Abstract
Background
Late‐onset infection is the most common serious complication associated with hospital care for newborn infants. Because confirming the diagnosis by microbiological culture typically takes 24 to 48 hours, the serum level of the inflammatory marker C‐reactive protein (CRP) measured as part of the initial investigation is used as an adjunctive rapid test to guide management in infants with suspected late‐onset infection.
Objectives
To determine the diagnostic accuracy of serum CRP measurement in detecting late‐onset infection in newborn infants.
Search methods
We searched electronic databases (MEDLINE, Embase, and Science Citation Index to September 2017), conference proceedings, previous reviews, and the reference lists of retrieved articles.
Selection criteria
We included cohort and cross‐sectional studies evaluating the diagnostic accuracy of serum CRP levels for the detection of late‐onset infection (occurring more than 72 hours after birth) in newborn infants.
Data collection and analysis
Two review authors independently assessed eligibility for inclusion, evaluated the methodological quality of included studies, and extracted data to estimate diagnostic accuracy using hierarchical summary receiver operating characteristic (SROC) models. We assessed heterogeneity by examining variability of study estimates and overlap of the 95% confidence interval (CI) in forest plots of sensitivity and specificity.
Main results
The search identified 20 studies (1615 infants). Most were small, single‐centre, prospective cohort studies conducted in neonatal units in high‐ or middle‐income countries since the late 1990s. Risk of bias in the included studies was generally low with independent assessment of index and reference tests. Most studies used a prespecified serum CRP threshold level as the definition of a 'positive' index test (typical cut‐off level between 5 mg/L and 10 mg/L) and the culture of a pathogenic micro‐organism from blood as the reference standard.
At median specificity (0.74), sensitivity was 0.62 (95% CI 0.50 to 0.73). Heterogeneity was evident in the forest plots but it was not possible to conduct subgroup or meta‐regression analyses by gestational ages, types of infection, or types of infecting micro‐organism. Covariates for whether studies used a predefined threshold or not, and whether studies used a standard threshold of between 5 mg/L and 10 mg/L, were not statistically significant.
Authors' conclusions
The serum CRP level at initial evaluation of an infant with suspected late‐onset infection is unlikely to be considered sufficiently accurate to aid early diagnosis or select infants to undergo further investigation or treatment with antimicrobial therapy or other interventions.
Plain language summary
C‐reactive protein for diagnosing infection in newborn infants
Review question
We reviewed studies that assessed whether measuring the blood level of C‐reactive protein (CRP) helped to make an earlier diagnosis of serious infections in newborn infants.
Background
Newborn infants, especially sick or preterm infants, are at risk of developing severe infections (such as bloodstream infections) during their stay on neonatal units. Infections are often difficult to diagnose early with certainty, and quick tests such as measuring the blood level of a protein that responds to infection (called CRP) are sometimes used to help make an earlier diagnosis. We aimed to assess the evidence for the accuracy of this test.
Study characteristics
We found 20 studies that assessed the accuracy of measuring the blood level of CRP to diagnose infections in newborn infants. These studies were similar enough to justify a combined analysis of their findings.
Key results
The combined analysis indicated that a positive CRP test correctly identified infants with infection about six times out of 10.
Conclusion
Measuring the blood level of CRP is not sufficiently accurate to help early diagnosis of infection in newborn infants.
Summary of findings
Summary of findings'. 'Should serum CRP levels be used to diagnose late‐onset infection in newborn infants?
| Question: Should serum CRP levels be used to diagnose late‐onset infection in newborn infants? | |||||||||
| Study design: prospective or retrospective cohorts and cross‐sectional studies. We excluded case reports and studies of case‐control design. | |||||||||
|
Sensitivity at median specificity: 0.62 (95% CI 0.50
to 0.73) Median specificity: 0.74 | |||||||||
|
№ of studies (infants): 20 (1615) № of true positives: 617 № of true negatives: 998 | |||||||||
| Outcome | Factors that may decrease certainty of evidence | Effect per 1000 infants tested | Test accuracy quality of evidence | ||||||
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 20% | Pretest probability of 40% | Pretest probability of 60% | ||
| True positives (infants with late‐onset infection) | Not serious | Not serious | Serious | Not serious | None | 124 (100 to 146) | 248 (200 to 292) | 372 (300 to 438) | ⊕⊕⊕⊝ Moderate |
| False negatives (infants incorrectly classified as not having late‐onset infection) | 76 (54 to 100) | 152 (108 to 200) | 228 (162 to 300) | — | |||||
| True negatives (infants without late‐onset infection) | Not serious | Not serious | Serious | Not serious | None | 592 (‐ to ‐) | 444 (‐ to ‐) | 296 (‐ to ‐) | ⊕⊕⊕⊝ Moderate |
| False positives (infants incorrectly classified as having late‐onset infection) | 208 (‐ to ‐) | 156 (‐ to ‐) | 104 (‐ to ‐) | — | |||||
CI: confidence interval.
Background
Target condition being diagnosed
Late‐onset infection in newborn infants
Late‐onset infection (occurring more than 72 hours after birth) is the most common serious complication associated with intensive care for newborn infants (McGuire 2004). The incidence of late‐onset infection is inversely related to gestational age at birth and has increased as survival rates for preterm infants have improved (van den Hoogen 2010). About 20% of very preterm infants experience an episode of late‐onset infection reflecting their level and duration of exposure to invasive procedures and intensive care (Vergnano 2011; Berrington 2012; Oeser 2014). Central line‐associated bloodstream infection (CLABSI) is a major subtype of late‐onset infection that is associated with the use of central vascular catheters (CVC) to deliver drugs, fluids, or parenteral nutrition for newborn infants (Benjamin 2001; Butler‐O'Hara 2012; Shalabi 2015). Other putative risk factors include receipt of broad‐spectrum antibiotics and of histamine type 2‐receptor antagonists (Shane 2014; Tsai 2014a). However, interunit variation in the incidence of late‐onset infection is not fully explained by case‐mix and may relate to uptake and use of care or infection control practices (Wong 2012).
Microbiology of late‐onset infection
The common causes of late‐onset infections in sick or preterm newborn infants are coagulase‐negative staphylococci, other Gram‐positive cocci (Staphylococcus aureus (S aureus), enterococci), Gram‐negative bacilli (mainly enteric bacilli), and fungi (predominantly Candida species) (Stoll 2003; Zaidi 2005; Gordon 2006; Muller‐Pebody 2011; Hornik 2012; Shane 2013). Preterm infants, particularly very preterm infants, with late‐onset infection have a higher risk of mortality and a range of important morbidities including bronchopulmonary dysplasia, necrotising enterocolitis, retinopathy of prematurity, and need for intensive care and prolonged hospitalisation than comparable infants without infection (Shah 2014). Late‐onset infection is associated with higher rates of adverse neurodevelopmental outcomes including visual, hearing, and cognitive impairment, and cerebral palsy (Stoll 2004; Bassler 2009).
Antimicrobial resistance
Delayed treatment of bacterial or fungal late‐onset infections may increase the risk of morbidity and mortality in newborn infants. However, because clinical signs of infection in neonates can be non‐specific, empirical treatment of all infants with suspected infection will result in the administration of unnecessary courses of antibiotics (Dong 2015). Such widespread use, particularly of broad‐spectrum antibiotics, is associated with accelerated selective pressure and the emergence of drug resistance through mechanisms such as extended‐spectrum B‐lactamase production (de Man 2000; Muller‐Pebody 2011; Tsai 2014b).
Index test(s)
Diagnosing late‐onset infection in preterm infants
Given the difficulty in establishing an early diagnosis based on clinical features alone, and the high level of associated morbidity and mortality, several 'biomarkers' of infection have been proposed and adopted as tests to determine whether late‐onset infection is more or less likely in newborn infants in whom it is suspected (Shane 2013; Gilfillan 2017). The most commonly used and established of these is the serum level of C‐reactive protein (CRP). CRP is an acute‐phase reactant synthesised by hepatocytes in response to inflammatory cytokines, particularly interleukin (IL)‐6, generated by white blood cells reacting to microbial pyrogens such as lipopolysaccharide (Steel 1994). The major physiological role of serum CRP is to bind to microbial polysaccharides and immune complexes and activate the classical complement cascade (Volanakis 2001).
CRP levels can be measured in laboratories within about one hour using a very small volume of serum (20 µL). Serum levels are usually very low (undetectable at the lower limits of sensitivity of standard laboratory analysis, typically 5 mg/L to 10 mg/L), but rise to detectable concentrations following an infectious or inflammatory stimulus over 12 to 24 hours. In infants exposed to infectious inflammatory stimuli, serum CRP levels may rise by more than 100‐fold, declining with a half‐life of about 18 to 24 hours when the stimulus ceases (Ehl 1999). Many non‐infectious inflammatory stimuli including chemical or physical irritation such as extravasation of hypertonic or irritant solutions may also cause serum CRP levels to rise in newborn infants (Hofer 2013).
Clinical pathway
The use of biomarkers in general, and CRP in particular, as adjunctive diagnostic tests in neonatal care settings occurs within three broad clinical contexts.
Diagnosing infection: the serum CRP level is used to determine whether late‐onset infection is less or more likely in infants in whom there is a clinical suspicion based on signs such as unstable temperature, respiratory instability (apnoea, desaturation), enteral feed intolerance, or general concern that the infant appears unwell.
Screening for infection: serum CRP levels are monitored at intervals to detect infants in whom an infection may be developing before being clinically suspected. In clinical practice, this approach is usually targeted to infants considered to be at an elevated risk of acquiring an infection because of specific risk factors such as the presence of a CVC.
Monitoring response to treatment: serial measurement of serum CRP is also used to track the course of late‐onset infection and assess the response to antimicrobial treatment, including acting as indicator for stopping antibiotics when a previously elevated serum CRP level has returned to 'normal' (Ehl 1997).
This review addressed the diagnostic accuracy of the serum CRP level in the first scenario (i.e. diagnosing infection) only. As detection of an elevated serum CRP level (index test) would be used to trigger application of the reference test in the other scenarios, it is not possible to measure diagnostic accuracy.
Prior test(s)
Serum CRP is typically measured at the initial assessment of an infant with suspected late‐onset infection, usually alongside other tests including laboratory culture of a blood sample to culture micro‐organisms ('blood culture').
Role of index test(s)
Because the microbiological culture of a potentially pathogenic organism (the reference standard) from a blood sample takes about 24 to 48 hours to complete, the purpose of measuring the serum CRP level is to help make a more immediate assessment of the overall likelihood that an infant is truly infected. In current clinical practice, the main aims are to help decide (Pammi 2015):
whether the likelihood of infection justifies further invasive tests (such as examination of the cerebrospinal fluid (CSF) to diagnose or exclude meningitis);
whether it is appropriate to administer antibiotics or other antimicrobial therapy immediately;
whether other interventions such as removing a CVC that is potentially a nidus for infection are justified.
Alternative test(s)
Several other biomarkers of infection or inflammation have been evaluated in neonatal care settings and different areas of clinical practice. These include haematological indices (peripheral total white blood cell count, neutrophil count, and immature‐to‐total neutrophil ratio (I/T‐ratio); serum procalcitonin; the acute phase protein serum amyloid A; several proinflammatory cytokines such as IL‐6, IL‐8, and tumour necrosis factor (TNF); and other markers of immune activation (Fowlie 1998; Malik 2003). These alternative biomarkers are generally more expensive to measure than CRP and are not as well established in routine practice (Hedegaard 2015). More recent developments include biomarkers based on detecting microbial DNA ('molecular biomarkers') and computer‐based integration of physiological markers such as heart rate variability to detect infants with a developing infection (Dong 2015).
Rationale
Serum CRP level may be a useful adjunctive diagnostic biomarker for late‐onset infection in newborn infants if it has acceptable levels of accuracy. Currently, in the absence of robust evidence to inform guideline or protocol development, clinical practice varies greatly with regard to the role of serum CRP in diagnostic algorithms for late‐onset infection (Dong 2015; Pammi 2015). Most studies examining the accuracy of CRP and other biomarkers of late‐onset infection have been conducted in single centres and, therefore, are limited by the small sample size. A systematic review to identify, quality‐appraise, and synthesise the data in meta‐analyses could help clarify the evidence‐base to inform policy and practice, and future research.
Objectives
To determine the diagnostic accuracy of serum CRP measurement in detecting late‐onset infection in newborn infants.
Secondary objectives
To investigate heterogeneity of test accuracy in the included studies (see: Investigations of heterogeneity).
Possible sources of heterogeneity include between‐study variation in the demographic characteristics of study participants (e.g. term versus preterm infants), use of different cut‐off values of serum CRP levels used to define a positive test (e.g. 5 mg/L to 10 mg/L versus higher values), and subtypes of late‐onset infection (e.g. CLABSI versus non‐CLABSI).
Methods
Criteria for considering studies for this review
Types of studies
Cohort and cross‐sectional studies evaluating the diagnostic accuracy of serum CRP for the detection of late‐onset infection (more than 72 hours after birth) in newborn infants were eligible for inclusion. Studies using CRP along with another biomarker were eligible provided data on the diagnostic performance of CRP alone could be extracted. We have not included case‐control studies as this design is unlikely to allow valid assessment of diagnostic test accuracy in this clinical context.
For inclusion in meta‐analyses, a study of diagnostic accuracy needed to provide sufficient data to construct the '2×2' diagnostic table (true positive, false positive, true negative, and false negative) showing the cross‐classification of disease status (microbiologically confirmed infection) and test outcome (serum CRP level). If study reports did not provide data sufficient to construct the diagnostic table, we contacted the corresponding authors to seek the missing data, where appropriate and practicable.
Studies investigating early‐onset infection (diagnosed before 72 hours postnatally) were not eligible. Studies investigating both early‐ and late‐onset infection were eligible provided separate data could be extracted for late‐onset infection. If not reported, we contacted the corresponding author to request unpublished data on late‐onset infection if the article indicated that these data may have been collected.
Participants
Hospitalised newborn infants aged more than 72 hours until the first discharge home after birth were eligible. We excluded studies where the participants were young infants cared for at home or in another community setting who then presented to a primary or secondary healthcare facility with possible infection.
We considered infants across all gestational ages and planned to conduct subgroup analyses by gestational age: term (greater than 37 weeks' gestation), preterm (less than 37 weeks' gestation), and very preterm (less than 32 weeks' gestation).
Index tests
Serum CRP level: we accepted the threshold for the index test as defined by individual studies (expected typically to be in the range 5 mg/L to 10 mg/L).
Target conditions
Microbiologically confirmed late‐onset infection (more than 72 hours after birth) including bacteraemia, fungaemia, meningitis, osteomyelitis, septic arthritis, and peritonitis.
We excluded data on the diagnostic accuracy of CRP in infants with 'suspected' or 'probable' infection (also referred to as 'clinical sepsis'), that is, infection suspected because of clinical or laboratory features and findings but not confirmed by microbiological culture of pathogens. If studies reported data on infants with clinical sepsis as well as 'microbiologically confirmed' infection, we extracted the data according to microbiological culture status rather than according to the authors' definition of clinical sepsis. We planned to exclude studies of infants diagnosed with pneumonia based on clinical and radiological features even if supported by microbiological culture of bacteria or fungi from endotracheal aspirates. Although it may be argued that excluding suspected or probable infection is inconsistent with normal clinical practices, the lack of a widely acceptable and reliable clinical definition makes it less valid to incorporate as an eligibility criterion for this review. We contacted the corresponding author to request unpublished data as necessary, where appropriate and practicable.
Reference standards
Infection diagnosed more than 72 hours after birth, confirmed by culture from a normally sterile site: CSF, blood (from peripheral sites, not from indwelling catheters), bone or joint, peritoneum, pleural space, or findings on autopsy examination consistent with microbial infection (microbiological confirmation or morphological findings consistent with infection). Because 'false‐positive' results due to skin contaminants were possible, we excluded cases where infection was attributed to diphtheroids, micrococci, propionibacteria, or a mixed microbial flora (where data were available).
We planned to examine specific infections with the following organisms, if data were available: coagulase‐negative staphylococci, other bacteria (Gram‐negative bacilli, S aureus, enterococci), and fungi.
We did not include urinary tract infections because these are uncommon in newborn infants unless associated with bacteraemia and because diagnosis requires urine obtained by sterile urethral catheterisation or suprapubic bladder tap which are sampling methods employed rarely in current practice.
We excluded any studies in which the reference standard incorporated the index test, that is, 'infection' was defined as a positive microbiological culture and raised serum CRP level.
Search methods for identification of studies
We used the standard Cochrane Neonatal search strategy adapted for studies of diagnostic test accuracy (Beynon 2013).
Electronic searches
We searched MEDLINE, Embase, and Science Citation Index: see Appendix 1. The initial search was carried out in May 2015 and updated in September 2017.
Searching other resources
We examined the reference lists of all studies identified as potentially relevant.
We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2017), the European Society for Pediatric Research (1995 to 2017), the UK Royal College of Paediatrics and Child Health (2000 to 2017), and the Perinatal Society of Australia and New Zealand (2000 to 2017). Studies reported only as abstracts were eligible if sufficient information was available from the report or from contact with the authors to fulfil the inclusion criteria.
Data collection and analysis
Two review authors screened all titles and abstracts identified by our search strategy for relevance to the inclusion criteria of this review as detailed in Criteria for considering studies for this review. All references were managed in an EndNote library and then exported to Covidence for study selection.
Selection of studies
We retrieved the full text of all identified articles that were deemed relevant to the review and evaluated them against our inclusion eligibility. Two review authors independently assessed studies for eligibility for inclusion. We resolved disagreements by discussion with a third review author.
Data extraction and management
One review author extracted the following data.
Author and year of publication.
Study design including sample size, type of recruitment (prospective or retrospective).
Study population characteristics and the clinical context in which the test was evaluated.
Definition of reference standard.
CRP threshold used.
Information regarding quality assessment items of the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool (Assessment of methodological quality).
Data to enable derivation of 2×2 tables of the number of true positives, false positives, false negatives, and true negatives.
One review author extracted data and a second review author checked them. We resolved any discrepancies by discussion and arbitration by a third review author if required.
When contacting corresponding authors to request additional data or information, we took a pragmatic approach to manage resources sensibly. We attempted to contact authors of reports published since 2005 by email only. If there was no contact email address after a reasonable amount of online research, we did not attempt to contact authors by other means such as telephone or post. If our email remained unanswered after one week, we sent one follow‐up email. If data could not be obtained after this, a team decision was made to exclude the study. Authors of studies published before 2005 were only contacted if an email address was provided as part of the publication. We reported all excluded studies, with reasons for exclusion given (Characteristics of excluded studies table).
Assessment of methodological quality
We assessed methodological quality of each included study following guidance from the Cochrane Screening and Diagnostic Test Methods Group, which is adapted from the QUADAS‐2 tool (Whiting 2011: see Table 2). The four domains assessed for risk of bias were patient selection, index test, reference standard, and flow and timing. Applicability concerns were assessed in the first three domains. In each domain, we answered the signalling questions with 'Yes', 'No', or 'Unclear' and for each domain judged the risk of bias as 'Low', 'High', or 'Unclear' risk.
1. Risk of bias and applicability items and criteria for their assessment (QUADAS‐2).
| Item | Criteria for their assessment |
| Domain 1: participant selection | |
| Describe methods of participant selection (prior testing, presentation, intended use of index test and setting) | |
| A. Risk of bias | |
| Was a consecutive or random sample of participants enrolled? | 'Yes' if described enrolling a consecutive or random
sample of newborns prior to discharge from
hospital 'No' if criteria for 'yes' not achieved 'Unclear' if the study did not describe the method of enrolment |
| Did the study avoid inappropriate exclusions? | 'Yes' if exclusions were detailed and review authors
reached consensus on the appropriateness of any
exclusion 'No' if inappropriate exclusions were reported 'Unclear' if insufficient information was provided |
| Could the selection of participants have introduced bias? | A judgement of low, high, or unclear risk of bias was made based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Is there concern that the included participants did not match the review question? | A judgement of low, high, or unclear concern about applicability will be made based on how closely the sample matches a population of newborn infants with suspected infection. |
| Domain 2: index test | |
| Describe the index test and how it was conducted and interpreted. | |
| A. Risk of bias | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | 'Yes' if the serum CRP level was measured before the
microbial culture result was available. 'No' if reference standard results were available to those who ordered or interpreted the serum CRP level. 'Unclear' if insufficient information was provided. |
| If a threshold was used, was it prespecified? | 'Yes' if a threshold was prespecified. 'No' if authors selected a cut‐off value based on the analysis of data collected. 'Unclear' if insufficient information was provided. |
| Could the conduct or interpretation of the index test have introduced bias? | A judgement of low, high, or unclear risk of bias will be made based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Was there concern that the index test, its conduct, or interpretation differ from the review question? | A judgement of low, high, or unclear concern about applicability will be made based on a balanced assessment of the information detailed under 'index test' description. |
| Domain 3: reference standard | |
| Describe the reference standard(s) and how they were conducted and interpreted. | |
| A. Risk of bias | |
| Was the reference standard likely to correctly classify the target condition? | 'Yes' if an appropriate reference standard (as
defined in the protocol) was used. 'No' if an inappropriate reference standard (not defined in the protocol) was used. 'Unclear' if the reference standard used was not clearly specified. |
| Were the reference standard results interpreted without knowledge of the results of the index test? | 'Yes' if the person undertaking the reference test
did not know the results of the microbial
culture. 'No' if the CRP level results were interpreted with prior knowledge of the index test result. 'Unclear' if insufficient information provided. |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | A judgement of low, high, or unclear risk of bias was made based on a balanced assessment of the responses to the above signalling questions. |
| B. Concerns about applicability | |
| Was there concern that the target condition as defined by the reference standard did not match the question? | A judgement of low, high, or unclear concern about applicability was made based on setting, population, risks, prevalence??? |
| Domain 4: flow and timing | |
| Describe any participants who did not receive the index test or reference standard or who were excluded from the 2×2 table (refer to flow diagram) and describe the time interval and any interventions between index test and reference standard(s). | |
| A. Risk of bias | |
| Did all infants receive a reference standard? | 'Yes' if the study specifically stated that all
infants received blood culture, lumbar puncture, or
other biopsy to identify infection (including
autopsy examination). 'No' if the study only assessed suspected or probable infection (not confirmed microbiologically) 'Unclear' if insufficient information was provided. |
| Were all infants included in the analysis? | 'Yes' if the study had no withdrawals or the
withdrawals were clearly described. 'No' if the number of participants contributing to the 2×2 table did not match the number of participants recruited and no reasons for exclusions were described. 'Unclear' if information was not enough to establish the flow of participants. |
| Could the patient flow have introduced bias? | A judgement of low, high, or unclear risk of bias was made based on a balanced assessment of the responses to the above signalling questions. |
We excluded studies at high risk of incorporation bias, that is, those studies in which a raised CRP level was part of the diagnostic criteria for infection.
One review author assessed study quality, which a second review author checked. We resolved any disagreements by discussion, with referral to a third review author as necessary.
We summarised overall quality of evidence using GRADE methodology recommended for diagnostic tests (Singh 2012).
Statistical analysis and data synthesis
We constructed 2×2 diagnostic tables for all studies based on dichotomous data from the reference standard (infected or not infected) and index test (the cut‐off level for serum CRP for a positive result (suggestive or diagnostic of late‐onset infection) as defined by each study). Only the test taken at the same time as the reference standard was used. We created forest plots with 95% confidence intervals (CI) for sensitivity and specificity for each study using Review Manager 5 (Review Manager 2014).
Since reported threshold levels for a positive test differed across studies, we fitted the data in a hierarchical summary receiver operating characteristic (HSROC) model that assumed accuracy and thresholds vary between studies (Rutter 2001). Analyses were conducted in SAS using the NLMIXED procedure (version 9.4, SAS Institute, Cary, NC, USA).
Investigations of heterogeneity
We assessed heterogeneity by examining forest plots of sensitivity and specificity across studies for variability of study estimates and overlap of the 95% CI.
Threshold (cut‐off) values
We examined the effect of using different threshold (cut‐off) levels of serum CRP to define a positive index test in studies. We expected most studies to use a value between 5 mg/L and 10 mg/L to define a positive test result but that some may have used higher or lower levels. We investigated the effect of studies reporting different thresholds using meta‐regression analyses (with a categorical covariate: standard threshold 5 mg/L to 10 mg/L versus any other threshold). We assessed the effect of studies reporting a predefined threshold (categorical covariate: reporting predefined threshold versus not reporting predefined threshold).
Other possible sources of heterogeneity
We planned to examine the effect of population subgroups in meta‐regression analyses (including categories as additional covariates). If there were sufficient data, we hoped to provide summary estimates for each subgroup and assess the statistical significance of differences between these subgroups: gestational age at birth (term, preterm, and very preterm infants), pathogens or putative pathogens (coagulase‐negative staphylococci, Gram‐negative bacilli, S aureus, enterococci, fungi), and subtypes of late‐onset infection (CLABSI versus non‐CLABSI).
Sensitivity analyses
If sufficient data were available, we planned to explore whether study methodological quality affected the results by removing studies considered at higher risk of bias across key domains (selection, verification).
Post hoc, we removed one study using a very high threshold (considered an outlier) in sensitivity analyses to investigate the impact on estimates.
Assessment of reporting bias
We assessed publication bias using funnel plots (the natural logarithm of the diagnostic odds ratio by 1/effective sample size) and Deeks' test in Stata 13 using the midas commands (Deeks 2005).
Results
Results of the search
The flow of studies through the review process is illustrated in Figure 1.
1.
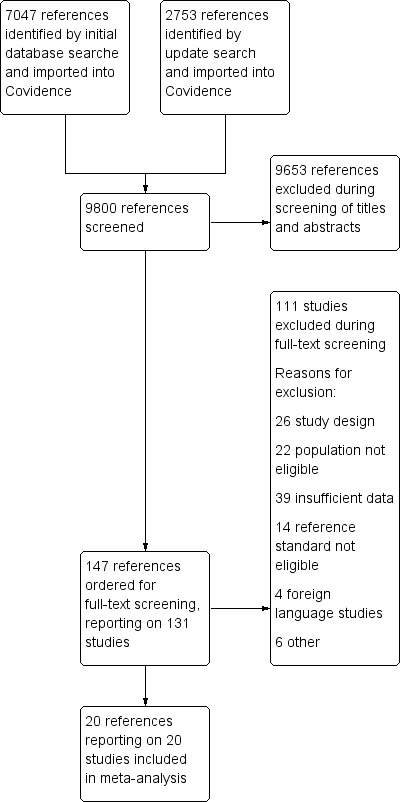
Study flow diagram.
We identified 9800 records in our electronic database searches up to September 2017. We excluded 9653 of these on screening the title and abstract. We screened the full text of 147 reports of 131 studies and excluded 111 of these (see Characteristics of excluded studies table).
We included 20 studies reported in 20 papers (total number of studied infants: 1615). Studies were published between 1990 and 2015 with most (15/20) published since 2000. Two studies were cohorts assembled retrospectively (Doellner 1998; Fendler 2008); the remaining 18 studies included prospectively observed cohorts.
Most studies (16/20) were carried out in high‐income countries in Europe (Bohnhorst 2012; Decembrino 2015; Doellner 1998; Fendler 2008; Jacquot 2009; Kipfmueller 2015; Kordek 2014; Verboon‐Maciolek 2006), Asia (Chan 1997; Ng 1997; Choo 2012), North America (Benitz 1998; Pynn 2015), South America (Bustos 2012), or Australasia (Seibert 1990; Sherwin 2008). Four studies were conducted in low‐ and middle‐income countries (Aminullah 2001; Boo 2008; Kumar 2010; Hisamuddin 2015). All but one of the studies were single‐centre investigations. Benitz 1998 was a three‐centre study.
Sample sizes ranged from 11 to 184 infants. Four studies had a sample size of 25 or fewer (Doellner 1998; Aminullah 2001; Choo 2012; Kipfmueller 2015). Nine studies included between 40 and 100 infants (Verboon‐Maciolek 2006; Boo 2008; Fendler 2008; Sherwin 2008; Jacquot 2009; Bustos 2012; Kordek 2014; Decembrino 2015; Hisamuddin 2015). Seven studies had sample sizes of 100 or more infants (Seibert 1990; Chan 1997; Ng 1997; Benitz 1998; Kumar 2010; Bohnhorst 2012; Pynn 2015).
In 16 studies, participants were preterm (or very low birth weight) infants predominantly. Four studies did not report the gestational age range of the included infants but it is likely that most participants were preterm or low birth weight (Aminullah 2001; Kumar 2010; Decembrino 2015; Hisamuddin 2015).
Twelve studies only investigated infants with late‐onset infection (Seibert 1990; Ng 1997; Doellner 1998; Aminullah 2001; Verboon‐Maciolek 2006; Fendler 2008; Sherwin 2008; Jacquot 2009; Bohnhorst 2012; Kordek 2014; Kipfmueller 2015; Pynn 2015). The remaining studies included infants with early‐onset and late‐onset infection. We were able to extract data on infants with late‐onset infection (or have these data provided by the primary investigators).
Where reported, most studies (9/12) defined late‐onset as occurring more than 72 hours after birth, but across studies the definition ranged from 48 hours to six days after birth. We included these studies as this is consistent with the range of definitions that exist in clinical practice and research (Dong 2015).
Fourteen studies used a prespecified threshold of serum CRP level to determine the threshold level (cut‐off) for a positive test. These thresholds ranged from 1 mg/L to 12 mg/L with most studies (12/14) using a cut‐off level between 5 mg/L and 10 mg/L. None of the studies reported sensitivity and specificity at multiple thresholds.
Six studies determined the CRP threshold level retrospectively (by modelling the area under the receiver operating curve). These studies determined the following thresholds:
Fendler 2008: 2.2 mg/L;
Decembrino 2015: 6 mg/L;
Benitz 1998: 10 mg/L;
Verboon‐Maciolek 2006: 14 mg/L;
Sherwin 2008: 18 mg/L;
Bustos 2012: 111 mg/L.
Methodological quality of included studies
Participant selection
1. Risk of bias
The included studies were at low risk of participant selection bias. While the details of the recruitment process were not always reported, we judged that, on the whole, studies avoided inappropriate exclusions. We specified in our protocol that case‐control studies would be excluded from the review as this design would not have been appropriate for the review question.
2. Concerns regarding applicability
Based on the information reported, we judged that the participants and the setting of the included studies were applicable to our review question.
Index test
1. Risk of bias
Overall, the risk that the conduct or interpretation of the index test (serum CRP level) could have introduced bias was low. The serum CRP level was measured in infants presenting with clinical features of late‐onset infection before the results of the reference standard were known. Most (14/20) studies prespecified threshold of CRP level consistent with current clinical practice (1 mg/L to 12 mg/L). The other studies determined the optimal threshold post hoc but, with the exception of one outlier (Bustos 2012), this threshold was similar to the range used in studies that prespecified the threshold (2 mg/L to 18 mg/L).
Two studies were at unclear risk that the conduct or interpretation of the index test (serum CRP level) could have introduced bias as we were unable to determine whether or not the index test results were interpreted without knowledge of the reference standard (Benitz 1998; Boo 2008). In one study, the risk of bias introduced by the conduct of the index test was high because the outcome of the reference standard (blood culture) was known before the interpretation of the CRP level (Fendler 2008).
2. Concerns regarding applicability
Across all studies, the index test, its conduct, and its interpretation were applicable to our review question. While there was expected variation in clinical practice between studies, we did not deem this to be of a magnitude that would cause concerns regarding applicability.
Reference standard
1. Risk of bias
As per our inclusion criteria, all studies used microbiological culture of a potential pathogen from blood or a normally sterile body fluid as the reference standard for late‐onset infection. Twelve studies reported some description of the infecting micro‐organisms (Chan 1997; Ng 1997; Benitz 1998; Aminullah 2001; Verboon‐Maciolek 2006; Boo 2008; Fendler 2008; Sherwin 2008; Jacquot 2009; Bohnhorst 2012; Bustos 2012; Pynn 2015). These included coagulase‐negative staphylococci (typically representing about 50% of the total cases), S aureus, enterococci, Streptococcus agalacticae, Gram‐negative bacillia, and fungi (typically Candida spp.).
Four studies included a clinical and radiological diagnosis of pneumonia within the case definition for the reference standard (Ng 1997; Benitz 1998; Doellner 1998; Sherwin 2008). Two of these studies reported no cases of pneumonia (Ng 1997; Doellner 1998). The other studies reported one (Benitz 1998) and two (Sherwin 2008) cases of pneumonia and it was unclear whether these infants had a concordant positive microbiological culture from blood or a normally sterile body fluid. We did not consider this deviation from the reference standard definition as a sufficient source of bias to justify excluding the studies. In one study, "urinary sepsis" was eligible for inclusion in case definition, but no episodes of urinary tract infection were reported (Kumar 2010).
We excluded all studies in which the index test (CRP level) was part of the reference standard, that is, those in which a raised CRP level and a positive blood culture was needed for a formal diagnosis of infection. As such, across the included studies, there was a low risk that the reference standard, its conduct, or its interpretation could have introduced bias.
2. Concerns regarding applicability
In all studies, the condition under investigation was infection in newborn infants. Across the included studies, there was some variation with regards to the babies' ages at the time of first clinical suspicion of sepsis and their gestational age. However, through contact with authors, we were able to obtain data for only those babies with late‐onset infection, even for those studies that also included younger infants with early‐onset infection. We were confident that the target condition investigated in the included studies matched our review question.
Flow and timing
1. Risk of bias
All studies used blood samples taken at the initial investigation of each infant to determine the serum CRP level and for the blood culture. Due to the nature of the reference standard, the blood culture results followed 24 to 48 hours after the index test, depending on laboratory procedure. Across all studies, there was a low risk that the patient flow might have introduced bias.
Overall risk of bias
The methodological quality of the included studies was good and the risk of bias minimal (Figure 2).
2.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Findings
See Figure 3 for the summary receiver operating curve and Figure 4 for forest plots.
3.
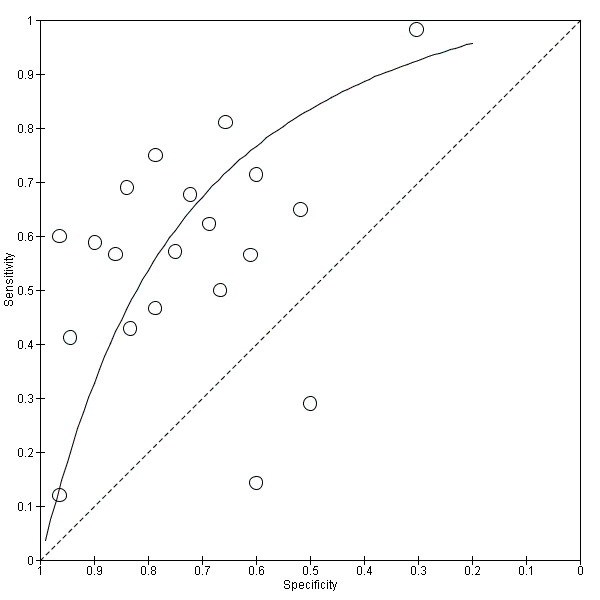
Summary receiver operating characteristic (SROC) plot of C‐reactive protein for neonatal infection. Study estimates of sensitivity and specificity are shown with the SROC curve.
4.
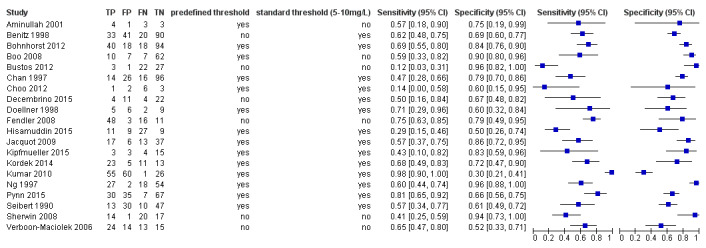
Forest plot: sensitivity and specificity of C‐reactive protein for diagnosing late‐onset infection.
We calculated estimates of sensitivity at fixed values of specificity (median, lower and upper quartiles reported in the included studies) on the SROC curve. At median reported specificity (0.74), sensitivity was 0.62 (95% CI 0.50 to 0.73); at the lower quartile reported specificity (0.61), sensitivity was 0.76 (95% CI 0.65 to 0.84); at the upper quartile reported specificity (0.85), sensitivity was 0.44 (95% CI 0.32 to 0.57). GRADE quality of evidence was moderate, downgraded from high for inconsistency (Table 1).
We used these data for sensitivity (0.62) and median reported specificity (0.74) to estimate post‐test probabilities after a 'positive' or 'negative' CRP test for a range of pretest probabilities in infants being evaluated for possible late‐onset infection and receiving a CRP test (Table 3).
2. Post‐test probabilities for late‐onset infection for a sample of population prevalences.
| Pretest probability | Post‐test probability after a positive result | Post‐test probability after a negative result |
| 0.2 | 0.37 | 0.11 |
| 0.3 | 0.51 | 0.18 |
| 0.4 | 0.61 | 0.26 |
| 0.5 | 0.70 | 0.34 |
| 0.6 | 0.78 | 0.44 |
The prevalence of late‐onset infection in the included studies ranged from 20% to 82% (median 40%, interquartile range 27% to 61%). We applied the diagnostic test accuracy estimates for sensitivity (0.62) and median specificity (0.74) from our meta‐analysis to a hypothetical cohort of 1000 neonates with a prevalence of infection of 20%, 40%, or 60%:
20%: on average, 76 cases of infection would be missed and 208 would be wrongly diagnosed with infection;
40%: 152 cases of infection would be missed and 156 wrongly diagnosed with infection;
60%: 228 cases would be missed and 104 wrongly diagnosed with infection.
Investigation of heterogeneity and sensitivity analyses
It was not possible to conduct any subgroup or meta‐regression analyses by gestational age (most participants were preterm infants of a range of gestational ages; some studies included term infants, but subgroup data were not available), subtypes of late‐onset infection (most studies did not report CLABSI or other subtypes of late‐onset infection), or types of pathogen or putative pathogen (studies included a range of pathogens, but subgroup data were generally not available).
Threshold values
We examined the effect of using different threshold (cut‐off) levels of serum CRP to define a positive index test in studies. All but one of the studies used a serum CRP level threshold between 1 mg/L and 18 mg/L, whether predefined or determined post‐hoc (see below), to define a positive test result. Most studies (13/20) used a threshold between 5 mg/L and 10 mg/L.
When covariates on thresholds above or below 5 mg/L to 10 mg/L were added, likelihood ratio tests found no statistically significant difference in goodness of fit for any of these models compared with those without covariates.
Reporting a predefined threshold versus not reporting a predefined threshold
Six studies did not report using apredefined threshold. Of these, four studies did not report a standard threshold. There were no statistically significant differences in goodness of fit between any of these models including a covariate for predefined threshold compared with models without covariates.
Sensitivity analyses
One study was an outlier and reported using a cut‐off level of 111 mg/L (Bustos 2012). Removing the study had limited impact on effect estimates. At median specificity reported in the included studies (0.72), sensitivity was 0.65 (95% CI 0.53 to 0.75); at the lower quartile for specificity (0.60) sensitivity was 0.76 (95% CI 0.65 to 0.84); at the upper quartile for specificity (0.84) sensitivity was 0.48 (95% CI 0.36 to 0.60).
Publication bias
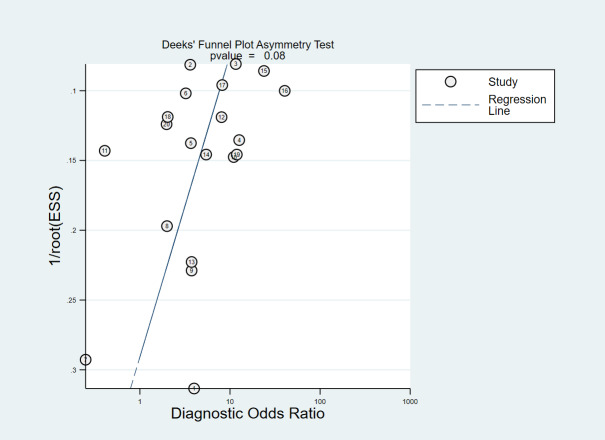
We assessed publication bias using a funnel plot and Deeks test (Deeks 2005). Visual assessment of the funnel plot did not identify important asymmetry and was not statistically significant (Figure 5).
5.
Deeks' Funnel Plot Asymmetry Test.
Discussion
Summary of main results
We identified 20 cohort studies reporting the test accuracy of serum CRP for diagnosing late‐onset infection in newborn infants. We calculated sensitivity (0.62, 95% CI 0.50 to 0.71) at the median specificity reported in our included studies (0.74). The analyses demonstrated inconsistency, that is, heterogeneity in the estimates of sensitivity and specificity. However, because of limited data availability, we were unable to explore whether the source of this variation was due to between‐study differences in the population of infants (preterm versus term infants), the subtypes of infection (such as CLABSI), or the infecting micro‐organisms.
Applying these data to a hypothetical cohort of 1000 newborn infants being evaluated for possible late‐onset infection, we estimated that, if the prevalence of true infection was 40% (the median prevalence in the included studies), then, on average, 152 cases of infection would be missed (false negative) and 156 non‐infected infants would be wrongly diagnosed (false positive). Therefore, serum CRP levels are unlikely to be considered sufficiently accurate as a triage test to select infants for further tests or interventions. These findings are similar to those in a systematic review and meta‐analysis of the accuracy of elevated serum CRP levels for diagnosing serious infection in children (aged one month to 18 years) with febrile illness (Van den Bruel 2011).
Strengths and weaknesses of the review
Index test
The serum CRP threshold level for a 'positive' test used in the included studies was typically between 5 mg/L and 10 mg/L, consistent with current use in clinical practice. In most studies, the threshold was defined a priori, and the estimates of test performance based on this predefined cut‐off for a positive test. Six studies did not predefine a threshold for positivity. The investigators performed post hoc analyses to determine the optimal cut‐off for test performance, that is, the level of serum CRP that maximised the area under the receiver operator curve. Five of these studies calculated levels between 2.2 mg/L and 18 mg/L (with two studies finding optimal cut‐offs between 5 mg/L and 10 mg/L). One study was an outlier with an optimal cut‐off of 111 mg/L (confirmed with primary investigator). Neither the published study nor any unpublished data we received from the authors explained this unexpectedly high cut‐off. However, in a sensitivity analysis without this study, there was not a substantial impact on estimates of sensitivity at median, upper, or lower quartiles of specificity reported in the included studies.
Reference standard
Our reference standard was microbiologically confirmed late‐onset infection (more than 72 hours after birth) including bacteraemia, fungaemia, meningitis, osteomyelitis, septic arthritis, and peritonitis. The definition of late‐onset infection used in the included studies varied. Some studies additionally included urinary tract infection or radiologically confirmed pneumonia within their case definition. These studies typically had none or very few participants with these diagnoses in the absence of bacteraemia, and we made a consensus decision to include their data rather than to exclude the full study.
Similarly, we accepted the primary study authors' definition of late‐onset infection with regards to the infant's age when evaluated. Definitions ranged from 48 hours to six days after birth. While this is a deviation from our proposed definition of more than 72 hours after birth, we made a pragmatic consensus decision to include studies in order to reflect the available evidence as well as variation in the precise definition of 'late‐onset' that exists in clinical practice (Haque 2005).
While variations in case‐definition may have contributed to differences in the rates of confirmed infection in studied cohorts, it is likely that between‐study differences in thresholds for investigating suspected infection are also important factors. We were unable to explore this possibility as we had insufficient data to determine how eligibility for inclusion criteria were applied in practice. Studies of diagnostic test accuracy in this context typically accept that variation in clinical decisions exists. However, though potentially contributing to heterogeneity in estimates of test performance, this pragmatic approach may make study findings more generally applicable.
There are concerns about how fully the reference standard defines all of those infants who truly have bloodstream infection. Microbiological cultures may not detect cases of bacteraemia or fungaemia if an insufficient volume of the infant's blood is incubated ('false negative'). Conversely, microbiological cultures may also generate 'false positive' results if blood sampling techniques allow entry of contaminating micro‐organisms (typically coagulase‐negative staphylococci from the infant's skin) (Oeser 2013). Insufficient data were available to undertake a subgroup analysis of infection with coagulase‐negative staphylococci versus other bacteria to explore whether test accuracy was affected by the likelihood of identified micro‐organisms representing true bloodstream infections. However, any such analysis may be confounded by between‐species differences in the capacity of micro‐organisms to trigger inflammatory cascades and generation of CRP.
Since these potential sources of verification bias may affect estimates of test performance, some studies required additional evidence of infection (usually clinical signs such as episodes of apnoea or temperature instability) for infants to meet the reference standard. However, some studies used a reference standard that incorporated the index test, that is, the serum CRP level was part of the definition of 'infection' in addition to microbiological culture of a pathogen from blood or a normally sterile body fluid. We excluded these studies because of their risk of bias; index test accuracy would be overestimated if the reference standard could only be met by infants with an elevated serum CRP level.
Search strategy
We undertook a comprehensive literature search for studies using a strategy designed by an information specialist. We did not use 'study type' filters as these increase the risk of relevant studies being missed due to inconsistent indexing in electronic databases (Wilczynski 2007). As with systematic reviews of interventional studies, publication bias may exist if studies which did not indicate good test performance were not submitted or accepted for publication (Leeflang 2014). Visual assessment of the funnel plot identified no important asymmetry and was not statistically significant (P = 0.1) and may indicate heterogeneity rather than publication bias.
We contacted study authors to obtain missing or unpublished data. While several authors provided additional information, many did not and this resulted in the exclusion of studies that might have been eligible for inclusion. We excluded 39 studies due to insufficient data. Similarly, we excluded four studies published in languages other than English as we did not have the resources to obtain a reliable translation.
We employed review methods to reduce the risk of reviewer error and bias, including independent and duplicate study selection as well as checking of data extraction and risk of bias assessments. We assessed the risk of bias of included studies using the QUADAS‐2 tool. Overall, studies were at low risk of bias but some use of the 'unclear' category was unavoidable due to missing detail in study reports. We excluded case‐control studies as this design is unlikely to allow valid assessment of diagnostic test accuracy in this clinical context. As described above, we excluded studies at high risk of incorporation bias (serum CRP level part of the reference standard) as these studies overestimate test performance. The only 'high' risk of bias was identified in a retrospective study in which the result of the reference standard was known before the index test was performed (although the laboratory test result was not likely to have been affected by this knowledge).
Heterogeneity
Heterogeneity is a well‐recognised feature in reviews of diagnostic test accuracy (Reitsma 2005). We observed between‐study variation in the estimates of sensitivity and specificity in the forest plot (Figure 3). We have not determined the degree to which this heterogeneity exceeded that expected due to chance (Naaktgeboren 2016). Our study selection, data extraction, and assessment of study quality was limited by missing details on procedures and infant characteristics, such as gestational age, blood sampling techniques, and methods used to determine the serum CRP level. Because of these data limitations, we were unable to explore sources of heterogeneity by subgroup analyses by gestational age (term versus preterm infants), type of infection (CLABSI versus other infections), type of infecting organism (coagulase‐negative staphylococci versus other pathogens), or type of laboratory technique used to measure serum CRP levels (high‐sensitivity CRP versus standard methods).
Setting
Most included studies assessed the accuracy of elevated serum CRP levels for diagnosing late‐onset infection in preterm infants in neonatal units in high‐ or middle‐income countries. While these data are likely to be applicable to preterm infants cared for in modern neonatal units in high‐ and (some) middle‐income countries, the review findings are less likely to be generalised to resource‐limited settings in low‐ or middle‐income countries where late‐onset infection in newborn infants differs with regard to epidemiology, microbiology, pathogenesis, treatment options, and outcomes (Vergnano 2005; Zea‐Vera 2015).
Applicability of findings to the review question
The review findings are specific to the accuracy of the serum CRP level in determining whether infection is less or more likely in infants in whom there is a clinical suspicion based on signs for other findings. The review does not address whether serial monitoring of the serum CRP level may be useful in screening well neonates for infection before it is suspected clinically, or in assessing the response to treatment, including helping to decide whether to stop antibiotics (Ehl 1997).
The timely diagnosis of late‐onset infection based on clinical features and signs in newborn infants, particularly very preterm infants, remains challenging (Verstraete 2015). This analysis suggests that the serum CRP level as an adjunctive triage test for late‐onset infection is not sufficiently accurate to determine which infants should receive treatment with antimicrobial agents or further tests. Applying the likelihood ratios derived from the meta‐analyses to a hypothetical cohort of infants with suspected late‐onset infection indicates that the test would generate a substantial number of both false‐negative and false‐positive results across a range of plausible prevalences (see Table 3 for post‐test probabilities across range of pretest probabilities). For example, if the estimated pretest probability of infection for a given infant was 40% (the median for the included studies), then adding in the serum CRP level to the assessment would generate a post‐test probability of 26% for a negative test (does not 'rule out' infection) and a post‐test probability of 61% for a positive test (does not 'rule in' infection).
This suboptimal diagnostic performance of serum CRP is consistent with estimates of its accuracy in other contexts including for diagnosing serious infection in children (aged one month to 18 years) with febrile illness (Van den Bruel 2011). The possible explanations for this lack of diagnostic accuracy include the potential for false‐positive results if CRP levels are elevated by triggers such as inflammation due to extravasation, cholestasis, or gastrointestinal pathology (Hofer 2013). Conversely, serum CRP levels may not rise, or rise only slowly, in some infected infants, particularly very preterm infants with coagulase‐negative staphylococcal bacteraemia (false‐negative results) (Lai 2015; Gilfillan 2017).
Authors' conclusions
Implications for practice.
The serum C‐reactive protein (CRP) level at initial evaluation of an infant with suspected late‐onset infection does not aid early diagnosis and is not likely to be considered a sufficiently accurate test to select infants who would undergo further investigation or be treated with antimicrobial therapy or other interventions.
Implications for research.
Given the poor performance of serum CRP in this context, research efforts might focus on other serum biomarkers, such as procalcitonin, that are elevated more quickly in response to infection or inflammation (Gilfillan 2017). Newer methods using molecular markers to identify pathogenic micro‐organisms (such as real‐time polymerase chain reaction or microarray techniques) are worthy of further research. These new techniques can provide results more quickly than standard microbiological culture (six to eight hours versus 24 to 36 hours), and evidence of their diagnostic accuracy is accumulating (Pammi 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2019 | Amended | Edited typo in PLS |
History
Protocol first published: Issue 3, 2016 Review first published: Issue 1, 2019
| Date | Event | Description |
|---|---|---|
| 16 January 2019 | Amended | Added correct Sources of support. |
Acknowledgements
We thank Ms Kath Wright for developing and managing the electronic search strategy. We thank the authors of the primary studies who supplied unpublished information or data.
Appendices
Appendix 1. Search strategy
MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
Via Ovid
1 exp Infant, Newborn/ (507086)
2 Premature Birth/ (7396)
3 (neonat$ or neo nat$).ti,ab. (204089)
4 (newborn$ or new born$ or newly born$).ti,ab. (135419)
5 (preterm or preterms or pre term or pre terms).ti,ab. (50291)
6 (preemie$ or premie or premies).ti,ab. (121)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (12210)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (26659)
9 (lbw or vlbw or elbw).ti,ab. (5938)
10 infan$.ti,ab. (345004)
11 (baby or babies).ti,ab. (54493)
12 "Intensive Care Units, Neonatal"/ (10315)
13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (854743)
14 C‐Reactive Protein/ (32529)
15 c‐reactive protein.ti,ab. (44280)
16 CRP.ti,ab. (30090)
17 Interleukin‐6/ (48141)
18 IL‐6.ti,ab. (71459)
19 interleukin‐6.ti,ab. (35713)
20 acute phase reactant$.ti,ab. (3064)
21 Biological Markers/ (172550)
22 biomarker$.ti,ab. (113562)
23 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (371276)
24 exp Sepsis/ (95928)
25 sepsis.ti,ab. (68288)
26 infection$.ti,ab. (1022808)
27 exp Bacterial Infections/ (745229)
28 (bacteraemia or bacteremia).ti,ab. (22257)
29 (fungaemia or fungemia).ti,ab. (1664)
30 exp Candidiasis/ (27375)
31 candidiasis.ti,ab. (12078)
32 exp Meningitis/ (48258)
33 meningitis.ti,ab. (42079)
34 Pneumonia, Bacterial/ (8792)
35 Urinary Tract Infections/ (32771)
36 Catheter‐related Infections/ (2533)
37 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (1653493)
38 13 and 23 and 37 (3447)
39 exp animals/ not humans.sh. (4037496)
40 38 not 39 (3184)
Embase via Ovid
1 exp Infant/ (898706)
2 Prematurity/ (76784)
3 (neonat$ or neo nat$).ti,ab. (253737)
4 (newborn$ or new born$ or newly born$).ti,ab. (162735)
5 (preterm or preterms or pre term or pre terms).ti,ab. (66105)
6 (preemie$ or premie or premies).ti,ab. (165)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (15808)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (31872)
9 (lbw or vlbw or elbw).ti,ab. (7572)
10 infan$.ti,ab. (406724)
11 (baby or babies).ti,ab. (71539)
12 newborn intensive care/ (22338)
13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (1220384)
14 C Reactive Protein/ (98704)
15 c‐reactive protein.ti,ab. (59724)
16 CRP.ti,ab. (53596)
17 Interleukin 6/ (140947)
18 IL‐6.ti,ab. (98082)
19 interleukin‐6.ti,ab. (41784)
20 acute phase reactant$.ti,ab. (4241)
21 Biological Marker/ (150561)
22 biomarker$.ti,ab. (170601)
23 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (472866)
24 exp Sepsis/ (181816)
25 newborn sepsis/ (4927)
26 sepsis.ti,ab. (96170)
27 infection$.ti,ab. (1249750)
28 exp Bacteremia/ (35078)
29 exp Fungemia/ (4805)
30 (bacteraemia or bacteremia).ti,ab. (27114)
31 (fungaemia or fungemia).ti,ab. (2019)
32 exp Candidiasis/ (40057)
33 candidiasis.ti,ab. (15095)
34 exp Meningitis/ (75363)
35 meningitis.ti,ab. (49821)
36 Pneumococcal meningitis/ (908)
37 Urinary Tract Infection/ (75050)
38 Catheter infection/ (11655)
39 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (1501019)
40 13 and 23 and 39 (5912)
41 animal/ (1656079)
42 human/ (15731222)
43 41 not (41 and 42) (1247837)
44 40 not 43 (5877)
Science Citation Index via Web of Science
| # 4 | 1,256 | #3 AND #2 AND #1 Indexes=SCI‐EXPANDED Timespan=1900‐2015 |
||||
| # 3 | 194,557 |
TOPIC: (sepsis) ORTOPIC: ("bacterial infection*") ORTOPIC: (bacteremia or bacteraemia) ORTOPIC: (fungaemia or fungemia) ORTOPIC:(candidiasis) ORTOPIC: (meningitis) ORTOPIC: ("pneumococcal meningitis") ORTOPIC: ("urinary tract infection*") ORTOPIC: ("catheter infection*") Indexes=SCI‐EXPANDED Timespan=1900‐2015 |
||||
| # 2 | 241,196 |
TOPIC: ("c reactive protein") ORTOPIC: ("Interleukin 6") ORTOPIC: ("acute phase reactants") ORTOPIC: ("biological markers") ORTOPIC:(biomarker*) Indexes=SCI‐EXPANDED Timespan=1900‐2015 |
||||
| # 1 | 616,040 |
TOPIC: ((neonat* or neo nat* or newborn* or
new born* or newly born*)) ORTOPIC: ((preterm or preterms or pre term or
pre terms or preemie* or premie* or premies))
ORTOPIC: ("premature birth" or "premature
delivery") ORTOPIC: (low birthweight* or "low birth
weight*") ORTOPIC: (lbw or vlbw or elbw) ORTOPIC: (infant or infants or infancy or baby
or babies) ORTOPIC: ("neonatal intensive care") Indexes=SCI‐EXPANDED Timespan=1900‐2015 |
||||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 CRP | 20 | 1615 |
1. Test.
CRP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aminullah 2001.
| Study characteristics | |||
| Patient sampling | Prospective investigation of newborn infants (birth weight > 1 kg) with suspected late‐onset (> 72 hours) infection | ||
| Patient characteristics and setting | Newborn infants with birth weight > 1 kg (gestational age range not reported) in 1 neonatal unit in Indonesia | ||
| Index tests | Serum CRP level > 12 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Serum CRP measured at initial evaluation, reference standard determined over 24–48 hours subsequently | ||
| Comparative | |||
| Notes | 1999 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Benitz 1998.
| Study characteristics | |||
| Patient sampling | Prospective evaluation of all infants with suspected infection | ||
| Patient characteristics and setting | Newborn infants (mean gestational age at birth 32 weeks), 3 NICU in USA | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Suspected or confirmed (microbiological culture of blood, CSF, or joint aspiration) late‐onset infection ("without consideration of CRP levels") | ||
| Flow and timing | Serum CRP measured at initial evaluation, reference standard determined over 24–48 hours subsequently | ||
| Comparative | |||
| Notes | The study included 'pneumonia' within the reference standard definition, but the investigators reported only 1 case of pneumonia in the entire study cohort. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bohnhorst 2012.
| Study characteristics | |||
| Patient sampling | Prospective evaluation of all neonates (term and preterm) with suspected infection after day 4 | ||
| Patient characteristics and setting | Newborn infants (mean gestational age at birth 28 weeks), 1 NICU in Germany | ||
| Index tests | Serum CRP level > 10 mg/L (defined a priori) | ||
| Target condition and reference standard(s) | Unclear if index test (serum CRP level) informed decisions to perform more tests (e.g. CSF sampling and culture) or diagnosis of 'suspected' infection if blood was negative. | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | Uncertain whether knowledge of initial serum CRP level affected care and investigation pathway. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Boo 2008.
| Study characteristics | |||
| Patient sampling | Prospective evaluation of newborn infants with suspected infection; not stated if consecutive or randomly sampled cohort | ||
| Patient characteristics and setting | Newborn infants (median gestation at birth 32 weeks)
with suspected infection after day 2, NICU in
Malaysia. (1/18 infants evaluated on day 1 after birth) |
||
| Index tests | Serum CRP level > 1 mg/L (defined a priori)* | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | *The unit for measuring serum CRP level was 'mg/mL' in the report but this was confirmed by the investigators to be a typographical error and should have been mg/L | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bustos 2012.
| Study characteristics | |||
| Patient sampling | Prospective evaluation of newborn infants with suspected infection; not stated if consecutive or randomly sampled cohort | ||
| Patient characteristics and setting | Neonates with clinically suspected late‐onset sepsis (gestational age 23–35 weeks) in 1 NICU in Chile | ||
| Index tests | Serum CRP level > 111 mg/L (calculated post hoc) | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Chan 1997.
| Study characteristics | |||
| Patient sampling | Prospective sample of VLBW infants; unclear whether randomly selected | ||
| Patient characteristics and setting | VLBW infants (mean gestation at birth 28 weeks) with suspected infection after day 3, 1 NICU in Singapore | ||
| Index tests | Serum CRP > 10 mg/L (defined a priori) | ||
| Target condition and reference standard(s) | Infection confirmed by blood, CSF, or joint aspiration microbiological culture | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Choo 2012.
| Study characteristics | |||
| Patient sampling | Prospective cohort of neonates (unclear whether consecutive or random sample) | ||
| Patient characteristics and setting | Newborn infants (> 29 weeks' gestation at birth) in 1 NICU in Korea | ||
| Index tests | Serum CRP > 10 mg/L (defined a priori) | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | We thank the investigators for providing data for infants with suspected late‐onset infection. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Decembrino 2015.
| Study characteristics | |||
| Patient sampling | Prospective cohort of infants (consecutive enrolment) | ||
| Patient characteristics and setting | Infants in 1 NICU in Italy (gestational age or age at study entry not reported) | ||
| Index tests | Serum CRP level > 6 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | We thank Dr Decembrino for providing unpublished data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Doellner 1998.
| Study characteristics | |||
| Patient sampling | Retrospective cohort study of newborn infants with suspected late‐onset (> 48 hours) infection during first week after birth | ||
| Patient characteristics and setting | Newborn infants (mean gestation 38 weeks) admitted to 1 NICU in Norway | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Neonatal infection confirmed by positive blood culture (some infants may have had an additional diagnosis of pneumonia*) | ||
| Flow and timing | Blood samples for CRP and blood culture were taken at the same time, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | The study authors provided additional information for
construction of 2×2 tables for infants with
late‐onset (> 48 hours) infection. (*The authors were unable to clarify how many infants who met the reference standard had pneumonia.) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Fendler 2008.
| Study characteristics | |||
| Patient sampling | Retrospective analysis of preterm neonates treated for suspected late‐onset infection | ||
| Patient characteristics and setting | Preterm infants (median gestational age 29 weeks) with suspected late‐onset infection who had all investigations (biomarkers and blood culture) assessed at the same time in 1 NICU in Poland | ||
| Index tests | Serum CRP level. Threshold of 2.2 mg/L determined retrospectively with Youden method on an ROC curve | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | Data from 5 eligible infants not available for
analysis. Further data provided by primary investigators. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hisamuddin 2015.
| Study characteristics | |||
| Patient sampling | Prospective study of consecutive infants with suspected sepsis* | ||
| Patient characteristics and setting | Infants (birth weight > 1500 g, gestational age > 32 weeks) with suspected sepsis in 1 NICU in Pakistan | ||
| Index tests | Serum CRP level > 5 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours | ||
| Comparative | |||
| Notes | * This study included both infants with early‐onset
sepsis (0–5 days after birth) and infants with
late‐onset sepsis (≥ 6 days after birth). The
authors provided unpublished data on infants aged
> 6 days. We thank Dr Hisam for providing unpublished data. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Jacquot 2009.
| Study characteristics | |||
| Patient sampling | Prospective study of all newborn infants with clinical suspicion of late‐onset infection | ||
| Patient characteristics and setting | Neonates (median gestation 28 weeks) evaluated for suspected infection, 1 NICU in France | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection* | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | *29/30 infants had blood culture confirmed infection, and 1 infant was classified as infection based on clinical features but without microbiological confirmation. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kipfmueller 2015.
| Study characteristics | |||
| Patient sampling | Prospective study of VLBW infants with suspected late‐onset infection | ||
| Patient characteristics and setting | VLBW infants (mean gestational age 28 weeks) in 1 NICU in Germany | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | Non‐infected group consisted of infants with clinical suspicion of infection without a positive blood culture. 1 indicator of 'clinical suspicion' was detection of a serum CRP level > 10 mg/L. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kordek 2014.
| Study characteristics | |||
| Patient sampling | Prospective study of all newborn infants with clinical suspicion of late‐onset infection | ||
| Patient characteristics and setting | Newborn infants (median gestational age 30 weeks) in 1 NICU in Poland | ||
| Index tests | Serum CRP level > 5 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kumar 2010.
| Study characteristics | |||
| Patient sampling | Prospective investigation of newborn infants evaluated for possible late‐onset infection | ||
| Patient characteristics and setting | Newborn infants (gestational age range not described) in 1 NICU in Kenya | ||
| Index tests | Serum CRP level > 5 mg/L | ||
| Target condition and reference standard(s) | Blood or CSF culture‐confirmed infection (plus urine culture*) | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | *Urinary sepsis was eligible for inclusion in case definition, but no episodes of urine infection were reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ng 1997.
| Study characteristics | |||
| Patient sampling | Prospective study of all VLBW infants evaluated for possible late‐onset infection | ||
| Patient characteristics and setting | VLBW infants (mean gestational age 30 weeks) in 1 NICU in Hong Kong | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection* | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | *Radiologically diagnosed pneumonia was eligible for inclusion in case definition, but no episodes of pneumonia were reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pynn 2015.
| Study characteristics | |||
| Patient sampling | Prospective observational study of neonates with suspected late‐onset infection | ||
| Patient characteristics and setting | Newborn infants (median gestation 28 weeks) in 1 NICU in USA | ||
| Index tests | Serum CRP level > 10 mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection* | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | *Definition of coagulase‐negative staphylococcal bacteraemia required 2 positive blood cultures | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Seibert 1990.
| Study characteristics | |||
| Patient sampling | Prospective study of very preterm infants evaluated for suspected late‐onset (> 72 hours) infection | ||
| Patient characteristics and setting | Very preterm newborn infants (23–31 weeks' gestation) in 1 NICU in Australia | ||
| Index tests | Serum CRP level > 10mg/L | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sherwin 2008.
| Study characteristics | |||
| Patient sampling | Prospective cohort study of neonates with suspected late‐onset infection | ||
| Patient characteristics and setting | Neonates (23–42 weeks' gestation) in 1 NICU in New Zealand | ||
| Index tests | Serum CRP level > 18 mg/L* determined retrospectively with reference to ROC curve | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection** | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | *Presumed typographical error in table describing
sensitivity and specificity; threshold reported in
pg/mL, rather than mg/L. **2/117 participating infants were diagnosed with pneumonia; unclear from the published data whether these infants had suspected late‐ or early‐onset sepsis and whether or not they had a positive blood culture. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Verboon‐Maciolek 2006.
| Study characteristics | |||
| Patient sampling | Prospective cohort study of infants with suspected late‐onset infection | ||
| Patient characteristics and setting | Preterm neonates (median gestation 29 weeks) in 1 NICU in the Netherlands | ||
| Index tests | Serum CRP level > 14 mg/L determined retrospectively with reference to ROC curve | ||
| Target condition and reference standard(s) | Blood culture‐confirmed infection | ||
| Flow and timing | Index test and reference standard samples were drawn simultaneously, blood culture result available over 24–48 hours. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
CRP: C‐reactive protein; CSF: cerebrospinal fluid; NICU: neonatal intensive care unit; ROC: receiver operating characteristic; VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboud 2010 | Study design not eligible. Case‐control study |
| Adhikari 1986 | Insufficient data. Participants' ages unclear, unable to decide if late‐onset or early‐onset sepsis was investigated. Decided not to try and contact the authors due to age of study. |
| Adib 2012 | Insufficient data. Data for infants with late‐onset sepsis (> 72 hours) were not reported separately. These data were requested from the authors but not provided. |
| Adly 2014 | Insufficient data. Published data was not sufficient to assess eligibility for inclusion. Additional data were requested from the authors but not provided. |
| Ahmed 2005 | Study design not eligible. Case‐control study |
| Ahmed 2013 | Insufficient data. Abstract only. Could not locate authors for full data. |
| Ainbender 1982 | Insufficient data. Decided not to contact the authors due to age of study. |
| Al‐Zwaini 2009 | Insufficient data |
| Alexejew 1990 | Study design not eligible. Review article |
| Aliefendioglu 2014 | Study design not eligible. Case‐control study |
| Alt 1982 | Study design not eligible. Case‐control study |
| Amato 1984 | Reference standard incorporated the index test. |
| Ammo 2008 | Insufficient data. Abstract only. We were unable to locate the full text or contact the authors to request further data. |
| Ananina 1963 | Insufficient data. Decided not to contact the authors due to age of study. |
| Ang 1990 | Insufficient data. Data not extractable. Could not locate authors for clarification. |
| Anwer 2000 | Population not eligible. Unclear from paper if late‐onset or early‐onset sepsis was investigated. We decided not to try and contact the authors due to age of study. |
| Apostolou 2002 | Insufficient data. We decided not to contact the authors due to age of study. |
| Arani 2013 | Population not eligible. Early‐onset sepsis |
| Arati 2014 | Insufficient data to populate 2×2 table. No email address available for corresponding author |
| Arayici 2014 | Study design not eligible. Case‐control study |
| Arnon 2004 | Population not eligible. Comparison of CRP levels in babies who died of sepsis and those who survived. |
| Arnon 2005 | Study design not eligible. Case‐control study |
| Arnon 2007 | Population not eligible. Early‐onset sepsis |
| Athhan 2002 | Insufficient data. We decided not to contact the authors due to age of study. |
| Athiraman 2011 | Insufficient data for. Could not locate authors for additional information. |
| Ayazi 2014 | Insufficient data. Published data did not allow extraction of information on infants with late‐onset sepsis only. This information was requested from the authors but not provided. |
| Aydin 2012 | Study design not eligible. Case‐control study |
| Aydin 2013 | Study design not eligible. Case‐control study |
| Baptista‐Gonzales 1989 | Population not eligible. Early‐onset sepsis |
| Baruti‐Gafurri 2010 | Study design not eligible. Case‐control study |
| Batfalsky 2012 | Study design not eligible. Case‐control study |
| Beceiro Mosquera 2009 | Population not eligible. Early‐onset sepsis |
| Berger 1995 | Insufficient data. Could not locate authors for additional information. |
| Berrington 2014 | Other. CRP not investigated |
| Bhandari 2008 | Other. No mention of CRP in the paper |
| Bhargava 2011 | Study design not eligible. Case‐control study |
| Bhargava 2013 | Study design not eligible. Case‐control study |
| Blanco 1996 | Insufficient data. We decided not to contact the authors due to age of study. |
| Blommendahl 2002 | Insufficient data. We decided not to contact the authors due to age of study. |
| Boonkasidecha 2013 | Insufficient data. Data from both early‐onset sepsis and late‐onset sepsis included in the study. We contacted authors for late‐onset sepsis‐only data but received no reply. |
| Boskabadi 2010 | Study design not eligible. Case‐control study |
| Bressan 2010 | Population not eligible. Participants were admitted from the community. |
| Buck 1994 | Insufficient data. We decided not to contact the authors due to age of study. |
| Bustos Betanzo 2007 | Study design not eligible. Case‐control study |
| Catal 2014 | Study design not eligible. Case‐control study |
| Ceccon 2006 | Foreign language study. The abstract published in English contained insufficient information to assess eligibility. We contacted the authors but received no reply. |
| Cekmez 2011 | Study design not eligible. Case‐control study |
| Celik 2010 | Reference standard incorporated the index test |
| Celik 2012 | Reference standard incorporated the index test |
| Cesur 2009 | Study design not eligible. Case‐control study |
| Cetinkaya 2009 | The reference standard incorporated the index test |
| Chen 2009 | Population not eligible. Participants were admitted to hospital from the community. |
| Davis 2015 | Reference standard not eligible. This study used the NEO‐KISS classification (German surveillance system for nosocomial infections in VLBW infants) as the reference standard. However, as blood culture results are part of the NEO‐KISS classification, we contacted the authors to request diagnostic data for CRP using blood culture results as the reference standard. We received no reply. |
| Dhanalakshmi 2015 | Published data insufficient to assess eligibility for inclusion. Additional data requested from the authors but not provided. |
| Dorado Moles 2007 | Other. Case‐control study of infants with suspected infection compared with infants with other neonatal conditions not due to infection (respiratory distress, encephalopathy). |
| Duhan 2016 | Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Edgar 1994 | Insufficient data. Data for infants with late‐onset sepsis (> 72 hours) were not reported separately. These data were requested from the authors but not provided. We thank Dr Edgar for responding to our emails and trying to find the requested data. |
| Edgar 2010 | Insufficient data. Data for infants with late‐onset sepsis (> 72 hours) were not reported separately. These data were requested from the authors but not provided. We thank Dr Edgar for responding to our emails and trying to find the requested data. |
| El Shimi 2017 | Reference standard incorporated the index test |
| El‐Sonbaty 2016 | Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Enguix 2001 | Study design not eligible. Case‐control study |
| Escobar 2015 | Population not eligible. Early‐onset sepsis |
| Fattah 2017 | Case‐control study |
| Franz 1999 | Reference standard incorporated the index test |
| Ganesan 2016 | Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Garland 2003 | Insufficient data. We decided not to contact the authors due to age of study. |
| Goldfinch 2015 | Insufficient data. Abstract only, full text not found |
| Gorbe 2007 | Foreign language study. The abstract did not contain sufficient information for inclusion. We were unable to reach the authors to request further data. |
| Gura 2003 | Foreign language study. Insufficient data contained in abstract. Decided not to contact the authors due to age of study. |
| Hegadi 2015 | Insufficient data to populate 2×2 table. No email address available for corresponding author |
| Khair 2012 | Population not eligible. Early‐onset and late‐onset sepsis combined. Late‐onset sepsis‐only data requested from authors but not provided. |
| Khassawneh 2007 | Insufficient data. Published data did not allow extraction of information on infants with late‐onset sepsis only. This information was requested from the authors but not provided. |
| Kite 1988 | Population not eligible. Combination of early‐onset and late‐onset sepsis. Unable to extract appropriate data after feedback from authors. We thank Dr Kite for his time and effort in replying to our email and trying to provide the requested data. |
| Kocabas 2007 | Case‐control study. No data on infants who had a negative sepsis evaluation |
| Krauel 1987 | Population not eligible. Not exclusively late‐onset sepsis and mixed population. Decided not to contact the authors due to age of study. |
| Krediet 1992 | Insufficient data. This study included infants with pneumonia but without a positive blood culture in the 'sepsis' group. We contacted the authors to request data on infants with positive blood culture only but received no reply. |
| Kuster 1998 | Other. Sensitivity and specificity calculations were based on CRP measurements taken before the onset on sepsis. We decided not to try to contact the authors due to age of study. |
| Laborada 2003 | Population not eligible. Combination of early‐onset and late‐onset sepsis. Decided not to try and contact authors due to age of study. |
| Lai 2015 | Insufficient data. Study did not collect diagnostic data for CRP |
| Liu 2016 | Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Lu 2016 | The reference standard incorporated the index test |
| Magudumana 2000 | Insufficient data. Unable to extract appropriate data. Decided not to try and contact authors due to age of study. |
| Manucha 2002 | All participants < 4 days old ('early‐onset' infection) |
| Matesanz 1980 | Insufficient data. No usable data. Decided not to try and contact the authors due to age of study. |
| Mehr 2001 | Insufficient data. No usable data. Decided not to try and contact the authors due to age of study. |
| Mustafa 2005 | Population not eligible. Combination of early‐onset and late‐onset sepsis. Could not locate author to request late‐onset sepsis only data. |
| Nakamura 1989 | Population not eligible. Combination of early‐onset and late‐onset sepsis. Could not locate author to request late‐onset sepsis only data. |
| Noto 2012 | Insufficient data. Abstract only. Authors' contact details not provided. |
| Nuntnarumit 2002 | Reference standard not eligible. The reference standard used in this paper included both positive and negative blood culture. We were unable to locate the authors to request positive blood culture only data. |
| Okulu 2015 | Reference standard incorporated index test |
| Omran 2018 | Case‐control study. Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Park 2014 | The reference standard incorporated the index test |
| Perez Solis 2006 | Study design not eligible. Case‐control study |
| Pourcyrous 1989 | Insufficient data. Abstract only. Decided not to try and contact the authors due to age of study. Could not locate full text. |
| Prasad 2015 | Insufficient data. Authors contacted but did not provide additional data. |
| Qin 2017 | Included only infants with early‐onset sepsis (< 72 hours) |
| Russell 1992 | The reference standard incorporated the index test |
| Sakha 2008 | Population not eligible. Combination of late‐onset and early‐onset sepsis. Could not locate author to request late‐onset sepsis only data. |
| Sarafidis 2017 | Study did not use a threshold of CRP to distinguish between infants with 'high' or 'low' CRP. |
| Sharma 1993 | Population not eligible. Unclear how many participants had late‐onset sepsis. Decided not to contact authors due to age of study. |
| Stein 2015 | Population not eligible. Participants were admitted to hospital from the community. |
| Topuz 2012 | Foreign language paper. The abstract published in English did not contain sufficient data to assess eligibility. We contacted the authors to request further information but received no reply. |
| Turner 2006 | Population not eligible. Combination of confirmed sepsis and clinical sepsis (without a positive blood culture). Authors were contacted and informed us that the requested data were no longer available. |
| Tyagi 2016 | Insufficient data to assess eligibility and to construct 2×2 table (investigators contacted October 2017) |
| Ussat 2015 | The reference standard incorporated the index test |
| Vazzalwar 2005 | Case‐control study |
| Wu 2013 | The reference standard incorporated the index test |
| Yao 2016 | Case‐control study |
| Ye 2017 | Case‐control study |
| Zarkesh 2015 | Population not eligible. Infants were admitted to hospital from the community. |
| Zhao 2015 | Comparison of CRP levels before and after antibiotic treatment |
CRP: C‐reactive protein; VLBW: very low birth weight.
Characteristics of studies awaiting classification [ordered by study ID]
Ohlin 2010.
| Study characteristics | |||
| Patient sampling | Prospective cohort | ||
| Patient characteristics and setting | Newborn infants with suspected infection (mostly infants with early‐onset infection, but some with late‐onset) | ||
| Index tests | Serum C‐reactive protein level > 10 mg/L | ||
| Target condition and reference standard(s) | Microbiologically confirmed infection | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Awaiting data for infants with late‐onset infection | ||
Differences between protocol and review
On the advice of a referee, we used a hierarchical summary receiver operating characteristic model rather than the proposed bivariate random‐effects approach because included studies did not use a common cut‐off.
We included studies in which infants with urinary tract infection or radiologically confirmed pneumonia fulfilled the case definition for late‐onset infection. These studies typically had none or very few participants with these diagnoses in the absence of bacteraemia.
We accepted the primary study authors' definition of late‐onset infection with regards to the infant's age when evaluated (range from 48 hours to six days after birth).
Contributions of authors
All authors contributed to the development of the protocol and final review.
Sources of support
Internal sources
University of York, UK.
External sources
-
NIHR, UK.
- This report is independent research funded by a UK National Institute for Health Research Grant (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
JVEB: none.
NM: none.
JC: none.
WM: none.
Edited (no change to conclusions)
References
References to studies included in this review
Aminullah 2001 {published data only}
- Aminullah A, Sjachroel DN, Hadinegoro SR, Madiyono B. The role of plasma C‐reactive protein in the evaluation of antibiotic treatment in suspected neonatal sepsis. Medical Journal of Indonesia 2001;10(1):16‐21. [Google Scholar]
Benitz 1998 {published data only}
- Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C‐reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998;102(4):E41. [PUBMED: 9755278] [DOI] [PubMed] [Google Scholar]
Bohnhorst 2012 {published data only}
- Bohnhorst B, Lange M, Bartels DB, Bejo L, Hoy L, Peter C. Procalcitonin and valuable clinical symptoms in the early detection of neonatal late‐onset bacterial infection. Acta Paediatrica 2012;101(1):19‐25. [DOI: 10.1111/j.1651-2227.2011.02438.x; PUBMED: 21824193] [DOI] [PubMed] [Google Scholar]
- Bohnhorst B, Lange M, Bejo L, Bartels DB, Hoy L, Peter C. Value of clinical symptoms and combination rules of procalcitonin, C‐reactive protein, and interleukin 6 in the diagnosis of neonatal sepsis. Klinische Padiatrie 2010;222:S5. [Google Scholar]
Boo 2008 {published data only}
- Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi‐quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Medical Journal 2008;49(3):204‐8. [PubMed] [Google Scholar]
Bustos 2012 {published data only}
- Bustos BR, Araneda CH. Procalcitonin for the diagnosis of late onset sepsis in newborns of very low birth weight [Procalcitonina para el diagnóstico de la sepsis tardía en recién nacidos de muy bajo peso de nacimiento]. Revista Chilena de Infectologia 2012;29(5):511‐6. [DOI: 10.4067/S0716-10182012000600005; PUBMED: 23282492] [DOI] [PubMed] [Google Scholar]
Chan 1997 {published data only}
- Chan DK, Ho LY. Usefulness of C‐reactive protein in the diagnosis of neonatal sepsis. Singapore Medical Journal 1997;38(6):252‐5. [PUBMED: 9294338] [PubMed] [Google Scholar]
Choo 2012 {published and unpublished data}
- Choo YK, Cho HS, Seo IB, Lee HS. Comparison of the accuracy of neutrophil CD64 and C‐reactive protein as a single test for the early detection of neonatal sepsis. Korean Journal of Pediatrics 2012;55(1):11‐7. [DOI: 10.3345/kjp.2012.55.1.11; PUBMED: 22359525] [DOI] [PMC free article] [PubMed] [Google Scholar]
Decembrino 2015 {published and unpublished data}
- Decembrino L, Amici M, Pozzi M, Silvestri A, Stronati M. Serum calprotectin: a potential biomarker for neonatal sepsis. Journal of Immunology Research 2015;2015:147973. [DOI: 10.1155/2015/147973; PUBMED: 26380313] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doellner 1998 {published and unpublished data}
- Doellner H, Arntzen KJ, Haereid PE, Aag S, Austgulen R. Interleukin‐6 concentrations in neonates evaluated for sepsis. Journal of Pediatrics 1998;132(2):295‐9. [PUBMED: 9506644] [DOI] [PubMed] [Google Scholar]
- Doellner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C‐reactive protein, interleukin‐6, soluble tumour necrosis factor receptors and soluble adhesion molecules. Journal of Clinical Epidemiology 2001;54(12):1251‐7. [PUBMED: 11750194] [DOI] [PubMed] [Google Scholar]
Fendler 2008 {published data only}
- Fendler WM, Piotrowski AJ. Procalcitonin in the early diagnosis of nosocomial sepsis in preterm neonates. Journal of Paediatrics and Child Health 2008;44(3):114‐8. [DOI: 10.1111/j.1440-1754.2007.01230.x; PUBMED: 17927729] [DOI] [PubMed] [Google Scholar]
Hisamuddin 2015 {published and unpublished data}
- Hisamuddin E, Hisam A, Wahid S, Raza G. Validity of C‐reactive protein (CRP) for diagnosis of neonatal sepsis. Pakistan Journal of Medical Sciences 2015;31(3):527‐31. [DOI: 10.12669/pjms.313.6668; PUBMED: 26150837] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacquot 2009 {published data only}
- Jacquot A, Labaune JM, Baum TP, Putet G, Picaud JC. Rapid quantitative procalcitonin measurement to diagnose nosocomial infections in newborn infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(5):F345‐8. [DOI: 10.1136/adc.2008.155754; PUBMED: 19439432] [DOI] [PubMed] [Google Scholar]
Kipfmueller 2015 {published data only}
- Kipfmueller F, Schneider J, Prusseit J, Dimitriou I, Zur B, Franz AR, et al. Role of neutrophil CD64 index as a screening marker for late‐onset sepsis in very low birth weight infants. PloS One 2015;10(4):e0124634. [PUBMED: 10.1371/journal.pone.0124634; PUBMED: 25894336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kordek 2014 {published data only}
- Kordek A, Loniewska B, Podraza W, Nikodemski T, Rudnicki J. Usefulness of estimation of blood procalcitonin concentration versus C‐reactive protein concentration and white blood cell count for therapeutic monitoring of sepsis in neonates. Postepy Higieny i Medycyny Doswiadczalnej (Online) 2014;68:1516‐23. [PUBMED: 25531715] [DOI] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar R. Validation of C‐Reactive Protein [Masters thesis]. Nairobi: University of Nairobi, 2006. [Google Scholar]
- Kumar R, Musoke R, Macharia WM, Revathi G. Validation of C‐reactive protein in the early diagnosis of neonatal sepsis in a tertiary care hospital in Kenya. East African Medical Journal 2010;87(6):255‐61. [PUBMED: 23057268] [DOI] [PubMed] [Google Scholar]
Ng 1997 {published data only}
- Ng PC, Cheng SH, Chui KM, Fok TF, Wong MY, Wong W, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C‐reactive protein in preterm very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;77(3):F221‐7. [PUBMED: 9462194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pynn 2015 {published data only}
- Pynn JM, Parravicini E, Saiman L, Bateman DA, Barasch JM, Lorenz JM. Urinary neutrophil gelatinase‐associated lipocalin: potential biomarker for late‐onset sepsis. Pediatric Research 2015;78(1):76‐81. [DOI: 10.1038/pr.2015.62; PUBMED: 25806716] [DOI] [PubMed] [Google Scholar]
Seibert 1990 {published data only}
- Seibert K, Yu VY, Doery JC, Embury D. The value of C‐reactive protein measurement in the diagnosis of neonatal infection. Journal of Paediatrics and Child Health 1990;26(5):267‐70. [PUBMED: 2265018] [DOI] [PubMed] [Google Scholar]
Sherwin 2008 {published data only}
- Sherwin C, Broadbent R, Young S, Worth J, McCaffrey F, Medlicott NJ, et al. Utility of interleukin‐12 and interleukin‐10 in comparison with other cytokines and acute‐phase reactants in the diagnosis of neonatal sepsis. American Journal of Perinatology 2008;25(10):629‐36. [DOI: 10.1055/s-0028-1090585; PUBMED: 18850512] [DOI] [PubMed] [Google Scholar]
Verboon‐Maciolek 2006 {published data only}
- Verboon‐Maciolek MA, Thijsen SF, Hemels MA, Menses M, Loon AM, Krediet TG, et al. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatric Research 2006;59(3):457‐61. [DOI: 10.1203/01.pdr.0000200808.35368.57; PUBMED: 16492989] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboud 2010 {published data only}
- Aboud MI, Waise MW, Shakerdi LA. Procalcitonin as a marker of neonatal sepsis in intensive care units. Iranian Journal of Medical Sciences 2010;35(3):205‐10. [Google Scholar]
Adhikari 1986 {published data only}
- Adhikari M, Coovadia HM, Coovadia YM, Smit SY, Moosa A. Predictive value of C‐reactive protein in neonatal septicaemia. Annals of Tropical Paediatrics 1986;6(1):37‐40. [PUBMED: 2428291] [DOI] [PubMed] [Google Scholar]
Adib 2012 {published data only (unpublished sought but not used)}
- Adib M, Bakhshiani Z, Navaei F, Saheb Fosoul F, Fouladi S, Kazemzadeh H. Procalcitonin: a reliable marker for the diagnosis of neonatal sepsis. Iranian Journal of Basic Medical Sciences 2012;15(2):777‐82. [PUBMED: 23493845] [PMC free article] [PubMed] [Google Scholar]
- Adib M, Ostadi V, Navaei F, Saheb Fosoul F, Oreizi F, Shokouhi R, et al. Evaluation of CD11b expression on peripheral blood neutrophils for early detection of neonatal sepsis. Iranian Journal of Allergy, Asthma, and Immunology 2007;6(2):93‐6. [PUBMED: 17563410] [PubMed] [Google Scholar]
Adly 2014 {published data only}
- Adly AA, Ismail EA, Andrawes NG, El‐Saadany MA. Circulating soluble triggering receptor expressed on myeloid cells‐1 (sTREM‐1) as diagnostic and prognostic marker in neonatal sepsis. Cytokine 2014;65(2):184‐91. [DOI: 10.1016/j.cyto.2013.11.004; PUBMED: 24290866] [DOI] [PubMed] [Google Scholar]
Ahmed 2005 {published data only}
- Ahmed Z, Ghafoor T, Waqar T, Ali S, Aziz S, Mahmud S. Diagnostic value of C‐reactive protein and haematological parameters in neonatal sepsis. Journal of the College of Physicians and Surgeons‐‐Pakistan : JCPSP 2005;15(3):152‐6. [DOI: ; PUBMED: 15808093] [PubMed] [Google Scholar]
Ahmed 2013 {published data only}
- Ahmed A, Baki A, Begum T, Nahar N. Evaluation of procalcitonin as a marker of neonatal sepsis. Intensive Care Medicine 2013;39:S75. [Google Scholar]
Ainbender 1982 {published data only}
- Ainbender E, Cabatu EE, Guzman DM, Sweet AY. Serum C‐reactive protein and problems of newborn infants. Journal of Pediatrics 1982;101(3):438‐40. [PUBMED: 7108668] [DOI] [PubMed] [Google Scholar]
Alexejew 1990 {published data only}
- Alexejew B, Hendrick W, Braun W, Domula M, Winiecki P. The significance of various hematological parameters for the early diagnosis of bacterial infections in premature and full‐term neonates. 3. Discussion of the study results. Padiatrie und Grenzgebiete 1990;29(6):455‐79. [PubMed] [Google Scholar]
Aliefendioglu 2014 {published data only}
- Aliefendioglu D, Gursoy T, Caglayan O, Aktas A, Ovali F. Can resistin be a new indicator of neonatal sepsis?. Pediatrics and Neonatology 2014;55(1):53‐7. [DOI: 10.1016/j.pedneo.2013.04.012; PUBMED: 23820264] [DOI] [PubMed] [Google Scholar]
Alt 1982 {published data only}
- Alt R, Willard D, Messer J, Metais P, Goester C, Mark JJ. Value of C‐reactive protein in neonatal bacterial infections [Interet de la c reactive proteine dans les infections bacteriennes neonatales]. Archives Francaises de Pediatrie 1982;39(10):811‐3. [PUBMED: 7168617] [PubMed] [Google Scholar]
Al‐Zwaini 2009 {published data only}
- Al‐Zwaini EJ. C‐reactive protein: a useful marker for guiding duration of antibiotic therapy in suspected neonatal septicaemia?. Eastern Mediterranean Health Journal 2009;15(2):269‐75. [PUBMED: 19554972] [PubMed] [Google Scholar]
Amato 1984 {published data only}
- Amato M, Ruckstuhl C, Muralt G. Serum C‐reactive protein in preterm infants [C‐reaktives protein im serum fruhgeborener]. Schweizerische Medizinische Wochenschrift 1984;114(12):412‐4. [PUBMED: 6719082] [PubMed] [Google Scholar]
Ammo 2008 {published data only}
- Ammo K, Salacity G. CRP and ESR as a diagnostic marker in detection of neonatal sepsis. Pakistan Paediatric Journal 2008;32(1):15‐22. [Google Scholar]
Ananina 1963 {published data only}
- Ananina NV. Significance of the electrophoretic investigation of the blood proteins and C‐reactive protein in the diagnosis of sepsis in the newborn. Pediatriia 1963;42:19‐24. [PUBMED: 14148317] [PubMed] [Google Scholar]
Ang 1990 {published data only}
- Ang AT, Ho NK, Chia SE. The usefulness of CRP and I/T ratio in early diagnosis of infections in Asian newborns. Journal of the Singapore Paediatric Society 1990;32(3‐4):159‐63. [PUBMED: 2133755] [PubMed] [Google Scholar]
Anwer 2000 {published data only}
- Anwer SK, Mustafa S. Rapid identification of neonatal sepsis. Journal of the Pakistan Medical Association 2000;50(3):94‐8. [PUBMED: 10795470] [PubMed] [Google Scholar]
Apostolou 2002 {published data only}
- Apostolou M, Dimitriou H, Kaleyias J, Perdikogianni C, Stiakaki E, Costalos C, et al. Levels of soluble ICAM‐1 in premature and full‐term neonates with infection. Mediators of Inflammation 2002;11(2):95‐8. [DOI: 10.1080/09629350220131944; PUBMED: 12061430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arani 2013 {published data only}
- Arani MH, Movahedian A, Arani MG, Adinah M, Mosayebi Z. Predictive value of interleukin‐6 (IL6) in term neonates with early sepsis during 2010‐2011. Jundishapur Journal of Microbiology 2013;6(10):e8580. [Google Scholar]
Arati 2014 {published data only}
- Arati K, Nagarathnamma T, Benakappa A. Comparative evaluation of blood culture, estimation of immunoglobulin M (IgM) and C‐reactive protein detection in the diagnosis of neonatal sepsis. International Journal of Current Research 2014;6(2):5094‐7. [Google Scholar]
Arayici 2014 {published data only}
- Arayici S, Kadioglu Simsek G, Conploat FE, Oncel M, Uras N, Oguz S, et al. Can base excess be used for early diagnosis of neonatal sepsis in preterm newborns?. Archives of Diseases in Childhood 2014;99:A418‐9. [Google Scholar]
Arnon 2004 {published data only}
- Arnon S, Litmanovitz I, Regev R, Lis M, Shainkin‐Kestenbaum R, Dolfin T. The prognostic virtue of inflammatory markers during late‐onset sepsis in preterm infants. Journal of Perinatal Medicine 2004;32(2):176‐80. [DOI: 10.1515/JPM.2004.032; PUBMED: 15085896] [DOI] [PubMed] [Google Scholar]
Arnon 2005 {published data only}
- Arnon S, Litmanovitz I, Regev R, Bauer S, Lis M, Shainkin‐Kestenbaum R, et al. Serum amyloid A protein is a useful inflammatory marker during late‐onset sepsis in preterm infants. Biology of the Neonate 2005;87(2):105‐10. [DOI: 10.1159/000081979; PUBMED: 15539766] [DOI] [PubMed] [Google Scholar]
- Arnon S, Litmanovitz I, Regev R, Lis M, Shainkin‐Kestenbaum R, Dolfin T. Serum amyloid A protein in the early detection of late‐onset bacterial sepsis in preterm infants. Journal of Perinatal Medicine 2002;30(4):329‐32. [DOI: 10.1515/JPM.2002.048; PUBMED: 12235722] [DOI] [PubMed] [Google Scholar]
Arnon 2007 {published data only}
- Arnon S, Litmanovitz I, Regev RH, Bauer S, Shainkin‐Kestenbaum R, Dolfin T. Serum amyloid A: an early and accurate marker of neonatal early‐onset sepsis. Journal of Perinatology 2007;27(5):297‐302. [DOI: 10.1038/sj.jp.7211682; PUBMED: 17344924] [DOI] [PubMed] [Google Scholar]
Athhan 2002 {published data only}
- Athhan F, Akagunduz B, Genel F, Bak M, Can D. Procalcitonin: a marker of neonatal sepsis. Journal of Tropical Pediatrics 2002;48(1):10‐4. [PUBMED: 11866330] [DOI] [PubMed] [Google Scholar]
Athiraman 2011 {published data only}
- Athiraman N, Agarwal R, Krishnamoorthy S, Guy M. Can we rely on procalcitonin in the diagnosis of late onset neonatal sepsis?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(Suppl 1):Fa40. [Google Scholar]
Ayazi 2014 {published data only}
- Ayazi P, Mahyar A, Daneshi MM, Jahanihashemi H, Esmailzadehha N, Mosaferirad N. Comparison of serum IL‐1beta and C reactive protein levels in early diagnosis and management of neonatal sepsis. Le Infezioni in Medicina 2014;22(4):296‐301. [PUBMED: 25551845] [PubMed] [Google Scholar]
Aydin 2012 {published data only}
- Aydin B, Zenciroglu A, Dilli D, Erol S, Ozyazici E, Karadag N, et al. The predictive values on mean platelet volume (MPV) in the diagnosis of neonatal sepsis. Archives of Disease in Childhood 2012;97(Suppl 2):A338. [Google Scholar]
Aydin 2013 {published data only}
- Aydin B, Dilli D, Zenciroglu A, Kaya O, Bilaloglu E, Okumus N, et al. Comparison of a rapid bed‐side test with a central laboratory analysis for C‐reactive protein in newborn infants with suspicion of sepsis. Clinical Laboratory 2013;59(9‐10):1045‐51. [PUBMED: 24273927] [DOI] [PubMed] [Google Scholar]
Baptista‐Gonzales 1989 {published data only}
- Baptista‐Gonzales HA, Ibarra‐Camacho A, Cedillo RE, Torres‐Alipi BI. Usefulness of C‐reactive protein for the diagnosis of neonatal sepsis. Boletin Medico del Hospital Infantil de Mexico 1989;46(8):543‐6. [PUBMED: 2803537] [PubMed] [Google Scholar]
Baruti‐Gafurri 2010 {published data only}
- Baruti‐Gafurri Z, Pacarizi H, Zhubi B, Begoli L, Topciul V. The importance of determining procalcitonin and C reactive protein in different stages of sepsis. Bosnian Journal of Basic Medical Sciences 2010;10(1):60‐4. [DOI: 10.17305/bjbms.2010.2737; PUBMED: 20192933] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batfalsky 2012 {published data only}
- Batfalsky A, Lohr A, Heussen N, Neunhoeffer F, Orlikowsky TW. Diagnostic value of an interleukin‐6 bedside test in term and preterm neonates at the time of clinical suspicion of early‐ and late‐onset bacterial infection. Neonatology 2012;102(1):37‐44. [DOI: 10.1159/000336632; PUBMED: 22507910] [DOI] [PubMed] [Google Scholar]
Beceiro Mosquera 2009 {published data only}
- Beceiro Mosquera J, Sivera Monzo CL, Oria de Rueda Salguero O, Olivas Lopez de Soria C, Herbozo Nory C. Usefulness of a rapid serum interleukin‐6 teste combined with C‐reactive protein to predict sepsis in newborns with suspicion of infection [Utilidad de un test rápido de interleuquina‐6 sérico combinado con proteína C reactiva para predecir la sepsis en recién nacidos con sospecha de infección]. Anales de Pediatria (Barcelona, Spain : 2003) 2009;71(6):483‐8. [DOI: 10.1016/j.anpedi.2009.07.027; PUBMED: 19811958] [DOI] [PubMed] [Google Scholar]
Berger 1995 {published data only}
- Berger C, Uehlinger J, Ghelfi D, Blau N, Fanconi S. Comparison of C‐reactive protein and white blood cell count with differential in neonates at risk for septicaemia. European Journal of Pediatrics 1995;154(2):138‐44. [PUBMED: 7720743] [DOI] [PubMed] [Google Scholar]
Berrington 2014 {published data only}
- Berrington JE, Hearn RI, Hall C, Stewart CJ, Cummings SP, Embleton ND. Proportionate reduction in uncertainty of late onset infection in preterm infants by neutrophil CH64 measurement. Fetal and Pediatric Pathology 2014;33(1):16‐22. [DOI: 10.3109/15513815.2013.842270; PUBMED: 24093544] [DOI] [PubMed] [Google Scholar]
Bhandari 2008 {published data only}
- Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics 2008;121(1):129‐34. [DOI: 10.1542/peds.2007-1308; PUBMED: 18166566] [DOI] [PubMed] [Google Scholar]
Bhargava 2011 {published data only}
- Bhargava M, Sindhuri U, Saluja S, Saraf A, Dayanand S, Simon‐Lopez R. Volume, conductivity, and scatter properties of leukocytes (VCS Technology) is a highly sensitive and specific predictor of blood culture proven neonatal sepsis. International Journal of Laboratory Hematology 2011;33(Suppl 1):55‐6. [Google Scholar]
Bhargava 2013 {published data only}
- Bhargava M. Utility of automated blood cell analyzer‐derived indices (VCS technology) in the rapid diagnosis of infectious, inflammatory, and neoplastic disorders part I: neonatal sepsis, malaria, and dengue. Indian Journal of Hematology & Blood Transfusion 2013;29(4):244. [Google Scholar]
- Bhargava M, Saluha S, Sindhuri U, Saraf A, Sharma P. Elevated mean neutrophil volume + CRP is a highly sensitive and specific predictor of neonatal sepsis. International Journal of Laboratory Hematology 2014;36(1):e11‐4. [DOI: 10.1111/ijlh.12120; PUBMED: 23795566] [DOI] [PubMed] [Google Scholar]
Blanco 1996 {published data only}
- Blanco A, Solis G, Arranz E, Coto GD, Ramos A, Telleria J. Serum levels of CD14 in neonatal sepsis by Gram‐positive and Gram‐negative bacteria. Acta Paediatrica 1996;85(6):728‐32. [PUBMED: 8816213] [DOI] [PubMed] [Google Scholar]
Blommendahl 2002 {published data only}
- Blommendahl J, Janas M, Laine S, Miettinen A, Ashorn P. Comparison of procalcitonin with CRP and differential white blood cell count for diagnosis of culture‐proven neonatal sepsis. Scandinavian Journal of Infectious Diseases 2002;34(8):620‐2. [PUBMED: 12238581] [DOI] [PubMed] [Google Scholar]
Boonkasidecha 2013 {published data only}
- Boonkasidecha S, Panburana J, Chansakulporn S, Benjasuwantep B, Kongsomboon K. An optimal cut‐off point of serum C‐reactive protein in prediction of neonatal sepsis. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2013;96(Suppl 1):S65‐70. [PUBMED: 23724458] [PubMed] [Google Scholar]
Boskabadi 2010 {published data only}
- Boskabadi H, Maamouri G, Afshari JT, Ghayour‐Mobarhan M, Shakeri MT. Serum interleukin 8 level as a diagnostic marker in late neonatal sepsis. Iranian Journal of Pediatrics 2010;20(1):41‐7. [PUBMED: 23056680] [PMC free article] [PubMed] [Google Scholar]
Bressan 2010 {published data only}
- Bressan S, Andreola B, Cattelan F, Zangardi T, Perilongo G, Dalt L. Predicting severe bacterial infections in well‐appearing febrile neonates: laboratory markers accuracy and duration of fever. Pediatric Infectious Disease Journal 2010;29(3):227‐32. [DOI: 10.1097/INF.0b013e3181b9a086; PUBMED: 19949364] [DOI] [PubMed] [Google Scholar]
Buck 1994 {published data only}
- Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin‐6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 1994;93(1):54‐8. [PUBMED: 8265324] [PubMed] [Google Scholar]
Bustos Betanzo 2007 {published data only}
- Bustos Betanzo RO. Procalcitonin, C‐reactive protein and leukocyte count in very‐low birth weight infants with late neonatal sepsis. Anales de Pediatria (Barcelona, Spain : 2003) 2007;66(5):541‐2. [PUBMED: 17517212] [DOI] [PubMed] [Google Scholar]
Catal 2014 {published data only}
- Catal F, Tayman C, Tonbul A, Akca H, Kara S, Tatli MM, et al. Mean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants. Clinical Laboratory 2014;60(7):1193‐200. [PUBMED: 25134389] [DOI] [PubMed] [Google Scholar]
Ceccon 2006 {published data only (unpublished sought but not used)}
- Ceccon M, Vaz FA, Diniz EM, Okay TS. Analysis of the use of interleukin 6 and 8 and C‐reactive protein for the diagnosis and therapeutic follow‐up of newborns with late onset sepsis admitted to the neonatal intensive care unit in Sao Paulo, Brazil. Pediatric Research 2003;53(4):312A‐3A. [Google Scholar]
- Ceccon ME, Vaz FA, Diniz EM, Okay TS. Interleukin 6 and C‐reactive protein for the diagnosis of late onset sepsis in the newborn infant [Interleucina 6 e proteína c reativa no diagnóstico de sepse tardia no recém‐nascido]. Revista de Associacao Medica Brasileira 2006;52(2):79‐85. [PUBMED: 16767331] [DOI] [PubMed] [Google Scholar]
Cekmez 2011 {published data only}
- Cekmez F, Canpolat FE, Cetinkaya M, Aydinoz S, Aydemir G, Karademir F, et al. Diagnostic value of resistin and visfatin, in comparison with C‐reactive protein, procalcitonin and interleukin‐6 in neonatal sepsis. European Cytokine Network 2011;22(2):113‐7. [DOI: 10.1684/ecn.2011.0283; PUBMED: 21636351] [DOI] [PubMed] [Google Scholar]
Celik 2010 {published data only (unpublished sought but not used)}
- Celik IH, Demirel FG, Uras N, Oguz SS, Erdeve O, Biyikli Z, et al. What are the cut‐off levels for IL‐6 and CRP in neonatal sepsis?. Journal of Clinical Laboratory Analysis 2010;24(6):407‐12. [DOI: 10.1002/jcla.20420; PUBMED: 21089127] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik IH, Demirel G, Uras N, Oguz ES, Erdeve O, Dilme U. The role of serum interleukin‐6 and C‐reactive protein levels for differentiating aetiology of neonatal sepsis. Archivos Argentinos de Pediatria 2015;113(6):534‐7. [DOI] [PubMed] [Google Scholar]
Celik 2012 {published data only (unpublished sought but not used)}
- Celik HT, Portakal O, Yigit S, HasCelik G, Korkmaz A, Yurdakok M. Comparison of the efficacy of serum C‐reactive protein, procalcitonin, interleukin‐6 levels and new leukocyte parameters in the diagnosis of neonatal sepsis. Clinical Chemistry and Laboratory Medicine 2014;52(Suppl 1):S856. [Google Scholar]
- Celik HT, Portakal O, Yigit S, Hascelik G, Korkmaz A. Yurdakoek M. Efficacy of new lycocyte parameters versus serum C‐reactive protein, procalcitonin, and interleukin‐6 in the diagnosis of neonatal sepsis. Pediatrics International 2016;58(2):119‐25. [DOI: 10.1111/ped.12754; PUBMED: 26190096] [DOI] [PubMed] [Google Scholar]
- Celik T, Portakal O, Yigit S, Hascelik G, Korkmaz A, Yurdakok M. Comparison of the efficacy of C‐reactive protein, procalcitonin, interleukin‐6 levels and new leukocyte parameters in the diagnosis of neonatal sepsis. Archives of Disease in Childhood 2012;97:A12. [DOI] [PubMed] [Google Scholar]
Cesur 2009 {published data only}
- Cesur S, Irmak H, Esras Z, Yasar H, Sengul A, Dilme U, et al. Prognostic value of serum TNF‐alpha, IL‐10, leptin, and CRP levels in newborns with septicemia. Mikrobiyoloji Bulteni 2009;43(3):607‐12. [PUBMED: 20084913] [PubMed] [Google Scholar]
Cetinkaya 2009 {published data only}
- Cetinkaya M, Ozkan H, Koksal N, Celebi S, Hacimustafaoglu M. Comparison of serum amyloid A concentrations with those of C‐reactive protein and procalcitonin in diagnosis and follow‐up of neonatal sepsis in premature infants. Journal of Perinatology 2009;29(3):225‐31. [DOI: 10.1038/jp.2008.207; PUBMED: 19078972] [DOI] [PubMed] [Google Scholar]
- Köksal N, Harmanci R, Cetinkaya M, Hacimustafaoğlu M. Role of procalcitonin and CRP in diagnosis and follow‐up of neonatal sepsis. Turkish Journal of Pediatrics 2007;49(1):21‐9. [PUBMED: 17479640] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen HL, Hung CH, Tseng HI, Yang RC. Circulating chemokine levels in febrile infants with serious bacterial infections. Kaohsiung Journal of Medical Sciences 2009;25(12):633‐9. [DOI: 10.1016/S1607-551X(09)70568-6; PUBMED: 19951848] [DOI] [PubMed] [Google Scholar]
- Chen HL, Hung CH, Tseng HI, Yang RC. Plasma IP‐10 as a predictor of serious bacterial infection in infants less than 4 months of age. Journal of Tropical Pediatrics 2009;55(2):103‐8; Corrected and republished in: Journal of Tropical Pediatrics: 2011: 57(2): 145‐51. [DOI: 10.1093/tropej/fmn092; PUBMED: 18952629] [DOI] [PubMed] [Google Scholar]
Davis 2015 {published data only}
- Davis J, Christie S, Fairley D, Coyle P, Tubman R, Shields MD. Performance of a novel molecular method in the diagnosis of late‐onset sepsis in very low birth weight infants. PloS One 2015;10(8):e0136472. [DOI: 10.1371/journal.pone.0136472; PUBMED: 26305781] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhanalakshmi 2015 {published data only (unpublished sought but not used)}
- Dhanalakshmi V, Sivakumar ES. Comparative study in early neonates with septicemia by blood culture, staining techniques and C‐reactive protein (CRP). Journal of Clinical and Diagnostic Research 2015;9(3):DC12‐5. [DOI: 10.7860/JCDR/2015/12437.5725; PUBMED: 25954618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorado Moles 2007 {published data only}
- Dorado Moles MJ, Figueredo Delgado MA, Fernández Pérez C, Moro Serrano M. T lymphocyte immunophenotype as a diagnostic marker of late‐onset neonatal sepsis [Inmunofenotipificación de linfocitos T como marcador diagnóstico de sepsis neonatal tardía]. Anales de Pediatria (Barcelona, Spain : 2003) 2007;67(6):536‐43. [PUBMED: 18053517] [DOI] [PubMed] [Google Scholar]
Duhan 2016 {published data only}
- Duhan A, Berwal A, Raikwar P, Punia A, Beniwal K, Kamra HT. Utility of haematological parameters in detection of neonatal sepsis. JKIMSU 2016;5(3):98‐106. [Google Scholar]
Edgar 1994 {published data only (unpublished sought but not used)}
- Edgar JD, Wilson DC, McMillan SA, Crockard AD, Halliday MI, Gardiner KR, et al. Predictive value of soluble immunological mediators in neonatal infection. Clinical Science 1994;87(2):165‐71. [PUBMED: 7924161] [DOI] [PubMed] [Google Scholar]
Edgar 2010 {published data only (unpublished sought but not used)}
- Edgar JD, Gabriel V, Gallimore JR, McMillan SA, Grant J. A prospective study of the sensitivity, specificity and diagnostic performance of soluble intercellular adhesion molecule 1, highly sensitive C‐reactive protein, soluble E‐selectin and serum amyloid A in the diagnosis of neonatal infection. BMC Pediatrics 2010;10:22. [DOI: 10.1186/1471-2431-10-22; PUBMED: 20398379] [DOI] [PMC free article] [PubMed] [Google Scholar]
El Shimi 2017 {published data only}
- Shimi MS, Shady NM, Hamed GM, Shedeed NS. Significance of neutrophilic CD64 as an early marker for detection of neonatal sepsis and prediction of disease outcome. Journal of Maternal‐fetal & Neonatal Medicine 2017;30(14):1709‐14. [DOI: 10.1080/14767058.2016.1223030; PUBMED: 27578316] [DOI] [PubMed] [Google Scholar]
El‐Sonbaty 2016 {published data only (unpublished sought but not used)}
- El‐Sonbaty MM, AlSharany W, Youness ER, Mohamed NA, Abdel‐Hamid TA, Abdel‐Razek ARA. Diagnostic utility of biomarkers in diagnosis of early stages of neonatal sepsis in neonatal intensive care unit in Egypt. Egyptian Pediatric Association Gazette 2016;64(2):91‐6. [Google Scholar]
Enguix 2001 {published data only}
- Enguix A, Rey C, Concha A, Medina A, Coto D, Dieguez MA. Comparison of procalcitonin with C‐reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensive Care Medicine 2001;27(1):211‐5. [PUBMED: 11280637] [DOI] [PubMed] [Google Scholar]
Escobar 2015 {published data only}
- Escobar LA, Ruiz JS, Gil‐Ruiz Gil‐Esparza MA, Cardenas Rebollo JM, Diaz‐Parreno AS, Llana Martin I. C‐reactive protein utility as an indicator of proven neonatal sepsis. Journal of Perinatal Medicine 2015;43:338. [Google Scholar]
Fattah 2017 {published data only}
- Fattah MA, Omer AF, Asaif S, Manlulu R, Karar T, Ahmed A, et al. Utility of cytokine, adhesion molecule and acute phase proteins in early diagnosis of neonatal sepsis. Journal of Natural Science, Biology, and Medicine 2017;8(1):32‐9. [DOI: 10.4103/0976-9668.198362; PUBMED: 28250672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franz 1999 {published data only}
- Franz AR, Kron M, Pohlandt F, Steinbach G. Comparison of procalcitonin with interleukin 8, C‐reactive protein and differential white blood cell count for the early diagnosis of bacterial infections in newborn infants. Pediatric Infectious Disease Journal 1999;18(8):666‐71. [PUBMED: 10462333] [DOI] [PubMed] [Google Scholar]
- Franz AR, Sieber S, Pohlandt F, Kron M, Steinbach G. Whole blood interleukin 8 and plasma interleukin 8 levels in newborn infants with suspected bacterial infection. Acta Paediatrica 2004;93(5):648‐53. [PUBMED: 15174789] [DOI] [PubMed] [Google Scholar]
- Franz AR, Steinbach G, Kron M, Pohlandt F. Reduction of unnecessary antibiotic therapy in newborn infants using interleukin‐8 and C‐reactive protein as markers of bacterial infections. Pediatrics 1999;104(3 Pt 1):447‐53. [PUBMED: 10469768] [DOI] [PubMed] [Google Scholar]
Ganesan 2016 {published data only (unpublished sought but not used)}
- Ganesan P, Shanmugam P, Sattar SB, Shankar SL. Evaluation of IL‐6, CRP and hs‐CRP as early markers of neonatal sepsis. Journal of Clinical and Diagnostic Research 2016;10(5):13‐7. [DOI: 10.7860/JCDR/2016/19214.7764; PUBMED: 27437213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2003 {published data only}
- Garland SM, Bowman ED. Reappraisal of C‐reactive protein as a screening tool for neonatal sepsis. Pathology 2003;35(3):240‐3. [PUBMED: 14506969] [DOI] [PubMed] [Google Scholar]
Goldfinch 2015 {published data only}
- Goldfinch C, Komran T, Kotsanas D, Burgner D, Tan K. Should inflammatory markers inform the decision to perform a lumbar puncture in infants with suspected neonatal sepsis?. Journal of Paediatrics and Child Health 2015;51(Suppl 1):84. [Google Scholar]
Gorbe 2007 {published data only (unpublished sought but not used)}
- Gorbe E, Jaeger J, Nagy B, Harmath A, Hauzman E, Hruby E, et al. Assessment of serum interleukin‐6 with a rapid test. The diagnosis of neonatal sepsis can be established or ruled out. Orvosi Hetilap 2007;148(34):1609‐14. [DOI: 10.1556/OH.2007.27991; PUBMED: 17702690] [DOI] [PubMed] [Google Scholar]
Gura 2003 {published data only}
- Gura A, Oygur N, Ongun H, Saka O, Yegin O. E‐selectin as an early indicator and follow‐up parameter in late neonatal sepsis [Yenidogan gec sepsisinde erken tani ve tedavi kriteri olarak E‐selektin]. Cocuk Saglili ve Hastalkiklari Dergisi 2003;46(4):261‐6. [Google Scholar]
Hegadi 2015 {published data only}
- Hegadi SS, Kalpana S. Comparative evaluation of blood culture and C‐reactive protein detection in the diagnosis of neonatal sepsis. International Journal of Pharma and Bio Sciences 2015;6(2):B1366‐71. [Google Scholar]
Khair 2012 {published data only (unpublished sought but not used)}
- Khair KB, Rahman MA, Sultana T, Roy CK, Rahman MQ, Ahmed AN. Early diagnosis of neonatal septicemia by hematologic scoring system, C‐reactive protein and serum haptoglobin. Mymensingh Medical Journal : MMJ 2012;21(1):85‐92. [PUBMED: 22314460] [PubMed] [Google Scholar]
Khassawneh 2007 {published data only (unpublished sought but not used)}
- Khassawneh M, Hayajneh WA, Kofahi H, Khader Y, Amarin Z, Daoud A. Diagnostic markers for neonatal sepsis: comparing C‐reactive protein, interleukin‐6 and immunoglobulin M. Scandinavian Journal of Immunology 2007;65(2):171‐5. [DOI: 10.1111/j.1365-3083.2006.01878.x; PUBMED: 17257222] [DOI] [PubMed] [Google Scholar]
Kite 1988 {published data only (unpublished sought but not used)}
- Kite P, Millar MR, Gorham P, Congdon P. Comparison of five tests used in diagnosis of neonatal bacteraemia. Archives of Disease in Childhood 1988;63(6):639‐43. [PUBMED: 3389893] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocabas 2007 {published data only}
- Kocabas E, Sarikcioglu A, Aksaray N, Seydaoglu G, Seyhun Y, Yaman A. Role of procalcitonin, C‐reactive protein, interleukin‐6, interleukin‐8 and tumor necrosis factor‐alpha in the diagnosis of neonatal sepsis. Turkish Journal of Pediatrics 2007;49(1):7‐20. [PUBMED: 17479639] [PubMed] [Google Scholar]
Krauel 1987 {published data only}
- Krauel J, Salvado A, Mira A, Orellana N, Calvet I, Molina V, et al. Simultaneous determination of total and immature neutrophil C‐reactive protein in normal, diseased, and infected newborn infants. Anales Espanoles de Pediatria 1987;27(4):257‐60. [PUBMED: 3426018] [PubMed] [Google Scholar]
Krediet 1992 {published data only}
- Krediet T, Gerards L, Fleer A, Stekelenburg G. The predictive value of CRP and I/T‐ratio in neonatal infection. Journal of Perinatal Medicine 1992;20(6):479‐85. [PUBMED: 1293275] [DOI] [PubMed] [Google Scholar]
Kuster 1998 {published data only}
- Kuster H, Weiss M, Willeitner AE, Detlefsen S, Jeremias I, Zbojan J, et al. Interleukin‐1 receptor antagonist and interleukin‐6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet 1998;352(9136):1271‐7. [DOI: 10.1016/S0140-6736(98)08148-3; PUBMED: 9788457] [DOI] [PubMed] [Google Scholar]
Laborada 2003 {published data only}
- Laborada G, Rego M, Jain A, Guliano M, Stavola J, Ballabh P, et al. Diagnostic value of cytokines and C‐reactive protein in the first 24 hours of neonatal sepsis. American Journal of Perinatology 2003;20(8):491‐501. [DOI: 10.1055/s-2003-45382; PUBMED: 14703598] [DOI] [PubMed] [Google Scholar]
Lai 2015 {published data only}
- Lai MY, Tsai MH, Lee CW, Chiang MC, Lien R, Fu RH, et al. Characteristics of neonates with culture‐proven bloodstream infection who have low levels of C‐reactive protein (≤10 mg/L). BMC Infectious Diseases 2015;15:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016 {published data only (unpublished sought but not used)}
- Liu S, Hou Y, Cui H. Clinical values of the early detection of serum procalcitonin, C‐reactive protein and white blood cells for neonates with infectious diseases. Pakistan Journal of Medical Sciences 2016;32(6):1326‐9. [DOI: 10.12669/pjms.326.11395; PUBMED: 28083019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016 {published data only}
- Lu Q, Duan H, Yu J, Yao Y. Are global coagulation and platelet parameters useful markers for predicting late‐onset neonatal sepsis?. Clinical Laboratory 2016;62(1‐2):73‐9. [PUBMED: 27012035] [DOI] [PubMed] [Google Scholar]
- Yao Y, Tu Y, Lu Q. Values of C‐reactive protein, percentage of neutrophils and mean platelet volume in early diagnosis of neonatal sepsis. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2015;17(5):425‐9. [PUBMED: 26014688] [PubMed] [Google Scholar]
Magudumana 2000 {published data only}
- Magudumana MO, Ballot DE, Cooper PA, Trusler J, Cory BJ, Viljoen E, et al. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. Journal of Tropical Pediatrics 2000;46(5):267‐71. [PUBMED: 11077934] [DOI] [PubMed] [Google Scholar]
Manucha 2002 {published data only}
- Manucha V, Rusia U, Sikka M, Faridi MM, Madan N. Utility of haematological parameters and C‐reactive protein in the detection of neonatal sepsis. Journal of Paediatrics and Child Health 2002;38(5):459‐64. [PUBMED: 12354261] [DOI] [PubMed] [Google Scholar]
Matesanz 1980 {published data only}
- Matesanz JL, Malaga S, Santos F, Nuno F, Ramos A, Crespo M. Diagnostic value of C reactive protein in neonatal sepsis. Anales Espanoles de Pediatria 1980;13(8):671‐8. [PUBMED: 7436148] [PubMed] [Google Scholar]
Mehr 2001 {published data only}
- Mehr SS, Doyle LW, Rice GE, Vervaart P, Henschke P. Interleukin‐6 and interleukin‐8 in newborn bacterial infection. American Journal of Perinatology 2001;18(6):313‐24. [DOI: 10.1055/s-2001-17857; PUBMED: 11607849] [DOI] [PubMed] [Google Scholar]
Mustafa 2005 {published data only}
- Mustafa S, Farooqui S, Waheed S, Mahmood K. Evaluation of C‐reactive protein as early indicator of blood culture positivity in neonates. Pakistan Journal of Medical Sciences 2005;21(1):69‐73. [Google Scholar]
Nakamura 1989 {published data only}
- Nakamura H, Uetani Y, Nagata T, Yamasaki T. Serum C‐reactive protein in the early diagnosis of neonatal septicemia and bacterial meningitis. Acta Paediatrica Japonica; Overseas Edition 1989;31(5):567‐71. [PUBMED: 2515735] [DOI] [PubMed] [Google Scholar]
Noto 2012 {published data only}
- Noto A, Fanos V, Puxeddu E, Irmesi R, Puddu M, Ottonello G, et al. Soluble CD14‐subtype (sCD14‐ST) presepsin in critically ill preterm and term newborns for the early assessment of neonatal sepsis: preliminary results. Clinical Chemistry 2012;58(10 Suppl 1):A142‐3. [Google Scholar]
Nuntnarumit 2002 {published data only}
- Nuntnarumit P, Pinkaew O, Kitiwanwanich S. Predictive values of serial C‐reactive protein in neonatal sepsis. Journal of the Medical Association of Thailand 2002;85(Suppl 4):S1151‐8. [PUBMED: 12549789] [PubMed] [Google Scholar]
Okulu 2015 {published data only (unpublished sought but not used)}
- Okulu E, Arsan A, Akin IM, Ates C, Alan S, Kilic A, et al. Serum levels of soluble urokinase plasminogen activator receptor in infants with late‐onset sepsis. Journal of Clinical Laboratory Analysis 2015;29(5):347‐52. [DOI: 10.1002/jcla.21777; PUBMED: 25043869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omran 2018 {published data only (unpublished sought but not used)}
- Omran A, Maaroof A, Saleh MH, Abdelwahab A. Salivary C‐reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. Jornal de Pediatria 2018;94(1):82‐7. [DOI: 10.1016/j.jped.2017.03.006; PUBMED: 28734690] [DOI] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park IH, Lee SH, Yu ST, Oh YK. Serum procalcitonin as a diagnostic marker of neonatal sepsis. Korean Journal of Pediatrics 2014;57(10):451‐6. [DOI: 10.3345/kjp.2014.57.10.451; PUBMED: 25379046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez Solis 2006 {published data only}
- Perez Solis D, Lopez Sastre JB, Coto Cotallo GD, Dieguez Junquera MA, Deschamps Mosquera EM, Crespo Hernandez M. Procalcitonin for the diagnosis of nosocomial neonatal sepsis [Procalcitonina para el diagnóstico de sepsis neonatal de origen nosocomial]. Anales de Pediatria (Barcelona, Spain : 2003) 2006;64(4):349‐53. [PUBMED: 16606572] [DOI] [PubMed] [Google Scholar]
Pourcyrous 1989 {published data only}
- Pourcyrous M, Bada H, Jennings W, Korones S. C‐reactive protein‐ the most useful acute phase reactants for the early diagnosis of neonatal bacterial infection. Pediatric Research 1989;25(4):A280. [Google Scholar]
Prasad 2015 {published data only}
- Prasad A, Arora P, Chavan R. Role of hematological profile in early diagnosis of neonatal sepsis – one year cross sectional study in a tertiary care centre. Indian Journal of Hematology and Blood Transfusion 2015;31(1 Suppl 1):S18. [Google Scholar]
Qin 2017 {published data only}
- Qin DJ, Tang ZS, Chen SL, Xu XM, Mao SG, Zhang SF. Value of combined determination of neutrophil CD64 and procalcitonin in early diagnosis of neonatal bacterial infection. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2017;19(8):872‐6. [PUBMED: 28774361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 1992 {published data only}
- Russell GA, Smyth A, Cooke RW. Receiver operating characteristic curves for comparison of serial neutrophil band forms and C reactive protein in neonates at risk of infection. Archives of Disease in Childhood 1992;67(7 Spec No):808‐12. [PUBMED: 1519980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakha 2008 {published data only}
- Sakha K, Husseini MB, Seyyedsadri N. The role of the procalcitonin in diagnosis of neonatal sepsis and correlation between procalcitonin and C‐reactive protein in these patients. Pakistan Journal of Biological Sciences 2008;11(14):1785‐90. [PUBMED: 18817217] [DOI] [PubMed] [Google Scholar]
Sarafidis 2017 {published data only}
- Sarafidis K, Chatziioannou AC, Thomaidou A, Gika H, Mikros E, Benaki D, et al. Urine metabolombics in neonates with late‐onset sepsis in a case‐control study. Scientific Reports 2017;7:45506. [DOI: 10.1038/srep45506; PUBMED: 28374757] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 1993 {published data only}
- Sharma A, Kutty CV, Sabharwal U, Rathee S, Mohan H. Evaluation of sepsis screen for diagnosis of neonatal septicemia. Indian Journal of Pediatrics 1993;60(4):559‐63. [PUBMED: 8262592] [DOI] [PubMed] [Google Scholar]
Stein 2015 {published data only}
- Stein M, Schachter‐Davidov A, Babai I, Tasher D, Somekh E. The accuracy of C‐reactive protein, procalcitonin, and s‐TREM‐1 in the prediction of serious bacterial infection in neonates. Clinical Pediatrics 2015;54(5):439‐44. [DOI: 10.1177/0009922814553435; PUBMED: 25294884] [DOI] [PubMed] [Google Scholar]
Topuz 2012 {published data only (unpublished sought but not used)}
- Topuz S, Ovali F. Comparison of C‐reactive protein and procalcitonin in the diagnosis of neonatal sepsis. Nobel Medicus 2012;8(1):72‐6. [Google Scholar]
Turner 2006 {published data only}
- Turner D, Hammerman C, Rudensky B, Schlesinger Y, Schimmel MS. The role of procalcitonin as a predictor of nosocomial sepsis in preterm infants. Acta Paediatrica 2006;95(12):1571‐6. [DOI: 10.1080/08035250600767811; PUBMED: 17129964] [DOI] [PubMed] [Google Scholar]
Tyagi 2016 {published data only (unpublished sought but not used)}
- Tyagi T, Kelkar A, Doshi P, Mani NS. Utility of VCS parameters for early detection of neonatal sepsis. Indian Journal of Hematology & Blood Transfusion 2016;32(Suppl 2):S420. [Google Scholar]
Ussat 2015 {published data only}
- Ussat M, Vogtman C, Gebauer C, Pulzer F, Thome U, Knuepfer M. The role of elevated central‐peripheral temperature difference in early detection of late‐onset sepsis in preterm infants. Early Human Development 2015;91(12):677‐81. [DOI: 10.1016/j.earlhumdev.2015.09.007; PUBMED: 26513628] [DOI] [PubMed] [Google Scholar]
Vazzalwar 2005 {published data only (unpublished sought but not used)}
- Vazzalwar R, Pina‐Rodrigues E, Puppala BL, Angst DB, Schweig L. Procalcitonin as a screening test for late‐onset sepsis in preterm very low birth weight infants. Journal of Perinatology 2005;25(6):397‐402. [DOI: 10.1038/sj.jp.7211296; PUBMED: 15830005] [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu TW, Tabangin M, Kusano R, Ma Y, Ridsdale R, Akinbi H. The utility of serum hepcidin as a biomarker for late‐onset neonatal sepsis. Journal of Pediatrics 2013;162(1):67‐71. [DOI: 10.1016/j.jpeds.2012.06.010; PUBMED: 22796049] [DOI] [PubMed] [Google Scholar]
Yao 2016 {published data only}
- Yao A, Liu J, Chang J, Deng C, Hu Y, Yu F, et al. Clinical practice of procalcitonin and hypersensitive C‐reactive protein test in neonatal infection. Pakistan Journal of Pharmaceutical Sciences 2016;29(2 Suppl):753‐6. [PUBMED: 27113303] [PubMed] [Google Scholar]
Ye 2017 {published data only}
- Ye Q, Du L, Shao WX, Shang S. Utility of cytokines to predict neonatal sepsis. Pediatric Research 2017;81(4):616‐21. [DOI: 10.1038/pr.2016.267; PUBMED: 27997530] [DOI] [PubMed] [Google Scholar]
Zarkesh 2015 {published data only}
- Zarkesh M, Sedaghat F, Heidarzadeh A, Tabrizi M, Bolooki‐Moghadam K, Ghesmati S. Diagnostic value of IL‐6, CRP, WBC, and absolute neutrophil count to predict serious infection in febrile infants. Acta Medica Iranica 2015;53(7):408‐11. [PUBMED: 26520627] [PubMed] [Google Scholar]
Zhao 2015 {published data only}
- Zhao FX, Liu GH, Zhang J. Value of IL‐6 and IL‐8 in the diagnosis of neonatal sepsis. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2015;17(12):1311‐5. [PUBMED: 26695671] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ohlin 2010 {published data only}
- Ohlin A, Bjorkqvist M, Montgomery SM, Schollin J. Clinical signs and CRP values associated with blood culture results in neonates evaluated for suspected sepsis. Acta Paediatrica 2010;99(11):1635‐40. [DOI: 10.1111/j.1651-2227.2010.01913.x; PUBMED: 20560896] [DOI] [PubMed] [Google Scholar]
Additional references
Bassler 2009
- Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009;123(1):313‐8. [DOI: 10.1542/peds.2008-0377; NCT00009646; PUBMED: 19117897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2001
- Benjamin DK Jr, Miller W, Garges H, Benjamin DK, McKinney RE Jr, Cotton M, et al. Bacteremia, central catheters, and neonates: when to pull the line. Pediatrics 2001;107(6):1272‐6. [PUBMED: 11389242] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. Journal of Pediatrics 2012;160(1):49‐53.e1. [DOI: 10.1016/j.jpeds.2011.06.046; PUBMED: 21868028] [DOI] [PubMed] [Google Scholar]
Beynon 2013
- Beynon R, Leeflang MM, McDonald S, Eisinga A, Mitchell RL, Whiting P, et al. Search strategies to identify diagnostic accuracy studies in MEDLINE and EMBASE. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.MR000022.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler‐O'Hara 2012
- Butler‐O'Hara M, D'Angio CT, Hoey H, Stevens TP. An evidence‐based catheter bundle alters central venous catheter strategy in newborn infants. Journal of Pediatrics 2012;160(6):972‐7.e2. [DOI: 10.1016/j.jpeds.2011.12.004; PUBMED: 22240109] [DOI] [PubMed] [Google Scholar]
de Man 2000
- Man P, Verhoeven BA, Verbrugh HA, Vos MC, Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet 2000;355(9208):973‐8. [PUBMED: 10768436] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58:882‐93. [DOI] [PubMed] [Google Scholar]
Dong 2015
- Dong Y, Speer CP. Late‐onset neonatal sepsis: recent developments. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(3):F257‐63. [DOI: 10.1136/archdischild-2014-306213; PUBMED: 25425653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehl 1997
- Ehl S, Gering B, Bartmann P, Hogel J, Pohlandt F. C‐reactive protein is a useful marker for guiding duration of antibiotic therapy in suspected neonatal bacterial infection. Pediatrics 1997;99(2):216‐21. [PUBMED: 9024449] [DOI] [PubMed] [Google Scholar]
Ehl 1999
- Ehl S, Gehring B, Pohlandt F. A detailed analysis of changes in serum C‐reactive protein levels in neonates treated for bacterial infection. European Journal of Pediatrics 1999;158(3):238‐42. [PUBMED: 10094447] [DOI] [PubMed] [Google Scholar]
Fowlie 1998
- Fowlie PW, Schmidt B. Diagnostic tests for bacterial infection from birth to 90 days‐‐a systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;78(2):F92‐8. [PUBMED: 9577277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilfillan 2017
- Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Human Development 2017;105:25‐33. [DOI: 10.1016/j.earlhumdev.2016.12.002; PUBMED: 28131458] [DOI] [PubMed] [Google Scholar]
Gordon 2006
- Gordon A, Isaacs D. Late onset neonatal Gram‐negative bacillary infection in Australia and New Zealand: 1992‐2002. Pediatric Infectious Disease Journal 2006;25(1):25‐9. [PUBMED: 16395098] [DOI] [PubMed] [Google Scholar]
Haque 2005
- Haque KN. Definitions of bloodstream infection in the newborn. Pediatric Critical Care Medicine 2005;6(3 Suppl):S45‐9. [DOI: 10.1097/01.PCC.0000161946.73305.0A; PUBMED: 15857558] [DOI] [PubMed] [Google Scholar]
Hedegaard 2015
- Hedegaard SS, Wisborg K, Hvas AM. Diagnostic utility of biomarkers for neonatal sepsis – a systematic review. Infectious Diseases 2015;47(3):117‐24. [DOI: 10.3109/00365548.2014.971053; PUBMED: 25522182] [DOI] [PubMed] [Google Scholar]
Hofer 2013
- Hofer N, Müller W, Resch B. Chapter 4: the role of C‐reactive protein in the diagnosis of neonatal sepsis. In: Resch B editor(s). Neonatal Bacterial Infection. London: InTech, 2013. [Google Scholar]
Hornik 2012
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very‐low‐birth‐weight infants from a large group of neonatal intensive care units. Early Human Development 2012;88(Suppl 2):S69‐74. [DOI: 10.1016/S0378-3782(12)70019-1; PUBMED: 22633519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2014
- Leeflang MM. Systematic reviews and meta‐analyses of diagnostic test accuracy. Clinical Microbiology and Infection 2014;20(2):105‐13. [DOI: 10.1111/1469-0691.12474; PUBMED: 24274632] [DOI] [PubMed] [Google Scholar]
Malik 2003
- Malik A, Hui CP, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C‐reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Archives of Pediatrics & Adolescent Medicine 2003;157(6):511‐6. [DOI: 10.1001/archpedi.157.6.511; PUBMED: 12796229] [DOI] [PubMed] [Google Scholar]
McGuire 2004
- McGuire W, Clerihew L, Fowlie PW. Infection in the preterm infant. BMJ 2004;329(7477):1277‐80. [DOI: 10.1136/bmj.329.7477.1277; PUBMED: 15564261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller‐Pebody 2011
- Muller‐Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M, iCAP Group (Improving Antibiotic Prescribing in Primary Care). Empirical treatment of neonatal sepsis: are the current guidelines adequate?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F4‐8. [DOI: 10.1136/adc.2009.178483; PUBMED: 20584804] [DOI] [PubMed] [Google Scholar]
Naaktgeboren 2016
- Naaktgeboren CA, Ochodo EA, Enst WA, Groot JAH, Hooft L, Leeflang MM, et al. Assessing variability in results in systematic reviews of diagnostic studies. BMC Medical Research Methodology 2016;16:6. [DOI: 10.1186/s12874-016-0108-4; PUBMED: 26772804] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oeser 2013
- Oeser C, Lutsar I, Metsvaht T, Turner MA, Heath PT, Sharland M. Clinical trials in neonatal sepsis. Journal of Antimicrobial Chemotherapy 2013;68(12):2733‐45. [DOI: 10.1093/jac/dkt297; PUBMED: 23904558] [DOI] [PubMed] [Google Scholar]
Oeser 2014
- Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal Infection Surveillance Network (neonIN). Neonatal invasive fungal infection in England 2004‐2010. Clinical Microbiology and Infection 2014;20(9):936‐41. [DOI: 10.1111/1469-0691.12578; PUBMED: 24479862] [DOI] [PubMed] [Google Scholar]
Pammi 2015
- Pammi M, Weiman LE. Late‐onset sepsis in preterm infants: update on strategies for therapy and prevention. Expert Review of Anti‐infective Therapy 2015;13(4):487‐504. [DOI] [PubMed] [Google Scholar]
Pammi 2017
- Pammi M, Flores A, Versalovic J, Leeflang MMG. Molecular assays for the diagnosis of sepsis in neonates. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD011926.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI: 10.1016/j.jclinepi.2005.02.022; PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [PUBMED: 11568945] [DOI] [PubMed] [Google Scholar]
Shah 2014
- Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS, Canadian Neonatal Network. Risk factors and outcomes of late‐onset bacterial sepsis in preterm neonates born at < 32 weeks' gestation. American Journal of Perinatology 2014;32(7):675‐82. [DOI: 10.1055/s-0034-1393936; PUBMED: 25486288] [DOI] [PubMed] [Google Scholar]
Shalabi 2015
- Shalabi M, Adel M, Yoon E, Aziz K, Lee S, Shah PS, Canadian Neonatal Network. Risk of infection using peripherally Inserted central and umbilical catheters in preterm neonates. Pediatrics 2015;136(6):1073‐9. [DOI: 10.1542/peds.2015-2710; PUBMED: 26574592] [DOI] [PubMed] [Google Scholar]
Shane 2013
- Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. American Journal of Perinatology 2013;30(2):131‐41. [DOI: 10.1055/s-0032-1333413; PUBMED: 23297182] [DOI] [PubMed] [Google Scholar]
Shane 2014
- Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. Journal of Infection 2014;68 Suppl 1:S24‐32. [PUBMED: 24140138] [DOI] [PubMed] [Google Scholar]
Singh 2012
- Singh S, Chang SM, Matchar DB, Bass EB. Chapter 7: grading a body of evidence on diagnostic tests. Journal of General Internal Medicine 2012;27(Suppl 1):S47‐55. [DOI: 10.1007/s11606-012-2021-9; PUBMED: 22648675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steel 1994
- Steel DM, Whitehead AS. The major acute phase reactants: C‐reactive protein, serum amyloid P component and serum amyloid A protein. Immunology Today 1994;15(2):81‐8. [DOI: 10.1016/0167-5699(94)90138-4; PUBMED: 8155266] [DOI] [PubMed] [Google Scholar]
Stoll 2003
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Seminars in Perinatology 2003;27(4):293‐301. [PUBMED: 14510320] [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292(19):2357‐65. [DOI: 10.1001/jama.292.19.2357; PUBMED: 15547163] [DOI] [PubMed] [Google Scholar]
Tsai 2014a
- Tsai MH, Chu SM, Lee CW, Hsu JF, Huang HR, Chiang MC, et al. Recurrent late‐onset sepsis in the neonatal intensive care unit: incidence, clinical characteristics and risk factors. Clinical Microbiology and Infection 2014;20(11):O928‐35. [DOI: 10.1111/1469-0691.12661; PUBMED: 24796697] [DOI] [PubMed] [Google Scholar]
Tsai 2014b
- Tsai MH, Chu SM, Hsu JF, Lien R, Huang HR, Chiang MC, et al. Risk factors and outcomes for multidrug‐resistant Gram‐negative bacteremia in the NICU. Pediatrics 2014;133(2):e322‐9. [DOI: 10.1542/peds.2013-1248; PUBMED: 24420803] [DOI] [PubMed] [Google Scholar]
Van den Bruel 2011
- Bruel A, Thompson MJ, Haj‐Hassan T, Stevens R, Moll H, Lakhanpaul M, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011;342:d3082. [PUBMED: 21653621] [DOI] [PubMed] [Google Scholar]
van den Hoogen 2010
- Hoogen A, Gerards LJ, Verboon‐Maciolek MA, Fleer A, Krediet TG. Long‐term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology 2010;97(1):22‐8. [DOI: 10.1159/000226604; PUBMED: 19571584] [DOI] [PubMed] [Google Scholar]
Vergnano 2005
- Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(3):F220‐4. [DOI: 10.1136/adc.2002.022863; PUBMED: 15846011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9‐F14. [DOI: 10.1136/adc.2009.178798; PUBMED: 20876594] [DOI] [PubMed] [Google Scholar]
Verstraete 2015
- Verstraete EH, Blot K, Mahieu L, Vogelaers D, Blot S. Prediction models for neonatal health care‐associated sepsis: a meta‐analysis. Pediatrics 2015;135(4):e1002‐14. [DOI: 10.1542/peds.2014-3226; PUBMED: 25755236] [DOI] [PubMed] [Google Scholar]
Volanakis 2001
- Volanakis JE. Human C‐reactive protein: expression, structure, and function. Molecular Immunology 2001;38(2‐3):189‐97. [PUBMED: 11532280] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI: 10.7326/0003-4819-155-8-201110180-00009; PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
Wilczynski 2007
- Wilczynski NL, Haynes RB. Indexing of Diagnostic Accuracy Studies in MEDLINE and EMBASE. AMIA Annual Symposium Proceedings 2007;11:801‐5. [PUBMED: 18693947] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong J, Dow K, Shah PS, Andrews W, Lee S. Percutaneously placed central venous catheter‐related sepsis in Canadian neonatal intensive care units. American Journal of Perinatology 2012;29(8):629‐34. [DOI: 10.1055/s-0032-1311978; PUBMED: 22566117] [DOI] [PubMed] [Google Scholar]
Zaidi 2005
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital‐acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175‐88. [DOI: 10.1016/S0140-6736(05)71881-X; PUBMED: 15794973] [DOI] [PubMed] [Google Scholar]
Zea‐Vera 2015
- Zea‐Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. Journal of Tropical Pediatrics 2015;61(1):1‐13. [DOI: 10.1093/tropej/fmu079; PUBMED: 25604489] [DOI] [PMC free article] [PubMed] [Google Scholar]