Abstract
Background/Objectives
Major organ- and tissue-specific metabolic rate (Ki) values were initially estimated using in vivo methods, and values reported by Elia1 were subsequently supported by statistical analysis. However, the majority of work to date on this topic has addressed individuals of European descent, whereas population variability in resting energy metabolism has been reported. We aimed to estimate Ki values in South Asian females.
Subjects/Methods
This cross-sectional study recruited 70 healthy young women of South Asian ancestry. Brain and organs were measured using magnetic resonance imaging, skeletal muscle mass by dual-energy X-ray absorptiometry, fat mass by the 4-component model, and whole-body resting energy expenditure by indirect calorimetry. Organ and tissue Ki values were estimated indirectly using regression analysis through the origin. Preliminary analysis suggested overestimation of heart mass, hence the modeling was repeated with a literature-based 22.5% heart mass reduction.
Results
The pattern of derived Ki values across organs and tissues matched that previously estimated in vivo, but the values were systematically lower. However, adjusting for the overestimation of heart mass markedly improved the agreement.
Conclusions
Our results support variability in Ki values among organs and tissues, where some are more metabolically ‘expensive’ than others. Initial findings suggesting lower organ/tissue Ki values in South Asian women were likely influenced by heart mass estimation bias. The question of potential ethnic variability in organ- and tissue-specific energy metabolism requires further investigation.
Introduction
Resting energy expenditure (REE) is an important index of individual and population energy requirements. Whole-body REE (or, equivalently, resting metabolic rate) can be viewed as the sum of specific energy expenditures of individual body organs and tissues, which demonstrate considerable variability in magnitude. In 1992, for example, Elia1 published tissue-specific expenditure, or ‘Ki’ values (kcal/kg/day) for the brain, heart, kidneys, liver, skeletal muscle mass (SMM), adipose tissue (AT), and a residual tissue component, based on arterio-venous catheterization studies. Whereas the energy expenditure of the heart and kidneys was estimated to be 440 kcal/kg/day, the equivalent metabolic rate of muscle at rest was just 13 kcal/kg/day. The relative contribution of different organs and tissues to whole-body lean mass therefore has major implications for total REE.
Measuring organ/tissue Ki values by in vivo arterio-venous methods in human subjects is technically difficult, 2,3 and has not subsequently been reported in the literature. With the use of indirect calorimetry and imaging methods, a number of authors have collected REE and organ size data and utilized indirect statistical models to assess the applicability of Elia’s Ki values in various cohorts of adult men and women.3–7 In 2010, Wang and colleagues developed a stepwise univariable regression method, and reported validation of Elia’s values in young adults recruited in Kiel, Germany.3
Ethnicity and/or geography-related REE variability has long been discussed in the literature, with, for example, the suggestion that tropical and non-tropical peoples differ in average REE.8–11 Evidence also suggests ethnic differences in organ and tissue mass (e.g. smaller organ size in African Americans12 and South Asians13,14 than Europeans). Adjusting for tissue mass has been shown to reduce ethnic differences in REE.12,15–17 However, the method developed by Wang et al.3 to assess organ and tissue-specific resting energy rates has not been replicated in non-European populations. The present study aimed to utilize indirect calorimetry, state-of-the-art body composition methods, and the statistical method developed by Wang and coworkers to assess the applicability of Elia’s Ki values to young women of South Asian ancestry.
Materials and Methods
Participants
Power analysis demonstrated that at an alpha level of 0.05, a sample size of 70 would yield 80% power to detect a correlation of 0.33, which represents a medium effect size.18
We sought to recruit in London, UK women of South Asian (Indian, Pakistani, Bangladeshi, Sri Lankan) ancestry; aged 20-28 years; healthy; nulliparous; term-born; with body mass index (BMI) in the range 17-28 kg/m2. Determination of ethnicity was based on subjects’ self-identification and confirmed by maternal and paternal grandparents also being Indian, Pakistani, Bangladeshi or Sri Lankan. The age range and single sex were chosen to avoid phenotypic variability associated with sexual dimorphism, pubertal growth, and aging.
Individuals born at ≥37 weeks’ gestation were recruited to control for the possibility that variability in body composition and/or metabolism outcomes would have developed in association with pre-term birth. Individuals were excluded if they reported health conditions with the potential to affect growth or metabolism; smoking; or contraindications for magnetic resonance imaging (MRI). The BMI range was set to avoid very underweight or obese women. In general, Asian populations demonstrate lower median BMI compared to non-Asians,19 and increased adiposity or altered metabolism may occur below a BMI of 30 kg/m2,19,20 therefore the upper BMI cutoff for recruitment was set at 28 kg/m2.
Flyers, posters and online advertisements were used in recruitment. Data were collected from March 2015 to May 2016 at UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust, London, UK. Ethical approval was granted by the Camden and Kings Cross NHS Research Ethics Committee of the Health Research Authority. Upon joining the study all participants gave written, informed consent.
Height and weight
Duplicate measures of height were taken to the nearest 0.1cm using a wall-mounted stadiometer (Holtain, Dyfed, UK), with subjects standing barefoot. Weight was taken in duplicate to the nearest 0.01kg.
Fat mass and skeletal muscle mass
Fat mass (FM) was derived using the 4-component model of body composition assessment, as described previously.21 Dual-energy X-ray absorptiometry (DXA; Lunar Prodigy, GE Medical Systems, Madison, WI, USA) was used to quantify SMM. Subjects underwent a scan of approximately 5 minutes’ duration, with the mass of lean, non-fat tissue in the arms and legs provided directly by the DXA system software (Encore, v14.10.022). The basis for using these data as a measure of whole-body SMM, and further details of the method, have been described previously.22
Resting energy expenditure
A Deltatrac II indirect calorimeter (Datex-Engstrom Corp, Helsinki, Finland) was used to measure subjects’ whole-body REE in a post-absorptive state, in a thermoneutral environment. Subjects were not measured at a specified point in their menstrual cycle. However, day-of-cycle at data collection (estimated using subject-reported information on recent menstruation and general cycle length) was found not to be associated with measured REE, and thus rejected as a potential confounder.
Following gas calibration of the metabolic cart, O2 consumption and CO2 production were assessed continuously for 25 minutes as subjects rested, supine, on a hospital cot under a ventilated plastic canopy. Using these data, REE was calculated in kcal/24hr units using the equation of Weir: (3.941 x VO2) + (1.106 x VCO2).23
MRI acquisition
High-resolution 3D imaging of the brain, chest and abdomen was undertaken using a 3T Siemens Magnetom Prisma scanner (Siemens, Erlangen, Germany). The following were acquired: a T1-weighted MPRAGE sequence for brain volume (TR = 2000ms, TE = 2.74ms, flip angle = 8º, voxel size = 1.0 x 1.0 x 1.0mm isotropic, slices = 240, duration = 5min); a T2-weighted, turbo spin echo SPACE sequence for the abdomen (TR = 2000ms, TE = 220ms, flip angle = variable, voxel size = 1.5 x 1.5 x 1.5mm isotropic, slices = 144, duration = 7min); and for the chest, a T2-weighted TrueFISP sequence with breath-hold (TR = 475ms, TE = 1.53ms, flip angle = 47º, voxel size = 1.5 x 1.5 x 4.0mm, gap = 0, slices = 42, duration = 20sec).
Brain and body organ volumes
To derive brain volume (summed cerebral and cerebellar gray and white matter, and subcortical structures including the amygdala, hippocampus, pallidum, thalamus and striatum), T1-weighted MR images were processed and segmented with FreeSurfer (v5.3) and FIRST (FMRIB Software Library v5.0), as described in detail elsewhere.24–26 Specifically, FIRST was used to segment subcortical structures, due to limitations of FreeSurfer for this purpose.
The heart, kidneys and liver were manually segmented by MKS from raw MRI data using the open-source OsiriX DICOM viewer (v8.5).27 Regions of interest were drawn around the organs of interest in contiguous image slices in each subject dataset. The software automatically calculated organ volume by summing the voxels in the regions of interest and multiplying by the slice thickness. Duplicate organ volumes were derived on different days, and averaged. The technical error of measurement28 for the duplicate measures was 1.9% for the heart, 1.1% for the left kidney, 0.7% for the right kidney, and 0.7% for the liver.
Preliminary analysis demonstrated unexpectedly high values for heart mass in comparison with published data.5,29,30 Heart mass/volume measurement bias may vary across autopsy/dissection or in vivo MRI protocols, thus potentially resulting in outcomes which are not comparable across studies. Moreover, preliminary statistical modeling indicated that Elia’s Ki values lay outside the 95% confidence intervals (CIs) associated with our organ/tissue Ki values, which could be due to our overestimation of heart mass relative to Elia’s protocol.
Few studies have compared MRI with autopsy-derived organ volumes, however one such study by Prodhomme and colleagues31 reported MRI-derived heart volume in infants at post-mortem imaging to be 22.5% greater on average than ‘real heart volume’ measured at autopsy. As we could not change our measurement protocol for heart mass, we explored the effect of reducing measured heart mass by 22.5%, to see how it affected the Ki values.
Statistics
Brain, heart, liver and kidney volumes were converted to mass using the following published density values in g/cm3: 1.036 (brain); 1.06 (heart and liver); and 1.05 (kidneys).32
We followed the statistical approach developed by Wang and colleagues3 to assess the applicability of Elia’s published Ki values. First, body mass was treated as the sum of 7 body components:
| (1) |
where M is the mass of the specific body component. In contrast to Wang et al.,3 we used a measure of FM, rather than AT. Previous authors have used FM rather than AT in similar models.5,33 Residual mass comprises tissues including blood, bone, skin, stomach and intestines, connective tissue, and lungs.3,4 It was calculated as body mass minus the sum of masses for brain, heart, liver, kidneys, SMM and FM.
By definition, whole-body REE is the sum of the products of each body component mass and its corresponding Ki value:
| (2) |
where Ki is the specific resting metabolic rate in kcal/kg/day for body component i, and Mi is the mass of the component in kilograms. Hence the resting energy expenditure for organ i is given by
| (2a) |
where the summation omits element i.
Energy expenditure for each organ/tissue was calculated using measured masses and Elia’s Ki values, for example:
| (3) |
Following Wang et al.3, least-squares univariable regression through the origin was then used to estimate Ki:
| (4) |
The estimate of Ki could then be compared with Elia’s value, of e.g. 240 kcal/kg/day for brain. These steps (Equations 3 and 4) were repeated for each organ and tissue.
Statistical analyses were performed using the R language for statistical computing34 in RStudio (v1.1.419) with two-tailed significance tests at an alpha level of 0.05.
Results
Seventy women were recruited, the majority of them students attending universities in and around London. Fifty-one percent of the sample reported Indian ethnicity, 11% Pakistani, 11% Bangladeshi and 11% Sri Lankan; the remainder reported mixed South Asian ancestry.
Two subjects misreported their height and weight at recruitment, resulting in a measured BMI range of 17-30 kg/m2. One subject reported a gestational age of 34 weeks; all other participants were born at 37-42 weeks’ gestation.
Table 1 details descriptive characteristics for the sample. Heart mass was missing for one subject, and REE for two subjects.
Table 1. Sample statistics for age, anthropometry, organ mass and resting energy expenditure in this study, and in previously-reported all-female study samples (South Asian or European cohorts) and a mixed-sex sample.
| This study | All-female studies (South Asian): Mean ± SD or Range | All-female studies (European): Mean ± SD | Mixed-sex study (European): Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Subject characteristic | n | Mean ± SD | Sahni et al., 19941 n = 87 | Singh et al., 20041 n = 204 | Illner et al., 20002 n = 13 | De la Grandmaison et al., 20011 n = 329 | Davis et al., 20152 n = 14 | Wang et al., 20102 n = 43 (f=27) |
| Age (yr) | 70 | 24 ± 2 | 21 - 25 | 21 - 30 | 25 ± 2 | 49 ± 20 | 37 ± 12 | 26 ± 2.0 |
| Weight (kg) | 70 | 57.8 ± 9.3 | 48.1 ± 7.6 | 62.8 ± 9.5 | 58.0 ± 13.2 | 53.9 ± 6.4 | 70.0 ± 11.3 | |
| Height (cm) | 70 | 161.2 ± 6.6 | 170.0 ± 6.0 | 161.0 ± 7.5 | 155.0 ± 7.0 | 174.0 ± 6.0 | ||
| BMI (kg/m2) | 70 | 22.3 ± 3.5 | 22.1 ± 2.5 | 22.5 ± 4.5 | 22.4 ± 2.6 | 23.0 ± 2.7 | ||
| Fat mass (kg) | 70 | 20.3 ± 6.7 | 19.2 ± 6.0 | 16.6 ± 6.7 | ||||
| Skeletal muscle mass (kg) | 70 | 15.3 ± 2.2 | 17.9 ± 2.5 | 25.0 ± 5.9 | ||||
| Brain (kg) | 70 | 1.08 ± 0.08 | 1.21 ± 0.11 | 1.5 ± 0.1 | 1.33 ± 0.11 | |||
| Heart (kg) | 69 | 0.53 ± 0.09 | 0.23 ± 0.04 | 0.24 ± 0.05 | 0.3 ± 0.0 | 0.31 ± 0.08 | 0.44 ± 0.04 | 0.31 ± 0.09 |
| Liver (kg) | 70 | 1.21 ± 0.21 | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.48 ± 0.36 | 1.3 ± 0.23 | 1.35 ± 0.23 | |
| Kidneys (kg) | 70 | 0.29 ± 0.05 | 0.26 ± 0.05 | 0.2 ± 0.0 | 0.27 ± 0.08 | 0.25 ± 0.04 | 0.28 ± 0.06 | |
| Residual mass (kg) | 69 | 19.2 ± 2.1 | 19.7 ± 2.1 | 22.8 ± 3.9 | ||||
| Resting energy expenditure (kcal/24hr) | 68 | 1337 ± 184 | 1372 ± 163 | 1547 ± 241 | ||||
Autopsy study
MRI study
Table 1 also presents previously published average organ masses for five all-female cohorts and one mixed-sex cohort for comparison with the current study. Our average liver mass was smaller, and heart mass larger, than the results of Illner et al.,5 Grandmaison et al.,28 and Davis et al.,29 whilst kidney mass was similar across studies. Our average heart mass was approximately double that reported in two female South Asian cohorts35,36 and nearly double that of the mixed-sex cohort of Wang et al.3 Age, height, weight, FM and SMM differed somewhat across the cohorts (in particular the mixed-sex cohort3, as would be expected), although BMI averages were similar.
Table 2 shows organ/tissue Ki value estimates and their associated 95% CIs.
Table 2. Estimates of organ- and tissue-specific metabolic rate (Ki) values (kcal/kg/day), and effect of heart mass adjustment.
| Organ/tissue | Ki value (95% CI) | Ki value (95% CI) (heart mass less 22.5%, following Prodhomme et al.) | Ki value (Elia) |
|---|---|---|---|
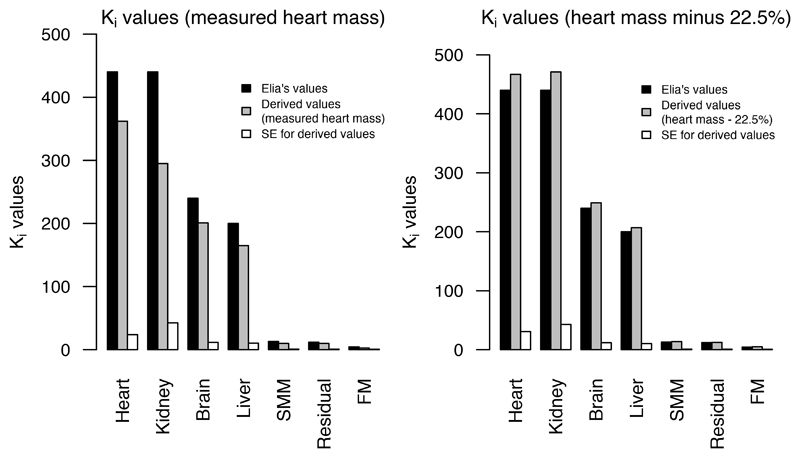
| Heart | 362 (315, 409) | 467 (406, 528) | 440 |
| Kidneys | 295 (210, 380) | 471 (385, 556) | 440 |
| Brain | 201 (178, 224) | 249 (226, 273) | 240 |
| Liver | 165 (144, 185) | 207 (186, 228) | 200 |
| Skeletal muscle mass | 10 (8.7, 12.0) | 13.7 (12.1, 15.4) | 13 |
| Fat mass | 2.6 (1.4, 3.8) | 5.0 (3.8, 6.2) | 4.5 |
| Residual mass | 10 (8.5, 11.1) | 12.5 (11.2, 13.8) | 12 |
Table 2 also provides results from the sensitivity analysis, where measured heart mass was reduced by 22.5% (ref. 31). With this reduction, mean heart mass was 0.41 ± 0.07 kg. Our Ki values better matched Elia’s when using adjusted heart mass, as shown in Figure 1.
Figure 1.
Ki values (kcal/kg/day) derived using measured heart mass and heart mass minus 22.5%, compared with Elia’s values
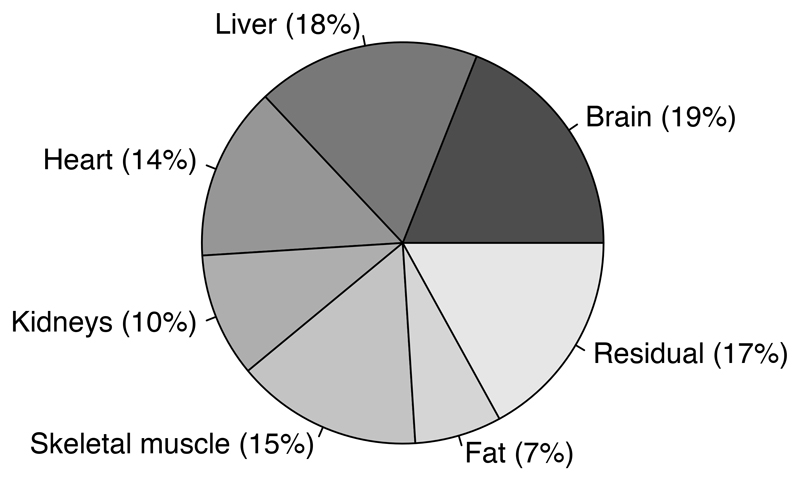
Finally, Figure 2 is a pie chart demonstrating the percentage contribution of each of the 7 organs and tissues to total REE, following calculation of the products of Ki and Mi. The contribution of brain and liver to REE is roughly double that of fat and the kidneys, which demonstrate the smallest contribution of the 7 body components.
Figure 2.
Percentage contribution of organs and tissues to total resting energy expenditure (kcal/day) in females, calculated using Ki values reported by Elia and masses measured in the current study, including adjusted heart mass
Discussion
This study has extended the analysis by Wang and colleagues3 to test the applicability of Elia’s1 Ki values to young South Asian women in the UK. Our Ki values demonstrated a similar pattern to Elia’s, in that they ranked the organs and tissues in the same order (i.e. values for heart, kidney, brain and liver were larger than those for SMM, which was in turn larger than FM). However, our values were significantly lower.
Of the internal organs measured for this study, the heart was the most difficult to extract from MR images using manual segmentation due to relatively ambiguous boundaries with surrounding tissues. As this may have led to over-estimation of MRI heart volume, we tried reducing our estimates by 22.5% based on previously published data.31 The latter study involved infants at post mortem and hence was not ideally matched to the present study of adults. However, it does provide clear evidence of the bias in heart volume based on MRI.
Our average heart mass was greater than that reported in previous female cohorts of both South Asian and European ethnicity, and also greater than that reported in a mixed-sex cohort (see Table 1). These comparisons would suggest that heart mass in our cohort is double that reported for South Asian women of similar age, and nearly double that for males and females of European origin (who, as noted above, have been suggested to in fact have larger organ size on average than South Asians).13,14 At the same time, the comparison studies demonstrated larger values than ours for the brain and the liver, and similar values for the kidneys.3,5,29,30,36
Of course, comparisons among these studies also have limitations. The studies in South Asian women (both from the Chandigarh region of Northwest India) rely on autopsy data, which may be particularly difficult to compare with MRI studies due to differences in technique and protocol. With respect to the heart, MRI and ‘real’ volume outcomes may differ due to the presence of blood in the ventricles.31,37 Comparing MRI studies to one another may similarly be difficult when variation exists in sample size, body size/composition, and the software and protocols utilized to extract organ mass. Our average measured heart mass, for example, was 77% larger than that reported by Illner et al.5 and Wang et al.3, but just 21% larger than that reported by Davis and colleagues.30 Finally, it is problematic to compare body composition between an all-female sample and a mixed-sex sample, although this was done here with a focus on heart mass to consider the possibility of overestimation.
Assuming that heart mass was overestimated using our protocol, we explored whether reducing heart mass by 22.5% (ref. 31) altered the results of our Ki value analysis. Indeed, repeating Wang et al.’s analysis following heart mass adjustment yielded Ki values very similar to Elia’s published values.
Reports from the 1930s indicated ethnic and/or geographical variability in REE,9,38 for example Tamil women in South India having lower average metabolism than white American women.39 Individuals of Japanese, Filipino and Bengalese ethnicity demonstrated negative deviations in REE, as assessed against contemporary normative standards, of 6.4%, 8.3% and 9%, respectively.40 Henry and Rees11 concluded that individuals in tropical regions could be characterized generally as having lower REE than individuals in temperate regions.
However, subsequent studies suggested that differences in body composition (i.e. variation in the relative proportion of high-metabolic rate organs and lower-metabolic rate SMM and FM) could explain ethnic/geographical REE differences.12,16,40–43 Whereas ethnic differences in REE tend to persist when controlling for overall weight or BMI, they are abolished when fat-free mass, which includes SMM and organs, is taken into account. Differences in average REE between Indian females and Australian females of European descent were rendered non-significant with adjustment for both fat-free mass and FM.16
It is possible that organ/tissue Ki values vary by ethnic ancestry and/or geography for reasons unrelated to body composition. Our initial results suggest that Ki values, or the metabolic ‘cost’ of six organs and tissues and a residual component, might indeed be different in South Asian women compared to other cohorts. It is more likely, however, that bias in the estimation of heart mass, or potentially another organ or tissue, explains inconsistencies between our estimated values and Elia’s.
In conclusion, we cannot definitively determine whether our Ki values for South Asian women are inconsistent with those reported by Elia, as we have shown that potential error in the measurement of organ mass may impact results when using the method of Wang and coworkers.3 Despite the difficulties of in vivo Ki value assessment, such methods may be necessary to more firmly elucidate ethnic variability in tissue-specific metabolic rates, if it exists.
The current results suggest that the heart may be the least reproducible organ to determine with MRI using manual measurements. Software designed to assist segmentation and/or the use of advanced imaging methods (e.g. ref. 44) may increase accuracy in heart volume/mass estimation, even as investigators must remain cautious when comparing MRI with autopsy-derived measurements.
Nevertheless, our results support the rankings in Elia’s reported Ki values, irrespective of the heart mass variable used: organs and tissues appear variably ‘expensive’, with the brain and visceral organs demonstrating higher specific metabolic rates than SMM, FM or residual mass. High-cost organs such as the heart and kidney have a specific REE more than 30 times greater than an equivalent amount of muscle tissue at rest (although it has not been sufficiently appreciated that skeletal muscle comprises a greater proportion of total REE than the kidneys when mass is taken into account; see Figure 2). Variability in the size of organ/tissue components therefore contributes to variability in energy expenditure associated with fat-free mass, which in turn accounts for much of the variance in whole-body REE.45 This suggests that patterns of growth and development in early life may influence adult REE, as shown previously in an elderly European population.46
Acknowledgements
This study was supported in part by a Dissertation Fieldwork Grant awarded by the Wenner-Gren Foundation to MKS.
Funding: OJA was funded by a National Institute for Health Research (NIHR) Clinician Scientist Fellowship award (NIHR-CS-012-002), and supported by Great Ormond Street Hospital (GOSH) Children’s Charity and NIHR GOSH Biomedical Research Centre. SE was supported by Great Ormond Street Hospital Children’s Charity and NIHR GOSH Biomedical Research Centre. TJC was funded by Medical Research Council grant MR/M012069/1. This article presents independent research funded by the NIHR and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. None of the funders were involved in the design or interpretation of the results.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, editors. Energy metabolism: tissue determinants and cellular corollaries. Raven Press; New York, NY: 1992. pp. 61–80. [Google Scholar]
- 2.Elia M. The inter-organ flux of substrates in fed and fasted man, as indicated by arterio-venous balance studies. Nutr Res Rev. 1991;4:3–31. doi: 10.1079/NRR19910005. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369–1377. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 5.Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Müller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab. 2000;278:E308–E315. doi: 10.1152/ajpendo.2000.278.2.E308. [DOI] [PubMed] [Google Scholar]
- 6.Bosy-Westphal A, Reinecke U, Schlörke T, Illner K, Kutzner D, Heller M, et al. Effect of organ and tissue masses on resting energy expenditure in underweight, normal weight and obese adults. Int J Obes. 2004;28:72–79. doi: 10.1038/sj.ijo.0802526. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Heller M, Later W, et al. Evaluation of specific metabolic rates of major organs and tissues: comparison between men and women. Am J Hum Biol. 2011;23:333–338. doi: 10.1002/ajhb.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Almeida AO. L’emission de chaleur. Le metabolisme basal et le metabolisme minimum de l’homme noir tropical. J Physiol Pathol Gen. 1921;18:958–964. [Google Scholar]
- 9.Benedict FG. The racial elements in human metabolism. Am J Phys Anthropol. 1932;16:463–473. [Google Scholar]
- 10.Shetty PS, Soares MJ, Sheela ML. Basal Metabolic Rates of South Indian Males. FAO; Bangalore, India: 1988. [Google Scholar]
- 11.Henry CJK, Rees DG. New predictive equations for the estimation of basal metabolic rate in tropical peoples. Eur J Clin Nutr. 1991;45:177–185. [PubMed] [Google Scholar]
- 12.Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African Americans than in white adults. Am J Clin Nutr. 2006;83:1062–1067. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohli A, Aggarwal N. Normal organ weights in Indian adults. Medico Legal Update Int J. 2006;6:49–52. [Google Scholar]
- 14.Wells JCK, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS. The elevated susceptibility to diabetes in India: an evolutionary perspective. Front Pub Health. 2016;4:145. doi: 10.3389/fpubh.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S. ICMR Special Report No. 43. New Delhi: 1962. Studies in energy metabolism. [Google Scholar]
- 16.Soares MJ, Piers LS, O’Dea K, Shetty PS. No evidence for an ethnic influence on basal metabolism: an examination of data from India and Australia. Br J Nutr. 1998;79:333–341. doi: 10.1079/bjn19980057. [DOI] [PubMed] [Google Scholar]
- 17.Javed F, He Q, Davidson LE, Thornton JC, Albu J, Boxt L, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr. 2010;91:907–912. doi: 10.3945/ajcn.2009.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Unni US, Ramakrishnan G, Raj T, Kishore RP, Thomas T, Vaz M, et al. Muscle mass and functional correlates of insulin sensitivity in lean young Indian men. Eur J Clin Nutr. 2009;63:1206–1212. doi: 10.1038/ejcn.2009.32. [DOI] [PubMed] [Google Scholar]
- 21.Wells JCK, Haroun D, Williams JE, Nicholls D, Darch T, Eaton S, et al. Body composition in young female eating-disorder patients with severe weight loss and controls: evidence from the four-component model and evaluation of DXA. Eur J Clin Nutr. 2015;69:1330–1335. doi: 10.1038/ejcn.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 23.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perini TA, de Oliveira GL, dos Santos Ornellas J, de Oliveira FP. Technical error of measurement in anthropometry. Rev Bras Med Esporte. 2005;11:86–90. [Google Scholar]
- 29.De la Grandmaison GL, Clairand I, Durigon M. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic Sci Int. 2001;119:149–154. doi: 10.1016/s0379-0738(00)00401-1. [DOI] [PubMed] [Google Scholar]
- 30.Davis ML, Stitzel JD, Gayzik FS. Thoracoabdominal organ volumes for small women. Traffic Injury Prevention. 2015;16:611–617. doi: 10.1080/15389588.2014.988787. [DOI] [PubMed] [Google Scholar]
- 31.Prodhomme O, Seguret F, Martrille L, Pidoux O, Cambonie G, Couture A, et al. Organ volume measurements: comparison between MRI and autopsy findings in infants following sudden unexpected death. Arch Dis Child Fetal Neonatal Ed. 2012;97:F434–F438. doi: 10.1136/fetalneonatal-2011-301309. [DOI] [PubMed] [Google Scholar]
- 32.Duck FA. Physical Properties of Tissues. Academic; New York, NY: 1990. [Google Scholar]
- 33.Geisler C, Hübers M, Granert O, Müller MJ. Contribution of structural brain phenotypes to the variance in resting energy expenditure in healthy Caucasian subjects. J Appl Physiol. 2017 doi: 10.1152/japplphysiol.00690.2017. e-pub ahead of print 21 September 2017. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2016. https://www.R-project.org/ [Google Scholar]
- 35.Sahni D, Jit I, Sanjeev Weights of the heart in Northwest Indian adults. Am J Hum Biol. 1994;6:419–423. doi: 10.1002/ajhb.1310060402. [DOI] [PubMed] [Google Scholar]
- 36.Singh D, Bansal YS, Sreenivas M, Pandey AN, Tyagi S. Weights of human organs at autopsy in Chandigarh zone of Northwest India. J Indian Acad Forens Med. 2004;26:97–99. [Google Scholar]
- 37.Thayyil S, Schievano S, Robertson NJ, Jones R, Chitty LS, Sebire NJ, et al. A semi-automated method for non-invasive internal organ weight estimation by post-mortem magnetic resonance imaging in fetuses, newborns and children. Eur J Radiol. 2009;72:321–326. doi: 10.1016/j.ejrad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan BT, Vareed C. Basal metabolism of young college students, men and women in Madras. Indian J Med Res. 1932;20:831–858. [Google Scholar]
- 39.Mason ED, Benedict FG. The basal metabolism of South Indian women. Indian J Med Res. 1931;19:75–98. [Google Scholar]
- 40.Dubois EF. Basal metabolic rate in health and disease. Lea and Febiger; Philadelphia, PA: 1936. [Google Scholar]
- 41.Ferro-Luzzi A, Petracchi C, Kuriyan R, Kurpad AV. Basal metabolism of weight-stable chronically undernourished men and women: lack of metabolic adaptation and ethnic differences. Am J Clin Nutr. 1997;66:1086–1093. doi: 10.1093/ajcn/66.5.1086. [DOI] [PubMed] [Google Scholar]
- 42.Wouters-Adriaens MPE, Westerterp KR. Low resting energy expenditure in Asians can be attributed to body composition. Obesity. 2008;16:2212–2216. doi: 10.1038/oby.2008.343. [DOI] [PubMed] [Google Scholar]
- 43.Song LLT, Venkataraman K, Gluckman P, Chong YS, Chee MWL, Khoo CM, et al. Smaller size of high metabolic rate organs explains lower resting energy expenditure in Asian-Indian than Chinese men. Int J Obes. 2016;40:633–638. doi: 10.1038/ijo.2015.233. [DOI] [PubMed] [Google Scholar]
- 44.Bustamante M, Gupta V, Forsberg D, Carlhäll C-J, Engvall J, Ebbers T. Automated multi-atlas segmentation of cardiac 4D flow MRI. Med Image Anal. 2018 doi: 10.1016/j.media.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Müller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3:113–122. doi: 10.1046/j.1467-789x.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 46.Kensara OA, Wooton SA, Phillips DIW, Patel M, Hoffman DJ, Jackson AA, et al. Substrate-energy metabolism and metabolic risk factors for cardiovascular disease in relation to fetal growth and adult body composition. Am J Physiol Endocrinol Metab. 2006;291:E365–E371. doi: 10.1152/ajpendo.00599.2005. [DOI] [PubMed] [Google Scholar]