Abstract
N-Methyl D-Aspartate Receptors (NMDAR) are central mediators of glutamate actions underlying learning and memory processes including those required for extinction of fear and fear-related behaviors. Consistent with this view, in animal models, antagonists of NMDAR typically impair fear extinction, whereas partial agonists have facilitating effects. Promoting NMDAR function has thus been recognized as a promising strategy towards reduction of fear symptoms in patients suffering from anxiety disorders and post-traumatic disorder (PTSD). Nevertheless, application of these drugs in clinical trials, has proved of limited utility. Here we summarize recent advances in our knowledge of NMDAR pharmacology relevant for fear extinction, focusing on molecular, cellular, and circuit aspects of NMDAR function as they relate to fear extinction at the level of behavior and cognition. We also discuss how these advances from animal models might help to understand and overcome the limitations of existing approaches in human anxiety disorders and how novel, more specific, and personalized approaches might help advance future therapeutic strategies.
Keywords: NMDAR subunits, pharmacology, synaptic plasticity, network oscillations, fear extinction
Introduction
Fear responses to stimuli that reliably signal danger are rapidly acquired, however when danger is no longer present such responses typically subside, a phenomenon known as fear extinction. Behaviorally, fear extinction is very robust and essential for the cessation of ongoing fear responses rooted in past stressful experiences. Failure to extinguish fear is seen as a key contributing factor in anxiety and stress-related disorders, and prolonged exposures to fear-provoking but harmless situations have been an important component of cognitive behavioral therapies for anxiety and stress-related disorders (Abramowitz 2013; Craske et al. 2014; Pittig et al. 2016). Because existing psychotherapies are not effective in all patients, it has been suggested that superior outcomes might be achieved using cognitive enhancer drugs as adjunct treatment (Singewald et al. 2015; Wenk and Olton 1989). As key mediators of glutamate actions underlying learning, memory, and their modulation by stress (Graybeal et al. 2012), NMDAR are seen as promising cognitive enhancers and therapeutics for anxiety symptoms of affective disorders (Collingridge et al. 2013; Kaplan and Moore 2011). This promise has received ample support from research in animal models and from early human studies, as summarized and discussed in depth in several outstanding reviews (Amaral and Roesler 2008; Davis 2011; Myers and Davis 2007). However, several rodent reports cautioned that boosting NMDAR function with drugs such as D-cycloserine (DCS) is effective only in rats showing within-session extinction (Bolkan and Lattal 2014; Weber et al. 2007) and in fast extinguishers, who have elevated GluN1 but decreased GluN2A and 2B subunit levels in several brain areas (King et al. 2018). In line with the rodent findings reporting lack of DCS efficacy in slow extinguishers, meta analyses revealed that DCS efficacy in various patient populations was modest if any (McGuire et al. 2017; Ori et al. 2015). Here we summarize recent advances in the neurobiology and pharmacology of NMDAR, discuss challenges in using NMDAR-targeting drugs as treatment option, and propose future directions that might help to overcome existing limitations.
As Myers and Davis noted in a seminal review on the neurobiology of fear extinction (2011), this term is used with different meanings, which can be confusing. To clarify, we use the term fear extinction to refer to the reduction of fear-related behavior to stimuli that no longer predict danger (e.g. environmental context-shock or cue-shock). We use the term “extinction paradigm” to refer to the task that induces fear reduction (nonreinforced presentation of stimuli that used to signal danger), and the term “extinction learning” to refer to the learning processes that mediate reduction of behavioral and (in humans) affective components of fear.
Is there an Extinction Learning Process?
There is a general agreement that fear extinction is based on learning that cues and situations associated with past stressful events no longer predict such outcomes (Bouton 2004; Maren and Quirk 2004; Milad and Quirk 2002; Myers et al. 2011), however the nature of the learning process is not well understood. To date, the most prevalent views have revolved around simple classical conditioning (ranging from the formation of competing excitatory associations, inhibitory learning, and safety learning), which rely on lower limbic implicit memory systems, and processing of negative valence as a function of threat expectancy (Craske et al. 2018; Jovanovic et al. 2012; Weisman and Rodebaugh 2018). While these types of learning are likely to account for extinction of some fear-related responses and behaviors, a role for more complex, higher order associative learning processes, has been proposed for fear extinction of hippocampus-dependent learning (Grillon 2009; Otto et al. 2016). An additional process, alternative to associative learning has so far been overlooked. It is noteworthy that repetition of events across time does not only result in the formation of simple or higher order associations that could lead to fear extinction, but also tends to transform episodic memories into semantic memories (Greenberg and Verfaellie 2010). Re-living negative experiences is a key feature of episodic memories (Tulving et al., 1985), thus transformation to semantic memory could alleviate some of the key symptoms of stress-related anxiety disorders while leaving the memory of facts intact. Consistent with the ideas on higher cognitive function in fear extinction, episodic and semantic memory systems are predominantly hippocampal/temporal and prefrontal cortical (Wheeler and McMillan 2001), and are likely to engage different mechanisms in memory processing than implicit, subcortical memory systems. Interestingly, NMDAR antagonism has discrete effects on episodic and semantic memory recollection (Morgan and Curran 2006), suggesting that better understanding of the role of individual NMDAR subunits in episodic and semantic memory circuits (Squire 1992) or processing modes (Rajah and McIntosh 2005), could facilitate the development of novel therapeutic strategies.
A related issue, which is of particular relevance for translation from animal research to patients, is that most research so far has been performed under conditions when fear extinction is not impaired. We have recently observed that NMDAR antagonists have completely opposite effects on conditioned freezing behavior in mice trained to reliably predict the presence or absence of shock relative to mice exposed to partial reinforcement with random trials (Huh et al. 2009; Leaderbrand et al. 2014). In the latter case, uncertainty about shock occurrence is accompanied by resistance to fear extinction, which could be more relevant for patient populations. Paradoxically, NMDAR antagonists facilitate fear extinction in this model, probably due to co-activation of various MMDAR subunits, suggesting that in some populations attenuation rather than activation of NMDAR signaling may be beneficial.
Overall, it is likely that a multitude of learning mechanisms occurring at various neuroanatomical levels contribute to behavioral phenomenon of fear extinction. This point is important because we need cognitive enhancers for learning process(es) that are most likely to facilitate fear extinction in a particular individual (e.g., those that malfunction, rather than those that show compensatory activation).
Targeting the Relevant NMDAR Subunit
NMDA receptors are tetrameric protein complexes composed of a combination of two glycine-binding GluN1 subunits and two glutamate-binding GluN2 subunits (Chen and Wyllie 2006; Dingledine et al. 1999). Four different GluN2 subunits (GluN2A-D) have been identified with distinct expression and functional profiles (Erreger et al. 2004; Furukawa and Kurokawa 2007). A third subunit, GluN3, can also assemble with GluN1 subunits, however, GluN1/3 heteromeres do not bind glutamate because they only possess glycine binding sites (Perez-Otano et al. 2016). We will therefore focus on the subunits forming “conventional” glutamate-binding NMDAR.
The NMDAR complex has binding sites for numerous agonists, antagonists, and modulators that differentially affect NMDAR function. The main binding sites for endogenous ligands are: (1) glutamate (and NMDA) binding site on GluN2, (2) glycine modulatory site on GluN1, (3) polyamine modulatory site (both subunits), (4) voltage-independent Zn2+ binding site (high affinity for GluN2A), (5) voltage-dependent Mg2+ binding site (transmembrane pore formed by all subunits), (6) and neurosteroid binding sites (GluN2) (Fig. 1). It has long been known that there are also allosteric sites in the transmembrane pore formed by all subunits [binding sites for dissociative antagonists, e.g. MK-801, CPP, ketamine, phencyclidine (PCP)] and allosteric sites in the N-terminal domain of vGluN2 (binding sites for GluN2-specific antagonists, e.g. ifenprodil) (Fig. 2A) (Kemp and McKernan 2002).
Fig. 1. Schematic of the NMDAR complex.
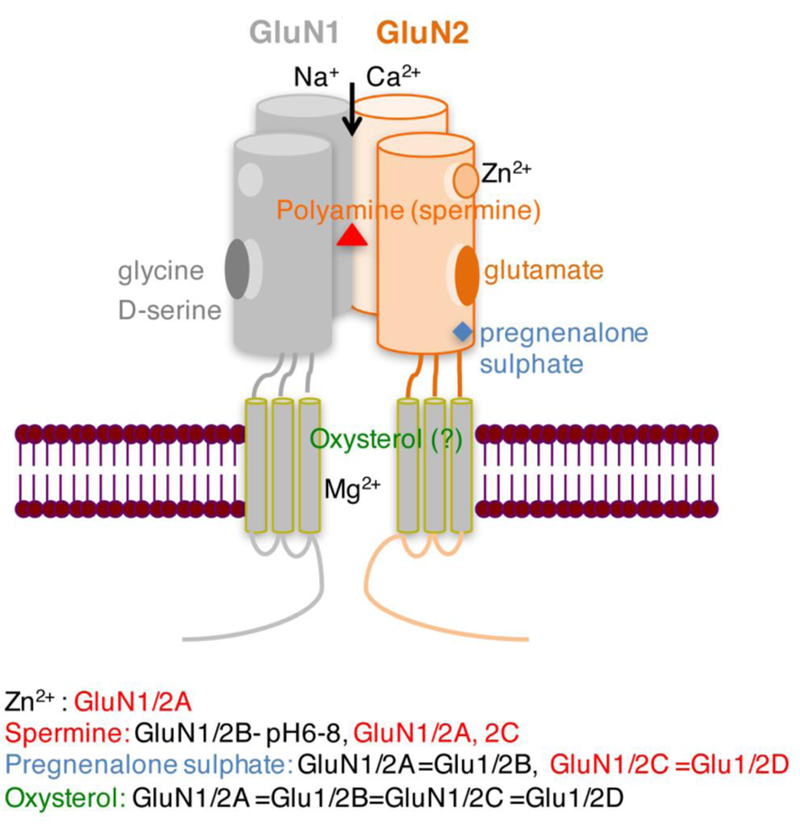
Binding sites for endogenous ligands and modulators in the N-terminal and ligand-binding domains are illustrated. Inhibitory effects on GluN are depicted in red, stimulatory in black. Different colors depict different binding sites.
Fig. 2. Antagonists and allosteric modulators of NMDAR.
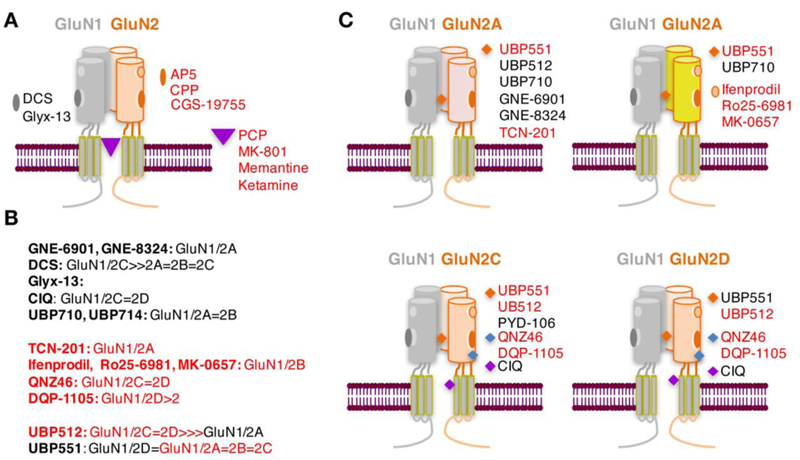
A, Non-selective competitive and dissociative NMDAR antagonists. B, Subunit selectivity of positive (black) and negative (red) NMDAR modulators. C, Binding sites for NMDAR subunit specific allosteric modulators. Summarized based on recently reviewed data (Hackos and Hanson 2017; Yao and Zhou 2017; Zhu and Paoletti 2015).
Most pharmacological approaches to manipulate NMDAR function, including their role in fear extinction, have so far utilized NMDAR antagonists acting as classical, competitive glutamate antagonists [such as AP5( APV) and CGS-10755] (Dravid et al. 2008), dissociative antagonists (mainly MK-801), preferential GluN2A antagonists (e.g., NVP-AAM007) and specific GluN2B antagonists (e.g., ifenprodil, Ro-256981). Consistent with the idea that fear extinction requires learning, which in turn depends on NMDAR activity, these NMDAR antagonists impair fear extinction across different rat and mouse models of cued, contextual, and trace fear conditioning paradigms, suggesting that GluN1/2A and 2B contribute to fear extinction (Furini et al. 2014; Myers and Davis 2007; Radulovic and Tronson 2010; Singewald et al. 2015). In line with the same reasoning, positive modulators of NMDAR, most commonly DCS, have a facilitating effect, resulting in an accelerated fear extinction (Singewald et al. 2015). This effect of DCS has traditionally been assigned to its partial agonist activity at the glycine binding site of the GluN1 subunit, however, recent work has shown that in addition to acting as a partial agonist at GluN1/2A, 2B and 2D receptor complexes, DCS also acts as a superagonist at GluN1/2C receptors (Jessen et al. 2017; Sheinin et al. 2001). Interestingly, CIQ ((3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone), a positive modulator of GluN1/2C and 2D complexes also enhances fear extinction after injection into the basolateral amygdala (BLA) (Ogden et al. 2014), further supporting a role of these subunits.
Over a decade our approaches to enhance fear extinction using controlled activation of NMDAR have been hampered by the limited availability of NMDAR subunit-specific positive modulators. However, there has been a recent burst in the development of such compounds, which has not only broadened the repertoire of subunit specific drugs (Fig. 2B,C), but also revealed novel mechanisms of NMDAR modulation. These mechanisms, range from dependence on endogenous ligand binding to dependence on receptor subunit composition, as recently analyzed in depth (Zhu and Paoletti, 2015; Hackos and Hanson, 2017; Yao and Zhou, 2017) and are depicted here to illustrate this recent progress (Fig. 2). Thus, an array of compounds displaying specificity for GluN2A-D subunits is now available for research on fear extinction. In addition to GluN2B, allosteric modulators of GluN2A and GluN2C seem promising based on scarce initial findings (Leaderbrand et al. 2014; Ogden et al. 2014). These compounds often have mixed agonist/antagonist properties with respect to various subunits and could help to tune NMDAR responses rather than exert simple effects. Research utilizing these new compounds is likely to reveal novel applications of drugs targeting specific NMDAR subunits in different phases of fear extinction as they relate to the underlying learning process, recency of the fear-inducing memories, and other factors.
Disrupting Specific NMDAR Signaling Complexes
An additional layer of complexity of NMDAR function is related to the tissue-, cell-, and synapse-specific coupling of different NMDAR subunits to signaling molecules (protein kinases, phosphatases, cytoskeletal regulators) of the postsynaptic density. These large multifunctional protein complexes are the actual mediators of long-term glutamatergic effects on neuronal plasticity, and are important spatial and temporal determinants of NMDAR activation and function. Many of these pathways play important roles in the conditioning and extinction of fear, as discussed in detail earlier (Tronson et al. 2011). In general, GluN1/2A and GluN1/2A/2B heteromers are predominantly found in the synapse, whereas GluN1/2B heterodimers are predominantly extrasynaptic (Thomas et al. 2006). This suggests that the former population primarily responds to signals arriving from presynaptic excitatory neurons, whereas the latter is more responsive to signals released from non-neural cells, such as astrocytes or microglia. The subcellular localization of NMDAR is important because activation of GluN1/2A and GluN1/2B heterodimers can have completely opposite effects on neuronal signal propagation, synaptic plasticity, and learning. For example, synaptic GluN1/2A activate extraxellular signal-regulated kinase (ERK) signaling, which is required for both conditioning and extinction of context fear (Fischer et al. 2007; Gao et al. 2011; Gao et al. 2010), whereas coactivation of synaptic and extrasynaptic NMDAR shuts off these pathways (Hardingham 2009; Leveille et al. 2008), and induces resistance to fear extinction (Huh et al. 2009; Leaderbrand et al. 2014).
Further specialization can be found at the level of the same NMDAR heterodimer forming multiprotein complexes with different signaling pathways (Fig. 3A). Interference with these complexes appears to affect learning and affective processes with higher specificity when compared to administration of receptor agonists or antagonists (Gao C 2013; Radulovic and Tronson 2010; Tronson et al. 2011). For example, disrupting the interaction between GluN2A and IQGAP1 specifically causes memory encoding impairments without affecting anxiety- or depression-like behavior (Gao et al. 2011). On the other hand, targeting the interaction between GluN2B and receptor for activated C kinase 1 (RACK1), which inhibits NR2B function (Yaka et al. 2002), enhances fear extinction without affecting depression-like behavior (Corcoran et al. 2015).
Fig. 3. Interaction of NMDAR with intracellular signaling pathways.
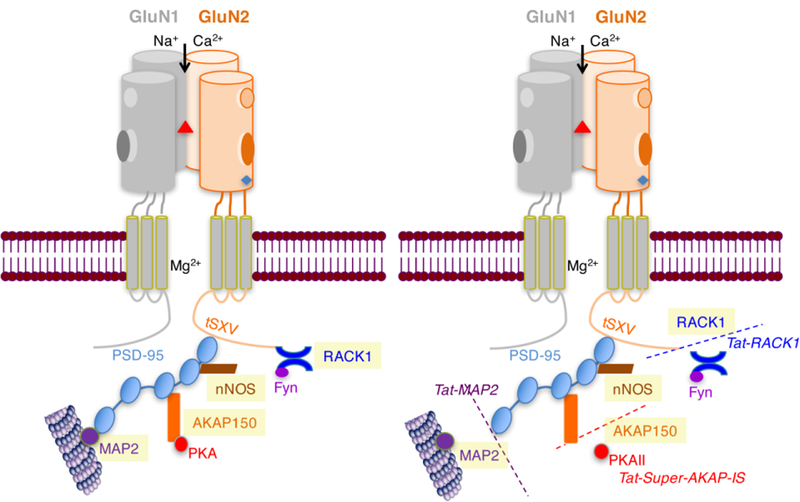
In the C-terminal intracytoplasmic tail, interaction with the postsynaptic density protein-95 (PSD-95) through the tSXV amino acid motif are depicted. Although both GluN1 and GluN2 contain this motif, PSD-95 has mainly been detected in association with GluN2 subunits (Bassand et al. 1999). Some of the main signaling pathways triggered through this interaction are RACK1 - Receptor of activated protein C kinase 1 (RACK1) (Yaka et al. 2002), A-kinase anchoring protein 150 (AKAP150) (Jurado et al. 2010), microtubule-associated protein 2 (MAP2) (Buddle et al. 2003), and neuronal nitric oxide synthase (nNOS) (Hillier et al. 1999). Disrupting specific interactions with interfering peptides can facilitate fear extinction without affecting depression-like behavior (Corcoran et al. 2015).
Site-specific disruption of NMDAR interactions with individual signaling proteins, such as RACK1, protein kinase A (PKA), and others is easily achieved by interference approaches with cell-permeable small peptides (Fig. 3B). These competing peptides contain short sequences of specific recognition sites between GluN subunits and their interacting partners, and their fusion to the transduction domain of the HIV-1 transactivating regulatory protein (Tat) makes them cell permeant, effective in vivo, and highly specific (Faruque et al. 2009; Peitz et al. 2002). Compared to small molecule drugs, peptides exhibit better efficacy, higher specificity, and lower toxicity. Compared to protein-based pharmaceuticals, peptides are relatively inexpensive and can easily be synthesized to exacting standards of purity and reproducibility between batches (Diaz-Eufracio et al. 2018; Otvos 2008). Overall, however, peptide-based approaches in mental health are lagging significantly behind the fields of immunology and oncology because they have been hampered by the short bioavailability of peptides and their limited passage through the blood-brain barrier. With innovative application strategies, in particular the use of nanotechnology, peptide-based drugs could emerge as first choice as contemporary therapeutics as well as probes for molecular imaging. The preclinical success of the tetrapeptide Glyx-13 supports this view (Vasilescu et al. 2017).
The Challenge of Cells and Circuits
There is increasing evidence that NMDAR are not only expressed on excitatory neurons but also on interneurons. In fact, all NMDAR subunits appear to be expressed in cortical interneurons (Hadzic et al. 2017). Depending on the interneuron type, activation of NMDAR can either enhance inhibition (e.g., if acting via parvalbumin-positive interneurons) or induce disinhibition (e.g., if acting via vasoactive intestinal peptide-positive interneurons) of excitatory neurons. It is hypothesized that the anxiolytic and antidepressant effects of ketamine might be due, at least in part, by inhibition of NMDAR expressed in prefrontal cortical interneurons (Zanos and Gould 2018). Whether a similar mechanism could contribute to fear extinction remains to be shown. In addition to excitatory and inhibitory neurons, NMDAR are expressed on other brain cell types. Particularly interesting is the high expression of GluN2C (Ravikrishnan et al. 2018) in astrocytes, a non-neural cell population that notably contributes to learning processes (Suzuki et al. 2011). Further characterization of the cell-specific actions of different NMDAR allosteric modulators will likely improve our ability to predict their behavioral effects in general and in fear extinction.
GluN2 receptor subunits differ significantly in their anatomical localization within fear and memory circuits (Fig. 4) (Monyer et al. 1994; Ozawa et al. 1998). Whereas GluN2A are most abundant in the neocortex, hippocampus, and cerebellum, GluN2B show similar hippocampal levels, but considerably higher levels in the amygdala and specific thalamic nuclei ((Fukaya et al. 2003; Thomas et al. 1996; Watanabe et al. 1993). The standard fear extinction circuit involves the basolateral amygdala (BLA), ventromedial prefrontal cortex (vmPFC) and the hippocampus (Maren and Quirk 2004; Sotres-Bayon et al. 2004). The lateral septum, bed nucleus of the stria terminalis also appear to play important roles (Davis et al. 1997; Zoicas et al. 2014). Thus, targeting GluN2A subunits appears to be a reasonable approach for hippocampal-cortical circuits whereas GluN2B would be expected to show higher efficacy at the level of subcortical nuclei. As for GluN2C and 2D, these subunits have been traditionally viewed as predominantly cerebellar and diencephalic, respectively (Buller et al. 1994; Watanabe et al. 1992). Therefore antagonists selective for these subunits were not expected to play major roles in fear regulation. However, recent findings suggest otherwise. The circuit mediating contextual fear extinction, a model of episodic-like memories in rodents, has recently been extended to the retrosplenial cortex (RSC) and thalamus (Corcoran et al. 2013; Kwapis et al. 2014; Marchand et al. 2014). These brain areas, which are functionally and neuroanatomically associated with the hippocampus, seem to have an unusually high content of GluN2C (Karavanova et al. 2007). While expression in the thalamus and hippocampus appears to be predominantly astrocytic, the cellular localization of GluN2C in RSC iremains to be clarified because newly developed reporter mouse lines show neuronal (Karavanova et al. 2007)and asctrocytic (Ravikrishnan et al. 2018) expression, respectively. In either case, GluN2C are likely to play a stronger regulatory role in fear extinction through the thalamic-hippocampal-RSC circuit (Fig. 4). Thus, the neuroanatomical source of fear extinction failure could be a key determinant of the efficacy of an NMDAR modulator.
Fig. 4. Distribution of NMDAR in hippocampal memory circuits involved in fear regulation.
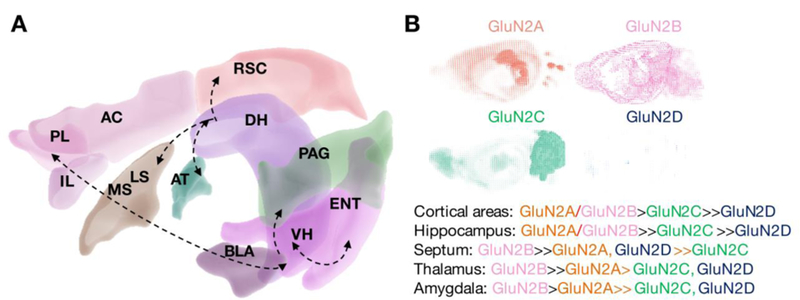
A, Brain areas involved in fear conditioning and extinction (based on (Kwapis et al. 2015; Laurent and Westbrook 2008; Maren and Quirk 2004; Sevenster et al. 2018) highlighting inter-regional interactions as well as differential connectivity of dorsal and ventral hippocampus to anterior cortices and amygdala. B, distribution of NMDAR subunits in these brain areas. Neuroanatomical and gene expression data are from Brain Explorer, Allen Brain Institute. Overview on GluN2 subunit distribution summarizes previous findings obtained with in situ hybridization analyses (Thomas et al. 1996; Watanabe et al. 1993). Abbreviations: ACC, anterior cingulate cortex; AT, anterior thalamic nuclei; BLA, basolateral amygdala; DH, dorsal hippocampus; ENT, entorhinal cortex; IL, infralimbic cortex; LS, lateral septum; MS, medial septum; VH, ventral hippocampus; PAG, periaquaeductal gray matter; PL, prelimbic cortex; RSC, retrosplenial cortex. Other areas include the bed nucleus of the stria terminalis, other amygdalar nuclei as well as the orbitofrontal cortex. These regions were excluded from the figure for reasons of clarity.
Taken together, the broad distribution of NMDAR subunits across cell types and circuits could be a significant complicating factor in predicting drug actions in fear extinction. Restoring the cellular and circuit dysbalance of excitation and inhibition could require NMDAR manipulations adjusted to regional NMDAR changes. Important advances in neuroimaging now enable us to determine the excitation/inhibition ratio as well as to visualize the open channel or glutamate binding sites of NMDAR in the human brain (Carletti et al. 2017; Legon et al. 2016; Tamborini et al. 2016). This could significantly facilitate the choice of therapeutics in the future.
NMDAR-Mediated Synaptic Plasticity and Network Activity Relevant for Fear Extinction
As noted above, NMDAR function is thought to be essential for synaptic plasticity underlying learning and memory (Volianskis et al. 2015), with tissue-specific and subunit-dependent effects (Shipton and Paulsen 2014). There is evidence that fear extinction is initiated by NMDAR-dependent bursting of vmPFC neurons (Burgos-Robles et al. 2007) and maintained by long-term depression (LTD) (Carretero-Guillen et al. 2015; Dalton et al. 2012). In addition to LTD, recent findings also implicate long-term potentiation (LTP) as a synaptic correlate of fear extinction (Table 1). Interestingly, GluN2-mediated memory enhancement relevant for fear extinction correlates with enhanced LTP in the 10–100 Hz range while requiring intact LTD capacity at the 1–5 Hz range (Jacobs et al. 2014). Overall, although subunit-, cell-, region-, and paradigm-specific effects of NMDAR are found, GluN manipulations affecting fear extinction at the behavioral level typically exert profound impact on synaptic plasticity as well.
Table 1.
Synaptic NMDAR effects related to fear extinction
| Receptor/drug | Species | Process | Effect | Circuit | Reference |
|---|---|---|---|---|---|
| GluN (DCS) | rat | LFS-primed LTP | Correlate of fear extinction |
hippocampus- mPFC |
(Inoue et al. 2013) |
| GluN1/2B (Glyx-13) |
rat | LTP-like metaplasticity |
Correlate of fear extinction- CFC |
PFC, hippocampus |
(Burgdorf et al. 2015a) |
| GluN1/2B (Ro25–6981) |
rat | LTD | Correlate of fear extinction- DFC |
Lateral amygdala |
(Dalton et al. 2012) |
| GluN | mouse | SC-CA1 LTP | Correlate of fear extinction- CFC |
Hippocampus | (Kim et al. 2011) |
| GluN1 | mouse | stimulation- evoked synaptic NMDAR- mediated currents |
Correlate of fear extinction- DFC |
IL | (Holmes et al. 2012) |
| Aquaporin-4 | mouse | Enhanced GluN2B- dependent CA3- CA1 LTD |
Correlate of fear extinction- DFC |
Local astrocytes |
(Wu et al. 2017) |
It is increasingly recognized that efficient learning processes also require coordinated intra- and inter-regional activity in memory-processing networks. Typically, studies in rodents show increased theta synchronization between areas of the fear circuit (such as amygdala, mPFC, hippocampus, RSC, anterior thalamus), which decreases during successful extinction. Consistent with this pattern, human studies with patients suffering from PTSD show increased inter-network synchronization, compared with controls, although in the gamma (30–80 Hz) and high-gamma range (80–150 Hz) (Dunkley et al. 2015). Thus, PTSD patients appear to be hypersynchronous in brain rhythms that are posited to be active in the formation and retrieval of autobiographical memories in humans (Canolty et al. 2006). Understanding the roles of NMDAR and the actions of NMDAR modulators on network oscillations can therefore be of high relevance for developing effective therapies.
One of the most consistent effects of NMDAR antagonists on network activity is their ability to significantly increase the power of gamma oscillations (Table 2). Acute administration of MK-801 alters the power, peak frequency, dynamics and periodicity of hippocampal neuronal oscillations in the alpha and gamma frequency bands (Lemercier et al. 2017). Strong increase in gamma power is induced by nonselective NMDAR antagonists and by antagonism of GluN2A subunit containing NMDAR. In contrast, selective blockade of GluN2B, 2C, or 2D subunit-containing receptors had minor effects (Kocsis 2012). Similarly, entrainment of BLA principal neurons in an oscillatory generation of inhibitory activity depends primarily on activation of GluN2A-NMDAR (Aroniadou-Anderjaska et al. 2018). Mice lacking the NR1 NMDAR subunit only in fast-spiking, parvalbumin-containing interneurons exhibit reduced sensitivity to the effects of NMDAR antagonists (Carlen et al. 2012), suggesting that the effects of NMDAR antagonists on local oscillations are mediated, at least in part, by inhibitory neurons. In addition to inducing gamma oscillations, MK-801 also abolishes theta synchronization in the hippocampal-RSC-ACC circuit during recall of context-fear memory (Miller et al. 2017). However, this effect seems to be more relevant for impairments of memory retrieval rather than extinction, because it is only transient while the drug is active and fear recovers afterwards. Whether these effects can be translated to PTSD patients, and how NMDAR agonists and modulators affect network synchronization warrants much more research in this area both in experimental animals and in human patient populations. Nevertheless, this is an important future research direction because analyses of oscillatory activity could be potential indicators of drug actions and successful treatments.
Table 2.
Network effects related to fear extinction
| Network activity |
Species | Effect | Paradigm | CS | Circuit | Reference |
|---|---|---|---|---|---|---|
| Theta coupling |
rat | increased | Fear conditioning: retrieval |
cue context |
LA, hippocampus |
(Seidenbecher et al. 2003) |
| Theta (4 Hz) coupling |
rat | increased | Fear conditioning: retrieval |
cue | Amygdala PFC |
(Karalis et al. 2016) |
| Theta coupling |
mouse | increased | Fear conditioning: retrieval |
context | RSC, hippocampus, ACC, AT |
(Miller et al. 2017) |
| Theta coupling |
mouse | increased | Fear conditioning: retrieval |
cue | lateral amygdala- mPFC and CA1-mPFC |
(Lesting et al. 2011) |
| Gamma to theta coupling |
mouse | increased | Fear conditioning: retrieval |
cue | BLA | (Stujenske et al. 2014) |
| Theta and gamma coupling |
mouse | decreased | Fear extinction |
context | RSC, AT | (Corcoran et al. 2016) |
| Theta coupling |
mouse | decreased | Fear extinction |
cue | Lateral amygdala- mPFC and CA1-mPFC |
(Lesting et al. 2011) |
| gamma oscillations |
mouse | Increased spontaneous recovery |
Fear extinction |
cue | amygdala | (Courtin et al. 2014) |
| Gamma to theta coupling |
mouse | increased | Fear extinction |
cue | BLA and mPFC |
(Stujenske et al. 2014) |
Challenges and future directions
It is obvious that targeting NMDAR complexes has the potential to translate into development of effective therapies for anxiety disorders. This might not always be due to primary NMDAR dysfunction, but also secondary changes of NMDAR expression and function caused by other factors that adversely affect fear extinction, such as various genetic abnormalities (Table 3). However, there is much more to be researched before we have developed a comprehensive understanding of NMDAR function in physiological and pathophysiological fear regulation.
Table 3.
Genetic abnormalities associated with GluN dysfunction and impaired extinction
| Gene/drug | Modification | Species | GluN deficit | Circuit | Effect | Reference |
|---|---|---|---|---|---|---|
| serine racemase |
single nucleotide polymorphism rs4523957 |
human | Predicted reduced D- serine levels |
n/a | Associated with PTSD |
(Balu et al. 2018) |
| BDNF | Val66Met Polymorphism |
mouse | Suppressed GluN transmission |
CEm amygdala |
Impaired fear extinction |
(Galvin et al. 2015) |
| 129S1/SvImJ (S1) strain |
Inbred strain | mouse | Possibly Suppressed GluN transmission (DCS reverses deficit) |
n/a | Resistance to fear extinction |
(Whittle et al. 2013) |
| PI3K knockout |
Transgene | mouse | Impaired GluN transmission |
Hippocampus | Impaired fear extinction |
(Kim et al. 2011) |
| GRIN1 knockout |
Transgene | mouse | GluN1 deficiency in PFC excitatory neurons |
PFC | Enhanced fear extinction |
(Vieira et al. 2015) |
| GRIN1A | Transgene | mouse | CA1- specific knockout |
Hippocampus | Enhances fear renewal |
(Hirsch et al. 2015) |
| Aquaporin-4 | Knockout | mouse | Enhanced GluN2B transmission |
Astrocytes | Enhanced fear extinction |
(Wu et al. 2017) |
Abnormal extinction is only one of many processes that could account for fear and anxiety symptomatology. Unfortunately, NMDAR often play opposite roles in some of these processes. For example, feelings of anxiety are believed to result from increased glutamatergic neurotransmission in emotion-processing brain regions (Martin et al. 2010). Therefore, drugs such as benzodiazepines, which reduce excitation by increasing inhibition, have been used over decades as anxiolytics. It is possible that paradoxical enhancements of fear extinction with NMDAR antagonists and inhibitors of glutamate release, such as riluzole (Sugiyama et al. 2018), could be due to a similar mechanism. However, as discussed above, enhanced glutamatergic activation is also required for extinction learning, thus post-extinction NMDAR antagonism in most cases impairs fear extinction. Ideally, one would need to reduce anxiety symptoms while promoting extinction, so this is obviously a challenge. Also, although ketamine and NMDAR open channel antagonists might have strong anxiolytic actions, one needs to be cautious because these dissociative drugs could enhance the recall of state-dependent stress-related memories (Clifton et al. 2018) and thus worsen the symptoms of stress-related disorders. One approach to overcome this challenge could lie in the better characterization of the roles of individual NMDAR signaling complexes because one can achieve much higher functional and behavioral specificity by interfering at this level of NMDAR signaling.
One of the important future goals of both animal and human research will be to improve our understanding of the dynamics of NMDAR complexes in vivo. All of the NMDAR features discussed above are activity- and experience-dependent (Kopp et al. 2007; Rebola et al. 2010) and also change in response to psychotropic drugs such as fluoxetine and citalopram (Ampuero et al. 2010; Burghardt et al. 2013).Therefore, the composition, localization, and function of NMDAR complexes is likely to differ during physiological relative to pathophysiological conditions. Animal studies could advance the field by extending the focus on the role of NMDAR complexes in extinction-resistant models in addition to studying physiological extinction processes (what is most commonly the case). This can be researched in more depth at the behavioral level, with genetic and environmental rodent models, and at the cellular level, by better understanding of the role of NMDAR in the firing of extinction susceptible and extinction-resistant cells [e.g. in the amygdala (An et al. 2012) or periaquaeductal gray matter (Fragale et al. 2016)]. Human studies, on the other hand, will soon be able to take advantage of novel radiolabeled ligands targeting the open channel or glutamate binding sites of NMDAR (Carletti et al. 2017; Tamborini et al. 2016). Thereby, the application of NMDAR subunit modulators in patient populations will be much better informed and adjusted to individual needs. Advances in designing subunit-specific ligands could also facilitate research related to the role of episodic to semantic memory transfer in affected individuals.
We also need more research focusing on the potential role of NMDAR in alpha oscillations. It was only recently shown that the dissociative antagonists MK-801, PCP, and ketamine significantly reduce of the power of hippocampal alpha oscillations (Kealy et al. 2017). More research in this area could be highly relevant because associations between aberrant alpha patterns and affective disorders, including PTSD, have been found both in response to specific cognitive and emotional tasks and during a resting state (Eidelman-Rothman et al. 2016).
Lastly, it seems clear that NMDAR modulators will not always be effective in fear extinction. There is consistent evidence that manipulations of NMDAR become less effective or ineffective with changes of the extinction context (Langton and Richardson 2009). We therefore need to improve our understanding of NMDAR-independent processes and other neuronal mediators (involving or not involving glutamate) driving the fear extinction circuits.
Acknowledgements.
The authors acknowledge that this work was supported by NIH grants MH108837 and MH078064 to J.R. and by the National Natural Science Foundation of China grant 81471101 to C.G.
Footnotes
Compliance with ethical standards
N/A
Conflict of interest. The authors declare that they have no competing interests.
References
- Abramowitz JS (2013) The practice of exposure therapy: relevance of cognitive-behavioral theory and extinction theory. Behav Ther 44: 548–58. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Roesler R (2008) Targeting the NMDA receptor for fear-related disorders. Recent Pat CNS Drug Discov 3: 166–78. [DOI] [PubMed] [Google Scholar]
- Ampuero E, Rubio FJ, Falcon R, Sandoval M, Diaz-Veliz G, Gonzalez RE, Earle N, Dagnino-Subiabre A, Aboitiz F, Orrego F, Wyneken U (2010) Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience 169: 98–108. [DOI] [PubMed] [Google Scholar]
- An B, Hong I, Choi S (2012) Long-term neural correlates of reversible fear learning in the lateral amygdala. J Neurosci 32: 16845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Braga MFM (2018) Oscillatory Synchronous Inhibition in the Basolateral Amygdala and its Primary Dependence on NR2A-containing NMDA Receptors. Neuroscience 373: 145–158. [DOI] [PubMed] [Google Scholar]
- Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M (1999) Differential interaction of the tSXV motifs of the NR1 and NR2A NMDA receptor subunits with PSD-95 and SAP97. Eur J Neurosci 11: 2031–43. [DOI] [PubMed] [Google Scholar]
- Bolkan SS, Lattal KM (2014) Opposing effects of D-cycloserine on fear despite a common extinction duration: interactions between brain regions and behavior. Neurobiol Learn Mem 113: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2004) Context and behavioral processes in extinction. Learning and Memory 11: 485–94. [DOI] [PubMed] [Google Scholar]
- Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM (2003) Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res 978: 38–50. [DOI] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT (1994) The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci 14: 5471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE (2013) Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry 73: 1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53: 871–80. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313: 1626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH (2012) A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 17: 537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti R, Tacconi S, Mugnaini M, Gerrard P (2017) Receptor distribution studies. Curr Opin Pharmacol 35: 94–100. [DOI] [PubMed] [Google Scholar]
- Carretero-Guillen A, Pacheco-Calderon R, Delgado-Garcia JM, Gruart A (2015) Involvement of hippocampal inputs and intrinsic circuit in the acquisition of context and cues during classical conditioning in behaving rabbits. Cereb Cortex 25: 1278–89. [DOI] [PubMed] [Google Scholar]
- Chen PE, Wyllie DJ (2006) Pharmacological insights obtained from structure-function studies of ionotropic glutamate receptors. Br J Pharmacol 147: 839–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton NE, Thomas KL, Hall J (2018) The effect of ketamine on the consolidation and extinction of contextual fear memory. J Psychopharmacol 32: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, Monaghan DT, Bortolotto ZA, Molnar E, Lodge D, Jane DE (2013) The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Jovasevic V, Guedea AL, Kassam F, Radulovic J (2015) Regulation of fear extinction versus other affective behaviors by discrete cortical scaffolding complexes associated with NR2B and PKA signaling. Transl Psychiatry 5: e657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Radulovic J (2013) Extinction of remotely acquired fear depends on an inhibitory NR2B/PKA pathway in the retrosplenial cortex. J Neurosci 33: 19492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, Vervliet B (2018) State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B (2014) Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 58: 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG (2012) NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology 62: 797–806. [DOI] [PubMed] [Google Scholar]
- Davis M (2011) NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13: 463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y (1997) Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci 352: 1675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Eufracio BI, Naveja JJ, Medina-Franco JL (2018) Protein-Protein Interaction Modulators for Epigenetic Therapies. Adv Protein Chem Struct Biol 110: 65–84. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–61. [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF (2008) Activation of recombinant NR1/NR2C NMDA receptors. J Physiol 586: 4425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Doesburg SM, Jetly R, Sedge PA, Pang EW, Taylor MJ (2015) Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res 234: 172–81. [DOI] [PubMed] [Google Scholar]
- Eidelman-Rothman M, Levy J, Feldman R (2016) Alpha oscillations and their impairment in affective and post-traumatic stress disorders. Neurosci Biobehav Rev 68: 794–815. [DOI] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF (2004) Glutamate receptor gating. Crit Rev Neurobiol 16: 187–224. [DOI] [PubMed] [Google Scholar]
- Faruque OM, Le-Nguyen D, Lajoix AD, Vives E, Petit P, Bataille D, Hani EH (2009) Cell-permeable peptide-based disruption of endogenous PKA-AKAP complexes: a tool for studying the molecular roles of AKAP-mediated PKA subcellular anchoring. Am J Physiol Cell Physiol 296: C306–16. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J (2007) Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem 87: 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Khariv V, Gregor DM, Smith IM, Jiao X, Elkabes S, Servatius RJ, Pang KC, Beck KD (2016) Dysfunction in amygdala-prefrontal plasticity and extinction-resistant avoidance: A model for anxiety disorder vulnerability. Exp Neurol 275 Pt 1: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M (2003) Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A 100: 4855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini C, Myskiw J, Izquierdo I (2014) The learning of fear extinction. Neuroscience and biobehavioral reviews 47: 670–83. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Kurokawa J (2007) Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias. Pharmacology and Therapeutics 115: 106–15. [DOI] [PubMed] [Google Scholar]
- Gao C, Frausto SF, Guedea AL, Tronson NC, Jovasevic V, Leaderbrand K, Corcoran KA, Guzman YF, Swanson GT, Radulovic J (2011) IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J Neurosci 31: 8533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J (2010) Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus 20: 1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CTN, Radulovic J (2013) Modulation of behavior by scaffolding proteins of the postsynaptic density. Neurobiology of Learning and memory in press [DOI] [PMC free article] [PubMed]
- Graybeal C, Kiselycznyk C, Holmes A (2012) Stress-induced deficits in cognition and emotionality: a role of glutamate. Curr Top Behav Neurosci 12: 189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Verfaellie M (2010) Interdependence of episodic and semantic memory: evidence from neuropsychology. J Int Neuropsychol Soc 16: 748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C (2009) D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biological Psychiatry 66: 636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackos DH, Hanson JE (2017) Diverse modes of NMDA receptor positive allosteric modulation: Mechanisms and consequences. Neuropharmacology 112: 34–45. [DOI] [PubMed] [Google Scholar]
- Hadzic M, Jack A, Wahle P (2017) Ionotropic glutamate receptors: Which ones, when, and where in the mammalian neocortex. J Comp Neurol 525: 976–1033. [DOI] [PubMed] [Google Scholar]
- Hardingham GE (2009) Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans 37: 1147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA (1999) Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 284: 812–5. [PubMed] [Google Scholar]
- Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J (2009) Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learn Mem 16: 273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Cui Z, Feng R, Wang H, Wang D, Tsien JZ (2014) Molecular and genetic determinants of the NMDA receptor for superior learning and memory functions. PLoS One 9: e111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen M, Frederiksen K, Yi F, Clausen RP, Hansen KB, Brauner-Osborne H, Kilburn P, Damholt A (2017) Identification of AICP as a GluN2C-Selective N-Methyl-d-Aspartate Receptor Superagonist at the GluN1 Glycine Site. Mol Pharmacol 92: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M (2012) Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Biou V, Malenka RC (2010) A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat Neurosci 13: 1053–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA (2011) The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav 99: 217–28. [DOI] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A (2007) Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Molecular and Cellular Neurosciences 34: 468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Commins S, Lowry JP (2017) The effect of NMDA-R antagonism on simultaneously acquired local field potentials and tissue oxygen levels in the brains of freely-moving rats. Neuropharmacology 116: 343–350. [DOI] [PubMed] [Google Scholar]
- Kemp JA, McKernan RM (2002) NMDA receptor pathways as drug targets. Nat Neurosci 5 Suppl: 1039–42. [DOI] [PubMed] [Google Scholar]
- King G, Graham B, Richardson R (2018) T14. Individual Differences in Extinction and Relapse: Who, Why, and What Can We Do? Biological Psychiatry 83 (Supplement): S134. [Google Scholar]
- Kocsis B (2012) Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry 71: 987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Luthi A (2007) Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology 53: 1–9. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ (2014) Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol Learn Mem 113: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ (2015) The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory 123: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton JM, Richardson R (2009) The role of context in the re-extinction of learned fear. Neurobiol Learn Mem 92: 496–503. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF (2008) Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learning and Memory 15: 657–66. [DOI] [PubMed] [Google Scholar]
- Leaderbrand K, Corcoran KA, Radulovic J (2014) Co-activation of NR2A and NR2B subunits induces resistance to fear extinction. Neurobiol Learn Mem 113: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Punzell S, Dowlati E, Adams SE, Stiles AB, Moran RJ (2016) Altered Prefrontal Excitation/Inhibition Balance and Prefrontal Output: Markers of Aging in Human Memory Networks. Cereb Cortex 26: 4315–4326. [DOI] [PubMed] [Google Scholar]
- Lemercier CE, Holman C, Gerevich Z (2017) Aberrant alpha and gamma oscillations ex vivo after single application of the NMDA receptor antagonist MK-801. Schizophr Res 188: 118–124. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A (2008) Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J 22: 4258–71. [DOI] [PubMed] [Google Scholar]
- Marchand A, Faugere A, Coutureau E, Wolff M (2014) A role for anterior thalamic nuclei in contextual fear memory. Brain Struct Funct 219: 1575–86. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nature reviews Neuroscience 5: 844–52. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB (2010) The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin Lab Med 30: 865–91. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Wu MS, Piacentini J, McCracken JT, Storch EA (2017) A Meta-Analysis of D-Cycloserine in Exposure-Based Treatment: Moderators of Treatment Efficacy, Response, and Diagnostic Remission. J Clin Psychiatry 78: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–4. [DOI] [PubMed] [Google Scholar]
- Miller AMP, Frick BJ, Smith DM, Radulovic J, Corcoran KA (2017) Network oscillatory activity driven by context memory processing is differently regulated by glutamatergic and cholinergic neurotransmission. Neurobiol Learn Mem 145: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–40. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV (2006) Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 188: 408–24. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA Jr., Davis M (2011) Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36: 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M (2007) Mechanisms of fear extinction. Mol Psychiatry 12: 120–50. [DOI] [PubMed] [Google Scholar]
- Ogden KK, Khatri A, Traynelis SF, Heldt SA (2014) Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology 39: 625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori R, Amos T, Bergman H, Soares-Weiser K, Ipser JC, Stein DJ (2015) Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. Cochrane Database Syst Rev: CD007803. [DOI] [PMC free article] [PubMed]
- Otto MW, Kredlow MA, Smits JAJ, Hofmann SG, Tolin DF, de Kleine RA, van Minnen A, Evins AE, Pollack MH (2016) Enhancement of Psychosocial Treatment With D-Cycloserine: Models, Moderators, and Future Directions. Biol Psychiatry 80: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L Jr. (2008) Peptide-based drug design: here and now. Methods Mol Biol 494: 1–8. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K (1998) Glutamate receptors in the mammalian central nervous system. Prog Neurobiol 54: 581–618. [DOI] [PubMed] [Google Scholar]
- Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F (2002) Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc Natl Acad Sci U S A 99: 4489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Larsen RS, Wesseling JF (2016) Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat Rev Neurosci 17: 623–35. [DOI] [PubMed] [Google Scholar]
- Pittig A, van den Berg L, Vervliet B (2016) The key role of extinction learning in anxiety disorders: behavioral strategies to enhance exposure-based treatments. Curr Opin Psychiatry 29: 39–47. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC (2010) Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 21: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR (2005) Overlap in the functional neural systems involved in semantic and episodic memory retrieval. Journal of cognitive neuroscience 17: 470–82. [DOI] [PubMed] [Google Scholar]
- Ravikrishnan A, Gandhi PJ, Shelkar GP, Liu J, Pavuluri R, Dravid SM (2018) Region-specific Expression of NMDA Receptor GluN2C Subunit in Parvalbumin-Positive Neurons and Astrocytes: Analysis of GluN2C Expression using a Novel Reporter Model. Neuroscience 380: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Srikumar BN, Mulle C (2010) Activity-dependent synaptic plasticity of NMDA receptors. J Physiol 588: 93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Visser RM, D’Hooge R (2018) A translational perspective on neural circuits of fear extinction: Current promises and challenges. Neurobiol Learn Mem 155: 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M (2001) Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 41: 151–8. [DOI] [PubMed] [Google Scholar]
- Shipton OA, Paulsen O (2014) GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci 369: 20130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ (2015) Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149: 150–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE (2004) Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 11: 525–35. [DOI] [PubMed] [Google Scholar]
- Squire LR (1992) Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. Journal of cognitive neuroscience 4: 232–43. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Yamada M, Saitoh A, Oka JI, Yamada M (2018) Administration of riluzole to the basolateral amygdala facilitates fear extinction in rats. Behav Brain Res 336: 8–14. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144: 810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborini L, Chen Y, Foss CA, Pinto A, Horti AG, Traynelis SF, De Micheli C, Mease RC, Hansen KB, Conti P, Pomper MG (2016) Development of Radiolabeled Ligands Targeting the Glutamate Binding Site of the N-Methyl-d-aspartate Receptor as Potential Imaging Agents for Brain. J Med Chem 59: 11110–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL (2006) Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95: 1727–34. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Davis S, Hunt SP, Laroche S (1996) Alterations in the expression of specific glutamate receptor subunits following hippocampal LTP in vivo. Learn Mem 3: 197–208. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J (2011) Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci: In press [DOI] [PMC free article] [PubMed]
- Vasilescu AN, Schweinfurth N, Borgwardt S, Gass P, Lang UE, Inta D, Eckart S (2017) Modulation of the activity of N-methyl-d-aspartate receptors as a novel treatment option for depression: current clinical evidence and therapeutic potential of rapastinel (GLYX-13). Neuropsychiatr Dis Treat 13: 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL (2015) Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res 1621: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M (1992) Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3: 1138–40. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M (1993) Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol 338: 377–90. [DOI] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R (2007) Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem 87: 476–82. [DOI] [PubMed] [Google Scholar]
- Weisman JS, Rodebaugh TL (2018) Exposure therapy augmentation: A review and extension of techniques informed by an inhibitory learning approach. Clin Psychol Rev 59: 41–51. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Olton DS (1989) Cognitive enhancers: potential strategies and experimental results. Prog Neuropsychopharmacol Biol Psychiatry 13 Suppl: S117–39. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, McMillan CT (2001) Focal retrograde amnesia and the episodic-semantic distinction. Cogn Affect Behav Neurosci 1: 22–36. [DOI] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D (2002) NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A 99: 5710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Zhou Q (2017) Enhancing NMDA Receptor Function: Recent Progress on Allosteric Modulators. Neural Plast 2017: 2875904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Paoletti P (2015) Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol 20: 14–23. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID (2014) Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology 39: 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


