of the Environment and Policy Assessment and Observation. BMC Public Health2017; Vol. 17, issue 1:680. [DOI] [PMC free article] [PubMed]
- Vecchiarelli S, Prelip M, Slusser W, Weightman H, Neumann C. Using participatory action research to develop a school‐based environmental intervention to support healthy eating and physical activity. American Journal of Health Education 2005;36(1):35‐42. [Google Scholar]
- Veldhuis L, Struijk MK, Kroeze W, Oenema A, Renders CM, Bulk‐Bunschoten AMW, et al. 'Be active, eat right', evaluation of an overweight prevention protocol among 5‐year‐old children: design of a cluster randomised controlled trial. BMC Public Health 2009;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano E, Viggiano A, Vicidomini C, Costanzo A, Andreozzi E, Romano V, et al. Kaledo, a new educational board‐game for nutrition education: cluster randomized trial of healthy lifestyle promotion. Obesity Facts 2012;5:260. [Google Scholar]
- Vio F, Salinas J, Montenegro E, González CG, Lera L. Impact of a nutrition education intervention in teachers, preschool and basic school‐age children in Valparaiso region in Chile. Nutricion Hospitalaria 2014;29(6):1298‐304. [DOI] [PubMed] [Google Scholar]
- Rauber F, Hoffman DJ, Vitolo MR. Diet quality from pre‐school to school age in Brazilian children: a 4‐year follow‐up in a randomised control study. British Journal of Nutrition 2014;111(3):499‐505. [DOI] [PubMed] [Google Scholar]; Valmorbida JL, Vitolo MR. Factors associated with low consumption of fruits and vegetables by preschoolers of low socio‐economic level. Jornal de Pediatria 2014;90:464‐71. [DOI] [PubMed] [Google Scholar]; Vitolo MR, Rauber F, Campagnolo PD, Feldens CA, Hoffman DJ. Maternal dietary counseling in the first year of life is associated with a higher healthy eating index in childhood. Journal of Nutrition 2010;140(11):2002‐7. [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Louzada ML, Rauber F. Positive impact of child feeding training program for primary care health professionals: a cluster randomized field trial. Revista Brasileira de Epidemiologia [Brazilian journal of epidemiology]2014; Vol. 17, issue 4:873‐86. [DOI] [PubMed]
- Wald ER, Ewing LJ, Moyer SCL, Eickhoff JC. An interactive web‐based intervention to achieve healthy weight in young children. Clinical Pediatrics 2017;Epub ahead of print:No pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K, Filion AJ, Darlington G, Morrongiello B, Haines J. Parents and tots together: adaptation of a family‐based obesity prevention intervention to the Canadian context. Canadian Journal of Diabetes 2015;39:S72‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink B, Just DR, Hanks AS, Smith LE. Pre‐sliced fruit in school cafeterias: children's selection and intake. American Journal of Preventive Medicine 2013;44(5):477‐80. [DOI] [PubMed] [Google Scholar]
- Wansink B, Just D, Dollahite J, Latimer L, Thomas L, Hill T, et al. Smarter lunchrooms ‐ does changing environments really give more nutritional bang for the buck?. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S198‐9. [Google Scholar]
- Ward DS, Vaughn AE, Bangdiwala KI, Campbell M, Jones DJ, Panter AT, et al. Integrating a family‐focused approach into child obesity prevention: rationale and design for the My Parenting SOS study randomized control trial. BMC Public Health 2011;11:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DS, Vaughn AE, Mazzucca S, Burney R. Translating a child care based intervention for online delivery: development and randomized pilot study of Go NAPSACC. BMC Public Health 2017;17(1):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Herrera M‐L, Gibson EL. Modifying children’s food preferences: the effects of exposure and reward on acceptance of an unfamiliar vegetable. European Journal of Clinical Nutrition 2003;57(2):341‐8. [DOI] [PubMed] [Google Scholar]
- Warschburger P, Gmeiner M, Morawietz M, Rinck M. Battle of plates: a pilot study of an approach‐avoidance training for overweight children and adolescents. Public Health Nutrition 2018;21(2):426‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Nelson M. The National School Fruit Scheme produces short‐term but not longer‐term increases in fruit consumption in primary school children. British Journal of Nutrition 2005;93:537‐42. [DOI] [PubMed] [Google Scholar]
- Baur L. Effectiveness of a home‐based early intervention on children's BMI at age two years: randomised controlled trial. Obesity Facts 2012;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wen LM, Baur LA, Rissel C, Wardle K, Alperstein G, Simpson JM. Early intervention of multiple home visits to prevent childhood obesity in a disadvantaged population: a home‐based randomised controlled trial (Healthy Beginnings Trial). BMC Public Health 2007;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: Randomised controlled trial. BMJ 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Healthy Beginnings trial: the journey from the beginning. Obesity Research and Clinical Practice 2013;7:e2. [Google Scholar]
- Wen LM, Baur LA, Simpson JM, Rissel C, Flood VM. Effectiveness of an early intervention on infant feeding practices and "tummy time": a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2011;165(8):701‐7. [DOI] [PubMed] [Google Scholar]
- Wen J, Wang NR, Zhao Y, Fan X, Ye Y. Effect of eating behavior intervention on infants in the urban area of Chongqing, China. Zhongguo Dangdai Erke Zazhi 2013;15:361‐3. [PubMed] [Google Scholar]
- Wen LM, Rissel C, Baur LA, Hayes AJ, Xu H, Whelan A, et al. A 3‐arm randomised controlled trial of Communicating Healthy Beginnings Advice by Telephone (CHAT) to mothers with infants to prevent childhood obesity. BMC Public Health 2017;17(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengreen H, Aguilar S, Madden G. Incentivizing children’s intake of fruits and vegetables at school: a U.S. evaluation of the Food Dudes program. Journal of Nutrition Education and Behavior 2013;45(4 Supplement):S33. [DOI] [PubMed] [Google Scholar]
- Whaley SE, McGregor S, Jiang L, Gomez J, Harrison G, Jenks E. A WIC‐based intervention to prevent early childhood overweight. Journal of Nutrition Education and Behavior 2010;42(3S):S47‐S51. [DOI] [PubMed] [Google Scholar]
- Whiteside‐Mansell L, Swindle TM. Together we inspire smart eating: a preschool curriculum for obesity prevention in low‐income families. Journal of Nutrition Education and Behavior 2017;49(9):789‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinha‐Bettoni R, Orito A, Löwik M, McLean C, Muehlhoff E. Increasing fruit and vegetable consumption among schoolchildren: efforts in middle‐income countries. Food & Nutrition Bulletin 2013;34(1):75‐94. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Han H, Johnson WD, Martin CK, Newton RL Jr. Modification of the school cafeteria environment can impact childhood nutrition. Results from the Wise Mind and LA Health studies. Appetite 2013;61(1):77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Hartell B, Qu S, Martinez R. A one‐year innovative fruit and vegetable sampling program for WIC children: Willow Comes to WIC. Journal of the Academy of Nutrition and Dietetics 2016;116(9):A10. [Google Scholar]
- Wyatt KM, Lloyd JJ, Abraham C, Creanor S, Dean S, Densham E, et al. The Healthy Lifestyles Programme (HeLP), a novel school‐based intervention to prevent obesity in school children: study protocol for a randomised controlled trial. Trials 2013;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Wolfenden L, Wyse R, Bowman J, McElduff P, Duncan S. A randomised controlled trial and mediation analysis of the 'Healthy Habits', telephone‐based dietary intervention for preschool children. International Journal of Behavioral Nutrition and Physical Activity 2013;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wyse R, Campbell KJ, Brennan L, Wolfenden L. A cluster randomised controlled trial of a telephone‐based intervention targeting the home food environment of preschoolers (The Healthy Habits Trial): the effect on parent fruit and vegetable consumption. International Journal of Behavioral Nutrition & Physical Activity 2014;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y, Hartlieb KB, Danford C, Catherine JKL. Effectiveness of nutrition intervention in a selected group of overweight and obese African‐American preschoolers. Journal of Racial & Ethnic Health Disparities2017; Vol. 11:11. [DOI] [PubMed]
- Yin Z, Parra‐Medina D, Cordova A, He M, Trummer V, Sosa E, et al. Miranos! Look at us, we are healthy! An environmental approach to early childhood obesity prevention. Childhood Obesity 2012;8(5):429‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoong SL, Grady A, Wiggers J, Flood V, Rissel C, Finch M, et al. A randomised controlled trial of an online menu planning intervention to improve childcare service adherence to dietary guidelines: a study protocol. BMJ Open2017; Vol. 7, issue 9:e017498. [DOI] [PMC free article] [PubMed]
- Young L. Under 5 Energize: Improving child nutrition and physical activity through early childhood centres [Thesis]. Auckland: Auckland University of Technology, 2017. [Google Scholar]
- Adams J, Molyneux M, Squires L. Sustaining an obesity prevention intervention in preschools. Health Promotion Journal of Australia 2011;22(1):6‐10. [DOI] [PubMed] [Google Scholar]; Adams J, Zask A, Dietrich U. Tooty Fruity Vegie in Preschools: an obesity prevention intervention in preschools targeting children's movement skills and eating behaviours. Health Promotion Journal of Australia 2009;20(2):112‐9. [DOI] [PubMed] [Google Scholar]; Zask A, Adams JK, Brooks LO, Hughes DF. Tooty Fruity Vegie: an obesity prevention intervention evaluation in Australian preschools. Health Promotion Journal of Australia 2012;23(1):10‐5. [DOI] [PubMed] [Google Scholar]
- Zeinstra GG, Renes RJ, Koelen MA, Kok FJ, Graaf C. Offering choice and its effect on Dutch children's liking and consumption of vegetables: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91(2):349‐56. [DOI] [PubMed] [Google Scholar]
- Zhou G, Gan Y, Hamilton K, Schwarzer R. The role of social support and self‐efficacy for planning fruit and vegetable intake. Journal of Nutrition Education and Behavior2016; Vol. 49, issue 2:100‐6. [DOI] [PubMed]
- Zhou N, Cheah CSL, Li Y, Liu J, Sun S. The role of maternal and child characteristics in Chinese children's dietary intake across three groups. Journal of Pediatric Psychology 2017;31:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalma A, Zota D, Kouvari M, Kastorini CM, Veloudaki A, Ellis‐Montalban P, et al. Daily distribution of free healthy school meals or food‐voucher intervention? Perceptions and attitudes of parents and educators. Appetite 2018;120:627‐35. [DOI] [PubMed] [Google Scholar]; Zota D, Dalma A, Petralias A, Lykou A, Kastorini CM, Yannakoulia M, et al. Promotion of healthy nutrition among students participating in a school food aid program: a randomized trial. International Journal of Public Health 2016;61(5):583–92. [DOI] [PubMed] [Google Scholar]
- Zotor FB, Amuna P. The food multimix concept: new innovative approach to meeting nutritional challenges in Sub‐Saharan Africa. Proceedings of the Nutrition Society 2008;67(1):98‐104. [DOI] [PubMed] [Google Scholar]
- Østbye T, Krause KM, Stroo M, Lovelady CA, Evenson KR, Peterson BL, et al. Parent‐focused change to prevent obesity in preschoolers: results from the KAN‐DO study. Preventive Medicine 2012;55(3):188‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]; Østbye T, Zucker NL, Krause KM, Lovelady CA, Evenson KR, Peterson BL, et al. Kids and adults now! Defeat Obesity (KAN‐DO): rationale, design and baseline characteristics. Contemporary Clinical Trials 2011;32(3):461‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Hull PC, Emerson JS, Schmidt D, Vylegzhanina V, Quirk M, Mulvaney S, et al. Nashville Children Eating Well (CHEW) for Health: smartphone application for WIC‐participating families. Journal of Nutrition Education and Behavior 2014;46(4 Supplement):S202. [Google Scholar]
- Shahriarzadeh F, Kelishadi R, Fatehizadeh M, Hassanzadeh A, Askari G. The effect of motivational interviewing and healthy diet on anthropometric indices and blood pressure in overweight and obese school children. Journal of Isfahan Medical School 2017;35(426):412‐21. [Google Scholar]
References to ongoing studies
- Belanger M, Humbert L, Vatanparast H, Ward S, Muhajarine N, Chow A F, et al. A multilevel intervention to increase physical activity and improve healthy eating and physical literacy among young children (ages 3‐5) attending early childcare centres: the Healthy Start‐Depart Sante cluster randomised controlled trial study protocol. BMC Public Health 2016;16(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy‐Herb HE, Horodynski M, Contreras D, Kerver J, Kaciroti N, Stein M, et al. Effectiveness of differing levels of support for family meals on obesity prevention among Head Start preschoolers: the Simply Dinner study. BMC Public Health 2017;17(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle C, Hillesund ER, Omholt ML, Overby NC. Early food for future health: a randomized controlled trial evaluating the effect of an eHealth intervention aiming to promote healthy food habits from early childhood. BMC Public Health 2017;17(1):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennink‐Kaminski H, Vaughn AE, Hales D, Moore RH, Luecking CT, Ward DS. Parent and child care provider partnerships: protocol for the Healthy Me, Healthy We (HMHW) cluster randomized control trial. Contemporary Clinical Trials 2017;8:8. [DOI] [PubMed] [Google Scholar]
- Horodynski MA, Baker S, Coleman G, Auld G, Lindau J. The Healthy Toddlers Trial Protocol: an intervention to reduce risk factors for childhood obesity in economically and educationally disadvantaged populations. BMC Public Health 2011;11:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN45864056. First food for infants (randomized controlled trial evaluating a cooking intervention to improve parental cooking skills and thereby improve dietary intake in infants aged 6‐12 months). https://doi.org/10.1186/ISRCTN45864056 (date applied 9 May 2016).
- ISRCTN98064772. A cluster randomized web‐based intervention trial among one‐year‐old‐children in kindergarten to reduce food neophobia and promote healthy diets. https://doi.org/10.1186/ISRCTN98064772 (date applied 18 May 2017). [DOI] [PMC free article] [PubMed]
- Kobel S, Wartha O, Wirt T, Dreyhaupt J, Lammle C, Friedemann EM, et al. Design, implementation, and study protocol of a kindergarten‐based health promotion intervention. Biomed Research International 2017;2017:4347675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Daniels LM, Reiter AR. Learning to like vegetables during breastfeeding: a randomized clinical trial of lactating mothers and infants. American Journal of Clinical Nutrition 2017;106(1):67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03003923. A randomised control trial of an educational and taste‐exposure intervention to promote vegetable intake in preschool aged children. https://clinicaltrials.gov/ct2/show/NCT03003923 (first posted 28 December 2016).
- NCT03229629. What promotes healthy eating? The roles of information, affordability, accessibility, gender, and peers on food consumption. clinicaltrials.gov/ct2/show/NCT03229629 (first posted 25 July 2017).
- NTR6572. Baby's first bites (The What and How in Weaning: a randomised controlled trial to assess the effects of vegetable‐exposure and responsive feeding on vegetable acceptance in infants and toddlers). http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6572 (date registered NTR 17 July 2017).
- Seguin RA, Morgan EH, Hanson KL, Ammerman AS, Jilcott Pitts SB, Kolodinsky J, et al. Farm Fresh Foods for Healthy Kids (F3HK): an innovative community supported agriculture intervention to prevent childhood obesity in low‐income families and strengthen local agricultural economies. BMC Public Health 2017;17(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobko T, Tse M, Kaplan M. A randomized controlled trial for families with preschool children ‐ promoting healthy eating and active playtime by connecting to nature. BMC Public Health 2016;16(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt RG, Draper AK, Ohly HR, Rees G, Pikhart H, Cooke L, et al. Methodological development of an exploratory randomised controlled trial of an early years' nutrition intervention: the CHERRY programme (Choosing Healthy Eating when Really Young). Maternal & Child Nutrition 2014;10(2):280‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar A, Vaughn AE, Fallon M, Hennessy E, Burney R, Østbye T, et al. Providers' response to child eating behaviors: a direct observation study. Appetite 2016;105:534‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]; Østbye T, Mann C, Namenek Brouwer R, Vaughn A, Bartlett R, Ward D. The keys to healthy family child care homes (KEYS) intervention study: design, rationale and baseline characteristics. Obesity Reviews 2014;15:238. [Google Scholar]; Østbye T, Mann CM, Vaughn AE, Namenek Brouwer RJ, Benjamin Neelon SE, Hales D, et al. The keys to healthy family child care homes intervention: study design and rationale. Contemporary Clinical Trials 2015;40:81‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50(2):179‐211. [Google Scholar]
- Antova T, Pattenden S, Nikiforov B, Leonardi GS, Boeva B, Fletcher T, et al. Nutrition and respiratory health in children in six Central and Eastern European countries. Thorax 2003;58(3):231‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Bureau of Statistics. 4364.0.55.007 ‐ Australian Health Survey: Nutrition First Results – Food and Nutrients, 2011‐12. Canberra: Australian Bureau of Statistics, 2014. [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, New Jersey: Prentice Hall, 1986. [Google Scholar]
- Blanchette L, Brug J. Determinants of fruit and vegetable consumption among 6‐12 year old children and effective interventions to increase consumption. Journal of Human Nutrition and Dietetics 2005;18(6):431‐43. [DOI] [PubMed] [Google Scholar]
- Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, et al. Critical review: vegetables and fruit in the prevention of chronic diseases. European Journal of Nutrition 2012;51(6):637‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett H. Increasing fruit and vegetable consumption among British primary school children: a review. Health Education 2003;103(2):99‐109. [Google Scholar]
- Campbell KJ, Hesketh KD. Strategies which aim to positively impact on weight, physical activity, diet and sedentary behaviours in children from zero to five years. A systematic review of the literature. Obesity Reviews 2007;8:327‐38. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Strategies to Prevent Obesity and Other Chronic Diseases. www.cdc.gov/obesity/downloads/fandv_2011_web_tag508.pdf. Atlanta: U.S. Department of Health and Human Services, 2011 (accessed 31st August 2017).
- Ciliska D, Miles E, O'Brien MA, Turl C, Tomasik HH, Donovan U, et al. Effectiveness of community‐based interventions to increase fruit and vegetable consumption. Journal of Nutrition Education 2000;32(6):341‐52. [Google Scholar]
- The Cochrane Collaboration. CRS (Cochrane Register of Studies). community.cochrane.org/tools/data‐management‐tools/crs2017.
- The Cochrane Collaboration. Cochrane Crowd. crowd.cochrane.org2017.
- Contento I, Balch GI, Bronner YL, Lytle LA, Maloney SK, Olson CM, et al. The effectiveness of nutrition education and implications for nutrition education policy, programs, and research: a review of research. Journal of Nutrition Education 1995;27(6):277‐418. [Google Scholar]
- Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC. Tracking of obesity‐related behaviours from childhood to adulthood: a systematic review. Maturitas 2011;70:266‐84. [DOI] [PubMed] [Google Scholar]
- Sa J, Lock K. Will European agricultural policy for school fruit and vegetables improve public health? A review of school fruit and vegetable programmes. European Journal of Public Health 2008;18(6):558‐68. [DOI] [PubMed] [Google Scholar]
- Delgado‐Noguera M, Tort S, Martínez‐Zapata MJ, Bonfill X. Primary school interventions to promote fruit and vegetable consumption: a systematic review and meta‐analysis. Preventive Medicine 2011;53:3‐9. [DOI] [PubMed] [Google Scholar]
- Elkan R, Kendrick D, Hewitt M, Robinson JJ, Tolley K, Blair M, et al. The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature. Health Technology Assessment 2000;4(13):1‐339. [PubMed] [Google Scholar]
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living Systematic Reviews: 1. Introduction ‐ the Why, What, When and How. Journal of Clinical Epidemiology (in press).
- Evans CE, Christian MS, Cleghorn CL, Greenwood DC, Cade JE. Systematic review and meta‐analysis of school‐based interventions to improve daily fruit and vegetable intake in children aged 5 to 12 y. American Journal of Clinical Nutrition 2012;96(4):889‐901. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon ML, Stolley MR, Schiffer L, Horn L, KauferChristoffel K, Dyer A. Two‐year follow‐up results for Hip‐Hop to Health Jr.: a randomized controlled trial. The Journal of Pediatrics 2005;146(5):618‐25. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon ML, Stolley MR, Schiffer L, Horn L, KauferChristoffel K, Dyer A. Hip‐Hop to Health Jr. for Latino preschool children. Obesity (Silver Spring) 2006;14(9):1616‐52. [DOI] [PubMed] [Google Scholar]
- Fjeldsoe B, Neuhaus M, Winkler E, Eakin E. Systematic review of maintenance of behaviour change following physical activity and dietary interventions. Health Psychology 2011;30(1):99‐109. [DOI] [PubMed] [Google Scholar]
- Forastiere F, Pistelli R, Sestini P, Fortes C, Renzoni E, Rusconi F, et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. Thorax 2000;55(4):283‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosomatic Medicine 2011;73(4):323‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Stables G. Environmental interventions to promote vegetable and fruit consumption among youth in school settings. Preventive Medicine 2003;37(6 Pt 1):593‐610. [DOI] [PubMed] [Google Scholar]
- Hartley L, Iabinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009874.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector D, Shrewsbury V. Building solutions for preventing childhood obesity. Module 2: Interventions to increase consumption of fruit and vegetables. NSW Centre for Overweight and Obesity, Sydney2008.
- Hendrie GA, Lease HJ, Bowen J, Baird DL, Cox DN. Strategies to increase children's vegetable intake in home and community settings: a systematic review of literature. Maternal & Child Nutrition 2017;13(1):[Epub 29 February 2016]. [DOI: 10.1111/mcn.12276] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy HM. Comparison of five teacher actions to encourage children’s new food acceptance. Annals of Behavioral Medicine 1999;21(1):20‐6. [DOI] [PubMed] [Google Scholar]
- Hesketh KD, Campbell KJ. Interventions to prevent obesity in 0‐5 year olds: an updated systematic review of the literature. Obesity 2010;18(Suppl. 1):S27‐35. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- Hopewell S, Wolfenden L, Clarke M. A survey of adverse event reporting in systematic reviews. Journal of Clinical Epidemiology 2008;61(6):597‐602. [DOI] [PubMed] [Google Scholar]
- Howerton MW, Bell S, Dodd KW, Berrigan D, Stolzenberg‐Solomon R, Nebeling L. School‐based nutrition programs produced a moderate increase in fruit and vegetable consumption: Meta and pooling analyses from 7 studies. Journal of Nutrition Education and Behavior 2007;39:186‐96. [DOI] [PubMed] [Google Scholar]
- Inchley J, Currie D, Young T, Samdal O, Torsheim T, Augustson L, et al. Growing up unequal: gender and socioeconomic differences in young people's health and well‐being. Health Behaviour in School‐aged Children (HBSC) study: international report from the 2013/2014 survey. Copenhagen: WHO Regional Office for Europe, 2016. [Google Scholar]
- Jaime PC, Lock K. Do school based food and nutrition policies improve diet and reduce obesity?. Preventive Medicine 2009;48(1):45‐53. [DOI] [PubMed] [Google Scholar]
- Jones R, Sinn N, Campbell KJ, Hesketh K, Denney‐Wilson E, Morgan PJ, et al. The importance of long‐term follow‐up in child and adolescent obesity prevention interventions. International Journal of Pediatric Obesity 2011;6(3‐4):178‐81. [DOI] [PubMed] [Google Scholar]
- Klepp KI, Pérez‐Rodrigo C, Bourdeaudhuij I, Due PP, Elmadfa I, Haraldsdóttir J, et al. Promoting fruit and vegetable consumption among European schoolchildren: rationale, conceptualization and design of the Pro Children Project. Annals of Nutrition and Metabolism 2005;49(4):212‐20. [DOI] [PubMed] [Google Scholar]
- Knai C, Pomerleau J, Lock K, McKee M. Getting children to eat more fruit and vegetables: A systematic review. Preventive Medicine 2006;42(2):85‐95. [DOI] [PubMed] [Google Scholar]
- Lapinleimu H, Viikari J, Jokinen E, Salo P, Routi T, Leino A, et al. Prospective randomised trial in 1062 infants of diet low in saturated fat and cholesterol. Lancet 1995;345(8948):471‐6. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Version 5.1.0. The Cochrane Collaboration, 2011.
- Lien N, Lytle L, Klepp KI. Stability in consumption of fruit, vegetables and sugary foods in a cohort from age 14 to 21. Preventive Medicine 2001;33(3):217‐26. [DOI] [PubMed] [Google Scholar]
- Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bulletin of the World Health Organization 2005;83(2):100‐8. [PMC free article] [PubMed] [Google Scholar]
- Maynard M, Gunnell D, Emmett PM, Frankel S, Davey Smith G. Fruit, vegetables, and antioxidants in childhood and risk of adult cancer: the Boyd Orr cohort. Journal of Epidemiology and Community Health 2003;57(3):218‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Khatibzadeh S, Shi P, Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys worldwide. BMJ 2015;5:e008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Longitudinal changes in diet from childhood into adulthood with respect to risk of cardiovascular diseases: The Cardiovascular Risk in Young Finns Study. European Journal of Clinical Nutrition 2004;58(7):1038‐45. [DOI] [PubMed] [Google Scholar]
- Miller M, Stafford H. An Intervention Portfolio to Promote Fruit and Vegetable Consumption: The Process and Portfolio. Melbourne: National Public Health Partnership, 2000. [Google Scholar]
- National Cancer Institute. Usual Dietary Intakes: Food Intakes, US Population, 2007‐10. Usual Dietary Intakes: Food Intakes, US Population, 2007‐10. US, National Cancer Institute, 2015. [Google Scholar]
- National Health and Medical Research Council. Australian Dietary Guidelines. Canberra: National Health and Medical Research Council, 2013. [Google Scholar]
- Ness AR, Maynard M, Frankel S, Smith GD, Frobisher C, Leary SD, et al. Diet in childhood and adult cardiovascular and all cause mortality: the Boyd Orr cohort. Heart 2005;91(7):894‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N, Biddle SJH, Gorely T. Family correlates of fruit and vegetable consumption in children and adolescents: a systematic review. Public Health Nutrition 2008;12(2):267‐83. [DOI] [PubMed] [Google Scholar]
- Peters Jacqueline, Sinn Natalie, Campbell Karen, Lynch John. Parental influences on the diets of 2–5‐year‐old children: systematic review of interventions. Early Child Development and Care 2012;182:837‐57. [Google Scholar]
- Peñalvo JL, Santos‐Beneit G, Sotos‐Prieto M, Martínez R, Rodríguez C, Franco M, et al. A cluster randomized trial to evaluate the efficacy of a school‐based behavioral intervention for health promotion among children aged 3 to 5. BMC Public Health 2013;13:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalvo JL, Sotos‐Prieto M, Santos‐Beneit G, Pocock S, Redondo J, Fuster V. The Program SI! intervention for enhancing a healthy lifestyle in preschoolers: first results from a cluster randomized trial. BMC Public Health 2013;13:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalvo J L, Santos‐Beneit G, Sotos‐Prieto M, Bodega P, Oliva B, Orrit X, et al. The SI! program for cardiovascular health promotion in early childhood: a cluster‐randomized trial. Journal of the American College of Cardiology 2015;66:1525‐34. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClimente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, Illinois: Dow Jones Irwin, 1984. [Google Scholar]
- Rasmussen M, Krølner R, Klepp KI, Lytle L, Brug J, Bere E, et al. Determinants of fruit and vegetable consumption among children and adolescents: a review of the literature. Part 1: quantitative studies. International Journal of Behavioral Nutrition and Physical Activity 2006;3:22. [DOI: 10.1186/1479-5868-3-22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoie‐Roskos MR, Wengreen H, Durward C. Increasing fruit and vegetable intake among children and youth through gardening‐based interventions: a systematic review. Journal of the Academy of Nutrition and Dietetics 2017;117(2):240‐50. [DOI] [PubMed] [Google Scholar]
- Simmonds MM, Salanti G, McKenzie J, Elliott JE, Living Systeamtic Review Network. Living Systematic Reviews 3: Statistical methods for updating meta‐analyses. Journal of Clinical Epidemiology (in press). [DOI] [PubMed]
- Tedstone A, Aviles M, Shetty P, Daniels L. Effectiveness of interventions to promote healthy eating in preschool children aged 1 to 5 years: a review. Health Education Authority, London1998; Vol. 65.
- U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015‐2020 Dietary Guidelines for Americans. 8th Edition. Washington: U.S. Department of Health and Human Services, 2015. [Google Scholar]
- Cauwenberghe E, Maes L, Spittaels H, Lenthe FJ, Brug J, Oppert JM, et al. Effectiveness of school‐based interventions in Europe to promote healthy nutrition in children and adolescents: systematic review of published and 'grey' literature. British Journal of Nutrition 2010;103(6):781‐97. [DOI] [PubMed] [Google Scholar]
- Horst K, Oenema A, Ferreira I, Wendel‐Vos W, Giskes K, Lenthe F, et al. A systematic review of environmental correlates of obesity related dietary behaviors in youth. Health Education Research 2007;22(2):203‐26. [DOI] [PubMed] [Google Scholar]
- Wallace BC, Noel‐Storr A, Marshall IJ, Cohen AM, Smalheiser NR, Thomas J. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association : JAMIA2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Waters E, Silva‐Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, et al. Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD001871.pub2] [DOI] [PubMed] [Google Scholar]
- Whitehead WE. Control groups appropriate for behavioral interventions. Gastroenterology 2004;126(1 Suppl 1):S159‐63. [DOI] [PubMed] [Google Scholar]
- Williams CL, Strobino BA, Bollella M, Brotanek J. Cardiovascular risk reduction in preschool children: the “Healthy Start” project. Journal of the American College of Nutrition 2004;23(2):117‐23. [DOI] [PubMed] [Google Scholar]
- Wolfenden L, Wiggers J, Tursan d'Espaignet E, Bell AC. How useful are systematic reviews of child obesity interventions. Obesity Reviews 2010;11(2):159‐65. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a Joint FAO/ WHO Expert Consultation. Technical Report Series, No. 916. Geneva: World Health Organization, 2003. [PubMed] [Google Scholar]
- Currie C, Roberts C, Morgan A, Smith R, Settertobulte W, Samdal O, et al. Young Peoples Health in Context. Health Behaviour in School‐aged Children (HBSC) Study: International Report from the 2001/2002 Survey. Denmark: World Health Organization, 2004. [Google Scholar]
- World Health Organization. Fruit and Vegetables for Health: Report of a Joint FAO / WHO Workshop, 1‐3 September, 2004 Kobe Japan. www.who.int/dietphysicalactivity/publications/fruit_vegetables_report.pdf2004.
- World Health Organization. Global Status Report on Non Communicable Diseases 2010. Geneva: WHO, 2011. [Google Scholar]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health: Promoting fruit and vegetable consumption around the world. www.who.int/dietphysicalactivity/fruit/en/ (accessed 31st August 2017).
- Yngve A, Wolf A, Poortvliet E, Elmadfa I, Brug J, Ehrenblad B, et al. Fruit and vegetable intake in a sample of 11 year old children in 9 European countries: the Pro Children cross‐sectional Survey. Annals of Nutrition and Metabolism 2005;49(4):236‐45. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Hodder RK, Stacey FG, Wyse RJ, O'Brien KM, Clinton‐McHarg T, Tzelepis F, et al. Interventions for increasing fruit and vegetable consumption in children aged five years and under. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD008552.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder RK, Stacey FG, O'Brien KM, Wyse RJ, Clinton‐McHarg T, Tzelepis F, et al. Interventions for increasing fruit and vegetable consumption in children aged five years and under. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD008552.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenden L, Wyse RJ, Britton BI, Campbell KJ, Hodder RK, Stacey FG, et al. Interventions for increasing fruit and vegetable consumption in preschool aged children. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008552] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenden L, Wyse RJ, Britton BI, Campbell KJ, Hodder RK, Stacey FG, et al. Interventions for increasing fruit and vegetable consumption in children aged 5 years and under. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


