Abstract
Introduction:
The purpose of this systematic review is to provide supporting evidence for the clinical practice guideline for the treatment of obstructive sleep apnea (OSA) in adults using positive airway pressure (PAP).
Methods:
The American Academy of Sleep Medicine commissioned a task force of experts in sleep medicine. A systematic review was conducted to identify studies that compared the use of PAP with no treatment as well as studies that compared different PAP modalities. Meta-analyses were performed to determine the clinical significance of using PAP in several modalities (ie, continuous PAP, auto-adjusting PAP, and bilevel PAP), to treat OSA in adults. In addition, meta-analyses were performed to determine the clinical significance of using an in-laboratory versus ambulatory strategy for the initiation of PAP, educational and behavioral interventions, telemonitoring, humidification, different mask interfaces, and flexible or modified pressure profile PAP in conjunction with PAP to treat OSA in adults. Finally, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used to assess the evidence for making recommendations.
Results:
The literature search resulted in 336 studies that met inclusion criteria; 184 studies provided data suitable for meta-analyses. The data demonstrated that PAP compared to no treatment results in a clinically significant reduction in disease severity, sleepiness, blood pressure, and motor vehicle accidents, and improvement in sleep-related quality of life in adults with OSA. In addition, the initiation of PAP in the home demonstrated equivalent effects on patient outcomes when compared to an in-laboratory titration approach. The data also demonstrated that the use of auto-adjusting or bilevel PAP did not result in clinically significant differences in patient outcomes compared with standard continuous PAP. Furthermore, data demonstrated a clinically significant improvement in PAP adherence with the use of educational, behavioral, troubleshooting, and telemonitoring interventions. Systematic reviews for specific PAP delivery method were also performed and suggested that nasal interfaces compared to oronasal interfaces have improved adherence and slightly greater reductions in OSA severity, heated humidification compared to no humidification reduces some continuous PAP-related side effects, and pressure profile PAP did not result in clinically significant differences in patient outcomes compared with standard continuous PAP.
Citation:
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301–334.
Keywords: obstructive sleep apnea, OSA, positive airway pressure, PAP
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder affecting 26% of the United States adult population1 and is associated with adverse health outcomes including excessive sleepiness, impaired quality of life (QOL), increased motor vehicle crashes (MVC), and cardiovascular events.2,3 Despite the advent of an array of treatment options, positive airway pressure (PAP) remains the primary treatment modality for OSA since the approach was introduced in 1981.4 Since the publication of the previous American Academy of Sleep Medicine (AASM) PAP practice parameters,5–7 the scientific literature on the effects of PAP on clinical outcomes in adults with OSA has grown substantially. Research on improving PAP adherence, a major barrier to maximizing the effectiveness of PAP therapy, and advancements in device technology to improve patient comfort have continued to evolve. The objective of this systematic review is to examine the clinical utility of PAP to treat OSA in adults given these recent advancements in technology and knowledge. The AASM commissioned a task force (TF) of content experts to conduct this review. This review is intended to provide supporting evidence for the new clinical practice guideline on the use of PAP for the treatment of OSA in adults,8 and to replace the previously published AASM systematic review on the use of PAP treatment for sleep-related breathing disorders.9 This review addresses the initial management of patients with OSA without major medical comorbidities. This review does not address the initiation and management of PAP in patients with obesity hypoventilation syndrome, sleep-related hypoventilation, or those with concurrent forms of OSA and central sleep apnea. Prior reviews provided evidence in support of previously published AASM practice parameters regarding the efficacy of various modes of PAP therapy for central sleep apnea and hypoventilation syndromes,10,11 and are not considered in the scope of this review.
BACKGROUND
OSA is a common sleep disorder affecting 26% of adults, with 10% estimated to have moderate to severe disease.1 Untreated OSA is associated with multiple adverse health outcomes including daytime sleepiness and decreased QOL as well as increased risk of MVC, systemic hypertension, diabetes, coronary artery disease, stroke, atrial fibrillation, congestive heart failure, and mortality.1 OSA is defined by repetitive upper airway collapse and arousals from sleep, traditionally quantified with testing during sleep by the apnea-hypopnea index (AHI), respiratory disturbance index (RDI) or respiratory event index (REI). Common risk factors for OSA include obesity, advanced age, male gender, post-menopausal status in women, race, and craniofacial dysmorphisms.1 Obesity is a prominent risk factor for OSA as demonstrated by reductions in OSA severity with weight loss interventions12,13 and the concurrent rise in the prevalence of OSA as obesity rates have risen.1 Specifically, recent data from the Wisconsin Sleep Cohort estimate that 17% of men and 9% of women aged 50 to 70 years have at least moderate to severe OSA.1 Furthermore, individuals of African American, Asian, or Hispanic race/ethnicity are at higher risk for OSA compared with similarly-aged Caucasians.14,15
An important and well-recognized direct consequence of OSA is excessive daytime sleepiness, which can interfere with productivity both at home and in the workplace, and has been associated with an increased risk of MVC.16 OSA has been associated with QOL impairment, based upon global questionnaires like the Short Form of the Medical Outcomes Survey (SF-36), as well as those more specific to sleep-related domains, such as the Functional Outcomes of Sleep Questionnaire (FOSQ), Quebec Sleep Questionnaire (QSQ), and the Calgary Sleep Apnea Quality of Life Index (SAQLI). Although results have varied, studies have also found associations between OSA and impaired cognition, with more consistent deficits in executive function and vigilance.17
OSA is also associated with a number of systemic disorders. It is strongly linked with cardiovascular diseases such as congestive heart failure, stroke, atrial fibrillation and ischemic heart disease, and may have a causal role in the development of systemic hypertension.18 Although the evidence is conflicting and sometimes confounded by obesity, OSA has been shown to impair insulin sensitivity and predict incident type 2 diabetes mellitus (T2DM).19
The pathogenic role of upper airway collapse was initially described in the 1960's,20,21 and for more than a decade tracheostomy was the only effective treatment. PAP has become the primary therapy used to treat adult OSA across the spectrum of disease severity. Continuous positive airway pressure (CPAP) therapy as a treatment modality was first described in 1981.4 This form of treatment applied a constant pressure throughout the respiratory cycle to splint the airway open. Subsequently, bilevel PAP (BPAP), a modality which delivers a higher inspiratory PAP (IPAP) relative to the expiratory PAP (EPAP) was also found to be effective in the treatment of OSA. A theoretical advantage of BPAP was that a lower EPAP could be applied that would increase tolerance to PAP treatment of OSA. With advancements in technology, flow sensors were integrated into PAP devices to assess the presence of obstructive breathing events. Computer algorithms were then developed and incorporated into CPAP devices to dynamically increase CPAP when obstructive breathing events were detected, and to periodically reduce the delivered pressure when no events were detected for some period of time, ie auto-adjusting PAP (APAP). Auto-adjusting computer algorithms were subsequently developed for BPAP (auto-BPAP). APAP in the ambulatory setting is increasingly being utilized as an alternative to traditional in-laboratory PAP titrations for the initiation and continued treatment of OSA.
Regardless of these technological advancements, the continuous application of PAP during sleep when the airway is vulnerable to collapse is critical. To maximize clinical benefit, most clinicians recommend utilization of PAP therapy for the entire sleeping period, though lesser utilization may have benefits for some individuals. Although PAP use for at least 4 hours during sleep per a 24-hour period is commonly used to clinically define minimal acceptable levels of adherence, current evidence suggests a continuous dose-response relationship between hours of use and therapeutic response.22,23
Given challenges in optimizing PAP adherence, approaches to making PAP more comfortable are desirable. Technological advances in PAP therapy have occurred over time to promote patient comfort and potentially improve adherence to treatment. A variety of mask interfaces available continues to evolve with design advances in nasal masks, nasal pillows, full face masks, and oral masks. This greater variety of mask configurations has allowed for better individualization of the interface to a patient to reduce leak and improve comfort. PAP manufacturers have also addressed the common side effect of nasal dryness by designing in-line humidifiers, which were first passive but now include heated systems. These have become standard to include with PAP therapy in many markets. The current generation of PAP devices also integrates modified pressure profiles and is offered as a standard feature. This option transiently lowers the treatment pressure during expiration, with some systems also modifying the inspiratory pressure profile, to increase patient comfort without compromising airway patency. Prior to the development of modified pressure profile technologies, BPAP was and continues to be used for similar reasons.
Given evidence that patients overestimate their usage of PAP, objective adherence monitoring has been another major advance in PAP technology. Initially, based on a meter built into the machine,24 the development of removable cards to record PAP usage increased the ability of providers to track patient adherence. Internet-based applications combined with built-in modems now allow for remote monitoring of usage. The adoption of adherence requirements for insurance coverage by many payors has made objective adherence monitoring a standard of care in the United States.
Because device improvements have only had a modest impact on adherence,25 more attention is being given to educational and behavioral interventions to improve patient adherence. Observational data have demonstrated that increased knowledge of OSA and its long-term impacts, as well as the beneficial effects of PAP predict adherence, raising interest in educational interventions.26 Similar data suggest that decisions about PAP usage are made very early after treatment initiation suggesting any such intervention needs to be delivered early to maximize effect.27 Based on efficacy in changing behaviors in other conditions and settings such as sleep behaviors in insomnia, abstinence in addiction disorders, and medication adherence in chronic medical diseases, there has been interest in developing behavioral interventions such as cognitive behavioral therapy or motivational enhancement to improve PAP adherence. A major challenge, however, has been developing an intervention intensive enough to be effective, but not so expensive as to reduce feasibility in clinical practice. In this milieu, the use of telemonitoring of adherence has gained substantial interest. By identifying those patients who are having the greatest difficulties in real-time, interventions can be individually tailored and quickly deployed to those who will benefit the most.
Finally, the overall concerns of rising healthcare costs have impacted the delivery of OSA care. More patients are being diagnosed based on home sleep apnea tests and in this setting the use of APAP has the potential to allow for rapid initiation of treatment at lower costs in the uncomplicated patient. These devices detect flow and/or impedance and based on manufacturer-specific algorithms, adjust pressure in real-time in an effort to deliver the lowest pressure needed to maintain airway patency.28,29 While originally developed to improve comfort, the technology has increasingly been utilized as an alternative to in-laboratory PAP titration. Long-term use of APAP has the potential benefit of obviating adjustments in pressure settings over time in response to changes in OSA severity. However, as the algorithms are designed to continually lower pressure until respiratory events return, there is the potential for incomplete treatment of OSA.29
With these key issues in mind, this systematic review provides a comprehensive update of the latest evidence for the use of PAP to treat adult patients with OSA.
METHODS
Expert Task Force
The AASM commissioned a TF composed of both board-certified sleep medicine specialists and experts with proficiency in the use of PAP in adults with OSA to develop this systematic review. The TF was required to disclose all potential conflicts of interest (COI) per the AASM's COI policy prior to being appointed to the TF, and throughout the research and writing of this paper. In accordance with the AASM's COI policy, TF members with a Level 1 conflict were not allowed to participate. TF members with a Level 2 conflict were required to recuse themselves from any related discussion or writing responsibilities. All relevant COI are listed in the disclosure statement.
PICO Questions
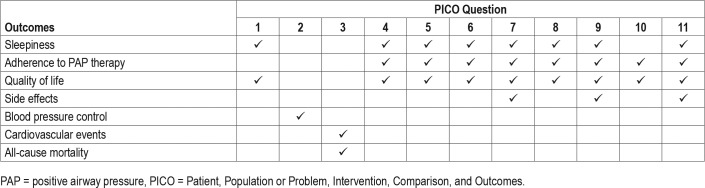
PICO (Patient, Population or Prob lem, Intervention, Comparison, and Outcomes) questions were developed based on a review of the existing AASM practice parameters on the use of PAP and a review of systematic reviews, meta-analyses, and guidelines published since 2005. The AASM Board of Directors approved the final list of PICO questions presented in Table 1 before the literature search was performed. To develop the PICO questions, the TF identified commonly used PAP interventions and alternative approaches and strategies for the implementation of PAP in the treatment of adults with OSA. The TF then developed a list of patient-oriented, clinically relevant outcomes to determine whether CPAP, compared to no treatment, alternative PAP modes, and concurrent strategies designed to enhance acceptance and use of PAP for OSA treatment should be recommended for clinical practice. The TF rated the relative importance of each outcome to determine which outcomes were critical for decision-making. A summary of these “critical” outcomes by PICO is presented in Table 2. Several additional clinical outcomes considered of importance for the clinical management of OSA and related comorbidities were also examined including the AHI/RDI/REI, hemoglobin A1c, fasting glucose, blood pressure, left ventricular ejection fraction (LVEF), neurocognitive function, MVC, hospitalizations, cardiovascular events, and mortality.
Table 1.
PICO questions.
Table 2.
Critical outcomes by PICO question.
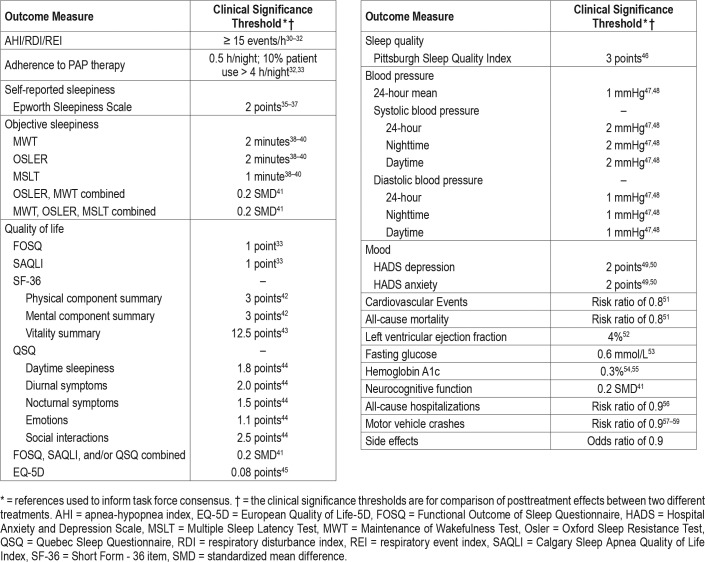
The TF set a clinical significance threshold for each outcome to determine whether the mean changes in the outcomes assessed were clinically significant. The clinical significance threshold was defined as the minimum level of improvement in the outcome of interest that would be considered clinically important to clinicians and patients. Outcomes which met the clinical significance threshold but were not statistically significant resulted in reductions in the grading of the evidence quality and reduced the strength of the recommendation. A summary of the clinical significance thresholds for the clinical outcome measures is presented in Table 3. Clinical significance thresholds were determined based on a TF literature review of commonly used thresholds. Where no clearly established threshold values could be determined, the TF used the literature review, clinical judgment, and experience to establish a clinical significance threshold based on consensus.
Table 3.
Summary of clinical significance thresholds for outcome measures.
Literature Searches, Evidence Review and Data Extraction
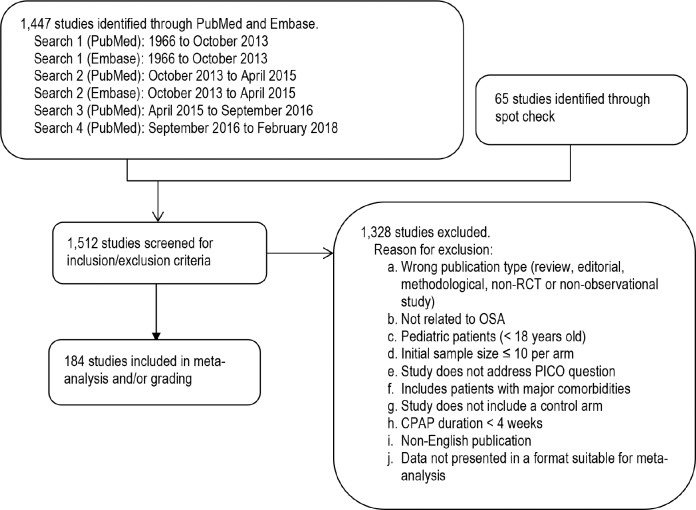
The TF performed an extensive review of the scientific literature to retrieve articles that addressed the PICO questions. Separate literature searches were performed by the AASM research staff for each PICO question using the PubMed and Embase databases (see Figure 1). The key terms, search limits, and inclusion/exclusion criteria specified by the TF are detailed in the supplemental material. Randomized controlled trials (RCTs) and observational studies that were cited in the prior AASM PAP practice parameters5,7 were included for data analysis only if they met the current inclusion criteria.
Figure 1. Evidence base flow diagram.
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, PICO = Patient, Population or Problem, Intervention, Comparison, and Outcomes, RCT = randomized controlled trial.
The initial literature search of English publications in PubMed and Embase was performed in October 2013 and was limited to RCTs. A second literature search was performed in April 2015 using broader search terms to identify additional articles in PubMed and Embase from October 2013 to April 2015 (see supplemental material). This search was conducted using broader search terms in an effort to capture more relevant articles than the initial PICO-targeted searches. In addition, for PICO questions 1 (MVC only), 3, and 8, where the evidence based on RCTs was low or not available, the TF also searched for observational studies with both an intervention and control group relevant to the specific PICO. A third literature search limited to PubMed was performed in September 2016 to identify studies that were published since the second literature search to update the body of evidence for the review. A fourth search also limited to PubMed was conducted in February 2018 to update the evidence prior to publication. These searches identified a total of 1,447 unique articles. Lastly, the TF reviewed previously published guidelines, systematic reviews, and meta-analyses to spot check for references that may have been missed during the prior searches. The TF identified 65 additional articles for a total of 1,512 articles that were screened for inclusion/exclusion in the guideline.
The TF set inclusion and exclusion criteria, which are presented in the supplemental material and summarized in Figure 1. All abstracts were reviewed based on inclusion/ exclusion criteria by two TF members. Any discrepancies between the reviewers were discussed and resolved by the Chair. A total of 184 studies were determined to be suitable for meta-analysis and/or grading.
Meta-Analysis
Meta-analysis was performed on outcomes of interest, when possible, for each PICO question. Comparisons of CPAP to no treatment and the comparative efficacy of alternative types of PAP devices used to treat OSA in adult patients were performed. For the purposes of our analyses, PAP devices were categorized into the following categories: CPAP, APAP, BPAP, and modified pressure profile PAP. Mask interfaces were categorized as nasal PAP, nasal pillow PAP, oral PAP, and oronasal PAP. Education and behavioral interventions were categorized as education, education plus troubleshooting, and behavioral interventions. Telemonitoring was defined as the remote monitoring of PAP parameters such as PAP usage, residual OSA severity, excessive mask leaks, and PAP settings, during treatment initiation and follow-up. Treatment delivery strategies were categorized as home APAP-initiated (ambulatory) or in-laboratory initiated CPAP and treatment with APAP or fixed CPAP. There was insufficient evidence to perform meta-analyses for some outcome measures and comparisons within some of the PICO questions, including side effects data.
Meta-analysis was performed using Review Manager 5.3 software by pooling data across studies for each outcome measure. Posttreatment data were used for meta-analysis, except where change values were determined to be more meaningful to the reader (eg, blood pressure [BP], LVEF, neurocognitive outcomes, and driving proficiency). Standardized mean differences (SMD) were used for outcomes when the TF determined interpretation of effect size would be more clinically meaningful than posttreatment or change values (eg, combined Maintenance of Wakefulness Test [MWT] and Oxford Sleep Resistance Test [OSLER], combined FOSQ, QSQ, and SAQLI, neurocognitive measures, and driving simulator outcomes). The pooled results for each continuous outcome measure are expressed as the mean difference or standardized mean difference between the intervention and comparator. The pooled results for dichotomous outcome measures are expressed as the odds ratio or risk ratio between the intervention and comparator. All analyses were performed using a random effects model with results displayed as a forest plot. Interpretation of clinical significance for the outcomes of interest was conducted by comparing the mean difference in effect of each treatment approach to the clinical significance threshold (see Table 3).
GRADE Assessment for Developing Recommendations
The assessment of evidence quality was performed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process.60 The TF assessed the following four components to determine the direction and strength of a recommendation: quality of evidence, balance of beneficial and harmful effects, patient values and preferences, and resource use, as described below.
Quality of evidence – based on an assessment of the overall risk of bias (randomization, blinding, allocation concealment, selective reporting), imprecision (95% confidence interval relative to the clinical significance threshold), inconsistency (I2 cutoff of 50%), indirectness (study population), and risk of publication bias (funding sources), the TF determined their overall confidence that the estimated effect found in the body of evidence was representative of the true treatment effect that typical adult patients with OSA would see. The overall quality of the evidence was based on outcomes that the TF deemed critical for decision making.
Benefits versus harms – based on the meta-analysis (if data were available), analysis of any harms/side effects reported within the accepted literature, and the clinical expertise of the TF, the TF determined if the beneficial outcomes of the intervention outweighed any harmful side effects.
Patient values and preferences – based on the clinical expertise of the TF members and any data published on the topic relevant to patient preferences, the TF determined if patient values and preferences would be generally consistent across the majority of patients, and if patients would use the intervention based on the relative harms and benefits identified.
Resource use – based on the clinical expertise of the TF members, the TF judged resource use to be important for determining whether to recommend the use of a specific PAP device type or approach to patient care over another for the treatment of adults with OSA.
A summary of each GRADE domain is provided after the detailed evidence review. As this guideline focuses on providing recommendations on the indications for PAP therapy in adult patients with OSA, rather than the use of specific components or accessories of the PAP device, recommendations for PICOs 7–9 (Table 1) were not included. A summary of the systematic review and meta-analyses of the evidence for these PICO questions can be found in the “Additional Considerations” section, as these factors are still important for clinicians to consider in the context of their individual patient's circumstances when initiating PAP therapy.
Public Comment and Final Approval
A draft of the guideline and systematic review was made available for public comment for a two-week period on the AASM website. The TF took into consideration all the comments received and made decisions about whether to revise the draft based on the comments. The revised guideline and systematic review were submitted to the AASM Board of Directors for subsequent approval. This review reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
THE USE OF POSITIVE AIRWAY PRESSURE
The aims of the current literature reviews and data analyses were focused on addressing 11 questions pertaining to the use of PAP to treat OSA in adults. Below are detailed summaries of the evidence identified in the literature searches and the statistical analyses performed by the TF. Each evidence summary is accompanied by a discussion of the quality of evidence, balance of benefits and harms, patient values and preferences, and resource use considerations that contributed to the development of the recommendations provided in the accompanying clinical practice guideline.8
Continuous Positive Airway Pressure Therapy Versus No Therapy
This section addresses PICO questions 1–3 (see Table 1) and resulted in three recommendations (see Recommendations 1–3 in the companion clinical practice guideline).8 A total of 80 RCTs34,36,61–132 and 15 non-randomized studies133–152 investigated the use of PAP to improve one or more of the following outcomes: OSA severity, sleepiness, QOL, sleep quality, mood, neurocognitive function, MVC, blood pressure, left ventricle ejection fraction, fasting glucose, hemoglobin A1c, incident cardiovascular events, and incident mortality. Participants in the studies were from clinic-based populations and were predominantly male, obese, with moderate to severe OSA and self-reported sleepiness. RCTs were reviewed for all outcomes with the exception of MVC, for which non-randomized studies were reviewed. Both RCTs and non-randomized studies were reviewed for the outcomes of incident cardiovascular events and incident mortality. For the RCTs, participants were randomized to a control intervention utilizing sham CPAP, conservative measures or no intervention, sham surgery, placebo tablet, or nasal dilator strips. For each outcome, important differences in patient population or study design from the general description above are noted below. Several meta-analyses were performed to assess the efficacy of PAP for the treatment of OSA in adults as compared with no therapy. The meta-analyses are provided in the supplemental material, Figure S1 through Figure S57. Summary of Findings tables are provided in the supplemental material, Table S1 through Table S3. A summary of the evidence for each outcome is provided below.
OSA Severity
The efficacy of PAP in reducing OSA severity in adults was evaluated using a meta-analysis of studies that reported on the AHI or RDI. For this analysis, the two measures were considered equivalent. All studies were RCTs, with 3 studies using a randomized, cross-over design.61,65,84 Participants were randomized to CPAP or a control intervention. The control interventions utilized included sham CPAP (n = 5),34,66,74,84,93 conservative measures (advice on weight loss or good sleep habit counseling), no intervention (n = 3),78,82,92 sham surgery (n = 1),90 placebo tablet (n = 1),65 and nasal dilator strips (n = 1)61. The duration of intervention was at least 1 month (range: 1–6 months).
A meta-analysis of 11 RCTs34,61,65,66,74,78,82,84,90,92,93 demonstrated a clinically significant mean difference in OSA severity of −23 events/h (95% CI: −29 to −18 events/h) with PAP (see supplemental material, Figure S1). An additional meta-analysis of these studies comparing OSA severity before and after CPAP treatment demonstrated a clinically significant mean reduction in OSA severity of −29 events/h (95% CI: −37 to −20 events/h) or an AHI reduction of 86% with PAP (see supplemental material, Figure S2). The mean pretreatment AHI was 32.7 ± 12.6 events/h and the posttreatment AHI was 4.1 ± 5.6 events/h. Overall, the analyses support the conclusion that CPAP is effective in reducing OSA severity as measured by the AHI or RDI, across the spectrum of OSA severity. The quality of evidence for OSA severity was high.
Sleepiness
Meta-analyses on sleepiness outcomes were performed analyzing both self-reported sleepiness as determined by the Epworth Sleepiness Scale (ESS) and objective sleepiness as determined by the Multiple Sleep Latency Test (MSLT), MWT, and the OSLER. Participants were self-reportedly sleepy with the exception of 5 studies that recruited non-sleepy participants evaluated after at least 1 month of intervention (range: 4–12 months follow-up).63,76,85,106,112 Seven studies recruited participants with concomitant hypertension36,63,68,86,100,106,112 and one study89 recruited participants with concomitant T2DM. The control intervention employed was predominantly sham PAP or no PAP with one study85 using nasal dilator strips, one study112 using sleep hygiene and counseling, and one study36 asked participants to continue with their normal medication.
A meta-analysis of 38 RCTs34,61–90,106,112,114–116,150,153 demonstrated a clinically significant reduction in self-reported sleepiness of −2.4 points in the ESS score (95% CI: −2.8 to −1.9 points) in participants on PAP compared to controls (see supplemental material, Figure S3). A sub-analysis of the 5 studies63,85,86,106,112 recruiting only non-sleepy participants with OSA demonstrated an ESS reduction of −1.0 points (95% CI: −0.7 to −1.4 points) that the TF judged to not be clinically significant. A meta-analysis of 7 RCTs using the MWT or OSLER sleep latency to assess objective wakefulness demonstrated a clinically significant SMD in objective sleepiness of 0.5 (95% CI: 0.2 to0.8) with the use of PAP65,71,73,76,77,81,89 (see supplemental material, Figure S4). In contrast, a meta-analysis of 7 RCTs63,64,69,70,82,85,91 using the MSLT to assess objective sleepiness demonstrated no clinically significant difference in sleep latency with the use of PAP (see supplemental material, Figure S5). Overall, the analyses support the conclusion that treatment of OSA with CPAP results in clinically significant improvements in self-reported sleepiness and the ability to maintain wakefulness, particularly in sleepy patients with OSA. The overall quality of evidence for sleepiness was high.
Quality of Life
The efficacy of PAP in improving sleep-related QOL in adults with OSA was evaluated using meta-analyses combining studies that reported on the FOSQ (n = 8),34,63,65,72,82–84,90 and the SAQLI (n = 5),36,78,81,87,89 In addition, meta-analyses were also performed for the SF-36 component summary scores, specifically the mental component score (n = 12),36,63,64,71,76,78,83,87,90,116,150,151 the physical component score (n = 11),63,64,70,76,78,83,87,90,116,150,151 and the vitality score (n = 8)36,64,71,78,83,87,150,151 to assess the efficacy of PAP to improve general QOL.
The studies were performed with participants who had moderate to severe OSA and/or were self-reportedly sleepy, with the exception of one study that recruited non-sleepy participants with mild to moderate OSA63 and two studies71,72 that specifically recruited participants with mild OSA and symptoms of sleepiness. All studies were RCTs, with 5 studies64,65,71,72,84 using a randomized, cross-over design. Participants were randomized to PAP or a control intervention. Sham PAP (n = 8),34,63,76,83,84,87,89,150 conservative measures (advice on weight loss or good sleep habit counseling) or no intervention (n = 6),36,78,82,116,150,151 placebo tablet (n = 4),64,65,71,72 and sham surgery (n = 1)90 were utilized as control interventions. The length of the intervention was at least 1 month (range: 1–48 months follow-up).
The meta-analyses for QOL are presented in the supplemental material, Figure S6 through Figure S9. A total of 19 RCTs investigated the efficacy of PAP to improve QOL in adults with OSA.34,36,63–65,71,72,76,78,81–84,87,89,90,116,150,151 Meta-analysis of some measures of QOL demonstrated a clinically significant difference with PAP while others did not. A meta-analysis of 8 RCTs34,63,65,72,82–84,90 reporting on FOSQ and 5 RCTs36,78,81,87,89 reporting on SAQLI demonstrated a clinically significant SMD of 0.3 (95% CI: 0.1 to 0.5). Meta-analyses of RCTs reporting on QOL using the SF-36 physical component summary score,63,64,71,76,78,83,87,90,116,150,151 the mental component summary score,36,63,64,71,76,78,83,87,90,116,150,151 and the vitality score36,64,71,78,83,87,150,151 demonstrated no clinically significant improvement in QOL using PAP. Overall, the analyses suggest that PAP is effective in improving sleep-related QOL, but not overall QOL in adults with OSA. The quality of evidence for QOL ranged from moderate to high, depending on the measure employed, and was downgraded due to imprecision.
Blood Pressure (All Participants)
A total of 26 RCTs measured BP before and after PAP therapy.36,63–66,68,75,79,82,86,89,94–98,100–102,104,105,112,116,117,120,148 Of these, 5 specifically recruited hypertensive participants,68,86,100,112,148 and 579,97,98,117,120 focused on resistant hypertension (participants treated with ≥ 3 antihypertensive medications). The majority of RCTs studied mixed populations of normotensives and hypertensives, many of whom were treated with antihypertensive drugs. Three trials recruited only normotensive participants.72,94,95 Most RCTs did not specify sleepiness status a priori, however, a few RCTs were limited to non-sleepy63,86,112 or sleepy94,101 participants, with most studies63,86,112 primarily based on the ESS. Trial participants were often concurrently treated with anti-hypertensive agents at study enrollment, but medication use was not explicitly considered in participant selection or outcome assessment. Several control conditions were utilized, ranging from sham PAP,63,66,68,75,86,89,94,100–102,104,120 to usual care,36,82,97,98,112,116,148 to an oral placebo tablet64,65 to no treatment while maintaining antihypertensive medications79,95,96,105,117 for comparison to PAP. The intervention duration ranged from 1 month to 1 year. Many studies utilized 24-hour (or 48-hour) ambulatory BP measurements, considered to be the most accurate method to diagnose hypertension and the best predictor of future cardiovascular risk.154 Some studies utilized office or lab-based measurements limited to the daytime hours67,82 and one study36 utilized home daytime measurements.
Meta-analyses were performed on several measures of BP including: nighttime systolic and diastolic BP; daytime systolic and diastolic BP; 24-hour systolic and diastolic BP; and 24-hour mean BP (see supplemental material, Figure S10 through Figure S33). Studies that did not describe a systematic method of BP ascertainment were excluded from the meta-analyses.
For the entire participant sample, meta-analysis demonstrated that PAP therapy was associated with a clinically significant reduction in nighttime systolic blood pressure (SBP) and diastolic blood pressure (DBP) of −4.2 mmHg (95% CI: −6.0 to −2.5 mmHg), and −2.3 mmHg (95% CI: −3.7 to −0.9 mmHg), respectively (see supplemental material, Figure S10 and Figure S11). Clinically significant reductions in daytime SBP and DBP of −2.8 mmHg (95% CI: −4.3 to −1.2 mmHg) and −2.0 mmHg (95% CI: −3.0 to −0.9 mmHg), respectively were observed (see supplemental material, Figure S12 and Figure S13). In addition, PAP therapy was also associated with a clinically significant reduction in 24-hour SBP and DBP of −1.5 mmHg (95% CI: −2.3 to −0.7 mmHg) and −1.6 mmHg (95% CI: −2.2 to −0.9 mmHg), respectively (see supplemental material, Figure S14 and Figure S15). Lastly, a meta-analysis demonstrated a clinically significant reduction in 24-hour mean BP of −2.6 mmHg (95% CI: −3.9 to −1.4 mmHg) with PAP therapy (see supplemental material, Figure S16).
As described above, many trials studied heterogeneous populations with respect to characteristics that may differentially influence the BP-lowering response to PAP therapy. For example, most studies described a minimum requirement for moderate to severe OSA, while only two63,95 specified severe OSA defined by an AHI > 30 events/h. Two studies included participants with an AHI of 5–30 events/h,64,65 and none exclusively recruited participants with mild OSA (AHI of 5–15 events/h). Nightly PAP adherence was variable and commonly in the range of what most clinicians would deem suboptimal. One study suggested greater BP reduction with increased CPAP adherence.155 However, whether this reflects the effect of PAP treatment or patient adherence with therapies in general, remains unclear. Some trials63,95 used fixed CPAP titrated during PSG in the sleep laboratory and some36,94 used APAP, while others68,97 used CPAP derived from a night on APAP. Distinguishing between these modes may be important in light of some studies suggesting differential effects of CPAP and APAP on BP.156,157
The BP reductions associated with RCTs of PAP therapy found in these meta-analyses, if sustained, would result in substantial reductions in long-term cardiovascular risk.158 The TF only considered the impact of PAP versus no treatment on BP in adults with OSA and did not compare PAP to antihypertensive drugs, which has been considered in some recent trials.159,160
Overall, the analyses suggest that PAP use reduces BP in adults with OSA, particularly in participants with moderate to severe OSA. The quality of evidence for BP in all participant types with OSA ranged from moderate to high, depending on the time and type of BP measured, and was downgraded due to imprecision.
Blood Pressure (Resistant Hypertensive Participants)
A total of 5 of the 27 RCTs reported on the effects of PAP therapy on BP in participants with OSA and comorbid resistant hypertension at baseline.79,97,98,117,120 Participants had predominantly moderate to severe OSA. Meta-analyses demonstrated the mean estimate of the effect of PAP therapy were clinically significant reductions in nighttime SBP and DBP (−3.3 mmHg [95% CI: −6.1 to −0.4 mmHg] and −2.2 mmHg [95% CI: −4.4 to 0.0 mmHg], respectively), daytime DBP (−1.1 mmHg [95% CI: −3.4 to +1.1 mmHg]), and 24-h SBP and DBP, (−2.2 mmHg [95% CI: −5.1 to +0.8 mmHg], and −2.1 mmHg [95% CI: −4.1 to 0.0 mmHg], respectively) with CPAP therapy but not a clinically significant reduction in daytime SBP (see supplemental material, Figure S17 through Figure S22).
Overall, the analyses suggest that PAP use reduces nighttime and 24h blood pressure in adults with predominantly moderate to severe OSA and resistant hypertension. The quality of evidence for BP in resistant hypertensive participants with OSA was moderate due to imprecision.
Blood Pressure (Hypertensive Participants)
A total of 5 of the 27 RCTs reported on the effects of PAP therapy on BP in participants with OSA and comorbid hyper-tension at baseline.68,86,100,112,148 Participants had predominantly moderate to severe OSA. Meta-analyses demonstrated clinically significant reductions in nighttime SBP and DBP of −3.9 mmHg [95% CI: −6.5 to −1.4 mmHg] and −3.0 mmHg [95% CI: −5.3 to −0.8 mmHg], respectively (see supplemental material, Figure S23 and Figure S24). Clinically significant reductions in daytime SBP and DBP of −2.7 mmHg [95% CI: −4.9 to −0.5 mmHg], and −2.4 mmHg [95% CI: −3.9 to −0.9] respectively, were observed (see supplemental material Figure S25 and Figure S26). In addition, PAP therapy was also associated with clinically significant reductions in 24-hour SBP and DBP of −2.5 mmHg [95% CI: −4.3 to −0.8 mmHg] and −2.2 mmHg [95% CI: −3.4 to −1.0 mmHg] with PAP therapy, respectively (see supplemental material, Figure S27 and Figure S28). Lastly, meta-analysis demonstrated a clinically signifi-cant reduction in 24-hour mean BP of −2.2 mmHg [95%CI: −3.6 to −0.7 mmHg], (see supplemental material, Figure S29).
Overall, the analyses suggest that PAP use reduces BP in adults with OSA and hypertension. The quality of evidence for BP in hypertensive participants was moderate due to imprecision.
Blood Pressure (Normotensive Participants)
A total of 3 of the 27 RCTs reported on the effects of PAP therapy on BP in normotensive participants at baseline.72,94,95 Meta-analyses demonstrated no clinically significant reduction in daytime or nighttime SBP and DBP (see supplemental material, Figure S30 through Figure S33). However, one study demonstrated a clinically significant reduction in 24-hour DBP of −1.4 mmHg [95% CI: −3.2 to 0.4 mmHg] with PAP therapy.72
Overall, the analyses suggest that PAP use does not reduce blood pressure in normotensive adults with OSA. The quality of evidence for BP in normotensive participants was low due to very high imprecision.
Cardiovascular Events
The TF reviewed both RCT and non-randomized data regarding the effects of PAP on cardiovascular event rate. Six RCTs assessed the impact of PAP therapy on cardiovascular event rate, which were variably defined by composite outcomes.36,81,106–108,116 The studies recruited participants with at least moderate OSA severity (AHI > 15–20 events/h), middle to older age, predominantly male and overweight to obese, followed for an average of 3 to 5 years. Studies of participants examining incident cardiovascular events106 and recurrent cardiovascular events107,108,116 were included for analysis. The largest trial to date showed no clinically significant impact of CPAP therapy on secondary prevention in adults with established cardiovascular disease.116 The meta-analysis also did not demonstrate a clinically significant reduction in the rate of cardiovascular events occurring with the use of PAP (see supplemental material, Figure S34).
Eleven non-randomized studies assessed the impact of PAP on cardiovascular event rate.133,134,136–141,143–145 Most studies included participants that were male, middle-aged, overweight to obese with predominantly moderate to severe OSA, and mean follow-up time ranged from 1 to 10.1 years. A notable exception was one study that reported on women only.147 The majority of studies measured composite outcomes of fatal and non-fatal cardiovascular events,134,136–140,143–145 while two studies133,141 were limited to incident arrhythmias. Two studies were also limited to participants with heart failure.142,146 Meta-analysis of 11 non-RCTs133,134,136–141,143–145 demonstrated a clinically significant reduction in cardiovascular events with a risk ratio of 0.5 (95% CI: 0.3 to 0.7) with the use of PAP (see supplemental material, Figure S35).
The vulnerability of non-randomized studies to bias is worth highlighting, as reliance on such studies often contributes to downgrading of recommendations. Comorbidities among study cases and controls are often imbalanced and may be difficult to control for. In many instances, the control groups were comprised of participants who refused PAP therapy,134,143 raising questions of adherence with other medical therapies that may impact outcomes. Non-systematic ascertainment of study participant characteristics, and the outcomes typical of non-randomized studies, may be biased in the data abstraction process.161 Furthermore, non-randomized studies are much more prone to publication bias. Finally, since many of the studies were published more than a decade ago, including the largest,143 it is unknown what impact interim advances in cardiovascular disease therapies may have on the benefit of treating OSA with PAP.
One area of controversy in reconciling the discrepant findings between the non-randomized studies and RCTs is that, in general, PAP adherence was lower in the RCTs than in the non-randomized studies. Whether the greater effect of PAP on lowering cardiovascular event rate in non-randomized studies reflects a beneficial effect of a higher adherence to CPAP or alternatively, a non-specific effect of being adherent with other medical treatments or healthier lifestyles remains to be answered.162 Furthermore, whether greater PAP use would have demonstrated a beneficial impact on cardiovascular event reduction is unknown, but was suggested in secondary analyses performed in several studies.106,108,116 Two reasons for the lower adherence in RCTs may be the inclusion of less symptomatic/sleepy participants and exclusion of participants with the most severe disease116— given that symptoms and OSA severity are predictors of PAP adherence.163 In addition, the benefits of PAP on cardiovascular event risk may be greater in more symptomatic and more severe disease, which are the groups that were excluded from the RCTs.
The quality of evidence for cardiovascular event rate ranged from low to moderate, based on the types of studies pooled for meta-analysis, and was downgraded due to study type and imprecision.
All-Cause Mortality
The TF reviewed both RCT and non-randomized data regarding the effects of PAP on all-cause mortality. Four RCTs assessed the impact of PAP therapy on all-cause mortality.106–108,116 The studies recruited participants with at least moderate OSA severity (AHI > 15–20 events/h), middle to older age, predominantly male and overweight to obese, followed for an average of 3 to 5 years. cardiovascular prevention studies106,107,108,116 were included for analysis. The largest trial to date showed no clinically significant impact of CPAP therapy on mortality in adults with established cardiovascular disease.116 The meta-analysis did not demonstrate a clinically significant reduction in all-cause mortality with the use of PAP (see supplemental material, Figure S36).
Nine non-randomized trials reported on mortality associated with the use of PAP versus control conditions in participants with or without heart failure (see supplemental material, Figure S37).137–140,142–144,146,147 A meta-analysis of these studies demonstrated a clinically significant reduction in risk with a risk ratio of 0.40 (95% CI: 0.24 to 0.69). When studies were stratified into subgroups based on the presence or absence of heart failure, meta-analyses demonstrated clinically signifi-cant reductions in the risk ratio for mortality of 0.2 (95% CI: 0.1 to 0.5) and 0.4 (95% CI: 0.2 to 0.7) for heart failure142,146 and no heart failure participants,137,139,143,144,147 respectively (see supplemental material, Figure S38 and Figure S39).
Like the evidence review for cardiovascular events, the analyses are inconclusive regarding the effects of PAP in reducing all-cause mortality in adults with OSA, in part related to differences in patient populations studied and PAP adherence between randomized and non-randomized studies. The quality of evidence for mortality was low due to study type and imprecision.
Overall Quality of Evidence
The outcomes of sleepiness, sleep-related QOL, and blood pressure were determined by the TF to be critical for decision-making. The overall quality of evidence for recommendation 1, based on the critical outcome of sleepiness, was high. The overall quality of evidence for recommendation 2, based on the critical outcome of sleep-related QOL, was moderate due to imprecision. The overall quality of evidence for recommendation 3, based on the critical outcome of BP, was moderate due to imprecision.
Benefits Versus Harms
The potential benefits of CPAP based on the meta-analyses performed include reduction in OSA severity, improvement in patient symptoms, particularly sleepiness, sleep-related QOL, MVCs, and reduction in BP. The potential for cardiovascular and mortality benefits cannot be ruled out. These potential benefits should be considered in the context of the potential harms of CPAP. Direct side effects that have been reported with the use of PAP are presented in the supplemental material, Table S16.9 These side effects can result in sleep disruption and poor sleep quality thereby reducing patient adherence to CPAP, and should be carefully monitored and managed by a clinician. There are also some concerns about the development of treatment-emergent central sleep apnea associated with PAP in general, however, patient harm has yet to be demonstrated. The TF judged that the potential benefits of CPAP outweighed the harms in those patients with excessive daytime sleepiness, other symptoms impairing sleep-related QOL, or with hypertension.
Patient Values and Preferences
The TF judged that the majority of sleepy patients and most patients with reduced sleep-related QOL with OSA of any severity would consider a trial of PAP therapy given the rapid reversibility of side effects. The TF recognizes that individual patients, despite their symptoms, may choose not to pursue CPAP treatment due to concerns about side effects. A balanced discussion between a patient and their clinical provider about the consequences of excessive sleepiness and other OSA symptoms, the benefits and harms of CPAP, and consideration of alternative therapies such as weight loss, positional therapy, oral appliance therapy or surgical interventions, can help guide individual treatment decisions.
The TF also judged that most patients with OSA and hyper-tension would want their OSA treated to help reduce BP as the benefits may include reduction in cardiovascular risk. Patients experiencing symptoms of OSA (eg, excessive sleepiness) may be more accepting of CPAP therapy, with the possibility of secondary benefits related to cardiovascular risk reduction. Non-sleepy patients with OSA, however, may have a more nuanced view of whether to pursue treatment of OSA, particularly given the efficacy of alternative antihypertensive treatments. The TF recognizes that some non-sleepy patients will place a high value on any intervention that potentially reduces long-term cardiovascular events, including CPAP therapy.
The meta-analysis of non-sleepy OSA participants, demonstrated a statistically but not clinically significant reduction in ESS. However, given the limitations of the ESS in assessing sleepiness,164,165 patients with OSA who are non-sleepy or minimally symptomatic as assessed by the ESS, may experience clinically important improvements in sleepiness. As such, the clinician assessment of sleepiness should not be solely based on the ESS. For patients with a normal ESS but other signs of sleepiness, a short-term therapeutic trial of CPAP may be reasonable to assess symptomatic benefits. For other non-sleepy patients, based on the ESS, the uncertainty of any cardiovascular benefit, may lead them to decline treatment of OSA, regardless of their OSA severity. For example, some RCTs that selectively recruited non-sleepy participants demonstrated no benefits in BP63,86,106 or cardiovascular risk reduction106,108 with CPAP. Given the absence of high-quality evidence for the use of PAP to treat non-sleepy adults with OSA, conservative management of OSA in non-sleepy patients, with monitoring for development of OSA symptoms over time may be appropriate.
Resource Use
In general, cost-effectiveness analyses have demonstrated that CPAP is a cost-effective therapy compared to no therapy. In one systematic review performed by the Canadian Agency for Drugs and Technologies in Health,166 two studies167,168 were identified demonstrating the cost effectiveness of CPAP. The first study167 demonstrated an incremental cost-effectiveness ratio (ICER) of CDN$15,915 per quality-adjusted life year (QALY) with CPAP therapy while another study168 performed for the National Institute of Health and Clinical Excellence (NICE) in the United Kingdom demonstrated an ICER for CPAP therapy compared to dental devices or lifestyle advice that ranged from £4,413–£20,585 depending on the OSA severity. The TF judged that resource use is justified for CPAP for the treatment of OSA in adults to improve patient sleepiness and sleep-related QOL. The TF did not identify cost-effectiveness studies regarding PAP therapy and outcomes related to blood pressure. Hypertension is highly prevalent, affecting nearly one-third of the United States adult population. Depending upon patterns of provider recognition, perceived value, and patient acceptance, resource use may be substantial. Cost analyses are therefore needed. In light of comparative trials highlighting the efficacy of antihypertensive medications in those with OSA, as well as potential synergy with PAP therapy, modeling of this relationship in such analyses will be important.
Other Outcomes
The TF considered several other outcomes to be important but not critical for decision-making for the development of the recommendations. These outcomes included neurocognitive function, mood, MVC, fasting glucose, hemoglobin A1c, LVEF, and incident hospitalizations. A summary of the findings for each of these outcomes is presented below.
Neurocognitive Function
For neurocognitive outcomes, two experts outside of the TF were consulted to assist grouping of neurocognitive tests into appropriate domains, which was finalized through a consensus process. The efficacy of PAP in improving neuro-cognitive function in adults with OSA was evaluated using meta-analyses of studies that reported on several sub-domains of executive function (shifting, updating, and fluid reasoning) and the domains of processing speed, attention and vigilance, memory, and intelligence (see supplemental material, Tables S17). A total of 9 RCTs investigated the efficacy of PAP for improvement in neurocognitive function as measured across these domains.63,65,70,71,81,82,90,114,115 Two studies recruited elderly participants (age ≥ 65 years).81,114 One study71 recruited sleepy participants with mild OSA and one study63 recruited non-sleepy participants with mild to moderate OSA. None of the studies selectively enrolled participants with concurrent mild cognitive impairment or dementia. However, one study of participants with predominantly severe OSA had baseline impairments in short-term memory and executive function compared to a historical control group matched on age and educational background.114 Sham PAP (n = 1),63 conservative measures (advice on weight loss or good sleep habit counseling) (n = 3),81,82,114 and placebo tablet (n = 3)65,70,71 were utilized as control interventions. The intervention lasted for at least 1 month (range: 1–12 months follow-up).
Meta-analyses performed to assess neurocognitive function are presented in the supplemental material, Figure S40 through Figure S46. The meta-analyses demonstrated no clinically significant differences between PAP and control groups in any of the domains of neurocognitive function tested, which included executive function, processing speed, attention and vigilance, memory, and intelligence.
Overall, the analyses suggest that CPAP does not appear to improve neurocognitive function in adults with OSA. The quality of evidence for neurocognitive function ranged from low to high and was downgraded due to imprecision in certain domains.
Mood
The efficacy of PAP in improving mood, specifically anxiety and depression, in adults with OSA was evaluated using meta-analyses of 5 studies that reported on the Hospital Anxiety and Depression Scale (HADS anxiety and HADS depression).70,71,81,116,130 The studies identified for the systematic review did not specifically recruit participants with comorbid anxiety or depression.
Three studies were performed with participants who had moderate to severe OSA and were self-reportedly sleepy,70,81,130 one study recruited minimally sleepy participants with mild to moderate OSA,116 and one study71 recruited participants specifically with mild OSA and symptoms of sleepiness. Two studies recruited only older participants.81,130 All studies were RCTs, with 2 studies using a randomized, cross-over design.70,71 Participants were randomized to CPAP or a control intervention. No intervention (n = 3)81,116,130 or placebo tablet (n = 2)72 were utilized as controls. The length of the intervention was for at least 1 month (range: 1–4 years follow-up).
Meta-analyses of the HADS anxiety scale and HADS depression scale scores demonstrated no clinically significant improvements in mood using CPAP; however, the studies did not specifically enroll participants with anxiety or depression at baseline (see supplemental material, Figure S47 and Figure S48). The quality of evidence for depression and anxiety was high.
Motor Vehicle Crashes
The efficacy for CPAP in improving MVC in adults with OSA was evaluated using meta-analyses examining the relative risk reduction of obstacles hit during driving simulation in 4 RCTs63,70,71,82 and MVC in ten non-randomized studies118,119,121–123,125–128,132 (see supplemental material, Figure S49 through Figure S51).
In studies that used driving simulator data and had control participants with untreated OSA, all but one study63 was performed with participants that were self-reportedly sleepy. One study63 recruited non-sleepy participants with mild to moderate OSA and one study71 recruited participants specifically with mild OSA with symptoms of sleepiness. Sham CPAP (n = 1),63 conservative measures (advice on weight loss or good sleep habit counseling) (n = 2),82,85 and placebo tablet (n = 3)64,70,71 were utilized as control interventions. The duration of the intervention was for at least 1 month (range: 1–6 months follow-up) in the RCTs. Meta-analyses of RCTs did not demonstrate a clinically significant reduction in obstacles hit or percent obstacles hit using a driving simulator (see supplemental material, Figure S49 and Figure S50). Extrapolation of results from driving simulators to real world driving should be made with caution given variations in simulators and protocols for testing and differences in participant motivations when driving in simulated versus real world conditions.
For studies examining MVC risk reduction, the TF included 10 non-randomized studies with pre- and post-CPAP assessment of MVC by self-report or objective reports and performed a meta-analyses on these studies.118,119,121–123,125–128,132 Participants had predominantly moderate to severe OSA and were self-reportedly sleepy,119 ESS or another tool,118,121,125,127,128,132 or data122,123 was not reported. Most studies compared participants for a period pre-CPAP intervention to post-CPAP intervention. Two studies compared changes in MVC in participants with OSA before and after CPAP to a non-OSA control group followed over time to control for secular trends,118,123 while one study122 compared changes in MVC to participants with OSA declining CPAP use also followed over time. All studies included were of non-commercial motor vehicle operators. A separate AASM TF has reviewed data from studies of commercial motor vehicle drivers.169 Outcome assessment was through self-report,119,121,125,127,128,132 data from transportation offices,122,123 or data118 from auto insurers. Follow-up varied ranging up to 2 years before enrollment to 6 years after (range 2–6 years) or prospective follow-up after enrollment between 6–12 months.
Meta-analyses of the 10 non-randomized studies118,119,121–123,125–128,132 comparing participants with OSA before and after CPAP treatment demonstrated a mean crash rate risk ratio of 0.3 (95% CI: 0.2 to 0.4) (see supplemental material, Figure S51), which was considered to be clinically significant. Overall, the analyses suggest that CPAP use results in a reduction in crash rates in adults with OSA as assessed by both objective MVC data and self-report from questionnaires. The quality of evidence for MVC ranged from low to moderate. The quality of evidence from RCTs for the use of PAP to reduce MVC was downgraded due to imprecision and was moderate. The quality of evidence from observational studies for the use of PAP to reduce MVC was low and was downgraded due to study design.
Fasting Glucose and Hemoglobin A1c
A total 8 RCTs assessed fasting glucose before and after 6 to 12 weeks of PAP therapy in primarily obese, male participants with at least moderate to severe OSA.67,74,88,89,93,129,131,153 Five studies included participants without diabetes,67,74,88,93,153 3 studies89,129,131 included participants with T2DM, and one study recruited participants from an obesity surgery clinic.153 All of the trials individually failed to demonstrate a clinically signifi-cant reduction in fasting glucose with PAP (see supplemental material, Figure S52). Despite the lack of improvement in fasting glucose levels, there have been several trials in those without diabetes suggesting CPAP therapy for comorbid OSA may reduce insulin resistance.170–172 Whether this translates into a reduction in risk of incident T2DM is unclear.
The efficacy of PAP in reducing HbA1c in adults with OSA was evaluated using a meta-analysis of 4 RCTs.89,129,131,153 The studies were performed in primarily clinic-based populations. Participants in three of four studies had T2DM and were predominantly male, obese, with moderate to severe OSA.89,129,131 One study recruited severely obese participants without T2DM with severe OSA.153 Three of the four studies recruited participants that as a group were not self-reportedly sleepy based on the ESS.129,131,153 The mean baseline HbA1c ranged from 5.7 to 8.5%. Participants were randomized to CPAP or a control intervention, which included either sham CPAP89 or usual care129,131,153 for diabetes management. Participant follow-up ranged from 3–6 months. Of the four studies, only one showed a significant reduction in HbA1c.129 A meta-analysis of the four studies did not demonstrate a clinically significant improvement in HbA1c with PAP (see supplemental material, Figure S53). Mean CPAP use across participants in three of the four studies ranged from 3.6–5.4 h/night.89,129,131 Whether interventions that lead to greater PAP use could demonstrate an improvement in glycemic control remains unknown.173
Overall, the TF judged that analyses do not support that PAP reduces fasting glucose or HbA1C in adults with OSA with or without T2DM. The quality of evidence for the efficacy of PAP to reduce fasting glucose and hemoglobin A1c was high in finding no clinically significant reduction.
Left Ventricle Ejection Fraction
Eight RCTs measuring LVEF by echocardiography or radionuclide ventriculography compared the efficacy of PAP versus control conditions (see supplemental material, Figure S54 through Figure S56).93,94,99,102,109,110,113,124 Studies on participants with heart failure99,102,109,110 recruited from cardiology or heart failure clinics while studies of participants without heart failure93,94,113 recruited primarily from sleep clinics. Participants were largely male, between the age of 50–60, obese, with severe OSA, and the intervention lasted for at least 1 month (mean 2.7 months; range: 1 to 6-month follow-up). Either sham PAP93,94,102,110 or no PAP99,109,113 was employed as the control intervention.
Meta-analysis of all participants in these studies showed no clinically significant improvement in LVEF.93,94,99,102,109,110,113,124 When limited to participants with heart failure, a meta-analysis of 5 RCTs demonstrated no clinically significant improvement in LVEF.99,102,109,110,124 In addition, a meta-analysis of the 3 RCTs that were conducted in participants without heart failure demonstrated no clinically significant improvement in LVEF.93,94,113
Overall, the analyses suggest that PAP does not result in clinically significant improvements in LVEF in adults with OSA either with or without comorbid heart failure. The quality of evidence for LVEF was moderate due to imprecision.
Hospitalization
Two non-randomized studies reported on all-cause hospitalizations associated with PAP versus control conditions.135,140 A meta-analysis of these studies did not demonstrate a signifi-cant reduction in hospitalizations associated with PAP therapy compared with control conditions (see supplemental material, Figure S57). Overall, the analyses did not support that CPAP reduced the risk of hospitalization in adults with OSA, although very few studies were identified that met criteria for analysis. The quality of evidence for hospitalizations was very low due to study type and imprecision.
APAP at Home Versus In-Laboratory PAP Titration for Initiation of PAP
This section addresses PICO question 4 (see Table 1) and resulted in one recommendation (see Recommendation 4 in the companion clinical practice guideline).8 A total of 10 RCTs were identified that compared initiation of PAP using home APAP versus an in-laboratory PAP titration in improving one or more of the following outcomes: AHI/RDI, adherence to PAP therapy, sleepiness, and QOL.35,174–182 Participants were predominantly middle-aged males with sleepiness and moderate to severe OSA. Studies included only participants with high clinical suspicion of moderate to severe OSA. Most studies reviewed excluded participants with the following comorbidities or conditions: congestive heart failure, chronic opiate use, significant lung disease such as chronic obstructive pulmonary disease, neuromuscular disease, history of uvulopalatopharyngoplasty, sleep-related oxygen requirements, or expectation for nocturnal arterial oxyhemoglobin desaturation due to conditions other than OSA, including hypoventilation syndromes and central sleep apnea syndromes. For the in-laboratory titration protocol, 6 studies used only full night in-laboratory titration and 4 studies used a combination of full night and split-night in-laboratory titration. Most participants in these studies using home APAP had mask fittings and education on PAP use at a sleep center. Some studies also offered daytime nap acclimatization. Follow-up by trained staff early during the treatment period was common. All studies used the home APAP device in auto-adjustment mode for a brief period (2–7 nights) and then switched to a fixed pressure (90th or 95th percentile). Several meta-analyses were performed to assess the impact of home APAP versus in-laboratory PAP titration for the initiation of OSA treatment. The meta-analyses are provided in the supplemental material, Figure S58 through Figure S61. A Summary of Findings table is included in Table S4 of the supplemental material. A summary of the evidence for each outcome is provided below.
OSA Severity
The impact of PAP in reducing OSA severity in adults with OSA who initiated PAP using home APAP was evaluated using a meta-analysis of 3 RCTs that reported on the AHI.174,178,179 Participants were randomized to in-laboratory CPAP titration versus home APAP followed by conversion to a fixed CPAP pressure based on PAP monitoring data, with outcomes assessed after at least 6 weeks of treatment (range 6 to 12 weeks). The meta-analysis demonstrated no clinically significant difference in residual OSA severity (see supplemental material, Figure S58) when PAP was initiated using home APAP compared to in-laboratory titration.174,178,179 Residual OSA severity was obtained from the in-laboratory PSG178,179 or from the PAP device174 in these studies.
Overall, the analysis demonstrated similar effects on OSA severity in adults with OSA when PAP is initiated via home APAP or in-laboratory PAP titration. The quality of evidence for OSA severity was high.
Adherence
Adherence to PAP in adults with OSA, after initiation of PAP using home APAP versus an in-laboratory titration, was evaluated using a meta-analysis of 10 RCTs that reported on adherence.35,174–182 Participants were randomized to a home-based pathway that included APAP versus an in-laboratory CPAP titration with outcomes assessed after at least 1 month of treatment (range 1 month to 6 months). The meta-analysis demonstrated no clinically significant difference in PAP adherence when comparing treatment initiation using home APAP versus in-laboratory titration (see supplemental material, Figure S59).
Overall, the analysis demonstrated similar levels of PAP adherence in adults with OSA with PAP initiation by either home APAP or in-laboratory titration. The quality of evidence for adherence was high.
Sleepiness
The impact of PAP initiation using APAP versus an in-laboratory titration for the treatment of self-reported sleepiness in adults was evaluated using a meta-analysis of 9 RCTs that reported on the ESS.35,174–180,182 Participants with high clinical suspicion for OSA and without comorbid conditions were randomized to home APAP versus in-laboratory CPAP titration with outcomes assessed after at least 1 month of treatment (range 1 month to 3 months). The meta-analysis demonstrated no clinically significant difference in self-reported sleepiness when PAP therapy was initiated using home APAP compared to an in-laboratory titration (see supplemental material, Figure S60).
Overall, the analysis suggests that initiation of therapy using home APAP compared to an in-laboratory titration in adults with OSA results in similar effects on sleepiness. The quality of evidence for sleepiness was high.
Quality of Life
Meta-analyses of RCTs that reported on the SAQLI,178,180,182 FOSQ,175,176,180 and SF-36 component summary scores175,180 were performed to assess the impact of PAP initiation using home APAP versus an in-laboratory titration on QOL. Participants were randomized to a home-based pathway that included APAP versus in-laboratory CPAP titration with outcomes assessed after 3 months. A meta-analysis combining 2 RCTs measuring sleep-related QOL with FOSQ,175,176 2 RCTs measuring sleep-related QOL with SAQLI,178,182 and one RCT180 measuring both FOSQ and SAQLI demonstrated no clinically significant difference in sleep-related QOL when comparing PAP initiation using home APAP versus an in-laboratory titration (see supplemental material, Figures S61). Two RCTs175,180 demonstrated no clinically significant difference in general QOL as assessed by the SF-36 mental component summary, physical component summary, and vitality scores when comparing home APAP versus an in-laboratory titration (see supplemental material, Table S4).
Overall, the analysis suggests that PAP initiation in adults with OSA using a home APAP or in-laboratory titration have similar effects on both sleep-related and general QOL. Overall, the quality of evidence for QOL was moderate. The quality of evidence for the SF-36 physical and mental component summary scores was low due to very high imprecision. The quality of evidence for SF-36 vitality was high. The quality of evidence for combined FOSQ and SAQLI was moderate.
Side Effects
No studies were identified that reported on side effects of either strategy.
Overall Quality of Evidence
The outcomes of adherence to PAP therapy, sleepiness, and QOL were determined by the TF to be critical for decision-making. The overall quality of evidence was high.
Benefits Versus Harms
The potential benefits of PAP initiation using home APAP over in-laboratory titration are a reduced time to initiation of therapy, particularly in areas with limited laboratory resources, reduced time away from home, lower overall cost, and greater access to care. Despite the greater cost-effectiveness of home-based APAP initiation,175,177,179 out-of-pocket costs to patients may be lower with either split or whole-night in-laboratory PAP titration due to payor coverage policies in certain instances. The potential harms of initiating therapy with APAP at home after adequate patient education is provided are difficulties in identifying and immediately addressing problems related to mask fit or leak. However, similar issues could occur with an in-laboratory titration approach once the patient is using PAP in the home setting. In such instances, initiating therapy with APAP at home may delay or obscure recognition of these conditions and reduce adherence to therapy. There was no evidence of poor treatment efficacy (based on AHI) or reduced PAP adherence in the home APAP arm and the side effects of PAP that have been reported were deemed by the TF as likely independent of PAP initiation strategy. There are some concerns about the development of treatment-emergent central sleep apnea associated with PAP in general, however, patient harm has yet to be demonstrated. Nevertheless, the TF determined that the potential benefits of PAP initiation using either APAP at home or in-laboratory PAP titration in adults outweigh the potential harms and burdens of doing neither.
Patient Values and Preferences
Both review of available data and clinical expertise of the TF was used to assess patient values and preferences. Only one randomized trial was identified that assessed patient preference of an ambulatory/home pathway versus an in-laboratory diagnostic and treatment pathway.178 In that study, 62% of participants randomized to the in-laboratory pathway would have preferred home management, compared to 6% of participants in the ambulatory group who would have preferred in-laboratory based management.
The TF considered issues of patient access for home APAP and in-laboratory PAP titration. From a logistical standpoint, home APAP setup requires one step after diagnosis of OSA—a visit to educate on APAP use and provision of the APAP device. In-laboratory PAP titration typically requires two steps—one visit for the titration study and another for PAP education and provision of the PAP device. However, there are some situations where a single visit may suffice such as when a split-night study is performed, or immediate dispensation of equipment is available at the laboratory. Regional variations in the time to get scheduled for an additional sleep study for PAP titration and navigating the healthcare system for PAP setup after titration can be substantial, which would generally favor home APAP. On the other hand, in some regions, the health care system creates barriers that make APAP difficult to implement and may take longer to perform than an in-laboratory titration strategy followed by PAP setup. The motivation to address OSA symptoms is greatest when patients first seek OSA evaluation. Behavior change theory informs clinicians that overcoming some level of ambivalence and motivation to begin treatment varies depending on other life challenges competing for attention (eg, job or family demands, other health issues). Delays in initiating PAP therapy can substantially increase chances of loss to follow-up or poor adherence due to loss of engagement and motivation.
The TF also recognized that clinicians may need to consider patient burdens associated with in-laboratory CPAP titration or home APAP. For example, with respect to in-laboratory CPAP titration, some patients may find it difficult to spend a night away from home due to shiftwork, child-care or adult-care responsibilities, or transportation challenges between home and the testing facility that make APAP at home more convenient. In contrast, for some patients with issues of comprehension, anxiety or physical limitation, in-laboratory CPAP titration may be more favorable as a sleep technologist can provide education and other intervention during this initial introduction to PAP therapy.
Given this discussion, the TF determined that the majority of well-informed patients would most likely choose the more convenient, accessible, and cost-effective intervention, particularly when adequate education on PAP with mask fittings and daytime acclimatization by trained staff are available. Determination of which strategy is ideal for an individual patient should be based on patient preferences and abilities, the sleep clinician's judgement, anticipated or known previous difficulty with PAP treatment, and availability of resources and cost of each strategy in a particular region.
Resource Use
Six studies evaluated cost,35,175,177,179–181 of which three35,180,181 reported a slightly reduced cost for a combined home-based diagnostic and treatment pathway, and three175,177,179 reported a lower cost for home APAP compared to in-laboratory titrations. The cost reduction ranged from 25–84% in favor of treatment arms including APAP in the home. Of note, one study180 demonstrated lower cost using a home-based pathway compared to the laboratory pathway where > 50% of the studies were split-night studies. While in-laboratory titration costs include infrastructure and overnight staffing, resources for education and training of patients are required for APAP initiation. The availability of resources and cost of each strategy may vary by region. The TF judged that resource use is justified for home APAP titration in the initiation of therapy for patients without significant comorbidities with established diagnoses of OSA, while recognizing that in some regions due to patient access and patient preference that in-laboratory CPAP titration may be a more effective use of resources.
APAP Versus CPAP
This section addresses PICO question 5 (see Table 1) and resulted in one recommendation (see Recommendation 5 in the companion clinical practice guideline).8 A total of 26 RCTs were identified that investigated the effects of ongoing treatment with APAP compared with fixed CPAP in reducing side effects and improving one or more of the following outcomes: AHI/RDI, adherence to PAP therapy, sleepiness, QOL, and neurocognitive function.156,179,183–206 Participants were predominantly male, with previously untreated moderate to severe OSA and no major medical comorbidities. Participants were randomized to CPAP versus APAP for at least 1 month up to a maximum of 6 months (median 2 months). In the studies reviewed, participants with conditions that increased the risk of central sleep apnea (eg, congestive heart failure or opiate use), hypoventilation syndromes (eg, significant lung disease such as chronic obstructive pulmonary disease), neuromuscular disease, sleep-related oxygen requirements or the expectation of nocturnal arterial oxyhemoglobin desaturations, or a history of uvulopalatopharyngoplasty (which potentially could affect inspiratory airflow patterns and the response of some APAP algorithms) were usually excluded. Thus, results of meta-analyses should not be extrapolated to participants with OSA and these comorbidities or situations. For each outcome, important differences in participant population or study design from the general description reported above are provided. Meta-analyses were performed to assess the effects of APAP compared with CPAP in improving several clinical outcomes. Results of these meta-analyses are provided in the supplemental material, Figure S62 through Figure S74. Side effect data were not sufficiently standardized to permit meta-analysis, but a description of side effect findings is provided. A Summary of Findings table is provided in the supplemental material, Table S5. A summary of the evidence for each outcome is provided below.
OSA Severity
A meta-analysis of 21 RCTs that reported on OSA severity was performed (see supplemental material, Figure S62).156,179,183,184,187,188,191,193–197,199–204,206–208 Residual OSA severity was obtained in a majority of studies from PSG recordings on treatment, while several studies reported OSA severity from the PAP device microprocessor.193,199,200,202,205,206 Meta-analyses demonstrated no clinically significant differences in residual AHI between APAP and CPAP.
Overall, the analysis supports the conclusion that CPAP and APAP in adults with OSA have similar effects on OSA severity as measured by the AHI or RDI, across the spectrum of OSA severity. The quality of evidence for this outcome was high.
Adherence
Adherence to APAP versus CPAP was evaluated using meta-analyses of 23 studies (see supplemental material, Figure S63 through Figure S65).156,179,183–196,198,199,202–206 A meta-analysis of all 23 RCTs demonstrated no clinically significant difference in average hours of use in adults with OSA treated with APAP compared to CPAP. In addition, a meta-analysis of 6 of these studies demonstrated no clinically significant difference in percent of nights PAP was used.186,189,191,192,206,208 Furthermore, a meta-analysis of 2 RCTs demonstrated that the difference in the percent of nights PAP therapy was used > 4 hours with APAP versus CPAP was not clinically significant.185,193
Overall, the analyses support the conclusion that adherence to APAP and CPAP in adults with OSA is similar. The quality of evidence for this outcome ranged from moderate to high, being downgraded due to imprecision.
Sleepiness
The efficacy of APAP versus CPAP for the treatment of sleepiness in adults with OSA was evaluated using meta-analyses of 19 studies179,183–199,202 that reported on the ESS, 4 studies193,196,199,200 that reported on the OSLER, and 2 studies197,201 that reported on the MWT. Meta-analyses of studies reporting ESS and combining studies reporting either the OSLER or MWT demonstrated no clinically significant mean differences in self-reported or objective sleepiness (see supplemental material, Figure S66 and Figure S67).
Overall, the analyses support the conclusion that APAP and CPAP in adults with OSA have similar effects on daytime sleepiness. The overall quality of evidence for sleepiness ranged from moderate to high and was downgraded due to imprecision.
Quality of Life
Meta-analyses of studies that reported on the SAQLI,198,200 the FOSQ,188,202 and SF-36 component summary scores190,193,196,199,205,208 were performed to evaluate the efficacy of APAP compared to CPAP in improving QOL in adults with OSA. Meta-analyses demonstrated no clinically significant differences in QOL as measured by the combined SAQLI/ FOSQ, or by the SF-36 physical component summary, mental component summary, or vitality scores (see supplemental material, Figure S68 through Figure S71).
Overall, the analyses support the conclusion that APAP and CPAP in adults with OSA have similar effects on sleep-related and general QOL. The overall quality of evidence for QOL ranged from moderate to high and was downgraded due to imprecision.
Neurocognitive Function
Two RCTs studied participants' attention span using the psychomotor vigilance test (PVT).188,199 Participants were randomized to APAP versus CPAP in a parallel design for 6 months188 or in a cross-over study199 for 6 weeks per arm. Meta-analyses of these studies demonstrated no clinically significant differences in attention as measured by either mean reaction time or lapses on the PVT (see supplemental material, Figure S72 and Figure S73).
Overall, the analyses support the conclusion that APAP and CPAP in adults with OSA have similar effects on attention. The quality of evidence for neurocognitive function was moderate due to imprecision.
Side Effects
The efficacy of APAP versus CPAP in reducing PAP-related side effects in adults with OSA was evaluated. However, data were not reported in a sufficiently standardized format to perform a meta-analysis. A total of 11 studies reported data on side effe cts,179,183,186,188,191,193–196,198,205 with 6 of the studies179,183,188,191,195,196 reporting no clinically significant differences in side effects between APAP and CPAP. A total of 5 studies reported differences in side effects between APAP and CPAP.186,193,194,198,208 In 4 of these studies,186,193,198,208 there was less pressure discomfort with APAP than CPAP, and in at least 2 of the studies, less nasal irritation186,205 or machine noise193,205 with APAP than CPAP. On the other hand, in one study more discomfort due to pressure variation with APAP was noted.194
Overall, differences in side effects between APAP and CPAP in adults with OSA were minor and were judged by the TF to not be clinically significant (see supplemental material, Table S5). The TF noted that in clinical practice, side effects may differ between APAP and CPAP for individual patients, and that a trial of the alternate modality may be warranted when treatment intolerance due to side effects occurs. The overall quality of evidence for side effects was low due to imprecision and heterogeneity.
Overall Quality of Evidence
The outcomes of adherence to PAP therapy, sleepiness, and QOL were determined by the TF to be critical for decision-making. The overall quality of evidence was moderate due to imprecision.
Benefits Versus Harms
A potential benefit of APAP over CPAP is the ability to automatically adjust therapeutic pressures as OSA severity changes with weight fluctuations, nighttime alcohol consumption, body position, seasonal variations (eg, upper respiratory tract infections), and changes in upper airway anatomy. Potential disadvantages of APAP, which may be observed for some patients, include sleep disruption from pressure fluctuations or the return of sleep disordered breathing events when the PAP level is lowered by internal device algorithms. Furthermore, inappropriate or inadvertent increases in pressure may result in the development of treatment-emergent central sleep apnea or periodic breathing in certain patients, however, patient harm has yet to be demonstrated. The present meta-analyses demonstrated no clinically significant differences in most of the critical outcomes assessed between APAP and CPAP, and no substantial harm was identified for APAP compared with CPAP. Thus, the TF judged that the balance of benefit versus harm does not strongly favor either intervention. The TF therefore concluded that either APAP or CPAP should be used to treat adult OSA.
Patient Values and Preferences
Patient preference for APAP compared with CPAP was assessed in a total of 9 RCTs.183,184,187,189,191–193,198,199 The proportion of participants favoring APAP (see supplemental material, Figure S74) varied considerably between studies, with no consistent pattern of preference emerging. Based on this variability in patient preference between studies, the similarity of clinical outcomes with APAP versus CPAP, and variations in economic considerations and health care access, patients should discuss with their sleep clinician which form of PAP is best suited to their individual needs.
Resource Use
The TF did not perform a systematic review to identify cost-effectiveness studies of APAP versus CPAP devices. However, in some regions of the world, market and other factors may lead to differences in cost between APAP and fixed CPAP (as discussed in the section on APAP at home versus in-laboratory PAP titration for initiation of PAP for the treatment of adults with OSA), which may therefore impact the feasibility of APAP-based treatment. Over the long-term, APAP therapy may reduce costs due to reduced need for patient visits and in-laboratory titrations as pressure requirements change over time. However, the magnitude of any hypothetical costs savings has not been studied.
BPAP Versus CPAP
This section addresses PICO question 6 (see Table 1) and resulted in one recommendation (see Recommendation 6 in the companion clinical practice guideline).8 A total of 5 RCTs compared the use of BPAP to CPAP to improve one or more of the following outcomes: AHI, adherence to PAP therapy, sleepiness, neurocognitive function, QOL, and reduction of side effects.209–213 Participants were predominantly male, middle aged, referred to sleep clinics without concomitant medical or psychiatric disorders with moderate to severe OSA, randomized to CPAP versus BPAP for durations of either 1 month, 3 months, or 12 months of PAP use. Only one study included participants who had previously showed nonadherence with CPAP (< 4 h/ night),209 whereas the other 4 studies included PAP naïve participants.210–213 The average therapeutic pressure reported in the studies was ∼10 cm H2O and none of the studies specifically selected participants with high PAP requirements. Four of the studies implemented modified pressure profile technology.209–211,213 Two studies employed the use of auto-BPAP.211,213 One study employed the use of flexible BPAP.209 One study employed the use of a novel BPAP device.210 This device was modified so that the inspiratory pressure was slightly reduced near the end of inspiration, and the expiratory pressure slightly reduced near the beginning of expiration. One study employed the use of standard BPAP.212 All 5 studies titrated CPAP and BPAP pressure levels during an attended laboratory study.209–213 Meta-analyses were performed to assess the effects of BPAP compared with CPAP in improving AHI, PAP adherence, and daytime sleepiness (see supplemental material, Figure S75 through Figure S77). There were insufficient data available to perform meta-analyses for QOL, neurocognitive function, or side effects; however, data from individual studies were reviewed. A Summary of Findings table is presented in the supplemental material, Table S6. A summary of the evidence for each outcome is provided below.
OSA Severity
The efficacy of BPAP compared to CPAP in reducing OSA severity in adults with OSA was evaluated in a meta-analysis of 2 RCTs.210,213 Participants were randomized to BPAP versus CPAP for a duration of 1 month or 3 months of PAP use. Meta-analysis of the 2 RCTs210,213 did not demonstrate a clinically significant difference in residual AHI with treatment using BPAP compared to CPAP (see supplemental material, Figure S75). The mean difference in residual AHI between BPAP and CPAP was −2.2 events/h (95% CI: −5.1 to 0.7 events/h).
Overall, the analyses suggest that BPAP compared to CPAP in adults with OSA similarly reduces AHI in adults with OSA. The quality of evidence for OSA severity was low due to imprecision and potential publication bias from industry funding.
Adherence
Adherence to BPAP compared to CPAP for the treatment of adult OSA was evaluated using a meta-analysis of 4 studies210–213 in PAP naïve participants and one study209 in CPAP nonadherent participants that reported on adherence. Participants were randomized to BPAP versus CPAP for at least 4 weeks up to a maximum of 1 year of PAP use. The meta-analysis demonstrated no clinically significant difference in adherence with BPAP compared with CPAP (see supplemental material, Figure S76) in the 4 studies that used BPAP as the first line therapy.210–213 The study using BPAP with a modified pressure profile as a rescue therapy in participants nonadherent to CPAP after ≥ 2 weeks demonstrated a clinically significant increase in the point estimate adherence of 0.8 h/night (95% CI: −0.03 to 1.6 h/night) in the BPAP compared to the CPAP group, although the precision was very low.209
Overall, the analyses suggest that BPAP conferred no clinically significant advantage over CPAP in adults with OSA in improving adherence, except potentially as rescue therapy for participants' nonadherent to CPAP. The quality of evidence for adherence was low due to imprecision and potential publication bias from industry funding.
Sleepiness
The efficacy of BPAP compared to CPAP for the treatment of sleepiness in adults with OSA was evaluated using a meta-analysis of 3 RCTs that reported on the ESS.210,211,213 Participants were randomized to BPAP versus CPAP for a duration of 1 month210 or 3 months211,213 of PAP use. The meta-analysis demonstrated no clinically significant difference in self-reported sleepiness in adults with OSA treated with BPAP compared to CPAP (see supplemental material, Figure S77). However, the studies demonstrated that both BPAP and CPAP use improve self-reported sleepiness compared to before initiation of treatment.
Overall, the analyses suggest that BPAP compared to CPAP in adults with OSA similarly reduces sleepiness. The quality of evidence for self-reported sleepiness was low due to potential publication bias from industry funding and imprecision.
Quality of Life
There were insufficient data available to perform a meta-analysis of the impact of BPAP compared to CPAP on QOL in adults with OSA. However, the TF reviewed available data from 2 RCTs that reported on the FOSQ.209,210 Participants were randomized to BPAP versus CPAP for a duration of 1 month210 and 3 months209 of PAP use. One of the studies examined the effects of BPAP compared to CPAP on QOL in participants previously intolerant of CPAP, while the other study recruited participants naïve to PAP, thus they were not combined for meta-analysis. Neither study209,210 demonstrated a clinically significant difference in QOL between BPAP and CPAP as assessed by the FOSQ (see supplemental material, Table S6).
Overall, the analyses suggest that BPAP compared to CPAP in adults with OSA results in similar effects on sleep-related QOL. The quality of evidence for sleep-related QOL was very low due to imprecision and potential publication bias from industry funding.
Side Effects
There were insufficient data available to perform a meta-analysis of side effects. Side effects have been reported with the use of both BPAP and CPAP. These include but are not limited to nasal dryness or irritation, dry mouth, sore throat, sinus infection, and poor sleep quality. These side effects can impact patient adherence with PAP and should be carefully monitored. Participants in the one RCT of CPAP versus BPAP that reported side effects followed participants for 1 year.212 This study reported no clinically significant difference in side effects with similar complaints in both groups with regard to mask discomfort, machine noise, and nasal stuffiness.212 In addition, in one other available study,213 there was no difference between BPAP and CPAP treatment in sleep quality as assessed by the Pittsburgh Sleep Quality Index (see supplemental material, Table S6).
Overall, the quality of evidence for side effects was low due to imprecision and potential publication bias from industry funding.
Overall Quality of Evidence
The outcomes of adherence to PAP therapy, sleepiness, and QOL were determined by the TF to be critical for decision-making. The overall quality of evidence was downgraded to very low due to imprecision and potential bias due to industry funding.
Benefits Versus Harms
There is no expected advantage of BPAP over CPAP in reducing OSA severity, which was confirmed in the meta-analysis of the limited studies available. Thus, in general, the benefits of BPAP are similar to CPAP. A potential benefit of BPAP over CPAP is improved comfort due to a lower pressure during exhalation, which may then improve patient adherence and consequentially improve OSA-related outcomes. However, improved adherence to BPAP was not observed in the available studies that predominantly recruited PAP naïve patients. Only one study, which assessed participants that were CPAP intolerant, suggests that BPAP might improve adherence, although the precision in this study was very low and precluded any definitive statements.209 Finally, a small subset of patients with high PAP requirement that cannot be provided by CPAP devices, but can be provided by BPAP devices, would benefit from BPAP use. Potential harms of BPAP are, in general, like CPAP with a few notable additional considerations including the potential for suboptimal improvement in the residual AHI from an inappropriately low expiratory pressure setting and the substantially higher cost of BPAP devices. The potential benefit of a lower expiratory pressure may be less relevant since modified pressure profiles in current PAP devices perform a similar function.
Given the available data, the TF judged that the potential harms and burden of BPAP outweighed the potential benefit in adults with OSA. Therefore, the TF concluded that in general, clinicians should use CPAP over BPAP in the routine initiation of treatment of adults with OSA.
Patient Values and Preferences
The TF determined that the majority of patients would prefer their OSA be treated with CPAP rather than BPAP based on the similar benefits of treatment with BPAP and CPAP, and the potential for increased cost of BPAP, risk for incomplete treatment, and the availability of modest expiratory pressure reduction in most CPAP devices manufactured today. However, the TF also recognized that BPAP may be of benefit in some CPAP intolerant patients, despite the use of modified pressure profile. In addition, some patients may require BPAP when therapeutic pressure settings are higher than what can be delivered by a CPAP device. In these situations, BPAP devices may be needed for optimal treatment and can be utilized during an initial or subsequent in-laboratory PAP titration study. For specific patients who are unable to tolerate CPAP, due to high pressure requirements, a trial of BPAP may be offered either during the in-laboratory titration or following a period of demonstrated non-acceptance.
Resource Use
The TF recognized that there are significant differences in the cost of BPAP and CPAP devices between countries and medical systems, with small differences in some regions and larger differences in others. While the TF did not identify cost-effectiveness studies and did not undertake a comprehensive cost comparison of BPAP versus CPAP devices, the TF determined based on its collective clinical experience that the cost of BPAP could result in greater resource use.
Educational, Behavioral, and Troubleshooting Interventions With PAP Versus PAP Alone
This section addresses PICO question 10 (see Table 1) and resulted in two recommendation (see Recommendations 7 and 8 in the companion clinical practice guideline).8 A total of 18 RCTs were identified that evaluated the use of some combination of an educational, behavioral, or troubleshooting intervention as an adjunct to initiation of PAP therapy compared to PAP therapy with standard of care alone on PAP adherence, sleepiness, and QOL.207,214–230 Data were not available to assess the effect of these interventions on sleepiness or QOL. Given the substantial heterogeneity in the interventions assessed, the TF decided to divide interventions into one of three broad categories: (1) educational interventions—interventions focused primarily on providing information at initiation of PAP about what OSA is, the downstream consequences of the disorder, what PAP therapy involves, and the potential benefits of PAP therapy; (2) behavioral interventions—interventions focused on behavior change related to use of PAP therapy using strategies such as cognitive behavioral therapy or motivational enhancement; and (3) troubleshooting interventions—interventions focused on close patient communication to identify PAP-related problems and to initiate potential solutions. Of note, both behavioral and troubleshooting interventions include some amount of patient education to motivate the behavior change or understand how to address problems; the TF considered this an integral part of the behavioral or troubleshooting intervention.
All studies included in this assessment compared at least one of these interventions to a standard of care which varied substantially across studies in terms of the level and intensity of care provided, resulting in heterogeneity across studies. Similarly, the intensity of the intervention varied substantially across studies. Overall, there were 7 RCTs207,214,215,220,221,229,230 identified that compared a pure educational intervention versus usual care, 6 RCTs214–219 that compared a behavioral intervention versus usual care, and 9 RCTs207,220,222–228 that compared a troubleshooting with education intervention versus usual care. Although several studies had more than one intervention arm, the TF did not compare the effectiveness of different interventions against each other. Meta-analyses were performed to assess the efficacy of educational, behavioral, and troubleshooting interventions as an ancillary treatment when combined with PAP therapy to increase PAP adherence, thereby improving symptom control in the treatment of OSA as compared with PAP therapy without such adjunctive intervention. The meta-analyses are provided in the supplemental material, Figure S78 through Figure S82. Summary of Findings tables are also included in the supplemental material, Table S7 through Table S9. A summary of the evidence for each outcome by intervention is provided below.
Adherence (Educational Interventions)
A total of 7 RCTs were identified assessing the impact of a pure educational intervention as an adjunct to PAP therapy to improve adherence with PAP.207,214,215,220,221,229,230 The delivery of education varied substantially and included being given written materials, watching a video, or face-to-face didactic sessions. All studies included participants with a mean AHI in the severe range and follow-up ranged from 1 month to 1 year. Meta-analysis of all 7 studies demonstrated a clinically signifi-cant difference in PAP usage of 0.6 h/night (95% CI: +0.0 to 1.1 h/night) (see supplemental material, Figure S78). One trial was excluded from the meta-analysis as the study had undue leverage (weighting) due to standard deviations reported for PAP adherence that were much lower than what the TF would expect in typical OSA populations being treated with PAP.231
The magnitude of effect observed in that study was not different from that seen in the meta-analysis of the other studies. A meta-analysis of 3 RCTs214,221,229 demonstrated that the mean estimate of the impact of an education intervention on obtaining PAP usage > 4 h/night was clinically significant, with an odds ratio of 1.2 (95% CI: 0.8 to 1.9) (see supplemental material, Figure S79).
Overall, these results demonstrate a clinically significant improvement in PAP adherence in adults with OSA with an educational intervention compared to usual care. The quality of evidence for adherence was moderate due to imprecision.
Adherence (Behavioral Interventions)
The efficacy of a behavioral intervention as an adjunct to PAP therapy to improve adherence was evaluated based on 6 RCTs.214–219 There was substantial heterogeneity in terms of the type of intervention (motivational enhancement, cognitive behavioral therapy, stage matched intervention), delivery of intervention (individual, group, peer), and duration of intervention. Follow-up ranged from 1 month to 1 year. A meta-analysis of all 6 RCTs demonstrated a clinically significant difference in PAP usage of 1.2 h/night (95% CI: 0.3 to 2.0 h/night) (see supplemental material, Figure S80). Similarly, a meta-analysis of 5 of these RCTs86,214,216–218 reporting on obtaining PAP usage > 4 h/night found behavioral interventions were associated with a clinically significant odds ratio of 3.1 (95% CI: 1.7 to 5.9) for being adherent (see supplemental material, Figure S81).
Overall, these results demonstrate a clinically significant improvement in PAP adherence in adults with OSA with behavioral interventions compared to usual care. The quality of evidence for behavioral interventions to increase PAP adherence ranged from moderate to high, depending on the measure employed, due to imprecision.
Adherence (Troubleshooting Interventions)
A total of 9 RCTs evaluated the efficacy of education combined with troubleshooting interventions as an adjunct to PAP therapy to improve adherence.207,220,222–228 Substantial heterogeneity was found in the delivery of the intervention including home visits, phone calls from medical or non-medical personnel, automated phone calls, and inquiries via computer. While most studies relied on participants reporting problems, at least one228 used objective data obtained from the PAP device itself. Follow-up assessments ranged from 1 month to 1 year. Meta-analysis demonstrated a clinically significant difference in PAP usage of 0.7 h/night (95% CI: 0.2 to 1.1 h/night) (see supplemental material, Figure S82).
Overall, the results demonstrate a clinically significant improvement in PAP adherence in adults with OSA with troubleshooting combined with education interventions compared to usual care. The quality of evidence for troubleshooting combined with education interventions to increase PAP adherence was moderate due to imprecision.
Overall Quality of Evidence
The overall quality of evidence based on the critical outcome of adherence for the use of educational, behavioral, and troubleshooting interventions was downgraded to moderate due to imprecision.
Benefits Versus Harms
The benefits of all three types of interventions include increased adherence with PAP therapy along with the presumed downstream effects of increased adherence, such as better control of OSA symptoms.181 In addition, increased knowledge and mastery of CPAP therapy would lead to improvements in psychological well-being. Educational interventions, which are typically one-time sessions providing information about OSA and PAP therapy have minimal burden and are relatively easy to implement in virtually all healthcare settings. Behavioral and troubleshooting interventions do impose burdens on the patient including time required and cost to receive the intervention. In addition, the behavioral and troubleshooting interventions may cause a sense of loss of privacy or discomfort to patients. Finally, these interventions require development of infrastructure and expertise that may not be readily available in some healthcare settings. Overall, the TF judged that the benefits of an educational intervention strongly outweigh any potential harms or burdens, while the benefits of a behavioral and/or troubleshooting intervention likely outweigh the harms and burdens of such interventions in most patients.
Patient Values and Preferences
Based on their clinical expertise the TF determined that the vast majority of patients would want an educational intervention provided with PAP therapy, and the majority of patients would want a behavioral and/or troubleshooting intervention to facilitate improved adherence with PAP therapy.
Resource Use
The TF did not identify cost-effectiveness studies evaluating educational, troubleshooting, and behavioral interventions. The cost of implementing educational, troubleshooting, and behavioral interventions by health care providers will vary depending on the complexity of the intervention. For example, educational interventions can range from providing patients with literature to review regarding the diagnosis and treatment of OSA to dedicated one-on-one sessions with a respiratory therapist on how to use PAP therapy. Behavioral therapy interventions may require the most resources given the need for trained behavioral specialists to implement the intervention, the patient's time, and the length and number of sessions needed for a successful program. However, this increased resource use may be offset by the increase in PAP adherence obtained and the relative improvement in patient symptoms. The TF judged that resource use for educational, troubleshooting, and behavioral interventions is warranted to ensure adequate PAP adherence.
Telemonitoring Versus No Telemonitoring Interventions With PAP
This section addresses PICO question 11 (see Table 1) and resulted in one recommendation (see Recommendation 9 in the companion clinical practice guideline).8 A total of 5 RCTs were identified that evaluated the use of remote monitoring of PAP variables to trigger early interventions versus no such system as an adjunct to PAP therapy for the treatment of adults with OSA.228,232–235 Outcomes assessed included adherence to PAP therapy, sleepiness, QOL and PAP-associated side effects. All studies evaluated outcomes at 2–3 months after PAP initiation. However, details about the triggers for intervention and the intensity of the intervention used when poor usage patterns were identified varied greatly across studies likely resulting in heterogeneity of results. Some studies only triggered interventions based on low usage while other studies also triggered interventions for high mask leak, high delivered pressures, and/or high residual AHI. The intervention triggered by concerning PAP data also varied substantially, ranging from text messages to telephone calls, in-person visits with sleep staff, and even in-person visits with a sleep physician. Nevertheless, meta-analyses were performed to assess the efficacy of telemonitoring guided interventions as an ancillary treatment when combined with PAP therapy to increase PAP adherence and thereby improve symptom control in the treatment of OSA in adults as compared with PAP therapy without such an intervention (see supplemental material, Figure S83 and Figure S84). Meta-analysis demonstrated a clinically significant improvement in PAP adherence with the use of telemonitoring. A Summary of Findings table is also included in the supplemental material, Table S10. A summary of the evidence for each outcome is provided below.
Adherence
The efficacy of an intervention guided by remote monitoring of PAP therapy to improve PAP adherence was evaluated using a meta-analysis of 5 RCTs that reported on hours per night of PAP usage.228,232–235 These studies used data from the PAP machine to guide the intervention. Four of the five studies enrolled newly diagnosed participants with OSA with minimal comorbidity and follow-up was short ranging from 1 to 3 months.228,232–235 The meta-analysis demonstrated a clinically significant increase in PAP usage of 1.0 h/night (95% CI: 0.5 to 1.4 h/night) (see supplemental material, Figure S83). A potential explanation for the increase in adherence with telemonitoring is access to real-time assistance from a clinical provider to address PAP-related issues for patients rather than waiting for an appointment to see a clinician. An alternative explanation is that daily monitoring motivates patient to have an increased sense of accountability for their care or to their health care provider.228 These mechanisms have yet to be fully evaluated.
Overall, the analyses demonstrated a clinically significant improvement in adherence in adults with OSA using telemonitoring compared to usual care. The quality of evidence for PAP adherence was high.
Sleepiness
The efficacy of a telemonitoring guided intervention as part of PAP therapy in adult participants with OSA was evaluated using a meta-analysis of 3 RCTs that reported on self-reported sleepiness using the ESS.228,232,234 Studies used interventions that relied on data from the PAP machine. The meta-analysis did not demonstrate a clinically significant reduction in the ESS with the telemonitoring guided intervention as compared to no such intervention (see supplemental material, Figure S84).
Overall, the analyses did not demonstrate a clinically significant improvement in sleepiness in adults with OSA using tele-monitoring compared to usual care. The quality of evidence for self-reported sleepiness was moderate due to imprecision.
Side Effects
Two RCTs were identified that assessed the impact of a telemonitoring guided PAP adherence intervention on PAP-induced side effects; however, data were not reported in a sufficiently standardized format to allow for a meta-analysis.228,235 Side effects assessed included CPAP discomfort, difficulty exhaling, mask leaks, aerophagia, allergic reactions to device components, headache, facial pain or bruises, mouth dryness, or nasal congestion. Both studies demonstrated no clinically significant difference in the frequency of PAP-related side effects, with the exception of one study which suggested that telemonitoring was associated with fewer complaints of a dry mouth (see supplemental material, Table S10).228 The quality of evidence was low due to imprecision.
Quality of Life
The efficacy of adding a telemonitoring guided PAP intervention to PAP therapy on QOL in adult participants with OSA was evaluated in 2 RCTs, one that reported on QOL using the FOSQ232, and the other235 using the EQ5D. In the first trial232, the intervention relied on self-reported problems from the participant to guide management rather than data from the PAP device. In this study, no clinically significant increase in QOL was observed with the telemonitoring guided intervention (see supplemental material, Table S10).232 In the other trial,235 interventions were performed by a clinician based on alerts generated by the PAP monitoring system. Clinically signifi-cant improvements in QOL with CPAP were observed in both telemonitoring and control groups; however, there was no difference in the magnitude of improvement between those who received telemonitoring and those who did not. The quality of evidence for QOL was low due to imprecision.
Overall Quality of Evidence
The outcomes of adherence to PAP therapy, sleepiness, and side effects were determined by the TF to be critical for decision-making. The overall quality of evidence based on the critical outcomes was downgraded to moderate due to imprecision.
Benefits Versus Harms
The benefits of a telemonitoring guided adherence intervention are improvements in PAP adherence to improve control of OSA symptoms and reduce the need for office visits, which might reduce healthcare costs. The primary potential harm to a patient of a telemonitoring guided adherence intervention is the potential loss of privacy, as data on PAP usage are saved on servers owned by PAP manufacturers and may be subject to changes in privacy guarantees. Furthermore, for some patients, increased communication with a health care provider or healthcare medical equipment company may be perceived as intrusive or seem more impersonal which could result in reduced patient satisfaction. Overall, the TF deemed the harms of telemonitoring were minor for most patients and outweighed by the potential benefits.
Patient Values and Preferences
Patient satisfaction with telemonitoring guided adherence interventions was assessed in two of the reviewed studies, with discrepant findings.232,235 In one study, participants in the tele-monitoring group indicated a greater likelihood of continuing to use CPAP compared to the usual care group, while both groups were highly satisfied with their care and were not concerned about being wirelessly monitored. In the other RCT study, participants in the telemonitoring group reported low to moderate overall satisfaction compared to those in the usual care arm (26% versus 4% respectively) with additional concerns about privacy when being identified as being nonadherent or that non-hospital personnel had access to their PAP data. Despite this, 63% of those in the telemonitoring arm placed a high or very high value on the usefulness of the telemonitoring assessment.235 Based on their clinical expertise, the TF judged that the benefits of telemonitoring guided adherence interventions outweigh the harms, and that most patients would want a telemonitoring system as part of a PAP treatment program given the improved PAP adherence, though some may have concerns of privacy or feel less satisfaction with their care.
Resource Use
For some health systems and clinical practices, there may be increased costs associated with a telemonitoring adherence intervention. Some telemonitoring systems also allow patients to self-monitor if they are comfortable with using a patient portal with a computer or smartphone or tablet. The cost of resources spent to implement such programs may be offset by potential savings due to less frequent healthcare visits. One of the studies reviewed suggested that telemonitoring may be a more cost-effective approach with an ICER of €17,359 per QALY. The TF judged that resource use for telemonitoring interventions is warranted to ensure adequate PAP adherence.
ADDITIONAL CONSIDERATIONS
The TF considered whether advancements in specific PAP delivery methods result in clinically significant improvement in patient outcomes compared with standard delivery methods and modalities. The TF reviewed evidence for advancements in PAP delivery methods including the use of modified pressure profiles, different mask interfaces, and heated humidification. The findings of the evidence review for these delivery methods are presented below.
Modified Pressure Profile PAP Versus Standard CPAP
This section addresses PICO question 7 (see Table 1). A total of 7 RCTs investigated the use of modified pressure profile PAP to improve clinical outcomes and reduce side effects.188,236–241 One of these studies used a cross-over design.240 Participants were predominantly male, obese, with moderate to severe OSA, and self-reportedly sleepy. The intervention was administered for a period of at least 1 month (range: 1–6 months). Meta-analyses were performed to assess the impact of modified pressure profile PAP for the treatment of OSA in adults as compared with standard PAP (see supplemental material, Figure S85 through Figure S90). The outcomes analyzed were adherence to PAP therapy, sleepiness, neurocognitive function, QOL, and side effects. A Summary of Findings is also included in the supplemental material, Table S11. A summary of the evidence for each outcome is provided below.
Adherence
The effect of modified pressure profile PAP in adults with OSA on PAP adherence was evaluated using a meta-analysis of 6 RCTs that reported on the number of hours per night the device was used.188,236–240 All 6 studies were performed in participants who were naïve to CPAP, had not used CPAP in the past year, or were not clearly specified. The meta-analysis demonstrated no clinically significant difference in adherence for participants that received modified pressure profile PAP versus standard PAP (see supplemental material, Figure S85). One additional RCT that was reviewed but could not be included in the meta-analysis (data on standard deviation was not provided) reported no clinically significant difference in adherence for participants that received modified pressure profile PAP versus standard PAP.241 One of the included studies allowed participants after the RCT ended to cross-over to using a modified pressure profile PAP in an open-label study for 3 months and demonstrated an increase in adherence in those participants that had had low adherence on standard PAP (< 4 h/night), suggesting that participants with poor adherence might increase their PAP use once transitioned to modified pressure profile PAP.239 The meta-analysis demonstrated no clinically significant improvement in adherence in adults with OSA with modified pressure profile PAP compared to standard PAP in adult patients with OSA, however, the possibility of benefits in patients demonstrating poor adherence remains to be demonstrated.
Sleepiness
A meta-analysis of 5 RCTs188,236–239 demonstrated no clinically significant difference in self-reported sleepiness between participants on modified pressure profile PAP compared to standard PAP (see supplemental material, Figure S86). One additional RCT that was reviewed but could not be included in the meta-analysis reported no significant difference in ESS with modified pressure profile PAP compared to standard PAP.241 The meta-analysis demonstrated no benefit in adults with OSA of modified pressure profile PAP compared to standard PAP in reducing sleepiness in adult patients with OSA.
Quality of Life
The efficacy of modified pressure profile PAP versus standard PAP on sleep-related QOL was evaluated based on two studies188,236 that reported on the FOSQ, one study237 that reported on the SAQLI, and two studies236,237 that reported on the Pittsburgh Sleep Quality Index. Meta-analysis demonstrated no clinically significant difference in sleep-related QOL (see supplemental material, Figure S87 and Figure S88). One study236 reporting global QOL using SF-36 MCS, PCS, and vitality scores found no significant difference in any of these measures. The meta-analyses demonstrated no clinically significant improvements in sleep-related QOL, general QOL measures, and sleep quality in adults with OSA with modified pressure profile PAP compared to standard PAP in adult patients with OSA.
Neurocognitive Function
The efficacy of modified pressure profile PAP versus standard PAP for improvement in neurocognitive function was evaluated using meta-analyses of 3 RCTs that reported on attention and vigilance using the psychomotor vigilance test (PVT).188,236,238 Meta-analysis demonstrated a clinically signifi-cant standardized mean difference of 0.3 (95% CI: 0.0 to 0.6) in PVT reaction time in favor of standard PAP over modified pressure profile and a clinically significant standardized mean difference in PVT lapses of 0.2 (95% CI: −0.2 to 0.7) (see supplemental material, Figure S89 and Figure S90). Given the absence of testing of other important neurocognitive domains and findings from the present meta-analyses, together with imprecision of the point estimate of effect, there was insufficient evidence demonstrating that neurocognitive function in adults with OSA is improved with modified pressure profile PAP compared to standard PAP in adults with OSA.
Side Effects
The efficacy of modified pressure profile PAP versus standard PAP in reducing PAP-related side effects in adults with OSA was evaluated; however, data were not reported in a sufficiently standardized format to perform meta-analyses except for the outcome of sleep quality. Only 3 RCTs reported data on side effects.188,239,241 One study had participants answer a questionnaire that assessed a broad range of side effects including mouth dryness, eye watering, chest pressure, cold sensation, frequent awakening, mask leak, and machine noise.241 At 7 weeks, there were no significant differences in any of these side effects between the modified pressure profile PAP and standard PAP groups.241 Another study assessed participant side effects and comfort, but did not specify what was assessed.239 This study reported no differences in side effects or participant comfort between the groups at 3 months.239 The third study assessed mask comfort and sleep quality using a visual analog scale.188 There were no differences in mask comfort between the groups; however, there was a trend in sleep quality being worse in the modified pressure profile PAP group at 90 and 180 days after the start of therapy.188 Overall, there were no clinically significant differences in side effects or in participant preference188,240 between modified pressure profile PAP and standard PAP. Nevertheless, analyses from at least one study239 suggest that certain patient populations, particularly poorly adherent patients, may benefit from modified pressure profile PAP, though this requires further investigation.
Nasal PAP Versus Intranasal PAP Versus Oral PAP Versus Oronasal PAP
This section addresses PICO question 8 (see Table 1). A total of 11 studies (3 observational studies and 8 RCTs) were identified which evaluated the effects of different PAP interfaces on reducing AHI, improving adherence to PAP therapy, sleepiness and QOL and reducing side effects.208,242–251 Participants in the 8 RCTs were predominantly middle-aged males without major medical comorbidities with moderate to severe OSA who were treated with each interface for at least 1 and up to 8 weeks (median duration of 4 weeks) in either parallel or crossover designs. Participants were previously untreated except for one study243 in which participants established on PAP treatment for > 6 months were randomized to intra-nasal versus nasal treatment. Generally, participants in these studies were not selected based on specific side effects (eg, nasal congestion, oral dryness) or mask interface intolerance, except for one study where participants with significant nasal resistance were excluded.248 Data on adherence for nasal versus oronasal interfaces were also analyzed from 3 non-randomized studies.249–251 Participants were predominantly male without major medical comorbidities, with previously untreated moderate to severe OSA, and were treated for at least 3 weeks up to 24 months. Meta-analyses were performed comparing different interfaces to standard nasal interfaces for the outcomes of OSA severity, adherence, and self-reported sleepiness (see supplemental material, Figure S91 through Figure S98). A Summary of Findings is also included in the supplemental material, Tables S12–S14. A summary of the evidence for each outcome is provided below.
OSA Severity
The efficacy of intra-nasal compared to nasal interfaces for the treatment of OSA severity in adults was evaluated using a meta-analysis of 3 cross-over RCTs; two of 3–4 weeks duration208,242 involving newly treated participants with a range of PAP pressures, and one for a 1-week period243 in participants previously established on nasal PAP treatment at ≥ 12 cm H2O for > 6 months. There was no clinically significant difference in AHI (see supplemental material, Figure S91).
The efficacy of oronasal compared to nasal interfaces for the treatment of OSA severity in previously untreated adults was evaluated using a meta-analysis of 2 cross-over RCTs; one of 3 weeks duration245 and one248 of 4 weeks duration. Residual AHI was higher with oronasal than nasal interfaces, although this difference was not clinically significant (see supplemental material, Figure S95).
There was insufficient evidence to perform a meta-analysis on OSA severity for oral versus nasal interfaces. One RCT employing a 4-week cross-over design246 demonstrated no clinically significant differences in AHI with oral compared with nasal interfaces.
Adherence
The efficacy of intra-nasal compared with nasal interfaces for improving adherence to PAP therapy was evaluated using meta-analyses of 2 cross-over RCTs of 3–4 weeks duration208,242 involving newly treated participants with a range of PAP pressures, and one RCT243 for 1 week periods in participants previously established on nasal PAP treatment at ≥ 12 cm H2O for > 6 months. There was no clinically significant difference in mean adherence208,242,243 and percent nights of CPAP use208,242 with intra-nasal interfaces compared with nasal interfaces (see supplemental material, Figure S92 and Figure S93).
The efficacy of oronasal compared with nasal interfaces for improving adherence was evaluated in meta-analyses of 3 cross-over RCTs of 3 to 4 weeks duration,244,245,248 which demonstrated a clinically significant improvement in adherence of 0.6 h/night (95% CI: −0.2 to 1.3 h/night) with nasal interface compared with oronasal interface (see supplemental material, Figure S96). A meta-analysis was performed of 3 non-randomized studies in which participants were predominantly male without major medical comorbidities, with previously untreated moderate to severe OSA, treated for at least 3 weeks up to 24 months.249–251 This also demonstrated a clinically signifi-cant difference in adherence of 0.7 h/night (95% CI: 0.2 to 1.2 h/night) in favor of nasal interfaces (see supplemental material, Figure S97).
There was insufficient evidence to perform meta-analysis for the effects on adherence for oral versus nasal interfaces. The literature search identified one 8-week parallel arm RCT247 that demonstrated a clinically significant difference in adherence with a mean difference of 0.9 h/night (95% CI: −0.7 to 2.5 h/night) in favor of oral interfaces.
The meta-analyses demonstrated clinically significant improvements in adherence in adults with OSA with nasal interfaces compared to oronasal interfaces. The evidence review comparing adherence between oral versus nasal interfaces was limited to one study and suggested increased adherence in adults with OSA with an oral compared to a nasal interface. However, based on the clinical experience of the TF, most patients have difficulties using an oral interface over the long-term.
Sleepiness
The efficacy of intra-nasal compared with nasal interfaces for improving self-reported sleepiness was evaluated using a meta-analysis of two crossover studies, one employing a 3 week duration208 and one employing a 4 week duration242, that demonstrated no clinically significant difference in self-reported sleepiness between intra-nasal and nasal interfaces as assessed with the ESS (see supplemental material, Figure S94).
The efficacy of oronasal versus nasal interfaces was evaluated using a meta-analysis of 2 RCTs.244,248 The meta-analysis demonstrated no clinically significant difference in self-reported sleepiness between the interfaces as assessed with the ESS (see supplemental material, Figure S98). There was insufficient evidence to perform meta-analysis for the effects on self-reported sleepiness for oral versus nasal interfaces. One RCT246 demonstrated no clinically significant difference in self-reported sleepiness with oral interfaces compared with nasal interfaces. The meta-analyses demonstrated no clinically significant differences in self-reported sleepiness between the different mask interfaces.
Quality of Life
There was insufficient evidence to perform meta-analysis for the effects of the various interface types on QOL. Only one RCT208 was identified that met inclusion criteria which assessed the effect of intra-nasal versus nasal interfaces on QOL over 3 weeks each in a cross-over RCT. QOL was assessed with the FOSQ and no clinically significant difference in QOL was found comparing intra-nasal versus nasal interfaces.208 No RCT evidence was available to assess the effects of oronasal or oral interfaces compared to nasal interfaces on QOL. There was insufficient evidence demonstrating differences in QOL improvement on PAP with any mask interface.
Side Effects
The efficacy of the various mask interfaces in reducing PAP-related side effects in adults with OSA was evaluated. However, sufficient standardized data were not available to perform a meta-analysis for any of the interface types. A well-sealed interface is necessary for effective delivery of PAP, and mask and/or mouth leak may adversely impact treatment efficacy. Side effects have been reported with all forms of PAP interface and may adversely impact adherence. Side effects may differ between interface type and between individuals for a given interface. Improvements of air leak and other side effects through interface selection may have beneficial effects on treatment adherence and efficacy.
For intra-nasal versus nasal interfaces, side effect data were reported from 2 cross-over RCTs of 3-week and 4-week duration208,242 involving newly treated participants with a range of PAP pressures, or for 1 week periods243 in participants previously established on nasal PAP treatment at ≥ 12 cm H2O for > 6 months. An overall multi-item side effect score favored intra-nasal interfaces in one study of newly treated participants,208 but there were no clinically significant differences in overall side effects between interfaces for the other 2 studies.242,243 Individual side effects including pressure sensation on the face, skin irritation, claustrophobia and obtrusiveness were in general less for intra-nasal interfaces in the 3 studies, while nasal interfaces were scored as being less obtrusive. There were no clinically significant differences between interfaces for nasal or oral congestion or dryness. In one study,208 overall mask satisfaction scores were significantly higher for intra-nasal interfaces while in the other 2 studies242,243 which determined participant preference, there was no clinically significant difference between intra-nasal versus nasal interfaces either for newly treated or previously treated participants. Overall, differences in side effects were not clinically signifi-cant between the two interfaces.
For oronasal versus nasal interfaces, two 4-week cross-over RCTs244,248 and two non-RCTs249,250 evaluating treatment periods of up to 24 months (6 months, mean of 4.5 months, respectively), reported data on side effects. In one cross-over RCT,244 19 of 20 participants rated the nasal interface as more comfortable. Higher scores for nasal and throat dryness but not nasal stuffiness were clinically significant with the nasal interface while higher scores of self-reported mask leak, sore eyes, claustrophobia and difficulty exhaling were clinically significant with the oronasal interface. All participants chose the nasal interface for long-term treatment. In another cross-over RCT,248 mask noise and leak were greater with oronasal masks which were also reported to be harder to fit and hold in place, while other side effects did not differ. Participant preference favored nasal over oronasal masks with 21 of 33 participants selecting the nasal mask option, and only 4 choosing the oronasal mask option. In one of the non-RCTs, oronasal dryness was more prevalent with oronasal than nasal interfaces (80% versus 46%, respectively).250 In a non-RCT of 2,311 participants in whom 62% were using nasal and 26% oronasal interfaces, there were greater reports in symptoms of eye irritation, dry mouth, choking sensation and psychologically perceived inconvenience with oronasal interfaces, while there were no clinically signifi-cant differences between oronasal and nasal interfaces in nasal congestion, headache, aerophagia, or family tolerance of treatment. In a multivariate analysis, PAP nonadherence in this cohort was independently associated with use of the oronasal interface.249 In these non-RCT cohorts, oronasal interfaces were least often chosen by participants for long-term treatment compared with nasal and intra-nasal interfaces.249,250 Overall, there are clinically important differences in side effects with oronasal compared with nasal interfaces. The increased side effects appear to result in a patient preference for nasal over oronasal interfaces and translate into a clinically significant reduction in adherence with oronasal compared to nasal PAP (see Adherence above). In light of this, as well as the tendency to increased residual OSA severity (see OSA Severity above) and in some studies increased pressure requirements with oro-nasal compared to nasal interfaces,248,252–257 nasal or intranasal mask interfaces may be preferred over oronasal interfaces for the routine initiation of PAP therapy in adults with OSA. However, patient factors will vary, and interface selection should be based on individual patient preference and tolerance.
For oral versus nasal interfaces, side effect data were reported in two 8-week parallel arm RCTs246,247 and two non-RCTs249,250 evaluating participants over 6 months treatment. For both RCTs, oral interfaces were associated with more oral dryness, excess salivation, lip and gum discomfort, while nasal interfaces were associated with more self-reports of air leaks, nasal dryness and strap/mask discomfort, with no differences in interface dislodgement.246,247 In one RCT,246 there was a trend for participants to prefer nasal (71%) over oral (29%) interfaces. In the non-RCTs, oral interfaces were associated with significantly more upper airway dryness and “rainout” (condensation) than nasal interfaces.249,250 In one study in which participants selected mask interface for initial titration and later long-term use, 27% chose oral versus 66% nasal initially, while long-term, after 6 months, 43% of those who initially selected an oral interface switched to nasal, while no one who initially selected a nasal interface switched masks.250 For individual patients there may be clinically important differences in side effects with oral compared with nasal interfaces, which on average lead patients to select nasal over oral interfaces, but ultimately interface selection should be based on individual patient preference and tolerance.
Humidified PAP Versus No Humidified PAP
This section addresses PICO question 9 (see Table 1). A total of 9 RCTs were identified that evaluated the use of PAP with humidification versus PAP without humidification to improve one or more of the following outcomes: adherence to PAP therapy, sleepiness, QOL, or PAP-related side effects including nasal discharge, nasal congestion, dry nose, epistaxis, and dry mouth/throat.258–266 All studies evaluated only participants with OSA who were naïve to PAP. The mean AHI in nearly all studies was in the severe range. The duration of treatment for most studies was only 3–4 weeks, although one study did follow participants to 1 year.258 Most studies utilized heated humidification, except for 2 studies where one study259 compared both heated and cold pass-over humidification to no humidification and one study263 that did not specify the form of humidification used. Meta-analyses were performed to assess the efficacy of humidification as an ancillary treatment when combined with PAP to increase PAP adherence and QOL and reduce sleepiness and PAP-related side effects in the treatment of OSA in adults as compared with PAP therapy without humidification (see supplemental material, Figure S99 through Figure S106). A Summary of Findings is also included in the supplemental material, Table S15. A summary of the evidence for each outcome is provided below.
Adherence
The efficacy of humidification with PAP therapy to improve PAP adherence was evaluated using a meta-analysis of 9 RCTs that reported on hours per night of PAP usage.258–266 The meta-analysis demonstrated no clinically significant difference in PAP usage in adults with OSA with the addition of humidification (see supplemental material, Figure S99).
Sleepiness
The efficacy of humidification when added to PAP for the treatment of OSA in reducing sleepiness in adults was evaluated using a meta-analysis of 8 RCTs.258–264,266 All of the included studies assessed self-reported sleepiness using the ESS. The meta-analysis demonstrated no clinically significant difference in self-reported sleepiness in adults with OSA with humidification as compared to no humidification (see supplemental material, Figure S100).
Quality of Life
A meta-analysis of 3 RCTs that reported on the effect of humid-ification on QOL using the SAQLI,258 QSQ,265 or FOSQ266 was performed to evaluate the efficacy of adding humidification to PAP for the improvement of QOL in adults with OSA. Meta-analysis demonstrated no clinically significant difference in QOL in adults with OSA with or without humidification (see supplemental material, Figure S101).
Side Effects
Meta-analyses were conducted for each identified PAP-related side effect whenever possible. The studies assessed only participants with newly diagnosed OSA with no prior history of treatment and only one study266 specifically recruited individuals with nasal symptoms. Meta-analyses of 2 RCTs260,262 demonstrated clinically significant reduction in the odds of nasal discharge, dry nose, epistaxis, and dry mouth/throat with the use of humidified PAP while meta-analyses of 3 RCTs260,262,263 demonstrated clinically significant reduction in the odds of nasal congestion and dry mouth with the use of humidified PAP (see supplemental material, Figure S102 through Figure S106). In one study, while overall nasopharyngeal scores were not significantly different, complaints of dry or sore throat were significantly less with humidified than non-humidified PAP.266 Another study reported clinically significant reductions in the incidence of sinus pain/headache, sore throat, hoarse voice, and “smell” with odds ratios of 0.4 (95% CI: 0.1 to 1.4), 0.3 (95% CI: 0.1 to 1.6), 0.8 (95% CI: 0.2 to 3.2), and 0.7 (95% CI: 0.2 to 2.3) with humidification, respectively.260 However, this study reported no clinically significant differences in cough or sinus infection.260 Potential adverse effects of humidification including “rain out” (condensation) of water into the PAP circuit or participants' face, nose or mouth with excessive humidity settings, or the inconvenience of purchasing distilled water and additional cleaning requirements were not reported on. Five studies259–262,264 reported on participant preference or satisfaction with the use of humidification, with participants on average either preferring heated humidification260,264 or expressing no clear preference261,262 for heated humidification. Overall, review of the data suggests a clinically significant reduction in the incidence of CPAP-related side effects with humidification, which together with the widespread availability of integrated humidifiers on current PAP devices, favors the routine use of humidification. However, some patients may determine that the absence of humidification results in no untoward side effects and the decision to use humidification should be based on individual patient preference and tolerance.
DISCUSSION AND FUTURE DIRECTIONS
The systematic review performed by the TF identified many areas that merit further investigation to determine effects on patient outcomes and inform clinical decision-making.
Effect of PAP on OSA-Related Outcomes
More work in both short-term and long-term studies is needed to determine the efficacy of PAP therapy to improve key outcomes currently associated with OSA including symptoms outside of excessive sleepiness (eg, nocturia, insomnia, or sexual dysfunction) impaired cognition and mood, reduced QOL, increased MVCs, as well as hypertension, cardiovascular disease, and metabolic disorders (eg, pre-diabetes and diabetes).
Neurocognitive Outcomes and Mood
Although the quality of evidence for neurocognitive outcomes was rated as moderate, the number of studies available for review was small and these studies focused on participants without baseline deficits in neurocognitive function. Therefore, RCTs targeted at addressing whether PAP improves neurocognitive outcomes in patients with OSA with baseline cognitive impairment would be highly informative. Consensus on core validated assessment tools for each neurocognitive domain should be reached on measures to be routinely included in future PAP intervention studies with sufficient power to detect meaningful changes. Similarly, trials evaluating the impact of PAP on mood in those with baseline depression or anxiety should be a priority.
Motor Vehicle Crashes
The quality of evidence regarding reductions in MVC with PAP intervention was of low to moderate quality due to issues of study design ascertainment of the outcome. The current evidence base has been used to set public policy on driving restrictions for drivers on the state level and safety-sensitive personnel (eg, commercial motor vehicle drivers, pilots, railroad workers, etc.) at a federal level. Despite these policies, high quality data is currently not available to indicate which patients with OSA are most likely to experience a reduction in MVC risk from CPAP. Although randomized trials in this area are not feasible, larger scale, prospective studies with appropriate control groups and objective ascertainment of MVC through insurers or registries could and should be performed to further inform development of appropriate public policies. Additional studies in commercial motor vehicle drivers and other safety sensitive occupations (eg airline pilots or railroad workers) are specifically needed given that public policy decisions focus on these populations. Development of more sensitive biomarkers using driving simulators or other techniques that are highly correlated and validated against real world crash risk are needed, which can then be applied in treatment trials of patients with OSA.
Hypertension
Regarding hypertension, progress has been made in determining the beneficial effects of PAP. Relative to prior guidelines, the TF judged that PAP should be used for patients with OSA and hypertension. The BP lowering effects, though small at the patient level, may be meaningful at a population health level. However, there are still several knowledge gaps that remain to be addressed. Long-term studies are needed to determine the benefits of PAP on hypertension and hypertension-related outcomes (eg, chronic kidney disease, congestive heart failure, and stroke) for periods more than a year, particularly in patients with resistant hypertension. Furthermore, data are needed on whether patients with milder forms of OSA derive the same BP lowering benefits as patients with moderate to severe OSA. Additional investigations as to whether non-sleepy patients with OSA derive similar benefits to sleepy patients with OSA may help determine in future guidelines whether the strength of the recommendation can be increased or not. Given recent small, short-term trials showing that drug therapy lowers BP more robustly than CPAP in those with OSA and hypertension and that effects may be synergistic between anti-hypertensive medications and PAP,159,160 future trials should explore how OSA screening and treatment with PAP might best integrate into current guidelines on the approach to treating hypertension.
Cardiovascular Events and Metabolic Outcomes
The TF found conflicting data regarding PAP-related effects for cardiovascular events and no significant PAP-related effects on the metabolic outcomes reviewed (ie, fasting glucose, hemoglobin A1c). Non-randomized data suggest that PAP reduces cardiovascular events, while randomized control trial data have not shown any benefit. While non-randomized cohort studies are known to over-estimate therapy-related effects compared to randomized controlled designs, current RCTs are limited by several factors: the extent of PAP adherence obtained (eg, 3–4 h/night of PAP use), the severity of OSA in the patient sample, and restriction of recruitment to non-sleepy patients (which likely contributes to sub-optimal PAP adherence). The RCT data currently available, therefore, are more informative with respect to PAP-related effectiveness, than on the efficacy of PAP in OSA on reducing cardiovascular events. Efficacy studies on the effects of PAP on metabolic disorders including diabetes, but in particular for pre-diabetes are needed. A major challenge in the implementation of such trials will be ensuring long-term adherence to PAP for time periods long enough for adequately powered studies. Studies incorporating known strategies to increase adherence (educational, behavioral, troubleshooting and telemonitoring interventions, mask optimization, etc.), development of novel methods to improve PAP adherence (see “Adherence Strategies”, below), and innovative strategies to optimize PAP adherence in the long term need to be developed as an integral component of large scale RCTs examining key outcomes. Furthermore, understanding the dose-response relationship of PAP for various cardiovascular and metabolic outcomes is needed.
Patient Groups and Comorbidities
From the review, the TF believes that additional research is needed to answer important questions regarding the foregoing outcomes in specific patient populations, through inclusion of traditionally under-represented groups including minorities, women, and older people. More work through both high-quality observational studies and RCTs are needed to determine whether treatment of OSA with PAP improves neurocognitive, cardiovascular, and metabolic outcomes in these under-represented groups. Another research priority is the development of methods to increase PAP adherence in groups that traditionally have low adherence (eg, African-Americans, adolescents, the cognitively impaired) to ensure health equity. Patient groups with comorbidities that are highly prevalent in OSA and for which OSA treatment may reduce the risk of additional events or disease progression that merit further investigation include those with a history of stroke, myocardial infarction, heart failure, and atrial fibrillation. In addition, comparative effectiveness studies are needed to assess the effects of PAP compared to other OSA treatments on OSA-related outcomes. Identifying patient subgroups that may benefit from one intervention compared to another will help to realize the goal of precision medicine.
PAP Modalities and In-Laboratory Versus APAP Strategies
While the TF recommended either APAP or CPAP should be used in the treatment of OSA, there are additional questions to be addressed. Recent data suggest that the blood pressure reduction reported with APAP may not be as robust with CPAP—whether this difference has clinical relevance is unclear.156,157 In addition, studies are needed in patients with co-morbid conditions commonly seen in sleep clinic populations (eg, obstructive and restrictive lung disease, CHF, pulmonary hypertension, neuromuscular disease, co-existing central sleep apnea, etc.) to determine the benefits, risks, and contraindications of APAP versus in-laboratory based PAP. Existing APAP algorithms could prove to be suboptimal, for example, in patients with markedly altered respiratory mechanics, requiring the development of modified and/or enhanced algorithms. Further research will also be required to determine whether application of existing automated BPAP algorithms may be preferable to CPAP or APAP in such patients and whether further development of automated PAP algorithms is required.
More work is also needed to establish the cost-effectiveness of CPAP compared to APAP as well as PAP compared to other therapies in the long-term treatment of OSA. The impact of mask leak on APAP effectiveness and patient adherence also needs to be determined. In addition, little remains known about patient preferences for strategies using either CPAP or APAP, which would be informative for future guidelines.
Review of data regarding BPAP for the treatment of OSA led the TF to recommend that BPAP should not be used in the routine treatment of OSA. Further research, however, should clarify patient groups that might benefit from BPAP. For example, research evaluating the benefit, risks, contraindications, and outcomes of BPAP in nonadherent patients or patients deemed to be at high risk of nonadherence are needed. Such data would inform if BPAP should be used as initial therapy or rescue therapy in certain subgroups.
Adherence Strategies
All medical therapies have challenges with patient adherence. PAP therapy for OSA has unique challenges as available data indicate that optimal benefit is derived from continued use throughout the patient's sleeping period while clinical trials continue to demonstrate suboptimal group adherence ranging from 3–5 h/night. Post-hoc analyses of recent RCTs focusing on cardiovascular events suggest better outcomes with more consistent PAP use across the night. However, these analyses are confounded by concerns as to whether PAP-adherent patients are also more adherent to non-PAP therapies for healthy lifestyle habits (eg, smoking cessation, regular exercise) or co-morbid disorders (eg, beta-blockers for coronary artery disease, statins for hyperlipidemia, or anti-hypertensives for hyper-tension), which may explain the positive findings. Given this, substantial work remains to be done to determine the optimal combination of strategies to maximize adherence including PAP-related technologies (eg, cloud-based monitoring systems), mask interfaces, educational, behavioral and troubleshooting interventions. Effective adherence approaches can then be deployed in future RCTs examining the potential benefits of PAP for OSA-related outcomes. Of note, virtually all research on increasing adherence has evaluated outcomes at 3 months or less, despite evidence that usage continues to wane over time long-term. Evaluation of strategies to maintain adherence long term is a critical research priority. The quality of evidence with respect to mask interfaces remains low. RCTs should be performed examining effectiveness of different mask types on OSA severity, pressure requirements, side effects, and adherence. Such studies will need to carefully consider whether patient factors such as nasal obstruction and related symptoms may affect efficacy of therapy and adherence. Furthermore, given differences in facial structure, studies of mask type need to be conducted across a broad range of racial and ethnic groups. A better understanding of the effects of humidification in promoting adherence is needed and which patient subgroups are most likely to benefit (eg, all patients, nonadherent patients, nasal obstruction, or oro-nasal dryness). This would have implications for patient preference and the cost-effectiveness of these interventions. RCTs examining whether APAP, mask type, humidification and modified pressure profiles improve adherence and clinically relevant outcomes in poorly adherent patients or patient at high risk of nonadherence are needed.
Significant progress has been made in examining educational, behavioral, troubleshooting, and telemonitoring strategies that can improve adherence to PAP. Future research should focus on strategies for identification of patient-factors that place them at risk for nonadherence prior to PAP use, and the development and validation of specific algorithms (eg, number of hours, significant leaks, residual AHI, residual central events, or some combination) to utilize with telemonitoring in order to identify nonadherent patients early after initiation of PAP. Comparative effectiveness studies and implementation research of these strategies will be needed to develop a comprehensive adherence program that can be deployed into routine clinical care as well as for all long-term randomized clinical trials of CPAP.
DISCLOSURE STATEMENT
Dr. Indu Ayappa disclosed that she receives royalties from patents held on some PAP devices. Dr. Ayappa's potential conflict was managed by requesting that she refrain from participation on any discussions pertaining to devices for which she may hold a patent. She was also asked to refrain from voting on recommendations pertaining to those devices. Dr. Sanjay Patel disclosed that he receives compensation from Bayer Pharmaceuticals to assess the impact of OSA and its treatment on pulmonary hypertension and other cardiovascular outcomes. He was asked to refrain from participation on any discussion pertaining to the use of PAP to treat hypertension. He was also asked to refrain from voting on recommendations pertaining to the use of PAP to treat hypertension. Mr. Harrod is employed by the American Academy of Sleep Medicine. No other task force members had any relevant conflicts of interest to disclose.
ACKNOWLEDGMENTS
The task force thanks Dr. Romola Bucks (University of Western Australia) and Dr. Gerry Taylor (Case Western Reserve University) for lending their expertise in determining how neurocognitive outcomes should be addressed in this guideline.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- APAP
auto-adjusting positive airway pressure
- BP
blood pressure
- BPAP
bilevel positive airway pressure
- CPAP
continuous positive airway pressure
- DBP
diastolic blood pressure
- EPAP
expiratory positive airway pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HADS
Hospital Anxiety and Depression Scale
- IPAP
inspiratory positive airway pressure
- LVEF
left ventricular ejection fraction
- MSLT
Multiple Sleep Latency Tes
- MVC
motor vehicle crashes
- MWT
Maintenance of Wakefulness Test
- OSA
obstructive sleep apnea
- OSLER
Oxford Sleep Resistance Test
- PAP
positive airway pressure
- PICO
Patient, Population or Problem, Intervention, Comparison, and Outcomes
- QOL
quality of life
- QSQ
Quebec Sleep Questionnaire
- RCT
randomized controlled trial
- RDI
respiratory disturbance index
- REI
respiratory event index
- SAQLI
Calgary Sleep Apnea Quality of Life Index
- SBP
systolic blood pressure
- SF-36
Short Form of the Medical Outcomes Survey
- SMD
standardized mean differences
- T2DM
type 2 diabetes mellitus
- TF
task force
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 5.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 7.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343. doi: 10.5664/jcsm.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 10.Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6(5):491–509. [PMC free article] [PubMed] [Google Scholar]
- 11.Aurora RN, Bista SR, Casey KR, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553–1562. doi: 10.5665/sleep.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96–102. doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 17.Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol. 2016;31(2):186–193. doi: 10.1093/arclin/acv087. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 19.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state-ofthe-art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gastaut H, Tassinari CA, Duron B. [Polygraphic study of diurnal and nocturnal (hypnic and respiratory) episodal manifestations of Pickwick syndrome] Rev Neurol (Paris) 1965;112(6):568–579. [PubMed] [Google Scholar]
- 21.Jung R, Kuhlo W. Neurophysiological studies of abnormal night sleep and the Pickwickian syndrome. Prog Brain Res. 1965;18:140–159. doi: 10.1016/s0079-6123(08)63590-6. [DOI] [PubMed] [Google Scholar]
- 22.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 25.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009;(4):CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 28.Farre R, Navajas D, Montserrat JM. Technology for noninvasive mechanical ventilation: looking into the black box. ERJ Open Res. 2016;2(1) doi: 10.1183/23120541.00004-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu K, Roisman G, Aouf S, Escourrou P. All APAPs are not equivalent for the treatment of sleep disordered breathing: a bench evaluation of eleven commercially available devices. J Clin Sleep Med. 2015;11(7):725–734. doi: 10.5664/jcsm.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushida CA, Rao S, Guilleminault C, et al. Cervical positional effects on snoring and apneas. Sleep Res Online. 1999;2(1):7–10. [PubMed] [Google Scholar]
- 31.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 32.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 33.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(6):501–508. doi: 10.1164/rccm.200810-1558OC. [DOI] [PubMed] [Google Scholar]
- 36.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67(12):1090–1096. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 37.Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomised controlled trial. Sleep Med. 2009;10(9):1056–1058. doi: 10.1016/j.sleep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Alakuijala A, Maasilta P, Bachour A. The Oxford Sleep Resistance test (OSLER) and the Multiple Unprepared Reaction Time Test (MURT) detect vigilance modifications in sleep apnea patients. J Clin Sleep Med. 2014;10(10):1075–1082. doi: 10.5664/jcsm.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163(5):565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavior Sciences. Hillsdale, NJ: L. Eribaum Associates; 1988. [Google Scholar]
- 42.Ware JE, Kosinski M, Bjorner JB, et al. User's Manual for the SF-36 v2 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 43.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40(2):577–591. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacasse Y, Bureau M, Series F. A new standardised and self-administered quality of life questionnaire specific to obstructive sleep apnoea. Thorax. 2004;59:494–499. doi: 10.1136/thx.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 48.Turnbull F Blood Pressure Lowering Treatment Trialists Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 49.Chan KS, Aronson Friedman L, Bienvenu OJ, et al. Distribution-based estimates of minimal important difference for hospital anxiety and depression scale and impact of event scale-revised in survivors of acute respiratory failure. Gen Hosp Psychiatry. 2016;42:32–35. doi: 10.1016/j.genhosppsych.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Group NS, McMurray JJ, Holman RR, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 52.Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366(14):1319–1327. doi: 10.1056/NEJMcp1013127. [DOI] [PubMed] [Google Scholar]
- 55.Look ARG, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 57.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294(9):1025–1033. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 58.Hingson R, Heeren T, Winter M. Effects of recent 0.08% legal blood alcohol limits on fatal crash involvement. Inj Prev. 2000;6(2):109–114. doi: 10.1136/ip.6.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tippetts AS, Voas RB, Fell JC, Nichols JL. A meta-analysis of .08 BAC laws in 19 jurisdictions in the United States. Accid Anal Prev. 2005;37(1):149–161. doi: 10.1016/j.aap.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Morgenthaler TI, Deriy L, Heald JL, Thomas SM. The evolution of the AASM clinical practice guidelines: another step forward. J Clin Sleep Med. 2016;12(1):129–135. doi: 10.5664/jcsm.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amaro AC, Duarte FH, Jallad RS, Bronstein MD, Redline S, Lorenzi-Filho G. The use of nasal dilator strips as a placebo for trials evaluating continuous positive airway pressure. Clinics (Sao Paulo) 2012;67(5):469–474. doi: 10.6061/clinics/2012(05)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 63.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 64.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(6):773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 65.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 66.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107(1):68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 67.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29(4):720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 68.Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 69.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52(2):114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53(5):341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 72.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 73.Hack M, Davies RJ, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000;55(3):224–231. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67(12):1081–1089. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 75.Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. 2006;61(12):1083–1090. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 77.Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32(6):1488–1496. doi: 10.1183/09031936.00026608. [DOI] [PubMed] [Google Scholar]
- 78.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–359. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 80.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1459–1463. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 81.McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–812. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 82.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164(6):939–943. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 83.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164(4):608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 84.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184(3):355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 85.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3 Pt 1):858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 86.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 87.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31(11):1551–1558. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sivam S, Phillips CL, Trenell MI, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40(4):913–918. doi: 10.1183/09031936.00177011. [DOI] [PubMed] [Google Scholar]
- 89.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128(6):848–861. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 91.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 92.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson. 2010;12:50. doi: 10.1186/1532-429X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112(3):375–383. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 95.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 96.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57(3):549–555. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 97.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28(10):2161–2168. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 98.Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–1494. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 99.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 100.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 101.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax. 2008;63(7):578–583. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 102.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9(6):660–666. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 103.Jones A, Vennelle M, Connell M, et al. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep Med. 2013;14(12):1260–1265. doi: 10.1016/j.sleep.2013.08.786. [DOI] [PubMed] [Google Scholar]
- 104.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 105.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 107.Parra O, Sanchez-Armengol A, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24(1):47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 108.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with non-sleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 109.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 110.Smith LA, Vennelle M, Gardner RS, et al. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007;28(10):1221–1227. doi: 10.1093/eurheartj/ehm131. [DOI] [PubMed] [Google Scholar]
- 111.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45(12):2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 112.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 113.Craig S, Kylintireas I, Kohler M, et al. Effect of CPAP on cardiac function in minimally symptomatic patients with OSA: results from a subset of the MOSAIC randomized trial. J Clin Sleep Med. 2015;11(9):967–973. doi: 10.5664/jcsm.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalmases M, Sole-Padulles C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214–1223. doi: 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 115.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 117.Muxfeldt ES, Margallo V, Costa LM, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65(4):736–742. doi: 10.1161/HYPERTENSIONAHA.114.04852. [DOI] [PubMed] [Google Scholar]
- 118.Barbe F, Sunyer J, de la Pena A, et al. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74(1):44–49. doi: 10.1159/000094237. [DOI] [PubMed] [Google Scholar]
- 119.Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, von Wichert P. Risk of traffic accidents in patients with sleep-disordered breathing: reduction with nasal CPAP. Eur Respir J. 1996;9(12):2606–2611. doi: 10.1183/09031936.96.09122606. [DOI] [PubMed] [Google Scholar]
- 120.de Oliveira AC, Martinez D, Massierer D, et al. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: a randomized, double-blind, clinical trial. Am J Respir Crit Care Med. 2014;190(3):345–347. doi: 10.1164/rccm.201403-0479LE. [DOI] [PubMed] [Google Scholar]
- 121.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep. 1996;19(5):378–381. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- 122.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161(3 Pt 1):857–859. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 123.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56(7):508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hall AB, Ziadi MC, Leech JA, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130(11):892–901. doi: 10.1161/CIRCULATIONAHA.113.005893. [DOI] [PubMed] [Google Scholar]
- 125.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep. 2000;23(3):383–389. [PubMed] [Google Scholar]
- 126.Karimi M, Hedner J, Habel H, Nerman O, Grote L. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish Traffic Accident Registry data. Sleep. 2015;38(3):341–349. doi: 10.5665/sleep.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komada Y, Nishida Y, Namba K, Abe T, Tsuiki S, Inoue Y. Elevated risk of motor vehicle accident for male drivers with obstructive sleep apnea syndrome in the Tokyo metropolitan area. Tohoku J Exp Med. 2009;219(1):11–16. doi: 10.1620/tjem.219.11. [DOI] [PubMed] [Google Scholar]
- 128.Krieger J, Meslier N, Lebrun T, et al. Accidents in obstructive sleep apnea patients treated with nasal continuous positive airway pressure: a prospective study. The Working Group ANTADIR, Paris and CRESGE, Lille, France. Association Nationale de Traitement a Domicile des Insuffisants Respiratoires. Chest. 1997;112(6):1561–1566. doi: 10.1378/chest.112.6.1561. [DOI] [PubMed] [Google Scholar]
- 129.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 130.Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46(1):142–151. doi: 10.1183/09031936.00064214. [DOI] [PubMed] [Google Scholar]
- 131.Shaw JE, Punjabi NM, Naughton MT, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016;194(4):486–492. doi: 10.1164/rccm.201511-2260OC. [DOI] [PubMed] [Google Scholar]
- 132.Yamamoto H, Akashiba T, Kosaka N, Ito D, Horie T. Long-term effects nasal continuous positive airway pressure on daytime sleepiness, mood and traffic accidents in patients with obstructive sleep apnoea. Respir Med. 2000;94(1):87–90. doi: 10.1053/rmed.1999.0698. [DOI] [PubMed] [Google Scholar]
- 133.Abe H, Takahashi M, Yaegashi H, et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25(1):63–69. doi: 10.1007/s00380-009-1164-z. [DOI] [PubMed] [Google Scholar]
- 134.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 135.Cai Q, Tan H, Singer J. Impact of positive airway pressure among obstructive sleep apnea patients. Am J Manag Care. 2012;18(6):e225–e233. [PubMed] [Google Scholar]
- 136.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 137.Capodanno D, Milazzo G, Cumbo M, et al. Positive airway pressure in patients with coronary artery disease and obstructive sleep apnea syndrome. J Cardiovasc Med (Hagerstown) 2014;15(5):402–406. doi: 10.2459/JCM.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 138.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(14):1310–1314. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 139.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 140.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2015;169(5):647–654.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 141.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 142.Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133(3):690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 143.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 144.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/ hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20(6):1511–1518. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 145.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 146.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 147.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 148.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ng SS, Liu EK, Ma RC, et al. Effects of CPAP therapy on visceral fat thickness, carotid intima-media thickness and adipokines in patients with obstructive sleep apnoea. Respirology. 2017;22(4):786–792. doi: 10.1111/resp.12963. [DOI] [PubMed] [Google Scholar]
- 150.Zhao YY, Wang R, Gleason KJ, et al. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep. 2017;40(4) doi: 10.1093/sleep/zsx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lewis EF, Wang R, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: a Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59–67. doi: 10.1016/j.ahj.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barcelo A, Morell-Garcia D, Salord N, et al. A randomized controlled trial: branched-chain amino acid levels and glucose metabolism in patients with obesity and sleep apnea. J Sleep Res. 2017;26(6):773–781. doi: 10.1111/jsr.12551. [DOI] [PubMed] [Google Scholar]
- 153.Salord N, Fortuna AM, Monasterio C, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep. 2016;39(1):35–41. doi: 10.5665/sleep.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 155.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 156.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131(5):1393–1399. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 157.Pepin JL, Tamisier R, Baguet JP, et al. Fixed-pressure CPAP versus auto-adjusting CPAP: comparison of efficacy on blood pressure in obstructive sleep apnoea, a randomised clinical trial. Thorax. 2016;71(8):726–733. doi: 10.1136/thoraxjnl-2015-207700. [DOI] [PubMed] [Google Scholar]
- 158.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 159.Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 160.Thunstrom E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193(3):310–320. doi: 10.1164/rccm.201505-0998OC. [DOI] [PubMed] [Google Scholar]
- 161.Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed. Chicago, IL: American Medical Association; 2015. [Google Scholar]
- 162.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Campbell AJ, Neill AM, Scott DAR. Clinical reproducibility of the Epworth Sleepiness Scale for patients with suspected sleep apnea. J Clin Sleep Med. 2018;14(5):791–795. doi: 10.5664/jcsm.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith S, Rossdale J, Serry Y, Sekaran A, Drakatos P, Steier J. Multiple dimensions of excessive daytime sleepiness. J Thorac Dis. 2018;10(Suppl 1):S170–S176. doi: 10.21037/jtd.2017.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Canadian Agency for Drugs and Technologies in Health. CPAP Treatment for Adults with Obstructive Sleep Apnea: Review of the Clinical and Cost-Effectiveness and Guidelines. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2013. [PubMed] [Google Scholar]
- 167.Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34(6):695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weatherly HL, Griffin SC, Mc Daid C, et al. An economic analysis of continuous positive airway pressure for the treatment of obstructive sleep apnea-hypopnea syndrome. Int J Technol Assess Health Care. 2009;25(1):26–34. doi: 10.1017/S0266462309090047. [DOI] [PubMed] [Google Scholar]
- 169.Gurubhagavatula I, Sullivan S, Meoli A, et al. Management of obstructive sleep apnea in commercial motor vehicle operators: recommendations of the AASM sleep and transportation safety awareness task force. J Clin Sleep Med. 2017;13(5):745–758. doi: 10.5664/jcsm.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35(5):617–625. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med. 2015;192(1):96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35(1):138–145. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 173.Ioachimescu OC, Anthony J, Jr, Constantin T, Ciavatta MM, McCarver K, Sweeney ME. VAMONOS (Veterans Affairs' Metabolism, Obstructed and Non-Obstructed Sleep) study: effects of CPAP therapy on glucose metabolism in patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13(3):455–466. doi: 10.5664/jcsm.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31(10):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 175.Cross MD, Vennelle M, Engleman HM, et al. Comparison of CPAP titration at home or the sleep laboratory in the sleep apnea hypopnea syndrome. Sleep. 2006;29(11):1451–1455. doi: 10.1093/sleep/29.11.1451. [DOI] [PubMed] [Google Scholar]
- 176.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183(9):1238–1244. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 177.McArdle N, Singh B, Murphy M, et al. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax. 2010;65(7):606–611. doi: 10.1136/thx.2009.116756. [DOI] [PubMed] [Google Scholar]
- 178.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157–166. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 179.Planes C, D'Ortho MP, Foucher A, et al. Efficacy and cost of home-initiated auto-nCPAP versus conventional nCPAP. Sleep. 2003;26(2):156–160. doi: 10.1093/sleep/26.2.156. [DOI] [PubMed] [Google Scholar]
- 180.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309(10):997–1004. doi: 10.1001/jama.2013.1823. [DOI] [PubMed] [Google Scholar]
- 182.Hui DS, Ng SS, Tam WWS. Home-based approach is non-inferior to hospital-based approach in managing patients with suspected obstructive sleep apnoea syndrome. Am J Respir Crit Care Med. 2018;197(9):1233–1234. doi: 10.1164/rccm.201711-2185LE. [DOI] [PubMed] [Google Scholar]
- 183.d'Ortho MP, Grillier-Lanoir V, Levy P, et al. Constant vs. automatic continuous positive airway pressure therapy: home evaluation. Chest. 2000;118(4):1010–1017. doi: 10.1378/chest.118.4.1010. [DOI] [PubMed] [Google Scholar]
- 184.Galetke W, Anduleit N, Richter K, Stieglitz S, Randerath WJ. Comparison of automatic and continuous positive airway pressure in a night-by-night analysis: a randomized, crossover study. Respiration. 2008;75(2):163–169. doi: 10.1159/000097767. [DOI] [PubMed] [Google Scholar]
- 185.Hudgel DW, Fung C. A long-term randomized, cross-over comparison of auto-titrating and standard nasal continuous airway pressure. Sleep. 2000;23(5):645–648. [PubMed] [Google Scholar]
- 186.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004;27(8):1512–1517. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 187.Hussain SF, Love L, Burt H, Fleetham JA. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea-hypopnea. Respir Med. 2004;98(4):330–333. doi: 10.1016/j.rmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 188.Kushida CA, Berry RB, Blau A, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34(8):1083–1092. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marrone O, Resta O, Salvaggio A, Giliberti T, Stefano A, Insalaco G. Preference for fixed or automatic CPAP in patients with obstructive sleep apnea syndrome. Sleep Med. 2004;5(3):247–251. doi: 10.1016/j.sleep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 190.Meurice JC, Cornette A, Philip-Joet F, et al. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med. 2007;8(7–8):695–703. doi: 10.1016/j.sleep.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 191.Nolan GM, Doherty LS, Mc Nicholas WT. Auto-adjusting versus fixed positive pressure therapy in mild to moderate obstructive sleep apnoea. Sleep. 2007;30(2):189–194. doi: 10.1093/sleep/30.2.189. [DOI] [PubMed] [Google Scholar]
- 192.Noseda A, Kempenaers C, Kerkhofs M, Braun S, Linkowski P, Jann E. Constant vs auto-continuous positive airway pressure in patients with sleep apnea hypopnea syndrome and a high variability in pressure requirement. Chest. 2004;126(1):31–37. doi: 10.1378/chest.126.1.31. [DOI] [PubMed] [Google Scholar]
- 193.Nussbaumer Y, Bloch KE, Genser T, Thurnheer R. Equivalence of autoadjusted and constant continuous positive airway pressure in home treatment of sleep apnea. Chest. 2006;129(3):638–643. doi: 10.1378/chest.129.3.638. [DOI] [PubMed] [Google Scholar]
- 194.Randerath WJ, Schraeder O, Galetke W, Feldmeyer F, Ruhle KH. Autoadjusting CPAP therapy based on impedance efficacy, compliance and acceptance. Am J Respir Crit Care Med. 2001;163(3 Pt 1):652–657. doi: 10.1164/ajrccm.163.3.2006168. [DOI] [PubMed] [Google Scholar]
- 195.Resta O, Carratu P, Depalo A, et al. Effects of fixed compared to automatic CPAP on sleep in obstructive sleep apnoea syndrome. Monaldi Arch Chest Dis. 2004;61(3):153–156. doi: 10.4081/monaldi.2004.694. [DOI] [PubMed] [Google Scholar]
- 196.Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short-term trial of two autoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med. 2003;168(12):1506–1511. doi: 10.1164/rccm.200304-542OC. [DOI] [PubMed] [Google Scholar]
- 197.Series F, Marc I. Importance of sleep stage- and body position-dependence of sleep apnoea in determining benefits to auto-CPAP therapy. Eur Respir J. 2001;18(1):170–175. doi: 10.1183/09031936.01.98103501. [DOI] [PubMed] [Google Scholar]
- 198.To KW, Chan WC, Choo KL, Lam WK, Wong KK, Hui DS. A randomized cross-over study of auto-continuous positive airway pressure versus fixed-continuous positive airway pressure in patients with obstructive sleep apnoea. Respirology. 2008;13(1):79–86. doi: 10.1111/j.1440-1843.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 199.Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS) Sleep. 2010;33(2):267–271. doi: 10.1093/sleep/33.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.West SD, Jones DR, Stradling JR. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax. 2006;61(3):226–231. doi: 10.1136/thx.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Meurice JC, Marc I, Series F. Efficacy of auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1996;153(2):794–798. doi: 10.1164/ajrccm.153.2.8564134. [DOI] [PubMed] [Google Scholar]
- 202.Berry RB, Sriram P. Auto-adjusting positive airway pressure treatment for sleep apnea diagnosed by home sleep testing. J Clin Sleep Med. 2014;10(12):1269–1275. doi: 10.5664/jcsm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fietze I, Glos M, Moebus I, Witt C, Penzel T, Baumann G. Automatic pressure titration with APAP is as effective as manual titration with CPAP in patients with obstructive sleep apnea. Respiration. 2007;74(3):279–286. doi: 10.1159/000100364. [DOI] [PubMed] [Google Scholar]
- 204.Konermann M, Sanner BM, Vyleta M, et al. Use of conventional and self-adjusting nasal continuous positive airway pressure for treatment of severe obstructive sleep apnea syndrome: a comparative study. Chest. 1998;113(3):714–718. doi: 10.1378/chest.113.3.714. [DOI] [PubMed] [Google Scholar]
- 205.Massie CA, McArdle N, Hart RW, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167(1):20–23. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 206.Teschler H, Wessendorf TE, Farhat AA, Konietzko N, Berthon-Jones M. Two months auto-adjusting versus conventional nCPAP for obstructive sleep apnoea syndrome. Eur Respir J. 2000;15(6):990–995. doi: 10.1034/j.1399-3003.2000.01503.x. [DOI] [PubMed] [Google Scholar]
- 207.Meurice JC, Ingrand P, Portier F, et al. A multicentre trial of education strategies at CPAP induction in the treatment of severe sleep apnoeahypopnoea syndrome. Sleep Med. 2007;8(1):37–42. doi: 10.1016/j.sleep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 208.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest. 2003;123(4):1112–1118. doi: 10.1378/chest.123.4.1112. [DOI] [PubMed] [Google Scholar]
- 209.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3(7):706–712. [PMC free article] [PubMed] [Google Scholar]
- 210.Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep. 2003;26(7):864–869. doi: 10.1093/sleep/26.7.864. [DOI] [PubMed] [Google Scholar]
- 211.Powell ED, Gay PC, Ojile JM, Litinski M, Malhotra A. A pilot study assessing adherence to auto-bilevel following a poor initial encounter with CPAP. J Clin Sleep Med. 2012;8(1):43–47. doi: 10.5664/jcsm.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(2 Pt 1):443–449. doi: 10.1164/ajrccm.151.2.7842204. [DOI] [PubMed] [Google Scholar]
- 213.Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients--a pilot study. Sleep Breath. 2012;16(3):773–779. doi: 10.1007/s11325-011-0574-1. [DOI] [PubMed] [Google Scholar]
- 214.Aloia MS, Smith K, Arnedt JT, et al. Brief behavioral therapies reduce early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav Sleep Med. 2007;5(2):89–104. doi: 10.1080/15402000701190549. [DOI] [PubMed] [Google Scholar]
- 215.Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2013;36(11):1655–1662. doi: 10.5665/sleep.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Deng T, Wang Y, Sun M, Chen B. Stage-matched intervention for adherence to CPAP in patients with obstructive sleep apnea: a randomized controlled trial. Sleep Breath. 2013;17(2):791–801. doi: 10.1007/s11325-012-0766-3. [DOI] [PubMed] [Google Scholar]
- 217.Lai AY, Fong DY, Lam JC, Weaver TE, Ip MS. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest. 2014;146(3):600–610. doi: 10.1378/chest.13-2228. [DOI] [PubMed] [Google Scholar]
- 218.Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna S. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med. 2013;9(6):543–550. doi: 10.5664/jcsm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30(5):635–640. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 220.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997;20(4):284–289. doi: 10.1093/sleep/20.4.284. [DOI] [PubMed] [Google Scholar]
- 221.Wang W, He G, Wang M, Liu L, Tang H. Effects of patient education and progressive muscle relaxation alone or combined on adherence to continuous positive airway pressure treatment in obstructive sleep apnea patients. Sleep Breath. 2012;16(4):1049–1057. doi: 10.1007/s11325-011-0600-3. [DOI] [PubMed] [Google Scholar]
- 222.Damjanovic D, Fluck A, Bremer H, Muller-Quernheim J, Idzko M, Sorichter S. Compliance in sleep apnoea therapy: influence of home care support and pressure mode. Eur Respir J. 2009;33(4):804–811. doi: 10.1183/09031936.00023408. [DOI] [PubMed] [Google Scholar]
- 223.Nilius G, Cottin U, Domanski U, et al. Effects of intensive outpatient training on the adherence of CPAP therapy for patients with OSA. Somnologie. 2012;16:251–256. [Google Scholar]
- 224.DeMolles DA, Sparrow D, Gottlieb DJ, Friedman R. A pilot trial of a telecommunications system in sleep apnea management. Med Care. 2004;42(8):764–769. doi: 10.1097/01.mlr.0000132353.99209.fe. [DOI] [PubMed] [Google Scholar]
- 225.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med. 1999;159(4 Pt 1):1096–1100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 226.Hui DS, Chan JK, Choy DK, et al. Effects of augmented continuous positive airway pressure education and support on compliance and outcome in a Chinese population. Chest. 2000;117(5):1410–1416. doi: 10.1378/chest.117.5.1410. [DOI] [PubMed] [Google Scholar]
- 227.Taylor Y, Eliasson A, Andrada T, Kristo D, Howard R. The role of telemedicine in CPAP compliance for patients with obstructive sleep apnea syndrome. Sleep Breath. 2006;10(3):132–138. doi: 10.1007/s11325-006-0059-9. [DOI] [PubMed] [Google Scholar]
- 228.Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012;35(4):477–481. doi: 10.5665/sleep.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guralnick AS, Balachandran JS, Szutenbach S, et al. Educational video to improve CPAP use in patients with obstructive sleep apnoea at risk for poor adherence: a randomised controlled trial. Thorax. 2017;72(12):1132–1139. doi: 10.1136/thoraxjnl-2017-210106. [DOI] [PubMed] [Google Scholar]
- 230.Sarac S, Afsar GC, Oruc O, Topcuoglu OB, Salturk C, Peker Y. Impact of patient education on compliance with positive airway pressure treatment in obstructive sleep apnea. Med Sci Monit. 2017;23:1792–1799. doi: 10.12659/MSM.902075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Falcone VA, Damiani MF, Quaranta VN, Capozzolo A, Resta O. Polysomnograph chart view by patients: a new educational strategy to improve CPAP adherence in sleep apnea therapy. Respir Care. 2014;59(2):193–198. doi: 10.4187/respcare.02491. [DOI] [PubMed] [Google Scholar]
- 232.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. J Med Internet Res. 2007;9(2):e14. doi: 10.2196/jmir.9.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hoet F, Libert W, Sanida C, Van den Broecke S, Bruyneel AV, Bruyneel M. Telemonitoring in continuous positive airway pressure-treated patients improves delay to first intervention and early compliance: a randomized trial. Sleep Med. 2017;39:77–83. doi: 10.1016/j.sleep.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 234.Hwang D, Chang JW, Benjafield AV, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The Tele-OSA randomized trial. Am J Respir Crit Care Med. 2018;197(1):117–126. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
- 235.Turino C, de Batlle J, Woehrle H, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01128-2016. [DOI] [PubMed] [Google Scholar]
- 236.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33(4):523–529. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep. 2013;36(2):229–236. doi: 10.5665/sleep.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C-Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep Breath. 2008;12(4):393–396. doi: 10.1007/s11325-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 239.Pepin JL, Muir JF, Gentina T, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest. 2009;136(2):490–497. doi: 10.1378/chest.08-2646. [DOI] [PubMed] [Google Scholar]
- 240.Leidag M, Hader C, Keller T, Meyer Y, Rasche K. Mask leakage in continuous positive airway pressure and C-Flex. J Physiol Pharmacol. 2008;59(Suppl 6):401–406. [PubMed] [Google Scholar]
- 241.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130(4):1018–1024. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 242.Ryan S, Garvey JF, Swan V, Behan R, McNicholas WT. Nasal pillows as an alternative interface in patients with obstructive sleep apnoea syndrome initiating continuous positive airway pressure therapy. J Sleep Res. 2011;20(2):367–373. doi: 10.1111/j.1365-2869.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- 243.Zhu X, Wimms AJ, Benjafield AV. Assessment of the performance of nasal pillows at high CPAP pressures. J Clin Sleep Med. 2013;9(9):873–877. doi: 10.5664/jcsm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax. 1998;53(4):290–292. doi: 10.1136/thx.53.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ebben MR, Narizhnaya M, Segal AZ, Barone D, Krieger AC. A randomised controlled trial on the effect of mask choice on residual respiratory events with continuous positive airway pressure treatment. Sleep Med. 2014;15(6):619–624. doi: 10.1016/j.sleep.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 246.Anderson FE, Kingshott RN, Taylor DR, Jones DR, Kline LR, Whyte KF. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep. 2003;26(6):721–726. doi: 10.1093/sleep/26.6.721. [DOI] [PubMed] [Google Scholar]
- 247.Khanna R, Kline LR. A prospective 8 week trial of nasal interfaces vs. a novel oral interface (Oracle) for treatment of obstructive sleep apnea hypopnea syndrome. Sleep Med. 2003;4(4):333–338. doi: 10.1016/s1389-9457(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 248.Rowland S, Aiyappan V, Hennessy C, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med. 2018;14(1):101–108. doi: 10.5664/jcsm.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One. 2013;8(5):e64382. doi: 10.1371/journal.pone.0064382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Beecroft J, Zanon S, Lukic D, Hanly P. Oral continuous positive airway pressure for sleep apnea: effectiveness, patient preference, and adherence. Chest. 2003;124(6):2200–2208. doi: 10.1378/chest.124.6.2200. [DOI] [PubMed] [Google Scholar]
- 251.Bachour A, Vitikainen P, Virkkula P, Maasilta P. CPAP interface: satisfaction and side effects. Sleep Breath. 2013;17(2):667–672. doi: 10.1007/s11325-012-0740-0. [DOI] [PubMed] [Google Scholar]
- 252.Kaminska M, Montpetit A, Mathieu A, Jobin V, Morisson F, Mayer P. Higher effective oronasal versus nasal continuous positive airway pressure in obstructive sleep apnea: effect of mandibular stabilization. Can Respir J. 2014;21(4):234–238. doi: 10.1155/2014/408073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Andrade RG, Madeiro F, Piccin VS, et al. Impact of acute changes in CPAP flow route in sleep apnea treatment. Chest. 2016;150(6):1194–1201. doi: 10.1016/j.chest.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 254.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep. 2011;34(7):951–955. doi: 10.5665/SLEEP.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ebben MR, Oyegbile T, Pollak CP. The efficacy of three different mask styles on a PAP titration night. Sleep Med. 2012;13(6):645–649. doi: 10.1016/j.sleep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 256.Deshpande S, Joosten S, Turton A, et al. Oronasal masks require a higher pressure than nasal and nasal pillow masks for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2016;12(9):1263–1268. doi: 10.5664/jcsm.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bakker JP, Neill AM, Campbell AJ. Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: a pilot investigation of pressure requirement, residual disease, and leak. Sleep Breath. 2012;16(3):709–716. doi: 10.1007/s11325-011-0564-3. [DOI] [PubMed] [Google Scholar]
- 258.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest. 2005;128(4):2151–2158. doi: 10.1378/chest.128.4.2151. [DOI] [PubMed] [Google Scholar]
- 259.Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116(2):403–408. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 260.Neill AM, Wai HS, Bannan SP, Beasley CR, Weatherall M, Campbell AJ. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. Eur Respir J. 2003;22(2):258–262. doi: 10.1183/09031936.03.00035603. [DOI] [PubMed] [Google Scholar]
- 261.Ryan S, Doherty LS, Nolan GM, McNicholas WT. Effects of heated humidification and topical steroids on compliance, nasal symptoms, and quality of life in patients with obstructive sleep apnea syndrome using nasal continuous positive airway pressure. J Clin Sleep Med. 2009;5(5):422–427. [PMC free article] [PubMed] [Google Scholar]
- 262.Salgado SM, Boleo-Tome JP, Canhao CM, et al. Impact of heated humidification with automatic positive airway pressure in obstructive sleep apnea therapy. J Bras Pneumol. 2008;34(9):690–694. doi: 10.1590/s1806-37132008000900009. [DOI] [PubMed] [Google Scholar]
- 263.Sommer JU, Kraus M, Birk R, Schultz JD, Hormann K, Stuck BA. Functional short- and long-term effects of nasal CPAP with and without humidification on the ciliary function of the nasal respiratory epithelium. Sleep Breath. 2014;18(1):85–93. doi: 10.1007/s11325-013-0853-0. [DOI] [PubMed] [Google Scholar]
- 264.Worsnop CJ, Miseski S, Rochford PD. Routine use of humidification with nasal continuous positive airway pressure. Intern Med J. 2010;40(9):650–656. doi: 10.1111/j.1445-5994.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 265.Ruhle KH, Franke KJ, Domanski U, Nilius G. Quality of life, compliance, sleep and nasopharyngeal side effects during CPAP therapy with and without controlled heated humidification. Sleep Breath. 2011;15(3):479–485. doi: 10.1007/s11325-010-0363-2. [DOI] [PubMed] [Google Scholar]
- 266.Soudorn C, Muntham D, Reutrakul S, Chirakalwasan N. Effect of heated humidification on CPAP therapy adherence in subjects with obstructive sleep apnea with nasopharyngeal symptoms. Respir Care. 2016;61(9):1151–1159. doi: 10.4187/respcare.04536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.